Abstract
Microbial exopolysaccharides (EPSs) are receiving growing interest today, owing to their diversity in chemical structure and source, multiple functions, and immense potential applications in many food and non-food industries. Their health-promoting benefits for humans deserve particular attention because of their various biological activities and physiological functions. The aim of this paper is to provide a comprehensive review of microbial EPSs, covering (1) their chemical and biochemical diversity, including composition, biosynthesis, and bacterial sources belonging mainly to lactic acid bacteria (LAB) or probiotics; (2) their technological and analytical aspects, especially their production mode and characterization; (3) their biological and physiological aspects based on their activities and functions; and (4) their current and future uses in medical and pharmaceutical fields, particularly for their prebiotic, anticancer, and immunobiotic properties, as well as their applications in other industrial and agricultural sectors.
Keywords: exopolysaccharides, probiotics, lactic acid bacteria, immunobiotics, pharmaceutical applications
1. Introduction
Microbial exopolysaccharides (EPSs) are extracellular carbohydrate-based biopolymers produced by various kinds of microorganisms such as bacteria, fungi, yeasts, and microalgae [1,2,3,4]. Bacterial EPSs are synthesized through different pathways and then secreted, either in bound form around the cell surface (capsular EPSs) or in free form into the medium of cell growth (slime EPSs). Once released in the surrounding medium, EPSs associate with other biomolecules like proteins, lipids, and uronic acid derivatives to form an extracellular matrix [5]. Consequently, the term EPS has been used to characterize polysaccharides that represent approximately 40 to 95% of the extracellular polymeric substances secreted by microorganism into the surrounding environment [6]. According to their monosaccharide content, EPSs are mainly classified as homopolysaccharides (HoPSs), that is, polysaccharides with one kind of sugar unit (e.g., glucose or fructose), or heteropolysaccharides (HePSs), those containing different types of monomer residues (e.g., D-glucose, D-galactose, and L-rhamnose), and may include non-carbohydrate groups (e.g., phosphate, acetyl, or amine groups). EPSs also vary in molecular mass (~0.5–2.0 × 106 mol/g), size, sugar linkage pattern, branching degree, and neutral or ionic functional groups [7].
Owing to the diversity in chemical structure, various microbial sources, and multiple properties and functions, as well as the environmental compatibility and non-toxicity, EPSs find a wide range of applications in many areas of food, including dairy products and beverages [7,8,9], and non-food areas such as cosmetics, textiles, environmental, agricultural, medical, and pharmaceutical industries [10,11,12].
Among EPS-producing microorganisms that have recently received special attention from scientific and industrial communities are LABs [7,8,12]. In fact, more than 40 species of LAB, i.e., microorganisms being generally recognized as safe (GRAS), or having the Qualified Presumption of Safety (QPS), have been reported to produce a wide range of EPSs without health risk [7]. While currently used for their hydrocolloid techno-functionalities as thickeners, stabilizers, emulsifiers, and gelling and water-binding agents in fermented food (e.g., yoghurt and cheese), and pharmaceutical product formulations (e.g., excipients, drug delivery agents), microbial EPSs have also been reported to provide numerous health-promoting benefits for humans, owing to their various biological activities and physiological functions [5,10,11,12]. Antioxidant, antihypertension, hypocholesterolemic, antiviral, antitumor, and immunomodulating activities are some examples currently reported in the literature [7,10,12]. More emergent and relevant functions of EPSs from LABs are their potential use as immunobiotic adjuvants and smart drug delivery systems in vaccine preparation and theranostic design, respectively, which should deserve much more particular attention now and in the future [11,13].
The goal of the present paper is to review the diversity of microbial EPSs regarding (1) chemical and biochemical aspects, including composition, biosynthesis, and bacterial sources belonging mainly to LABs or probiotics; (2) technological and analytical aspects, especially their production mode and characterization; (3) biological and physiological aspects based on their activities and functions; and (4) the present and future medical and pharmaceutical applications in connection to their prebiotic, antioxidant, anticancer, and immunomodulation activities against intestinal infections, by particularly emphasizing their role as immunobiotics.
2. Microbial EPS
2.1. Chemical Structure, Composition, and Types of EPS
EPS composition and chemical structure have been widely investigated by different analytical techniques in terms of monosaccharide units, molecular mass and size, linkage between monomers (glycoside bounds), functional groups (ionic or non-ionic EPSs), branching structure, and microstructure [14,15,16]. Table 1 summarizes different variants and classification criteria in the microbial EPS molecular structure, with some examples of producing microorganisms.
Table 1.
Classification and examples of microbial EPS.
| Monomers | Substituents | Linkage | Branching | Charge | HoPS | HePS | |
|---|---|---|---|---|---|---|---|
| Bacterial EPS | |||||||
| LAB EPS | |||||||
| Glc | β(1→3) | Linear | Neutral | β-D-glucan | |||
| α(1→3) | Linear | Mutan | |||||
| α(1→3) | Linear | Neutran | |||||
| α(1→6); α(1→4) | Branched | Reuteran | |||||
| α(1→6); α(1→3) | Branched | Dextran | |||||
| α(1→6); α(1→3) | Linear | Alternan | |||||
| Fru | β(2→1) | Linear | Fructan | ||||
| Gal | Linear | Polygalactan | |||||
| Fru | β(2→6); β(2→1) | Branched | Levan | ||||
| Glc; Gal | Branched | Kefiran | |||||
| Non-LAB EPS | |||||||
| Glc | Neutral | Curdulan | |||||
| Glc; Man; GlcA | Ace; Pyr | β(1→4) | Branched | Anionic | Xanthan | ||
| GulA; ManA | Ace | Branched | Alginate | ||||
| Glc; Rha; GlcA | Ace; Gly | Linear | Anionic | Gellan | |||
| GlcN | β(1→4); β(1→3) | Linear | Anionic | Hyaluronic acid | |||
| Man | Neutral | Xylinan | |||||
| Glc | β(1→4) | Branched | Neutral | Cellulose | |||
| Fungi EPS | |||||||
| Glc | α(1→4); α(1→6) | Linear | Neutral | Pullulan | |||
| Amino sugar | Ace; Gal-N, R-COOH | Anionic | Emulsan | ||||
Glucose (Glc); fructose (Fru); galactose (Gal); mannose (Man); glucuronic acid (GlcA); guluronic acid (GulA); mannuronic acid (ManA); rhamnose (Rha); glucosamine (GlcN); acetate (Ace); pyruvate (Pyr); glycerate (Gly); galactosamine (Gal-N).
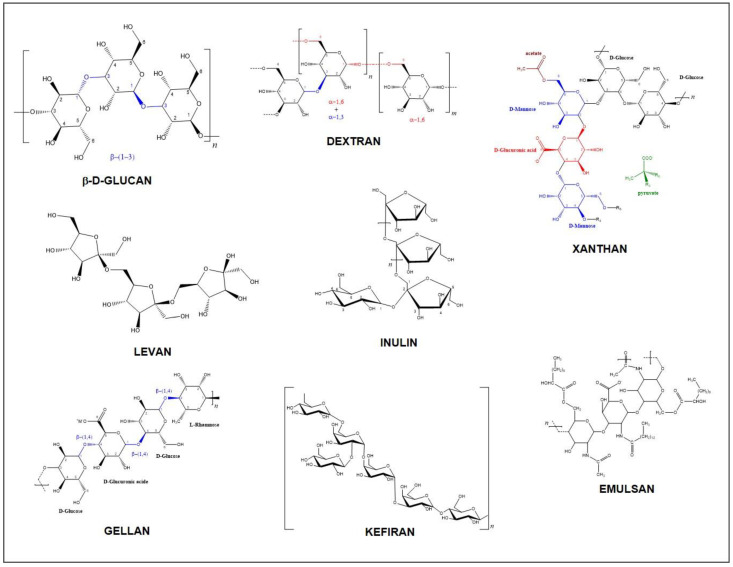
Their classification and name are attributed as a function of these chemical structure criteria. Based on the EPS primary structure, i.e., the monosaccharide composition, it is possible first to distinguish HoPSs from HePSs, that is, EPSs having only one or more type of sugar units. Second, the linkages between units and the position of the carbon involved in the bond C1, C3-α/β, C1, C4- α/β, or C1, C6- α/β, named glycosidic linkages, allow the sub-classification of EPSs. Third, the neutral or ionic nature of functional groups and the degree of chain branching are other sub-criteria of EPS categories within HoPSs and HePSs. Furthermore, uncommon variants such as the oligomeric repeating with the same units (e.g., pentameric of galactose), with three to eight monosaccharide derivative different units (HePSs), or with an approximately equal proportion of glucose and galactose subunits (e.g., kefiran), are among the structural criteria of bacterial EPSs [17]. Some examples of EPS chemical structure are shown in Figure 1.
Figure 1.
EPS chemical structure examples.
2.2. Biosynthesis and Producing Microorganisms
2.2.1. Biosynthesis of EPS
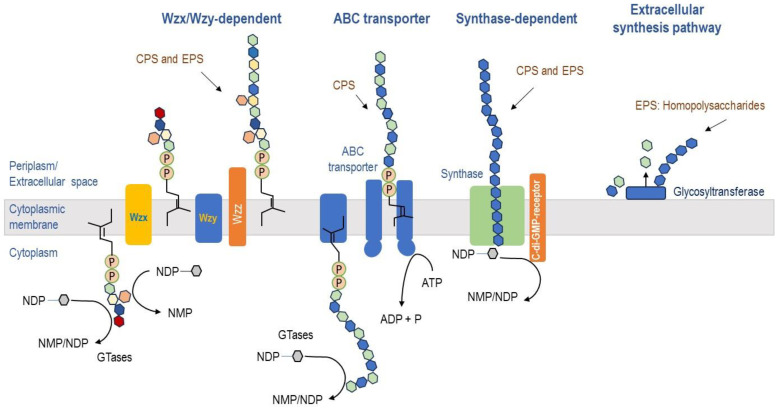
Despite the wide variety of EPS chemical structures, microorganisms produce EPSs via four different pathways: the Wzx/Wzy-dependent pathway, the ATP-binding cassette (ABC) transporter pathway, the synthase-dependent pathway, and extracellular synthesis using a single glycosyltransferase (Figure 2). In the same species of microorganisms, two or more pathways can coexist to produce different macromolecules [18].
Figure 2.
Biosynthesis of polysaccharides in microorganisms. Wzx/Wzy-dependent pathway: Responsible for synthesizing lipopolysaccharide O-antigen polysaccharides in Gram-negative bacteria, as well as capsular polysaccharides (CPSs) and EPSs in both Gram-negative and Gram-positive bacteria. ATP-binding ABC transporter pathway: Facilitates the synthesis of CPS specifically in Gram-negative bacteria. Synthase-dependent pathway: Involved in the synthesis of both CPSs and EPSs in Gram-negative and Gram-positive bacteria. Extracellular synthesis via a single glycosyltransferase: Responsible for the synthesis of EPSs that fall under the category of HoPS.
HoPSs are biosynthesized by specific glycosyltransferase or fructosyltransferase through two reactions [19].These are the sucrose hydrolysis and the transfer glucosyl (or fructosyl) to the glucan (fructan) polymer chain or oligosaccharide synthesis before creating the final EPS. While the glycosyltransferase catalyzes the biosynthesis of glucans (e.g., dextran, mutan, alternane, or reuteran), the fructosyltransferase synthesizes fructans (e.g., levan and inulin) [20].
The so-called Wzx/Wzy-dependent pathway relates to Gram-positive bacteria [18]. Here, individual repeating units are linked to an undecaprenol diphosphate anchor (C55) at the inner membrane, which is assembled by several glycosyltransferases and translocated across the cytoplasmic membrane by flippase, a Wzx protein. In the next step, their polymerization by the Wzy protein occurs in the periplasmic space before they are released to the cell surface. The transport of polymerized repeat units to the cell surface depends on the polysaccharide co-polymerase (PCP) and the outer membrane polysaccharide export (Outer Polysaccharide Export (OPX) or Outer Membrane Auxiliary (OMA)) families. All polysaccharides synthesized by the Wzx/Wzy path display diverse monosaccharide composition, including in their chemical structure up to four or five sugars. They are therefore classified as HePSs (e.g., xanthan) [16].
The ABC transporter pathway mainly occurs in Gram-negative bacteria for CPS production [18]. Such polysaccharides do not really represent EPSs since they are still attached to the cell surface. CPS, synthesized through ABC transport-dependent pathway, is assembled by a single glycosyltransferase on the cytoplasmic surface of the inner membrane. This process yields HoPS or HePS when multiple glycosyltransferases are involved for the assembly process [16]. The synthesized polysaccharides are exported through an efflux pump complex, which includes ABC transporters that span the inner membrane, periplasmatic proteins of the PCP and OPX families, and the outer membrane [18].
The synthase-dependent pathway is involved in the synthesis of both CPS and EPS. This pathway utilizes a single synthase complex to perform both polymerization and transport, secreting complete polymer chains directly onto the membrane and cell walls [18]. In the absence of membrane anchors, a receptor protein for signaling molecules, such as bis-(3′-5′)-cyclic dimeric guanosine monophosphate (c-di-GMP), may help initiate polysaccharide assembly. In Gram-negative bacteria, polysaccharides are often transported across the outer membrane [21]. The synthase-dependent pathway is commonly used to assemble HoPSs that require only one type of sugar precursor, as seen in biosynthesis of curdlan, cellulose, alginates, and hyaluronic acid [16].
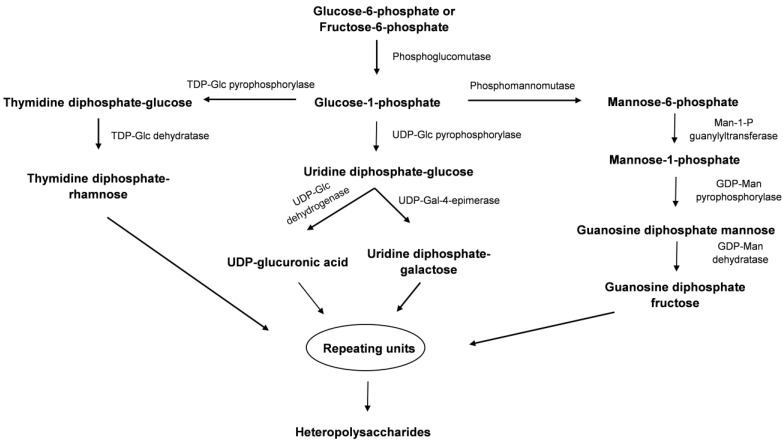
In the biosynthesis of EPSs through the Wzx/Wzy-dependent, ABC transporter-dependent, or synthase-dependent pathways, polysaccharides are synthesized from nucleotide diphosphate sugars [21]. These sugar nucleotides are produced through a multistep process starting with glycolytic intermediates such as glucose-6-phosphate or fructose-6-phosphate.
Initially, glucose-6-phosphate is converted into glucose-1-phosphate (Glc-1-P) by phosphoglucomutase. Glc-1-P is then utilized to form sugar nucleotides like uridine diphosphate-glucose (UDP-Glc) and thymidine diphosphate-glucose (TDP-Glc) through the action of UDP-Glc pyrophosphorylase and TDP-Glc pyrophosphorylase, respectively. These sugar nucleotides further transform into other forms: UDP-Glc is converted to uridine diphosphate-galactose (UDP-Gal) by UDP-Gal-4-epimerase, or to uridine diphosphate-glucuronic acid (UDP-GlcA) by UDP-Glc dehydrogenase. TDP-Glc is converted into thymidine diphosphate-rhamnose (TDP-Rha) via the action of TDP-Glc dehydratase. Additionally, Glc-1-P can be converted into guanosine diphosphatefructose (GDP-Fruc) through a series of three intermediates—mannose-6-phosphate, mannose-1-phosphate, and GDP-mannose—by the sequential action of phosphomannomutase (PMM), Man-1-P guanylyltransferase, GDP-mannose pyrophosphorylase, and GDP-mannose dehydratase (Figure 3). The formation of these sugar nucleotides (TDP-Rha, UDP-Gal, UDP-GlcA, and GDP-Fruc) is crucial for EPS biosynthesis, as they serve as precursors for the repeating units that contribute to the diversity of EPS structures [15].
Figure 3.
Outline of biosynthesis of HePS. The abbreviations are TDP: thymidine diphosphate; UDP: uridine diphosphate; GDP: guanosine diphosphate; Man-1-P: mannose-1-phosphate; Glc: glucose; Gal: galactose; Man: mannose.
2.2.2. Producing Microorganisms
EPSs can be generated by a diverse range of microorganisms. These compounds are found outside the cell wall, where they may either adhere to cells, forming capsules, or be secreted into the extracellular environment, forming a slime layer [22]. EPSs are produced by various genera of archaea, bacteria, fungi, and algae, with these microorganisms predominantly falling into mesophilic, thermophilic, and halophilic categories [23]. Among these, bacteria, fungi, yeasts, and microalgae are the most common producers [24], as listed in Table 2.
Table 2.
Microbial EPS producers: bacteria, fungi, and microalgae.
| Microorganism | Polymer | Sugar Monomers | Non-Sugar Residues | Mw (Da) | Reference |
|---|---|---|---|---|---|
| BACTERIA | |||||
| Agrobacterium sp. | Curdlan | Glc | --- | 5.3 × 104–2.0 × 106 | [25] |
| Azotobacter vinelandii; Pseudomonas aeruginosa | Alginate | GulA, ManA | Ace | (0.3–1.3) × 106 | [26,27,28] |
| Bacillus subtilis; Halomonas sp.; Zymomonas sp. | Levan | Fru | --- | 2 × 106 | [29,30] |
| Enterobacter A47 | FucoPol | Fuc, Gal, Glc, GlcA | Ace, Pyr, Succ | (1.7–5.8) × 106 | [31] |
| Klebsiella pneumoniae | Fucogel | GalA, Fuc, Gal | Ace | 4 × 104 | [29] |
| Acetobacter sp.; Glucanoacetobacter sp.; Rhizobium sp.; Sarcina sp. | Bacterial cellulose | Glc | --- | ~106 | [29,32,33] |
| Lactobacillus sp.; Leuconostoc sp.; Streptococcus sp. | Dextran | Glc | --- | 103–107 | [34] |
| Pseudomonas oleovorans | GalactoPol | Gal, Glc, Man, Rha | Ace, Pyr, Succ | (1.0–5.0) × 106 | [35,36] |
| Sphingomonas paucimobilis | Gellan | Glc, Rha, GlcA | Gly, Ace | 5.2 × 105 | [37] |
| Streptococcus zooepidemicus | Hyaluronic acid | GlcNAc, GlcA | --- | (2.0–3.0) × 103 | [38] |
| Xanthomonas sp. | Xanthan | Glc, Man, GlcA | Pyr, Ace | 2.0 × 106–5.0 × 107 | [27] |
| FUNGI | |||||
| Tuber borchii | --- | Glc | --- | 92 × 103 | [39] |
| Colletotrichum alatae LCS1 | --- | Man, Gal, Rha, Ara, Glc, Fuc | --- | --- | [40] |
| Aureobasidium pullulans | Pullulan | Glc | --- | 4.8 × 104–2.2 × 106 | [2,41] |
| Penicillium janthinellum N29 | --- | Gal, Man | --- | 10.24 × 103 | [42] |
| Monascus purpureus | --- | Fuc, Gal, Glc, Man, GalA, GlcA | --- | 3.2 × 105 | [43] |
| Penicillium citrinum | --- | Ara, Gal, Glc, Man, GalA, GlcA | --- | 1.58 × 105 | |
| Aspergillus versicolor | --- | Ara, Gal, Glc, Man, Xyl, GalA, GlcA | --- | 1.14 × 105 | |
| Fusarium merismoides A6 | --- | Man, Glc, Gal, Rib | --- | (5.14–6.50) × 104 | [44] |
| Ganoderma lucidum | --- | Gal, Man, Glc, Ara, Rha | --- | 2.08 × 104 | [45] |
| Sclerotium sp. | Scleroglucan | Glc | --- | 1.3 × 105–6.0 × 106 | [46] |
| Schizophyllum commune 227E.32 | Schizophyllan | Glc | --- | 1.1 × 106 | [47] |
| MICROALGAE | |||||
| Anabaena augstmalis | --- | Glc, Gal, Man, Xyl, Fuc, Rha, Gal-N, GlcN, GalA, GlcA | Sulf | n.a. | [48] |
| Dunaliella tertiolecta | --- | Glc | --- | n.a. | [49] |
| Scenedesmus acuminatus | --- | Gal, GlcN, Man | --- | High (>50 × 103) and low molecular weight (<3 × 103) | [50] |
| Phormidium autumnale | --- | Rha, Rib, Man, Glc, Fuc, Gal, Ara, GalA, GlcA | Sulf | n.a. | [48,51] |
| Porphyridium sordidum | --- | Fuc, Rha, Ara, Gal, Glc, Xyl, GlcA | Sulf | 14 × 105 | [52] |
| Rhodella sp. | --- | Xyl, Gal, Glc, Rha, Ara, GlcA | Sulf | n.a. | [53] |
| Synechocystis aquatilis | --- | Fuc, Glc, Rha, Xyl, Man, GlcN, GalA, GlcA | Sulf | n.a. | [48] |
| CYANOBACTERIA | |||||
| Spirulina platensis | --- | Fru, Rha, Rib, Man, Gal, GalA, Glc, Xyl | Sulf, Ca | [54] | |
| Nostoc sp. | --- | Ara, Glc, Man, Xyl, GlcA | lactyl | 214 × 103 | [55] |
| Anabaena sp. CCC 745 | --- | Glc, Rha, GlcA | 19.57 × 103 30.29 × 103 |
[56] | |
| Nostoc cf. linckia | --- | Glc, Gal, Xyl, Man, GlcA | lactyl | 1.31 × 105 | [57] |
| Gloeocapsa gelatinosa | --- | Glc, Gal, Ara, Fuc, Xyl, Rha, Man, GlcA, GalA | 67.2 × 103 598.3 × 103 |
[58] | |
Fucose (Fuc); arabinose (Ara); xylose (Xyl); ribose (Rib); galacturonic acid (GalA); succinate (Succ); phosphate (Phosp); sulfate (Sulf); n.a (not available).
