Abstract
Background
Enteric hepatitis A virus (HAV) infections during childhood are often asymptomatic but may cause severe illness in adults. To improve public health surveillance we assessed the applicability of sewage monitoring during an HAV outbreak at a primary school.
Methods
Between October 19 and December 27, 2022, five symptomatic HAV cases were notified to the Public Health Service Amsterdam; all attended the same primary school. Passive samplers, small absorbent tools, were deployed in sewage near the school from November 14, 2022, to March 22, 2023. The absorbents were subjected to RNA extraction, HAV PCR testing, and, if positive, sequencing. PCR and sequencing were also performed on plasma and feces samples of HAV cases.
Results
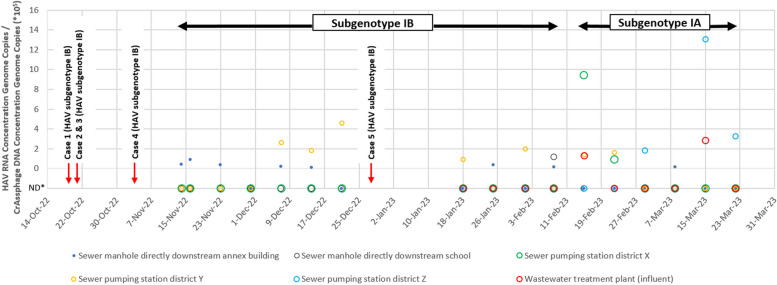
In 22 out of 88 (25%) of sewage samples, HAV RNA was detected. All HAV-RNA-positive sewage samples until 8 February 2023 were subgenotype IB, matching the strain detected in all cases. Another strain of HAV (subgenotype IA) was detected in sewage from 15 February 2023 onwards, without associated cases.
Conclusions
Passive sampler-based sewage monitoring is an effective method to rapidly detect HAV shedding linked to diagnosed cases. It detects unnoticed viral infections and allows monitoring of outbreaks. This suggests that passive sampler-based monitoring is a promising tool supporting the public health response during HAV and other outbreaks.
Keywords: Sewage sampling, Passive samplers, Hepatitis A virus, Outbreak detection, Sewage monitoring
Background
Hepatitis A virus (HAV) is the cause of acute hepatitis A infection. During early childhood the symptoms can vary from asymptomatic to mild disease, but in adults it may cause severe illness with jaundice and liver failure that, in rare cases, can be fatal [1]. In 2021, 3,864 HAV cases were reported by 30 countries in the European Economic Area; people 45 years of age or older accounted for almost a third of the cases [2]. Transmission mainly takes place via the faecal-oral route via person-to-person contact and through consumption of contaminated food or water [3, 4]. The incubation period of hepatitis A ranges from 15 to 50 days with a mean of 30 days [5]. HAV infection is a notifiable disease in the Netherlands; its incidence is low, with 85 cases reported in 2022 [6] and a considerable proportion occurring in men who have sex with men [7]. It can be contracted by unvaccinated residents travelling to high or intermediate endemic countries. Upon return to the Netherlands HAV infection can result in silent transmission among children, due to a predominantly immune-naïve population regarding hepatitis A virus, a relatively long incubation period and an often asymptomatic course of infection. This may result in HAV-clusters that pose health threats for specific risk groups such as people at occupational risk of exposure [8]. These include unprotected staff in schools or child day care centres who have not been vaccinated or not previously been exposed to the virus. The risk of infection is increased in children who travel to endemic HAV countries, often for visits to family in their parents’ countries of birth [9, 10].
Sewage monitoring using passive samplers is a method for tracking and analysing the presence of various substances such as genomic material of pathogens in sewage. The first passive sampler described was a folded gauze attached to a string which was submerged in a sewer to detect Salmonella typhi [11]. In addition to gauze, electronegative membranes and cotton swabs are considered appropriate absorbing material to be placed within passive samplers [12]. Passive samplers absorb faecal matter, including viruses, from the sewage and they are strategically placed within sewer systems to collect sewage samples over time as introduced during the coronavirus disease (COVID-19) pandemic [13]. This monitoring approach might also detect silent transmission of other infections, such as HAV.
In October 2022 three Dutch children attending the same school in Amsterdam were diagnosed with HAV; they had recently returned from a visit to an HAV endemic country. To monitor potential ongoing transmission, sewage monitoring around the school was initiated. This study focuses on the application of this targeted sewage monitoring, using passive samplers, to detect ongoing circulation of HAV.
Methods
Setting of the study
On 18 October 2022 a case of HAV was notified by an Amsterdam hospital to the Public Health Service (GGD) Amsterdam. After source and contact tracing, a second and third case were notified on 20 October 2022 and a fourth case on 3 November 2022 by the Regional Laboratory of the GGD Amsterdam. A fifth case was notified by the same hospital on 27 December 2022. All five cases were attending the same primary school. No faeces of other children at the school was collected to monitor transmission, as this was not a standard procedure in the outbreak protocol.
