Abstract
Latent membrane protein 2A (LMP2A) is expressed in latent Epstein-Barr virus (EBV) infection. We have demonstrated that Nedd4 family ubiquitin-protein ligases (E3s), AIP4, WWP2/AIP2, and Nedd4, bind specifically to two PY motifs present within the LMP2A amino-terminal domain. In this study, LMP2A PY motif mutant viruses were constructed to investigate the role of the LMP2A PY motifs. AIP4 was found to specifically associate with the LMP2A PY motifs in EBV-transformed lymphoblastoid cell lines (LCLs), extending our original observation to EBV-infected cells. Mutation of both of the LMP2A PY motifs resulted in an absence of binding of AIP4 to LMP2A, which resulted in an increase in the expression of Lyn and the constitutive hyperphosphorylation of LMP2A and an unknown 120-kDa protein. In addition, there was a modest increase in the constitutive phosphorylation of Syk and an unidentified 60-kDa protein. These results indicate that the PY motifs contained within LMP2A are important in regulating phosphorylation in EBV-infected LCLs, likely through the regulation of Lyn activity by specifically targeting the degradation of Lyn by ubiquination by Nedd4 family E3s. Despite differences between PY motif mutant LCLs and wild-type LCLs, the PY motif mutants still exhibited a block in B-cell receptor (BCR) signal transduction as measured by the induction of tyrosine phosphorylation and BZLF1 expression following BCR activation. EBV-transformed LCLs with mutations in the PY motifs were not different from wild-type LCLs in serum-dependent cell growth. Protein stability of LMP1, which colocalizes with LMP2A, was not affected by the LMP2A-associated E3s.
Epstein-Barr virus (EBV) is a potentially oncogenic herpesvirus persisting in B lymphocytes of most adult humans (for reviews, see references 15, 16, and 26). B lymphocytes infected with EBV in vitro are immortalized and are termed lymphoblastoid cell lines (LCLs), and they provide a model of in vivo latent infection (15, 16, 26). These EBV-transformed LCLs express a restricted set of latency-associated viral products, including six nuclear proteins (EBNAs), three integral membrane proteins (latent membrane protein 1 [LMP1], LMP2A, and LMP2B), and two small RNAs (EBERs) (15, 16). However, EBNA1, LMP1, and LMP2A are the proteins consistently detected in nasopharyngeal carcinoma tumor biopsies and EBV-related malignancies (for reviews, see references 1, 26, and 30). The LMP2A mRNA is also consistently detected by PCR analysis in peripheral B lymphocytes from humans with latent EBV infections (23–25, 31). Thus, LMP2A is suspected of having an important role in vivo for EBV latency and persistence (16).
In primary B lymphocytes, B-cell receptor (BCR) activation leads to signal transduction cascades, including the recruitment and activation of cellular protein tyrosine kinases (PTKs) (5, 8). The Src family PTKs, Lyn, Fyn, and Blk, are activated following BCR stimulation, which is followed by activation and binding of the Syk PTK to the BCR through an interaction of Syk SH2 domains with the immunoreceptor tyrosine-based activation motifs contained within BCR (J. C. Cambier, Letter, Immunol. Today, 16:110, 1995). Interestingly, both the Src family PTKs and the Syk PTK are also associated with LMP2A (11, 12).
LMP2A is expressed in aggregates in the plasma membrane of latently infected B cells (18). Most of the anti-phosphotyrosine reactivity within these B cells is associated with LMP2A aggregates (17, 18). The Src family Lyn PTK binds to tyrosine 112, whereas the Syk PTK binds to the LMP2A immunoreceptor tyrosine-based activation motif present at tyrosines 74 and 85 of the LMP2A amino-terminal domain via SH2-phosphotyrosine interactions (11, 12). The association of LMP2A with the Src family and the Syk PTKs is essential for the LMP2A-mediated block of BCR signal transduction observed in EBV-immortalized LCLs grown in culture (11, 12). Thus, the amino terminus of LMP2A appears to act as a functional decoy for BCR-associated proteins, which results in the down-modulation of BCR-mediated signal transduction.
Also contained within the amino-terminal domain of LMP2A are two PY motifs (PPPPY). These motifs are conserved in clinical EBV isolates and in the EBV-related herpesvirus papio (4, 9). PY motifs interact with WW domains through a consensus sequence of xPPxY (6). Our previous studies indicated that the LMP2A PY motifs specifically associate with Nedd4 family ubiquitin-protein ligases, such as AIP4, WWP2/AIP2, and Nedd4 (14). The ubiquitin proteolytic pathway plays a crucial role in the degradation of short-lived and regulatory proteins important in various cellular processes (7, 13). The ubiquitin-protein ligase (E3) facilitates the sequential transfer of ubiquitin to target proteins. Different E3s are important in determining the selectivity of ubiquitin-mediated protein degradation. Proteins ligated to polyubiquitin chains are usually degraded by the 26S proteasome in an ATP-dependent manner. Nedd4 family E3s may ubiquitinate LMP2A and LMP2A-associated proteins through the interaction of the LMP2A PY motif and the WW domain of the Nedd4 family E3s. Indeed, the rapid turnover of LMP2A is observed in LMP2A-expressing BJAB cell lines (14). Furthermore, Lyn is ubiquinated and there is a reduction and rapid turnover in the Lyn protein in LMP2A-expressing cell lines (14, 32), suggesting that LMP2A and Lyn may be degraded through the ubiquitin-proteasome pathway.
