Dear editor,
Pneumocystis jirovecii pneumonia (PJP) is a severe fungal opportunistic infection occurring in immunocompromised patients, commonly associated with Human Immunodeficiency Virus (HIV) in the past and nowadays increasingly diagnosed in non-HIV patients with immune suppression. Severe PJP requiring admission to the intensive care unit is associated with mortality rates > 50%, and several factors have been associated with reduced survival including age, a non-HIV status, invasive mechanical ventilation and the admission SOFA score [1, 2]. Whether additional prognostic factors might help identify high-risk patients at an early stage of ICU stay remains undefined. To address this issue, we retrospectively analyzed (study protocol approved by our ethical committee, CER-VD Nr 2020-00201) the clinical and early (admission—day 0- and day 1) arterial blood gas (ABG) variables, including values of methemoglobin (MetHb) and carboxyhemoglobin (HbCO), in a cohort of PJP patients admitted to our multidisciplinary ICU between 2006 and 2019. The primary outcome was mortality at day 60. Data were compared between survivors and non survivors using the Wilcoxon’s rank sum test and the Pearson’s chi-squared test, and univariate logistic regression analyses were done to evaluate associations between variables and 60-day mortality. We also performed a multivariable analysis incorporating invasive mechanical ventilation at day 1 as a possible confounder, with blood gas data at day 1 (PaCO2, HbCO and MetHb) as explanatory co-variables. The impact of blood gas variables on 60-day survival was further assessed using Kaplan–Meier plots and log-rank test analysis.
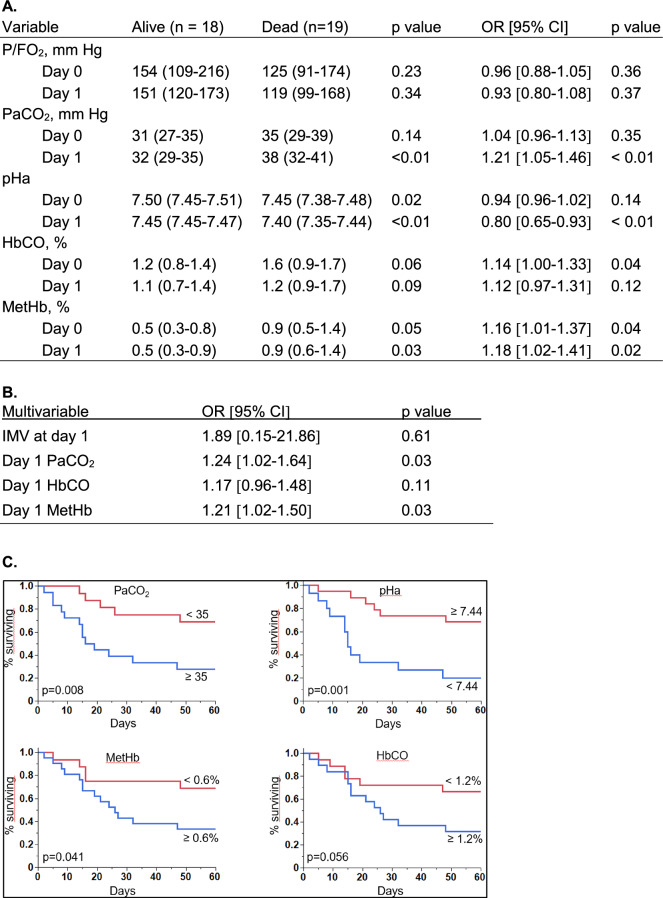
A total of 37 patients with confirmed Pneumocystis jirovecii infection (except in one patient in whom no sample could be obtained, but with typical clinical/radiological presentation and a positive beta-glucan test) were included. Underlying diagnoses were malignancy (n = 21), chronic immune-mediated inflammatory disease (n = 8), HIV (n = 5), solid organ (n = 4) or bone marrow transplantation (n = 5), with more than 1 condition present in 6 patients. Most patients had been treated prior to admission with one or more immune suppressive therapies. The 60-day mortality was 51% (19/37 patients). Non-survivors were significantly older but did not differ from survivors with respect to gender and underlying diagnoses. All patients received non-invasive and/or invasive respiratory support, and non-survivors required significantly more often invasive mechanical ventilation (79 vs. 39%, p < 0.05). ABG analyses showed that non-survivors had higher PaCO2 (day 1), lower pHa and higher MetHb as well as a trend for higher HbCO (day 0 and day 1). In contrast, P/F O2 was comparable in survivors and non-survivors at the two time-points. In univariate analyses, day 0 HbCO and MetHb, and day 1 PaCO2, pHa and MetHb were significantly associated with 60-day mortality (Fig. 1A). In multivariable analysis, PaCO2 and MetHb at day 1 remained significantly associated with 60-day mortality (Fig. 1B). Kaplan–Meier analyses showed that patients with higher MetHb and lower pHa at day 0 (not shown), as well as higher HbCO, PaCO2 and MetHb, as well as lower pHa at day 1 (Fig. 1C) had significantly shorter survival.
