Abstract
The Kaposi's sarcoma-associated herpesvirus (KSHV) K1 gene encodes a polypeptide bearing an immunoreceptor tyrosine-based activation motif (ITAM) that is constitutively active for ITAM-based signal transduction. Although ectopic overexpression of K1 in cultured fibroblasts can lead to growth transformation, in vivo this gene is primarily expressed in lymphoid cells undergoing lytic infection. Here we have examined function of K1 in the setting of lytic replication, through the study of K1 mutants lacking functional ITAMs. Expression of such mutants in BJAB cells cotransfected with wild-type K1 results in dramatic inhibition of K1 signal transduction, as judged by impaired activation of Syk kinase and phospholipase C-γ2 as well as by diminished expression of a luciferase reporter gene dependent upon K1-induced calcium and Ras signaling. Thus, the mutants behave as dominantly acting inhibitors of K1 function. To assess the role of K1 in lytic replication, we introduced these K1 mutants into BCBL-1 cells, a B-cell lymphoma line latently infected with KSHV, and induced lytic replication by ectopic expression of the KSHV ORF50 transactivator. Expression of lytic cycle genes was diminished up to 80% in the presence of a K1 dominant negative mutant. These inhibitory effects could be overridden by tetradecanoyl phorbol acetate treatment, indicating that inhibition was not due to irreversible cell injury and suggesting that other signaling events could bypass the block. We conclude that ITAM-dependent signaling by K1 is not absolutely required for lytic reactivation but functions to modestly augment lytic replication in B cells, the natural reservoir of KSHV.
Infection with Kaposi's sarcoma (KS)-associated herpesvirus (KSHV; also known as human herpesvirus 8) is a key factor in the pathogenesis of KS, a complex endothelial neoplasm commonly seen in human immunodeficiency virus-positive subjects (reviewed in references 8, 14, and 33). KSHV infection precedes the development of the tumor (15) and is strongly predictive of increased KS risk (29). Viral DNA and transcripts are found predominantly in spindle cells, endothelial cells thought to be the principal tumor cell in KS (2, 36). Phylogenetically, KSHV is a member of the lymphotropic (γ) family of herpesviruses; in keeping with this, viral DNA is generally found in CD19+ B cells in lymph nodes and in peripheral blood mononuclear cells in infected individuals (1). KSHV is also associated with two rare B-cell lymphoproliferative disorders, primary effusion lymphoma (PEL) and multicentric Castleman's disease (4, 31, 35).
Like all herpesviruses, KSHV displays both latent and lytic cycles of infection. In KS tumors and in PEL cells, KSHV is found predominantly in a latent state, with transcription limited to a small subset of viral genes and no production of progeny virus particles (31, 36). Lytic infection does occur in a small subset of KS and PEL cells. BCBL-1, a cultured PEL cell line, also displays latent KSHV expression in most cells and represents an in vitro model for KSHV latency (31, 36).Treatment of these cells with phorbol esters (or other stimulants, including calcium ionophores and gamma interferon) induces lytic replication and permits experimental study of reactivation from latency (7, 31)
Although latent KSHV gene expression certainly plays a role in KS and PEL development, accumulating evidence increasingly suggests a role for lytic replication as well. Treatment of AIDS patients with ganciclovir, a drug that blocks lytic but not latent KSHV infection (20), sharply decreases incident KS (28). Moreover, the KSHV viral load in peripheral blood mononuclear cells increases during the progression to clinical KS, indicating a close correlation between enhanced lytic reactivation of KSHV and disease pathogenesis (3, 39). These and other findings suggest that lytic replication may play a more prominent role in KS development than might have been suspected on the basis of tumorigenesis by other herpesviruses. Several models for how lytic reactivation might relate to KS tumorigenesis have recently been proposed (5, 21).
One gene often mentioned as having a potential role in KS pathogenesis is the viral K1 gene, which encodes a type I membrane glycoprotein (22). Its map position directly adjacent to the terminal repeats at the left end of the genome is syntenic with the STP and tip genes of herpesvirus saimiri (HVS), which encode two known transforming proteins of HVS, and the Epstein-Barr virus (EBV) transforming protein LMP-1. Though it has no sequence homology to those proteins, K1 can transform rodent fibroblasts in vitro (albeit at low efficiency) and can restore tumorigenicity to HVS mutants from which STP has been deleted (25). However, K1 behaves kinetically like an early or immediate-early lytic cycle gene, being upregulated during lytic induction of cultured PEL cells but not being blocked by inhibition of viral DNA replication (22). Moreover, K1 transcripts have not been identified by in situ hybridization in latently infected cells (unpublished data of K. Staskus, M. Lagunoff, and D. Ganem). These observations do not accord well with the simple notion that K1 functions as a dominantly acting, cell-autonomous oncogene.
Although its ectodomain is highly variable (save for 12 conserved cysteines), the cytoplasmic tail of K1 is nearly invariant and bears two tyrosines in a motif reminiscent of an immunoreceptor tyrosine-based activation motif (ITAM) (24, 41). ITAMs are found on the cytoplasmic domain of molecules associated with the B- and T-cell antigen receptors (and other immune system receptors) and are required for transducing receptor-ligand binding events to intracellular signals (17, 32). After receptor engagement, both tyrosines in the ITAM are phosphorylated by Src family kinases, allowing the binding of the Syk family kinases to the ITAM (10, 18, 19). Syk family kinase binding leads to many downstream signaling events, including activation of the Ras/mitogen-activated protein kinase cascade culminating in AP-1 activity (38). ITAM signaling also leads to phosphorylation of phospholipase C-γ (PLC-γ), resulting in Ca2+ release, activation of the phosphatase calcineurin, and subsequent nuclear accumulation of the activated NFATc transcription factor (38). It is likely that other signaling pathways are also engaged, directly or indirectly.
ITAMs are generally silent in the ground state, becoming activated only upon receptor cross-linking, and the K1 ITAM behaves in this fashion in heterologous fusion proteins (23, 24). However, the wild-type (WT) K1 molecule is constitutively activated for ITAM signaling, even in the absence of exogenous cross-linking ligands (23). This is likely due to the ability of the K1 ectodomain to homomultimerize, though the presence of a ubiquitous cell surface ligand for K1 has not been formally excluded (23). Signaling depends upon the ITAM: mutation in its conserved tyrosine residues strongly inhibits this activity and deletions across the ITAM abolish signaling (23).
