Abstract
Infection of susceptible mice with the low-neurovirulence Theiler's murine encephalomyelitis virus strain BeAn results in an inflammatory demyelinating disease similar to multiple sclerosis. While the majority of virus antigen is detected in central nervous system macrophages (Mφs), few infiltrating Mφs are infected. We used the myelomonocytic precursor M1 cell line to study BeAn virus-Mφ interactions in vitro to elucidate mechanisms for restricted virus expression. We have shown that restricted BeAn infection of M1 cells differentiated in vitro (M1-D) results in apoptosis. In this study, BeAn infection of gamma interferon (IFN-γ)-activated M1-D cells also resulted in apoptosis but with no evidence of virus replication or protein expression. RNase protection assays of M1-D cellular RNA revealed up-regulation of Fas and the p55 chain of the tumor necrosis factor alpha (TNF-α) receptor transcripts with IFN-γ activation. BeAn infection of activated cells resulted in increased caspase 8 mRNA transcripts and the appearance of TNF-α-related apoptosis-inducing ligand (TRAIL) 4 h postinfection. Both unactivated and activated M1-D cells expressed TRAIL receptors (R1 and R2), but only activated cells were killed by soluble TRAIL. Activated cells were also susceptible to soluble FasL- and TNF-α-induced apoptosis. The data suggest that IFN-γ-activated M1-D cell death receptors become susceptible to their ligands and that the cells respond to BeAn virus infection by producing the ligands TNF-α and TRAIL to kill the susceptible cells. Unactivated cells are not susceptible to FasL or TRAIL and require virus replication to initiate apoptosis. Therefore, two mechanisms of apoptosis induction can be triggered by BeAn infection: an intrinsic pathway requiring virus replication and an extrinsic pathway signaling through the death receptors.
Theiler's murine encephalomyelitis virus (TMEV) is a member of the family Picornaviridae, genus Cardiovirus, and a natural enteric pathogen of mice. TMEV has been divided into two groups based on neurovirulence following intracerebral inoculation of genetically susceptible mice. Infection with BeAn, a low-neurovirulence strain, results in a chronic, demyelinating disease of the central nervous system (CNS). Persistence of BeAn virus leads to activation of major histocompatibility complex class II-restricted CD4+ Th1 lymphocytes directed at virus epitopes (11, 13, 14, 33, 34) and immunopathologic damage of myelin. This animal model has been used as an experimental analogue of multiple sclerosis.
During TMEV persistence, the major virus antigen burden resides in CNS macrophages (Mφs) (12, 29, 37); however, only a relatively small percentage of Mφs infiltrating demyelinating lesions contain detectable virus antigen (29), and an infected Mφ produces only 1 to 5 PFU (12). To investigate virus-Mφ interactions that might lead to restricted virus replication, we have used the myelomonocytic precursor cell line, M1. Recently, it was reported that the M1 cell line was susceptible to virus infection only after differentiation into Mφ-like cells (M1-D cells) (22). BeAn infection of M1-D cells is highly restricted and results in apoptotic cell death that requires virus replication. In the present study, M1-D cells were treated with gamma interferon (IFN-γ) prior to BeAn virus infection to determine whether activated Mφs secreting antiviral cytokines would inhibit BeAn virus replication and to explain, at least in part, the low virus titers in the spinal cords of infected mice. As expected, BeAn-infected, IFN-γ-activated M1-D cells showed no evidence of virus replication; unforeseen was the observation that activated M1-D cells died by apoptosis.
We describe experiments to define the mechanism(s) by which activated M1-D cells die after BeAn infection. Soluble FasL, tumor necrosis factor alpha (TNF-α), and TNF-α apoptosis-inducing ligand (TRAIL) induced significant cell death in M1-D cells after IFN-γ activation. RNase protection assay (RPA) showed increased Fas and TNF-R p55 (p55 chain of the TNF-α receptor) mRNA transcript levels with activation alone and increased TRAIL mRNA with activation and infection.
One possible scenario suggested by the data is that IFN-γ activation sensitizes these cells to death-inducing ligands. Virus infection after activation also causes increased IFN-α/β (22) and results in up-regulation of TRAIL and increased secretion of TNF-α, both of which result in cell death. Thus, depending on the activation state, BeAn virus induces apoptosis in Mφs either through an intrinsic mechanism requiring virus replication or through an extrinsic mechanism involving the death receptors and their ligands.
MATERIALS AND METHODS
Cells, viruses, and reagents.
M1-D cells were differentiated from the M1 cell line with supernatants from L929 and P388D1 cells as previously described (22) and maintained in complete medium containing 10% fetal bovine serum. Cells were either left untreated or treated with 100 U of IFN-γ (Sigma, St. Louis, Mo.) per ml for 24 h unless otherwise specified. Soluble TRAIL was a generous gift of James Pan (Genentech, South San Francisco, Calif.), TNF-α was purchased from Sigma, and soluble FasL was purchased from Alexis Corporation (San Diego, Calif.). TNF-α was analyzed by the mouse TNF-α immunoassay kit, Quantikine M, from R&D Systems (Minneapolis, Minn.). The inhibitors soluble TNF-α RII (sTNF-RII) and recombinant human TRAIL-R2:Fc (rhTRAIL-R2:Fc) were obtained from Oncogene Research Products (Cambridge, Mass.) and from Alexis Corporation, respectively. The origin and passage history of the BeAn virus stock have been described (39). Virus titers of clarified lysates of infected cells were determined by standard plaque assay on BHK-21 cells (39).
Virus infections.
After virus adsorption at a multiplicity of infection of 10, or as indicated, for 45 min at 24°C, M1-D cells were washed with phosphate-buffered saline, pH 7.2, and incubated in complete RPMI medium containing 5% fetal bovine serum at 37°C in a 5% CO2 atmosphere for the indicated times.
MTT assay.
Cell viability was determined by the conversion of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to blue formazan crystals as previously described (23). Briefly, 104 cells were incubated for 2 to 4 h with 50 μl of MTT (1 mg/ml) 20 to 24 h after infection and/or treatment with cytokines. Formazan crystals were dissolved in 100 μl of dimethyl sulfoxide, and the optical density at 560 nm (OD560) was read on a UV Max microplate reader (Molecular Diagnostics, Palo Alto, Calif.). Statistical analysis was performed on quadruplicate samples using Cricket Graph 1.3 unless otherwise indicated.
Flow cytometry.
