Abstract
Background
Intraoperative hemorrhage in cardiac surgery increases risk of morbidity and mortality. Low pre-operative and perioperative levels of fibrinogen, a key clotting factor, are associated with severity of hemorrhage and increased transfusion of blood components. The ability to supplement fibrinogen during hemorrhagic resuscitation is delayed 45–60 min because cryoprecipitated antihemophilic factor (cryo AHF) is stored frozen, due to a short post-thaw shelf life. Pathogen Reduced Cryoprecipitated Fibrinogen Complex (INTERCEPT Fibrinogen Complex, IFC) can be kept thawed, at room temperature, for up to 5 days, making it possible to be immediately available for hemorrhaging patients. This trial will investigate if earlier correction of acquired hypofibrinogenemia with IFC in hemorrhaging cardiac surgery patients reduces the total number of perioperatively transfused allogeneic blood products (red blood cells, plasma, and platelets) as compared to cryo AHF.
Methods
This is a single center, prospective, cluster randomized trial with an adaptive design. Acquired hypofibrinogenemia will be assessed by rotational thromboelastometry (ROTEM) and the threshold for cryo AHF/IFC transfusion defined as FIBTEM A10 ≤ 10 mm in bleeding patients. IFC/cryo AHF will be randomized by 1-month blocks. Cardiac surgery patients will be enrolled in the study if they have an eligible procedure and at least one dose of a cryo AHF/IFC product (approximately 2 g fibrinogen) is transfused. Data from the electronic health record, including the blood bank and lab information systems, will be prospectively collected from the health system’s data warehouse.
Discussion
This trial aims to provide evidence of the clinical efficacy of utilizing readily available thawed IFC during acute bleeding in the cardiac surgery setting compared to traditional cryo AHF.
Trial registration
ClinicalTrials.gov NCT05711524. Feb 3, 2023.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13063-024-08398-x.
Keywords: Cardiac surgery, Blood transfusion, Cryoprecipitate, Pathogen reduction, FIBTEM, Intercept fibrinogen complex
Background
Intraoperative hemorrhage in cardiac surgery results in substantial increases in post-operative morbidity and mortality. Without the ability to adequately prevent, or provide rapid control of, hemorrhage, the need for re-exploration, prolonged ICU stay, ventilation for > 24 h, high volumes of blood products and associated risks, and risk of mortality, increase significantly [1, 2]. Protocolized assessment of coagulopathy and delivery of blood components have improved outcomes and reduced overall component use. The pathophysiology of cardiac surgery-related bleeding is often multifactorial and frequently includes acquired hypofibrinogenemia (fibrinogen level < 200 mg/dL).
Fibrinogen, a key protein in clot formation, is the first clotting factor to reach critically low levels during hemorrhage [3]. Acquired hypofibrinogenemia is associated with excessive perioperative bleeding in children [4] and adults [5], and guidelines recommend treatment with either cryoprecipitated AHF (cryo AHF) or fibrinogen concentrate [6, 7] in cases of excessive bleeding and acquired hypofibrinogenemia. Pre-operative fibrinogen levels in cardiac surgery are independently associated with post-operative blood loss, re-exploration, and RBC transfusion [8]. Similarly, post-operative fibrinogen levels below 200 mg/dL are an independent risk factor for severe hemorrhage [9]. The ability to identify hypofibrinogenemia faster with viscoelastic testing, and treat it perioperatively, is associated with reduced blood component utilization and improved mortality rates in cardiovascular surgery [10, 11]. Fibrinogen replacement with cryo AHF has traditionally occurred later in hemorrhagic resuscitation, since it is stored frozen, and not thawed until after it is ordered, leading to 45–60 min delay in availability. Pathogen Reduced Cryoprecipitated Fibrinogen Complex (INTERCEPT Fibrinogen Complex, IFC) can be stored thawed in the blood bank or operating room and then be ready for immediate transfusion for up to 5 days post-thaw [12].
IFC was approved by the United States Food and Drug Administration (FDA) and commercially available in 2021. Given the relatively recent addition of this option, the impact of treating hypofibrinogenemia faster in cardiac surgery is unknown. The approval process of IFC did not require clinical study, and therefore no real-world or clinical data exist directly comparing traditional cryo AHF and IFC use in the perioperative setting. This clinical trial attempts to compare the two products in a real-world setting. In the United States, fibrinogen concentrate, which can also be prepared more rapidly than traditional cryo AHF, is frequently used off-label for acquired hypofibrinogenemia in bleeding patients. Previous trials comparing cryo AHF and fibrinogen concentrate have shown no major differences in outcomes [13–17] in many different clinical settings. While this study will not compare IFC to fibrinogen concentrate, IFC has the potential added benefit over fibrinogen concentrate of containing the other coagulation factors found in cryo AHF (von Willebrand factor and factors VIII and XIII) which may also become deficient in bleeding patients. All three products (fibrinogen concentrate, IFC, and traditional cryo) contain concentrated factor XIII.
Objective
The aim of this study is to investigate if use of IFC during cardiac surgery-related bleeding can reduce the total number of allogeneic blood products transfused as compared to traditional cryo AHF.
Methods/design
Study design
A single-center, prospective, cluster randomized, non-blinded study with adaptive design, comparing the clinical and financial effectiveness of cryo AHF and IFC during cardiac surgery.
