Abstract
Background
Hypercholesterolemia has been identified as an independent predictor of cardiovascular disease (CVD). Inclisiran, an innovative small interfering RNA agent, is anticipated to result in a notable reduction of approximately 50% in low-density lipoprotein cholesterol (LDL-C) levels. Given its transformative impact, this study scrutinized the eligibility of the US population for inclisiran treatment and evaluated its potential effects on hypercholesterolemia and the primary prevention of CVD.
Methods
This study applied the eligibility criteria from the ORION 10 and 11 trials to the 1999–2018 National Health and Nutrition Examination Survey (NHANES) dataset to estimate the size of the eligible population for atherosclerotic cardiovascular disease (ASCVD) and ASCVD-risk equivalents. Utilizing the reduction in LDL-C levels from ORION 10, this study predicted the impact of inclisiran on LDL-C levels among ASCVD patients. Similarly, leveraging the changes in lipid levels from ORION 11, this study predicted inclisiran’s effect on the 10-year change in CVD risk and preventable CVD events in the ASCVD-risk equivalents population, employing the Framingham CVD Risk Score.
Results
The study identified 579 ASCVD patients (5 million) and 382 ASCVD-risk equivalents (2.66 million) who met the eligibility criteria from ORION 10 and 11. Among the ASCVD population, 3.5 million (70.2%) would achieve a ≥ 50% reduction in LDL-C levels after treatment. Furthermore, 4.6 million (91.3%) would achieve LDL-C levels < 70 mg/dL, and 3.8 million (75%) would achieve LDL-C levels < 55 mg/dL after treatment. For the ASCVD-risk equivalents population, the estimated 10-year CVD risk would decrease from 25.3 to 17.7%, an absolute reduction of 7.6% and a relative reduction of 30% following inclisiran treatment, potentially preventing 202,353 CVD events over a decade, including 138,084 coronary heart disease cases, 37,351 strokes, and 23,894 congestive heart failure cases.
Conclusions
Inclisiran has the potential to substantially reduce the prevalence of hypercholesterolemia and prevent nearly 200,000 CVD events in eligible US adults.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12944-024-02294-8.
Keywords: Inclisiran, Cardiovascular disease, NHANES, LDL-C, Hypercholesterolemia
Introduction
Over the past six decades, there has been a notable increase in mean life expectancy, along with a decline in lifetime risk of atherosclerotic cardiovascular disease (ASCVD), as evidenced by the Framingham Study [1]. However, ASCVD remains the leading cause of mortality worldwide [2]. Hypercholesterolemia, particularly elevated levels of low-density lipoprotein cholesterol (LDL-C), is a pivotal risk factor for ASCVD [3]. Despite the widespread use of statin therapy, either alone or in combination with other lipid-lowering agents, a significant proportion of patients continue to have elevated LDL-C levels [4]. In the era of monoclonal antibodies targeting proprotein convertase subtilisin/kexin type 9 (PCSK9), which are known for their profound LDL-C reduction both as standalone therapies and in combination with statins [5], inclisiran has emerged as a promising therapeutic option. This double-stranded small interfering RNA (siRNA) exerts its action by targeting and degrading the mRNA of PCSK9 [6]. Inclisiran received approval from the European Medicines Agency (EMA) [7] in 2020 for LDL-C reduction, followed by approval from the Food and Drug Administration (FDA) [8] in 2021. In early 2023, the FDA extended inclisiran’s approved indications to include adults with high LDL-C levels and those at heightened risk of ASCVD [9].
Numerous studies, including the four-year open-label extension trial ORION-3, consistently demonstrate that twice-yearly administration of inclisiran results in a substantial reduction of approximately 50% in LDL-C levels. This effect is also observed when inclisiran is used alongside maximally tolerated oral lipid-lowering therapy across diverse populations. Notably, those populations include individuals with heterozygous familial hypercholesterolemia, ASCVD, and other equivalent risk profiles [10–14]. Regarding safety, a comprehensive analysis from seven clinical trials highlights inclisiran’s favorable tolerability profile across a diverse patient population during long-term treatment, with no significant safety concerns [15]. Furthermore, a patient-level pooled analysis from the ORION-9, -10, and − 11 trials offers early insights into the potential cardiovascular benefits associated with LDL-C reduction through inclisiran therapy. This analysis suggests promising potential for reducing major adverse cardiovascular events, further highlighting inclisiran’s role in cardiovascular risk reduction [16].
Despite extensive research, the precise number of eligible individuals in the United States for inclisiran therapy is still unknown, and the drug’s effect on LDL-C levels within this population, as well as its effectiveness in the primary prevention of cardiovascular disease (CVD), has not yet been fully clarified. Therefore, the objective of this study is: (1) to identify the eligible US population for inclisiran treatment based on the stringent eligibility criteria established in the ORION 10 and 11 trials, (2) to estimate inclisiran’s population-wide impact on LDL-C levels among individuals with ASCVD, and (3) to quantify the potential reduction in CVD events due to inclisiran therapy in individuals with ASCVD-risk equivalents. These findings could provide valuable insights for ongoing clinical trials such as ORION 4 and VICTORION-2 PREVENT, which aim to assess the cardiovascular outcomes associated with inclisiran treatment [17, 18].