After blocking nonspecific antibody binding with 10% goat serum and/or 2 μl of anti-CD32/16 (FcR) (PharMingen, San Diego, Calif.), cells were incubated with polyclonal rabbit anti-BeAn antiserum (1:1,000), polyclonal rabbit anti-TRAIL antiserum (1:100)(Alexis Corporation), or hamster anti-FasL monoclonal antibody (1:100) (PharMingen). Secondary antibodies were fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit immunoglobulin G (IgG) F(ab')2 (1:200) (Cappel/Organon Teknikon, Durham, N.C.) and biotinylated anti-hamster IgG cocktail (1:100) (PharMingen). A 1:200 dilution of avidin-FITC (PharMingen) was used as the fluorochrome for the biotinylated antibody. Cytoplasmic antigen was detected as previously described (21). After staining, cells were fixed in 1% paraformaldehyde and analyzed on a FACSCalibur (Becton Dickinson, Palo Alto, Calif.). Data were evaluated using CELLQuest 3.1f supplied with the instrument. Annexin V staining using Annexin V-FITC apoptosis detection kit (Sigma) was performed according to the manufacturer's instructions.
Western blot analysis.
Cell lysates were prepared in buffer containing 50 mM Tris-HCl, pH 7.4, 1% NP-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EGTA, 1 mM phenylmethylsulfonyl fluoride, and 100 μl of protease inhibitor cocktail (Sigma). Cell lysates were clarified by low-speed centrifugation to remove nuclei and debris, and protein content was determined with Bio-Rad DC protein assay kit (Hercules, Calif.) according to the manufacturer's instructions. Samples (40 μg/lane) were electrophoresed on 10 to 12% polyacrylamide gels and transferred to ProBlot membranes (Applied Biosystems, Foster City, Calif.). Membranes were blocked overnight with Tris-buffered saline containing 5% nonfat dry milk and 0.02% Tween 20, washed extensively, and incubated with rabbit polyclonal anti-human TRAIL-R1 or R2 (1:500) (BioSource International, Camarillo, Calif.), anti-human TRAIL (1:1,000) (Alexis Corporation) or anti-caspase 8 (1:100) (Santa Cruz Biotechnology, Santa Cruz, Calif.). After a washing, the blots were incubated with horseradish peroxidase-conjugated goat anti-rabbit antibody (Sigma), washed again, and analyzed by enhanced chemiluminescence (ECL) using SuperSignal West Dura as a substrate (Pierce, Rockford, Ill.).
Assay for viral RNA replication.
Viral RNA replication was assayed by incorporation of [3H]uridine in the presence of actinomycin D as previously described (23). Briefly, after virus adsorption, 2 × 104 cells were incubated in a 96-well plate with 100 μl of complete medium containing 5 μg of actinomycin D per ml and 10 μCi of [3H]uridine (ICN; 15 to 25 Ci/mmol) per ml. At the indicated times, samples were harvested with a PHD Cell Harvester (Cambridge Technologies, Watertown, Mass.) and radioactivity was determined using a Beckman scintillation counter (LS5000TD; Palo Alto, Calif.). The mean and standard deviation of quadruplicate samples were calculated using Cricket Graph 1.3.
Immunoprecipitation of virus capsid proteins.
Immunoprecipitation of [35S]methionine-labeled virus capsid proteins was as previously described (23). Briefly, 106 cells were incubated with 100 μCi of l-[35S]methionine (ICN; 100 Ci/mmol) for 18 h postinfection (p.i.) and lysed in radioimmunoprecipitation assay buffer. Lysates were clarified by centrifugation, and protein content was determined as described above. After preclearing with normal rabbit serum, BeAn proteins were immunoprecipitated with 5 μl of polyclonal anti-BeAn rabbit antiserum and protein G-coupled Sepharose beads (Sigma). Samples were solubilized in sample buffer and electrophoresed on SDS-polyacrylamide gels.
Assay for caspase activity.
Caspase protease activity was measured in cell lysates by release of aminomethylcoumarin from the substrate peptide, acetyl-Asp-Glu-Val-Asp (DEVD), as previously described (22).
RPA.
RNA was isolated from M1-D cells using TRIZOL Reagent (Life Technologies, Grand Island, N.Y.) according to the manufacturer's instructions. The RiboQuant Multi-Probe RNase protection assay (RNA) (PharMingen) was used according to the manufacturer's instructions to analyze RNA expression of the bcl-2 family (mAPO-1) and the TNF superfamily members (mAPO-2). Probes were synthesized using [α-35S]UTP instead of [α-32P]UTP. Densitometry scans were done on the STORM 860 PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.) using ImageQuant version 5.0 software. The numbers were derived as ratios of the housekeeping gene, L32.
RESULTS
BeAn virus-induced apoptosis of IFN-γ-activated M1-D cells.
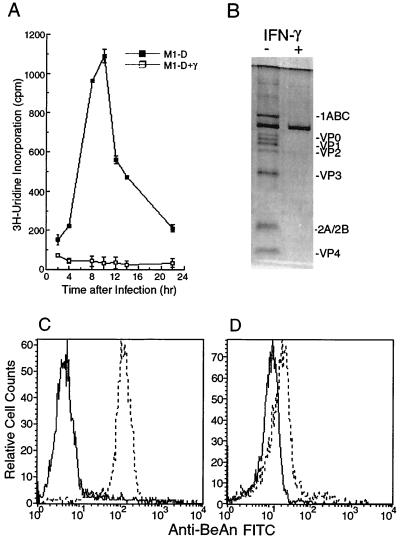
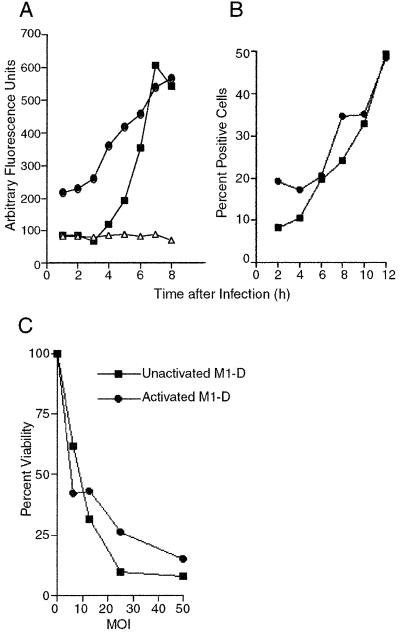
While viral RNA replication (Fig. 1A), expression of capsid proteins (Fig. 1B), and production of infectious virus (22) were measurable in M1-D cells, there was no evidence of viral RNA replication after activation with IFN-γ as measured by [3H]uridine incorporation (Fig. 1A) or of viral protein expression as assayed by immunoprecipitation (Fig. 1B). Mean fluorescence intensities of BeAn virus antigen staining by flow cytometry in M1-D cells and activated M1-D cells were 145 and 18, respectively (Fig. 1C and D) (mean fluorescence intensities for uninfected control cells were 4.6 and 9.7). Although flow cytometry analysis indicated the presence of low levels of viral proteins in activated M1-D cells, this probably represents residual virus and not newly synthesized viral proteins. Plaque assays of lysates from unactivated and activated M1-D cells showed that 2 to 2.5 PFU were produced in each M1-D cell (four samples from two separately differentiated populations done in duplicate) and showed consistent titers of <0.1 PFU for each activated cell. Since activated M1-D cells produce 10-fold higher levels of IFN-α/β than do M1-D cells (22), the lack of BeAn replication and protein expression in activated M1-D cells was not surprising (Fig. 1A, B, and D). However, activated M1-D cells showed apoptotic changes after BeAn infection including cell rounding, surface blebbing, and condensed nuclei and loss of adherence (not shown). To confirm the mechanism of virus-induced cell death of activated M1-D cells, caspase activity and annexin V binding were assayed as early indicators of apoptosis (32, 42, 47). Caspase activity increased fivefold in both unactivated and activated infected M1-D cells by 8 h p.i. compared to uninfected controls (Fig. 2A). The kinetics of caspase induction were similar in the two cell populations beginning ≈4 h p.i., peaking at 8 and 10 h and declining to undetectable levels by 24 h (data not shown). The kinetics of annexin V binding paralleled that of caspase activity, beginning 4 to 6 h p.i. (Fig. 2B). Together these results indicate that both unactivated and activated M1-D cells died by apoptosis after BeAn virus infection (summarized in Table 1).
