Abstract
Background
While death in old age is inevitable, premature death at younger ages is within our control. Premature mortality (death < 70 years) is a crucial indicator of health status and access to healthcare, with variations observed across regions. In North Africa and the Middle East, ischemic heart disease (IHD), road injuries, stroke, and chronic kidney disease are projected to be the main causes of premature mortality. Unfortunately, few studies have been conducted on premature mortality worldwide. This study aimed to analyze the causes of premature death and associated risk factors within the Pars Cohort Study.
Methods
The Pars Cohort Study is a prospective cohort study conducted in Fars Province, Iran, involving 9,264 individuals aged 40–75 years, 53.8% of whom were women. We assessed participants from baseline (2012–2014) to 2021. The data were gathered through interviews, biological samples, and physical examinations. The causes of premature mortality, hazard ratios (HRs), and population attributable fraction (PAF) with 95% confidence intervals (95% CIs) for the variables were calculated.
Results
Out of 388 deaths, 54% were premature. The most common causes of premature death included IHD (40%), stroke (11%), road traffic injuries (6%), lower respiratory infections (5%), and COVID-19 (3%). The predictive factors [adjusted HRs (95% CIs)] associated with premature mortality included age [year, 1.07 (1.04, 1.10)], tobacco [1.43 (0.96, 2.11)], opium [2.12 (1.39, 3.24)], hypertension [1.52 (1.10, 2.12)], waist circumference [cm, 1.03 (1.00, 1.05)], female sex [0.30 (0.19, 0.47)], education [> 8 years vs. no formal schooling, 0.46 (0.24, 0.88)], being married [0.60 (0.37, 0.97)], physical activity [3rd vs. 1st tertile, 0.38 (0.26, 0.57)], hip circumference [cm, 0.96 (0.92, 0.99)], estimated GFR [mL/min/1.73 m², 0.99 (0.978, 0.999)], and wealth score [4th vs. 1st quartile, 0.54 (0.32, 0.90)]. The PAF (95% CI) for all modifiable predictors was 0.83 (0.62, 0.92).
Conclusions
The predominant causes of premature mortality were IHD and stroke. To mitigate premature deaths, it is recommended to address both socioeconomic and behavioral factors simultaneously.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-024-19583-7.
Keywords: Premature mortality, Pars cohort study, Risk factor, Protective factor, Prevention, Noncommunicable disease, Cardiovascular disease
Introduction
Death in old age is inevitable, but death at younger ages is controllable [1]. Premature death, defined as death under 70 years of age [2], is an important indicator of health status and access to healthcare [3]. Premature death has significant negative psychological and socioeconomic impacts on affected families and societies. Studies have demonstrated that the loss of parents in childhood has many devastating consequences, including substance abuse, depression, violent behavior, education dropouts, and a decrease in employment [4].
Premature death prevalence and causes vary by region. For example, more than 85% of premature deaths due to noncommunicable diseases (NCDs) occur in low- and middle-income countries. Cardiovascular diseases (CVDs), cancers, chronic respiratory diseases, and diabetes account for more than 80% of all premature NCD deaths worldwide [5, 6]. A study has forecasted that by 2040, life expectancy will continue to vary worldwide, ranging from more than 85 years in four countries (Japan, Singapore, Spain, and Switzerland) to less than 65 years in certain African countries. In North Africa and the Middle East, ischemic heart disease, road injuries, stroke, and chronic kidney disease are projected to be the main causes of premature mortality [7]. Therefore, comprehensive studies are needed in different regions to evaluate the rates and predictors of premature deaths. This information will help healthcare decision-makers plan cost-effective interventions and allocate sufficient budgetary resources in each region [8]. Unfortunately, few studies have been conducted on this topic worldwide, especially among low- to middle-income countries [9–11].
The Pars Cohort Study (PCS) is a population-based prospective cohort study in Iran that, with its detailed medical, demographic, and socioeconomic data, provides a platform for investigating premature mortality [12]. We aimed to analyze the causes of premature death and associated risk factors among the PCS participants.
Methods
Design, setting, and population
The detailed methodological design of the PCS, including the interview questionnaire, has been described previously [12]. The PCS is an ongoing prospective cohort study conducted in the Valashahr region of Fars Province, Iran. All rural residents of the Valashahr district, within the age range of 40 to 75 years, were invited to participate in interviews, undergo physical examinations, and contribute biological samples as part of the study. Temporary residents of the district and unwillingness to participate were the only exclusion criteria for the PCS. A total of 9,264 individuals (with a 95% participation rate) were recruited at baseline (2012–2014) for this population-based study.
The ethics committees of the Digestive Diseases Research Institute (DDRI) and Shiraz University of Medical Sciences (SUMS) approved the study protocol, and the ethics code of this study is IR.TUMS.SHARIATI.REC.1402.001. The completion and signing of the informed consent form were done in the presence of a third party, and their information was kept completely confidential.
Data collection
The data were collected through interviews, biological samples, and physical examinations. Trained interviewers applied detailed questionnaires to collect demographic data, medical history, and lifestyle information, including physical activity and wealth status. Physicians and nurses measured anthropometric indicators such as height, weight, waist and hip circumference, and blood pressure. In this study, our primary focus was on modifiable risk factors associated with an increased risk of premature mortality.