In this study, the LMP2A PY mutations examined in previous in vitro studies (14) were incorporated into the EBV genome. LCLs containing these mutations were isolated to investigate the functional importance of the LMP2A PY motifs. Each LMP2A PY motif, PY1 (56 to 60 amino acids [aa]) and PY2 (97 to 101 aa), was changed from PPPPY to PAAPY by PCR-mediated mutagenesis (Fig. 1A) using a previously described strategy (11). Recombinant viruses were used to infect purified B lymphocytes in culture to generate EBV-infected LCLs (11). Multiple LCLs transformed by the PY motif mutants were identified and are characterized in this study.
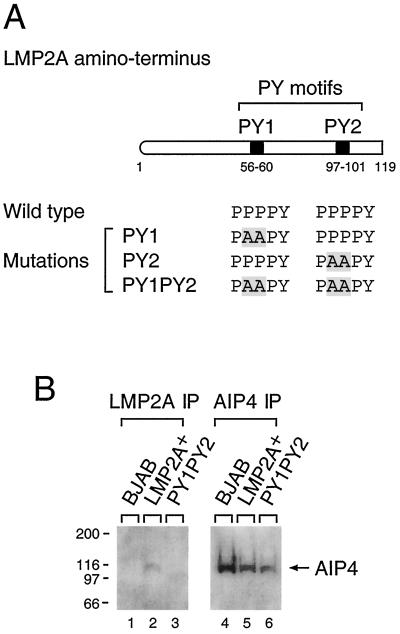
FIG. 1.
Site-directed mutation of the LMP2A PY motifs and association of LMP2A and AIP4 in LMP2A PY1PY2 LCLs. (A) The first 119 amino acids of the LMP2A amino-terminal domain is shown schematically, with each PY motif indicated by a box. PY motifs were mutated from PPPPY to PAAPY, which was previously shown to block binding of the WW domain-containing Nedd4 family ubiquitin E3s to LMP2A (15). These mutations were made to determine the function of the LMP2A PY motifs. A PvuII site was incorporated at the mutation site to allow for identification of LCLs containing the LMP2A point mutation. The restriction site addition was silent with regard to the LMP2A amino acid sequence. (B) Triton X-100 lysates from BJAB cells and LCLs (10 7 cells) were immunoprecipitated (IP) with anti-LMP2A (lanes 1 to 3) or anti-AIP4 (lanes 4 to 6) antibody. Precipitated proteins were separated on sodium dodecyl sulfate–7.5% polyacrylamide gel electrophoresis gels and immunoblotted with anti-AIP4 antibody. The position of AIP4 is indicated by an arrow. Molecular mass standards (in kilodaltons) are indicated at the left.
AIP4 binds to the LMP2A PY motifs in vivo.
Previous studies using the amino terminus of LMP2A expressed in bacteria demonstrated that each of the LMP2A PY motifs is sufficient for the in vitro association of LMP2A with WW domain-containing E3s, such as AIP4, WWP2/AIP2, and Nedd4 (14, 19). Of these E3s, AIP4, which bound LMP2A in cells of B-cell origin, was the most abundant E3 (14). Therefore, to verify the in vivo interaction of LMP2A PY motifs with the Nedd4 family E3s, the binding of AIP4 to LMP2A was investigated in wild-type LCLs and PY1PY2 LCLs. Rabbit polyclonal sera directed against AIP4 was derived using a glutathione S-transferase–fusion protein containing the unique domain of AIP4 (100 to 285 aa). The antisera demonstrated specific recognition of the AIP4–glutathione S-transferase fusion protein and an epitope-tagged version of AIP4, as well as AIP4 in Triton X-100 lysates from BJAB cells and LCLs (data not shown). Using these antisera, the interaction of AIP4 with LMP2A was investigated by immunoprecipitation of cell lysates from wild-type-infected LCLs and PY1PY2 LCLs using anti-LMP2A antibody followed by immunoblotting with the AIP4 antiserum (Fig. 1B). AIP4 was detected only in the LMP2A immunoprecipitates in wild-type-infected LCLs, whereas it was not detected in the PY1PY2 LCL or in BJAB cells which do not contain LMP2A (Fig. 1B). Therefore, the two PPPPY sequences contained within the LMP2A amino terminus are functional PY motifs for in vivo AIP4 binding.
Protein levels of LMP2A and Lyn in LMP2A PY1PY2 LCLs.
Previous studies had demonstrated that the expression of LMP2A in EBV-negative BJAB cells led to the rapid turnover of the Lyn PTK, whereas the protein level of the Syk PTK and tubulin was unchanged (14). In addition, the LMP2A protein was also rapidly turned over when compared to Syk and tubulin (14). Thus, protein levels of LMP2A, Lyn, Syk, and tubulin were investigated by immunoblottings in wild-type-infected LCLs and LCLs infected with the LMP2A PY1PY2 mutant. When compared to wild-type-infected LCLs, there was approximately a twofold increase, as determined by densitometry, in the protein level of LMP2A in the PY1PY2 mutant-infected LCLs (Fig. 2A). However, LMP2A protein levels tended to be inconsistent and some mutant LCLs demonstrated similar levels of LMP2A protein when compared to the wild type (data not shown).
FIG. 2.
