Abstract
The 100K protein has a number of critical roles vital for successful completion of the late phases of the adenovirus (Ad) life cycle. We hypothesized that the introduction of deletions within the 100K gene would allow for the production of a series of new classes of Ad vector, including one that is replication competent but blocked in the ability to carry out many late-phase Ad functions. Such a vector would have potential for several gene therapy applications, based upon its ability to increase the copy number of the transgene encoded by the vector (via genome replication) while decreasing the side effects associated with Ad late gene expression. To efficiently produce 100K-deleted Ad ([100K−]Ad) vectors, an E1- and 100K-complementing cell line (K-16) was successfully isolated. Transfection of an [E1−,100K−]Ad vector genome into the K-16 cells readily yielded high titers of the vector. After infection of noncomplementing cells, we demonstrated that [100K−]Ad vectors have a significantly decreased ability to express several Ad late genes. Additionally, if the E1 gene was present in the infected noncomplementing cells, [100K−]Ad vectors were capable of replicating their genomes to high copy number, but were significantly blocked in their ability to efficiently encapsidate the replicated genomes. Injection of an [E1−,100K−]Ad vector in vivo also correlated with significantly decreased hepatotoxicity, as well as prolonged vector persistence. In summary, the unique properties of [100K−]Ad vectors suggest that they may have utility in a variety of gene therapy applications.
Helper virus-independent, E1-deleted adenovirus ([E1−]Ad)-based gene transfer vectors exhibit many positive attributes, including a large transgene-encoding capacity, a relative ease of high-titer production to clinical grades, and the ability to infect a wide range of tissue types. Despite the fact that [E1−]Ad vectors are significantly blocked in their ability to replicate (relative to a wild-type Ad), low-level replication and/or gene expression derived from [E1−]Ad vectors can limit their usefulness (2, 18). To overcome this problem, we previously demonstrated that [E1−]Ad vectors incorporating additional deletions in the Ad E2b genes (polymerase and/or pTP) rendered [E1−,E2b−]Ad vectors truly replication incompetent (2, 10, 11). As a result, [E1−,E2b−]Ad vector-derived late gene expression was also significantly diminished, since Ad late gene expression is only initiated after Ad genome replication has occurred (19).
Despite the problems associated with Ad replication, a potentially useful Ad vector might be one that can replicate its genome to very high levels after infection of a cell. This feature could be capitalized upon in efforts to either amplify transgene expression encoded by the vector and/or induce cytopathic effects as a consequence of high-level Ad replication and/or infectious virus production. For example, E1a-positive, E1b-deleted ([E1a+,E1b−])Ad vectors have been described; the E1b deletion restricts E1a-dependent vector replication (and generation of infectious vector) to cancer cells only, resulting in their death (3, 9). There is evidence, however, that [E1a+,E1b−]Ad vectors can also replicate in noncancerous cells, potentially limiting the risk/benefit ratio of [E1a+,E1b−]Ad-based cancer therapies (17). In a recent attempt to address the latter concerns, Ad vectors have been developed that are protease deleted (14). Protease-deleted [E1+]Ad viruses can also replicate but are blocked in their ability to produce infectious virus, due to inadequate maturation of viral capsid proteins during the late phase of the Ad life cycle. Importantly, however, both [E1b−] and protease-deleted Ad vectors are fully capable of producing wild-type levels of the Ad late genes once replication has occurred (14). The late genes are numerous and include the hexon, 100K, penton, and fiber proteins. Some of the toxicity normally associated with the expression of these proteins (particularly penton) may potentially limit the overall usefulness of both of these types of replicating Ad vector.
It is with these considerations that we have targeted the 100K gene of the Ad for deletion. After Ad replication occurs, transcription is initiated from the major late promoter (MLP), and activation of the MLP results in the generation of the L4 transcript, which encodes the Ad 100K protein. The 100K gene fully encompasses 10% of the Ad genome, reflective of the vital role 100K plays in various aspects of the Ad life cycle. Functions of the 100K protein include the transport of newly synthesized hexon monomers (the major structural protein of the Ad capsid) from the cytoplasm to the nucleus and trimerization of hexon monomers (4). Without this activity, hexon monomers are degraded in the cytoplasm (15). 100K also acts as a “scaffolding platform” for the assembly of virus capsids, although the 100K protein has not been found to be physically incorporated into mature Ad capsids (13). 100K can also interact with a number of RNA transcripts, both vector and host cell derived, preferentially allowing for translation of Ad-derived late-gene transcripts (1, 12, 16). Considering these critical roles, we hypothesized that deletions within the 100K gene might allow for the production of Ad-based vectors with significantly altered characteristics. In this report we now demonstrate that [100K−] vectors can be produced to high titer and that the altered biology of vectors incorporating these deletions might be capitalized upon in several gene therapy applications.
MATERIALS AND METHODS
Production of E1- and 100K-expressing cell lines.