Methods
Study sample
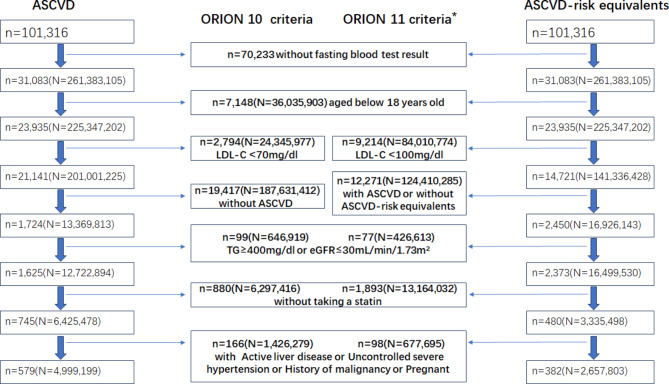
The study focused on two cohorts within the US population: individuals diagnosed with ASCVD and those at equivalent ASCVD risk. These cohorts were identified using the publicly available US National Health and Nutrition Examination Survey (NHANES) database from 1999 to 2018 (ClinicalTrials.gov ID: NCT00005154). Prior consent for research use of data was obtained from all NHANES participants. The study identified the ASCVD sample and the ASCVD-risk equivalent sample by applying the inclusion and exclusion criteria from the ORION 10 and 11 trials to the NHANES database (Fig. 1).
Fig. 1.
Screening and weighting of eligible samples from the NHANES 1999–2018 dataset based on ORION-10 and ORION-11 trial criteria. Note Lowercase n represents the sample size; uppercase N represents the weighted US population. *ORION-11 criteria apply only to ASCVD-risk equivalent subjects. Abbreviations ASCVD, atherosclerotic cardiovascular disease; LDL-C, low-density lipoprotein cholesterol; TG, triglycerides; eGFR, estimated glomerular filtration rate
Inclusion and exclusion criteria
The inclusion criteria were: (1) Participants of all genders, aged 18 years or older; (2) Participants with a history of ASCVD (including coronary heart disease (CHD), heart attack, stroke or peripheral arterial disease (PAD)) for the ASCVD sample, or those without ASCVD but with equivalent risk factors (e.g., type 2 diabetes, or a 10-year cardiovascular event risk ≥ 20% as assessed by the Framingham Risk Score) for the ASCVD-risk equivalent sample; (3) Serum LDL-C levels ≥ 1.8 mmol/L (≥ 70 mg/dL) in the ASCVD sample, or ≥ 2.6 mmol/L (≥ 100 mg/dL) in the ASCVD-risk equivalent sample; (4) Fasting triglyceride levels < 4.52 mmol/L (< 400 mg/dL); (5) Estimated glomerular filtration rate (eGFR) > 30 mL/min/1.73 m^2 using Cockcroft-Gault equation; (6) Currently receiving statin therapy.
The exclusion criteria were: (1) New York Heart Association Class IV heart failure or last recorded left ventricular ejection fraction < 25%; (2) Uncontrolled severe hypertension (systolic > 180 mmHg or diastolic > 110 mmHg despite therapy); (3) Alanine aminotransferase (ALT) or aspartate aminotransferase (AST) levels > 3 times the upper limit of normal, or total bilirubin levels > 2 times the upper limit of normal; (4) Severe non-cardiovascular disease reducing life expectancy to < 2 years; (5) History of malignancy; (6)Pregnancy or positive urine pregnancy test; (7) Ongoing treatment with PCSK9 inhibitors.
Weighting
Samples meeting the criteria at each step were weighting throughout the screening process. Since fasting blood tests were required for all eligible participant, the study combined the Fasting Subsample 2-Year Mec Weight from 1999 to 2018 to derive the definitive 20-Year Fasting Subsample Mec Weight [19]. These calculated weights were used to determine the population estimates for the United States.
Variables
Variables were selected to align with those used in the ORION trials. Age, gender, and ethnicity were extracted from the NHANES Demographic Data. Data on ASCVD (including CHD, heart attack and stroke), hypertension, diabetes, and smoking status were obtained from the Questionnaire Data, while PAD, defined by an ankle-brachial index < 0.9, was obtained from the Examination Data. Lipid levels, following the same statistical methods and units as in the ORION trials, were retrieved from the Laboratory Data. Baseline data from NHANES samples were then compared with those from the ORION trials for analysis.
Estimate the effect of inclisiran on LDL-C levels in the ASCVD population
The study estimated the number of patients achieving a reduction in LDL-C levels of over 50%, based on the proportion observed in the ORION 10 trial. These estimations were stratified by sex and ethnicity. The study also estimated the number of patients achieving LDL-C levels below 70 or 55 mg/dL, as recommended by the 2018 ACC/AHA [20] and 2019 ESC/EAS [21] Guidelines. These projections were based on the average LDL-C reductions observed in the ORION 10 trial and were stratified by sex and ethnicity for comprehensive analysis.
Estimate the effect of inclisiran on CVD risk in the ASCVD-risk equivalent population
The study used the Framingham risk scores, a Cox regression model developed by D’Agostino et al. [22], to estimate the baseline 10-year risk of CVD events in the ASCVD-risk equivalent population. Using NHANES sample weighting, the study estimated the population size in millions. The study then repeated this process with risk factor adjustments from the ORION 11 trial to estimate the “post-treatment” 10-year CVD risk. The risk difference before and after inclisiran treatment, multiplied by the eligible population size, estimated the number of preventable CVD events. These analyses were stratified by sex and ethnicity.
Statistics and ethics
Quantitative variables are presented as mean ± standard deviation for normally distributed data and as median with interquartile range (in parentheses) for non-normally distributed data. Qualitative variables are reported as frequencies with percentages (in parentheses). Statistical comparisons of quantitative variables were made using Student’s t-test for normally distributed data and the Wilcoxon rank-sum test for non-normally distributed data. The chi-squared test was used for comparisons involving qualitative variables. All analyses were conducted using SAS version 9.4. This study used publicly available de-identified data and was therefore exempt from institutional review board approval.
