Abstract
Since June 2021 in France, patients with haemophilia A with anti-factor VIII inhibitors and patients with severe haemophilia A without anti-factor VIII inhibitors have the choice between the community and the hospital pharmacy for dispensing emicizumab (Hemlibra®). This study aims to investigate patient-centred access to treatment by evaluating and comparing the dimensions of the Penchansky and Thomas model, between community and hospital pharmacies. The evaluation of access to treatment was based on the dimensions of the Penchansky and Thomas model: accessibility, availability, acceptability, accommodation and affordability. These were assessed using appropriate and specific indicators in the study context and calculated for patients choosing community pharmacy or hospital pharmacy for emicizumab dispensing. Geographical data collected as part of the national ‘PASO DOBLE DEMI’ study were used for this analysis. The findings reveal that dispensing emicizumab in community pharmacies improves accessibility by significantly reducing travel time. The availability of healthcare services is also improved due to the territorial coverage of community pharmacies. The extended opening hours and low waiting time also contribute to better access to emicizumab in community pharmacies. The dimension of acceptability must be improved, and further investigations are needed to address the affordability dimension. Several months after emicizumab became available in French community pharmacies, access to treatment has significantly improved, reducing the burden of this rare chronic disease for patients and their careers. These results suggest that this innovative model could be extended to other medicines and even other therapeutic areas.
Key points.
Access to emicizumab is improved by the dual dispensing circuit in France.
Accessibility is improved by reducing patient travel time to community pharmacy.
Territorial coverage of community pharmacies tends to increase availability.
Pre-existing relations with community pharmacists increase acceptability.
Extended opening hours make a better accommodation in community pharmacy.
Introduction
Haemophilia A is a rare constitutional bleeding disorder related to a total or partial deficiency of clotting factor VIII (FVII). The severity of the disease is determined by the level of FVIII deficiency: severe (FVIII level <1%), moderate (1% < FVIII level <5%) and mild (5% < FVIII level <40%). The frequency and intensity of bleeding depends on severity. Severe haemophilia causes mainly internal bleeding such as hemarthrosis and haematoma and external bleeding [1, 2]. Injection of clotting factor represents the conventional treatment for haemophilia A. Recently, an alternative therapeutic strategy has been developed with emicizumab (Hemlibra®), a bispecific monoclonal antibody. Commercialized since 2018, this prophylactic treatment can be administered subcutaneously for patients with haemophilia A with FVIII inhibitors or patients without FVIII inhibitors with severe form, or moderate form with a severe bleeding phenotype [3].
The dispensing of clotting factors only by hospital pharmacies represented access constraints for patients and their caregivers [4]. Recent studies reported that travel time to hospital pharmacy was the most unsatisfactory criteria for patients and their caregivers. They requested a change in the treatment dispensing circuit [5].
The antibody emicizumab was initially available only in French hospital pharmacies. Since June 2021 in France, people with haemophilia A had the choice between the community and the hospital pharmacy for dispensing emicizumab. This dual dispensing circuit improved significantly the overall satisfaction of patients and carers, especially active workers, parents of children with haemophilia and patients living in rural areas [6]. To maintain the continuity, quality and safety of the healthcare pathway, the French Reference Centre for Haemophilia coordinate the dual circuit of dispensing by identifying and linking all the stakeholders (i.e. patients and carers, hospital pharmacists and community pharmacists who join the new circuit of dispensing). This coordination scheme enables community pharmacists to identify all the other stakeholders involved in the healthcare pathway, as well as the hospital-based experts. To assist community pharmacists with the fundamental skills required to dispense emicizumab, the HEMOPHAR e-learning program was also developed by the French Reference Centre for Haemophilia and proposed to volunteer community pharmacists chosen by patients or carers [7, 8].
Access to healthcare services represents a crucial determinant in reducing territorial inequalities and improving the quality of life for patients, especially for rare and chronic diseases such as haemophilia which requires lifelong treatment and constant vigilance. However, measuring access to healthcare services remains a complex concept as exemplified by the variety of interpretations depending on the authors [9]. Penchansky and Thomas defined access as the match between patients’ needs and the capacity of health systems to meet them, using a multidimensional concept composed of five interrelated dimensions: accessibility, acceptability, availability, affordability and accommodation [10]. Accessibility and availability represent the spatial dimensions of access. Acceptability, accommodation, and affordability represent the aspatial dimensions of access [10, 11].
