Abstract
Introduction
Inborn errors of immunity (IEI) are disorders that present a health issue, especially in developing countries where there is a high rate of consanguineous marriages and an increasing rate of diagnosis. One of these disorders is Bare Lymphocyte Syndrome II (BLS II) which is a rare and genetically complex disease that has high morbidity and mortality. The exact genotypic and phenotypic characteristics are still poorly characterized especially in developing countries.
Case Presentation
Here, we report the first case of BLS II in a seven-month-old Sudanese female with recurrent chest infections, dermatitis, persistent diarrhea, and failure to thrive. The patient’s all four sisters and three paternal uncles died in early infancy. Laboratory investigations revealed low CD3+, CD4+, and CD8+ lymphocytes, along with normal CD19+ and CD16+ lymphocytes, and low serum IgM and IgA levels. Genetic analysis revealed two CIITA variants; c.2296C >G p. (Pro766Ala) and c.439+1G >A.
Conclusion
Further bioinformatics, immunological and clinical workups supported a pathogenic effect of both mutations affecting the function of CIITA protein, and suggesting a compound heterozygote mutation. The patient was started on prophylactic antibiotics and regular intravenous immunoglobulin replacement therapy. The prognosis of this disease is poor in most of the cases, with only a few reported cases surviving until adulthood.
Keywords: inborn errors of immunity, bare lymphocyte syndrome II, CIITA gene, compound heterozygote mutation, primary immunodeficiency
Introduction
Primary immunodeficiency disorders (PID), or inborn errors of immunity (IEI), include a heterogeneous group of conditions caused by defects in the immune functions. There are more than 480 genes that have been associated with these disorders. One of these is the Major Histocompatibility Complex II (MHC II) deficiency or bare lymphocyte syndrome II (BLS II) which is an extremely rare autosomal recessive, but genetically complex disease characterized by an early onset of severe and recurrent infections.1 Only about 200 cases of BLS II have been reported. Almost two-thirds of the patients were reported from North African countries, where there is a high prevalence of consanguineous marriages.2 BLS II patients are vulnerable to a variety of bacterial, viral, fungal, and protozoan infections, with the most prevalent pathogens being Salmonella, Pneumocystis jirovecii, Cryptosporidium species, Herpes simplex virus (HSV), and Cytomegalovirus (CMV). Developmental delay, early mortality, and recurrent infections, mostly affecting the lung and gastrointestinal system are reported, while chronic diarrhea and recurrent pneumonia affect nearly all of these patients.3
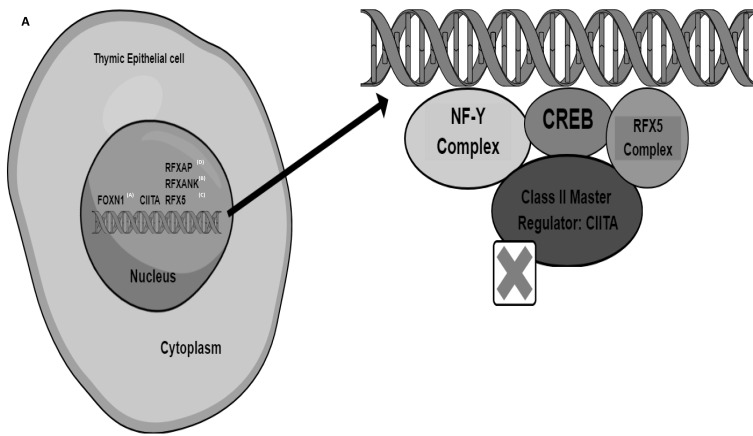
Even though the mutations in the genes associated with this disease are found outside the MHC II locus, BLS II is distinct because it appears as the lack of MHC II protein expression on specific cell surfaces.4 Antigen-presenting cells (APCs) including dendritic cells and macrophages exhibit reduced antigen presentation by HLA-DQ, HLA-DR, and HLA-DP molecules because of the lack of the MHC II protein resulting from the transcription factor mutations.5 Also, the consequent impaired CD4+ T-cell development, as well as the lack of Th-cell-dependent antibody generation by B-cells, these transcription factor alterations can affect both humoral and cell-mediated immunity.6 MHC II is preferentially expressed in certain cell types, including thymic epithelial cells, APCs, and other cell types activated by interferon-γ.7 This