Results
Characteristics of the participants
The study identified 31,083 individuals who underwent fasting blood tests from a total of 101,316 NHANES participants. Using the Fasting Subsample 20-Year MEC Weight, the study applied sample weighting, resulting in a weighted sample size of 261 million individuals, representing the entire US population. After further screening, the study identified 579 ASCVD participants, representing 5 million US adults who met the eligibility criteria of the ORION 10 trial, and 382 ASCVD-risk equivalent participants, representing 2.66 million US adults who met the eligibility criteria of the ORION 11 trial (Fig. 1).
Descriptive statistics on demographic and cardiovascular risk factors were obtained from the NHANES database and compared to the inclisiran group data from the ORION trials. While the mean age across the ORION trials and NHANES sample was similar, significant differences were found in the distribution of sex and ethnicity. Specifically, the ASCVD sample had a lower proportion of males and Whites compared to the ORION 10 trial, while the ASCVD-risk equivalent sample had a higher proportion of males and a lower proportion of Whites compared to the ORION 11 trial. Regarding cardiovascular risk factors, the ASCVD sample had a lower prevalence of hypertension and diabetes but a higher prevalence of smoking compared to the ORION 10 trial. Similarly, the ASCVD-risk equivalent sample had a lower prevalence of diabetes but a higher prevalence of other CVD risk factors compared to the ORION 11 trial. These disparities may be due to differences in background, objectives, and methodologies used during baseline data collection. Regarding blood lipid levels, no significant differences were found between the ASCVD sample and the ORION 10 trial. However, the ASCVD-risk equivalent sample exhibited lower levels of LDL-C, non-High-density lipoprotein cholesterol (non-HDL-C), total cholesterol (TC), and triglycerides (TG) compared to the ORION 11 trial. These differences are primarily due to the limited participant pool in the ORION 11 trial, as individuals with higher risk profiles are more likely to participate in clinical trials, thus influencing the observed lipid levels (Table 1).
Table 1.
Demographic and clinical characteristics of the NHANES samples and the ORION trials
| Characteristic | ASCVD sample (n = 579) |
Control one† (n = 781) |
ASCVD-risk equivalent sample (n = 382) |
Control two‡ (n = 98) |
|---|---|---|---|---|
| Age — yr | 67 ± 10.2 | 66.4 ± 8.9 | 63.6 ± 11.5 | 62.7 ± 10.6 |
| Male sex — no.(%) | 359(62)*** | 535(68.5) | 219(57.3) *** | 45(45.9) |
| White race — no.(%) | 341(58.9) *** | 653(83.6) | 142(37.2) *** | 94(95.9) |
| Cardiovascular risk factors — no.(%) | ||||
| ASCVD | 579(100) | 781(100) | 0(0) | 0(0) |
| Current smoker | 127(21.9) *** | 123(15.7) | 68(17.8) | 19(19.4) |
| Hypertension | 438(75.7) *** | 714(91.4) | 264(69.1) | 67(68.4) |
| Diabetes | 190(32.8) *** | 371(47.5) | 208(54.5) *** | 66(67.3) |
|
10-year predicted CVD risk ≥ 20% |
/ | / | 287(75.1) *** | 54(55.1) |
| Lipid measures — mg/dL | ||||
| LDL cholesterol | 103.2 ± 28 | 104.5 ± 39.6 | 128.8 ± 25.8*** | 143.1 ± 65.7 |
| Total cholesterol | 181.6 ± 35.9 | 180.6 ± 46.1 | 210.2 ± 30.9*** | 232 ± 69.6 |
| Non-HDL cholesterol | 131.2 ± 34.3** | 134 ± 44.5 | 158.6 ± 31.1*** | 177.9 ± 69.6 |
| HDL cholesterol | 50.3 ± 14.3** | 46.6 ± 14.3 | 51.7 ± 13.7* | 50.3 ± 15.5 |
| Triglycerides | 125 (87–175) | 127 (92–181) | 136 (95–188)*** | 159 (115–204) |
Quantitative variables are presented as mean ± standard deviation, except for triglycerides, which are reported as median (interquartile range) due to its non-normal distribution. Qualitative variables are expressed as numbers (percentages). The characteristics of the inclisiran groups in the ORION trials are provided for comparison with the NHANES eligible samples
