Abstract
Objective
To determine whether weekly oral vitamin D supplementation influences grip strength, explosive leg power, cardiorespiratory fitness or spirometric lung volumes in Mongolian schoolchildren.
Methods
Multicentre, randomised, placebo-controlled clinical trial conducted in children aged 6–13 years at baseline attending 18 schools in Ulaanbaatar. The intervention was weekly oral doses of 14 000 IU vitamin D3 (n=4418) or placebo (n=4433) for 3 years. Outcome measures were grip strength, standing long jump distance and serum 25-hydroxyvitamin D (25(OH)D) concentrations (determined in all participants), peak oxygen uptake (VO2peak, determined in a subset of 632 participants using 20 m multistage shuttle run tests) and spirometric outcomes (determined in a subset of 1343 participants).
Results
99.8% of participants had serum 25(OH)D concentrations <75 nmol/L at baseline, and mean end-study 25(OH)D concentrations in children randomised to vitamin D versus placebo were 77.4 vs 26.7 nmol/L (mean difference 50.7 nmol/L, 95% CI 49.7 to 51.4). However, vitamin D supplementation did not influence mean grip strength, standing long jump distance, VO2peak, spirometric lung volumes or peak expiratory flow rate, either overall or within subgroups defined by sex, baseline 25(OH)D concentration <25 vs ≥25 nmol/L or calcium intake <500 vs ≥500 mg/day.
Conclusion
A 3-year course of weekly oral supplementation with 14 000 IU vitamin D3 elevated serum 25(OH)D concentrations in Mongolian schoolchildren with a high baseline prevalence of vitamin D deficiency. However, this intervention did not influence grip strength, explosive leg power, peak oxygen uptake or spirometric lung volumes, either overall or in subgroup analyses.
Trial registration number
Keywords: Nutrition, Fitness testing, Children's health and exercise
WHAT IS ALREADY KNOWN ON THIS TOPIC
Observational studies have reported that vitamin D deficiency associates with reduced muscle strength and peak oxygen uptake in children, but randomised controlled trials (RCT) of vitamin D supplementation to improve grip strength and cardiorespiratory fitness in this age group have yielded conflicting results.
WHAT THIS STUDY ADDS
This phase 3 multicentre RCT of vitamin D supplementation, conducted in Mongolian schoolchildren with a high baseline prevalence of asymptomatic vitamin D deficiency, found that a 3-year course of weekly oral supplementation with 14 000 IU vitamin D3 was effective in elevating serum 25-hydroxyvitamin D concentrations. However, this intervention did not influence participants’ grip strength, long jump distance, peak oxygen uptake, spirometric lung volumes or peak expiratory flow rate, either overall or in subgroup analyses.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Taken together with results from another phase 3 randomised controlled trial of vitamin D supplementation conducted in South African children, our findings do not suggest a role for weekly oral vitamin D supplementation to enhance muscle strength, peak oxygen uptake or respiratory function in schoolchildren in whom rickets has been excluded.
Introduction
Muscular strength and exercise tolerance in childhood are positive correlates of physical and mental health that are associate with reduced cardiometabolic risk later in life.1,4 Vitamin D plays a key role in supporting normal development and function of skeletal muscle, the lung and the heart.5,7 Vitamin D deficiency is common among children living in higher-income and lower-income countries alike8,11 and has been reported to be associated with lower muscle strength, poorer cardiorespiratory fitness and lower spirometric lung volumes in children and adolescents participating in observational studies.12,14 Numerous randomised controlled trials (RCTs) of vitamin D supplementation to improve muscle strength and power have been conducted in adults, with meta-analysis revealing small positive impacts.15 Fewer RCTs have been conducted in children, and these have yielded inconsistent results. One trial in male soccer players in Tunisia aged 8–15 years has reported improvements in jump, sprint and shuttle run outcomes16 while another conducted in girls aged 12–14 year-olds in the UK reported a statistically significant improvement in efficiency of movement, with trends towards improvements in jumping velocity and grip strength.17 Other RCTs conducted in children and adolescents living in Denmark,18 19 the USA,20 Israel21 and Lebanon22 have reported null overall effects for outcomes including grip strength, leg press strength and swimming performance. No such trials have yet been conducted in Asia; moreover, there is a lack of large, multicentre trials examining the impact of prolonged (greater than 1 year) vitamin D supplementation on muscle strength, cardiorespiratory fitness and spirometric outcomes in children with a high baseline prevalence of vitamin D deficiency, regardless of setting.
Given these limitations of the existing evidence base, we took the opportunity to generate new data in this area during the conduct of a phase 3 RCT of vitamin D supplementation in 8851 schoolchildren aged 6–13 years living in Mongolia who were given weekly oral vitamin D supplementation over 3 years.23,25 The primary aim of the trial was to test whether this intervention reduced the risk of incident tuberculosis infection; null results for this outcome have been reported elsewhere.23 This paper reports findings for secondary outcomes that were investigated to test the hypotheses that vitamin D supplementation would improve grip strength and standing long jump distance, measured at annual intervals in all participants; peak oxygen uptake (VO2peak), estimated using 20 m shuttle run tests performed at annual intervals in a subset of 632 participants; and spirometric lung volumes, measured at 3-year follow-up in a subset of 1343 participants.
Methods
Study design, setting, participants, randomisation and intervention
We conducted a parallel two-arm individually randomised placebo-controlled trial in 18 public schools in Ulaanbaatar, Mongolia, as previously described.23,25 The study protocol is available in online supplemental file 1. The primary outcome of the trial was the acquisition of latent tuberculosis infection; the current manuscript reports the effects of the intervention on prespecified secondary outcomes relating to grip strength, explosive leg power, peak oxygen uptake and spirometric outcomes. Principal inclusion criteria were age 6–13 years at screening and attendance at a participating school; principal exclusion criteria were a positive QuantiFERON-TB Gold in-tube assay (QFT) result, presence of conditions associated with vitamin D hypersensitivity (primary hyperparathyroidism or sarcoidosis) or immunocompromise (taking immunosuppressant medication or cytotoxic therapy), use of vitamin D supplements, signs of rickets (all participants were screened for rickets via physical examination by a paediatrician), or intention to move from Ulaanbaatar within 4 years of enrolment. Trial staff who determined whether subjects were eligible for inclusion in the trial were unaware which group the next subject would be allocated to, that is, allocation was concealed. Eligible participants were individually randomised to receive a weekly capsule containing 14 000 IU (350 µg) vitamin D3 or a weekly capsule of placebo (olive oil) for 3 years, with a one-to-one allocation ratio and stratification by school of attendance. Capsules containing vitamin D3 had identical appearance and taste to placebo capsules. The dose was chosen on the basis of results of another RCT showing that a weekly oral administration of 14 000 IU vitamin D3 to schoolchildren aged 10–17 years for 1 year resulted in a 52.5 nmol/L increase in mean 25(OH)D concentration without inducing adverse events.26
The randomisation list was computer generated by a statistician (Dr Polyna Khudyakov, Sage Therapeutics, Cambridge, Massachusett, USA) prior to the start of recruitment. This list was held by the data monitoring committee during the conduct of the trial: concealment of allocation was achieved by ensuring that no trial staff (including those who assessed eligibility) had access to the trial randomisation list. All participants and their parents/guardians, and all trial personnel, including principal investigators and all those who had contact with study participants, including those who assessed outcomes, were blinded to participant allocation during the conduct of the trial. The trial is registered with ClinicalTrials.gov (NCT02276755).
Baseline procedures
At baseline, participants’ parents were asked to complete questionnaires detailing their socioeconomic circumstances, lifestyle and dietary factors influencing vitamin D status, and intake of foods previously shown to be major contributors to dietary calcium intake in urban Mongolia.27 For all participants, height was then measured using a portable stadiometer (SECA, Hamburg, Germany), weight was measured using a Digital Floor Scale (SECA), grip strength was measured as described elsewhere28 using a portable dynamometer (Takei Digital Grip Strength Dynamometer, Model T.K.K.5401, with the best of two readings for the dominant hand recorded, except where injury precluded measurement, where strength of the other hand was measured), and standing long jump distance was measured as described elsewhere29 using a DiCUNO measuring tape, with the best of two readings recorded. Validity and reliability of the Takei Digital Grip Strength Dynamometer for measurement of grip strength, and of the standing long jump for measurement of lower body muscular power have both been demonstrated in schoolchildren studied in other settings.30 31 In a subset of 620 children who additionally participated in the exercise substudy, a 20 m multistage shuttle run test was administered as described below to determine VO2peak. A blood sample was drawn from all participants at baseline for separation and storage of serum for determination of baseline 25(OH)D concentrations as described below.