Case definitions
A suspected case was defined as a person attending or working at the primary school with at least one of the following symptoms after 8 October 2022: jaundice, discoloured stool, dark urine, fever, loss of appetite, nausea, diarrhoea, fatigue and abdominal pain. A confirmed case was defined as a person with immunoglobulin M (IgM) anti-HAV detected in the serum, or a positive polymerase chain reaction (PCR) of faeces or plasma, and with reported fever or jaundice.
Specimen collection
EDTA-plasma of two suspected cases was obtained on 17 October 2022 (case #1) and on 22 December 2022 (case #5) by the local hospital and faeces specimens of three suspected cases were collected on 20 October 2022 (cases #2 and #3) and 3 November 2022 (case #4) by the GGD for routine HAV PCR diagnostic testing. All PCR positive specimens were sent to the National Institute for Public Health and the Environment (RIVM) for sequencing analysis.
Outbreak investigation
Confirmed cases and their parents or guardians were interviewed by telephone by trained nurses using a standardized questionnaire on the day their positive test result became available, or the next day. The questions included a list of symptoms experienced, HAV vaccination status, visited locations, history of recent travel abroad, mapping of the family situation and attendance to the school main building or annex building. The GGD regularly contacted the school during the outbreak to enquire if other children had developed symptoms that could be related to HAV infection.
HAV sewage monitoring methods
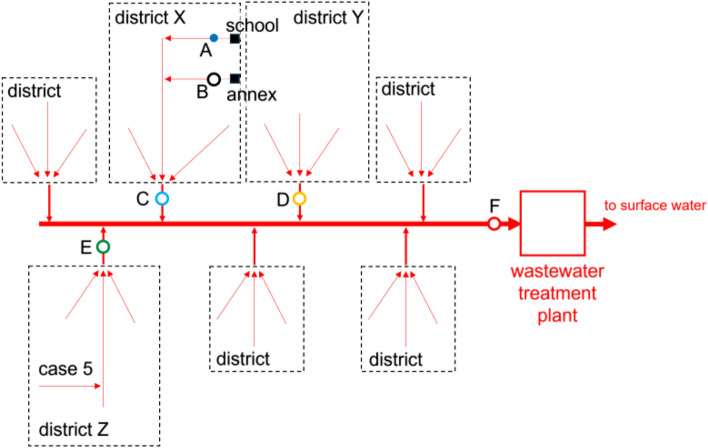
On 9 November 2022 it was decided to employ sewage monitoring. After city sewage employees shared the map of the city sewage flows, the sewage sampling locations were identified that same day. HAV sewage monitoring using 3-D printed torpedo passive samplers with cotton tips (Fig. 1) was implemented on 10 November 2022, 23 days after the notification of the first case, until 21 December 2022 in district X and Y (Fig. 2) in two sewer manholes that serviced the two buildings of the school and two sewer pumping stations that serviced the city area (± 20,000 residents) around the school of the cases [14]. When a new HAV case (case #5) was notified to the GGD Amsterdam on 27 December 2022, the sewage monitoring was expanded to district Z from 18 January 2023 until 22 March 2023 with one sewer pumping station near the home address of case #5 and one wastewater treatment plant that collected sewage of both the school and the residence of case #5. In the first week one passive sampler was deployed per manhole and sewer pumping station for 96 h; after the first week passive samplers were deployed for 48 h. When collected, samples including torpedo and the passive sampler material (inside a Zip bag) were transported to the laboratory of KWR Water Research Institute (Nieuwegein, Netherlands) on ice and processed the same day. All HAV PCR positive sewage extracts were sent to the RIVM for sequencing analysis.
Fig. 1.
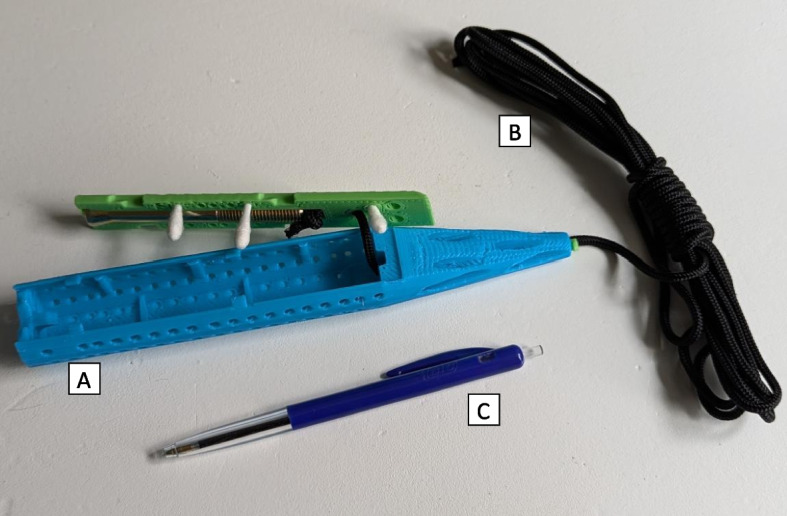
Passive samplers used for sewage sampling around Amsterdam school, the Netherlands, November 2022 – March 2023. A 3-D printed torpedo passive sampler with cotton tips B Suspension cord C: Pen for reference
Fig. 2.
