Abstract
Cytochrome P450 enzymes (P450s) play a critical role in drug metabolism, with the CYP3A subfamily being responsible for the biotransformation of over 50% of marked drugs. While CYP3A enzymes are known for their extensive catalytic versatility, one intriguing and less understood function is the ability to mediate carbon–carbon (C–C) bond cleavage. These uncommon reactions can lead to unusual metabolites and potentially influence drug safety and efficacy. This review focuses on examining examples of C–C bond cleavage catalyzed by CYP3A, exploring the mechanisms, physiological significance, and implications for drug metabolism. Additionally, examples of CYP3A-mediated ring expansion via C–C bond cleavages are included in this review. This work will enhance our understanding of CYP3A-catalyzed C–C bond cleavages and their mechanisms by carefully examining and analyzing these case studies. It may also guide future research in drug metabolism and drug design, improving drug safety and efficacy in clinical practice.
Keywords: CYP3A, carbon–carbon bond cleavage, drug metabolism
1. Introduction
Cytochrome P450 enzymes (P450s) represent a diverse and essential class of thiolate-ligated heme proteins characterized by their remarkable catalytic versatility [1,2]. These enzymes play pivotal roles in various physiological functions, spanning specialized biosynthetic pathways to xenobiotic detoxification [3]. Their catalytic repertoire includes a broad spectrum of oxidations crucial for cellular function, such as hydroxylation, dealkylation, deamination, dehalogenation, cyclization [4,5], carbon–nitrogen (C–N) bond formation [6], and C–C bond cleavage [7,8,9]. Understanding the mechanisms and substrate specificity of P450 enzymes is crucial for elucidating their roles in physiology and pharmacology, as well as for the development of novel therapeutic interventions and biotechnological applications [3]. P450s play a crucial role in the phase I metabolism of xenobiotics, accounting for approximately 75% of drug metabolism [10]. However, mammalian drug-metabolizing P450-mediated C–C bond cleavages are uncommon in drug metabolism, although P450-mediated C–C bond cleavages in sterol metabolism, including CYP51 [11,12], CYP11A [13], CYP17A [14], CYP19A [15], and CYP24A1 [16] and bacterial P450s (CYP125, CYP152L1, CYP152A1, and CYP107H1) catalyzed C–C bond cleavage in fatty acid metabolism, have been reported [17,18,19,20,21]. The exact mechanisms underlying these cleavages remain incompletely understood.
The CYP3A subfamily including CYP3A4 and CYP3A5 is a prominent member of the P450 family, mainly expressed in the liver and intestine [22,23]. CYP3A enzymes play a crucial role in the metabolism of a wide range of xenobiotics and endogenous compounds, contributing to the biotransformation of over 50% of marketed drugs [24,25,26,27]. The primary role of CYP3A in drug metabolism underscores its clinical significance, as variations in CYP3A activity can result in drug–drug interactions, influencing drug efficacy and toxicity [28]. CYP3A-involved C–C bond cleavages have been reported. Determining CYP3A-mediated unusual C–C bond cleavage reactions may improve drug efficacy and safety as well as elucidate the pathophysiology of various diseases [8,29]. This review focuses on CYP3A-mediated C–C bond cleavages with the proposed possible mechanisms in the previous report, which will provide insights for the scientists working on the P450-mediated uncommon reactions and drug development. The drugs included in this review are listed in Table 1.
Table 1.
Drugs and the types of C–C bond cleavage.
| Drug Name | Indications | C–C Bond Cleavage Type |
|---|---|---|
| Plitidepsin | Antitumor and antiviral activities | C(sp3)-C(sp3) [30,31,32,33] |
| IKRO-105714 | Alleviate atopic dermatitis | C(sp3)-C(sp3) [34,35] |
| Noscapine | Cough suppressing | C(sp3)-C(sp3) [36] |
| Tipranavir | Anti-HIV agent | C(sp3)-C(sp3) [37,38] |
| Evatanepag | Treats tibial fractures. | C(sp3)-C(sp3) [39] |
| Olanexidine | Antiseptic agent | C(sp3)-C(sp3) [40,41] |
| Nabumetone | Treats pain and arthritis | C(sp3)-C(sp3) [42,43,44,45] |
| Pexidartinib | Treats tenosynovial giant cell tumor | C(sp2)-C(sp3) [46] |
| Nefazodone | Treats depression | C(sp2)-C(sp3) [47] |
| Indacaterol | Treats chronic obstructive pulmonary disease and asthma | C(sp2)-C(sp3) [48] |
| DPC 963 | Anti-HIV agent | Ring-open [49,50] |
| Tetramethylpiperidine | Building blocks of drugs | Ring-open [51,52,53] |
| Cipargamin | Anti-Malarial | Ring-open [54,55] |
2. CYP3A Mediates C(sp3)-C(sp3) Bond Cleavages
2.1. Plitidepsin
Plitidepsin (also known as Aplidin), a marine-derived cyclic depsipeptide, has shown potent antitumor activity against a variety of cancer cell lines, including melanoma, ovarian, renal, prostate, and breast cancers [31,33]. Its effectiveness in treating non-small-cell lung and colon cancers has made it a promising candidate in phase II clinical trials [32].
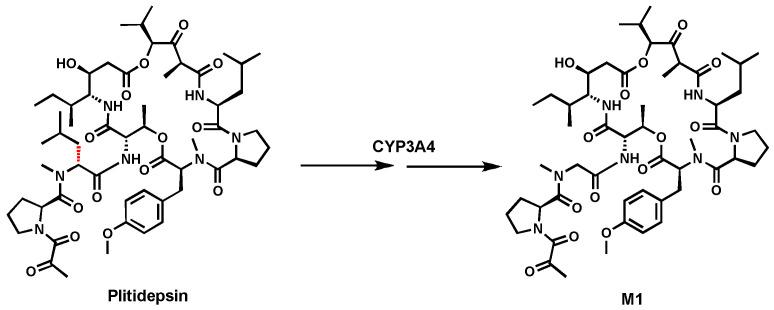
Previous metabolism studies of Plitidepsin in human liver microsomes (HLM) and human P450 isoenzymes indicated that CYP3A4-mediated C-dealkylation occurred leading to M1 (Figure 1) [30]. The exact mechanism of this C–C bond cleavage is not fully understood [30]. C-dealkylation metabolite was also observed in the urine and feces of patients treated with Plitidepsin [56]. More studies are needed to understand the cleavage.
Figure 1.
CYP3A4 mediates C(sp3)-C(sp3) bond cleavage in Plitidepsin.
2.2. KRO-105714
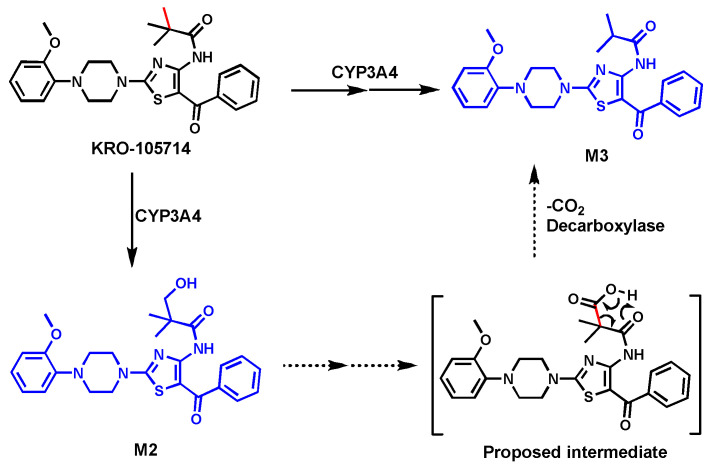
KRO-105714 was developed due to its anti-atopic dermatitis (eczema) activity by robust suppression of the sphingosylphosphorylcholine (SPC) receptor [35]. Experiments with a specific CYP3A4 inhibitor (ketoconazole at 2 µM) and in HLM and human P450 isoforms revealed that CYP3A4 was the primary enzyme contributing to the formation of monohydroxylated (M2), O-demethylated, and C-demethylated KRO-105714 (M3) metabolites [34].
The metabolite (M3) formed by uncommon C–C bond cleavage was confirmed by its synthetic standard. In CYP3A4 isoform, alcohol M2 can be readily converted to M3. Thus, the pathway leading to C-demethylation likely involves the initial hydroxylation of KRO-105714 to generate an alcohol M2. The M2 was further oxidized to acid, which undergoes the elimination of CO2 to form M3 (Figure 2). In the biological system, other enzymes like decarboxylase may be involved in the M3 formation. The exact mechanism of the C–C bond cleavage is still not clear.