Follow-up procedures and outcomes
During school terms, study participants had weekly face-to-face visits at which study capsules were administered and adverse events were recorded. During school holidays, children were either given a single bolus dose of up to 36 000 IU (shorter holidays), study staff travelled to participants’ homes to administer medication, or parents were supplied with sufficient trial medication to cover the holiday period, along with instructions on its storage and administration. Using the same methods as at baseline, all participants were reassessed at 12-month, 24-month and 36-month follow-ups for grip strength and long jump distance. Exercise substudy participants additionally completed 20 m multistage shuttle run tests at 12-month, 24-month and 36-month follow-up, as described below. Spirometry substudy participants additionally underwent spirometry testing at 36-month follow-up using a portable spirometer (spirolab III, Medical International Research, Rome, Italy) and performed according to ERS/ATS standards32 to assess % predicted forced expiratory volume in 1 s (FEV1), % predicted forced vital capacity (FVC), % predicted FEV1/FVC, % predicted peak expiratory flow rate (PEFR) and % predicted forced expiratory flow over the middle one-half of the FVC (FEF25–75).
Measurement of vitamin D status
25(OH)D concentrations were determined in serum samples from baseline and 3-year follow-up using an enzyme-linked fluorescent assay (VIDAS 25OH Vitamin D total, bioMérieux, Marcy-l’Étoile, France). Non-zero 25(OH)D values were standardised using a published method,33 using a set of 40 serum samples provided by DEQAS (the Vitamin D External Quality Assessment Scheme, http://www.deqas.org/). The total coefficient of variation (CV) was 7.9%, the mean bias was 7.7% and the limit of quantitation (LOQ) was 20.2 nmol/L. Values below the LOQ were classified as <20.2 nmol/L.
Exercise test
A 20 m multistage shuttle run test was conducted using freely available recorded instruction (Shuttle run bleep test, www.bleeptests.com). Two lines were marked 20 m apart and an audible ‘beep’ signalled to participants the speed required to run between them. Participants’ number of completed laps was used to derive their estimated VO2peak using a published formula.34
Sample size and statistical methods
All analyses were conducted by using STATA V.IC 15.1 (StataCorp). The sample size calculation for the main trial was based on the power needed to detect a clinically significant effect of the intervention on the primary endpoint (incident latent tuberculosis infection) as described previously.20 For the exercise substudy, assuming an SD for VO2peak of 6.5 mL/kg/min at 3-year follow-up,34 we calculated that a total of 334 participants (167 per arm) would need to be recruited and followed up to have 80% power to detect a clinically significant difference of 2 mL/kg/min between arms with 5% alpha. Allowing for a 20% loss to follow-up, we originally estimated that a total of 420 participants (210 per arm) would need to be recruited for the exercise substudy. Subsequently, we became concerned that rates of loss to follow-up might be higher than originally anticipated, and consequently, the target sample size for this substudy was increased to 614. Ultimately, the exercise substudy over-recruited slightly (n=632). Spirometry was performed for a subset of children who participated in a bone health substudy, powered to detect an effect of the intervention on the radial speed of sound Z-scores as described elsewhere.25
Estimated calcium intakes were calculated on the basis of parental responses to a food frequency questionnaire, as described elsewhere.24 Anthropometric measurements and data on participants’ age and sex were used to compute Z-scores for height-for-age and BMI-for-age, using the Canadian Pediatric Endocrine Group who2007 Shiny App (https://cpeg-gcep.shinyapps.io/who2007_cpeg/) based on WHO 2007 growth reference data for 5–19 years.35 Serum 25(OH)D values were adjusted for seasonal variation prior to analysis using a sinusoidal model.36 Outcomes measured over multiple years’ follow-up were analysed overall and in each subgroup using mixed models for repeated measures with fixed effects for treatment and time and treatment-by-time interaction, adjusted for school of attendance and random effects for individuals. Adjusted treatment mean differences at different time points are presented with 95% CIs, and significance tests were conducted for the treatment effect at each time point and the overall treatment-by-time interaction. Where overall p values were less than 0.05, we applied a Benjamini Hochberg procedure with a 10% false discovery rate37 to the relevant family of p values to adjust for multiple comparisons. Outcomes measured at end-study only were analysed using general linear models, adjusted for school of attendance. Prespecified subgroup analyses were conducted according to participants’ sex (males vs females), baseline deseasonalised 25(OH)D concentration (<25 nmol/L vs ≥25 nmol/L) and estimated calcium intake (<500 mg/day vs ≥500 mg/day). The primary p values for outcome modelling were the overall p values, that is, those associated with the interaction between follow-up time point and treatment allocation.
Patient and public involvement
We consulted children and their parents/guardians on questionnaire design and acceptability of clinical measurements prior to implementation of the trial. Study findings were disseminated via community engagement events.
Equity, diversity and inclusion statement
This trial recruited a group of participants who are under-represented in research (schoolchildren in Mongolia). Participation was open to children of any gender, race/ethnicity/culture and socioeconomic level. The author team is gender-balanced and nationality-balanced and includes junior researchers and perspectives from multiple disciplines. Subgroup analyses were conducted to detect gender differences in response to vitamin D supplementation.
Results
Participants
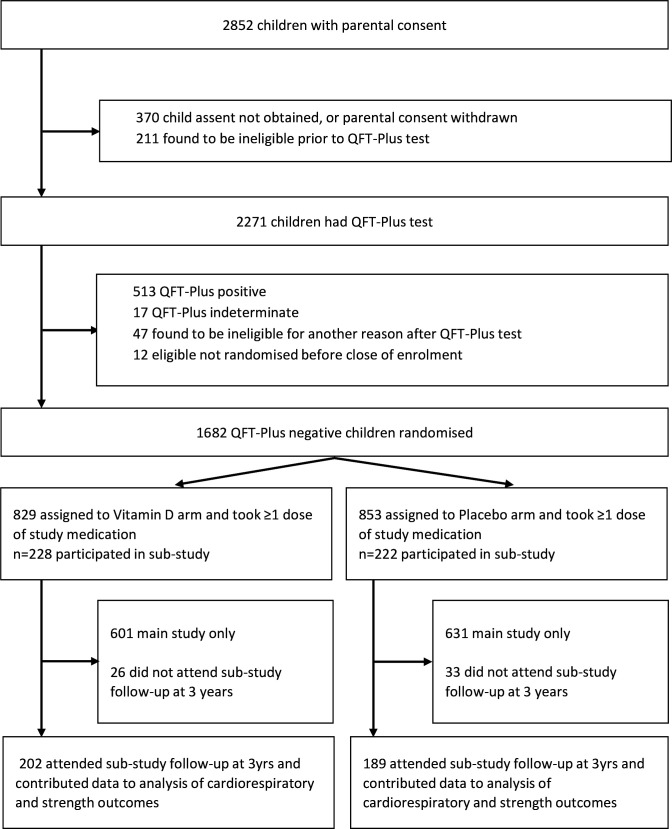
11 475 children were invited to participate in the study from September 2015 to March 2017 inclusive, of whom 9814 underwent QFT testing: 8851 QFT-negative children were randomly assigned to receive vitamin D or placebo (4418 vs 4433, respectively) as previously described.23 Of these, 632 children (302 vs 330 randomised to vitamin D vs placebo, respectively) also participated in the exercise substudy, and 1343 children (666 vs 677 randomised to vitamin D vs placebo, respectively) underwent spirometry at 3-year follow-up (figure 1). Table 1 shows participants’ baseline characteristics: overall, mean age was 9.4 years and 49.3% were female. Mean deseasonalised baseline serum 25(OH)D concentration was 29.7 nmol/L (SD 10.5). Baseline characteristics were well balanced between participants randomised to vitamin D versus placebo, both for those in the main trial and for participants contributing data to the exercise and spirometry substudies. Mean serum 25(OH)D concentration at 3-year follow-up was higher in the vitamin D group versus the placebo group (77.4 vs 26.7 nmol/L, respectively; mean difference 50.7 nmol/L, 95% CI 49.7 to 51.4). 8549 participants contributed data to the analysis of grip strength; 8539 to the analysis of long jump distance; 611 to the analysis of cardiorespiratory fitness and 1343 to the analysis of spirometric outcomes (figure 1).