What is the role of K1 signaling in the viral life cycle? Since K1 appears to be a lytic cycle gene, we hypothesized that its signaling function may play an important role in lytic viral growth (22). To test this notion, we have generated dominant negative mutant versions of K1 and examined their effect on viral replication in induced BCBL-1 cells. Here we show that inhibition of K1 function by this strategy reduces lytic reactivation of KSHV at a relatively early stage, prior to the onset of viral DNA synthesis. Since ITAM signaling functions primarily in lymphoid cells, our findings suggest that K1 may have evolved to augment lytic reactivation in B cells.
MATERIALS AND METHODS
Cells.
BCBL-1 is an established KSHV-infected, EBV-free, human B-cell line that is maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum, penicllin, streptomycin, glutamine, sodium bicarbonate, and β-mercaptoethanol. More than 95% of BCBL-1 cells maintain KSHV in the latent state, with the remaining few percent undergoing lytic replication (31, 36). Raji and BJAB cells are also human B cells, EBV infected and uninfected, respectively, and are maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum, penicillin, streptomycin, and glutamine.
Plasmids and antibodies.
All of the K1-containing plasmids were described previously (23). Briefly, Flag-K1 contains the WT sequences of K1 from amino acid 19 (immediately after the predicted signal sequence) to the stop codon cloned downstream of the sequences that encode the CD8 signal sequence linked to the Flag epitope, all under the control of the EF-1α promoter in the pCDEF3 backbone (16). The Y>F mutants have exchanged one or both of the tyrosines in the ITAM, amino acids 271 and 282, for phenylalanines. K1ΔC is truncated after amino acid 266. The ORF50 and 50ΔSTAD expression vectors were described previously (26, 27). The NFAT-luc plasmid containing three NFATc/AP-1 sites and the AP-1–luc plasmid containing seven AP-1 sites from the interleukin 2 promoter were described previously, as were the Syk and the RasN17 expression vectors (9, 13, 34, 40). Finally, green fluorescent protein (GFP) was expressed under the contol of the the cytomegalovirus (CMV) immediate-early promoter. The antibody to the Flag epitope (M2) was purchased from Sigma (St. Louis, Mo.), the 4G10 antibody directed against phosphotyrosine-containing proteins was purchased from Upstate Biotechnology (Lake Placid, N.Y.), and the rabbit antisera recognizing PLC-γ2 were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.). The mouse monoclonal antibody recognizing the Syk tyrosine kinase was a kind gift of D. Chu and A. Weiss. The mouse monoclonal antibody recognizing ORF59 was a kind gift of L. Wu and B. Forghani.
Luciferase assays.
BJAB cells (2 × 107) were electroporated in serum-free media with the indicated plasmids at 960 μF and 250 V. For the signal transduction assays (Fig. 1), 20 μg of the test plasmid and 15 μg of NFAT-luc plasmid were added. For the dominant negative signal transduction assays, 4 μg of Flag-K1 was added, as was 15 μg of NFAT-luc with 0, 8, 16, or 32 μg of K1ΔC (so equivalent amounts of DNA in each transfection empty vector were added as needed). Twenty-four hours after transfection, cells were counted and 105 live cells were added to a single well in a 96-well plate. Three wells from each transfection were unstimulated, while three had tetradecanoyl phorbol acetate (TPA) and ionomycin added to them to stimulate AP-1 and NFATc for transfection efficiency controls. Six hours after stimulation, cells were lysed and luciferase activity was read in a 96-well plate luminometer. Each bar is an average of at least three experiments, each measured in triplicate.
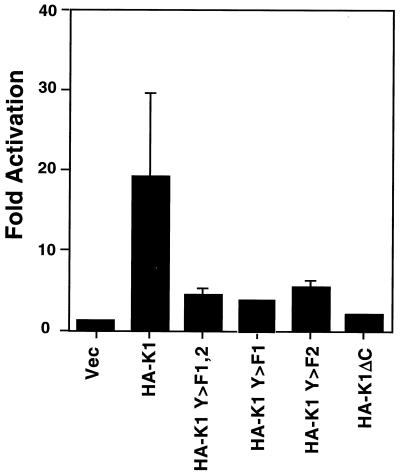
FIG. 1.
K1 induces signal transduction in BJAB, an EBV-negative human B-cell line. BJAB cells were electroporated with a plasmid expressing the indicated K1 or K1 mutant expression vector and a plasmid with luciferase under the control of three composite NFATc/AP-1 sites from the interleukin 2 promoter. Luciferase activity is plotted as fold activation over the activity of the empty vector transfection, which is set to 1. The bars represent an average of three separate experiments, each measured in triplicate. Vec, vector.
Immunoprecipitations and immunoblotting.
Raji cells (6 × 107 in three cuvettes) were electroporated at 960 μF and 250 V in serum-free media. No Flag-K1, 10 μg of Flag-K1, or 10 μg of Flag-K1 with 20 μg of HA-K1 Y>F1,2 was transfected with 5 μg of a Syk expression vector per cuvette. Cells were harvested 18 h after electroporation and were lysed in 1% Triton X-100 lysis buffer (1% Triton X-100, 10 mM Tris [pH 7.5], 150 mM NaCl, 1 mM EDTA, 1 mM NaVO4, and a protease inhibitor cocktail). The pellet was removed after centrifugation, and 4G10 antibody was added to the supernatants for 1 h, followed by protein A/G agarose for 2 to 6 h. The agarose was spun down (the supernatants were saved for immunoblotting) and then washed five times in the Triton X-100 lysis buffer. Immunoprecipitations and supernatants were separated by sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis (SDS–10% PAGE); electroblotted to a polyvinylidene difluoride membrane; blocked overnight with 5% milk in Tris-buffered saline containing 0.1% Tween 20; blotted with primary murine monoclonal antibody to the Flag epitope, the Syk tyrosine kinase, or rabbit polyclonal sera raised to PLC-γ2; washed; blotted with secondary anti-mouse or anti-rabbit antibody conjugated to horseradish peroxidase; and detected by chemiluminescence as recommended by the manufacturer of enhanced chemiluminescence (Amersham Pharmacia).
BCBL-1 lytic replication assay.
