Abstract
Simple Summary
The survival of patients with colorectal cancer liver metastasis can be prognosticated based on the presence or absence of desmoplastic histopathologic growth patterns. Patient survival is improved if the liver tumor has at least a 50% desmoplastic histopathologic growth pattern. Determing the patients tumor histopathological features is essential to prognosis outcomes and treatment management.
Abstract
Introduction: Colorectal cancer liver metastasis (CRCLM) remains a lethal diagnosis, with an overall 5-year survival rate of 5–10%. Two distinct histopathological growth patterns (HGPs) of CRCLM are known to have significantly differing rates of patient survival and response to treatment. We set out to review the results of 275 patients who underwent liver resection for CRCLM at the McGill University Health Center (MUHC) and analyze their clinical outcome, mutational burden, and pattern of cancer progression in light of their HGPs, and to consider their potential effect on surgical decision making. Methods: We performed a retrospective multivariate analysis on clinical data from patients with CRCLM (n = 275) who underwent liver resection at the McGill University Health Center (MUHC). All tumors were scored using international consensus guidelines by pathologists trained in HGP scoring. Results: A total of 109 patients (42.2%) were classified as desmoplastic and angiogenic, whereas 149 patients (57.7%) were non-desmoplastic and vessel co-opting. The 5-year survival rates for angiogenic patients compared with vessel co-opting patients were 47.1% and 13%, respectively (p < 0.0001). Multivariate analysis showed patients with vessel co-opting CRCLM had a higher incidence of extrahepatic metastatic disease (p = 0.0215) compared with angiogenic CRCLM. Additionally, KRAS mutation status was a marker of increased likelihood of disease recurrence (p = 0.0434), as was increased number of liver tumors (p = 0.0071) and multiple sites of extrahepatic metastatic disease (p < 0.0001). Conclusions: Multivariate analysis identified key clinical prognostic and molecular features correlating with the two HGPs. Determining liver tumor HGPs is essential for patient prognostication and treatment optimization.
Keywords: liver metastasis, colorectal cancer, histopathologic growth patterns, liver resection
1. Introduction
Approximately 600,000 people die annually of colorectal cancer worldwide, and two-thirds of these deaths are related to liver metastases [1]. The liver is one of the most common sites of colorectal cancer metastases. Approximately 50% of patients will have liver metastases during the course of their disease [2]. The prognosis and treatment of patients with colorectal liver metastasis (CRCLM) has improved dramatically during the past decades [3,4]. This has occurred due to simultaneous advancements in the surgical treatment of CRCLM patients, improvement in modern chemotherapy protocols that downsize tumors and allow resection, and the development of modern antiangiogenic and immunologic drugs.
CRCLM can be characterized by three types of histopathological growth pattern (HGP), defined by the spatial interaction of tumor cells with the surrounding parenchyma at the tumor interface. The desmoplastic HGP (dHGP) is characterized by a capsule of desmoplastic stroma at the tumor interface, separating it from the liver parenchyma. The replacement HGP (rHGP) is characterized by tumor cells infiltrating the hepatic plates of the adjacent parenchyma. In the pushing HGP (pHGP), tumor cells compress the surrounding liver parenchyma but do not infiltrate it [5]. While liver metastases with dHGP depend mostly on angiogenesis to become vascularized, liver metastases with rHGP mostly depend on vessel co-option to supply their nutrients and oxygen. Consequently, liver metastases with a replacement HGP are resistant to antiangiogenic drugs [6,7,8] and have reduced overall survival [9,10]
Multiple studies have demonstrated that liver tumor HGPs are significantly correlated with patient prognosis [6,7,8,11,12,13,14,15,16,17,18,19,20]. While dHGP has consistently been shown to provide a survival benefit compared with non-dHGP, assessment of patient survival with predominantly rHGP vs. pHGP has had mixed results [13,18,19,20,21]. Patients with predominantly dHGP liver tumors are associated with angiogenesis, have more limited liver disease, salvageable recurrences [7], and improved overall survival [8], while patients with non-dHGP liver tumors, which are associated with vessel co-option, do not respond to antiangiogenic drugs [6,22,23,24,25,26], have higher rates of R1 resection, and have reduced overall survival [9,10,27,28,29,30,31,32,33,34].
In this article we will compare the clinical characteristics and survival outcomes of CRCLM patients with dHGP and non-dHGP liver tumors who underwent liver resection at a high-volume hepatobiliary center. We aim to provide new insights regarding the role of liver tumor HGPs in predicting disease free interval, overall survival, and optimal treatment strategy in colorectal cancer patients. An overview of the clinical differences between desmoplastic and non-desmoplastic histopathologic growth patterns are summarized in Table 1 and Supplementary Table S1.
Table 1.
Summary of clinical differences between desmoplastic and non-desmoplastic histopathologic growth patterns.
| Desmoplastic | Non-Desmoplastic | |
|---|---|---|
| Survival analysis | Increased OS, MS, DFS, PFS | Decreased OS, MS, DFS, PFS |
| Surgical outcomes | Increased rate of successful re-resection for recurrent disease | Increased risk of R1 resection; increased risk of incomplete resection |
| Response to systemic chemotherapy | Good response to chemotherapy | Decreased response |
| Response to targeted therapy | Good response | Poor response to anti-VEGF and anti-EGFR therapy |
| Disease recurrence | Lower rate of overall disease recurrence; increased rate of hepatic recurrence compared with extrahepatic recurrence |
Increased rate of overall recurrence; increased rate of extrahepatic recurrence compared with hepatic recurrence |
| Immune landscape | Increased lymphocyte infiltration; increased numbers of CD3+ and CD8+ immune cells |
Decreased lymphocyte infiltration; adaptive immune phenotype with neutrophils present |
| Primary colon tumor | Lower tumor budding score; pushing colon tumor margin |
High tumor budding score; infiltrative colon tumor margin |
OS—overall survival, MS—median survival, DFS—disease-free survival, PFS—progression-free survival.
1.1. Histopathologic Growth Patterns and Primary Tumor Characteristics
The primary CRC tumor may predict the HGP of future CRCLMs. Rajageneshan et al. compared primary CRC tumors with invasive margins characterized as “pushing” as opposed to “infiltrative” with liver tumor in the same patients. They found that patients with “pushing” primary tumors more often developed liver metastases with capsules, while patients with “infiltrative” type primary tumors were more likely to develop non-encapsulated liver tumor. DFS was improved in the “pushing” type but there was no significant difference in OS between the two groups [35].
RHGP liver tumors are associated with high tumor budding scores and infiltrating growth patterns in the primary CRC. Wu et al. assessed features of primary CRC tumors including histology and genetic mutations to see whether it was possible to predict the HGP of CRCLM. Wu also characterized primary tumors in patients with dHGP liver tumors to be associated with expanding growth patterns with low tumor budding scores and a Crohn’s disease-like response. Infiltrative growth patterns in primary CRC and thus rHGP liver metastases were associated with worse OS (p = 0.0337) [36].
1.2. Histopathologic Growth Patterns, Immune Scores, and Immunotherapy
Recently, work has gone into the development of Immunoscore for the prognostication of different cancers, including CRC. Immunoscore is a standardized scoring system based on lymphocyte populations and densities, such as CD3+ and CD8+, measured at the tumor center and the invasive margin. Scores range from 0 to 4, with higher values associated with longer survival [23]. Immunoscore has been validated as a reliable prognostic predictor for patients with colon cancer stages I-III [37,38]. Studies have found the score to also be reliable following surgical resection of CRCLM with patients who have a high Immunoscore having prolonged RFS and OS [23]. Liang et al. developed a scoring system combining liver tumor HGP, Immunoscore, and CRS after they found the densities of CD+3 and CD+8 immune cells were higher in dHGP compared with non-dHGPs liver tumors [23].
CRCLM tumors have been evaluated for individual tumor immune phenotypes and have demonstrated vessel co-opting tumors to be associated with lower immune reactions compared with desmoplastic tumors. Stremitzer et al. evaluated immune phenotypes of CRCLM patients by analyzing the results of patients who were treated with perioperative bevacizumab-based chemotherapy and found that desmoplastic liver tumors were associated with an “inflamed” immune phenotype, shown by the presence of CD8-positive immune cells at the tumor interface and the tumor itself. In contrast, replacement HGP were found to have no CD8-positive T-cell infiltration in the tumor and deemed to have a “non-inflamed” or “desert” immune phenotype. The desmoplastic HGP was associated with better radiologic response compared with the replacement HGP subgroup, along with having better histological response rates and longer RFS and OS [23].
