Abstract
Morbilliviruses comprise measles virus, canine distemper virus, rinderpest virus, and several other viruses that cause devastating human and animal diseases accompanied by severe immunosuppression and lymphopenia. Recently, we have shown that human signaling lymphocyte activation molecule (SLAM) is a cellular receptor for measles virus. In this study, we examined whether canine distemper and rinderpest viruses also use canine and bovine SLAMs, respectively, as cellular receptors. The Onderstepoort vaccine strain and two B95a (marmoset B cell line)-isolated strains of canine distemper virus caused extensive cytopathic effects in normally resistant CHO (Chinese hamster ovary) cells after expression of canine SLAM. The Ako vaccine strain of rinderpest virus produced strong cytopathic effects in bovine SLAM-expressing CHO cells. The data on entry with vesicular stomatitis virus pseudotypes bearing measles, canine distemper, or rinderpest virus envelope proteins were consistent with development of cytopathic effects in SLAM-expressing CHO cell clones after infection with the respective viruses, confirming that SLAM acts at the virus entry step (as a cellular receptor). Furthermore, most measles, canine distemper, and rinderpest virus strains examined could any use of the human, canine, and bovine SLAMs to infect cells. Our findings suggest that the use of SLAM as a cellular receptor may be a property common to most, if not all, morbilliviruses and explain the lymphotropism and immunosuppressive nature of morbilliviruses.
Morbilliviruses are highly contagious pathogens that cause some of the most devastating viral diseases of humans and animals worldwide (15, 28). They include measles virus (MV), canine distemper virus (CDV), rinderpest virus (RPV), and peste des petits ruminants virus. Although live attenuated vaccines have effectively reduced their incidences, morbillivirus infections still present a major threat to the health of humans and animals. There are, for example, roughly 30 million cases of measles and 1 million deaths associated with measles per year worldwide (11). Furthermore, emerging infectious diseases of marine mammals have been found to be caused by new morbilliviruses, such as phocine (seal), dolphin, and porpoise distemper viruses (13, 21, 26, 32, 48).
Morbilliviruses are enveloped, nonsegmented negative-strand RNA viruses and constitute a genus within the family Paramyxoviridae. They cause fever, coryza, conjunctivitis, gastroenteritis, and pneumonia in their respective host species. The major sites of viral propagation are lymphoid tissues, and acute diseases are usually accompanied by profound lymphopenia and immunosuppression, leading to secondary and opportunistic infections (1, 15, 24, 28). While CDV and phocine distemper virus often invade the central nervous systems of their hosts (46), encephalitis is not common in MV and RPV infections.
The host range of CDV includes all species of the families Canidae (e.g., dog), Procyonidae (e.g., raccoon), and Mustelidae (e.g., ferret). The recent outbreaks of distemper in seals in Lake Baikal (47), in lions in the Serengeti National Park (36), and in leopards and other large cats in zoos (3) have underscored the ability of CDV to invade new host species. Virus isolation is usually done by cocultivation of lymphocytes from suspect dogs with mitogen-stimulated dog lymphocytes (2). Field isolates of CDV also replicate in dog or ferret macrophages (9, 27) as well as in primary dog brain cell cultures (52). Cell lines such as Vero (African green monkey kidney) cells do not allow the propagation of field isolates, whereas cell culture-adapted CDV strains such as the Onderstepoort vaccine strain are able to replicate in many cell lines (1). It is known that virulence for the natural host may be lost when CDV is adapted to cell culture (17).
Rinderpest, one of the oldest recorded plagues of livestock, is still the cause of great economic loss in Africa, the Middle East, and parts of Asia. The host range of RPV includes domestic cattle, water buffalo, sheep, goats, and pigs (28). In cattle, target cells for RPV are epithelial cells, activated lymphocytes, and macrophages (34, 37, 49). Virus isolation is carried out routinely in primary bovine kidney cell cultures or a Theileria parva-transformed bovine lymphocyte cell line (38).
Cellular receptors are one of the major determinants of the host range and tissue tropism of a virus. Recently we have reported that human signaling lymphocyte activation molecule (SLAM; also known as CD150), a membrane glycoprotein expressed on some lymphocytes and dendritic cells (12, 40), is a cellular receptor for MV (45). Since the tissue distribution of human SLAM can explain the pathology of measles, we proposed that selective infection and destruction of SLAM-positive cells may be a principal mechanism of the immunosuppressive nature of morbilliviruses in general (45). Furthermore, the marmoset B cell line B95a, which is commonly used to isolate MV from clinical specimens (22) and expresses a high level of SLAM on the cell surface (45), has been shown to be very sensitive to CDV and RPV (20, 23).
In this study, we examined whether SLAM can act as a cellular receptor for CDV and RPV. Our results confirmed our proposition that the use of SLAM as a cellular receptor is a trait common to MV, CDV, and RPV. Furthermore, we found that these three morbilliviruses can use SLAMs of nonhost species as receptors.
MATERIALS AND METHODS
Viruses.
The Onderstepoort vaccine strain of CDV was propagated on Vero cells. The HA7 and 851 strains of CDV were isolated using B95a cells from dogs with distemper, and they have been passaged five to seven times on B95a cells. These CDV strains were kindly provided by the staff of Division of Veterinary Microbiology, Kyoto Biken Laboratories, Uji, Japan. The Edmonston and KA strains of MV were propagated on Vero and B95a cells, respectively (43). The Onderstepoort and Edmonston strains were titrated on Vero cells, and the B95a-isolated CDV strains and KA strain were titrated on B95a cells. The Ako strain of lapinized-avianized RPV (14) was kindly provided by Hidetoshi Ikeda, National Institute of Animal Health, Tsukuba, Japan, and was grown and titrated on Vero cells.
Molecular cloning of canine and bovine SLAM cDNAs.