The aim of this study is to investigate patient-centred access to emicizumab by comparing the dimensions of access whether the patient chooses the community or the hospital pharmacy.
Methods
Study design
This ancillary study is based on the national retrospective survey, as part of the PASO DOBLE DEMI study [7]. The eligible population was adult patients, carers of adult patients, or legal representatives of children (<18 years old) with severe haemophilia A with or without inhibitors, treated with emicizumab and living in France. The data collection lasted 5 months; from 13 September 2022 to 09 January 2023.
Two specific e-questionnaires were initially developed by a team composed of pharmacists, a methodologist, and a health geographer, and validated by a scientific committee dedicated to the PASO DOBLE DEMI study, with the participation of the French haemophilia association. Volunteer adult respondents completed the e-questionnaire by flashing the QR code or using the short URL address, from any terminal.
Access dimensions
Evaluation of access to treatment was based on the five dimensions of the Penchansky and Thomas model: accessibility, availability, acceptability, accommodation and affordability [10]. The dimension of affordability represents the relationship between the prices of health services and the insurance or deposit requirements of providers and the income, ability to pay and health insurance coverage of patients. In France, the cost of emicizumab is fully covered by the national health insurance system, without any advance payment for patients, so this dimension was not addressed in this study. The four other dimensions were evaluated by appropriate indicators (Table 1) specific to this study and calculated both for patients who choose the community pharmacy and patients who choose the hospital pharmacy for dispensing emicizumab.
Table 1.
Specific definitions of the four-dimension of access adapted from the covariates collected in the PASO DOBLE DEMI study.
| Definitions | Indicators | Methods for evaluation |
|---|---|---|
| Accessibility: The relationship between the location of the health service and the location of patients, taking account of patients’ transportation resources and travel time, distance, and cost. | A1: Means of transportation. | Questionnaire items: |
| A2: Door-to-door travel time from residence or from workplace. | ‘What is the estimated door-to-door travel time to your hospital pharmacy?’ | |
| A3: Calculated travel time from the preferred location of the patient to the pharmacy. | ‘What transportation do you use to get to your hospital pharmacy?’ | |
| A4: Estimated travel time savings for respondents who chose the community pharmacy. | ‘According to your estimates, what is the saving in travel time compared with hospital pharmacy?’ | |
| Availability: The relationship between the type of health services and the amount of resources available, according to the needs and the number of patients. | B1: Pharmacy density per 100 000 inhabitants in France. | Open-source FINESS database including all community pharmacies in France (updated in 2023) [The French Ministry of Health and Solidarity (Ministère des Solidarités et de la Santé), 2023] |
| Open-source INSEE database including regional repartition of the population (updated in 2021) [The National Institute of Statistics and Economic Studies (Institut National des Statistiques et Études Économiques—INSEE), 2023]. | ||
| Open-source list of regional health agencies including hospital pharmacies in France authorized for hospital dispensation of emicizumab. | ||
| Acceptability: The relationship of patients’ attitudes about personal and practice characteristics of health providers, as well as to health provider attitudes about acceptable personal characteristics of patients. | C1: % of patients who reported a pre-existing relationship with the pharmacist as motivation for choosing the mode of dispensation. |
|
| C2: % of patients reported the proposal to exchange in a patient confidential zone at the pharmacy. | ||
| Accommodation: The relationship between how services are organised to receive patients and patients’ ability to adapt to that organisation. | D1: % of patients who reported traffic conditions as motivation for choosing their mode of dispensation. |
|
| D2: % of patients who reported parking conditions as motivation for choosing their mode of dispensation. | ||
| D3: % of patients who reported opening hours as motivation for choosing their mode of dispensation. | ||
| D4: Attendance time of patients in the pharmacy | ||
| D5: Estimated attendance time savings for respondents who chose the community pharmacy |
To collect anonymous data, a specific module was developed in ArcGIS Survey 123 (Version 3.17.54) to calculate the travel time between the patient’s preferred place to the hospital pharmacy or to the community pharmacy, without saving the respective postal addresses and guaranteeing anonymity of responses. Each respondent filled in his postal address directly in the e-questionnaire, and the module automatically calculated the travel time between the two locations. The default parameters were based on travel by car and on average traffic conditions. Each travel time was calculated instantaneously, and the patient’s address was immediately deleted in order to remain compliant with the processing of sensitive data as defined in accordance with regulatory authorizations. If the patient had not provided his postal address or the postal address of the pharmacy, the travel time value was missing data.