Figure 2.
C-Demethylation of KRO-105714.
2.3. Noscapine
Noscapine is a non-sedating alkaloid used as an antitussive (cough suppressant) and for various cancers [57]. Due to controversies regarding its drug safety [36], the metabolites and in vivo hepatotoxicity through bioactivation were intensely studied [58,59,60,61].
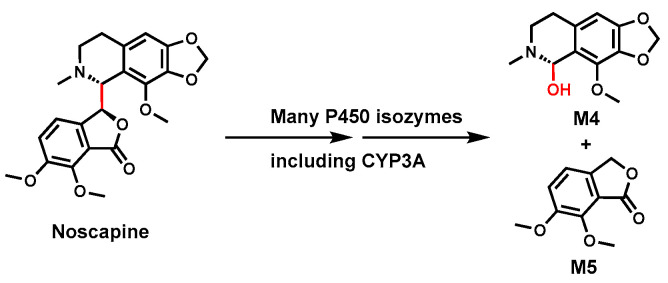
As shown in Figure 3, two unusual Noscapine metabolites, hydrocotarnine (M4) and meconine (M5), were observed in the urine samples from animal models (rats and rabbits) and human subjects administered with Noscapine [62]. The formation of these metabolites indicated that one C–C bond in Noscapine is cleaved during its metabolism [63]. In vitro studies using HLM and human P450 isoforms suggested that multiple P450s are involved in this C–C bond cleavage. Among these enzymes, CYP3A4 plays a significant role in Noscapine metabolism [36]. The mechanism (s) of this rare C–C bond cleavage remains unknown, and the mechanism likely involves P450-mediated oxidation to form an unstable intermediate leading to bond cleavage.
Figure 3.
CYP3A4 mediates C(sp3)-C(sp3) bond cleavage in Noscapine.
2.4. Tipranavir
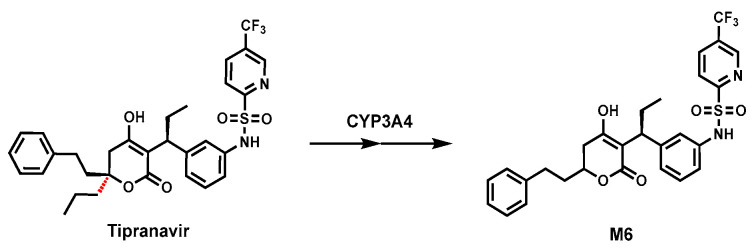
Tipranavir (TPV), a non-peptidic protease inhibitor, is commonly used to treat drug-resistant HIV infections in combination with ritonavir (RTV, CYP3A inhibitor) [37]. Our previous study revealed that an unusual dealkylated metabolite (M6) resulted from C–C bond cleavage in TPV metabolism in mice (Figure 4). Using chemical inhibitors (RTV) in HLM and human P450 isozymes, CYP3A4 was identified as a key enzyme for this C–C bond cleavage reaction [38,64]. M6 was also observed in the incubation of TPV with cDNA recombinant human CYP3A4. The precise mechanism of dealkylation is still unclear, and further research is required to determine the mechanism of the C–C bond cleavage in TPV.
Figure 4.
C-Depropylation of Tipranavir.
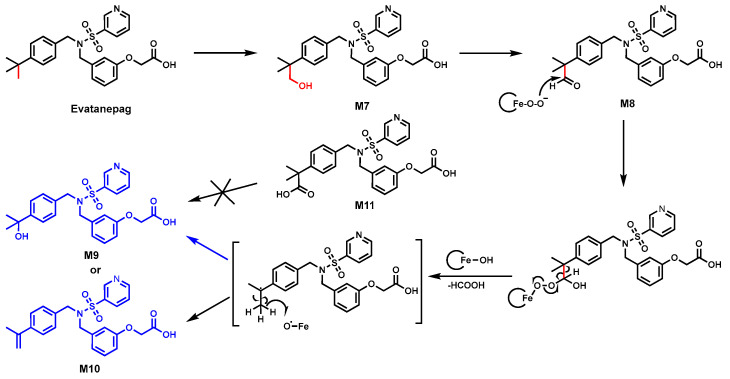
2.5. Evatanepag
Evatanepag (also known as CP-533,536), an EP2 receptor-selective prostaglandin E2 agonist, shows promise for aiding bone fracture healing [65]. In HLM, two C-demethylation-related metabolites, M9 and M10, were identified (Figure 5) by LC-MS, and their structures were confirmed by NMR. CYP3A4/5 and CYP2C8 were determined as the major enzymes responsible for the C–C bond cleavage using CYP3A4/5 inhibitors (ketoconazole) and a CYP2C8 inhibitor (quercetin).
Figure 5.
Proposed mechanism for demethylation of the t-butyl group in Evatanepag.
The proposed mechanism, as shown in Figure 5, involved the oxidation of the tert-butyl moiety to form alcohol M7, which was further oxidized into aldehyde M8. The resulting M8 produced a CYP3A4/5 mediated carbon-centered radical, which could either undergo oxygen rebound to yield M9 or hydrogen abstraction to create the olefin M10 [39]. Moreover, the possibility of carboxylic acid metabolite as the key intermediate was ruled out because incubating M11 with either HLM or CYP3A/2C8 isoforms did not generate C-demethylated metabolites.
2.6. Olanexidine
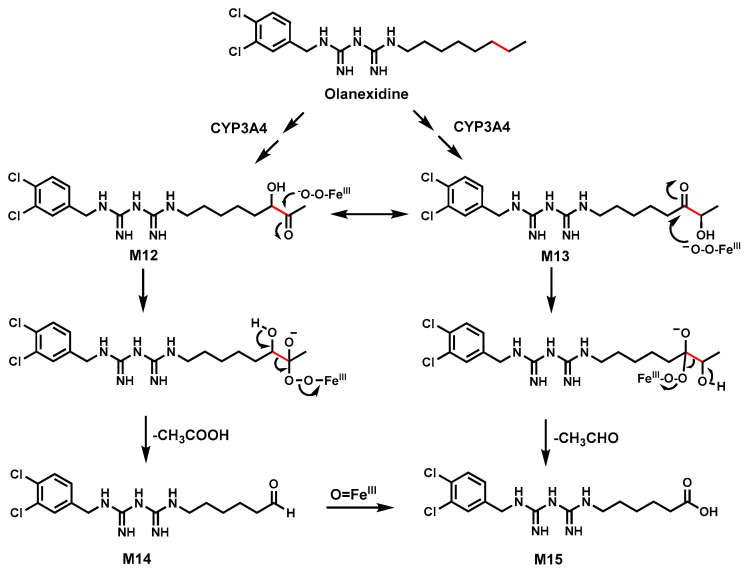
Olanexidine is an antiseptic containing a biguanide group with a long aliphatic side chain [40,66]. Incubation of Olanexidine in HLM, rat, or dog liver S9 fractions revealed that it could be metabolized into several shortened side chain metabolites (M14 and M15) via C–C bond cleavages (Figure 6) [41].
Figure 6.
The proposed mechanism for carbon–carbon bond cleavage in Olanexidine.
CYP3A4 is the major enzyme involved in the C–C bond cleavage [40,41].
The proposed mechanism for C–C bond cleavage in Olanexidine involves the formation of a ketol intermediate M12 or M13.
A ferric peroxide (FeIII-O-OH) complex is trapped by the electrophilicity of the carbonyl group, leading to a hydroperoxide intermediate. This intermediate subsequently triggers C–C bond cleavage. M12 led to aldehyde M14 by the loss of carboxylic acid, which could be further oxidized to acid M15, while M13 could directly proceed to acid M15 (Figure 6) [41,67]. Incubating ketol intermediates M12 and M13 in HLM and P450s generated the metabolites M14 and M15, supporting the proposed mechanism of C–C bond cleavage in Olanexidine.