Schematics of sewage sampling locations around Amsterdam school, the Netherlands, November 2022 – March 2023. A Sewer manhole directly downstream of the school main building. B Sewer manhole directly downstream of the annex building. C Sewer pumping station district X. D Sewer pumping station district Y. E Sewer pumping station district Z. F Wastewater treatment plant (influent)
Laboratory analysis
Diagnostics human specimens
Routine HAV PCR testing was performed on blood (two) and faeces (three) specimens of suspected cases using specimen type specific protocols. In short, for faeces specimens ribonucleic acid (RNA) was extracted from 250 ul faeces suspension using the Nordiag Arrow (Isogen Life Science, De Meern, the Netherlands) according to manufacturer’s instructions. For EDTA-plasma 200 µl of plasma was used for RNA extraction using TriPure Isolation Reagent (Roche Diagnostics, Germany) according to manufacturer’s instructions, spiked with 5 µl of Phocine herpes virus (PhHV) as an internal control. The residual pellet was dissolved in 500 µl 0.2 u/ml T10-RNAsin (Sigma Aldrich, USA). From extracted RNA via both methods, 5 µl was used in a hepatitis A real-time PCR on a Rotor Gene Q Real Time PCR system(Qiagen), using the Quantinova Pathogen + IC kit (Qiagen, cat.no. 208652) using primers HAV F-San6 (5’-GCTCTCCCCTTGCCCTAG-3’), HAV R-San4 (5’-TCCCCAATTTAGACTCCTACAGC-3’) and HAV probe San12 ([FAM]-CGGGGTCAACTCCATGATTAGCATGG- [15]) according to manufacturer’s protocol. Resulting S-curves were visually checked by a trained medical molecular microbiologist and the final diagnostic result clinically authorized by the medical microbiologist.
Analysis sewage samples
The cotton swab (still containing a small volume of wastewater) from the passive samplers was placed in 1.5 ml lysis buffer from the Biomerieux Nuclisens kit (Biomerieux, Amersfoort, the Netherlands) and vortexed four times for four seconds and the lysis buffer was transferred to a 2 ml centrifuge tube. To the tube with the cotton swab 1.5 ml fresh lysis buffer was added and vortexed again four times for four seconds and the lysis buffer was transferred to a new 2 ml centrifuge tube. Total contact time in the Nuclisens buffer was 10 min. The 2 ml centrifuge tubes were centrifuged for 1 min at 13.000xg and the supernatant (2.8–2.9 mL) was pooled and processed using the semi-automated KingFisher mL Purification System (Thermo Scientific, Bleiswijk, The Netherlands) [16]. Extracted nucleic acid was eluted in a volume of 100 µl. The digital droplet reverse transcription PCR (RT-PCR) method of Persson et al. was used to quantify HAV in the nucleic acid extracts [17]. CrAssphage was used as index of the human faecal load captured by the passive sampler and quantified from the same extracts as previously described [18]. Since the amount of human faecal matter captured can vary from (passive) sample to sample, the amount of CrAssphage captured by each passive sampler was used as index for the amount of human faecal matter captured. The observed concentration of HAV RNA in the extract of the passive sampler was normalized by dividing this through the observed CrAssphage concentration in the same extract. RT-ddPCR reactions performed on PCR grade and RNAse free distilled water and RNA extracted from PCR grade and RNAse free distilled water were used as negative controls. RT-ddPCR performed on both approximately 500 and 50 genome copies of HAV were used as positive controls. Assays were performed in technical duplicates, each in 20 µl reaction volumes containing the reagents from the One-Step Advance RT-ddPCR for probes: 5 µl RTddPCR One-Step Advanced Supermix, 2 µl Reverse Transcriptase, 1 µl DTT (300 mM) supplemented with 3.7 µl PCR grade and RNAse free water (Applied Biosystems, Fisher Scientific, Landsmeer, the Netherlands) and 5 µl sample-RNA. Primers and probe were the same as Persson et al., 2021, and were added as 1 µl HepA-F (500 nM), 1.8 µl HepA-R (900 nM), probe 0.5 µl FAM-labeled HepA-P (250 nM) with minor groove binder-eclipse quencher. The BioRad QX200 droplet generator partitioned sample-RNA and reagents in (at least) 77,000 droplets. The temperature profile used for RTddPCR was as follows: 60 min. 50 °C, 10 min 95 °C, 50 cycles with 30 s. 95 °C and 1 min. 60 °C followed by 10 min. 98 °C, 30 min. 4 °C and hold at 12 °C. Samples were scanned using the QX200 system (BioRad) and analyzed using the QuantaSoft-Analysis software (BioRad). For each sample, the number of negative and HAV ddPCR positive droplets were recorded and used to determine the HAV concentration. HAV RNA (kindly provided by ViroScience at Erasmus Medical Center, Rotterdam, the Netherlands) was used as positive control with each batch of samples, in two concentrations, yielding at least 500 and 50 positive droplets. PCR blancs (with 5 µl PCR grade water as sample) were run with each sample batch. When positive for RNA of HAV (when at least 1.2 genome copies per reaction), 25 µl of nucleic acid extract of the sewage samples were shipped to RIVM for sequencing.
