Abstract
In recent years, research has unveiled the significant role of hydrogen sulfide (H2S) in many physiological and pathological processes. The role of endogenous H2S, H2S donors, and inhibitors has been the subject of studies that have aimed to investigate this intriguing molecule. The mechanisms by which H2S contributes to different diseases, including inflammatory conditions, cardiovascular disease, viral infections, and neurological disorders, are complex. Despite noteworthy progress, several questions remain unanswered. H2S donors and inhibitors have shown significant therapeutic potential for various diseases. This review summarizes our current understanding of H2S-based therapeutics in inflammatory conditions, cardiovascular diseases, viral infections, and neurological disorders.
Keywords: hydrogen sulfide, H2S donors, H2S inhibitors, inflammatory conditions, cardiovascular diseases, viral infections, neurological disorders, therapeutic applications, molecular pathways
1. Introduction
This review aims to understand the role of H2S distribution, biosynthesis, mechanistic pathways, and H2S-based therapeutics in different diseases—inflammatory conditions, cardiovascular disorders, viral diseases, and central nervous system diseases. In modern biology, a fascinating theme is that small molecules can contribute to intricate physiological and pathological functions in living organisms [1]. Hydrogen sulfide (H2S) was traditionally believed to be a toxic, flammable gas with an unpleasant rotten egg odor [2]. However, recent research has unveiled that H2S is an important cellular signaling molecule, marking a significant paradigm shift in research. The recognition of H2S as an important contributor to both physiological and pathological processes underscore the importance and impact of this gas. H2S is the third gasotransmitter, followed by nitric oxide (NO) and carbon monoxide (CO). It exhibits various physiological actions, including apoptosis, cytoprotection, vasodilation, angiogenesis, modulation of inflammatory responses, antioxidant properties, regulation of vascular tone, enhancing cell survival, and regulation of cellular metabolism [3,4,5]. Pathological conditions, including inflammatory conditions (such as arthritis/joint inflammation, sepsis, and acute pancreatitis), cardiovascular disorders, viral diseases, and central nervous system diseases, may arise due to the disruption in its endogenous production or metabolism. By examining the underlying mechanisms and therapeutic potential of H2S donors and inhibitors, this article summarizes our current understanding of the role of H2S in health and disease.
2. Synthesis and Distribution of H2S
Three enzymes—cystathionine-β-synthase (CBS), cystathionine-γ-lyase (CTH, CGL, or CSE), and 3-mercaptopyruvate sulfurtransferase (3-MST or MPST)—are primarily responsible for H2S biosynthesis (Figure 1). Some other enzymatic or non-enzymatic pathways can also produce H2S under normal or pathological conditions.
Figure 1.
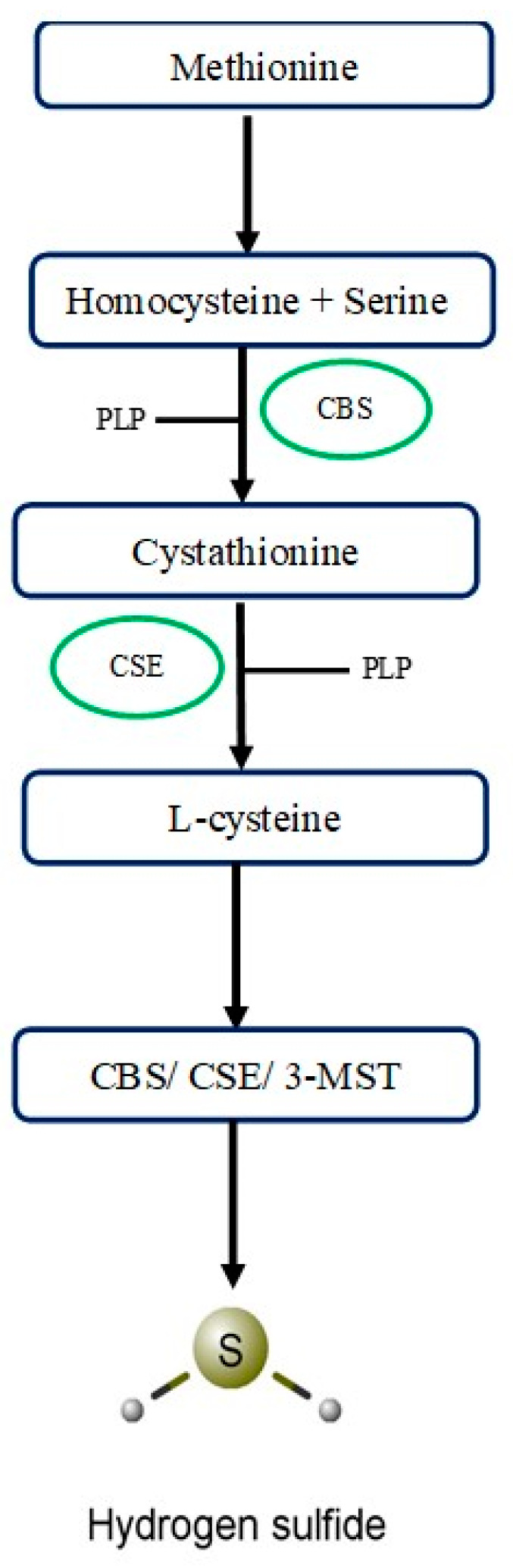
An overview of H2S biosynthesis. This pathway shows the biosynthesis of H2S. Pyridoxal 5’-phosphate (PLP) acts as a co-factor for Cystathionine β-synthase (CBS) and Cystathionine γ-lyase (CSE) dependent H2S synthesis.
2.1. Cystathionine-β-Synthase
CBS is also termed as beta-thionase, serine sulfhydrase, and L-serine hydrolyase. This enzyme is encoded by the cystathionine-β-synthase gene present on chromosome 21 in humans [6]. It is predominantly present in different parts of the brain (including the cerebral cortex, cortical astrocytes, corpus callosum, neuronal cells, hippocampus, olfactory bulb, and cerebellum) and in the pancreas (acinar cells) [7]. CBS is predominantly present in the cytosol, but it has also been found in mitochondria. Levels of CBS in mitochondria are increased after hypoxia [8].
2.2. Cystathionine-γ-Lyase
CSE is also referred to as homoserine dehydratase, cysteine desulfhydrase, cystine desulfhydrase, and γ-cystathionase. This enzyme in humans is encoded by the CSE gene present on chromosome 1. This gene is highly expressed in several organs, including the liver, lungs, uterus, and kidneys. A small amount of CSE contributes to H2S synthesis in the brain as well [9,10].
2.3. 3-Mercaptopyruvate Sulfurtransferase
3-MST is also called human liver rhodanese 2, β-mercaptopyruvate sulfurtransferase, thiosulfate sulfurtransferase 2, and tRNA thiouridin modification protein. This enzyme is encoded by a 3-mercaptopyruvate sulfurtransferase gene present on chromosome 22 in humans [11]. It has been reported in several studies that 3-MST is predominantly present in mitochondria. Studies from neurons, heart, liver, endothelial, and epithelial cells have shown that an equal concentration of 3-MST is present in cytosol as well as mitochondria [12,13].
Figure 1 provides an overview of the synthesis of H2S in the body.
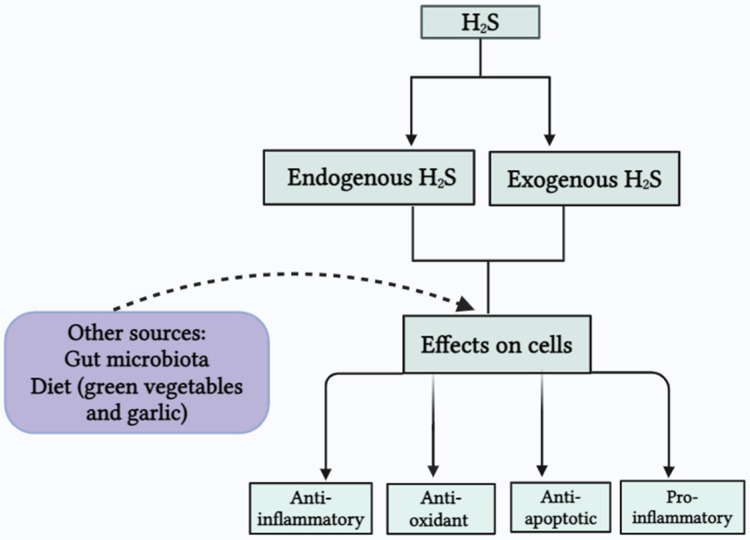
2.4. Other Sources of H2S
In addition to these enzymatic sources, H2S can also be produced by gut microbiota and through different natural dietary sources. Natural sources, including sulforaphane and erucin (which are abundantly present in green vegetables) and diallyl disulfide and diallyl trisulfide (DATS), are found in garlic [14,15]. Some bacteria, including Desulfotomaculum, Desulfovibrio, and Desulfobacter, are abundantly present in the colon and are known as sulfate-reducing bacteria [16,17]. Many bacterial strains, such as Staphylococcus, Salmonella, Escherichia coli, Corynebacterium, and Enterobacter, also have sulfite reductase. Furthermore, several anaerobic bacterial species, e.g., Clostridia and Bacillus, also play a role in the conversion of cysteine into H2S, ammonia, and pyruvate through cysteine desulfhydrases [18].
Figure 2 provides an overview of the sources of H2S in the body and its effects on cells.
Figure 2.
Endogenous and exogenous H2S and effects on cells.
3. H2S Donors
H2S-releasing compounds (Table 1) are widely used to understand the biological pathways of H2S and develop H2S-targeting therapeutics. In recent decades, the development of H2S donors has been a rapidly expanding area of research [19,20]. These donors are designed to release H2S by various mechanisms. According to the literature, the role of H2S, using H2S donors, appears to be controversial. In some conditions, H2S has been shown to have pro-inflammatory actions, whereas some studies have pointed to the anti-inflammatory effects of H2S [21,22]. These differences in effects of H2S can be attributed to several factors such as the nature of H2S donors, experimental models, method of administration, timing, site, and dosage of the agent. H2S donors are classified into two classes—fast H2S-releasing compounds and slow-H2S-releasing compounds. Among fast H2S-releasing compounds inorganic sulfide salts, including sodium hydrogen sulfide (NaHS) and sodium sulfide (Na2S), are commonly used. These inorganic salts have protective effects in neurological and cardiovascular disorders but show pro-inflammatory and deleterious effects in conditions such as sepsis [23,24]. Despite their protective effect, the main problem with these H2S donors is their quick, complete, and uncontrollable release in an aqueous solution. This quick release results in the loss of H2S in solution, and therefore the efficacy of these salts is compromised [25,26]. Among slow-H2S-releasing compounds, perthiol-based donors, thioamino acids (thioglycine and thiovaline), photolabile H2S donors, thioamide- and aryl isothiocyanate-based donors, S-aroylthiooximes, S-propargyl-cysteine (SPRC), dithioperoxyanhydrides, morpholin-4-ium 4-methoxyphenyl-morpholino-phosphinodithioate (GYY4137, H2S-releasing NSAIDs, e.g., H2S-diclofenac (ACS-15), sodium thiosulfate (Na2S2O3), and garlic-derived sulfur-containing compounds diallyl disulfide (DADS), and allicin are used [27]. These compounds can be used for therapeutic purposes due to their reduced toxicity, effective biological functions, and controlled H2S release. H2S donors exert their effects through several mechanisms, such as combating oxidative stress, protein persulfidation, and interacting with metal-containing proteins. H2S has inherent antioxidative activities, which decrease the production of ROS by neutralizing oxidative stress. This activity preserves the redox balance between cells and prevents cell damage [28,29]. Persulfidation is a reversible biochemical process in which H2S interacts with cysteine in proteins to make persulfide bonds. This modification is similar to phosphorylation and nitrosylation, which regulate the structure and function of proteins [30]. Furthermore, H2S interacts with several metal-containing proteins, such as chromoprotein, ferritin, molybdenum protein, zinc protein, and cuproprotein. Interaction of H2S with these proteins can affect their conformation and function through different mechanisms. These mechanisms may include the modification of coordination number, the binding ability of metals, and oxidation state [31]. These mechanisms help in mitigating inflammatory, viral, cardiovascular, and neurological disorders.
Table 1.
Mechanism of action and effects of common H2S donors in different diseases.
| H2S Donor | Mechanism of Action | Disease Models | Effects on Cells | References |
|---|---|---|---|---|
| S-propargyl-cysteine (SPRC) | Ameliorates Nrf2-ARE pathway | In rheumatoid arthritis | Anti-inflammatory | [31] |
| Suppression of pro-inflammatory cytokines | In pancreatitis-induced acute lung injury | Anti-inflammatory | [32] | |
| Decreases the ROS formation, Bax caspase 3 and 9, increase Bcl-2 expression | Myocardial infarction and heart failure | Antioxidant Promotes angiogenesis |
[33] | |
| Morpholin-4-ium 4-methoxyphenyl-morp holino-phosphinodithioate (GYY4137) |
Inhibition of P13/Akt/TLR4 pathway | Atherosclerosis | Maintain mitochondrial function | [34] |
| Suppression of pro-inflammatory cytokines and chemokines | RSV-infected cells | Anti-inflammatory | [35] | |
| Activation of Nrf2-ARE pathway and suppression of NOS2 and COX-2 expression. | In osteoarthritis | Antioxidant | [36] | |
| Decreased upregulation of iNOS, COX2, NF-кB, and STAT 3 | Endotoxemia | Anti-inflammatory | [37] | |
| Increased TGF-β expression and decreased IFN-γ and IL-17 production | Multiple sclerosis | Anti-inflammatory | [38] | |
| AP-39 | Modulation of AMPK/UCP2 pathway and decrease in ROS | Ischemia–reperfusion injury and cardiotoxicity | Antioxidant Maintain mitochondrial function |
[39] |
| Decrease in MPO and IL-6 levels, increase in IL-10 levels | Acute lung injury | Anti-inflammatory | [40] | |
| Allicin and Diallyl disulfide (DADS) | Activates Nrf2/ARE pathway | Cardiac hypertrophy and Alzheimer’s disease | Antioxidant | [41,42,43] |
| Inhibition of pro-inflammatory cytokines | In viral-associated lung injury | Anti-inflammatory | [44] | |
| Suppression of p-AKT, NOS2, and PI3K levels | In osteoarthritis | Mitigate pain | [45] | |
| S-diclofenac | Suppression of NF-κB pathway | Pancreatic and acute lung injury in acute pancreatitis | Anti-inflammatory | [46] |
| Stabilizes P53, P21, P53AIPI, and Bax | Atherosclerosis | Antiproliferative | [36] | |
| Inhibition of COX enzymes, NO production, and decreased MPO activity | Carrageenan induced hind-paw edema | Anti-inflammatory | [47] | |
| NaHS | Activation of ERK and NF-κB signaling pathways | Sepsis-induced organ damage | Pro-inflammatory | [48] |
| Activation of NF-κB and Src-family kinase signaling pathways | Acute pancreatitis | Pro-inflammatory | [49] | |
| Activation of cGMP/PKG signaling pathway | Myocardial infarction | Antioxidant | [50] | |
| Inhibition of mitochondrial transmembrane potential loss | Parkinson’s disease | Relieve mitochondrial dysfunction | [51] | |
| Inhibits IkB-a degradation and NF-κB nuclear translocation |
Atherosclerosis | Decreases expression of adhesion molecules (specifically ICAM-1) | [52] |
4. H2S Synthesis Inhibitors
To investigate the functions of H2S, scientists have used different approaches to inhibit H2S synthesis, i.e., by pharmacological agents or by H2S-producing gene silencing through siRNA. Different pharmacological agents, including aminooxyacetic acid (AOAA), hydroxylamine (HA), propargylglycine (PAG), trifluoroalanine, and β-cyanoalanine (BCA), are commonly used as H2S synthesis inhibitors [53,54]. Among these, AOAA is considered a specific inhibitor of CBS, whereas PAG and BCA selectively target the CSE activity. PAG exists in two isoforms, i.e., L-isomer and D-isomer. The L-isomer is used to inhibit CSE production, whereas the D-isomer is believed to be a nephrotoxin [55,56]. In addition to these conventional pharmacological inhibitors, novel H2S-targeting inhibitors, including aurintricarboxylic acid (NSC4056), S-3-carboxpropyl-L-cysteine (CPC), and 2-arylidene hydrazinecarbodithioates, have been developed. These compounds show significant inhibition of CSE, but not CBS, in a dose-dependent manner [57]. Furthermore, the expression of the CSE gene is inhibited through the small interfering RNA (siRNA). Our group has used siRNA as a targeted probe to suppress the expression of CSE in monocytes/macrophages, thereby reducing the production of H2S in a mouse model of sepsis. Using this approach, we showed that CSE gene silencing through siRNA inhibits H2S production in macrophages. Inhibition of H2S production by CSE gene silencing decreases the levels of pro-inflammatory chemokines and cytokines in the liver and lungs [58]. In addition to pharmacological inhibition and gene silencing, H2S synthesis can also be inhibited by some alternative pathways. These pathways include modulation in levels of cysteine (substrate for H2S synthesis) and co-factor S-adenosylmethionine (SAM), and decreasing the levels of homocysteine [59,60].