2.7. Nabumetone
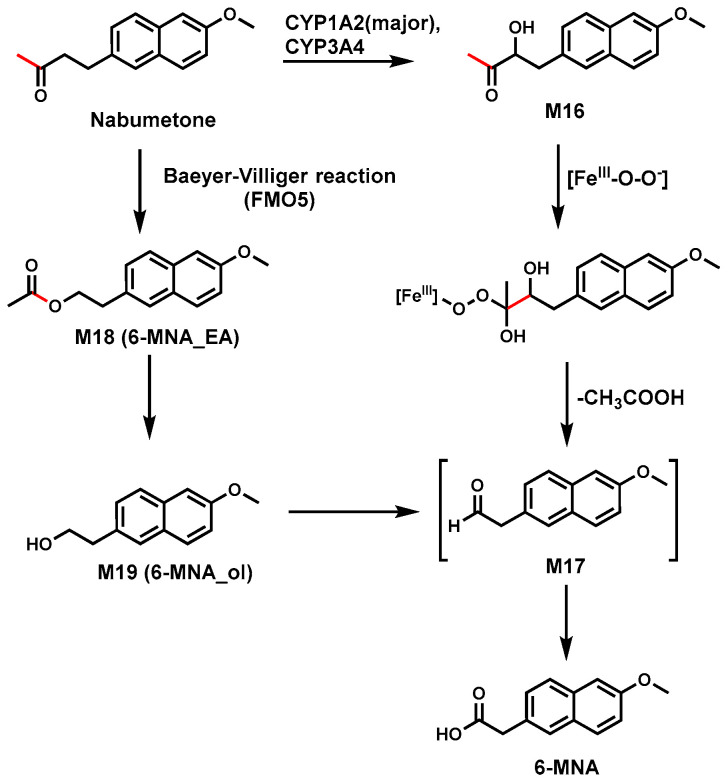
Nabumetone is a widely used non-steroidal anti-inflammatory prodrug [68]. In vitro studies with HLM and P450 isoforms found that CYP3A4 and CYP1A2 are responsible for the formation of acid 6-MNA [42,43,44,69]. The proposed mechanism is that Nabumetone was oxidized to M16, followed by the addition of a nucleophile, the peroxyl anion (FeIII-O-O-) intermediate, to the carbonyl group. The resulting tetrahedral intermediate releases carboxylic acid to form an aldehyde M17, which was converted to acid 6-MNA (Figure 7) [44].
Figure 7.
Proposed mechanism for the C–C bond cleavage in Nabumetone.
A recent study also demonstrated that 6-MNA could be produced via Baeyer–Villiger oxidation catalyzed by flavin-containing monooxygenase isoform 5 (FMO5). Nabumetone was first oxidized to ester (M18) by FMO5, followed by hydrolysis to produce primary alcohol (M19) [45]. The carboxylesterase (CES) inhibiting the formation of 6-MNA in the S9 fractions indicated CES may play a role in the ester hydrolysis as the alcohol is the precursor of 6-MNA formation. The alcohol was oxidized to the intermediate aldehyde M17 and further converted to 6-MNA. As to which pathway plays the dominant role, more studies are needed.
3. CYP3A Mediates C(sp2)-C(sp3) Bond Cleavage
3.1. Pexidartinib
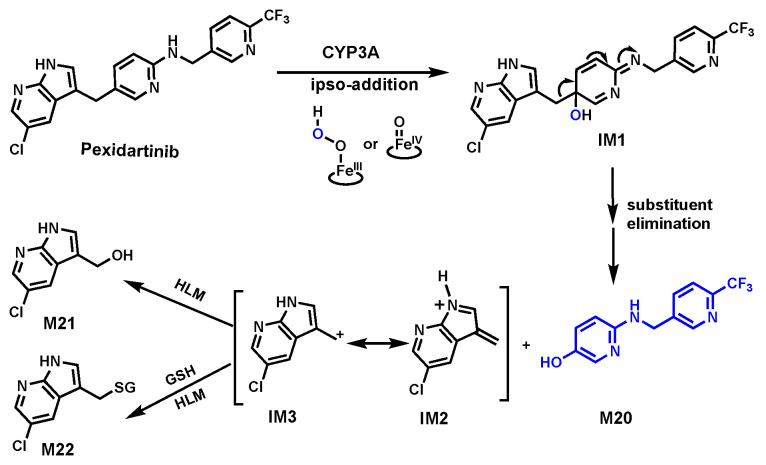
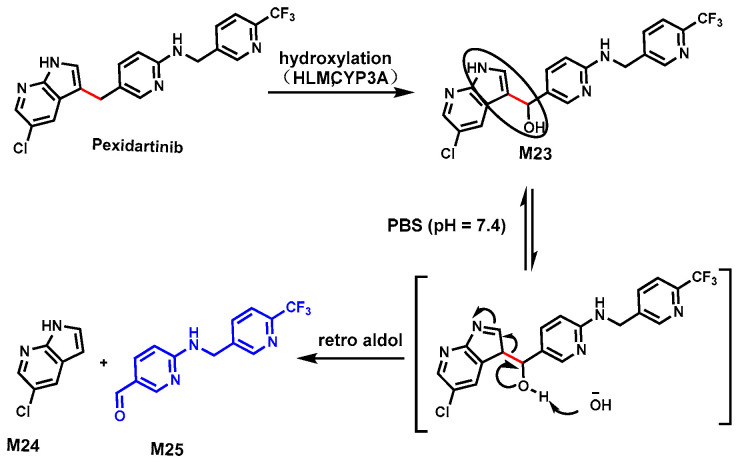
Pexidartinib (PEX) is a kinase inhibitor used to treat tenosynovial giant cell tumors, especially when surgery is not a suitable option [70]. However, cases of serious liver toxicity in some patients raised concerns about its safety [71]. In HLM, four metabolites of PEX (M20, M21, M22, and M23) indicated C–C bond cleavages occurred (Figure 8 and Figure 9). The structures of these metabolites were confirmed based on their exact masses, MS/MS fragmentation patterns, and their standard compounds. CYP3A was determined as a primary enzyme responsible for cleavage using human P450 isozymes and chemical inhibitors (ketoconazole, a CYP3A inhibitor) in HLM [46,72].
Figure 8.
CYP3A-mediated C–C bond cleavage in Pexidartinib via ipso-addition of oxygen.
Figure 9.
CYP3A-mediated C–C bond cleavages in Pexidartinib via retro aldol.
This study revealed two distinct pathways for C–C bond cleavage in PEX metabolism. The first pathway, as shown in Figure 8, involved CYP3A-activated oxygen added to the 5-alkylated position of a 5-alkylated N-protected pyridin-2-amine to form the intermediate IM1. The substituent elimination of IM1 leads to C–C bond cleavage, yielding phenol M20 and intermediates IM2 or IM3. The formation of M21 and M22 indicated that the intermediates IM2 or IM3 exist. In H218O and 18O2, enriched incubation systems suggested that the source of oxygen in M20 is from the CYP3A-activated oxygen. All the evidence supports that the C–C bond cleavage occurred through a CYP3A-mediated oxygen ipso-addition mechanism.
In the second pathway, as shown in Figure 9, a pseudo-retro-aldol mechanism was proposed. We found that the formation of alcohol M23 was mediated by CYP3A. The beta-hydroxy enamine moiety of M23 (circled) can undergo a pseudo-retro-aldol reaction to produce aldehyde M25 and 5-chloro-7-azaindole (M24). Both metabolites were detected in the incubation system with HLM and CYP3A4 using a PEX substrate. Alcohol M23 as a substrate more efficiently generated M24 and M25, suggesting that M23 is on the kinetic pathway of M25 formation from PEX.
The safety of the metabolites generated from these unusual metabolic pathways is unknown. Thus, this research not only sheds light on the mechanism of novel and rare CYP3A-mediated C–C bond cleavages but also provides valuable insights for drug safety evaluations [46].
