Abstract
Herpes simplex viruses (HSV) have developed several immunoevasive strategies. Here we demonstrate a novel mechanism by which HSV type 1 may interfere with the immune response through infection of immature dendritic cells (DC) and selective downmodulation of costimulatory molecules. In our study we show productive infection of immature monocyte-derived DC, which closely resemble sessile Langerhans cells, by sequential expression of immediate-early, early, and late viral proteins and of glycoprotein D mRNA, as well as production of infectious virus of moderate titers. Infection was cytopathic, with the progressive loss of 20 to 45% of cells from 24 to 48 h after infection, with no more than 80% of DC found to be infected. These results are in contrast to those of previous findings of nonpermissive or abortive infection of monocytes and mature monocyte-derived DC. Infection of immature DC also led to selective and asynchronous downregulation of CD1a, CD40, CD54 (ICAM-1) (12 h postinfection), CD80 (24 h postinfection), and CD86 (48 h postinfection) but not of CD11c or major histocompatibility complex class I and II molecules when compared to DC exposed to UV-inactivated virus. Thus, we propose that productive infection of epidermal Langerhans cells in vivo may lead to delayed activation of T cells, allowing more time for replication of HSV type 1 in epidermal cells.
Herpes simplex virus type 1 (HSV-1) initially infects epidermal or mucosal tissues where it replicates and enters the cutaneous sensory axons. It is then retrogradely transported to the neurons of dorsal root ganglia where latent infection is established. Sporadic reactivation of latent virus and transmission to peripheral sites leads to asymptomatic viral shedding or recurrent herpes lesions (41, 50). Symptomatic recurrences are dependent on the levels of local immunity at the periphery and are principally controlled by cell-mediated responses via direct T-cell effector function and cytokine release (24, 34, 37, 45). CD4+ T cells are the initial infiltrating cells in the recurrent lesion, followed by CD8+ cells (9). An immediate-early gene product, ICP47, inhibits major histocompatibility complex (MHC) class I-mediated antigen presentation by binding via its N terminus to the transporter-associated protein (TAP) (1, 13, 18, 49, 52). This ICP47-TAP complex prevents translocation of the MHC class I-processed peptide complex to the cell surface, which would prevent recognition by CD8+ T cells. However, gamma interferon secreted by CD4 cells reverses the MHC class I downregulation and stimulates MHC class II expression, allowing targeting of HSV-infected epidermal cells by both CD4+ and CD8+ cytotoxic T cells (33). The persistence of HSV-specific CD8+ clones in seropositive individuals, however, suggests their complementary importance to CD4+ T cells in the control of recurrences (35, 36). The early appearance of virus-specific T-cell effectors depends on prompt signaling from antigen-presenting cells, such as dendritic cells (DC), B cells, or macrophages. The strategic positioning of Langerhans cells in the skin and mucosa identifies them as the most likely antigen-presenting DC to have the earliest contact with the incoming virus.
DC form a network of bone marrow-derived antigen-presenting cells that are required for the initiation of adaptive immune responses. DC can be found in virtually all peripheral tissues and are characterized by the CD1a+ HLA-DR+ CD80+ CD86+ phenotype (3, 17). The capacity of DC to stimulate T cells is closely related to their maturation stage (40). Immature DC, exemplified by epidermal Langerhans cells, act as sentinels and are highly specialized at antigen uptake and processing but are poor stimulators of primary immune responses (3, 17, 43). A variety of immune stimuli induce phenotypic and functional changes to resting DC as they migrate out of the tissues and into secondary lymphoid organs. Mature DC no longer take up and process antigen but upregulate MHC class I and II costimulatory and adhesion molecules, effectively boosting their ability to present processed peptides to antigen-specific T cells. During the process of maturation, DC also express CD83, a maturation marker not found on resting immature DC such as Langerhans cells (54). Terminal maturation of DC is induced upon contact with T cells via CD40 ligation (6). Experimental studies of human Langerhans cells have been hampered to an extent by low cell yield and purity from skin. Generation of DC from peripheral blood monocytes represents a well characterized and highly reproducible model for studies of the role of DC in viral infections. Fully functional immature DC that closely simulate Langerhans cells found in skin and mucosa can be generated by 5 to 7 days of culture with granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4). Further maturation can be induced by the addition of tumor necrosis factor alpha (43, 53).