Analysis
Baseline characteristics were described by counts and percentages for categorical variables, medians and interquartile range for continuous variables, mentioned in brackets in the following order in the results section; community at first, and then hospital pharmacy. Bivariate analysis was assessed using the Pearson chi-square test for categorical variables and the nonparametric Wilcoxon rank test for continuous variables. When the numbers per group were less than five respondents, the P-value was not provided, indicated by an ‘X’ in tables. Statistical analysis was performed using R 4.1.1 software. The level of significance was set at a P-value < .05. Geographical analysis was performed using Qgis (version 3.34.3).
Ethics statement
The study complies with the reference methodology for studies and evaluations in the health field. It did not require the patient’s written consent to participate. The ethics committee of the Hospices Civils de Lyon approved the study (n°2022-06-01 obtained on 14 June 2022) and the study was registered in ClinicalTrials.gov (NCT05450640).
Results
The PASO DOBLE DEMI study included 175 respondents from all regions of France, including 123 respondents in community pharmacies and 52 respondents in hospital pharmacies [6].
Evaluation of accessibility
The car remains the main mode of transportation to go to the pharmacy; 66.7% of patients in community pharmacies tend to use the car and 26.8% get around by walking, whereas 88.5% of patients in hospital pharmacies use their car and 5.8% the public transports. We observed that 84.6% of patients reach the community pharmacy within 10 minutes from their residence, compared to 26.9% of patients in the hospital pharmacy (P < .0001). From the workplace, the travel time within 10 minutes is not different between patients in community and hospital pharmacies (P = .6714). Median access time to the community pharmacy was inferior compared to hospital pharmacy (P < .0001). We observed that 70% of patients estimate that they saved more than 15 minutes of travel time (Table 2).
Table 2.
Evaluation and comparison of the accessibility dimension between community and hospital pharmacies.
| Community pharmacy n=123 | Hospital pharmacy n=52 | P-value | |
|---|---|---|---|
| A1: Means of transportation | |||
| Car | 66.7% n=82 | 88.5% n=46 | .0053 |
| Walking | 26.8% n=33 | 1.9% n=1 | X |
| Public transport | 1.6% n=2 | 5.8% n=3 | X |
| A2: Door-to-door travel time | |||
| From residence | |||
| ≤10 minutes | 84.6% n=104 | 26.9% n=14 | <.0001 |
| 10–30 minutes | 13.8% n=17 | 48.0% n=25 | <.0001 |
| >30 minutes | 1.6% n=2 | 23.1% n=12 | X |
| From workplace | |||
| ≤10 minutes | 17.1% n=21 | 21.2% n=11 | .6714 |
| 10–30 minutes | 18.7% n=23 | 28.8 % n=15 | .1980 |
| >30 minutes | 11.4% n=14 | 3.8% n=2 | X |
| A3: Calculated travel time | |||
| Median (quartile 1; quartile 3) in minutes | 3 [2; 5] n=75 | 22 [11.75; 55] n=40 | <.0001 |
| A4: Estimated travel time savings for respondents who chose the community pharmacy | |||
| ≤15 minutes | 30.0% n=37 | X | X |
| >15 minutes | 70.0% n=86 | X | X |
Evaluation of availability
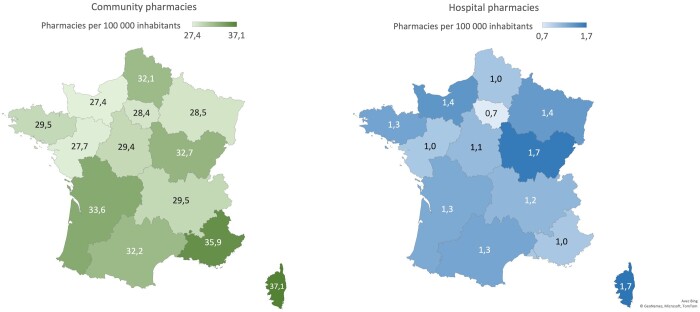
The density of community pharmacies is more important than the density of hospital pharmacies which are mainly located in University Hospital Centers, with respectively 31.1 and 1.2 pharmacies per 100 000 inhabitants. The density of community and hospital pharmacies remains consistent across regions (Fig. 1). For example, the Ile-de-France region is characterized by a weak density of hospital pharmacies less than one pharmacy (i.e. 0.7) per 100 000 inhabitants, whereas the patients would benefit from one of the most-dense distribution with 28.4 community pharmacies per inhabitants.