† Control 1: ASCVD patients treated with inclisiran in the ORION-10 trial
‡ Control 2: ASCVD-risk equivalent subjects treated with inclisiran in the ORION-11 trial
*P < 0.05, **P < 0.01, ***P < 0.001
Abbreviations NHANES, National Health and Nutrition Examination Survey; ASCVD, atherosclerotic cardiovascular disease; CVD, cardiovascular disease; LDL, low-density lipoprotein; HDL, high-density lipoprotein
Changes in LDL-C levels
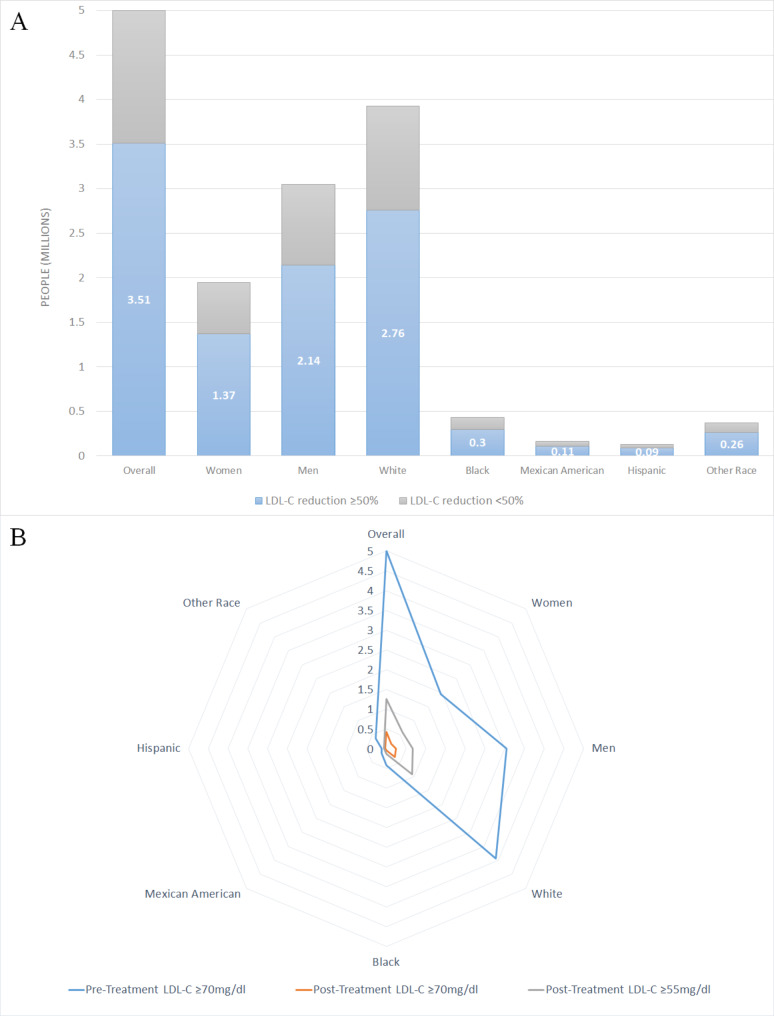
The study estimated the number of ASCVD patients achieving ≥ 50% reduction in LDL-C levels after inclisiran or placebo treatment. Extrapolating the findings of the ORION 10 trial, where 72.8% of inclisiran-treated patients experienced ≥ 50% reductions in LDL-C, this study predicted that approximately 3.64 million patients within the ASCVD cohort of 5 million would achieve similar reductions. Even accounting for potential placebo effects, the analysis indicated that roughly 3.51 million patients would still achieve LDL-C reductions of ≥ 50%. This includes an estimated 2.14 million men, 1.37 million women, as well as 2.76 million White individuals, 0.3 million Black individuals, 0.11 million Mexican American individuals, and 0.09 million Hispanic individuals (Fig. 2A, Table S1).
Fig. 2.
Number of ASCVD patients with LDL-C reductions following inclisiran treatment, based on placebo-adjusted effects from the ORION-10 trial. (A) Patients achieving at least a 50% reduction in LDL-C. The blue areas indicate those with LDL-C reductions of ≥ 50%, while the gray areas represent those with reductions of < 50%. (B) Patients reaching LDL-C levels below 70 or 55 mg/dL. The area between the blue and orange lines represents patients achieving LDL-C < 70 mg/dL, and the area between the blue and gray lines represents those achieving LDL-C < 55 mg/dL. Abbreviations LDL-C, low-density lipoprotein cholesterol
The study also estimated the number of US adults within the ASCVD population expected to achieve LDL-C levels < 70 or 55 mg/dL following inclisiran treatment. The results indicated that 4.57 million (91.3%) of patients achieved LDL-C < 70 mg/dL after inclisiran treatment. This includes 2.79 million (91.7%) men and 1.77 million (90.8%) women, as well as 3.63 million (92.5%) White individuals, 0.37 million (87.6%) Black individuals, 0.14 million (88.5%) Mexican American individuals, and 0.09 million (76.1%) Hispanic individuals. Additionally, 3.75 million (75%) achieved LDL-C < 55 mg/dL, comprising 2.38 million (77.9%) men and 1.38 million (70.5%) women, with 3 million (76.6%) White individuals, 0.28 million (66.8%) Black individuals, 0.11 million (67.3%) Mexican American individuals, and 0.06 million (48%) Hispanic individuals (Fig. 2B, Table S2).
Changes in 10‑year CVD risk
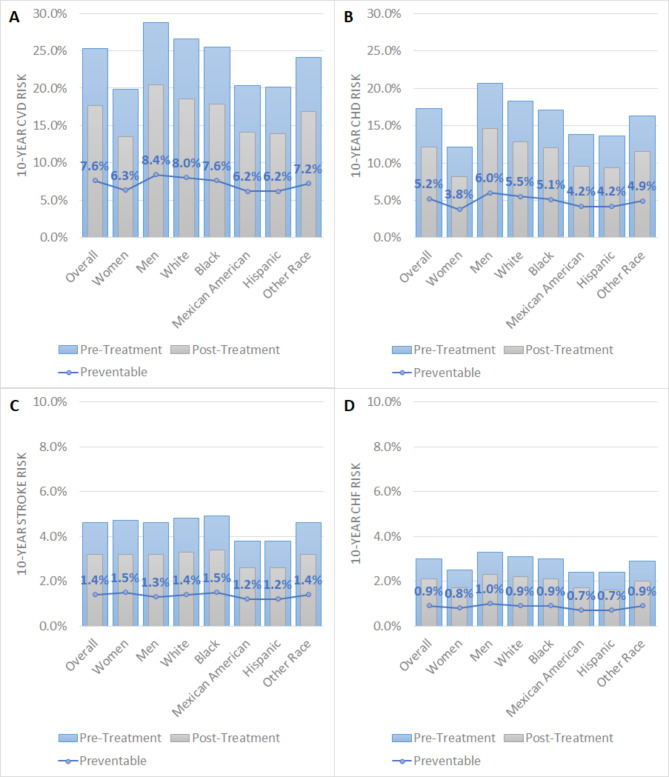
The study estimated the 10-year CVD risk before and after inclisiran treatment in the ASCVD-risk equivalent population using the Framingham risk scores. Prior to treatment, the average risk for the entire population was 25.3%, with men having a higher risk of 28.8%. Among all racial groups, White individuals exhibited the highest average risk at 26.6%. Following inclisiran treatment, an overall absolute risk reduction of 7.6% and a relative risk reduction of 30% were observed. Notably, substantial risk reductions were seen among men (8.4%) and White individuals (8%). Furthermore, the study predicted a total of 202,353 preventable CVD events, including 138,084 cases of CHD, 37,351 strokes, and 23,894 cases of congestive heart failure (CHF) (Fig. 3A-D, Table S3).