Expression of LMP2A, Lyn, and Syk in LMP2A PY1PY2 and Y112F LCLs. (A) The protein levels of LMP2A, Lyn, Syk, and tubulin in BJAB cells (lanes 1) and in LMP2A− (lanes 2), LMP2A+ (lanes 3 and 4), and PY1PY2 (lanes 5 and 6) LCLs were analyzed. (B) Levels of Lyn and tubulin expression in LMP2A+ (lanes 1), PY1PY2 (lanes 2), and LMP2A Y112F (lanes 3 to 5) LCLs were analyzed. Triton X-100 lysates from the various cell lines were separated on sodium dodecyl sulfate–7.5% polyacrylamide gel electrophoresis gels, transferred to Immobilon membranes, and immunoblotted with anti-LMP2A, anti-Lyn, anti-Syk, or anti-tubulin antibody, followed by incubation with horseradish peroxidase-conjugated secondary antibody and enhanced chemiluminescence detection. Tubulin served as a protein loading control. The positions of LMP2A, Lyn (both the 56- and 53-kDa forms), Syk, and tubulin are indicated by arrows.
In contrast, changes in Lyn protein levels were much more dramatic in the PY1PY2 LCLs when compared to LMP2A+ LCLs. Compatible with previous results with LMP2A-expressing BJAB cells, LMP2A+ LCLs demonstrated a reduction in Lyn protein expression when compared to LMP2A− LCLs or BJAB cells (Fig. 2A). This decrease of Lyn expression was not observed in PY1PY2 LCLs; rather, the Lyn protein level in the PY1PY2 LCLs was equal to that of LMP2A− LCLs or BJAB cells (Fig. 2A). In contrast to the results obtained with Lyn, Syk protein levels were not changed in the LCLs with or without the PY1PY2 mutation (Fig. 2A).
Next, the levels of Lyn protein in PY1PY2 LCLs were compared to Lyn protein levels in Y112F LCLs (12). Y112F LCLs contain a tyrosine-to-phenylalanine mutation at tyrosine 112 within the LMP2A amino-terminal domain. This mutation results in a loss of binding of Lyn to LMP2A, which results in an increase of Lyn protein levels to wild-type levels (12). The protein level of Lyn was similar in both the PY1PY2 LCLs and the Y112F LCLs, demonstrating that both the single tyrosine-to-phenylalanine mutation and the double PY mutations have similar effects on Lyn protein levels (Fig. 2B).
In addition, the levels of other proteins known to be important in BCR signal transduction were also tested. These included BLNK, Btk, Shc, PI3K p85, PLCγ 2, and Cas p130. However, as with Syk and tubulin, there was no change in the protein level of any of these proteins in the wild-type LCLs when compared to PY1PY2 LCLs (data not shown). Therefore, the LMP2A effect on Lyn protein level appears to be specific. Single mutation of either PY motif had little effect on the protein levels of Lyn or LMP2A in LCLs infected with the appropriate mutant (data not shown). In summary, mutation of both LMP2A PY motifs results in a restoration of Lyn protein levels to that seen in LMP2A− LCLs and in EBV− BJAB cells and a possibly modest increase in LMP2A protein levels when compared to wild-type-infected LCLs.
Phosphorylation in LMP2A PY1PY2 LCLs.
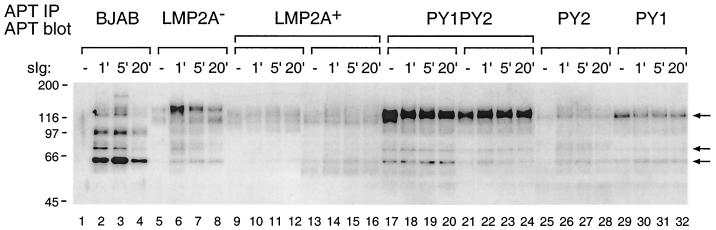
Induction of tyrosine phosphorylation is one of the earliest known biochemical events following BCR cross-linking in B lymphocytes. LMP2A functions to block BCR-mediated tyrosine phosphorylation in LMP2A+ LCLs. To determine the effect of the PY motif mutations on tyrosine phosphorylation following BCR cross-linking, LCLs with mutations in the LMP2A PY motifs were tested. The LCLs used in these experiments were verified for surface immunoglobulin (Ig) expression by flow cytometry (data not shown). The induction of tyrosine phosphorylation was analyzed at 1, 5, and 20 min after BCR cross-linking. Cell lysates of LMP2A PY motif mutant LCLs and control cells were immunoprecipitated with anti-phosphotyrosine antibody followed by an immunoblotting with anti-phosphotyrosine monoclonal antibody. As previously reported, tyrosine phosphorylation was induced in the BJAB cells and the LMP2A− LCL but not in the wild-type LCL (Fig. 3). In contrast, the PY1PY2 LCLs showed an apparent hyperphosphorylation of a protein with a molecular mass of approximately 120 kDa and a modest increase in phosphorylation of 75- and 60-kDa proteins prior to BCR stimulation when compared to wild-type LCLs (Fig. 3). This pattern remained unchanged following BCR cross-linking (Fig. 3). This apparent hyperphosphorylation of the 120-kDa protein was particularly impressive in being the dominant phosphoprotein present in any of the samples from the various cell lines. Interestingly, the level of constitutive phosphorylation in PY1 or PY2 single-mutation LCLs was reduced when compared to the PY1PY2 LCLs, although there was some constitutive phosphorylation of the 120-kDa protein in PY1 LCLs (Fig. 3). In summary, despite the observed differences in patterns of phosphorylation prior to BCR cross-linking, both wild-type LCLs and the PY1, PY2, and PY1PY2 LCLs all exhibited a block in BCR signal transduction, as measured by the induction of tyrosine phosphorylation following BCR cross-linking (Fig. 3). In addition, a 120-kDa protein was dramatically hyperphosphorylated in PY1PY2 LCLs.
FIG. 3.