Figure 1.
Map of France representing the number of community pharmacies per 100 000 inhabitants per region (left) and the number of hospital pharmacies per 100 000 inhabitants per region (right).
Evaluation of acceptability
We observed that 26.0% of the patients in community pharmacies motivated their choice because of the pre-existing relationship with their pharmacist. However, we also observed that 36.5% of patients in the hospital pharmacy stayed at the hospital for the same reason (P=.2233). In community pharmacy, 44.7% of patients were invited to exchange within a confidential area, compared to 65.4% of patients in hospital pharmacy (P=.0421).
Evaluation of accommodation
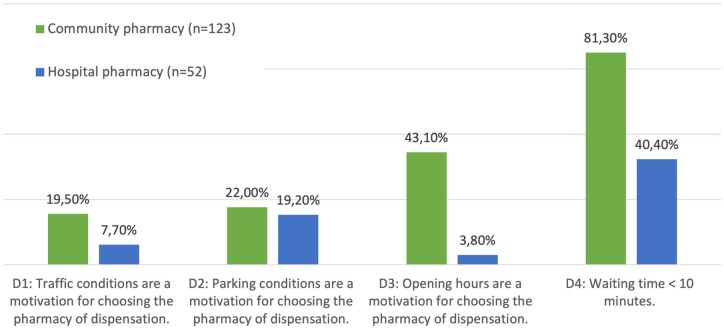
To go to their community pharmacy, 19.5% of patients find that driving conditions are easier, compared to 7.7% of patients in hospital pharmacy. Parking conditions at the community pharmacy are considered easier by 22.0% of patients, whereas 19.2% in hospital pharmacy. Also, 43.1% of patients are motivated by extended opening hours in community pharmacies, compared to 3.8% in hospital pharmacies. The waiting time was significantly lower in community pharmacies compared to hospital pharmacies (P < .0001) (Fig. 2). Concerning the estimated attendance time savings for respondents who chose the community pharmacy, 20.3% estimated less than 10 minutes, 53.7% between 10 and 20 minutes and 26% more than 20 minutes.
Figure 2.
Evaluation of the four criteria of the accommodation dimension and comparison between French community and hospital pharmacies.
Discussion
This model is innovative in comparison to other medicines in rare diseases, especially subcutaneous biotherapies, which are dispensed in community pharmacies without any specific coordination or support. The new patient pathway and dual circuit allow patients to choose where to obtain emicizumab, which is a significant benefit. Furthermore, the French Haemophilia Reference Centre was responsible for the coordination of the patient pathway and the support of the community pharmacists, in particular by offering an e-learning training programme designed to assist them with the fundamental skills required to dispense emicizumab safely.
Since emicizumab became available in pharmacies in June 2021, access has improved. The dense network of community pharmacies in France allows for greater availability and improves patient accessibility to treatment. Accommodation criteria represent a motivation for patients to choose community pharmacies. The dimension of acceptability (i.e. confidentiality in community pharmacies) must be improved. A medico-economic analysis will be conducted to address the financial aspect of access, which will complete the study. This improvement in access through community pharmacy dispensing has resulted in patient satisfaction and a reduction in the burden of disease. The patients expressed their desire to extend access to other medicines in community pharmacies to ensure equitable and equal access for all people with haemophilia. The success of the coordination for all the stakeholders, the high satisfaction of patients and carers, and the HEMOPHAR e-learning program for effective training and skill development among community pharmacists in the context of the emicizumab dual dispensing circuit may facilitate the extension of this new pathway of dispensation to other medicines and may open the way towards other therapeutic areas. Indeed, other treatments could be made available in community pharmacies, particularly in rare diseases, reaching institutional expectations and enhancing the access to treatment for patients [8].