3.2. Nefazodone
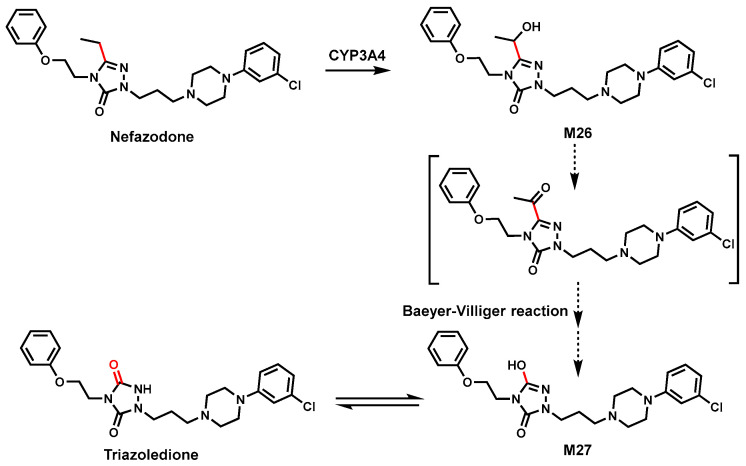
Nefazodone is an atypical antidepressant medication but was withdrawn from some countries due to rare liver toxicity [73]. In HLM, a specific breakdown pathway of Nefazodone involving C–C bond cleavage was identified (Figure 10). Incubating Nefazodone in HLM with CYP3A4 inhibitor and P450s isoenzymes both in vitro [74] and in vivo [75,76] revealed that the metabolite triazoledione formed by a CYP3A4-mediated C(sp2)-C(sp3) bond cleavage (Figure 10).
Figure 10.
CYP3A4 mediates C–C bond cleavage in Nefazodone.
The mechanism was proposed as an initial hydroxylation of the ethyl side chain of Nefazodone to form alcohol M26, which undergoes C–C bond scission to generate intermediate alcohol M27. Possibly, M26 was further oxidized to ketone intermediate, which proceeded to M27 by the Baeyer–Villiger reaction. It is well known that M27 is tautomerized to a ketone, triazoledione in the body [47]. The exact mechanism of this C–C bond cleavage is still unclear.
3.3. Indacaterol
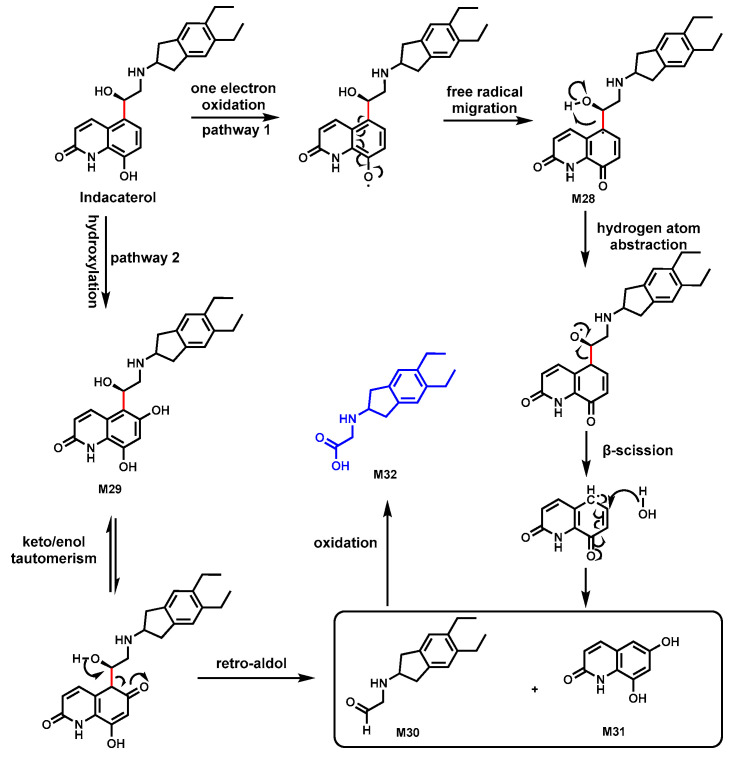
Indacaterol, a long-acting inhaled β2-adrenergic receptor agonist, is used for the treatment of chronic obstructive pulmonary disease [77]. The metabolite M32 from Indacaterol was identified as a unique metabolic product characterized by an unusual C–C bond cleavage linking the hydroxy-quinolinone and diethyl-indanyl-aminoethanol moieties.
The first potential mechanism pathway involved a one-electron oxidation of the hydroxyquinolinone moiety resulting in the formation of a semiquinone imine radical intermediate M28 (Figure 11). This radical would then migrate to the carbon adjacent to the hydroxyquinolinone group where a hydrogen atom abstraction could occur, followed by a β-scission that leads to the breaking of the C–C bond, forming M30 and M31. The second pathway suggested that an initial hydroxylation at the hydroxyquinolinone M29 happened, followed by a retro-aldol reaction, resulting in the cleavage of the C–C bond. The cleavage formed aldehyde M30, which could then be oxidized to yield the carboxylic acid M32 [48].
Figure 11.
Proposed mechanism of M30 formation from Indacaterol.
CYP3A4 could be the primary enzyme responsible for the formation of metabolite M32 based on the observation that CYP3A4 inhibitor ketoconazole increases serum Indacaterol area under the curve [48].
4. CYP3A Mediates Miscellaneous C–C Bond Cleavage
4.1. DPC 963
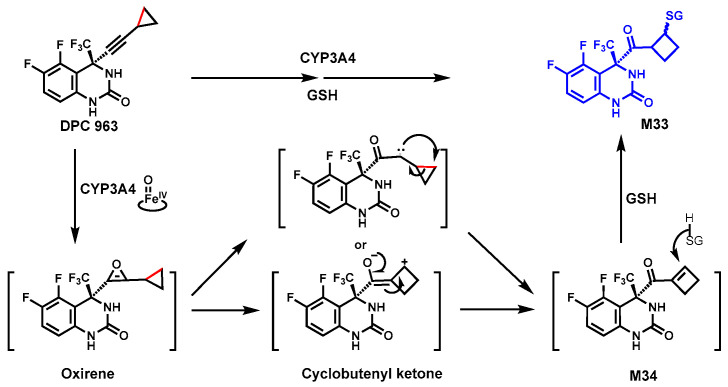
DPC 963 is a non-nucleoside human immunodeficiency virus-1 reverse transcriptase inhibitor (NNRTI) [78]. In rat bile and human liver microsomes, the diastereomeric glutathione adducts M33 were identified (Figure 12), implying that a C–C bond cleavage occurred during the metabolism. The chemical structure of M33 was confirmed by NMR. Using anti-rat P450 antibodies, selective chemical inhibitors, and human P450 isoform enzymes, CYP3A was identified as a major enzyme contributing to the formation of glutathione adducts.
Figure 12.
CYP3A4-mediated ring expansion of the cyclopropyl ring in DPC 963.
The proposed mechanism of M33 formation involved multiple steps (Figure 12): CYP3A4-mediated oxidation of the triple bond to form the putative oxirene, followed by ring expansion to form a butyl cation via C–C bond cleavage or rearrangement to form the reactive cyclobutenyl ketone [79,80,81]. These active species were converted to an α,β-unsaturated ketone intermediate M34, which reacts with GSH to form the M33 [49]. However, the exact mechanism is still unclear.
The formations of GSH adducts are often considered as detoxification in vivo. Overproduction of the reactive species may cause adverse effects; thus, avoiding co-administration of CYP3A inducers may improve DPC 963 efficacy and safety.
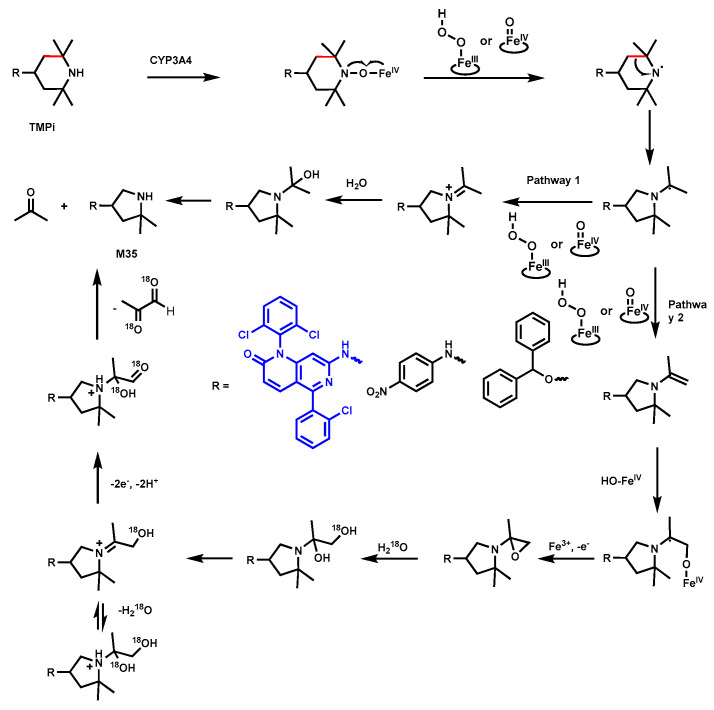
4.2. 2,2,6,6-Tetramethylpiperidine
2,2,6,6-Tetramethylpiperidine (TMPi) is a building block with the widespread use of piperidine derivatives in drug development. Researchers discovered an uncommon ring-shrinked metabolite M35, indicating that the TMPi ring undergoes a C–C bond cleavage and ring contraction. NMR spectroscopy experiments confirmed the structure of contracted pyrrolidine ring M35 (Figure 13). Additional experiments with anti-P450 monoclonal antibodies and P450 isoenzymes suggested that the CYP3A4 primarily contributed to this metabolic transformation [51]. The potential mechanism is proposed as a nitroxide radical derived from TMPi interacts with heme iron, leading to homolytic scission of the N-O bond and subsequent C–C bond cleavage, resulting in the contraction of the piperidine ring to M35.