contrasts with the broadly expressed MHC I in normal cells. Additionally, the MHC II enhanceosome, a cell-specific multiprotein complex, controls the expression of the MHC II genes.7 The X, X2, Y, and W/S boxes are the 4 cis-acting components of the MHC-II promoter.8 The heterotrimeric RFX complex binds the X-box. Cyclic-AMP-responsive element-binding protein (CREB) binds the X2 element.8,9 The primary CCAAT-box-binding protein complex NF-Y binds to the Y-box, an inverted CCAAT region.8,10 On the other hand, a yet unidentified component binds the W/S box. Through a variety of protein–protein interactions, these protein complexes work together to form a platform that resembles an enhanceosome and enhances the recruitment of Class II transactivator (CIITA) protein8 (Figure 1). The CIITA and the multiprotein RFX complex were found to be the two important regulatory factors that activate the transcription of the genes producing MHC II in investigations involving the isolation of the gene from patients with MHC II deficiency.11 The direct binding to the promoters of MHC II genes, the RFX complex, made up of regulatory factor X-5 (RFX5), RFX-associated protein (RFXAP), and RFXAP-containing ankyrin repeat (RFXANK) (Figure 1), works with other pleiotropic elements to form the MHC II enhanceosome.12
Figure 1.
The Genetic Groups Associated with Bare Lymphocyte Syndrome II and The Major Histocompatibility Complex II Enhanceosome.
Notes: This figure shows the four distinct genetic groups that are associated with BLS II in the upper-left corner (labeled A), designated as groups A-D, which correspond to CIITA, RFXANK, RFX5, and RFXAP, respectively, depending on the affected transcription factor. These genes map to chromosomes 19p12, 16p13, 1q21.1–21.3 and 13q14 for RFXANK, CIITA, RFX5 and RFXAP, respectively. CIITA, which accounts for approximately 9% of the reported BLS II. In the right corner, cyclic-AMP-responsive element-binding protein (CREB) binds the X2 element. The primary CCAAT-box-binding protein complex NF-Y binds to the Y-box, RFX complex binds the X-box. By attaching to the RFX complex and initiating transcription, the inducible factor CIITA regulates the expression of the MHC class II genes. The RFX complex, which is widely expressed, consists of the RFXANK, RFX5, and RFXAP proteins as subunits. This complex attaches directly to the promoters of MHC class II genes and forms the MHC class II expression enhanceosome with the help of additional pleiotropic factors.
As a result, BLS II is divided into four groups, designated as groups A to D, which correspond to CIITA, RFXANK, RFX5, and RFXAP, respectively, depending on the affected transcription factor (Figure 1).2 The genes of these proteins map to chromosomes 19p12, 16p13, 1q21.1–21.3, and 13q14 for RFXANK (Ensembl: ENSG00000064490), CIITA (ENSG00000179583), RFX5 (ENSG00000143390) and RFXAP (ENSG00000133111), respectively. CIITA is expressed in professional APCs via CIITA promoters I and III, while non-professional APCs can also control the expression of MHC II and CIITA. Interferon is needed to synthesize CIITA through promoter IV and transform MHC class II negative monocytes into active APCs expressing MHC class II on their surfaces.13 The majority (75.3%) of all known BLS II with gene mutations are due to defects in the RFXANK gene, while the CIITA genetic mutations are much rare and account for about 9% of all BLS II cases.3 Here we report a case with a possible compound heterozygous CIITA mutation, resulting in BLS II. To our knowledge, this is the first documented case of a confirmed BLS II from Sudan.