Tyrosine phosphorylation following BCR cross-linking in LMP2A PY motif mutant LCLs. BJAB cells and LCLs (107 cells) were untreated (−) or treated with anti-human surface Ig (sIg) antibodies for the indicated times (1, 5, or 20 min), lysed in Triton X-100 lysis buffer, and immunoprecipitated (IP) with anti-phosphotyrosine antibody (PY20). Precipitated proteins were separated on sodium dodecyl sulfate–7.5% polyacrylamide gel electrophoresis gels and immunoblotted with horseradish peroxidase-conjugated anti-phosphotyrosine antibody (RC20). The hyperphosphorylated 120-, 75-, and 60-kDa proteins are indicated by arrows. Molecular mass standards are indicated (in kilodaltons).
Tyrosine phosphorylation of LMP2A, Lyn, and Syk in LMP2A PY1PY2 LCLs.
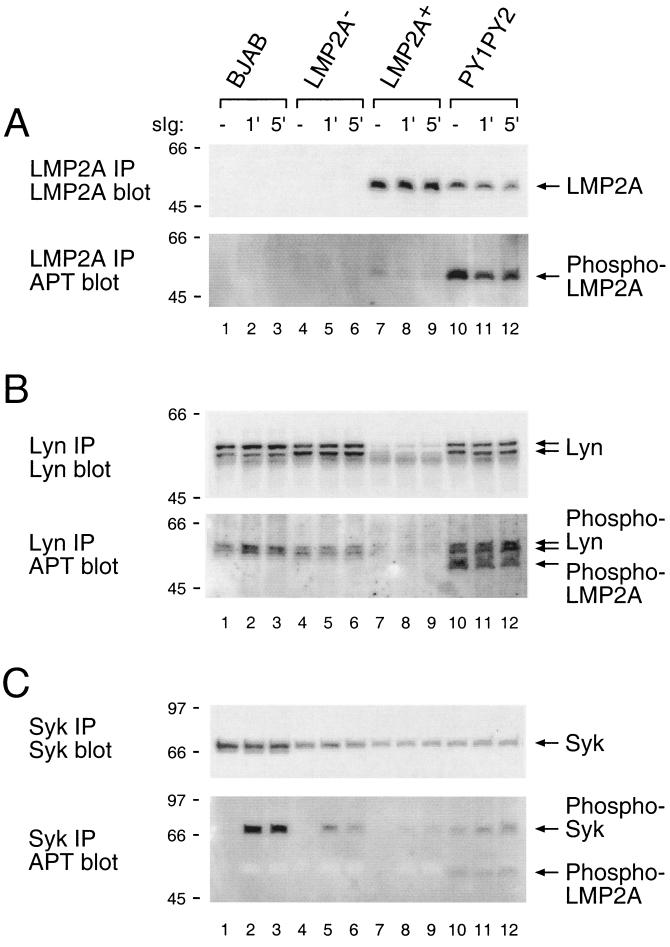
To further characterize tyrosine-phosphorylated proteins in the PY1PY2 LCLs, tyrosine phosphorylation of specific proteins was examined in the PY1PY2 LCLs before and after BCR cross-linking. To determine the level of LMP2A phosphorylation, cell lysates from PY1PY2 LCLs were immunoprecipitated with anti-LMP2A antibody followed by an immunoblotting with anti-LMP2A antibody to verify similar amounts of LMP2A in the immunoprecipitation or with anti-phosphotyrosine antibody to determine the level of phosphorylation of LMP2A (Fig. 4A). Both wild-type and mutant LMP2A were found at nearly the same level in the LMP2A immunoprecipitation (Fig. 4A). More interestingly, LMP2A in the PY1PY2 LCLs exhibited a much greater level of phosphorylation when compared to LMP2A in wild-type LCLs, indicating that the PY1PY2 mutation caused hyperphosphorylation of LMP2A (Fig. 4A).
FIG. 4.
Expression and tyrosine phosphorylation of LMP2A, Lyn, and Syk after BCR cross-linking in LMP2A PY1PY2 LCLs. BJAB cells and LCLs (107 cells) were untreated (−) or treated with anti-human Ig antibodies for the indicated times (1 or 5 min), lysed in Triton X-100 lysis buffer, and immunoprecipitated (IP) with anti-LMP2A (A), anti-Lyn (B), or anti-Syk (C) antibody. Precipitated proteins were separated on sodium dodecyl sulfate–7.5% polyacrylamide gel electrophoresis gels and transferred to Immobilon membranes. To determine the amount of each protein in the immunoprecipitation (top), membranes were immunoblotted with anti-LMP2A (A), anti-Lyn (B), or anti-Syk (C) antibody. To determine the level of tyrosine phosphorylation (bottom), membranes were immunoblotted with horseradish peroxidase-conjugated anti-phosphotyrosine antibody (RC20). Relevant proteins are indicated with arrows, and molecular mass standards are shown (in kilodaltons).
Next, Lyn phosphorylation was determined by the same procedure described above. As already shown in Fig. 2, Lyn expression was remarkably decreased in the LMP2A+ LCL, whereas this decrease in Lyn protein levels was not observed in the PY1PY2 LCL (Fig. 4B). In the PY1PY2 LCLs, both the amount of Lyn protein and the degree of phosphorylation were similar to what was observed in BJAB cells or in LMP2A− LCLs for Lyn (Fig. 4B). Of particularly interest was the appearance of a band corresponding in size to that of LMP2A. LMP2A has previously been shown to bind tightly to Lyn (12). This band was identified as being LMP2A by stripping the blot and reprobing with LMP2A-specific antibodies (data not shown). The considerable increase of LMP2A bound to Lyn is likely due to the increased levels of Lyn in the PY1PY2 LCLs and the increased level of LMP2A phosphorylation which may enhance Lyn binding to LMP2A. Previous studies have indicated that the interaction of Lyn with LMP2A requires the phosphorylation of LMP2A (12).
