Abstract
Herpes simplex virus (HSV) nucleocapsids acquire an envelope by budding through the inner nuclear membrane, but it is uncertain whether this envelope is retained during virus maturation and egress or whether mature progeny virions are derived by deenvelopment at the outer nuclear membrane followed by reenvelopment in a cytoplasmic compartment. To resolve this issue, we used immunogold electron microscopy to examine the distribution of glycoprotein D (gD) in cells infected with HSV-1 encoding a wild-type gD or a gD which is retrieved to the endoplasmic reticulum (ER). In cells infected with wild-type HSV-1, extracellular virions and virions in the perinuclear space bound approximately equal amounts of gD antibody. In cells infected with HSV-1 encoding an ER-retrieved gD, the inner and outer nuclear membranes were heavily gold labeled, as were perinuclear enveloped virions. Extracellular virions exhibited very little gold decoration (10- to 30-fold less than perinuclear virions). We conclude that the envelope of perinuclear virions must be lost during maturation and egress and that mature progeny virions must acquire an envelope from a post-ER cytoplasmic compartment. We noted also that gD appears to be excluded from the plasma membrane in cells infected with wild-type virus.
Herpesvirus nucleocapsids assemble in the nuclei of infected cells and acquire an envelope by budding through the inner nuclear membrane, but the subsequent route of virus maturation and egress has been a matter of controversy. Over 30 years ago, Stackpole (19) proposed that enveloped virions in the perinuclear space fused with the outer nuclear membrane, releasing into the cytoplasm naked nucleocapsids which acquired a final envelope by budding into a late cytoplasmic compartment. The observation that infectious herpes simplex virions accumulated within cells in the absence of a functional Golgi apparatus (11) implied that virions in the perinuclear space were infectious and suggested that the Golgi apparatus was required merely for egress of these virions. This “single envelopment” pathway, in which perinuclear enveloped virions are transported to the cell surface via the secretory pathway and the envelope glycoproteins are processed in situ, has the virtue of simplicity and became widely accepted as the route of egress of herpes simplex virus (HSV) (e.g., see reference 17). Studies of other alphaherpesviruses, notably varicella-zoster virus and pseudorabies virus, have, however, supported the view that the final envelope is acquired in a cytoplasmic compartment, thus favoring the “two-step envelopment” route of egress (6, 8, 12, 13, 22, 24). Indeed, several observations are inconsistent with the view that HSV acquires its final envelope from the nuclear membrane: the phospholipid composition of secreted virions is different from that of the nuclear membrane (21); naked nucleocapsids, not enveloped virions, are observed in axons during virus egress (10, 15, 16); and a major tegument component, VP22, is observed apparently exclusively in the cytoplasm of live virus-infected cells (4). A detailed analysis of the evidence for and against the alternative routes of egress is provided by Enquist et al. (5).
In an attempt to resolve this controversy, we constructed HSVs in which glycoprotein D (gD) or gH were targeted to the endoplasmic reticulum (ER) by addition of the ER retrieval signal KKXX to the C-terminal cytoplasmic domain, and we reported that secreted progeny virions were devoid of the targeted molecules (3, 23). The simplest interpretation of these findings is that the virus acquires its final envelope from a cytoplasmic compartment from which an ER-retrieved molecule would be excluded. It is possible, however, that the KKXX motif could result in reduced trafficking of the molecule to the inner nuclear membrane or could exclude the molecule from the budding process, and in either case the targeted molecule would be excluded from progeny virions regardless of the route of egress. Formal proof that progeny virions are enveloped in the cytoplasm requires us to demonstrate that enveloped virions in the perinuclear space contain the ER-targeted glycoprotein but that this molecule is absent in progeny virus. Here, we report immunogold electron microscopic studies which show that this is the case: cells infected with an HSV-1 mutant encoding an ER-retrieved gD produce perinuclear enveloped virions which contain gD, but the extracellular progeny virions have lost this molecule.