FIG. 1.
Characteristics of BeAn virus infection in M1-D and IFN-γ-activated M1-D cells. (A) BeAn RNA replication in unactivated (■) or IFN-γ-activated (□) M1-D cells. Cells were incubated in 100 μl of medium containing 1 μCi of [3H]uridine and 0.5 μg of actinomycin D and harvested at 2-h intervals. Data are presented as means ± standard deviations of 6 samples. (B) Immunoprecipitation of BeAn virus capsid proteins from lysates of [35S]methionine-labeled BeAn-infected unactivated and IFN-γ-activated M1-D cells. One million cell equivalents were lysed in lysis buffer (see Materials and Methods), precleared with normal rabbit serum, and immunoprecipitated with rabbit polyclonal anti-BeAn antiserum. Immunoprecipitated proteins were resolved on a 12% polyacrylamide gel; virus proteins are indicated. The experiment was repeated twice with similar results. (C and D) Representative flow cytometry histograms of permeabilized uninfected (solid line) and infected (dashed line) unactivated (C) and IFN-γ-activated (D) M1-D cells 6 h p.i. stained for BeAn virus antigen.
FIG. 2.
Evidence for apoptosis in unactivated and IFN-γ-activated M1-D cells. (A) Kinetics of caspase activity in BeAn-infected unactivated (■) and IFN-γ-activated (●) M1-D cells and uninfected control cells (▵). Lysates from 106 cells were analyzed for the ability to cleave the DEVD-aminomethylcoumarin substrate. Data are means of duplicate samples with microtiter plate background values subtracted and are representative of two kinetic experiments. Caspase activity was measured in three other preparations of M1-D cells at 8.5 h p.i. with similar results. (B) Kinetics of annexin V binding to BeAn-infected, unactivated (■) and activated (●) M1-D cells. Background values for uninfected cells were 8.2% ± 2.1% and 17.0% ± 6.5%, respectively. Values are representative of two kinetic experiments. (C) MTT assay of cell viability with increasing multiplicities of infection of BeAn virus in unactivated (■) and activated (●) M1-D cells. The results shown are representative of four independent experiments. Quadruplicate samples were averaged, and the results are expressed as percentages of uninfected controls. The standard deviation for the OD readings was less than 0.1 for all samples.
TABLE 1.
Summary of BeAn virus infection in unactivated and IFN-γ-activated M1-D cells
| Cells | Differentiation state | Infectiona | Apoptosisb |
|---|---|---|---|
| M1-D | Mφ-like, unactivated | Restricted | Yes |
| IFN-γ M1-D | Mφ-like, activated | None | Yes |
Infection as determined by viral RNA replication, virus antigen expression, and infectious virus titer.
Apoptosis measured by morphology, annexin-V binding, and caspase 3 activity.
Up-regulation of TRAIL in BeAn-infected, IFN-γ-activated M1-D cells.
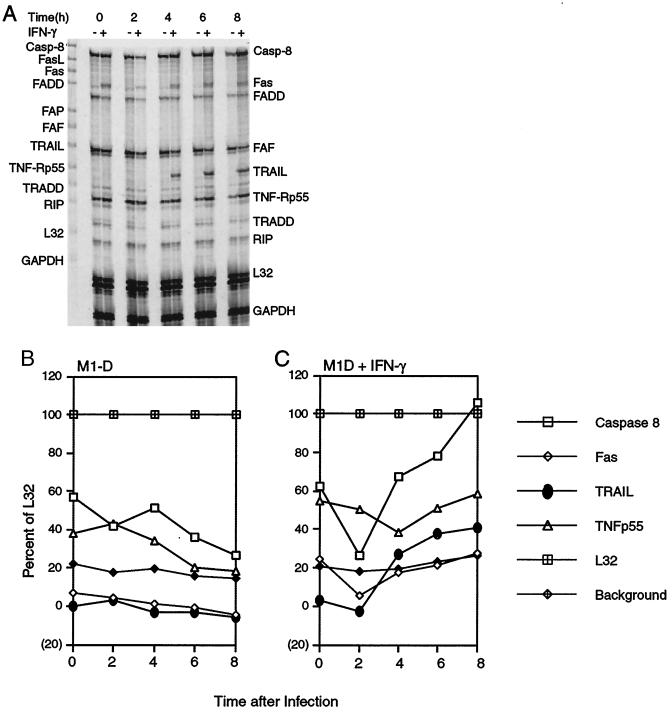
To determine whether the TNF family of proteins was altered during IFN-γ activation and/or BeAn virus infection, total RNA was isolated at various times p.i. and analyzed by RPA. mRNA levels of three TNF family members (FAF, TRADD, and RIP) (Fig. 3A) did not change under the conditions tested, and that of FasL was not expressed. Densitometric analysis relative to the housekeeping gene, L32, revealed that caspase 8 and TNF-R p55 mRNA levels decreased with time after infection of unactivated M1-D cells (Fig. 3B). IFN-γ activation alone up-regulated Fas and TNF-R p55 mRNAs, neither of which changed after infection (Fig. 3C). Caspase 8 mRNA expression increased almost twofold after infection of activated cells, consistent with its role as an initiator caspase in apoptosis (42). TRAIL mRNA was expressed in activated M1-D cells beginning 4 h p.i. and increased 1.5-fold by 8 h (Fig. 3A and C). Neither IFN-γ activation nor BeAn virus infection alone was sufficient to initiate TRAIL mRNA transcription in unactivated M1-D cells (Fig. 3A and C). Both signals were required to up-regulate TRAIL mRNA, and its transcription correlated with the kinetics of apoptosis (Fig. 2B).
FIG. 3.