Finally, the induction of Syk phosphorylation prior to and following BCR cross-linking was determined. Syk was immunoprecipitated from each LCL, and the amount of Syk in each immunoprecipitate was approximately equal in each LCL (Fig. 4C). There was very little induction of Syk phosphorylation in the LMP2A+ LCL following BCR cross-linking when compared to the LMP2A− LCL (Fig. 4C), but there was a modest increase in Syk constitutive phosphorylation in the PY1PY2 LCLs when compared to wild-type LCLs, and this increase remained following BCR cross-linking (Fig. 4C). As observed with the Lyn immunoprecipitations, LMP2A was readily detected in the Syk immunoprecipitates in the PY1PY2 LCLs (Fig. 4C). This band was verified as LMP2A by stripping the blot and reprobing with LMP2A-specific antibody (data not shown). As with Lyn, this increased binding of Syk to LMP2A is likely due to the enhanced phosphorylation of LMP2A in the PY1PY2 LCLs. The binding of Syk to LMP2A has also been shown to be dependent on LMP2A phosphorylation (11).
In order to identify the hyperphosphorylated 120-kDa protein in the PY1PY2 LCLs, anti-phosphotyrosine immunoprecipitates from PY1PY2 LCLs were immunoblotted with antibodies against proteins which are phosphorylated following BCR stimulation and are known to be approximately 120 kDa in size. Antibodies directed against RasGAP (120 kDa), Cbl (120 kDa), and Cas (130 kDa) were negative in immunoblottings using the anti-phosphotyrosine immunoprecipitates (data not shown). Thus, mutation of both LMP2A PY motifs results in the hyperphosphorylation of LMP2A and a modest increase in Syk phosphorylation and this phosphorylation does not change following BCR cross-linking. Thus, the 75-kDa protein identified in the previous section may be Syk, whereas the identities of the 60- and 120-kDa proteins await determination.
BCR cross-linking does not induce BZLF1 expression in LMP2A PY1PY2 LCLs.
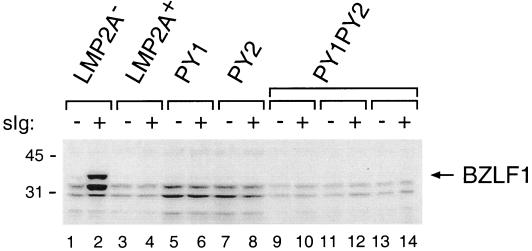
Previous studies have shown that lytic EBV replication can be induced in LCLs with deletions of genes for LMP2A or LCLs which have specific mutations which block the association of either Lyn or Syk with LMP2A (11, 12). To determine if mutation of the LMP2A PY motifs altered this LMP2A function, LCLs were either untreated or treated for 48 h with goat anti-human Ig to cross-link the BCR and then were analyzed for BZLF1 expression by immunoblotting with BZLF1-specific monoclonal antibody (33). BZLF1 is the immediate-early transactivator of EBV lytic replication and is expressed upon switch from EBV latent infection to lytic replication (3). The induction of BZLF1 expression was evident only in the LMP2A− LCL after BCR cross-linking but not in wild-type LCLs as previously described (22) or in any of the LMP2A PY motif mutants (Fig. 5). This indicates that the LMP2A PY motifs are not required for blocking the induction of EBV lytic replication through BCR-mediated signal transduction.
FIG. 5.
Induction of BZLF1 expression in LMP2A PY motif mutant LCLs. LCLs (4 × 106 cells) were untreated (−) or treated (+) with anti-human surface Ig (sIg) antibody for 48 h. Triton X-100 lysates from LCLs were separated on sodium dodecyl sulfate–7.5% polyacrylamide gel electrophoresis gels and immunoblotted with anti-BZLF1 antibody, followed by incubation with horseradish peroxidase-conjugated secondary antibody and enhanced chemiluminescence detection. Molecular mass standards are indicated (in kilodaltons).
Comparative growth of LMP2A PY1PY2 LCLs.
Although routine passage of the PY1PY2 LCLs did not indicate any growth phenotype in tissue culture, the growth of PY1PY2 LCLs was investigated at various cell concentrations and in media supplemented with 10, 1, 0.1, or 0% fetal bovine serum. PY1PY2 LCLs, LMP2A− LCLs, and the wild-type LCLs were plated, and growth was assayed 10 days later by microscopic examination and monitoring of medium pH (Table 1). As previously reported, there was no difference in growth between wild-type and LMP2A− LCLs (20, 21, 28). The growth characteristics for the PY1PY2 LCLs were very similar to those of the control LCLs (Table 1). All LCLs were unable to grow or grew poorly in wells seeded with 2.5 × 104 cells in 0.1% or lower serum or seeded with 5 × 103 cells in 1% or lower serum. Two PY1PY2 LCLs seeded at 105 cells grew somewhat better than the control LCLs in 0% serum, but the other PY1PY2 LCLs behaved similarly to the control LCLs. Therefore, there was no apparent difference in growth between the PY1PY2 LCLs and control LCLs.
TABLE 1.