Figure 1. Trial profile. QFT, QuantiFERON-TB Gold in-tube assay.
Table 1. Participants’ baseline characteristics by allocation and overall: main trial, exercise substudy and spirometry substudy.
| Main trial | Exercise substudy | Spirometry substudy | |||||||
| Placebo | Vitamin D | Total | Placebo | Vitamin D | Total | Placebo | Vitamin D | Total | |
| N=4433 | N=4418 | N=8851 | N=330 | N=302 | N=632 | N=677 | N=666 | N=1343 | |
| Mean age, years (SD) (range) | 9.4 (1.6)(5.9–13.7) | 9.4 (1.6)(5.8–14.2) | 9.4 (1.6)(5.8–14.2) | 9.5 (1.5)(6.5–12.8) | 9.5 (1.5)(5.8–13.9) | 9.5 (1.5)(5.8–13.9) | 9.5 (1.5)(6.5–13.0) | 9.5 (1.6)(6.4–13.3) | 9.5 (1.6)(6.4–13.3) |
| Sex, n (%) | |||||||||
| Female | 2224 (50.2) | 2142 (48.5) | 4366 (49.3) | 166 (50.3) | 141 (46.7) | 307 (48.6) | 325 (48.0) | 333 (50.0) | 658 (49.0) |
| Male | 2209 (49.8) | 2276 (51.5) | 4485 (50.7) | 164 (49.7) | 161 (53.3) | 325 (51.4) | 352 (52.0) | 333 (50.0) | 685 (51.0) |
| Ethnic origin | |||||||||
| Khalkh, n (%) | 4103 (92.6) | 4062 (91.9) | 8165 (92.2) | 300 (90.9) | 275 (91.1) | 575 (91.0) | 628 (92.8) | 607 (91.1) | 1235 (92.0) |
| Other, n (%) | 330 (7.4) | 356 (8.1) | 686 (7.8) | 30 (9.1) | 27 (8.9) | 57 (9.0) | 49 (7.2) | 59 (8.9) | 108 (8.0) |
| Parental education* | |||||||||
| Secondary school or lower, n (%) | 2457 (55.4) | 2401 (54.3) | 4858 (54.9) | 201 (60.9) | 171 (56.6) | 372 (58.9) | 417 (61.6) | 398 (59.8) | 815 (60.7) |
| University/polytechnic, n (%) | 1976 (44.6) | 2017 (45.7) | 3993 (45.1) | 129 (39.1) | 131 (43.4) | 260 (41.1) | 260 (38.4) | 268 (40.2) | 528 (39.3) |
| Type of residence | |||||||||
| House with central heating, n (%) | 1083 (24.4) | 1110 (25.1) | 2193 (24.8) | 62 (18.8) | 54 (17.9) | 116 (18.4) | 100 (14.8) | 99 (14.9) | 199 (14.8) |
| Ger (Yurt), n (%) | 1628 (36.7) | 1643 (37.2) | 3271 (37.0) | 112 (33.9) | 106 (35.1) | 218 (34.5) | 277 (40.9) | 290 (43.5) | 567 (42.2) |
| House without central heating, n (%) | 1722 (38.8) | 1665 (37.7) | 3387 (38.3) | 156 (47.3) | 142 (47.0) | 298 (47.2) | 300 (44.3) | 277 (41.6) | 577 (43.0) |
| Home ownership, n (%) | |||||||||
| No | 963 (21.7) | 925 (20.9) | 1888 (21.3) | 77 (23.3) | 55 (18.2) | 132 (20.9) | 164 (24.2) | 157 (23.6) | 321 (23.9) |
| Yes | 3470 (78.3) | 3493 (79.1) | 6963 (78.7) | 253 (76.7) | 247 (81.8) | 500 (79.1) | 513 (75.8) | 509 (76.4) | 1022 (76.1) |
| Mean monthly household income, US dollars (SD)† | 845.8 (603.7) | 850.6 (553.8) | 848.2 (579.3) | 769.5 (411.6) | 844.1 (515.3) | 805.1 (465.2) | 751.5 (441.2) | 778.0 (427.8) | 764.6 (434.7) |
| Calcium intake† | |||||||||
| <500 mg/day, n (%) | 2628 (64.6) | 2599 (63.7) | 5227 (64.2) | 190 (63.8) | 179 (63.3) | 369 (63.5) | 423 (63.4) | 435 (66.1) | 858 (64.8) |
| ≥500 mg/day, n (%) | 1437 (35.4) | 1481 (36.3) | 2918 (35.8) | 108 (36.2) | 104 (36.7) | 212 (36.5) | 244 (36.6) | 223 (33.9) | 467 (35.2) |
| Mean BMI-for-age z-score (SD)† | 0.2 (1.1) | 0.2 (1.0) | 0.2 (1.1) | 0.2 (1.1) | 0.1 (1.1) | 0.2 (1.1) | 0.1 (1.0) | 0.1 (1.1) | 0.1 (1.0) |
| Mean height-for-age z-score (SD)† | −0.3 (1.0) | −0.3 (1.0) | −0.3 (1.0) | −0.3 (1.0) | −0.3 (1.1) | −0.3 (1.0) | −0.4 (0.9) | −0.3 (1.0) | −0.4 (1.0) |
| Mean serum 25(OH)D, nmol/L (SD)2 3 | 29.7 (10.5) | 29.5 (10.5) | 29.7 (10.5) | 28.6 (11.5) | 27.5 (10.5) | 28.1 (11.0) | 28.5 (10.5) | 28.1 (10.7) | 28.3 (10.6) |
| Serum 25(OH)D category†‡ | |||||||||
| <25 nmol/L, n (%) | 1424 (32.1) | 1395 (31.6) | 2819 (31.8) | 126 (38.2) | 116 (38.4) | 242 (38.3) | 253 (37.4) | 232 (34.8) | 485 (36.1) |
| 25–49.9 nmol/L, n (%) | 2809 (63.4) | 2827 (64.0) | 5636 (63.7) | 189 (57.3) | 174 (57.6) | 363 (57.4) | 399 (58.9) | 408 (61.3) | 807 (60.1) |
| 50–74.9 nmol/L, n (%) | 193 (4.4) | 187 (4.2) | 380 (4.3) | 14 (4.2) | 11 (3.6) | 25 (4.0) | 25 (3.7) | 26 (3.9) | 51 (3.8) |
| ≥75 nmol/L, n (%) | 7 (0.2) | 9 (0.2) | 16 (0.2) | 1 (0.3) | 1 (0.3) | 2 (0.3) | – | – | – |
| Mean maximal grip strength, kg (SD)† | 12.0 (4.0) | 12.0 (4.0) | 12.0 (4.0) | 12.2 (3.9) | 12.4 (3.8) | 12.3 (3.9) | 12.0 (3.9) | 12.3 (4.1) | 12.1 (4.0) |
| Mean standing long jump distance, m (SD)† | 107.9 (21.8) | 108.2 (22.6) | 108.0 (22.2) | 106.7 (21.6) | 108.8 (21.2) | 107.7 (21.5) | 107.4 (21.7) | 107.9 (23.0) | 107.6 (22.4) |
| Mean VO2peak, mL/kg/min (SD)† | – | – | – | 43.8 (3.1) | 44.2 (3.3) | 44.0 (3.2) | – | – | – |
Highest educational level attained by either parent;.