Compared with dHGP CRCLM, vessel co-oping rHGP had less lymphocytic infiltration and less expression of immune-related genes such as CD8A/CD8B, GZMA/GZMB, and PRF1 [23]. Therefore, it is possible that non-dHGP liver tumors will have a decreased response to immunotherapy compared with dHGP, resulting in a worse prognosis.
1.3. Vessel Co-Option and Genetics
Identifying mutations in liver tumors of patients with CRCLM can guide management by predicting responses to targeted therapy. Thus far, there is no documented association between liver HGP and actionable mutations in the liver tumor. However, a number of genes identified via RNA-sequencing have been associated with vessel co-option and rHGP. Lazaris et al. identified a number of differentially expressed genes using RNA-sequencing data taken from rHGP lesions that were associated with cell migration, cell motility, proteolysis, and wound healing. These included CXCL9, LOXL4, PTHLH, TMEM156, and TNFRSF12A. When compared with dHGP, LOXL4 was significantly upregulated in vessel co-opting lesions (p = 0.0015) [39]. Currently, LOXL4 has not been studied in CRCLM, but it is known to function as a catalyst for the crosslinking of collagen and elastin in extracellular matrix remodeling, which may contribute to cancer metastasis [37].
The mutational burden of primary CRC tumors may predict the HGP of future liver metastases. Wu et al. [23] found primary CRC tumors with infiltrating growth patterns were associated with rHGP liver tumors. Both infiltrating-type primary CRC lesions and rHPG CRCLM were associated with mutations in APC (⅗) and TP53 (⅗), KRAS, FAT4, DNH5, SMAD, ERBB2, ERBB3, LRP1, and SDK1 (⅕). On the other hand, dHGP was associated with primary CRC tumors with expanding growth patterns and mutations in APC (⅘); TP53 (⅗); KRAS, PIK3CA, and FAT4 (⅖); and BRCA-1, BRCA2, BRAF, and DNAH5 (⅕). They also found PIK3CA, a mutation in the PIK3 pathway that improves cell proliferation, leading to carcinogenesis and being associated with angiogenesis. The PIK3CA mutation was present in 40% of CRCLM with dHGP, which may support desmoplastic tumor growth via angiogenesis [23].
An overview of the clinical differences between desmoplastic and non-desmoplastic histopathologic growth patterns are summarized in Table 1 and Supplementary Table S1.
2. Methods
2.1. Patient Selection
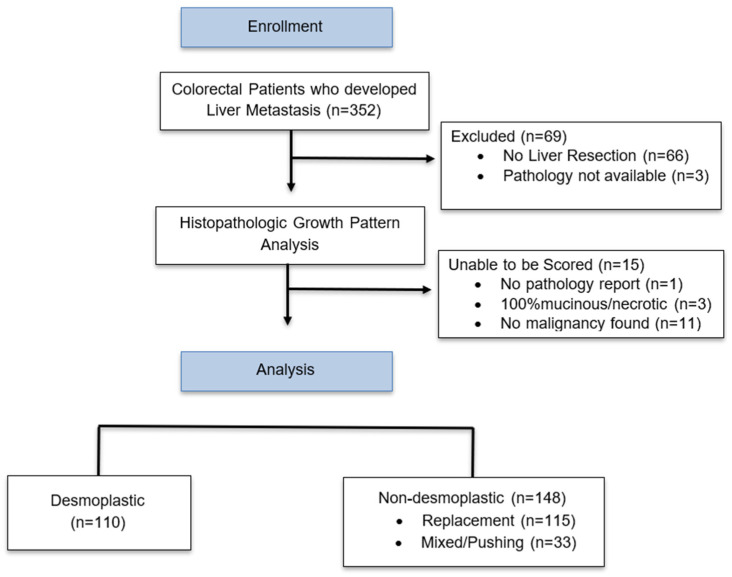
We performed a retrospective data collection on all patients 18 years and older, with CRCLM, who had consented to participate in the McGill University Liver disease biobank research program. The study participants were all living in the province of Quebec at the time of recruitment into the McGill University Liver disease biobank. Of the consented patients, we excluded those who did not undergo surgical resection of their liver metastases, as the histopathologic growth pattern could not be determined via biopsy alone. There were 352 patients with colorectal liver metastases who consented and were included in the liver biobank. Of those, 277 patients, 176 male (63.5%) and 101 female (36.4%), underwent liver resection. The liver resections were completed between January 2009 and December 2020 at McGill University Health Center (MUHC). Sixty-six patients who only underwent liver biopsy were excluded from the analysis, as a biopsy is insufficient for determining tumor HGPs. Of the 277 patients, 258 had their liver tumors scored for HGPs. A total of 109 patients (42.2%) were designated as desmoplastic and 149 patients (57.7%) were designated as non-desmoplastic at their initial liver resection. The HGPs of non-desmoplastic patients were either rHGP, pHGP, or mixed (Figure 1). All patients were followed until death. Loss of follow up was defined as no patient contact for 12 months. Patient data were updated and reviewed through July 2022.
Figure 1.
Flowchart of patient enrollment and stratification.
All patients were intended to be followed until death. Loss of follow up was defined as no patient contact for 12 months. Patient data was updated and reviewed through July 2022.
2.2. Determination of Tumor Histopathologic Growth Pattern
Liver tumor HGPs were scored by AL and ZG, who were both involved in outlining the international consensus guidelines for scoring the HGPs of liver metastasis [12]. HGPs were scored either immediately following surgical resection, or retrospectively scored by reviewing all relevant H&E slides of each patient’s entire resected liver tumor. All resected liver tumors from each patient were evaluated for HGP using the international consensus guidelines [12]. There was no minimum section required for pathologic assessment and the entire tumor interface was evaluated. Tumors with greater than 50% of a specific growth pattern, i.e., dHGP, rHGP, or pHGP, were designated predominately HGP. If a patient had multiple liver tumors with different dominant growth patterns, or if a patient had liver tumors with >2 HGPs, the patient would then be designated as “mixed” and grouped with the non-desmoplastic patients. There was no minimum section required for pathologic assessment.
2.3. Next Generation Sequencing
Genomic sequencing was performed either as a send-out test for specific PCR testing of KRAS, NRAS with or without BRAF, or using the Illumina AmpliSeq focus panel for solid tumors utilized by the McGill University Pathology Department. NGS and/or PCR testing was performed on both the primary colorectal tumor and at least one metastatic liver tumor. Tissue sections were assessed on tumor regions with more than 40% viability. All patients in this study were MSI stable.
2.4. Statistical Analysis
Categorical data were reported both as absolute numbers and corresponding percentages and compared using the Chi-squared test. Non-parametric continuous data were reported as median values with corresponding standard deviation values (SD). Kaplan–Meier analysis with log-rank tests were used to estimate survival curves. Univariate and multivariate Cox proportional hazard regression survival analyses were performed and reported as hazard ratios (HRs) with corresponding 95% confidence intervals (CIs) and p-values. An alpha value of 0.05 was used to indicate statistical significance. The statistical calculations were performed using SAS version 9.4 statistical software.
3. Results
At McGill University Health center, there were 352 patients with colorectal liver metastasis who consented for participation in the liver biobank research program. Of these patients, 277 underwent liver resection between January 2009 and December 2020 at McGill University Health Center. All 277 patients had their liver tumors evaluated for histopathologic growth patterns, but 19 patients were unable to have their liver tumors scored due to completely mucinous tumors, or no evidence of residual malignancy. Of the remaining 258 patients, 109 had desmoplastic tumors (42.2%) and 149 patients had non-desmoplastic tumors (57.7%). There was no significant difference between desmoplastic and non-desmoplastic patients when looking at gender, age of diagnosis, BMI, or incidence of synchronous disease. Unfortunately, the race and ethnicity of patients were not at the time being consistently documented in the electronic medical record of patients at the MUHC and therefore were not included in the statistical analysis. There was no significant difference between the desmoplastic and non-desmoplastic groups when comparing the size and location of the primary tumor; however, there was a significant difference in the size of the liver metastasis. Non-desmoplastic tumors were slightly larger than desmoplastic tumors when measuring the tumor’s greatest dimension. The mean greatest dimension of desmoplastic liver tumors was 3.34 cm (SD 2.35) and the mean greatest dimension of non-desmoplastic tumors was 3.86 cm (SD 2.78) (p = 0.047). There was a trend towards a higher number of tumors in patients with non-desmoplastic HGP (p = 0.0581). The mean number of tumors in patients with desmoplastic HGPs was 3.07 (SD 2.35) and the mean number of liver tumors in patients with non-desmoplastic HGPs was 3.4 (SD 2.03) (Table 2).
Table 2.