Fig. 1.
Arterial blood gas data at day 0 and day 1. A P/FO2, PaCO2, pHa, HbCO and MetHb at day 0 and day 1 in survivors and non-survivors (median, interquartile rage) and their univariate association with 60-day mortality. B Multivariable analysis of factors associated with 60-day mortality. C Kaplan–Meier plots illustrating the proportion of survivors in the 60-day observation period, as a function of PaCO2, (in mmHg), pHa, MetHb and HbCO at day 1, dichotomized according to their median values in the whole cohort. For continuous variables, odds ratios (OR) and 95% confidence intervals (CI) were calculated per unit change (P/FO2: 10 mm Hg; PaCO2: 1 mm Hg; pHa: 0.01 pH unit; HbCO: 0.1%; MetHb: 0.1%). Note: At day 0, P/FO2 missing in 1 patient (survivor), HbCO and MetHB not measured in 3 patients (2 survivors, 1 non survivors). At day 1: ABG not obtained in 3 patients (1 non-survivor, 2 survivors), in whom HbCO and MetHb were obtained from central venous blood gas analysis. IMV invasive mechanical ventilation
Our study found that several ABG variables obtained during the first 24 h of ICU admission may provide important early prognostic information in patients with PJP. The higher PaCO2 and lower pHa in non-survivors could either reflect the development of respiratory fatigue or increased dead space ventilation, which would be consistent with the negative impact of higher dead space fraction in other forms of acute respiratory failure [3]. Non-survivors also displayed higher levels of MetHb, whose values at day 0 and day 1 were significantly associated with 60-day mortality. MetHb formed from Hb autooxidation is normally maintained at very low levels due to the activity of MetHb reductase, but may increase in critically ill patients with sepsis, or treated with inhaled NO or prooxidant drugs such as dapsone [4]. Since none of our patients received these therapies at the time of MetHb measurements, we propose that MetHb formation could reflect more severe lung inflammation, favoring a greater pro-oxidant environment fostering hemoglobin oxidation. We also noted, to a lesser extent, that HbCO was higher in non-survivors and that its value at admission was associated with 60-day mortality. Endogenous CO formation results from the activity of heme oxygenase (HO), and pulmonary HO induction has been reported in a variety of lung inflammatory diseases [5]. Therefore, we speculate that the early increase of HbCO in PJP non-survivors could reflect such an induction of HO due to more severe lung inflammation. In summary, we found that, in patients admitted to the ICU for acute respiratory failure due to Pneumocystis jirovecii pneumonia, several early (first 24 h) changes in arterial blood gases present a significant association with 60-day mortality. These include a higher PaCO2, a lower arterial pH, higher values of methemoglobin and (to a lesser extent) carboxyhemoglobin. Whether the recognition of these early prognostic variables could alter patient management and result in a better outcome should require further studies.
Acknowledgements
None
Author contributions
AV: Experimental design; investigation; data curation; formal analysis; writing: original draft, JB: Formal analysis; writing: review and editing, ZL: Formal analysis; writing: review and editing, OM: Formal analysis; writing: review and editing, LL: Formal analysis; writing: original draft, review and editing.
Funding
None to declare.
Availability of data and materials
All data generated or analyzed during this study are included in this article.
Declarations
Ethics approval and consent to participate
The study was approved by our local ethical committee (Commission cantonale d'éthique de la recherche sur l’être humain, CER-VD, project number 2020–00201).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lecuyer R, Issa N, Camou F, Lavergne RA, Gabriel F, Morio F, et al. Characteristics and prognosis factors of Pneumocystis jirovecii pneumonia according to underlying disease: a retrospective multicenter study. Chest. 2024;165:1319–29. [DOI] [PubMed] [Google Scholar]
- 2.Giacobbe DR, Dettori S, Di Pilato V, Asperges E, Ball L, Berti E, et al. Pneumocystis jirovecii pneumonia in intensive care units: a multicenter study by ESGCIP and EFISG. Crit Care. 2023;27:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kallet RH, Zhuo H, Ho K, Lipnick MS, Gomez A, Matthay MA. Lung injury etiology and other factors influencing the relationship between dead-space fraction and mortality in ARDS. Respir Care. 2017;62:1241–8. [DOI] [PubMed] [Google Scholar]
- 4.Belzer A, Krasowski MD. Causes of acquired methemoglobinemia—a retrospective study at a large academic hospital. Toxicol Rep. 2024;12:331–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagasawa R, Hara Y, Murohashi K, Aoki A, Kobayashi N, Takagi S, et al. Serum heme oxygenase-1 measurement is useful for evaluating disease activity and outcomes in patients with acute respiratory distress syndrome and acute exacerbation of interstitial lung disease. BMC Pulm Med. 2020;20:310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article.