Sequencing human specimens and sewage samples
Sequencing analysis was performed on nucleic acid extracts originating from HAV PCR positive blood and faeces specimens and sewage samples (22). For sequence based typing we routinely use the HAVNet protocol [19]. Briefly, cDNA synthesis and nested-PCR were used to obtain a 460bp fragment of the VP1-P2A junction, which was sequenced by Sanger sequence analysis. For serum samples and faeces, the protocol was used exactly as described. For sewage samples the yield of fragment was not enough for Sanger sequencing. These samples were analysed on the Oxford Nanopore platform, with sequences assembled using Canu, and trimmed to the 460bp fragment between the primers used for amplification. For Oxford Nanopore based sequence analysis we used the ligation sequencing kit (SQK-LSK109) with the Native Barcoding Expansion 96 kit (EXP-NBD196), and we used flowcell FLO-MIN106D R9.4.1. Resulting sequences were compared by alignment and phylogenetic analysis with sequences from reference strains from known geographic origin.
Results
Description of cases
From 18 October 2022 to 27 December 2022, five hepatitis A cases were associated with the outbreak. All five cases were confirmed by HAV PCR testing and they were all infected with the same strain of subgenotype IB (Table 1). The age range of the cases was 0 to 12 years. The five confirmed cases all attended different school classes; three of the five cases also attended the annex of the school. Case #1 and #4 were siblings, and cases #2 and #3 were also siblings. None of the five confirmed cases had been vaccinated against HAV and four confirmed cases reported recent travel to Somalia or Morocco, both known to be HAV endemic [20]. For cases #1–3, their travel occurred before the incubation period, making it unlikely to be the source of infection. Case #4 was identified through source and contact tracing related to case #1. It was discovered that case #4 had experienced a prior fever, which had not been addressed as it resolved on its own. This case was recognized as the missing link to Somalia, likely serving as the initial source of the outbreak.
Table 1.
Characteristics of hepatitis A virus cases attending Amsterdam school, the Netherlands, October—December 2022
| Case | Age (years) | Sex | Region of origina | Recentb travel history | Symptomatology | HAV vaccination status | Date start symptoms | Diagnostics | HAV typing | |
|---|---|---|---|---|---|---|---|---|---|---|
| Specimen taken | Specimen result | |||||||||
| 1 (index) | 5–8 | F | Eastern Mediterranean | Somalia | Jaundice, dark urine, discolored stool, fever, loss of appetite, nausea, fatigue | Not vaccinated | 15 Oct 2022 | 17 Oct 2022 | 18 Oct 2022 | Type 1B |
| 2 | 0–4 | F | Eastern Mediterranean | Morocco | Jaundice, abdominal pain, discolored stool | Not vaccinated | Unknown | 20 Oct 2022 | 20 Oct 2022 | Type 1B |
| 3 | 5–8 | M | Eastern Mediterranean | Morocco | Fever, diarrhea | Not vaccinated | 26 Sep 2022 | 20 Oct 2022 | 20 Oct 2022 | Type 1B |
| 4 | 9–12 | F | Eastern Mediterranean | Somalia | Fever | Not vaccinated | 15 Oct 2022 | 3 Nov 2022 | 3 Nov 2022 | Type 1B |
| 5 | 5–8 | M | Eastern Mediterranean | None reported | Jaundice, dark urine, discolored stool, loss of appetite, nausea | Not vaccinated | 19 Dec 2022 | 22 Dec 2022 | 27 Dec 2022 | Type 1B |
aContinent of birth of at least one parent
bIn last three months
Sewage monitoring
HAV sewage monitoring around the school (Fig. 2) from 10 November 2022 until 22 March 2023 resulted in the collection and analysis of 88 sewage samples. Of these, 22 (25%) were positive for RNA of HAV. In the first weeks of the sewage monitoring HAV RNA was only detected in low concentrations in sewage of the sewer manhole of the annex building, later also in the sewage of the sewer manhole of the school main building and sewer pumping stations in districts X, Y and Z (Fig. 3). Eight out of 17 sewage samples taken from the sewer manhole of the annex building and one out of 17 sewage samples taken from the sewer manhole of the school main building were positive for HAV RNA. Of the sewage samples taken from sewer pumping stations in districts X and Y, two and seven out of 17 were positive for HAV RNA, respectively. Three and two out of 10 sewage samples taken from sewer pumping station in district Z and the wastewater treatment plant were positive for HAV RNA, respectively.
Fig. 3.
HAV concentrations and strain typing in sewage samples around Amsterdam school, November 2022 – March 2023. *Non-Detect
Genotyping showed that the HAV RNA positive sewage samples all contained the same subgenotype IB up to 8 February 2023 and this subgenotype was indistinghuisable from the outbreak strain. From 15 February 2023 onwards, the subgenotype IB was no longer detected in sewage samples, but another subgenotype (IA) was detected in 10 positive sewage samples. During the subgenotype IB outbreak, RNA of HAV was detected most frequently in the sewer manhole directly downstream of the school annex building and the sewer pumping station in district Y (both seven times), and once from the sewer manhole of the school main building. The average detected concentration of HAV RNA genome copies/CrAssphage DNA genome copies was 2.24*105, with a minimum of 0.12*105 and a maximum of 13.06*105. In the sewer pumping station in district Y the highest concentration of subgenotype IB HAV RNA was observed, from 9 until 21 December 2022. One week later, case #5 was diagnosed. Based on time, place and subgenotype we considered this case part of the outbreak. From 15 February 2023 onwards, HAV subgenotype IA was detected mostly in different sewage sampling sites: district X and Z and the wastewater treatment plant. Following the first detection of subgenotype IA strain on 15 February 2023, an increased concentration of HAV RNA was detected in five out of six sewage monitoring locations until 22 March 2023, whereafter it was decided to end sewage monitoring since no new hepatitis A cases had been notified to the GGD Amsterdam since 27 December 2022.