Case Presentation
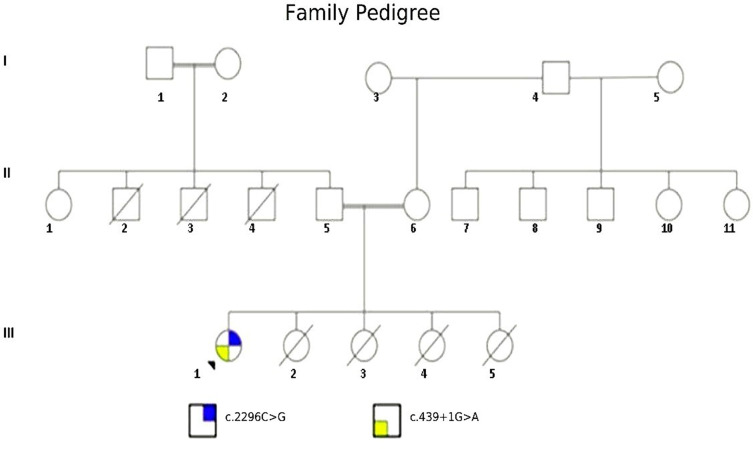
A 7-month-old Sudanese female (the proband: III-1, Figure 2) from consanguineous parents (II-5 and II-6: Figure 2) was referred to the pediatric immunology department at the Tropical Diseases Teaching Hospital-Omdurman for the screening of a possible immunodeficiency syndrome. Before presenting to our unit, the patient had sepsis due to Pseudomonas species that was only sensitive to ciprofloxacin and was treated for about two weeks. On presentation, the patient had recurrent chest infections, chronic diarrhea, persistent oral thrush, and dermatitis since the early neonatal period. Clinical examination revealed a wasted child with extensive seborrheic dermatitis and oral thrush.
Figure 2.
Family Pedigree.
Notes: This figure shows the family pedigree of the proband (III-1), all the patient sisters (III-2, III-3, III-4, and III-5) died in early infancy, the patient’s father reported that three of his brothers died (II-2, II-3, and II-4); however, the father does not remember exactly if they are two or three brothers due to recall bias.
Investigations
Microbiological investigations of the skin revealed a Methicillin-resistant Staphylococcus aureus infection that was only sensitive to Ciprofloxacin and Septrin. The patient had four female siblings who all died with similar clinical presentations before the age of three months and without a definitive diagnosis. Flow cytometry testing for lymphocyte subsets revealed low CD3+, CD4+, and CD8+ T cell counts, normal CD19+ cells, normal CD16+ cells, and a preserved CD4+/CD8+ ratio (Table 1). Immunoglobulin level tests showed decreased IgA and IgM with normal IgG levels. Specific antibody-based vaccine response tests and advanced flow cytometry tests were not available in Sudan at the time of the patient’s presentation. Following these results, the patient was started on prophylactic antibiotics and regular intravenous immunoglobulin (IVIG) for replacement.
Table 1.
Flow Cytometry Testing Results
| Cell Type | Patient Count | Normal Range (Cells/cmm) |
|---|---|---|
| CD3+ T-cell | 715 | 1900–5900 |
| CD4+ | 537 | 1400–4300 |
| CD8+ | 190 | 500–1700 |
| CD19+ | 966 | 610–2600 |
| CD16+ | 223 | 160–950 |
| CD4/CD8 ratio | 1.62 | 0.8–2.4 |
Materials and Methods
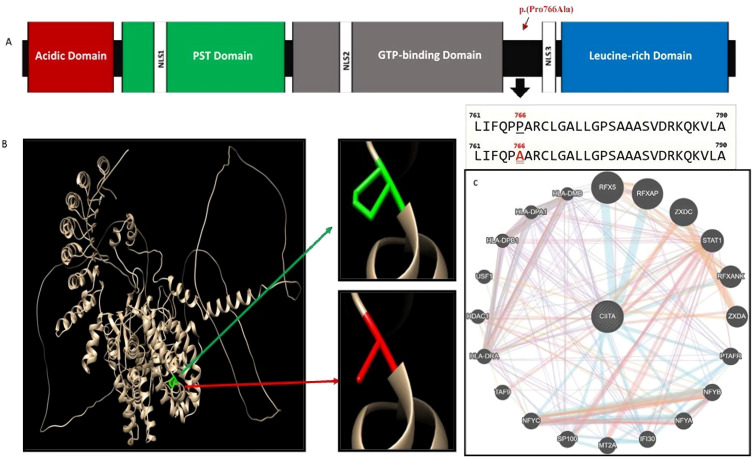
Next-generation exome sequencing targeting known primary immunodeficiency genes was done using the Illumina platform provided by CENTOGENE (Rostock, Germany). It included 41 Mb of the human coding exome along with the mitochondrial genome. In addition, an in-house bioinformatics pipeline (PolyPhen, Align-GVDG, SIFT (Sorting Intolerant from Tolerant) and MutationTaster), was used to compare the generated data with the reference sequence to detect and annotate different variants. This was further refined by including the relevant variants available in databases like gnomAD and ClinVar. Variants were then categorized according to the five classes ranging from pathogenic to benign. The platform used four databases to predict allele frequencies of the identified mutations which are Genome Aggregation Database (gnomAD), Exome Sequencing Project (ESP), 1000Genome project (1000G), and CentoMD® (latest database available). To elucidate the structural changes on the protein created by the missense mutation, we predicted the 3D structure of the MHC class II transactivator protein (ID: NM_001286402.1) using Alphafold 2 through ColabFold v 1.5.5. Then we visualized the wild and mutant amino acids using Chimera software as illustrated in (Figure 3A and B).