Bacteria
The ability to produce polysaccharides is common among various bacteria, including species from the Lactobacillus, Streptococcus, Xanthomonas, and Acetobacter families. Bacteria can produce both HoPSs and HePSs.
Dextran, a polymer of D-glucose, is primarily produced by several genera of LAB such as Leuconostoc, Streptococcus, Weisella, Pediococcus, and Lactobacillus [59]. Commercial dextran is biosynthesized by the non-pathogenic bacterium Leuconostoc mesenteroides NRRL B-512 [60]. The Food and Drug Administration (FDA) approves dextran for use in food, cosmetic, and medical applications [59]. Another important glucan is curdlan, which is produced by a variety of bacteria such as Agrobacterium spp., Pseudomonas spp., and Bacillus spp. [61]. Curdlan is a linear, insoluble HoPS composed of 400–500 D-glucose residues linked by β-(1–3)-glucosidic linkages [62]. Water-soluble derivatives of curdlan are utilized in various applications, including as immune recognition sites of dectin-1, and in anti-HIV agents to inhibit Human Immunodeficiency Virus (HIV) infection [63].
Levan is a natural fructan found in various plants and microorganism species. This HoPS is made up of D-fructofuranosyl residues linked together by β -(2–6) bonds. Levan is synthesized outside the cell and can be produced by fermentation of sucrose by bacteria such as Zymomonas mobilis or B. subtilis [62,64]. Alternatively, it can be synthesized enzymatically using levansucrase with sucrose as the substrate. Both Gram-positive bacteria, like Bacillus species, and Gram-negative bacteria, such as Z. mobilis, are known to produce levansucrase [19].
Xanthan is among the most studied HePS. It is synthesized by Xanthomonas campestris. Xanthan features a backbone of glucose with side chains made up of a trisaccharide unit consisting of two mannose residues alternating with glucuronic acid [65]. This EPS typically has repeating units of 2–8 monomers, and exhibits a high molecular weight, ranging from 500 to 2000 kDa, depending on the bacterial genus and species [19]. Xanthan is available in various purity grades for use in food, pharmaceutical, and oil recovery industries [24].
Fungi and Yeasts
EPS production is widely distributed among fungi such as the members of the genera Aureobasidium, Candida, and Cryptococcus. The most studied fungal origin polysaccharides are pullulan, scleroglucan, and yeast-glucans. In addition, mushroom polysaccharides such as lentinan, ganoderan, and schizophyllan also have high biological and medicinal properties, which have been gaining increasing attention in recent decades.
Pullulan is produced through the fermentation of black yeast, such as Aureobasidium pullulans. It is currently utilized in the food and pharmaceutical industries due to its unique properties [66]. Structurally, pullulan is a (α-1,4)→(α-1,6)-glucan composed of maltotriose units. In each maltotriose unit, three glucose molecules are linked by α-1,4 glycosidic bonds, while consecutive maltotriose units are connected by α-1,6 glycosidic bonds. Pullulan plays a significant role in protecting the fungal cell from desiccation, and aids in the transport of molecules within the cell [67].
Scleroglucan, the largest β-glucan, is a high-molecular-weight, nonionic polysaccharide produced by fungi like Botrytis cinerea, Schizophyllum commune, Sclerotium rolfsii, Sclerotium glucanicum, and Epicoccum nigrum. Its structure consists of a (1,3)-β-D-glucopyranosyl backbone with (1,6)-β-D-glucopyranosyl branching residues [68]. Scleroglucan and some derivatives are used in pharmaceutical applications, and in particular for the formulation of modified-release dosage forms [69].
Mushroom polysaccharides include a large group of fungal polysaccharides. Lentinan, a β-(1,3) glucan with β-(1,6)-D-glucose side chains (branching at every third main chain unit), is one of the most important mushroom polysaccharides produced by Lentinus edodes [70]. Another example is schizophyllan, an extracellular β-(1,3), β-(1,6) glucan from the filamentous fungus Schizophyllum commune. Schizophyllan shares a similar structure with lentinan regarding the side chains and the branching frequency. They also exhibit comparable immunomodulatory and anticancer properties [71]. Ganoderan from Ganoderma lucidum, along with grifolan from Grifola frondosa and pleuran from Pleurotus ostreatus, are other β-(1,3), β-(1,6)-branched mushroom glucans known for their immunomodulatory properties [72]. Additionally, Ganoderma lucidum produces a similar immunostimulating endopolysaccharide in submerged cultures [73]. Other medicinal mushroom polysaccharides include krestin (PSK), a HePS proteoglucan from Trametes versicolor [74]. Agaricus blazei and Agaricus brasiliensis produce various EPSs such as glucans and proteoglycans which are safe as functional foods in managing obesity and diabetes [75]. Proteoglycans derived from Cordyceps species (C. militaris and C. sinensis), which contain glucose, galactose, arabinose, and several amino acid residues, have been investigated for their immunomostimulating and hypolipidemic properties [61,76]. Additionally, research is ongoing into the biological activities of other mushroom polysaccharides [77].
Cell-wall β-glucans from S. cerevisiae (Y-BG), commercially known as ZymoSan or Zymocel, feature a main chain of β-(1,3)-glucose with β-(1,6)-glucose branches. This immunomodulating and prophylactic β-glucan (proteoglucan) may also include mannans and amino acids. It has been approved for use in the EU by the European Food Safety Authority (EFSA) and in the USA by the FDA [72]. The commercial yeast β-glucan Betafectin (Poly-[1-6]-D-glucopyranosyl-[1-3]-D-glucopyranose) modulates the gut microbiota, promoting the growth of beneficial probiotic bacteria, which in turn produce immunostimulating agents such as short-chain fatty acids (SCFAs) [78].
Microalgae and Cyanobacteria
EPSs produced by microalgae exhibit complex chemical structures, ranging from HoPSs, which contain glucose or galactose, to HePSs, which include a variety of different sugar monomers. EPSs from microalgae present a high diversity of sugar monomers. The presence of rare sugars (such as fucose, rhamnose, and ribose), along with uronic acids and sulfates, is common in microalgal EPSs, contributing to their unique properties.
Porphyridium sp. is one of the microalgae exploited for commercial EPS production. Common microalgae species known for producing EPS include Arthrospira platensis, Aphanizomenon, Chlorella vulgaris, Dunaliella salina, and Porphyridium cruentum [24]. Spirulina platensis produces sulfated polysaccharides, such as Calcium spinilan, which inhibit tumor invasion and metastasis [79].
EPSs produced by cyanobacteria are primarily composed of high-molecular-weight HePSs. Cyanobacterial EPSs can be classified into two main types: those associated with the cell surface (e.g., slime, sheath, and capsules) and those released into the surrounding medium, referred to as released polysaccharides (RPSs) [80]. It is widely acknowledged that the majority of the cyanobacterial EPSs are composed of CPSs, with very few identified as RPSs [81].
Cyanobacterial EPSs have unique characteristics due to the presence of uronic acids and sulfate groups, which confer a negative charge, classifying them as anionic polysaccharides. Compared to bacterial EPSs, cyanobacterial EPSs exhibit a higher degree of monosaccharide diversity, typically containing six or more different monosaccharides. Glucose, xylose, arabinose, galactose, and fucose are the most commonly monosaccharides constituting cyanobacterial EPSs [82]. Methyl sugars and amino sugars have also been reported in the chemical structure of cyanobacterial EPSs [83]. In addition to sulfate groups, which are unique to archaea and eukaryotes, other possible groups include succinyl, pyruvyl, and methyl residues [84]. Common cyanobacteria species that produce EPSs include Nostoc spp., Anabaena spp., Phormidium spp., and Microcystis spp. [48].
There is growing interest in the large-scale production of cyanobacterial EPSs due to their potential industrial applications such as their use as gums, bio-flocculants, soil conditioners, and biosorbents. Spirulan, Immulan, Nostoflan, and Emulcyan are some examples of commercially available cyanobacterial EPS produced by Arthrospira platensis, Aphanotece halophytica, Nostoc flagelliforme, and Phormidium, respectively [84]. Compared to other Gram-negative bacteria, cyanobacteria possess a more significant, thicker peptidoglycan layer, with a greater degree of crosslinking between polysaccharide chains. These unique features of cyanobacterial cell walls make them particularly effective in removing heavy metal from wastewater and outperforming other Gram-negative bacteria. For instance, significant heavy metal removal has been observed with EPSs from Nostoc muscorum and Cyanothece sp. CCY 0110 [85,86].
2.3. Production and Analytical Characterization
2.3.1. Production
The process of producing microbial EPSs varies, depending on the species and growth conditions. Environmental changes influence microorganisms, thereby affecting enzyme activity (inhibition or stimulation), protein synthesis (induction and repression), and cell shape. For example, bacteria can produce EPSs in concentrations ranging from 0.29 to 100 g/L in a short time (0.5–7 days), compared to fungi, which have longer cultivation durations (2–32 days) [24]. Controlling cultivation factors such as pH, temperature, dissolved oxygen concentration, aeration, and mixing and stirrer speed is crucial for achieving reproductible bioprocess performance [24].
At the lab scale, the cultivation mode is determined by whether EPS production is linked to microbial growth, as with gellan, or occurs independently of growth, as seen with curdlan [87,88]. Most microbial EPS production processes utilize either simple batch cultures or single-pulse fed-batch cultures, typically after the nitrogen source in the medium has been depleted [81]. Other cultivation methods, such as fed batch and continuous culture, have also been proposed. Continuous culture systems are generally more productive; however, there is a greater risk of contamination and they may lead to the development of genetic variants with lower yields [89].
At both lab and industrial scales, the synthesis of EPSs is often conducted in stirred tank bioreactors (STRs) or air lift bioreactors (ALRs) [90,91]. The packed bed bioreactor is another type of fermenter configuration [92]. The advantages of STRs are good mixing properties and volumetric productivities, whereas ALRs have the advantage of reduced energy input with efficient heat transfer [93].
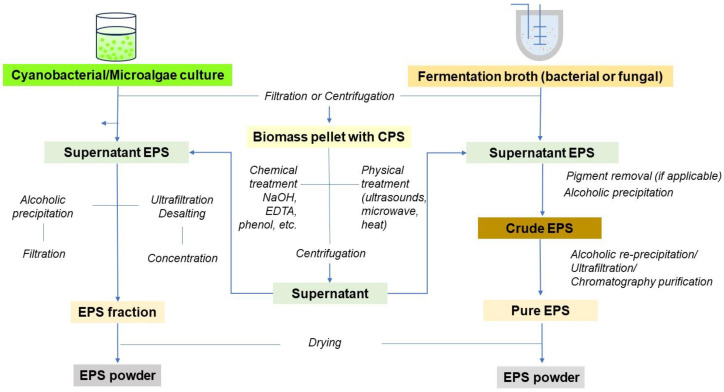
The method chosen for recovering EPSs from the cultivation broth depends on the characteristics of the producing organisms, the type of polysaccharide involved, and the desired level of purity [24]. The downstream processing involves multiple steps: first, cell removal through centrifugation or filtration, followed by the recovery of the polymer from the cell-free supernatant. The polymer is then precipitated by adding a water-miscible non-polar solvent such as acetone, ethanol, or isopropanol. The resulting precipitate can be separated from the solvent–water mixture and dried. Additional procedures such as re-precipitation with diluted aqueous solutions, deproteinization by chemical or enzymatic methods, and membrane processes can be employed to further remove contaminants [24,94,95]. The general scheme of EPS downstream processing is shown in Figure 4.
Figure 4.
Workflow of EPS production.
Notably, microbial EPSs were never able to find a suitable place in the polymer market due to their very high production costs. The cost-effectiveness of manufacturing microbial EPSs is influenced by various factors, including production volume, the final product’s value, and the availability of affordable raw materials. EPSs have been evaluated for numerous applications, with their feasibility often dependent on whether fermentation offers a more cost-effective solution than chemical synthesis. For small-scale or waste-utilizing processes, solid-state fermentation can be economical, though it faces scalability challenges. In contrast, submerged and continuous fermentation methods, while offering higher yields, require more energy and investment. Additionally, the required downstream processing, especially to obtain high-purity EPSs, can be expensive and labor-intensive when conducted concurrently.
To improve the industrial-scale production of microbial biopolymers, it is recommended to optimize fermentation conditions, leverage biotechnological tools like genetic and metabolic engineering, and investigate cost-effective fermentation substrates [96]. Furthermore, using complex media for growth is economically impractical due to the high cost of ingredients such as yeast extract, peptone, and salts, which are needed in large amounts. An alternative is to use agricultural waste as a raw material for EPS production, which can lower manufacturing costs. This method is also environmentally beneficial, as it helps manage waste that would otherwise incur disposal costs and potentially cause environmental issues if sent to landfills or soil. By utilizing agro-industrial wastes as substrates, the process becomes more cost-effective and reduces reliance on nonrenewable resources while minimizing the environmental impact of industrial activities.
2.3.2. Purification and Structural Identification
A series of analytical tools have been used for the purification, structural identification, and quantification of bacterial EPS [5]. In summary, the first step begins by its recovery or extraction from the culture media, generally by precipitation. Once more concentrated substrate is available, the following step is to further purify the EPS-based compounds to precisely determine its composition and chemical structure. A quantitative analysis is also performed to determine the yield of the microorganism in producing EPSs. Further qualitative analyses such as the microstructure and surface property determination may complete its structural characterization. Table 3 summarizes the different techniques frequently used, as well as their functions in the purification and structural characterization of bacterial EPS.
Table 3.
Different techniques frequently used and their functions in qualitative and quantitative EPS analyses.
| Recovery/Purification | Functions | Reference |
|---|---|---|
| Heating—Sonication | Recovery of CPS | [97] |
| Precipitation | ||
| Dialysis | Removing simple carbohydrates | |
| Ion-exchange chromatography | Final purification before quantification | |
| Size Exclusion Chromatography (SEC) | ||
| Preparative Sodium Dodecyl Sulfate—Polyacrylamide Gel (SDS-PAGE) | ||
| Qualitative analysis | ||
| Ultraviolet (UV) spectroscopy | Detection of nucleic acids and proteins | [7] |
| Fourier Transform—Infrared (FT-IR) spectroscopy | Detection of functional group; configuration α or β; fingerprints | [5] |
| Gel permeation chromatography or SEC-Multi-Angle Light Scattering (MALS) | Molecular mass detection | |
| Gas chromatography coupled to mass spectrometry | Monosaccharide composition | |
| High performance Anion exchange chromatography (HPAEC) | Linkage and composition | [5] |
| Near Magnetic Resonance (NMR) spectroscopy | linkage pattern | |
| Confocal laser scanning microscopy | Microstructure analysis | [97] |
| Scanning electron microscopy | ||
| Transmission electron microscopy | ||
| Atomic force microscopy | [7] | |
| Differential scanning calorimetry (DSC) | Structural analysis | |
| Thermogravimetric analysis (TGA) | [5] | |
| X-Ray diffraction (XRD) | ||
| Laser light scattering/electrophoretic analysis | Physico-chemical properties | [98] |
| Quantitative analysis | ||
| Gravimetrics | [97] | |
| Colorimetrics | ||
| SEC-Refractive Index (RI) | ||
| Near Infrared (NIR) spectroscopy |
3. Properties and Functions
3.1. Physiological Functions
In bacterial cells, EPSs play an important role in the formation and maintenance of complex microbial communities, such as flocs and biofilms [99]. They participate in the composition of the biofilm layer surrounding microorganism cells. For instance, glycocalyx is a component of EPSs that is essential for the formation of biofilms. EPSs influence the stability of biofilms by mediating interactions between polysaccharide chains [99]. EPSs also enhance cell adhesion to solid surfaces, including the intestinal mucosa [100].
In addition to its role in adhesion, biofilm formation plays a crucial role in the adaptation of bacteria to various physical and chemical conditions in their environment. Microorganisms produce EPSs in response to both biotic stress (e.g., competition) and abiotic stress factors (e.g., changes temperature, light intensity, pH, and salinity). This production is also a strategy of adaptation to an extreme environment, as seen in acidophilic or thermophilic species. EPSs form a protective polymer layer around microbial cells, particularly in harsh conditions. Extreme environmental conditions have stimulated microorganisms to develop various adaptive strategies to counteract the adverse effects of extreme temperatures, high salt concentrations, high and low pH, and radiation. Among these strategies, EPS biosynthesis is one of the most protective mechanisms [101]. The high-water content of the polysaccharide layer enhances resistance to osmotic stress, while its anionic properties help capture essential minerals and nutrients. EPSs can assist in metal degradation due to their chelating properties [19]. The polysaccharide envelope also regulates the diffusion of molecules between extracellular and intracellular media. This diffusion activity can help some bacteria resist surfactants and antibiotics [102,103].
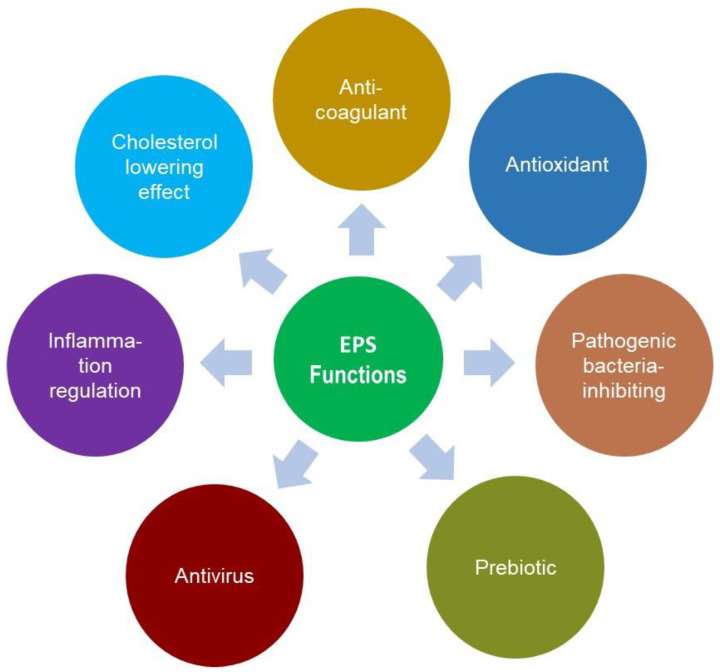
The physiological functions of microbial polysaccharides are extremely diverse and depend on their monosaccharide component and structure. EPSs may contribute to human health (Figure 5) via their prebiotic, anticancer, anti-ulcer, immunomodulatory, or cholesterol-lowering effects [104].