The importance of DC in viral infections stems from their superiority over other antigen-presenting cells in stimulating cytotoxic T-lymphocyte responses and maintaining protective antiviral immunity (10, 28, 29, 31). Because of their role in initiating antiviral immune responses, DC represent an ideal target for immune evasion by viruses. Several viruses interfere with DC function, especially measles virus (14, 16), vaccinia virus (11, 12), and dengue virus (51).
The main sites of HSV-1 replication in vivo are epidermal keratinocytes and the oropharyngeal epithelium. The resting epidermal DC (Langerhans cells) are thus likely to encounter the virus at the onset of primary or recurrent infections. Cytopathic infection of immature DC by HSV-1 at the site of infection could impair the immune response, leading to more extensive infection of sensory nerve endings and consequently to a greater load of latent neuronal infection within the dorsal root ganglia. A recent study has shown that abortive or nonproductive infection of mature monocyte-derived DC (MDDC) by HSV-1 is able to modulate their function through inhibition of T-cell stimulatory function (25). However, as immature DC (Langerhans cells) are more relevant to the pathogenesis of recurrent HSV infection, the aim of our study was to determine the susceptibility of immature MDDC to productive HSV-1 infection in vitro and its effects on MDDC phenotype.
MATERIALS AND METHODS
Generation of immature MDDC.
Blood was obtained from healthy individuals from Parramatta Blood Bank (NSW, Australia). The HSV-1 serostatus of the donors was unknown. Peripheral blood mononuclear cells were obtained by Ficoll-Hypaque density gradient separation (Amersham Pharmacia Biotech, Uppsala, Sweden). Monocytes were enriched by counter-current elutriation in a 5-ml Elutriator chamber (Elutriator JE-5.0 rotor from Beckman, Palo Alto, Calif.) as previously described (23, 32). On average, pooled monocyte fractions from elutriation were >75% CD14+ and 15% CD3+ of an average total cell population of 108 cells. To increase purity, the cells were incubated with paramagnetic beads (Dynal) conjugated to CD14 monoclonal antibody (Pharmingen, San Diego, Calif.). The positively selected cells were seeded at a density of 5 × 106 cells/well. Immature DC were generated over 6 to 7 days in complete medium consisting of RPMI 1640 with 2.05 mM l-glutamine (JRH Biosciences, Lenexa, Kans.) and 10% heat-inactivated fetal calf serum (CSL, Parkville, Australia) supplemented with 200 U of GM-CSF/ml and 250 U of IL-4 (Schering-Plough, Kenilworth, N.J.)/ml. Every 2 days, 50% of the medium was removed and replaced with fresh complete medium supplemented with GM-CSF and IL-4. At day 6 or 7 of culture, cells were routinely checked for expression of DC markers prior to infection experiments. Routinely, the cells were ≥95% CD1a+ CD11c+, with no detectable CD3+ cells or CD14 or CD83 expression as determined by flow cytometry (described in Results).
Virus.
A clinical strain of HSV-1 (MC1) was passaged in HEp-2 cells and typed with fluorescein isothiocyanate (FITC)-conjugated anti-HSV-1 type-specific monoclonal antibody (Trinity Biotech, Bray, Ireland). Titers were determined by plaque-forming assay. All stocks were stored at −80°C until use.
HSV-1 infection of immature MDDC.
DC in 24-well plates were infected with HSV-1 at different multiplicities of infection: 0.1, 0.5, 1, 5, and 10 PFU/cell or mock infected for 1 h at 37°C. The cells were washed three times with phosphate-buffered saline (PBS) to remove unbound virus, resuspended in complete medium with GM-CSF and IL-4, and seeded at 106 cells/ml in 24-well plates. The cells were collected at various time points.
Detection of HSV-1 antigens in immature MDDC by immunofluorescence-confocal microscopy and flow cytometry.