Marital status was categorized as married and non-married. The alcohol, opium, and tobacco variables were defined as the use of the substance at least once a week for 6 months or more. Diabetes was characterized by a self-report of a known case of diabetes mellitus or a fasting blood sugar ≥ 126 mg/dL. Physical activity was calculated based on the metabolic equivalent of task per minute per week according to the World Health Organization (WHO) guidelines and categorized into tertiles [13]. The education variable was divided into 4 groups based on the number of years of education: no formal schooling; 1–5 years; 6–8 years; and over 8 years. Brachial blood pressure was measured in a sitting position after 5 min of rest on each arm twice, 2 min apart. The second measurement on the side that had the highest value was considered the blood pressure variable [14]. A systolic blood pressure of ≥ 140 mm Hg, a diastolic blood pressure of ≥ 90 mm Hg [15], or a self-reported history of hypertension confirmed by a physician was considered hypertension. Non-high-density lipoprotein cholesterol (non-HDL-C) is the result of subtracting the amount of HDL-C from the total cholesterol level and is considered an indicator of dyslipidemia, which is classified into three groups: low (< 130), moderate (≥ 130 and < 160), and high (≥ 160 mg/dL) [16, 17]. The wealth score was calculated via multiple correspondence analysis, in which the required data were collected by asking detailed questions about the ownership of a house, a car, household appliances such as a TV, computer, and refrigerator, and the size of the house. The wealth score was divided into quartiles [18]. The estimated glomerular filtration rate (eGFR) was calculated using the chronic kidney disease epidemiology (CKD-Epi) equation based on participants’ serum creatinine levels and categorized as low (< 60), moderate (60–90), and high (≥ 90 mL/min/1.73 m²) [19, 20]. Body-mass index (weight in kilograms divided by the square of height in meters, in kg/m², BMI) and waist and hip circumferences (in centimeters) were measured using a standard protocol. The waist circumference was divided into two groups based on the appropriate cut-off (95 cm) for the Iranian population [21, 22].
Follow-up and mortality assessment
The follow-up period extended from the date of the first interview to the date of the last interview (up to December 31, 2021), age 70 years, or premature death (whichever occurred earlier). In this study, death before the age of 70 years was defined as premature death—the outcome variable. All study participants were followed up annually by telephone and with a follow-up form, which included 40 items, such as vital signs, hospitalizations, deaths, and the occurrence of new diseases. In the case of death, the follow-up team collected all medical records, including paraclinical reports related to the diagnosis and treatment of the deceased individual. If the data were incomplete, the PCS team visited each participant’s home address, interviewed their relatives, and completed the verbal autopsy questionnaire. The verbal autopsy questionnaire showed both good validity and reliability in a large-scale follow-up study among the Iranian population [23]. Two internists independently reviewed all documents of the deceased person and determined the exact cause of death based on the 10th revision of the International Classification of Diseases (ICD-10) [24]. In cases of discrepancies, an experienced internist reviewed all the documents together with the judgment of the other two experts and announced the final diagnosis of the cause of death.
Statistical methods
The study was conducted according to the methods and data sources of the WHO for causes of death at the country level from 2000 to 2019 [25]. The garbage ICD codes used included C76, C80, I26.9, I46, I50, R00-94, R96-99, Y10-34 (Y12.9), C55, A40-41 (A41.9), J96, and N18. After the redistribution of garbage codes, the top 5 causes of death were identified in three categories: <70 years, ≥ 70 years, and total. Because coronavirus disease 2019 (COVID-19) was common in recent years, we considered COVID-19 as a separate code and did not combine it with other respiratory infections. To clarify the associations between predictor variables and premature death, Cox proportional hazard regression models were used to determine hazard ratios (HRs) and 95% confidence intervals (CIs). For this analysis, we excluded participants aged ≥ 70 years and those with a history of chronic diseases, including ischemic heart disease (IHD), stroke, and cancer, at baseline. In this model, unadjusted, age- and sex-adjusted, and fully adjusted HRs were calculated for each variable. In the mutual model, HRs were adjusted for probable confounders that included continuous (age at baseline, waist and hip circumferences, eGFR, and non-HDL-C) and categorical (sex, ethnicity, marital status, education, wealth score, history of alcohol, tobacco, and opium use, physical activity, hypertension, and diabetes) variables. We used waist and hip circumferences in the models as measures of obesity because a large cohort of Iranian participants with the same age range showed that the significant impact of visceral adiposity becomes evident only when considering both waist circumference as a risk factor and hip circumference as a protective factor in the models [26]. Consequently, hip-adjusted waist circumference was utilized as the adiposity variable in our analysis. The proportional hazard assumption was verified using the PH test.
We used the “punafcc” command in Stata to calculate the PAF for modifiable factors based on mutual regression models. For this analysis, the reference distribution was set so that each participant was either not exposed to the risk factor or belonged to the category associated with the lowest risk of premature death (such as the highest level of education or eGFR ≥ 90).
Stata statistical software (version 17, Stata Inc, College Station, Texas, USA) was used for data analysis. In addition to Stata, Excel (Microsoft Office Excel 2007) was used for data processing. We used complete case analysis, and P < 0.05 and 95% CIs not including one were considered statistically significant. Additionally, ChatGPT was used in some sections of the article for paraphrasing.
Results
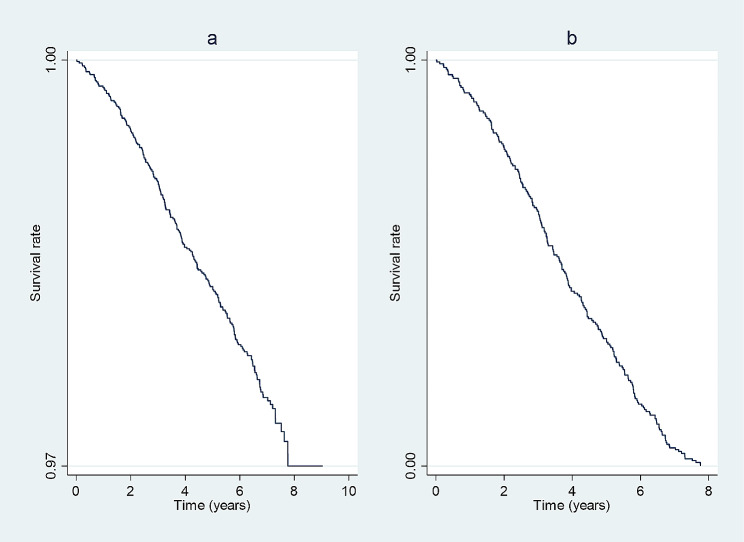
During the follow-up, with a median (interquartile range boundaries, IQR) of 6.90 (6.27–7.37) years and a loss-to-follow-up rate of 0.59% (55 participants), a total of 388 deaths occurred, 213 (54%) of which were premature. Noncommunicable diseases (NCDs), communicable, maternal, perinatal, and nutritional conditions (CMPN), and injuries constituted 79%, 11%, and 8% of premature deaths, respectively. Within the NCD, CMPN, and injury groups, 63%, 50%, and 50%, respectively, occurred before the age of 70. Figure 1 demonstrates the survival rates during follow-up among the total population (a) and participants with premature deaths (b).