Figure 5.
Physiological functions of EPS.
On the other hand, EPSs represent a category of potential bioactive polymers with health-promoting functions such as anticancer, antioxidant, antimicrobial, antibiofilm, antihypertensive, antiulcer, hypocholesterolemia, and immunomodulatory activities [7,8,10]. Another category of application is the potential use of EPSs as therapeutic agent carriers (drug, probiotic, etc.) and delivery nano- and microsystems [11]. They play roles as both a protectant and a controlled release system, owing to their various physicochemical and functional properties, such as their adhesion and water-binding capacities, and hydrogel-forming properties, while being biodegradable and safe. One promising application of EPSs is their use mainly under derivative forms as antigen-carriers or adjuvant systems in vaccine preparations [105], gene delivery vectors in gene therapy [106,107], and encapsulation agents for improving cell stability and viability, for instance, in the case of alginate beads for encapsulating fibroblast cells [108] and probiotic bacterium strains [109].
EPSs have a positive impact on gut microbiota. The most well-known mechanisms by which EPSs interact with the gut microbiota are linked to their prebiotic effects and their ability to inhibit microbial pathogens, thus helping to regulate the microbiota. Gut microbiota ferment EPSs, producing SCFAs, which lower pH levels and promote the growth and diversity of gut microbial taxa [110,111]. The monosaccharides released from the degradation of EPSs influence the composition of the microbiota through cross-feeding interactions. For instance, EPSs from Bifidobacterium longum E44 and Bifidobacterium animalis subsp. lactis R1 can alter the metabolism of Bacteroides fragilis when it grows in their presence [112]. Additionally, EPSs from Bifidobacterium breve UCC2003 exhibit antagonistic effects by protecting the host against pathogens, indicating a role for EPSs in providing the health benefits typically linked to probiotic strains and in modulating the immune system [113]. Moreover, EPSs can inhibit pathogenic bacteria, promote probiotics (good bacteria), and maintain the balance of intestinal microflora, due to their viscosity and rheological properties [114,115].
EPS exhibit antimicrobial properties with resistance to both Gram-positive and Gram-negative pathogens. EPS-Ca6 produced by Lactobacillus sp. Ca6 indicates significant antibacterial activity against pathogenic bacteria such as Salmonella enterica ATCC 43972 and Micrococcus luteus [116]. EPS-DN1 from L. kefiranofaciens DN1 has an inhibitory effect on Listeria monocytogenes and Salmonella enteritidis. Such inhibition increases with increasing EPS concentration [117]. EPSs can inhibit the growth of pathogens by (a) increasing their competition inhibition against pathogenic bacteria in hosts; (b) combining with signaling molecules related to biofilms or glycocalyx receptors in the pathogen surface that hinder the formation of biofilms; or (c) disrupting membrane integrity and loss of soluble proteins [118].
EPSs are also directly or indirectly related to lowering cholesterol [118]. Those produced by Enterococcus faecium K1 and Lactoplantibacillus plantarum BR2 have been shown to lower the cholesterol level (48.81%) compared to a negative control [119]. Similarly, in in vitro tests, a 45% reduction in cholesterol was achieved by EPS from L. plantarum BR2 [120]. Several hypotheses about the cholesterol-lowering mechanism via EPSs have been proposed based on in vitro and animal experiments. These include bile removal, cholesterol assimilation and conversion, co-precipitation effects (between hydrolyzed bile salts and cholesterol), and the promotion of short-chain fatty acid production to lower cholesterol [121,122].
The strong anticoagulant activity of EPS–sulfate derivatives has been demonstrated [123,124]. Heparin Cofactor II (HC II) is a strong inhibitor of thrombin in blood clotting pathways, and these EPS derivatives may interact with HC II to express anticoagulant activity. The sulfated EPS provides an acidic environment and facilitates the inhibitory effects of HC II on thrombin. The sulfated regions and stereochemistry of EPSs can activate HC II through an allosteric mechanism [118].
Microbial EPSs are also capable of performing very good antioxidant activity. The EPS from Lactobacillus gasseri FR4 shows good free radical activity and hydroxyl and superoxide radical capture activities, depending on EPS concentration [125]. The antioxidant mechanism of EPSs is due to the hydrolysis of these biological molecules when exposed to acid, due to active hydroxyl hemiacetates. These active substances provide electrons to free radicals, which turn into stable forms, and eventually reduce the concentration of free radicals [118]. In addition, EPS increases the activity of superoxide effutase, serum catalase, and hepatic glutathione S-transferase in vivo, and reduces the serum malondialdehyde concentration and the activity of monoamine oxidase, showing excellent antioxidant and antiaging effect evidences [126,127].
Some EPSs also exhibit a strong immune response and show great potential for fighting inflammation and tumors. Dextran may increase the expression of interferon-1 and interferon-γ in salmon kidneys [128]. A high dose of EPS333 (a HePS) isolated from Streptococcus thermophilus was shown to stimulate macrophages to release nitric oxide (NO) and increase cellular immune response [129]. The antitumor activity of EPSs is based on immunomodulation, not only in indirect ways, but also by a direct killing effect on tumor cells. Some EPSs may indirectly activate macrophages to enhance their phagocytic capabilities by (a) facilitating the secretion of pro-inflammatory factors (e.g., IL-1 IL-6, and IL-12), as well as interferon (INF-λ), (b) inhibiting the production of anti-inflammatory factors (e.g., IL-10), and (c) ultimately stimulating interaction between immune and tumor cells [129]. For direct killing effects, it has been shown that EPSs can significantly inhibit the proliferation of HepG-2 and BGC-823 tumor cells, especially HT-29 tumor cells [130].
Different antiviral properties of EPSs, including high antiretroviral activity (anti-acquired immunodeficiency syndrome), have been reported in the literature [12,131]. For instance, EPSs extracted from L. plantarum LRCC5310 can effectively control rotavirus infection [132]. The antiviral activity of sulfated EPSs is related to the structure, which interacts with the signaling system, receptors, or enzymes, and to the negatively charged properties of these polymers [118]. Heparan Sulfate (HS), a receptor involved in viral infection, exists on the surface of cells. Meanwhile, sulfated polysaccharide shows a structural similarity with HS, and for this reason, it can inhibit competitive combination between HS and viruses, contributing to protection against other pathogens [133].
The principal biological and health-promoting properties of EPSs are listed in Table 4.
Table 4.
Biological properties and health-promoting effects of EPS.
| Biological Properties and Health Benefits | EPS | Source | Reference |
|---|---|---|---|
| Anticancer activity and Anticancer adjuvant | |||
| Antitumor activity by the activation of defender cells against cancer cells | β-glucans-based EPS | Aureobasidium pullulans | [134] |
| Anticancer activity against human colon, liver, embryonic kidney, breast cancer cell lines | Levans | Lactobacili, Bifidobacteria | [135] |
| Antiproliferative effect | Lactobacillus pantheris TCP102 | [136] | |
| Antitumor activity on HepG-2, BGC-823, HT-29 cancerous cells | L. plantarum 70810 | [137] | |
| Induced cytotoxicity in colon cancer cell lines | Limosilactobacillus fermentum YL-11 | [138] | |
| Apoptotic, antiangiogenic effects, and autophagy | Bacillus sonorensis, Rhodococcus pyridinivorans ZZ47 | [139,140] | |
| Immunomodulatory activity | |||
| Immunomodulation effects through interaction with macrophage receptors | β-glucans-based EPS | [141] | |
| Immunomodulation by human macrophage activation (cytokine production) | β-glucans | P. parvulus | [142] |
| Modulate the immune system (innate and adaptive response) | LAB | [143] | |
| Suppressors of the immune response | Lactobacillus confusus TISTR 1498 | [144,145] | |
| Stimulation of antigen presenting cells (e.g., dendritic cells) | Limosilactobacillus reuteri L26, L. reuteri DSM17938 | [146] | |
| Stimulate production of cytokines by macrophages | Nostoc sp. | [55] | |
| Maintaining the immune balance in states of inflammation and/or infection | Lactobacilli | [147] | |
| Improving allergic responses and suppressing allergen specific IgE synthesis | Leuconostoc citreum L3C1E7 | [148] | |
| Suppression the pro-inflammation and promotion of regulatory cytokine | S. thermophilus, Bacillus licheniformis, Leu. mesenteroides | [149] | |
| Activation of T lymphocytes and monocytes | S. thermophilus, Bifidobacterium breve | [150,151] | |
| Evasion of potentially damaging immune responses | Bifidobacterium breve | [151] | |
| Restoration of the mucosal barrier | Lactobacillus helveticus KLDS1. 8701 | [152] | |
| Immunostimulator | Levan (β-2, 6-fructan) | B. subtilis natto | [153,154] |
| Antiviral effects | |||
| Antiviral activity on avian influenza and adenovirus | Levans | B. subtilis (honey) | [155] |
| Anti-AIDS | Curdlan | Agrobacterium sp. | [156] |
| Antiviral against human hepatitis B | Curdulan sulfate | [157] | |
| Effects against enveloped viruses | Dextran sulfate | Leu. mesenteroides B512F | [158] |
| Antiviral and antibacterial activities | β-glucans-based EPS | Fungi | [159] |
| Cholesterol lowering and anti-hypertensive properties | |||
| Hypoglycemic and hypolipidemic activities | Cordyceps militaris. | [160] | |
| Lowering blood cholesterol | Lactiplantibacillus paraplantarum NCCP 962 | [161] | |
| Modulation of lipid metabolism | Lactobacilli | [162] | |
| Cholesterol-lowering properties and inhibit α-amylase | L. plantarum RJF4 | [137] | |
| Antihypertensive effects | S. thermophilus and Lactobacillus bulgaricus | [163] | |
| Anti-diabetes type 2 and hypocholesterolemia | |||
| Anti-diabetes | Pseudomonas sp. strain AHG22 | [164] | |
| Antibiofilm agents | |||
| Ability to repress biofilm | Lactobacillus helveticus MB2-1 | [165] | |
| Limiting the biofilm formation on medical devices | L. fermentum, Leu. citreum, Leu. Mesenteroides, Leu. Pseudomesenteroides, Ped. pentosaceus | [166] | |
| Antiadhesive and antibiofilm activities against oral S. Aureus strains | Lactobacilli | [167] | |
| Antibiofilm activity | L. plantarum-12 | [168] | |
| Anti-ulcer effects | |||
| Gastro-protective effect | L. plantarum E1K2R2 | [169] | |
| Inhibition of the adhesion of H. pylori | Lacticaseibacillus paracasei | [170] | |
| Antioxidant activities | |||
| Antioxidant and antiproliferative activities against human gastric | Levan | Pantoea agglomerans ZMR7 | [171] |
| Removal of free radicals scavenging activities | Halomonas elongata | [172] | |
| Scavenging of reactive oxygen species (ROS) and reduction in lipid peroxidation | Bacillus velezensis SN-1 | [173] | |
| Inhibition of H2O2 induced apoptosis | L. plantarum C88 | [137] | |
| Ferrous ion chelation | Gloeocapsa gelatinosa | [58] | |
| Prebiotic activities | |||
| Prebiotic effect | HePS | L. paracasei | [174] |
| Bifidogenic effect | Dextran | Leu. mesenteroides | [175] |
| Pathogen Antagonism | |||
| Prevent binding of pathogenic bacteria to mucus | HePS | Probiotic LAB | [176] |
| Reduce the adherence of pathogen to Caco-2 cells surfaces | L. casei NA-2 | [177] | |
| Inhibit the biofilm formation of a number of pathogens | B. licheniformis, Leu. mesenteroides | [175,178,179] | |
| Modulate microbial biofilms | Man-Glu | Lactobacillus | [180] |
| Enhancing intestinal barrier function | Man-Glu-Rib | L. plantarum | [181] |
| Anti-obesity effects | |||
| Improved human gut microbiota | Glucan | Weissella cibaria | [182] |
| Anti-skin irritation | |||
| Anti-inflammation | Dextran | Leuconostocaceae | [183] |
| Anti-Alzheimer’s disease | |||
| Treating Alzheimer’s illness to obviate side effects of synthetic drugs | ManA-Glc-Man-Rha | Streptomyces | [184] |
| Neuroprotective agent against amyloid beta1–42-induced apoptosis in SH-SY5Y cells | Man-Glc-Fru-Ace-Glc-N | Lactobacillus delbrueckii ssp. bulgaricus B3 | [185] |
3.2. Physico-Chemical Properties and Functionalities
EPSs are natural metabolites produced by bacteria when their environmental conditions become unfavorable or extreme, due to different existing stress conditions. These compounds play essentially protective and adaptation roles with regards to high variations in temperature, osmotic pressure, pH, and radiation, but also against pathogen microorganisms. EPS confer to their producing bacteria various fundamental properties such as the aggregation and adhesion capacity to the surface by the capsule form [7,186], biofilm formation or destruction on the solid substrates [187], the ability to colonize in host tissues [188], uptake of nutriments through emulsifying properties [189], and protection from external system by chelating action (binding activities) of toxic elements such as heavy metals [190]. These properties enable the bacteria producing EPSs to compete with pathogens when the energy and nutritional starvation conditions occur since most microorganisms are unable to grow, and even survive, under extreme environment conditions [5]. In nature, EPSs are biodegradable and harmful while being unusable as a carbon source by their own bacterial producers [5].
Their natural and fundamental properties are dependent on their chemical structure, but also on their state after bacterial secretion, either cell surface-bound (capsular EPSs) or cell-free biopolymer (slime EPSs), in the liquid medium. The diversity in chemical structure (composition, branched degree, linkage pattern, charge, etc.), molecular weight (MW), and physical state generates a wide range of EPS techno-functionalities, such as solubility and rheology, which can be exploitable in many industrial applications. With different MWs, the same type of EPS can generate various product viscoelasticity, owing to the change in macromolecular conformation in solution, and therefore the volume occupied by a polymer chain. For instance, dextran having a high MW and stronger viscoelastic properties affects the bread characteristics more positively than that having a low MW [191].
The bacterial capacity to aggregate, adhere, emulsify, and form biofilm on the solid surface is affected by the functional groups of EPSs (e.g., amphiphilic EPSs) surrounding the cell membrane, whereas their thickening, viscosifying, gelling, stabilizing, water-binding, and heavy metal absorbent (chelating) powers arise rather from their hydrocolloid and bulk-related properties, such as the molecular weight, water solubility, ionized state, and branching degree. Such techno-functionalities are valuable in both food and non-food product formulations. Table 5 illustrates some examples of bacterial EPS and their fundamental properties and techno-functionalities.
Table 5.
Bacterial EPS and their properties and techno-functionalities.
| EPS | Activities | Producers | Reference |
|---|---|---|---|
| α-D-glucan | Viscosifier | Levilactobacillus brevis HDE-9 | [192] |
| Water holding capacity | Enterococcus hirae OL616073 | [193] | |
| Levan | Enhancer of Bifidobacteria growth | Lactobacillus sanfranciscensis | [194] |
| Dextran | Enhancer of probiotic growth | W. cibaria | [195] |
| Promote colonization of strains | Leuconostoc lactis | [196] | |
| Kefiran | Enhancer of Bifidobacteria growth | Lactobacillus kefiranofaciens | [197] |
| Emulsifying and flocculating activities | L. kefiranofaciens | [197] | |
| Galactan | Emulsifying capacity and stability; flocculating activity at a wide range of pH | Weissella confusa | [198,199] |
| Anionic EPS | Heavy metal absorption activity (binding agents, bioabsorbents) | Lactobacilli | [200] |
| [Man:Glc]-EPS | Antibiofilm against pathogens | L. fermentum LB-69 | [201] |
| [Gal:Glc:Man]-EPS | Probiofilm | L. plantarum KF5 | [202] |
| [Glc:Man 1:7.01] [Man:Glc:Rha 7.45: 1.00: 2.34] |
a protector against oxidative stresses or reducing power or agent against free radicals | L. lactis subsp. lactis IMAU11823 | [203] |
| [Glu:Rib:Man:Xyl 1.0:16.4:6.6:6.5] [Rib:Man:Xyl:GA:Ara 7.1:1.6:4.8:1.0:9.0] |
Inhibitor of lipid peroxidation | Lactobacillus sp. | [204] |
| [Uronic acid]-EPS | Protector of cell membrane against lipid peroxidation | L. plantarum LP6 | [137] |
| [Gal-Glc-Man]-EPS | Inhibition of H2O2 | Bacillus sp. S-1 | [205] |
| [Se]-EPS | Antioxidant activity | L. lactis | [206] |
| [Glc-Man-Gal-Fuc-Glc-NH2]-EPS | Antioxidant effects | B. coagulans RK-02 | [207] |
| Gellan | Gelling (flexible elastic gel) and emulsifying activities | Sphingomonas elodea | [208] |
| Pullulan | Coating protector effect against mold | Aureobasidium pullulans | [209] |
4. Current and Future Applications of EPSs
4.1. Applications in Pharmaceutical and Medical Fields
Microbial EPSs and their derivatives have a wide range and numerous potential and commercial applications as biomaterial components, bioingredients, and bioactive agents in medical and pharmaceutical areas [11]. Their health-promoting values in humans have generated particular attention within scientists and industries because of their various biological activities and physiological functions, besides their biocompatibility, biodegradability, and non-toxicity [5]. Dextran as a blood plasma volume expander for controlling wounds [210], an alginate for tissue-engineering of bio-artificial organs [211] and its sodium derivative as an antacid protector [212], and pullulan as a drug carrier or coating agent [213] are among a few examples of commercialized EPS applications as medical devices and biopharmaceuticals. Moreover, some bacterial and fungal EPSs, particularly those from LABs, are largely used as universal “health bioingredients” for supplements or functional foods, and excipients for biopharmaceutical formulations of tablets, capsules, creams, gels, and suspensions, owing not only to their various techno-functionalities (water binding, viscosifying, thickening, emulsifying, stabilizing, and gelling capacities) but also to their GRAS status [9]. Xanthan, cellulose and derivatives, gellan, and levan are some examples of bio-excipients and health ingredients, among others, which are used as thickeners, suspension stabilizers in pharmaceutical creams, and disintegrating agents in tablets for oral, ophthalmic, and nasal drug formulations [7,11,12]. Illustrative examples of commercialized EPS are provided in Table 6.
Table 6.
Examples of bacterial EPSs manufactured at the industrial scale.
| EPS | Microorganism Producer | Manufacturer | Brand Name | Reference |
|---|---|---|---|---|
| Xanthan | Xanthomonas campestris | CPKelco, San Diego, CA, USA | Keldent | [214] |
| Xantural | ||||
| Gellan | Pseudomonas elodea | CPKelco, San Diego, CA, USA | Gelzan | |
| Kelcogel | ||||
| Hyaluronic acid | Streptococcus zooepidemicus Equi | Contipro Biotech, Dolni Dobrouc, Czech Republic | Sodium hyaluronate | [215] |
| Pullulan | Aureabasidium pullulans | Nagase Viita (Hayashibara Co., Ltd., Okayama, Japan) | Pullulan | [216] |
| Cellulose | Gluconacetobacter xylinum | fzmb GmbH, Research Centre of Medical Technology and Biotechnology | Nanomasque | [217] |
| Axcelon Demacare Inc, North York, ON, Canada | Nanoderm | |||
| Dextran | Leu. mesenteroides | Meito Sangyo, Nagoya, Japan | Dextran sulfate Na | [218] |
Table 7 summarizes the main current commercial applications in medical and pharmaceutical applications of EPSs and their derivatives reported in the literature to date.
Table 7.
