Abstract
Atmospheric methane (CH4) acts as a key contributor to global warming. As CH4 is a short-lived climate forcer (12 years atmospheric lifespan), its mitigation represents the most promising means to address climate change in the short term. Enteric CH4 (the biosynthesized CH4 from the rumen of ruminants) represents 5.1% of total global greenhouse gas (GHG) emissions, 23% of emissions from agriculture, and 27.2% of global CH4 emissions. Therefore, it is imperative to investigate methanogenesis inhibitors and their underlying modes of action. We hereby elucidate the detailed biophysical and thermodynamic interplay between anti-methanogenic molecules and cofactor F430 of methyl coenzyme M reductase and interpret the stoichiometric ratios and binding affinities of sixteen inhibitor molecules. We leverage this as prior in a graph neural network to first functionally cluster these sixteen known inhibitors among ~54,000 bovine metabolites. We subsequently demonstrate a protocol to identify precursors to and putative inhibitors for methanogenesis, based on Tanimoto chemical similarity and membrane permeability predictions. This work lays the foundation for computational and de novo design of inhibitor molecules that retain/ reject one or more biochemical properties of known inhibitors discussed in this study.
Keywords: enteric methanogenesis, bromoform, enzyme inhibition, emissions mitigation, climate change
Graphical Abstract

INTRODUCTION
Greenhouse gases (GHGs) are atmospheric gases that possess the potential to absorb and retain infrared radiation in the atmosphere, hence trapping heat and causing a rise in temperature of the earth’s surface 1,2. Prominent GHGs encompass carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O), as well as a selection of fluorinated gases1–4. GHGs have been one of the world’s major climate change drivers over generations since their emissions degrade the atmospheric layer. This results in global warming due to anthropogenic activities. Inclusive of these activities are enteric CH4 emissions from ruminant livestock, the release of CO2 from fossil fuel use, land use change, and landfills.
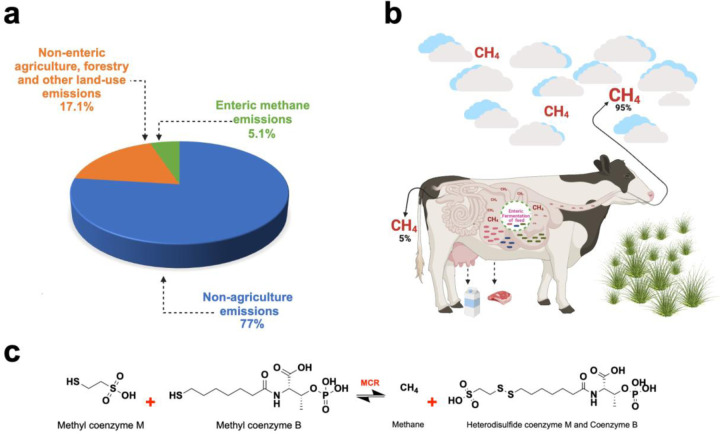
According to the sixth assessment report by the Intergovernmental Panel on Climate Change (IPCC)5, there were 59 Gt of CO2-equivalence (CO2-e) emitted globally in 2019. Emissions from Agriculture, Forestry and Other Land Use (AFOLU) represented 22% of these emissions. Enteric CH4 emissions accounted for 5.1% of total global GHGs, 23% of AFOLU, and 27.2% of total CH4 emissions (Figure 1a). As CH4 has a short atmospheric lifespan (approximately 12 years), in periods where emission rates are reduced to a large enough degree, there will be less atmospheric CH4, resulting in lower warming. Accordingly, rapidly declining CH4 emissions can reduce temperature equivalent to the removal of atmospheric CO2. As such, reductions in CH4 emissions represent the most promising means to address climate change in the short term 6. This nuance of CH4 emissions in general and the relative contribution of enteric CH4 to total CH4 emissions make enteric CH4 mitigation particularly important 7,8.
Figure 1.
A comprehensive schematic illustrating the distribution of greenhouse gas emissions, focusing specifically on methane and detailing its biochemical synthesis and release mechanisms. a) Global representation of GHGs emissions with distributions centered on methane by sector as gathered from literature b) The entire enteric fermentation of carbohydrate (cellulose) feed as a mechanism of methane release. c) Biochemical reaction and the rate-limiting step in enteric methane synthesis catalyzed by MCR enzyme.
Due to increasing production efficiency, the carbon footprint of milk production was reduced by 40%, from 33.6 to 19.9 g CH4/kg milk in recent years, and reductions of 16.3% of enteric CH4 per unit of beef produced for 2007 relative to 1977 9. While these reductions in carbon footprint from the dairy and beef industry are commendable, recent pledges of carbon neutrality by industries and companies have increased since the Paris Climate Agreement10. These types of commitments require reductions in absolute emissions rather than reductions in emissions per unit of product. Accordingly, enteric CH4 mitigation is highly needed by both the dairy and beef industries.
Methanogenesis is methane biosynthesis, irrespective of its emission source. Methane is a key natural secondary metabolite of enteric fermentation in the rumen of ruminants upon the digestion of consumed feed 11. Conditions favoring the production of enteric CH4 are designated to achieve homeostasis in the presence of excess hydrogen for maximum energy production. Methanogens (methanogenic archaea) are the predominant mediators of methanogenesis within the rumen. In agreement with the above, a study reported methanogens are influenced by other microbial members, primarily bacteria 12. Methanogenic interactions with bacteria, fungi, and protozoa influence enteric fermentation, the main metabolic reaction that leads to CH4 production. Therefore, methanogens represent a key target for investigating metabolic processes for CH4 mitigation.
Significantly, enteric CH4 production has been a conventional marker for farming productivity as CH4 is an associated product for carbohydrate utilization in ruminants. The quest for essential and volatile fatty acid production in livestock dietary metabolism has leveraged this gross implication of CH4 production in the four-chambered stomach of herbivorous grazing mammals13. As a natural result of excess hydrogen production in ruminants, CH4 is released into the atmosphere through either eructation (95%) or flatulence (5%)1 (Figure 1b). Following the stepwise biochemical reaction of CH4 biogenesis in ruminants, the enzyme Methyl Coenzyme M Reductase (MCR) produced from methanogenic archaea plays a key role. MCR catalyzes the final but rate-limiting step between methyl-coenzyme B (CoB-HS) and methyl-coenzyme M (CH3-S-COM) to release a heterodisulfide Coenzyme M and Coenzyme B (COM-S-S-COB) and CH4 as products14 (Figure 1c). The entire biochemical process is labeled methanogenesis for reference15.
Methanogenesis mitigation strategies and approaches have been conceptualized, designed, and deployed for a green and CH4-reduced ecosystem. Currently, several CH4 mitigation strategies are being explored by the agricultural sector. Options such as increasing feeding levels, decreasing dietary forage-to-concentrate ratios 16,17, and improving feed quality and digestibility have been promising options. However, these strategies often reduce enteric CH4 emission on a per product produced basis and have only demonstrated reductions by around 16.3% 18. Mitigation options that reduce absolute emissions have also been investigated and include genetic and breeding selection19, feeding tanniferous forages 20 providing electron sinks 21 and supplementing fat 16,17. These options have been shown to reduce enteric CH4 emissions by around 10% 18.
The mitigation options that have demonstrated the largest enteric CH4 mitigation potential are direct methanogenesis inhibitors. These include 3-nitrooxypropanol (3-NOP) and bromoform (CHBr3)-containing seaweeds (Asparagopsis spp.). 3-nitrooxypropanol has been shown to reduce enteric CH4 by 25–30% 22 and Asparagopsis seaweeds have reduced enteric CH4 by 80–98% 23,24. 3-nitrooxypropanol and CHBr3 from red seaweed have been suggested to inhibit methanogenesis by competitively binding and providing an agonistic effect on CH3-S-COM hence hindering the final and rate-limiting step in enteric methanogenesis 1,25,26. More specifically, halogenated compounds such as CHBr3 competitively displace other natural substrates that tend to interact with the Ni(I) ion of F430 coenzyme M. This results in methyl transfer inhibition and a reduction in CH3-S-COM mediated CH4 release26.