Ad5-derived DNA was used as a template for the PCR amplification of the 100K open reading frame using an EcoRI-tailed forward primer, 5′-GCGGAATTCGATCATGGAGTCAGTCGAG-3′, and an XbaI-tailed reverse primer, 5′-GCCTCTAGAGTCCCATCTACGGTTGGG-3′. One hundred nanograms of each primer was included in a reaction mixture containing 10 mM KCl, 10 mM (NH4)2SO4, 20 mM Tris·HCl (pH 8.75), 2 mM MgSO4, 0.1% Triton X-100, 0.1 mg of bovine serum albumin/ml, 25 mM (each) deoxynucleoside triphosphates, 2.5 U of a high-fidelity Taq polymerase (Stratagene, La Jolla, Calif.), and 100 ng of Ad5 genomic DNA. After denaturation for 3 min at 95°C, the reaction mixture was subjected to a limited number of PCR amplification cycles consisting of DNA denaturation at 95°C for 30 s, primer annealing at 55°C for 45 s, and Taq-mediated extension at 72°C for 1.5 min. This same PCR was utilized to screen genomic DNA from G-418-resistant cells for the presence of 100K-specific DNA sequences (see below). The PCR yielded the predicted ∼2.3-kb 100K-specific product, which was digested with EcoRI and XbaI and directionally ligated into the XbaI and EcoRI sites within pcDNA3 (Invitrogen, Carlsbad, Calif.), generating pcDNA3/100K. In this manner, the 100K open reading frame was placed under the expressional control of a CMV enhancer-promoter element. Two micrograms of the pcDNA3/100K plasmid was linearized with ClaI restriction enzyme digestion and transfected into 293 cells ([E1+]) by the calcium phosphate method. Transfected cells were placed into medium containing 800 μg of G-418/ml, and clonal isolates of G-418-resistant cells were serially expanded. The subclones were screened for the ability to transcomplement the growth of the temperature-sensitive (ts) Ad5 100K mutant, H5ts116 (kindly supplied by H. Ginsberg, Columbia University, New York, N.Y.) at the nonpermissive temperature of 39°C. Of approximately 35 G-418 resistant cell lines, 1 (referred to as K-16) was found to be consistently capable of effectively transcomplementing growth of H5ts116 at 39°C.
Construction of an [E1−,100K−]Ad vector.
The pAdEasy-1 plasmid (8) was used as a template for the generation of 100K deletions within the Ad5 genome (8). Briefly, pAdEasy-1 was digested with BamHI, and the subfragment containing the right end of the Ad5 genome (Ad5 sequences 21696 to 35995) was isolated and subcloned into BamHI-digested pcDNA3, yielding pAdEΔBamHI. The latter was digested with NheI to release a 687-bp fragment within the 100K gene (Ad5 sequences 24999 to 25686) and self-ligated to generate pAdEΔBamHI/Δ100K. The pAdEΔBamHI/Δ100K plasmid was then digested with BamHI and ligated to the large BamHI subfragment of pAdEasy-1, generating pAdEΔ100K.
A 3.1-kb SalI fragment encompassing the bacterial β-galactosidase (lacZ) gene (kindly provided by W. Koch, Duke University) was ligated into the SalI site of pShuttleCMV, generating pShuttleCMVlacZ (8). The lacZ-carrying shuttle plasmid was linearized with PmeI and coelectroporated with pAdEΔ100K into Escherichia coli BJ5183. In this manner, targeted recombination between the two plasmids generated the full-length [E1−,E3−,100K−]AdlacZ vector genome within a bacterial plasmid. Similarly, we coelectroporated the pShuttleCMVlacZ plasmid with pAdEasy-1 to generate the [E1−,E3−]AdlacZ vector-containing plasmid.
Ten micrograms of the respective plasmids was digested with PacI and transfected either into 293 cells (for generation of the [E1−,E3−]AdlacZ vector) or into K-16 cells (for generation of the [E1−,E3−,100K−]AdlacZ vector). Within 1 week of transfection, extensive cytopathic effects were visible in both cell lines, indicating widespread vector growth and amplification. The infected cells were harvested and freeze-thawed, and the vectors were amplified. After infection of 60 150-mm-diameter tissue culture plates, the respective vectors were purified and twice banded on CsCl2 gradients, and titers for lacZ-transducing units were determined as previously described (2).
Replication assays.
The indicated cell lines were infected at a multiplicity of infection (MOI) of 5 with the respective vectors and incubated for 2 or 20 h at 37°C, and total DNA was harvested. Ten micrograms of each sample was digested with EcoRV and electrophoresed through a 0.7% agarose gel, and the vector DNA was visualized after ethidium bromide staining.
One-step, limited-burst assay.
Indicated cell lines were infected at the indicated MOIs with the respective vectors, and total virus yield was measured by 5-bromo-4-chloro-3-indoyl-β-d-galactopyranoside (X-Gal) staining of C-7 cells infected with serial dilutions of the vector-containing lysates, as previously described (2).
Protein analysis of Ad-infected cell lines.
Indicated cell lines were infected with each of the vectors at an MOI of 5. Twenty hours postinfection, the medium was replaced with methionine-free medium supplemented with [35S]methionine at 90 mCi/ml. The cells were harvested 3 h later, rinsed in phosphate-buffered saline (PBS), and lysed in 50 mM Tris-HCl (pH 6.8), 4% sodium dodecyl sulfate, and 2% β-mercaptoethanol. The protein content of the cell lysates was determined against a protein standard curve via the Bradford assay, and 75 μg of each cell extract was electrophoresed in a sodium dodecyl sulfate–6.0% polyacrylamide gel. The gel was Coomassie stained and photographed. Duplicate gels were dried down and subjected to autoradiography, and the respective proteins were identified based upon their characteristic molecular weights and analyzed using the SCION image analysis software package.
Detection of 100K RNA sequences.
Total cellular RNA was isolated, electrophoretically separated, and ethidium bromide stained to confirm equivalent loading of the samples. The RNA samples were transferred to a nylon membrane and probed with the 2.3-kb, 100K-specific, 32P-labeled Ad subfragment derived from digestion of pcDNA3/100K with EcoRI and XbaI. The nylon membrane was exposed to autoradiography film, and the image was photographed.
DNA isolation and analysis.
Southern blot analysis was performed as follows. DNA was extracted from either liver tissues or tissue culture cells as previously described (11). Twenty micrograms of total liver DNA from infected mice was digested with EcoRI, electrophoretically separated, and transferred to a nylon membrane. Liver DNA isolated from noninfected animals was spiked with an [E1−]AdlacZ virus genome as a positive control. The membrane was hybridized to a [α-32P]dCTP-labeled DNA probe (the ∼5,300-bp BstXI subfragment of Ad5). The membrane was washed, exposed to autoradiography films, and photographed.
Noncompetitive, quantitative, Ad-specific PCR.