Total RNA was extracted from canine peripheral blood mononuclear cells (PBMCs) at 2 h after stimulation with 2.5 μg of phytohemagglutinin per ml and used for reverse transcription with oligo(dT) primers. The conditions used for PBMC stimulation were based on induction kinetics of human SLAM mRNA and protein (5, 12). To amplify the cDNA encoding canine SLAM, we performed PCR using various combinations of the primers for human and marmoset SLAMs. Using a sense primer for the open reading frame (ORF) of B95a SLAM (5′-GAGGGGTGGTATTTTATGACC-3′) and an antisense primer for the 3′ untranslated region of human SLAM (5′-AAGAAACATCACCAGGGAGTTG-3′), we successfully amplified a DNA fragment. Direct sequencing revealed that it had strong homology to human and B95a SLAM cDNAs (12, 45). Using the 5′ RACE system, version 2.0 (Life Technologies), we obtained the sequence of the 5′ untranslated region for it. After obtaining all this information, we performed PCR using cDNA of phytohemagglutinin-stimulated canine PBMCs, the primers 5′-AATGAATTCCCTGTCTCCCTGGCCGAT-3′ and 5′-TCTTGCGGCCGCCTTCAGAAAGTCCCTTCACTG-3′ (restriction sites are underlined), and KOD-Plus polymerase (Toyobo Biochemicals), which has a high proofreading activity. The amplified DNA was sequenced in both strands and found to contain an ORF which had strong homology to human SLAM (77% identity at the nucleotide level in the ORF). This canine SLAM cDNA was subcloned into the eukaryotic expression vector pCAGGS (31), and the resulting construct was named pCAGDogSLAM. The signal sequence of canine SLAM was predicted using SignalP software, version 2.0 (30). The canine SLAM cDNA whose 5′ untranslated region and signal sequence were deleted was subcloned behind the sequence encoding the immunoglobulin (Ig) κ leader sequence and 17 amino acid residues containing the influenza virus hemagglutinin (HA) epitope (NH2-YPYDVPDYAGAQPARSP-COOH; the HA epitope is underlined) of the expression vector pDisplay (Invitrogen). The fragment containing the Ig leader sequence, HA tag, and canine SLAM was further subcloned into pCAGGS (pCAGDogSLAMtag), which was expected to direct the expression of canine SLAM with the HA tag on eukaryotic cells.
Bovine SLAM cDNA was cloned similarly using total RNA extracted from bovine PBMCs at 3 h after stimulation with 25 ng of phorbol 12-myristate 13-acetate and 1 μg of ionomycin per ml (5, 12). A DNA fragment was successfully amplified by PCR using a sense primer for the ORF of canine SLAM (5′-TGGAAAACCTGACCCTGAGGAT-3′) and an antisense primer for the 3′ untranslated region of human SLAM described above. It had strong homology to human, marmoset, and canine SLAM cDNAs (12, 45). After obtaining the 5′ untranslated region sequence for it, we performed PCR using cDNA of stimulated bovine PBMCs, the primers 5′-AATGAATTCCTTATCCTCACTGGCTGATG-3′ and 5′-TCTTGCGGCCGCCTTCGGAAAGTCCTTTCAC-3′ (restriction sites are underlined), and KOD-Plus polymerase. The clone obtained contained an ORF which had strong homology to human SLAM (78% identity at the nucleotide level in the ORF). This bovine SLAM cDNA clone was modified so as to direct the expression of bovine SLAM with the HA tag on eukaryotic cells as described above, and the plasmid was named pCAGCowSLAMtag.
Expression plasmids.
cDNA encoding marmoset SLAM was cloned into pCAGGS, and the construct was named pCAGB95aSLAM (marmoset SLAM cDNA was obtained from B95a cells) (45). The plasmids expressing the H protein (pCXN2H) and F protein (pCXN2F) of the MV Edmonston strain and the H protein of the MV KA strain (pCXN2KAH) have been described (43). pCVSVG expressing the vesicular stomatitis virus (VSV) G protein was kindly provided by M. A. Whitt. cDNA clones encoding the CDV envelope proteins were obtained by reverse transcriptase PCR of total RNA extracted from Vero cells infected with the Onderstepoort strain or B95a cells infected with the HA7 strain. Primers used were 5′-TTGGTACCAACTTAGGGCTCAGGTAGTCC-3′ and 5′-TTTAGCATGCTGGAGATGGTTTAATTCAATCG-3′ for the H genes, and 5′-CAGGTACCAGCAAGCCAACAGGTCAACCA-3′ and 5′-TTTAGCATGCAATCACGTAATCATGGTCAGTC-3′ for the F gene (restriction sites are underlined). cDNAs encoding the H protein and F protein of the Onderstepoort strain and the H protein of the HA7 strain were subcloned into pCAGGS, and the resulting constructs were named pCAGOPH, pCAGOPF, and pCAGHA7H, respectively. pvRVH (51) and pBac-F (7) contain the H and F genes of the Kabete O strain of RPV, respectively, and were kindly provided by T. Yilma. The H and F genes recovered from pvRVH and pBac-F were subcloned into pCAGGS, and the constructs were named pCAGKOH and pCAGKOF, respectively.
Cells.
CHO (Chinese hamster ovary) cell clones were generated by transfecting CHO cells with pCXN2 (31) plus pCAGB95aSLAM, pCAGDogSLAMtag, pCAGCowSLAMtag, or pCAGGS. CHO.SLAM is the CHO cell clone stably expressing human SLAM (45). CHO cell clones were grown in RPMI 1640 medium supplemented with 7% heat-inactivated fetal bovine serum, 0.15% sodium bicarbonate, and 0.5 mg of G418 per ml. Vero and B95a cells were grown as described elsewhere (44).
Virus infections of cells.
Cells were plated in 24-well plates and infected with MV, CDV, or RPV strains. At 1 h after infection, the cells were washed and replenished with fresh medium. The cells were observed under a microscope at 24 h after infection with MV or CDV strains or at 12 h after infection with the Ako strain of RPV. RPV infection was performed at the special facility of National Institute of Animal Health, Tsukuba, Japan. When the effects of antibody on viral infections were examined, cells were plated in 96-well flat-bottom plates and cultured overnight. Then, the culture medium was replaced with one containing 10 μg of IPO-3 (Kamiya Biomedical) per ml, 10 μg of mouse control monoclonal antibody (MAb) per ml, or no antibody. After 1 h of incubation, the cells were infected with a virus and incubated for 1 h. After washing, the cells were replenished with the fresh medium containing the same antibody as before. The cells were observed at 24 h after infection.
Immunofluorescence staining.
Cells were stained with IPO-3, anti-influenza virus HA epitope MAb 12CA5 (Boehringer Mannheim), or mouse control antibody, and then stained with fluorescein isothiocyanate (FITC)-labeled goat anti-mouse IgG. The stained cells were analyzed on a FACScan machine (Becton Dickinson).
VSV pseudotypes.