Amongst the methanogenesis inhibitors investigated and implemented, a data-driven deep-dive with precise molecular modeling of the atomic-level biochemistry of these inhibition mechanisms has remained largely elusive. Empirical approaches thus far have not provided enough biochemical information in order to design novel inhibitor molecules which can posit a high affinity of binding to rumen MCR bound to its cognate cofactor F430 aside 3-NOP1,2. Here, we employ in silico approaches to interpret the stoichiometric ratios (i.e., biophysical flooding) and binding affinities (i.e., biochemical trapping) of all well-documented inhibitor molecules against the redox-active nickel (Ni(I)) tetrahydrocorphin, coenzyme F430 cofactor of MCR.
METHODS
Selection of Methanogenic Protein Structure and Inhibitor Compounds.
A data mining sweep through reported literature was performed encompassing Google Scholar, GenBank 27, and UniProt 28 databases to pinpoint the methanogenic archaea Methyl-coenzyme M reductase enzyme responsible for enteric CH4 biosynthesis. Studies focused on the biochemical mechanism of the MCR enzyme, inhibitor molecules, and structural insights of both MCR and the inhibitor molecules were shortlisted. Based on the structural insight, a high-resolution, X-ray diffracted crystallized MCR (PDB Accession ID: 5G0R) was identified2 and downloaded from the Research Collaboratory for Structural Bioinformatics Protein Data Bank (RCSB PDB)29. Protein structure visualization, characterization, and determination of active site residues within a 5Å distance from the cofactor F430 were investigated using PyMOL30,31. A library of inhibitor molecules was downloaded from PubChem32 after a deep literature search for inhibitor molecules with or without experimental data from the above-mentioned literature databases. The MolView server33 was used to generate structures for inhibitors that were not available in PubChem.
Molecular docking and molecular dynamics (MD) simulations.
Molecular docking and MD simulations were conducted to explore further insights into the binding poses and proximities for CH4 inhibition amongst selected 16 individual inhibitor molecules with the Ni(I) of F430 cofactor of MCR. Rigid molecular docking was performed using AutoDock Vina34 to explore the binding interactions of the selected inhibitors out of a library of literature-derived small molecule compounds and the cofactor F430 of MCR (PDB ID: 5G0R). Protein and ligand preparation steps were conducted using AutoDockTools34. Using the gradient-based local search genetic algorithm built in AutoDock Vina 34, the docking energy scores and rankings of binding poses of each inhibitor molecule to the active-site of the MCR enzyme were obtained. Illustrations of inhibitor-MCR complex were generated using PyMOL30,31. Molecular dynamics of the respective top-scored conformations of MCR- cofactor F430-anti methanogen ternary complexes were set up using GROMACS 2023 macromolecular modeling package with CHARMM36 forcefield35 (see SI for details).
Stoichiometric ratio and binding affinity analysis.
The stoichiometric ratio and distribution of inhibitor molecules within the catalytic groove of MCR at the surface of cofactor F430 were analyzed. All ligands’ poses within an electron transfer range (<5Å) with bound Ni(I) of the cofactor F430 were selected. The number of such poses for each inhibitor represents the maximum biophysically permissible stoichiometric ratio against inhibitor molecule type.
Structural comparison of MCR inhibitors with ruminant specific metabolite databases.
The 16 inhibitors explored against MCR enzyme were compared for similarities in molecular fragments within two ruminant specific metabolite databases - a) Milk Composition Database (MCDB) 36 and b) Bovine Metabolome Database (BMDB) 37, containing 2,360 and 51,682 entries, respectively. The structural information of metabolites was downloaded in Structure-Data File (SDF) format and further processed to obtain canonical Simplified Molecular Input Line Entry System (SMILES) representation using RDKit38. These SMILES strings were used as input for a GNN to generate molecular embeddings, providing a standardized and machine-readable representation of the complex molecular structures present in milk and bovine metabolites. Initially, the RDKit cheminformatics package was utilized to extract each metabolite’s atomic identities and structural information into features as nodes and edges. These features were then passed into a simple GNN architecture containing 58 input neurons, corresponding to the different atoms present in the structure databases, a hidden layer with 64 neurons, and 128 output neurons. This GNN framework generated molecular embeddings as tensors with dimensions (N×128), where N represents the number of atoms in each metabolite, and 128 is the dimensionality of the embedding space. These tensors were subsequently averaged across the atomic dimension to produce a unified 128-dimensional vector representation for each molecule. The high-dimensional embeddings were reduced to two dimensions using t-distributed Stochastic Neighbor Embedding (t-SNE). t-SNE parameters were optimized, with perplexity set to the minimum value between 30 and the total number of molecules in the database. This dimensionality reduction facilitated the visualization of molecular relationships, enabling the identification of structural similarities and potential functional associations among the metabolites.
Validation of clustered potential inhibitors via Tanimoto chemical similarity analysis and Haddock.
We employed Tanimoto similarity analysis, utilizing Morgan fingerprints, to assess the similarity between selected metabolites and the set of MCR inhibitors39,40. Bovine metabolites were categorized into groups of two, those with the highest similarity (Likely Inhibitors Molecules; LIMs) and those with the lowest similarity (Unlikely Inhibitor Molecules; UIMs) with the 16 known MCR inhibitors. Categorization was done based on clustering proximity. Additionally, we performed molecular docking studies using HADDOCK on five of the nearest and five of the farthest metabolites, targeting the enzyme MCMI reductase41. The active residues from the enzyme were selected for docking with the chosen metabolites (as detailed in S1 Figure). We predicted the expected membrane permeability of randomly chosen 16 Likely and Unlikely Inhibitor Molecules (LIMs/ UIMs), using an established supervised machine learning protocol42. Herein the SMILES representation of each molecule is one-hot encoded using an encoder-decoder setup and mapped to the respective membrane permeabilities (preferentially trained on colorectal adenocarcinoma cell membrane; Caco-2). The above protocol is housed within the KNIME suite of ML platforms43.
RESULTS AND DISCUSSION
MCR from Methanothermobacter marburgensis and diverse inhibitors identified as key targets for methanogenesis inhibition.
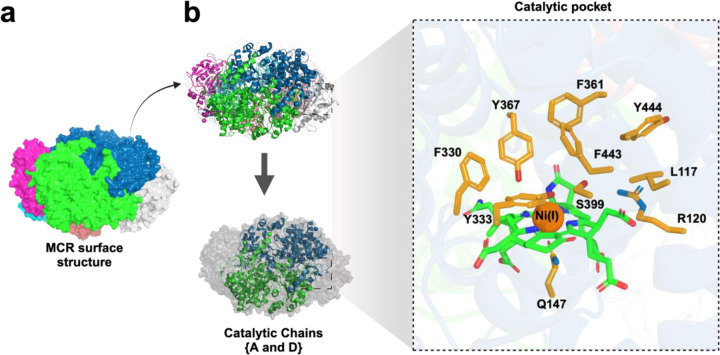
We selected an x-ray defined crystal structure of MCR protein with a Ni-methyl species that is a proposed catalytic intermediate in MCR. The methyl group of methyl-coenzyme M stated usually situates at least a 2.1 Å proximal to the Ni(I) of the MCR coenzyme F430 for a successful catalysis to materialize. A rearrangement of the substrate channel has been posited to bring together substrate species; however, Ni (III)-methyl formation alone does not lead to any observable structural changes in the channel 2. Given this, studies with biochemical and structural analysis of the MCR from Methanothermobacter marburgensis were focused upon with the assumption that the last step of CH4 production in ruminants is the rate-limiting step of methanogenesis 44. A recent experimental study 2 of the inhibitory properties of 3-NOP with the 3D structure of MCR (PDB ID: 5G0R) deciphered at a high resolution of 1.25 Å was selected for our study. In agreement with previous literature 44–46, the MCR protein selected is a 273 kDa hexameric protein (Figure 2) with two catalytic subunits that are 50Å apart. The MCR protein has a deep active site pocket with a substrate groove that runs ~ 30Å from to the protein’s surface1. The activity of MCR, as reported by computational analysis from experimental data44,47, demonstrated the enzyme remains active only when its Ni ion in the tetrapyrrole derivative of the cofactor F430 has a +1-oxidation state, therefore catalyzing the last CH4-production step of methanogenesis in the rumen of livestock such as cattle, sheep, and goats1,48,49.