Examples of current medical and pharmaceutical applications of EPSs and their derivatives.
| Medical and Pharmaceutical Applications | EPS | Source | Reference |
|---|---|---|---|
| Blood plasma volume expander, blood plasma substitute | Dextran | Leu. mesenteroides | [219] |
| Excipients, thickener and suspension stabilizer, drug-controlled release carrier | Xanthan | Xanthomonas campestris | [220] |
| Tablets disintegrant, thickener, emulsion stabilizing agents | Alginate |
Pseudomonas aeruginosa
Azotobacter vinelandii |
[221,222] |
| Antiacid protector in capsules | Na-alginate | ||
| Disintegrating agent, drug-controlled release, gelling agent, wound dressing | Gellan |
Sphingomonas elodea
Sphingomonas paucimobilis |
[223,224] |
| Binding and film-forming properties, coating agent for oxygen impermeability | Pullulan | Aurebasidium pullulans | [225] |
| Vitreous substitution during eye surgery, intraarticular injections in osteoarthritis | Hyaluronic acid | Streptococcus equi | [226,227] |
Two main future pharmaceutical applications of microbial EPSs, especially those from LAB, emerge through the recent literature overview.
4.1.1. EPSs as Immunobiotic Agents
EPSs can act as immunobiotic agents by playing some roles in tolerogenic activities of the producing LAB or probiotics. The immune-modulatory capacity of these immunobiotics is highlighted by our recent knowledge of the regulatory arm of immunity [228,229,230]. Indeed, many LAB strains activate the tolerogenic arm of immune reaction, as opposed to the inflammatory one, induced by pathogenic microbes. These pathogens include Escherichia coli O157: H7 (EHEC), Salmonella, Listeria monocytogenes, Campylobacter, and HIV-1 or Hepatitis B Virus (HBV). It has been shown in vivo that acidic EPSs and neutral EPSs are involved in the modulation of innate antiviral immune response in intestinal epithelium cells [231]. Different mechanisms have been reported in the literature for explaining EPS immunomodulation activities (Table 8).
Table 8.
Examples of EPS immunomodulation activity mechanisms.
| Mechanism | EPS Producing LAB | References |
|---|---|---|
| Interaction with dendritic cells and macrophages | Lactobacillus bulgaricus | [232] |
| Enhancing the proliferation of lymphocytes | ||
| Induction of nitric oxide secretion in vitro | L. plantarum JLK0142 | [233] |
| Enhancing the phagocytic potential of macrophages | ||
| Increasing IgA concentrations in the intestinal mucosa | ||
| Stimulating lymphocyte proliferation |
Thanks to their tolerogenic activities, some EPSs from LABs find a new potential application for the preparation of antiviral and antibacterial vaccines. It has already been announced in the past that EPSs could be used as novel adjuvant systems by enhancing vaccine-induced protection to target challenging pathogens, such as new pandemic viruses and resistant bacteria [11]. To date, immunobiotic agents including LAB strains and their metabolites like EPSs are used through their biological activities, either as preventive or curative treatments, to control inflammatory pathologies [234,235,236,237], or as an adjuvant for vaccine Human Immunodeficiency Virus type 1 (HIV-1) vaccine [238]. Indeed, it has been shown that a vaccine preparation containing inactivated Simian Immunodeficiency Virus (SIV) as the active principle, and L. plantarum (ATCC8014) as the adjuvant, when administered orally in Rhesus macaques, protected all these animals from SIV infection [238]. Recently, EPS from L. casei has been shown to increase the effectiveness of the foot-and-mouth disease vaccine [239]. Moreover, owing to their intrinsic innocuousness, long-term viability in host organisms, and their immune-modulatory capacity, some strains of EPS-producing LABs have been used as vectors to elicit immune responses against bacterial (e.g., E. coli and H. pylori) or viral (e.g., Influenza virus, SARS-CoV, and HIV) pathogens [240,241,242,243,244,245].
Considering the ongoing knowledge of the tolerogenic arm of immune reaction, and the ongoing discovery of new LAB strains and new genus commensal microbes, these data anticipate that immunobiotic agents, including both producing LABS and their metabolites such as EPSs, will constitute new bioactive agents of the preventive and/or therapeutic arsenal to use in medicine.
4.1.2. EPSs as Smart Delivery Systems
Another future promising application of bacterial EPSs in pharmaceutics is in the field of smart drug delivery systems, particularly when both diagnostic and therapy strategies (theranostics) can be combined [246]. For instance, EPS-coated magnetic material nanoparticles have a potential use in theranostics, such as in the case of the super paramagnetic iron oxide nanoparticles with crosslinked dextran coating (CLIO). These nanosystems can be used in photodynamic therapy by irradiating atheroma cells in carotid arteries [247].
4.2. Other Industrial and Agricultural Applications
In addition to their pharmaceutical and medical applications, EPSs have other industrial interests, driven by their unique physicochemical properties and potential as eco-friendly, sustainable alternatives to chemical-based polymers. These applications include textiles [248], bioplastics [249], and petroleum [250]. Welan gum shows particularly great promise in petroleum engineering, especially in polymer flooding for enhanced oil recovery (EOR) in high-salinity and high-temperature reservoirs. This potential stems from its ability to thicken aqueous solutions and its strong viscosifying properties [251]. Additionally, chemical modifications to this biopolymer can further improve its thermoviscosifying performance, solubility, and resistant to bacterial degradation, making it highly suitable for EOR applications in harsh environments [252].
In sustainable agricultural, EPSs are essential for effectively managing both farming practices and environmental health. They improve soil properties, helping to create a moist environment, aggregate soil particles, and protect plant cells from environmental stress and predators [50,253]. EPS has been examined for its capacity to bind soil particles together, functioning like a glue thanks to its ionic charges and viscous texture [254]. In wastewater treatment, EPSs are employed for their biofilm-forming, emulsifying, flocculating, and coagulating properties, which facilitate the decolorization of pollutants and the bioremediation of heavy metals [255]. Table 9 provides a summary of recent studies on EPS applications in various areas.
Table 9.
Recent studies of EPS applications.
| Uses | EPS | Properties | Reference |
|---|---|---|---|
| Textiles | Xanthan | Improve adhesive properties of textile | [256] |
| Oil Recovery | Xanthan | Reduce oil spreading | [257] |
| Modified xanthan | Enhance oil recovery | [258] | |
| Bioremediation | KO-EPS | Chelate iron | [259] |
| Agricultural | Xanthan | Improve water absorption and water retention capacity | [260] |
| EPS | Antifungal activity | [261] | |
| Packaging | Pullulan | Antifungal activity | [262] |
Microbial exopolysaccharides (EPSs) have a wide range of applications, but for human use, they must either meet the GRAS standard or have an affordable method to neutralize any toxic components, particularly in environmental applications such as municipal and water treatment. The regulatory approval process for EPS-based products varies as a function of their intended use. Key regulatory organizations that ensure the safety, efficacy, and quality of these products include the FDA in the USA, and EFSA and European Medical Agency (EMA) in the EU [4,263].
Industries in the food, pharmaceutical, and cosmetic sectors must adhere to strict regulatory frameworks that include safety, labeling, and Good Manufacturing Practice (GMP) standards to gain market approval. For instance, when dealing with biomass derived from microalgae, there may be concerns about toxicity due to potential contaminants like toxins, heavy metals, or pathogens. It is crucial to assess the potential for harmful substances when choosing microalgae for food products. To comply with legal and regulatory requirements, microalgae biomass and related products must undergo thorough safety evaluations before they can be marketed [264].
5. Conclusions
Overviewing the advances in microbial EPSs appears today to be relevant owing to their high diversity in sources and chemical structures, as well as the wide range and multiple functions of such biopolymers for health benefits. Bacterial, fungi, yeast, and microalgae EPSs find numerous applications in pharmaceutical and biomedical areas as biomaterials (e.g., tissue engineering of bio-artificial organs), bio-therapeutic agents (e.g., antiviral, antioxidant, and anticancer activities), and bio-excipients or “bio-ingredients” (e.g., thickeners, emulsifiers, and stabilizers) for drug formulations. Some EPSs are already produced and commercialized at the industrial scale (e.g., xanthan, dextran, and gellan), whereas others (e.g., kefiran and levan) are still in research and development stages, while having a lot of potential future applications. Those from lactic bacterial species (Lactobacilli, Bifidobacteria, etc.), or probiotics in general terms, are gaining particular attention because of their GRAS and QPS status, which allow them to be easily used in many sectors from a juridical viewpoint. Moreover, the current trends to use natural, biocompatible, non-toxic, and biodegradable active compounds for health prevention (e.g., strengthening our immune system) and therapy (e.g., fighting cancers and viral epidemics) are favorable in research and development advances, as well as for the commercialization of existing and new bioactive polymers from bacterial EPSs. While the present review indicates the significant knowledge and use of bacterial EPSs in biomedical and pharmaceutical areas to date, efforts should be continued to improve their production strategies. In fact, their high production cost is the main limiting factor in the advance of microbial EPS use. However, some compensation may come from the high interest and added values of their promising biological activities, such as immunobiotic adjuvants for preparing anti-microbial vaccines and anti-AIDS drugs in pharmaceutical and biomedical areas. Another future promising application of bacterial EPSs is in the field of smart drug delivery systems, particularly when both diagnostic and therapy strategies (theranostics) can be combined. Microbial EPSs, and particularly LAB EPSs, could have diverse and growing applications for our modern society in the near future.
Despite their potential, ongoing challenges in production and purification processes may hinder their scalability and commercial viability due to high costs and low yields. Thus, numerous studies aim to address these challenges and develop practical solutions. Among these are the utilization of affordable substrates and the isolation of novel strains. To enhance microbial productivity and optimize the use of extracellular polymeric substances (EPSs) in industrial and medical biotechnology, it is crucial to clarify the relationships between metabolic pathways and biosynthetic mechanisms. To uncover new EPS biosynthesis routes and understand the fundamentals of EPS production, advanced omics technologies—such as genome sequencing, functional genomics, protein structure analysis, and emerging bioinformatics tools—are being utilized. Additionally, ongoing research aims to maximize EPS production by investigating how various process variables influence biosynthesis and by identifying the most effective extraction and purification methods.
Abbreviations
|
ABC: ATP-Binding Cassette Ace: Acetate AIDS: Acquired Immunodeficiency Syndrome ALR: Air Lift Bioreactors Ara: Arabinose c-di-GMP: Cyclic Dimeric Guanosine Monophosphate CLIO: Cross-linked Dextran Coating CPS: Capsular Polysaccharide DSC: Differential Scanning Calorimetry EFSA : European Food Safety Authority EMA: European Medical Agency EOR: Enhanced Oil Recovery EPS: Exopolysaccharides FDA: Food Safety Authority and Fru: Fructose FT-IR: Fourier Transform—Infrared Fuc: Fucose Gal: Galactose GalA: Galacturonic acid Gal-N: Galactosamine GDP-Fruc: Guanosine Diphosphate Fructose Glc: Glucose Glc-1-P: Glucose-1-phosphate GlcA: Glucuronic acid GlcN: Glucosamine Gly: Glycerate GRAS: generally recognized as safe GulA: Guluronic acid HC: Heparin Cofactor HePS: Heteropolysaccharides HIV-1: Human Immunodeficiency Virus type 1 HoPS: Homopolysaccharides HPAEC: High performance Anion exchange chromatography HS: Heparin sulfate IgE: Immunoglobulin E IL: Interleukin INF: Interferon LAB: Lactic acid Bacteria MALS: Multi-Angle Light Scattering Man: Mannose (Man) Man-1-P: Mannose-1-phosphate ManA: Mannuronic acid MW: Molecular weight NIR: Near Infrared NMR: Near Magnetic Resonance NMP: Nucleoside Monophosphate NDP: Nucleoside Diphosphate OMA: Outer Membrane Auxiliary OPX: Outer Polysaccharide Export PCP: Polysaccharide co-polymerase Phosp: Phosphate Pyr: Pyruvate QPS: Qualified Presumption of Safety Rha: Rhamnose RI: Refractive Index Rib: Ribose (Rib) ROS: Reactive Oxygen Species SCFA: Short Chain Fatty acids SDS-PAGE: Sodium Dodecyl Sulfate—Polyacrylamide Gel SEC: Size Exclusion Chromatography SIV: Simian immunodeficiency virus STR: Stirred Tank Bioreactors Succ: Succinate Sulf: Sulfate TDP: Thymidine Diphosphate TGA: Thermogravimetric analysis UDP: Uridine Diphosphate UV: Ultraviolet XRD: X-Ray diffraction Xyl: Xylose |
Author Contributions
Conceptualization, H.L.R. and H.-T.N.; Figure creation, H.L.R., H.-T.N. and H.N.R.; writing—original draft preparation, H.L.R., H.-T.N., T.-T.P., P.-T.N., H.L.-B. and H.N.R.; writing—review and editing, H.L.R.; Funding acquisition, H.L.R. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research has received funding from the European Union’s Horizon research and innovation programme under grant agreement N° 101081802 (CONSERWA project).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Costa J.A.V., Lucas B.F., Alvarenga A.G.P., Moreira J.B., de Morais M.G. Microalgae Polysaccharides: An Overview of Production, Characterization, and Potential Applications. Polysaccharides. 2021;2:759–772. doi: 10.3390/polysaccharides2040046. [DOI] [Google Scholar]
- 2.Hamidi M., Okoro O.V., Milan P.B., Khalili M.R., Samadian H., Nie L., Shavandi A. Fungal Exopolysaccharides: Properties, Sources, Modifications, and Biomedical Applications. Carbohydr. Polym. 2022;284:119152. doi: 10.1016/j.carbpol.2022.119152. [DOI] [PubMed] [Google Scholar]
- 3.Rahbar Saadat Y., Yari Khosroushahi A., Pourghassem Gargari B. Yeast Exopolysaccharides and Their Physiological Functions. Folia Microbiol. 2021;66:171–182. doi: 10.1007/s12223-021-00856-2. [DOI] [PubMed] [Google Scholar]
- 4.Sørensen H.M., Rochfort K.D., Maye S., MacLeod G., Brabazon D., Loscher C., Freeland B. Exopolysaccharides of Lactic Acid Bacteria: Production, Purification and Health Benefits towards Functional Food. Nutrients. 2022;14:2938. doi: 10.3390/nu14142938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rana S., Upadhyay L.S.B. Microbial Exopolysaccharides: Synthesis Pathways, Types and Their Commercial Applications. Int. J. Biol. Macromol. 2020;157:577–583. doi: 10.1016/j.ijbiomac.2020.04.084. [DOI] [PubMed] [Google Scholar]
- 6.Branda S.S., Vik Å., Friedman L., Kolter R. Biofilms: The Matrix Revisited. Trends Microbiol. 2005;13:20–26. doi: 10.1016/j.tim.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Xu Y., Cui Y., Yue F., Liu L., Shan Y., Liu B., Zhou Y., Lü X. Exopolysaccharides Produced by Lactic Acid Bacteria and Bifidobacteria: Structures, Physiochemical Functions and Applications in the Food Industry. Food Hydrocoll. 2019;94:475–499. doi: 10.1016/j.foodhyd.2019.03.032. [DOI] [Google Scholar]
- 8.Lynch K.M., Zannini E., Coffey A., Arendt E.K. Lactic Acid Bacteria Exopolysaccharides in Foods and Beverages: Isolation, Properties, Characterization, and Health Benefits. Annu. Rev. Food Sci. Technol. 2018;9:155–176. doi: 10.1146/annurev-food-030117-012537. [DOI] [PubMed] [Google Scholar]
- 9.Torino M.I., Font de Valdez G., Mozzi F. Biopolymers from Lactic Acid Bacteria. Novel Applications in Foods and Beverages. Front. Microbiol. 2015;6:834. doi: 10.3389/fmicb.2015.00834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andrew M., Jayaraman G. Structural Features of Microbial Exopolysaccharides in Relation to Their Antioxidant Activity. Carbohydr. Res. 2020;487:107881. doi: 10.1016/j.carres.2019.107881. [DOI] [PubMed] [Google Scholar]
- 11.Moscovici M. Present and Future Medical Applications of Microbial Exopolysaccharides. Front. Microbiol. 2015;6:1012. doi: 10.3389/fmicb.2015.01012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rahbar Saadat Y., Yari Khosroushahi A., Pourghassem Gargari B. A Comprehensive Review of Anticancer, Immunomodulatory and Health Beneficial Effects of the Lactic Acid Bacteria Exopolysaccharides. Carbohydr. Polym. 2019;217:79–89. doi: 10.1016/j.carbpol.2019.04.025. [DOI] [PubMed] [Google Scholar]
- 13.Eze C.O., Berebon D.P., Gugu T.H., Anazodo F.I., Okorie J.E., Eze C.O., Berebon D.P., Gugu T.H., Anazodo F.I., Okorie J.E. Lactobacillus—A Multifunctional Genus. IntechOpen; London, UK: 2022. Lactobacillus Exopolysaccharide: An Untapped Biopolymer. [Google Scholar]