Fig. 3.
Changes in 10-year CVD risk for ASCVD-risk equivalent populations before and after treatment, based on placebo-adjusted effects of inclisiran from the ORION-11 trial. (A) 10-year CVD risk. (B) 10-year CHD risk. (C) 10-year stroke risk. (D) 10-year CHF risk. Abbreviations CVD, cardiovascular disease; CHD, coronary heart disease; CHF, congestive heart failure
Discussion
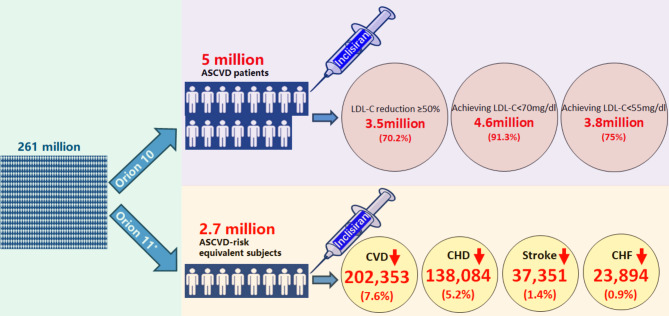
The study highlights that approximately 5 million US adults with ASCVD and 2.66 million with ASCVD-risk equivalents meet the eligibility criteria for inclisiran treatment based on the ORION 10 and 11 trials, respectively. In the ASCVD population, an estimated 3.5 million individuals could expect ≥ 50% reductions in LDL-C levels. Additionally, 4.6 million (91.3%) or 3.8 million (75%) individuals would achieve LDL-C levels below 70 or 55 mg/dL after inclisiran treatment, respectively. In the ASCVD-risk equivalent population, the estimated 10-year CVD risk decreased from 25.3 to 17.7% after inclisiran treatment. This corresponds to a 7.6% absolute and a 30% relative reduction in risk. Over 10 years, inclisiran could prevent an estimated 202,353 CVD events, including 138,084 cases of CHD, 37,351 strokes, and 23,894 cases of CHF. These findings underscore the significant potential impact of inclisiran on the broader US population (Fig. 4).
Fig. 4.
Central illustration. Based on the inclusion and exclusion criteria of the ORION-10 and ORION-11 trials, 5 million ASCVD patients and 2.7 million ASCVD-risk equivalent individuals were identified from a population of 261 million Americans. Among the ASCVD patients, 3.5 million (70.2%) could achieve LDL-C reductions of ≥ 50%, 4.6 million (91.3%) could reach LDL-C levels below 70 mg/dL, and 3.8 million (75%) could reach levels below 55 mg/dL with inclisiran treatment. In the ASCVD-risk equivalent population, inclisiran could potentially prevent 202,353 (7.6%) CVD events, including 138,084 (5.2%) CHD events, 37,351 (1.4%) strokes, and 23,894 (0.9%) CHF events over a 10-year period. Note Each small white man icon approximately represents 330,000 Americans. *ORION-11 criteria apply only to ASCVD-risk equivalent subjects. Abbreviations ASCVD, atherosclerotic cardiovascular disease; CVD, cardiovascular disease; LDL-C, low-density lipoprotein cholesterol; CHD, coronary heart disease; CHF, congestive heart failure
Hypercholesterolemia is a significant contributor to the global health burden, with cardiovascular morbidity increasing notably with age, primarily driven by elevated LDL-C levels [23, 24]. Statins remain the cornerstone pharmacotherapy for reducing the risk of future ASCVD events [25]. Pioneering work by Dubuc et al. illuminated the complex relationship between dietary cholesterol intake and PCSK9 expression, establishing PCSK9 as a cholesterol-regulated gene [26]. Mechanistically, statins inhibit cholesterol biosynthesis, which in turn increases PCSK9 transcription, leading to accelerated degradation of LDL receptors [27–30]. Paradoxically, this pathway can maintain or even elevate serum LDL-C levels. Supporting murine studies have confirmed these findings, demonstrating that PCSK9 deficiency is associated with lower plasma cholesterol levels and enhanced sensitivity to statin therapy [31]. Moreover, genetic studies in humans revealed that individuals with non-functional PCSK9 variants have a significantly reduced lifetime risk of cardiovascular events [32], underscoring the therapeutic potential of PCSK9 inhibition for LDL-C reduction.
Through significant efforts by the pharmaceutical industry, two distinct and effective PCSK9 inhibitory therapies have emerged: PCSK9 monoclonal antibodies (PCSK9mAbs) and siRNA targeting PCSK9. Although both exhibit comparable lipid-lowering effects [13, 33, 34], PCSK9mAbs require frequent subcutaneous administration, while siRNA therapies necessitate fewer injections, potentially improving patient adherence. As the pioneer siRNA drug targeting PCSK9, inclisiran has gained considerable attention and has been extensively evaluated for its efficacy and safety. However, no large-scale studies involving more than 10,000 participants have been reported to date.