Figure 3.
(A-C) Computational Analysis and Visualization of the CIITA Protein and its gene–gene Interactions.
Notes: Figure 3A: This schematic figure shows the CIITA protein, with a length of 1130 amino acids. This protein comprises several regions including an N-terminal acidic domain, a proline-, serine-, and threonine-rich (PST) domain, a GTP-binding site, and at least four nuclear localization sequences (NLSs), and leucine-rich regions (LRRs). These components can bind with various factors. The first variant is highlighted in red is p.(Pro766Ala) located after the GTP-binding domain (red arrow) but before LRRs, and the other variant c.439+1G >A (SNP identifier rs373613022) is a splicing variant, which is predicted to disrupt the highly conserved donor splice site and leads to an inframe deletion of the respective exon (not shown). Figure 3B: The structural effect of the predicted missense mutation (c.2296C>G/ p.(Pro766Ala)) on the CIITA gene of MHC class II transactivator protein, as visualized using Chimera software version 1.16. The green color indicates the wild amino acid, while the red indicates the mutant amino acids. Figure 3C: Gene–gene interactions of CIITA gene as predicted by GeneMANIA server (https://genemania.org). Different line colors reflect different types of interactions between the genes as predicted by the software. As illustrated in Figure 1, the CIITA gene interacts with several other genes to perform the regulation functions. By using the GeneMANIA server, the CIITA gene appeared to be associated with more genes like PTAFR, STAT1, and TAF9, among many others, all of which could be affected by CIITA mutations (Figure 3B).
Result
Genotyping analysis revealed two compound heterozygous variants in the CIITA gene. The first was c.2296C>G p.(Pro766Ala) causing an amino acid change from Proline to Alanine at position 766 (Figure 3A, B and Table 2). This variant was not reported in the literature and has unknown allele frequency. It was reported as a variant of unknown significance. The other variant c.439+1G>A (Table 2), was predicted by the platform to disrupt the highly conserved donor splice site in the gene leading to distributed CIITA protein. The allele frequency of this variant according to different databases is (gnomAD: 0.000024, ESP: 0.000077, 1000G: 0, CentoMD: 0.000022). These structural changes are predicted to have a detrimental effect on the function of CIITA protein indicating a possible damaging effect by Polyphen, and MutationTaster software despite a general final classification as a variant of uncertain significance (VUS) by the American College of Medical Genetics and Genomics (ACMG) guidelines. Despite being reported as a VUS in the NGS result, the c.439+1G>A variant has been classified pathogenic in clinvar database (RCV001379491) and with a dbSNP ID (rs373613022). Thus the two variants have damaging effect; p.Pro766Ala was predicted to have possible damaging effect predicted by polyphen, and as a disease causing by the mutation taster algorithm. The c.439+1G>A may disrupt the highly conserved donor splice site causing an inframe deletion of the respective exon and also predicted as a disease causing by mutation taster (Table 2).Thus, both variants has possible damaging effect, but one variant is classified pathologic and the second is a novel VUS.