A total of 400 ng of liver DNA derived from each of the [E1−,E3−,100K−]AdlacZ-infected mice was subjected to PCR with the Ad-specific primers 5′-GGTAGCACCACTGCAGAGCTTC-3′ and 5′-GGTCACAAGGGCGTCTCCAAG-3′, generating a 348-bp product, in the buffer described above under the following cycling conditions: 94°C for 3 min, followed by 22 cycles of 94°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 1 min. Equivalent amounts of liver DNA derived from mock-infected mice were similarly amplified after being spiked with increasing amounts of Ad DNA to generate a standard curve. The amounts of Ad-specific PCR product derived from amplification of the infected liver samples were then determined after comparison to the standard curve data. To further normalize the assay and be sure that the amount of Ad-specific PCR product generated was from equivalent amounts of template, identical amplifications of the experimental DNA samples were carried out utilizing primers specific for the glyceraldehyde-3-phosphate deydrogenase (G3PDH) gene (5′-ACCACAGTCCATCGGATCAC-3′ and 5′-TCCACCACCCTGTTGCTGTA-3′, generating a 452-bp product) for 16 cycles of amplification and compared to the amounts of G3PDH-specific amplification product derived from standard amounts of mock-infected murine liver DNA. All Ad genome copy numbers were then normalized to G3PDH concentrations. All PCR products were visualized by ethidium bromide staining of 1.5% agarose gels after electrophoretic separation and quantitated with the freeware version of the SCION imaging software, as previously described (11).
Animal injections, X-Gal staining, and AST-ALT analyses.
Adult (7 to 9 weeks old) C57BL/6 mice (Jackson Laboratories, Bar Harbor, Maine) were intravenously injected (retro-orbitally) with either PBS (mock) or PBS containing 4 × 109 lacZ-transducing units of each of the respective vectors. Animals were killed, and liver tissues were harvested for DNA analysis or processed for X-Gal substrate staining (indicating lacZ expression) as previously described (10). Serial plasma samples derived from the infected mice were analyzed for evidence of Ad vector-induced hepatitis by monitoring aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels using the respective transaminase kits, per the manufacturer's guidelines (Sigma, St. Louis, Mo.). Statistical analysis were performed using Student's t test. All animal procedures were done in accordance with Duke University Institutional Animal Care and Use Committee guidelines.
RESULTS
Production of E1- and 100K-expressing cell lines.
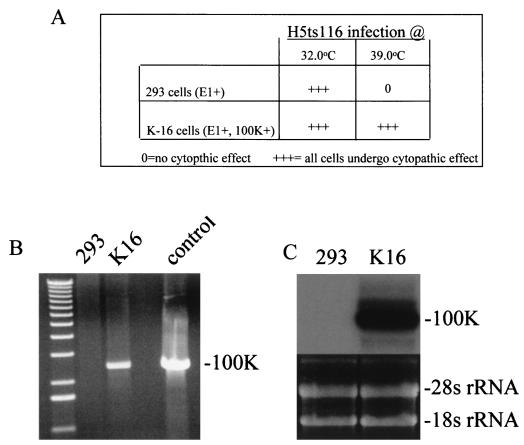
To fully investigate the impact that lack of 100K activities might have upon Ad vector biology, significant deletions within the 100K gene needed to be engineered. To enable the isolation of these vectors to high quantity (and in a helper virus-independent fashion), cell lines that could transcomplement the growth of [100K−]Ad were isolated. To accomplish this, human 293 cells ([E1+]) were transfected with a 100K expression plasmid (pcDNA3/100K) (see Materials and Methods for full details). Cells that had successfully integrated the pcDNA3/100K plasmid were initially identified by their ability to grow in high concentrations of G-418. We determined whether the G-418-resistant cell lines expressed adequate amounts of 100K by assessing their ability to transcomplement the growth of a 100K ts Ad mutant, at the nonpermissive temperature of 39oC. Of several G-418-resistant clones, one (K-16) was found to consistently allow for evidence of growth of the ts mutant H5ts116 at the nonpermissive temperature, based upon visualization of virus-induced cytopathic effects noted in the cells after infection at 39°C (Fig. 1A). DNA isolated from the K-16 cells was evaluated by a PCR specific for sequences residing within the pcDNA3/100K plasmid; K-16-derived DNA demonstrated the presence of the 100K-specific sequences, in contrast to the lack of such sequences in the parental 293 cells (Fig. 1B). Furthermore, total RNA derived from the K-16 cells contained large amounts of 100K-specific mRNA, compared to the lack of such transcripts in 293 cells (Fig. 1C). Unfortunately, utilization of 100K-specific monoclonal antibodies was not able to detect 100K-specific peptide within protein extracts derived from K-16 cells (data not shown). At this time we cannot discern whether the lack of sensitivity was simply due to technical difficulties with the antibodies utilized or to low levels of 100K protein expression within the cell lines (see results below).
FIG. 1.
(A) Cytopathic effects in infected cells. The mutant virus H5ts116 was utilized to infect various G-418-resistant cell lines at the permissive temperature of 32°C or the nonpermissive temperature of 39°C. The K-16 cell line supported growth of the virus, as evidenced by the onset of cytopathic effect (+++) at both temperatures. 0 = no cytopathic effect. (B) Detection of 100K-specific DNA sequences within K-16 cells and not 293 cells. A 100K-specific PCR product at ∼2.3 kb was only detected when DNA isolated from the G-418-resistant K-16 cells was utilized as a template. The first lane contains a 1-kb DNA ladder, while the control lane utilized pcDNA3/100K as a positive control template. See Materials and Methods for full experimental details. (C) Detection of 100K-specific RNA sequences within K-16 cells. A 100K-specific mRNA was detected only in RNA isolated from G-418-resistant K-16 cells. The lower half of the figure depicts the amounts of RNA loaded in the gel prior to transfer to the nylon membrane, demonstrating that both samples contained equal amounts of intact RNA. See Materials and Methods for full experimental details.