Figure 13.
Carbon–carbon bond cleavage in Tetramethyl-piperidine.
Evidence for Pathway 1 is based on the generation of acetone in the incubation of TMPi with CYP3A4, whereas Pathway 2 provides a possible explanation for the incorporation of two oxygen atoms into the molecule from the water of the incubation medium using a different H218O ratio before its final degradation to 2,2-dimethylpyrrolidine. This transformation could occur through CYP3A4 catalysis or other heme proteins, suggesting a heme-catalyzed process [51]. Thus, in the study of the metabolism of TMPi-containing drugs, these uncommon metabolites should be monitored.
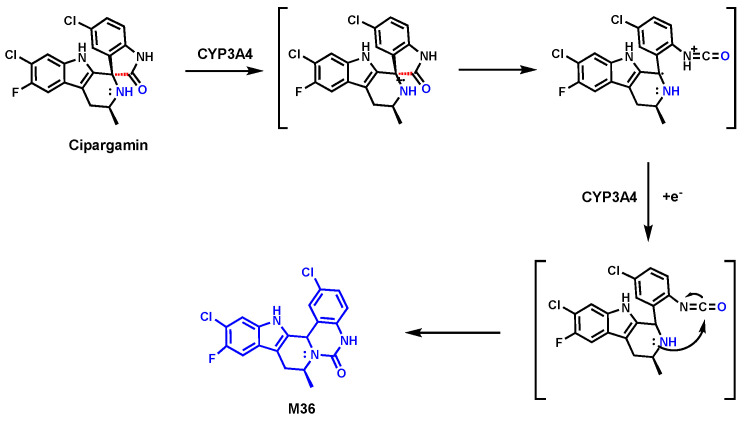
4.3. Cipargamin
Cipargamin (also known as KAE-609) is a drug candidate under clinical development by Novartis and is currently in Phase II for Malaria [82]. In the metabolic study of Cipargamin in healthy male subjects [54], rats, and dogs, a notable metabolite M36 (Figure 14) was found. In the proposed mechanism, the C–C bond cleavage-mediated ring expansion process is involved in the formation of M36 [55]. Briefly, an initial oxidation step by CYP3A4 results in the formation of a radical cation. Following the ring opening via C–C bond cleavage, subsequent recyclization produced metabolite M36 (Figure 14) [55]. CYP3A4 is mainly responsible for C–C bond cleavage in the formation of M36. The exact mechanism of this C–C bond cleavage is still unknown.
Figure 14.
C–C bond cleavage in Cipargamin.
5. Summary
In the pharmaceutical field, identifying the uncommon reactions in drug metabolism, like C–C bond cleavages, is challenging but essential for understanding drug interactions and improving drug safety. In this review, we especially summarize the drugs in which CYP3A-mediated C–C bond cleavage occurred, including C(sp3)-C(sp3), C(sp2)-C(sp3), and other uncommon reactions, along with the available mechanisms. Determining the mechanisms of the C–C bond cleavages could provide hints for predicting this type of uncommon reaction. The insights gained from studying CYP3A-mediated carbon–carbon (C–C) bond cleavage have significant implications for drug design, safety, and pharmacokinetics. Overall, a thorough investigation of drug metabolism will ensure the safe and effective use of pharmaceuticals, including unusual reactions like CYP3A-mediated C–C bond cleavages. Additionally, investigating the detailed mechanisms underlying C–C bond cleavage will further expand our understanding of CYP3A functions.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the National Institute of Diabetes and Digestive and Kidney (R01-DK121970) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R61/R33HD099995) to Feng Li.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Guengerich F.P. Mechanisms of Cytochrome P450-Catalyzed Oxidations. ACS Catal. 2018;8:10964–10976. doi: 10.1021/acscatal.8b03401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meunier B., de Visser S.P., Shaik S. Mechanism of oxidation reactions catalyzed by cytochrome p450 enzymes. Chem. Rev. 2004;104:3947–3980. doi: 10.1021/cr020443g. [DOI] [PubMed] [Google Scholar]
- 3.Werck-Reichhart D., Feyereisen R. Cytochromes P450: A success story. Genome Biol. 2000;1:reviews3003.1. doi: 10.1186/gb-2000-1-6-reviews3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng Q., Lamb D.C., Kelly S.L., Lei L., Guengerich F.P. Cyclization of a cellular dipentaenone by Streptomyces coelicolor cytochrome P450 154A1 without oxidation/reduction. J. Am. Chem. Soc. 2010;132:15173–15175. doi: 10.1021/ja107801v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacKenzie K.R., Zhao M., Barzi M., Wang J., Bissig K.D., Maletic-Savatic M., Jung S.Y., Li F. Metabolic profiling of norepinephrine reuptake inhibitor atomoxetine. Eur. J. Pharm. Sci. 2020;153:105488. doi: 10.1016/j.ejps.2020.105488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He F., Mori T., Morita I., Nakamura H., Alblova M., Hoshino S., Awakawa T., Abe I. Molecular basis for the P450-catalyzed C-N bond formation in indolactam biosynthesis. Nat. Chem. Biol. 2019;15:1206–1213. doi: 10.1038/s41589-019-0380-9. [DOI] [PubMed] [Google Scholar]
- 7.Guengerich F.P. Common and uncommon cytochrome P450 reactions related to metabolism and chemical toxicity. Chem. Res. Toxicol. 2001;14:611–650. doi: 10.1021/tx0002583. [DOI] [PubMed] [Google Scholar]
- 8.Bolleddula J., Chowdhury S.K. Carbon-carbon bond cleavage and formation reactions in drug metabolism and the role of metabolic enzymes. Drug Metab. Rev. 2015;47:534–557. doi: 10.3109/03602532.2015.1086781. [DOI] [PubMed] [Google Scholar]
- 9.Guengerich F.P., Yoshimoto F.K. Formation and Cleavage of C-C Bonds by Enzymatic Oxidation-Reduction Reactions. Chem. Rev. 2018;118:6573–6655. doi: 10.1021/acs.chemrev.8b00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao M., Ma J., Li M., Zhang Y., Jiang B., Zhao X., Huai C., Shen L., Zhang N., He L., et al. Cytochrome P450 Enzymes and Drug Metabolism in Humans. Int. J. Mol. Sci. 2021;22:12808. doi: 10.3390/ijms222312808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hargrove T.Y., Wawrzak Z., Guengerich F.P., Lepesheva G.I. A requirement for an active proton delivery network supports a compound I-mediated C-C bond cleavage in CYP51 catalysis. J. Biol. Chem. 2020;295:9998–10007. doi: 10.1074/jbc.RA120.014064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCarty K.D., Tateishi Y., Hargrove T.Y., Lepesheva G.I., Guengerich F.P. Oxygen-18 Labeling Reveals a Mixed Fe-O Mechanism in the Last Step of Cytochrome P450 51 Sterol 14alpha-Demethylation. Angew. Chem. Int. Ed. Engl. 2024;63:e202317711. doi: 10.1002/anie.202317711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henry H.L. The 25(OH)D(3)/1alpha,25(OH)(2)D(3)-24R-hydroxylase: A catabolic or biosynthetic enzyme? Steroids. 2001;66:391–398. doi: 10.1016/S0039-128X(00)00158-6. [DOI] [PubMed] [Google Scholar]
- 14.Mak P.J., Duggal R., Denisov I.G., Gregory M.C., Sligar S.G., Kincaid J.R. Human Cytochrome CYP17A1: The Structural Basis for Compromised Lyase Activity with 17-Hydroxyprogesterone. J. Am. Chem. Soc. 2018;140:7324–7331. doi: 10.1021/jacs.8b03901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hackett J.C., Brueggemeier R.W., Hadad C.M. The final catalytic step of cytochrome p450 aromatase: A density functional theory study. J. Am. Chem. Soc. 2005;127:5224–5237. doi: 10.1021/ja044716w. [DOI] [PubMed] [Google Scholar]