BCBL-1 cells (2 × 107) growing to log phase were electroporated (960 μF, 210 V) with 10 μg of the ORF50 expression vector; 8 μg of the CMV-GFP plasmid; and 20 μg of empty vector, K1 Y>F1,2, K5, the 50ΔSTAD expression vector, or a RasN17 expression plasmid. When used, FK506 was added to a concentration of 100 ng/ml immediately after electroporation, while 20 ng of TPA/ml was added 8 h after electroporation to allow time for expression of plasmids first. Forty hours after electroporation, cells were washed in phosphate-buffered saline (PBS); fixed in 4% paraformaldehyde; washed in PBS with 1% bovine serum albumin, 0.02% saponin (to permeabilize the cells), and 0.1% sodium azide; incubated with mouse monoclonal antibody to ORF59 in the PBS-saponin buffer; washed; incubated with secondary goat anti-mouse phycoerythrin antibody; washed; and run on a Facscalibur flow cytometer. Gating on only live cells, one laser filter was set to measure GFP while the other was set for phycoerythrin. As controls, cells transfected without GFP were used to mark untransfected cell limits, while cells that were transfected with GFP without the ORF50 expression plasmid were used to mark the limits of lytic versus latent cells.
RESULTS
K1 induces signaling in BJAB cells.
In earlier work, it was shown that K1 signaling is active in avian B cells and in the human B-cell line Raji, which is also latently infected with EBV (23). Because EBV also encodes modifiers of ITAM signaling, we sought to examine the ability of K1 to induce signal transduction in a human B-cell line (BJAB) that lacks EBV infection. Accordingly, an expression vector that uses the strong EF-1α promoter to direct expression of a K1 open reading frame tagged with a hemagglutinin epitope (HA-K1) was transfected into BJAB cells together with a luciferase reporter driven by three composite NFATc/AP-1 sites. Four mutants of K1 were also tested: a C-terminal truncation of K1(K1ΔC) and K1 mutants with either tyrosine or both tyrosines in the ITAM sequence mutated to phenylalanine (K1Y>F1, K1 Y>F2, and K1 Y>F1,2). Cells were transfected by electroporation, were incubated for 24 h, and were then either left unstimulated or were stimulated (as a control for transfection efficiency) for 6 h with TPA and ionomycin, which activate AP-1 and NFAT, respectively. Luciferase activity was then measured; the luciferase activity of cells transfected with the control vector was set to 1, and the fold activation observed in cells transfected with the K1 plasmids was calculated (Fig. 1). The WT HA-K1 expression vector induced nearly 20-fold greater activation of luciferase than that of the empty vector, while the K1 mutant expression vectors displayed strong reductions in luciferase activity. As in Raji cells, the phenotype was strongest with the deletion mutant K1ΔC. These results indicate that in human B cells, K1 signaling behaves similarly in the presence or absence of EBV (23).
K1ΔC blocks K1 signaling and phosphorylation.
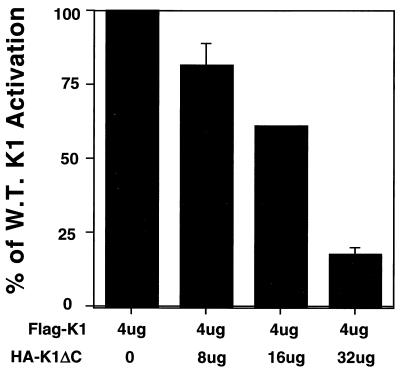
Since K1's multimerization domain is extracellular (23) and thus distinct from its (cytosolic) signaling domain, we reasoned that mutants bearing only one such domain—like K1ΔC and the K1 Y>F mutants—might act as transdominant inhibitors of WT K1 signaling. To test this, BJAB cells were transfected with the NFATc/AP-1 luciferase plasmid and a fixed amount of the WT Flag-K1 expression vector and were also tagged with the Flag epitope linked to the the CD8 signal sequence (23) and increasing amounts of a hemagglutinin-tagged K1ΔC expression vector. Empty vector plasmid DNA was used to adjust the transfections such that the same amount of DNA was used in each transfection, and 24 h after transfection, cells were lysed and assayed for luciferase activity. The luciferase activity of cells transfected with Flag-K1 and empty vector was set to 100%, and the levels of luciferase activity in cells cotransfected with K1ΔC were expressed relative to this value. Figure 2 shows that there was a dose-dependent decrease in luciferase activity in the presence of K1ΔC, indicating that K1ΔC could block WT K1 signaling. The cell surface quantity of Flag-K1 was equivalent or even slightly higher in the cells transfected with two- and fourfold the amount of K1ΔC and was slightly lower in the cells transfected with eightfold-excess K1ΔC, as measured by cell surface staining and flow cytometry analysis (data not shown). Nearly identical results were seen when K1ΔC was replaced with the K1Y>F mutants (data not shown).
FIG. 2.
A K1 mutant with a deletion of the C-terminal 23 amino acids (including the entire ITAM) inhibits WT K1 signaling in a dose-dependent fashion. BJAB cells were electroporated with 4 μg of the Flag-K1 expression plasmid and an increasing amount in micrograms (two-, four-, and eightfold more than the WT plasmid) of the K1ΔC expression vector. The luciferase activity of cells with no K1ΔC was set at 100%, and the percent decrease in signaling when the K1ΔC plasmid is added is plotted. Each bar represents an average of three experiments, each measured in triplicate.