5. H2S in Inflammatory Conditions
Inflammation is a protective response of the body to harmful stimuli, including infection, irradiation, and poisonous chemicals, and functions by eliminating these harmful stimuli and starting the healing process [61,62]. Among fast H2S-releasing compounds, inorganic sulfide salts (NaHS and Na2S) show pro-inflammatory effects in sepsis by the upregulation of several pro-inflammatory mediators. On the other hand, slow H2S-releasing donors show anti-inflammatory effects by several intricate signaling pathways. These pathways include suppression of the release of inflammatory regulators, which mitigates the inflammatory response. Slow H2S-releasing donors act by inhibiting the activation of NF-κB. This inhibition decreases the release of inflammatory cytokines and reduces the infiltration of neutrophils to the site of the inflammation [63].
5.1. H2S and Arthritis/Joint Inflammation
Gout and rheumatoid arthritis (RA) are considered the most common forms of joint inflammation. The role of H2S in joint inflammation is controversial; in some cases, H2S acts as a pro-inflammatory mediator, while in others it has been reported to have anti-inflammatory effects. In clinical RA, some advancements have been made to understand the contribution of H2S, but more research is needed to clearly define its role. In order to address this gap in knowledge, our group has shown that H2S, has pro-inflammatory actions in clinical arthritis. Levels of H2S were elevated in the synovial fluid of patients with RA and gout when compared with plasma H2S levels in the same patients. Furthermore, in the case of RA, there was a significant correlation between synovial fluid H2S levels and the disease activity score. This find-ing underscores the critical role of H2S in clinical arthritis and points to its potential as a therapeutic target [64]. Another common form of arthritis that significantly affects elderly people in particular is osteoarthritis (OA) [65]. In hind-paw edema induced by carrageenan (a standard method to cause acute joint inflammation), increased levels of H2S synthesis with inflammation were observed. Prophylactic administration of PAG showed a decrease in hind-paw inflammation in a concentration-dependent manner [66]. Moreover, treatment with D-penicillamine (an anti-rheumatoid drug) downregulates the expression levels of CSE. It is proposed that this inhibition contributes to its efficacy against RA [67]. These findings suggest that H2S plays an important role in mediating inflammation in joints. On the other hand, slow H2S-releasing compounds show anti-inflammatory activity against joint inflammation. ACS 15 (a slow H2S-releasing derivative of the NSAID diclofenac) shows strong anti-inflammatory activity against carrageenan-induced hind-paw edema in comparison to its precursor NSAID [47]. In RA, SPRC also shows anti-inflammatory activity by ameliorating the Nrf2-ARE (nuclear-factor-E2-related factor-2) signaling pathway [31]. In joint inflammation, H2S regulates inflammation through several mechanisms, including (1) decreasing the expression of pro-inflammatory mediators, e.g., interleukins (IL) and tumour necrosis factor (TNF), etc.; and (2) via the inhibition of important signaling pathways, including phosphatidylinositol 3-kinase (PI3K), mitogen-activated protein kinase (MAPK), and nuclear factor-κB (NF-κB). The anti-inflammatory action of H2S in OA patients depends on both the timing of H2S administration and its concentration. Furthermore, whether the action of H2S in RA is pro- or anti-inflammatory is debatable [68,69]. There is mounting evidence suggesting a correlation between H2S production and the development of bone. The anti-inflammatory action of H2S in OA has been observed. It has been found that the levels of H2S, 3-MST mRNA expression, and proteins were decreased in OA patients compared to healthy individuals. The levels of other H2S-producing enzymes (CSE and CBS) were, however, not affected. These findings suggest that a significant decrease in the production of 3-MST in the cartilage of OA patients may contribute to the decreased production of H2S. This, in turn, may lead to the development of OA [70]. Another H2S donor GYY4137 (a modified form of Lawesson’s reagent) gained attention in scientific research due to its slow H2S-releasing ability and sustainability in aqueous solution in comparison to sulfide salts [71]. In in vitro studies, GYY4137 has been reported to have strong anti-inflammatory and cartilage-protective effects in human synoviocytes (HFLS) and articular chondrocytes (HAC). In complete Freund’s adjuvant (CFA)-induced joint inflammation in mice, the anti-inflammatory effects of GYY4137 are associated with the timing of administration [72]. In rats, the levels of H2S were increased 30 min after the administration of GYY4137 and persisted for approximately 3 h [73]. Allicin (a sulfur-containing compound in garlic) exhibits anti-inflammatory properties and can act as a therapeutic agent for joint inflammation [74]. ATB-346 (naproxen-derived H2S donor) is also useful to alleviate pain induced by OA [75]. Slow H2S-releasing compounds such as AP39 have been developed, which particularly affect mitochondrial function. During oxidative stress, AP39 has been reported to show antioxidative and cytoprotective effects. Interestingly, these effects of AP39 are observed at a concentration between 30 to 100 nM, which is considerably lower in comparison to GYY4137, NaHS, and Na2S. This shows that the efficacy of AP39 is higher than other H2S donors even at lower concentrations [76]. This finding suggests the therapeutic potential of H2S-releasing compounds that particularly affect mitochondrial function. Therefore, development of H2S-based drugs can contribute to the treatment of joint inflammation.
5.2. H2S and Sepsis
Sepsis is defined as a serious life-threatening complication that results in organ dysfunction due to dysregulation of the host’s immune response to an infection [77]. It has been reported that development of sepsis is not only confined to the recognition of microbial or host patterns and inflammation. It also severely affects microcirculation, immune tissues, endothelial tissues, microglial cells, neurons, and parenchymal tissues [78,79]. Sepsis is considered to be one of the leading causes of death, which usually affects about 30 million people annually. Sepsis remains a major health concern globally due to the increasing incidence in recent years [80,81]. Sepsis is a systemic disorder that affects the vascular system, cardiovascular system, lungs, central nervous system, and liver [82]. The role of H2S in sepsis has been widely studied, and it has been shown that it plays a significant role in the pathogenesis of this condition. The role of H2S in sepsis is, however, subject to a lot of discussion in the field. Some studies found that an increased level of H2S in sepsis can exacerbate inflammation and organ damage, whereas controlled H2S release can show protective effects. It was observed that there is a correlation between sepsis and H2S levels. In rodents, cecal ligation and puncture (CLP) is a standard and clinically relevant model to induce sepsis. Following CLP-induced sepsis in mice, increased levels of plasma H2S and upregulation of the expression of CSE were observed. It was found that administration of PAG (prophylactically and therapeutically) in CLP-induced mice reduced MPO activity and changes in lung and liver tissues and improved sepsis-associated inflammation. On the other hand, following treatment with NaHS, sepsis-induced organ damage was exacerbated [83]. Administration of dexamethasone in a mouse model of LPS (lipopolysaccharide)-induced endotoxemia significantly decreased the activity of iNOS, the expression of CSE, and ultimately the plasma levels of H2S [84]. In mice with CLP-induced sepsis, increased levels of H2S-activated NF-κB signaling pathways were observed. This increase in turn upregulated the expression of chemokines (MCP-1 and MCP-2), adhesion molecules (P-selectin, E-selectin, ICAM-1, and VCAM-1), and cytokines (TNF-α, IL-1β, and IL-6) [85]. Furthermore, it was shown that the ERK pathway played a significant role in activating the NF-κB signaling pathway during sepsis. Treatment with ERK inhibitor (PD98059) inhibited the activity of NF-κB and sepsis-induced systemic inflammation, as well as decreasing organ damage. Inhibition of CSE with PAG administration reduced the levels of H2S and showed a better prognosis in the liver and lungs, whereas higher H2S levels caused a hemodynamic collapse [86]. In an in vitro study, an effect of treatment with FW1256 (slow-H2S releasing compound) to the LPS-treated mouse macrophage cell line RAW 264.7 was observed. It was found that FW1256 decreased the production of IL-6, COX2, PGE2, iNOS, TNF-α, and IL-Iβ and also inhibited the activity of NF-κB [87]. Moreover, in LPS-treated THP-1 macrophages, prophylactic treatment with different doses of NaHS (0.01 mM, 0.1 mM, and 1 mM) has been reported to decrease pro-inflammatory cytokine production. The anti-inflammatory effect of NaHS was attributed to epigenetic modifications in histones [88].
Administration of NaHS to the cell line (U937) showed upregulation of pro-inflammatory cytokines, including IL-1b, IL-6, and TNF-a, through ERK and NF-кB signaling pathways [48]. Furthermore, in another study, treatment of cell line RAW264.7 with LPS showed overexpression of CSE, chemokines, and cytokines, whereas CSE gene silencing decreased the production of these pro-inflammatory mediators [89]. Pro-inflammatory roles of H2S were also observed in LPS-induced endotoxemia in rats and mice [90,91]. In LPS-induced endotoxemic rats, ACS-15 showed anti-inflammatory actions by preventing the production of endogenous H2S [92]. This finding suggests that slow H2S-releasing donors can exhibit anti-inflammatory actions by blocking the synthesis of endogenous H2S through negative feedback [93]. GYY4137 also shows anti-inflammatory actions against LPS-induced endotoxemia in the rat model by inhibiting NF-кB activation, iNOS, and COX2 expression. Treatment with Na2S2O3 also decreased liver and lung injury and neuroinflammation and preserved mitochondrial functions in the mice model. The anti-inflammatory effects of Na2S2O3 were associated with the presence of CSE, as in CSE−/− mice, Na2S2O3 did not exhibit anti-inflammatory effects. This finding points to the role of CSE in regulating the protective actions of Na2S2O3 [94]. Furthermore, we have shown that liver sinusoidal endothelial cells (LSECs) also contribute to the pro-inflammatory effects of H2S in sepsis. The liver sinusoid is important for maintaining the function of the liver, but its homeostasis is disturbed in endotoxemia and sepsis. It is believed that in endotoxemia H2S shows vasoconstriction in liver sinusoids [95,96]. In wild-type (WT) mice, increased gap formation and defenestration in LSECs was observed due to increased H2S levels compared to CSE knockout mice. It was also observed that a key factor in H2S-induced liver sinusoidal dysfunction is the activation of the substance P-tachykinin receptor 1 axis and the ERK1/2-NF-κB p65 pathway [97,98]. Furthermore, in septic patients and in mice with LPS-induced endotoxemia, similar dysregulation of H2S has been observed [99,100,101].
Substance P (SP) and neurogenic inflammation are important mediators that contribute to the inflammatory action of H2S in sepsis. Treatment with capsaicin (responsible for the depletion of SP from sensory neurons) alleviates H2S-induced lung inflammation in mice. In sepsis, the levels of SP and the expression of pulmonary PPT-A (preprotachykinin A—the gene encoding SP) are upregulated. Administration of PAG downregulates the expression of the PPT-A gene and the levels of SP in lungs, but NaHS administration elevates SP levels [102]. Furthermore, PPT-A gene deletion and prophylactic treatment with SP inhibitor (L703606) shows that SP interacts with H2S and plays an important role in the pro-inflammatory effects of H2S. Moreover, H2S triggers systemic inflammation and multiple organ dysfunction in sepsis through transient TRPV-1 (transient receptor potential vanilloid-1)-mediated neurogenic inflammation [103]. We have shown that in sepsis, H2S regulates TRPV-1-mediated neurogenic inflammation by increasing the production of SP and by activating the ERK–NF-κB signaling pathway [104]. In sepsis, H2S activates TRPV-1, which in turn upregulates the PGE metabolite and COX2 levels, resulting in acute lung injury [105]. However, treatment with capsazepine, an antagonist of TRPV-1, also mitigates lung inflammation induced by H2S. These findings suggest that SP and neurogenic inflammatory pathways play an important role in inflammation caused by H2S [106].
These studies highlight the complex and biphasic role of H2S in sepsis, where it can show either pro- or anti-inflammatory effects. These effects depend upon several factors, including H2S release rate, concentration, and its interaction with specific signaling pathways and the cellular environment.
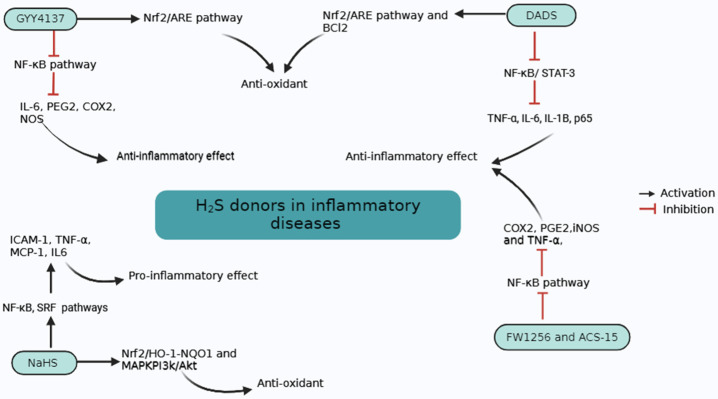
Figure 3 summarizes our current understanding of the role of H2S in inflammatory diseases and mechanisms of action of H2S donors in these diseases.
Figure 3.
Mechanisms of action of H2S donors in inflammatory diseases. GYY4137 and DADS inhibit NF-κB pathways and activate the Nrf2/ARE pathway, which inhibits pro-inflammatory cytokines and activates antioxidant pathways, respectively. NaHS activates NF-κB and SRF pathways and activates pro-inflammatory mediators. NaHS also activates antioxidant pathways. FW1256 and ACS-15 also suppress the NF-κB pathway and show anti-inflammatory effects.
5.3. Acute Pancreatitis
Excessive consumption of alcohol, gallstones, and increased levels of triglycerides in the blood usually result in acute inflammation of the pancreas, known as acute pancreatitis (AP) [107]. This condition is generally associated with increased circulating levels of digestive enzymes (amylase and lipase) and abdominal pain [108,109]. Inflammatory responses in severe cases of AP trigger both localized as well as systemic complications, which makes the treatment of AP even more complicated [110,111]. Localized pathological hallmarks of AP include infected necrosis, acute necrotic collections (ANCs), acute peripancreatic fluid collections (APFCs), pseudocysts, and walled-off necrosis (WON). Systemic pathological hallmarks of severe AP are shock, and kidney and respiratory failure. In AP, the most common contributing factor to death is believed to be the multiple organ dysfunction syndrome (MODS) [112]. A major component of MODS is lung injury, that is clinically manifested as acute respiratory distress syndrome (ARDS). It has been observed that production of H2S is significantly increased in AP. H2S acts as a pro-inflammatory mediator in AP due to the abundance of CSE and CBS in the pancreas [113]. Different approaches (such as silencing of the CSE gene by siRNA, inhibition of H2S synthesis by pharmacological agents, and deletion of the CSE gene) were used to check the pro-inflammatory effects of H2S in AP [114]. In an in vivo study in cerulein-induced AP (cerulein is a cholecystokinin analogue commonly used to induced pancreatitis) in mice, inhibition of CSE by PAG decreased H2S synthesis, which led to decreased inflammation. This decrease in inflammation was associated with the downregulation of MIP-1α, MIP-2, and MCP-1 [22]. Furthermore, it was observed that H2S may exacerbate AP by the PI3K/Akt/Sp1 signaling pathway [115]. In addition to H2S, levels of ammonia in plasma and tissues were also elevated in severe cases of AP. CBS also plays a role in the progression of AP. In in vivo experiments, AOAA administration to mice helps to mitigate AP and associated acute lung injury [116]. Treatment with slow H2S-releasing compounds, including SPRC, and S-diclofenac, showed anti-inflammatory effects against pancreatic and lung injury during AP. The protective role of these compounds can be due to the suppression of the NF-κB signaling pathway [32,46]. Administration of NaHS leads to the activation of ICAM-1 expression and the adhesion of neutrophils to the isolated acinar cells treated with cerulein. This process is mediated by the activation of NF-κB and Src-family kinase signaling pathways [49]. In addition to cytokines, adhesion molecules, and chemokines, H2S also plays a key role in inflammation associated with AP through its interaction with SP. Treatment with NaHS showed a significant increase in levels of SP in the plasma and inflammation in lungs in a mouse model of AP [106]. In PPT-A−/− mice, however, there was significant protection against AP-associated lung inflammation. In addition, administration of CP-96345, an antagonist of neurokinin-1 receptor-NK-IR (SP receptor) showed a protective response against lung inflammation triggered by H2S. Administration of PAG decreased the elevated levels of SP in the lungs, pancreas, and plasma in severe cases of AP. In addition, in mice with AP, PAG treatment also decreased the levels of expression of NK-1R and PPT-A in the lungs and pancreas. These findings indicate that the pro-inflammatory effects of H2S in AP can be regulated through SP [117]. Additionally, studies with isolated pancreatic acini suggest that H2S enhances the Toll-like receptor 4 pathway in AP and NF-κB activity through SP [118]. These studies show that H2S plays a crucial role in the progression of AP, and it can act as a potential therapeutic target.