TRAIL mRNA expression in M1-D cells. (A) RPA of RNA isolated from unactivated and IFN-γ-activated M1-D cells at 2-h intervals after BeAn virus infection. Probes are shown in the far left lane; protected RNA bands are labeled on the right and were identified by size according to manufacturer's instructions. The time course was repeated twice, and samples were subjected to RPA twice with similar results. (B and C) Densitometry scans of RPA illustrated in panel A: caspase 8 (□), Fas (◊), TRAIL (●), TNF-R p55 (▵), L32 housekeeping gene ( ), and background (
), and background ( ) in unactivated (B) and IFN-γ-activated (C) M1-D cells.
) in unactivated (B) and IFN-γ-activated (C) M1-D cells.
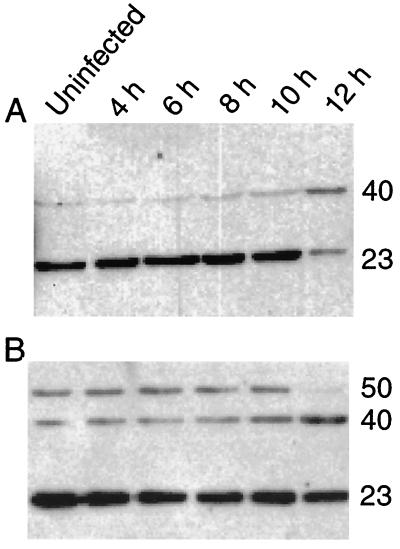
Since the increase in caspase 8 mRNA in activated M1-D cells was unexpected, immunoblotting of cell lysates harvested 4 to 8 h p.i. with a polyclonal rabbit anti-caspase 8 antibody that detects both the proenzyme and the cleavage products was performed. Procaspase 8 (50 to 55 kDa) was easily detected in activated M1-D cells (Fig. 4B). The cleavage products (∼40 and 23 kDa) were detected in all lysates, even in the uninfected cells (Fig. 4A). However, only upon longer exposure (with higher background) was procaspase 8 detected in unactivated cells (not shown). Although some apoptotic cells are always observed in M1-D cell cultures, the high amount of cleavage products in uninfected cells remains unexplained.
FIG. 4.
Caspase 8 expression in M1-D cells. Lysates from unactivated (A) or IFN-γ-activated (B) M1-D cells at various times after infection are shown. Mouse caspase 8 zymogen is ∼50 to 55 kDa, while cleaved products recognized by this antibody are ∼40 and 23 kDa as shown. The ECL was exposed for 2 min; the zymogen can be seen in unactivated M1-D cells on longer exposure (20 min); however, background levels are much higher. Cleavage products are seen in the uninfected cell lysates from both cell populations (A and B). More of the procaspase 8 is seen in the IFN-γ-activated M1-D cells than in the unactivated cells.
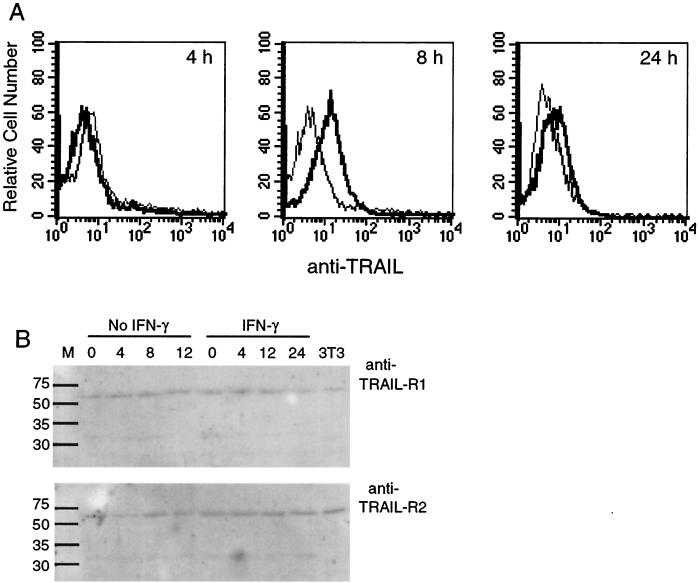
Flow cytometry confirmed TRAIL protein expression 8 h p.i. in activated M1-D cells decreasing by 24 h p.i. (Fig. 5A), and the results were confirmed by Western blotting (not shown). FasL was not detectable by flow cytometry even in the presence of metalloprotease inhibitors (not shown).
FIG. 5.
TRAIL protein expression in M1-D cells. (A) Flow cytometry of cytoplasmic TRAIL expression in unactivated (light line) and IFN-γ-activated M1-D cells (bold line) 4, 8, and 24 h p.i., respectively. (B) Western blot of unactivated and IFN-γ-activated M1-D cell lysates prepared from samples harvested 4, 8, 12, and 24 h p.i. Polyclonal anti-human TRAIL-R1 (62 kDa) and R2 (62 kDa) (1:500) followed by horseradish peroxidase-conjugated goat anti-rabbit IgG antibody (1:50,000) were used for detection with SuperSignal West Dura substrate by ECL. 3T3 cell lysate was used as a positive control as suggested by the manufacturer.
Western blot analysis of the TRAIL receptors R1 (DR4) and R2 (DR5) revealed a 62-kDa band in unactivated and activated M1-D cells regardless of infection (Fig. 5B). Staining uninfected mouse 3T3 cells with rabbit polyclonal antibodies to human TRAIL-R1 and -R2 showed that the antibodies cross-reacted with mouse receptors for TRAIL (Fig. 5B). A BLAST search for mouse TRAIL receptor sequences revealed a single expressed sequence tag clone with homology to human TRAIL-R2 (DR5) but no sequence homology to TRAIL-R1 (Mouse EST Project, Washington University, St. Louis, Mo.). Whether the two proteins identified as mouse TRAIL receptors are actually two distinct proteins or cross-reactive species remains to be determined. Nonetheless, M1-D cells expressed TRAIL receptor proteins prior to activation and infection.
Effects of sTRAIL, FasL, and TNF-α on M1-D cell viability.