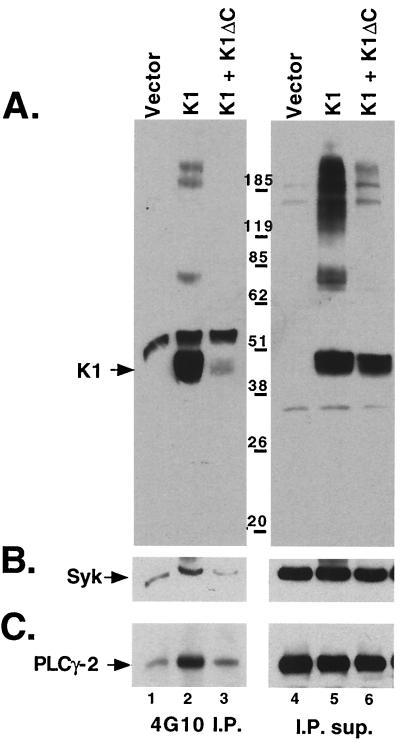
K1 is tyrosine phosphorylated in vivo, and expression of K1 leads to hyperphosphorylation of the Syk tyrosine kinase as well as PLC-γ2 in B cells (23). To examine how K1ΔC blocked K1 signaling, Raji cells were transfected with small amounts of a Syk kinase expression vector along with empty vector, Flag-K1, or Flag-K1 plus a vector expressing untagged K1ΔC. Eighteen hours after transfection, cell extracts were immunoprecipitated with an antiphosphotyrosine antibody (4G10), fractionated by SDS-PAGE, and first immunoblotted with anti-Flag epitope antibody (FLAG M2). As expected, in the empty vector-transfected cells, no K1 was immunoprecipitated by 4G10, while Flag-tagged WT K1 was efficiently precipitated by 4G10 in the Flag-K1-expressing cells, indicating that it had become tyrosine phosphorylated (Fig. 3A). By contrast, in the presence of K1ΔC a dramatic reduction of phosphorylated Flag-K1 was detected (Fig. 3A), even though there was slightly higher surface expression of Flag-K1 in these cells, as determined by flow cytometry (data not shown). Very similar results were seen when K1Y>F1,2 was used in place of K1ΔC (data not shown). This clearly indicates that the K1 dominant negative mutants prevent full-length WT K1 from being phosphorylated.
FIG. 3.
K1ΔC inhibits phosphorylation of WT K1 and K1-induced phosphorylation of the Syk tyrosine kinase and of PLC-γ2. All panels are immunoblots of 4G10 (antiphosphotyrosine) immunoprecipitations from Raji cells transfected with empty vector (lane 1), Flag-K1 expression plasmid (lane 2), and the same amount of the Flag-K1 expression plasmid with double the amount of the K1ΔC expression plasmid (lane 3). The immunoprecipitations (I.P.) and their supernatants (I.P. sup.; lanes 4 to 6) were separated by SDS-PAGE and transferred to a polyvinylidene difluoride membrane. Membranes were immunoblotted with Flag M2 antibody recognizing the Flag-tagged K1 protein (A), anti-Syk antibody (B), and anti-PLC-γ2 (C). K1 is indicated with an arrow.
Expression of K1 leads to hyperphosphorylation of the Syk tyrosine kinase as well as of PLC-γ2 (23). To examine the effects of the K1 dominant negative mutants on downstream activation events induced by K1, we probed the same immunoblots shown in Fig. 3A with an anti-Syk antibody and (subsequently) with an anti-PLC-γ2 antibody (Fig. 3B and C). As previously seen, in control Raji cells small amounts of Syk and PLC-γ2 were immunoprecipitated by the 4G10 antibody, while significantly larger amounts were immunoprecipitated by 4G10 in the presence of the Flag-K1 expression. By contrast, coexpression of K1ΔC with WT K1 nullified this increase, resulting in levels of Syk and PLC-γ 2 phosphorylation that were near background levels. Similar results were also seen with a K1 Y>F1,2 expression vector in place of the K1ΔC expression plasmid (not shown).
A K1 dominant negative mutant reduces lytic replication.
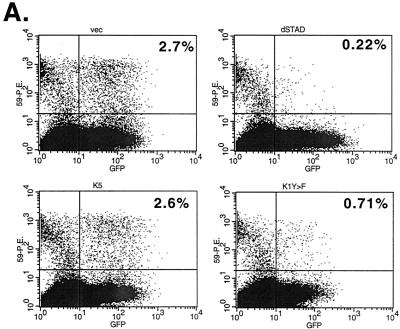
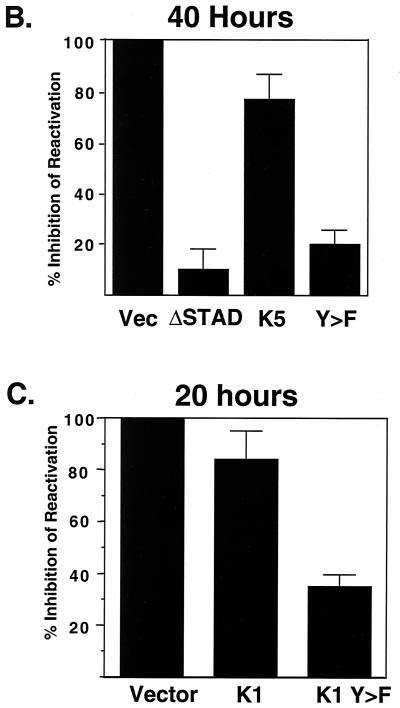
To examine the role of K1 dominant negative mutants on lytic replication of KSHV, we employed an assay based on reactivation of lytic KSHV replication from latency in the BCBL-1 cell line. Lytic KSHV reactivation was induced by ectopic expression of the viral transactivator ORF50, the key regulator of the latent-to-lytic switch (27, 37). Reactivation was assessed by measurement of the accumulation of viral proteins specific to the early (ORF59) and late (K8.1) phases of the lytic cycle, as judged by flow cytometry. Specifically, BCBL-1 cells were electroporated with a CMV-GFP expression vector (to mark transfected cells), an ORF50 expression vector to induce lytic replication, and a plasmid encoding a K1 dominant negative mutant (or control plasmids). Forty or 20 h posttransfection, cells were fixed in 4% paraformaldehyde for 30 min, washed and permeabilized in 0.02% saponin, and stained with a mouse monoclonal anti-ORF59 antibody. ORF59 expresses the KSHV homolog of other herpesvirus polymerase accessory proteins and is a nuclear protein that is expressed with delayed early kinetics (6). The cells were then incubated with phycoerythrin-labeled anti-mouse secondary antibody to label the BCBL-1 cells that were undergoing lytic replication. Cells were then examined by flow cytometry, by looking at GFP expression in one laser filter and the ORF59-phycoerythrin expression in the other. As seen in Fig. 4, approximately 2 to 5% of the cells express GFP; of these, 2 to 4% express ORF59, indicating ORF50-mediated reactivation. As a control, the test plasmid was replaced with a dominant negative mutant of ORF50, 50ΔSTAD, which was previously shown to efficiently and specifically block ORF50-induced reactivation of KSHV in BCBL-1 cells (26). As expected, very few transfected BCBL-1 cells (0.1 to 0.2%) underwent lytic replication in the presence of 50ΔSTAD. When the K1 dominant negative mutant expression plasmids were transfected in this system, there was a decrease in the number of BCBL-1 cells undergoing lytic replication, with only 0.5 to 1% of the transfected cells expressing detectable ORF59 protein. As a control for the specificity of this inhibition, we also examined the effects of similar overexpression of the viral K5 protein. Like K1, K5 is a membrane protein produced early in lytic infection that accumulates principally in the endoplasmic reticulum (11). As shown in Fig. 4, K5 expression only slightly reduced lytic reactivation. To allow assessment of the magnitude and reproducibility of these effects, a summary of five different experiments is shown in Fig. 4B. The percentage of transfected cells expressing ORF59 in the control transfected cells was set at 0% inhibition, and the percent inhibition by different expression plasmids was plotted. K1 Y>F1,2 inhibited approximately 80% of lytic induction by ORF50. Interestingly, overexpression of WT K1 also produced a milder (twofold) inhibition of lytic reactivation. This may indicate some nonspecific toxicity of K1 expression but more likely indicates that prolonged constitutive signaling by K1 is associated with desensitization of the signaling pathway. In support of this, K1 had little effect on KSHV reactivation in this assay when the cells were harvested at 20 to 24 h after transfection, while K1 Y>F still had a significant effect at this time point (Fig. 4C). At 40 h postinfection, the K1 dominant negative mutant showed similar effects on another marker of lytic reactivation, K8.1, an envelope protein expressed at late times postreactivation (however, the overall number of live cells expressing K8.1 was lower than the number of live cells expressing ORF59, making this a less robust assay for the inhibitory activity of the mutants; data not shown).