Figure 2.
Illustration of the crystal structure of Methyl Coenzyme M Reductase (MCR) (PBD accession ID: 5G0R) from Methanothermobacter marburgensis and the six-chain hexameric complex. a) Each chain of MCR crystal structure has been indicated with six colors. b) Catalytically active chains (A and D) of MCR are shown in green and blue, while other non-catalytic chains are shown in gray. The location of the cofactor F430 in the enzyme structure for both catalytic chains are indicated.
All literature-based reported inhibitor compounds for enteric methanogenesis inhibition were collected. Sixteen distinct molecular compounds were selected, including statins, pterins, nitro-ol/esters, Coenzyme-B analogs (COBs), and CHBr3 (see Figure 3). Three statins (atorvastatin, rosuvastatin, and simvastatin), four nitro-ol/esters (2-nitroethanol, 2-nitropropanol, 3-nitropropionate and 3- NOP), five Coenzyme B analogs (COBs) (N-5-mercaptopentanoylthreonine phosphate: CoB5, N-6-mercaptohexanoylthreonine phosphate: CoB6, N-7-mercaptoheptanoylthreonine phosphate: COB7, N-8-mercaptooctanoylthreonine phosphate: CoB8, and N-9-mercaptononanoylthreonine phosphate: CoB9, and three Pterins (pterin B53 (2,6-diamino-5-nitrosopyrimidin-4(3H)-one), pterin B54 (4-{3-[(2-amino-5-nitroso-6-oxo-1,6-dihydropyrimidin-4-yl)amino]propoxy}benzoic acid) and pterin B55 (2-amino-8-sulfanyl-1,9-dihydro-6H-purin-6-one)) were studied in comparison with CHBr3 using detailed molecular modeling and thermodynamic assessment of binding interactions with MCR in the presence of cofactor F430.
Figure 3.
Representation of all selected anti-methanogenic molecules (inhibitors) structures adopted for this study. a. Bromoform molecule. b. Group of Pterins. c. Group of Nitro- alcohols and esters. d. Group of Coenzyme B analogs. e. Group of Statins or HMG-CoA reductase inhibitors.
Nitro-ol/ester compounds outperform other inhibitors in MCR binding affinity and stoichiometry.
The top-scoring (strongest) binding poses were analyzed to evaluate the ligands’ binding affinities, interactions, and potential binding modes with no superimpositions within 5Å. No superimposition criterion was imposed to infer the maximum number of inhibitor molecules that can simultaneously invade and yet remain biochemically bound within catalytic distances of cofactor F430 within the MCR enzyme pocket. The number of inhibitor molecules thus obtained is a representation of the maximum permissible stoichiometry of the inhibitor on a per-molecule basis with the MCR enzyme. Consequently, the inhibitor molecule poses that were accounted for were the ones within the electron transfer range with the Ni(I) of the tetrapyrrole of F430 in MCR. The least likely inhibitor molecule from the binding affinities records were HMG-CoA reductase inhibitors (statins) with rosuvastatin, simvastatin, and atorvastatin having positive (i.e., overall repulsive binding interactions with MCR). They had +55.5, +54.7 and 79.7 kcal/mol binding energy scores, respectively – reflecting they are unlikely to stay bound and/ or inhibit catalysis sustainably, even though they are shape compatible for the MCR pocket and might temporally occlude the pocket. It is noteworthy that the MCR-binding affinity values observed with the statins numerically correlate (R2 = 0.82) with the molecular weights of each statin due to the tube-like shape of the binding pocket of MCR. Next, the coenzyme B analogs ranked as poor, albeit stable inhibitors from the affinity values from the top three docking poses per inhibitor, with CoB5 having the lowest affinity value (3.9 kcal/mol). However, the third best group of inhibitors was the pterins, with pterinB55 being the best amongst them at 2.17 kcal/mol, while the worst of that group was pterinB54 with an affinity binding of 15.52 kcal/mol. Best as desired, inhibitor molecules surfaced as the nitro-ol/ester group of molecules with mean affinity values ranging from −2.87 to −5.37 kcal/mol (see Figure 4). The CHBr3 molecule scored an average affinity value of 1.33 kcal/mol, with the best individual CHBr3 molecule having a 0.2 kcal/mol but stoichiometrically having three poses with no superimposition (Figure 4).
Figure 4:
Illustration of all three selected poses of bromoform interacting with Ni(I) of F430 in MCR protein and graphical representation of the stoichiometric ratio of individual inhibitors docked to the active site of MCR enzyme in the close vicinity of F430. The dashed lines indicate the distances, in Å, between Ni(I) and bromoform. Cyan: for the distances of the first bromoform molecule. Gold: for the distances of the second bromoform molecule. Magenta: for the distances of the third bromoform molecule. The distances of other inhibitor molecules from Ni(I) are represented in supplementary information (see Figure S2-S17). b. Scatter plot representation of the mean binding affinity values of top three conformations of inhibitor molecules docked to F430 of MCR. c. Representation of all positive conformations of inhibitor molecules accurately posed within a 5Å range.
Energetics for each inhibitor (in silico affinity value scores) were calculated based on the best conformations docked at the active site. The relatively small CHBr3 and nitro-ol/ester compounds were observed to be comparable with each other and better than the other anti-methanogenic compounds due to their larger molecular size; however, fragments that interacted with F430 need to be analyzed further for more insights. Affinity values of each inhibitor (selected poses) were plotted for the compounds which are correlated with the stoichiometric ratio plot (Figure 4). Apart from CHBr3, which has more experimental evidence, nitro-ol/ester compounds could be stable enough for competitive inhibition. Molecular dynamics for these compounds warrant further investigation into their anti-methanogenic capabilities.
Ni(I) ion mobility and steric clashes hinder stable MCR-cofactor F430 complex simulations.
The MCR enzyme is a hexameric enzyme with two catalytic grooves, each guarded by three chains (Figure 2). The active form of cofactor F430 has a tetrapyrrole ring with Ni(I) held at its center. To reduce the computational cost without compromising the quality of MD simulation, we focused on one catalytic groove, which encompasses chains A, C, and D along with cofactor F430. The force field parameters for MCR are taken from CHARMM36, while cofactor F430 is parametrized using ATB 50. Equilibration of the three chains of enzyme along with the cofactor F430 with Ni(I) in an orthohedral TIP3P water box resulted in cofactor F430 moving out of the solvation box and Ni(I) moving away from cofactor F430 into the bulk solvent (SI Figure A). Selection of orthohedral simulation box is to reduce the solvent molecules with the aim to reduce computational cost. The undesirable shifting of cofactor F430 in orthohedral box may be a result of the edge effect due to poor solvation, hence we controlled it by using a cubic water box, with 2.8 times increase in number of solvent molecules. Nevertheless, the tendency of Ni(I) to behave as a solvent ion continued to pose difficulty in modeling MCR-cofactor F430 complex (SI Figure B). We attempted to control the relative movement of Ni(I) with respect to cofactor F430 by imposing movement restrictions, which resulted in unfeasibly unstable energy due to steric clashes. Adding inhibitor molecules to a non-equilibrated enzyme-cofactor complex further worsened the instability of the whole simulation system, resulting in an unphysical simulation box.