HSV-1- and mock-infected cells were fixed with 4% paraformaldehyde (EM Sciences, Fort Washington, Pa.) for 15 min and permeabilized in 0.05% Tween-20 (Sigma, St. Louis, Mo.). Nonspecific binding was blocked with 5% normal mouse serum (Sigma) for 15 min. Cells were spotted on poly-l-lysine-coated (Sigma) coverslips and air-dried. Primary monoclonal antibodies against HSV-1 proteins ICP4 (Fitzgerald Industries International, Concord, Mass.), ICP8 (a kind gift from D. Knipe, Harvard Medical School, Boston, Mass.), gD (Fitzgerald Industries International), and FITC-conjugated anti-gC (Trinity Biotech) were used at 1:50 dilution. Unconjugated antibodies were detected with a goat anti-mouse FITC-conjugated antibody (Becton Dickinson, San Jose, Calif.) for 20 min. Labeled cells were washed three times in PBS and mounted on 95% glycerol. The cells were examined by confocal laser-scanning microscopy, and the images were analyzed by Optiscan software. For flow cytometry, cells were collected at 0, 12, 24, 36, and 48 h postinfection (p.i.).
DC phenotype was evaluated with a panel of FITC- and phycoerythrin-conjugated monoclonal antibodies using FACSCalibur (Becton Dickinson). The monoclonal antibodies used were against CD1a, CD14, CD40, CD54, CD80, CD83, CD86, HLA-A, -B, -C (Pharmingen), and HLA-DR (Becton Dickinson) as well as isotype-matched controls. For the intracellular detection of antigens, cells were fixed with 4% paraformaldehyde and permeabilized in 0.05% Tween-20. Monoclonal antibodies directed against viral antigens were used as mentioned above.
Quantification of infectious HSV-1 virions by plaque-forming assay.
DC were infected with 0.1, 1, 5, or 10 PFU of HSV-1/cell. The cell-free supernatants were collected at 0, 6, 12, 24, or 48 h p.i. and stored at −80°C until use. Supernatants were serially diluted (10−1 to 10−6), and 100 μl of each dilution was added to 105 fibroblasts (MRC-5; CSL, Parkville, Victoria, Australia) in 24-well plates in triplicate for 1 h. The supernatant was removed, and fresh minimal essential medium containing 0.9% carboxymethyl cellulose (Sigma) and 1% fetal calf serum was added. After 40 to 48 h, the medium was removed and the cells were fixed with 2% paraformaldehyde for 5 min and stained with 0.1% crystal violet for a further 5 min. The monolayers were washed twice with water and examined under 20× magnification, and plaque numbers were quantified in triplicate. The virus titer was expressed as PFU/milliliter.
RESULTS
Generation of immature MDDC.
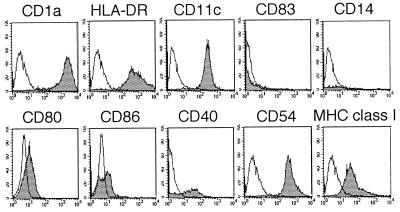
Immature MDDC were generated in vitro over 6 to 7 days by culturing monocytes in GM-CSF and IL-4. Routinely, ≥95% of cells displayed a CD1a+ CD11c+ HLA-DR+ CD14− CD83− phenotype, as assessed by flow cytometry (Fig. 1).
FIG. 1.
Phenotypic analysis of immature MDDC by flow cytometry. DC were derived from peripheral blood monocytes cultured for 6 to 7 days in the presence of GM-CSF and IL-4. Clear histograms show background staining with isotype control antibodies, and shaded histograms show specific staining.
HSV-1-infected immature MDDC express viral proteins.