6. H2S and Diseases of the Cardiovascular System
CVD (cardiovascular disease) includes all diseases related to the heart and the vascular system. CVDs are considered to be the leading cause of death globally, which results in an increased economic burden [119]. CVD can be caused by physical inactivity, a sedentary lifestyle, and poor dietary patterns, etc. [120]. A substantial body of evidence has revealed that H2S can improve and play a protective role in many diseases, including atherosclerosis, cardiac hypertrophy, ischemia–reperfusion injury, myocardial infarction, cardiac arrhythmia, and heart failure (HF) [121]. It has been reported that H2S has a beneficial role in cardiovascular diseases. H2S plays a cardioprotective role by regulating several mechanisms, such as alteration in mitochondrial autophagy, inhibiting pro-inflammatory cytokines, inhibiting endothelial mesenchymal transition, and preventing lipid peroxidation and antioxidative stress. [122,123]. H2S is a key regulator in maintaining the oxidation/reduction balance by neutralizing ROS in CVD. It shows antioxidant properties by altering L-cysteine amino acid residues at different signaling molecules, including HIF-1α, keap1/Nrf2, and NF-κB [124]. Ischemia–reperfusion (I/R) injury often leads to the destruction of myocardial tissues and eventually HF. Reperfusion helps in the treatment of ischemia but also activates a complex series of reactions that cause oxidative damage and inflammation, which eventually causes cell injury [125]. It has been reported that the exogenous administration of H2S in ischemic rat hearts resulted in the activation of the JAK2 pathway, which increased the phosphorylation and activation of STAT3 [126]. In in vivo experiments, prophylactic treatment with sulfur dioxide (SO2) decreased I/R-induced heart damage, while elevated antioxidant activity and upregulation of CSE expression were observed [127]. In a clinical study, individuals with ACS (acute coronary syndrome) showed significantly lower levels of H2S. Expression of CX3CL1 and CCL2 (chemokines) was upregulated as compared to non-CAD (coronary artery disease) and angina patients [128]. Furthermore, an increased risk of CVD was observed in chronic hemodialysis patients due to the activation of protein kinase C beta II (PKCβII), overexpression of adhesion molecules (VCAM-1 and ICAM-1), and downregulation of CSE [129]. In ApoE knockout mice and Ox-LDL-stimulated human aortic endothelial cells (HAEC), aortic and plasma H2S levels were reduced during atherosclerosis [130]. An effect of H2S donor (NaHS) and endogenous H2S inhibitor (PAG) in atherosclerosis has been reported. In an in vivo study, treatment with NaSH significantly increased the levels of plasma H2S in the ApoE−/− mouse model. This increase in plasma H2S levels reduced the size of atherosclerotic plaques and levels of ICAM-1 in plasma and the aorta, which improved atherosclerosis. On the other hand, treatment with PAG decreased the levels of plasma H2S. This decrease in plasma H2S levels increased plaque size and levels of ICAM-1 in plasma and exacerbated atherosclerosis. In an in vitro study of TNF-α treated human umbilical vein endothelial cells (HUVECs), prophylactic treatment with NaHS inhibited the expression of ICAM-1 [52]. In rats, the release of myocardial enzyme and myocardial infarct size was prevented by H2S. This prevention was due to the inhibition of oxidation, activation of Sirt1/PGC1α signaling pathways, and attenuation of the activation of inflammatory cytokines [131,132]. Furthermore, in in vivo experiments, H2S showed a protective role in the development of myocardial injury by angiogenesis through activating the vascular endothelial growth factor (VEGF) and hindering the activation of parastatin [133]. It was found that plasma and aortic H2S levels were significantly decreased in the high-fat diet (HFD) cardiomyopathy mouse model [134]. Cardiac remodeling is influenced substantially by elevated levels of homocysteine in blood (hyperhomocysteinemia). Hyperhomocysteinemia plays a role in the progression of myocardial hypertrophy by the formation of the MEF2C-HDAC1 (myocyte-specific enhancer factor 2C/histone deacetylases) complex. This complex leads to the inactivation of MEF2C and suppresses miR-133a expression in the heart muscles. On the other hand, H2S activates MEF2C and initiates miR-133a, which results in the prevention of cardiac hypertrophy [135]. In in vivo experiments, it was observed that mice deficient in CBS (CBS+/−) showed upregulation of CSE expression, which suggests that a negative correlation exists between the regulation of these two enzymes [136]. Prolonged activation of angiotensin II (a hormone responsible for regulating blood pressure) can initiate cardiac remodeling. Renin-angiotensin-aldosterone-based pharmacological agents are used to treat cardiac remodeling. Among these agents, angiotensin receptor blockers and ACE (angiotensin-converting enzyme) inhibitors are commonly used [137]. H2S inhibits the activity of angiotensin II in heart tissues, which mitigates myocardial hypertrophy and fibrosis [138]. It has been reported that Cx43 levels in cardiomyocytes were upregulated by endoge-nous H2S, suggesting that H2S contributes to the maintenance of arrhythmia and car-diac health [139]. Angiotensin II activates the Kruppel-like factor 5 (KLF5) gene, which in turn results in cardiac remodeling. KLF5 is a key regulator in cardiac remodeling and affects different conditions, such as angiogenesis, cardiac fibrosis, thickening of arterial walls, and enlargement of the heart. In an in vivo study, a decrease in the thickening of the arterial wall was observed in vascular-induced injury in KLF5−/− mice. In addition, H2S downregulates the expression of the KLF5 gene, which results in alleviating cardiac hypertrophy [140]. In in vitro studies, upon the exposure of human atrial fibroblasts (HAF) to H2S, a decrease in arterial fibrosis was observed [141]. At a molecular level, the CSE/H2S pathway modulates eNOS expression by the PKC βII/Akt signaling pathway and shows cardioprotective effects against atherosclerosis [142]. CBS gene mutations also play an important role in the progression of CVD and endothelial dysfunction by interacting with the mitochondrial function [143]. A transmembrane protein Cx43 (connexin) is primarily present in the ventricle and exhibits a significant link with cardiac arrhythmias [144]. Moreover, plasma and myocardial levels of H2S were decreased during cardiac failure. Furthermore, after transverse aortic constriction (TAC), increased abnormal cardiac function and dilation were seen in CSE−/− mice in comparison to CSE+/+ mice. On the other hand, after TAC, CSE overexpressing-mice exhibited cardioprotective effects by using endogenous H2S. Due to the limited research, however, there is no evidence concerning the expression of 3-MST and CBS transgenic mice [10]. In in vivo experiments, upon exposure to 4-carboxyphenyl isothiocyanate (4-CPI), a significant decrease in ventricular arrythmias and myocardial infarct size was observed in rat heart [145]. In addition, treatment of isolated rat hearts with another H2S donor AP39 attenuated IR injury by reducing harmful oxidative molecules and blocking the opening of mitochondrial permeability transition pore (PTP) [146]. Alpha lipoic acid (ALA), a novel organosulfur compound, also showed cardioprotective effects in post-I/R arrhythmias by regulating KATP channels. This effect of ALA can be due to the direct release of sulfur and H2S together [147]. In isolated pig and mice hearts, zofenopril (ACE inhibitor) alleviates myocardial infarct size (post-IR injury) by increasing H2S and NO production [148]. Administration of NaHS in rabbit hearts activated the cGMP/PKG signaling pathway, which helped in decreasing the size of myocardial infarcts [50]. In mice with left coronary artery (LCA) occlusion-induced HF, improved left ventricular function and a significant decrease in myocardial hypertrophy were seen after administration of Na2S [149]. Administration of SPRC upregulated the expression of CSE and increased the H2S concentration, which showed protective effects against myocardial infarction by decreasing ROS production [150]. Furthermore, another H2S donor GYY4137 has been reported to provide cardioprotective effects in atherosclerosis by regulating the PI3K/Akt/TLR4 signaling pathway [34]. GYY4137 shows beneficial effects by inhibiting myocardial fibrosis, reducing oxidative stress and inflammation [151]. In high fructose-fed insulin-resistant rats, it was demonstrated that administering a freshly prepared garlic homogenate, known to produce H2S upon interacting with cellular proteins, activates the myocardial Nrf2 via the PI3K/Akt pathway. This activation helps reduce cardiac hypertrophy and oxidative stress by enhancing the antioxidant defense system [41]. A novel H2S donor SG1002 maintained vascular homeostasis by preventing mitochondrial dysfunction and improving myocardial vascular density in individuals with HF [152]. Recently, it has been found that SG1002 upregulates the expression of H2S-producing enzymes, which activates the nuclear Nrf2 pathway. Activation of the Nrf2 pathway decreases the oxidative stress as well as ROS formation and protects against cardiac hypertrophy and HF [153]. Furthermore, in ACS patients, intravenous administration of Na2S2O3 (inorganic sodium salt with thiosulfate ions–a clinically approved drug) up to 15g is considered safe [154]. Many novel therapeutic targets, such as mitofusin 2 (Mfn2-mitochondrial protein), still need to be explored in CVD. H2S plays a significant role in regulating Mfn2, and its abnormal function can result in IR, dilated cardiomyopathy, and HF [155,156]. Although H2S is known to regulate Mfn2, currently there are no data on the use of H2S donors specifically in the treatment of Mfn2-related cardiovascular diseases. Targeting this protein with H2S donors could be an important and innovative area for future research [157].
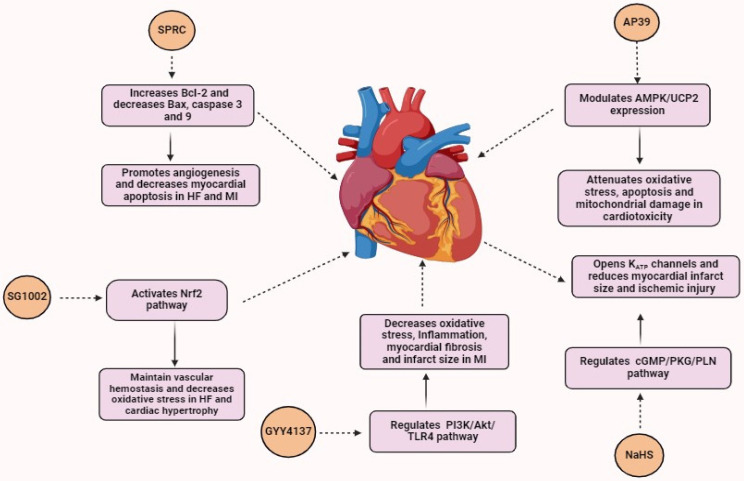
Figure 4 summarizes our understanding of the role of H2S in CVD and the mechanism by which it acts.
Figure 4.
Role of H2S donors in CVD. SPRC promotes angiogenesis and decreases myocardial apoptosis in HF and MI. SG1002 maintains vascular hemostasis and decreases oxidative stress in HF and cardiac hypertrophy. GYY4137 decreases oxidative stress, inflammation, myocardial fibrosis, and infarct size in MI. NaHS opens up KATP channels and reduces myocardial infarct size and ischemic injury. AP39 attenuates oxidative stress, apoptosis, and mitochondrial damage in cardiotoxicity.
7. H2S and Viral Diseases
Viral infections are considered a major cause of mortality as well as a major economic burden globally in the clinical setting. Members of the Paramyxoviridae family, such as hMPV (human metapneumovirus), RSV (respiratory syncytial virus), and NiV (Nipah virus, a zoonotic virus) contribute to major respiratory tract infections. These infections include pneumonia, asthma progression, bronchiolitis, and cough [158,159]. Despite the severe infections caused by these contagious viruses, currently there are no vaccines or effective therapeutic strategies except prophylactic immunization for RSV. It was found that in AECs (airway epithelial cells) these viruses activate several regulators of inflammation, such as cytokines and chemokines, which contribute to disease development [160]. In infected cells, gene expression of these regulators is mediated by the activation of IRF-3 (interferon regulatory factor 3) and NF-κB signaling pathways. In in vitro experiments, AECs were infected with RSV to check the role of endogenous H2S levels in viral infections. In RSV-infected AECs, the expression of CSE was downregulated while the decomposition of H2S was increased compared to normal cells. Furthermore, increased expression of pro-inflammatory cytokines and viral particles was measured after the inhibition of endogenous H2S by PAG. This finding sheds light on the protective role of endogenous H2S in viral replication. The underlying mechanism of endogenous H2S in preventing viral infection is not clearly understood. It has been observed that in viral infections, levels of Nrf2–ARE pathway-associated antioxidant enzymes and ROS are significantly increased, which results in impaired antioxidant responses. To control the infections caused by respiratory viruses, activation of the Nrf2 pathway and induction of long-term antioxidant activity can be a potential strategy [161]. H2S activates several antioxidant mechanisms, including NADPH oxidase enzyme inhibition and increased activation of Nrf2 translocation to the nucleus. It suggests the role of endogenous H2S in protecting against pulmonary viral infections. Despite directly activating antioxidant mechanisms, H2S also contributes to antioxidant activity through various indirect effects. For example, increased levels of glutathione (GSH) (an important compound with antioxidant activity as well as an efficient scavenger of ROS) are maintained by endogenous H2S [162]. GSH is reported as an important compound to inhibit viral infections. However, in HSV-1 (herpes simplex virus type 1), levels of GSH were surprisingly decreased, whereas after GSH administration, viral replication was inhibited [163]. Recently, it has been highlighted that GSH shows therapeutic potential against COVID-19 by interacting with human proteins such as TMPRSS2 (transmembrane serine protease 2) and ACE2 (angiotensin-converting enzyme 2). These proteins are important in the adhesion and entry of viral cells in a host cell [164]. Furthermore, a close relationship between endogenous H2S levels and mortality rate among 74 SARS-CoV-2 patients has been observed. The findings of this study suggest that levels of H2S were significantly elevated on days 1 and 7 in patient survivors on the 28th day compared to non-survivors. Specifically, a total sulfide pool (which measures H2S levels) below 150.44 μM at the outset was associated with an elevenfold increase in the risk of dying within 28 days. A reduction in H2S levels of more than 36% from day 1 to day 7 correlated with higher rate of 28-day mortality. This finding indicates that lower H2S levels during SARS-CoV-2 infection may exacerbate the infection [165]. In RSV-infected mice, the administration of GYY4137 reduced the activation of different factors, including GM-CSF (granulocyte-macrophage colony-stimulating factor) and G-CSF (granulocyte-colony stimulating factor). However, a decrease in levels of pro-inflammatory mediators, including TNF-α, IL-6, IL-1, KC, IFN-α, IFN-β, MCP-1, MIP-1α, and -β was also observed. In in vivo experiments, in RSV-infected mice a significant decrease in RSV titer and neutrophilia in lungs was observed after the treatment with GYY4137 in comparison to normal controls [166]. Treatment with DADS has elevated the level of GSH in lung tissues and prevented the release of pro-inflammatory cytokines (IL-6, IL-8, and TNF). Inhibition of pro-inflammatory cytokines was linked to decreased recruitment of inflammatory cells in the lungs notably reducing neutrophil infiltration [44]. In mice with LPS-induced acute lung injury, it has been observed that sulforaphane (novel H2S donor belonging to isothiocyanate) inhibited the release of pro-inflammatory cytokines [167]. The transcription factor Nrf2 played an important role in the lung protection mediated by sulforaphane by enhancing mitochondrial function and energy metabolism. These findings suggest that exogenous administration of H2S can protect host cells from severe viral infections [168]. Although various studies have indicated the antiviral effects of H2S, these effects are not universal. For example, rotavirus infection was not inhibited by XM-01 (a cysteine-based perthiol derivative), which suggests that H2S does not have a protective effect against non-enveloped viruses [169]. This limitation is associated with the mechanism of action of XM-01, i.e., by inhibition of membrane fusion (enveloped viruses), whereas non-enveloped viruses penetrate host cells via endocytosis. These results suggest that H2S can be considered a potential therapeutic target against infections caused by enveloped viruses. The role of H2S has also been studied in several other respiratory tract infections [170]. In rats, administration of NaHS significantly improved ventilator-induced lung injury (VILI) by inhibiting ATF4 nuclear expression and PERK phosphorylation. In L2 cells, NaHS treatment decreased the endoplasmic reticulum (ER) stress and autophagy, which improved VILI [171]. During cellular stress conditions, 4-PBA (4-phenylbutyric acid; ER-stress inhibitor) and NaHS inhibited the activation of ERK, NF-κB, p65, MAPK, p38, and JNK pathways [172]. H2S alleviates VILI by neutralizing the generation of ROS through the PI3K/Akt signaling pathway [173]. H2S also plays a significant role in pulmonary physiology and in the development of asthma. Decreased enzymatic H2S production can exacerbate the onset of asthma [174]. On the other hand, high doses of H2S (300 ppm) can enter blood circulation through the pleural membrane and result in vascular changes and hypoxemia [175]. In clinical studies, it has been found that exogenous administration of H2S downregulates the phosphorylation of p38 and ERK1/2 pathways. Inhibition of these pathways results in the suppression of pro-inflammatory mediators and cell proliferation [176]. Furthermore, clinical trials have revealed that higher levels of serum H2S are linked to improved lung function (as measured by forced expiratory volume 1.0) in asthma patients. Higher levels of H2S in serum were associated with lower numbers of neutrophils in the sputum, which indicates reduced inflammation in the airways of asthma patients [177]. However, Na2S2O3 also shows antiviral and anti-inflammatory activities through activating the Nrf2/ARE pathway. This compound is widely used in the treatment of calciphylaxis and cyanide poisoning. It can also serve as an important therapeutic agent for the treatment of SARS-CoV-2 [178]. In children, Na2S2O3 significantly reduced acute lung injury associated with pneumonia [179]. Furthermore, Na2S2O3 inhalation also decreased acute lung injury associated with SARS-CoV-2 infection [180]. These findings suggest that the development of H2S-based therapeutics can be a potential target in the treatment of viral infections.