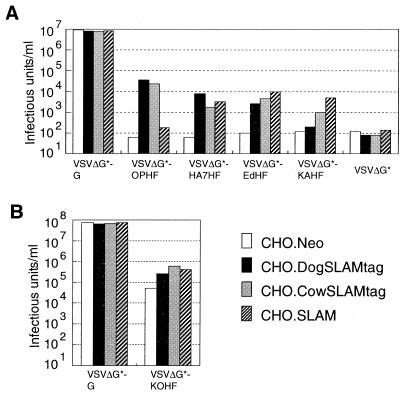
The preparation and titration of VSV pseudotypes were done essentially as described previously (44) with some modifications. We used CHO cells instead of 293T cells to prepare the pseudotype viruses used for Fig. 6A, because 293T cells transfected with pCAGOPH plus pCAGOPF developed extensive cell fusion, which could not be inhibited by the fusion block peptide (Z-D-Phe-Phe-Gly) (35, 44). For this reason, titers of VSV pseudotypes bearing MV envelope proteins and VSV G protein were lower than those in our previous reports (44, 45) or in Fig. 6B. CHO cells were transfected with pCVSVG, pCAGOPH plus pCAGOPF, pCAGHA7H plus pCAGOPF, pCXN2H plus pCXN2F, pCXN2KAH plus pCXN2F, or pCAGGS by using Lipofectamine Plus (Life Technologies). At 32 h after transfection, the cells were infected with VSVΔG*-G (42) (a gift of M. A. Whitt) at a multiplicity of infection (MOI) of 1 (titrated on CHO cells) for 1 h at 37°C. The cells were washed with medium without fetal bovine serum seven times and then replenished with fresh medium. After 16 h of incubation at 37°C in a CO2 incubator, culture fluid and scraped cell debris were collected, treated by one cycle of freezing-thawing, and sonicated. The suspensions containing pseudotype viruses were clarified by low-speed centrifugation and stored at −80°C. They were designated VSVΔG*-G, VSVΔG*-OPHF, VSVΔG*-HA7HF, VSVΔG*-EdHF, VSVΔG*-KAHF, and VSVΔG*, respectively. To prepare the pseudotype viruses used for Fig. 6B (VSVΔG*-G and VSVΔG*-KOHF), we transfected 293T cells with pCVSVG or pCAGKOH plus pCAGKOF. When VSVΔG*-KOHF was prepared, culture medium was supplemented with the fusion block peptide from 3 h after lipofection to immediately before infection with VSVΔG*-G, in order to prevent 293T cells from fusing to each other upon transfection.
FIG. 6.
Infectivities of pseudotype viruses on CHO cells expressing canine, bovine, or human SLAM. The indicated CHO cell clones were infected with VSVΔG*-G, VSVΔG*-OPHF, VSVΔG*-HA7HF, VSVΔG*-EdHF, VSVΔG*-KAHF, or VSVΔG* (A) or with VSVΔG*-G or VSVΔG*-KOHF (B), and infectious titers were measured by counting the number of GFP-expressing cells.
For titrations, 104 cells of each CHO cell clone in 100 μl of fresh culture medium were sedimented in the well of 96-well flat-bottom plates. After overnight incubation, 50 μl of serially diluted virus stock was added to each well, followed by incubation at 37°C in a CO2 incubator. At 24 h after infection, infectious units of pseudotype virus stocks were determined by counting the number of green fluorescence protein (GFP)-expressing cells under a fluorescence microscope.
Nucleotide sequence accession number.
The GenBank accession numbers for canine and bovine SLAM cDNA sequences are AF325357 and AF329970, respectively.
RESULTS
Cell tropism of CDV strains.
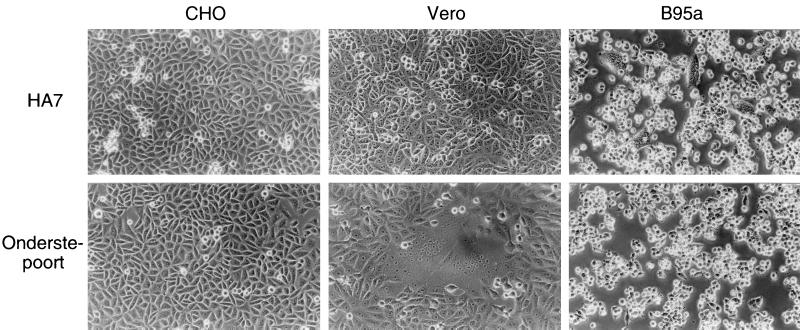
The Onderstepoort vaccine strain of CDV was derived from the virus, which had been isolated from a natural case of distemper and serially passaged in ferrets. The ferret-passaged virus was then adapted to chicken embryos, after which it was called the Onderstepoort strain (16). This strain has been grown on Vero cells. B95a-isolated CDV strains HA7 and 851 have been passaged on B95a cells only five to seven times after isolation from dogs with distemper.
We inoculated CHO, Vero, and B95a cells with the Onderstepoort and HA7 strains at an MOI of 0.1 and observed them at 24 h postinfection (Fig. 1). No cytopathic effects (CPEs) were found in CHO cells infected with either strain. The HA7 strain caused CPEs in B95a cells but not in Vero cells, whereas the Onderstepoort strain caused CPEs in Vero cells but not in B95a cells. Syncytium formation is a CPE characteristic of all morbilliviruses. Longer incubation (up to 96 h) did not affect the presence or absence of CPEs in the cells, although the observed CPEs became stronger. Another B95a-isolated CDV strain, 851, showed the same results as the HA7 strain (data not shown). Thus, the chicken embryo-adapted vaccine strain and B95a-isolated strains of CDV had distinct abilities to cause CPEs in different cell lines.
FIG. 1.
Cell tropism of CDV strains. CHO, Vero, and B95a cells were infected with the HA7 strain or Onderstepoort strain of CDV at an MOI of 0.1. Cells were observed at 24 h after infection.
B95a-isolated CDV strains cause CPEs in CHO cells expressing marmoset SLAM.
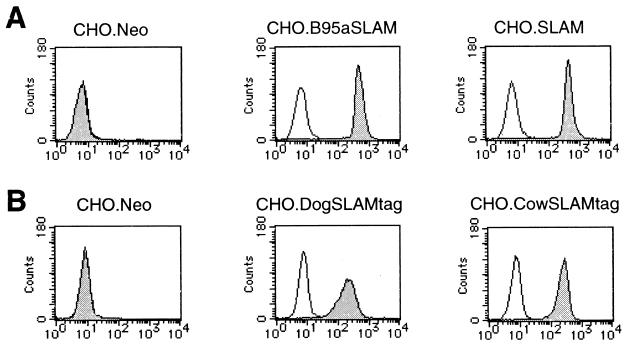
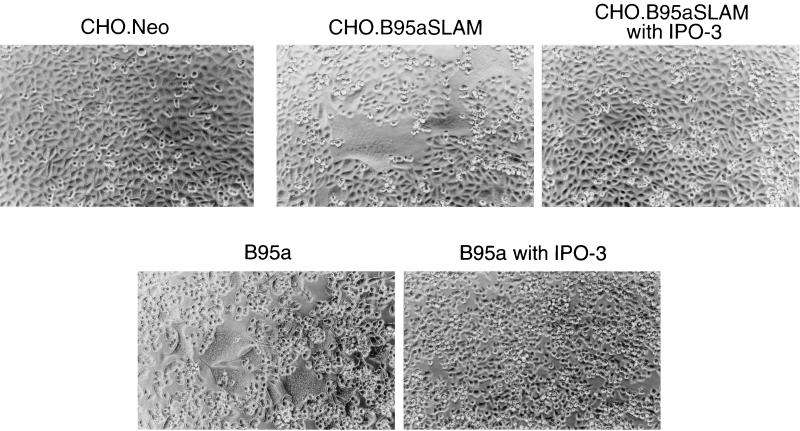
Wild-type strains of MV isolated in B95a cells are able to use marmoset SLAM as a cellular receptor (45). We thought that B95a-isolated CDV strains might also use marmoset SLAM to infect B95a cells. To test this idea, we generated the CHO cell clone stably expressing marmoset SLAM of B95a cells (CHO.B95aSLAM) as well as a control CHO cell clone (CHO.Neo). The cell surface expression of marmoset SLAM was confirmed by flow cytometry (Fig. 2A). At 24 h after infection, the B95a-isolated HA7 strain caused apparent CPEs in CHO.B95aSLAM cells but not in CHO.Neo cells (Fig. 3). Development of CPEs in CHO.B95aSLAM cells was completely blocked by treating the cells with anti-human SLAM MAb IPO-3 (Fig. 3). IPO-3 has been shown to block development of CPEs in susceptible cells (including B95a cells) infected with wild-type MV strains (45). The isotype control did not affect CPEs (data not shown). IPO-3 also completely blocked CPEs in B95a cells infected with the HA7 strain (Fig. 3). Another B95a-isolated strain, 851, showed exactly the same results as the HA7 strain, while the Onderstepoort vaccine strain caused CPEs on neither CHO.Neo nor CHO.B95aSLAM cells (data not shown). These results suggest that marmoset SLAM also acts as a cellular receptor for B95a-isolated CDV strains and that marmoset SLAM is probably the only CDV receptor on B95a cells.