Missing values: monthly household income missing for 2 participants in the main trial (1 randomised to placebo, 1 to vitamin D); calcium intake missing for 706 participants in the main trial (368 randomised to placebo, 338 to vitamin D), 51 participants in the exercise substudy (32 randomised to placebo, 19 to vitamin D) and 18 participants in the spirometry substudy (18 randomised to placebo, 10 to vitamin D); BMI-for-age z-score missing for one1 participant in the main trial (randomised to placebo); height-for-age z-score missing for one1 participant in the main trial (randomised to placebo); 25(OH)D missing for 5 participants in the main trial (4 randomised to placebo, and 1 to vitamin D); grip strength missing for 6 participants in the main trial (2 randomised to placebo, 4 to vitamin D) and 1 participant in the spirometry substudy (randomised to placebo); long jump distance missing for 38 participants in the main trial (23 randomised to placebo, 15 to vitamin D), 2 participants in the exercise substudy (both randomised to placebo) and 3 participants in the spirometry substudy (2 randomised to placebo, and 1 to vitamin D); VO2 peak missing for 17 participants in the exercise substudy (11 randomised to placebo, and 6 to vitamin D).
Baseline 25(OH)D values deseasonalised.
BMIbody mass index25(OH)D25-hydroxyvitamin DSOSspeed of soundVO2peakpeak oxygen consumption
Outcomes
Allocation to vitamin D versus placebo did not influence mean grip strength, either overall or in subgroups defined by male versus female sex, baseline 25(OH)D concentration <25 vs ≥25 nmol/L or estimated calcium intake <500 vs ≥500 mg/day (table 2). Similarly, no effect of the intervention was seen on long jump distance, either overall or by subgroup, after correction for multiple comparison testing (table 3). Among exercise substudy participants, allocation to vitamin D versus placebo did not influence mean VO2peak, either overall or within subgroups defined by male versus female sex, baseline 25(OH)D concentration<25 vs ≥25 nmol/L or estimated calcium intake <500 vs ≥500 mg/day (table 4). Among participants who underwent spirometry at 3-year follow-up, allocation to vitamin D versus placebo did not influence % predicted FEV1, FVC, FEV1/FVC, PEFR or FEF25–75 after correction for multiple comparison testing, either overall or within subgroups defined by male versus female sex, baseline 25(OH)D concentration <25 vs ≥25 nmol/L or estimated calcium intake <500 vs ≥500 mg/day (table 5).
Table 2. Mean grip strength in main trial participants at 1, 2 and 3 years follow-up by allocation: overall and by subgroup.
| Vitamin D arm: mean strength, kg (SD) (n) | Placebo arm: mean strength, kg (SD) (n) | Adjusted mean difference (95% CI)* | P for time point | Overall p value | P for interaction | |
| Overall | ||||||
| 1 year | 15.0 (4.9) (4219) | 14.9 (4.8) (4229) | 0.03 (−0.19, 0.25) | 0.79 | 0.999 | -- |
| 2 years | 17.1 (5.5) (3802) | 17.3 (5.5) (3829) | −0.09 (−0.31, 0.13) | 0.41 | ||
| 3 years | 20.0 (6.4) (4074) | 20.0 (6.4) (4054) | 0.01 (−0.21, 0.23) | 0.94 | ||
| By sex | ||||||
| Male | ||||||
| 1 year | 15.6 (5.0) (2184) | 15.5 (4.8) (2103) | 0.10 (−0.22, 0.42) | 0.53 | 0.90 | 0.55 |
| 2 years | 17.9 (5.8) (1952) | 18.0 (5.8) (1902) | −0.07 (−0.40, 0.26) | 0.67 | ||
| 3 years | 21.4 (7.2) (2100) | 21.4 (7.1) (2010) | −0.00 (−0.32, 0.32) | 0.99 | ||
| Female | ||||||
| 1 year | 14.2 (4.7) (2035) | 14.3 (4.7) (2126) | −0.10 (−0.38, 0.18) | 0.47 | 0.48 | |
| 2 years | 16.2 (5.1) (1850) | 16.5 (5.1) (1927) | −0.19 (−0.47, 0.09) | 0.19 | ||
| 3 years | 18.6 (5.1) (1974) | 18.7 (5.2) (2044) | −0.10 (−0.38, 0.18) | 0.5 | ||
| By baseline 25(OH)D concentration† | ||||||
| <25 nmol/L | ||||||
| 1 year | 15.5 (5.2) (1342) | 15.5 (5.3) (1352) | 0.05 (−0.33, 0.44) | 0.78 | 0.73 | 0.75 |
| 2 years | 17.7 (5.9) (1214) | 17.9 (6.0) (1226) | −0.05 (−0.44, 0.34) | 0.81 | ||
| 3 years | 20.4 (6.7) (1291) | 20.5 (6.7) (1309) | 0.14 (−0.24, 0.53) | 0.47 | ||
| ≥25 nmol/L | ||||||
| 1 year | 14.7 (4.7) (2876) | 14.6 (4.5) (2873) | 0.08 (−0.17, 0.33) | 0.53 | 0.77 | |
| 2 years | 16.8 (5.3) (2587) | 16.9 (5.3) (2600) | −0.05 (−0.31, 0.21) | 0.71 | ||
| 3 years | 19.8 (6.3) (2782) | 19.8 (6.2) (2742) | 0.02 (−0.24, 0.27) | 0.91 | ||
| By calcium intake | ||||||
| <500 mg/day | ||||||
| 1 year | 15.0 (4.8) (1463) | 14.9 (4.8) (1422) | 0.12 (−0.16, 0.40) | 0.41 | 0.51 | 0.20 |
| 2 years | 17.2 (5.6) (1355) | 17.2 (5.6) (1305) | −0.00 (−0.29, 0.29) | 0.998 | ||
| 3 years | 20.2 (6.4) (1444) | 20.0 (6.5) (1410) | 0.18 (−0.10, 0.46) | 0.21 | ||
| ≥500 mg/day | ||||||
| 1 year | 14.8 (5.0) (2572) | 15.0 (4.7) (2596) | 0.12 (−0.16, 0.40) | 0.41 | 0.38 | |
| 2 years | 16.8 (5.5) (2336) | 17.3 (5.5) (2400) | −0.00 (−0.29, 0.29) | 0.998 | ||
| 3 years | 19.7 (6.5) (2549) | 20.1 (6.2) (2561) | 0.18 (−0.10, 0.46) | 0.21 | ||
Adjusted for baseline value and school of attendance.
Deseasonalised values.
Nnumber25(OH)D25-hydroxyvitamin D
Table 3. Mean long jump distance in main trial participants at 1, 2 and 3 years follow-up by allocation: overall and by subgroup.