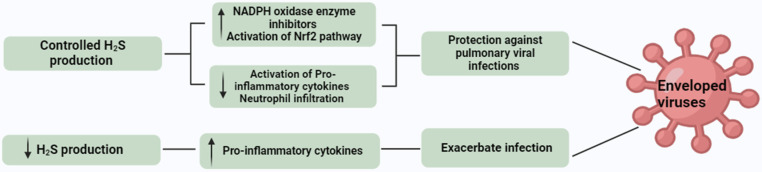
Figure 5 summarizes our current understanding of the role of H2S in viral infections.
Figure 5.
Role of H2S in viral infections. Controlled production of H2S provides protection against pulmonary viral infections. Decreased H2S production exacerbates infection.
8. H2S and CNS Diseases
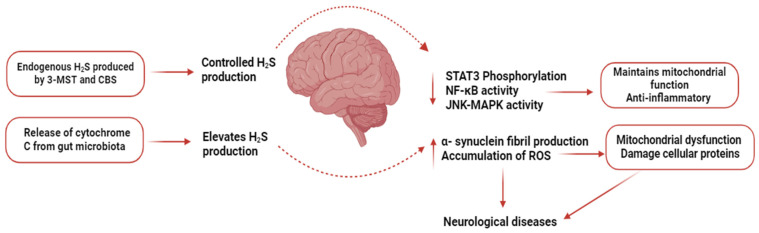
Central nervous system-related diseases are usually caused by any damage to peripheral nerves, the brain, spinal cord, and neurodegeneration. Globally, these diseases are considered to be the second major cause of morbidity as well as mortality and affect billions of people annually [181]. Neurodegenerative disorders such as Huntington’s disease (HD), Alzheimer’s disease (AD), and Parkinson’s disease (PD) are characterized by the degeneration of nerve cells. CBS and 3-MST are key enzymes for the production of H2S in CNS, while 90% of H2S is synthesized by 3-MST in the brain [182]. 3-MST is abundantly present in neurons, and it is responsible for the regulation of mitochondrial H2S for neurodegenerative processes [183]. CBS, on the other hand, is present in supporting cells, i.e., microglia, astrocytes, and oligodendrocytes. CBS is responsible for neurological functions, including synaptogenesis, maintaining the permeability of the blood–brain barrier, and neurogenesis [184,185]. H2S increases long-term potential (LTP) by activating N-methyl-D-aspartate (NMDA) receptors in neurons. NMDA receptors help in preventing oxidative damage and dysfunction of mitochondria through ROS and neutralizing free radicals [186]. H2S also triggers calcium homeostasis in astrocytes, regulates apoptotic signaling in neurons, and activates anti-inflammatory responses in microglia and astrocytes [187,188]. Findings of these studies emphasize that H2S acts as a neuromodulator and shows a protective response against neurodegenerative disorders. These neuroprotective effects are due to its anti-neuroinflammatory, antioxidant, and anti-apoptotic properties. AD is a neurological disorder that is associated with impaired cognitive functions and loss of memory. This condition commonly occurs due to pathological changes such as increased phosphorylation of tau proteins and accumulation of β-amyloid plaque in the brain and correlated with oxidative stress [189]. It has been suggested that amyloid-beta (Aβ) inhibits EAAT3-excitatory amino acid transporter 3 (transporter of cysteine), which disrupts H2S signaling. Consequently, this disruption in H2S signaling leads to neuronal degeneration and oxidative stress [190,191]. It has been reported that in AD individuals, levels of H2S in the brain and plasma were significantly reduced in comparison to the normal control group, which emphasizes the neuroprotective role of H2S in AD [192]. However, a negative correlation exists between levels of plasma H2S and the progression of AD. In the trans-sulfuration pathway, H2S is produced from homocysteine by the catalytic action of CBS, whereas SAM acts as a co-factor for CBS [193]. Although in AD patients, levels of homocysteine were higher, and levels of SAM were lower, no difference in the levels of CBS between AD patients and normal individuals was observed. These findings indicate that lower levels of H2S may be due to the reduced activity of CBS, which supports the hypothesis that the CBS-H2S signaling pathway is affected by AD [194,195]. In a mouse model of AD, it was found that H2S shows neuroinflammatory effects through different pathways, for example, by inhibiting phosphorylation of STAT3, preventing NF-κB activation pathways in the hippocampus, triggering cathepsin S (Cat S), and maintaining the function of mitochondria by the JNK-MAPK signaling pathway in microglia [196,197]. Contributions of CSE and CBS to health and disease are relatively well documented, but more research is needed to explore the role of 3-MST in pathophysiological conditions. In the AD mouse model, it has been observed that the 3MST/H2S pathway plays a significant role in memory and cognitive function. In the hippocampus and cortex regions, the activity of 3-MST was significantly decreased. Treatment with 3-MST substrate (sulfanegen) significantly protected against memory loss and cognitive impairment caused by 3-MST [198]. Another neurodegenerative disorder caused by a deficiency in dopaminergic neurons in the SN (substantia nigra) and loss of dopamine in the striatum is termed PD [199]. Cognition impairment is commonly caused by neuro-pathological hallmark events, such as the deposition of misfolded proteins, oxidative stress, abnormal mitochondrial function, and neuroinflammation [200]. A substantial body of evidence suggest that H2S protects PD patients from oxidative stress by exerting an antioxidant effect [201]. In a mouse model of PD, it was found that H2S regulates mitochondrial function by activating the JNK-MAPK pathway, which alleviates cell apoptosis [202]. Controlled production of CBS-mediated H2S exerts anti-neuroinflammatory effects in astrocytes, Whereas increased synthesis of H2S produced from gut microbiota releases cytochrome C, which elevates α-synuclein fibril production and triggers the accumulation of ROS [203,204]. These findings suggest that the neuroprotective effect of H2S is concentration-dependent. Another neurodegenerative disorder characterized by a mutation in the huntingtin gene and its deposits in neurons, which specifically targets corpus striatum, is known as HD [205]. Destruction in the corpus striatum affects the motor activity of the brain and results in cognitive impairment, involuntary movements of muscles (such as chorea), and changes in behavior [206]. Defective huntingtin proteins affect many cellular as well as metabolic activities, such as mitochondrial activity, DNA repair, redox homeostasis, nuclear-cytoplasmic transport, autophagy, transcription, and translation [207,208,209]. Among these processes, H2S regulates several, and many studies have shown that disruption in cysteine metabolism causes HD. Despite many treatment options being available for patients, the prognosis of neurodegenerative diseases is compromised. The neuroprotective role of H2S specifically in 3xTg (triple transgenic—PSEN1 mutation M146V, MAPT P301L tau mutation, and Swedish APPKM670/671 NL mutation, a widely used model to induce AD) mice was observed [210]. It was observed that daily intraperitoneal administration of GYY4137 for three months resulted in improvement in both cognitive as well as motor function. Administration of NaHS with sulfur water intraperitoneally for 12 weeks improved brain functions in a 3xTg-AD mouse model [211]. Furthermore, a significant decrease in the deposition of β-amyloid plaques was observed in parts of the brain (hippocampus and cortex). This decrease was associated with the inhibition of ERK, P38, and JNK signaling pathways against hyperphosphorylation of tau proteins and neuroinflammation. Allicin also exhibits neuroprotective effects in AD patients. It inhibits the activity of butyrylcholinesterase and acetylcholinesterase (enzymes responsible for decreasing the levels of acetylcholine and in the progression of AD). In AD, the use of allicin slows down neuronal death, decreases the level of tau proteins, and improves impaired cognitive functions [42,43]. In another study, it was observed that administration of AP39 in APP/PS1 double-transgenic mice (AD model) by intraperitoneal injection for six months daily improved mitochondrial and cellular functions in neurons. Furthermore, AP39 inhibited mitochondrial DNA damage, increased antioxidative activity, and increased the generation of ATP within neurons in comparison to a normal control group [212]. In an in vivo study, in a 6-hydroxydopamine (6-OHDA)-induced PD rat model, it has been found that natural H2S levels were reduced in the SN. Conversely, administration of external H2S was found to elevate these levels and prevent movement disorders. This was achieved by restoring the levels of tyrosine-hydroxylase-positive neurons in the SN. This effect is potentially due to enhanced leptin signaling and elevated malondialdehyde levels in the striatum. Furthermore, the administration of NaHS protected SH-SY5Y cells from mitochondrial transmembrane potential loss, apoptosis, and oxidative stress induced by the neurotoxin 1-methyl-4-phenylpyridinium (MPP+) in a PD mice model [51]. In the 1980s and 1990s, AOAA was used for the treatment of HD and tinnitus in clinical studies. It was found that AOAA did not show strong therapeutic effects, which limits its development in a clinical setting. However, AOAA did not show any adverse effects during these clinical trials either [213,214]. Abnormal mitochondrial function and oxidative stress are believed to be the main causes of HD, AD, and PD. H2S provides protection against neurological disorders through the upregulation of GSH, Nrf2, SIRT-1, GSK3β, and Keap1 and downregulation of pro-inflammatory mediators, JNK-MAPK, and Bax caspase 3/9/12 signaling pathways. Regulation of these pathways provides anti-apoptotic, antioxidative, and anti-inflammatory effects. These pathways can also affect the activities of proteins by sulfhydration [4]. These findings suggest that controlled H2S production shows a neuroprotective role, and H2S donors can be used as potential agents for treating neurodegenerative disorders.
Figure 6 summarizes our current understanding of the role of H2S in neurological disorders.
Figure 6.
Role of H2S in neurological disorders. Controlled H2S production helps in the maintenance of mitochondrial function and provides anti-inflammatory effects, whereas increased H2S production results in mitochondrial dysfunction and damages cellular proteins.
9. Uses of H2S Donors in the Clinical Setting
In preclinical studies, H2S has been widely recognized for its therapeutic potential in different conditions. However, translating this knowledge to the clinic has been a challenge for researchers due to its pharmacokinetic limitations. For instance, Na2S and NaHS are commonly used in research, but their immediate and uncontrollable release limits their usefulness in clinical settings. Organosulfur garlic-containing compounds (DATS and DADS) exert vasoactive properties. Their use in clinical settings is also limited due to their short lifespan and unstable nature [14]. Over the past decades, researchers have developed many H2S-releasing compounds for clinical settings that have been tested for their usefulness in different clinical conditions (Table 2).
Table 2.
Development of H2S-based therapeutics in the clinical setting.
| Therapeutic Compound | Pharmacological Profile | Targeted Condition | Clinical Trial Phase | References |
|---|---|---|---|---|
| IK-1001 | Injection of Na2S (stabilized form) | Mitigation of cardiac complications during coronary artery bypass surgery | Discontinued after phase II | [215] |
| Na2S2O3 | Sodium salt (inorganic) with thiosulfate ions | Cyanide intoxication, cisplatin toxicities, and calciphylaxis in end-stage renal disease | Approved | [179,216] |
| Zofenopril | ACE inhibitor in conjunction with a H2S donor | Hypertension | Approved | [217,218] |
| GIC-1001 | Analgesic effect of trimebutine with H2S release | Visceral pain during sedation-free, full colonoscopy | Clinical phase II (pending) | [219] |
| SG1002 | H2S-releasing prodrug | Heart failure | Phase I | [152] |
| ATB-346 | H2S-releasing naproxen-derived compound | Inflammatory conditions | Phase II | [220] |
| Alpha lipoic acid | Organosulfur compound | Hypertension, cardioprotection, diabetes, and obesity | Phase II | [221] |
| ATB-340 | H2S releasing aspirin-derived compound | Anti-coagulant for CVD | Discontinued | [222] |
10. Gaps in Knowledge and the Way Forward
As described above, H2S acts as a pro- and anti-inflammatory mediator, antioxidant, and vasodilator. Also, H2S significantly contributes to different disease conditions, such as inflammatory conditions, neurological disorders, cardiovascular diseases, and viral infections. The precise mechanisms by which H2S significantly affects the host are, however, not clearly understood. Investigating the complex molecular mechanisms of H2S in diseases will be helpful for the development of novel H2S-based therapeutics. We now have a better understanding of CSE and CBS, but more research is needed to explore the effects of 3-MST as well. We need better H2S synthesis inhibitors and donors in order to translate these approaches to clinical settings. Some important questions that need to be addressed as the field develops are related to the following: (1) the concentration of H2S to be administered, (2) the timing of administration, (3) the nature of H2S-based compounds, (4) the pharmacokinetics of H2S-based drugs, and (5) the sensitivity and specificity of H2S.
11. Conclusions
The recognition of H2S as a critical signaling molecule marks a significant paradigm shift in biomedical research. The diverse roles of H2S in health and disease underscore the importance of continued research to fully understand the mechanisms by which it acts and therapeutic potential of this knowledge. The development of H2S donors and inhibitors provides promising strategies for treating a wide range of conditions, including inflammatory diseases, cardiovascular diseases, viral infections, and neurological diseases. The translation of H2S donors from pre-clinical to clinical settings can lead to novel therapeutic strategies for different diseases. However, further research is needed to assess the dosage of H2S donors and inhibitors as well as the timing of intervention. This approach will lead to the translation of knowledge on H2S from bench to bedside.