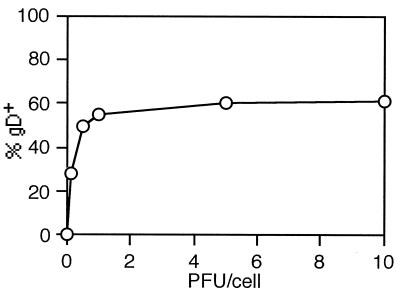
In order to determine the susceptibility of immature MDDC to HSV-1 infection, MDDC were infected with HSV-1 at 0.1, 0.5, 1, 5, and 10 PFU/cell and analyzed for expression of a late HSV-1 protein, gD, by flow cytometry and confocal microscopy at time points ranging from 12 to 48 h p.i. Controls included mock-infected cells and isotype-matched antibody controls. DC stained positive for the viral antigen at all infectious doses tested, but the rate of expression reached a plateau after infection with 5 PFU/cell, where approximately 75% of cells were infected, indicating that a proportion of the population could not be productively infected even after high multiplicity of infection (Fig. 2). HSV-1 immediate-early protein ICP4 was detected in the cytoplasm as early as 2 h p.i. and peaked at about 4 h p.i. (Fig. 3A). This was followed by expression of the early protein ICP8 (Fig. 3B). No late HSV-1 proteins could be detected at these time points (data not shown). By 10 h p.i., approximately 60% of DC showed intracellular staining for gB and gD (Fig. 3C), but 2 h later these were mostly found on the cell surface (Fig. 3D). Weak intracellular expression of gC, another late protein, could be detected at 12 h p.i. in 25% of cells. Surface expression of gC, however, could not be detected before 15 h p.i., when approximately 60% of cells were positive (Fig. 3E). This sequential expression of immediate-early, early, and late viral proteins correlates with the cascade fashion of HSV replication reported in susceptible cell lines (19, 20). Viral proteins were found on the surface of the cells as late as 48 h p.i. (Fig. 4A). Viral replication was not followed further due to the cytopathic effects of HSV infection. Cytopathic effects characteristic of HSV-1 infection could be seen as early as 12 h p.i. Swelling and loss of dendrites were observed at all infectious doses. Cell viability was determined by the trypan blue exclusion method. Over the next 12 h, cell numbers decreased and by 24 h p.i., less than 70% of DC were viable (Fig. 4B). By 48 h p.i. cell viability had decreased to 50%. The cytopathic effect was dependent on viral replication, as mock-infected DC and DC exposed to UV-inactivated HSV-1 retained their viability and morphology (Fig. 4B). These results indicate that HSV-1 infects immature MDDC efficiently and induces cell death in a portion of these cells by 48 h.
FIG. 2.
Intracellular expression of late HSV-1 glycoprotein gD by immature MDDC as a function of infectious dose. Immature MDDC were infected with HSV-1 at 0.1 to 10 PFU/cell and were analyzed by flow cytometry 12 h later. Mock-infected cells served as a control. The results are representative of three independent experiments.
FIG. 3.
Intracellular expression of HSV-1 proteins by immature MDDC. Immature MDDC were infected with HSV-1 at 5 PFU/cell and analyzed for expression of immediate-early (ICP4), early (ICP8), and late (gB, gC, and gD) HSV-1 antigens at selected times. (A) ICP4 at 4 h p.i., showing cytoplasmic staining; (B) cytoplasmic staining with ICP8 at 6 h p.i.; (C) gD at 10 h p.i., showing cytoplasmic staining; and gB at 12 h p.i. (D) and gC staining at 18 h p.i.(E), showing cell surface staining. Mock-infected cells did not stain for any of the tested antibodies (not shown). Bar, 10 μm.
FIG. 4.
Kinetics of viral antigen expression, cell viability, and infectious virus production. Immature MDDC were infected with HSV-1 at 5 PFU/cell (A and C) or 1 and 5 PFU/cell (B). Vertical bars represent standard deviation. (A) Kinetics of viral antigen expression by immature MDDC. DC were analyzed for expression of late viral antigens (gC and gD) at 0, 12, 24, 36, and 48 h p.i. by flow cytometry and confocal laser-scanning microscopy. (B) Viability of HSV-1-infected immature MDDC. Viability was analyzed by the trypan blue exclusion method at 0, 12, 24, and 48 h p.i. White bar, uninfected DC; stippled bar, DC stimulated with UV-inactivated HSV-1; shaded bar, DC infected with 1 PFU of HSV-1/cell; solid bar, DC infected with 5 PFU of HSV-1/cell. (C) Infectious virus production by immature MDDC. Cell culture supernatants were collected at 0, 12, 24, 36, and 48 h p.i. Cell-free infectious virus was quantified by plaque assay.
HSV-1-infected immature MDDC release infectious virus.
To determine whether the infection of DC was productive, immature MDDC were infected with HSV-1 at a multiplicity of infection of 5 and washed three times with PBS; the supernatants were collected from uninfected and infected cell suspensions at various time points. The presence and titer of infectious virus were determined by plaque assay. No infectious virus was detectable at 0 and 6 h p.i. HSV-1 was first detected at 12 h p.i., and the viral titer peaked at 24 h p.i. (Fig. 4C). The subsequent 50% decrease in virus titer was compatible with the loss of 40 to 50% of cells. No cytopathic effects were observed in fibroblasts cultured with supernatants from mock-infected cells (data not shown). These results indicate that infection of immature MDDC by HSV-1 results in a complete replication cycle, with the release of infectious viral progeny.