- 16.Jones G., Prosser D.E., Kaufmann M. 25-Hydroxyvitamin D-24-hydroxylase (CYP24A1): Its important role in the degradation of vitamin D. Arch. Biochem. Biophys. 2012;523:9–18. doi: 10.1016/j.abb.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Sivaramakrishnan S., Ouellet H., Matsumura H., Guan S., Moenne-Loccoz P., Burlingame A.L., Ortiz de Montellano P.R. Proximal ligand electron donation and reactivity of the cytochrome P450 ferric-peroxo anion. J. Am. Chem. Soc. 2012;134:6673–6684. doi: 10.1021/ja211499q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shoji O., Fujishiro T., Nakajima H., Kim M., Nagano S., Shiro Y., Watanabe Y. Hydrogen peroxide dependent monooxygenations by tricking the substrate recognition of cytochrome P450BSbeta. Angew. Chem. Int. Ed. Engl. 2007;46:3656–3659. doi: 10.1002/anie.200700068. [DOI] [PubMed] [Google Scholar]
- 19.Matsunaga I., Sumimoto T., Ueda A., Kusunose E., Ichihara K. Fatty acid-specific, regiospecific, and stereospecific hydroxylation by cytochrome P450 (CYP152B1) from Sphingomonas paucimobilis: Substrate structure required for alpha-hydroxylation. Lipids. 2000;35:365–371. doi: 10.1007/s11745-000-533-y. [DOI] [PubMed] [Google Scholar]
- 20.Fang B., Xu H., Liu Y., Qi F., Zhang W., Chen H., Wang C., Wang Y., Yang W., Li S. Mutagenesis and redox partners analysis of the P450 fatty acid decarboxylase OleT(JE) Sci. Rep. 2017;7:44258. doi: 10.1038/srep44258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cryle M.J., De Voss J.J. Carbon-carbon bond cleavage by cytochrome p450(BioI)(CYP107H1) Chem. Commun. 2004;35:86–87. doi: 10.1039/B311652B. [DOI] [PubMed] [Google Scholar]
- 22.Thummel K.E., Wilkinson G.R. In vitro and in vivo drug interactions involving human CYP3A. Annu. Rev. Pharmacol. Toxicol. 1998;38:389–430. doi: 10.1146/annurev.pharmtox.38.1.389. [DOI] [PubMed] [Google Scholar]
- 23.Shimada T., Yamazaki H., Mimura M., Inui Y., Guengerich F.P. Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: Studies with liver microsomes of 30 Japanese and 30 Caucasians. J. Pharmacol. Exp. Ther. 1994;270:414–423. [PubMed] [Google Scholar]
- 24.Klyushova L.S., Perepechaeva M.L., Grishanova A.Y. The Role of CYP3A in Health and Disease. Biomedicines. 2022;10:2686. doi: 10.3390/biomedicines10112686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rittle J., Green M.T. Cytochrome P450 compound I: Capture, characterization, and C-H bond activation kinetics. Science. 2010;330:933–937. doi: 10.1126/science.1193478. [DOI] [PubMed] [Google Scholar]
- 26.Denisov I.G., Makris T.M., Sligar S.G., Schlichting I. Structure and chemistry of cytochrome P450. Chem. Rev. 2005;105:2253–2277. doi: 10.1021/cr0307143. [DOI] [PubMed] [Google Scholar]
- 27.Zhang R.X., Dong K., Wang Z., Miao R., Lu W., Wu X.Y. Nanoparticulate Drug Delivery Strategies to Address Intestinal Cytochrome P450 CYP3A4 Metabolism towards Personalized Medicine. Pharmaceutics. 2021;13:1261. doi: 10.3390/pharmaceutics13081261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Waterschoot R.A., ter Heine R., Wagenaar E., van der Kruijssen C.M., Rooswinkel R.W., Huitema A.D., Beijnen J.H., Schinkel A.H. Effects of cytochrome P450 3A (CYP3A) and the drug transporters P-glycoprotein (MDR1/ABCB1) and MRP2 (ABCC2) on the pharmacokinetics of lopinavir. Br. J. Pharmacol. 2010;160:1224–1233. doi: 10.1111/j.1476-5381.2010.00759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhattacharyya S., Sinha K., Sil P.C. Cytochrome P450s: Mechanisms and biological implications in drug metabolism and its interaction with oxidative stress. Curr. Drug Metab. 2014;15:719–742. doi: 10.2174/1389200215666141125121659. [DOI] [PubMed] [Google Scholar]
- 30.Brandon E.F., van Ooijen R.D., Sparidans R.W., Lazaro L.L., Heck A.J., Beijnen J.H., Schellens J.H. Structure elucidation of aplidine metabolites formed in vitro by human liver microsomes using triple quadrupole mass spectrometry. J. Mass Spectrom. 2005;40:821–831. doi: 10.1002/jms.863. [DOI] [PubMed] [Google Scholar]
- 31.Brandon E.F., Sparidans R.W., van Ooijen R.D., Meijerman I., Lazaro L.L., Manzanares I., Beijnen J.H., Schellens J.H. In vitro characterization of the human biotransformation pathways of aplidine, a novel marine anti-cancer drug. Investig. New Drugs. 2007;25:9–19. doi: 10.1007/s10637-006-7589-7. [DOI] [PubMed] [Google Scholar]
- 32.Le Tourneau C., Faivre S., Ciruelos E., Dominguez M.J., Lopez-Martin J.A., Izquierdo M.A., Jimeno J., Raymond E. Reports of clinical benefit of plitidepsin (Aplidine), a new marine-derived anticancer agent, in patients with advanced medullary thyroid carcinoma. Am. J. Clin. Oncol. 2010;33:132–136. doi: 10.1097/COC.0b013e318199fb6e. [DOI] [PubMed] [Google Scholar]
- 33.Nuijen B., Bouma M., Henrar R.E., Manada C., Bult A., Beijnen J.H. Compatibility and stability of aplidine, a novel marine-derived depsipeptide antitumor agent, in infusion devices, and its hemolytic and precipitation potential upon i.v. administration. Anti-Cancer Drugs. 1999;10:879–887. doi: 10.1097/00001813-199911000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Song M., Lee D., Kim S., Bae J.S., Lee J., Gong Y.D., Lee T., Lee S. Identification of metabolites of N-(5-benzoyl-2-(4-(2-methoxyphenyl)piperazin-1-yl)thiazol-4-yl)pivalamide including CYP3A4-mediated C-demethylation in human liver microsomes with high-resolution/high-accuracy tandem mass. Drug Metab. Dispos. 2014;42:1252–1260. doi: 10.1124/dmd.114.057570. [DOI] [PubMed] [Google Scholar]
- 35.Yoon S.B., Lee C.H., Kim H.Y., Jeong D., Jeon M.K., Cho S.A., Kim K., Lee T., Yang J.Y., Gong Y.D., et al. A novel sphingosylphosphorylcholine and sphingosine-1-phosphate receptor 1 antagonist, KRO-105714, for alleviating atopic dermatitis. J. Inflamm. 2020;17:20. doi: 10.1186/s12950-020-00244-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fang Z.Z., Krausz K.W., Li F., Cheng J., Tanaka N., Gonzalez F.J. Metabolic map and bioactivation of the anti-tumour drug noscapine. Br. J. Pharmacol. 2012;167:1271–1286. doi: 10.1111/j.1476-5381.2012.02067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mehandru S., Markowitz M. Tipranavir: A novel non-peptidic protease inhibitor for the treatment of HIV infection. Expert Opin. Investig. Drugs. 2003;12:1821–1828. doi: 10.1517/13543784.12.11.1821. [DOI] [PubMed] [Google Scholar]
- 38.Li F., Wang L., Guo G.L., Ma X. Metabolism-mediated drug interactions associated with ritonavir-boosted tipranavir in mice. Drug Metab. Dispos. 2010;38:871–878. doi: 10.1124/dmd.109.030817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prakash C., Wang W., O’Connell T., Johnson K.A. CYP2C8- and CYP3A-mediated C-demethylation of (3-[(4-tert-butylbenzyl)-(pyridine-3-sulfonyl)-amino]-methyl-phenoxy)-acetic acid (CP-533,536), an EP2 receptor-selective prostaglandin E2 agonist: Characterization of metabolites by high-resolution liquid chromatography-tandem mass spectrometry and liquid chromatography/mass spectrometry-nuclear magnetic resonance. Drug Metab. Dispos. 2008;36:2093–2103. doi: 10.1124/dmd.108.022897. [DOI] [PubMed] [Google Scholar]