In an initial series of experiments, we infected Vero cells with HSV-1 strain SC16 at a multiplicity of infection (MOI) of 10 and examined thin sections of fixed embedded cells at various times after infection during the productive phase (0 to 16 h). We found that after 8, 12, or 16 h, most cells contained many capsids in the nucleus and many cytoplasmic and extracellular enveloped virions, but perinuclear enveloped virions and virions budding at the inner nuclear membrane were extremely rare—most sections contained no virions of this type. It appears that in Vero cells, strain SC16 buds through the inner nuclear membrane and traverses the perinuclear space very rapidly, and it was apparent that it would be difficult or impossible to collect adequate data on the composition of perinuclear virions using this virus-cell system. Therefore, we examined infected BL1 cells (7) since these cells are reported to accumulate perinuclear virions (G. Kousoulas, personal communication), and we found that these cells, when infected with strain SC16, contained a few perinuclear enveloped virions in most cell profiles. These cells were therefore used in subsequent experiments. This, clearly, is a compromise. BL1 cells are derived from Vero cells and carry HSV-1 sequences corresponding to nucleotides 107951 to 113323, which contain the UL51, UL52, and UL53 open reading frames. The presence of these virus sequences may modify the virus replication cycle, but BL1 cells have been used as complementing cell lines for UL52 mutants (7), and we found that they produced the same virus yields, with the same kinetics, as Vero cells following infection with HSV-1 strain SC16 at a high MOI. We therefore consider it very unlikely that these cells are grossly aberrant in the envelopment and processing of HSV virions. Furthermore, it is important to note that previous work has established that gD-KKXX and gH-KKXX are excluded from extracellular virions in BHK or Vero cells (3, 23). The main purpose of this study was to establish that the KKXX motif did not exclude molecules from the envelope of particles in the perinuclear space.
Monolayers of BL1 cells were infected at an MOI of 10 with HSV-1 strain SC16 or with SC16gDKKXX, a recombinant virus encoding an ER-targeted gD (23). After a 1-h absorption period, residual inoculum was removed using a low pH wash, and after 14 h, the monolayers were harvested by scraping. The infected cells were pelleted, and small droplets (0.5 μl) of packed cells were mounted onto resin films 3 mm by 2 mm by 15 μm in dimension, which were quench-frozen by plunging into liquid propane cooled in liquid nitrogen. Alternatively, monolayers of BL1 cells were grown on Formvar-coated 100-mesh gold transmission-electron microscope grids and infected at an MOI of 10 as described above. After 14 h, the grids were blotted dry and snap-frozen in liquid propane as described above. Subsequent low-temperature embedding and antibody labeling was performed as described in detail by Skepper (18). The frozen cells were transferred into a Leica AFS freeze substitution unit in vials of frozen dry methanol containing 0.5% uranyl acetate and were maintained at −90°C for 24 h, −70°C for 24 h, and −50°C for 24 h. The cells were infiltrated for 3 days with Lowicryl HM20, which was then polymerized by UV irradiation for 48 h. Sections 50 nm thick were cut using a Leica Ultracut-S and mounted on Formvar-coated nickel grids. The sections were incubated overnight in a mixture of anti-gD monoclonal antibodies LP2, LP14, and AP7 (14), each at approximately 100 μg/ml in 10 mM Tris-buffered saline (TBS) (pH 7.4) containing 0.1% Tween 20, 0.1% Triton X-100, 10% fetal bovine serum, and 10% normal goat serum (antibody diluent). The sections were then washed six times in TBS and incubated for 1 h in goat anti-mouse immunoglobulin G conjugated to 10-nm-diameter gold particles (British Biocell, Cardiff, United Kingdom) diluted 1:100 in antibody diluent buffered to pH 8.5 and without normal goat serum. They were then rinsed six times with TBS, rinsed twice with deionized water, and stained with uranyl acetate and lead citrate before examination using a Philips CM100 transmission electron microscope.
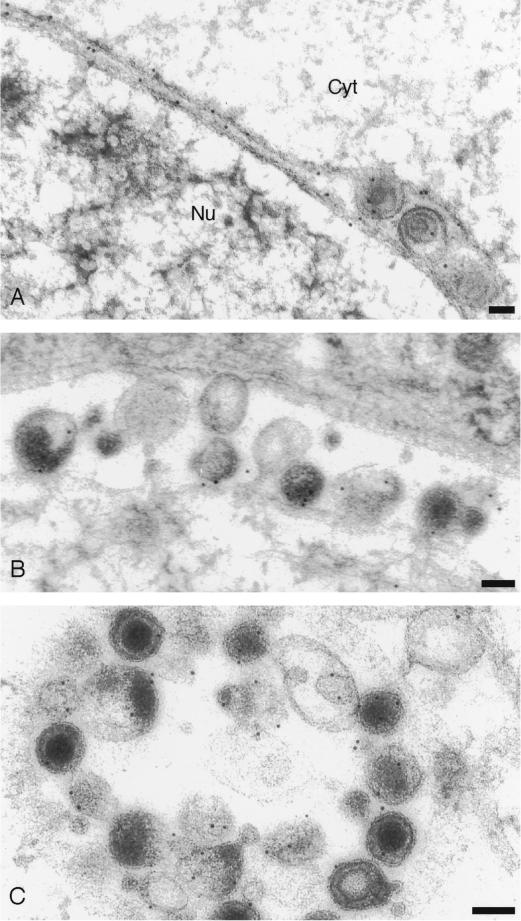
In cells infected with wild-type virus, gD was widely distributed (Fig. 1). Extracellular virions and enveloped virions in the perinuclear space and in cytoplasmic compartments were invariably gold labeled, as were the nuclear membrane and cytoplasmic membranes. Gold particles associated with enveloped virions in the perinuclear and extracellular space were counted (Table 1), and the frequency of gold particles per virion was similar in these two compartments (approximately two particles per virion).