Production of [E1−,100K−]Ad vectors in K-16 cells.
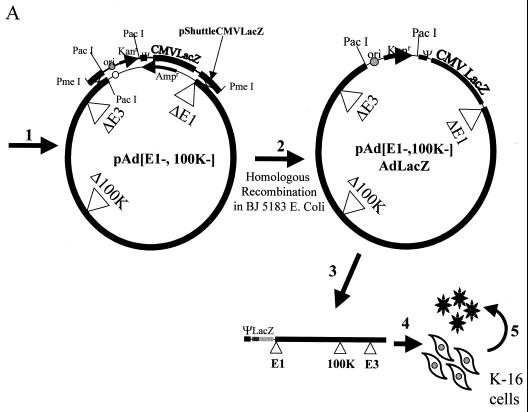
To facilitate the construction of [100K−]Ad vectors, we modified a previously described method for [E1−,E3−]Ad vector production (8). Reconstruction of pAdEasy-1 was undertaken (as described in Materials and Methods) to introduce an extensive deletion within the 100K gene. The new plasmid was referred to as pAdEΔ100K (Fig. 2A). Recombination between a shuttle plasmid (containing the right end of the Ad genome juxtaposed to a CMV-lacZ transgene cassette) with pAdEΔ100K allowed us to generate the full-length [E1−,E3−,100K−]AdlacZ vector genome within a bacterial plasmid. PacI restriction enzyme digestion of the plasmid, followed by transfection into K-16 cells, resulted in a productive infection as evidenced by the rapid onset of widespread cytopathic effects and subsequent high-level amplification and purification by cesium chloride banding. Final concentrations of the purified [E1−,E3−,100K−]AdlacZ vector were similar to those achieved with growth of [E1−,E3−]Ad vectors in 293 cells (data not shown). Titers of the [E1−,E3−,100K−]AdlacZ vector derived from this stock were determined for the number of lacZ-transducing units and utilized for all subsequent experiments described below.
FIG. 2.
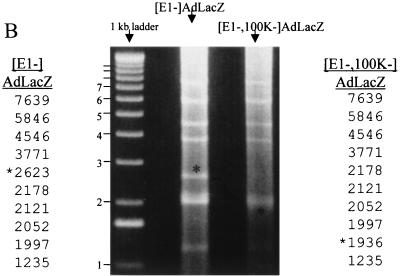
(A) Assembly of [100K−]Ad vectors by homologous recombination in bacterial plasmids. Construction of [E1−,E3−,100K−]AdlacZ was as follows: step 1, coelectroporate linearized pShuttleCMVlacZ with p[E1−,E3−,100K−]Ad into E. coli BJ5183; step 2, screen kanamycin-resistant colonies for identification of clones containing recombinant pAd[E1−,E3−,100K−]AdlacZ plasmid; step 3, linearize the p[E1−,E3−,100K−]AdlacZ plasmid with PacI, releasing the Ad inverted terminal repeat elements; step 4, transfect PacI-linearized p[E1−,E3−,100K−]AdlacZ into K-16 cells for virus growth; and step 5, serially propagate virus for conventional Ad vector amplification and purification. (B) Confirmation of [E1−,E3−,100K−]AdlacZ genome integrity. K-16 cells were identically infected at an MOI of 5 with each of the indicated vectors, total DNA was harvested 20 h after infection, and nearly equivalent amounts were digested with EcoRV. The genomes of the two vectors are identical except for the altered migration of the indicated subfragment (∗) in the [E1−,E3−,100K−]AdlacZ vector; the latter fragment encompasses the 100K deletion.
Confirmation of genome integrity as well as the replication potential of [E1−,E3−,100K−]AdlacZ was compared to the [E1−,E3−]AdlacZ vector (Fig. 2B). The results demonstrated that the two vector genomes were identical except for the presence of the 100K deletion. The results also confirmed the stability of [100K−] vector genomes despite repeated cycles of replication and amplification. The latter findings are mandatory features, required for generation and utilization of clinical grades of these vectors in the future. Finally, both vectors appeared to be capable of replicating their respective genomes to near-identical levels in this experiment.
High-level growth of [100K−] vectors.
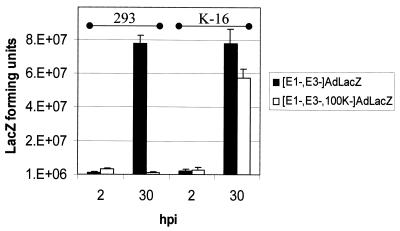
Repeated 30-h, one-step burst assays demonstrated that infections of 293 cells with the [E1−,E3−]AdlacZ vectors yielded amounts of vector near to those obtained after infection of K-16 cells with the [E1−,E3−,100K−]AdlacZ vector (Fig. 3). Although these experiments demonstrated that there was a slight reduction in the absolute yields of the [E1−,E3−,100K−]AdCMVlacZ from K16 cells compared to the yield of the [E1−,E3−]AdlacZ vector in 293 cells, in practice this did not significantly affect our ability to produce high-titer stocks of the [E1−,100K−]AdlacZ vector. In contrast, yields of the [100K−] vector were significantly reduced when identical infections of 293 cells were simultaneously attempted (Fig. 3). The results confirmed that high-level growth of the [100K−] vector was critically dependent upon the transcomplementation of 100K functions provided by the K-16 cell line. As an additional control, we simultaneously infected 293, C-7, or K-16 cells with an [E1−,E2b−]AdlacZ vector; these vectors are only capable of being grown to high titers when transcomplemented for both E1 and E2b functions in C-7 cells (2). The [E1−,E2b−]AdlacZ vector was blocked in growth after infection of 293 or K-16 cells (to a degree similar to that when the [E1−,100K−]AdlacZ vector was grown in 293 or C-7 cells) and only grew to high levels when transcomplemented in C-7 cells.