Overall survival using Cox regression.
| Characteristics | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | p-Value | Hazard Ratio | 95% CI | p-Value | |
| Liver tumor histology | ||||||
| Desmoplastic | 1.00 | 1.00 | ||||
| Non-desmoplastic | 1.70 | 1.23–2.34 | 0.0010 | 1.54 | 1.09–2.15 | 0.0133 |
| Synchronous presentation | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 4.30 | 2.79–6.65 | <0.0001 | 4.13 | 2.63–6.48 | <0.0001 |
| Neoadjuvant chemotherapy before primary resection | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 1.87 | 1.36–2.55 | <0.0001 | 1.80 | 1.30–2.50 | 0.0004 |
| Adjuvant chemotherapy after liver resection | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 1.51 | 0.82–2.83 | 0.1819 | 1.54 | 0.80–2.98 | 0.1960 |
| TNM stage of primary tumor | ||||||
| 1 | 1.00 | 1.00 | ||||
| 2 | 1.67 | 0.39–7.13 | 0.4875 | 1.82 | 0.42–7.79 | 0.4208 |
| 3 | 2.96 | 0.43–12.09 | 0.1299 | 2.76 | 0.67–11.29 | 0.1588 |
| 4 | 4.98 | 1.20–20.69 | 0.0273 | 5.92 | 1.41–24.77 | 0.0149 |
| KRAS | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 1.43 | 0.99–2.09 | 0.0600 | 1.56 | 1.03–2.36 | 0.0373 |
| Location of primary tumor | ||||||
| Bilateral | 1.00 | 1.00 | ||||
| Right | 3.75 | 0.91–15.39 | 0.0670 | 3.32 | 0.79–14.00 | 0.1022 |
| Left | 2.26 | 0.55–9.32 | 0.2592 | 2.00 | 0.48–8.34 | 0.3442 |
| Rectum | 2.53 | 0.62–10.34 | 0.1950 | 2.13 | 0.52–8.78 | 0.2975 |
| Volume of primary tumor | ||||||
| 1.003 | 0.995–1.010 | 0.4924 | 1.002 | 0.995–1.010 | 0.5749 | |
| Greatest dimension of liver tumor | ||||||
| 0.968 | 0.912–1.028 | 0.4822 | 0.3673 | 0.907–1.029 | 0.2803 | |
| Development of extra-hepatic metastatic disease | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 1.82 | 1.29–2.57 | 0.0006 | 1.88 | 1.3–2.72 | 0.0007 |
| Multiple of extra-hepatic metastatic site | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 1.96 | 1.44–2.67 | <0.0001 | 2.02 | 1.46–2.80 | <0.0001 |
| Liver metastatic recurrence | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 1.46 | 1.02–2.10 | 0.0403 | 1.81 | 1.13–2.88 | 0.0127 |
| Never fully resected | 3.42 | 2.25–5.22 | <0.0001 | 1.32 | 0.79–2.22 | 0.2871 |
| Liver resection with and without BEV | ||||||
| Chemonaive | 1.00 | 1.00 | ||||
| Neoadjuvent | 2.26 | 1.42–3.59 | 0.0005 | 1.93 | 0.97–3.82 | 0.0602 |
| BEV | 1.68 | 1.02–2.76 | 0.0415 | 2.98 | 1.47–6.02 | 0.0024 |
| Number of clinically relevant mutations in liver tumors | ||||||
| 0 | 1.00 | 1.00 | ||||
| 1 | 1.76 | 1.10–2.81 | 0.0177 | 2.06 | 1.24–3.43 | 0.0053 |
| 2 | 1.20 | 0.64–2.27 | 0.5666 | 1.45 | 0.73–2.87 | 0.2912 |
| 3 | 13.42 | 1.71–105.69 | 0.0136 | 16.81 | 2.00–141.45 | 0.0094 |
| Number of clinically relevant mutations in primary tumor | ||||||
| 0 | 1.00 | 1.00 | ||||
| 1 | 1.42 | 0.80–2.52 | 0.2326 | 1.74 | 0.93–3.28 | 0.0838 |
| 2 | 1.21 | 0.55–2.64 | 0.6412 | 1.62 | 0.72–3.67 | 0.2459 |
| 3 | 39.98 | 3.56–448.71 | 0.0028 | 63.79 | 4.62–881.05 | 0.0019 |
Models are all adjusted for age at first diagnosis, gender, and BMI. Significant values are highlighted in bold.
3.1. Systemic Therapy
Overall, 24.3% (N = 25) of dHGP patients were chemo-naive and 19.9% (N = 27) of non-dHGP patients were chemo-naive. For the remaining dHGP patients, 35.9% (N = 28) received neoadjuvant bevacizumab compared with 41.3% (n = 45) of the non-dHGP cohort. There was no significant difference in the number of patients who were chemo-naive or who received neoadjuvant chemotherapy in the dHGP and non-dHGP groups. There was also no difference in the number of patients who received neoadjuvant bevacizumab (Table 2). The most common systemic chemotherapy regimens were FOLFOX (N = 170), followed by FOLFIRI (N = 18) and XELODA (N = 9).
3.2. Mutation Analysis
We compared the number of mutations found in the liver tumor and found in the primary tumor of patients with dHGP and non-dHGP. There was no significant difference in the incidence of KRAS, NRAS, or BRAF mutations identified in the liver tumors or in the primary tumors in the desmoplastic group compared with the non-desmoplastic group (Supplemental Table S2). There was no significant difference in the incidence of overall number of mutations found in the liver tumors or the primary tumors when comparing the desmoplastic and non-desmoplastic groups (Supplemental Table S2).
We also evaluated the mutational burden of the primary tumors. In our analysis, right-sided colon tumors had a statistically higher incidence of liver tumors with KRAS mutations (p = 0.0241) when compared against left-sided tumors and rectal tumors (Supplemental Table S3). There was a significant difference in PIK3CA mutational burden based on the location of the primary tumor (p = 0.0139), with most PIK3CA mutations located in right-sided primary tumors. Seven out of the ten patients with PIK3CA mutations had right-sided colon tumors (Supplemental Table S1). When looking at the overall mutational burden of the liver tumors, there was a significant difference in the location of the associated primary tumor (p = 0.0042); however, there was no significant difference in the overall mutational burden of the primary tumor (p = 0.4026). Also, there was no significant difference in the location of the primary tumor of desmoplastic vs. non-desmoplastic liver tumors (p = 0.8261) (Supplemental Table S3).
A subgroup survival analysis was performed for all patients with right-sided primary tumors. Interestingly, when comparing overall survival from the time of liver metastasis diagnosis, the median survival of dHGP patients (N = 31) was 60 months, and the median survival of non-dHGP patients (N = 37) was 39 months (p = 0.0231) (Supplemental Figure S1).
3.3. Survival Analysis
The median survival for the entire cohort of 258 patients who had their liver tumor HGP scored was 66 months from initial diagnosis of CRC and 49 months from diagnosis of CRCLM. A total of 71.3% of patients presented with synchronous disease. Synchronous disease was defined as the presence of liver metastasis within 1 year of CRC diagnosis.
The median survival for patients with dHGP liver tumors from diagnosis of CRC and CRCLM was 79 and 61 months, respectively, ranging from 11 to 192 months. The median survival for patients with non-dHGP from diagnosis or CRC and CRCLM was 56 and 43 months, respectively, ranging from 3 to 146 months. For patients with dHGP, the 3 and 5 year OS after CRC diagnosis was 73.8% and 45.8%, respectively, and the 3 and 5 year OS after diagnosis of CRCLM was 65.4% and 34.6%, respectively. For patients with non-dHGP, the 3 and 5 year OS after diagnosis of CRC was 62.3% and 39.8%, respectively, and the 3 and 5 year OS after diagnosis of CRCLM was 47.1% and 13%, respectively.