Table 2.
Summary of Bioinformatics Analysis of Two Variants in the CIITA Gene
| Variants | Computational Analysis | Classification |
|---|---|---|
|
NM_001286402.1: c.2296C>G |
PolyPhen: Possibly damaging Align-GVDG: C15 SIFT:- MutationTaster: Disease-causing |
Missense Uncertain significance |
|
NM_001286402.1: c.439+1G>A |
PolyPhen:- Align-GVDG: N/A SIFT: N/A MutationTaster: Disease causing |
Splicing Uncertain significance |
Also, the overall clinical picture indicate a diagnosis of BLS II, for which immunoglobulin replacement therapy and hematopoietic stem cell transplantation (HSCT) are the main modes of treatment. The platform recommends parental targeted testing to ascertain the phase (cis or trans) of the reported variants, however, the parents refused genetic testing. They were counseled about the disease and the need for lifelong follow-up under the care of clinical immunology and infectious diseases specialists. The patient needs three weekly IVIG replacements with antibiotics prophylaxis until HSCT is organized abroad. She remained asymptomatic with no further infections until the time of writing this manuscript.
Discussion
The exact molecular defects have not been fully identified in BLS II. The defects are genetically complex and may involve variants in the MHC II promoter complex DNA-binding regulatory factors (CIITA, RFX5, RFXAP, and RFXANK) (Figure 1). Our patient has CIITA genetic variant, which is located on chromosome 16,14 and was the first patient with BLS II to be identified in Sudan. CIITA is considered a potent regulator of the immune response and has been described as a “master controller” of MHC-II genes.15 The protein has been shown to promote INF-induced MHC expression by being an essential part of MHC II enhanceosome. The latter is described as a higher-order nucleoprotein complex made stable through the cooperative binding of RFX, X2BP and NF-Y.15 CIITA belongs to the family of proteins that contain leucine-rich region (LRR), CIITA interacts with P-TEFb, phosphorylates RNA polymerase II, and recruits elements of the general transcription machinery such TFIID and TFIIB. Additionally, it attracts coactivators of chromatin remodelling such BRG1, PCAF, CBP, and p300.8 Also, CIITA is described to have acetyltransferase and kinase activities that facilitate both MHC I and MHC II expression, as well as activating the transcription of non-MHC genes, a function that required further clarification and research.15
The CIITA gene’s C-terminal region functions as a transcriptional activator and provides MHC II promoter specificity. CIITA has three isoforms, each of which participates in a protein-protein interaction in the LRR. The LRR domain is significant in CIITA movement to the nucleus and in controlling its transactivation functions.16 Due to a malfunctioning LRR region, the majority of malfunctioning CIITA proteins are unable to bind to the enhanceosome complex in the MHC II gene promoter, while the majority of the CIITA gene variants that have been reported result in premature stop codons that truncate proteins, or missense mutations that produce inactive proteins.3 New evidence suggests that the CIITA gene has complex gene–gene interactions (Figure 3C), and possible antiviral activity by inducing resistance in human cell lines and controlling the expression of the p41 isoform of the invariant chain (CD74). Here, Ebola glycoprotein processing carried out by cathepsin is interfered with by CD74 p41, preventing viral fusion and entry. Coronaviruses can also be prevented from entering the endosomes by CD74 p41.17
In this rare case, the patient’s family originated from Sudan, an East African region with highly diverse demographics, and genetic patterns.18 There, consanguineous marriages constitute roughly 67% of marriages in some regions.19,20 The present case describes a seven-month-old female with a family history of consanguinity and infantile deaths in two generations (Figure 2: II, III). The recurrent infections and failure to thrive in the patient together with the abnormal lymphocyte subsets analysis and serum immunoglobulin levels, were indicative of an underlying immune deficiency. No genetic testing results were available for other members of the family as the patient’s parents refused genetic screening due to the high anxiety21 that manifested after obtaining the genetic results of their daughter, and the previous multiple offspring losses they have. Therefore, it was not possible to identify the paternal and maternal variants or their carrier status.22 The family pedigree also shows two 1st degree consanguineous marriages in the patient’s parents, and paternal grandparents (Figure 2: II-5, II-6, I-1, and I-2), which increases the possibility of occurrence of autosomal recessive conditions like BLS II.