FIG. 3.
Growth of modified vectors only in 100K-transcomplementing cell lines. The indicated cell lines were infected at an MOI of 5 and incubated for the indicated time periods. Infectious virus (as determined by assessing total lacZ-transducing units yielded from two identical infections) during viral eclipse (2 h postinfection [2 hpi]) and after virus replication (30 hpi) were compared.
Replication and late gene expression of [E1−,E3−,100K−]Ad vectors.
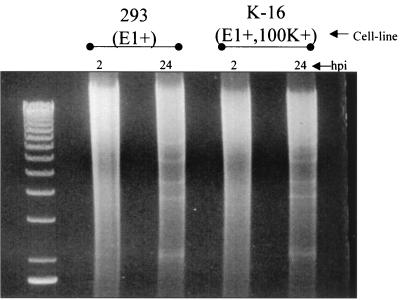
Our original hypothesis predicted that Ad vectors with the 100K gene deleted should not be affected in their ability to replicate their genomes (in the presence of normal amounts of E1 activity), since the primary functional roles for the 100K protein are not expressed until after Ad replication occurs. We therefore infected 293 cells ([E1+]) or K-16 cells ([E1+,100K+]) with the [E1−,E3−,100K−]AdlacZ vector and evaluated vector replication (Fig. 4). Whereas both cell lines had barely detectable levels of input vector DNA 2 h after infection, high levels of vector-specific DNA sequences (superimposed upon the cellular DNA genomic smear) were readily detected in both cells lines 20 h after infection. The results confirmed that Ad genomes with the 100K gene deleted were fully capable of replicating in the presence of the Ad E1 proteins. The replication results also demonstrated that Ad genome replication could be effectively uncoupled from the production of infectious virus simply by deletion of 100K gene functions, since infection of 293 cells with the [E1−,E3−,100K−]AdlacZ vector yielded low levels of virus (Fig. 3).
FIG. 4.
Replication of [E1−,E3−,100K−]AdlacZ in the presence of E1. 293 or K-16 cells were infected at an MOI of 5 with [E1−,E3−,100K−]AdlacZ, and total DNA was electrophoretically separated and visualized after ethidium bromide staining of the gel.
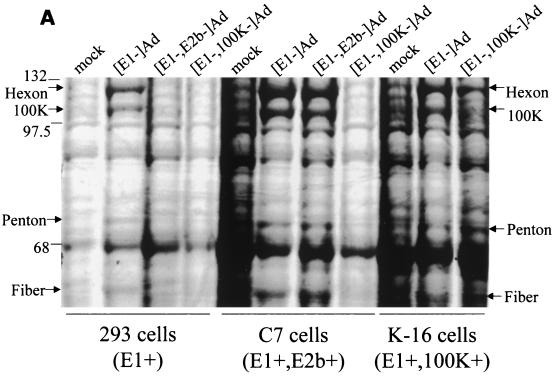
Proteins derived from K-16 cells or several noncomplementing cell lines infected with the [E1−,E3−,100K−]AdlacZ vector were next compared to identical infections with other classes of modified Ad vectors. Whereas infection of 293, C-7, or K-16 cells with the [E1−,E3−]AdlacZ vector resulted in detection of high levels of the hexon, 100K, penton, and fiber proteins 24 h postinfection, identical infections with the [E1−,E3−,100K−]AdlacZ vector resulted in a significant decrease in the absolute amounts of each of these proteins in 293 or C-7 cells, a defect that was normalized for all proteins except 100K, when the [100K−] vector infected K-16 cells (Fig. 5A). Interestingly, 100K protein was not detected by this method with any of the cell lines tested, suggesting that even though K-16 cells express low levels of 100K (relative to that in a wild-type infection), the small amounts actually expressed are adequate to transcomplement the hexon, penton, and fiber expression defect of the [E1−,E3−,100K−]AdlacZ vector. The results indirectly suggest that wild-type Ad may actually express excessive amounts of the 100K peptide, much more than is required to assemble significant amounts of infectious virus, a point elaborated upon in the discussion.
FIG. 5.
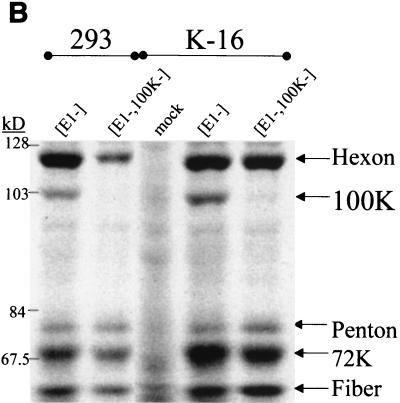
[E1−,E3−,100K−]Ad late gene expression analysis. The indicated cell lines were infected at an MOI of 5 with the respective vectors. Duplicate infections were carried out, and proteins were either not labeled or [35S]methionine radiolabeled as described in Materials and Methods. Identical amounts of all proteins derived from the infections were extracted, electrophoretically separated, and visualized by either Coomassie staining (A) or autoradiography (B) of the gels. The locations of the hexon, 100K, penton, and fiber Ad late proteins are indicated.
The hexon, 100K, penton, and fiber protein level defects were also analyzed after a radiolabeling of viral proteins during the late phase of vector infection of 293 or K-16 cells (Fig. 5B). These experiments demonstrated that there was at least a 65% decline (based upon quantitative image analysis of Fig. 5B) in the amount of hexon that was radiolabeled in 293 cells infected with the [E1−,E3−,100K−]AdlacZ vector, compared to infection of 293 cells infected with identical amounts of the [E1−,E3−]AdlacZ vector. Importantly, K-16 cells infected with the [E1−,E3−,100K−]AdlacZ vector demonstrated a nearly normalized restoration of hexon radiolabeling. The results suggest that lack of hexon accumulation in 293 cells infected with the [E1−,E3−,100K−]AdlacZ vector was due to a lack of adequate synthesis of hexon, possibly related to the known influences 100K protein has upon late mRNA translation rates (1, 12, 16). The assay also confirmed an absence of 100K protein when the [E1−,E3−,100K−]AdlacZ vector infected either 293 or K-16 cells. Despite lack of 100K detection, the latter results again confirmed that K-16 cells express adequate amounts of 100K, since these cells adequately transcomplemented the hexon expression defect of [E1−,E3−,100K−]AdlacZ.