Microbiological investigations
Sequencing analysis of all blood and faeces specimens that were positive for HAV RNA identified HAV subgenotype IB strain which matched the strain of the sewage samples until 8 February 2023 (Table 1 & Fig. 3). The HAV subgenotype IB strain was genetically closely related to strains previously isolated from travellers returning from Somalia. Case #4 had returned from Somalia a couple of weeks prior to onset of symptoms, suggesting Somalia as the most likely country of acquisition. HAV subgenotype IA strain was identified in sewage samples from 15 February until 22 March 2023, but no HAV suspected cases or diagnoses with an epidemiological or geographical link to the school were notified to the GGD during this period or in the following months.
Outbreak control measures
GGD Amsterdam issued a letter to the school management on 24 October 2022 informing them, that a pupil had been notified with HAV. The school forwarded this letter to all parents or guardians of the pupils. Parents, guardians and school staff were advised to be vigilant for HAV related symptoms in their children and pupils and to contact the general practitioner should children display any symptoms. Parents or guardians were also informed that the majority of HAV infections is asymptomatic and measures of transmission prevention were provided. Subsequently, GGD Amsterdam organized an HAV vaccination campaign at the school on 26 October 2022. Out of 234 pupils 33 (14%) were vaccinated; none of the school staff wanted to be vaccinated. An information session at the school was organized on 6 March 2023 about vaccines, their benefits, and to dispel any misconceptions that might exist. This did not lead to additional vaccination uptake among pupils and school staff.
Discussion
This study demonstrates to our knowledge for the first time the application of passive samplers to detect HAV RNA in sewage while monitoring an outbreak. The described sewage monitoring approach enabled public health authorities to detect and sequence HAV RNA in sewage around the school of the first case and to match the identified HAV strains in sewage samples with the human specimens of all identified cases. Prior studies in endemic regions conducted sewage monitoring as a surveillance tool to track HAV circulation and other studies detected HAV in sewage using grab sampling: a method that involves sewage collection at one point in time [21–32]. Here, we introduced and evaluated another approach: passive sampler-based sewage monitoring that can detect HAV for a defined period of time. The sewage data showed that silent transmission of HAV was ongoing after the identified outbreak, and indicated that the outbreak was over by showing that the outbreak HAV strain disappeared from the sewage around the school and the city district. Interestingly, the sewage monitoring detected circulation of another HAV strain (subgenotype IA) indicating silent transmission of another HAV strain in that city area. Identifying the source of this strain was outside the scope of this outbreak investigation, but the positive signal from the sewage could have been combined with other epidemiological approaches to benefit infectious disease control.
The described sewage monitoring technique shows the potential of using passive samplers in sewage to become an added value for outbreak investigation and management of hepatitis A or potentially other infectious diseases by offering a relatively new, non-invasive and near-real-time approach to monitor (re)emerging infectious diseases. Compared to autosamplers that collect wastewater, passive samplers are simple in design and operation, are easy to deploy and require minimal maintenance. In line with our findings, studies have shown that passive samplers are a powerful tool for viral detection and can provide robust information for infectious disease control [33, 34]. Passive sampler-based sewage monitoring is a promising tool for future outbreak responses because public health authorities can take prompt action to control the spread of the disease, such as implementing increased testing, contact tracing, isolation measures and vaccination campaigns when the pathogens are detected in sewage. It can also potentially lead to improved targeting of public health interventions: sewage monitoring using passive samplers can provide information on the location of outbreaks, help to identify areas with more cases (hot-spots) and monitor trends over time [35, 36].
The organized vaccination campaign targeting the pupils and staff of the school resulted in a low vaccination uptake of 14%. The pre-outbreak vaccination coverage was estimated to be very low by the youth health care physician affiliated with the school. Next to hesitancy of the school to participate in the promotion of vaccination, the relatively short window for campaign announcement, coinciding with the autumn holidays from 15 till 23 October 2022 could have attributed to the low uptake. Possibly the education efforts of the GGD were not appropriate or suitable, or not timely, for the parents or guardians of the pupils of this school. We think the effect of vaccination on the course of this outbreak at the school was probably minimal. Low impact of vaccination was also observed in an HAV outbreak report at a British school in 2019 [37]; it suggests that outbreaks in these settings may be effectively managed without resorting to vaccination campaigns.