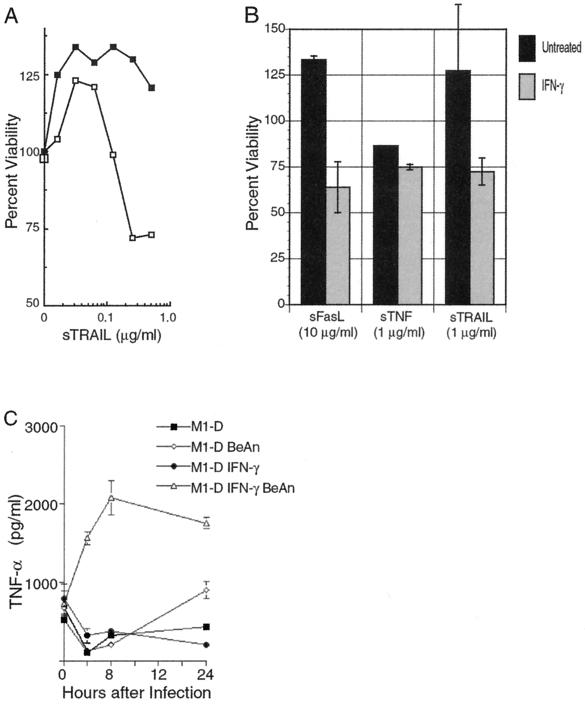
To determine whether TRAIL receptors were functional in unactivated and activated M1-D cells, soluble TRAIL (sTRAIL) was added to cell cultures and cell viability was analyzed by the MTT assay. Cell death in activated M1-D cells was induced in a dose-dependent manner and averaged 28% (72% viability) at 1 μg of soluble TRAIL per ml (Fig. 6A). Interestingly, unactivated M1-D cells proliferated in the presence of TRAIL, averaging 125% above control values. These results were consistent in two separate experiments using two independently derived M1-D cell populations. The low biological activity of TRAIL in these experiments probably reflects the absence of artificial trimerization, which is required for full biological activity of TRAIL, TNF-α, and FasL (5, 45, 46). Nevertheless, TRAIL at higher concentrations significantly decreased cell viability over control cultures in a dose-dependent fashion (Fig. 6A).
FIG. 6.
Ability of soluble death ligands to induce apoptosis in unactivated and IFN-γ-activated M1-D cells. (A) Percent viability, measured by the MTT assay, of unactivated (■) and IFN-γ-activated (□) M1-D cells treated with increasing amounts of soluble TRAIL. Formazan crystal formation was measured 20 to 22 h after treatment with soluble TRAIL. Quadruplicate samples were averaged, and the results are expressed as percentages of values for uninfected controls (P < 0.02). Results are representative of three independent experiments. (B) Percent viability of unactivated (black columns) and IFN-γ-activated (gray columns) M1-D cells with soluble TRAIL, FasL, and TNF-α measured by the MTT assay. Data are presented as means ± standard deviations (SD) of four independent measurements with two different M1-D cell populations. Concentrations are shown. (C) TNF-α in supernatants of M1-D cells measured by immunoassay. Supernatants collected at time zero were harvested 24 h after the initial cultures were plated. Symbols: ■, uninfected M1-D; ◊, BeAn-infected M1-D; ●, IFN-γ-activated, uninfected M1-D; ▵, IFN-γ-activated, BeAn-infected M1-D. Means of quadruplicate samples and SD are shown.
Since both Fas and TNF-R p55 RNA were upregulated with activation, their respective ligands, soluble FasL and TNF-α, were examined for their effects. When soluble FasL and TNF-α were added to activated M1-D cell cultures, viability levels similar to those using sTRAIL were observed, although 10-fold more FasL than TRAIL or TNF-α was required to induce equivalent levels of cell death (Fig. 6B). In unactivated M1-D cells, TNF-α killed to levels comparable to that for activated M1-D cells, whereas both TRAIL and FasL stimulated cells to proliferate. The stimulatory activity for Fas-FasL interaction has been reported in T cells and human monocytes (28, 31).
Another possible mechanism of apoptosis induction is signaling through TNF-R, because TNF-R p55 mRNA was up-regulated after infection (Fig. 3). Since soluble TNF-α was as effective as TRAIL and FasL in killing activated M1-D cells and activated Mφs are known to secrete TNF-α (1), TNF-α levels in the supernatant of cell cultures were measured by an immunoassay. Supernatants collected 24 h after IFN-γ treatment showed a 1.5-fold increase in TNF-α concentration compared to untreated cells (Fig. 6C). Activated M1-D cell supernatants collected 24 h p.i. secreted 8.5-fold more TNF-α compared to uninfected, activated cells. TNF-α levels in activated M1-D cells rose quickly and plateaued at 8 h p.i. Uninfected, IFN-γ-activated cells showed little change after the initial 24-h accumulation of TNF-α (shown on the graph as 0 h). TNF-α levels in supernatants from unactivated, BeAn-infected M1-D cells increased 2.1-fold 24 h p.i. compared to uninfected cells.
To assess the relative contributions of TRAIL, FasL, and TNF-α in BeAn virus-induced apoptosis, antibodies to the three molecules were added to cell cultures at concentrations varying from 10 to 0.001 μg/ml. (Three sources of anti-TRAIL antibodies were used.) Incubation with anti-TRAIL, anti-TNF-α, or anti-FasL did not inhibit apoptosis of infected, unactivated M1-D cells, but potentiated cell death of infected, IFN-γ-activated cells (not shown). The antibodies were not toxic when tested on uninfected cells. Although inhibition of apoptosis with ligand-specific antibodies has been reported in the literature (9, 10, 25, 26, 48), we were unable to duplicate the response with the above-mentioned antibodies. The significance of these data is not clear in light of the ability of soluble ligands to induce apoptosis in activated M1-D cells. Perhaps the Fc receptors on these Mφ-like cells are providing another cell death-inducing signal.
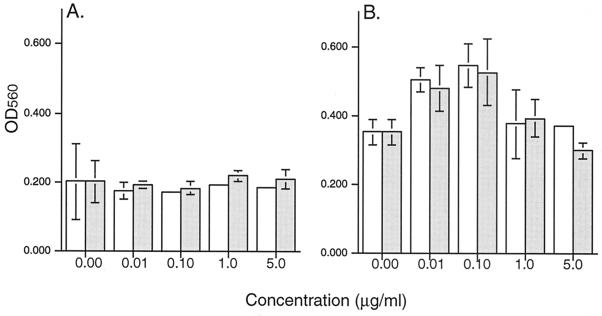
Another approach to assess the relative contributions of TNF-α and TRAIL is to use the corresponding receptor molecules to neutralize ligand function. sTNF-RII and rhTRAIL-R2:Fc were added to M1-D cultures 4 h p.i., and cell viability was measured by the MTT assay (Fig. 7). Infected, unactivated M1-D cells were not protected by these molecules; however, both soluble receptors protected activated M1-D cells from virus-induced cell death by 36 to 52% in a dose-dependent fashion. Addition of inhibitors 1 h p.i. had no effect.
FIG. 7.
Inhibition of BeAn virus-induced cell death by TNF-RII and TRAIL-R2. BeAn-infected unactivated (A) or IFN-γ-activated (B) M1-D cells were incubated with increasing concentrations of either sTNF-RII (open bars) or rhTRAIL-R2:Fc (gray bars), and cell viability was assayed by MTT 20 h p.i. Results are shown as mean OD560 and standard deviations of quadruplicate samples. The inhibitors protected IFN-γ-activated M1-D cells, whereas no protection occurred in the unactivated M1-D cells. This experiment was repeated with similar trends.