| Vitamin D arm: mean distance, m (SD) (n) | Placebo arm: mean distance, m (SD) (n) | Adjusted mean difference (95% CI)* | P for time point | Overall p value | P for interaction | |
| Overall | ||||||
| 1 year | 117.6 (22.4) (4206) | 117.1 (21.9) (4201) | 0.47 (−0.50, 1.45) | 0.34 | 0.33 | -- |
| 2 years | 124.2 (23.5) (3790) | 124.0 (22.8) (3809) | 0.42 (−0.57, 1.41) | 0.41 | ||
| 3 years | 132.0 (26.4) (4012) | 131.3 (25.4) (3988) | 0.76 (−0.22, 1.74) | 0.13 | ||
| By sex | ||||||
| Male | ||||||
| 1 year | 125.9 (22.2) (2177) | 124.9 (21.6) (2094) | 0.71 (−0.66, 2.08) | 0.31 | 0.17 | 0.04† |
| 2 years | 134.2 (23.7) (1944) | 133.1 (23.0) (1895) | 1.10 (−0.30, 2.50) | 0.13 | ||
| 3 years | 144.4 (26.9) (2065) | 142.9 (25.8) (1984) | 1.32 (−0.06, 2.70) | 0.06 | ||
| Female | ||||||
| 1 year | 108.8 (18.9) (2029) | 109.4 (19.2) (2107) | −0.40 (−1.53, 0.72) | 0.48 | 0.18 | |
| 2 years | 113.6 (18.0) (1846) | 115.0 (18.6) (1914) | −1.01 (−2.17, 0.14) | 0.09 | ||
| 3 years | 118.8 (18.3) (1947) | 119.9 (18.9) (2004) | −0.73 (−1.87, 0.41) | 0.21 | ||
| By baseline 25(OH)D concentration‡ | ||||||
| <25 nmol/L | ||||||
| 1 year | 119.2 (22.6) (1339) | 119.3 (22.1) (1343) | 0.32 (−1.42, 2.05) | 0.72 | 0.78 | 0.54 |
| 2 years | 125.6 (24.1) (1212) | 126.1 (23.9) (1219) | 0.51 (−1.26, 2.28) | 0.58 | ||
| 3 years | 133.3 (26.8) (1268) | 133.3 (25.4) (1288) | 0.80 (−0.96, 2.55) | 0.37 | ||
| ≥25 nmol/L | ||||||
| 1 year | 116.9 (22.2) (2866) | 116.1 (21.7) (2854) | 0.65 (−0.51, 1.81) | 0.27 | 0.23 | |
| 2 years | 123.5 (23.2) (2577) | 123.0 (22.2) (2587) | 0.48 (−0.71, 1.67) | 0.43 | ||
| 3 years | 131.4 (26.2) (2743) | 130.4 (25.3) (2697) | 0.85 (−0.33, 2.02) | 0.16 | ||
| By calcium intake | ||||||
| <500 mg/day | ||||||
| 1 year | 117.7 (22.0) (1461) | 117.3 (21.9) (1412) | 0.34 (−0.90, 1.59) | 0.59 | 0.42 | 0.84 |
| 2 years | 124.3 (23.3) (1355) | 123.8 (22.6) (1301) | 0.55 (−0.72, 1.81) | 0.40 | ||
| 3 years | 131.8 (26.2) (1422) | 131.1 (25.2) (1382) | 0.65 (−0.60, 1.89) | 0.31 | ||
| ≥500 mg/day | ||||||
| 1 year | 117.5 (22.8) (2562) | 116.7 (21.9) (2579) | 0.96 (−0.76, 2.67) | 0.28 | 0.49 | |
| 2 years | 124.0 (23.9) (2329) | 124.3 (23.1) (2385) | 0.31 (−1.44, 2.05) | 0.73 | ||
| 3 years | 132.5 (27.0) (2510) | 131.7 (25.7) (2527) | 1.30 (−0.42, 3.03) | 0.14 | ||
Adjusted for baseline value and school of attendance.
After correction for multiple comparisons testing using the Benjamini and Hochberg method and a false discovery rate of 10%, this P-p value for interaction did not remain significant (critical threshold of significance for family of P-p values was <0.03).
Deseasonalised values.
Nnumber25(OH)D25-hydroxyvitamin D
Table 4. Mean VO2peak in exercise substudy participants at 1, 2 and 3 years follow-up by allocation: overall and by subgroup.
| Vitamin D arm: mean VO2peak, ml/kg/min (SD) (n) | Placebo arm: mean VO2peak, ml/kg/min (SD) (n) | Adjusted mean difference (95% CI)* | P for time point | Overall p value | P for interaction | |
| Overall | ||||||
| 1 year | 43.2 (3.7) (278) | 43.0 (3.4) (296) | 0.27 (−0.30, 0.84) | 0.36 | 0.28 | -- |
| 2 years | 41.9 (3.9) (268) | 41.9 (3.7) (289) | 0.20 (−0.37, 0.78) | 0.48 | ||
| 3 years | 41.3 (4.2) (268) | 41.3 (4.1) (286) | 0.05 (−0.53, 0.62) | 0.87 | ||
| By sex | ||||||
| Male | ||||||
| 1 year | 45.0 (3.1) (151) | 44.5 (3.1) (144) | 0.61 (−0.09, 1.32) | 0.09 | 0.06 | 0.06 |
| 2 years | 43.9 (3.1) (144) | 43.5 (3.3) (143) | 0.55 (−0.17, 1.26) | 0.13 | ||
| 3 years | 43.6 (3.0) (143) | 43.2 (3.8) (143) | 0.46 (−0.25, 1.18) | 0.20 | ||
| Female | ||||||
| 1 year | 41.1 (3.3) (127) | 41.6 (3.1) (152) | −0.32 (−1.05, 0.40) | 0.38 | 0.47 | |
| 2 years | 39.7 (3.5) (124) | 40.2 (3.4) (146) | −0.40 (−1.13, 0.32) | 0.28 | ||
| 3 years | 38.7 (3.8) (125) | 39.4 (3.5) (143) | −0.68 (−1.41, 0.04) | 0.07 | ||
| By baseline 25(OH)D concentration† | ||||||
| <25 nmol/L | ||||||
| 1 year | 43.0 (4.2) (103) | 43.0 (3.2) (113) | −0.11 (−1.05, 0.83) | 0.82 | 0.44 | 0.06 |
| 2 years | 41.3 (4.0) (104) | 41.9 (3.7) (107) | −0.62 (−1.56, 0.32) | 0.20 | ||
| 3 years | 40.8 (4.6) (100) | 41.3 (4.1) (108) | −0.64 (−1.59, 0.30) | 0.18 | ||
| ≥25 nmol/L | ||||||
| 1 year | 43.4 (3.5) (175) | 43.0 (3.5) (183) | 0.50 (−0.22, 1.22) | 0.18 | 0.050 | |
| 2 years | 42.4 (3.7) (164) | 41.8 (3.7) (182) | 0.71 (−0.02, 1.43) | 0.06 | ||
| 3 years | 41.6 (3.9) | 41.3 (4.1) (178) | 0.46 (−0.26, 1.19) | 0.21 | ||
| By calcium intake | ||||||
| <500 mg/day | ||||||
| 1 year | 43.2 (3.9) (98) | 42.8 (3.5) (102) | 0.36 (−0.41, 1.14) | 0.36 | 0.18 | 0.60 |
| 2 years | 42.0 (4.1) (99) | 41.5 (3.7) (102) | 0.48 (−0.30, 1.25) | 0.23 | ||
| 3 years | 41.2 (4.4) (96) | 41.2 (4.2) (102) | 0.13 (−0.64, 0.91) | 0.74 | ||
| ≥500 mg/day | ||||||
| 1 year | 43.5 (3.4) (169) | 43.3 (3.5) (174) | 0.27 (−0.68, 1.22) | 0.58 | 0.80 | |
| 2 years | 42.1 (3.4) (161) | 42.4 (3.7) (179) | 0.01 (−0.94, 0.96) | 0.99 | ||
| 3 years | 41.5 (3.9) (167) | 41.5 (4.0) (176) | 0.08 (−0.88, 1.03) | 0.88 | ||
Adjusted for baseline value and school of attendance.
Deseasonalised values.
Nnumber25(OH)D25-hydroxyvitamin DVO2peakpeak oxygen consumption
Table 5. Spirometric outcomes at 3-year follow-up by allocation: overall and by subgroup.