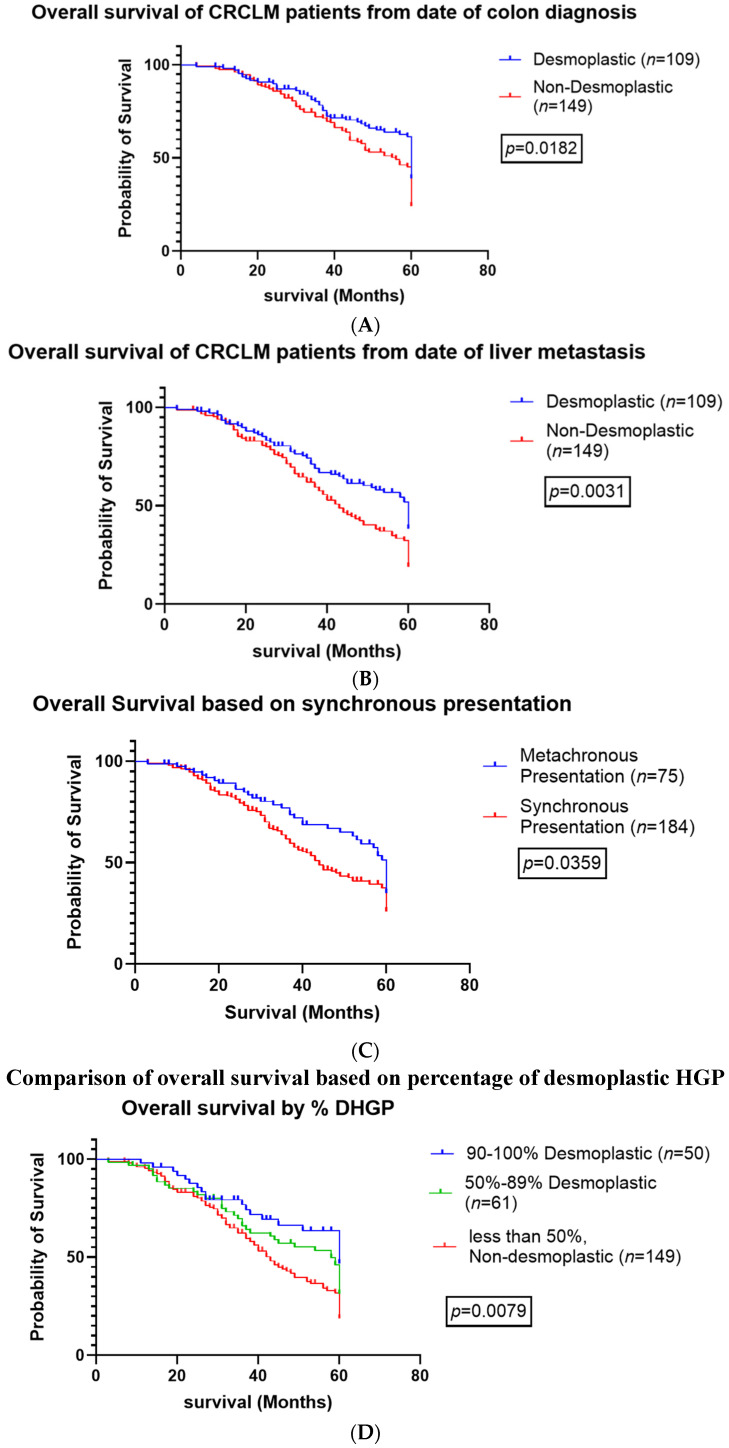
Kaplan–Meier curves found the dHGP patients to have a significantly greater OS compared with non-dHGP patients when evaluating survival from diagnosis of CRC (p = 0.0182) and CRCLM (p < 0.0031) (Figure 2A and Figure 2B, respectively). Patients with synchronous disease had worse overall survival regardless of HGP (p < 0.0359) (Figure 2C).
Figure 2.
Overall survival based on histopathologic growth patterns. (A). Overall survival of patients based on the date of primary colon cancer diagnosis. (B). Overall survival of patients based on the date of liver metastasis diagnosis (C). Overall survival based on synchronous presentation of colon cancer with liver metastasis (D). Overall survival based on percent of tumor DHGP.
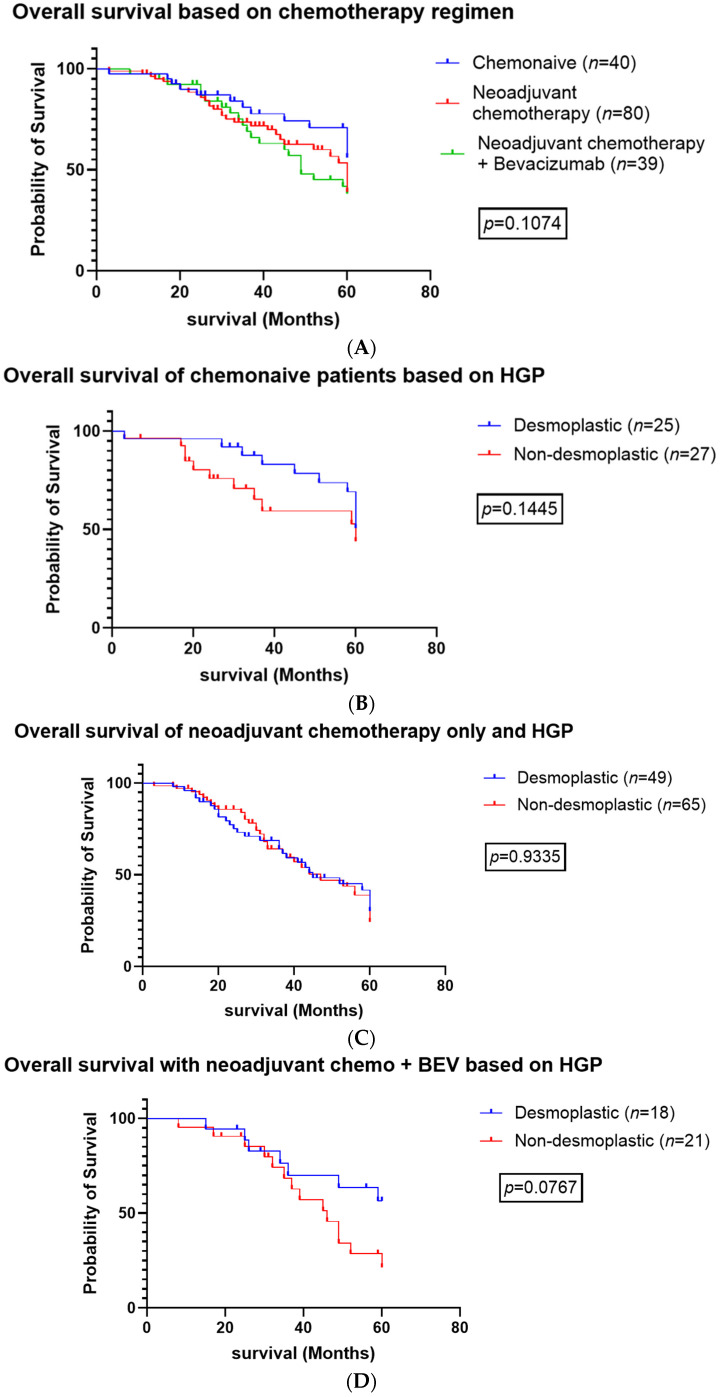
There was no significant difference between the proportion of dHGP and non-dHGP groups who received neoadjuvant chemotherapy (p = 0.4123) or neoadjuvant chemotherapy + bevacizumab (p = 0.4565), as seen in Table 2. There was no significant difference between the survival curves evaluating chemotherapy regimens (chemo-naive vs. neoadjuvant chemotherapy vs. neoadjuvant chemotherapy + bevacizumab) (Figure 3A, p = 0.1074). There was no significant difference in OS for dHGP patients and non-dHGP patients who were chemo-naive or who received neoadjuvant chemotherapy (p = 0.1445) or neoadjuvant chemotherapy + bevacizumab (p = 0.9335) (Figure 3B and Figure 3C, respectively). There was a trend towards significance when comparing the OS of patients who received neoadjuvant chemotherapy + Bevacizumab (p = 0.0767) with dHGP patients exhibiting improved overall survival compared with non-dHGP patients (Figure 3D). We excluded patients who required two-staged hepatectomy.
Figure 3.
Overall survival comparing systemic chemotherapy regimens. (A). Overall survival of patients based on chemotherapy regimens (B). Overall survival of chemonaive patients based on histopathologic growth patterns (C). Overall survival of patients who received neoadjuvant chemotherapy without Bevacizumab based histopathologic growth patterns (D). Overall survival survival of patients who received neoadjuvant chemotherapy with Bevacizumab based histopathologic growth patterns.
Interestingly, when we evaluated the overall survival of patients based on their percent desmoplastic HGP, we found a statistically significant difference between patients with 90–100% desmoplastic HGP, patients with 50–89% desmoplastic HGP, and patients with under 50% HGP (or non-desmoplastic HGP, (p = 0079) (Figure 2D).