FIG. 4.
K1 Y>F1,2 inhibits reactivation of KSHV in BCBL-1 cells. (A) Primary flow cytometry data from BCBL-1 cells transfected with an ORF50 expression vector to induce reactivation; a GFP expression vector to mark the transfected cells; and either empty vector, 50ΔSTAD, a K5 expression vector, or a K1Y>F1,2 expression vector. Cells were fixed and stained for flow cytometry 40 h after electroporation. The x axis is set for GFP detection, while the y axis is set for ORF59 antibody conjugated to phycoerythrin. Each dot in the right quadrants of each panel represents a transfected cell expressing GFP, the lower right being latent and the upper right indicating BCBL-1 cells undergoing lytic replication. The upper left quadrant represents spontaneous reactivation in untransfected cells. The percentage in the upper right quadrant is the percentage of transfected cells that are undergoing lytic replication. (B) The histogram represents a summary of five to seven experiments done as for panel A, 40 h after transfection. The level of reactivation in cells transfected with ORF50, GFP, and empty vector is set at 100%, and the relative level of reactivation in cells transfected with 50ΔSTAD (8%), K5 (90%), and K1Y>F1,2 (21%) compared to that for vector is plotted. (C) Summary of three experiments performed and plotted as for panel B except that the cells were harvested 20 h after transfection.
K1 Y->F-induced block in lytic replication can be overcome by TPA.
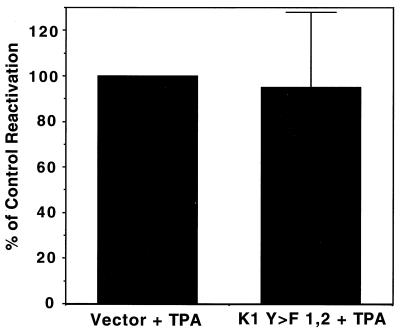
To further exclude that the block in lytic replication by the K1 dominant negative mutant was due to general toxicity to the cell, we replaced the ORF50 expression plasmid (the reactivation inducer) with TPA (31). TPA has pleiotropic effects on B cells. It is known to induce AP-1 activity through activation of Ras pathways among other signal transduction effects and can also induce AP-1 activity in BJAB cells transfected with K1 dominant negative mutants (data not shown). Therefore, TPA not only induces reactivation of KSHV but also many of the same signal transduction pathways that K1 induces, though generally downstream of points at which K1 induces these pathways. Therefore, TPA should overcome the block in lytic reactivation induced by the K1 dominant negative mutants. This was tested by electroporating BCBL-1 cells with a CMV-GFP plasmid and either a K1 dominant negative plasmid or a control vector. Eight hours after transfection, the cells were stimulated with TPA. Forty hours posttransfection, cells were fixed and stained as before for ORF59 expression. There was no significant difference between the amount of reactivation induced by the empty vector and the K1 Y>F1,2 expression plasmid (Fig. 5). This clearly indicates that the K1 dominant negative mutant is inhibiting replication through a pathway that can be bypassed by TPA treatment and also excludes that the inhibition was due to generalized toxicity, since cells expressing the K1 mutant retain their ability to support lytic replication in the presence of TPA.
FIG. 5.
TPA overcomes the block in lytic replication indcued by K1Y>F1,2. BCBL-1 cells were electroporated as done before, with a GFP expression vector and with or without a K1 Y>F1,2 expression vector. Eight hours after electroporation, TPA was added to the cells; after 40 h, flow cytometry was done as for Fig. 4. The level of induction of the empty vector-transfected cells was set at 100%, and the level of K1 Y>F1,2 was compared.
FK506 and RasN17 do not block reactivation.