| Vitamin D arm: mean value (SD) (n) | Placebo arm: mean value (SD) (n) | Adjusted mean difference (95% CI)* | P value | P for interaction | |
| % predicted FEV1 | |||||
| Overall | 101.5 (11.1) (666) | 100.8 (11.0) (679) | 0.75 (−0.44, 1.93) | 0.22 | -- |
| By sex | |||||
| Male | 99.5 (10.2) (333) | 100.0 (10.9) (354) | −0.45 (−2.05, 1.16) | 0.58 | 0.04† |
| Female | 103.4 (11.5) (333) | 101.7 (11.1) (325) | 2.06 (0.31, 3.81) | 0.021 | |
| By baseline 25(OH)D concentration‡ | |||||
| <25 nmol/L | 102.0 (10.7) (232) | 101.0 (11.3) (253) | 1.31 (−0.67, 3.29) | 0.20 | 0.71 |
| ≥25 nmol/L | 101.2 (11.3) (434) | 100.7 (10.8) (426) | 0.63 (−0.86, 2.12) | 0.41 | |
| By calcium intake | |||||
| <500 mg/day | 101.2 (10.9) (223) | 100.7 (10.7) (244) | 0.73 (−0.73, 2.19) | 0.33 | 0.96 |
| ≥500 mg/day | 102.1 (11.5) (435) | 101.1 (11.6) (425) | 0.88 (−1.26, 3.01) | 0.42 | |
| % predicted FVC | |||||
| Overall | 101.6 (11.2) (666) | 101.3 (11.5) (677) | 0.34 (−0.88, 1.55) | 0.59 | -- |
| By sex | |||||
| Male | 100.2 (10.0) (333) | 101.5 (10.9) (352) | −1.13 (−2.71, 0.46) | 0.16 | 0.017 |
| Female | 103.0 (12.1) (333) | 101.2 (12.1) (325) | 1.82 (−0.05, 3.70) | 0.06 | |
| By baseline 25(OH)D concentration‡ | |||||
| <25 nmol/L | 102.2 (11.5) (232) | 101.0 (11.9) (253) | 1.36 (−0.75, 3.46) | 0.21 | 0.34 |
| ≥25 nmol/L | 101.3 (11.1) (434) | 101.5 (11.2) (424) | −0.07 (−1.57, 1.43) | 0.92 | |
| By calcium intake | |||||
| <500 mg/day | 101.5 (11.2) (223) | 101.4 (11.4) (244) | 0.23 (−1.30, 1.77) | 0.77 | 0.87 |
| ≥500 mg/day | 101.6 (11.3) (435) | 101.1 (11.6) (423) | 0.57 (−1.53, 2.68) | 0.59 | |
| % predicted FEV1/FVC | |||||
| Overall | 98.0 (6.3) (666) | 97.6 (6.4) (679) | 0.49 (−0.19, 1.17) | 0.16 | -- |
| By sex | |||||
| Male | 97.0 (6.3) (333) | 96.2 (6.6) (353) | 0.72 (−0.25, 1.69) | 0.15 | 0.49 |
| Female | 99.1 (6.2) (333) | 99.0 (6.0) (326) | 0.25 (−0.68, 1.18) | 0.60 | |
| By baseline 25(OH)D concentration‡ | |||||
| <25 nmol/L | 97.9 (6.2) (232) | 98.4 (6.4) (254) | −0.27 (−1.40, 0.85) | 0.64 | 0.08 |
| ≥25 nmol/L | 98.1 (6.4) (434) | 97.1 (6.4) (425) | 0.96 (0.10, 1.82) | 0.028 | |
| By calcium intake | |||||
| <500 mg/day | 97.8 (6.4) (223) | 97.4 (6.6) (244) | 0.51 (−0.37, 1.38) | 0.26 | 0.99 |
| ≥500 mg/day | 98.5 (6.3) (435) | 98.0 (6.0) (425) | 0.45 (−0.68, 1.58) | 0.43 | |
| % predicted PEFR | |||||
| Overall | 95.2 (14.1) (666) | 93.6 (13.6) (680) | 1.69 (0.21, 3.18) | 0.026 | -- |
| By sex | |||||
| Male | 94.2 (13.4) (333) | 93.1 (14.0) (354) | 1.08 (−0.98, 3.13) | 0.30 | 0.33 |
| Female | 96.3 (14.8) (333) | 94.1 (13.2) (326) | 2.42 (0.24, 4.59) | 0.030 | |
| By baseline 25(OH)D concentration‡ | |||||
| <25 nmol/L | 96.0 (13.4) (232) | 93.5 (13.9) (254) | 2.67 (0.19, 5.15) | 0.035 | 0.47 |
| ≥25 nmol/L | 94.8 (14.5) (434) | 93.6 (13.5) (426) | 1.30 (−0.58, 3.18) | 0.18 | |
| By calcium intake | |||||
| <500 mg/day | 94.3 (13.9) (223) | 92.8 (13.2) (244) | 1.76 (0.93, 3.59) | 0.060 | 0.97 |
| ≥500 mg/day | 97.1 (14.7) (435) | 95.1 (14.2) (426) | 1.47 (−1.18, 4.12) | 0.28 | |
| % predicted FEF25–75 | |||||
| Overall | 102.4 (21.9) (667) | 100.7 (21.8) (680) | 1.47 (1.19, 3.82) | 0.22 | -- |
| By sex | |||||
| Male | 99.8 (21.2) (334) | 98.0 (22.2) (354) | 1.17 (−2.14, 4.48) | 0.49 | 0.73 |
| Female | 105.0 (22.3) (333) | 103.6 (20.9) (326) | 2.11 (−1.20, 5.42) | 0.21 | |
| By baseline 25(OH)D concentration‡ | |||||
| <25 nmol/L | 102.0 (21.6) (232) | 101.4 (21.7) (254) | 1.19 (−2.69, 5.08) | 0.55 | 0.79 |
| ≥25 nmol/L | 102.7 (22.1) (435) | 100.3 (21.8) (426) | 1.84 (−1.14, 4.81) | 0.23 | |
| By calcium intake | |||||
| <500 mg/day | 100.9 (20.2) (223) | 99.9 (21.8) (244) | 0.92 (−1.94, 3.77) | 0.53 | 0.55 |
| ≥500 mg/day | 105.6 (24.9) (436) | 102.5 (21.7) (426) | 2.38 (−1.88, 6.65) | 0.27 | |
Adjusted for school of attendance.
After correction for multiple comparisons testing using the Benjamini and Hochberg method and a false discovery rate of 10%, this P-p value for interaction did not remain significant (critical threshold of significance for family of P-p values was <0.007).
Deseasonalised values.
FEF25–75forced mid-expiratory flowFEV1forced expiratory volume in 1 sFVCforced vital capacityNnumber25(OH)D25-hydroxyvitamin DPEFRpeak expiratory flow rate
Discussion
We present findings of the largest RCT to investigate the effects of vitamin D on muscle strength, peak oxygen uptake and spirometric lung volumes in children. Vitamin D deficiency was highly prevalent among the study population at baseline, and the intervention was highly effective in elevating serum 25(OH)D concentrations among participants who were randomised to receive it. However, this was not associated with any effect on any physiological outcome investigated, either overall or in subgroups defined by baseline vitamin D status, sex or calcium intake.
Our findings contrast with those of observational studies reporting associations between low vitamin D status and reduced muscle strength and cardiorespiratory fitness,12 13 and chime with results of smaller RCTs, conducted in populations with lower prevalence of vitamin D deficiency, that have yielded null results.18,22 They are also consistent with the lack of effect seen for muscle strength and exercise outcomes in a similar trial conducted in South Africa,38 and for other ‘non-classical’ outcomes in trials of weekly vitamin D supplementation in children.23,2539 Inconsistency between positive findings of observational versus null findings from interventional studies may reflect effects of confounding or bias in the former.42 Lack of subgroup effects in participants with lower baseline vitamin D status or calcium intake suggests that neither of these factors modified the effects of vitamin D supplementation on outcomes investigated. We highlight that our null findings do not have relevance for children with symptomatic vitamin D deficiency since those who were found to have signs of rickets were excluded from the trial, as it would not have been ethical to randomise them to placebo.
Our study has several strengths. The large sample size and low rates of loss to follow-up maximised our power to detect the effects of the intervention, and the high prevalence of vitamin D deficiency and low calcium intake at baseline allowed us to rule out effects even in subgroups who might have been expected to derive particular benefit from vitamin D replacement. The intervention was highly effective in elevating serum 25(OH)D concentrations into the physiological range. We also assessed a comprehensive range of outcomes relating to muscle and cardiorespiratory fitness, with assessments at more than one time point to allow detection of any effects that may have differed with varying duration of supplementation.