A key question emerges: what proportion of eligible Americans could benefit from inclisiran, and how effectively could it manage their LDL-C levels? This study estimates that approximately 5 million adults with ASCVD meet the eligibility criteria for inclisiran treatment based on the ORION-10 trial. The study focused on LDL-C reductions of at least 50% and two LDL-C thresholds (70 and 55 mg/dL) as primary outcomes. These targets were selected in accordance with the 2018 ACC/AHA Guidelines [20], which recommends that ASCVD patients undergo lipid-lowering therapy to achieve a minimum of 50% LDL-C reduction. The guidelines also suggest intensifying or combining therapies if LDL-C levels remain above 70 mg/dL after treatment. Additionally, the 2019 ESC/EAS Guidelines [21] recommend an LDL-C target below 55 mg/dL for ASCVD patients. This study estimates that approximately 3.5 million individuals would likely achieve LDL-C reductions of ≥ 50%, with 4.57 million reaching LDL-C levels below 70 mg/dL, and 3.75 million reaching levels below 55 mg/dL following inclisiran therapy. A detailed analysis of LDL-C reductions, stratified by sex and ethnicity, showed that men and White individuals derived the most significant benefits. This outcome is largely attributable to the demographic composition of the eligible population, which predominantly consists of men and White individuals. Furthermore, the analysis identified that Hispanics had the highest baseline LDL-C and TC levels. Given their reported suboptimal adherence to statin therapy [35], this group may experience substantial benefits from inclisiran treatment.
To date, several studies have attempted to estimate the impact of inclisiran on CVD prevention. One such study utilized a Markov model to project the health benefits of inclisiran treatment in individuals aged 50 and older with pre-existing ASCVD in England. The analysis specifically focused on the prevention of cardiovascular events and related mortality. The results indicated that over a 10-year period, inclisiran treatment could potentially prevent 138,647 cardiovascular events, yielding a cost-effectiveness ratio of 1.03 [36]. In another study, the addition of inclisiran to statin therapy was compared to statin therapy alone. The analysis projected that, among 1000 subjects over their lifetimes in Australia, the addition of inclisiran would prevent 235 non-fatal myocardial infarctions and 114 coronary revascularizations [37]. However, uncertainties remain regarding the assumptions inherent in these methodological approaches, and substantial deviations between predicted and actual eligible populations for treatment are likely. In this investigation, weighting the NHANES database helps address these limitations. Notably, all previous studies have exclusively focused on patients with ASCVD. As primary prevention in ASCVD gains increasing importance [38], it warrants further investigation. However, there is a lack of studies assessing the impact of inclisiran on primary prevention. This study aims to estimate the number of individuals without ASCVD but meeting the ASCVD-risk equivalent criteria outlined in the ORION 11 trial, and to evaluate inclisiran’s potential in preventing CVD in this group. Using Framingham risk scores, the analysis suggests that up to 202,353 cardiovascular events (7.6%) could potentially be prevented over a decade in the United States. Detailed examination reveals that men (8.4%) and White individuals (8%) benefit the most. This is likely due to the higher susceptibility of men to CVD compared to women [39], and the more pronounced variation in risk factors among men. Regarding race, Whites have the highest prevalence of risk factors and CVD rates [40, 41], making them the predominant ASCVD-risk equivalents. Consequently, they stand to gain the most from primary prevention strategies involving inclisiran.
Strengths and limitations
This study has several notable strengths. Firstly, it utilizes the comprehensive NHANES database, which includes a diverse sample of US adults. By employing the database’s sample weighting functionality, the study effectively assesses the population-level impact of inclisiran on ORION-eligible US adults and explores CVD prevention across various demographic groups. Although NHANES relies on self-reported measures for certain variables, including CVD status, previous research has confirmed the reliability of these self-reports [42]. Secondly, by extrapolating the LDL-C-lowering effects observed in the ORION 10 trial to the NHANES data, this study estimates the number of ASCVD patients who could achieve target LDL-C levels with inclisiran. This provides valuable insights for national lipid management strategies. Thirdly, this study includes individuals with ASCVD-risk equivalents, a population often overlooked in previous research, which has primarily focused on inclisiran’s effects in secondary prevention of CVD. The incorporation of data on ASCVD-risk equivalent individuals from the ORION 11 trial, as reported by Professor Kausik [14], is innovative. This enables an estimation of inclisiran’s potential for primary prevention of CVD in the US population by integrating these data with the NHANES database.
However, this study has certain limitations that should be acknowledged. Firstly, the NHANES database does not include specific indicators for familial hypercholesterolemia (FH), limiting the ability to fully assess the national impact of inclisiran on this condition. Furthermore, the absence of detailed data on statin dosage and tolerability required the use of “currently receiving statin therapy” as a criterion instead of “receiving a maximally tolerated dose”. This substitution may overestimate the number of individuals eligible for the ORION trials and overlook those with ASCVD who are statin-intolerant. Secondly, although the ORION eligibility criteria were applied to identify the NHANES sample, differences between the NHANES sample and the ORION trial participants likely exist. For example, the NHANES sample includes a lower proportion of White individuals compared to the ORION 10 trial, which may lead to an overestimation of inclisiran’s efficacy at the national level. This potential overestimation could stem from the fact that White individuals, who generally exhibit higher adherence to statin or other lipid-lowering treatments, might show reduced sensitivity to inclisiran. Lastly, although the Framingham Risk Score is widely used to estimate cardiovascular events, its accuracy may be limited by its inability to account for certain factors such as family history and other risk-enhancing elements. Moreover, since the score was primarily derived from long-term observations of a predominantly White cohort, its application in predicting cardiovascular events across the diverse US population may reduce its precision and generalizability.
Conclusions
This study suggests that inclisiran treatment in eligible US adults has the potential to significantly reduce hypercholesterolemia and prevent nearly 200,000 CVD events. These findings provide compelling evidence for policymakers, supporting the integration of inclisiran into healthcare strategies for patients with hypercholesterolemia. Additionally, the results advocate for the adoption of inclisiran to enhance both primary and secondary CVD prevention strategies, aiming to reduce cardiovascular mortality and improve the overall longevity of the population.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was analyzed using the data provided by the National Health and Nutrition Examination 1999–2018. The authors would like to thank the NHANES participants and the staff members for their contribution to data collection and for making the data publicly available.