HSV-1 downregulates expression of adhesion and costimulatory molecules on immature MDDC.
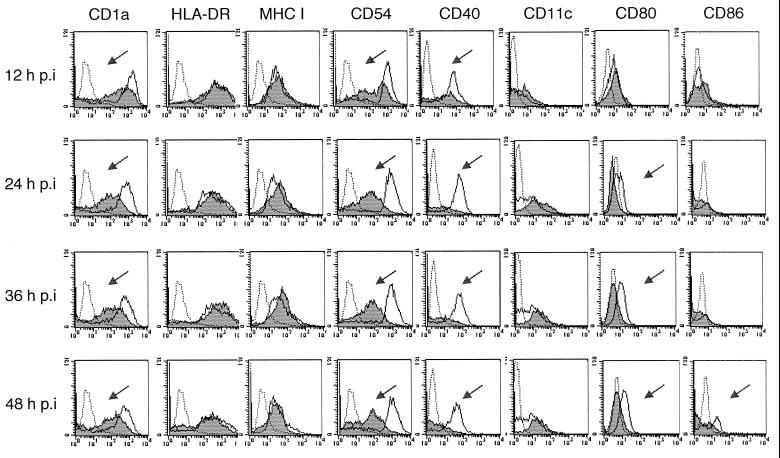
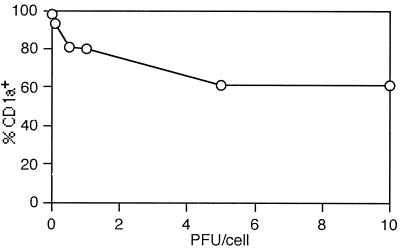
To investigate the effect of HSV-1 infection on the DC phenotype, the expression of costimulatory molecules CD80, CD86, and CD40, adhesion molecule CD54, and MHC class I and II molecules by immature MDDC before and after HSV-1 infection was examined by flow cytometry. The cells were infected with HSV-1 at a multiplicity of infection of 5 or mock infected and analyzed at 0, 12, 24, 36, and 48 h p.i. However, no increase in surface expression of any of the maturation markers was noticed at any time point. Conversely, the levels of CD1a, CD80, CD40, and CD54 decreased markedly when compared to those of mock-infected controls (Fig. 5). Downregulation of CD1a, CD40, and CD54 was evident as early as 12 h p.i. and that of CD80 occurred by 24 h p.i. Surface expression of CD86 remained unchanged initially, but it decreased after 24 h p.i., concomitant with the reduction in cell viability (Fig. 5). The reduction in CD1a expression was proportional to the infectious dose of HSV-1 (Fig. 6). The maximal effect, a 55% reduction in CD1a expression, was seen with 10 PFU/cell, corresponding to 80% of infected cells. Double-staining experiments indicate that CD1a was downregulated in both infected and uninfected MDDC (results not shown). Inhibition of surface expression of costimulatory molecules and CD54 in MDDC was dependent on viral replication. The costimulatory molecules were upregulated in response to stimulation with UV-inactivated HSV-1 (data not shown). The inhibition of expression of adhesion and costimulatory molecules was not only asynchronous but selective rather than global: surface levels of CD11c and MHC class II molecules remained unaffected (Fig. 5). Unlike most other cell types, downregulation of MHC class I by HSV-1 infection was not evident at any time point in immature MDDC (Fig. 5). These results indicate that HSV-1 infection modulates expression of costimulatory and adhesion molecules on the surface of immature MDDC.
FIG. 5.
Phenotypic changes after HSV-1 infection of immature MDDC detected by flow cytometry. DC were infected with HSV-1 at 5 PFU/cell and analyzed at selected times by flow cytometry for surface expression of CD1a, CD11c, CD54, costimulatory molecules, and MHC class I and II molecules. Mock-infected DC were tested in parallel. Dotted histograms indicate background staining with isotype control antibodies, white histograms represent mock-infected cells, and shaded histograms represent HSV-1-infected DC. Arrows show downregulation of surface markers on infected immature MDDC.