FIG. 2.
CHO cell clones stably expressing SLAMs of various species. (A) CHO.Neo, CHO.B95aSLAM, and CHO.SLAM cells were stained with IPO-3 (solid profile) or mouse control IgG antibody (open profile), followed by staining with FITC-labeled goat anti-mouse IgG. (B) CHO.Neo, CHO.DogSLAMtag, and CHO.CowSLAMtag cells were stained with anti-influenza virus HA epitope MAb 12CA5 (solid profile) or mouse control IgG antibody (open profile), followed by staining with FITC-labeled goat anti-mouse IgG.
FIG. 3.
CDV infection of marmoset SLAM-expressing CHO and B95a cells. CHO.Neo, CHO.B95aSLAM, and B95a cells were either untreated or treated with IPO-3 and then infected with the HA7 strain of CDV at an MOI of 0.5 (0.1 for B95a cells). Cells were observed at 24 h after infection.
Molecular cloning of canine and bovine SLAM cDNAs.
We reasoned that B95a-isolated CDV strains use the dog homologue of SLAM as a cellular receptor and that these CDV strains have been successfully isolated in B95a cells because they can use homologous marmoset SLAM to infect B95a cells. To test this idea, we isolated a putative canine SLAM cDNA clone from canine PBMCs based on the sequences of human and marmoset SLAM cDNAs. This clone was predicted to encode a membrane protein having strong homology to human SLAM (12) (65% identity at the amino acid level) (Fig. 4). Four cysteine residues in the extracellular C2 domain and three tyrosine-based signaling motifs in the cytoplasmic tail were also conserved between the molecules. From these results, we concluded that this cDNA clone encodes canine SLAM. Using a similar approach, we also isolated a bovine SLAM cDNA clone from bovine PBMCs, and its predicted amino acid sequence is shown in Fig. 4. At the amino acid level, bovine SLAM has 65 and 69% identity to human and dog homologues, respectively.
FIG. 4.
Predicted amino acid sequences of canine, bovine, and human SLAMs. Amino acid sequences of canine, bovine, and human SLAMs are aligned. Residues having similarity are shaded (dark shading, identical residues; light shading, conservative changes). The predicted signal peptides of respective SLAMs and transmembrane domain of human SLAM are underlined. Potential N-linked glycosylation sites are circled. Cysteine residues predicted to make disulfide bonds in the Ig C2 domain are indicated by asterisks. Tyrosine-based signaling motifs are boxed. Spaces (indicated by dashes) were introduced for optimal comparison.
Expression of SLAM allows CDV and RPV strains to cause CPEs in CHO cells. Anti-human SLAM MAb IPO-3 did not react to canine and bovine SLAMs when cells were transiently transfected with cDNA clones encoding them (data not shown). To detect the cell surface expression of these molecules, we constructed plasmids expressing the membrane-bound form of canine and bovine SLAM fused to the influenza virus HA tag at the N terminus (pCAGDogSLAMtag and pCAGCowSLAMtag). We transfected CHO cells with either plasmid plus pCXN2 containing the neo gene and selected stable clones in the presence of G418, followed by immunofluorescence staining with anti-HA epitope MAb (Fig. 2B). We used the clone expressing the highest level of canine SLAM (CHO.DogSLAMtag) and one expressing the highest level of bovine SLAM (CHO.CowSLAMtag) in the following experiments.
We inoculated CHO.DogSLAMtag cells with CDV strains. Within 24 h after infection, both the HA7 and Onderstepoort strains produced extensive CPEs in CHO.DogSLAMtag cells but not in CHO.Neo cells (Fig. 5). We also observed that CHO cells transiently transfected with pCAGDogSLAM (expressing the authentic canine SLAM without the HA tag) developed CPEs after infection with either strain (data not shown). We then examined whether these CDV strains can cause CPEs in CHO cells expressing human SLAM. The CHO cell clone stably expressing human SLAM (CHO.SLAM) (Fig. 2A) has been described (45). The HA7 strain, but not the Onderstepoort strain, caused CPEs in CHO.SLAM cells (Fig. 5), which were significantly weaker than CPEs in CHO.DogSLAMtag cells. The 851 strain showed the same results on all CHO cell clones as the HA7 strain (data not shown).
FIG. 5.
CDV and RPV infections of SLAM-expressing CHO cell clones. CHO.Neo, CHO.DogSLAMtag, CHO.SLAM, and CHO.CowSLAMtag cells were infected with the HA7 or Onderstepoort strain of CDV or Ako strain of RPV at an MOI of 0.1. Cells were observed at 24 h after infection with CDV strains or at 12 h after infection with the RPV strain.
We next examined whether expression of bovine SLAM allows RPV to cause CPEs in CHO cells. The lapinized vaccine strain of RPV had been produced by virus passage in rabbits (29). This strain was then adapted to chicken embryos to obtain the Ako vaccine strain of RPV (14). We further passaged it four times on Vero cells to obtain the virus stock used for this experiment. We inoculated CHO.CowSLAMtag cells with the Ako vaccine strain. At 12 h after infection, they developed extensive syncytia and the majority of cells were detached from the plates (Fig. 5). On the other hand, CHO.Neo and Vero cells did not show any sign of CPEs at 12 h after infection (Fig. 5 and data not shown). CHO.SLAM cells developed weaker but apparent CPEs (Fig. 5). After longer incubation (more than 15 h), infected CHO.Neo and Vero cells started to develop syncytia, but CPEs in CHO.CowSLAMtag and CHO.SLAM cells were still much stronger.