Author Contributions
Conceptualization, M.B.; supervision, M.B.; writing—original draft preparation, A.S.; writing—review and editing, A.S. and M.B.; project management, M.B. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Rodrigues L.M., Gregório J., Wehrwein E. Contemporary views on the future of physiology—A report from the 2019 P-MIG focus group. Front. Physiol. 2023;14:1176146. doi: 10.3389/fphys.2023.1176146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munteanu C., Rotariu M., Turnea M.A., Anghelescu A., Albadi I., Dogaru G., Silișteanu S.C., Ionescu E.V., Firan F.C., Ionescu A.M., et al. Topical Reappraisal of Molecular Pharmacological Approaches to Endothelial Dysfunction in Diabetes Mellitus Angiopathy. Curr. Issues Mol. Biol. 2022;44:3378–3397. doi: 10.3390/cimb44080233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huerta de la Cruz S., Medina-Terol G.J., Tapia-Martínez J.A., Silva-Velasco D.L., Beltran-Ornelas J.H., Sánchez-López A., Sancho M., Centurión D. Hydrogen sulfide as a neuromodulator of the vascular tone. Eur. J. Pharmacol. 2023;940:175455. doi: 10.1016/j.ejphar.2022.175455. [DOI] [PubMed] [Google Scholar]
- 4.Zhou L., Wang Q. Advances of H2S in Regulating Neurodegenerative Diseases by Preserving Mitochondria Function. Antioxidants. 2023;12:652. doi: 10.3390/antiox12030652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Z., Xia H., Sharp T.E., 3rd, LaPenna K.B., Elrod J.W., Casin K.M., Liu K., Calvert J.W., Chau V.Q., Salloum F.N., et al. Mitochondrial H2S Regulates BCAA Catabolism in Heart Failure. Circ. Res. 2022;131:222–235. doi: 10.1161/CIRCRESAHA.121.319817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zuhra K., Augsburger F., Majtan T., Szabo C. Cystathionine-β-Synthase: Molecular Regulation and Pharmacological Inhibition. Biomolecules. 2020;10:697. doi: 10.3390/biom10050697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tamizhselvi R., Moore P.K., Bhatia M. Hydrogen sulfide acts as a mediator of inflammation in acute pancreatitis: In vitro studies using isolated mouse pancreatic acinar cells. J. Cell. Mol. Med. 2007;11:315–326. doi: 10.1111/j.1582-4934.2007.00024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szabo C., Coletta C., Chao C., Módis K., Szczesny B., Papapetropoulos A., Hellmich M.R. Tumor-derived hydrogen sulfide, produced by cystathionine-β-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc. Natl. Acad. Sci. USA. 2013;110:12474–12479. doi: 10.1073/pnas.1306241110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vitvitsky V., Thomas M., Ghorpade A., Gendelman H.E., Banerjee R. A functional transsulfuration pathway in the brain links to glutathione homeostasis. J. Biol. Chem. 2006;281:35785–35793. doi: 10.1074/jbc.M602799200. [DOI] [PubMed] [Google Scholar]
- 10.Kondo K., Bhushan S., King A.L., Prabhu S.D., Hamid T., Koenig S., Murohara T., Predmore B.L., Gojon G., Sr., Gojon G., Jr., et al. H2S protects against pressure overload-induced heart failure via upregulation of endothelial nitric oxide synthase. Circulation. 2013;127:1116–1127. doi: 10.1161/CIRCULATIONAHA.112.000855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunham I., Shimizu N., Roe B.A., Chissoe S., Hunt A.R., Collins J.E., Bruskiewich R., Beare D.M., Clamp M., Smink L.J., et al. The DNA sequence of human chromosome 22. Nature. 1999;402:489–495. doi: 10.1038/990031. [DOI] [PubMed] [Google Scholar]
- 12.Peleli M., Bibli S.I., Li Z., Chatzianastasiou A., Varela A., Katsouda A., Zukunft S., Bucci M., Vellecco V., Davos C.H., et al. Cardiovascular phenotype of mice lacking 3-mercaptopyruvate sulfurtransferase. Biochem. Pharmacol. 2020;176:113833. doi: 10.1016/j.bcp.2020.113833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shibuya N., Mikami Y., Kimura Y., Nagahara N., Kimura H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J. Biochem. 2009;146:623–626. doi: 10.1093/jb/mvp111. [DOI] [PubMed] [Google Scholar]
- 14.Benavides G.A., Squadrito G.L., Mills R.W., Patel H.D., Isbell T.S., Patel R.P., Darley-Usmar V.M., Doeller J.E., Kraus D.W. Hydrogen sulfide mediates the vasoactivity of garlic. Proc. Natl. Acad. Sci. USA. 2007;104:17977–17982. doi: 10.1073/pnas.0705710104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kashfi K., Olson K.R. Biology and therapeutic potential of hydrogen sulfide and hydrogen sulfide-releasing chimeras. Biochem. Pharmacol. 2013;85:689–703. doi: 10.1016/j.bcp.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carbonero F., Benefiel A.C., Gaskins H.R. Contributions of the microbial hydrogen economy to colonic homeostasis. Nat. Rev. Gastroenterol. Hepatol. 2012;9:504–518. doi: 10.1038/nrgastro.2012.85. [DOI] [PubMed] [Google Scholar]
- 17.Rabus R., Venceslau S.S., Wöhlbrand L., Voordouw G., Wall J.D., Pereira I.A. A post-genomic view of the ecophysiology, catabolism and biotechnological relevance of sulphate-reducing prokaryotes. Adv. Microb. Physiol. 2015;66:55–321. doi: 10.1016/bs.ampbs.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Awano N., Wada M., Mori H., Nakamori S., Takagi H. Identification and functional analysis of Escherichia coli cysteine desulfhydrases. Appl. Environ. Microbiol. 2005;71:4149–4152. doi: 10.1128/AEM.71.7.4149-4152.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martelli A., Testai L., Marino A., C Breschi M., Da Settimo F., Calderone V. Hydrogen sulphide: Biopharmacological roles in the cardiovascular system and pharmaceutical perspectives. Curr. Med. Chem. 2012;19:3325–3336. doi: 10.2174/092986712801215928. [DOI] [PubMed] [Google Scholar]
- 20.Zhao Y., Biggs T.D., Xian M. Hydrogen sulfide (H 2 S) releasing agents: Chemistry and biological applications. Chem. Commun. 2014;50:11788–11805. doi: 10.1039/C4CC00968A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li L., Bhatia M., Zhu Y.Z., Zhu Y.C., Ramnath R.D., Wang Z.J., Anuar F.B.M., Whiteman M., Salto-Tellez M., Moore P.K. Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. FASEB J. 2005;19:1196–1198. doi: 10.1096/fj.04-3583fje. [DOI] [PubMed] [Google Scholar]
- 22.Tamizhselvi R., Moore P.K., Bhatia M. Inhibition of hydrogen sulfide synthesis attenuates chemokine production and protects mice against acute pancreatitis and associated lung injury. Pancreas. 2008;36:e24–e31. doi: 10.1097/MPA.0b013e31816857bb. [DOI] [PubMed] [Google Scholar]
- 23.Giuliani D., Ottani A., Zaffe D., Galantucci M., Strinati F., Lodi R., Guarini S. Hydrogen sulfide slows down progression of experimental Alzheimer’s disease by targeting multiple pathophysiological mechanisms. Neurobiol. Learn. Mem. 2013;104:82–91. doi: 10.1016/j.nlm.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Calvert J.W., Elston M., Nicholson C.K., Gundewar S., Jha S., Elrod J.W., Ramachandran A., Lefer D.J. Genetic and pharmacologic hydrogen sulfide therapy attenuates ischemia-induced heart failure in mice. Circulation. 2010;122:11–19. doi: 10.1161/CIRCULATIONAHA.109.920991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao Y., Pacheco A., Xian M. Medicinal Chemistry: Insights into the Development of Novel H2S Donors. Handb. Exp. Pharmacol. 2015;230:365–388. doi: 10.1007/978-3-319-18144-8_18. [DOI] [PubMed] [Google Scholar]
- 26.Whiteman M., Li L., Rose P., Tan C.H., Parkinson D.B., Moore P.K. The effect of hydrogen sulfide donors on lipopolysaccharide-induced formation of inflammatory mediators in macrophages. Antioxid. Redox Signal. 2010;12:1147–1154. doi: 10.1089/ars.2009.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montanaro R., Vellecco V., Torregrossa R., Casillo G.M., Manzo O.L., Mitidieri E., Bucci M., Castaldo S., Sorrentino R., Whiteman M., et al. Hydrogen sulfide donor AP123 restores endothelial nitric oxide-dependent vascular function in hyperglycemia via a CREB-dependent pathway. Redox Biol. 2023;62:102657. doi: 10.1016/j.redox.2023.102657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu W., Cui C., Cui C., Chen Z., Zhang H., Cui Q., Xu G., Fan J., Han Y., Tang L. Hepatocellular cystathionine γ lyase/hydrogen sulfide attenuates nonalcoholic fatty liver disease by activating farnesoid X receptor. Hepatology. 2022;76:1794–1810. doi: 10.1002/hep.32577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dongó E., Beliczai-Marosi G., Dybvig A.S., Kiss L. The mechanism of action and role of hydrogen sulfide in the control of vascular tone. Nitric Oxide. 2018;81:75–87. doi: 10.1016/j.niox.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 30.Yuan S., Shen X., Kevil C.G. Beyond a gasotransmitter: Hydrogen sulfide and polysulfide in cardiovascular health and immune response. Antioxid. Redox Signal. 2017;27:634–653. doi: 10.1089/ars.2017.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu W.J., Jia W.W., Liu X.H., Pan L.L., Zhang Q.Y., Yang D., Shen X.Y., Liu L., Zhu Y.Z. S-propargyl-cysteine attenuates inflammatory response in rheumatoid arthritis by modulating the Nrf2-ARE signaling pathway. Redox Biol. 2016;10:157–167. doi: 10.1016/j.redox.2016.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sidhapuriwala J.N., Hegde A., Ang A.D., Zhu Y.Z., Bhatia M. Effects of S-propargyl-cysteine (SPRC) in caerulein-induced acute pancreatitis in mice. PLoS ONE. 2012;7:e32574. doi: 10.1371/journal.pone.0032574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang C., Kan J., Liu X., Wang S., Zhu Y. Cardioprotective effect of controlled release sprc to heart failure of rats after myocardial infarction and its possible mechanism. Heart. 2012;98:E232. doi: 10.1136/heartjnl-2012-302920o.3. [DOI] [Google Scholar]
- 34.Zhou X., An G., Lu X. Hydrogen sulfide attenuates the development of diabetic cardiomyopathy. Clin. Sci. 2015;128:325–335. doi: 10.1042/CS20140460. [DOI] [PubMed] [Google Scholar]
- 35.Li H., Ma Y., Escaffre O., Ivanciuc T., Komaravelli N., Kelley John P., Coletta C., Szabo C., Rockx B., Garofalo Roberto P., et al. Role of Hydrogen Sulfide in Paramyxovirus Infections. J. Virol. 2015;89:5557–5568. doi: 10.1128/JVI.00264-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vaamonde-García C., Burguera E.F., Vela-Anero Á., Hermida-Gómez T., Filgueira-Fernández P., Fernández-Rodríguez J.A., Meijide-Faílde R., Blanco F.J. Intraarticular Administration Effect of Hydrogen Sulfide on an In Vivo Rat Model of Osteoarthritis. Int. J. Mol. Sci. 2020;21:7421. doi: 10.3390/ijms21197421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li L., Salto-Tellez M., Tan C.-H., Whiteman M., Moore P.K. GYY4137, a novel hydrogen sulfide-releasing molecule, protects against endotoxic shock in the rat. Free. Radic. Biol. Med. 2009;47:103–113. doi: 10.1016/j.freeradbiomed.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 38.Lazarević M., Battaglia G., Jevtić B., Đedović N., Bruno V., Cavalli E., Miljković Đ., Nicoletti F., Momčilović M., Fagone P. Upregulation of Tolerogenic Pathways by the Hydrogen Sulfide Donor GYY4137 and Impaired Expression of H2S-Producing Enzymes in Multiple Sclerosis. Antioxidants. 2020;9:608. doi: 10.3390/antiox9070608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang B., Li Y., Liu N., Liu B. AP39, a novel mitochondria-targeted hydrogen sulfide donor ameliorates doxorubicin-induced cardiotoxicity by regulating the AMPK/UCP2 pathway. PLoS ONE. 2024;19:e0300261. doi: 10.1371/journal.pone.0300261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahmad A., Szabo C. Both the H2S biosynthesis inhibitor aminooxyacetic acid and the mitochondrially targeted H2S donor AP39 exert protective effects in a mouse model of burn injury. Pharmacol. Res. 2016;113:348–355. doi: 10.1016/j.phrs.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 41.Padiya R., Chowdhury D., Borkar R., Srinivas R., Pal Bhadra M., Banerjee S.K. Garlic attenuates cardiac oxidative stress via activation of PI3K/AKT/Nrf2-Keap1 pathway in fructose-fed diabetic rat. PLoS ONE. 2014;9:e94228. doi: 10.1371/journal.pone.0094228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vecchio I., Sorrentino L., Paoletti A., Marra R., Arbitrio M. The state of the art on acetylcholinesterase inhibitors in the treatment of Alzheimer’s disease. J. Cent. Nerv. Syst. Dis. 2021;13:11795735211029113. doi: 10.1177/11795735211029113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hao Z., Hai P., Yue W. Effect of Allicin on the Expression of tau Protein in Transgenic Mice Brain with Alzheimer’s Disease. Nat. Prod. Res. Dev. 2016;28:685. [Google Scholar]
- 44.Liu Y., Li A., Feng X., Sun X., Zhu X., Zhao Z. Pharmacological Investigation of the Anti-Inflammation and Anti-Oxidation Activities of Diallyl Disulfide in a Rat Emphysema Model Induced by Cigarette Smoke Extract. Nutrients. 2018;10:79. doi: 10.3390/nu10010079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Batallé G., Bai X., Pouso-Vázquez E., Roch G., Rodríguez L., Pol O. The Recovery of Cognitive and Affective Deficiencies Linked with Chronic Osteoarthritis Pain and Implicated Pathways by Slow-Releasing Hydrogen Sulfide Treatment. Antioxidants. 2021;10:1632. doi: 10.3390/antiox10101632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhatia M., Sidhapuriwala J.N., Sparatore A., Moore P.K. Treatment with H2S-releasing diclofenac protects mice against acute pancreatitis-associated lung injury. Shock. 2008;29:84–88. doi: 10.1097/shk.0b013e31806ec26. [DOI] [PubMed] [Google Scholar]
- 47.Sidhapuriwala J., Li L., Sparatore A., Bhatia M., Moore P.K. Effect of S-diclofenac, a novel hydrogen sulfide releasing derivative, on carrageenan-induced hindpaw oedema formation in the rat. Eur. J. Pharmacol. 2007;569:149–154. doi: 10.1016/j.ejphar.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 48.Zhang H., Zhi L., Moochhala S., Moore P.K., Bhatia M. Hydrogen sulfide acts as an inflammatory mediator in cecal ligation and puncture-induced sepsis in mice by upregulating the production of cytokines and chemokines via NF-kappaB. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;292:L960–L971. doi: 10.1152/ajplung.00388.2006. [DOI] [PubMed] [Google Scholar]
- 49.Tamizhselvi R., Koh Y.H., Sun J., Zhang H., Bhatia M. Hydrogen sulfide induces ICAM-1 expression and neutrophil adhesion to caerulein-treated pancreatic acinar cells through NF-kappaB and Src-family kinases pathway. Exp. Cell Res. 2010;316:1625–1636. doi: 10.1016/j.yexcr.2010.02.044. [DOI] [PubMed] [Google Scholar]
- 50.Bibli S.I., Andreadou I., Chatzianastasiou A., Tzimas C., Sanoudou D., Kranias E., Brouckaert P., Coletta C., Szabo C., Kremastinos D.T., et al. Cardioprotection by H2S engages a cGMP-dependent protein kinase G/phospholamban pathway. Cardiovasc. Res. 2015;106:432–442. doi: 10.1093/cvr/cvv129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu L., Wang J., Wang H. Hydrogen sulfide alleviates oxidative stress injury and reduces apoptosis induced by MPP+ in Parkinson’s disease cell model. Mol. Cell. Biochem. 2020;472:231–240. doi: 10.1007/s11010-020-03801-y. [DOI] [PubMed] [Google Scholar]
- 52.Wang Y., Zhao X., Jin H., Wei H., Li W., Bu D., Tang X., Ren Y., Tang C., Du J. Role of hydrogen sulfide in the development of atherosclerotic lesions in apolipoprotein E knockout mice. Arterioscler. Thromb. Vasc. Biol. 2009;29:173–179. doi: 10.1161/ATVBAHA.108.179333. [DOI] [PubMed] [Google Scholar]
- 53.Szabó C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 54.Wang R. Physiological implications of hydrogen sulfide: A whiff exploration that blossomed. Physiol. Rev. 2012;92:791–896. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- 55.Asimakopoulou A., Panopoulos P., Chasapis C.T., Coletta C., Zhou Z., Cirino G., Giannis A., Szabo C., Spyroulias G.A., Papapetropoulos A. Selectivity of commonly used pharmacological inhibitors for cystathionine β synthase (CBS) and cystathionine γ lyase (CSE) Br. J. Pharmacol. 2013;169:922–932. doi: 10.1111/bph.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maekawa M., Okamura T., Kasai N., Hori Y., Summer K.H., Konno R. D-amino-acid oxidase is involved in D-serine-induced nephrotoxicity. Chem. Res. Toxicol. 2005;18:1678–1682. doi: 10.1021/tx0500326. [DOI] [PubMed] [Google Scholar]