3.4. Extrahepatic and Recurrent Disease
There was a significantly higher incidence of extrahepatic metastatic disease in non-desmoplastic patients compared with desmoplastic (p = 0.0009) and a statistically higher incidence of pulmonary metastatic disease (p = 0.0006) (Table 1). There was a trend towards a higher incidence of multiple sites of metastatic disease in non-desmoplastic patients (p = 0.1575) with 42.1% of non-desmoplastic patients having multiple sites of recurrent disease compared with 33.3% of desmoplastic patients. When evaluating liver-specific recurrence rates, 44% of desmoplastic patients never experienced liver recurrence, compared with only 30.6% of non-desmoplastic patients. Also, 22.4% of non-desmoplastic patients were unable to have their liver tumors fully resected, compared with only 13% of desmoplastic patients (Table 2) (p = 0.0375)
Factors associated with recurrent metastatic liver disease and inability to fully resect liver tumors included larger size of liver tumor (4.05 cm vs. 2.94 cm vs. 3.9 cm, p = 0.0037), higher number of liver tumors at initial diagnosis (3.52 vs. 2.23 vs. 4.74, p < 0.0001), synchronous presentation (p = 0.0018) and receiving neoadjuvant chemotherapy prior to colon resection (p = 0.0120) or liver resection (p = 0.0024), having non-desmoplastic HGP (p = 0.0375) development of pulmonary metastasis (p < 0.0001), and extrahepatic metastasis (p < 0.0001) (Supplemental Table S4).
3.5. Univariate and Multivariate Analysis
Regarding univariate analysis (UVA) controlling for patient age, gender, and BMI, higher OS was associated with dHGP (p = 0.0010), metachronous presentation (p < 0.0001), and chemo-naive status prior to undergoing colon resection (p < 0.0001). Regarding multivariate analysis (MVA), only metachronous presentation (p = 0.002) and chemo-naive status prior to colon resection (p = 0.011) were statistically significant for improved OS. Development of extrahepatic metastasis, multiple sites of extrahepatic metastasis, and pulmonary metastasis were all associated with worse OS regarding both UVA and MVA (Table 2).
Cancer recurrence was associated with non-desmoplastic HGP (p = 0.0001) and presence of neoadjuvant chemotherapy prior to colon resection or liver resection (p = 0.0100 and p = 0.024, respectively) regarding both UVA and MVA controlling for patient age, gender, and BMI. Regarding UVA, KRAS status was associated with cancer recurrence (0.0392), but not regarding MVA. Regarding both UVA and MVA, development of extrahepatic metastatic disease, pulmonary metastatic disease, and multiple sites of metastatic disease were all associated with increased risk of cancer recurrence following liver resection (Table 3).
Table 3.
Cancer recurrence using cox regression.
| Characteristics | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | p-Value | Hazard Ratio | 95% CI | p-Value | |
| Number of liver tumors at 1st diagnosis | ||||||
| 1.103 | 1.045–1.163 | 0.0003 | 1.095 | 1.035–1.158 | 0.0016 | |
| Greatest dimension of liver tumor | ||||||
| 1.034 | 0.954–1.056 | 0.8781 | 0.999 | 0.947–1.054 | 0.9685 | |
| Development of extra-hepatic metastatic disease | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 2.03 | 1.49–2.78 | <0.0001 | 2.10 | 1.50–2.93 | <0.0001 |
| Development of pulmonary metastasis | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 2.02 | 1.50–2.72 | <0.0001 | 2.01 | 1.46–2.93 | <0.0001 |
| Liver metastasis recurrence | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 1.37 | 0.99–1.89 | 0.0543 | 1.35 | 0.96–1.90 | 0.0866 |
| Multiple of extra-hepatic metastatic site | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 2.09 | 1.57–2.77 | <0.0001 | 2.16 | 1.60–2.91 | <0.0001 |
| Resection with and without BEV | ||||||
| Chemo-naive | 1.00 | 1.00 | ||||
| Neoadjuvent | 1.89 | 1.27–2.80 | 0.0018 | 1.61 | 1.07–2.42 | 0.0230 |
| BEV | 1.27 | 0.83–1.95 | 0.2748 | 1.07 | 0.68–1.69 | 0.7589 |
Models are all adjusted for age at first diagnosis, gender, and BMI. Significant values are highlighted in bold.
4. Discussion
This study aimed to evaluate the prognostic differences between desmoplastic and non-desmoplastic HGP in the setting of CRCLM. We retrospectively analyzed all the HGPs in patients at McGill University Health Center with CRCLM who underwent liver resection.
According to our results, dHGP was independently associated with improved OS and a lower overall incidence of cancer recurrence. Our study showed a dramatic improvement in both 3 and 5 year survival for patients with predominantly dHGP after diagnosis of CRCLM, as demonstrated by reporting a median 3 year OS of 65.4% for dHGP compared with 47.1% for non-dHGP and a median 5 year OS of 34.6% for dHGP patients compared with 13% for non-dHGP patients.
Galjart et al. published the largest single center study to date investigating the prognosis of CRCLM patients based on their liver tumor HGP [8]. They also reported improved survival in patients with angiogenic dHGP liver tumors, (78% 5-year survival). However, they used a cut-off value of 100% to categorize dHGPs. While many other groups have previously reported improved survival for patients with high percentages of dHGP, we were able to demonstrate improved outcomes for patients with >50% dHGP according to the international consensus guidelines for scoring CRCLM, and as previously documented by Frentzas et al. [6]. This is significant because the majority of patients develop multiple liver tumors with mixed phenotypes. We have demonstrated that patients with CRCLM demonstrating at least 50% of the interface demonstrating a desmoplastic ring can have a similar improved prognosis as patients with a “pure” desmoplastic phenotype. We were also able to show that there was a significant difference in survival between patients with different dHGP cut-off points (p = 0.0079, Figure 2D). Patients had improved OS with both 50–89% dHGP and >90% dHGP compared with non-desmoplastic HGPs. Including patients with 50–89% dHGP allowed us to double our dHGP cohort while still improving overall patient survival.
When we performed univariate and multivariate analysis comparing patients based on their systemic chemotherapy regimens, patients who received neoadjuvant chemotherapy with or without bevacizumab prior to undergoing liver resection had a worse overall survival (p = 0.1074) (Figure 3). Half of the chemo-naive cohort were dHGP (n = 20) compared with non-dHGP (n = 20), and 42% (n = 21) had a single liver lesion. We hypothesize that the chemo-naive patients had a lower burden of disease compared with patients who received either neoadjuvant chemotherapy + bevacizumab or neoadjuvant chemotherapy alone. Therefore, clinicians may have opted to perform upfront surgical resection. Interestingly, there was a near significance in improved overall survival comparing patients who received neoadjuvant chemotherapy with bevacizumab (p = 0.0767, Figure 3). This cohort mirrored Frentzas et al.’s cohort, as we selected patients who had complete R0 resections of the liver metastasis.
Unfortunately, the majority of patients with CRCLM develop recurrent disease. This was especially true in our cohort, which exhibited significantly higher rates of synchronous disease compared with other publications. The rate of synchronous disease was 71% in our cohort (Table 2). We were able to demonstrate a different pattern in disease recurrence among dHGP patients and non-dHGP patients. In UVA and MVA, non-dHGP was associated with cancer recurrence (p < 0.0001, HR 1.92 and p = 0.0001, HR1.83, respectively, Table 2). Patients with non-dHGP were more likely to develop extrahepatic metastasis (p = 0.0006, HR 1.82 on UVA; p = 0.0007, HR 1.88 on MVA) and were more likely to develop pulmonary metastasis (p < 0.0001, HR 2.02 and 2.01 on UVA and MVA, respectively, Table 3) and develop multiple sites of extrahepatic metastasis (p < 0.0001, HR 2.09 and 2.16 on UVA and MVA, respectively, Table 3). There was a higher percentage of non-dHGP patients who developed disease at multiple extrahepatic locations (33.3% vs. 42.1%) (Supplemental Table S1), but it was not significant (p = 0.1575). These findings are complemented by Neirop et al., who previously reported that patients with dHGP were more likely to develop liver recurrence and were more likely to have recurrent liver disease amenable to re-operation [10]. We think this is a large part of the reason for patient survival improving for patients with desmoplastic tumors. Since desmoplastic tumors are more likely to recur in the liver, patients are more likely to have repeat liver operations or procedures—such as RFA—to control the recurrence. This is in contrast with patients with non-desmoplastic tumors who are more likely to have extra-hepatic disease recurrence, which is non-resectable. These findings suggest that overall survival in patients with CRCLM is driven more by rHGP and vessel co-option than dHGP angiogenesis.