Since B95a-isolated CDV strains and the RPV vaccine strain caused CPEs in CHO.SLAM cells, we further examined the abilities of MV, CDV, and RPV strains to cause CPEs in CHO cells expressing SLAMs of nonhost species. We inoculated CHO.Neo, CHO.SLAM, CHO.DogSLAMtag, and CHO.CowSLAMtag cells with the Edmonston strain and B95a-isolated KA strain of MV, the Onderstepoort strain and B95a-isolated HA7 and 851 strains of CDV, and the Ako vaccine strain of RPV. Developments of CPEs in the CHO cell clones are summarized in Table 1. All viruses except the Onderstepoort strain caused CPEs in CHO.SLAM, CHO.DogSLAMtag, and CHO.CowSLAMtag cells, although CPEs caused by a virus were strongest in CHO cells expressing SLAM of its host species. The Onderstepoort strain produced CPEs in CHO.DogSLAMtag and CHO.CowSLAMtag cells but not in CHO.SLAM cells. Development of CPEs in CHO, Vero, and B95a cells inoculated with these morbillivirus strains is also shown in Table 1.
TABLE 1.
Development of CPEs in various cell lines infected with different strains of morbillivirusesa
| Cells | Development of CPEs inb
|
|||||
|---|---|---|---|---|---|---|
| MV strains
|
CDV strains
|
RPV strain Ako | ||||
| Edmonston | KA | Onderstepoort | HA7 | 851 | ||
| CHO | − | − | − | − | − | ND |
| Vero | ++ | − | + | − | − | −c |
| B95a | ++ | ++ | − | + | + | ND |
| CHO.Neo | − | − | − | − | − | −c |
| CHO.SLAM | ++ | ++ | − | ++ | ++ | ++ |
| CHO.DogSLAMtag | ++ | + | ++ | ++ | ++ | ++ |
| CHO.CowSLAMtag | ++ | ++ | ++ | + | ++ | +++ |
Cell lines were infected with each morbillivirus strain at an MOI of 0.1, and development of CPEs was assessed under a microscope at 24 h after infection with MV and CDV strains and at 12 h after infection with RPV. The susceptibilities of CHO, Vero, and B95a cells to MV strains have been described elsewhere (43). Longer incubation did not affect the presence or absence of CPEs in cells except those infected with the Ako strain.
−, no syncytia found; +, syncytia found in some of the fields; ++, syncytia found in most fields; +++, the majority of cells detached from the plate because of extensive cell fusion; ND, not done.
Cells developed CPEs after more than 15 h of incubation.
SLAM acts at the virus entry step as revealed by VSV pseudotypes.
The results thus far described do not necessarily exclude the possibility that SLAM acts only at the postentry step of the virus life cycle to allow efficient virus replication and/or cell fusion. To confirm that SLAM operates at the virus entry step (as a receptor), we used the VSV pseudotype system (42, 44, 45). VSVΔG* is the recombinant VSV in which the coding region of the G envelope protein is replaced by the modified GFP gene, and thus, it is not infectious unless the envelope proteins are provided in trans (42). The infectivity of a virus using envelope proteins supplied in trans can be determined by counting the number of GFP-expressing cells. We first prepared six types of pseudotypes: VSVΔG*-G, bearing the VSV G protein; VSVΔG*-OPHF, bearing the hemagglutinin (H) and fusion (F) proteins of the Onderstepoort strain; VSVΔG*-HA7HF, bearing the H protein of the CDV HA7 strain and the F protein of the Onderstepoort strain; VSVΔG*-EdHF, bearing the H and F proteins of the MV Edmonston strain; VSVΔG*-KAHF, bearing the H protein of the MV KA strain and the F protein of the Edmonston strain; and VSVΔG*, bearing no envelope protein. The H protein of a morbillivirus mediates receptor binding and confers cell tropism, whereas the F protein has membrane fusion activity (15, 41).
Figure 6A shows the infectivities of these pseudotype viruses on CHO cells expressing canine, human, or bovine SLAM. VSVΔG*-G, which can infect all mammalian cells (42), and VSVΔG* were used as positive and negative controls, respectively. Infectivity titers of VSV pseudotypes bearing CDV envelope proteins (VSVΔG*-OPHF and VSVΔG*-HA7HF) were more than 100 times higher on CHO.DogSLAMtag cells than on CHO.Neo cells. They were also higher on CHO.SLAM and CHO.CowSLAMtag cells than on CHO.Neo cells, although infectivity titer of VSVΔG*-OPHF on CHO.SLAM cells was not significantly different from that of VSVΔG*. VSVΔG*-EdHF showed higher titers on all CHO cell clones expressing SLAMs than on CHO.Neo cells, and VSVΔG*-KAHF exhibited higher titers on CHO.SLAM and CHO.CowSLAMtag cells than on CHO.Neo cells. Although the infectivity titer of VSVΔG*-KAHF was not significantly higher on CHO.DogSLAMtag cells than on CHO.Neo cells, it was indeed higher (103.4 infectious units per ml) on CHO cells expected to express the authentic canine SLAM without the HA tag (CHO.DogSLAM).
The VSV pseudotype bearing RPV envelope proteins (VSVΔG*-KOHF) was prepared using the H and F genes of the cell culture-adapted strain derived from the Kabete O strain of RPV (18, 50). The infectivity titer of VSVΔG*-KOHF was more than 10 times higher on CHO.CowSLAMtag cells than on CHO.Neo cells (Fig. 6B). VSVΔG*-KOHF also showed 5 to 10 times higher titers on CHO.DogSLAMtag and CHO.SLAM cells than on CHO.Neo cells. Its background infectivity titer on CHO.Neo cells was high, unlike titers of VSV pseudotypes bearing MV or CDV envelope proteins on CHO.Neo cells (Fig. 6A). During the adaptation to cell culture, the Kabete O strain may have come to use, besides SLAM, a ubiquitously expressed molecule(s) (thus present on CHO cells) as a cellular receptor. This interpretation was supported by the finding that all CHO, Vero, 293T (human kidney), and L (mouse fibroblast) cells developed syncytia after transfection with the H and F genes of the Kabete O strain (data not shown).
All these results with VSV pseudotypes bearing MV, CDV, or RPV envelope proteins are consistent with development of CPEs in CHO cell clones after infection with MV, CDV, and RPV. Thus, these morbilliviruses could use SLAMs of all three species to infect cells as virus or VSV pseudotype, although human, canine, and bovine SLAMs appeared to act most efficiently as receptors for MV, CDV, and RPV, respectively.