- 57.Li M., Liu Y., Deng Y., Pan L., Fu H., Han X., Li Y., Shi H., Wang T. Therapeutic potential of endogenous hydrogen sulfide inhibition in breast cancer (Review) Oncol. Rep. 2021;45:68. doi: 10.3892/or.2021.8019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Badiei A., Chambers S.T., Gaddam R.R., Bhatia M. Cystathionine-γ-lyase gene silencing with siRNA in monocytes/macrophages attenuates inflammation in cecal ligation and puncture-induced sepsis in the mouse. J. Biosci. 2016;41:87–95. doi: 10.1007/s12038-016-9598-9. [DOI] [PubMed] [Google Scholar]
- 59.Banjac A., Perisic T., Sato H., Seiler A., Bannai S., Weiss N., Kölle P., Tschoep K., Issels R., Daniel P. The cystine/cysteine cycle: A redox cycle regulating susceptibility versus resistance to cell death. Oncogene. 2008;27:1618–1628. doi: 10.1038/sj.onc.1210796. [DOI] [PubMed] [Google Scholar]
- 60.Cramer S.L., Saha A., Liu J., Tadi S., Tiziani S., Yan W., Triplett K., Lamb C., Alters S.E., Rowlinson S. Systemic depletion of L-cyst (e) ine with cyst (e) inase increases reactive oxygen species and suppresses tumor growth. Nat. Med. 2017;23:120–127. doi: 10.1038/nm.4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Medzhitov R. Inflammation 2010: New adventures of an old flame. Cell. 2010;140:771–776. doi: 10.1016/j.cell.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 62.Ferrero-Miliani L., Nielsen O., Andersen P., Girardin S. Chronic inflammation: Importance of NOD2 and NALP3 in interleukin-1β generation. Clin. Exp. Immunol. 2007;147:227–235. doi: 10.1111/j.1365-2249.2006.03261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Han N.-R., Ko S.-G., Park H.-J., Moon P.-D. Hydrogen Sulfide Downregulates Oncostatin M Expression via PI3K/Akt/NF-κB Signaling Processes in Neutrophil-like Differentiated HL-60 Cells. Antioxidants. 2023;12:417. doi: 10.3390/antiox12020417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Muniraj N., Stamp L.K., Badiei A., Hegde A., Cameron V., Bhatia M. Hydrogen sulfide acts as a pro-inflammatory mediator in rheumatic disease. Int. J. Rheum. Dis. 2017;20:182–189. doi: 10.1111/1756-185X.12472. [DOI] [PubMed] [Google Scholar]
- 65.Martel-Pelletier J., Barr A.J., Cicuttini F.M., Conaghan P.G., Cooper C., Goldring M.B., Goldring S.R., Jones G., Teichtahl A.J., Pelletier J.P. Osteoarthritis. Nat. Rev. Dis. Primers. 2016;2:16072. doi: 10.1038/nrdp.2016.72. [DOI] [PubMed] [Google Scholar]
- 66.Bhatia M., Sidhapuriwala J., Moochhala S.M., Moore P.K. Hydrogen sulphide is a mediator of carrageenan-induced hindpaw oedema in the rat. Br. J. Pharmacol. 2005;145:141–144. doi: 10.1038/sj.bjp.0706186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brancaleone V., Esposito I., Gargiulo A., Vellecco V., Asimakopoulou A., Citi V., Calderone V., Gobbetti T., Perretti M., Papapetropoulos A., et al. D-Penicillamine modulates hydrogen sulfide (H2S) pathway through selective inhibition of cystathionine-γ-lyase. Br. J. Pharmacol. 2016;173:1556–1565. doi: 10.1111/bph.13459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fox B., Schantz J.T., Haigh R., Wood M.E., Moore P.K., Viner N., Spencer J.P., Winyard P.G., Whiteman M. Inducible hydrogen sulfide synthesis in chondrocytes and mesenchymal progenitor cells: Is H2S a novel cytoprotective mediator in the inflamed joint? J. Cell. Mol. Med. 2012;16:896–910. doi: 10.1111/j.1582-4934.2011.01357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kloesch B., Liszt M., Krehan D., Broell J., Kiener H., Steiner G. High concentrations of hydrogen sulphide elevate the expression of a series of pro-inflammatory genes in fibroblast-like synoviocytes derived from rheumatoid and osteoarthritis patients. Immunol. Lett. 2012;141:197–203. doi: 10.1016/j.imlet.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 70.Burguera E.F., Vela-Anero Á., Gato-Calvo L., Vaamonde-García C., Meijide-Faílde R., Blanco F.J. Hydrogen sulfide biosynthesis is impaired in the osteoarthritic joint. Int. J. Biometeorol. 2020;64:997–1010. doi: 10.1007/s00484-019-01823-w. [DOI] [PubMed] [Google Scholar]
- 71.Li L., Whiteman M., Guan Y.Y., Neo K.L., Cheng Y., Lee S.W., Zhao Y., Baskar R., Tan C.-H., Moore P.K. Characterization of a Novel, Water-Soluble Hydrogen Sulfide–Releasing Molecule (GYY4137) Circulation. 2008;117:2351–2360. doi: 10.1161/CIRCULATIONAHA.107.753467. [DOI] [PubMed] [Google Scholar]
- 72.Li L., Fox B., Keeble J., Salto-Tellez M., Winyard P.G., Wood M.E., Moore P.K., Whiteman M. The complex effects of the slow-releasing hydrogen sulfide donor GYY4137 in a model of acute joint inflammation and in human cartilage cells. J. Cell. Mol. Med. 2013;17:365–376. doi: 10.1111/jcmm.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hao Y., Wang H., Fang L., Bian J., Gao Y., Li C. H2S Donor and Bone Metabolism. Front. Pharmacol. 2021;12:661601. doi: 10.3389/fphar.2021.661601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qian Y.Q., Feng Z.H., Li X.B., Hu Z.C., Xuan J.W., Wang X.Y., Xu H.C., Chen J.X. Downregulating PI3K/Akt/NF-κB signaling with allicin for ameliorating the progression of osteoarthritis: In vitro and vivo studies. Food Funct. 2018;9:4865–4875. doi: 10.1039/C8FO01095A. [DOI] [PubMed] [Google Scholar]
- 75.Dief A.E., Mostafa D.K., Sharara G.M., Zeitoun T.H. Hydrogen sulfide releasing naproxen offers better anti-inflammatory and chondroprotective effect relative to naproxen in a rat model of zymosan induced arthritis. Eur. Rev. Med. Pharmacol. Sci. 2015;19:1537–1546. [PubMed] [Google Scholar]
- 76.Szczesny B., Módis K., Yanagi K., Coletta C., Le Trionnaire S., Perry A., Wood M.E., Whiteman M., Szabo C. AP39, a novel mitochondria-targeted hydrogen sulfide donor, stimulates cellular bioenergetics, exerts cytoprotective effects and protects against the loss of mitochondrial DNA integrity in oxidatively stressed endothelial cells in vitro. Nitric Oxide. 2014;41:120–130. doi: 10.1016/j.niox.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gotts J.E., Matthay M.A. Sepsis: Pathophysiology and clinical management. BMJ. 2016;353:i1585. doi: 10.1136/bmj.i1585. [DOI] [PubMed] [Google Scholar]
- 78.Deutschman C.S., Tracey K.J. Sepsis: Current Dogma and New Perspectives. Immunity. 2014;40:463–475. doi: 10.1016/j.immuni.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 79.Opal S.M., van der Poll T. Endothelial barrier dysfunction in septic shock. J. Intern. Med. 2015;277:277–293. doi: 10.1111/joim.12331. [DOI] [PubMed] [Google Scholar]
- 80.Rudd K.E., Kissoon N., Limmathurotsakul D., Bory S., Mutahunga B., Seymour C.W., Angus D.C., West T.E. The global burden of sepsis: Barriers and potential solutions. Crit. Care. 2018;22:232. doi: 10.1186/s13054-018-2157-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rudd K.E., Johnson S.C., Agesa K.M., Shackelford K.A., Tsoi D., Kievlan D.R., Colombara D.V., Ikuta K.S., Kissoon N., Finfer S. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet. 2020;395:200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Caraballo C., Jaimes F. Organ Dysfunction in Sepsis: An Ominous Trajectory From Infection To Death. Yale J. Biol. Med. 2019;92:629–640. [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang H., Zhi L., Moore P.K., Bhatia M. Role of hydrogen sulfide in cecal ligation and puncture-induced sepsis in the mouse. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006;290:L1193–L1201. doi: 10.1152/ajplung.00489.2005. [DOI] [PubMed] [Google Scholar]
- 84.Li L., Whiteman M., Moore P.K. Dexamethasone inhibits lipopolysaccharide-induced hydrogen sulphide biosynthesis in intact cells and in an animal model of endotoxic shock. J. Cell. Mol. Med. 2009;13:2684–2692. doi: 10.1111/j.1582-4934.2008.00610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang H., Zhi L., Moochhala S.M., Moore P.K., Bhatia M. Endogenous hydrogen sulfide regulates leukocyte trafficking in cecal ligation and puncture-induced sepsis. J. Leukoc. Biol. 2007;82:894–905. doi: 10.1189/jlb.0407237. [DOI] [PubMed] [Google Scholar]
- 86.Coletta C., Szabo C. Potential role of hydrogen sulfide in the pathogenesis of vascular dysfunction in septic shock. Curr. Vasc. Pharmacol. 2013;11:208–221. [PubMed] [Google Scholar]
- 87.Huang C.W., Feng W., Peh M.T., Peh K., Dymock B.W., Moore P.K. A novel slow-releasing hydrogen sulfide donor, FW1256, exerts anti-inflammatory effects in mouse macrophages and in vivo. Pharmacol. Res. 2016;113:533–546. doi: 10.1016/j.phrs.2016.09.032. [DOI] [PubMed] [Google Scholar]
- 88.Rios E.C., Szczesny B., Soriano F.G., Olah G., Szabo C. Hydrogen sulfide attenuates cytokine production through the modulation of chromatin remodeling. Int. J. Mol. Med. 2015;35:1741–1746. doi: 10.3892/ijmm.2015.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Badiei A., Rivers-Auty J., Ang A.D., Bhatia M. Inhibition of hydrogen sulfide production by gene silencing attenuates inflammatory activity of LPS-activated RAW264. 7 cells. Appl. Microbiol. Biotechnol. 2013;97:7845–7852. doi: 10.1007/s00253-013-5080-x. [DOI] [PubMed] [Google Scholar]
- 90.Collin M., Anuar F.B., Murch O., Bhatia M., Moore P.K., Thiemermann C. Inhibition of endogenous hydrogen sulfide formation reduces the organ injury caused by endotoxemia. Br. J. Pharmacol. 2005;146:498–505. doi: 10.1038/sj.bjp.0706367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fiorucci S., Orlandi S., Mencarelli A., Caliendo G., Santagada V., Distrutti E., Santucci L., Cirino G., Wallace J. Enhanced activity of a hydrogen sulphide-releasing derivative of mesalamine (ATB-429) in a mouse model of colitis. Br. J. Pharmacol. 2007;150:996–1002. doi: 10.1038/sj.bjp.0707193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li L., Rossoni G., Sparatore A., Lee L.C., Del Soldato P., Moore P.K. Anti-inflammatory and gastrointestinal effects of a novel diclofenac derivative. Free. Radic. Biol. Med. 2007;42:706–719. doi: 10.1016/j.freeradbiomed.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 93.Bhatia M. H2S and inflammation: An overview. Chem. Biochem. Pharmacol. Hydrog. Sulfide. 2015;230:165–180. doi: 10.1007/978-3-319-18144-8_8. [DOI] [PubMed] [Google Scholar]
- 94.Merz T., McCook O., Brucker C., Waller C., Calzia E., Radermacher P., Datzmann T. H2S in Critical Illness-A New Horizon for Sodium Thiosulfate? Biomolecules. 2022;12:543. doi: 10.3390/biom12040543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Norris E.J., Feilen N., Nguyen N.H., Culberson C.R., Shin M.C., Fish M., Clemens M.G. Hydrogen sulfide modulates sinusoidal constriction and contributes to hepatic micorcirculatory dysfunction during endotoxemia. Am. J. Physiol.—Gastrointest. Liver Physiol. 2013;304:G1070–G1078. doi: 10.1152/ajpgi.00395.2012. [DOI] [PubMed] [Google Scholar]
- 96.Poisson J., Lemoinne S., Boulanger C., Durand F., Moreau R., Valla D., Rautou P.-E. Liver sinusoidal endothelial cells: Physiology and role in liver diseases. J. Hepatol. 2017;66:212–227. doi: 10.1016/j.jhep.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 97.Gaddam R.R., Fraser R., Badiei A., Chambers S., Cogger V.C., Le Couteur D.G., Ishii I., Bhatia M. Cystathionine-gamma-lyase gene deletion protects mice against inflammation and liver sieve injury following polymicrobial sepsis. PLoS ONE. 2016;11:e0160521. doi: 10.1371/journal.pone.0160521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gaddam R.R., Chambers S., Fraser R., Cogger V.C., Le Couteur D.G., Ishii I., Bhatia M. Cystathionine-Gamma-Lyase-Derived hydrogen sulfide-regulated substance P modulates liver sieve fenestrations in Caecal ligation and puncture-induced sepsis. Int. J. Mol. Sci. 2019;20:3191. doi: 10.3390/ijms20133191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gaddam R.R., Chambers S., Murdoch D., Shaw G., Bhatia M. Circulating levels of hydrogen sulfide and substance P in patients with sepsis. J. Infect. 2017;75:293–300. doi: 10.1016/j.jinf.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 100.Liu S., Wang X., Pan L., Wu W., Yang D., Qin M., Jia W., Xiao C., Long F., Ge J. Endogenous hydrogen sulfide regulates histone demethylase JMJD3-mediated inflammatory response in LPS-stimulated macrophages and in a mouse model of LPS-induced septic shock. Biochem. Pharmacol. 2018;149:153–162. doi: 10.1016/j.bcp.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 101.Bee N., White R., Petros A.J. Hydrogen sulfide in exhaled gases from ventilated septic neonates and children: A preliminary report. Pediatr. Crit. Care Med. 2017;18:e327–e332. doi: 10.1097/PCC.0000000000001223. [DOI] [PubMed] [Google Scholar]
- 102.Zhang H., Hegde A., Ng S.W., Adhikari S., Moochhala S.M., Bhatia M. Hydrogen Sulfide Up-Regulates Substance P in Polymicrobial Sepsis-Associated Lung Injury1. J. Immunol. 2007;179:4153–4160. doi: 10.4049/jimmunol.179.6.4153. [DOI] [PubMed] [Google Scholar]
- 103.Ang S.F., Moochhala S.M., Bhatia M. Hydrogen sulfide promotes transient receptor potential vanilloid 1-mediated neurogenic inflammation in polymicrobial sepsis. Crit. Care Med. 2010;38:619–628. doi: 10.1097/CCM.0b013e3181c0df00. [DOI] [PubMed] [Google Scholar]
- 104.Ang S.F., Moochhala S.M., MacAry P.A., Bhatia M. Hydrogen sulfide and neurogenic inflammation in polymicrobial sepsis: Involvement of substance P and ERK-NF-κB signaling. PLoS ONE. 2011;6:e24535. doi: 10.1371/journal.pone.0024535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ang S.-F., Sio S.W.S., Moochhala S.M., MacAry P.A., Bhatia M. Hydrogen Sulfide Upregulates Cyclooxygenase-2 and Prostaglandin E Metabolite in Sepsis-Evoked Acute Lung Injury via Transient Receptor Potential Vanilloid Type 1 Channel Activation. J. Immunol. 2011;187:4778–4787. doi: 10.4049/jimmunol.1101559. [DOI] [PubMed] [Google Scholar]
- 106.Bhatia M., Zhi L., Zhang H., Ng S.W., Moore P.K. Role of substance P in hydrogen sulfide-induced pulmonary inflammation in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006;291:L896–L904. doi: 10.1152/ajplung.00053.2006. [DOI] [PubMed] [Google Scholar]
- 107.Fonseca Sepúlveda E.V., Guerrero-Lozano R. Acute pancreatitis and recurrent acute pancreatitis: An exploration of clinical and etiologic factors and outcomes. J. Pediatr. Rio. J. 2019;95:713–719. doi: 10.1016/j.jped.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 108.Ghatak R., Masso L., Kapadia D., Kulairi Z.I. Medication as a cause of acute pancreatitis. Am. J. Case Rep. 2017;18:839. doi: 10.12659/AJCR.903327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Quinlan C., Gallagher T., Moran N., Dass G., Sweeney B., Conlon K., Ridgway P. Acute pancreatitis in the paediatric population: Different disease, same management? Ir. J. Med. Sci. 2011;180:S254–S255. doi: 10.1016/j.pan.2012.03.034. [DOI] [Google Scholar]
- 110.Zerem E. Treatment of severe acute pancreatitis and its complications. World J. Gastroenterol. WJG. 2014;20:13879. doi: 10.3748/wjg.v20.i38.13879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Balthazar E.J. Acute pancreatitis: Assessment of severity with clinical and CT evaluation. Radiology. 2002;223:603–613. doi: 10.1148/radiol.2233010680. [DOI] [PubMed] [Google Scholar]
- 112.Banks P.A., Bollen T.L., Dervenis C., Gooszen H.G., Johnson C.D., Sarr M.G., Tsiotos G.G., Vege S.S. Classification of acute pancreatitis—2012: Revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 113.Bhatia M., Wong F.L., Fu D., Lau H.Y., Moochhala S.M., Moore P.K. Role of hydrogen sulfide in acute pancreatitis and associated lung injury. FASEB J. 2005;19:623–625. doi: 10.1096/fj.04-3023fje. [DOI] [PubMed] [Google Scholar]