Each month all patients in the hospital will get either IFC or cryo AHF depending on the randomization scheme. Study month clusters will include all patients in the hospital, regardless of study eligibility, for whom cryo AHF/IFC is ordered. The pooled doses of IFC and cryo AHF are considered clinically equivalent products by our investigators and institution. The study will only include patients meeting inclusion criteria. This design was used to improve adherence to the study protocol, minimize disruption to the blood bank staff, and avoid delays in patient care due to time needed for individual randomization.
The SPIRIT reporting guidelines for clinical trial protocols were followed [18]. See SPIRIT checklist and figure.
Setting
This clinical trial occurs in a quaternary care academic medical center (862 beds) in New York City. The hospital performs a large number of highly complex cardiac surgeries (approximately 1000) each year, with an array of services that spans from robotic surgery to cardiac transplants.
Outcomes
The primary outcome will be the total number of non-cryo AHF/IFC blood components (RBCs, platelets, plasma) transfused during the admission within the first 30 days after surgery.
The secondary outcomes, which are all routinely available in the Electronic Health Record (EHR) per standard of care, will include:
Number of cryo AHF/IFC or fibrinogen concentrate products used perioperatively (defined as anesthesia start time to three days post-procedure)
Number of RBC, plasma, and platelet products used perioperatively (defined as anesthesia start time to 3 days post-procedure)
Time from OR start time to start of cryo AHF/IFC transfusion
Time from cryo AHF/IFC order to start of transfusion
Number of cryo AHF/IFC units wasted by blood bank per month during study periods
Laboratory measures, as available in the EHR, related to the indications for fibrinogen supplementation: pre- and post-transfusion FIBTEM amplitude (A10) and maximum clot firmness (MCF) and Clauss fibrinogen level within 10 min to 1 h after the end of the first cryo AHF/IFC transfusion
Highest and lowest fibrinogen level or ROTEM FIBTEM MCF within 24 h after surgery
Volume in drains (e.g., chest tube for CV surgery) at 24 h or until removed, whichever is sooner
Time from end of bypass pump until end of surgery (reflects time to hemostasis)
Length of stay (OR, ICU, and hospital)
Need for post-operative ventilator therapy (yes/no)
Time on ventilator
Overall cost of cryo AHF vs. IFC, when factoring wastage, during each 1-month period
Adverse events: fevers, infections, transfusion reactions within 5 days of surgery start time, need for surgical re-exploration, multiorgan system failure
Fibrinogen level most proximal to end of procedure
Study population
All patients > 18 years who receive a cryoprecipitate product during cardiothoracic surgery will be screened for the study.
Inclusion criteria
Adult patients undergoing cardiovascular surgery who receive intraoperative cryo AHF/IFC during the study period. Cryo AHF/IFC must be transfused in the primary procedure. Cardiovascular surgery includes the following procedures: coronary artery bypass grafting, valve repair or replacement, open thoracic aortic and thoracoabdominal aortic surgery, atrial or ventricular septal defects, ventricular assist device implantation or revision, or any combination of the above. Pre-emptive ordering of cryo AHF/IFC prior to the onset of bleeding will not result in exclusion, as this is a common operative practice.
Exclusion criteria
Pregnant women and pediatric patients (< 18 years). Cardiac transplant patients or patients transferred after surgery at other hospitals. Patients with known coagulopathies or congenital dysfibrinogenemia or afibrinogenemia. Patients who do not receive cryo AHF/IFC in the OR, or only receive during re-exploration, follow-up procedures, or post-operatively. Patients who receive a product in error or receive both IFC and cryo AHF due to treatment spanning different product randomized months. Patient who receives less than 1 pool (5 units/ ~ 2 g fibrinogen per dose) of cryo AHF or less than 1 FC20 (~ 2 g fibrinogen per dose) IFC.
Since the trial compares two standard-of-care products and uses standard-of-care treatment, there were no restrictions on concomitant care or other experimental interventions.
Recruitment
Data from the electronic health record and blood bank and lab information systems will be prospectively collected from the health system’s data warehouse during the study and included in the data analysis. Due to the cluster randomization strategy, all subjects who meet study criteria are automatically enrolled.
Interventions
The pooled doses of IFC and cryo AHF are considered clinically equivalent products by our investigators and institution.
Cryoprecipitated antihemophilic factor (cryo AHF)
Pooled cryo AHF is a plasma-derived product from 5 blood donors. Cryo AHF is the conventional method of fibrinogen replacement for acquired hypofibrinogenemia in bleeding patients in the United States. When stored frozen, it has a shelf life of 1 year; however, it must be thawed immediately prior to use due to a 4–6 h post-thaw shelf life. The product must be transfused within 4–6 h of thawing due to a risk of bacterial contamination [19]. Due to the need to thaw, issue, and transport cryo AHF from the blood bank to the operating room, order-to-transfusion time routinely requires up to 60 min with an efficient system.