Serum-dependent cell growth of LMP2A PY mutant LCLsa
| Cell line | Growthb of cells in various serum concn (%)
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 × 105 cells
|
2.5 × 104 cells
|
5 × 103 cells
|
||||||||||
| 10 | 1 | 0.1 | 0 | 10 | 1 | 0.1 | 0 | 10 | 1 | 0.1 | 0 | |
| LMP2A− ES1 | +++ | +++ | ++ | − | +++ | ++ | − | − | +++ | − | − | − |
| LMP2A+ 65 | +++ | +++ | ++ | − | +++ | ++ | − | − | +++ | − | − | − |
| LMP2A+ 5 | +++ | +++ | + | + | +++ | + | − | − | +++ | − | − | − |
| PY1PY2 9.16 | +++ | ++ | + | − | +++ | + | − | − | +++ | − | − | − |
| PY1PY2 34 | +++ | +++ | ++ | ++ | +++ | +++ | − | − | +++ | − | − | − |
| PY1PY2 5 | +++ | +++ | ++ | + | +++ | + | − | − | +++ | − | − | − |
| PY1PY2 7 | +++ | +++ | +++ | ++ | +++ | ++ | − | − | +++ | − | − | − |
Cells were plated in 96-well tissue culture plates at either 105, 2.5 × 104, or 5 × 103 cells per well in 10, 1, 0.1, or 0% serum. The wells were scored 10 days after plating.
Growth was scored according to the following guidlines: +++, large clumps, acidic media, clear growth; ++, medium clumps, possible acidification of media, probable growth; +, small clumps, neutral or basic media, little growth; −, no clumps, neutral or basic media, no growth.
LMP1 stability in LMP2A PY1PY2 LCLs.
Previous studies indicated that LMP1 and LMP2A colocalize in EBV-infected LCLs grown in tissue culture (18). Recently, it was reported that LMP1 is specifically ubiquinated and that this ubiquination is important for LMP1 degradation (2). Although previous studies indicated that there was no difference in the level of LMP1 protein in LMP2A− LCLs or in wild-type LMP2A+ LCLs, indicating no apparent effect of LMP2A on LMP1 protein levels (20, 21), it was of interest to confirm that LMP2A did not direct the ubiquitination of LMP1 by association with E3 ubiquitin ligases. LMP1 levels were therefore investigated by immunoblotting in PY1PY2 LCLs and a variety of LCLs containing tyrosine-to-phenylalanine point mutations in LMP2A which had previously been described (10, 11, 29). Figure 6 shows similar LMP1 expression in all LCLs. The Y112F LMP2A mutation blocked LMP2A phosphorylation and the binding of Lyn to LMP2A. Both Y74F and Y85F blocked the binding of Syk to LMP2A, but this did not affect overall LMP2A phoshorylation. All three of the mutations resulted in a nonfunctional LMP2A in regard to the LMP2A function of blocking B-cell signal transduction. The other LMP2A tyrosines as of yet have no identified function (29). In summary, these results confirm the previous experimental results (20, 21) demonstrating that expression of LMP2A does not affect the protein level of LMP1. In addition, they indicate that LMP2A phosphorylation or interaction with Nedd4 family E3s, Syk, or Lyn does not have any role in LMP1 protein levels.
FIG. 6.
Expression of LMP1 in LMP2A PY motif and tyrosine motif mutant LCLs. The protein levels of LMP1 was analyzed in various LMP2A phenylalanine-to-tyrosine-mutation LCLs. Triton X-100 lysates from PY1PY2 (lanes 1 and 2), Y23F (lanes 3 and 4), Y23F/Y31F (lane 5), Y31F (lane 6), Y60F (lanes 7 and 8), Y64F (lanes 9 and 10), Y74F (lanes 11 and 12), Y85F (lanes 13 and 14), Y101F (lanes 15 and 16), and Y112F (lanes 17 and 18) LCLs were separated on sodium dodecyl sulfate–7.5% polyacrylamide gel electrophoresis gels and immunoblotted with anti-LMP1 antibody. The position of LMP1 is indicated by an arrow, and molecular mass standards are indicated (in kilodaltons).
Ligand-induced receptor activation requires membrane recruitment of signaling proteins stimulated by tyrosine phosphorylation of docking proteins (for review, see reference 27). Most docking proteins contain a membrane-targeting region, multiple tyrosine phosphorylation sites that function as binding sites for SH2 domains of a variety of signaling proteins, and specific domains such as PTB domains that are responsible for complex formation with cell surface receptors (27). In this regard, LMP2A resembles a constitutively active receptor bound to its relevant docking protein. The 12 LMP2A transmembrane domains mediate constitutive aggregation of LMP2A while the eight tyrosines, which are phosphorylated independently of ligand binding, and the two PY motifs of LMP2A provide binding sites for a variety of proteins involved in cell signaling (11, 12, 14), much like an activated receptor. Relevant to this study is the binding of the SH2 domain-containing Lyn and the WW domain-containing Nedd4 family E3s to LMP2A, which results in the specific degradation of Lyn. This degradation is dependent on Lyn binding to LMP2A since the phenylalanine-to-tyrosine mutation at amino acid 112 of LMP2A, which does not bind Lyn, has Lyn protein levels similar to those of the PY1PY2 LCLs and LCLs with a null mutation in LMP2A. Interestingly, we observed no difference in the level of the Syk PTK which also binds LMP2A via an SH2-phoshotyrosine interaction (14). There was also no change in protein levels for BLNK, Btk, PI3K p85, PLCγ 2, and Cas p130, indicating that the LMP2A effect on Lyn was relatively specific. Recently, in another study, it was reported that transiently transfected Lyn and Syk are specifically ubiquitinated in LMP2A-expressing HEK 293 cells (32). Although the observed Lyn ubiquitination is compatible with our results, the Syk ubiquitination, as noted by these investigators, did not result in lower levels of Syk similar to our results (14). The significance of this difference is not known but will require future investigation of the Nedd4 family E3s association with LMP2A.