Fig. 1.
Kaplan-Meier graphs assessing the survival rates during follow-up among the total population (a) and participants with premature deaths (b)
The most common causes of death in both age categories (< 70 and ≥ 70 years) were IHD and stroke. Other causes of premature death included road traffic injuries (RTIs), lower respiratory infections, and COVID-19. The top 5 causes of death based on age categories are presented in Table 1, with a detailed classification of death causes provided in Supplementary Table 1, Additional File 1.
Table 1.
The top 5 causes of death in the pars Cohort Study (2012–2021)
| < 70 years (n = 213) |
≥ 70 years (n = 170) |
All (n = 388)a |
||||
|---|---|---|---|---|---|---|
| Ranks | Causes | Number (%) | Causes | Number (%) |
Causes | Number (%) |
| 1 | Ischemic heart disease | 85 (39.90) | Ischemic heart disease | 47 (27.65) | Ischemic heart disease | 135 (34.79) |
| 2 | Stroke | 24 (11.26) | Stroke | 21 (12.35) | Stroke | 45 (11.60) |
| 3 | Road traffic injuries | 12 (5.63) | Road traffic injuries | 12 (7.06) | Road traffic injuries | 24 (6.19) |
| 4 | Lower respiratory infections | 10 (4.69) | Stomach cancer | 7 (4.12) | Lower respiratory infections | 17 (4.38) |
| 5 | COVID-19b | 7 (3.29) | Prostate cancer | 6 (3.53) | Stomach cancer | 11 (2.84) |
Numbers were obtained based on WHO methods after the redistribution of garbage ICD-10 codes, including 60 cardiovascular disease cases (I26.9, I46, I50), 5 cancer cases (C55, C76, C80), 1 external cause of morbidity and mortality (Y12.9), 21 infectious and parasitic cases (A41.9), 15 cases of symptoms, signs, and ill-defined conditions (R00-R94, R96-R99), 3 cases of respiratory disease (J96), and 6 cases of genitourinary disease (N18). a The age of death in 5 participants was undetermined. b COVID-19 indicates coronavirus disease.
Of the total participants (n = 9264), 46.16% were male. The mean (SD) age of all cohort participants at baseline was 52.64 ± 9.68 years. After excluding individuals aged ≥ 70 years at baseline and those with a history of IHD, stroke, or cancer (for determining HRs and PAF purposes), the remaining population was 7668, and 176 premature deaths occurred. The baseline characteristics of the participants are presented in Table 2. The HRs (95% CIs) for the relationships between variables and premature death are shown in Table 3. Based on the fully adjusted HRs (95% CIs), the risk of premature death was greater in older individuals [1.07 (1.04, 1.10)], tobacco smokers [1.43 (0.96, 2.11)], opium consumers [2.12 (1.39, 3.24)], those with hypertension [1.52 (1.10, 2.12)], and those with a greater waist circumference [1.03 (1.00, 1.05)]. The protective factors included female sex [0.30 (0.19, 0.47)], higher education levels [> 8 years vs. no formal schooling, 0.46 (0.24, 0.88)], being married [0.60 (0.37, 0.97)], higher physical activity [moderate and high vs. low, 0.50 (0.34, 0.74) and 0.38 (0.26, 0.57), respectively], hip circumference [0.96 (0.92, 0.99)], wealth score [4th vs. 1st quartile, 0.54 (0.32, 0.90)], and eGFR [0.99 (0.978, 0.999)].
Table 2.