Evaluating access to treatment is a significant challenge for rare chronic diseases, such as haemophilia, requiring long-term treatment throughout a patient’s life. The results of the present study highlight that dispensing emicizumab in community pharmacies improves access to treatment. Despite the impact of the obstacles of access to medicines in the significant burden of patients and caregivers [12], there have been few studies investigating the multidimensional access to medicines for patients with rare diseases. To the best of our knowledge, this study is the first French national survey to explore the four dimensions of access to medicine for people with haemophilia. This work is also in line with the French policy orientations. Since 2004, the government has implemented three successive National Plans for Rare Diseases and one of the main objectives was to improve access to treatments for rare diseases [13]. The third edition of the guidelines for the management of haemophilia mentioned the notion of access and referred that people with haemophilia must have access to safe and effective treatment with optimal efficiency in the prevention of bleeding and treatment of any spontaneous, breakthrough, or trauma-related bleeding diseases [2].
An important innovation is the development of two e-questionnaires, which are the first of their kind in the haemophilia field and include questions on multidimensional access to medicines. This study includes a novel module that calculates a patient’s travel time to the pharmacy. The module can be adapted and reused in other research projects; for ethical reasons, the exact address of the patient’s work or home was immediately deleted to avoid the possible identification of rare disease patients. If the patient did not provide their residence address or the pharmacy address, it was impossible to retrieve the information necessary to calculate the travel time. This may have limited certain analyses that required the exact location of the patient.
The provision of emicizumab in community pharmacies has removed the main barrier of access to treatment that was a major source of patient dissatisfaction, with a travel time-saving of more than 15 minutes for most patients [5, 14]. Providing the treatment closer to the patient, i.e. in a community pharmacy, improves accessibility and satisfaction with travel time, reducing the burden of illness [6, 15]. Dispensing emicizumab in a community pharmacy saves patients time, simplifies their organization, and also allows patients to walk to the pharmacy instead of driving [16].
The availability of community pharmacies is better than that of hospital pharmacies with a larger density of locations whatever the region, especially as not all hospital pharmacies really dispense emicizumab or other haemophilia medicines [4, 5], and because of regulatory quotas of French community pharmacies [17]. Thanks to this high density, patients have access to a wide range of community pharmacies throughout France, in every region [18].
Moreover, community pharmacies have extended opening hours and reduced waiting times for patients, with estimated attendance time saving between 10 and 20 minutes for most patients. Proximity to their place of residence or workplace constitutes a motivation because more convenient for patients, especially for parents of children with haemophilia [6, 19]. Barriers to accessing hospital pharmacies also include conditions for circulation to the hospital and hospital parking for patients travelling by car [5].
Because of the influence of the pre-existing relationship with their hospital pharmacist [20], some patients choose to stay in the hospital despite geographical and accommodation obstacles. This acceptability is an essential dimension in ensuring that a person uses a service, but it is a subjective concept that depends strongly on patient expectations and patient satisfaction [19]. The relationship between patients and community pharmacists is highly satisfactory [6]. However, most patients have reported a lack of confidentiality in community pharmacies, in contrast to hospital pharmacies. Protecting privacy can be a complex issue which can be improved with organisational solutions. It is crucial to continue working on this matter, particularly now that these pharmacies are caring for patients with rare diseases. Pharmacists must adapt to offer an exchange of information without compromising confidentiality [21].
Emicizumab is an expensive treatment in France, but the cost of this treatment is fully covered by French health insurance. Therefore, the affordability dimension was not studied as it does not limit patient access in France, unlike in other countries [22, 23]. Assessing the indirect costs for patients treated with emicizumab, such as travel costs to pharmacies, absenteeism from work, and loss of productivity, could be of interest [24]. To improve access to drugs for rare diseases, studies are increasingly assessing the socio-economic burden and overall costs [25]. A medico-economic study of the evolution of the care pathway and in particular the indirect costs associated with emicizumab will be carried out to assess the affordability of access.