DISCUSSION
In this study, we showed that CDV and RPV use SLAMs of their host species as cellular receptors, like another morbillivirus, MV. The Onderstepoort vaccine strain and two B95a-isolated strains of CDV caused extensive CPEs in normally resistant CHO cells after expression of canine SLAM. The Ako vaccine strain of RPV produced strong CPEs in bovine SLAM-expressing CHO cells, although after longer incubation, it also caused weaker CPEs in all cell lines examined. Furthermore, most morbillivirus strains were found to cause CPEs in cells expressing human, canine, and bovine SLAMs. Only the Onderstepoort vaccine strain of CDV could not induce CPEs in cells expressing human SLAM. The data obtained with the VSV pseudotypes bearing MV, CDV, or RPV envelope proteins were consistent with developments of CPEs in SLAM-expressing CHO cell clones after infection with respective viruses, confirming that SLAM acts at the virus entry step (as a receptor).
Since the Onderstepoort strain that has been passaged on chicken embryos and Vero cells possesses the ability to use canine SLAM as a receptor, the precursor of this strain must have been using it as a receptor in the dog from which this strain was derived. Similarly, the original RPV from which the Ako vaccine strain was derived after many passages in rabbits, chicken embryos, and Vero cells must have been using bovine SLAM as a receptor in cattle. It seems that the Onderstepoort and Ako strains had adapted to chicken embryos and Vero cells using an alternate receptor(s) during passages on these cells. On the other hand, B95a-isolated CDV strains could not grow in Vero cells. It is likely that the H proteins of the precursor viruses of these B95a-isolated strains were able to bind to marmoset SLAM on B95a cells with little, if any, change in the sequences, whereas the H protein of the Onderstepoort strain had lost the ability to interact with marmoset and human SLAMs through its adaptation to use the alternate receptor(s) on chicken embryos and Vero cells. This explains why the Onderstepoort strain failed to infect B95a cells as well as CHO cells expressing human or marmoset SLAM. The Ako and Kabete O strains of RPV also seem to have adapted to use a molecule(s) expressed on many types of cells. However, the presence of SLAMs on the cell surface significantly enhanced infectivities of these RPV strains. The lapinized strain (L strain) of RPV, the precursor to the Ako strain, remains virulent for rabbits, but the adaptation of the L strain to Vero cells in vitro results in a diminution of virulence (19). It has been reported that B95a was the only host cell system available for the propagation of the L strain and the propagation of the virus in B95a cells preserved its pathogenicity for rabbits (23).
SLAM is constitutively expressed on immature thymocytes, CD45ROhigh memory T cells and a proportion of B cells, and it is rapidly induced on T and B cells following activation, in humans (5, 12, 40) and mice (10). It is also expressed on dendritic cells (33). Although we have not been able to systematically examine the distribution of SLAMs in dogs and cattle, it may be selectively expressed in lymphoid tissues. In fact, we isolated cDNAs for canine and bovine SLAMs from mitogen-stimulated PBMCs of respective animals. Thus, our finding would explain the lymphotropism of CDV and RPV as well as lymphopenia and immunosuppression caused by infection with these viruses. It remains, however, to be determined whether SLAM is also involved in infections of nonlymphoid organs such as the brain, lungs, and gastrointestinal tract. A previous study has reported that an unidentified molecule encoded on human chromosome 19 is involved in cell fusion induced by the Onderstepoort strain (41). Since the human SLAM gene is located on chromosome 1 (4), SLAM cannot be the molecule implicated. CD9, another molecule implicated in infection with Vero cell-adapted CDV strains, has been shown to act at postentry steps such as cell-cell fusion and virus release but not as a cellular receptor (39).
On the basis of phylogenetic analysis of morbilliviruses, it is thought that when cattle were domesticated, they passed a morbillivirus, a progenitor of modern RPV, to humans, which eventually evolved into MV. Similarly, carnivores could have contracted a morbillivirus infection from their ruminant prey, which then evolved into CDV (6). MV and RPV are closely related, and CDV and phocine distemper virus are the most distantly related to MV and RPV among morbilliviruses (15, 28). Furthermore, among all viral proteins, the H protein is the least conserved among CDV, RPV, and MV (37% identity between CDV and MV) (8). Thus, the finding that these three morbilliviruses use SLAMs as cellular receptors suggests that the usage of SLAM as a receptor has been maintained from the ancestral virus, accounting for an essential part of the pathogenesis of morbillivirus infections. We predict that probably most, if not all, members of morbilliviruses use SLAMs of their respective host species as cellular receptors.
Recently, B95a is commonly used to isolate morbilliviruses from clinical specimens (20, 22, 23). A high level of SLAM expression on B95a cells (45) appears to be a reason for its usefulness. However, mitogen-stimulated canine PBMCs or SLAM-positive canine cell lines, if available, may be more appropriate for CDV isolation, because they will express canine rather than marmoset SLAM. B95a has been shown to be very sensitive to both virulent field virus and vaccine strains of RPV (23, 25). A T. parva-transformed bovine lymphocyte cell line has also been used for RPV isolation (38). It would be interesting to determine whether bovine SLAM is expressed on this cell line. Recently, new morbilliviruses have been found in various mammals (13, 21, 26, 32, 48). It may be useful to attempt the isolation of these viruses using the cells expressing SLAMs of their host species, such as mitogen-stimulated PBMCs.
We found that MV, CDV, and RPV strains could use SLAMs of their nonhost species as receptors, albeit at reduced efficiencies. Despite sequence differences, the structure required for the interaction with morbillivirus H proteins may be well conserved among SLAMs of many different species. This should be taken into account in planning MV eradication because other morbilliviruses may infect humans lacking sufficient anti-MV immunity. Morbilliviruses have been grouped together by their sequence relatedness and lack of neuraminidase activity. Now the use of SLAM as a cellular receptor may be included in their characteristic properties.
ACKNOWLEDGMENTS
We are grateful to Hidetoshi Ikeda, who provided the RPV vaccine strain and the facility to work with it. We thank Michael A. Whitt, Tilahun Yilma, Shirou Mohri, and Yutaka Nakano for the VSVΔG*system, RPV cDNA clones, dog blood samples, and cow blood samples, respectively. We also thank the staff of Division of Veterinary Microbiology, Kyoto Biken Laboratories, for providing CDV strains.
This work was supported by grants from the Ministry of Education, Science and Culture of Japan and from the Organization for Drug ADR Relief, R&D Promotion and Product Review of Japan.