As atomic scale MD simulation of MCR enzyme-cofactor F430-inhibitor ternary complex is a challenging venture involving multiple steps of optimization, equilibration, and analyses involving a huge computational cost, we intend to implement the knowledge we gained in optimizing the simulation box for a future follow-up study to compare the structural and thermodynamic underpinnings of MCR inhibition 51.
MCR inhibitors cluster together when compared with ruminant specific metabolite databases.
Spatially adjacent molecules to the inhibitor cluster in the reduced-dimensionality space emerge as putative inhibitors or precursors of anti-methanogenic compounds. Since it is unclear what characteristics define a good inhibitor, as all 16 molecules are very different from each other in shape and chemistry, binding energy calculations can tell if a molecule is a good inhibitor, but this information alone is insufficient to design a new inhibitor. This necessitates the identification of common structural and chemical features that unify these 16 molecules while simultaneously distinguishing them when put in context with other bovine metabolites. Since the number of molecular features required to identify such a cluster is unknown due to paucity of data in experimental literature, we chose to use a latent encoder of molecular signatures using a graph neural network (GNN) whose encodings when projected onto a 2D space, exhibits clustering of these 16 molecules close to each other and disparate from others. While other functional clusters have not been investigated in context with bovine metabolism and signal transduction, we are able to ascribe the clustering of all these 16 validated anti-methanogenic molecules to represent the loci in the 2D t-SNE space as responsible for anti-methanogenicity (Figure 5).
Figure 5.
Two-dimensional t-SNE projection of molecular signatures reveals clustering of methanogenesis inhibitors. a) and b) Visualization of 16 known MCR inhibitors (Red) in relation to their four nearest neighbors (Black) selected from the Milk Composition Database (MCDB). c) and d) Similar visualization with four proximal metabolites (Black) identified in the Bovine Metabolome Database (BMDB).
Proximal molecules to this functional cluster from the two databases emerge as putative inhibitors or precursors to anti-methanogenic molecules. Notably, molecules such as butyrate, 2-hydroxybutyric acid, and biotin were identified as potential candidates. Previous studies in the field address the success of computational tools for the prediction of inhibitors for various enzymes. From the discovery of novel QoI fungicides for cytochrome b inhibition in Peronophythora litchi 52 and the successful elucidation of antimicrobials for downy mildew pathogenicity in cucumber using in silico docking 53. Over the period of advancement, the use of ML-based tools54,55 dominates the race of drug or ligand prediction after several successes. On this note, our team’s next steps are to leverage generative AI frameworks like Drug-large language models (LLM) or Chemistry42 in subsequent studies 51 to computationally predict potential inhibitors using the putative inhibitors as templates and couple it with in vitro inhibitor assays to test the efficiency of such predictions.
Validation of clustered potential inhibitors via Tanimoto chemical similarity analysis and HADDOCK.
We demonstrate that the LIMs (likely inhibitors) exhibited significantly higher Tanimoto similarity scores with the known sixteen inhibitor molecules compared to the UIMs (unlikely inhibitors) metabolites (Figure 6 (a) and (b)). We conducted a t-test that yielded a p value of 0.0003 indicating that LIMs have a significantly higher chemical similarity (Tanimoto score) to the known inhibitors, compared to UIMs. This provides interpretability to our neural clustering (Figure 5). The chemical similarity trends, however, did not correlate with the HADDOCK computational docking scores (i.e., binding free enthalpies) with the MCR enzyme, as distal metabolites (UIMs) often resulted in tighter MCR binding (SI Table 3). This can be ascribed to the lack of appropriate biochemical microenvironment in a static docking simulation which ignores entropic effects of solvent molecules (see details on attempted MD simulations; Supplementary Information Figure S18). The complexity of the dynamics of this quaternary system (an enzyme, a F430 cofactor, a Ni(I) metal ion, and an inhibitor) when interfaced with explicit water molecules becomes intractable as seen in our attempt to perform the MD simulation (due to the paucity of all appropriate non-bonded parameters). This even more alludes to the lack of fidelity in available docking protocols which are not poised to handle co-docking setups with more than two moving pieces. Despite the accurate identification of the key (active) residues involved (S1 Figure) in substrate stabilization, HADDOCK results were thus not contributive to explaining the true energetics of the system. Overall, these findings suggest that chemical similarity, as measured by Tanimoto scores, is likely to be a more reliable predictor of MCR inhibition potential than inhibitor binding affinity.
Figure 6.
Tanimoto chemical similarity analysis between the LIM and UIMs relative to sixteen MCR inhibitors. (a) The sixteen inhibitors are represented at the periphery of the spider plot. The red-shaded area indicating the similarity of the proximal LIMs while the gray-shaded area represents the farther UIMs. b) Box plots illustrate the similarity of seven LIMs and nine UIMs relative to the 16 known MCR inhibitors. The red boxes represent LIMs, while the gray boxes represent UIMs. A p value of 0.003 indicates that the LIMs exhibit a statistically significant higher similarity to the known sixteen inhibitors compared to UIMs.
Membrane permeable metabolites are likely to inhibit the methane emission in ruminant.
MCR is mostly associated with the membrane56, and recent findings confirm its localization near the cytoplasmic membrane57. This indicates the necessity of membrane permeability for effective inhibition. For instance, bromoform and 3-NOP are established MCR inhibitors26,58. Bromoform is known to penetrate cell membranes rapidly, achieving diffusion within nanoseconds at low concentrations59. 3-NOP has been shown to significantly reduce methane emissions in dairy cows, leading to its approval for commercial use by the FDA58,60.
In our analysis, bromoform was predicted to exhibit high membrane permeability, albeit with low computational prediction confidence. Among putative inhibitors (without further property screening) candidates like CSCC(N)C(=O)O (S-methyl cysteine) and CCC(O)C(=O)O (4-hydroxybutyric acid) emerge as highly permeable with high confidence (Table 1). They have high chemical similarities (median Tanimoto scores ~0.10, ~0.13 respectively) with the known sixteen metabolites. While S-methyl cysteine is a known anti-oxidant, anti-inflammatory61, and is biologically regarded as safe62–64 and hence a promising target for experimental testing, 4-hydroxybutyric acid (Drugbank id: DB01440) is known to be a therapeutic drug and can lead to cytotoxicity65 above when administered beyond threshold. This makes the latter a less promising experimental target. It indicates the necessity to build additional bio-aware filters into computational predictive models beyond chemical similarity, membrane permeability and ability to approach Ni(I) before taking computationally predicted molecules to experimental testing for MCR. This is exemplified, as 3-NOP is predicted to have low permeability (even though with low confidence) (Table 1) which is contrary to experimental knowledge. Given its established use as a commercial feed additive, 3-NOP should have exhibited high membrane permeability in our predictions. One potential reason could be lack of 3-NOP-type molecules in the existing databases, making the prediction low confidence anyway (Table 1). Therefore, there is a clear need for a more precise Caco-2 membrane permeability predictor with biochemical awareness. Future work may involve developing advanced models, such as nonlinear regression or gradient-boosted trees66, leveraging data on 511 known metabolites with permeabilities across 11 representative membranes.