Abbreviations
- ASCVD
Atherosclerotic cardiovascular disease
- LDL-C
Low-density lipoprotein cholesterol
- PCSK9
Proprotein convertase subtilisin/kexin type 9
- siRNA
Small interfering RNA
- CVD
Cardiovascular disease
- NHANES
National health and nutrition examination survey
- CHD
Coronary heart disease
- PAD
Peripheral arterial disease
- eGFR
Estimated glomerular filtration rate
- non-HDL-C
Non-High-density lipoprotein cholesterol
- TC
Total cholesterol
- TG
Triglycerides
- CHF
Congestive heart failure
- PCSK9mAbs
PCSK9 monoclonal antibodies
Author contributions
YX, YZ and SS designed the study and revised the manuscript. HL and SZ wrote the main manuscript text, HL and JW performed the statistical analysis, SZ and SS prepared Fig. 1, JW, JH and SS prepared Figs. 2 and 3, HL and SS prepared Fig. 4. All authors read and approved the final manuscript.
Funding
This study was financially supported by the National Nature Science Foundation of China (82170388, 82370300), Shanghai Technology Research Leader Program (21XD1434700), the Cardiac rehabilitation fund by the International Medical Exchange Foundation (Z-2019-42-1908-3) and Grant for the construction of Innovative Flagship Hospital for Integrated Traditional Chinese and Western Medicine (No., ZY(2021–2023)-0205-05).
Data availability
Data used in this study are publicly available from the National Health and Nutrition Examination Survey: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.
Declarations
Ethics approval and consent to participate
This study utilized de-identified publicly available data and does not qualify for institutional review board review. The National Health and Nutrition Examination Survey obtains consent to participate from all subjects.
Consent for publication
Not applicable. No individual data or images are used in this manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Haonan Li and Song Zhao contributed equally to this work.
Contributor Information
Shengfeng Shi, Email: hmssf@sohu.com.
Yi Zhang, Email: yizshcn@gmail.com.
References
- 1.Vasan RS, Enserro DM, Xanthakis V, Beiser AS, Seshadri S. Temporal trends in the remaining lifetime risk of Cardiovascular Disease among Middle-aged adults across 6 decades: the Framingham Study. Circulation. 2022;145(17):1324–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global regional. National disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: a systematic analysis for the global burden of Disease Study 2015. Lancet (London England). 2016;388(10053):1603–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pirillo A, Casula M, Olmastroni E, Norata GD, Catapano AL. Global epidemiology of dyslipidaemias. Nat Reviews Cardiol. 2021;18(10):689–700. [DOI] [PubMed] [Google Scholar]
- 4.Jones PH, Nair R, Thakker KM. Prevalence of dyslipidemia and lipid goal attainment in statin-treated subjects from 3 data sources: a retrospective analysis. J Am Heart Assoc. 2012;1(6):e001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Räber L, Ueki Y, Otsuka T, et al. Effect of Alirocumab added to high-intensity statin therapy on coronary atherosclerosis in patients with Acute myocardial infarction: the PACMAN-AMI Randomized Clinical Trial. JAMA. 2022;327(18):1771–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitzgerald K, White S, Borodovsky A, et al. A highly durable RNAi therapeutic inhibitor of PCSK9. N Engl J Med. 2017;376(1):41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aguilar-Salinas CA, Gómez-Díaz RA, Corral P. New therapies for primary hyperlipidemia. J Clin Endocrinol Metab. 2022;107(5):1216–24. [DOI] [PubMed] [Google Scholar]
- 8.Al Shaer D, Al Musaimi O, Albericio F, de la Torre BG. 2021 FDA TIDES (Peptides and Oligonucleotides) Harvest. Pharmaceuticals (Basel, Switzerland). 2022;15(2). [DOI] [PMC free article] [PubMed]
- 9.US FDA approves expanded indication for Novartis Leqvio®. (inclisiran). https://www.novartis.com/us-en/news/media-releases/us-fda-approves-expanded-indication-novartis-leqvio-inclisiran-include-treatment-adults-high-ldl-c-and-who-are-increased-risk-heart-disease. Accessed 6 Sep 2024.
- 10.Ray KK, Landmesser U, Leiter LA, et al. Inclisiran in patients at High Cardiovascular risk with elevated LDL cholesterol. N Engl J Med. 2017;376(15):1430–40. [DOI] [PubMed] [Google Scholar]
- 11.Ray KK, Troquay RPT, Visseren FLJ, et al. Long-term efficacy and safety of inclisiran in patients with high cardiovascular risk and elevated LDL cholesterol (ORION-3): results from the 4-year open-label extension of the ORION-1 trial. Lancet Diabetes Endocrinol. 2023;11(2):109–19. [DOI] [PubMed] [Google Scholar]
- 12.Raal FJ, Kallend D, Ray KK, et al. Inclisiran for the treatment of heterozygous familial hypercholesterolemia. N Engl J Med. 2020;382(16):1520–30. [DOI] [PubMed] [Google Scholar]
- 13.Ray KK, Wright RS, Kallend D, et al. Two phase 3 trials of Inclisiran in patients with elevated LDL cholesterol. N Engl J Med. 2020;382(16):1507–19. [DOI] [PubMed] [Google Scholar]
- 14.Ray KK, Kallend D, Leiter LA, et al. Effect of inclisiran on lipids in primary prevention: the ORION-11 trial. Eur Heart J. 2022;43(48):5047–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wright RS, Koenig W, Landmesser U, et al. Safety and Tolerability of Inclisiran for treatment of Hypercholesterolemia in 7 clinical trials. J Am Coll Cardiol. 2023;82(24):2251–61. [DOI] [PubMed] [Google Scholar]
- 16.Ray KK, Raal FJ, Kallend DG, et al. Inclisiran and cardiovascular events: a patient-level analysis of phase III trials. Eur Heart J. 2023;44(2):129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scicchitano P, Milo M, Mallamaci R, et al. Inclisiran in lipid management: a literature overview and future perspectives. Biomed Pharmacother. 2021;143:112227. [DOI] [PubMed] [Google Scholar]
- 18.Stoekenbroek RM, Kallend D, Wijngaard PL, Kastelein JJ. Inclisiran for the treatment of cardiovascular disease: the ORION clinical development program. Future Cardiol. 2018;14(6):433–42. [DOI] [PubMed] [Google Scholar]
- 19.Heeringa S, West BT, Berglund PA. Applied survey data analysis. Second edition. ed. Boca Raton, FL: CRC Press, Taylor & Francis Group; 2017.