No faeces of pupils without symptoms was collected to monitor silent transmission as this supplementary screening tool was not a standard procedure of the local outbreak protocol. Requesting faeces of pupils for source and contact tracing may be a more direct method to detect silent transmission [38]. This could also include children who do not defecate at school. However, obtaining faeces of asymptomatic children may be a challenge, is fully dependent on informed consent of parents or guardians, and may be hard to repeat on a weekly basis for a long period. If during a potential outbreak of HAV or another (a)symptomatic infectious agent it is not possible to collect these faeces samples, sewage monitoring using passive samplers would be recommended for detecting (silent) transmission. Future studies around hepatitis A outbreaks should try to collect faeces of asymptomatic persons as well as deploy passive samplers, so that the findings of both approaches can be compared, and relative advantages of each evaluated. If the use of passive samplers is validated, and perhaps shown to be simpler and more feasible for ongoing monitoring, their use may be incorporated in outbreak protocols. Future studies could also focus on the concentrations of DNA or RNA of HAV or other infectious agents in sewage by passive samplers: how does it relate to concentrations identified in grab sampling and might this be an indication for the number of infected individuals, as is done in SARS-CoV-2 sewage monitoring [39].
Conclusions
Passive sampler-based sewage monitoring offers an effective approach for swift identification of HAV shedding, which can be directly associated with diagnosed cases. This method is adept at uncovering viral shedding from asymptomatic cases, enabling the near-real-time tracking of outbreaks and monitoring the effectiveness of interventions. Consequently, it emerges as a highly promising tool for public health authorities, empowering them to tailor their response strategies during HAV and other infectious disease outbreaks. This is the case in not only high-income countries like the Netherlands, but also in middle- and low-income countries [32]. We recommend piloting this sewage monitoring method during future outbreaks of infectious diseases to assess the potential of passive sampler-based surveillance systems in local (resource limited) settings and contribute to more effective infectious disease monitoring and control strategies.
Acknowledgements
The authors would like to thank the sewage consortium members including M. de Graaf, Erasmus Medical Center, Rotterdam, the Netherlands; P. Bijkerk and G. Sips, GGD Rotterdam, Rotterdam, the Netherlands for their input and feedback during deployment and microbiological analysis of the passive samplers; D. McCarthy, Monash University, Melbourne, Australia, for providing the print code of the passive sampler; J. Cremer and B. van der Veer, RIVM, Bilthoven, the Netherlands, for the sequencing analysis of the human specimens; G. Elsinga, KWR, Nieuwegein, the Netherlands, for the ddPCR analysis; J. Guldemeester, Erasmus Medical Center, Rotterdam, the Netherlands, for providing HAV genome copies to use as positive controls; and N. Khawaja, GGD Amsterdam, Amsterdam, the Netherlands, for providing the case data.
Abbreviations
- HAV
Hepatitis A Virus
- COVID-19
Coronavirus Disease
- GGD
Public Health Service
- IgM
Immunoglobulin M
- PCR
Polymerase Chain Reaction
- RIVM
National Institute for Public Health and the Environment
- RT-PCR
Reverse Transcription Polymerase Chain Reaction
- RNA
Ribonucleic Acid
- WPG
Wet Publieke Gezondheid
Authors’ contributions
MJ, CW, EF and GM coordinated the investigations of the outbreak. MJ, RS, JL, MS, EF and GM contributed to data collection, case information and data analysis. HV, MW and GM were involved in the laboratory investigations. MJ and MS drafted the manuscript and all authors were involved in revising the manuscript. All authors reviewed and approved the final version.
Funding
Deployment and microbiological analysis of the passive samplers described in this study was funded by a sewage consortium consisting of GGD Amsterdam, Amsterdam; GGD Rotterdam, Rotterdam; Erasmus Medical Center, Rotterdam; KWR, Nieuwegein; Partners4UrbanWater, Nijmegen; IMD, Apeldoorn; STOWA, Amersfoort, all in the Netherlands and financially supported by the Water Technology Topconsortium for Knowledge and Innovation. The hours dedicated to this project by the first and third author were funded by the Ministry of Health, Welfare and Sport of the Dutch Government within the program ‘Strengthening infectious disease control and pandemic preparedness of the regional Public Health Services’ in 2023–2024. The Ministry of Health, Welfare and Sport of the Dutch Government had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Permission of the (guardians of the) cases for diagnostics and sequencing of the human specimens was not required because the GGD has legal permission, provided by Dutch national public health law (Wet Publieke Gezondheid (WPG))[40], to process patient information for surveillance and outbreak analysis of notifiable diseases and can request microbiological laboratories to perform additional testing (including whole genome sequencing) on available clinical materials (article 25 section 5 WPG). Therefore, medical ethical clearance for this study was not required.
Consent for publication
Not applicable, see section ‘Ethics approval and consent to participate’.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Collier MG, Tong X, Xu F. Hepatitis A hospitalizations in the United States, 2002–2011. Hepatology. 2015;61(2):481–5. [DOI] [PubMed] [Google Scholar]
- 2.https://www.ecdc.europa.eu/sites/default/files/documents/HEPA_AER_2021.pdf. Last Accessed on 6 Nov 2023.