Table 1:
Predicted Membrane Permeability and Confidence Levels of MCR Inhibitors and Near Metabolites Based on SMILES Codes.
| Ligand Type | Smiles | Confidence | Permeability | Papp (10e−6 cm/s) |
|---|---|---|---|---|
| Known MCR Inhibitors | BrC(Br)Br | Low | High | 16.22 |
| P(=O)(O)(O)O[C@@H]([C@H](NC(CCCCS)=O)C(=O)O)C | Low | Low | 1.22 | |
| P(=O)(O)(O)O[C@@H]([C@H](NC(CCCCCS)=O)C(=O)O)C | High | Low | 1.45 | |
| P(=O)(O)(O)O[C@@H]([C@H](NC(CCCCCCS)=O)C(=O)O)C | High | High | 49.76 | |
| P(=O)(O)(O)O[C@@H]([C@H](NC(CCCCCCCS)=O)C(=O)O)C | High | High | 21.4 | |
| P(=O)(O)(O)O[C@@H]([C@H](NC(CCCCCCCCS)=O)C(=O)O)C | High | Low | 4.26 | |
| [N+](=O)([O−])CCO | High | Low | 4.97 | |
| [N+](=O)([O−])C(CO)C | Low | Low | 1.44 | |
| [N+](=O)([O−])CCC(=O)[O−] | High | Low | 8.1 | |
| [N+](=O)([O−])OCCCO | Low | Low | 1.13 | |
| FC1=CC=C(C=C1)C=1N(C(=C(C1C1=CC=CC=C1)C(NC1=CC=CC=C1)=O)C(C)C)CC[C@H](C[C@H](CC(=O)O)O)O | High | Low | 1.42 | |
| FC1=CC=C(C=C1)C1=NC(=NC(=C1/C=C/[C@H](C[C@H](CC(=O)O)O)O)C(C)C)N(S(=O)(=O)C)C | High | High | 19.33 | |
| CC(C(=O)O[C@H]1C[C@H](C=C2C=C[C@@H]([C@@H]([C@@H]12)CC[C@H]1OC(C[C@@H](C1)O)=O)C)C)(CC)C | High | High | 18.06 | |
| NC1=NC(=C(C(N1)=O)N=O)N | Low | Low | 0.98 | |
| NC=1NC(C(=C(N1)NCCCOC1=CC=C(C(=O)O)C=C1)N=O)=O | High | High | 13.73 | |
|
| ||||
| Likely Inhibitor Molecules (LIM) | NC=1NC(C=2N=C(NC2N1)S)=O | Low | Low | 1.8 |
| CCC(O)C(=O)O | High | High | 12.59 | |
| CCCC(=O)O | High | High | 18.79 | |
| O=C(O)CCCC(=O)C(=O)O | Low | Low | 0.96 | |
| NCCCN | Low | Low | 1.29 | |
| O=C(O)CC1=CC=C(O)C=C1 | High | Low | 7.33 | |
| CSCC(N)C(=O)O | High | High | 18.25 | |
| O=C(O)CCCCC(=O)C(=O)O | High | Low | 3.05 | |
CONCLUSION
MCR enzyme inhibition is considered a direct strategy to reduce CH4 emission from ruminant livestock. Here, we computationally compared 16 small molecules reported to be explored as MCR inhibitors. Through molecular docking, we showed that CHBr3 and nitro-ol/ester compounds have a higher affinity to bind to cofactor F430 in the active site of MCR compared to statins, pterins, and COBs. In this study, we revealed that the reaction dynamics and the overall mechanistic understanding of the inhibition process is greatly influenced by the stoichiometry of the inhibitors in the active site. Specifically, the presence of three bromine atoms in bromoform makes it a highly effective halogenated compound for competitively inhibiting the interaction of natural substrates with the Ni(I) ion in the F430 cofactor in MCR enzyme. Notably, inhibitor stoichiometry does not only dictate the binding affinity as a factor for methane inhibition but also the extent of methyl transfer inhibition and, consequently, the reduction in methane (CH₄) release. In this study, we demonstrate that the stoichiometry of the inhibitors in the active site, as deduced from the non-superimposing docking poses within the active site groove, is directly proportional to the size of the inhibitor. It can be interpreted that smaller inhibitors have higher flooding effects within the active site. The GNN-powered t-SNE clustering indicated that all the 16 inhibitor molecules explored in this study have inherent similarities among themselves when compared to ruminant specific metabolites and reveal some potential candidates from these databases as anti-methanogenic agents and their precursors. Lastly, the challenges in setting up an atomic scale MD simulation box with MCR enzyme-cofactor F430 with an electrostatically bound Ni(I)-inhibitor ternary complex is discussed, indicating the importance of optimizing each component of the ternary complex solvated in a solvent box big enough to ultimately house all the components.
Supplementary Material
ACKNOWLEDGMENT
R.C. acknowledges support through Iowa State University Startup Grant, Building A World of Difference Faculty fellowship, CIRAS Applied Mini-Grant, and NSF 22–599, EPSCoR RII Track-1, Award Number DQDBM7FGJPC5 for partially funding this study. R.A. was funded by NIH R35GM143074 to T.J.M. T.J.M. is also supported by the Karen and Denny Vaughn Faculty Fellowship. The authors thank Bibek Acharya for helping set up the force fields for the inhibitors to be compatible for molecular dynamics simulations. We also acknowledge the use of Google Scholar for the significant literature search process as it greatly contributed to collation of relevant references used in this study.
ABBREVIATIONS
- CCR2
CC chemokine receptor 2
- CCL2
CC chemokine ligand 2
- CCR5
CC chemokine receptor 5
- TLC
thin layer chromatography
Footnotes
ASSOCIATED CONTENT
Supporting Information. The Supporting information is compiled and available free of charge at the link to be added later.
REFERENCES
- (1).Duin E. C.; Wagner T.; Shima S.; Prakash D.; Cronin B.; Yáñez-Ruiz D. R.; Duval S.; Rümbeli R.; Stemmler R. T.; Thauer R. K.; Kindermann M. Mode of Action Uncovered for the Specific Reduction of Methane Emissions from Ruminants by the Small Molecule 3-Nitrooxypropanol. Proc. Natl. Acad. Sci. U.S.A. 2016, 113 (22), 6172–6177. 10.1073/pnas.1600298113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Patra A. K.; Puchala R. Methane Mitigation in Ruminants with Structural Analogues and Other Chemical Compounds Targeting Archaeal Methanogenesis Pathways. Biotechnology Advances 2023, 69, 108268. 10.1016/j.biotechadv.2023.108268. [DOI] [PubMed] [Google Scholar]
- (3).Joch M.; Vadroňová M.; Výborná A.; Jochová K. Inhibition of in Vitro Rumen Methane Production by Three Statins. Annals of Animal Science 2022, 22 (1), 271–282. 10.2478/aoas-2021-0022. [DOI] [Google Scholar]
- (4).Hegarty Roger S; Cortez Passetti Ra; Dittmer Kyle; Yuxi Wang; Sadie Shelton; Jeremy Emmet-Booth; Eva Wollenberg; Tim McAllister; Sinead Leahy; Karen Beauchemin; Noel Gurwick. An Evaluation of Evidence for Efficacy and Applicability of Methane Inhibiting Feed Additives for Livestock; Edition 1; Climate Change, Agriculture and Food Security (CCAFS) and the New Zealand Agricultural Greenhouse Gas Research Centre (NZAGRC) initiative of the Global Research; Alliance (GRA); p 104. https://globalresearchalliance.org/wp-content/uploads/2021/12/An-evaluation-of-evidence-for-efficacy-and-applicability-of-methane-inhibiting-feed-additives-for-livestock-FINAL.pdf (accessed 1996-04-11). [Google Scholar]
- (5).Emissions Trends and Drivers. In Climate Change 2022 - Mitigation of Climate Change; Intergovernmental Panel On Climate Change (Ipcc), Ed.; Cambridge University Press, 2023; pp 215–294. 10.1017/9781009157926.004. [DOI] [Google Scholar]