In contrast to the patterns we observed for hexon and 100K, there was no evidence of significant decreases of radiolabeled penton or fiber proteins after [E1−,E3−,100K−]AdlacZ infection of 293 cells (Fig. 5B). The observations suggest that a lack of stable accumulation of the penton and fiber proteins occurred in [E1−,E3−,100K−]AdlacZ-infected 293 cells (see Fig. 5A), rather than from a direct effect of 100K upon the rates of expression and/or translation of penton or fiber per se. Further studies will be required to fully elucidate the mechanisms responsible for the latter observations.
The blockade to late gene expression exhibited by the [E1−,E3−,100K−]AdlacZ in 293 cells was also qualitatively similar to that observed when a completely replication-incompetent Ad vector ([E1−,E3−,E2b−]AdlacZ) was utilized to infect 293 cells ([E1+,E2b−]), as determined by Coomassie staining of infected cell proteins (Fig. 5A). The [E1−,E3−,E2b−]AdlacZ vector was previously demonstrated by our group to produce a profound replication blockade after infection of 293 cells, which is also responsible for a significant blockade to late gene expression derived from these vectors (2). The latter is due to the fact that cis activation of the Ad MLP and subsequent late gene expression derived from the MLP are both dependent upon Ad genome replication (19). The 100K vectors, however, retain the ability to replicate their genomes, in contrast to [E1−,E3−,E2b−]Ad vectors, when in the presence of high levels of E1 activity (Fig. 3) (2, 10).
Analysis of acute liver toxicity and in vivo persistence of [E1−,E3−,100K−]AdlacZ vector.
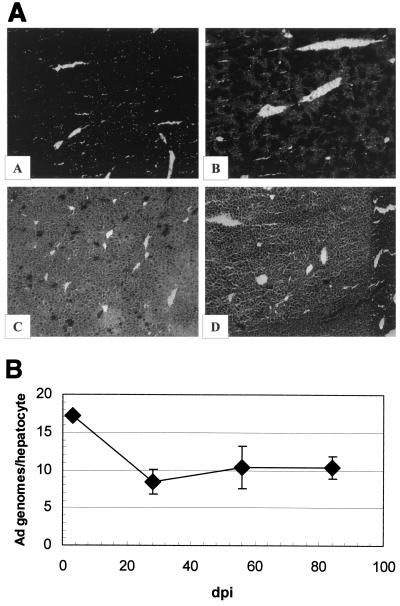
Since we were readily able to generate high titers of the 100K-deleted vector, in vivo studies were undertaken to further discern whether the late gene expression blockade afforded by deletion of 100K might reduce the acute hepatotoxicity of Ad vectors in vivo. We first demonstrated that the [E1−,E3−,100K−]AdlacZ vector could efficiently transduce hepatocytes in vivo, since >75% of the hepatocytes were demonstrated to express the lacZ gene 3 days after injection of 4 × 109 lacZ-forming units of the vector (Fig. 6A). The level of transduction was identical to that noted after injection of similar amounts of the [E1−,E3−]AdlacZ vector (data not shown). In contrast to results with the [E1−,E3−]AdlacZ vector, however, injection of the [E1−,E3−,100K−]AdlacZ vector resulted in significantly reduced levels of liver-derived plasma ALT at both 1 and 8 days postinjection, with AST levels also significantly lower 1 day postinjection (Fig. 7). The results demonstrated that deletion of 100K reduces the acute hepatotoxicity of Ad vectors that contain this deletion. The mechanisms for this are likely several and are detailed in the discussion.
FIG. 6.
[E1−,E3−,100K−]AdlacZ vector-derived transgene expression and persistence in vivo. (Panel A) In situ X-Gal staining of [E1−,E3−,100K−]AdlacZ-transduced murine liver. Liver samples were obtained from mice intravenously injected with the [E1−,E3−,100K−]AdlacZ vector and processed for in situ X-Gal staining as described in Material and Methods. Representative samples from each time point are presented. Magnification = 100×. Images show samples at 3 (A), 28 (B), 56 (C), and and 84 (D) dpi. (Panel B) Persistence of [E1−,E3−,100K−]AdlacZ vector genome DNA. Total DNA was extracted from the livers of [E1−,E3−,100K−]AdlacZ-infected mice at the indicated time points, and Ad vector genome copy numbers were determined by a noncompetitive, quantitative, Ad-specific PCR. All values were normalized to G3PDH copy number standards. The amounts of vector DNA present at 28, 56, or 84 dpi were not significantly different (P > 0.05). n = 1 at 3 dpi, n = 3 at 28 dpi, and n = 2 at 28 and 56 dpi.
FIG. 7.
Comparison of AST and ALT plasma levels after transduction of liver with [E1−,E3−]AdlacZ or [E1−,E3−,100K−]AdlacZ. A total of 4 × 109 lacZ-forming units of the respective vectors were intravenously injected into mice, and plasma samples were obtained from the animals at the indicated time points (n ≥ 4 at 1 and 3 dpi; n = 6 at 8 dpi). Similar levels of transduction were confirmed for both vectors after X-Gal staining of liver samples derived from the animals (see Fig. 6A and data not shown). Those time points that demonstrated levels of AST or ALT that were significantly different (P < 0.05; determined by two-tailed Student's t test) between the indicated vectors are indicated by an asterisk.