Limitations of this study include the retrospective nature of data collection and the lack of validation at an external site. We also had not been collecting race/ethnicity data on our liver disease biobank subjects until the end of 2021. We are in the process of adding race and ethnicity documentation for each subject. Additionally, molecular testing of liver tumors for all CRCLM patients was recently implemented at McGill University, therefore only 40% (n = 98) of our cohort was able to be analyzed for mutations. With uniform testing for multiple mutations, we hope to be able to determine an optimal targeted therapy approach for patients with different HGPs. So far, we have identified a higher incidence of mutations in non-dHGP patients and inability to fully resect the liver tumors (p = 0.0375, Table 4) and a higher incidence of KRAS mutations in patients with right-sided colon tumors (p = 0.0241 and p = 0.0197, Supplementary Table S3) and an association of PIK3CA mutations with right-sided primary tumors (p = 0.0139, Supplementary Table S3). It has been documented in numerous studies that right-sided colon tumors are associated with more advanced tumor stages, increased tumor sizes, and poor differentiation. All these findings are likely due to right-sided tumors becoming symptomatic later than left-sided tumors due to the wider lumen of the left colon and possibly due to the higher difficulty in identification of the right-sided tumors as they are far from the anal verge and unable to be identified on physical exam or sigmoidoscopy. Taking these factors together, right-sided colon tumors have been found in other studies to convey a worse overall survival compared with left-sided colon tumors. To date, the mechanistic link between KRAS mutation and tumor sidedness also remains to be assessed. These findings will need to be validated in future studies with a larger patient cohort. We do not believe these limitations affected the overall conclusions of this study.
Table 4.
Patient characteristics of desmoplastic vs. non-desmoplastic tumors.
| Desmoplastic | Non-Desmoplastic | p-Value | |
|---|---|---|---|
| n = 110 | n = 148 | ||
| Age at diagnosis, mean (SD) | 62.4 ± 10.22 | 60.57 ± 10.13 | 0.1250 |
| BMI, mean (SD) | 26.80 ± 5.66 | 26.96 ± 4.63 | 0.6221 |
| Mean size of primary tumor, cm (SD) | 13.79 ± 29.14 | 8.03 ± 11.76 | 0.1375 |
| Number of liver tumors at 1st diagnosis, mean (SD) | 3.07 ± 2.35 | 3.41 ± 2.03 | 0.0592 |
| Volume of primary tumor, mean (SD) | 13.79 ± 29.14 | 7.98 ± 11.69 | 0.1372 |
| Greatest dimension of liver tumor cm (SD) | 3.34 ± 2.35 | 3.86 ± 2.78 | 0.0470 |
| Number of liver tumors at initial diagnosis | 3.07 ± 2.35 | 3.40 ± 2.03 | 0.0581 |
| Synchronous presentation | 82 (74.5%) | 101 (68.2%) | 0.2702 |
| Systemic therapy | |||
| Chemo-naive | 25(24.3%) | 27 (19.9%) | 0.4123 |
| Neoadjuvant chemotherapy | 78 (75.7%) | 109 (80.1%) | 0.4123 |
| Neoadjuvant chemotherapy + bevacizumab | 28 (35.9%) | 45 (41.3%) | 0.4565 |
| Metastatic disease | |||
| Development of extrahepatic metastasis | 59 (53.6%) | 106 (73.6%) | 0.0009 |
| Multiple extrahepatic metastatic sites | 36 (33.3%) | 61 (42.1%) | 0.1575 |
| Development of pulmonary metastasis | 51 (46.4%) | 97 (67.8%) | 0.0006 |
| Liver metastatic recurrence | |||
| Yes | 46 (29.5%) | 69 (39.2%) | 0.0375 |
| No | 48 (44.4%) | 45 (30.6%) | |
| Never fully resected | 14 (13.0%) | 33 (22.4%) | |
N—patient number, SD—standard deviation. Categorical variables were compared using Chi-squared test. Significant values are highlighted in bold.
5. Future Directions
Our cohort population presented in this study is biased towards patients who were able to undergo at least partial resection of their metastatic liver tumors. However, in general, only 10–20% of patients with CRCLM are resectable [9]. There has been consistent evidence associating worse survival for patients with non-dHGP CRCLM. Currently, it is unknown why a patient develops dHGP vs. non-dHGP liver tumors. Thus, it is essential to accurately identify the biomarkers and characteristics of the tumor microenvironment of early developing liver tumors to guide treatment and identify optimal downstaging treatments.
Early HGP identification can guide treatment and identify optimal downstaging therapy to allow for liver resection. Development of imaging and liquid biopsy tools are essential to accurately identify the liver tumor HGP early in the disease course. Biomarkers identified by RNA sequencing in liquid biopsies could predict CRCLM HGP and guide systemic and immunologic therapies for downstaging. Liver tumor tissue biopsies might also be able to predict HGP in the future despite not providing the full tumor interface. A liver biopsy can provide enough tissue to perform next generation sequencing and provide information on the tumor immune population, which has been shown to differ between dHGP liver tumors and non-dHGP liver tumors, as previously discussed [9]. Radiologic features seen on MRIs of liver tumors in CRCLM have been able to predict the presence of KRAS mutations [38], and dHGPs appear to have a fibrous band encircling tumors visible on contrast-enhanced CT scans [40].
Neoadjuvant systemic therapy regimens could be tailored for patients based, in part, on their HGP. Patients with rHGP might benefit from more aggressive perioperative treatment without the addition of bevacizumab. Surgical planning could be influenced by pre-operatively knowing the tumor HGP. Patients with known rHGP might benefit from a surgeon intentionally performing anatomical liver resections or taking a wider margin to decrease the risk of performing an R1 resection. This might also lead to more two-staged hepatectomies for patients with bilateral disease and rHGP tumors due to the need to develop an adequate future liver remnant. On the other hand, patients with known dHGP might tolerate parenchymal sparing resections.
Additionally, liver transplantation for highly selected patients with unresectable liver-limited metastatic CRC has shown promising survival results [41,42,43]. The 5 year OS for patients with CRCLM following liver transplantation was 75% compared with a 76% 5 year OS for patients with HCC [41,42]. Patients with dHGP, who are less likely to have extra-hepatic metastatic disease and more likely to have liver-limited recurrent disease, would likely benefit from liver transplantation. Since dHGP liver tumors were found to have a higher immune response, it remains unclear what effect immunosuppression would have on cancer recurrence. Of note, the SECA investigators did not find a difference in the growth rate of lung nodules in patients with CRCLM treated with liver transplantation and immunosuppression compared with CRCLM patients who did not undergo liver transplantation [44]. Also, rapamycin demonstrated antiangiogenic qualities to inhibit tumor growth following hepatectomy for CRCLM [41], and sirolimus-based immunosuppression has demonstrated improved OS, specifically for patients with HCC following liver transplantation [45,46].
6. Conclusions
In conclusion, this study validates the improved prognostic value of the desmoplastic HGP in patients with CRCLM when categorizing patients according to the current international consensus guidelines for scoring HGPs in CRCLM. We recommend that all patients with CRCLM undergo HGP scoring of all liver tumors at each liver resection. Patients with CRCLM are highly complex and should be discussed at multidisciplinary team discussions where personalized treatment plans can be developed.
Acknowledgments
We thank Zu-Hua Gao (McGill University Health Center Pathology Department) for performing histopathologic growth pattern scoring for all liver tumors. We also thank RI-MUHC Liver Disease Biobank for providing CRCLM slides. This work was supported by Dana Massaro and Ken Verdoni Liver Metastases Research Fellowship.
Abbreviations
| BEV | bevacizumab |
| CDR | Crohn’s disease-like response |
| CRCLM | colorectal cancer liver metastasis |
| CRS | cancer risk score |
| DFS | disease free survival |
| HA | hyaluronic acid |
| HGP | histopathologic growth patterns |
| dHGP | desmoplastic histopathologic growth patterns |
| rHGP | replacement histopathologic growth patterns |
| IHC | immunohistochemistry |
| LM | liver metastasis |
| LR | liver resection |
| MSI | microsatellite instability |
| MVD | microvascular density |
| OS | overall survival |
| PFS | progression free survival |
| RFS | recurrence free survival |
| TBS | tumor budding score |
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers16183148/s1, Figure S1: Survival of Right-sided Primary tumors by Histopathologic Growth Pattern; Figure S2: Overall survival of entire cohort from diagnosis of liver metastasis; Table S1: Summary of papers reporting survival analysis of CRCLM based on liver tumor Histopathologic Growth Patterns; Table S2: Mutations in the primary tumor and the liver metastases; Table S3: Summary of mutations based on the location of the primary tumor; Table S4: Summary of exposures vs. liver metastasis recurrence;
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by L.K., S.K.P., O.Z. and A.L. The first draft of the manuscript was written by L.K. and all authors commented on previous versions of the manuscript. Pathology was reviewed by Z.-H.G. and P.M. provided overall direction and guidance of the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of McGill University Health Centre (MUHC REB) (Protocol code: 11-066-SDR and 2011-10-12).