- (6).Ocko I. B.; Sun T.; Shindell D.; Oppenheimer M.; Hristov A. N.; Pacala S. W.; Mauzerall D. L.; Xu Y.; Hamburg S. P. Acting Rapidly to Deploy Readily Available Methane Mitigation Measures by Sector Can Immediately Slow Global Warming. Environ. Res. Lett. 2021, 16 (5), 054042. 10.1088/1748-9326/abf9c8. [DOI] [Google Scholar]
- (7).Beck M. R.; Thompson L. R.; Campbell T. N.; Stackhouse-Lawson K. A.; Archibeque S. L. Implied Climate Warming Contributions of Enteric Methane Emissions Are Dependent on the Estimate Source and Accounting Methodology. Applied Animal Science 2022, 38 (6), 639–647. 10.15232/aas.2022-02344. [DOI] [Google Scholar]
- (8).Beck M. R.; Thompson L. R.; Rowntree J. E.; Thompson T. N.; Koziel J. A.; Place S. E.; Stackhouse-Lawson K. R. U.S. Manure Methane Emissions Represent a Greater Contributor to Implied Climate Warming than Enteric Methane Emissions Using the Global Warming Potential* Methodology. Front. Sustain. Food Syst. 2023, 7, 1209541. 10.3389/fsufs.2023.1209541. [DOI] [Google Scholar]
- (9).Capper J. L. The Environmental Impact of Beef Production in the United States: 1977 Compared with 2007. Journal of Animal Science 2011, 89 (12), 4249–4261. 10.2527/jas.2010-3784. [DOI] [PubMed] [Google Scholar]
- (10).Pineda A. C.; Faria P. Towards a Science - Based Approach to Climate Neutrality in the Corporate Sector; Discussion paper 1. https://sciencebasedtargets.org/resources/files/Towards-a-science-based-approach-to-climate-neutrality-in-the-corporate-sector-Draft-for-comments.pdf (accessed 2024-05-11). [Google Scholar]
- (11).Palangi V.; Lackner M. Management of Enteric Methane Emissions in Ruminants Using Feed Additives: A Review. Animals 2022, 12 (24), 3452. 10.3390/ani12243452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Henderson G.; Cox F.; Ganesh S.; Jonker A.; Young W.; Global Rumen Census Collaborators; Abecia L.; Angarita E.; Aravena P.; Nora Arenas G.; Ariza C.; Attwood G. T.; Mauricio Avila J.; Avila-Stagno J.; Bannink A.; Barahona R.; Batistotti M.; Bertelsen M. F.; Brown-Kav A.; Carvajal A. M.; Cersosimo L.; Vieira Chaves A.; Church J.; Clipson N.; Cobos-Peralta M. A.; Cookson A. L.; Cravero S.; Cristobal Carballo O.; Crosley K.; Cruz G.; Cerón Cucchi M.; De La Barra R.; De Menezes A. B.; Detmann E.; Dieho K.; Dijkstra J.; Dos Reis W. L. S.; Dugan M. E. R.; Hadi Ebrahimi S.; Eythórsdóttir E.; Nde Fon F.; Fraga M.; Franco F.; Friedeman C.; Fukuma N.; Gagić D.; Gangnat I.; Javier Grilli D.; Guan L. L.; Heidarian Miri V.; Hernandez-Sanabria E.; Gomez A. X. I.; Isah O. A.; Ishaq S.; Jami E.; Jelincic J.; Kantanen J.; Kelly W. J.; Kim S.-H.; Klieve A.; Kobayashi Y.; Koike S.; Kopecny J.; Nygaard Kristensen T.; Julie Krizsan S.; LaChance H.; Lachman M.; Lamberson W. R.; Lambie S.; Lassen J.; Leahy S. C.; Lee S.-S.; Leiber F.; Lewis E.; Lin B.; Lira R.; Lund P.; Macipe E.; Mamuad L. L.; Cuquetto Mantovani H.; Marcoppido G. A.; Márquez C.; Martin C.; Martinez G.; Eugenia Martinez M.; Lucía Mayorga O.; McAllister T. A.; McSweeney C.; Mestre L.; Minnee E.; Mitsumori M.; Mizrahi I.; Molina I.; Muenger A.; Muñoz C.; Murovec B.; Newbold J.; Nsereko V.; O’Donovan M.; Okunade S.; O’Neill B.; Ospina S.; Ouwerkerk D.; Parra D.; Pereira L. G. R.; Pinares-Patiño C.; Pope P. B.; Poulsen M.; Rodehutscord M.; Rodriguez T.; Saito K.; Sales F.; Sauer C.; Shingfield K.; Shoji N.; Simunek J.; Stojanović-Radić Z.; Stres B.; Sun X.; Swartz J.; Liang Tan Z.; Tapio I.; Taxis T. M.; Tomkins N.; Ungerfeld E.; Valizadeh R.; Van Adrichem P.; Van Hamme J.; Van Hoven W.; Waghorn G.; John Wallace R.; Wang M.; Waters S. M.; Keogh K.; Witzig M.; Wright A.-D. G.; Yamano H.; Yan T.; Yáñez-Ruiz D. R.; Yeoman C. J.; Zambrano R.; Zeitz J.; Zhou M.; Wei Zhou H.; Xia Zou C.; Zunino P.; Janssen P. H. Rumen Microbial Community Composition Varies with Diet and Host, but a Core Microbiome Is Found across a Wide Geographical Range. Sci Rep 2015, 5 (1), 14567. 10.1038/srep14567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Patra A.; Park T.; Kim M.; Yu Z. Rumen Methanogens and Mitigation of Methane Emission by Anti-Methanogenic Compounds and Substances. J Animal Sci Biotechnol 2017, 8 (1), 13. 10.1186/s40104-017-0145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Gunsalus R. P.; Wolfe R. S. Methyl Coenzyme M Reductase from Methanobacterium Thermoautotrophicum. Resolution and Properties of the Components. Journal of Biological Chemistry 1980, 255 (5), 1891–1895. 10.1016/S0021-9258(19)85966-5. [DOI] [PubMed] [Google Scholar]
- (15).Kurth J. M.; Op Den Camp H. J. M.; Welte C. U. Several Ways One Goal— Methanogenesis from Unconventional Substrates. Appl Microbiol Biotechnol 2020, 104 (16), 6839–6854. 10.1007/s00253-020-10724-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Beck M. R.; Thompson L. R.; White J. E.; Williams G. D.; Place S. E.; Moffet C. A.; Gunter S. A.; Reuter R. R. Whole Cottonseed Supplementation Improves Performance and Reduces Methane Emission Intensity of Grazing Beef Steers. The Professional Animal Scientist 2018, 34 (4), 339–345. 10.15232/pas.2018-01722. [DOI] [Google Scholar]
- (17).Beck M. R.; Thompson L. R.; Williams G. D.; Place S. E.; Gunter S. A.; Reuter R. R. Fat Supplements Differing in Physical Form Improve Performance but Divergently Influence Methane Emissions of Grazing Beef Cattle. Animal Feed Science and Technology 2019, 254, 114210. 10.1016/j.anifeedsci.2019.114210. [DOI] [Google Scholar]
- (18).Arndt C.; Hristov A. N.; Price W. J.; McClelland S. C.; Pelaez A. M.; Cueva S. F.; Oh J.; Dijkstra J.; Bannink A.; Bayat A. R.; Crompton L. A.; Eugène M. A.; Enahoro D.; Kebreab E.; Kreuzer M.; McGee M.; Martin C.; Newbold C. J.; Reynolds C. K.; Schwarm A.; Shingfield K. J.; Veneman J. B.; Yáñez-Ruiz D. R.; Yu Z. Full Adoption of the Most Effective Strategies to Mitigate Methane Emissions by Ruminants Can Help Meet the 1.5 °C Target by 2030 but Not 2050. Proc. Natl. Acad. Sci. U.S.A. 2022, 119 (20), e2111294119. 10.1073/pnas.2111294119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Dressler E. A.; Bormann J. M.; Weaber R. L.; Rolf M. M. Use of Methane Production Data for Genetic Prediction in Beef Cattle: A Review. Translational Animal Science 2024, 8, txae014. 10.1093/tas/txae014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Beck M. R.; Gregorini P. Animal Design Through Functional Dietary Diversity for Future Productive Landscapes. Front. Sustain. Food Syst. 2021, 5, 546581. 10.3389/fsufs.2021.546581. [DOI] [Google Scholar]