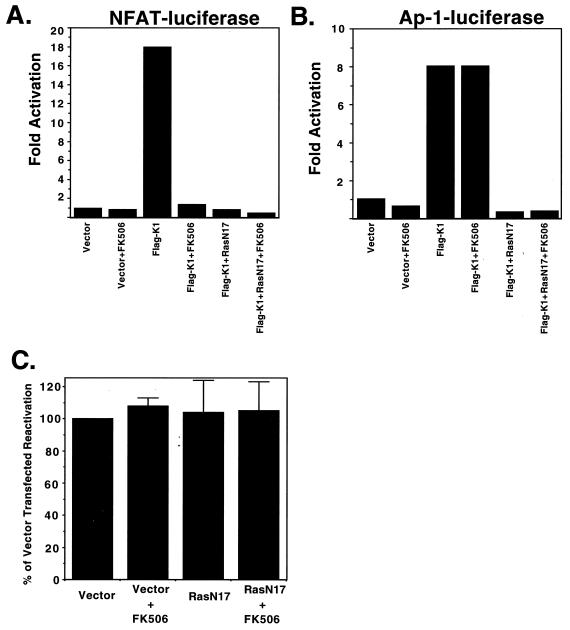
Our assay of ITAM signaling by K1 involves activation of a reporter plasmid harboring composite AP-1/NFATc binding sites and reflects the ability of ITAM signaling to activate both AP-1 and NFATc. Many stimuli are known to activate AP-1, including the Ras–Raf–mitogen-activated protein kinase pathway; NFATc is typically activated by calcineurin, a phosphatase activated by the Ca2+ transient produced by PLC-γ activity. In BJAB cells, FK506, an inhibitor of calcineurin, is able to block calcium-induced NFAT activation (Fig. 6A) but not AP-1 activation induced by WT K1 expression (Fig. 6B). RasN17 is a dominant negative mutant of Ras that blocks activation of AP-1 activity induced by K1 in transfected BJAB cells (Fig. 6B). Next, both inhibitors were used in the previously described reactivation assay in an effort to elucidate the pathway of K1 signaling that is necessary for lytic replication. Transfecting the RasN17 expression plasmid along with CMV-GFP and the ORF50 expression vector had no effect on the number of cells expressing markers of lytic replication, compared to the number of cells transfected with ORF50 and GFP expression plasmids alone (Fig. 6C). Addition of FK506 to BCBL-1 cells transfected with CMV-GFP and ORF50 also had no effect on lytic replication (Fig. 6C). FK506 was also added to the BCBL-1 cells transfected with RasN17, CMV-GFP, and the ORF50 expression vector, but again there was no effect on the number of cells undergoing lytic replication (Fig. 6C). At a minimum, this indicates that neither calcineurin nor Ras is a critical mediator of the K1-induced augmentation of lytic KSHV growth.
FIG. 6.
RasN17 and FK506 inhibit K1 signaling in BJAB cells but do not block KSHV reactivation in BCBL-1 cells. BJAB cells were transfected either with 3× NFAT/AP-1 luciferase (A) or with 7× AP-1 luciferase (B) and either empty vector, Flag-K1, RasN17, or Flag-K1 combined with RasN17. FK506 was added to half the cells immediately after electroporation. Luciferase activity is plotted as fold activation of luciferase activity over the empty vector-transfected cells. (C) BCBL-1 cells were transfected with ORF50, pCMV-GFP, and either empty vector or a RasN17 expression vector, and FK506 was added to half of each transfection. Flow cytometry was done as for Fig. 4. The percentage of transfected cell reactivation of vector-transfected and untreated cells was set to 100%, and the relative level of other conditions was plotted.
DISCUSSION
Reactivation of KSHV from its latent reservoir in lymphocytes is critical for viral dissemination within and between hosts and is suspected of playing an important role in the pathogenesis of KS (see above). The viral ORF50 transactivator is the key initiator of this reactivation (26, 27, 37); presumably, the cytokines and other signals thought to trigger lytic reactivation act to induce its expression, which is normally extinguished in latency. In earlier work (27) it was shown that K1 expression cannot by itself initiate reactivation. Here, through the use of dominant negative mutants of K1, we show that the protein may nonetheless play a role in this process. Inhibition of K1 signaling reproducibly generated a discernible but incomplete block to lytic cycle gene expression, with the number of cells undergoing lytic replication being reduced by 75 to 80% from that observed in the absence of K1 dominant negative mutants. Since the delayed-early marker ORF59 is affected by a K1 signaling blockade, we infer that the location of the block is early in the viral replication cascade, consistent with the expression of K1 mRNA early in the replicative cycle (22). This modest decrease in replication per single replicative cycle would likely result in a larger decrease in the growth of the virus upon serial passage. However, we emphasize that the block in lytic replication induced by the K1 dominant negative mutant is not complete. This leads us to infer that K1 signaling may not be an indispensable core function in viral replication but rather an accessory amplifier of lytic growth. This interpretation is consistent with earlier findings that in KS tumors, lytic reactivation can also be observed in a small subset of endothelial (spindle) cells (30, 36). Such cells lack Syk and are not expected to support ITAM signaling; in fact, we have been unable to detect upregulation of an AP-1/NFATc-dependent reporter gene in a cultured KS spindle cell line (SLK) cotransfected with WT K1 expression vectors (E. Jaehnig, M. Lagunoff, and D. Ganem, unpublished data). All of this is consistent with the view that K1 function is not essential for replication but enhances it in cells (e.g., B cells) that are competent for K1 signaling. Since B cells are the primary reservoir of KSHV in the human host, it makes sense that viral evolution should have selected for such a function.
The endogenous levels of K1 expression in virus-infected cells are very low (22). We believe that this is because sustained high levels of K1 expression inhibit K1 ITAM signaling via desensitization of the pathway. Similar results have been reported by others for the related R1 protein of the rhesus radinovirus (12). Indeed, when we overexpressed WT K1 in ORF50-reactivated BCBL-1 cells, a similar impact on lytic gene expression was observed—ORF59 levels were unchanged at 24 h but declined by 45% at 48 h posttransfection (Fig. 4C). Clearly, viral evolution has titrated K1 levels to those optimal for enhanced replication in permissive cells.
Although we have here dwelt mainly upon K1's function in vegetative viral growth, our results do not exclude other roles for K1 in KSHV biology. For example, it is possible that K1 signaling can induce expression of host genes or activation of host signaling molecules that might influence KS pathogenesis without directly affecting viral growth. For instance, K1 might upregulate the export of paracrine mediators of inflammation and angiogenesis, two key features of KS histopathology. If so, K1 could play an important role in KS pathogenesis, in a fashion similar to that posited for the viral G protein-coupled receptor (5, 21). Such non-cell-autonomous functions of K1 would not have been detected in our experiments.
The details of the signaling cascade underlying K1's stimulatory action remain to be elucidated. The ability of TPA stimulation to overcome the block imposed by K1 dominant negative mutants suggests either that phorbol esters can activate similar signaling pathways distal to K1's site(s) of action or that other signaling pathways activated by TPA can substitute for this stimulation. Similar statements apply to the observed failure of Ras and calcineurin inhibition to affect KSHV reactivation. The fact that RasN17 and FK506 together block AP-1/NFATc-luciferase activation by K1 in BJAB cells but do not inhibit KSHV reactivation in BCBL-1 cells indicates that either (i) these reagents were less efficient inhibitors in BCBL-1 cells; (ii) K1 can activate these transcription factors by other pathways in PEL cells; or (iii) other targets of K1 signaling are critical for the upregulation of KSHV replication (e.g., NF-κB, which can also be activated by K1 in BJAB cells (M. Lagunoff, unpublished). If the last hypothesis is correct, then the identification of those K1-sensitive targets may provide additional clues to the molecular basis of KSHV lymphotropism.