- 14.Amiri S., Rezaei Mokarram R., Sowti Khiabani M., Rezazadeh Bari M., Alizadeh Khaledabad M. Exopolysaccharides Production by Lactobacillus acidophilus LA5 and Bifidobacterium animalis subsp. lactis BB12: Optimization of Fermentation Variables and Characterization of Structure and Bioactivities. Int. J. Biol. Macromol. 2019;123:752–765. doi: 10.1016/j.ijbiomac.2018.11.084. [DOI] [PubMed] [Google Scholar]
- 15.Harutoshi T. Lactic Acid Bacteria-R & D for Food, Health and Livestock Purposes. InTech; London, UK: 2013. Exopolysaccharides of Lactic Acid Bacteria for Food and Colon Health Applications. [Google Scholar]
- 16.Schmid J., Sieber V., Rehm B. Bacterial Exopolysaccharides: Biosynthesis Pathways and Engineering Strategies. Front. Microbiol. 2015;6:496. doi: 10.3389/fmicb.2015.00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan K.-X., Chamundeswari V.N., Loo S.C.J. Prospects of Kefiran as a Food-Derived Biopolymer for Agri-Food and Biomedical Applications. RSC Adv. 2020;10:25339–25351. doi: 10.1039/D0RA02810J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeidan A.A., Poulsen V.K., Janzen T., Buldo P., Derkx P.M., Øregaard G., Neves A.R. Polysaccharide Production by Lactic Acid Bacteria: From Genes to Industrial Applications. FEMS Microbiol. Rev. 2017;41:S168–S200. doi: 10.1093/femsre/fux017. [DOI] [PubMed] [Google Scholar]
- 19.Donot F., Fontana A., Baccou J.C., Schorr-Galindo S. Microbial Exopolysaccharides: Main Examples of Synthesis, Excretion, Genetics and Extraction. Carbohydr. Polym. 2012;87:951–962. doi: 10.1016/j.carbpol.2011.08.083. [DOI] [Google Scholar]
- 20.Badel S., Bernardi T., Michaud P. New Perspectives for Lactobacilli Exopolysaccharides. Biotechnol. Adv. 2011;29:54–66. doi: 10.1016/j.biotechadv.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Yates L.E., Mills D.C., DeLisa M.P. Bacterial glycoengineering as a biosynthetic route to customized glycomolecules. In: Rapp E., Reichl U., editors. Advances in Glycobiotechnology. Springer International Publishing; Cham, Switzerland: 2021. pp. 167–200. [DOI] [PubMed] [Google Scholar]
- 22.Sutherland I.W. Advances in Microbial Physiology. Volume 23. Elsevier; Amsterdam, The Netherlands: 1982. Biosynthesis of microbial exopolysaccharides; pp. 79–150. [DOI] [PubMed] [Google Scholar]
- 23.Singha T.K. Microbial Extracellular Polymeric Substances: Production, Isolation and Applications. IOSR J. Pharm. 2012;2:271–281. [Google Scholar]
- 24.Freitas F., Torres C.A., Reis M.A. Engineering Aspects of Microbial Exopolysaccharide Production. Bioresour. Technol. 2017;245:1674–1683. doi: 10.1016/j.biortech.2017.05.092. [DOI] [PubMed] [Google Scholar]
- 25.Chaudhari V., Buttar H.S., Bagwe-Parab S., Tuli H.S., Vora A., Kaur G. Therapeutic and Industrial Applications of Curdlan with Overview on Its Recent Patents. Front. Nutr. 2021;8:646988. doi: 10.3389/fnut.2021.646988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohd Nadzir M., Nurhayati R.W., Idris F.N., Nguyen M.H. Biomedical Applications of Bacterial Exopolysaccharides: A Review. Polymers. 2021;13:530. doi: 10.3390/polym13040530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panchal R., Prajapati K., Prajapati M., Sharma S., Saraf M.S. Bacterial Exopolysaccharides: Types, Its Biosynthesis and Their Application in Different Fields. Acta Sci. Biotechnol. 2022;3:1–9. [Google Scholar]
- 28.Zhang P., Yuan L., Zeng J., Zou K., Liu B., Qing T., Feng B. Alginate Production of Pseudomonas Strains and Its Application in Preparation of Alginate-Biomass Hydrogel for Heavy Metal Adsorption. Int. J. Biol. Macromol. 2022;222:1511–1521. doi: 10.1016/j.ijbiomac.2022.09.252. [DOI] [PubMed] [Google Scholar]
- 29.Benhadda F., Zykwinska A., Colliec-Jouault S., Sinquin C., Thollas B., Courtois A., Fuzzati N., Toribio A., Delbarre-Ladrat C. Marine versus Non-Marine Bacterial Exopolysaccharides and Their Skincare Applications. Mar. Drugs. 2023;21:582. doi: 10.3390/md21110582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mummaleti G., Sarma C., Kalakandan S.K., Gazula H., Sivanandham V., Anandharaj A. Characterization of Levan Produced from Coconut Inflorescence Sap Using Bacillus Subtilis and Its Application as a Sweetener. LWT. 2022;154:112697. doi: 10.1016/j.lwt.2021.112697. [DOI] [Google Scholar]
- 31.Baptista S., Torres C.A.V., Sevrin C., Grandfils C., Reis M.A.M., Freitas F. Extraction of the Bacterial Extracellular Polysaccharide FucoPol by Membrane-Based Methods: Efficiency and Impact on Biopolymer Properties. Polymers. 2022;14:390. doi: 10.3390/polym14030390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wünsche J., Schmid J. Acetobacteraceae as Exopolysaccharide Producers: Current State of Knowledge and Further Perspectives. Front. Bioeng. Biotechnol. 2023;11:1166618. doi: 10.3389/fbioe.2023.1166618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang Y., Jiang G., Tian Y. Biological Activities and Applications of Exopolysaccharides Produced by Lactic Acid Bacteria: A Mini-Review. World J. Microbiol. Biotechnol. 2023;39:155. doi: 10.1007/s11274-023-03610-7. [DOI] [PubMed] [Google Scholar]
- 34.Jurášková D., Ribeiro S.C., Silva C.C. Exopolysaccharides Produced by Lactic Acid Bacteria: From Biosynthesis to Health-Promoting Properties. Foods. 2022;11:156. doi: 10.3390/foods11020156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balkrishna A., Agarwal V., Kumar G., Gupta A.K. Microbial Bioprospecting for Sustainable Development. Springer; Singapore: 2018. Applications of Bacterial Polysaccharides with Special Reference to the Cosmetic Industry; pp. 189–202. [Google Scholar]
- 36.Jeong J., Kim Y., Hu Y., Jung S. Bacterial Succinoglycans: Structure, Physical Properties, and Applications. Polymers. 2022;14:276. doi: 10.3390/polym14020276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang H., Lin J., Wang W., Li S. Biopolymers Produced by Sphingomonas Strains and Their Potential Applications in Petroleum Production. Polymers. 2022;14:1920. doi: 10.3390/polym14091920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Serra M., Casas A., Toubarro D., Barros A.N., Teixeira J.A. Microbial Hyaluronic Acid Production: A Review. Molecules. 2023;28:2084. doi: 10.3390/molecules28052084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen C.C., Nargotra P., Kuo C.H., Liu Y.C. High-Molecular-Weight Exopolysaccharides Production from Tuber brochii Cultivated by Submerged Fermentation. Int. J. Mol. Sci. 2023;24:4875. doi: 10.3390/ijms24054875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santra H.K., Banerjee D. Production, Optimization, Characterization and Drought Stress Resistance by β-Glucan-Rich Heteropolysaccharide From an Endophytic Fungi Colletotrichum Alatae LCS1 Isolated From Clubmoss (Lycopodium clavatum) Front. Fungal Biol. 2021;2:796010. doi: 10.3389/ffunb.2021.796010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen J., Lu Y., Liu L., Bai R., Zhang S., Hao Y., Xu F., Wei B., Zhao H. Characteristic Analysis and Fermentation Optimization of a Novel Aureobasidium pullulans RM1603 with High Pullulan Yield. J. Biosci. Bioeng. 2024;137:335–343. doi: 10.1016/j.jbiosc.2023.12.018. [DOI] [PubMed] [Google Scholar]
- 42.Shao Z., Tian Y., Liu S., Chu X., Mao W. Anti-Diabetic Activity of a Novel Exopolysaccharide Produced by the Mangrove Endophytic Fungus Penicillium janthinellum N29. Mar. Drugs. 2023;21:270. doi: 10.3390/md21050270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tao X., Chen X., Dong R., Wang G., Xu X., Yu Q., Chen Y., Wang X., Xie J. Characterization and Antioxidant Properties of Three Exopolysaccharides Produced by the Cyclocarya paliurus Endophytic Fungus. Int. J. Biol. Macromol. 2024;271:132110. doi: 10.1016/j.ijbiomac.2024.132110. [DOI] [PubMed] [Google Scholar]
- 44.Chen G., Xu Z., Wang F., Liu L., Wei Y., Li J., Zhang L., Zheng K., Wu L., Men X. Extraction, Characterization, and Biological Activities of Exopolysaccharides from Plant Root Soil Fungus Fusarium merismoides A6. Braz. J. Microbiol. 2023;54:199–211. doi: 10.1007/s42770-022-00842-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vadnerker P., Vyas T., Kapadia C. Characterization of Exopolysaccharide Produced by Ganoderma sp. TP and Its Immunomodulatory Properties. Romanian Biotechnol. Lett. 2022;28:3527–3535. doi: 10.25083/rbl/27.3/3527.3535. [DOI] [Google Scholar]
- 46.Castro S., Garay S., Espinoza-Carhuancho F., Medina J., Mendoza R., Mauricio F., Mayta-Tovalino F. Exploring the Potential of Probiotics in Dentistry: A Literature Review. Odovtos-Int. J. Dent. Sci. 2024;26:24–36. doi: 10.15517/ijds.2024.59138. [DOI] [Google Scholar]
- 47.Vanin A.P., Visentin E.Z., Fontana R.C., di Medeiros Leal M.C.B., de Avila e Silva S., Stokke B.T., Carbonero E.R., Camassola M. β-(1 → 3)(1 → 6)Glucan from Schizophyllum commune 227E.32: High Yield Production via Glucose/Xylose Co-Metabolization. Carbohydr. Polym. 2023;320:121176. doi: 10.1016/j.carbpol.2023.121176. [DOI] [PubMed] [Google Scholar]
- 48.Laroche C. Exopolysaccharides from Microalgae and Cyanobacteria: Diversity of Strains, Production Strategies, and Applications. Mar. Drugs. 2022;20:336. doi: 10.3390/md20050336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Babiak W., Krzemińska I. Extracellular Polymeric Substances (EPS) as Microalgal Bioproducts: A Review of Factors Affecting EPS Synthesis and Application in Flocculation Processes. Energies. 2021;14:4007. doi: 10.3390/en14134007. [DOI] [Google Scholar]
- 50.Moreira J.B., Kuntzler S.G., Bezerra P.Q.M., Cassuriaga A.P.A., Zaparoli M., da Silva J.L.V., Costa J.A.V., de Morais M.G. Recent Advances of Microalgae Exopolysaccharides for Application as Bioflocculants. Polysaccharides. 2022;3:264–276. doi: 10.3390/polysaccharides3010015. [DOI] [Google Scholar]
- 51.Visentin T.G., Guimarães B.M., Bastos R.G. Effects of Temperature, pH, and C/N Ratio of Sugarcane Wastewater Processing (Vinasse) on Phormidium autumnale Heterotrophic Cultivation. Algal Res. 2024;77:103349. doi: 10.1016/j.algal.2023.103349. [DOI] [Google Scholar]
- 52.Drira M., Elleuch J., Hlima H.B., Hentati F., Gardarin C., Rihouey C., Cerf D.L., Michaud P., Abdelkafi S., Fendri I. Optimization of Exopolysaccharides Production by Porphyridium sordidum and Their Potential to Induce Defense Responses in Arabidopsis thaliana against Fusarium oxysporum. Biomolecules. 2021;11:282. doi: 10.3390/biom11020282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Borjas Esqueda A., Gardarin C., Laroche C. Exploring the Diversity of Red Microalgae for Exopolysaccharide Production. Mar. Drugs. 2022;20:246. doi: 10.3390/md20040246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hayashi T., Hayashi K., Maeda M., Kojima I. Calcium Spirulan, an Inhibitor of Enveloped Virus Replication, from a Blue-Green Alga Spirulina platensis. J. Nat. Prod. 1996;59:83–87. doi: 10.1021/np960017o. [DOI] [PubMed] [Google Scholar]
- 55.Uhliariková I., Šutovská M., Barboríková J., Molitorisová M., Kim H.J., Park Y.I., Matulová M., Lukavský J., Hromadková Z., Capek P. Structural Characteristics and Biological Effects of Exopolysaccharide Produced by Cyanobacterium nostoc sp. Int. J. Biol. Macromol. 2020;160:364–371. doi: 10.1016/j.ijbiomac.2020.05.135. [DOI] [PubMed] [Google Scholar]
- 56.Tiwari O.N., Mondal A., Bhunia B., Bandyopadhyay T.K., Jaladi P., Oinam G., Indrama T. Purification, Characterization and Biotechnological Potential of New Exopolysaccharide Polymers Produced by Cyanobacterium Anabaena sp. CCC 745. Polymer. 2019;178:121695. doi: 10.1016/j.polymer.2019.121695. [DOI] [Google Scholar]
- 57.Uhliariková I., Matulová M., Košťálová Z., Lukavský J., Capek P. Lactylated Acidic Exopolysaccharide Produced by the Cyanobacterium Nostoc Cf. Linckia. Carbohydr. Polym. 2022;276:118801. doi: 10.1016/j.carbpol.2021.118801. [DOI] [PubMed] [Google Scholar]
- 58.Gongi W., Gomez Pinchetti J.L., Cordeiro N., Ouada H.B. Extracellular Polymeric Substances Produced by the Thermophilic Cyanobacterium Gloeocapsa gelatinosa: Characterization and Assessment of Their Antioxidant and Metal-Chelating Activities. Mar. Drugs. 2022;20:227. doi: 10.3390/md20040227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patel A., Prajapat J.B. Food and Health Applications of Exopolysaccharides Produced by Lactic Acid Bacteria. Adv. Dairy Res. 2013;1:107. [Google Scholar]
- 60.De belder A.N. CHAPTER 14—DEXTRAN. In: Whistler R.L., Bemiller J.N., editors. Industrial Gums (Third Edition) Academic Press; London, UK: 1993. pp. 399–425. [Google Scholar]
- 61.Yang B.-K., Ha J.-Y., Jeong S.-C., Das S., Yun J.-W., Lee Y.-S., Choi J.-W., Song C.-H. Production of Exo-Polymers by Submerged Mycelial Culture of Cordyceps Militaris and Its Hypolipidemic Effect. J. Microbiol. Biotechnol. 2000;10:784–788. [Google Scholar]
- 62.Shih L., Yu J.-Y., Hsieh C., Wu J.-Y. Production and Characterization of Curdlan by Agrobacterium sp. Biochem. Eng. J. 2009;43:33–40. doi: 10.1016/j.bej.2008.08.006. [DOI] [Google Scholar]
- 63.Yang M., Zhu Y., Li Y., Bao J., Fan X., Qu Y., Wang Y., Hu Z., Li Q. Production and Optimization of Curdlan Produced by Pseudomonas sp. QL212. Int. J. Biol. Macromol. 2016;89:25–34. doi: 10.1016/j.ijbiomac.2016.04.027. [DOI] [PubMed] [Google Scholar]
- 64.Monsan P., Bozonnet S., Albenne C., Joucla G., Willemot R.-M., Remaud-Siméon M. Homopolysaccharides from Lactic Acid Bacteria. Int. Dairy J. 2001;11:675–685. doi: 10.1016/S0958-6946(01)00113-3. [DOI] [Google Scholar]
- 65.Rosalam S., England R. Review of Xanthan Gum Production from Unmodified Starches by Xanthomonas comprestris sp. Enzyme Microb. Technol. 2006;39:197–207. doi: 10.1016/j.enzmictec.2005.10.019. [DOI] [Google Scholar]
- 66.Prajapati V.D., Jani G.K., Khanda S.M. Pullulan: An Exopolysaccharide and Its Various Applications. Carbohydr. Polym. 2013;95:540–549. doi: 10.1016/j.carbpol.2013.02.082. [DOI] [PubMed] [Google Scholar]
- 67.Cheng K.-C., Demirci A., Catchmark J.M. Pullulan: Biosynthesis, Production, and Applications. Appl. Microbiol. Biotechnol. 2011;92:29–44. doi: 10.1007/s00253-011-3477-y. [DOI] [PubMed] [Google Scholar]
- 68.Kırtel O., Avşar G., Erkorkmaz B.A., Öner E.T. Microbial Production of Food Ingredients and Additives. Elsevier; Amsterdam, The Netherlands: 2017. Microbial Polysaccharides as Food Ingredients; pp. 347–383. [Google Scholar]
- 69.Coviello T., Palleschi A., Grassi M., Matricardi P., Bocchinfuso G., Alhaique F. Scleroglucan: A Versatile Polysaccharide for Modified Drug Delivery. Molecules. 2005;10:6–33. doi: 10.3390/10010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu X., Yan H., Tang J., Chen J., Zhang X. Polysaccharides in Lentinus Edodes: Isolation, Structure, Immunomodulating Activity and Future Prospective. Crit. Rev. Food Sci. Nutr. 2014;54:474–487. doi: 10.1080/10408398.2011.587616. [DOI] [PubMed] [Google Scholar]
- 71.Giavasis I. Microbial Production of Food Ingredients, Enzymes and Nutraceuticals. Elsevier; Amsterdam, The Netherlands: 2013. Production of Microbial Polysaccharides for Use in Food; pp. 413–468. [Google Scholar]
- 72.Giavasis I., Seviour R.J., Hudman P., McNeil B. Advances in Food Bioproducts and Bioprocessing Technologies. CRC Press; Boca Raton, FL, USA: 2019. Fungal bioproducts for use in food: Polysaccharides, organic acids, and mycoprotein; pp. 511–548. [Google Scholar]
- 73.Babitskaya V.G., Shcherba V.V., Puchkova T.A., Smirnov D.A. Polysaccharides of Ganoderma Lucidum: Factors Affecting Their Production. Appl. Biochem. Microbiol. 2005;41:169–173. doi: 10.1007/s10438-005-0029-1. [DOI] [PubMed] [Google Scholar]
- 74.Meng X., Liang H., Luo L. Antitumor Polysaccharides from Mushrooms: A Review on the Structural Characteristics, Antitumor Mechanisms and Immunomodulating Activities. Carbohydr. Res. 2016;424:30–41. doi: 10.1016/j.carres.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 75.Yamanaka D., Liu Y., Motoi M., Ohno N. Royal Sun Medicinal Mushroom, Agaricus brasiliensis Ka21 (Higher Basidiomycetes), as a Functional Food in Humans. Int. J. Med. Mushrooms. 2013;15:335–343. doi: 10.1615/IntJMedMushr.v15.i4.10. [DOI] [PubMed] [Google Scholar]
- 76.Liu Y.-S., Wu J.-Y. Effects of Tween 80 and pH on Mycelial Pellets and Exopolysaccharide Production in Liquid Culture of a Medicinal Fungus. J. Ind. Microbiol. Biotechnol. 2012;39:623–628. doi: 10.1007/s10295-011-1066-9. [DOI] [PubMed] [Google Scholar]
- 77.Yu Y., Shen M., Song Q., Xie J. Biological Activities and Pharmaceutical Applications of Polysaccharide from Natural Resources: A Review. Carbohydr. Polym. 2018;183:91–101. doi: 10.1016/j.carbpol.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 78.Jayachandran M., Chen J., Chung S.S.M., Xu B. A Critical Review on the Impacts of β-Glucans on Gut Microbiota and Human Health. J. Nutr. Biochem. 2018;61:101–110. doi: 10.1016/j.jnutbio.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 79.Mishima T., Murata J., Toyoshima M., Fujii H., Nakajima M., Hayashi T., Kato T., Saiki I. Inhibition of Tumor Invasion and Metastasis by Calciumspirulan (Ca-SP), a Novel Sulfated Polysaccharide Derived from a Blue-Green Alga, Spirulina Platensis. Clin. Exp. Metastasis. 1998;16:541–550. doi: 10.1023/A:1006594318633. [DOI] [PubMed] [Google Scholar]
- 80.De Philippis R., Vincenzini M. Exocellular Polysaccharides from Cyanobacteria and Their Possible Applications. FEMS Microbiol. Rev. 1998;22:151–175. doi: 10.1016/S0168-6445(98)00012-6. [DOI] [Google Scholar]