- (21).Troy S. M.; Duthie C.-A.; Hyslop J. J.; Roehe R.; Ross D. W.; Wallace R. J.; Waterhouse A.; Rooke J. A. Effectiveness of Nitrate Addition and Increased Oil Content as Methane Mitigation Strategies for Beef Cattle Fed Two Contrasting Basal Diets1. Journal of Animal Science 2015, 93 (4), 1815–1823. 10.2527/jas.2014-8688. [DOI] [PubMed] [Google Scholar]
- (22).Dijkstra J.; Bannink A.; France J.; Kebreab E.; Van Gastelen S. Short Communication: Antimethanogenic Effects of 3-Nitrooxypropanol Depend on Supplementation Dose, Dietary Fiber Content, and Cattle Type. Journal of Dairy Science 2018, 101 (10), 9041–9047. 10.3168/jds.2018-14456. [DOI] [PubMed] [Google Scholar]
- (23).Roque B. M.; Venegas M.; Kinley R. D.; De Nys R.; Duarte T. L.; Yang X.; Kebreab E. Red Seaweed (Asparagopsis Taxiformis) Supplementation Reduces Enteric Methane by over 80 Percent in Beef Steers. PLoS ONE 2021, 16 (3), e0247820. 10.1371/journal.pone.0247820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Kinley R. D.; Tan S.; Turnbull J.; Askew S.; Roque B. M. Changing the Proportions of Grass and Grain in Feed Substrate Impacts the Efficacy of <I>Asparagopsis Taxiformis</I> to Inhibit Methane Production <I>in Vitro</I> AJPS 2021, 12 (12), 1835–1858. 10.4236/ajps.2021.1212128. [DOI] [Google Scholar]
- (25).Krone U. E.; Laufer K.; Thauer R. K.; Hogenkamp H. P. C. Coenzyme F430 as a Possible Catalyst for the Reductive Dehalogenation of Chlorinated C1 Hydrocarbons in Methanogenic Bacteria. Biochemistry 1989, 28 (26), 10061–10065. 10.1021/bi00452a027. [DOI] [PubMed] [Google Scholar]
- (26).Glasson C. R. K.; Kinley R. D.; De Nys R.; King N.; Adams S. L.; Packer M. A.; Svenson J.; Eason C. T.; Magnusson M. Benefits and Risks of Including the Bromoform Containing Seaweed Asparagopsis in Feed for the Reduction of Methane Production from Ruminants. Algal Research 2022, 64, 102673. 10.1016/j.algal.2022.102673. [DOI] [Google Scholar]
- (27).Sayers E. W.; Cavanaugh M.; Clark K.; Pruitt K. D.; Sherry S. T.; Yankie L.; Karsch-Mizrachi I. GenBank 2023 Update. Nucleic Acids Research 2023, 51 (D1), D141–D144. 10.1093/nar/gkac1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Apweiler R. UniProt: The Universal Protein Knowledgebase. Nucleic Acids Research 2004, 32 (90001), 115D – 119. 10.1093/nar/gkh131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Burley S. K.; Bhikadiya C.; Bi C.; Bittrich S.; Chao H.; Chen L.; Craig P. A.; Crichlow G. V.; Dalenberg K.; Duarte J. M.; Dutta S.; Fayazi M.; Feng Z.; Flatt J. W.; Ganesan S.; Ghosh S.; Goodsell D. S.; Green R. K.; Guranovic V.; Henry J.; Hudson B. P.; Khokhriakov I.; Lawson C. L.; Liang Y.; Lowe R.; Peisach E.; Persikova I.; Piehl D. W.; Rose Y.; Sali A.; Segura J.; Sekharan M.; Shao C.; Vallat B.; Voigt M.; Webb B.; Westbrook J. D.; Whetstone S.; Young J. Y.; Zalevsky A.; Zardecki C. RCSB Protein Data Bank (RCSB.Org): Delivery of Experimentally-Determined PDB Structures alongside One Million Computed Structure Models of Proteins from Artificial Intelligence/Machine Learning. Nucleic Acids Research 2023, 51 (D1), D488–D508. 10.1093/nar/gkac1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Seeliger D.; De Groot B. L. Ligand Docking and Binding Site Analysis with PyMOL and Autodock/Vina. J Comput Aided Mol Des 2010, 24 (5), 417–422. 10.1007/s10822-010-9352-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Yuan S.; Chan H. C. S.; Hu Z. Using PYMOL as a Platform for Computational Drug Design. WIREs Comput Mol Sci 2017, 7 (2), e1298. 10.1002/wcms.1298. [DOI] [Google Scholar]
- (32).Kim S.; Chen J.; Cheng T.; Gindulyte A.; He J.; He S.; Li Q.; Shoemaker B. A.; Thiessen P. A.; Yu B.; Zaslavsky L.; Zhang J.; Bolton E. E. PubChem 2023 Update. Nucleic Acids Research 2023, 51 (D1), D1373–D1380. 10.1093/nar/gkac956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Bergwerf H. MolView: An Attempt to Get the Cloud into Chemistry Classrooms. 2015. [Google Scholar]
- (34).Trott O.; Olson A. J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J Comput Chem 2010, 31 (2), 455–461. 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Huang J.; MacKerell A. D. CHARMM36 All-Atom Additive Protein Force Field: Validation Based on Comparison to NMR Data. J. Comput. Chem. 2013, 34 (25), 2135–2145. 10.1002/jcc.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Foroutan A.; Guo A. C.; Vazquez-Fresno R.; Lipfert M.; Zhang L.; Zheng J.; Badran H.; Budinski Z.; Mandal R.; Ametaj B. N.; Wishart D. S. Chemical Composition of Commercial Cow’s Milk. J. Agric. Food Chem. 2019, 67 (17), 4897–4914. 10.1021/acs.jafc.9b00204. [DOI] [PubMed] [Google Scholar]
- (37).Foroutan A.; Fitzsimmons C.; Mandal R.; Piri-Moghadam H.; Zheng J.; Guo A.; Li C.; Guan L. L.; Wishart D. S. The Bovine Metabolome. Metabolites 2020, 10 (6), 233. 10.3390/metabo10060233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Landrum G. RDKit: Open-Source Cheminformatics Software. http://www.rdkit.org (accessed 2024-04-15). [Google Scholar]
- (39).Zhou H.; Skolnick J. Utility of the Morgan Fingerprint in Structure-Based Virtual Ligand Screening. J. Phys. Chem. B 2024, 128 (22), 5363–5370. 10.1021/acs.jpcb.4c01875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Dominguez C.; Boelens R.; Bonvin A. M. J. J. HADDOCK: A Protein−Protein Docking Approach Based on Biochemical or Biophysical Information. J. Am. Chem. Soc. 2003, 125 (7), 1731–1737. 10.1021/ja026939x. [DOI] [PubMed] [Google Scholar]
- (41).Bajusz D.; Rácz A.; Héberger K. Why Is Tanimoto Index an Appropriate Choice for Fingerprint-Based Similarity Calculations? J Cheminform 2015, 7 (1), 20. 10.1186/s13321-015-0069-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Falcón-Cano G.; Molina C.; Cabrera-Pérez M. Á. Reliable Prediction of Caco-2 Permeability by Supervised Recursive Machine Learning Approaches. Pharmaceutics 2022, 14 (10), 1998. 10.3390/pharmaceutics14101998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Berthold M. R.; Cebron N.; Dill F.; Gabriel T. R.; Kötter T.; Meinl T.; Ohl P.; Thiel K.; Wiswedel B. KNIME - the Konstanz Information Miner: Version 2.0 and Beyond. SIGKDD Explor. Newsl. 2009, 11 (1), 26–31. 10.1145/1656274.1656280. [DOI] [Google Scholar]
- (44).Dey M.; Kunz R. C.; Van Heuvelen K. M.; Craft J. L.; Horng Y.-C.; Tang Q.; Bocian D. F.; George S. J.; Brunold T. C.; Ragsdale S. W. Spectroscopic and Computational Studies of Reduction of the Metal versus the Tetrapyrrole Ring of Coenzyme F 430 from Methyl-Coenzyme M Reductase. Biochemistry 2006, 45 (39), 11915–11933. 10.1021/bi0613269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Bonardi A. Chapter 5.2 - Methyl-Coenzyme M Reductase. In Metalloenzymes; Supuran C. T., Donald W. A., Eds.; Academic Press, 2024; pp 411–427. 10.1016/B978-0-12-823974-2.00034-6. [DOI] [Google Scholar]