Baseline characteristic of participants in the pars Cohort Study a
| Variable | Prevalence (total) (n = 7668) |
Prevalence (without premature death) (n = 7492) |
Prevalence (premature death) (n = 176) |
|---|---|---|---|
| Age (year) b | 50.52 (7.76) | 50.40 (7.72) | 55.69 (7.35) |
| Sex (Men) | 3538 (46.14) | 3424 (45.70) | 114 (64.77) |
| Ethnicity | |||
| Fars | 4310 (56.21) | 4220 (56.33) | 90 (51.14) |
| Turk | 2987 (38.95) | 2912 (38.87) | 75 (42.61) |
| Others | 371 (4.84) | 360 (4.81) | 11 (6.25) |
| Education | |||
| No formal schooling | 3419 (44.59) | 3311(44.19) | 108 (61.36) |
| years of education ≤ 5 | 2438 (31.79) | 2398 (32.01) | 40 (22.73) |
| 5 < years of education ≤ 8 | 889 (11.59) | 875 (11.68) | 14 (7.95) |
| 8 < years of education | 918 (11.97) | 904 (12.07) | 4 (7.95) |
| Marital status (Married) | 6895 (89.92) | 6745 (90.03) | 150 (85.23) |
| Physical activity | |||
| Low | 2294 (29.92) | 2219 (29.62) | 75 (42.61) |
| Moderate | 2558 (33.36) | 2510 (33.50) | 48 (27.27) |
| High | 2816 (36.72) | 2763 (36.88) | 53 (30.11) |
| Alcohol ever used | 165 (2.15) | 162 (2.16) | 3 (1.70) |
| Opium ever used | 625 (8.15) | 590 (7.88) | 35 (19.89) |
| Tobacco ever used | 1581 (20.62) | 1517 (20.25) | 64 (36.36) |
| Hypertension | 1899 (24.77) | 1832 (24.45) | 67 (38.07) |
| Diabetes | 881 (11.49) | 852 (11.37) | 29 (16.48) |
| BMI (kg/m2) | 25.85 (4.66) | 25.86 (4.66) | 25.25 (4.91) |
| Waist circumference (cm)b | 90.73 (12.10) | 90.72 (12.07) | 91.25 (13.04) |
| ≥ 95 cm | 2914 (38.00) | 2843 (37.95) | 71 (40.34) |
| Hip (cm)b | 95.81 (7.95) | 95.85 (7.95) | 94.14 (7.84) |
| Wealth score | |||
| First quartile (reference) | 1801 (23.49) | 1746 (23.30) | 55 (31.25) |
| Second quartile | 2169 (28.29) | 2113 (28.20) | 56 (31.82) |
| Third quartile | 1733 (22.60) | 1697 (22.65) | 36 (20.45) |
| Fourth quartile | 1964 (25.61) | 1935 (25.83) | 29 (16.48) |
| Non-HDL-C (mg/dL)b | 137.86 (39.10) | 137.78 (39.03) | 141.01 (41.96) |
| < 130 | 3384 (44.13) | 3316 (44.26) | 68 (38.64) |
| 130–159 | 2322 (30.28) | 2263 (30.21) | 59 (33.52) |
| ≥ 160 | 1950 (25.43) | 1902 (0.15) | 48 (27.27) |
| eGFR (mL/min/1.73m2)b | 74.90 (14.36) | 75.03 (14.23) | 69.26 (18.40) |
| < 60 | 1107 (14.44) | 1049 (14.00) | 58 (32.95) |
| 60–89 | 5393 (70.33) | 5300 (70.74) | 93 (52.84) |
| ≥ 90 | 1168 (15.23) | 1143 (15.26) | 25 (14.20) |
Non-HDL-C indicates non-high-density lipoprotein cholesterol and eGFR, estimated glomerular filtration rate
a After excluding individuals aged ≥ 70 years at baseline and those with a history of IHD, stroke, or cancer. Missing data included 4 cases in education, 39 in waist, 1 in wealth, and 12 in non-HDL categories. Data are presented as number (percentage) or mean (SD).
b These rows are demonstrated as mean (SD).
Table 3.
HRs (95% CIs) for relationships between variables and premature deaths in the pars Cohort Study a
| Variable | HR (95%CI) Crude |
p-value | HR (95%CI) Age and sex-adjusted |
p-value | HR (95%CI) Full adjustedb |
p-value |
|---|---|---|---|---|---|---|
| Age (year) | 1.11(1.09, 1.14) | < 0.001 | - | - | 1.07 (1.04, 1.10) | < 0.001 |
| Sex | - | - | ||||
| Men (reference) | 1 | - | 1 | |||
| Women | 0.46(0.33, 0.62) | < 0.001 | - | 0.30 (0.19, 0.47) | < 0.001 | |
| Ethnicity | ||||||
| Fars (reference) | 1 | 1 | 1 | |||
| Turk | 1.19 (0.87, 1.62) | 0.266 | 1.13 (0.83, 1.54) | 0.440 | 1(0.73, 1.39) | 0.982 |
| Others | 1.47 (0.78, 2.74) | 0.232 | 1.51 (0.80, 2.82) | 0.201 | 1.48 (0.77, 2.85) | 0.236 |
| Education | ||||||
| No formal schooling (reference) | 1 | 1 | 1 | |||
| ≤5 years of education | 0.50 (0.35, 0.72) | < 0.001 | 0.70 (0.47, 1.03) | 0.072 | 0.75 (0.50, 1.13) | 0.171 |
| 5 < years of education ≤ 8 | 0.47 (0.27, 0.83) | 0.009 | 0.52 (0.28, 0.94) | 0.030 | 0.56 (0.30, 1.04) | 0.068 |
| 8 < years of education | 0.46 (0.26, 0.80) | 0.006 | 0.46 (0.25, 0.85) | 0.012 | 0.46 (0.24, 0.88) | 0.020 |
| Marital status | ||||||
| Married | 0.61 (0.40, 0.94) | 0.024 | 0.50 (0.32, 0.80) | 0.004 | 0.60 (0.37, 0.97) | 0.036 |
| Other (reference) | 1 | 1 | 1 | |||
| Physical activity | ||||||
| Low (reference) | 1 | 1 | 1 | |||
| Moderate | 0.55 (0.38, 0.79) | 0.001 | 0.52 (0.36, 0.75) | < 0.001 | 0.50 (0.34, 0.74) | < 0.001 |
| High | 0.57 (0.40, 0.82) | 0.002 | 0.44 (0.30, 0.63) | < 0.001 | 0.38 (0.26, 0.57) | < 0.001 |
| Alcohol | ||||||
| Never (reference) | 1 | 1 | 1 | |||
| Ever used | 0.79 (0.25, 2.47) | 0.685 | 0.82 (0.26, 2.57) | 0.730 | 0.57 (0.18, 1.81) | 0.337 |
| Opium | ||||||
| Never (reference) | 1 | 1 | 1 | |||
| Ever used | 2.88 (1.99, 4.17) | < 0.001 | 2.44 (1.64, 3.63) | < 0.001 | 2.12 (1.39, 3.24) | 0.001 |
| Tobacco | ||||||
| Never (reference) | 1 | 1 | 1 | |||
| Ever used | 2.22 (1.63, 3.02) | < 0.001 | 1.59 (1.11, 2.29) | 0.012 | 1.43 (0.96, 2.11) | 0.076 |
| Hypertension | ||||||
| No (reference) | 1 | 1 | 1 | |||
| Yes | 1.93 (1.42, 2.63) | < 0.001 | 1.55 (1.13, 2.11) | 0.006 | 1.52 (1.10, 2.12) | 0.012 |
| Diabetes | ||||||
| No (reference) | 1 | 1 | 1 | |||