ACKNOWLEDGMENTS
We thank Laurent Coscoy for insightful discussion.
M.L. is supported by a special fellowship from the Leukemia and Lymphoma Society (formerly Leukemia Society of America). D.M.L. is supported by a fellowship from the Irvington Institute.
REFERENCES
- 1.Ambroziak J A, Blackbourn D J, Herndier B G, Glogan R G, Gullet J H, McDonald A R, Lennette E T, Levy J A. Herpesvirus-like sequences in HIV-infected and uninfected Kaposi's sarcoma patients. Science. 1995;268:582–583. doi: 10.1126/science.7725108. [DOI] [PubMed] [Google Scholar]
- 2.Boshoff C, Schulz T F, Kennedy M M, Graham A K, Fisher C, Thomas A, McGee J O, Weiss R A, O'Leary J J. Kaposi's sarcoma-associated herpesvirus infects endothelial and spindle cells. Nat Med. 1995;1:1274–1278. doi: 10.1038/nm1295-1274. [DOI] [PubMed] [Google Scholar]
- 3.Campbell T B, Borok M, Gwanzura L, MaWhinney S, White I E, Ndemera B, Gudza I, Fitzpatrick L, Schooley R T. Relationship of human herpesvirus 8 peripheral blood virus load and Kaposi's sarcoma clinical stage. AIDS. 2000;14:2109–2116. doi: 10.1097/00002030-200009290-00006. [DOI] [PubMed] [Google Scholar]
- 4.Cesarman E, Chang Y, Moore P S, Said J W, Knowles D M. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS- related body-cavity-based lymphomas. N Engl J Med. 1995;332:1186–1191. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- 5.Cesarman E, Mesri E A, Gershengorn M C. Viral G protein-coupled receptor and Kaposi's sarcoma: a model of paracrine neoplasia? J Exp Med. 2000;191:417–422. doi: 10.1084/jem.191.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan S R, Bloomer C, Chandran B. Identification and characterization of human herpesvirus-8 lytic cycle-associated ORF 59 protein and the encoding cDNA by monoclonal antibody. Virology. 1998;240:118–126. doi: 10.1006/viro.1997.8911. [DOI] [PubMed] [Google Scholar]
- 7.Chang J, Renne R, Dittmer D, Ganem D. Inflammatory cytokines and the reactivation of Kaposi's sarcoma-associated herpesvirus lytic replication. Virology. 2000;266:17–25. doi: 10.1006/viro.1999.0077. [DOI] [PubMed] [Google Scholar]
- 8.Chang Y, Moore P S. Kaposi's sarcoma (KS)-associated herpesvirus and its role in KS. Infect Agents Dis. 1996;5:215–222. [PubMed] [Google Scholar]
- 9.Chu D H, Spits H, Peyron J F, Rowley R B, Bolen J B, Weiss A. The Syk protein tyrosine kinase can function independently of CD45 or Lck in T cell antigen receptor signaling. EMBO J. 1996;15:6251–6261. [PMC free article] [PubMed] [Google Scholar]
- 10.Clark M R, Campbell K S, Kazlauskas A, Johnson S A, Hertz M, Potter T A, Pleiman C, Cambier J C. The B cell antigen receptor complex association of Ig-α and Ig-β with distinct cytoplasmic effectors. Science. 1992;258:123–126. doi: 10.1126/science.1439759. [DOI] [PubMed] [Google Scholar]
- 11.Coscoy L, Ganem D. Kaposi's sarcoma-associated herpesvirus encodes two proteins that block cell surface display of MHC class I chains by enhancing their endocytosis. Proc Natl Acad Sci USA. 2000;97:8051–8056. doi: 10.1073/pnas.140129797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Damania B, DeMaria M, Jung J U, Desrosiers R C. Activation of lymphocyte signaling by the R1 protein of rhesus monkey rhadinovirus. J Virol. 2000;74:2721–2730. doi: 10.1128/jvi.74.6.2721-2730.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feig L A, Cooper G M. Inhibition of NIH 3T3 cell proliferation by a mutant ras protein with preferential affinity for GDP. Mol Cell Biol. 1988;8:3235–3243. doi: 10.1128/mcb.8.8.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ganem D. KSHV and Kaposi's sarcoma: the end of the beginning? Cell. 1997;91:157–160. doi: 10.1016/s0092-8674(00)80398-0. [DOI] [PubMed] [Google Scholar]
- 15.Gao S J, Kingsley L, Hoover D R, Spira T J, Rinaldo C R, Saah A, Phair J, Detels R, Parry P, Chang Y, Moore P S. Seroconversion to antibodies against Kaposi's sarcoma-associated herpesvirus-related latent nuclear antigens before the development of Kaposi's sarcoma. N Engl J Med. 1996;335:233–241. doi: 10.1056/NEJM199607253350403. [DOI] [PubMed] [Google Scholar]
- 16.Goldman L A, Cutrone E C, Kotenko S V, Krause C D, Langer J A. Modifications of vectors pEF-BOS, pcDNA1 and pcDNA3 result in improved convenience and expression. BioTechniques. 1996;21:1013–1015. doi: 10.2144/96216bm10. [DOI] [PubMed] [Google Scholar]
- 17.Irving B, Weiss A. The cytoplasmic domain of the T cell receptor ζ chain is sufficient to couple to receptor-associated signal transduction pathways. Cell. 1991;64:891–901. doi: 10.1016/0092-8674(91)90314-o. [DOI] [PubMed] [Google Scholar]
- 18.Irving B A, Chan A C, Weiss A. Functional characterization of a signal transducing motif present in the T cell receptor ζ chain. J Exp Med. 1993;177:1093–1103. doi: 10.1084/jem.177.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwashima M, Irving B A, van Oers N S C, Chan A C, Weiss A. Sequential interactions of the TCR with two distinct cytoplasmic tyrosine kinases. Science. 1994;263:1136–1139. doi: 10.1126/science.7509083. [DOI] [PubMed] [Google Scholar]
- 20.Kedes D H, Ganem D. Sensitivity of Kaposi's sarcoma-associated herpesvirus replication to antiviral drugs. Implications for potential therapy. J Clin Investig. 1997;99:2082–2086. doi: 10.1172/JCI119380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirshner J R, Staskus K, Haase A, Lagunoff M, Ganem D. Expression of the open reading frame 74 (G-protein-coupled receptor) gene of Kaposi's sarcoma (KS)-associated herpesvirus: implications for KS pathogenesis. J Virol. 1999;73:6006–6014. doi: 10.1128/jvi.73.7.6006-6014.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lagunoff M, Ganem D. The structure and coding organization of the genomic termini of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) Virology. 1997;236:147–154. doi: 10.1006/viro.1997.8713. [DOI] [PubMed] [Google Scholar]