DISCUSSION
Apoptosis can be triggered by a number of stimuli, including viral infections (36) and cell stress (19) (intrinsic pathway), and cell surface signaling through the death receptors and their ligands (TNF-α, FasL, and TRAIL) (5) (extrinsic pathway). Our data indicate that both pathways are utilized in BeAn-infected Mφs depending on their activation state. Although poliovirus proteins 3C (7) and 2A (15) induce apoptosis directly, the mechanism of apoptosis induction by picornavirus infections has not been clearly defined. Using a yeast two-hybrid system, Henke et al. (20) demonstrated binding of coxsackievirus B3 VP2 to the proapoptotic siva protein; however, TMEV VP2 showed no such binding. Agol et al. (2, 3) described two competing death programs initiated with poliovirus infection: one dependent on caspases to initiate the apoptosis program and the other independent of caspases and resulting in cell death without the hallmarks of apoptosis (canonical cytopathic effect).
Our data show that BeAn virus induces caspase 3-dependent apoptosis in both unactivated and activated M1-D cells. However, BeAn-induced apoptosis in activated M1-D cells is not accompanied by virus replication, whereas apoptosis in unactivated cells requires virus replication. In addition, caspase 8 (an initiator caspase) mRNA expression increases with infection of activated cells, whereas it appears to be degraded in unactivated cells (Fig. 3A and C). The results of Western blotting for caspase 8 protein indicate that the protein is cleaved prior to infection in both unactivated and activated M1-D cells. Caspase 8, as well as the other 13 caspases, resides in the cytoplasm as inactive zymogens, which are cleaved upon activation to initiate the apoptosis program (19). Recent evidence has shown that caspase 8 is cleaved in activated T cells and may be involved in proliferation (4, 27). Los et al. (30) have postulated that caspase 8 may be involved in cell-cycle progression, another checkpoint assuring that only healthy cells progress through the cell cycle. Our data suggest that the caspase 8 proenzyme is already cleaved in M1-D cells. Thus, since M1-D cells have been differentiated in vitro, caspase 8 in these cells may be activated and function in the control of the cell cycle. A more detailed analysis of the caspase family members in M1-D as well as the precursor M1 cells will be required to resolve this issue. Nonetheless, it appears that activated M1-D cells have more of the unprocessed protein present than the unactivated cells, which supports the findings of the RPA.
Since IFN-γ activation sensitizes death receptors to signaling from their ligands (Fig. 4B), a phenomenon termed activation-induced cell death (24), and BeAn virus infection stimulates production of the ligands, a logical explanation for these data is that M1-D cells generate signals for their own demise. The only source of death-inducing ligands in this system is the activated M1-D cells themselves. Two candidate ligands for apoptosis induction in BeAn-infected, activated M1-D cells are TRAIL and TNF-α. After infection, TRAIL mRNA and protein expression are up-regulated and high levels of TNF-α are found in the supernatant (Fig. 3 and 6C, respectively). Thus, apoptosis through either ligand is possible. Inhibition of cell death by both sTNF-RII and rhTRAIL-R2 indicates that both molecules are used in this system. These data for cell death receptor and ligand expression are summarized in Table 2.
TABLE 2.
Summary of cell death receptor and ligand expression after BeAn virus infection
| Receptor or ligand | M1-D | IFN-γ-activated M1-D |
|---|---|---|
| TRAILabc | No | Yes |
| TRAIL R1/R2b | Yes | Yes |
| FasLc | No | No |
| Fasa | Yes | Yes |
| Secreted TNF-αd | Yes | Yes |
| TNF-α-Ra | Yes | Yes |
Measured by mRNA expression.
Measured by Western blot.
Measured by flow cytometry.
Measured by enzyme-linked immunosorbent assay.
Two cell death-signaling pathways have been described: an intrinsic pathway due to cellular stress which is dependent on mitochondrial release of cytochrome c and caspase 9 activation and controlled through the Bcl-2 family (17) and an extrinsic pathway which is dependent on ligand binding to death receptors, recruitment of FADD/Mort adaptor molecules, and caspase 8 activation (38). A recent report by Walczak et al. (44) describes experiments in which overexpression of Bcl-2 had no effect on TRAIL-induced apoptosis and expression of caspase 8 was necessary and sufficient for TRAIL-induced apoptosis. Sprick et al. (41) also reported that FADD/Mort1 and caspase 8 are essential for TRAIL-induced apoptosis. Additional evidence that the death receptors and their ligands are involved in BeAn virus-induced apoptosis in activated M1-D cells is that Bcl-2 overexpression had no effect either on caspase 3 activation or on cell viability (unpublished). RPA analysis for seven bcl-2 family members (bcl-W, bfl1, bcl-X, bak, bax, bcl-2, and bad) showed no change in expression with time after infection (unpublished). Therefore, in activated M1-D cells the intrinsic mitochondria-dependent apoptosis mechanism is not used and apoptosis occurs through the extrinsic pathway. Confirmation of this hypothesis awaits detailed analysis of the caspase cascade in these cells.
Recently, various investigators have reported TRAIL up-regulation after infection with several viruses including human cytomegalovirus in fibroblasts (40), measles virus in human monocyte-derived dendritic cells, monocytes, and CD3-activated T cells (43), and reovirus infection in fibroblasts (10). To our knowledge, this is the first report to show that a picornavirus up-regulates TRAIL in an activated Mφ-like cell line and broadens the range of viruses affecting the TRAIL apoptosis pathway.
It was recently reported that human monocyte-derived Mφs expressed TRAIL after IFN-γ and IFN-α treatment but resisted TRAIL-mediated apoptosis (18). IFN-γ and IFN-α by themselves did not up-regulate TRAIL expression in our system, but did sensitize M1-D cells to signaling from their death receptors. Additional signals, provided by BeAn virus infection, were required to upregulate TRAIL. Whether this discrepancy is due to species differences, use of primary versus tumor cells, or the different methods of Mφ differentiation induction is unclear.
IFNs (type 1 and 2) induce double-stranded RNA-dependent protein kinase (PKR), which mediates apoptosis (16). To assess the role of PKR in our system, we obtained undifferentiated M1 cells expressing a PKR mutation (p68Δ6 [6, 35]); however, we were unable to differentiate these cells in vitro (unpublished data). Infection of mouse peritoneal Mφs and CNS microglia from normal mice and mice with the PKR mutation could provide further information on apoptosis-signaling mechanisms in Mφs at different stages of activation. Until recently, we have been unable to infect peritoneal Mφs with BeAn virus; however, we are now examining the role of TRAIL, TNF-α, and Fas in apoptosis induction by infection of these cells. Continuous up-regulation of IFN-γ and TNF-α in the CNS has been reported during the course of TMEV-induced demyelinating disease (8), which may play a role in the immunopathology of the disease. Whether TRAIL also contributes to the immunopathology induced by TMEV is unknown but now merits further study.
ACKNOWLEDGMENTS
We thank J. Pan for soluble TRAIL, A. Kimchi for the M1 cells expressing the PKR mutation, H. Yagita for the N2B2 antibody, and Mark Trottier for critical review of the manuscript.