FIG. 1.
Immunogold labeling of cells infected with HSV-1 expressing wild-type gD. (A) Decoration of inner and outer nuclear membranes and particles in the perinuclear space; (B and C) decoration of extracellular particles. Bars, 100 nm.
TABLE 1.
Frequency of gold labeling in perinuclear and extracellular compartments
| Expt no. | Virus | Location | No. of enveloped virions | No. of gold particles | No. of gold particles/virion |
|---|---|---|---|---|---|
| 1 | Wild type | Perinuclear | 42 | 72 | 1.7 |
| Extracellular | 116 | 246 | 2.1 | ||
| SC16gD-KKXX | Perinuclear | 21 | 68 | 3.2 | |
| Extracellular | 70 | 18 | 0.26 | ||
| 2 | SC16gD-KKXX | Perinuclear | 9 | 32 | 3.6 |
| Extracellular | 143 | 15 | 0.10 |
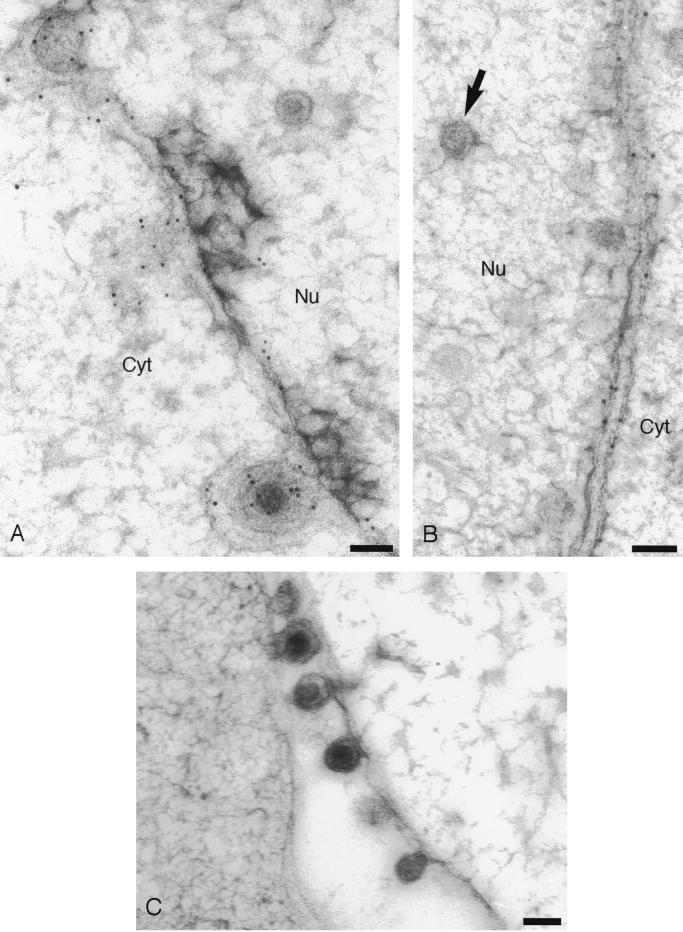
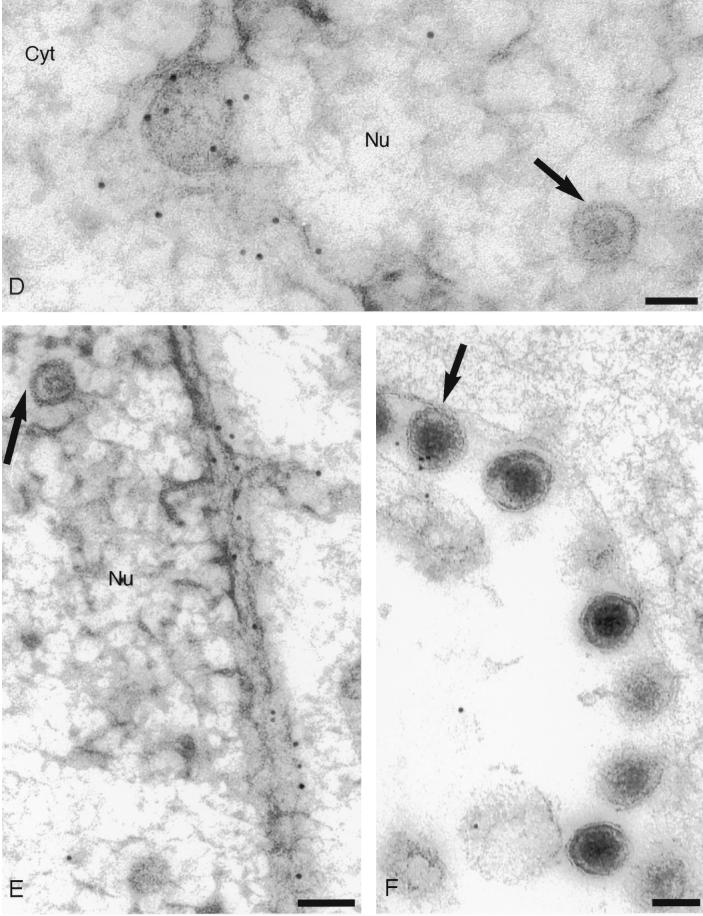
In contrast, cells infected with SC16gDKKXX showed a much more limited distribution of gD (Fig. 2). Inner and outer nuclear membranes were heavily decorated, and we occasionally observed decorated cisternae which probably correspond to ER membranes. Most cytoplasmic membranes, however, exhibited little or no decoration, consistent with the ER retrieval of gD expressed by this virus. Crucially, for the purpose of this study, enveloped particles in the perinuclear space were almost invariably associated with gold particles, whereas extracellular virions were not. Perinuclear virions were decorated with, on average, 3.2 gold particles per virion, whereas extracellular virions were associated with 0.26 gold particles/virion (Table 1). The experiment using SC16gDKKXX-infected BL1 cells was repeated, and similar results were obtained. Gold particles associated with perinuclear and extracellular virions were counted (Table 1), and on this occasion, perinuclear virions were labeled more heavily, by a factor of 30, than extracellular virions. We noted that the low level of labeling of extracellular SC16gDKKXX virions appeared asymmetric; the large majority of virions were unlabeled, but rare virions decorated with more than one gold particle were present (Fig. 2F). In contrast, wild-type extracellular virions were more randomly labeled (Fig. 1C). The source of extracellular gD-positive SC16gDKKXX virions is unclear, but previous work has shown that they contain endoglycosidase H-sensitive gD (23). It seems likely, therefore, that these rare virions are derived directly from the perinuclear space, either as a result of cell lysis or via a secretory pathway which bypasses the Golgi.
FIG. 2.
Immunogold labeling of cells infected with HSV-1 expressing an ER-targeted gD. (A) Decoration of particles in the perinuclear space; (B and E) decoration of inner and outer nuclear membranes; (D) enlargement of the particle shown in micrograph A that appears to be budding through the inner nuclear membrane; (C and F) extracellular particles. The arrows in micrographs B, D, and E indicate unenveloped capsids in the nucleus. The arrow in micrograph F indicates a very rare example of a decorated extracellular particle. Bars, 100 nm.
These data confirm previous findings, namely that an ER-targeted gD is excluded from progeny virions (23). Crucially, however, they show that this molecule is present on the inner nuclear membrane, is acquired during the initial budding event, and is present on perinuclear enveloped virions. It follows that the envelope present on perinuclear virions must be lost during the subsequent maturation and egress of these virus particles. In the face of these data, we consider that the single envelopment route of egress is untenable and that the final envelope is acquired in a cytoplasmic compartment.
Many questions remain to be answered. Since progeny virions assemble in the cytoplasm, it follows that the enveloped virions in the perinuclear space are of unknown composition. It has been noted that the tegument of alphaherpesvirus virions in the perinuclear space appears less electron dense than that of progeny virions (8, 19), and it appears that perinuclear virions lack the tegument protein VP22 (4). A study of the effects of monensin on HSV-infected cells implies that perinuclear virions are infectious (11), but similar studies of the effects of brefeldin A on pseudorabies virus infection suggest that these virions are noninfectious (22).