- 23.Lagunoff M, Majeti R, Weiss A, Ganem D. Deregulated signal transduction by the K1 gene product of Kaposi's sarcoma-associated herpesvirus. Proc Natl Acad Sci USA. 1999;96:5704–5709. doi: 10.1073/pnas.96.10.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee H, Guo J, Li M, Choi J-K, DeMaria M, Rosenzweig M, Jung J U. Identification of an immunoreceptor tyrosine-based activation motif of K1 transforming protein of Kaposi's sarcoma-associated herpesvirus. Mol Cell Biol. 1998;18:5219–5228. doi: 10.1128/mcb.18.9.5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee H, Veazey R, Williams K, Li M, Guo J, Neipel F, Fleckenstein B, Lackner A, Desrosiers R C, Jung J U. Deregulation of cell growth by the K1 gene of Kaposi's sarcoma-associated herpesvirus. Nat Med. 1998;4:435–440. doi: 10.1038/nm0498-435. [DOI] [PubMed] [Google Scholar]
- 26.Lukac D M, Kirshner J R, Ganem D. Transcriptional activation by the product of open reading frame 50 of Kaposi's sarcoma-associated herpesvirus is required for lytic viral reactivation in B cells. J Virol. 1999;73:9348–9361. doi: 10.1128/jvi.73.11.9348-9361.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lukac D M, Renne R, Kirshner J R, Ganem D. Reactivation of Kaposi's sarcoma-associated herpesvirus infection from latency by expression of the ORF 50 transactivator, a homolog of the EBV R protein. Virology. 1998;252:304–312. doi: 10.1006/viro.1998.9486. [DOI] [PubMed] [Google Scholar]
- 28.Martin D F, Kuppermann B D, Wolitz R A, Palestine A G, Li H, Robinson C A. Oral ganciclovir for patients with cytomegalovirus retinitis treated with a ganciclovir implant. Roche Ganciclovir Study Group. N Engl J Med. 1999;340:1063–1070. doi: 10.1056/NEJM199904083401402. [DOI] [PubMed] [Google Scholar]
- 29.Martin J N, Ganem D E, Osmond D H, Page-Shafer K A, Macrae D, Kedes D H. Sexual transmission and the natural history of human herpesvirus 8 infection. N Engl J Med. 1998;338:948–954. doi: 10.1056/NEJM199804023381403. [DOI] [PubMed] [Google Scholar]
- 30.Orenstein J M, Alkan S, Blauvelt A, Jeang K T, Weinstein M D, Ganem D, Herndier B. Visualization of human herpesvirus type 8 in Kaposi's sarcoma by light and transmission electron microscopy. AIDS. 1997;11:F35–F45. doi: 10.1097/00002030-199705000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Renne R, Zhong W, Herndier B, McGrath M, Abbey N, Kedes D, Ganem D. Lytic growth of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat Med. 1996;2:342–346. doi: 10.1038/nm0396-342. [DOI] [PubMed] [Google Scholar]
- 32.Romeo C, Seed B. Cellular immunity to HIV activated by CD4 fused to T cell or Fc receptor polypeptides. Cell. 1991;64:1037–1046. doi: 10.1016/0092-8674(91)90327-u. [DOI] [PubMed] [Google Scholar]
- 33.Schulz T F. Kaposi's sarcoma-associated herpesvirus (human herpesvirus-8) J Gen Virol. 1998;79:1573–1591. doi: 10.1099/0022-1317-79-7-1573. [DOI] [PubMed] [Google Scholar]
- 34.Shapiro V S, Mollenauer M N, Greene W C, Weiss A. c-Rel regulation of IL-2 gene expression may be mediated through activation of AP-1. J Exp Med. 1996;184:1663–1669. doi: 10.1084/jem.184.5.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, d'Agay M F, Clauvel J P, Raphael M, Degos L, et al. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood. 1995;86:1276–1280. [PubMed] [Google Scholar]
- 36.Staskus K A, Zhong W, Gebhard K, Herndier B, Wang H, Renne R, Beneke J, Pudney J, Anderson D J, Ganem D, Haase A T. Kaposi's sarcoma-associated herpesvirus gene expression in endothelial (spindle) tumor cells. J Virol. 1997;71:715–719. doi: 10.1128/jvi.71.1.715-719.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun R, Lin S F, Gradoville L, Yuan Y, Zhu F, Miller G. A viral gene that activates lytic cycle expression of Kaposi's sarcoma-associated herpesvirus. Proc Natl Acad Sci USA. 1998;95:10866–10871. doi: 10.1073/pnas.95.18.10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weiss A, Littman D R. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 39.Whitby D, Howard M R, Tenant-Flowers M, Brink N S, Copas A, Boshoff C, Hatzioannou T, Suggett F E, Aldam D M, Denton A S, et al. Detection of Kaposi sarcoma associated herpesvirus in peripheral blood of HIV-infected individuals and progression to Kaposi's sarcoma. Lancet. 1995;346:799–802. doi: 10.1016/s0140-6736(95)91619-9. [DOI] [PubMed] [Google Scholar]
- 40.Wu J, Katzav S, Weiss A. A functional T-cell receptor signaling pathway is required for p95vav activity. Mol Cell Biol. 1995;15:4337–4346. doi: 10.1128/mcb.15.8.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zong J C, Ciufo D M, Alcendor D J, Wan X, Nicholas J, Browning P J, Rady P L, Tyring S K, Orenstein J M, Rabkin C S, Su I J, Powell K F, Croxson M, Foreman K E, Nickoloff B J, Alkan S, Hayward G S. High-level variability in the ORF-K1 membrane protein gene at the left end of the Kaposi's sarcoma-associated herpesvirus genome defines four major virus subtypes and multiple variants or clades in different human populations. J Virol. 1999;73:4156–4170. doi: 10.1128/jvi.73.5.4156-4170.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]