Infections usually start in the first year of life and are associated with failure to thrive, diarrhea, and malabsorption.23 In this rare case, the patient presented with the typical symptoms of BLS II, including recurrent chest infections, persistent diarrhea, dermatitis, and recurrent oral thrush since the early neonatal period and family history on the paternal side. The patient’s decreased IgA and IgM levels may be due to impaired T cell effect on B cell activation; while the normal IgG could be of maternal origin. The absence of specific antibody testing in Sudan at the time of the study hampered further investigation into the patient’s immune responses to microbial antigens. MHC II expression on peripheral B cells and monocytes was not available to our patient, however, flow cytometry testing revealed a pan T cell lymphocytopenia (Table 1). Our patient showed a low CD8+ (Table 1) which was reported by other studies. In the case series by Hawary et al24 involving ten patients (two females and eight males) with MHC class II deficiency, two patients had low CD8+ levels, with one patient with RFXANK variant expressing extremely low CD8+ count 30 (500–1700 cells/μL), CD8+ lymphocytopenia in BLS II patients, due to CIITA and RFXAP, have been reported by Schmetterer et al25 and Dziembowska et al26 which may be reflective of the CIITA role in MHC-I expression,15 variability in genotype and phenotype relationship in patients with BLS II, or may be explained by the specific position and type of underlying mutations in the involved gene.24 The detection of HLA-DR using flow cytometric analysis was a limitation to reliably investigate this patient. Unfortunately, the investigation was unavailable in our setting, hence the urge to detect a genetic defect to confirm the diagnosis and plan the course of treatment.24 The parent’s reluctance to get genetic testing presented further limitations in interpreting the patient’s NGS report.
Also, in the study by Hawary et al, the patients had different profiles of clinical symptoms and signs, which affected their growth rate at varying degrees, furthermore despite most of them (70%) receiving intravenous immunoglobulin, all except one died a few months after the diagnosis. This could be due to the relatively late diagnosis compared with disease onset, which ranges from 5 to 13 months in most cases.24 This reflects the need for a screening program for the early detection of suspected cases, which could translate to a better response to IVIG therapy, faster HSCT, or even gene therapy. At the time of writing, our patient was in preparation for bone marrow transplantation, which could dramatically improve her life expectancy if successful. Another study done in India involved five patients with MHC II deficiency.27 Only one of the patients had a variant in the CIITA gene, the child had multiple respiratory infections with opportunistic microorganisms, which led to suspicion and, later, a diagnosis of BLS II at the age of 7 months. However, unlike our case, he did not belong to a consanguineous parent. Thus, BLS II should be considered in child with the clinical, genetic, and immunological features of the disease even if there is no consanguinity.
Although most patients with the disease die in early childhood, an adult male diagnosed with BLS II due to CIITA genetic variant, reportedly survived to the age of 23 years without receiving HSCT,28 while being kept on regular IVIG and antibiotics. Finally, it is important to note the importance of early diagnosis of such patients and genetic screening for PIDs in families with a history of repeated infection. The family in our case eventually sought medical advice after they lost four female children. Lastly, and to ensure the best possible outcomes for our patient and her family, the patient will be under the care and close follow-up at the pediatric immunology and the pediatric tropical and infectious diseases units.