Acknowledgements
We thank the scientific committee of the PASODOBLEDEMI I study: Béatrice Clairaz-Mahiou [community pharmacist, copresident of the French Society of Pharmaceutical Sciences (French: Société Francophone des Sciences Pharmaceutiques Officinales)], Yesim Dargaud (National Reference Center for Hemophilia, Lyon), Félicia Ferrera Bibas (cochair of the Provence-Alpes-Cote-d’Azur Unions for Health Professionals [French: Société Française de Pharmacie Clinique], Marseille), Nicolas Giraud [president of the French Hemophilia Association (French: Association Française des Hémophiles)], Rémi Varin (hospital pharmacist, Rouen), and Fabienne Volot (Expert Center Coordinator, Dijon). The authors thank the team from Roche-Chugai laboratories for their follow-up on the project (Loïc Bergougnoux, Aurélie de Lehvenfehlt).
Conflict of interest: None declared.
Contributor Information
Morgane Cabon, Haemophilia Treatment Centre and French Reference Centre on Haemophilia, Louis Pradel Hospital, Hospices Civils de Lyon, Bron, France.
Valérie Chamouard, Haemophilia Treatment Centre and French Reference Centre on Haemophilia, Louis Pradel Hospital, Hospices Civils de Lyon, Bron, France; Pharmaceutical Unit, Louis Pradel Hospital, Hospices Civils de Lyon, Bron, France.
Julie Freyssenge, Research on Healthcare Performance RESHAPE, INSERM U1290, University Claude Bernard Lyon 1, Lyon, France.
Laurie Fraticelli, Laboratory P2S (Health Systemic Process), UR 4129, Faculty of Medicine Laennec, University Claude Bernard Lyon 1, Lyon, France.
Funding
This study was promoted by the Hospices Civils of Lyon and founded by the laboratories Roche-Chugai.
Data availability
Upon reasonable request.
References
- 1. Blanchette VS, Key NS, Ljung LR, et al. Definitions in hemophilia: communication from the SSC of the ISTH. J Thromb Haemost 2014;12:1935–9. [DOI] [PubMed] [Google Scholar]
- 2. Srivastava A, Santagostino E, Dougall A, et al. World federation of hemophilia guidelines for the management of hemophilia, 3rd edition. Haemophilia 2020;26:1–158. [DOI] [PubMed] [Google Scholar]
- 3. The French National Authority for Health (Haute Autorité de Santé - HAS). HEMLIBRA (emicizumab) - hémophilie A [internet]. 2023. [cited 16 January 2024]. https://www.has-sante.fr/jcms/p_3443949/fr/hemlibra-emicizumab-hemophilie-a (16 January 2024, date last accessed).
- 4. Leroy V, Freyssenge J, Renard F, et al. Access to treatment among persons with hemophilia: a spatial analysis assessment in the Rhone-Alpes region, France. J Am Pharm Assoc 2019;59:797–803. [DOI] [PubMed] [Google Scholar]
- 5. Chamouard V, Fraticelli L, Freyssenge J, et al. PHAREO study: perceived and observed accessibility to therapeutic drugs used for treating patients with inherited bleeding disorders. J Clin Pharm Ther 2022;47:1667–75. [DOI] [PubMed] [Google Scholar]
- 6. Chamouard V, Freyssenge J, Duport G, et al. Evaluation of the care pathway in the context of the dispensing of emicizumab (Hemlibra) in community and hospital pharmacies in France: a patient satisfaction survey. Haemophilia 2023;29:1490–8. [DOI] [PubMed] [Google Scholar]
- 7. Fraticelli L, Freyssenge J, Promé-Combel E, et al. Evaluation of the care pathway in the context of the dispensing of emicizumab (Hemlibra) in community pharmacies in France: protocol for a cross-sectional study based on the Kirkpatrick model. JMIR Res Protoc 2023;12:e43091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chamouard V, Freyssenge J, Clairaz-Mahiou B, et al. Evaluation of an e-learning program for community pharmacists for dispensing emicizumab (Hemlibra) in France: nationwide cross-sectional study. JMIR Form Res 2024;8:e54656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Levesque JF, Harris MF, Russell G. Patient-centred access to health care: conceptualising access at the interface of health systems and populations. Int J Equity Health 2013;12:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Penchansky R, Thomas JW. The concept of access: definition and relationship to consumer satisfaction. Medical Care 1981;19:127–40. [DOI] [PubMed] [Google Scholar]
- 11. McLaughlin CG, Wyszewianski L. Access to care: remembering old lessons. Health Serv Res 2002;37:1441–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Coppola A, Cerbone AM, Mancuso G, et al. Confronting the psychological burden of haemophilia. Haemophilia 2011;17:21–7. [DOI] [PubMed] [Google Scholar]
- 13. The French Ministry of Health and Solidarity (Ministère des Solidarités et de la Santé). National plan for rare diseases 2018-2022 (Plan national maladies rares) [internet]. 2018. [cited 16 January 2024]. https://sante.gouv.fr/IMG/pdf/pnmr_3_v25-09pdf.pdf (16 January 2024, date last accessed).