The loss of the envelope from perinuclear virions and the release of nucleocapsids into the cytoplasm must involve a fusion event, but this cannot be mediated by those envelope proteins (gD, gB, and gH:L) known to be required for plasma membrane fusion (20) because virus mutants lacking these proteins are processed and secreted normally from infected cells. The UL20 gene product is a possible candidate for this fusion function because HSV-1 mutants lacking this gene accumulate in the perinuclear space (2). The site of final envelopment of alphaherpesviruses is uncertain. Studies of varicella-zoster virus favor the trans-Golgi network (6, 24), whereas a recent study of HSV-1 implicates a late endosomal compartment (9), but regardless of the site, the mechanism whereby some 10 or more envelope membrane proteins accumulate in the relevant compartment is entirely unknown. When expressed alone, different HSV-1 envelope proteins exhibit different trafficking properties. Thus, gE is localized to the trans-Golgi network (1), whereas gD appears to traffic to the cell surface via the default pathway (e.g., see reference 23). We noticed, however, that in the context of wild-type virus infection, gD is present at very low levels on the cell surface (Fig. 1B). To confirm this impression, we quantified gold decoration on nuclear membranes, cytoplasmic membranes, and plasma membranes in the same wild-type-infected cells. Table 2 shows that, per unit membrane length, plasma membranes contain about 50-fold less gD than cytoplasmic membranes. We conclude that in the context of virus infection, gD is excluded from the plasma membrane by a mechanism which is at present entirely obscure.
TABLE 2.
Labeling density of membranes in wild-type-infected cells
| Membrane | No. of gold particles (mean ± SD)/μm of membrane ina:
|
|
|---|---|---|
| gD antibody | Control antibody | |
| Nuclear | 5.9 ± 0.5 | 0.11 ± 0.02 |
| Cytoplasmic | 15.2 ± 3.1 | 0.15 ± 0.04 |
| Plasma | 0.26 ± 0.03 | 0.07 ± 0.01 |
The number of gold particles per micrometer of membrane were calculated for individual cell profiles. The mean and standard error are given for 10 profiles (using gD antibody) or 8 profiles (control antibody).
Acknowledgments
We thank Susanne Bell and Janet Powell for excellent technical support. We thank S. Weller for permission to use BL1 cells and K. Kousoulas for providing them.
This work was supported by the Wellcome Trust, United Kingdom.
REFERENCES
- 1.Alconada A, Bauer U, Sodeik B, Hoflack B. Intracellular traffic of herpes simplex virus glycoprotein gE: characterization of the sorting signals required for its trans-Golgi network localization. J Virol. 1999;73:377–387. doi: 10.1128/jvi.73.1.377-387.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baines J D, Ward P L, Campadelli-Fiume G, Roizman B. The UL20 gene of herpes simplex virus 1 encodes a function necessary for viral egress. J Virol. 1991;65:6414–6424. doi: 10.1128/jvi.65.12.6414-6424.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Browne H, Bell S, Minson T, Wilson D. An endoplasmic reticulum-retained herpes simplex virus glycoprotein H is absent from secreted virions: evidence for reenvelopment during egress. J Virol. 1996;70:4311–4316. doi: 10.1128/jvi.70.7.4311-4316.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elliott G, O'Hare P. Live-cell analysis of a green fluorescent protein-tagged herpes simplex virus infection. J Virol. 1999;73:4110–4119. doi: 10.1128/jvi.73.5.4110-4119.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Enquist L W, Husak P J, Banfield B W, Smith G A. Infection and spread of alphaherpesviruses in the nervous system. Adv Virus Res. 1998;51:237–347. doi: 10.1016/s0065-3527(08)60787-3. [DOI] [PubMed] [Google Scholar]
- 6.Gershon A A, Sherman D L, Zhenglun Z, Gabel C A, Ambron R T, Gershon M D. Intracellular transport of newly synthesized varicella-zoster virus: final envelopment in the trans-Golgi network. J Virol. 1994;68:6372–6390. doi: 10.1128/jvi.68.10.6372-6390.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldstein D J, Weller S K. An ICP6::lacZ insertional mutagen is used to demonstrate that the UL52 gene of herpes simplex virus type 1 is required for virus growth and DNA synthesis. J Virol. 1988;62:2970–2977. doi: 10.1128/jvi.62.8.2970-2977.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Granzow H, Weiland F, Jons A, Klupp B G, Mettenleiter T C. Ultrastructural analysis of the replication cycle of pseudorabies virus in cell culture: a reassessment. J Virol. 