This work was supported by NIH grant NS23349 and the Leiper Foundation.
REFERENCES
- 1.Adams D O, Hamilton T A. Molecular basis of macrophage activation diversity and its origins. In: Lewis C E, McGee J O D, editors. The natural immune system: the macrophage. New York, N.Y: IRL Press; 1992. pp. 77–114. [Google Scholar]
- 2.Agol V I, Belov G A, Bienz K, Egger D, Kolesnikova M S, Raikhlin N T, Romanova L I, Smirnova E A, Tolskaya E A. Two types of death of poliovirus-infected cells: caspase involvement in the apoptosis but not cytopathic effect. Virology. 1998;252:343–353. doi: 10.1006/viro.1998.9438. [DOI] [PubMed] [Google Scholar]
- 3.Agol V I, Belov G A, Bienz K, Egger D, Kolesnikova M S, Romanova L I, Sladkova L V, Tolskaya E A. Competing death programs in poliovirus-infected cells: commitment switch in the middle of the infectious cycle. J Virol. 2000;74:5534–5541. doi: 10.1128/jvi.74.12.5534-5541.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alam A, Cohen L Y, Aouad S, Sékaly R-P. Early activation of caspases during T lymphocyte stimulation results in selective substrate cleavage in nonapoptotic cells. J Exp Med. 1999;190:1879–1890. doi: 10.1084/jem.190.12.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashkenazi A, Dixit V M. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 6.Barber G N, Jagus R, Meurs E F, Hovanessian A G, Katze M G. Molecular mechanisms responsible for malignant transformation by regulatory and catalytic domain variants of the interferon-induced enzyme RNA-dependent protein kinase. J Biol Chem. 1995;270:17423–17428. doi: 10.1074/jbc.270.29.17423. [DOI] [PubMed] [Google Scholar]
- 7.Barco A, Feduchi E, Carrasco L. Poliovirus protease 3C(pro) kills cells by apoptosis. Virology. 2000;266:352–360. doi: 10.1006/viro.1999.0043. [DOI] [PubMed] [Google Scholar]
- 8.Begolka W S, Vanderlugt C L, Rahbe S M, Miller S D. Differential expression of inflammatory cytokines parallels progression of central nervous system pathology in two clinically distinct models of multiple sclerosis. J Immunol. 1998;161:4437–4446. [PubMed] [Google Scholar]
- 9.Bermudez L E, Parker A, Petrofsky M. Apoptosis of Mycobacterium avium-infected macrophages is mediated by both tumour necrosis factor (TNF) and Fas, and involves the activation of caspases. Clin Exp Immunol. 1999;116:94–99. doi: 10.1046/j.1365-2249.1999.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clarke P, Meintzer S M, Gibson S, Widmann C, Garrington T P, Johnson G L, Tyler K L. Reovirus-induced apoptosis is mediated by TRAIL. J Virol. 2000;74:8135–8139. doi: 10.1128/jvi.74.17.8135-8139.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clatch R J, Melvold R W, Miller S D, Lipton H L. Theiler's murine encephalomyelitis virus (TMEV)-induced demyelinating disease in mice is influenced by the H-2D region: correlation with TEMV-specific delayed-type hypersensitivity. J Immunol. 1985;135:1408–1414. [PubMed] [Google Scholar]
- 12.Clatch R J, Miller S D, Metzner R, Dal Canto M C, Lipton H L. Monocytes/macrophages isolated from the mouse central nervous system contain infectious Theiler's murine encephalomyelitis virus (TMEV) Virology. 1990;176:244–254. doi: 10.1016/0042-6822(90)90249-q. [DOI] [PubMed] [Google Scholar]
- 13.Gerety S J, Karpus W J, Cubbon A R, Goswami R G, Rundell M K, Peterson J D, Miller S D. Class II-restricted T cell responses in Theiler's murine encephalomyelitis virus-induced demyelinating disease. V. Mapping of a dominant immunopathologic VP2 T cell epitope in susceptible SJL/J mice. J Immunol. 1994;152:908–918. [PubMed] [Google Scholar]
- 14.Gerety S J, Rundell M K, Dal Canto M C, Miller S D. Class II-restricted T cell responses in Theiler's murine encephalomyelitis virus-induced demyelinating disease. VI. Potentiation of demyelination with and characterization of an immunopathologic CD4+ T cell line specific for an immunodominant VP2 epitope. J Immunol. 1994;152:919–929. [PubMed] [Google Scholar]
- 15.Goldstaub D, Gradi A, Bercovitch Z, Grosmann Z, Nophar Y, Luria S, Sonnenberg N, Kahana C. Poliovirus 2A protease induces apoptotic cell death. Mol Cell Biol. 2000;20:1271–1277. doi: 10.1128/mcb.20.4.1271-1277.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodbourn S, Didcock L, Randall R E. Interferons: cell signalling, immune modulation, antiviral responses and virus countermeasures. J Gen Virol. 2000;81:2341–2364. doi: 10.1099/0022-1317-81-10-2341. [DOI] [PubMed] [Google Scholar]
- 17.Green D R, Reed J C. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 18.Griffith T S, Wiley S R, Kubin M Z, Sedger L M, Maliszewski C R, Fanger N A. Monocyte-mediated tumoricidal activity via the tumor necrosis factor-related cytokine, TRAIL. J Exp Med. 1999;189:1343–1354. doi: 10.1084/jem.189.8.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grutter M G. Caspases: key players in programmed cell death. Curr Opin Struct Biol. 2000;10:649–655. doi: 10.1016/s0959-440x(00)00146-9. [DOI] [PubMed] [Google Scholar]
- 20.Henke A, Launhardt H, Klement K, Stelzner A, Zell R, Munder T. Apoptosis in coxsackievirus B3-caused diseases: interaction between the capsid protein VP2 and the proapoptotic protein siva. J Virol. 2000;74:4284–4290. doi: 10.1128/jvi.74.9.4284-4290.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jelachich M L, Bandyopadhyay P, Blum K, Lipton H L. Theiler's virus growth in murine macrophage cell lines depends on the state of differentiation. Virology. 1995;209:437–444. doi: 10.1006/viro.1995.1276. [DOI] [PubMed] [Google Scholar]