INTERCEPT Fibrinogen Complex (IFC)
Similar to cryo AHF, IFC is a plasma-derived product. The key difference is prior to cryoprecipitation of the plasma, it is pathogen reduced using the INTERCEPT Blood System for Pathogen Reduction (Cerus Corporation, Concord CA). This system uses amotosalen, which intercalates into the DNA and RNA of infectious organisms and lymphocytes and, upon UV-A irradiation, causes irreversible damage that inactivates potential pathogens and residual lymphocytes in the product, while leaving coagulation factors intact. Amotosalen is then removed from the product. The inactivation of pathogens allows thawed IFC to have a shelf life of 5 days without the risk of microbial contamination. The same pathogen reduction process has been in use in the United States since 2014 for platelet and plasma products. As IFC is a cryoprecipitate product and derivative of plasma, no safety differences are expected between the IFC and cryo AHF. Multiple studies have shown that fibrinogen is stable when thawed at room temperature for up to 35 days [20, 21]. The longer thawed shelf-life of this product should reduce product wastage which may offset the increased cost of IFC over cryo AHF. In vitro studies have shown no difference in efficacy between cryo AHF and IFC [22, 23]. The amount of fibrinogen, the most abundant protein in cryoprecipitate, contained in IFC and traditional cryo AHF is similar.
Randomization and treatment determination
Randomization is performed by month and all eligible patients treated during the month will be enrolled. During the IFC months, 4 doses of thawed IFC20 will be readily available to issue at all times on a room temperature shelf in the blood bank. One dose of cryo AHF will be thawed on order, per standard protocol.
Computer-generated random numbers were generated by the statistician to determine allocation sequence by month. No stratification was performed. Allocation is implemented by the supervisor of the blood bank. Each month the blood bank staff is notified regarding which product will be used. The freezer shelf where the in-use product is stored is kept open; the shelf where the other product is stored is blocked with tape covering the opening.
Goal directed therapy with viscoelastic testing (VET) is used to guide component transfusion determination. ROTEM Delta (Werfen, Bedford, MA) is available at point-of-care in the cardiac operating rooms (see ROTEM algorithm in Fig. 1). The fibrinogen assay available with ROTEM, the FIBTEM, can provide results to the operating room in 10 min, minimizing delay between sample, results, and order of a cryo AHF/IFC unit. The FIBTEM amplitude of clot firmness is available 10 min (A10) into the tracing (ROTEM®, Werfen, Bedford, MA). FIBTEM A10 has been shown to correlate with the laboratory measured (Clauss) fibrinogen level [24].
Fig. 1.
Weill Cornell Medicine ROTEM algorithm
Due to the delays in obtaining cryo AHF, institutional practice occasionally includes pre-emptive ordering in patients at high risk for bleeding prior to the onset of bleeding in the setting of expected or known hypofibrinogenemia (fibrinogen less than 250 mg/dL). If transfusion is subsequently not indicated (i.e., patient does not bleed), the products are returned. RBC transfusions are considered when hemoglobin is less than 7–8 g/dL (as measured by epoc® Blood Analysis System, Siemens Healthineers, Malvern, PA) or during uncontrolled hemorrhage.
Data collection and management
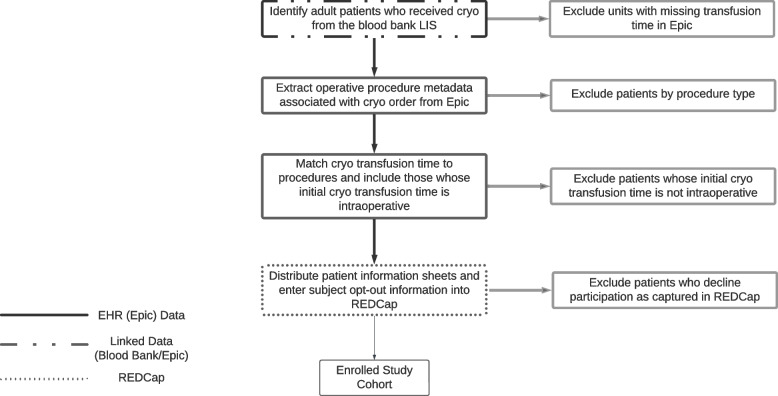
Data will be collected using automated scripts with Structured Query Language (SQL) queries of the hospital’s centralized data warehouse. Queries will extend into the backend databases of relevant clinical systems: blood bank data from SafetraceTx (Haemonetics, Boston, MA) and clinical data from Epic’s Clarity backend (Epic Systems, Verona, WI). Data will be extracted and stored in a Microsoft SQL database and imported into REDCap. Query of the blood bank database will identify all patients who received cryo AHF/IFC (the “qualifying cryo AHF/IFC”), and extract the associated transfusion start times from Epic using SQL queries. Queries will be run to identify the hospitalization and procedure from Epic associated with the qualifying cryo AHF/IFC order, and to exclude any patients with procedures that were not in our inclusion criteria procedure list (see Fig. 2). The included procedures list was generated by extracting all operative procedure types in which patients received a unit of cryo AHF perioperatively in the year prior to study initiation, since most of the eligible procedures would be captured in that time frame. Cardiac anesthesiologists manually annotated the generated list of procedures to validate those that should be included and to add any that may have been omitted using that approach.
Fig. 2.