REFERENCES
- 1.Appel M, Gillespie J. Canine distemper virus. Virol Monogr. 1972;11:1–96. [Google Scholar]
- 2.Appel M J, Summers B A. Pathogenicity of morbilliviruses for terrestrial carnivores. Vet Microbiol. 1995;44:187–191. doi: 10.1016/0378-1135(95)00011-x. [DOI] [PubMed] [Google Scholar]
- 3.Appel M J, Yates R A, Foley G L, Bernstein J J, Santinelli S, Spelman L H, Miller L D, Arp L H, Anderson M, Barr M, Pearce-Kelling S, Summers B A. Canine distemper epizootic in lions, tigers, and leopards in North America. J Vet Diagn Investig. 1994;6:277–288. doi: 10.1177/104063879400600301. [DOI] [PubMed] [Google Scholar]
- 4.Aversa G, Carballido J, Punnonen J, Chang C-C J, Hauser T, Cocks B G, de Vries J E. SLAM and its role in T cell activation and Th cell responses. Immunol Cell Biol. 1997;75:202–205. doi: 10.1038/icb.1997.30. [DOI] [PubMed] [Google Scholar]
- 5.Aversa G, Chang C-C, Carballido J M, Cocks B G, de Vries J E. Engagement of the signaling lymphocytic activation molecule (SLAM) on activated T cells results in IL-2-independent, cyclosporin A-sensitive T cell proliferation and IFN-gamma production. J Immunol. 1997;158:4036–4044. [PubMed] [Google Scholar]
- 6.Barrett T, Rossiter P B. Rinderpest: the disease and its impact on humans and animals. Adv Virus Res. 1999;53:89–110. doi: 10.1016/s0065-3527(08)60344-9. [DOI] [PubMed] [Google Scholar]
- 7.Bassiri M, Ahmad S, Giavedoni L, Jones L, Saliki J T, Mebus C, Yilma T. Immunological responses of mice and cattle to baculovirus-expressed F and H proteins of rinderpest virus: lack of protection in the presence of neutralizing antibody. J Virol. 1993;67:1255–1261. doi: 10.1128/jvi.67.3.1255-1261.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blixenkrone-Moller M. Biological properties of phocine distemper virus and canine distemper virus. APMIS Suppl. 1993;36:1–51. [PubMed] [Google Scholar]
- 9.Brugger M, Jungi T W, Zurbriggen A, Vandevelde M. Canine distemper virus increases procoagulant activity of macrophages. Virology. 1992;190:616–623. doi: 10.1016/0042-6822(92)90899-z. [DOI] [PubMed] [Google Scholar]
- 10.Castro A G, Hauser T M, Cocks B G, Abrams J, Zurawski S, Churakova T, Zonin F, Robinson D, Tangye S G, Aversa G, Nichols K E, de Vries J E, Lanier L L, O'Garra A. Molecular and functional characterization of mouse signaling lymphocytic activation molecule (SLAM): differential expression and responsiveness in Th1 and Th2 cells. J Immunol. 1999;163:5860–5870. [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Global measles control and regional elimination, 1998–1999. Morb Mortal Wkly Rep. 1999;48:1124–1130. [PubMed] [Google Scholar]
- 12.Cocks B G, Chang C-C J, Carballido J M, Yssel H, de Vries J E, Aversa G. A novel receptor involved in T-cell activation. Nature. 1995;376:260–263. doi: 10.1038/376260a0. [DOI] [PubMed] [Google Scholar]
- 13.Domingo M, Ferrer L, Pumarola M, Marco A, Plana J, Kennedy S, McAliskey M, Rima B K. Morbillivirus in dolphins. Nature. 1990;348:21. doi: 10.1038/348021a0. [DOI] [PubMed] [Google Scholar]
- 14.Furutani T, Kataoka T, Kurata K, Nakamura H. Studies on the Ako strain of lapinized-avianized rinderpest virus. I. Avianization of lapinized rinderpest virus. Bull Natl Inst Anim Health. 1957;32:117–135. [Google Scholar]
- 15.Griffin D E, Bellini W J. Measles virus. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1267–1312. [Google Scholar]
- 16.Haig D A. Canine distemper-immunization with avianized virus. Onderstepoort J Vet Res. 1956;17:19–53. [Google Scholar]
- 17.Harrison M J, Oxer D T, Smith F A. The virus of canine distemper in cell culture. II. Effect of serial passage in ferret kidney cell cultures and BS-C-1 cell cultures on the virulence of canine distemper virus. J Comp Pathol. 1968;78:133–139. doi: 10.1016/0021-9975(68)90089-3. [DOI] [PubMed] [Google Scholar]
- 18.Hsu D, Yamanaka M, Miller J, Dale B, Grubman M, Yilma T. Cloning of the fusion gene of rinderpest virus: comparative sequence analysis with other morbilliviruses. Virology. 1988;166:149–153. doi: 10.1016/0042-6822(88)90156-0. [DOI] [PubMed] [Google Scholar]
- 19.Ishii H, Yoshikawa Y, Yamanouchi K. Adaptation of the lapinized rinderpest virus to in vitro growth and attenuation of its virulence in rabbits. J Gen Virol. 1986;67:275–280. doi: 10.1099/0022-1317-67-2-275. [DOI] [PubMed] [Google Scholar]
- 20.Kai C, Ochikubo F, Okita M, Iinuma T, Mikami T, Kobune F, Yamanouchi K. Use of B95a cells for isolation of canine distemper virus from clinical cases. J Vet Med Sci. 1993;55:1067–1070. doi: 10.1292/jvms.55.1067. [DOI] [PubMed] [Google Scholar]
- 21.Kennedy S, Smyth J A, McCullough S J, Allan G M, McNeilly F, McQuaid S. Confirmation of cause of recent seal deaths. Nature. 1988;335:404. doi: 10.1038/335404a0. [DOI] [PubMed] [Google Scholar]
- 22.Kobune F, Sakata H, Sugiura A. Marmoset lymphoblastoid cells as a sensitive host for isolation of measles virus. J Virol. 1990;64:700–705. doi: 10.1128/jvi.64.2.700-705.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobune F, Sakata H, Sugiyama M, Sugiura A. B95a, a marmoset lymphoblastoid cell line, as a sensitive host for rinderpest virus. J Gen Virol. 1991;72:687–692. doi: 10.1099/0022-1317-72-3-687. [DOI] [PubMed] [Google Scholar]
- 24.Krakowka S, Higgins R J, Koestner A. Canine distemper virus: review of structural and functional modulations in lymphoid tissues. Am J Vet Res. 1980;41:284–292. [PubMed] [Google Scholar]
- 25.Lund B T, Barrett T. Rinderpest virus infection in primary bovine skin fibroblasts. Arch Virol. 2000;145:1231–1237. doi: 10.1007/s007050070122. [DOI] [PubMed] [Google Scholar]
- 26.McCullough S J, McNeilly F, Allan G M, Kennedy S, Smyth J A, Cosby S L, McQuaid S, Rima B K. Isolation and characterisation of a porpoise morbillivirus. Arch Virol. 1991;118:247–252. doi: 10.1007/BF01314034. [DOI] [PubMed] [Google Scholar]
- 27.Metzler A E, Higgins R J, Krakowka S, Koestner A. Virulence of tissue culture-propagated canine distemper virus. Infect Immun. 1980;29:940–944. doi: 10.1128/iai.29.3.940-944.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy F A, Gibbs E P J, Horzinek M C, Studdert M J. Veterinary virology. 3rd ed. San Diego, Calif: Academic Press; 1999. pp. 411–428. [Google Scholar]