FIG. 6.
Expression of CD1a by HSV-1-infected immature MDDC. DC were mock infected or infected with HSV-1 at 0.1, 0.5, 1, 5, or 10 PFU/cell and analyzed by flow cytometry 24 h later. Percent CD1a positivity was determined by comparing the mean fluorescence intensities to that of the isotype control. The results are representative of three independent experiments.
DISCUSSION
In this study, we have shown that the exposure of MDDC in their immature state to HSV-1 leads to productive infection as demonstrated by the sequential appearance of early and late viral proteins and by moderate levels of released infectious virions. The percentage of infected DC was proportional to the input multiplicity, but it never exceeded 80% of total DC population, even at 10 PFU/cell, indicating that a portion of DC were refractory to the infection. The exact phenotype of this resistant cell population and the reasons for its refractoriness to HSV infection are not yet known but are the subject of future investigations. There was a progressive loss of cell viability following the first cycle of infection, when approximately 65% of cells remained viable. Whether this effect is the result of necrosis or apoptosis is being investigated. Although apoptosis of infected cells is usually efficiently inhibited by HSV-1, its contribution to the decrease in cell viability should not be discounted, as the inhibitory effect is highly species and cell type specific (15, 21). This represents the first demonstration of productive HSV infection of DC, and it contrasts with those reported for freshly isolated peripheral blood monocytes and lymphocytes, which are innately resistant to HSV infection (2, 44, 48). In the latter cells, viral replication is inhibited early in the replicative cycle prior to immediate-early protein synthesis and no viral DNA synthesis is detectable. Infection of activated but not resting T cells may lead to clearance of effector T cells via the induction of apoptosis (38). Infected DC may thus contribute to viral pathogenesis by transferring the virus to naive or memory T cells during antigen presentation in lymph nodes or epidermis. Similarly, human immunodeficiency virus-infected DC serve as vehicles for transport and spread of virus early in the infection and thus contribute to disease pathogenesis (5). Interestingly, Kruse et al. (25) recently found that mature MDDC could be infected with HSV-1 but this was highly restricted, being nonproductive, unlike the infection of immature MDDC described here. The resistance of mature DC to productive infection has also been shown for influenza virus and dengue virus (7, 51), but the exact mechanism for this phenomenon remains to be elucidated. The nature of HSV-1 infection, however, suggests that DC (Langerhans cells) are most likely to encounter the virus in peripheral sites as sessile, immature cells. The surface characteristics of MDDC appear to be closer to those of Langerhans cells and other tissue DC than of blood DC, e.g., expression of C-type lectins (S. Turville, unpublished data), strengthening the relevance of our findings to natural HSV infection. These collective findings demonstrate the progression of susceptibility to HSV infection from nonpermissive infection of monocytes to productive infection of immature MDDC and the development of further restriction after differentiation to mature MDDC.
The kinetics of decreasing viability of infected DC indicate that the cytopathicity of HSV infection of immature MDDC is slow despite the high proportion of infected cells. Thus, it is possible that infected DC may continue to function for the first and critical 24 to 48 h after infection. However, a marked decrease in key functional molecules, especially CD40, CD54, CD80, and CD86, occurs within the first 24 h of infection. The effect was specific for HSV infection, as incubation of MDDC with UV-inactivated virus did not affect the normal progressive upregulation of these molecules over time. The most striking effects were seen with CD40 and CD54, with a 1 to 2 log decrease in the surface expression within 12 h of infection. Secretion of IL-12, one of the major Th1 cytokines, by DC is dependent on the CD40-CD40 ligand interaction (8, 47). It can be predicted that downregulation of CD40 expression on HSV-1-infected DC may lead to decreased production of IL-12. Moreover, CD54 participates in the inhibition of IL-4 secretion and thus skews the response toward the Th1 type (30). Thus, downmodulation of CD40 and CD54 may favor viral spread, which is mainly controlled by Th1 cytokine-induced T cells (9, 33). Recent findings by Salio et al. (42) indicate that DC infected with a mutant HSV-1 capable of a single cycle of replication did not change the expression of costimulatory molecules. Our data strongly indicate that their findings underestimate the extent of inhibition in true infection. A consequence of HSV infection is the loss of host cell protein synthesis due to degradation of mRNA caused by the endonuclease activity of the viral protein vhs (26, 27). This effect should be evident within the first 8 h of infection; however, no changes in expression of CD11c or of MHC class I and II molecules were observed in the cultures, thus ruling out global effects on protein synthesis. This suggests that HSV proteins target individual signal transduction pathways that control expression of costimulatory molecules. Experiments using a vhs mutant to clarify its contribution to negative changes in DC phenotype are under way. The persistence of MHC class I on the surface of infected DC might be partly explained by the inability of viral ICP47 to completely exert its effect due to inherently high levels of TAP in DC (22, 52). Similarly, HSV-1 infection of activated T lymphocytes did not inhibit MHC class I expression, suggesting a cell type-dependent mechanism (38).