| Yes | 1.52 (1.01, 2.28) | 0.043 | 1.30 (0.86, 1.95) | 0.213 | 1.03 (0.67, 1.59) | 0.895 |
| Waist (cm) | 1.05 (1.03, 1.07) | < 0.001 | 1.03 (1.01, 1.1) | 0.005 | 1.03 (1.00, 1.05) | 0.019 |
| Hip (cm) | 0.91 (0.88, 0.94) | < 0.001 | 0.95 (0.92, 0.98) | 0.004 | 0.96 (0.92, 0.99) | 0.018 |
| Wealth score | ||||||
| First quartile (reference) | 1 | 1 | 1 | |||
| Second quartile | 0.83 (0.57, 1.20) | 0.319 | 0.86 (0.59, 1.26) | 0.445 | 0.89 (0.61, 1.31) | 0.563 |
| Third quartile | 0.64(0.42, 0.98) | 0.039 | 0.71 (0.46, 1.08) | 0.110 | 0.77 (0.50, 1.21) | 0.257 |
| Fourth quartile | 0.45 (0.28, 0.70) | 0.001 | 0.53 (0.34, 0.85) | 0.008 | 0.54 (0.32, 0.90) | 0.017 |
| Non-HDL (mg/dl) | 1.00 (0.998, 1.010) | 0.432 | 1.00 (0.997, 1.004) | 0.549 | 1.00 (0.995, 1.003) | 0.632 |
| eGFR (ml/min/1.73 m2) | 0.97 (0.962, 0.982) | < 0.001 | 0.99 (0.975, 0.997) | 0.011 | 0.99 (0.978, 0.999) | 0.037 |
Non-HDL-C indicates non-high-density lipoprotein cholesterol and eGFR, estimated glomerular filtration rate
a After excluding participants with age ≥ 70 years at baseline and a history of ischemic heart disease, stroke, or cancers. b Adjusted for all variables in the table. c HRs for age, waist, hip, non-HDL, and eGFR which are quantity variables are calculated for 1 unit increase in the value of variable
Table 4 demonstrates the PAF for 11 modifiable risk factors, with the overall PAF (95% CI) for all variables being 0.83 (0.62, 0.92). Socioeconomic risk factors (i.e., education and wealth score) accounted for 0.61 (0.33, 0.77), and behavioral variables (i.e., physical activity and tobacco and opium use) accounted for 0.48 (0.34, 0.59) of the PAF. The highest PAFs were observed for education, physical activity, wealth score, and hypertension, respectively.
Table 4.
Population attributable fraction of modifiable variables for premature mortality in the pars Cohort Study (2012–2021)
| Variable | PAF |
|---|---|
| Education | 0.43 (0.04, 0.66) |
| Physical activity | 0.35 (0.21, 0.47) |
| Alcohol use | -0.01 (-0.05, 0.02) |
| Opiate use | 0.11 (0.07, 0.15) |
| Tobacco use | 0.11 (-0.002, 0.21) |
| Hypertension | 0.13 (0.05, 0.21) |
| Diabetes | 0.003 (-0.07, 0.07) |
| Non-HDL-C | 0.06 (-0.14, 0.22) |
| Waist circumference | 0.12 (-0.002, 0.23) |
| Wealth score | 0.33 (0.05, 0.52) |
| eGFR | -0.20 (-0.79, 0.19) |
| Behavioral variables a | 0.48 (0.34, 0.59) |
| Metabolic variables b | 0.14 (-0.34, 0.45) |
| Socioeconomic variables c | 0.61 (0.33, 0.77) |
| All variables | 0.83 (0.62, 0.92) |
Non-HDL-C indicates non-high-density lipoprotein cholesterol, and eGFR stands for estimated glomerular filtration rate. a Behavioral variables include alcohol, opium, and tobacco use, as well as physical activity. b Metabolic variables include non-HDL-C, hypertension, diabetes, eGFR, and waist circumference. c Socioeconomic variables include education and wealth score.
Discussion
Approximately 54% of the deaths in our study were considered premature. In each category—NCDs, CMPN conditions, and injuries—more than half of the deaths occurred prematurely. Among these premature deaths, 79% were attributed to NCDs, with vascular diseases such as IHD and stroke being the most common causes. The identified risk factors for premature death included older age, tobacco and opium consumption, hypertension, and increased waist circumference. Conversely, protective factors included being female, having higher education levels, being married, engaging in high physical activity, possessing a greater hip circumference, and having a greater wealth score. Socioeconomic variables, including education and wealth score, and behavioral variables, including opium and tobacco use and physical activity, exhibited the highest PAF, respectively. By effectively managing these modifiable risk factors, substantial reductions of 61% and 48% in premature deaths can be achieved, respectively.
Globally, some studies have investigated the prevalence of premature death. For example, in the Golestan Cohort Study (GCS) conducted in northeastern Iran, the rate of premature death among participants aged 40–70 years was 63.3% [9]. In our current investigation, a comparable 54% of deaths were deemed premature. In contrast, this rate was reported to be 4.5% in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort study conducted among nine European countries with the same age range [10]. Factors contributing to the elevated rates of premature deaths in this demographic may include both country-level and individual-level socioeconomic status. For example, rural residents with low levels of education (76% of participants had less than five years of education) and a low wealth score (52% falling within the first two quartiles of the wealth score) composed most of the participants in our study. Environmental factors, such as access to healthy food and venues for physical activity, and healthcare system factors are other important factors that affect premature mortality.