- 3.Takuissu GR, Kenmoe S, Ebogo-Belobo JT, Kengne-Nde C, Mbaga DS, Bowo-Ngandji A, et al. Occurrence of hepatitis A virus in water matrices: a systematic review and meta-analysis. Int J Environ Res Public Health. 2023;20(2):1054. [DOI] [PMC free article] [PubMed]
- 4.Chatziprodromidou IP, Dimitrakopoulou ME, Apostolou T, Katopodi T, Charalambous E, Vantarakis A. Hepatitis A and E in the Mediterranean: a systematic review. Travel Med Infect Dis. 2022;47: 102283. [DOI] [PubMed] [Google Scholar]
- 5.Khan OA, Heymann DL, American Public Health Association. Control of communicable diseases. Clinical practice. 21 ed. Washington, DC: American Public Health Association; 2020.
- 6.RIVM. Jaarlijkse meldingen per infectieziekte. https://www.rivm.nl/meldingsplicht-infectieziekten/overzicht-meldingen. Last Accessed on 3 Nov 2023.
- 7.Alberts CJ, Boyd A, Bruisten SM, Heijman T, Hogewoning A, Rooijen MV, et al. Hepatitis A incidence, seroprevalence, and vaccination decision among MSM in Amsterdam, the Netherlands. Vaccine. 2019;37(21):2849–56. [DOI] [PubMed] [Google Scholar]
- 8.Migueres M, Lhomme S, Izopet J. Hepatitis A: epidemiology, high-risk groups, prevention and research on antiviral treatment. Viruses. 2021;13(10):1900. [DOI] [PMC free article] [PubMed]
- 9.Heywood AE, Zwar N, Forssman BL, Seale H, Stephens N, Musto J, et al. The contribution of travellers visiting friends and relatives to notified infectious diseases in Australia: state-based enhanced surveillance. Epidemiol Infect. 2016;144(16):3554–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whelan J, Sonder G, van den Hoek A. Declining incidence of hepatitis A in Amsterdam (The Netherlands), 1996–2011: second generation migrants still an important risk group for virus importation. Vaccine. 2013;31(14):1806–11. [DOI] [PubMed] [Google Scholar]
- 11.Moore B. The detection of enteric carriers in towns by means of sewage examination. J R Sanit Inst. 1951;71(1):57–60. [DOI] [PubMed] [Google Scholar]
- 12.Habtewold J, McCarthy D, McBean E, Law I, Goodridge L, Habash M, et al. Passive sampling, a practical method for wastewater-based surveillance of SARS-CoV-2. Environ Res. 2022;204(Pt B):112058. [DOI] [PMC free article] [PubMed]
- 13.Schang C, Crosbie ND, Nolan M, Poon R, Wang M, Jex A, et al. Passive sampling of SARS-CoV-2 for wastewater surveillance. Environ Sci Technol. 2021;55(15):10432–41. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Verhagen R, Ahmed W, Metcalfe S, Thai PK, Kaserzon SL, et al. In situ calibration of passive samplers for viruses in wastewater. ACS ES&T Water. 2022;2(11):1881–90. [Google Scholar]
- 15.Aghaei AM, Gholami J, Sangchooli A, Rostam-Abadi Y, Olamazadeh S, Ardeshir M, et al. Prevalence of injecting drug use and HIV, hepatitis B, and hepatitis C in people who inject drugs in the Eastern Mediterranean region: a systematic review and meta-analysis. Lancet Glob Health. 2023;11(8):e1225–37. [DOI] [PubMed] [Google Scholar]
- 16.Heijnen L, Medema G. Surveillance of influenza A and the pandemic influenza A (H1N1) 2009 in sewage and surface water in the Netherlands. J Water Health. 2011;9(3):434–42. [DOI] [PubMed] [Google Scholar]
- 17.Persson S, Alm E, Karlsson M, Enkirch T, Norder H, Eriksson R, et al. A new assay for quantitative detection of hepatitis A virus. J Virol Methods. 2021;288:114010. [DOI] [PubMed] [Google Scholar]
- 18.Langeveld J, Schilperoort R, Heijnen L, Elsinga G, Schapendonk CEM, Fanoy E, et al. Normalisation of SARS-CoV-2 concentrations in wastewater: the use of flow, electrical conductivity and crAssphage. Sci Total Environ. 2023;865:161196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.HAVNET. Protocol Molecular detection and typing of VP1region of Hepatitis A Virus (HAV). https://www.rivm.nl/sites/default/files/2018-11/Typing%20protocol%20HAVNET%20VP1P2A%20a1a.pdf. Last Accessed on 8 Sep 2023.