Inclusion criteria procedure list
For patients passing initial screen, procedure and hospitalization metadata will be extracted, namely, admission and procedure start and stop times. Qualifying cryo AHF/IFC will be matched to procedure times, to exclude any patient who’s qualifying (only) cryo AHF/IFC was not given intraoperatively. The remaining patients will be considered eligible and will be imported into REDCap (Research Electronic Data Capture) with an automated R script. Any patient who opts-out of the study is marked as such in REDCap manually. REDCap is a free data management software system that is fully supported by the Weill Cornell Medicine Clinical and Translational Science Center (CTSC). It is a tool for the creation of customized, secure data management systems that include web-based data-entry forms, reporting tools, and a full array of security features including user and group based privileges, authentication using the institution’s Lightweight Directory Access Protocol (LDAP system) with a full audit trail of data manipulation and export procedures. Only investigators who have been authorized will have access to the data. REDCap is maintained on CTSC-owned servers that are backed up nightly and support encrypted (SSL-based) connections. Nationally, the software is developed, enhanced, and supported through a multi-institutional consortium led by the Vanderbilt University CTSA.
The following demographic variables will be collected: age, ethnicity, race, gender, underlying diseases, surgery type, medications, and other clinical parameters (see Table 1). Most clinical metrics will be collected via an automated daily export from the institution’s EHR. Metrics that cannot be reliably captured through informatic automation (e.g., thrombotic events) will be collected manually. After obtaining the information, we will remove details that can identify patients personally (deidentify). Study results will be reported in aggregate form only, so that no individuals can be identified. Information will be stored in REDCap to protect patient privacy. Regular manual quality assurance (QA) will be conducted to ensure that no eligible patients are missed with the automated identification method. Ongoing, periodic QA of the demographic and clinical data will be conducted to validate extracted data for accuracy, including ensuring that ineligible patients are not included.
Table 1.
Demographics and clinical variables collected
| Variables collected | Collection method (manual or automated) | |
|---|---|---|
| Demographics | Last name, first name | Automated |
| Medical record number | Automated | |
| Race | Automated | |
| Sex | Automated | |
| Age | Automated | |
| Ethnicity | Automated | |
| Date of birth | Automated | |
| Blood products | Time from cryo order to issue from blood bank (minutes) | Automated |
| Time from cryo order to start of transfusion (minutes) | Automated | |
| Time from OR start time to start of cryo transfusion (minutes) | Automated | |
| Number of cryo units wasted by blood bank that month | Automated | |
| Cryo unit type: | ||
| Traditional cryo | Automated | |
| Pathogen reduced cryo | Automated | |
| Number of units transfused perioperatively, start of anesthesia to 30 days post-op: | ||
| RBCs | Automated | |
| Platelets | Automated | |
| Plasma | Automated | |
| Cryo | Automated | |
| Fibrinogen concentrate | Automated | |
| Number of units transfused perioperatively, start of anesthesia to 24 h post-op: | ||
| RBCs | Automated | |
| Platelets | Automated | |
| Plasma | Automated | |
| Cryo | Automated | |
| Fibrinogen concentrate | Automated | |
| Number of units transfused perioperatively, start of anesthesia to 72 h post-op: | ||
| RBCs | Automated | |
| Platelets | Automated | |
| Plasma | Automated | |
| Cryo | Automated | |
| Fibrinogen concentrate | Automated | |
| Laboratory | Intraoperative (value and date-time of result): | |
| First FIBTEM A10 | Automated | |
| First FIBTEM MCF | Automated | |
| First EXTEM A10 | Automated | |
| First fibrinogen | Automated | |
| First EXTEM CT | Automated | |
| Intraoperative (lowest value and date-time of result): | ||
| Lowest FIBTEM A10 | Automated | |
| Lowest FIBTEM MCF | Automated | |
| Lowest EXTEM A10 | Automated | |
| Lowest fibrinogen | Automated | |
| Lowest EXTEM CT | Automated | |
| Post-operative (value and date-time of result): | ||
| Post-operative fibrinogen most proximal to end of procedure | Automated | |
| Within 24 h after surgery (value and date-time of result): | ||
| Maximum fibrinogen | Automated | |
| Minimum fibrinogen | Automated | |
| Clinical | Surgery name | Automated |
| Surgery date | Automated | |
| OR case ID | Automated | |
| Length of hospital stay | Automated | |
| Length of ICU stay | Automated | |
| Length of OR stay | Automated | |
| Need for ventilator after surgery | Automated | |
| Time on ventilator after surgery | Automated | |
| Estimated blood loss, intraop (mL) | Automated | |
| Volume in drains at 24 h or until removed | Automated | |
| Time from end of bypass pump until end of surgery | Automated | |
| Adverse events: | ||
| Thrombotic events within 7 days after start of surgery | Manual | |
| Need for surgical re-exploration | Manual | |
| Re-exploration OR case ID | Manual | |
| Re-exploration date | Manual | |
| Multiorgan system failure | Manual | |
| Adverse events within 5 days after start of surgery: | ||
| Fevers | Manual | |
| Infections | Manual | |
| Transfusion reaction information | Manual | |
Data will be queried via SQL queries from the R statistical software, and, where necessary, linked and manipulated before storage. Study data will be represented in an R Shiny dashboard (R Foundation, Vienna, Austria) with several tabs to represent new enrollees, patient-level aggregate data, figures and graphs summarizing the cohort, and statistical analyses associated with study primary and secondary endpoints. All aspects of the dashboard will update in real-time to include the latest extracted data. Clinical and blood utilization data will be collected largely with automated queries supplemented by manual data collection.