- 81.Delattre C., Pierre G., Laroche C., Michaud P. Production, Extraction and Characterization of Microalgal and Cyanobacterial Exopolysaccharides. Biotechnol. Adv. 2016;34:1159–1179. doi: 10.1016/j.biotechadv.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 82.Pereira S., Zille A., Micheletti E., Moradas-Ferreira P., De Philippis R., Tamagnini P. Complexity of Cyanobacterial Exopolysaccharides: Composition, Structures, Inducing Factors and Putative Genes Involved in Their Biosynthesis and Assembly. FEMS Microbiol. Rev. 2009;33:917–941. doi: 10.1111/j.1574-6976.2009.00183.x. [DOI] [PubMed] [Google Scholar]
- 83.Pathak J., Rajneesh R., Sonker A.S., Kannaujiya V.K., Sinha R.P. Marine Glycobiology. CRC Press; Boca Raton, FL, USA: 2016. Cyanobacterial extracellular polysaccharide sheath pigment, scytonemin: A novel multipurpose pharmacophore. [Google Scholar]
- 84.Cruz D., Vasconcelos V., Pierre G., Michaud P., Delattre C. Exopolysaccharides from Cyanobacteria: Strategies for Bioprocess Development. Appl. Sci. 2020;10:3763. doi: 10.3390/app10113763. [DOI] [Google Scholar]
- 85.Diengdoh O.L., Syiem M.B., Pakshirajan K., Rai A.N. Zn2+ Sequestration by Nostoc muscorum: Study of Thermodynamics, Equilibrium Isotherms, and Biosorption Parameters for the Metal. Environ. Monit. Assess. 2017;189:314. doi: 10.1007/s10661-017-6013-4. [DOI] [PubMed] [Google Scholar]
- 86.Mota R., Rossi F., Andrenelli L., Pereira S.B., De Philippis R., Tamagnini P. Released Polysaccharides (RPS) from Cyanothece sp. CCY 0110 as Biosorbent for Heavy Metals Bioremediation: Interactions between Metals and RPS Binding Sites. Appl. Microbiol. Biotechnol. 2016;100:7765–7775. doi: 10.1007/s00253-016-7602-9. [DOI] [PubMed] [Google Scholar]
- 87.Shakeri M., Naji-Tabasi S. Characterization and Optimization of Gellan Gum Production by Natural Sphingomonas sp. SM2. LWT. 2024;200:116164. doi: 10.1016/j.lwt.2024.116164. [DOI] [Google Scholar]
- 88.Liu Z., Xu Y., Wang Z., Zhu L., Li Z., Jiang Y., Zhan X., Gao M. Promoting Substrates Uptake and Curdlan Synthesis of Agrobacterium sp. by Attenuating the Exopolysaccharide Encapsulation. Carbohydr. Polym. 2023;315:120941. doi: 10.1016/j.carbpol.2023.120941. [DOI] [PubMed] [Google Scholar]
- 89.Seviour R.J., McNeil B., Fazenda M.L., Harvey L.M. Operating Bioreactors for Microbial Exopolysaccharide Production. Crit. Rev. Biotechnol. 2011;31:170–185. doi: 10.3109/07388551.2010.505909. [DOI] [PubMed] [Google Scholar]
- 90.Cao J., Zhang H.-J., Xu C.-P. Culture Characterization of Exopolysaccharides with Antioxidant Activity Produced by Pycnoporus sanguineus in Stirred-Tank and Airlift Reactors. J. Taiwan Inst. Chem. Eng. 2014;45:2075–2080. doi: 10.1016/j.jtice.2014.05.005. [DOI] [Google Scholar]
- 91.Usuldin S.R.A., Ilham Z., Jamaludin A.A., Ahmad R., Wan-Mohtar W.A.A.Q.I. Enhancing Biomass-Exopolysaccharides Production of Lignosus rhinocerus in a High-Scale Stirred-Tank Bioreactor and Its Potential Lipid as Bioenergy. Energies. 2023;16:2330. doi: 10.3390/en16052330. [DOI] [Google Scholar]
- 92.Jaswal A.S., Elangovan R., Mishra S. Synthesis and Molecular Characterization of Levan Produced by Immobilized Microbacterium paraoxydans. J. Biotechnol. 2023;373:63–72. doi: 10.1016/j.jbiotec.2023.07.003. [DOI] [PubMed] [Google Scholar]
- 93.Namdeo N., Kumar B., Jha H. Microbial Exopolysaccharides. CRC Press; Boca Raton, FL, USA: 2024. Bioreactor Design for the Production of Microbial Polysaccharides. [Google Scholar]
- 94.Finore I., Di Donato P., Mastascusa V., Nicolaus B., Poli A. Fermentation Technologies for the Optimization of Marine Microbial Exopolysaccharide Production. Mar. Drugs. 2014;12:3005–3024. doi: 10.3390/md12053005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kumar M.A., Anandapandian K.T.K., Parthiban K. Production and Characterization of Exopolysaccharides (EPS) from Biofilm Forming Marine Bacterium. Braz. Arch. Biol. Technol. 2011;54:259–265. doi: 10.1590/S1516-89132011000200006. [DOI] [Google Scholar]
- 96.Sanalibaba P., Cakmak G.A. Exopolysaccharides Production by Lactic Acid Bacteria. Appl. Microbiol. Open Access. 2016;2:1000115. doi: 10.4172/2471-9315.1000115. [DOI] [Google Scholar]
- 97.Leroy F., De Vuyst L. Advances in Production and Simplified Methods for Recovery and Quantification of Exopolysaccharides for Applications in Food and Health1. J. Dairy Sci. 2016;99:3229–3238. doi: 10.3168/jds.2015-9936. [DOI] [PubMed] [Google Scholar]
- 98.Nguyen H.T., Razafindralambo H., Blecker C., N’Yapo C., Thonart P., Delvigne F. Stochastic Exposure to Sub-Lethal High Temperature Enhances Exopolysaccharides (EPS) Excretion and Improves Bifidobacterium bifidum Cell Survival to Freeze–Drying. Biochem. Eng. J. 2014;88:85–94. doi: 10.1016/j.bej.2014.04.005. [DOI] [Google Scholar]
- 99.Higgins M.J., Novak J.T. Characterization of Exocellular Protein and Its Role in Bioflocculation. J. Environ. Eng. 1997;123:479–485. doi: 10.1061/(ASCE)0733-9372(1997)123:5(479). [DOI] [Google Scholar]
- 100.Yegorenkova I.V., Tregubova K.V., Matora L.Y., Burygin G.L., Ignatov V.V. Biofilm Formation by Paenibacillus polymyxa Strains Differing in the Production and Rheological Properties of Their Exopolysaccharides. Curr. Microbiol. 2011;62:1554–1559. doi: 10.1007/s00284-011-9896-2. [DOI] [PubMed] [Google Scholar]
- 101.Poli A., Di Donato P., Abbamondi G.R., Nicolaus B. Synthesis, Production, and Biotechnological Applications of Exopolysaccharides and Polyhydroxyalkanoates by Archaea. Archaea. 2011;2011:693253. doi: 10.1155/2011/693253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.O’Toole G., Kaplan H.B., Kolter R. Biofilm Formation as Microbial Development. Annu. Rev. Microbiol. 2000;54:49–79. doi: 10.1146/annurev.micro.54.1.49. [DOI] [PubMed] [Google Scholar]
- 103.Schwarzmann S., Boring J.R. Antiphagocytic Effect of Slime from a Mucoid Strain of Pseudomonas aeruginosa. Infect. Immun. 1971;3:762–767. doi: 10.1128/iai.3.6.762-767.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ruas-Madiedo P., Hugenholtz J., Zoon P. An Overview of the Functionality of Exopolysaccharides Produced by Lactic Acid Bacteria. Int. Dairy J. 2002;12:163–171. doi: 10.1016/S0958-6946(01)00160-1. [DOI] [Google Scholar]
- 105.Li W., Ji J., Rui X., Yu J., Tang W., Chen X., Jiang M., Dong M. Production of Exopolysaccharides by Lactobacillus helveticus MB2-1 and Its Functional Characteristics in Vitro. LWT Food Sci. Technol. 2014;59:732–739. doi: 10.1016/j.lwt.2014.06.063. [DOI] [Google Scholar]
- 106.Khan W., Hosseinkhani H., Ickowicz D., Hong P.-D., Yu D.-S., Domb A.J. Polysaccharide Gene Transfection Agents. Acta Biomater. 2012;8:4224–4232. doi: 10.1016/j.actbio.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 107.Zhang Y., Kong H., Fang Y., Nishinari K., Phillips G.O. Schizophyllan: A Review on Its Structure, Properties, Bioactivities and Recent Developments. Bioact. Carbohydr. Diet. Fibre. 2013;1:53–71. doi: 10.1016/j.bcdf.2013.01.002. [DOI] [Google Scholar]
- 108.Park M., Lee D., Hyun J. Nanocellulose-Alginate Hydrogel for Cell Encapsulation. Carbohydr. Polym. 2015;116:223–228. doi: 10.1016/j.carbpol.2014.07.059. [DOI] [PubMed] [Google Scholar]
- 109.Albadran H.A., Chatzifragkou A., Khutoryanskiy V.V., Charalampopoulos D. Development of Surfactant-Coated Alginate Capsules Containing Lactobacillus plantarum. Food Hydrocoll. 2018;82:490–499. doi: 10.1016/j.foodhyd.2018.04.035. [DOI] [Google Scholar]
- 110.Zhu C.-Z., Li D., Chen W.-J., Ban S.-N., Liu T., Wen H., Jiang M. Effects of Dietary Host-Associated Lactococcus lactis on Growth Performance, Disease Resistance, Intestinal Morphology and Intestinal Microbiota of Mandarin Fish (Siniperca chuatsi) Aquaculture. 2021;540:736702. doi: 10.1016/j.aquaculture.2021.736702. [DOI] [Google Scholar]
- 111.Walker A.W., Duncan S.H., McWilliam Leitch E.C., Child M.W., Flint H.J. pH and Peptide Supply Can Radically Alter Bacterial Populations and Short-Chain Fatty Acid Ratios within Microbial Communities from the Human Colon. Appl. Environ. Microbiol. 2005;71:3692–3700. doi: 10.1128/AEM.71.7.3692-3700.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rios-Covian D., Cuesta I., Alvarez-Buylla J.R., Ruas-Madiedo P., Gueimonde M., de los Reyes-Gavilán C.G. Bacteroides fragilis Metabolises Exopolysaccharides Produced by Bifidobacteria. BMC Microbiol. 2016;16:150. doi: 10.1186/s12866-016-0773-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fanning S., Hall L.J., Cronin M., Zomer A., MacSharry J., Goulding D., O’Connell Motherway M., Shanahan F., Nally K., Dougan G., et al. Bifidobacterial Surface-Exopolysaccharide Facilitates Commensal-Host Interaction through Immune Modulation and Pathogen Protection. Proc. Natl. Acad. Sci. USA. 2012;109:2108–2113. doi: 10.1073/pnas.1115621109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Balzaretti S., Taverniti V., Guglielmetti S., Fiore W., Minuzzo M., Ngo H.N., Ngere J.B., Sadiq S., Humphreys P.N., Laws A.P. A Novel Rhamnose-Rich Hetero-Exopolysaccharide Isolated from Lactobacillus paracasei DG Activates THP-1 Human Monocytic Cells. Appl. Environ. Microbiol. 2017;83:e02702-16. doi: 10.1128/AEM.02702-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bengoa A.A., Llamas M.G., Iraporda C., Dueñas M.T., Abraham A.G., Garrote G.L. Impact of Growth Temperature on Exopolysaccharide Production and Probiotic Properties of Lactobacillus paracasei Strains Isolated from Kefir Grains. Food Microbiol. 2018;69:212–218. doi: 10.1016/j.fm.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 116.Trabelsi I., Ktari N., Slima S.B., Triki M., Bardaa S., Mnif H., Salah R.B. Evaluation of Dermal Wound Healing Activity and in Vitro Antibacterial and Antioxidant Activities of a New Exopolysaccharide Produced by Lactobacillus sp. Ca6. Int. J. Biol. Macromol. 2017;103:194–201. doi: 10.1016/j.ijbiomac.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 117.Jeong D., Kim D.-H., Kang I.-B., Kim H., Song K.-Y., Kim H.-S., Seo K.-H. Characterization and Antibacterial Activity of a Novel Exopolysaccharide Produced by Lactobacillus kefiranofaciens DN1 Isolated from Kefir. Food Control. 2017;78:436–442. doi: 10.1016/j.foodcont.2017.02.033. [DOI] [Google Scholar]
- 118.Zhou Y., Cui Y., Qu X. Exopolysaccharides of Lactic Acid Bacteria: Structure, Bioactivity and Associations: A Review. Carbohydr. Polym. 2019;207:317–332. doi: 10.1016/j.carbpol.2018.11.093. [DOI] [PubMed] [Google Scholar]
- 119.Bhat B., Bajaj B.K. Hypocholesterolemic and Bioactive Potential of Exopolysaccharide from a Probiotic Enterococcus faecium K1 Isolated from Kalarei. Bioresour. Technol. 2018;254:264–267. doi: 10.1016/j.biortech.2018.01.078. [DOI] [PubMed] [Google Scholar]
- 120.Sasikumar K., Vaikkath D.K., Devendra L., Nampoothiri K.M. An Exopolysaccharide (EPS) from a Lactobacillus plantarum BR2 with Potential Benefits for Making Functional Foods. Bioresour. Technol. 2017;241:1152–1156. doi: 10.1016/j.biortech.2017.05.075. [DOI] [PubMed] [Google Scholar]
- 121.Ishimwe N., Daliri E.B., Lee B.H., Fang F., Du G. The Perspective on Cholesterol-lowering Mechanisms of Probiotics. Mol. Nutr. Food Res. 2015;59:94–105. doi: 10.1002/mnfr.201400548. [DOI] [PubMed] [Google Scholar]
- 122.Michael D.R., Davies T.S., Moss J.W.E., Calvente D.L., Ramji D.P., Marchesi J.R., Pechlivanis A., Plummer S.F., Hughes T.R. The Anti-Cholesterolaemic Effect of a Consortium of Probiotics: An Acute Study in C57BL/6J Mice. Sci. Rep. 2017;7:2883. doi: 10.1038/s41598-017-02889-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li H., Mao W., Hou Y., Gao Y., Qi X., Zhao C., Chen Y., Chen Y., Li N., Wang C. Preparation, Structure and Anticoagulant Activity of a Low Molecular Weight Fraction Produced by Mild Acid Hydrolysis of Sulfated Rhamnan from Monostroma latissimum. Bioresour. Technol. 2012;114:414–418. doi: 10.1016/j.biortech.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 124.Li N., Liu X., He X., Wang S., Cao S., Xia Z., Xian H., Qin L., Mao W. Structure and Anticoagulant Property of a Sulfated Polysaccharide Isolated from the Green Seaweed Monostroma angicava. Carbohydr. Polym. 2017;159:195–206. doi: 10.1016/j.carbpol.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 125.Rani R.P., Anandharaj M., Ravindran A.D. Characterization of a Novel Exopolysaccharide Produced by Lactobacillus Gasseri FR4 and Demonstration of Its in Vitro Biological Properties. Int. J. Biol. Macromol. 2018;109:772–783. doi: 10.1016/j.ijbiomac.2017.11.062. [DOI] [PubMed] [Google Scholar]
- 126.Guo Y., Pan D., Li H., Sun Y., Zeng X., Yan B. Antioxidant and Immunomodulatory Activity of Selenium Exopolysaccharide Produced by Lactococcus lactis subsp. Lactis. Food Chem. 2013;138:84–89. doi: 10.1016/j.foodchem.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 127.Pan D., Mei X. Antioxidant Activity of an Exopolysaccharide Purified from Lactococcus lactis subsp. Lactis 12. Carbohydr. Polym. 2010;80:908–914. doi: 10.1016/j.carbpol.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 128.Nácher-Vázquez M., Ballesteros N., Canales Á., Saint-Jean S.R., Pérez-Prieto S.I., Prieto A., Aznar R., López P. Dextrans Produced by Lactic Acid Bacteria Exhibit Antiviral and Immunomodulatory Activity against Salmonid Viruses. Carbohydr. Polym. 2015;124:292–301. doi: 10.1016/j.carbpol.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 129.Ren W., Xia Y., Wang G., Zhang H., Zhu S., Ai L. Bioactive Exopolysaccharides from a S. thermophilus Strain: Screening, Purification and Characterization. Int. J. Biol. Macromol. 2016;86:402–407. doi: 10.1016/j.ijbiomac.2016.01.085. [DOI] [PubMed] [Google Scholar]
- 130.Wang K., Li W., Rui X., Chen X., Jiang M., Dong M. Characterization of a Novel Exopolysaccharide with Antitumor Activity from Lactobacillus plantarum 70810. Int. J. Biol. Macromol. 2014;63:133–139. doi: 10.1016/j.ijbiomac.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 131.Singh P., Saini P. Food and Health Potentials of Exopolysaccharides Derived from Lactobacilli. Microbiol. Res. J. Int. 2017;22:1–14. doi: 10.9734/MRJI/2017/36935. [DOI] [Google Scholar]
- 132.Kim K., Lee G., Thanh H.D., Kim J.-H., Konkit M., Yoon S., Park M., Yang S., Park E., Kim W. Exopolysaccharide from Lactobacillus plantarum LRCC5310 Offers Protection against Rotavirus-Induced Diarrhea and Regulates Inflammatory Response. J. Dairy Sci. 2018;101:5702–5712. doi: 10.3168/jds.2017-14151. [DOI] [PubMed] [Google Scholar]
- 133.Liu J., Thorp S.C. Cell Surface Heparan Sulfate and Its Roles in Assisting Viral Infections. Med. Res. Rev. 2002;22:1–25. doi: 10.1002/med.1026. [DOI] [PubMed] [Google Scholar]
- 134.Liao Y., Wang R., Qin X., Ma X., Liu X., Jia S., Zhong C. A β-Glucan from Aureobasidium pullulans Enhanced the Antitumor Effect with Rituximab against SU-DHL-8. Int. J. Biol. Macromol. 2022;220:1356–1367. doi: 10.1016/j.ijbiomac.2022.09.106. [DOI] [PubMed] [Google Scholar]
- 135.Khalil M.A., Sonbol F.I., Al-Madboly L.A., Aboshady T.A., Alqurashi A.S., Ali S.S. Exploring the Therapeutic Potentials of Exopolysaccharides Derived from Lactic Acid Bacteria and Bifidobacteria: Antioxidant, Antitumor, and Periodontal Regeneration. Front. Microbiol. 2022;13:803688. doi: 10.3389/fmicb.2022.803688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sheng S., Fu Y., Pan N., Zhang H., Xiu L., Liang Y., Liu Y., Liu B., Ma C., Du R., et al. Novel Exopolysaccharide Derived from Probiotic Lactobacillus pantheris TCP102 Strain with Immune-Enhancing and Anticancer Activities. Front. Microbiol. 2022;13:1015270. doi: 10.3389/fmicb.2022.1015270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xiong J., Liu D., Huang Y. Exopolysaccharides from Lactiplantibacillus plantarum: Isolation, Purification, Structure–Function Relationship, and Application. Eur. Food Res. Technol. 2023;249:1431–1448. doi: 10.1007/s00217-023-04237-6. [DOI] [Google Scholar]
- 138.Li F., Jiao X., Zhao J., Liao X., Wei Y., Li Q. Antitumor Mechanisms of an Exopolysaccharide from Lactobacillus fermentum on HT-29 Cells and HT-29 Tumor-Bearing Mice. Int. J. Biol. Macromol. 2022;209:552–562. doi: 10.1016/j.ijbiomac.2022.04.023. [DOI] [PubMed] [Google Scholar]
- 139.Abdelnasser S.M., Abu-Shahba N. Bacillus sonorinses Derived Exopolysaccharide Enhances Cell Cycle Arrest, Apoptosis, Necrosis, Autophagy and COX-2 down Regulation in Liver Cancer Cells. Biotechnol. Rep. 2024;48:e00848. doi: 10.1016/j.btre.2024.e00848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Taşkaya A., Güvensen N.C., Güler C., Şancı E., Karabay Ü. Exopolysaccharide from Rhodococcus pyridinivorans ZZ47 Strain: Evaluation of Biological Activity and Toxicity. J. Agric. Prod. 2023;4:63–71. doi: 10.56430/japro.1307611. [DOI] [Google Scholar]