- (46).Ermler U.; Grabarse W.; Shima S.; Goubeaud M.; Thauer R. K. Crystal Structure of Methyl-Coenzyme M Reductase: The Key Enzyme of Biological Methane Formation. Science 1997, 278 (5342), 1457–1462. 10.1126/science.278.5342.1457. [DOI] [PubMed] [Google Scholar]
- (47).Cedervall P. E.; Dey M.; Pearson A. R.; Ragsdale S. W.; Wilmot C. M. Structural Insight into Methyl-Coenzyme M Reductase Chemistry Using Coenzyme B Analogues,. Biochemistry 2010, 49 (35), 7683–7693. 10.1021/bi100458d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Gottlieb K.; Wacher V.; Sliman J.; Pimentel M. Review Article: Inhibition of Methanogenic Archaea by Statins as a Targeted Management Strategy for Constipation and Related Disorders. Aliment Pharmacol Ther 2016, 43 (2), 197–212. 10.1111/apt.13469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Hill J.; McSweeney C.; Wright A.-D. G.; Bishop-Hurley G.; Kalantar-zadeh K. Measuring Methane Production from Ruminants. Trends in Biotechnology 2016, 34 (1), 26–35. 10.1016/j.tibtech.2015.10.004. [DOI] [PubMed] [Google Scholar]
- (50).Malde A. K.; Zuo L.; Breeze M.; Stroet M.; Poger D.; Nair P. C.; Oostenbrink C.; Mark A. E. An Automated Force Field Topology Builder (ATB) and Repository: Version 1.0. J. Chem. Theory Comput. 2011, 7 (12), 4026–4037. 10.1021/ct200196m. [DOI] [PubMed] [Google Scholar]
- (51).Chowdhury R.; Thompson L. R.; Frazier A. N.; Koziel J. A.; Beck M. R. Computational Approaches for Enteric Methane Mitigation Research: From Fermi Calculations to Artificial Intelligence Paradigms. Anim. Front. IN PRESS 2024. https://doi:10.1093/af/vfae025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Zhou Y.; Chen L.; Hu J.; Duan H.; Lin D.; Liu P.; Meng Q.; Li B.; Si N.; Liu C.; Liu X. Resistance Mechanisms and Molecular Docking Studies of Four Novel QoI Fungicides in Peronophythora Litchii. Sci Rep 2015, 5 (1), 17466. 10.1038/srep17466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Jhansirani N.; Devappa V.; Sangeetha C. G.; Sridhara S.; Shankarappa K. S.; Mohanraj M. Identification of Potential Phytochemical/Antimicrobial Agents against Pseudoperonospora Cubensis Causing Downy Mildew in Cucumber through In-Silico Docking. Plants 2023, 12 (11), 2202. 10.3390/plants12112202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Noviandy T. R.; Idroes G. M.; Hardi I. Machine Learning Approach to Predict AXL Kinase Inhibitor Activity for Cancer Drug Discovery Using Bayesian Optimization-XGBoost. 2024, 15 (1). [Google Scholar]
- (55).Yang S.; Li S.; Chang J. Discovery of Cobimetinib as a Novel A-FABP Inhibitor Using Machine Learning and Molecular Docking-Based Virtual Screening. RSC Adv. 2022, 12 (21), 13500–13510. 10.1039/D2RA01057G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Thauer R. K. Methyl (Alkyl)-Coenzyme M Reductases: Nickel F-430-Containing Enzymes Involved in Anaerobic Methane Formation and in Anaerobic Oxidation of Methane or of Short Chain Alkanes. Biochemistry 2019, 58 (52), 5198–5220. 10.1021/acs.biochem.9b00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Wrede C.; Walbaum U.; Ducki A.; Heieren I.; Hoppert M. Localization of Methyl-Coenzyme M Reductase as Metabolic Marker for Diverse Methanogenic Archaea. Archaea 2013, 2013, 1–7. 10.1155/2013/920241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Garcia F.; Muñoz C.; Martínez-Ferrer J.; Urrutia N. L.; Martínez E. D.; Saldivia M.; Immig I.; Kindermann M.; Walker N.; Ungerfeld E. M. 3-Nitrooxypropanol Substantially Decreased Enteric Methane Emissions of Dairy Cows Fed True Protein- or Urea-Containing Diets. Heliyon 2022, 8 (6), e09738. 10.1016/j.heliyon.2022.e09738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Shi J.; Cheng K.; Pororegov T.; Capponi S. Atomistic Simulations and Machine Learning Approaches to Investigate Bromoform Interactions with Cell Membranes: Implications for Seaweed-Based Methane Emission Reduction; 2024. [Google Scholar]
- (60).Alemu A. W.; Shreck A. L.; Booker C. W.; McGinn S. M.; Pekrul L. K. D.; Kindermann M.; Beauchemin K. A. Use of 3-Nitrooxypropanol in a Commercial Feedlot to Decrease Enteric Methane Emissions from Cattle Fed a Corn-Based Finishing Diet. Journal of Animal Science 2021, 99 (1), skaa394. 10.1093/jas/skaa394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Amano H.; Kazamori D.; Itoh K. Evaluation of the Effects of S-Allyl-L-Cysteine, S-Methyl-L-Cysteine, Trans-S-1-Propenyl-L-Cysteine, and Their N-Acetylated and S-Oxidized Metabolites on Human CYP Activities. Biological & Pharmaceutical Bulletin 2016, 39 (10), 1701–1707. 10.1248/bpb.b16-00449. [DOI] [PubMed] [Google Scholar]
- (62).Senthilkumar G. P. Study the Effect of S-Methyl L-Cysteine on Lipid Metabolism in an Experimental Model of Diet Induced Obesity. JCDR 2013. 10.7860/JCDR/2013/7304.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Jeelani G.; Nozaki T. Metabolomic Analysis of Entamoeba: Applications and Implications. Current Opinion in Microbiology 2014, 20, 118–124. 10.1016/j.mib.2014.05.016. [DOI] [PubMed] [Google Scholar]
- (64).Eyre M. D.; Phillips D. E.; Evans I. M.; Thompson A. The Nutritional Role of S ‐methyl‐ L ‐cysteine. J Sci Food Agric 1983, 34 (7), 696–700. 10.1002/jsfa.2740340705. [DOI] [PubMed] [Google Scholar]
- (65).Knox C.; Wilson M.; Klinger C. M.; Franklin M.; Oler E.; Wilson A.; Pon A.; Cox J.; Chin N. E. (Lucy); Strawbridge S. A.; Garcia-Patino M.; Kruger R.; Sivakumaran A.; Sanford S.; Doshi R.; Khetarpal N.; Fatokun O.; Doucet D.; Zubkowski A.; Rayat D. Y.; Jackson H.; Harford K.; Anjum A.; Zakir M.; Wang F.; Tian S.; Lee B.; Liigand J.; Peters H.; Wang R. Q. (Rachel); Nguyen T.; So D.; Sharp M.; da Silva R.; Gabriel C.; Scantlebury J.; Jasinski M.; Ackerman D.; Jewison T.; Sajed T.; Gautam V.; Wishart D. S. DrugBank 6.0: The DrugBank Knowledgebase for 2024. Nucleic Acids Research 2024, 52 (D1), D1265–D1275. 10.1093/nar/gkad976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Lomize A. L.; Hage J. M.; Schnitzer K.; Golobokov K.; LaFaive M. B.; Forsyth A. C.; Pogozheva I. D. PerMM: A Web Tool and Database for Analysis of Passive Membrane Permeability and Translocation Pathways of Bioactive Molecules. J. Chem. Inf. Model. 2019, 59 (7), 3094–3099. 10.1021/acs.jcim.9b00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.