Data management committee
A research committee meets weekly by video conference during the trial to audit the trial, review any study events, and to perform quality assurance on study data. The committee consists of a research coordinator, data engineer, statistician, principal investigator, transfusion medicine physician co-investigator, and occasionally anesthesiologist co-investigators. Since both products are FDA-approved and considered equivalent and standard of care, and this is a low-risk intervention, an independent data safety and monitoring committee was not required by the IRB. The day-to-day group running the trial includes the Director of Transfusion Medicine, the Clinical Laboratory Director, another transfusion medicine physician, and the study coordinator. Responsibilities include monitoring adherence to the study protocol, the safety of study subjects, and the integrity of study data. The hospital Transfusion Committee, which consists of hematologists, anesthesiologists, transfusion medicine professionals, nursing, and hospital administrators, provides study oversight and discusses the trial quarterly.
Public or patient involvement
There was no public or patient involvement in the design of the protocol since this was a low-risk intervention involving two blood components already approved by the national regulatory body (FDA).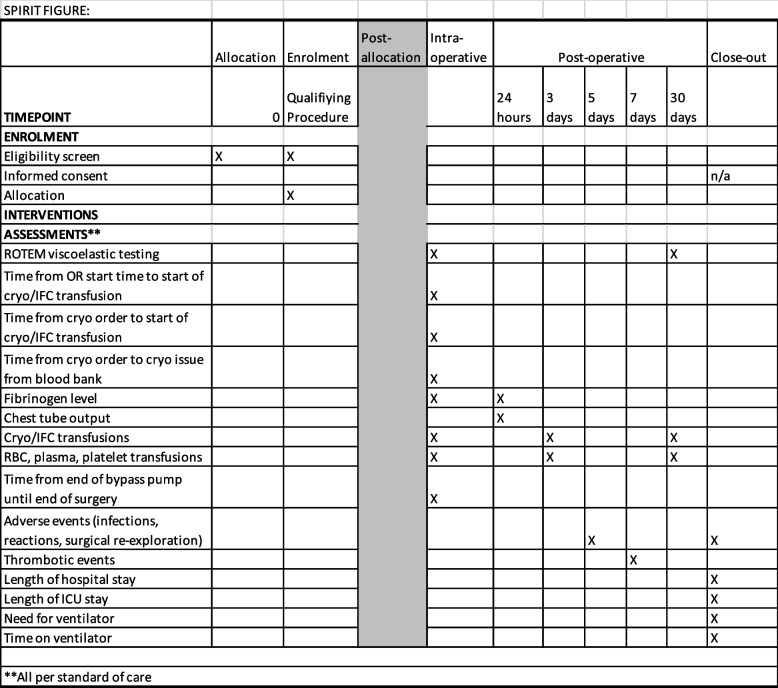
Consent and human research protection
Consent for inclusion in the study will not be obtained at the level of the individual patient before transfusion. IFC and cryo AHF are FDA-approved and considered standard of care. In addition, a minority of patients require fibrinogen replacement and it is not currently possible to adequately predict the need prior to surgery, making it challenging to consent ahead of time. At the time the decision is made to transfuse cryo AHF/IFC, the patient is bleeding and under general anesthesia; thus, there is no opportunity to consent in real time. No additional tests or evaluations will be performed during this study beyond those that are considered standard of care, including treatment of any adverse events related to cryoprecipitate transfusions.
All subjects will be retrospectively provided with a patient information sheet about the study after the transfusion in the operating room. The patient information sheet will be provided to the patient during, or immediately after, the primary hospitalization when eligibility determination is made by study personnel. If a patient wishes to withdraw their data from the study, they will be able to contact the study principal investigator by phone or e-mail. The only data that will be collected will be part of standard care. All decisions about when to transfuse will be made by clinical providers according to their clinical judgment and following institutional protocol, and are unrelated to the study.
Communication about protocol amendments, if any, will be sent to the IRB and if they affect patient safety, will be sent to patients’ listed address in the electronic health record. Any planned or unplanned deviations from the protocol will be reported to the IRB within 24 h of discovery. ClinicalTrials.gov will be updated as necessary.
This study was approved by the Biomedical Research Alliance of NY (BRANY) institutional review board (IRB) (Lake Success, NY) and the Weill Cornell IRB via a partnership with the Weill Cornell Medicine IRB. The trial is posted on ClinicalTrials.gov. ClinicalTrials.gov ID is NCT05711524.
Statistical analysis
Rationale for a cluster randomized trial
Individual patient efficacy clinical trials are useful to establish the clinical efficacy of an intervention amongst a carefully selected population, under optimal conditions, following detailed protocols. Such trials do not address questions about how an intervention, or logistical change, affects clinical practice in the real world.
The surgical care of patients in our hospital is undertaken using standardized procedures that optimize outcomes, such as standard pre-operative assessment and pre- and post-operative care pathways. The cardiac surgery service delivers care through standard institutional policies. The blood bank is tasked with responding to all blood needs throughout the hospital. Time to access is considered the most impactful variable to compare two FDA-approved blood products for fibrinogen replacement in acquired hypofibrinogenemia. Using a pragmatic design randomizing by month eliminates time-consuming randomization by patient and delays in patient care, as well as increasing study population inclusiveness.