- 141.Zhong X., Wang G., Li F., Fang S., Zhou S., Ishiwata A., Tonevitsky A.G., Shkurnikov M., Cai H., Ding F. Immunomodulatory Effect and Biological Significance of β-Glucans. Pharmaceutics. 2023;15:1615. doi: 10.3390/pharmaceutics15061615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Notararigo S., Varela E., Otal A., Antolín M., Guarner F., López P. Anti-Inflammatory Effect of an O-2-Substituted (1-3)-β-D-Glucan Produced by Pediococcus parvulus 2.6 in a Caco-2 PMA-THP-1 Co-Culture Model. Int. J. Mol. Sci. 2022;23:1527. doi: 10.3390/ijms23031527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yang S., Xu X., Peng Q., Ma L., Qiao Y., Shi B. Exopolysaccharides from Lactic Acid Bacteria, as an Alternative to Antibiotics, on Regulation of Intestinal Health and the Immune System. Anim. Nutr. 2023;13:78–89. doi: 10.1016/j.aninu.2023.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Abdalla A.K., Ayyash M.M., Olaimat A.N., Osaili T.M., Al-Nabulsi A.A., Shah N.P., Holley R. Exopolysaccharides as Antimicrobial Agents: Mechanism and Spectrum of Activity. Front. Microbiol. 2021;12:664395. doi: 10.3389/fmicb.2021.664395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang W., Ju Y., Liu N., Shi S., Hao L. Structural Characteristics of Microbial Exopolysaccharides in Association with Their Biological Activities: A Review. Chem. Biol. Technol. Agric. 2023;10:137. doi: 10.1186/s40538-023-00515-3. [DOI] [Google Scholar]
- 146.Kiššová Z., Schusterová P., Mudroňová D., Novotný J., Tkáčiková Ľ. Exopolysaccharides from Limosilactobacillus reuteri: Their Influence on in Vitro Activation of Porcine Monocyte-Derived Dendritic Cells-Brief Report. Vet. Res. Commun. 2024:1–7. doi: 10.1007/s11259-024-10445-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rajoka M.S.R., Wu Y., Mehwish H.M., Bansal M., Zhao L. Lactobacillus Exopolysaccharides: New Perspectives on Engineering Strategies, Physiochemical Functions, and Immunomodulatory Effects on Host Health. Trends Food Sci. Technol. 2020;103:36–48. doi: 10.1016/j.tifs.2020.06.003. [DOI] [Google Scholar]
- 148.Domingos-Lopes M.F.P., Nagy A., Stanton C., Ross P.R., Gelencsér E., Silva C.C.G. Immunomodulatory Activity of Exopolysaccharide Producing Leuconostoc citreum Strain Isolated from Pico Cheese. J. Funct. Foods. 2017;33:235–243. doi: 10.1016/j.jff.2017.03.054. [DOI] [Google Scholar]
- 149.Zhang J., Xiao Y., Wang H., Zhang H., Chen W., Lu W. Lactic Acid Bacteria-Derived Exopolysaccharide: Formation, Immunomodulatory Ability, Health Effects, and Structure-Function Relationship. Microbiol. Res. 2023;274:127432. doi: 10.1016/j.micres.2023.127432. [DOI] [PubMed] [Google Scholar]
- 150.Dargahi N., Johnson J.C., Apostolopoulos V. Immune Modulatory Effects of Probiotic Streptococcus Thermophilus on Human Monocytes. Biologics. 2021;1:396–415. doi: 10.3390/biologics1030023. [DOI] [Google Scholar]
- 151.Hickey A., Stamou P., Udayan S., Ramón-Vázquez A., Esteban-Torres M., Bottacini F., Woznicki J.A., Hughes O., Melgar S., Ventura M., et al. Bifidobacterium Breve Exopolysaccharide Blocks Dendritic Cell Maturation and Activation of CD4+ T Cells. Front. Microbiol. 2021;12:653587. doi: 10.3389/fmicb.2021.653587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liu Y., Zheng S., Cui J., Guo T., Zhang J., Li B. Alleviative Effects of Exopolysaccharide Produced by Lactobacillus helveticus KLDS1. 8701 on Dextran Sulfate Sodium-Induced Colitis in Mice. Microorganisms. 2021;9:2086. doi: 10.3390/microorganisms9102086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Young I.D., Latousakis D., Juge N. The Immunomodulatory Properties of β-2, 6 Fructans: A Comprehensive Review. Nutrients. 2021;13:1309. doi: 10.3390/nu13041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Xu Q., Yajima T., Li W., Saito K., Ohshima Y., Yoshikai Y. Levan (β-2, 6-Fructan), a Major Fraction of Fermented Soybean Mucilage, Displays Immunostimulating Properties via Toll-like Receptor 4 Signalling: Induction of Interleukin-12 Production and Suppression of T-Helper Type 2 Response and Immunoglobulin E Production. Clin. Exp. Allergy. 2006;36:94–101. doi: 10.1111/j.1365-2222.2006.02401.x. [DOI] [PubMed] [Google Scholar]
- 155.Wahab W.A.A., Shafey H.I., Mahrous K.F., Esawy M.A., Saleh S.A.A. Coculture of Bacterial Levans and Evaluation of Its Anti-Cancer Activity against Hepatocellular Carcinoma Cell Lines. Sci. Rep. 2024;14:3173. doi: 10.1038/s41598-024-52699-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Aquinas N., Bhat M.R., Selvaraj S. A Review Presenting Production, Characterization, and Applications of Biopolymer Curdlan in Food and Pharmaceutical Sectors. Polym. Bull. 2022;79:6905–6927. doi: 10.1007/s00289-021-03860-1. [DOI] [Google Scholar]
- 157.Ganie S.A., Rather L.J., Assiri M.A., Li Q. Recent Innovations (2020–2023) in the Approaches for the Chemical Functionalization of Curdlan and Pullulan: A Mini-Review. Int. J. Biol. Macromol. 2024;260:129412. doi: 10.1016/j.ijbiomac.2024.129412. [DOI] [PubMed] [Google Scholar]
- 158.Andreu S., von Kobbe C., Delgado P., Ripa I., Buzón M.J., Genescà M., Gironès N., del Moral-Salmoral J., Ramírez G.A., Zúñiga S. Dextran Sulfate from Leuconostoc mesenteroides B512F Exerts Potent Antiviral Activity against SARS-CoV-2 in Vitro and in Vivo. Front. Microbiol. 2023;14:1185504. doi: 10.3389/fmicb.2023.1185504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mirończuk-Chodakowska I., Kujawowicz K., Witkowska A.M. Beta-Glucans from Fungi: Biological and Health-Promoting Potential in the COVID-19 Pandemic Era. Nutrients. 2021;13:3960. doi: 10.3390/nu13113960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wu N., Ge X., Yin X., Yang L., Chen L., Shao R., Xu W. A Review on Polysaccharide Biosynthesis in Cordyceps militaris. Int. J. Biol. Macromol. 2024;260:129336. doi: 10.1016/j.ijbiomac.2024.129336. [DOI] [PubMed] [Google Scholar]
- 161.Afreen A., Ahmed Z., Khalid N., Ferheen I., Ahmed I. Optimization and Cholesterol-Lowering Activity of Exopolysaccharide from Lactiplantibacillus paraplantarum NCCP 962. Appl. Microbiol. Biotechnol. 2023;107:1189–1204. doi: 10.1007/s00253-023-12372-z. [DOI] [PubMed] [Google Scholar]
- 162.Zhao X., Zhong X., Liu X., Wang X., Gao X. Therapeutic and Improving Function of Lactobacilli in the Prevention and Treatment of Cardiovascular-Related Diseases: A Novel Perspective from Gut Microbiota. Front. Nutr. 2021;8:693412. doi: 10.3389/fnut.2021.693412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Al-Nabulsi A.A., Jaradat Z.W., Qudsi F.R.A., Elsalem L., Osaili T.M., Olaimat A.N., Esposito G., Liu S.-Q., Ayyash M.M. Characterization and Bioactive Properties of Exopolysaccharides Produced by Streptococcus thermophilus and Lactobacillus bulgaricus Isolated from Labaneh. LWT. 2022;167:113817. doi: 10.1016/j.lwt.2022.113817. [DOI] [Google Scholar]
- 164.Aloraini G.S., Albureikan M.O.I., Shahlol A.M.A., Shamrani T., Daghistani H., El-Nablaway M., Tharwat N.A., Elazzazy A.M., Basyony A.F., Ghareeb A. Biomedical and Therapeutic Potential of Marine-Derived Pseudomonas sp. Strain AHG22 Exopolysaccharide: A Novel Bioactive Microbial Metabolite. Rev. Adv. Mater. Sci. 2024;63:20240016. doi: 10.1515/rams-2024-0016. [DOI] [Google Scholar]
- 165.Ge Z., Wang D., Azi F., Zhao W., Wang P., Dong M., Wang J., Zhao Y., Zhao X. The Optimization of in Situ Exopolysaccharides Production in Lactobacillus helveticus MB2-1 Fermented Milk and Its Functional Characteristics in Vitro. Int. Dairy J. 2024;155:105969. doi: 10.1016/j.idairyj.2024.105969. [DOI] [Google Scholar]
- 166.Carvalho F.M., Teixeira-Santos R., Mergulhao F.J.M., Gomes L.C. The Use of Probiotics to Fight Biofilms in Medical Devices: A Systematic Review and Meta-Analysis. Microorganisms. 2020;9:27. doi: 10.3390/microorganisms9010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Giordani B., Parolin C., Vitali B. Lactobacilli as Anti-Biofilm Strategy in Oral Infectious Diseases: A Mini-Review. Front. Med. Technol. 2021;3:769172. doi: 10.3389/fmedt.2021.769172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Song Y., Sun M., Feng L., Liang X., Song X., Mu G., Tuo Y., Jiang S., Qian F. Antibiofilm Activity of Lactobacillus plantarum 12 Exopolysaccharides against Shigella flexneri. Appl. Environ. Microbiol. 2020;86:e00694-20. doi: 10.1128/AEM.00694-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Aliouche N., Sifour M., Kebsa W., Khennouf T., Ercan F., Ouled-Haddar H. Prophylactic Effect and Antiulcerogenic Potential of Probiotic Lactiplantibacillus plantarum E1K2R2 and Its Exopolysaccharide against Ibuprofen-Induced Acute Gastric Ulcer. Probiotics Antimicrob. Proteins. 2024;2024:1–16. doi: 10.1007/s12602-024-10321-4. [DOI] [PubMed] [Google Scholar]
- 170.Yu J., Chen Z., Zhou Q., Li P., Wu S., Zhou T., Gu Q. Exopolysaccharide from Lacticaseibacillus paracasei Alleviates Gastritis in Helicobacter Pylori-Infected Mice by Regulating Gastric Microbiota. Front. Nutr. 2024;11:1426358. doi: 10.3389/fnut.2024.1426358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Al-Qaysi S.A.S., Al-Haideri H., Al-Shimmary S.M., Abdulhameed J.M., Alajrawy O.I., Al-Halbosiy M.M., Moussa T.A.A., Farahat M.G. Bioactive Levan-Type Exopolysaccharide Produced by Pantoea agglomerans ZMR7: Characterization and Optimization for Enhanced Production. J. Microbiol. Biotechnol. 2021;31:696. doi: 10.4014/jmb.2101.01025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Erdal Altıntaş Ö., Toksoy Öner E., Çabuk A., Aytar Çelik P. Biosynthesis of Levan by Halomonas elongata 153B: Optimization for Enhanced Production and Potential Biological Activities for Pharmaceutical Field. J. Polym. Environ. 2023;31:1440–1455. doi: 10.1007/s10924-022-02681-1. [DOI] [Google Scholar]
- 173.Cao C., Bian Y., Cang W., Wu J., Wu R. Structural Characterization and Hepatoprotective Activity of Exopolysaccharide from Bacillus velezensis SN-1. J. Sci. Food Agric. 2023;103:738–749. doi: 10.1002/jsfa.12185. [DOI] [PubMed] [Google Scholar]
- 174.Lee M.-G., Joeng H., Shin J., Kim S., Lee C., Song Y., Lee B.-H., Park H.-G., Lee T.-H., Jiang H.-H. Potential Probiotic Properties of Exopolysaccharide-Producing Lacticaseibacillus paracasei EPS DA-BACS and Prebiotic Activity of Its Exopolysaccharide. Microorganisms. 2022;10:2431. doi: 10.3390/microorganisms10122431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bisson G., Comuzzi C., Giordani E., Poletti D., Boaro M., Marino M. An Exopolysaccharide from Leuconostoc mesenteroides Showing Interesting Bioactivities versus Foodborne Microbial Targets. Carbohydr. Polym. 2023;301:120363. doi: 10.1016/j.carbpol.2022.120363. [DOI] [PubMed] [Google Scholar]
- 176.Kavitake D., Tiwari S., Shah I.A., Devi P.B., Delattre C., Reddy G.B., Shetty P.H. Antipathogenic Potentials of Exopolysaccharides Produced by Lactic Acid Bacteria and Their Food and Health Applications. Food Control. 2023;152:109850. doi: 10.1016/j.foodcont.2023.109850. [DOI] [Google Scholar]
- 177.Ma L., Xu X., Peng Q., Yang S., Zhang Y., Tian D., Shi L., Qiao Y., Shi B. Exopolysaccharide from Lactobacillus casei NA-2 Attenuates Escherichia coli O157:H7 Surface Adhesion via Modulation of Membrane Surface Properties and Adhesion-Related Gene Expression. Microb. Pathog. 2022;173:105863. doi: 10.1016/j.micpath.2022.105863. [DOI] [PubMed] [Google Scholar]
- 178.Zammuto V., Spanò A., Agostino E., Macrì A., De Pasquale C., Ferlazzo G., Rizzo M.G., Nicolò M.S., Guglielmino S., Gugliandolo C. Anti-Bacterial Adhesion on Abiotic and Biotic Surfaces of the Exopolysaccharide from the Marine Bacillus Licheniformis B3-15. Mar. Drugs. 2023;21:313. doi: 10.3390/md21050313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.IS W.B. Biofilm Development and Approaches to Biofilm Inhibition by Exopolysaccharides. New Microbiol. 2022;45:227–236. [PubMed] [Google Scholar]
- 180.Giordani B., Naldi M., Croatti V., Parolin C., Erdoğan Ü., Bartolini M., Vitali B. Exopolysaccharides from Vaginal lactobacilli Modulate Microbial Biofilms. Microb. Cell Factories. 2023;22:45. doi: 10.1186/s12934-023-02053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Tao T., Zhang L., Yu T., Ma J., Lu S., Ren J., Li X., Guo X. Exopolysaccharide Production by Lactobacillus Plantarum T10 Is Responsible for the Probiotic Activity in Enhancing Intestinal Barrier Function in Vitro and in Vivo. Food Funct. 2024;15:3583–3599. doi: 10.1039/D4FO00526K. [DOI] [PubMed] [Google Scholar]
- 182.Xie Y., Pei F., Liu Y., Liu Z., Chen X., Xue D. Fecal Fermentation and High-Fat Diet-Induced Obesity Mouse Model Confirmed Exopolysaccharide from Weissella cibaria PFY06 Can Ameliorate Obesity by Regulating the Gut Microbiota. Carbohydr. Polym. 2023;318:121122. doi: 10.1016/j.carbpol.2023.121122. [DOI] [PubMed] [Google Scholar]
- 183.Pérez-Rivero C., López-Gómez J.P. Unlocking the Potential of Fermentation in Cosmetics: A Review. Fermentation. 2023;9:463. doi: 10.3390/fermentation9050463. [DOI] [Google Scholar]
- 184.Mahmoud M.G., Awady M.E.E., Selim M.S., Ibrahim A.Y., Ibrahim F.M., Mohamed S.S. Characterization of Biologically Active Exopolysaccharide Produced by Streptomyces sp. NRCG4 and Its Anti-Alzheimer Efficacy: In-Vitro Targets. J. Genet. Eng. Biotechnol. 2023;21:76. doi: 10.1186/s43141-023-00530-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sirin S., Aslim B. Characterization of Lactic Acid Bacteria Derived Exopolysaccharides for Use as a Defined Neuroprotective Agent against Amyloid Beta1–42-Induced Apoptosis in SH-SY5Y Cells. Sci. Rep. 2020;10:8124. doi: 10.1038/s41598-020-65147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Darilmaz D.O., Beyatli Y. Investigating Hydrophobicity and the Effect of Exopolysaccharide on Aggregation Properties of Dairy Propionibacteria Isolated from Turkish Homemade Cheeses. J. Food Prot. 2012;75:359–365. doi: 10.4315/0362-028X.JFP-11-225. [DOI] [PubMed] [Google Scholar]
- 187.Hooshdar P., Kermanshahi R.K., Ghadam P., Khosravi-Darani K. A Review on Production of Exopolysaccharide and Biofilm in Probiotics Like Lactobacilli and Methods of Analysis. Biointerface Res. Appl. Chem. 2020;10:6058–6075. doi: 10.33263/BRIAC105.60586075. [DOI] [Google Scholar]
- 188.Jahr H., Bahro R., Eichenlaub R. Genetics of phytopathology: Phytopathogenic bacteria. In: Esser K., Kadereit J.W., Lüttge U., Runge M., editors. Progress in Botany: Genetics Cell Biology and Physiology Systematics and Comparative Morphology Ecology and Vegetation Science. Springer; Berlin/Heidelberg, Germany: 1999. pp. 119–138. [Google Scholar]
- 189.Satpute S.K., Banat I.M., Dhakephalkar P.K., Banpurkar A.G., Chopade B.A. Biosurfactants, Bioemulsifiers and Exopolysaccharides from Marine Microorganisms. Biotechnol. Adv. 2010;28:436–450. doi: 10.1016/j.biotechadv.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 190.d’Abzac P., Bordas F., Joussein E., Van Hullebusch E.D., Piet N.L., Guibaud G. Metal Binding Properties of Extracellular Polymeric Substances Extracted from Anaerobic Granular Sludges. Environ. Sci. Pollut. Res. 2013;20:4509–4519. doi: 10.1007/s11356-012-1401-3. [DOI] [PubMed] [Google Scholar]
- 191.Zhang Y., Li D., Yang N., Jin Z., Xu X. Comparison of Dextran Molecular Weight on Wheat Bread Quality and Their Performance in Dough Rheology and Starch Retrogradation. LWT. 2018;98:39–45. [Google Scholar]
- 192.Du R., Yu L., Yu N., Ping W., Song G., Ge J. Characterization of Exopolysaccharide Produced by Levilactobacillus brevis HDE-9 and Evaluation of Its Potential Use in Dairy Products. Int. J. Biol. Macromol. 2022;217:303–311. doi: 10.1016/j.ijbiomac.2022.07.057. [DOI] [PubMed] [Google Scholar]
- 193.Kavitake D., Tiwari S., Devi P.B., Shah I.A., Reddy G.B., Shetty P.H. Production, Purification, and Functional Characterization of Glucan Exopolysaccharide Produced by Enterococcus Hirae Strain OL616073 of Fermented Food Origin. Int. J. Biol. Macromol. 2024;259:129105. doi: 10.1016/j.ijbiomac.2023.129105. [DOI] [PubMed] [Google Scholar]
- 194.Mummaleti G., Sarma C., Yarrakula S., Urla R., Gazula H. Production, Properties and Applications of Levan Polysaccharide. Food Humanit. 2024;3:100369. doi: 10.1016/j.foohum.2024.100369. [DOI] [Google Scholar]
- 195.Tintoré M., Cuñé J., Vu L.D., Poppe J., Van den Abbeele P., Baudot A., de Lecea C. A Long-Chain Dextran Produced by Weissella cibaria Boosts the Diversity of Health-Related Gut Microbes Ex Vivo. Biology. 2024;13:51. doi: 10.3390/biology13010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Besrour-Aouam N., Fhoula I., Hernández-Alcántara A.M., Mohedano M.L., Najjari A., Prieto A., Ruas-Madiedo P., López P., Ouzari H.-I. The Role of Dextran Production in the Metabolic Context of Leuconostoc and Weissella Tunisian Strains. Carbohydr. Polym. 2021;253:117254. doi: 10.1016/j.carbpol.2020.117254. [DOI] [PubMed] [Google Scholar]
- 197.Georgalaki M., Zoumpopoulou G., Anastasiou R., Kazou M., Tsakalidou E. Lactobacillus kefiranofaciens: From Isolation and Taxonomy to Probiotic Properties and Applications. Microorganisms. 2021;9:2158. doi: 10.3390/microorganisms9102158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Bibi A., Xiong Y., Rajoka M.S.R., Mehwish H.M., Radicetti E., Umair M., Shoukat M., Khan M.K.I., Aadil R.M. Recent Advances in the Production of Exopolysaccharide (EPS) from Lactobacillus spp. and Its Application in the Food Industry: A Review. Sustainability. 2021;13:12429. doi: 10.3390/su132212429. [DOI] [Google Scholar]