- 40.Umehara K., Shimokawa Y., Koga T., Ohtani T., Miyamoto G. Oxidative one-carbon cleavage of the octyl side chain of olanexidine, a novel antimicrobial agent, in dog liver microsomes. Xenobiotica. 2004;34:61–71. doi: 10.1080/00498250310001646335. [DOI] [PubMed] [Google Scholar]
- 41.Hu Y., Xiao Y., Rao Z., Kumar V., Liu H., Lu C. Carbon-carbon Bond Cleavage Catalyzed by Human Cytochrome P450 Enzymes: Alpha-ketol as the Key Intermediate Metabolite in Sequential Metabolism of Olanexidine. Drug Metab. Lett. 2021;14:41–53. doi: 10.2174/1872312813666191125095818. [DOI] [PubMed] [Google Scholar]
- 42.Turpeinen M., Hofmann U., Klein K., Murdter T., Schwab M., Zanger U.M. A predominate role of CYP1A2 for the metabolism of nabumetone to the active metabolite, 6-methoxy-2-naphthylacetic acid, in human liver microsomes. Drug Metab. Dispos. 2009;37:1017–1024. doi: 10.1124/dmd.108.025700. [DOI] [PubMed] [Google Scholar]
- 43.Nobilis M., Mikusek J., Szotakova B., Jirasko R., Holcapek M., Chamseddin C., Jira T., Kucera R., Kunes J., Pour M. Analytical power of LLE-HPLC-PDA-MS/MS in drug metabolism studies: Identification of new nabumetone metabolites. J. Pharm. Biomed. Anal. 2013;80:164–172. doi: 10.1016/j.jpba.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 44.Varfaj F., Zulkifli S.N., Park H.G., Challinor V.L., De Voss J.J., Ortiz de Montellano P.R. Carbon-carbon bond cleavage in activation of the prodrug nabumetone. Drug Metab. Dispos. 2014;42:828–838. doi: 10.1124/dmd.114.056903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsumoto K., Hasegawa T., Ohara K., Takei C., Kamei T., Koyanagi J., Takahashi T., Akimoto M. A metabolic pathway for the prodrug nabumetone to the pharmacologically active metabolite, 6-methoxy-2-naphthylacetic acid (6-MNA) by non-cytochrome P450 enzymes. Xenobiotica. 2020;50:783–792. doi: 10.1080/00498254.2019.1704097. [DOI] [PubMed] [Google Scholar]
- 46.Qin X., Wang Y., Ye Q., Hakenjos J.M., Wang J., Teng M., Guo L., Tan Z., Young D.W., Mackenzie K.R., et al. CYP3A Mediates an Unusual C(sp2)-C(sp3) Bond Cleavage via Ipso-Addition of Oxygen in Drug Metabolism. Angew. Chem. Int. Ed. Engl. 2024;136:e202405197. doi: 10.1002/ange.202405197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rotzinger S., Baker G.B. Human CYP3A4 and the metabolism of nefazodone and hydroxynefazodone by human liver microsomes and heterologously expressed enzymes. Eur. Neuropsychopharmacol. 2002;12:91–100. doi: 10.1016/S0924-977X(02)00005-6. [DOI] [PubMed] [Google Scholar]
- 48.Kagan M., Dain J., Peng L., Reynolds C. Metabolism and pharmacokinetics of indacaterol in humans. Drug Metab. Dispos. 2012;40:1712–1722. doi: 10.1124/dmd.112.046151. [DOI] [PubMed] [Google Scholar]
- 49.Chen H., Shockcor J., Chen W., Espina R., Gan L.S., Mutlib A.E. Delineating novel metabolic pathways of DPC 963, a non-nucleoside reverse transcriptase inhibitor, in rats. Characterization of glutathione conjugates of postulated oxirene and benzoquinone imine intermediates by LC/MS and LC/NMR. Chem. Res. Toxicol. 2002;15:388–399. doi: 10.1021/tx010153f. [DOI] [PubMed] [Google Scholar]
- 50.Chen H., Chen W., Gan L.S., Mutlib A.E. Metabolism of (S)-5,6-difluoro-4-cyclopropylethynyl-4-trifluoromethyl-3, 4-dihydro-2(1H)-quinazolinone, a non-nucleoside reverse transcriptase inhibitor, in human liver microsomes. Metabolic activation and enzyme kinetics. Drug Metab. Dispos. 2003;31:122–132. doi: 10.1124/dmd.31.1.122. [DOI] [PubMed] [Google Scholar]
- 51.Yin W., Mitra K., Stearns R.A., Baillie T.A., Kumar S. Conversion of the 2,2,6,6-tetramethylpiperidine moiety to a 2,2-dimethylpyrrolidine by cytochrome P450: Evidence for a mechanism involving nitroxide radicals and heme iron. Biochemistry. 2004;43:5455–5466. doi: 10.1021/bi035944q. [DOI] [PubMed] [Google Scholar]
- 52.Chen Y., Skiles G.L., Shou M., Hickman D., Hsieh F. A novel biotransformation of alkyl aminopyrrolidine to aminopiperidine ring by human CYP3A. Drug Metab. Dispos. 2011;39:1668–1673. doi: 10.1124/dmd.111.039750. [DOI] [PubMed] [Google Scholar]
- 53.Yin W., Doss G.A., Stearns R.A., Chaudhary A.G., Hop C.E., Franklin R.B., Kumar S. A novel P450-catalyzed transformation of the 2,2,6,6-tetramethyl piperidine moiety to a 2,2-dimethyl pyrrolidine in human liver microsomes: Characterization by high resolution quadrupole-time-of-flight mass spectrometry and 1H-NMR. Drug Metab. Dispos. 2003;31:215–223. doi: 10.1124/dmd.31.2.215. [DOI] [PubMed] [Google Scholar]
- 54.Huskey S.E., Zhu C.Q., Fredenhagen A., Kuhnol J., Luneau A., Jian Z., Yang Z., Miao Z., Yang F., Jain J.P., et al. KAE609 (Cipargamin), a New Spiroindolone Agent for the Treatment of Malaria: Evaluation of the Absorption, Distribution, Metabolism, and Excretion of a Single Oral 300-mg Dose of [14C]KAE609 in Healthy Male Subjects. Drug Metab. Dispos. 2016;44:672–682. doi: 10.1124/dmd.115.069187. [DOI] [PubMed] [Google Scholar]
- 55.Huskey S.E., Zhu C.Q., Lin M.M., Forseth R.R., Gu H., Simon O., Eggimann F.K., Kittelmann M., Luneau A., Vargas A., et al. Identification of Three Novel Ring Expansion Metabolites of KAE609, a New Spiroindolone Agent for the Treatment of Malaria, in Rats, Dogs, and Humans. Drug Metab. Dispos. 2016;44:653–664. doi: 10.1124/dmd.115.069112. [DOI] [PubMed] [Google Scholar]
- 56.van Andel L., Rosing H., Tibben M.M., Lucas L., Lubomirov R., Aviles P., Francesch A., Fudio S., Gebretensae A., Hillebrand M.J.X., et al. Metabolite profiling of the novel anti-cancer agent, plitidepsin, in urine and faeces in cancer patients after administration ofC-plitidepsin. Cancer Chemother. Pharmacol. 2018;82:441–455. doi: 10.1007/s00280-018-3637-1. [DOI] [PubMed] [Google Scholar]
- 57.Mahmoudian M., Rahimi-Moghaddam P. The anti-cancer activity of noscapine: A review. Recent Pat. Anti-Cancer Drug Discov. 2009;4:92–97. doi: 10.2174/157489209787002524. [DOI] [PubMed] [Google Scholar]
- 58.Porter R., Parry E.M., Parry J.M. Morphological transformation of an established Syrian hamster dermal cell with the anti-tussive agent noscapine. Mutagenesis. 1992;7:205–209. doi: 10.1093/mutage/7.3.205. [DOI] [PubMed] [Google Scholar]
- 59.Schuler M., Muehlbauer P., Guzzie P., Eastmond D.A. Noscapine hydrochloride disrupts the mitotic spindle in mammalian cells and induces aneuploidy as well as polyploidy in cultured human lymphocytes. Mutagenesis. 1999;14:51–56. doi: 10.1093/mutage/14.1.51. [DOI] [PubMed] [Google Scholar]