Analysis of the infected mice after prolonged periods of time was also carried out. For example, after intravenous injection of the [E1−,E3−,100K−]AdlacZ vector, the number of lacZ-positive hepatocytes decreased from 75 to 100% at 3 days postinfection (dpi) to <5% after 3 months (Fig. 6A). The lack of lacZ expression was not, however, due to lack of persistence of the [E1−,E3−,100K−]AdlacZ vector genome, since both Southern blot analysis (data not shown) and a quantitative Ad-specific PCR demonstrated persistence of the vector for up to 12 weeks in all injected animals (Fig. 6B). We concluded that lack of persistent lacZ expression in [E1−,E3−,100K−]AdlacZ-infected hepatocytes was not due to the loss of vector DNA but rather to CMV enhancer-promoter shutdown-related events, a result that is consistent with our previous studies with other modified Ad vectors in vivo (5, 11).
DISCUSSION
The widespread utilization of [E1−]Ad-based vectors for gene transfer has enabled researchers to explore the ramifications of exogenous gene expression in a variety of settings, both with classical tissue culture experiments as well as with a variety of animal models. In an effort to improve the efficacy of helper virus-independent Ad vectors, our group, as well as a number of others, has demonstrated that sequential elimination of Ad-encoded gene activities can impart additive improvements to several aspects of Ad vector biology (6, 7, 10, 11, 20, 21). These improvements include reduced toxicity, prolonged vector genome persistence, increased carrying capacity, and decreased propensity of the vectors to revert to a replication-competent, wild-type Ad during serial propagation. As part of our long-term goal to produce multiply deleted Ad vectors, we have now demonstrated that inactivating deletions in the 100K gene can be successfully introduced into Ad vectors without compromising their ability to be grown to high titers. We subsequently investigated the ramifications of this particular modification on overall Ad vector biology.
To grow such a virus in a helper virus-independent manner, we isolated the cell line K-16. K-16 cells were found to be fully capable of transcomplementing the growth of [E1−, E3−,100K−]AdCMVlacZ vectors despite expressing relatively low levels of 100K protein. The results indirectly suggested that during a wild-type infection, 100K may be present in an overabundance and that significantly lower levels of 100K protein can adequately carry out the multiple functions of 100K. Possibly, the relatively large amounts of 100K protein expressed during a wild-type Ad infection may be more of a reflection of high-level transcription of all genes transcribed from the MLP.
Beyond the obvious practical benefits afforded by the physical deletion of the 100K gene (increased carrying capacity and a decreased propensity to revert to a wild-type Ad during serial propagation), our studies demonstrated that [100K−]Ad vectors were fully capable of replicating their genomes to high levels in the presence of the E1 genes. However, elimination of 100K transcomplementation (i.e., demonstrated after [E1−,E3−,100K−]AdlacZ infection of 293 cells) significantly diminished the amounts of several of the late proteins that normally accumulate after Ad replication occurs, as well as preventing infectious virus production. Several reasons can be forwarded to explain why hexon expression appears to be affected by deletion of the 100K gene, based upon the known functions of 100K. For example, 100K plays several critical roles in facilitating translation of late mRNAs by direct physical interaction, therefore [100K−]Ad vectors may have a reduced ability to translate hexon mRNA (1, 12, 16). It is also known that the 100K protein physically associates with hexon monomers in the cytoplasm (facilitating their transport into the nucleus); failure of this transport mechanism also results in the degradation of hexon monomers in the cytoplasm (4, 15). We also noted that lack of 100K function also appeared to decrease the overall accumulation levels of penton and fiber proteins without affecting the relative rates of synthesis of these proteins. There are likely several mechanisms for the latter, likely secondary to lack of adequate hexon expression and/or capsid assembly by the [100K−] vector.
Incorporation of 100K deletions into [E1−,E3−]Ad vectors also correlated with diminished hepatotoxicity of the vectors in vivo, suggesting that Ad late gene expression contributes to acute Ad vector hepatotoxicity. Our previous results with [E1−,E3−,E2b−]Ad vectors are consistent with the results, since the latter have a significantly diminished capability to express multiple Ad late genes in noncomplementing cells and also have decreased hepatotoxicity in vivo (10, 11).
Although not formally tested, our results strongly suggested that [E1+,100K−]Ad vectors (potentially containing transgenes in the E3 region of the Ad genome) can now be constructed and capitalized upon in a variety of settings. Cells that are successfully transduced by the [E1+,100K−] vector would be subject to Ad genome replication and amplification of the carried transgene by virtue of the presence of the E1 genes within the vector, but due to deletion of the 100K gene, this class of vector would have a decreased ability to express Ad late genes and/or produce infectious vector, thereby decreasing some of the side effects associated with the latter. As a result, the amount of transgene expression within the infected cells (in vitro or in vivo) could potentially be greatly amplified, relative to identical infections attempted with nonreplicating Ad vectors. Importantly, this may allow for the use of smaller amounts of total virus to achieve a similar level of transgene expression.
Recently a report described the production of replication-competent Ad vectors with the protease gene deleted (14). This class of vector is similar to the 100K-deleted class of Ad vectors, in that protease-deleted vectors are also capable of replicating their genomes and incapable of producing infectious virus if the protease gene is not trans complemented. In contrast to our results with [100K−] vectors, however, protease-deleted vectors were demonstrated to express wild-type levels of all Ad late genes, despite full deletion of protease activities (14). Since we have demonstrated (in this and previous studies) that Ad-derived late gene expression can be associated with significant vector toxicity, the use of 100K deletions to facilitate Ad replication (in the absence of late gene expression) may be more desirable than similar attempts utilizing protease-deleted Ad vectors (10, 14). Studies in our laboratory are exploring each of these exciting possibilities.
ACKNOWLEDGMENTS
We thank C. Cepko for the gift of 100K-specific monoclonal antibodies. We thank B. K. Yeargan for special technical support. Use of the Duke University Tissue Culture Facility, housed in the Comprehensive Cancer Center, is acknowledged.