Informed Consent Statement
Written informed consent was obtained from all individual patients included in this study to participate in the McGill University Liver Disease Biobank, which exists to progress the understanding of liver disease and cancer development. Each patient consented to have their clinical data collected and anonymized and published.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
L.K received a fellowship from the Ken Verdoni Liver Metastases Fellowship.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M., Parkin D.M., Forman D., Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Schmoll H.J., Van Cutsem E., Stein A., Valentini V.G., Limelius B., Haustermans K., Nordlinger B., van de Velde C.J., Balmana J., Regula J., et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann. Oncol. 2012;23:2479–2516. doi: 10.1093/annonc/mds236. [DOI] [PubMed] [Google Scholar]
- 3.Lemke J., Cammerer G., Ganser J., Scheele J., Xu P., Sander S., Henne-Bruns D., Kornmann M. Survival and Prognostic Factors of Colorectal Liver Metastases after Surgical and Nonsurgical Treatment. Clin. Color. Cancer. 2016;15:e183–e192. doi: 10.1016/j.clcc.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Hubbard J.M., Grothey A. Colorectal cancer in 2014: Progress in defining first-line and maintenance therapies. Nat. Rev. Clin. Oncol. 2015;12:73–74. doi: 10.1038/nrclinonc.2014.233. [DOI] [PubMed] [Google Scholar]
- 5.Vermeulen P.B., Colpaert C., Salgado R., Royers R., Hellemans H., Van den Heuvel E., Goovaerts G., Dirix L.Y., Van Marck E. Liver metastases from colorectal adenocarcinomas grow in three patterns with different angiogenesis and desmoplasia. J. Pathol. 2001;195:336–342. doi: 10.1002/path.966. [DOI] [PubMed] [Google Scholar]
- 6.Frentzas S., Simoneau E., Bridgeman V.L., Vermeulen P.B., Foo S., Kostaras E., Nathan M., Wotherspoon A., Gao Z.H., Shi Y., et al. Vessel co-option mediates resistance to anti-angiogenic therapy in liver metastases. Nat. Med. 2016;22:1294–1302. doi: 10.1038/nm.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nierop P.M.H., Galjart B., Höppener D.J., van der Stok E.P., Coebergh van den Braak R.R.J., Vermeulen P.B., Grünhagen D.J., Verhoef C. Salvage treatment for recurrences after first resection of colorectal liver metastases: The impact of histopathological growth patterns. Clin. Exp. Metastasis. 2019;36:109–118. doi: 10.1007/s10585-019-09960-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galjart B., Nierop P.M.H., van der Stok E.P., van den Braak R.R.J.C., Höppener D.J., Daelemans S., Dirix L.Y., Verhoef C., Vermeulen P.B., Grünhagen D.J. Angiogenic desmoplastic histopathological growth pattern as a prognostic marker of good outcome in patients with colorectal liver metastases. Angiogenesis. 2019;22:355–368. doi: 10.1007/s10456-019-09661-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oliveira R.C., Alexandrino H., Cipriano M.A., Alves F.C., Tralhão J.G. Predicting liver metastases growth patterns: Current status and future possibilities. Semin. Cancer Biol. 2020;71:42–51. doi: 10.1016/j.semcancer.2020.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Nierop P.M.H., Höppener D.J., van der Stok E.P., Galjart B., Buisman F.E., Balachandran V.P., Jarnagin W.R., Kingham T.P., Allen P.J., Shia J., et al. Histopathological growth patterns and positive margins after resection of colorectal liver metastases. HPB. 2020;22:911–919. doi: 10.1016/j.hpb.2019.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moro C.F., Bozoky B., Gerling M. Growth patterns of colorectal cancer liver metastases and their impact on prognosis: A systematic review. BMJ Open Gastroenterol. 2018;5:e000217. doi: 10.1136/bmjgast-2018-000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Dam P.J., van der Stok E.P., Teuwen L.A., Van den Eynden G.G., Illemann M., Frentzas S., Majeed A.W., Eefsen R.L., Coebergh van den Braak R.R.J., Lazaris A., et al. International consensus guidelines for scoring the histopathological growth patterns of liver metastasis. Br. J. Cancer. 2017;117:1427–1441. doi: 10.1038/bjc.2017.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falcão D., Alexandrino H., Caetano Oliveira R., Martins J., Ferreira L., Martins R., Serôdio M., Martins M., Tralhão J.G., Cipriano M.A., et al. Histopathologic patterns as markers of prognosis in patients undergoing hepatectomy for colorectal cancer liver metastases—Pushing growth as an independent risk factor for decreased survival. Eur. J. Surg. Oncol. 2018;44:1212–1219. doi: 10.1016/j.ejso.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 14.Brunner S., von Moos R., Mey U., Camenisch Gross U., Freyholdt T., Cathomas R. Efficacy of triplet combination chemotherapy with oxaliplatin, irinotecan and capecitabine (OCX) in metastatic colorectal cancer in relation to RAS/RAF mutation status: Results of a retrospective analysis. Oncol. Res. Treat. 2014;37:646–652. doi: 10.1159/000368313. [DOI] [PubMed] [Google Scholar]
- 15.Siriwardana P.N., Luong T.V., Watkins J., Turley H., Ghazaley M., Gatter K., Harris A.L., Hochhauser D., Davidson B.R. Biological and Prognostic Significance of the Morphological Types and Vascular Patterns in Colorectal Liver Metastases (CRLM): Looking Beyond the Tumor Margin. Medicine. 2016;95:e2924. doi: 10.1097/MD.0000000000002924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinheiro R.S., Herman P., Lupinacci R.M., Lai Q., Mello E.S., Coelho F.F., Perini M.V., Pugliese V., Andraus W., Cecconello I., et al. Tumor growth pattern as predictor of colorectal liver metastasis recurrence. Am. J. Surg. 2014;207:493–498. doi: 10.1016/j.amjsurg.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 17.Nyström H., Naredi P., Berglund A., Palmqvist R., Tavelin B., Sund M. Liver-metastatic potential of colorectal cancer is related to the stromal composition of the tumour. Anticancer. Res. 2012;32:5183–5191. [PubMed] [Google Scholar]
- 18.Eefsen R.L., Vermeulen P.B., Christensen I.J., Laerum O.D., Mogensen M.B., Rolff H.C., Van Den Eynden G.G., Høyer-Hansen G., Osterlind K., Vainer B., et al. Growth pattern of colorectal liver metastasis as a marker of recurrence risk. Clin. Exp. Metastasis. 2015;32:369–381. doi: 10.1007/s10585-015-9715-4. [DOI] [PubMed] [Google Scholar]
- 19.Nielsen K., Rolff H.C., Eefsen R.L., Vainer B. The morphological growth patterns of colorectal liver metastases are prognostic for overall survival. Mod. Pathol. 2014;27:1641–1648. doi: 10.1038/modpathol.2014.4. [DOI] [PubMed] [Google Scholar]
- 20.Van den Eynden G.G., Bird N.C., Majeed A.W., Van Laere S., Dirix L.Y., Vermeulen P.B. The histological growth pattern of colorectal cancer liver metastases has prognostic value. Clin. Exp. Metastasis. 2012;29:541–549. doi: 10.1007/s10585-012-9469-1. [DOI] [PubMed] [Google Scholar]
- 21.Eefsen R.L., Van den Eynden G.G., Høyer-Hansen G., Brodt P., Laerum O.D., Vermeulen P.B., Christensen I.J., Wettergren A., Federspiel B., Willemoe G.L., et al. Histopathological growth pattern, proteolysis and angiogenesis in chemonaive patients resected for multiple colorectal liver metastases. J. Oncol. 2012;2012:907971. doi: 10.1155/2012/907971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rahbari N.N., Bork U., Schölch S., Reissfelder C., Thorlund K., Betzler A., Kahlert C., Schneider M., Ulrich A.B., Büchler M.W., et al. Metastatic Spread Emerging from Liver Metastases of Colorectal Cancer: Does the Seed Leave the Soil Again? Ann. Surg. 2016;263:345–352. doi: 10.1097/SLA.0000000000001341. [DOI] [PubMed] [Google Scholar]