Our study also has some limitations. Spirometry was assessed at a 3-year follow-up only: accordingly, analyses testing the effect of allocation to vitamin D versus placebo could not be adjusted for baseline. However, we have no reason to suspect a significance imbalance in baseline values between groups, as the randomisation process was effective in distributing all other baseline characteristics evenly between children randomised to intervention versus control arms. We acknowledge that the outcomes investigated in the current study were secondary, rather than primary, outcomes (although prespecified ones), and that multiple tests for statistical significance were performed. Statistically significant findings may, therefore, have arisen as a result of type 1 error. We addressed this issue by applying a correction for multiple comparison testing. We also highlight that our findings relate to effects of weekly vitamin D supplementation specifically. It remains technically possible that daily supplementation might have a different effect, although the fact that weekly supplementation is effective in suppressing serum concentrations of parathyroid hormone and alkaline phosphatase in study participants25 40 suggests that this dosing regimen exerts the same physiological effects as would be expected with daily supplementation. Further trials comparing effects of daily versus weekly supplementation would be needed to resolve this question definitively.
In conclusion, this large multicentre RCT of vitamin D supplementation, administered for 3 years to a population of children with low baseline 25(OH)D concentrations, did not show any effect of the intervention on muscle strength, cardiorespiratory fitness or spirometric outcomes. Taken together with null results from a similarly designed phase 3 RCT conducted in CapeTown, South Africa,38 our study does not suggest a role for weekly oral vitamin D supplementation to enhance muscle strength, peak oxygen uptake or respiratory function in schoolchildren in whom rickets has been excluded.
supplementary material
Acknowledgements
We thank all the children who participated in the trial, and their parents and guardians. We also thank independent members of the Data Safety Monitoring Board (Professor SM Fortune and Dr P.L. Williams, Harvard T.H. Chan School of Public Health; Professors MF Holick and CR Horsburgh, Boston University; Professor P Enkhbaatar, University of Texas and Dr E. Chadraa, Minnesota State University); independent members of the Trial Steering Committee (Professors W.C. Willett, Edward L. Giovannucci and B.R. Bloom, Harvard T.H. Chan School of Public Health; Dr N Naranbat, Gyals Medical Laboratory, Ulaanbaatar; and late Dr D Malchinkhuu, National Center for Maternal and Child Health of Mongolia); board members at the Mongolian Health Initiative (Dr J Tuyatsetseg, Mongolian University of Science and Technology; Dr J Amarsanaa, Happy Veritas Laboratory, Ulaanbaatar; Drs P Erkhembulgan and G Batbaatar, Mongolian National University of Medical Sciences; Professor M.C. Elliott, Harvard University and Mr Ts. Munh-Orgil, Member of the Mongolian Parliament); and the following individuals for advice and helpful discussions: Dr Winthrop Burr (Anadyne Psychotherapy Inc) and Dr Masae Kawamura at Qiagen, USA.
Footnotes
Funding: This study was supported by an award from the US National Institutes of Health, ref. 1R01HL122624-01.
Provenance and peer review: Not commissioned; internally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: This study involves human participants and the study was approved by institutional review boards of the Mongolian Ministry of Health, Mongolian National University and Harvard T. H. Chan School of Public Health (IRB # 14-0513). Participants gave informed consent to participate in the study before taking part.
Data availability free text: Anonymised data are available from corresponding authors on reasonable request, subject to terms of IRB and regulatory approval.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Contributor Information
Davaasambuu Ganmaa, Email: gdavaasa@hsph.harvard.edu.
Stephanie Hemmings, Email: S.Hemmings@brighton.ac.uk.
David A Jolliffe, Email: D.A.jolliffe@qmul.ac.uk.
Uyanga Buyanjargal, Email: uyangabegi@gmail.com.
Gantsetseg Garmaa, Email: gantsetseg.garmaa@gmail.com.
Unaganshagai Adiya, Email: unaganshagai@mnums.edu.mn.
Tumenulzii Tumurbaatar, Email: tumenulziitumurbaatar@gmail.com.
Khulan Dorjnamjil, Email: hulan.dr06@gmail.com.
Enkhtsetseg Tserenkhuu, Email: enkhtsetseg.mhi@gmail.com.
Sumiya Erdenenbaatar, Email: sumiya.mhisumiya@gmail.com.
Enkhjargal Tsendjav, Email: enkhjin2005@gmail.com.
Nomin Enkhamgalan, Email: nomin.enkhamgalan29@gmail.com.
Chuluun-Erdene Achtai, Email: chuluunerdene.a@eclinic.mn.
Yagaantsetseg Talhaasuren, Email: yagaanaa74@yahoo.com.
Tuya Byambasuren, Email: tuyabyambasuren@yahoo.com.
Erdenetuya Ganbaatar, Email: erdenetuya@mnums.edu.mn.
Erkhembulgan Purevdorj, Email: erkhemtoliun@gmail.com.
Adrian R Martineau, Email: a.martineau@qmul.ac.uk.
Data availability statement
Data are available on reasonable request.
References
- 1.Smith JJ, Eather N, Morgan PJ, et al. The health benefits of muscular fitness for children and adolescents: a systematic review and meta-analysis. Sports Med. 2014;44:1209–23. doi: 10.1007/s40279-014-0196-4. [DOI] [PubMed] [Google Scholar]
- 2.García-Hermoso A, Ramírez-Campillo R, Izquierdo M. Is Muscular Fitness Associated with Future Health Benefits in Children and Adolescents? A Systematic Review and Meta-Analysis of Longitudinal Studies. Sports Med. 2019;49:1079–94. doi: 10.1007/s40279-019-01098-6. [DOI] [PubMed] [Google Scholar]
- 3.Raghuveer G, Hartz J, Lubans DR, et al. Cardiorespiratory Fitness in Youth: An Important Marker of Health: A Scientific Statement From the American Heart Association. Circulation. 2020;142:e101–18. doi: 10.1161/CIR.0000000000000866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.García-Hermoso A, Ramírez-Vélez R, García-Alonso Y, et al. Association of Cardiorespiratory Fitness Levels During Youth With Health Risk Later in Life: A Systematic Review and Meta-analysis. JAMA Pediatr. 2020;174:952–60. doi: 10.1001/jamapediatrics.2020.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ceglia L. Vitamin D and its role in skeletal muscle. Curr Opin Clin Nutr Metab Care. 2009;12:628–33. doi: 10.1097/MCO.0b013e328331c707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lykkedegn S, Sorensen GL, Beck-Nielsen SS, et al. The impact of vitamin D on fetal and neonatal lung maturation. A systematic review. Am J Physiol Lung Cell Mol Physiol. 2015;308:L587–602. doi: 10.1152/ajplung.00117.2014. [DOI] [PubMed] [Google Scholar]
- 7.Hunter L, Ferguson R, McDevitt H. Vitamin D deficiency cardiomyopathy in Scotland: a retrospective review of the last decade. Arch Dis Child. 2020;105:853–6. doi: 10.1136/archdischild-2019-317794. [DOI] [PubMed] [Google Scholar]
- 8.Roth DE, Abrams SA, Aloia J, et al. Global prevalence and disease burden of vitamin D deficiency: a roadmap for action in low- and middle-income countries. Ann N Y Acad Sci. 2018;1430:44–79. doi: 10.1111/nyas.13968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voortman T, van den Hooven EH, Heijboer AC, et al. Vitamin D deficiency in school-age children is associated with sociodemographic and lifestyle factors. J Nutr. 2015;145:791–8. doi: 10.3945/jn.114.208280. [DOI] [PubMed] [Google Scholar]