- 29.Nakamura J, Miyamoto T. Avianization of lapinized rinderpest virus. Am J Vet Res. 1953;14:307–317. [PubMed] [Google Scholar]
- 30.Nielsen H, Engelbrecht J, Brunak S, von Heijne G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- 31.Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants by a novel eukaryotic vector. Gene. 1991;108:193–200. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 32.Osterhaus A D, Groen J, De Vries P, UytdeHaag F G, Klingeborn B, Zarnke R. Canine distemper virus in seals. Nature. 1988;335:403–404. doi: 10.1038/335403a0. [DOI] [PubMed] [Google Scholar]
- 33.Polacino P S, Pinchuk L M, Sidorenko S P, Clark E A. Immunodeficiency virus cDNA synthesis in resting T lymphocytes is regulated by T cell activation signals and dendritic cells. J Med Primatol. 1996;25:201–209. doi: 10.1111/j.1600-0684.1996.tb00017.x. [DOI] [PubMed] [Google Scholar]
- 34.Rey Nores J E, Anderson J, Butcher R N, Libeau G, McCullough K C. Rinderpest virus infection of bovine peripheral blood monocytes. J Gen Virol. 1995;76:2779–2791. doi: 10.1099/0022-1317-76-11-2779. [DOI] [PubMed] [Google Scholar]
- 35.Richardson C D, Scheid A, Choppin P W. Specific inhibition of paramyxovirus and myxovirus replication by oligopeptides with amino acid sequences similar to those at the N-termini of the F1 or HA2 viral polypeptides. Virology. 1980;105:205–222. doi: 10.1016/0042-6822(80)90168-3. [DOI] [PubMed] [Google Scholar]
- 36.Roelke-Parker M E, Munson L, Packer C, Kock R, Cleaveland S, Carpenter M, O'Brien S J, Pospischil A, Hofmann-Lehmann R, Lutz H, Mwamengele G L M, Mgasa M N, Machange G A, Summers B A, Appel M J G. A canine distemper virus epidemic in Serengeti lions (Panthera leo) Nature. 1996;379:441–445. doi: 10.1038/379441a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rossiter P B, Herniman K A, Gumm I D, Morrison W I. The growth of cell culture-attenuated rinderpest virus in bovine lymphoblasts with B cell, CD4+ and CD8+ alpha/beta T cell and gamma/delta T cell phenotypes. J Gen Virol. 1993;74:305–309. doi: 10.1099/0022-1317-74-2-305. [DOI] [PubMed] [Google Scholar]
- 38.Rossiter P B, Herniman K A, Wamwayi H M. Improved isolation of rinderpest virus in transformed bovine T lymphoblast cell lines. Res Vet Sci. 1992;53:11–18. doi: 10.1016/0034-5288(92)90077-f. [DOI] [PubMed] [Google Scholar]
- 39.Schmid E, Zurbriggen A, Gassen U, Rima B, ter Meulen V, Schneider-Schaulies J. Antibodies to CD9, a tetraspan transmembrane protein, inhibit canine distemper virus-induced cell-cell fusion but not virus-cell fusion. J Virol. 2000;74:7554–7561. doi: 10.1128/jvi.74.16.7554-7561.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sidorenko S P, Clark E A. Characterization of a cell surface glycoprotein IPO-3, expressed on activated human B and T lymphocytes. J Immunol. 1993;151:4614–4624. [PubMed] [Google Scholar]
- 41.Stern L B, Greenberg M, Gershoni J M, Rozenblatt S. The hemagglutinin envelope protein of canine distemper virus (CDV) confers cell tropism as illustrated by CDV and measles virus complementation analysis. J Virol. 1995;69:1661–1668. doi: 10.1128/jvi.69.3.1661-1668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takada A, Robinson C, Goto H, Sanchez A, Murti K G, Whitt M A, Kawaoka Y. A system for functional analysis of Ebola virus glycoprotein. Proc Natl Acad Sci USA. 1997;94:14764–14769. doi: 10.1073/pnas.94.26.14764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanaka K, Xie M, Yanagi Y. The hemagglutinin of recent measles virus isolates induces cell fusion in a marmoset cell line, but not in other CD46-positive human and monkey cell lines, when expressed together with the F protein. Arch Virol. 1998;143:213–225. doi: 10.1007/s007050050281. [DOI] [PubMed] [Google Scholar]
- 44.Tatsuo H, Okuma K, Tanaka K, Ono N, Minagawa H, Takade A, Matsuura Y, Yanagi Y. Virus entry is a major determinant of cell tropism of Edmonston and wild-type strains of measles virus as revealed by vesicular stomatitis virus pseudotypes bearing their envelope proteins. J Virol. 2000;74:4139–4145. doi: 10.1128/jvi.74.9.4139-4145.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tatsuo H, Ono N, Tanaka K, Yanagi Y. SLAM (CDw150) is a cellular receptor for measles virus. Nature. 2000;406:893–897. doi: 10.1038/35022579. [DOI] [PubMed] [Google Scholar]
- 46.Vandevelde M, Zurbriggen A. The neurobiology of canine distemper virus infection. Vet Microbiol. 1995;44:271–280. doi: 10.1016/0378-1135(95)00021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Visser I K, Kumarev V P, Orvell C, de Vries P, Broeders H W M, van de Bildt W, Groen J, Teppema J S, Burger M C, UytdeHaag F G, Osterhaus A D M E. Comparison of two morbilliviruses isolated from seals during outbreaks of distemper in north west Europe and Siberia. Arch Virol. 1990;111:149–164. doi: 10.1007/BF01311050. [DOI] [PubMed] [Google Scholar]
- 48.Visser I K, van Bressem M F, Barrett T, Osterhaus A D. Morbillivirus infections in aquatic mammals. Vet Res. 1993;24:169–178. [PubMed] [Google Scholar]
- 49.Wohlsein P, Trautwein G, Harder T C, Liess B, Barrett T. Viral antigen distribution in organs of cattle experimentally infected with rinderpest virus. Vet Pathol. 1993;30:544–554. doi: 10.1177/030098589303000608. [DOI] [PubMed] [Google Scholar]
- 50.Yamanaka M, Hsu D, Crisp T, Dale B, Grubman M, Yilma T. Cloning and sequence analysis of the hemagglutinin gene of the virulent strain of rinderpest virus. Virology. 1988;166:251–253. doi: 10.1016/0042-6822(88)90168-7. [DOI] [PubMed] [Google Scholar]
- 51.Yilma T, Hsu D, Jones L, Owens S, Grubman M, Mebus C, Yamanaka M, Dale B. Protection of cattle against rinderpest with vaccinia virus recombinants expressing the HA or F gene. Science. 1988;242:1058–1061. doi: 10.1126/science.3194758. [DOI] [PubMed] [Google Scholar]
- 52.Zurbriggen A, Vandevelde M, Dumas M, Griot C, Bollo E. Oligodendroglial pathology in canine distemper virus infection in vitro. Acta Neuropathol. 1987;74:366–373. doi: 10.1007/BF00687214. [DOI] [PubMed] [Google Scholar]