From our previous studies (14), in which we identified the association of LMP2A with AIP4 and other ubiquitin ligases of the Nedd4 family, and from the results presented in the present study, we hypothesize that rather than being a static complex, the LMP2A complex is dynamic. Our refined model hypothesizes that once LMP2A is synthesized, it becomes phosphorylated and activates Lyn, Syk, and potentially other SH2 domain-containing proteins. This LMP2A complex is able to deliver a B-cell survival and developmental signal, as observed in LMP2A transgenic animals, and block BCR signal transduction, as observed in wild-type LCLs grown in culture. Binding of LMP2A with AIP4 and other Nedd4 ubiquitin ligases would then be important for the specific internalization and degradation of these LMP2A complexes in a ubiquitin-dependent fashion (14). The internalization and degradation does not occur or is much reduced in the PY1PY2 LCLs, which results in an increase in Lyn protein levels since it is not targeted for degradation by the Nedd4 family E3s. This results in the hyperphosphophorylation of the unknown 120-kDa protein and LMP2A. The identification of the 120-kDa protein is currently being investigated since it will likely have an important role in LMP2A function and, quite possibly, normal BCR signal transduction.
Acknowledgments
We thank members of the Longnecker and Spear laboratories for providing invaluable advice and help.
M.I. is a special fellow of the Leukemia and Lymphoma Society. R.L. is a Stohlman Scholar of the Leukemia and Lymphoma Society and is supported by Public Health Service grants CA62234 and CA73507 from the National Cancer Institute and DE13127 from the National Institute of Dental and Craniofacial Research.
REFERENCES
- 1.Ambinder R F, Mann R B. Detection and characterization of Epstein-Barr virus in clinical specimens. Am J Pathol. 1994;145:239–252. [PMC free article] [PubMed] [Google Scholar]
- 2.Aviel S, Winberg G, Massucci M, Ciechanover A. Degradation of the Epstein-Barr virus latent membrane protein 1 (LMP1) by the ubiquitin-proteasome pathway. Targeting via ubiquitination of the N-terminal residue. J Biol Chem. 2000;275:23491–23499. doi: 10.1074/jbc.M002052200. [DOI] [PubMed] [Google Scholar]
- 3.Biggin M, Bodescot M, Perricaudet M, Farrell P. Epstein-Barr virus gene expression in P3HR1-superinfected Raji cells. J Virol. 1987;61:3120–3132. doi: 10.1128/jvi.61.10.3120-3132.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Busson P, Edwards R H, Tursz T, Raab-Traub N. Sequence polymorphism in the Epstein-Barr virus latent membrane protein (LMP)-2 gene. J Gen Virol. 1995;76:139–145. doi: 10.1099/0022-1317-76-1-139. [DOI] [PubMed] [Google Scholar]
- 5.Cambier J C, Pleiman C M, Clark M R. Signal transduction by the B cell antigen receptor and its coreceptors. Annu Rev Immunol. 1994;12:457–486. doi: 10.1146/annurev.iy.12.040194.002325. [DOI] [PubMed] [Google Scholar]
- 6.Chen H I, Sudol M. The WW domain of Yes-associated protein binds a proline-rich ligand that differs from the consensus established for Src homology 3-binding modules. Proc Natl Acad Sci USA. 1995;92:7819–7823. doi: 10.1073/pnas.92.17.7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciechanover A. The ubiquitin-proteasome proteolytic pathway. Cell. 1994;79:13–21. doi: 10.1016/0092-8674(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 8.DeFranco A L. Structure and function of the B cell antigen receptor. Annu Rev Cell Biol. 1993;9:377–410. doi: 10.1146/annurev.cb.09.110193.002113. [DOI] [PubMed] [Google Scholar]
- 9.Franken M, Annis B, Ali A N, Wang F. 5′ Coding and regulatory region sequence divergence with conserved function of the Epstein-Barr virus LMP2A homolog in herpesvirus papio. J Virol. 1995;69:8011–8019. doi: 10.1128/jvi.69.12.8011-8019.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fruehling S, Lee S K, Herrold R, Frech B, Laux G, Kremmer E, Grasser F A, Longnecker R. Identification of latent membrane protein 2A (LMP2A) domains essential for the LMP2A dominant-negative effect on B-lymphocyte surface immunoglobulin signal transduction. J Virol. 1996;70:6216–6226. doi: 10.1128/jvi.70.9.6216-6226.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fruehling S, Longnecker R. The immunoreceptor tyrosine-based activation motif of Epstein-Barr virus LMP2A is essential for blocking BCR-mediated signal transduction. Virology. 1997;235:241–251. doi: 10.1006/viro.1997.8690. [DOI] [PubMed] [Google Scholar]
- 12.Fruehling S, Swart R, Dolwick K M, Kremmer E, Longnecker R. Tyrosine 112 of latent membrane protein 2A is essential for protein tyrosine kinase loading and regulation of Epstein-Barr virus latency. J Virol. 1998;72:7796–7806. doi: 10.1128/jvi.72.10.7796-7806.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hochstrasser M. Protein degradation or regulation: Ub the judge. Cell. 1996;84:813–815. doi: 10.1016/s0092-8674(00)81058-2. [DOI] [PubMed] [Google Scholar]