- 199.Kavitake D., Balyan S., Devi P.B., Shetty P.H. Evaluation of Oil-in-Water (O/W) Emulsifying Properties of Galactan Exopolysaccharide from Weissella confusa KR780676. J. Food Sci. Technol. 2020;57:1579–1585. doi: 10.1007/s13197-020-04262-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kermanshahi R.K., Khaniki G.J., Goudarzi L. Biosorption of Cd+2 and Pb+2 by Exopolysaccharide Extracted from Lactobacillus Fermentum 6b; Adsorption Isotherm and Kinetic Studies. Iran. J. Public Health. 2023;52:622–632. doi: 10.18502/ijph.v52i3.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Werning M.L., Hernández-Alcántara A.M., Ruiz M.J., Soto L.P., Dueñas M.T., López P., Frizzo L.S. Biological Functions of Exopolysaccharides from Lactic Acid Bacteria and Their Potential Benefits for Humans and Farmed Animals. Foods. 2022;11:1284. doi: 10.3390/foods11091284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wang Y., Li C., Liu P., Ahmed Z., Xiao P., Bai X. Physical characterization of exopolysaccharide produced by Lactobacillus plantarum KF5 isolated from Tibet Kefir. Carbohydrate Polymers. 2010;82:895–903. doi: 10.1016/j.carbpol.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 203.Li M., Li W., Li D., Tian J., Xiao L., Kwok L.-Y., Li W., Sun Z. Structure Characterization, Antioxidant Capacity, Rheological Characteristics and Expression of Biosynthetic Genes of Exopolysaccharides Produced by Lactococcus lactis subsp. Lactis IMAU11823. Food Chem. 2022;384:132566. doi: 10.1016/j.foodchem.2022.132566. [DOI] [PubMed] [Google Scholar]
- 204.Tarique M., Ali A.H., Kizhakkayil J., Gan R.-Y., Liu S.-Q., Kamal-Eldin A., Ayyash M. Investigating the Biological Activities and Prebiotic Potential of Exopolysaccharides Produced by Lactobacillus delbrueckii and Lacticaseibacillus rhamnosus: Implications for Gut Microbiota Modulation and Rheological Properties in Fermented Milk. Food Hydrocoll. Health. 2023;4:100162. doi: 10.1016/j.fhfh.2023.100162. [DOI] [Google Scholar]
- 205.Hu X., Pang X., Wang P.G., Chen M. Isolation and Characterization of an Antioxidant Exopolysaccharide Produced by Bacillus sp. S-1 from Sichuan Pickles. Carbohydr. Polym. 2019;204:9–16. doi: 10.1016/j.carbpol.2018.09.069. [DOI] [PubMed] [Google Scholar]
- 206.Yang J., Yang H. Recent Development in Se-Enriched Yeast, Lactic Acid Bacteria and Bifidobacteria. Crit. Rev. Food Sci. Nutr. 2023;63:411–425. doi: 10.1080/10408398.2021.1948818. [DOI] [PubMed] [Google Scholar]
- 207.Kodali V.P., Perali R.S., Sen R. Purification and Partial Elucidation of the Structure of an Antioxidant Carbohydrate Biopolymer from the Probiotic Bacterium Bacillus coagulans RK-02. J. Nat. Prod. 2011;74:1692–1697. doi: 10.1021/np1008448. [DOI] [PubMed] [Google Scholar]
- 208.Abdl Aali R.A.K., Al-Sahlany S.T.G. Gellan Gum as a Unique Microbial Polysaccharide: Its Characteristics, Synthesis, and Current Application Trends. Gels. 2024;10:183. doi: 10.3390/gels10030183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Gniewosz M., Pobiega K., Kraśniewska K., Synowiec A., Chaberek M., Galus S. Characterization and Antifungal Activity of Pullulan Edible Films Enriched with Propolis Extract for Active Packaging. Foods. 2022;11:2319. doi: 10.3390/foods11152319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Nwodo U.U., Green E., Okoh A.I. Bacterial Exopolysaccharides: Functionality and Prospects. Int. J. Mol. Sci. 2012;13:14002–14015. doi: 10.3390/ijms131114002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Aravamudhan A., Ramos D.M., Nada A.A., Kumbar S.G. Chapter 4—Natural polymers: Polysaccharides and their derivatives for biomedical applications. In: Kumbar S.G., Laurencin C.T., Deng M., editors. Natural and Synthetic Biomedical Polymers. Elsevier; Oxford, UK: 2014. pp. 67–89. [Google Scholar]
- 212.McHugh D.J. Production, Properties and Uses of Alginates. Prod. Util. Prod. Commer. Seaweeds FAO Fish. Tech. Pap. 1987;288:58–115. [Google Scholar]
- 213.Mocanu G., Mihai D., Dulong V., Picton L., Lecerf D. New Anionic Amphiphilic Thermosensitive Pullulan Derivatives. Carbohydr. Polym. 2011;84:276–281. doi: 10.1016/j.carbpol.2010.11.034. [DOI] [Google Scholar]
- 214.CP Kelco Ingredients & Products. [(accessed on 29 August 2024)]. Available online: https://www.cpkelco.com/products/
- 215.Borschiver S., Vasconcelos R.C., Silva F.C., Freitas G.C., Santos P.E., do Bomfim R.O. Technology Roadmap for Hyaluronic Acid and Its Derivatives Market. Biofuels Bioprod. Biorefining. 2019;13:435–444. doi: 10.1002/bbb.1936. [DOI] [Google Scholar]
- 216.PULLULAN|Research and Development Stories|Research and Development|Nagase Viita Co., Ltd. [(accessed on 29 August 2024)]. Available online: https://group.nagase.com/viita/en/rd/story/03/
- 217.Emre Oz Y., Keskin-Erdogan Z., Safa N., Esin Hames Tuna E. A Review of Functionalised Bacterial Cellulose for Targeted Biomedical Fields. J. Biomater. Appl. 2021;36:648–681. doi: 10.1177/0885328221998033. [DOI] [PubMed] [Google Scholar]
- 218.Meito Sangyo | Dextrans and Dextran Derivatives. [(accessed on 29 August 2024)]. Available online: https://www.meito-sangyo.co.jp/kaseihin/index_e/dextran/
- 219.Dahiya D., Nigam P.S. Dextran of Diverse Molecular-Configurations Used as a Blood-Plasma Substitute, Drug-Delivery Vehicle and Food Additive Biosynthesized by Leuconostoc, Lactobacillus and Weissella. Appl. Sci. 2023;13:12526. doi: 10.3390/app132212526. [DOI] [Google Scholar]
- 220.Singhvi G., Hans N., Shiva N., Kumar Dubey S. Chapter 5—Xanthan gum in drug delivery applications. In: Hasnain M.S., Nayak A.K., editors. Natural Polysaccharides in Drug Delivery and Biomedical Applications. Academic Press; Cambridge, MA, USA: 2019. pp. 121–144. [Google Scholar]
- 221.Szekalska M., Puciłowska A., Szymańska E., Ciosek P., Winnicka K. Alginate: Current Use and Future Perspectives in Pharmaceutical and Biomedical Applications. Int. J. Polym. Sci. 2016;2016:7697031. doi: 10.1155/2016/7697031. [DOI] [Google Scholar]
- 222.Urtuvia V., Maturana N., Acevedo F., Peña C., Díaz-Barrera A. Bacterial Alginate Production: An Overview of Its Biosynthesis and Potential Industrial Production. World J. Microbiol. Biotechnol. 2017;33:198. doi: 10.1007/s11274-017-2363-x. [DOI] [PubMed] [Google Scholar]
- 223.Feketshane Z., Alven S., Aderibigbe B.A. Gellan Gum in Wound Dressing Scaffolds. Polymers. 2022;14:4098. doi: 10.3390/polym14194098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Muthukumar T., Song J.E., Khang G. Biological Role of Gellan Gum in Improving Scaffold Drug Delivery, Cell Adhesion Properties for Tissue Engineering Applications. Mol. Basel Switz. 2019;24:4514. doi: 10.3390/molecules24244514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Singh R.S., Kaur N., Singh D., Purewal S.S., Kennedy J.F. Pullulan in Pharmaceutical and Cosmeceutical Formulations: A Review. Int. J. Biol. Macromol. 2023;231:123353. doi: 10.1016/j.ijbiomac.2023.123353. [DOI] [PubMed] [Google Scholar]
- 226.Fallacara A., Baldini E., Manfredini S., Vertuani S. Hyaluronic Acid in the Third Millennium. Polymers. 2018;10:701. doi: 10.3390/polym10070701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Li Q., Qi X., Zhang Z. Intra-Articular Oxygen-Ozone versus Hyaluronic Acid in Knee Osteoarthritis: A Meta-Analysis of Randomized Controlled Trials. Int. J. Surg. 2018;58:3–10. doi: 10.1016/j.ijsu.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 228.Gershon R.K., Kondo K. Infectious Immunological Tolerance. Immunology. 1971;21:903. [PMC free article] [PubMed] [Google Scholar]
- 229.Gershon R.K., Kondo K. Cell Interactions in the Induction of Tolerance: The Role of Thymic Lymphocytes. Immunology. 1970;18:723. [PMC free article] [PubMed] [Google Scholar]
- 230.Sakaguchi S., Sakaguchi N., Asano M., Itoh M., Toda M. Immunologic Self-Tolerance Maintained by Activated T Cells Expressing IL-2 Receptor Alpha-Chains (CD25). Breakdown of a Single Mechanism of Self-Tolerance Causes Various Autoimmune Diseases. J. Immunol. 1995;155:1151–1164. doi: 10.4049/jimmunol.155.3.1151. [DOI] [PubMed] [Google Scholar]
- 231.Kanmani P., Albarracin L., Kobayashi H., Iida H., Komatsu R., Humayun Kober A.K.M., Ikeda-Ohtsubo W., Suda Y., Aso H., Makino S., et al. Exopolysaccharides from Lactobacillus delbrueckii OLL1073R-1 Modulate Innate Antiviral Immune Response in Porcine Intestinal Epithelial Cells. Mol. Immunol. 2018;93:253–265. doi: 10.1016/j.molimm.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 232.Makino S., Sato A., Goto A., Nakamura M., Ogawa M., Chiba Y., Hemmi J., Kano H., Takeda K., Okumura K., et al. Enhanced Natural Killer Cell Activation by Exopolysaccharides Derived from Yogurt Fermented with Lactobacillus Delbrueckii ssp. Bulgaricus OLL1073R-1. J. Dairy Sci. 2016;99:915–923. doi: 10.3168/jds.2015-10376. [DOI] [PubMed] [Google Scholar]
- 233.Wang J., Wu T., Fang X., Min W., Yang Z. Characterization and Immunomodulatory Activity of an Exopolysaccharide Produced by Lactobacillus Plantarum JLK0142 Isolated from Fermented Dairy Tofu. Int. J. Biol. Macromol. 2018;115:985–993. doi: 10.1016/j.ijbiomac.2018.04.099. [DOI] [PubMed] [Google Scholar]
- 234.Hougaard A.B., Pindstrup H., Arneborg N., Andersen M.L., Skibsted L.H. Free Radical Formation by Lactobacillus Acidophilus NCFM Is Enhanced by Antioxidants and Decreased by Catalase. Food Res. Int. 2016;79:81–87. doi: 10.1016/j.foodres.2015.12.003. [DOI] [Google Scholar]
- 235.Hsieh F.-C., Lan C.-C.E., Huang T.-Y., Chen K.-W., Chai C.-Y., Chen W.-T., Fang A.-H., Chen Y.-H., Wu C.-S. Heat-Killed and Live Lactobacillus Reuteri GMNL-263 Exhibit Similar Effects on Improving Metabolic Functions in High-Fat Diet-Induced Obese Rats. Food Funct. 2016;7:2374–2388. doi: 10.1039/c5fo01396h. [DOI] [PubMed] [Google Scholar]
- 236.Azad M.A.K., Sarker M., Wan D. Immunomodulatory effects of probiotics on cytokine profiles. BioMed Res. Int. 2018;2018:8063647. doi: 10.1155/2018/8063647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Wang A.N., Cai C.J., Zeng X.F., Zhang F.R., Zhang G.L., Thacker P.A., Wang J.J., Qiao S.Y. Dietary Supplementation with L Actobacillus Fermentum I5007 Improves the Anti-oxidative Activity of Weanling Piglets Challenged with Diquat. J. Appl. Microbiol. 2013;114:1582–1591. doi: 10.1111/jam.12188. [DOI] [PubMed] [Google Scholar]
- 238.Lu W., Chen S., Lai C., Guo W., Fu L., Andrieu J.-M. Induction of CD8+ Regulatory T Cells Protects Macaques against SIV Challenge. Cell Rep. 2012;2:1736–1746. doi: 10.1016/j.celrep.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 239.Xiu L., Zhang H., Hu Z., Liang Y., Guo S., Yang M., Du R., Wang X. Immunostimulatory Activity of Exopolysaccharides from Probiotic Lactobacillus Casei WXD030 Strain as a Novel Adjuvant in Vitro and in Vivo. Food Agric. Immunol. 2018;29:1086–1105. doi: 10.1080/09540105.2018.1513994. [DOI] [Google Scholar]
- 240.Hongying F., Xianbo W., Fang Y., Yang B., Beiguo L. Oral Immunization with Recombinant Lactobacillus Acidophilus Expressing the Adhesin Hp0410 of Helicobacter Pylori Induces Mucosal and Systemic Immune Responses. Clin. Vaccine Immunol. 2014;21:126–132. doi: 10.1128/CVI.00434-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Marcobal A., Liu X., Zhang W., Dimitrov A.S., Jia L., Lee P.P., Fouts T.R., Parks T.P., Lagenaur L.A. Expression of Human Immunodeficiency Virus Type 1 Neutralizing Antibody Fragments Using Human Vaginal Lactobacillus. AIDS Res. Hum. Retroviruses. 2016;32:964–971. doi: 10.1089/aid.2015.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Suebwongsa N., Lulitanond V., Mayo B., Yotpanya P., Panya M. Development of an Escherichia coli–Lactobacillus casei Shuttle Vector for Heterologous Protein Expression in Lactobacillus casei. Springerplus. 2016;5:169. doi: 10.1186/s40064-016-1760-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Tan T.S., Syed Hassan S., Yap W.B. Expression of Surface-bound Nonstructural 1 (NS 1) Protein of Influenza Virus A H5N1 on Lactobacillus casei Strain C1. Lett. Appl. Microbiol. 2017;64:446–451. doi: 10.1111/lam.12738. [DOI] [PubMed] [Google Scholar]
- 244.Wang J., Fang X., Wu T., Fang L., Liu C., Min W. In Vitro Immunomodulatory Effects of Acidic Exopolysaccharide Produced by Lactobacillus planetarium JLAU103 on RAW264.7 Macrophages. Int. J. Biol. Macromol. 2020;156:1308–1315. doi: 10.1016/j.ijbiomac.2019.11.169. [DOI] [PubMed] [Google Scholar]
- 245.Yu M., Qi R., Chen C., Yin J., Ma S., Shi W., Wu Y., Ge J., Jiang Y., Tang L. Immunogenicity of Recombinant Lactobacillus casei-expressing F4 (K88) Fimbrial Adhesin FaeG in Conjunction with a Heat-labile Enterotoxin A (LTAK 63) and Heat-labile Enterotoxin B (LTB) of Enterotoxigenic Escherichia Coli as an Oral Adjuvant in Mice. J. Appl. Microbiol. 2017;122:506–515. doi: 10.1111/jam.13352. [DOI] [PubMed] [Google Scholar]
- 246.Asgher M., Qamar S.A., Iqbal H.M.N. Microbial Exopolysaccharide-Based Nano-Carriers with Unique Multi-Functionalities for Biomedical Sectors. Biologia. 2021;76:673–685. doi: 10.2478/s11756-020-00588-7. [DOI] [Google Scholar]
- 247.Tassa C., Shaw S.Y., Weissleder R. Dextran-Coated Iron Oxide Nanoparticles: A Versatile Platform for Targeted Molecular Imaging, Molecular Diagnostics, and Therapy. Acc. Chem. Res. 2011;44:842–852. doi: 10.1021/ar200084x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Topal M., Arslan Topal E.I. Extracellular polymeric substances in textile industry. In: Muthu S.S., editor. Sustainable Approaches in Textiles and Fashion: Fibres, Raw Materials and Product Development. Springer Nature; Singapore: 2022. pp. 23–40. [Google Scholar]
- 249.Altamira-Algarra B., Rueda E., Lage A., San León D., Martínez-Blanch J.F., Nogales J., García J., Gonzalez-Flo E. New Strategy for Bioplastic and Exopolysaccharides Production: Enrichment of Field Microbiomes with Cyanobacteria. New Biotechnol. 2023;78:141–149. doi: 10.1016/j.nbt.2023.10.008. [DOI] [PubMed] [Google Scholar]
- 250.Abou-alfitooh S.A.M., El-hoshoudy A.N. Eco-Friendly Modified Biopolymers for Enhancing Oil Production: A Review. J. Polym. Environ. 2024;32:2457–2483. doi: 10.1007/s10924-023-03132-1. [DOI] [Google Scholar]
- 251.Gao C. Potential of Welan Gum to Enhance Oil Recovery. J. Pet. Explor. Prod. Technol. 2015;5:197–200. doi: 10.1007/s13202-014-0135-9. [DOI] [Google Scholar]
- 252.He S., Zhang M., Chen B., Wei X., Su X. Modification of Welan Gum with Poly(2-Oxazoline) to Obtain Thermoviscosifying Polymer for Enhanced Oil Recovery. Int. J. Biol. Macromol. 2024;263:130193. doi: 10.1016/j.ijbiomac.2024.130193. [DOI] [PubMed] [Google Scholar]
- 253.Sengupta S., Dey S. Microbial Exo-Polysaccharides (EPS): Role in Agriculture and Environment. Agric. Food. 2019;1:4–8. [Google Scholar]
- 254.Costa O.Y.A., Raaijmakers J.M., Kuramae E.E. Microbial Extracellular Polymeric Substances: Ecological Function and Impact on Soil Aggregation. Front. Microbiol. 2018;9:1636. doi: 10.3389/fmicb.2018.01636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Banerjee A., Sarkar S., Govil T., González-Faune P., Cabrera-Barjas G., Bandopadhyay R., Salem D.R., Sani R.K. Extremophilic Exopolysaccharides: Biotechnologies and Wastewater Remediation. Front. Microbiol. 2021;12:721365. doi: 10.3389/fmicb.2021.721365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Costa J., Baratto M.C., Spinelli D., Leone G., Magnani A., Pogni R. A Novel Bio-Adhesive Based on Chitosan-Polydopamine-Xanthan Gum for Glass, Cardboard and Textile Commodities. Polymers. 2024;16:1806. doi: 10.3390/polym16131806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Ahmed F., Hutton-Prager B. Xanthan Gum Modification to Surface and Interfacial Properties between Soil-Based Matrixes and Petroleum Oils to Minimize Soil Pollution. Appl. Res. 2024;3:e202400096. doi: 10.1002/appl.202400096. [DOI] [Google Scholar]
- 258.Fu X., Qin F., Liu T., Zhang X. Enhanced Oil Recovery Performance and Solution Properties of Hydrophobic Associative Xanthan Gum. Energy Fuels. 2022;36:181–194. doi: 10.1021/acs.energyfuels.1c02941. [DOI] [Google Scholar]
- 259.De Melo Teixeira L., da Silva Santos É., dos Santos R.S., Ramos A.V.G., Baldoqui D.C., Bruschi M.L., Gonçalves J.E., Gonçalves R.A.C., de Oliveira A.J.B. Production of Exopolysaccharide from Klebsiella oxytoca: Rheological, Emulsifying, Biotechnological Properties, and Bioremediation Applications. Int. J. Biol. Macromol. 2024;278:134400. doi: 10.1016/j.ijbiomac.2024.134400. [DOI] [PubMed] [Google Scholar]
- 260.Fortuna B., Logar J., Sorze A., Valentini F., Smolar J. Influence of Xanthan Gum-Based Soil Conditioners on the Geotechnical Properties of Soils. Appl. Sci. 2024;14:4044. doi: 10.3390/app14104044. [DOI] [Google Scholar]
- 261.Garmasheva I., Tomila T., Kharkhota M., Oleschenko L. Exopolysaccharides of Lactic Acid Bacteria as Protective Agents against Bacterial and Viral Plant Pathogens. Int. J. Biol. Macromol. 2024;276:133851. doi: 10.1016/j.ijbiomac.2024.133851. [DOI] [PubMed] [Google Scholar]
- 262.Zhao J., Wang Y., Liu Q., Wang Y., Long C. Pullulan-Based Coatings Carrying Biocontrol Yeast Mixed with NaCl to Control Citrus Postharvest Disease Decays. Pestic. Biochem. Physiol. 2024;205:106108. doi: 10.1016/j.pestbp.2024.106108. [DOI] [Google Scholar]
- 263.Srivastava G.K., Martinez-Rodriguez S., Md Fadilah N.I., Looi Qi Hao D., Markey G., Shukla P., Fauzi M.B., Panetsos F. Progress in Wound-Healing Products Based on Natural Compounds, Stem Cells, and MicroRNA-Based Biopolymers in the European, USA, and Asian Markets: Opportunities, Barriers, and Regulatory Issues. Polymers. 2024;16:1280. doi: 10.3390/polym16091280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Barros De Medeiros V.P., Da Costa W.K.A., Da Silva R.T., Pimentel T.C., Magnani M. Microalgae as Source of Functional Ingredients in New-Generation Foods: Challenges, Technological Effects, Biological Activity, and Regulatory Issues. Crit. Rev. Food Sci. Nutr. 2022;62:4929–4950. doi: 10.1080/10408398.2021.1879729. [DOI] [PubMed] [Google Scholar]