The most common causes of premature death in PCS participants were IHD, stroke, RTIs, lower respiratory infections, and COVID-19. Our findings align with the Global Burden of Disease (GBD) study, underscoring IHD, stroke, and RTIs as the primary contributors to premature mortality in Iran, mirroring patterns observed in upper-middle-income countries. The GBD study demonstrated that the specific causes contributing to premature death varied globally [27, 28]. Premature deaths in the EPIC cohort study were primarily attributed to cancer (50%) and circulatory diseases (22%). In contrast, cohort studies conducted in Iran, including the PCS, GCS, and Tehran Lipid and Glucose Study, indicated that CVDs are the leading cause of death, with RTIs ranking among the top five causes of premature death. The leading causes of premature death in GCS participants are IHD, stroke, RTIs, stomach cancer, and esophageal cancer [9]. The Tehran Lipid and Glucose Study cohort in Iran identified CVD, cancer, RTIs, sepsis, and pneumonia as the underlying causes of premature deaths [6, 11]. In this study, RTIs were the third leading cause of premature death. According to the Global Status Report on Road Safety 2018, 93% of the 1.35 million global road traffic deaths occurred in low- and middle-income countries in 2016 [29]. RTIs remain a public health concern in Iran, despite evidence of a decline in RTIs across all countries [30].
This study revealed that premature mortality due to the COVID-19 pandemic was one of the main causes of premature death, although only the first year of the pandemic was included in our analysis. The COVID-19 pandemic has had a significant impact on mortality worldwide. The GBD study revealed that while age-standardized mortality rates globally declined between 1950 and 2019 (a 62.8% decline), they increased considerably during the COVID-19 pandemic period (2020–21; by 5.1%) [31]. Unprecedented reversals in adult mortality and life expectancy trends at the global and national levels during the pandemic highlighted the importance of paying comprehensive attention to both communicable and noncommunicable diseases simultaneously.
Cohort studies play a pivotal role in uncovering modifiable risk factors for premature deaths. In the GCS, noteworthy protective factors included wealth score, physical activity, education, and fruit/vegetable consumption. Conversely, significant risk factors included opium use, tobacco consumption, diabetes, and hypertension. The cumulative impact of these factors accounted for 73% of the PAF. The factors associated with the highest PAF were wealth score, physical activity, and hypertension [9]. In our study, modifiable factors played a pivotal role, contributing to 83% of premature deaths. The Tehran Lipid and Glucose Study identified hypertension, diabetes, and current smoking as significant risk factors for premature mortality. Controlling these risk factors, particularly diabetes, hypertension, and smoking, has the potential to reduce mortality by more than 40% [11]. In the PCS, opium use, hypertension, and tobacco consumption were considered among the most important modifiable risk factors.
Globally, some studies have sought to assess premature deaths, revealing regional variations in the significance of risk factors. These disparities arise from differences in the strength of associations and variations in the prevalence of these factors across diverse regions. The EPIC cohort study conducted on middle-aged individuals in Western Europe focused on evaluating modifiable causes of premature death [10]. While smoking remains the primary contributor to premature mortality in Europe, other notable factors such as suboptimal dietary habits, overweight and obesity, hypertension, insufficient physical activity, and excessive alcohol consumption also play significant roles. The collective attributable fraction for these six risk factors was determined to be 57%.
In the PCS, the predominant cause of death was vascular disease, which included IHD and stroke. CVD accounts for a substantial proportion of global deaths, accounting for 31% annually, a trend partially attributed to population growth and aging [32]. Findings from the PURE cohort study indicated that a substantial 70% of CVD and associated deaths can be attributed to a limited set of modifiable factors [33]. Certain factors, such as high blood pressure and education, exert a widespread influence globally, while others, such as household air pollution and unhealthy eating habits, exhibit variability based on a country’s economic status. Metabolic elements emerged as the primary contributors to the risk of CVD, constituting 41.2% of the PAF, with hypertension representing the most significant portion at 22.3% of the PAF. The INTERSTROKE case-control study identified various factors associated with all strokes, including a history of hypertension or elevated blood pressure, regular physical activity, apolipoprotein (Apo)B/ApoA1 ratio, dietary habits, waist-to-hip ratio, psychosocial factors, current smoking status, cardiac causes, alcohol consumption, and diabetes mellitus. Together, these factors contributed to 90.7% of the population-attributable risk for all strokes globally [34]. A case-control study known as the INTERHEART study revealed that abnormal lipids, smoking, hypertension, diabetes, abdominal obesity, psychosocial factors, consumption of fruits, vegetables, alcohol, and regular physical activity are the primary contributors to the risk of myocardial infarction globally. Together, these nine risk factors were responsible for 90.4% of the total population-attributable risk [35].
In our study, opium exhibited the highest HR among the variables. Opium consumption is notably correlated with elevated risks of death from diverse causes, including circulatory diseases and cancer [36]. In the GCS study, long-term opiate consumption was related to increased cardiovascular death, independent of other known risk factors [37]. Opium consumption was also associated with an increased likelihood of developing various cancers [38].
This study possesses notable strengths attributable to its prospective design, extensive and precise follow-up data, meticulous death ascertainment, and the incorporation of objective measurements alongside self-reported data. A comprehensive evaluation was conducted, including wealth score, eGFR, and non-HDL. Noteworthy strengths of this investigation included the availability of data for confounder adjustment and the low loss-to-follow-up rate (0.59%). Several limitations characterize our study, including the limited sample size and the fact that the participants were solely from rural districts. The infrequent occurrence of premature death within our cohort may compromise the statistical power of our study, potentially impeding the detection of risk factors within specific subgroups. Furthermore, the absence of data on urban populations and individuals younger than 40 years can limit the generalizability of our findings. The impact of alcohol consumption could not be analyzed due to its low prevalence for religious reasons and absence from social habits. We recommend conducting further cohort studies on this subject to obtain comprehensive results and implement necessary interventions and policies based on the outcomes. Also, we recommend conducting additional cohort studies with repeated risk factor measurements, shorter interval follow-ups, and including quality of life to achieve a comprehensive and accurate perspective on health outcomes.