The main challenge of a cluster-randomized trial is the need for inflated sample size relative to a non-clustered design for statistical calculations. Individuals within a cluster tend to have a smaller degree of variation compared to the variation between clusters, which is measured statistically by the intra-cluster correlation coefficient (ICC) [25]. If the ICC is greater than 1, then this cluster effect will incorrectly [25] reduce the standard error of the model estimates if it is not properly accounted for in the analysis. This study will use randomization between intervention arms each month with a design known as the cluster randomized trial. This ensures that all research subjects in a given month will receive a particular product and reduces risk of incorrect product use. Each month, the blood bank will be randomized to issue either cryo AHF or IFC; the randomized product will be used as standard institutional policy for the calendar month. At the end of each month, the blood bank is re-randomized to a new standard product that is used for the subsequent calendar month. This design is methodologically rigorous and tests the effect of a change in standard policy as would occur in the clinical setting.
Sample size analysis
The primary endpoint of the study is total number of blood components other than cryo AHF or IFC (RBCs, platelets, plasma) used over the hospital admission within the first 30 days after start of surgery. The goal of the trial is to demonstrate a 20% reduction in this total blood product use for IFC compared to the total used with cryo AHF. To determine this effect size, and the patient variability associated with this outcome, data were collected from over 100 cardiovascular surgery cases at the study center during 6 months in 2021. Because the number of units used was clearly right-skewed and possibly log-normally distributed, the sample size determination was based on the hypothesized ratio of means (effect size) of 0.8. In addition, the coefficient of variation is the key determinant of variability with log-normal data, and this was seen with the recent data to be a value of 1.1.
The cluster in this study is the month in which the surgery is performed. The assumption is made that the intra-cluster correlation of the primary outcome within a given month should be very small, approximately 0.0001. As a result, this would have very little effect on the sample size (the Kish design effect is about 1.01 assuming approximately 100 subjects per month). With 80% power and 5% significance (two-sided), the goal number of subjects per group would be 251. It is possible that the effect size has been overestimated. A sample size re-estimation will be conducted after 60% of the subjects have been included in the trial. The method of Gould [26] will be used to re-evaluate the sample size.
Equivalency between cohorts [12]
In order to evaluate possible patient population clinical variation between the patients transfused with cryo AHF and IFC, a cardiac surgery operative risk score (e.g., STS risk score (STS.org) or EUROSCORE [27]) for each patient will be calculated and utilized to ensure the two groups are similar. In the final analysis, a propensity score will be used as a covariate to adjust for any remaining imbalances.
Evaluation of product superiority
The primary outcome of total number of blood components (RBCs, platelets, plasma) used over the admission and within the first 30 days after surgery will be evaluated using a two-sample t-test on the log-transformed values. If the log-transform does not result in an acceptable comparability to a normal distribution, the Wilcoxon rank-sum test will be used. This will be based on an intent-to-treat paradigm (all subjects randomized will be evaluated and in the group to which they were randomized). However, it is assumed that all of the subjects will be appropriately evaluated for this endpoint.
There are a number of secondary endpoints; the Wilcoxon rank-sum test will be used to evaluate the statistical significance of the majority, including number of cryo AHF/IFC or fibrinogen concentrate products used perioperatively, number of RBC, plasma, and platelet products used perioperatively, time from cryo AHF/IFC order to ready in the blood bank, time from cryo AHF/IFC order to start of infusion, number of cryo AHF/IFC units wasted by blood bank per day during study periods, laboratory measures for cryo AHF/IFC, volume in mediastinal drains, and costs of cryo AHF versus IFC. Fibrinogen level most proximal to end of procedure will be calculated by the Wilcoxon rank-sum test. A log-rank test, particularly if there is any censoring, will be used to evaluate length of stay (OR, ICU, and hospital) and time on ventilator. Many endpoints may also be evaluated after adjustment with selected covariates using a properly selected linear model (depending on the link function required). To control statistical significance for this number of endpoints, the Holm procedure will be used.
Interim analysis
An interim analysis for futility will be conducted on the primary endpoint when approximately 60% of the subjects have completed the trial at 1 year. During the interim analysis, we will decide if the study will continue or stop. This will involve the calculation of conditional power and a potential stopping rule of a value for this calculation of < 10%.
Discussion
Conducting randomized trials in bleeding patients with hypofibrinogenemia is challenging [28]. This study design was utilized to account for the unpredictability of hypofibrinogenemia and bleeding during cardiac surgery. Although the hospital standard is to use a ROTEM algorithm, ROTEM is not performed in all cases, due to limited staffing, lack of training, or lack of time. The ROTEM algorithm may not always be followed exactly. This is due to the timing of testing, the availability of blood products, knowledge of the ordering physician, and severity of bleeding. Cryo AHF/IFC is not always ordered according to hospital protocol, in response to test results indicating hypofibrinogenemia. It is instead pre-emptively ordered earlier during surgery, when bleeding is expected due to the complexity of the surgery or specific characteristics of the patient. This is a potential confounder for two outcomes: product usage and time from order to transfusion. Based on our institution’s experience, orders that appear to be placed pre-emptively, prior to bleeding, will be labeled as such, and analyses will be conducted both with and without patients who received cryo AHF/IFC via pre-emptive orders to compare with the results of patients who only had cryo AHF/IFC ordered after the onset of bleeding. It is not possible to fully differentiate pre-emptive ordering from on-demand ordering at the time of bleeding.