- 22.Jelachich M L, Bramlage C, Lipton H L. Differentiation of M1 myeloid precursor cells into macrophages results in binding and infection by Theiler's murine encephalomyelitis virus and apoptosis. J Virol. 1999;73:3227–3235. doi: 10.1128/jvi.73.4.3227-3235.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jelachich M L, Lipton H L. Theiler's murine encephalomyelitis virus kills restrictive but not permissive cells by apoptosis. J Virol. 1996;70:6856–6861. doi: 10.1128/jvi.70.10.6856-6861.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kabelitz D, Pohl T, Pechhold K. Activation-induced cell death (apoptosis) of mature peripheral T lymphocytes. Immunol Today. 1993;14:338–339. doi: 10.1016/0167-5699(93)90231-9. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan M J, Ray D, Mo R R, Yung R L, Richardson B C. TRAIL (Apo2 ligand) and TWEAK (Apo3 ligand) mediate CD4+ T cell killing of antigen-presenting macrophages. J Immunol. 2000;164:2897–2904. doi: 10.4049/jimmunol.164.6.2897. [DOI] [PubMed] [Google Scholar]
- 26.Kayagaki N, Yamaguchi N, Nakayama M, Takeda K, Akiba H, Tsutsui H, Okamura H, Nakanishi K, Okumura K, Yagita H. Expression and function of TNF-related apoptosis-inducing ligand on murine activated NK cells. J Immunol. 1999;163:1906–1913. [PubMed] [Google Scholar]
- 27.Kennedy N J, Kataoka T, Tschopp J, Budd R C. Caspase activation is required for T cell proliferation. J Exp Med. 1999;190:1891–1895. doi: 10.1084/jem.190.12.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiener P A, Davis P M, Rankin B M, Klebanoff S J, Ledbetter J A, Starling G C, Liles W C. Human monocytic cells contain high levels of intracellular Fas ligand. J Immunol. 1997;159:1594–1598. [PubMed] [Google Scholar]
- 29.Lipton H L, Twaddle G, Jelachich M L. The predominant virus antigen burden is present in macrophages in Theiler's murine encephalomyelitis virus-induced demyelinating disease. J Virol. 1995;69:2525–2533. doi: 10.1128/jvi.69.4.2525-2533.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Los M, Stroh C, Janicke R U, Engels I H, Schulze-Osthoff K. Caspases: more than just killers? Trends Immunol. 2001;22:31–34. doi: 10.1016/s1471-4906(00)01814-7. [DOI] [PubMed] [Google Scholar]
- 31.Lynch D H, Ramsdell F, Alderson M R. Fas and FasL in the homeostatic regulation of immune responses. Immunol Today. 1995;16:569–574. doi: 10.1016/0167-5699(95)80079-4. [DOI] [PubMed] [Google Scholar]
- 32.Martin S J, Reutelingsperger C P M, McGahon A J, Rader J A, van Schie R C A A, LaFace D M, Green D R. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller S D, Clatch R J, Pevear D C, Trotter J L, Lipton H L. Class II-restricted T cell responses in Theiler's murine encephalomyelitis virus (TMEV)-induced demyelinating disease. I. Cross-specificity among TMEV substrains and related picornaviruses, but not myelin proteins. J Immunol. 1987;138:3776–3784. [PubMed] [Google Scholar]
- 34.Miller S D, Gerety S J, Kennedy M K, Peterson J D, Trotter J L, Touhy V K, Waltenbaugh C, Dal Canto M C, Lipton H L. Class-II restricted T cell responses in Theiler's murine encephalomyelitis virus (TMEV)-induced demyelinating disease. III. Failure of neuroantigen-specific immune tolerance to affect the clinical course of demyelination. J Neuroimmunol. 1989;26:9–23. doi: 10.1016/0165-5728(90)90115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raveh T, Hovanessian A G, Meurs E F, Sonenberg N, Kimchi A. Double-stranded RNA-dependent protein kinase mediates c-Myc suppression induced by type I interferons. J Biol Chem. 1996;271:25479–25484. doi: 10.1074/jbc.271.41.25479. [DOI] [PubMed] [Google Scholar]
- 36.Razvi E S, Welch R M. Apoptosis in viral infections. Adv Virus Res. 1995;45:1–60. doi: 10.1016/s0065-3527(08)60057-3. [DOI] [PubMed] [Google Scholar]
- 37.Rossi C P, Delcroix M, Huitinga I, McAllister A, van Rooijen N, Claassen E, Brahic M. Role of macrophages during Theiler's virus infection. J Virol. 1997;71:3336–3340. doi: 10.1128/jvi.71.4.3336-3340.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roy S, Nicholson D W. Cross-talk in cell death signaling. J Exp Med. 2000;192:21–26. [PubMed] [Google Scholar]
- 39.Rozhon E J, Kratochvil J D, Lipton H L. Analysis of genetic variation in Theiler's virus during persistent infection in the mouse central nervous system. Virology. 1983;128:16–32. doi: 10.1016/0042-6822(83)90315-x. [DOI] [PubMed] [Google Scholar]
- 40.Sedger L M, Shows D M, Blanton R A, Peschon J J, Cosman R G, Wiley S R. IFN-gamma mediates a novel antiviral activity through dynamic modulation of TRAIL and TRAIL receptor expression. J Immunol. 1999;163:920–926. [PubMed] [Google Scholar]
- 41.Sprick M R, Weigand M A, Rieser E, Rauch C T, Juo P, Blenis J, Krammer P H, Walczak H. FADD/MORT1 and caspase-8 are recruited to TRAIL receptors 1 and 2 and are essential for apoptosis mediated by TRAIL receptor 2. Immunity. 2000;12:599–609. doi: 10.1016/s1074-7613(00)80211-3. [DOI] [PubMed] [Google Scholar]
- 42.Thornberry N A, Lazebnik Y A. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 43.Vidalain P O, Azocar O, Lamouille B, Astier A, Rabourdin-Combe C, Servet-Delprat C. Measles virus induces functional TRAIL production by human dendritic cells. J Virol. 2000;74:556–559. doi: 10.1128/jvi.74.1.556-559.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walczak H, Bouchon A, Stahl H, Krammer P H. Tumor necrosis factor-related apoptosis-inducing ligand retains its apoptosis-inducing capacity on Bcl-2-or Bcl-xL-overexpressing chemotherapy-resistant tumor cells. Cancer Res. 2000;60:3051–3057. [PubMed] [Google Scholar]
- 45.Walczak H, Miller R E, Ariail K, Gliniak B, Griffith T S, Kubin M, Chin W, Jones J, Woodward A, Le T, Smith C, Smolak P, Goodwin R G, Rauch C T, Schuh J C L, Lynch D H. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5:157–163. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 46.Wiley S R, Schooley K, Smolak P J, Din W S, Huang C-P, Nicholl J K, Sutherland G R, Smith T D, Rauch C, Smith C A, Goodwin R G. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 47.Zhang G, Gurtu V, Kain S R, Yan G. Early detection of apoptosis using a fluorescent conjugate of annexin V. Biotechniques. 1997;23:525–531. doi: 10.2144/97233pf01. [DOI] [PubMed] [Google Scholar]
- 48.Zhao S, Asgary Z, Wang Y, Goodwin R, Andreeff M, Younes A. Functional expression of TRAIL by lymphoid and myeloid tumour cells. Br J Haematol. 1999;106:827–832. doi: 10.1046/j.1365-2141.1999.01630.x. [DOI] [PubMed] [Google Scholar]