- 20.Badur S, Ozturk S, AbdelGhany M, Khalaf M, Lagoubi Y, Ozudogru O, et al. Hepatitis A in the Eastern Mediterranean Region: a comprehensive review. Hum Vaccin Immunother. 2022;18(5):2073146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aw TG, Gin KY. Environmental surveillance and molecular characterization of human enteric viruses in tropical urban wastewaters. J Appl Microbiol. 2010;109(2):716–30. [DOI] [PubMed] [Google Scholar]
- 22.Beji-Hamza A, Khelifi-Gharbi H, Hassine-Zaafrane M, Della Libera S, Iaconelli M, Muscillo M, et al. Qualitative and quantitative assessment of hepatitis A virus in wastewaters in Tunisia. Food Environ Virol. 2014;6(4):246–52. [DOI] [PubMed] [Google Scholar]
- 23.Bisseux M, Colombet J, Mirand A, Roque-Afonso AM, Abravanel F, Izopet J, et al. Monitoring human enteric viruses in wastewater and relevance to infections encountered in the clinical setting: a one-year experiment in central France, 2014 to 2015. Euro Surveill. 2018;23(7):17–00237. [DOI] [PMC free article] [PubMed]
- 24.Farkas K, Cooper DM, McDonald JE, Malham SK, de Rougemont A, Jones DL. Seasonal and spatial dynamics of enteric viruses in wastewater and in riverine and estuarine receiving waters. Sci Total Environ. 2018;634:1174–83. [DOI] [PubMed] [Google Scholar]
- 25.Hellmer M, Paxeus N, Magnius L, Enache L, Arnholm B, Johansson A, et al. Detection of pathogenic viruses in sewage provided early warnings of hepatitis A virus and norovirus outbreaks. Appl Environ Microbiol. 2014;80(21):6771–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiulia NM, Netshikweta R, Page NA, Van Zyl WB, Kiraithe MM, Nyachieo A, et al. The detection of enteric viruses in selected urban and rural river water and sewage in Kenya, with special reference to rotaviruses. J Appl Microbiol. 2010;109(3):818–28. [DOI] [PubMed] [Google Scholar]
- 27.La Rosa G, Libera SD, Iaconelli M, Ciccaglione AR, Bruni R, Taffon S, et al. Surveillance of hepatitis A virus in urban sewages and comparison with cases notified in the course of an outbreak, Italy 2013. BMC Infect Dis. 2014;14:419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Brien E, Nakyazze J, Wu H, Kiwanuka N, Cunningham W, Kaneene JB, et al. Viral diversity and abundance in polluted waters in Kampala. Uganda Water Res. 2017;127:41–9. [DOI] [PubMed] [Google Scholar]
- 29.Pellegrinelli L, Galli C, Binda S, Primache V, Tagliacarne C, Pizza F, et al. Molecular characterization and phylogenetic analysis of enteroviruses and hepatitis A viruses in sewage samples, Northern Italy, 2016. Food Environ Virol. 2019;11(4):393–9. [DOI] [PubMed] [Google Scholar]
- 30.Prevost B, Lucas FS, Goncalves A, Richard F, Moulin L, Wurtzer S. Large scale survey of enteric viruses in river and waste water underlines the health status of the local population. Environ Int. 2015;79:42–50. [DOI] [PubMed] [Google Scholar]
- 31.Yanez LA, Lucero NS, Barril PA, Diaz Mdel P, Tenaglia MM, Spinsanti LI, et al. Evidence of hepatitis A virus circulation in central Argentina: seroprevalence and environmental surveillance. J Clin Virol. 2014;59(1):38–43. [DOI] [PubMed] [Google Scholar]
- 32.Fantilli A, Cola GD, Castro G, Sicilia P, Cachi AM, de Los Angeles Marinzalda M, et al. Hepatitis A virus monitoring in wastewater: A complementary tool to clinical surveillance. Water Res. 2023;241:120102. [DOI] [PubMed] [Google Scholar]
- 33.Mejías-Molina C, Pico-Tomàs A, Beltran-Rubinat A, Martínez-Puchol S, Corominas L, Rusiñol M, et al. Effectiveness of passive sampling for the detection and genetic characterization of human viruses in wastewater. Environ Sci: Water Res Technol. 2023;9(4):1195–204. [Google Scholar]
- 34.Cha G, Graham KE, Zhu KJ, Rao G, Lindner BG, Kocaman K, et al. Parallel deployment of passive and composite samplers for surveillance and variant profiling of SARS-CoV-2 in sewage. Sci Total Environ. 2023;866:161101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haskell BR, Dhiyebi HA, Srikanthan N, Bragg LM, Parker WJ, Giesy JP, et al. Implementing an adaptive, two-tiered SARS-CoV-2 wastewater surveillance program on a university campus using passive sampling. Sci Total Environ. 2024;912:168998. [DOI] [PubMed] [Google Scholar]
- 36.Holland SC, Smith MF, Holland LA, Maqsood R, Hu JC, Murugan V, et al. Human adenovirus outbreak at a university campus monitored by wastewater and clinical surveillance. medRxiv. 2024. [DOI] [PubMed]
- 37.Wensley A, Smout E, Ngui SL, Balogun K, Blomquist P, Edelstein M, et al. An outbreak of hepatitis A virus infection in a secondary school in England with no undetected asymptomatic transmission among students. Epidemiol Infect. 2022;151:e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rump BO, Visser O, Te Wierik MJ, Vennema H, Fanoy EB. Use of PCR for detection of faecal HAV as a screening tool in an outbreak of hepatitis A in daycare centres. Epidemiol Infect. 2013;141(3):549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McMahan CS, Self S, Rennert L, Kalbaugh C, Kriebel D, Graves D, et al. COVID-19 wastewater epidemiology: a model to estimate infected populations. Lancet Planet Health. 2021;5(12):e874–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.https://wetten.overheid.nl/BWBR0024705/2021-07-17. Last Aaccessed on 3 Nov 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.