The power of a cluster randomized trial is generally lower than an individually randomized trial. This type of trial may be subject to selection bias, imbalance between study arms, and lack of generalizability.
Trial status
Patient recruitment began on April 1, 2023, and the current IRB protocol version number is 22–04024649 version#2 (July 5, 2023 version). A single protocol amendment was approved to allow patient information sheets to be sent to the patient’s home after discharge if the letter could not be delivered while they were an inpatient. Some patients had very short lengths of stay and delivering the memo prior to discharge was not possible. The projected end of patient recruitment is March 31, 2025, unless futility is determined at the interim analysis.
Supplementary Information
Additional file 1. SPIRIT 2013 Checklist.
Additional file 2. Patient Information Sheet.
Acknowledgements
The authors thank the NewYork-Presbyterian Hospital Transfusion Medicine Service, Anesthesiology Department, and Cardiothoracic Surgery Department for their assistance with this trial.
Patient public involvement
There was no patient public involvement in the process of developing this trial protocol.
Authors’ contributions
MMC is the primary investigator. She conceived the study, led the proposal, and developed the protocol. RAD, TC, NG, AS, and SR contributed to the conceptualization, methodology, and editing of the original draft. SR, AS, SO, TH, NK, AJ, DC, and DB will contribute to the data curation and original drafting. MF, NIG, PP, and CL will contribute to supervision of the study protocol. No professional writers were used for this manuscript or will be used for the final results manuscript.
Funding
This work is supported by Cerus Corporation, who manufactures IFC. The contact information for the study is Dr. Nadia Keltner: NKeltner@cerus.com. Dr. Keltner contributed to the protocol design and protocol manuscript drafting. Cerus will be able to review the final manuscript before submission.
Availability of data and materials
The study results will be published through conferences and scientific publications in medical journals related to transfusion medicine, anesthesiology, or cardiothoracic surgery. Deidentified data will be available for online provision upon reasonable request.
Declarations
Ethics approval and consent to participate
This study protocol has been reviewed and approved by the IRB of Weill Cornell Medicine through a collaboration with the BRANY IRB (Lake Success, NY). The need for consent was waived by the IRB.
Consent for publication
Not applicable.
Competing interests
MMC is a consultant for Cerus Corporation, Octapharma, Haemonetics, and CSL Behring. NK is a full-time employee of Cerus Corporation and owns stock in the company. TH is a consultant for Cerus Corporation, Octapharma, Grifols, and CSL Behring. Remaining authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Christensen MC, Krapf S, Kempel A, von Heymann C. Costs of excessive postoperative hemorrhage in cardiac surgery. J Thorac Cardiovasc Surg. 2009;138(3):687–93. 10.1016/j.jtcvs.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 2.Murphy GJ, Reeves BC, Rogers CA, Rizvi SIA, Culliford L, Angelini GD. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007;116(22):2544–52. 10.1161/CIRCULATIONAHA.107.698977. [DOI] [PubMed] [Google Scholar]
- 3.Hagemo JS, Stanworth S, Juffermans NP, et al. Prevalence, predictors and outcome of hypofibrinogenaemia in trauma: a multicentre observational study. Crit Care. 2014;18(2):R52. 10.1186/cc13798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faraoni D, Willems A, Savan V, Demanet H, De Ville A, Van der Linden P. Plasma fibrinogen concentration is correlated with postoperative blood loss in children undergoing cardiac surgery. A retrospective review. Eur J Anaesthesiol. 2014;31(6):317–26. 10.1097/EJA.0000000000000043. [DOI] [PubMed] [Google Scholar]
- 5.Karkouti K, Callum J, Crowther MA, et al. The relationship between fibrinogen levels after cardiopulmonary bypass and large volume red cell transfusion in cardiac surgery: an observational study. Anesth Anal. 2013;117(1):14. 10.1213/ANE.0b013e318292efa4. [DOI] [PubMed] [Google Scholar]
- 6.Rossaint R, Afshari A, Bouillon B, et al. The European guideline on management of major bleeding and coagulopathy following trauma: sixth edition. Crit Care. 2023;27(1):80. 10.1186/s13054-023-04327-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boer C, Meesters MI, Milojevic M, et al. 2017 EACTS/EACTA guidelines on patient blood management for adult cardiac surgery. J Cardiothorac Vasc Anesth. 2018;32(1):88–120. 10.1053/j.jvca.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 8.Waldén K, Jeppsson A, Nasic S, Backlund E, Karlsson M. Low preoperative fibrinogen plasma concentration is associated with excessive bleeding after cardiac operations. Ann Thorac Surg. 2014;97(4):1199–206. 10.1016/j.athoracsur.2013.11.064. [DOI] [PubMed] [Google Scholar]
- 9.Ranucci M, Pistuddi V, Baryshnikova E, Colella D, Bianchi P. Fibrinogen levels after cardiac surgical procedures: association with postoperative bleeding, trigger values, and target values. Ann Thorac Surg. 2016;102(1):78–85. 10.1016/j.athoracsur.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Pearse BL, Smith I, Faulke D, et al. Protocol guided bleeding management improves cardiac surgery patient outcomes. Vox Sang. 2015;109(3):267–79. 10.1111/vox.12279. (Research Support, Non-U.S. Gov’t). [DOI] [PubMed] [Google Scholar]
- 11.Hinton JV, Xing Z, Fletcher CM, et al. Association of perioperative cryoprecipitate transfusion and mortality after cardiac surgery. Ann Thorac Surg. 2023. 10.1016/j.athoracsur.2023.02.054. [DOI] [PubMed] [Google Scholar]
- 12.INTERCEPT Blood System for Cryoprecipitation [Package Insert]; For the manufacturing of pathogen reduced cryoprecipitated fibrinogen complex. Concord: Cerus Corporation; 2021.