To decrease premature death, several measures can be taken. The PolyPars study, which was conducted in the PCS to assess the effectiveness of the polypill (two antihypertensive agents, a statin, and aspirin) for primary and secondary prevention of CVD, demonstrated that it can safely halve the risk of major CVDs [39]. Also, paying simultaneous attention to socioeconomic and behavioral factors is recommended. Because opium and road traffic have impressive negative effects, new policies and public awareness on these topics are suggested.
Conclusion
54% of deaths were premature. NCDs constituted 79% of premature deaths. The most common causes of premature death were IHD, stroke, RTIs, lower respiratory infections, and COVID-19, respectively. The risk of premature death was greater in older individuals, tobacco and opium consumers, those with hypertension, and those with a greater waist circumference. The protective factors included female sex, higher education levels, being married, higher physical activity, hip circumference, and wealth score. Modifiable risk factors could reduce premature death by approximately 83%. Continued research, the application of the findings from this study, and the formulation of policies in alignment with those findings will collectively play a crucial role in enhancing life expectancy and potentially mitigating the societal burden of avoidable outcomes.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the study participants for their cooperation.
Abbreviations
- Cis
Confidence intervals
- CKD-Epi
Chronic Kidney Disease Epidemiology
- CMPN
Communicable, maternal, perinatal, and nutritional conditions
- CVD
Cardiovascular disease
- DDRI
Digestive Diseases Research Institute
- eGFR
Estimated glomerular filtration rate
- EPIC
European Prospective Investigation into Cancer and Nutrition
- GBD
Global Burden of Disease
- GCS
Golestan Cohort Study
- HR
Hazard ratio
- ICD-10
10th revision of the International Classification of Diseases
- IHD
Ischemic heart disease
- NCD
Noncommunicable diseases
- non-HDL-C
Non-high-density lipoprotein cholesterol
- PAF
Population attributable fraction
- PCS
Pars Cohort Study
- RTI
Road traffic injury
- SUMS
Shiraz University of Medical Sciences
- WHO
World Health Organization
Author contributions
RM, MN, FM, AG, SS, and HP conceptualized the study; FZ prepared the original draft; MN and RM contributed to the design of the study; and FZ, MN, and SS performed the analysis. FZ, MN, RM, and SS contributed to writing, reviewing, and editing the manuscript. MN and FZ contributed to the interpretation of the data. All authors reviewed and approved the final manuscript.
Funding
This work was funded by Shiraz University of Medical Sciences, Grant Number [910210].
Data availability
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The ethics committees of the Digestive Diseases Research Institute (DDRI) and Shiraz University of Medical Sciences (SUMS) approved the study protocol, and the ethics code of this study is IR.TUMS.SHARIATI.REC.1402.001. The completion and signing of the informed consent form were done in the presence of a third party, and their information was kept completely confidential.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Reza Malekzadeh, Email: malek@tums.ac.ir.
Mahdi Nalini, Email: mahdinalini@yahoo.com.
References
- 1.Chen JT, Rehkopf DH, Waterman PD, Subramanian SV, Coull BA, Cohen B, et al. Mapping and measuring social disparities in premature mortality: the impact of census tract poverty within and across Boston neighborhoods, 1999–2001. J Urban Health. 2006;83(6):1063–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Razeghian-Jahromi I, Ghasemi Mianrood Y, Dara M, Azami P. Premature death, underlying reasons, and preventive experiences in Iran: a narrative review. Arch Iran Med. 2023;26(7):403–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peto R, Lopez AD, Norheim OF. Halving premature death. Science. 2014;345(6202):1272. [DOI] [PubMed] [Google Scholar]
- 4.Ellis J, Dowrick C, Lloyd-Williams M. The long-term impact of early parental death: lessons from a narrative study. J R Soc Med. 2013;106(2):57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noncommunicable diseases World Health. Organisation2023 [ https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases
- 6.Ziamanesh F, Mohajeri Tehrani MR, Hemmatabadi M, Sharghi S, Fallahi B, Haghpanah V, et al. Design and implementation of a national quality registry of thyroid cancer in Iran: study protocol. Journal of Diabetes & Metabolic Disorders; 2023. [DOI] [PMC free article] [PubMed]
- 7.Foreman KJ, Marquez N, Dolgert A, Fukutaki K, Fullman N, McGaughey M, et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet. 2018;392(10159):2052–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Global regional. National incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the global burden of Disease Study 2016. Lancet. 2017;390(10100):1211–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nalini M, Oranuba E, Poustchi H, Sepanlou SG, Pourshams A, Khoshnia M, et al. Causes of premature death and their associated risk factors in the Golestan Cohort Study, Iran. BMJ Open. 2018;8(7):e021479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muller DC, Murphy N, Johansson M, Ferrari P, Tsilidis KK, Boutron-Ruault M-C, et al. Modifiable causes of premature death in middle-age in Western Europe: results from the EPIC cohort study. BMC Med. 2016;14:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eslami A, Naghibi Irvani SS, Ramezankhani A, Fekri N, Asadi K, Azizi F, et al. Incidence and associated risk factors for premature death in the Tehran lipid and glucose study cohort, Iran. BMC Public Health. 2019;19(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gandomkar A, Poustchi H, Moini M, Moghadami M, Imanieh H, Fattahi MR, et al. Pars cohort study of non-communicable diseases in Iran: protocol and preliminary results. Int J Public Health. 2017;62(3):397–406. [DOI] [PubMed] [Google Scholar]