1997;71:2072–2082. doi: 10.1128/jvi.71.3.2072-2082.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harley C A, Dasgupta A, Wilson D W. Characterization of herpes simplex virus-containing organelles by subcellular fractionation: role for organelle acidification in assembly of infectious particles. J Virol. 2001;75:1236–1251. doi: 10.1128/JVI.75.3.1236-1251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holland D J, Miranda-Saksena M, Boadle R A, Armati P, Cunningham A L. Anterograde transport of herpes simplex virus proteins in axons of peripheral human fetal neurons: an immunoelectron microscopy study. J Virol. 1999;73:8503–8511. doi: 10.1128/jvi.73.10.8503-8511.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson D C, Spear P G. Monensin inhibits the processing of herpes simplex virus glycoproteins, their transport to the cell surface and the egress of virions from infected cells. J Virol. 1982;43:1102–1112. doi: 10.1128/jvi.43.3.1102-1112.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones F, Grose C. Role of cytoplasmic vacuoles in varicella-zoster virus glycoprotein trafficking and virion envelopment. J Virol. 1988;62:2701–2711. doi: 10.1128/jvi.62.8.2701-2711.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klupp B G, Granzow H, Mettenleiter T C. Primary envelopment of pseudorabies virus at the nuclear membrane requires the UL34 gene product. J Virol. 2000;74:10063–10073. doi: 10.1128/jvi.74.21.10063-10073.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minson A C, Hodgman T C, Digard P, Hancock D C, Bell S E, Buckmaster E A. An analysis of the biological properties of monoclonal antibodies against glycoprotein D of herpes simplex virus and identification of amino acid substitutions that confer resistance to neutralisation. J Gen Virol. 1986;67:1001–1013. doi: 10.1099/0022-1317-67-6-1001. [DOI] [PubMed] [Google Scholar]
- 15.Miranda-Saksena M, Armati P, Boadle R A, Holland D J, Cunningham A L. Anterograde transport of herpes simplex virus type 1 in cultured, dissociated human and rat dorsal root ganglion neurons. J Virol. 2000;74:1827–1839. doi: 10.1128/jvi.74.4.1827-1839.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Penfold M E, Armati P, Cunningham A L. Axonal transport of herpes simplex virions to epidermal cells: evidence for a specialised mode of virus transport and assembly. Proc Natl Acad Sci USA. 1994;91:6529–6533. doi: 10.1073/pnas.91.14.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roizman B, Sears A. Herpes simplex viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2231–2295. [Google Scholar]
- 18.Skepper J N. Immunocytochemical strategies for electron microscopy: choice or compromise. J Microsc. 2000;199:1–36. doi: 10.1046/j.1365-2818.2000.00704.x. [DOI] [PubMed] [Google Scholar]
- 19.Stackpole C W. Herpes-type virus of the frog renal adenocarcinoma. I. Virus development in tumor transplants maintained at low temperature. J Virol. 1969;4:75–93. doi: 10.1128/jvi.4.1.75-93.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turner A, Bruun B, Minson A, Browne H. Glycoproteins gB, gD, and gHgL of herpes simplex virus type 1 are necessary and sufficient to mediate membrane fusion in a Cos cell transfection system. J Virol. 1998;72:873–875. doi: 10.1128/jvi.72.1.873-875.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Genderen I, Brandimarti R, Torrisi M, Campadelli Fiume G, van Meer G. The phospholipid composition of extracellular herpes simplex virions differs from that of host cell nuclei. Virology. 1994;200:831–836. doi: 10.1006/viro.1994.1252. [DOI] [PubMed] [Google Scholar]
- 22.Whealy M E, Card J P, Meade R P, Robbins A K, Enquist L W. Effect of Brefeldin A on alphaherpesvirus membrane protein glycosylation and virus egress. J Virol. 1991;65:1066–1081. doi: 10.1128/jvi.65.3.1066-1081.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whiteley A, Bruun B, Minson T, Browne H. Effects of targeting herpes simplex virus type I gD to the endoplasmic reticulum and trans-Golgi network. J Virol. 1999;73:9515–9520. doi: 10.1128/jvi.73.11.9515-9520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu Z, Gershon M D, Hao Y, Ambron R T, Gabel C A, Gershon A A. Envelopment of varicella zoster virus: targeting of viral glycoproteins to the trans-Golgi network. J Virol. 1995;69:7951–7959. doi: 10.1128/jvi.69.12.7951-7959.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]