- 114.Ang A.D., Rivers-Auty J., Hegde A., Ishii I., Bhatia M. The effect of CSE gene deletion in caerulein-induced acute pancreatitis in the mouse. Am. J. Physiol.—Gastrointest. Liver Physiol. 2013;305:G712–G721. doi: 10.1152/ajpgi.00044.2013. [DOI] [PubMed] [Google Scholar]
- 115.Liu Y., Liao R., Qiang Z., Zhang C. Pro-inflammatory cytokine-driven PI3K/Akt/Sp1 signalling and H2S production facilitates the pathogenesis of severe acute pancreatitis. Biosci. Rep. 2017;37:BSR20160483. doi: 10.1042/BSR20160483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shanmugam M., Jing Z., Bhatia M. Aminooxyacetate inhibits hydrogen sulfide and ammonium synthesis and protects mice in acute pancreatitis. Int. J. Integr. Biol. 2009;8:7–14. [Google Scholar]
- 117.Bhatia M., Sidhapuriwala J.N., Ng S.W., Tamizhselvi R., Moochhala S.M. Pro-inflammatory effects of hydrogen sulphide on substance P in caerulein-induced acute pancreatitis. J. Cell. Mol. Med. 2008;12:580–590. doi: 10.1111/j.1582-4934.2007.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tamizhselvi R., Shrivastava P., Koh Y.H., Zhang H., Bhatia M. Preprotachykinin-A gene deletion regulates hydrogen sulfide-induced toll-like receptor 4 signaling pathway in cerulein-treated pancreatic acinar cells. Pancreas. 2011;40:444–452. doi: 10.1097/MPA.0b013e31820720e6. [DOI] [PubMed] [Google Scholar]
- 119.Benjamin E.J., Blaha M.J., Chiuve S.E., Cushman M., Das S.R., Deo R., de Ferranti S.D., Floyd J., Fornage M., Gillespie C., et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lichtenstein A.H., Appel L.J., Brands M., Carnethon M., Daniels S., Franch H.A., Franklin B., Kris-Etherton P., Harris W.S., Howard B., et al. Diet and lifestyle recommendations revision 2006: A scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 121.Mani S., Li H., Untereiner A., Wu L., Yang G., Austin R.C., Dickhout J.G., Lhoták Š., Meng Q.H., Wang R. Decreased endogenous production of hydrogen sulfide accelerates atherosclerosis. Circulation. 2013;127:2523–2534. doi: 10.1161/CIRCULATIONAHA.113.002208. [DOI] [PubMed] [Google Scholar]
- 122.Chen D.B., Feng L., Hodges J.K., Lechuga T.J., Zhang H. Human trophoblast-derived hydrogen sulfide stimulates placental artery endothelial cell angiogenesis. Biol. Reprod. 2017;97:478–489. doi: 10.1093/biolre/iox105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu H.T., Zhou Z.X., Ren Z., Yang S., Liu L.S., Wang Z., Wei D.H., Ma X.F., Ma Y., Jiang Z.S. EndMT: Potential Target of H2S against Atherosclerosis. Curr. Med. Chem. 2021;28:3666–3680. doi: 10.2174/0929867327999201116194634. [DOI] [PubMed] [Google Scholar]
- 124.Scammahorn J.J., Nguyen I.T.N., Bos E.M., Van Goor H., Joles J.A. Fighting Oxidative Stress with Sulfur: Hydrogen Sulfide in the Renal and Cardiovascular Systems. Antioxidants. 2021;10:373. doi: 10.3390/antiox10030373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dhalla N.S., Elmoselhi A.B., Hata T., Makino N. Status of myocardial antioxidants in ischemia-reperfusion injury. Cardiovasc. Res. 2000;47:446–456. doi: 10.1016/S0008-6363(00)00078-X. [DOI] [PubMed] [Google Scholar]
- 126.Luan H.F., Zhao Z.B., Zhao Q.H., Zhu P., Xiu M.Y., Ji Y. Hydrogen sulfide postconditioning protects isolated rat hearts against ischemia and reperfusion injury mediated by the JAK2/STAT3 survival pathway. Braz. J. Med. Biol. Res. 2012;45:898–905. doi: 10.1590/S0100-879X2012007500090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jin H., Wang Y., Wang X., Sun Y., Tang C., Du J. Sulfur dioxide preconditioning increases antioxidative capacity in rat with myocardial ischemia reperfusion (I/R) injury. Nitric Oxide. 2013;32:56–61. doi: 10.1016/j.niox.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 128.Gao L., Xu Z., Yin Z., Chen K., Wang C., Zhang H. Association of hydrogen sulfide with alterations of monocyte chemokine receptors, CCR2 and CX3CR1 in patients with coronary artery disease. Inflamm. Res. 2015;64:627–635. doi: 10.1007/s00011-015-0844-7. [DOI] [PubMed] [Google Scholar]
- 129.Feng S.J., Li H., Wang S.X. Lower Hydrogen Sulfide Is Associated with Cardiovascular Mortality, Which Involves cPKCβII/Akt Pathway in Chronic Hemodialysis Patients. Blood Purif. 2015;40:260–269. doi: 10.1159/000439580. [DOI] [PubMed] [Google Scholar]
- 130.Leucker T.M., Nomura Y., Kim J.H., Bhatta A., Wang V., Wecker A., Jandu S., Santhanam L., Berkowitz D., Romer L., et al. Cystathionine γ-lyase protects vascular endothelium: A role for inhibition of histone deacetylase 6. Am. J. Physiol. Heart Circ. Physiol. 2017;312:H711–H720. doi: 10.1152/ajpheart.00724.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang Y., Peng L., Yu X. Protective effect of hydrogen sulfide on rats with myocardial ischemia/reperfusion injury and its mechanism. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2015;31:316–320. [PubMed] [Google Scholar]
- 132.Hu M.Z., Zhou B., Mao H.Y., Sheng Q., Du B., Chen J.L., Pang Q.F., Ji Y. Exogenous Hydrogen Sulfide Postconditioning Protects Isolated Rat Hearts From Ischemia/Reperfusion Injury Through Sirt1/PGC-1α Signaling Pathway. Int. Heart J. 2016;57:477–482. doi: 10.1536/ihj.15-506. [DOI] [PubMed] [Google Scholar]
- 133.Qipshidze N., Metreveli N., Mishra P.K., Lominadze D., Tyagi S.C. Hydrogen sulfide mitigates cardiac remodeling during myocardial infarction via improvement of angiogenesis. Int. J. Biol. Sci. 2012;8:430–441. doi: 10.7150/ijbs.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Barr L.A., Shimizu Y., Lambert J.P., Nicholson C.K., Calvert J.W. Hydrogen sulfide attenuates high fat diet-induced cardiac dysfunction via the suppression of endoplasmic reticulum stress. Nitric Oxide. 2015;46:145–156. doi: 10.1016/j.niox.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kesherwani V., Nandi S.S., Sharawat S.K., Shahshahan H.R., Mishra P.K. Hydrogen sulfide mitigates homocysteine-mediated pathological remodeling by inducing miR-133a in cardiomyocytes. Mol. Cell. Biochem. 2015;404:241–250. doi: 10.1007/s11010-015-2383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nandi S.S., Mishra P.K. H2S and homocysteine control a novel feedback regulation of cystathionine beta synthase and cystathionine gamma lyase in cardiomyocytes. Sci. Rep. 2017;7:3639. doi: 10.1038/s41598-017-03776-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhao W., Zhao J., Rong J. Pharmacological Modulation of Cardiac Remodeling after Myocardial Infarction. Oxidative Med. Cell. Longev. 2020;2020:8815349. doi: 10.1155/2020/8815349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shindo T., Manabe I., Fukushima Y., Tobe K., Aizawa K., Miyamoto S., Kawai-Kowase K., Moriyama N., Imai Y., Kawakami H., et al. Krüppel-like zinc-finger transcription factor KLF5/BTEB2 is a target for angiotensin II signaling and an essential regulator of cardiovascular remodeling. Nat. Med. 2002;8:856–863. doi: 10.1038/nm738. [DOI] [PubMed] [Google Scholar]
- 139.Huang J., Wang D., Zheng J., Huang X., Jin H. Hydrogen sulfide attenuates cardiac hypertrophy and fibrosis induced by abdominal aortic coarctation in rats. Mol. Med. Rep. 2012;5:923–928. doi: 10.3892/mmr.2012.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Meng G., Xiao Y., Ma Y., Tang X., Xie L., Liu J., Gu Y., Yu Y., Park C.M., Xian M., et al. Hydrogen Sulfide Regulates Krüppel-Like Factor 5 Transcription Activity via Specificity Protein 1 S-Sulfhydration at Cys664 to Prevent Myocardial Hypertrophy. J. Am. Heart Assoc. 2016;5:e004160. doi: 10.1161/JAHA.116.004160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Su H., Su H., Liu C.-H., Hu H.-J., Zhao J.-B., Zou T., Tang Y.-X. H2S inhibits atrial fibrillation-induced atrial fibrosis through miR-133a/CTGF axis. Cytokine. 2021;146:155557. doi: 10.1016/j.cyto.2021.155557. [DOI] [PubMed] [Google Scholar]
- 142.Xiong R., Lu X., Song J., Li H., Wang S. Molecular mechanisms of hydrogen sulfide against uremic accelerated atherosclerosis through cPKCβII/Akt signal pathway. BMC Nephrol. 2019;20:358. doi: 10.1186/s12882-019-1550-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhu C., Liu Q., Li X., Wei R., Ge T., Zheng X., Li B., Liu K., Cui R. Hydrogen sulfide: A new therapeutic target in vascular diseases. Front. Endocrinol. 2022;13:934231. doi: 10.3389/fendo.2022.934231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Roell W., Lewalter T., Sasse P., Tallini Y.N., Choi B.R., Breitbach M., Doran R., Becher U.M., Hwang S.M., Bostani T., et al. Engraftment of connexin 43-expressing cells prevents post-infarct arrhythmia. Nature. 2007;450:819–824. doi: 10.1038/nature06321. [DOI] [PubMed] [Google Scholar]
- 145.Testai L., Marino A., Piano I., Brancaleone V., Tomita K., Di Cesare Mannelli L., Martelli A., Citi V., Breschi M.C., Levi R., et al. The novel H2S-donor 4-carboxyphenyl isothiocyanate promotes cardioprotective effects against ischemia/reperfusion injury through activation of mitoK(ATP) channels and reduction of oxidative stress. Pharmacol. Res. 2016;113:290–299. doi: 10.1016/j.phrs.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 146.Karwi Q.G., Bornbaum J., Boengler K., Torregrossa R., Whiteman M., Wood M.E., Schulz R., Baxter G.F. AP39, a mitochondria-targeting hydrogen sulfide (H2S) donor, protects against myocardial reperfusion injury independently of salvage kinase signalling. Br. J. Pharmacol. 2017;174:287–301. doi: 10.1111/bph.13688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dudek M., Knutelska J., Bednarski M., Nowiński L., Zygmunt M., Bilska-Wilkosz A., Iciek M., Otto M., Żytka I., Sapa J., et al. Alpha lipoic acid protects the heart against myocardial post ischemia-reperfusion arrhythmias via KATP channel activation in isolated rat hearts. Pharmacol. Rep. 2014;66:499–504. doi: 10.1016/j.pharep.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 148.Donnarumma E., Ali M.J., Rushing A.M., Scarborough A.L., Bradley J.M., Organ C.L., Islam K.N., Polhemus D.J., Evangelista S., Cirino G., et al. Zofenopril Protects Against Myocardial Ischemia-Reperfusion Injury by Increasing Nitric Oxide and Hydrogen Sulfide Bioavailability. J. Am. Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nicholson C.K., Lambert J.P., Molkentin J.D., Sadoshima J., Calvert J.W. Thioredoxin 1 is essential for sodium sulfide-mediated cardioprotection in the setting of heart failure. Arterioscler. Thromb. Vasc. Biol. 2013;33:744–751. doi: 10.1161/ATVBAHA.112.300484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang Q., Wang X.L., Liu H.R., Rose P., Zhu Y.Z. Protective effects of cysteine analogues on acute myocardial ischemia: Novel modulators of endogenous H2S production. Antioxid. Redox Signal. 2010;12:1155–1165. doi: 10.1089/ars.2009.2947. [DOI] [PubMed] [Google Scholar]
- 151.Peake B.F., Nicholson C.K., Lambert J.P., Hood R.L., Amin H., Amin S., Calvert J.W. Hydrogen sulfide preconditions the db/db diabetic mouse heart against ischemia-reperfusion injury by activating Nrf2 signaling in an Erk-dependent manner. Am. J. Physiol. Heart Circ. Physiol. 2013;304:H1215–H1224. doi: 10.1152/ajpheart.00796.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Polhemus D.J., Li Z., Pattillo C.B., Gojon Sr G., Gojon Jr G., Giordano T., Krum H. A novel hydrogen sulfide prodrug, SG 1002, promotes hydrogen sulfide and nitric oxide bioavailability in heart failure patients. Cardiovasc. Ther. 2015;33:216–226. doi: 10.1111/1755-5922.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Islam R.K., Donnelly E., Donnarumma E., Hossain F., Gardner J.D., Islam K.N. H2S Prodrug, SG-1002, Protects against Myocardial Oxidative Damage and Hypertrophy In Vitro via Induction of Cystathionine β-Synthase and Antioxidant Proteins. Biomedicines. 2023;11:612. doi: 10.3390/biomedicines11020612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.de Koning M.-S.L., Assa S., Maagdenberg C.G., van Veldhuisen D.J., Pasch A., van Goor H., Lipsic E., van der Harst P. Safety and Tolerability of Sodium Thiosulfate in Patients with an Acute Coronary Syndrome Undergoing Coronary Angiography: A Dose-Escalation Safety Pilot Study (SAFE-ACS) J. Interv. Cardiol. 2020;2020:6014915. doi: 10.1155/2020/6014915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chakraborty P.K., Murphy B., Mustafi S.B., Dey A., Xiong X., Rao G., Naz S., Zhang M., Yang D., Dhanasekaran D.N. Cystathionine β-synthase regulates mitochondrial morphogenesis in ovarian cancer. FASEB J. 2018;32:4145. doi: 10.1096/fj.201701095R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chen L., Liu B., Qin Y., Li A., Gao M., Liu H., Gong G. Mitochondrial fusion protein Mfn2 and its role in heart failure. Front. Mol. Biosci. 2021;8:681237. doi: 10.3389/fmolb.2021.681237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kolluru G.K., Shackelford R.E., Shen X., Dominic P., Kevil C.G. Sulfide regulation of cardiovascular function in health and disease. Nat. Rev. Cardiol. 2023;20:109–125. doi: 10.1038/s41569-022-00741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hall C.B., Weinberg G.A., Iwane M.K., Blumkin A.K., Edwards K.M., Staat M.A., Auinger P., Griffin M.R., Poehling K.A., Erdman D., et al. The burden of respiratory syncytial virus infection in young children. N. Engl. J. Med. 2009;360:588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Escaffre O., Borisevich V., Rockx B. Pathogenesis of Hendra and Nipah virus infection in humans. J. Infect. Dev. Ctries. 2013;7:308–311. doi: 10.3855/jidc.3648. [DOI] [PubMed] [Google Scholar]
- 160.Bao X., Liu T., Spetch L., Kolli D., Garofalo R.P., Casola A. Airway epithelial cell response to human metapneumovirus infection. Virology. 2007;368:91–101. doi: 10.1016/j.virol.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Komaravelli N., Casola A. Respiratory Viral Infections and Subversion of Cellular Antioxidant Defenses. J. Pharmacogenomics Pharmacoproteomics. 2014;5:1000141. doi: 10.4172/2153-0645.1000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gojon G., Morales G.A. SG1002 and Catenated Divalent Organic Sulfur Compounds as Promising Hydrogen Sulfide Prodrugs. Antioxid. Redox Signal. 2020;33:1010–1045. doi: 10.1089/ars.2020.8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Palamara A.T., Perno C.F., Ciriolo M.R., Dini L., Balestra E., D’Agostini C., Di Francesco P., Favalli C., Rotilio G., Garaci E. Evidence for antiviral activity of glutathione: In vitro inhibition of herpes simplex virus type 1 replication. Antivir. Res. 1995;27:237–253. doi: 10.1016/0166-3542(95)00008-A. [DOI] [PubMed] [Google Scholar]
- 164.Kim D., Lee J.Y., Yang J.S., Kim J.W., Kim V.N., Chang H. The Architecture of SARS-CoV-2 Transcriptome. Cell. 2020;181:914–921.e10. doi: 10.1016/j.cell.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Renieris G., Katrini K., Damoulari C., Akinosoglou K., Psarrakis C., Kyriakopoulou M., Dimopoulos G., Lada M., Koufargyris P., Giamarellos-Bourboulis E.J. Serum hydrogen sulfide and outcome association in pneumonia by the SARS-CoV-2 coronavirus. Shock. 2020;54:633–637. doi: 10.1097/SHK.0000000000001562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ivanciuc T., Sbrana E., Ansar M., Bazhanov N., Szabo C., Casola A., Garofalo R.P. Hydrogen Sulfide Is an Antiviral and Antiinflammatory Endogenous Gasotransmitter in the Airways. Role in Respiratory Syncytial Virus Infection. Am. J. Respir. Cell Mol. Biol. 2016;55:684–696. doi: 10.1165/rcmb.2015-0385OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cho H.Y., Miller-DeGraff L., Blankenship-Paris T., Wang X., Bell D.A., Lih F., Deterding L., Panduri V., Morgan D.L., Yamamoto M., et al. Sulforaphane enriched transcriptome of lung mitochondrial energy metabolism and provided pulmonary injury protection via Nrf2 in mice. Toxicol. Appl. Pharmacol. 2019;364:29–44. doi: 10.1016/j.taap.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bazhanov N., Ivanciuc T., Wu H., Garofalo M., Kang J., Xian M., Casola A. Thiol-Activated Hydrogen Sulfide Donors Antiviral and Anti-Inflammatory Activity in Respiratory Syncytial Virus Infection. Viruses. 2018;10:249. doi: 10.3390/v10050249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pacheco A. Sulfur-Containing Compounds as Hydrogen Sulfide Donors and Broad-Spectrum Antiviral Agents. Washington State University; Washington, DC, USA: 2017. [Google Scholar]