- 23.Stremitzer S., Vermeulen P., Graver S., Kockx M., Dirix L., Yang D., Zhang W., Stift J., Wrba F., Gruenberger T., et al. Immune phenotype and histopathological growth pattern in patients with colorectal liver metastases. Br. J. Cancer. 2020;122:1518–1524. doi: 10.1038/s41416-020-0812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lazaris A., Amri A., Petrillo S.K., Zoroquiain P., Ibrahim N., Salman A., Gao Z.H., Vermeulen P.B., Metrakos P. Vascularization of colorectal carcinoma liver metastasis: Insight into stratification of patients for anti-angiogenic therapies. J. Pathol. Clin. Res. 2018;4:184–192. doi: 10.1002/cjp2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cremolini C., Milione M., Marmorino F., Morano F., Zucchelli G., Mennitto A., Prisciandaro M., Lonardi S., Pellegrinelli A., Rossini D., et al. Differential histopathologic parameters in colorectal cancer liver metastases resected after triplets plus bevacizumab or cetuximab: A pooled analysis of five prospective trials. Br. J. Cancer. 2018;118:955–965. doi: 10.1038/s41416-018-0015-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ibrahim N.S., Lazaris A., Rada M., Petrillo S.K., Huck L., Hussain S., Ouladan S., Gao Z.H., Gregorieff A., Essalmani R., et al. Angiopoietin1 Deficiency in Hepatocytes Affects the Growth of Colorectal Cancer Liver Metastases (CRCLM) Cancers. 2019;12:35. doi: 10.3390/cancers12010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Cutsem E., Nordlinger B., Adam R., Köhne C.H., Pozzo C., Poston G., Ychou M., Rougier P. European Colorectal Metastases Treatment Group. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur. J. Cancer. 2006;42:2212–2221. doi: 10.1016/j.ejca.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 28.Stessels F., Van den Eynden G., Van der Auwera I., Salgado R., Van den Heuvel E., Harris A.L., Jackson D.G., Colpaert C.G., van Marck E.A., Dirix L.Y., et al. Breast adenocarcinoma liver metastases, in contrast to colorectal cancer liver metastases, display a non-angiogenic growth pattern that preserves the stroma and lacks hypoxia. Br. J. Cancer. 2004;90:1429–1436. doi: 10.1038/sj.bjc.6601727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barnhill R., Vermeulen P., Daelemans S., van Dam P.J., Roman-Roman S., Servois V., Hurbain I., Gardrat S., Raposa G., Nicolas A., et al. Replacement and desmoplastic histopathological growth patterns: A pilot study of prediction of outcome in patients with uveal melanoma liver metastases. J. Pathol. Clin. Res. 2018;4:227–240. doi: 10.1002/cjp2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Held T., Verbeke C.S., Strobel O., Rutkowski W., Villard C., Moro C.F., Del Chiaro M., Büchler M., Heuchel R., Löhr M. Immunohistochemical profiling of liver metastases and matched-pair analysis in patients with metastatic pancreatic ductal adenocarcinoma. Pancreatology. 2019;19:963–970. doi: 10.1016/j.pan.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 31.Kuczynski E.A., Vermeulen P.B., Pezzella F., Kerbel R.S., Reynolds A.R. Vessel co-option in cancer. Nat. Rev. Clin. Oncol. 2019;16:469–493. doi: 10.1038/s41571-019-0181-9. [DOI] [PubMed] [Google Scholar]
- 32.Temido M.J., Caetano Oliveira R., Martins R., Serôdio M., Costa B., Carvalho C., Santos E., Ferreira L., Teixeira P., Cipriano M.A., et al. Prognostic Factors After Hepatectomy for Gastric Adenocarcinoma Liver Metastases: Desmoplastic Growth Pattern as the Key to Improved Overall Survival. Cancer Manag. Res. 2020;12:11689–11699. doi: 10.2147/CMAR.S264586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kozaka K., Sasaki M., Fujii T., Harada K., Zen Y., Sato Y., Sawada S., Minato H., Matsui O., Nakanuma Y. A subgroup of intrahepatic cholangiocarcinoma with an infiltrating replacement growth pattern and a resemblance to reactive proliferating bile ductules: ‘bile ductular carcinoma’. Histopathology. 2007;51:390–400. doi: 10.1111/j.1365-2559.2007.02735.x. [DOI] [PubMed] [Google Scholar]
- 34.Bohlok A., Vermeulen P., Leduc S., Latacz E., Botzenhart L., Richard F., De Schepper M., Geukens T., Lucidi V., Ignatiadis M., et al. Association between the histopathological growth patterns of liver metastases and survival after hepatic surgery in breast cancer patients. NPJ Breast Cancer. 2020;6:64. doi: 10.1038/s41523-020-00209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rajaganeshan R., Prasad R., Guillou P.J., Chalmers C.R., Scott N., Sarkar R., Poston G., Jayne D.G. The influence of invasive growth pattern and microvessel density on prognosis in colorectal cancer and colorectal liver metastases. Br. J. Cancer. 2007;96:1112–1117. doi: 10.1038/sj.bjc.6603677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu X., Lin H., Li S. Prognoses of different pathological subtypes of colorectal cancer at different stages: A population-based retrospective cohort study. BMC Gastroenterol. 2019;19:1–8. doi: 10.1186/s12876-019-1083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J., Chen C., Huang J., Xie Z., Chen X., Zheng Z., Li E., Zou H. The possibilities of LOXL4 as a prognostic marker for carcinomas. Amino Acids. 2023;55:1519–1529. doi: 10.1007/s00726-023-03343-9. [DOI] [PubMed] [Google Scholar]
- 38.Gültekin M.A., Türk H.M., Beşiroğlu M., Toprak H., Yurtsever I., Yilmaz T.F., Sharifov R., Uysal Ö. Relationship between KRAS mutation and diffusion weighted imaging in colorectal liver metastases; Preliminary study. Eur. J. Radiol. 2020;125:108895. doi: 10.1016/j.ejrad.2020.108895. [DOI] [PubMed] [Google Scholar]
- 39.Palmieri V., Lazaris A., Mayer T.Z., Petrillo S.K., Alamri H., Rada M., Jarrouj G., Park W.Y., Gao Z.H., McDonald P.P., et al. Neutrophils expressing lysyl oxidase-like 4 protein are present in colorectal cancer liver metastases resistant to anti-angiogenic therapy. J. Pathol. 2020;251:213–223. doi: 10.1002/path.5449. [DOI] [PubMed] [Google Scholar]
- 40.Cheng J., Wei J., Tong T., Sheng W., Zhang Y., Han Y., Gu D., Hong N., Ye Y., Tian J., et al. Prediction of Histopathologic Growth Patterns of Colorectal Liver Metastases with a Noninvasive Imaging Method. Ann. Surg. Oncol. 2019;26:4587–4598. doi: 10.1245/s10434-019-07910-x. [DOI] [PubMed] [Google Scholar]
- 41.Dueland S., Grut H., Syversveen T., Hagness M., Line P.D. Selection criteria related to long-term survival following liver transplantation for colorectal liver metastasis. Am. J. Transplant. 2020;20:530–537. doi: 10.1111/ajt.15682. [DOI] [PubMed] [Google Scholar]
- 42.Line P.D., Hagness M., Dueland S. The Potential Role of Liver Transplantation as a Treatment Option in Colorectal Liver Metastases. Can. J. Gastroenterol. Hepatol. 2018;2018:8547940. doi: 10.1155/2018/8547940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simoneau E., D’Angelica M., Halazun K.J. Liver transplantation for colorectal liver metastasis. Curr. Opin. Organ. Transplant. 2019;24:175–181. doi: 10.1097/MOT.0000000000000623. [DOI] [PubMed] [Google Scholar]
- 44.Grut H., Solberg S., Seierstad T., Revheim M.E., Egge T.S., Larsen S.G., Line P.D., Dueland S. Growth rates of pulmonary metastases after liver transplantation for unresectable colorectal liver metastases. Br. J. Surg. 2018;105:295–301. doi: 10.1002/bjs.10651. [DOI] [PubMed] [Google Scholar]
- 45.Rupertus K., Dahlem C., Menger M.D., Schilling M.K., Kollmar O. Rapamycin inhibits hepatectomy-induced stimulation of metastatic tumor growth by reduction of angiogenesis, microvascular blood perfusion, and tumor cell proliferation. Ann. Surg. Oncol. 2009;16:2629–2637. doi: 10.1245/s10434-009-0564-8. [DOI] [PubMed] [Google Scholar]
- 46.Toso C., Kneteman N.M., James Shapiro A.M., Bigam D.L. The estimated number of patients with hepatocellular carcinoma selected for liver transplantation using expanded selection criteria. Transpl. Int. 2009;22:869–875. doi: 10.1111/j.1432-2277.2009.00882.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.