- 20.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082–e1143. [DOI] [PMC free article] [PubMed]
- 21.Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. EUR HEART J. 2020;41(1):111–88. [DOI] [PubMed] [Google Scholar]
- 22.D’Agostino RB, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–53. [DOI] [PubMed] [Google Scholar]
- 23.Ference BA, Graham I, Tokgozoglu L, Catapano AL. Impact of lipids on Cardiovascular Health: JACC Health Promotion Series. J Am Coll Cardiol. 2018;72(10):1141–56. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Pletcher MJ, Vittinghoff E, et al. Association between Cumulative Low-Density Lipoprotein Cholesterol Exposure during Young Adulthood and Middle Age and Risk of Cardiovascular events. JAMA Cardiol. 2021;6(12):1406–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin J. Statins for the Prevention of Cardiovascular Disease. JAMA. 2022;328(8):786. [DOI] [PubMed] [Google Scholar]
- 26.Dubuc G, Chamberland A, Wassef H, et al. Statins upregulate PCSK9, the gene encoding the proprotein convertase neural apoptosis-regulated convertase-1 implicated in familial hypercholesterolemia. Arterioscler Thromb Vascular Biology. 2004;24(8):1454–9. [DOI] [PubMed] [Google Scholar]
- 27.Benjannet S, Rhainds D, Essalmani R, et al. NARC-1/PCSK9 and its natural mutants: zymogen cleavage and effects on the low density lipoprotein (LDL) receptor and LDL cholesterol. J Biol Chem. 2004;279(47):48865–75. [DOI] [PubMed] [Google Scholar]
- 28.Maxwell KN, Breslow JL. Adenoviral-mediated expression of Pcsk9 in mice results in a low-density lipoprotein receptor knockout phenotype. Proc Natl Acad Sci USA. 2004;101(18):7100–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maxwell KN, Fisher EA, Breslow JL. Overexpression of PCSK9 accelerates the degradation of the LDLR in a post-endoplasmic reticulum compartment. Proc Natl Acad Sci USA. 2005;102(6):2069–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park SW, Moon YA, Horton JD. Post-transcriptional regulation of low density lipoprotein receptor protein by proprotein convertase subtilisin/kexin type 9a in mouse liver. J Biol Chem. 2004;279(48):50630–8. [DOI] [PubMed] [Google Scholar]
- 31.Rashid S, Curtis DE, Garuti R, et al. Decreased plasma cholesterol and hypersensitivity to statins in mice lacking Pcsk9. Proc Natl Acad Sci USA. 2005;102(15):5374–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen JC, Boerwinkle E, Mosley TH, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354(12):1264–72. [DOI] [PubMed] [Google Scholar]
- 33.Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and Clinical outcomes in patients with Cardiovascular Disease. N Engl J Med. 2017;376(18):1713–22. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and Cardiovascular outcomes after Acute Coronary Syndrome. N Engl J Med. 2018;379(22):2097–107. [DOI] [PubMed] [Google Scholar]
- 35.Vulic D, Lee BT, Dede J, Lopez VA, Wong ND. Extent of control of cardiovascular risk factors and adherence to recommended therapies in US multiethnic adults with coronary heart disease: from a 2005–2006 national survey. Am J Cardiovasc Drugs. 2010;10(2):109–14. [DOI] [PubMed] [Google Scholar]
- 36.Ostwald DA, Schmitt M, Peristeris P, Gerritzen T, Durand A. The societal impact of Inclisiran in England: evidence from a Population Health Approach. Value Health. 2023;26(9):1353–62. [DOI] [PubMed] [Google Scholar]
- 37.Kam N, Perera K, Zomer E, Liew D, Ademi Z. Inclisiran as Adjunct lipid-lowering therapy for patients with Cardiovascular Disease: a cost-effectiveness analysis. PharmacoEconomics. 2020;38(9):1007–20. [DOI] [PubMed] [Google Scholar]
- 38.Cai T, Abel L, Langford O, et al. Associations between statins and adverse events in primary prevention of cardiovascular disease: systematic review with pairwise, network, and dose-response meta-analyses. BMJ. 2021;374:n1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanchi R, Perlman SE, Chernov C, in Cardiovascular Disease Risk Factors among New York City Adults. Gender and Race Disparities : New York City Health and Nutrition Examination Survey (NYC HANES) 2013–2014. Journal of urban health-bulletin of the New York Academy of Medicine. 2018;95(6):801–812. [DOI] [PMC free article] [PubMed]
- 40.Budoff MJ, Young R, Burke G, et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multi-ethnic study of atherosclerosis (MESA). Eur Heart J. 2018;39(25):2401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao Y, Isakadze N, Duffy E, et al. Secular trends in Risk profiles among adults with Cardiovascular Disease in the United States. J Am Coll Cardiol. 2022;80(2):126–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bergmann MM, Byers T, Freedman DS, Mokdad A. Validity of self-reported diagnoses leading to hospitalization: a comparison of self-reports with hospital records in a prospective study of American adults. Am J Epidemiol. 1998;147(10):969–77. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used in this study are publicly available from the National Health and Nutrition Examination Survey: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.