- 170.Khattak S., Zhang Q.-Q., Sarfraz M., Muhammad P., Ngowi E.E., Khan N.H., Rauf S., Wang Y.-Z., Qi H.-W., Wang D., et al. The Role of Hydrogen Sulfide in Respiratory Diseases. Biomolecules. 2021;11:682. doi: 10.3390/biom11050682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ge X., Sun J., Fei A., Gao C., Pan S., Wu Z. Hydrogen sulfide treatment alleviated ventilator-induced lung injury through regulation of autophagy and endoplasmic reticulum stress. Int. J. Biol. Sci. 2019;15:2872–2884. doi: 10.7150/ijbs.38315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Han W., Dong Z., Dimitropoulou C., Su Y. Hydrogen sulfide ameliorates tobacco smoke-induced oxidative stress and emphysema in mice. Antioxid. Redox Signal. 2011;15:2121–2134. doi: 10.1089/ars.2010.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Spassov S.G., Donus R., Ihle P.M., Engelstaedter H., Hoetzel A., Faller S. Hydrogen Sulfide Prevents Formation of Reactive Oxygen Species through PI3K/Akt Signaling and Limits Ventilator-Induced Lung Injury. Oxid. Med. Cell. Longev. 2017;2017:3715037. doi: 10.1155/2017/3715037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wang P., Zhang G., Wondimu T., Ross B., Wang R. Hydrogen sulfide and asthma. Exp. Physiol. 2011;96:847–852. doi: 10.1113/expphysiol.2011.057448. [DOI] [PubMed] [Google Scholar]
- 175.Derwall M., Francis R.C., Kida K., Bougaki M., Crimi E., Adrie C., Zapol W.M., Ichinose F. Administration of hydrogen sulfide via extracorporeal membrane lung ventilation in sheep with partial cardiopulmonary bypass perfusion: A proof of concept study on metabolic and vasomotor effects. Crit. Care. 2011;15:R51. doi: 10.1186/cc10016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Perry M.M., Hui C.K., Whiteman M., Wood M.E., Adcock I., Kirkham P., Michaeloudes C., Chung K.F. Hydrogen sulfide inhibits proliferation and release of IL-8 from human airway smooth muscle cells. Am. J. Respir. Cell. Mol. Biol. 2011;45:746–752. doi: 10.1165/rcmb.2010-0304OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Cai W.J., Wang M.J., Moore P.K., Jin H.M., Yao T., Zhu Y.C. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc. Res. 2007;76:29–40. doi: 10.1016/j.cardiores.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 178.Dai J., Teng X., Jin S., Wu Y. The Antiviral Roles of Hydrogen Sulfide by Blocking the Interaction between SARS-CoV-2 and Its Potential Cell Surface Receptors. Oxidative Med. Cell. Longev. 2021;2021:7866992. doi: 10.1155/2021/7866992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Farese S., Stauffer E., Kalicki R., Hildebrandt T., Frey B.M., Frey F.J., Uehlinger D.E., Pasch A. Sodium thiosulfate pharmacokinetics in hemodialysis patients and healthy volunteers. Clin. J. Am. Soc. Nephrol. 2011;6:1447–1455. doi: 10.2215/CJN.10241110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Evgen’ev M.B., Frenkel A. Possible application of H2S-producing compounds in therapy of coronavirus (COVID-19) infection and pneumonia. Cell Stress Chaperones. 2020;25:713–715. doi: 10.1007/s12192-020-01120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Feigin V.L., Nichols E., Alam T., Bannick M.S., Beghi E., Blake N., Culpepper W.J., Dorsey E.R., Elbaz A., Ellenbogen R.G. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:459–480. doi: 10.1016/S1474-4422(18)30499-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Polhemus D.J., Lefer D.J. Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease. Circ. Res. 2014;114:730–737. doi: 10.1161/CIRCRESAHA.114.300505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zhang F., Chen S., Wen J.-Y., Chen Z.-W. 3-Mercaptopyruvate sulfurtransferase/hydrogen sulfide protects cerebral endothelial cells against oxygen-glucose deprivation/reoxygenation-induced injury via mitoprotection and inhibition of the RhoA/ROCK pathway. Am. J. Physiol. Cell Physiol. 2020;319:C720–C733. doi: 10.1152/ajpcell.00014.2020. [DOI] [PubMed] [Google Scholar]
- 184.Nicholson C.K., Calvert J.W. Hydrogen sulfide and ischemia-reperfusion injury. Pharmacol. Res. 2010;62:289–297. doi: 10.1016/j.phrs.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Siracusa R., Fusco R., Cuzzocrea S. Astrocytes: Role and Functions in Brain Pathologies. Front. Pharmacol. 2019;10 doi: 10.3389/fphar.2019.01114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tabassum R., Jeong N.Y. Potential for therapeutic use of hydrogen sulfide in oxidative stress-induced neurodegenerative diseases. Int. J. Med. Sci. 2019;16:1386–1396. doi: 10.7150/ijms.36516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kumar M., Sandhir R. Hydrogen Sulfide in Physiological and Pathological Mechanisms in Brain. CNS Neurol. Disord. Drug Targets. 2018;17:654–670. doi: 10.2174/1871527317666180605072018. [DOI] [PubMed] [Google Scholar]
- 188.Aschner M., Skalny A.V., Ke T., da Rocha J.B., Paoliello M.M., Santamaria A., Bornhorst J., Rongzhu L., Svistunov A.A., Djordevic A.B., et al. Hydrogen Sulfide (H2S) Signaling as a Protective Mechanism against Endogenous and Exogenous Neurotoxicants. Curr. Neuropharmacol. 2022;20:1908–1924. doi: 10.2174/1570159X20666220302101854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bellenguez C., Küçükali F., Jansen I.E., Kleineidam L., Moreno-Grau S., Amin N., Naj A.C., Campos-Martin R., Grenier-Boley B., Andrade V., et al. New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat. Genet. 2022;54:412–436. doi: 10.1038/s41588-022-01024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hodgson N., Trivedi M., Muratore C., Li S., Deth R. Soluble oligomers of amyloid-β cause changes in redox state, DNA methylation, and gene transcription by inhibiting EAAT3 mediated cysteine uptake. J. Alzheimers Dis. 2013;36:197–209. doi: 10.3233/JAD-130101. [DOI] [PubMed] [Google Scholar]
- 191.Aoyama K., Suh S.W., Hamby A.M., Liu J., Chan W.Y., Chen Y., Swanson R.A. Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat. Neurosci. 2006;9:119–126. doi: 10.1038/nn1609. [DOI] [PubMed] [Google Scholar]
- 192.Liu X.Q., Liu X.Q., Jiang P., Huang H., Yan Y. Plasma levels of endogenous hydrogen sulfide and homocysteine in patients with Alzheimer’s disease and vascular dementia and the significance thereof. Zhonghua Yi Xue Za Zhi. 2008;88:2246–2249. [PubMed] [Google Scholar]
- 193.Yan Y., Yan Q., Qian L., Jiang Y., Chen X., Zeng S., Xu Z., Gong Z. S-adenosylmethionine administration inhibits levodopa-induced vascular endothelial growth factor-A expression. Aging. 2020;12:21290–21307. doi: 10.18632/aging.103863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Eto K., Asada T., Arima K., Makifuchi T., Kimura H. Brain hydrogen sulfide is severely decreased in Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2002;293:1485–1488. doi: 10.1016/S0006-291X(02)00422-9. [DOI] [PubMed] [Google Scholar]
- 195.Peng S.Y., Wu X., Lu T., Cui G., Chen G. Research progress of hydrogen sulfide in Alzheimer’s disease from laboratory to hospital: A narrative review. Med. Gas. Res. 2020;10:125–129. doi: 10.4103/2045-9912.296043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Liu Y.Y., Bian J.S. Hydrogen sulfide protects amyloid-β induced cell toxicity in microglia. J. Alzheimers Dis. 2010;22:1189–1200. doi: 10.3233/JAD-2010-101002. [DOI] [PubMed] [Google Scholar]
- 197.Cao L., Cao X., Zhou Y., Nagpure B.V., Wu Z.Y., Hu L.F., Yang Y., Sethi G., Moore P.K., Bian J.S. Hydrogen sulfide inhibits ATP-induced neuroinflammation and Aβ(1-42) synthesis by suppressing the activation of STAT3 and cathepsin S. Brain Behav. Immun. 2018;73:603–614. doi: 10.1016/j.bbi.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 198.Rao S.P., Xie W., Kwon Y.I.C., Juckel N., Xie J., Dronamraju V.R., Vince R., Lee M.K., More S.S. Sulfanegen stimulates 3-mercaptopyruvate sulfurtransferase activity and ameliorates Alzheimer’s disease pathology and oxidative stress in vivo. Redox Biol. 2022;57:102484. doi: 10.1016/j.redox.2022.102484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Moore D.J., West A.B., Dawson V.L., Dawson T.M. Molecular pathophysiology of Parkinson’s disease. Annu. Rev. Neurosci. 2005;28:57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]
- 200.Parkinson J. An essay on the shaking palsy. 1817. J. Neuropsychiatry Clin. Neurosci. 2002;14:223–236; discussion 222. doi: 10.1176/jnp.14.2.223. [DOI] [PubMed] [Google Scholar]
- 201.Shefa U., Kim M.S., Jeong N.Y., Jung J. Antioxidant and Cell-Signaling Functions of Hydrogen Sulfide in the Central Nervous System. Oxid. Med. Cell. Longev. 2018;2018:1873962. doi: 10.1155/2018/1873962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hu L.F., Lu M., Wu Z.Y., Wong P.T., Bian J.S. Hydrogen sulfide inhibits rotenone-induced apoptosis via preservation of mitochondrial function. Mol. Pharmacol. 2009;75:27–34. doi: 10.1124/mol.108.047985. [DOI] [PubMed] [Google Scholar]
- 203.Lee M., Schwab C., Yu S., McGeer E., McGeer P.L. Astrocytes produce the antiinflammatory and neuroprotective agent hydrogen sulfide. Neurobiol. Aging. 2009;30:1523–1534. doi: 10.1016/j.neurobiolaging.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 204.Murros K.E. Hydrogen Sulfide Produced by Gut Bacteria May Induce Parkinson’s Disease. Cells. 2022;11:978. doi: 10.3390/cells11060978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.McColgan P., Tabrizi S.J. Huntington’s disease: A clinical review. Eur. J. Neurol. 2018;25:24–34. doi: 10.1111/ene.13413. [DOI] [PubMed] [Google Scholar]
- 206.Ross C.A., Aylward E.H., Wild E.J., Langbehn D.R., Long J.D., Warner J.H., Scahill R.I., Leavitt B.R., Stout J.C., Paulsen J.S., et al. Huntington disease: Natural history, biomarkers and prospects for therapeutics. Nat. Rev. Neurol. 2014;10:204–216. doi: 10.1038/nrneurol.2014.24. [DOI] [PubMed] [Google Scholar]
- 207.Paul B.D., Sbodio J.I., Snyder S.H. Mutant Huntingtin Derails Cysteine Metabolism in Huntington’s Disease at Both Transcriptional and Post-Translational Levels. Antioxidants. 2022;11:1470. doi: 10.3390/antiox11081470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Morozko E.L., Smith-Geater C., Monteys A.M., Pradhan S., Lim R.G., Langfelder P., Kachemov M., Kulkarni J.A., Zaifman J., Hill A. PIAS1 modulates striatal transcription, DNA damage repair, and SUMOylation with relevance to Huntington’s disease. Proc. Natl. Acad. Sci. USA. 2021;118:e2021836118. doi: 10.1073/pnas.2021836118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Sbodio J.I., Snyder S.H., Paul B.D. Golgi stress response reprograms cysteine metabolism to confer cytoprotection in Huntington’s disease. Proc. Natl. Acad. Sci. USA. 2018;115:780–785. doi: 10.1073/pnas.1717877115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Giovinazzo D., Bursac B., Sbodio J.I., Nalluru S., Vignane T., Snowman A.M., Albacarys L.M., Sedlak T.W., Torregrossa R., Whiteman M. Hydrogen sulfide is neuroprotective in Alzheimer’s disease by sulfhydrating GSK3β and inhibiting Tau hyperphosphorylation. Proc. Natl. Acad. Sci. USA. 2021;118:e2017225118. doi: 10.1073/pnas.2017225118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Vandini E., Ottani A., Zaffe D., Calevro A., Canalini F., Cavallini G.M., Rossi R., Guarini S., Giuliani D. Mechanisms of Hydrogen Sulfide against the Progression of Severe Alzheimer’s Disease in Transgenic Mice at Different Ages. Pharmacology. 2019;103:50–60. doi: 10.1159/000494113. [DOI] [PubMed] [Google Scholar]
- 212.Zhao F.L., Fang F., Qiao P.F., Yan N., Gao D., Yan Y. AP39, a Mitochondria-Targeted Hydrogen Sulfide Donor, Supports Cellular Bioenergetics and Protects against Alzheimer’s Disease by Preserving Mitochondrial Function in APP/PS1 Mice and Neurons. Oxid. Med. Cell. Longev. 2016;2016:8360738. doi: 10.1155/2016/8360738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Perry T.L., Wright J.M., Hansen S., Allan B.M., Baird P.A., MacLeod P.M. Failure of aminooxyacetic acid therapy in Huntington disease. Neurology. 1980;30:772–775. doi: 10.1212/WNL.30.7.772. [DOI] [PubMed] [Google Scholar]
- 214.Guth P.S., Blair P., Norris C., Risey J., Reed H.T., Housley G., Briner W., Bryant G., Miller R. Evaluation of amino-oxyacetic acid as a palliative in tinnitus. Ann. Otol. Rhinol. Laryngol. 1990;99:74–79. doi: 10.1177/000348949009900113. [DOI] [PubMed] [Google Scholar]
- 215.Jha S., Calvert J.W., Duranski M.R., Ramachandran A., Lefer D.J. Hydrogen sulfide attenuates hepatic ischemia-reperfusion injury: Role of antioxidant and antiapoptotic signaling. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H801–H806. doi: 10.1152/ajpheart.00377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Peng T., Zhuo L., Wang Y., Jun M., Li G., Wang L., Hong D. Systematic review of sodium thiosulfate in treating calciphylaxis in chronic kidney disease patients. Nephrology. 2018;23:669–675. doi: 10.1111/nep.13081. [DOI] [PubMed] [Google Scholar]
- 217.Borghi C., Omboni S., Novo S., Vinereanu D., Ambrosio G., Ambrosioni E. Efficacy and safety of zofenopril versus ramipril in the treatment of myocardial infarction and heart failure: A review of the published and unpublished data of the randomized double-blind SMILE-4 study. Adv. Ther. 2018;35:604–618. doi: 10.1007/s12325-018-0697-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ambrosioni E., Borghi C., Magnani B. The effect of the angiotensin-converting–enzyme inhibitor zofenopril on mortality and morbidity after anterior myocardial infarction. New Engl. J. Med. 1995;332:80–85. doi: 10.1056/NEJM199501123320203. [DOI] [PubMed] [Google Scholar]
- 219.Paquette J.M., Rufiange M., Iovu Niculita M., Massicotte J., Lefebvre M., Colin P., Telmat A., Ranger M. Safety, tolerability and pharmacokinetics of trimebutine 3-thiocarbamoylbenzenesulfonate (GIC-1001) in a randomized phase I integrated design study: Single and multiple ascending doses and effect of food in healthy volunteers. Clin. Ther. 2014;36:1650–1664. doi: 10.1016/j.clinthera.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 220.Wallace J.L., Nagy P., Feener T.D., Allain T., Ditrói T., Vaughan D.J., Muscara M.N., De Nucci G., Buret A.G. A proof-of-concept, Phase 2 clinical trial of the gastrointestinal safety of a hydrogen sulfide-releasing anti-inflammatory drug. Br. J. Pharmacol. 2020;177:769–777. doi: 10.1111/bph.14641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Vajdi M., Noshadi N., Hassanizadeh S., Bonyadian A., Seyedhosseini-Ghaheh H., Askari G. The effects of alpha lipoic acid (ALA) supplementation on blood pressure in adults: A GRADE-assessed systematic review and dose-response meta-analysis of randomized controlled trials. Front. Cardiovasc. Med. 2023;10 doi: 10.3389/fcvm.2023.1272837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Pavlovskiy Y., Yashchenko A., Zayachkivska O. H2S donors reverse age-related gastric malfunction impaired due to fructose-induced injury via CBS, CSE, and TST expression. Front. Pharmacol. 2020;11:1134. doi: 10.3389/fphar.2020.01134. [DOI] [PMC free article] [PubMed] [Google Scholar]