- 13.Global Physical Activity Surveillance World Health Organisation2017 [updated 24. May 2017. http://www.who.int/chp/steps/GPAQ/en/
- 14.Vinyoles E, Tafalla M, Robledo V, Marco M, Porta I, Muñoz M-A, et al. Interarm blood pressure measurement and the reference-arm assignment variability. Blood Press Monit. 2019;24(5):259–63. [DOI] [PubMed] [Google Scholar]
- 15.Hypertension: World Health Organization. 2023 [ https://www.who.int/news-room/fact-sheets/detail/hypertension#:~:text=Overview,get%20your%20blood%20pressure%20checked
- 16.Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–88. [DOI] [PubMed] [Google Scholar]
- 17.Pencina KM, Thanassoulis G, Wilkins JT, Vasan RS, Navar AM, Peterson ED, et al. Trajectories of Non-HDL cholesterol across midlife: implications for Cardiovascular Prevention. J Am Coll Cardiol. 2019;74(1):70–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Islami F, Kamangar F, Nasrollahzadeh D, Aghcheli K, Sotoudeh M, Abedi-Ardekani B, et al. Socio-economic status and oesophageal cancer: results from a population-based case-control study in a high-risk area. Int J Epidemiol. 2009;38(4):978–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doganer YC, Rohrer JE, Aydogan U, Barcin C, Cayci T, Saglam K. Association of renal function, estimated by four equations, with coronary artery disease. Int Urol Nephrol. 2015;47(4):663–71. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.AZIZI F, Khalili D, Aghajani H, Esteghamati A, Hosseinpanah F, Delavari A, et al. Appropriate waist circumference cut-off points among Iranian adults: the first report of. the Iranian National Committee of Obesity; 2010. [PubMed]
- 22.AZIZI F, Hadaegh F, KHALILI D, Esteghamati A, HOSSEIN PF, Delavari A, et al. Appropriate definition of metabolic syndrome among Iranian adults. report of the Iranian National Committee of Obesity; 2010. [PubMed]
- 23.Khademi H, Etemadi A, Kamangar F, Nouraie M, Shakeri R, Abaie B, et al. Verbal autopsy: reliability and validity estimates for causes of death in the Golestan Cohort Study in Iran. PLoS ONE. 2010;5(6):e11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.International Statistical Classification of Diseases and Related Health Problems 10th Revision. World Health Organization [updated 25 My 2017. http://apps.who.int/classifications/icd10/browse/2016/en
- 25.WHO methods and. data sources for country-level causes of death 2000–2019 2020 [ https://cdn.who.int/media/docs/default-source/gho-documents/global-health-estimates/ghe2019_cod_methods.pdf
- 26.Nalini M, Sharafkhah M, Poustchi H, Sepanlou SG, Pourshams A, Radmard AR, et al. Comparing Anthropometric indicators of visceral and General Adiposity as determinants of overall and Cardiovascular Mortality. Arch Iran Med. 2019;22(6):301–9. [PMC free article] [PubMed] [Google Scholar]
- 27.Naghavi M, Abajobir AA, Abbafati C, Abbas KM, Abd-Allah F, Abera SF, et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the global burden of Disease Study 2016. Lancet. 2017;390(10100):1151–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moradi-Lakeh M, Sepanlou SG, Karimi SM, Khalili N, Djalalinia S, Karimkhani C, et al. Trend of Socio-Demographic Index and Mortality Estimates in Iran and its neighbors, 1990–2015; findings of the Global Burden of diseases 2015 study. Arch Iran Med. 2017;20(7):419–28. [PubMed] [Google Scholar]
- 29.Organization WH. Global status report on road safety 2015. World Health Organization; 2015.
- 30.Bazargan-Hejazi S, Ahmadi A, Shirazi S, Ainy E, Djalalinia S, Fereshtehnejad S-M et al. The burden of road traffic injuries in Iran and 15 surrounding countries: 1990–2016. Arch Iran Med. 2018;21(12). [PubMed]
- 31.Schumacher AE, Kyu HH, Aali A, Abbafati C, Abbas J, Abbasgholizadeh R et al. Global age-sex-specific mortality, life expectancy, and population estimates in 204 countries and territories and 811 subnational locations, 1950–2021, and the impact of the COVID-19 pandemic: a comprehensive demographic analysis for the global burden of Disease Study 2021. The Lancet. 2024. [DOI] [PMC free article] [PubMed]
- 32.WHO. Cardiovascular diseases fact sheet. WHO Geneva; 2017.
- 33.Yusuf S, Joseph P, Rangarajan S, Islam S, Mente A, Hystad P, et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet. 2020;395(10226):795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Donnell MJ, Chin SL, Rangarajan S, Xavier D, Liu L, Zhang H, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet. 2016;388(10046):761–75. [DOI] [PubMed] [Google Scholar]
- 35.Yusuf S, Hawken S, Ôunpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–52. [DOI] [PubMed] [Google Scholar]
- 36.Khademi H, Malekzadeh R, Pourshams A, Jafari E, Salahi R, Semnani S et al. Opium use and mortality in Golestan Cohort Study: prospective cohort study of 50 000 adults in Iran. BMJ. 2012;344. [DOI] [PMC free article] [PubMed]
- 37.Nalini M, Shakeri R, Poustchi H, Pourshams A, Etemadi A, Islami F, et al. Long-term opiate use and risk of cardiovascular mortality: results from the Golestan Cohort Study. Eur J Prev Cardiol. 2021;28(1):98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kamangar F, Shakeri R, Malekzadeh R, Islami F. Opium use: an emerging risk factor for cancer? Lancet Oncol. 2014;15(2):e69–77. [DOI] [PubMed] [Google Scholar]
- 39.Malekzadeh F, Gandomkar A, Poustchi H, Etemadi A, Roshandel G, Attar A et al. Effectiveness of polypill for primary and secondary prevention of cardiovascular disease: a pragmatic cluster-randomised controlled trial (PolyPars). Heart. 2024. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.