- 13.Kim J-H, Kim K-S, Kwon H-M, et al. Comparison of fibrinogen concentrate and cryoprecipitate on major thromboembolic events after living donor liver transplantation. J Clin Med. 2023;12(23):7496. 10.3390/jcm12237496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cushing MM, Haas T, Karkouti K, Callum J. Which is the preferred blood product for fibrinogen replacement in the bleeding patient with acquired hypofibrinogenemia-cryoprecipitate or fibrinogen concentrate? Transfusion. 2020;60 Suppl 3:S17–23. 10.1111/trf.15614. [DOI] [PubMed] [Google Scholar]
- 15.Downey LA, Andrews J, Hedlin H, et al. Fibrinogen concentrate as an alternative to cryoprecipitate in a postcardiopulmonary transfusion algorithm in infants undergoing cardiac surgery: a prospective randomized controlled trial. Anesthes Anal. 2020;130(3):740–51. 10.1213/ANE.0000000000004384. [DOI] [PubMed] [Google Scholar]
- 16.Callum J, Farkouh ME, Scales DC, et al. Effect of fibrinogen concentrate vs cryoprecipitate on blood component transfusion after cardiac surgery: the FIBRES randomized clinical trial. JAMA. 2019;322(20):1966–76. 10.1001/jama.2019.17312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roy A, Stanford S, Nunn S, et al. Efficacy of fibrinogen concentrate in major abdominal surgery - a prospective, randomized, controlled study in cytoreductive surgery for pseudomyxoma peritonei. J Thromb Haemost. 2020;18(2):352–63. 10.1111/jth.14665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan AW, Tetzlaff JM, Gøtzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586. 10.1136/bmj.e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramirez-Arcos S, Jenkins C, Sheffield WP. Bacteria can proliferate in thawed cryoprecipitate stored at room temperature for longer than 4 h. Vox Sanguinis. 2017;112(5):477–9. 10.1111/vox.12517. [DOI] [PubMed] [Google Scholar]
- 20.Thomson C, Sobieraj-Teague M, Scott D, Duncan E, Abraham S, Roxby D. Extending the post-thaw viability of cryoprecipitate. Transfusion. 2021;61(5):1578–85. 10.1111/trf.16366. [DOI] [PubMed] [Google Scholar]
- 21.Fenderson JL, Meledeo MA, Rendo MJ, et al. Hemostatic characteristics of thawed, pooled cryoprecipitate stored for 35 days at refrigerated and room temperatures. Transfusion. 2019;59(S2):1560–7. 10.1111/trf.15180. [DOI] [PubMed] [Google Scholar]
- 22.Cushing MM, Asmis LM, Harris RM, et al. Efficacy of a new pathogen-reduced cryoprecipitate stored 5 days after thawing to correct dilutional coagulopathy in vitro. Transfusion. 2019;59(5):1818–26. 10.1111/trf.15157. [DOI] [PubMed] [Google Scholar]
- 23.Cushing MM, Fitzgerald MM, Harris RM, Asmis LM, Haas T. Influence of cryoprecipitate, factor XIII, and fibrinogen concentrate on hyperfibrinolysis. Transfusion. 2017;57(10):2502–10. 10.1111/trf.14259. [DOI] [PubMed] [Google Scholar]
- 24.Solomon C, Rahe-Meyer N, Schöchl H, Ranucci M, Görlinger K. Effect of haematocrit on fibrin-based clot firmness in the FIBTEM test. Blood Transfus. 2013;11(3):412–8. 10.2450/2012.0043-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnup SJ, Forbes AB, Kahan BC, Morgan KE, McKenzie JE. Appropriate statistical methods were infrequently used in cluster-randomized crossover trials. J Clin Epidemiol. 2016;74:40–50. 10.1016/j.jclinepi.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 26.Williams GW, Davis RL, Getson AJ, et al. Monitoring of clinical trials and interim analyses from a drug sponsor’s point of view. Stat Med. 1993;12(5–6):481–92. 10.1002/sim.4780120513. [DOI] [PubMed] [Google Scholar]
- 27.Nashef SAM, Roques F, Sharples LD, et al. EuroSCORE II. Eur J Cardiothorac Surg. 2012;41(4):734–44. 10.1093/ejcts/ezs043. discussion 744-745. [DOI] [PubMed] [Google Scholar]
- 28.Cushing MM, Haas T. Fibrinogen concentrate for perioperative bleeding: what can we learn from the clinical trials? Transfusion. 2019;59(11):3295–7. 10.1111/trf.15437. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. SPIRIT 2013 Checklist.
Additional file 2. Patient Information Sheet.
Data Availability Statement
The study results will be published through conferences and scientific publications in medical journals related to transfusion medicine, anesthesiology, or cardiothoracic surgery. Deidentified data will be available for online provision upon reasonable request.