- 60.Aneja R., Katyal A., Chandra R. Modulatory influence of noscapine on the ethanol-altered hepatic biotransformation system enzymes, glutathione content and lipid peroxidation in vivo in rats. Eur. J. Drug Metab. Pharmacokinet. 2004;29:157–162. doi: 10.1007/BF03190592. [DOI] [PubMed] [Google Scholar]
- 61.Fang Z.Z., Zhang Y.Y., Ge G.B., Huo H., Liang S.C., Yang L. Time-dependent inhibition (TDI) of CYP3A4 and CYP2C9 by noscapine potentially explains clinical noscapine-warfarin interaction. Br. J. Clin. Pharmacol. 2010;69:193–199. doi: 10.1111/j.1365-2125.2009.03572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsunoda N., Yoshimura H. Metabolic fate of noscapine. II. Isolation and identification of novel metabolites produced by C-C bond cleavage. Xenobiotica. 1979;9:181–187. doi: 10.3109/00498257909038719. [DOI] [PubMed] [Google Scholar]
- 63.Tsunoda N., Yoshimura H. Metabolic fate of noscapine. III. Further studies on identification and determination of the metabolites. Xenobiotica. 1981;11:23–32. doi: 10.3109/00498258109045268. [DOI] [PubMed] [Google Scholar]
- 64.Li F., Gonzalez F.J., Ma X. LC–MS-based metabolomics in profiling of drug metabolism and bioactivation. Acta Pharm. Sin. B. 2012;2:118–125. doi: 10.1016/j.apsb.2012.02.010. [DOI] [Google Scholar]
- 65.Paralkar V.M., Borovecki F., Ke H.Z., Cameron K.O., Lefker B., Grasser W.A., Owen T.A., Li M., DaSilva-Jardine P., Zhou M., et al. An EP2 receptor-selective prostaglandin E2 agonist induces bone healing. Proc. Natl. Acad. Sci. USA. 2003;100:6736–6740. doi: 10.1073/pnas.1037343100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hagi A., Iwata K., Nii T., Nakata H., Tsubotani Y., Inoue Y. Bactericidal Effects and Mechanism of Action of Olanexidine Gluconate, a New Antiseptic. Antimicrob. Agents Chemother. 2015;59:4551–4559. doi: 10.1128/AAC.05048-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Umehara K., Kudo S., Hirao Y., Morita S., Uchida M., Odomi M., Miyamoto G. Oxidative cleavage of the octyl side chain of 1-(3,4-dichlorobenzyl)-5-octylbiguanide (OPB-2045) in rat and dog liver preparations. Drug Metab. Dispos. 2000;28:887–894. [PubMed] [Google Scholar]
- 68.Davies N.M., McLachlan A.J. Properties and features of nabumetone. Drugs. 2000;59:25–33. doi: 10.2165/00003495-200059991-00004. [DOI] [PubMed] [Google Scholar]
- 69.Lee C.A., Neul D., Clouser-Roche A., Dalvie D., Wester M.R., Jiang Y., Jones J.P., III, Freiwald S., Zientek M., Totah R.A. Identification of novel substrates for human cytochrome P450 2J2. Drug Metab. Dispos. 2010;38:347–356. doi: 10.1124/dmd.109.030270. [DOI] [PubMed] [Google Scholar]
- 70.Vaynrub A., Healey J.H., Tap W., Vaynrub M. Pexidartinib in the Management of Advanced Tenosynovial Giant Cell Tumor: Focus on Patient Selection and Special Considerations. OncoTargets Ther. 2022;15:53–66. doi: 10.2147/OTT.S345878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lewis J.H., Gelderblom H., van de Sande M., Stacchiotti S., Healey J.H., Tap W.D., Wagner A.J., Pousa A.L., Druta M., Lin C.C., et al. Pexidartinib Long-Term Hepatic Safety Profile in Patients with Tenosynovial Giant Cell Tumors. Oncologist. 2021;26:e863–e873. doi: 10.1002/onco.13629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li F., Qin X., Wang Y., Mackenzie K., Ye Q., Hakenjos J.M., Wang J., Teng M., Guo L., Tan Z., et al. Metabolomics reveals a unique CYP3A-mediated C(sp3)-C(sp2) bond cleavage via ipso-addition reaction in drug metabolism. ChemRxiv. 2023 doi: 10.26434/chemrxiv-2023-mjsz8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Choi S. Nefazodone (Serzone) withdrawn because of hepatotoxicity. CMAJ. 2003;169:1187. [PMC free article] [PubMed] [Google Scholar]
- 74.Fogelman S.M., Schmider J., Venkatakrishnan K., von Moltke L.L., Harmatz J.S., Shader R.I., Greenblatt D.J. O- and N-demethylation of venlafaxine in vitro by human liver microsomes and by microsomes from cDNA-transfected cells: Effect of metabolic inhibitors and SSRI antidepressants. Neuropsychopharmacology. 1999;20:480–490. doi: 10.1016/S0893-133X(98)00113-4. [DOI] [PubMed] [Google Scholar]
- 75.Greene D.S., Barbhaiya R.H. Clinical pharmacokinetics of nefazodone. Clin. Pharmacokinet. 1997;33:260–275. doi: 10.2165/00003088-199733040-00002. [DOI] [PubMed] [Google Scholar]
- 76.Barbhaiya R.H., Shukla U.A., Kroboth P.D., Greene D.S. Coadministration of nefazodone and benzodiazepines: II. A pharmacokinetic interaction study with triazolam. J. Clin. Psychopharmacol. 1995;15:320–326. doi: 10.1097/00004714-199510000-00003. [DOI] [PubMed] [Google Scholar]
- 77.Cazzola M., Bardaro F., Stirpe E. The role of indacaterol for chronic obstructive pulmonary disease (COPD) J. Thorac. Dis. 2013;5:559–566. doi: 10.3978/j.issn.2072-1439.2013.07.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Corbett J.W., Ko S.S., Rodgers J.D., Jeffrey S., Bacheler L.T., Klabe R.M., Diamond S., Lai C.M., Rabel S.R., Saye J.A., et al. Expanded-spectrum nonnucleoside reverse transcriptase inhibitors inhibit clinically relevant mutant variants of human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 1999;43:2893–2897. doi: 10.1128/AAC.43.12.2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kent U.M., Lin H.L., Mills D.E., Regal K.A., Hollenberg P.F. Identification of 17-alpha-ethynylestradiol-modified active site peptides and glutathione conjugates formed during metabolism and inactivation of P450s 2B1 and 2B6. Chem. Res. Toxicol. 2006;19:279–287. doi: 10.1021/tx050256o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lin H.L., Kent U.M., Hollenberg P.F. Mechanism-based inactivation of cytochrome P450 3A4 by 17 alpha-ethynylestradiol: Evidence for heme destruction and covalent binding to protein. J. Pharmacol. Exp. Ther. 2002;301:160–167. doi: 10.1124/jpet.301.1.160. [DOI] [PubMed] [Google Scholar]
- 81.Lin H.L., Hollenberg P.F. The inactivation of cytochrome P450 3A5 by 17alpha-ethynylestradiol is cytochrome b5-dependent: Metabolic activation of the ethynyl moiety leads to the formation of glutathione conjugates, a heme adduct, and covalent binding to the apoprotein. J. Pharmacol. Exp. Ther. 2007;321:276–287. doi: 10.1124/jpet.106.117861. [DOI] [PubMed] [Google Scholar]
- 82.White N.J., Pukrittayakamee S., Phyo A.P., Rueangweerayut R., Nosten F., Jittamala P., Jeeyapant A., Jain J.P., Lefevre G., Li R., et al. Spiroindolone KAE609 for falciparum and vivax malaria. N. Engl. J. Med. 2014;371:403–410. doi: 10.1056/NEJMoa1315860. [DOI] [PMC free article] [PubMed] [Google Scholar]