- 14. Zhou ZY, Riske B, Forsberg AD, et al. Self-reported barriers to hemophilia care in people with factor VIII deficiency. Am J Prev Med 2011;41:S346–53. [DOI] [PubMed] [Google Scholar]
- 15. Murteira R, Romano S, Teixeira I, et al. Real-world impact of transferring the dispensing of hospital-only medicines to community pharmacies during the COVID-19 pandemic. Value Health 2022;25:1321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bragazzi NL, Mansour M, Bonsignore A, et al. The role of hospital and community pharmacists in the management of COVID-19: towards an expanded definition of the roles, responsibilities, and duties of the pharmacist. Pharmacy (Basel) 2020;8:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chapter 1: Conditions for the practice of community pharmacy (Articles L568 à L595) (Chapitre 1 : Conditions de l’exercice de la pharmacie d’officine) [internet]. https://www.legifrance.gouv.fr/codes/section_lc/LEGITEXT000006072665/LEGISCTA000006155247/ (16 January 2024, date last accessed).
- 18. The French Chamber of Pharmacists (Ordre National des Pharmaciens). Pharmacist demography—overview at 1 January 2023 (Démographie des pharmaciens - Panorama au 1er janvier 2023) [internet]. 2023. [cited 25 January 2024]. https://www.ordre.pharmacien.fr/les-communications/focus-sur/les-autres-publications/demographie-des-pharmaciens-panorama-au-1er-janvier-2023 (25 January 2024, date last accessed).
- 19. Meehan SA, Leon N, Naidoo P, et al. Availability and acceptability of HIV counselling and testing services. A qualitative study comparing clients’ experiences of accessing HIV testing at public sector primary health care facilities or non-governmental mobile services in Cape Town, South Africa. BMC Public Health 2015;15:845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Adekunle OA, Olson AW, Schommer JC, et al. Influence of patient-pharmacist relationship on willingness to accept pharmacist-provided services. J Am Pharm Assoc 2023;63:760–8.e1. [DOI] [PubMed] [Google Scholar]
- 21. Hattingh HL, Emmerton L, Ng Cheong Tin P, et al. Utilization of community pharmacy space to enhance privacy: a qualitative study. Health Expect 2016;19:1098–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kullgren JT, McLaughlin CG, Mitra N, et al. Nonfinancial barriers and access to care for U.S. adults. Health Serv Res 2012;47:462–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nugent D, Kalnins W, Querol F, et al. Haemophilia experiences, results and opportunities (HERO) study: treatment-related characteristics of the population. Haemophilia 2015;21:e26–38. [DOI] [PubMed] [Google Scholar]
- 24. Brown LJ, La HA, Li J, et al. The societal burden of haemophilia A. III—The potential impact of emicizumab on costs of haemophilia A in Australia. Haemophilia 2020;26:21–9. [DOI] [PubMed] [Google Scholar]
- 25. Marshall DA, Gerber B, Lorenzetti DL, et al. Are we capturing the socioeconomic burden of rare genetic disease? A scoping review of economic evaluations and cost-of-illness studies. PharmacoEconomics 2023;41:1563–88. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Upon reasonable request.