- 14.Ikeda M, Ikeda A, Longan L C, Longnecker R. The Epstein-Barr virus latent membrane protein 2A PY motif recruits WW domain-containing ubiquitin-protein ligases. Virology. 2000;268:178–191. doi: 10.1006/viro.1999.0166. [DOI] [PubMed] [Google Scholar]
- 15.Kieff E. Epstein-Barr virus and its replication. In: Fields B N, Knipe D M, Howley P M, editors. Fundamental virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1109–1162. [Google Scholar]
- 16.Longnecker R. Molecular biology of Epstein-Barr virus. In: McCance D J, editor. Human tumor viruses. Washington, D.C.: American Society for Microbiology; 1998. pp. 133–172. [Google Scholar]
- 17.Longnecker R, Druker B, Roberts T M, Kieff E. An Epstein-Barr virus protein associated with cell growth transformation interacts with a tyrosine kinase. J Virol. 1991;65:3681–3692. doi: 10.1128/jvi.65.7.3681-3692.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Longnecker R, Kieff E. A second Epstein-Barr virus membrane protein (LMP2) is expressed in latent infection and colocalizes with LMP1. J Virol. 1990;64:2319–2326. doi: 10.1128/jvi.64.5.2319-2326.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Longnecker R, Merchant M, Brown M E, Fruehling S, Bickford J O, Ikeda M, Harty R N. WW- and SH3-domain interactions with Epstein-Barr virus LMP2A. Exp Cell Res. 2000;257:332–340. doi: 10.1006/excr.2000.4900. [DOI] [PubMed] [Google Scholar]
- 20.Longnecker R, Miller C L, Miao X Q, Marchini A, Kieff E. The only domain which distinguishes Epstein-Barr virus latent membrane protein 2A (LMP2A) from LMP2B is dispensable for lymphocyte infection and growth transformation in vitro; LMP2A is therefore nonessential. J Virol. 1992;66:6461–6469. doi: 10.1128/jvi.66.11.6461-6469.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Longnecker R, Miller C L, Tomkinson B, Miao X Q, Kieff E. Deletion of DNA encoding the first five transmembrane domains of Epstein-Barr virus latent membrane proteins 2A and 2B. J Virol. 1993;67:5068–5074. doi: 10.1128/jvi.67.8.5068-5074.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller C L, Lee J H, Kieff E, Longnecker R. An integral membrane protein (LMP2) blocks reactivation of Epstein-Barr virus from latency following surface immunoglobulin crosslinking. Proc Natl Acad Sci USA. 1994;91:772–776. doi: 10.1073/pnas.91.2.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyashita E M, Yang B, Babcock G J, Thorley-Lawson D A. Identification of the site of Epstein-Barr virus persistence in vivo as a resting B cell. J Virol. 1997;71:4882–4891. doi: 10.1128/jvi.71.7.4882-4891.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyashita E M, Yang B, Lam K M, Crawford D H, Thorley-Lawson D A. A novel form of Epstein-Barr virus latency in normal B cells in vivo. Cell. 1995;80:593–601. doi: 10.1016/0092-8674(95)90513-8. [DOI] [PubMed] [Google Scholar]
- 25.Qu L, Rowe D T. Epstein-Barr virus latent gene expression in uncultured peripheral blood lymphocytes. J Virol. 1992;66:3715–3724. doi: 10.1128/jvi.66.6.3715-3724.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rickinson A B, Kieff E. Epstein-Barr virus. In: Fields B N, Knipe D M, Howley P M, editors. Virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2397–2446. [Google Scholar]
- 27.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 28.Speck P, Kline K A, Cheresh P, Longnecker R. Epstein-Barr virus lacking latent membrane protein 2 immortalizes B cells with efficiency indistinguishable from that of wild-type virus. J Gen Virol. 1999;80:2193–2203. doi: 10.1099/0022-1317-80-8-2193. [DOI] [PubMed] [Google Scholar]
- 29.Swart R, Fruehling S, Longnecker R. Tyrosines 60, 64, and 101 of Epstein-Barr virus LMP2A are not essential for blocking B cell signal transduction. Virology. 1999;263:485–495. doi: 10.1006/viro.1999.9964. [DOI] [PubMed] [Google Scholar]
- 30.Thorley-Lawson D A, Miyashita E M, Khan G. Epstein-Barr virus and the B cell: that's all it takes. Trends Microbiol. 1996;4:204–208. doi: 10.1016/s0966-842x(96)90020-7. [DOI] [PubMed] [Google Scholar]
- 31.Tierney R J, Steven N, Young L S, Rickinson A B. Epstein-Barr virus latency in blood mononuclear cells: analysis of viral gene transcription during primary infection and in the carrier state. J Virol. 1994;68:7374–7385. doi: 10.1128/jvi.68.11.7374-7385.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winberg G, Matskova L, Chen F, Plant P, Rotin D, Gish G, Ingham R, Ernberg I, Pawson T. Latent membrane protein 2A of Epstein-Barr virus binds WW domain E3 protein-ubiquitin ligases that ubiquitinate B-cell tyrosine kinases. Mol Cell Biol. 2000;20:8526–8535. doi: 10.1128/mcb.20.22.8526-8535.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Young L, Alfieri C, Hennessy K, Evans H, O'Hara C, Anderson K C, Ritz J, Shapiro R S, Rickinson A, Kieff E. Expression of Epstein-Barr virus transformation-associated genes in tissues of patients with EBV lymphoproliferative disease. N Engl J Med. 1989;321:1080–1085. doi: 10.1056/NEJM198910193211604. [DOI] [PubMed] [Google Scholar]