- 10.Middelkoop K, Walker N, Stewart J, et al. Prevalence and Determinants of Vitamin D Deficiency in 1825 Cape Town Primary Schoolchildren: A Cross-Sectional Study. Nutrients. 2022;14:1263. doi: 10.3390/nu14061263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bater J, Bromage S, Jambal T, et al. Prevalence and Determinants of Vitamin D Deficiency in 9595 Mongolian Schoolchildren: A Cross-Sectional Study. Nutrients. 2021;13:4175. doi: 10.3390/nu13114175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bjarnadottir A, Kristjansdottir AG, Hrafnkelsson H, et al. Insufficient autumn vitamin D intake and low vitamin D status in 7-year-old Icelandic children. Public Health Nutr. 2015;18:208–17. doi: 10.1017/S1368980013003558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valtueña J, Gracia-Marco L, Huybrechts I, et al. Cardiorespiratory fitness in males, and upper limbs muscular strength in females, are positively related with 25-hydroxyvitamin D plasma concentrations in European adolescents: the HELENA study. QJM. 2013;106:809–21. doi: 10.1093/qjmed/hct089. [DOI] [PubMed] [Google Scholar]
- 14.Yao T-C, Tu Y-L, Chang S-W, et al. Serum 25-hydroxyvitamin D levels in relation to lung function and exhaled nitric oxide in children. J Pediatr. 2014;165:1098–103. doi: 10.1016/j.jpeds.2014.08.048. [DOI] [PubMed] [Google Scholar]
- 15.Beaudart C, Buckinx F, Rabenda V, et al. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2014;99:4336–45. doi: 10.1210/jc.2014-1742. [DOI] [PubMed] [Google Scholar]
- 16.Bezrati I, Ben Fradj MK, Hammami R, et al. A single mega dose of vitamin D3 improves selected physical variables in vitamin D-deficient young amateur soccer players: a randomized controlled trial. Appl Physiol Nutr Metab. 2020;45:478–85. doi: 10.1139/apnm-2019-0525. [DOI] [PubMed] [Google Scholar]
- 17.Ward KA, Das G, Roberts SA, et al. A randomized, controlled trial of vitamin D supplementation upon musculoskeletal health in postmenarchal females. J Clin Endocrinol Metab. 2010;95:4643–51. doi: 10.1210/jc.2009-2725. [DOI] [PubMed] [Google Scholar]
- 18.Thams L, Hvid LG, Stounbjerg NG, et al. Vitamin D supplementation and increased dairy protein intake do not affect muscle strength or physical function in healthy 6-8-year-old children: the D-pro randomized trial. Eur J Nutr. 2022;61:3613–23. doi: 10.1007/s00394-022-02912-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mortensen C, Mølgaard C, Hauger H, et al. Winter vitamin D3 supplementation does not increase muscle strength, but modulates the IGF-axis in young children. Eur J Nutr. 2019;58:1183–92. doi: 10.1007/s00394-018-1637-x. [DOI] [PubMed] [Google Scholar]
- 20.Wright CS, Laing EM, Pollock NK, et al. Serum 25-Hydroxyvitamin D and Intact Parathyroid Hormone Influence Muscle Outcomes in Children and Adolescents. J Bone Miner Res. 2018;33:1940–7. doi: 10.1002/jbmr.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dubnov-Raz G, Livne N, Raz R, et al. Vitamin D Supplementation and Physical Performance in Adolescent Swimmers. Int J Sport Nutr Exerc Metab. 2015;25:317–25. doi: 10.1123/ijsnem.2014-0180. [DOI] [PubMed] [Google Scholar]
- 22.El-Hajj Fuleihan G, Nabulsi M, Tamim H, et al. Effect of vitamin D replacement on musculoskeletal parameters in school children: a randomized controlled trial. J Clin Endocrinol Metab. 2006;91:405–12. doi: 10.1210/jc.2005-1436. [DOI] [PubMed] [Google Scholar]
- 23.Ganmaa D, Uyanga B, Zhou X, et al. Vitamin D Supplements for Prevention of Tuberculosis Infection and Disease. N Engl J Med. 2020;383:359–68. doi: 10.1056/NEJMoa1915176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ganmaa D, Bromage S, Khudyakov P, et al. Influence of Vitamin D Supplementation on Growth, Body Composition, and Pubertal Development Among School-aged Children in an Area With a High Prevalence of Vitamin D Deficiency: A Randomized Clinical Trial. JAMA Pediatr. 2023;177:32–41. doi: 10.1001/jamapediatrics.2022.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ganmaa D, Khudyakov P, Buyanjargal U, et al. Vitamin D supplements for fracture prevention in schoolchildren in Mongolia: analysis of secondary outcomes from a multicentre, double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2024;12:29–38. doi: 10.1016/S2213-8587(23)00317-0. [DOI] [PubMed] [Google Scholar]
- 26.Maalouf J, Nabulsi M, Vieth R, et al. Short- and long-term safety of weekly high-dose vitamin D3 supplementation in school children. J Clin Endocrinol Metab. 2008;93:2693–701. doi: 10.1210/jc.2007-2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bromage S, Daria T, Lander RL, et al. Diet and Nutrition Status of Mongolian Adults. Nutrients. 2020;12:1514. doi: 10.3390/nu12051514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.España-Romero V, Artero EG, Santaliestra-Pasias AM, et al. Hand span influences optimal grip span in boys and girls aged 6 to 12 years. J Hand Surg Am. 2008;33:378–84. doi: 10.1016/j.jhsa.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 29.Castro-Piñero J, Ortega FB, Artero EG, et al. Assessing muscular strength in youth: usefulness of standing long jump as a general index of muscular fitness. J Strength Cond Res. 2010;24:1810–7. doi: 10.1519/JSC.0b013e3181ddb03d. [DOI] [PubMed] [Google Scholar]
- 30.Trajković N, Rančić D, Ilić T, et al. Measuring handgrip strength in school children: inter-instrument reliability between Takei and Jamar. Sci Rep. 2024;14:1074. doi: 10.1038/s41598-024-51368-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernandez-Santos JR, Ruiz JR, Cohen DD, et al. Reliability and Validity of Tests to Assess Lower-Body Muscular Power in Children. J Strength Cond Res. 2015;29:2277–85. doi: 10.1519/JSC.0000000000000864. [DOI] [PubMed] [Google Scholar]
- 32.Graham BL, Steenbruggen I, Miller MR, et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med. 2019;200:e70–88. doi: 10.1164/rccm.201908-1590ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sempos CT, Betz JM, Camara JE, et al. General Steps to Standardize the Laboratory Measurement of Serum Total 25-Hydroxyvitamin D. J AOAC Int. 2017;100:1230–3. doi: 10.5740/jaoacint.17-0259. [DOI] [PubMed] [Google Scholar]
- 34.Matsuzaka A, Takahashi Y, Yamazoe M, et al. Validity of the Multistage 20-M Shuttle-Run Test for Japanese Children, Adolescents, and Adults. Pediatr Exerc Sci. 2004;16:113–25. doi: 10.1123/pes.16.2.113. [DOI] [Google Scholar]
- 35.de Onis M, Onyango AW, Borghi E, et al. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85:660–7. doi: 10.2471/blt.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sachs MC, Shoben A, Levin GP, et al. Estimating mean annual 25-hydroxyvitamin D concentrations from single measurements: the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr. 2013;97:1243–51. doi: 10.3945/ajcn.112.054502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat Soc Ser B. 1995;57:289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- 38.Middelkoop K, Walker N, Stewart J, et al. Influence of vitamin D supplementation on muscle strength and exercise capacity in South African schoolchildren: a randomised controlled trial (ViDiKids) BMJ Open Sport Exerc Med. 2024 doi: 10.1136/bmjsem-2024-002019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Middelkoop K, Stewart J, Walker N, et al. Vitamin D supplementation to prevent tuberculosis infection in South African schoolchildren: multicenter phase 3 double-blind randomized placebo-controlled trial (ViDiKids) Int J Infect Dis. 2023;134:63–70. doi: 10.1016/j.ijid.2023.05.010. [DOI] [PubMed] [Google Scholar]
- 40.Middelkoop K, Micklesfield LK, Walker N, et al. Influence of vitamin D supplementation on bone mineral content, bone turnover markers, and fracture risk in South African schoolchildren: multicenter double-blind randomized placebo-controlled trial (ViDiKids) J Bone Miner Res. 2024;39:211–21. doi: 10.1093/jbmr/zjae007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Middelkoop K, Micklesfield L, Stewart J, et al. Influence of vitamin D supplementation on growth, body composition, pubertal development and spirometry in South African schoolchildren: a randomised controlled trial (ViDiKids) BMJ Paediatr Open . 2024;8:e002495. doi: 10.1136/bmjpo-2024-002495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang MTM, Bolland MJ, Grey A. Reporting of Limitations of Observational Research. JAMA Intern Med. 2015;175:1571–2. doi: 10.1001/jamainternmed.2015.2147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on reasonable request.