A.A. received support from the Duke Children's Miracle Network, the Muscular Dystrophy Association (USA), and NIH grant DK52925.
REFERENCES
- 1.Adam S A, Dreyfuss G. Adenovirus proteins associated with mRNA and hnRNA in infected HeLa cells. J Virol. 1987;61:3276–3283. doi: 10.1128/jvi.61.10.3276-3283.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amalfitano A, Hauser M A, Hu H, Serra D, Begy C R, Chamberlain J S. Production and characterization of improved adenovirus vectors with the E1, E2b, and E3 genes deleted. J Virol. 1998;72:926–933. doi: 10.1128/jvi.72.2.926-933.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bischoff J R, Kim D H, Williams A, Heise C, Horn S, Muna M, Ng L, Nye J A, Sampsonjohannes A, Fattaey A, Mccormick F. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 4.Cepko C L, Sharp P A. Analysis of Ad5 hexon and 100K ts mutants using conformation-specific monoclonal antibodies. Virology. 1983;129:137–154. doi: 10.1016/0042-6822(83)90402-6. [DOI] [PubMed] [Google Scholar]
- 5.Ding E Y, Hodges B L, McVie-Wylie A J, Serra D, Pressley D, Chen Y T, Amalfitano A. Long term efficacy after [E1,polymerase-] adenovirus mediated transfer of the human acid-α-glucosidase gene into GSD-II knockout mice. Hum Gene Ther. 2001;12:955–965. doi: 10.1089/104303401750195917. [DOI] [PubMed] [Google Scholar]
- 6.Gao G P, Yang Y P, Wilson J M. Biology of adenovirus vectors with E1 and E4 deletions for liver-directed gene therapy. J Virol. 1996;70:8934–8943. doi: 10.1128/jvi.70.12.8934-8943.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorziglia M I, Lapcevich C, Roy S, Kang Q, Kadan M, Wu V, Pechan P, Kaleko M. Generation of an adenovirus vector lacking E1, E2a, E3, and all of E4 except open reading frame 3. J Virol. 1999;73:6048–6055. doi: 10.1128/jvi.73.7.6048-6055.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He T C, Zhou S, da Costa L T, Yu J, Kinzler K W, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heise C, Sampsonjohannes A, Williams A, Mccormick F, Vonhoff D D, Kirn D H. Onyx-015, an e1b gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nat Med. 1997;3:639–645. doi: 10.1038/nm0697-639. [DOI] [PubMed] [Google Scholar]
- 10.Hodges B L, Serra D, Hu H, Begy C A, Chamberlain J S, Amalfitano A. Multiply deleted [E1-, polymerase-, and pTP-] adenovirus vector persists despite deletion of the preterminal protein. J Gene Med. 2000;2:250–259. doi: 10.1002/1521-2254(200007/08)2:4<250::AID-JGM113>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 11.Hu H M, Serra D, Amalfitano A. Persistence of an [E1(−), polymerase(−)] adenovirus vector despite transduction of a neoantigen into immune-competent mice. Hum Gene Ther. 1999;10:355–364. doi: 10.1089/10430349950018805. [DOI] [PubMed] [Google Scholar]
- 12.Mathews M B. Control of translation in adenovirus infected cells. Enzyme. 1990;44:250–264. doi: 10.1159/000468763. [DOI] [PubMed] [Google Scholar]
- 13.Morin N, Boulanger P. Hexon trimerization occurring in an assembly-defective, 100k temperature-sensitive mutant of adenovirus 2. Virology. 1986;152:11–31. doi: 10.1016/0042-6822(86)90367-3. [DOI] [PubMed] [Google Scholar]
- 14.Oualikene W, Lamoureux L, Weber J M, Massie B. Protease-deleted adenovirus vectors and complementing cell lines: potential applications of single-round replication mutants for vaccination and gene therapy. J Virol. 2000;11:1341–1353. doi: 10.1089/10430340050032438. [DOI] [PubMed] [Google Scholar]
- 15.Philipson L. Adenovirus assembly. In: Ginsberg H S, editor. The adenoviruses. New York, N.Y: Plenum Press; 1984. pp. 309–338. [Google Scholar]
- 16.Riley D, Flint S J. RNA binding properties of a translational activator, the adenovirus L4 100-kilodalton protein. J Virol. 1993;67:3586–3595. doi: 10.1128/jvi.67.6.3586-3595.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rothmann T, Hengstermann A, Whitaker N J, Scheffner M, Zurhausen H. Replication of onyx-015, a potential anticancer adenovirus, is independent of p53 status in tumor cells. J Virol. 1998;72:9470–9478. doi: 10.1128/jvi.72.12.9470-9478.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinwaerder D S, Carlson C A, Lieber A. DNA replication of first-generation adenovirus vectors in tumor cells. Hum Gene Ther. 2000;11:1933–1948. doi: 10.1089/10430340050129549. [DOI] [PubMed] [Google Scholar]
- 19.Thomas G P, Mathews M B. DNA replication and the early to late transition in adenovirus infection. Cell. 1980;22:523–533. doi: 10.1016/0092-8674(80)90362-1. [DOI] [PubMed] [Google Scholar]
- 20.Wang Q, Greenburg G, Bunch D, Farson D, Finer M H. Persistent transgene expression in mouse liver following in vivo gene transfer with a delta-E1/delta-E4 adenovirus vector. Gene Ther. 1997;4:393–400. doi: 10.1038/sj.gt.3300404. [DOI] [PubMed] [Google Scholar]
- 21.Yeh P, Dedieu J F, Orsini C, Vigne E, Denefle P, Perricaudet M. Efficient dual transcomplementation of adenovirus E1 and E4 regions from a 293-derived cell line expressing a minimal E4 functional unit. J Virol. 1996;70:559–565. doi: 10.1128/jvi.70.1.559-565.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]