Conclusion
This rare case describes a seven-month-old female with a family history of repeated infantile death, who was found to have pan T-cell lymphocytopenia, decreased IgA and IgM, and two heterozygous variants in the CIITA gene, consistent with the diagnosis of BLS II. Due to the clinical presentation and complexity of the disease as well as it is the first CIITA defect reported in a Sudanese patient. It is also important to reflect the difficulties in getting and interpreting NGS results in countries with low reporting of PIDs, and highlights the importance of simple in-house bioinformatics, and clinical correlation in the interpretation of genetic results.
Funding Statement
No fund was obtained.
Data Sharing Statement
Data that support this case presentation is available upon contacting the corresponding author.
Consent for Publication
After obtaining the parent’s informed consent to publish this case, the Tropical Disease Teaching Hospital ethical committee in Omdurman, Sudan approved this study serial number (TDTH/B/231/13).
Disclosure
The authors report no conflict of interest.
References
- 1.Tangye SG, Al-Herz W, Bousfiha A, et al. Human inborn errors of immunity: 2022 update on the classification from the international union of immunological societies expert committee. J Clin Immunol. 2022;42(7):1473–1507. doi: 10.1007/s10875-022-01289-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lum SH, Anderson C, McNaughton P, et al. Improved transplant survival and long-term disease outcome in children with MHC class II deficiency. Blood J Am Soc Hematol. 2020;135(12):954–973. [DOI] [PubMed] [Google Scholar]
- 3.Cai YQ, Zhang H, Wang XZ, et al. A novel RFXANK mutation in a Chinese child with MHC II deficiency: case report and literature review. In: Open Forum Infectious Diseases. Oxford University Press US; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanna S, Etzioni A. MHC class I and II deficiencies. J Allergy Clin Immunol. 2014;134(2):269–275. [DOI] [PubMed] [Google Scholar]
- 5.Waldburger J-M, Masternak K, Muhlethaler-Mottet A, et al. Lessons from the bare lymphocyte syndrome: molecular mechanisms regulating MHC class II expression. Immunol Rev. 2000;178:148–165. [DOI] [PubMed] [Google Scholar]
- 6.Sage AP, Murphy D, Maffia P, et al. MHC Class II–restricted antigen presentation by plasmacytoid dendritic cells drives proatherogenic T cell immunity. Circulation. 2014;130(16):1363–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nekrep N, Fontes JD, Geyer M, Peterlin BM. When the lymphocyte loses its clothes. Immunity. 2003;18(4):453–457. [DOI] [PubMed] [Google Scholar]
- 8.León Machado JA, Steimle V. The MHC class II transactivator CIITA: not (quite) the odd-one-out anymore among NLR proteins. Int J Mol Sci. 2021;22(3):1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moreno CS, Beresford GW, Louis-Plence P, Morris AC, Boss JM. CREB regulates MHC class II expression in a CIITA-dependent manner. Immunity. 1999;10(2):143–151. [DOI] [PubMed] [Google Scholar]
- 10.Dorn A, Bollekens J, Staub A, Benoist C, Mathis D. A multiplicity of CCAAT box-binding proteins. Cell. 1987;50(6):863–872. doi: 10.1016/0092-8674(87)90513-7 [DOI] [PubMed] [Google Scholar]
- 11.Reith W, Mach B. The bare lymphocyte syndrome and the regulation of MHC expression. Ann Rev Immunol. 2001;19(1):331–373. doi: 10.1146/annurev.immunol.19.1.331 [DOI] [PubMed] [Google Scholar]
- 12.Al-Herz W, Alsmadi O, Melhem M, Recher M, Frugoni F, Notarangelo LD. Major histocompatibility complex class II deficiency in Kuwait: clinical manifestations, immunological findings and molecular profile. J Clin Immunol. 2013;33:513–519. doi: 10.1007/s10875-012-9831-8 [DOI] [PubMed] [Google Scholar]
- 13.Roche PA, Furuta K. The ins and outs of MHC class II-mediated antigen processing and presentation. Nat Rev Immunol. 2015;15(4):203–216. doi: 10.1038/nri3818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steimle V, Otten LA, Zufferey M, Mach B. Complementation cloning of an MHC class II transactivator mutated in hereditary MHC class II deficiency (or bare lymphocyte syndrome). Cell. 1993;75(1):135–146. doi: 10.1016/S0092-8674(05)80090-X [DOI] [PubMed] [Google Scholar]
- 15.Masternak K, Muhlethaler-Mottet A, Villard J, Zufferey M, Steimle V, Reith W. CIITA is a transcriptional coactivator that is recruited to MHC class II promoters by multiple synergistic interactions with an enhanceosome complex. Genes Dev. 2000;14(9):1156–1166. doi: 10.1101/gad.14.9.1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devaiah BN, Singer DS. CIITA and its dual roles in MHC gene transcription. Front Immunol. 2013;4:476. doi: 10.3389/fimmu.2013.00476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruchez A, Sha K, Johnson J, et al. MHC class II transactivator CIITA induces cell resistance to Ebola virus and SARS-like coronaviruses. Science. 2020;370(6513):241–247. doi: 10.1126/science.abb3753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdelmajeed O, Ali MMD, Erwa NH, et al. Autosomal recessive IL12RB1 mutation: a case report of a Sudanese child and his father. Front Immunol. 2023;14:1135824. doi: 10.3389/fimmu.2023.1135824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Temaj G, Nuhii N, Sayer J. The impact of consanguinity on human health and disease with an emphasis on rare diseases. J Rare Dis. 2022;1(1):2. doi: 10.1007/s44162-022-00004-5 [DOI] [Google Scholar]
- 20.Barbouche M-R, Mekki N, Ben-Ali M, Ben-Mustapha I. Lessons from genetic studies of primary immunodeficiencies in a highly consanguineous population. Front Immunol. 2017;8:737. doi: 10.3389/fimmu.2017.00737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jun M, Thongpriwan V, Choi KS. Experiences of prenatal genetic screening and diagnostic testing among pregnant Korean women of advanced maternal age. J Transcult Nurs. 2017;28(6):550–557. doi: 10.1177/1043659616662913 [DOI] [PubMed] [Google Scholar]
- 22.van den Heuvel LM, van den Berg N, Janssens ACJW, et al. Societal implications of expanded universal carrier screening: a scoping review. Eur J Hum Genet. 2023;31(1):55–72. doi: 10.1038/s41431-022-01178-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saleem MA, Arkwright PD, Davies EG, Cant AJ, Veys PA. Clinical course of patients with major histocompatibility complex class II deficiency. Arch Dis Childhood. 2000;83(4):356–359. doi: 10.1136/adc.83.4.356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El Hawary RE, Mauracher AA, Meshaal SS, et al. MHC-II deficiency among Egyptians: novel mutations and unique phenotypes. J Allergy Clin Immunol Pract. 2019;7(3):856–863. doi: 10.1016/j.jaip.2018.07.046 [DOI] [PubMed] [Google Scholar]
- 25.Schmetterer KG, Seidel MG, Körmöczi U, et al. Two newly diagnosed HLA class II-deficient patients identified by rapid vector-based complementation analysis reveal discoordinate invariant chain expression levels. Int Arch Allergy Immunol. 2010;152(4):390–400. doi: 10.1159/000288292 [DOI] [PubMed] [Google Scholar]
- 26.Dziembowska M, Fondaneche MC, Vedrenne J, et al. Three novel mutations of the CIITA gene in MHC class II-deficient patients with a severe immunodeficiency. Immunogenetics. 2002;53(10–11):821–829. [DOI] [PubMed] [Google Scholar]
- 27.Aluri J, Gupta M, Dalvi A, et al. Clinical, immunological, and molecular findings in five patients with major histocompatibility complex class II deficiency from India. Front Immunol. 2018;9:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsieh J, Mandola A, Betschel SD. A novel homozygous mutation in CIITA resulting in MHC class II deficiency in an adult patient. LymphoSign J. 2018;5:135. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data that support this case presentation is available upon contacting the corresponding author.