The capacity to infect DC is shared by several viruses (4, 11, 12, 14, 16, 39, 51), but the effect of these infections on DC function varies greatly. For instance, productive infection of DC with influenza virus leads to an increase in surface levels of costimulatory molecules and efficient stimulation of virus-specific T cells (7). However, measles virus infection leads to apoptosis of DC and an inability to secrete IL-12 following CD40 ligation (14, 16, 46). These reports clearly show that DC can be major targets for immune evasion by viruses.
The consequences of productive infection of immature DC have implications for the immune events in primary and recurrent herpes lesions. The only constitutive MHC class II-expressing cell in the epidermis is the Langerhans cell. Many of these cells may ingest viral antigens and present them to immigrating naive T cells, in primary infection, or to resident memory T cells in skin or lymph nodes, in recurrent infection. At least a proportion of these Langerhans cells may be productively infected by HSV and their function may be disturbed by downregulation of costimulatory molecules. The host, however, is usually able to counter the infection. It is likely that the balance of infected dysfunctional resting DC versus uninfected DC taking up antigen and migrating will ultimately be influenced by the cytokine environment. The competence of local immunity will determine the size and duration of the lesion in primary or recurrent infection and subsequently will influence the load of virus taken up by the sensory nerve endings and thus the proportion of latently infected neurons in dorsal root ganglia and the frequency of reactivation.
In conclusion, we have demonstrated a fully productive infection of immature MDDC by HSV-1 and the ability of HSV-1 to interfere with the expression of costimulatory molecules. This is yet another example of the many immune evasion mechanisms utilized by HSV in its battle against the host immune response.
ACKNOWLEDGMENTS
Z. Mikloska and L. Bosnjak contributed equally to this work.
We thank S. Turville for excellent advice and technical assistance.
This work was supported by National Health and Medical Research Council of Australia grant 107 373 (to A.L.C.).
REFERENCES
- 1.Ahn K, Meyer T H, Uebel S, Sempe P, Djaballah H, Yang Y, Peterson P A, Fruh K, Tampe R. Molecular mechanism and species specificity of TAP inhibition by herpes simplex virus ICP47. EMBO J. 1996;15:3247–3255. [PMC free article] [PubMed] [Google Scholar]
- 2.Albers I, Kirchner H, Domke-Opitz I. Resistance of human blood monocytes to infection with herpes simplex virus. Virology. 1989;169:466–469. doi: 10.1016/0042-6822(89)90174-8. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu Y J, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 4.Bender A, Albert M, Reddy A, Feldman M, Sauter B, Kaplan G, Hellman W, Bhardwaj N. The distinctive features of influenza virus infection of dendritic cells. Immunobiology. 1998;198:552–567. doi: 10.1016/S0171-2985(98)80078-8. [DOI] [PubMed] [Google Scholar]
- 5.Cameron P U, Freudenthal P S, Barker J M, Gezelter S, Inaba K, Steinman R M. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science. 1992;257:383–387. doi: 10.1126/science.1352913. [DOI] [PubMed] [Google Scholar]
- 6.Caux C, Massacrier C, Vanbervliet B, Dubois B, Van Kooten C, Durand I, Banchereau J. Activation of human dendritic cells through CD40 cross-linking. J Exp Med. 1994;180:1263–1272. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cella M, Salio M, Sakakibara Y, Langen H, Julkunen I, Lanzavecchia A. Maturation, activation, and protection of dendritic cells induced by double-stranded RNA. J Exp Med. 1999;189:821–829. doi: 10.1084/jem.189.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunningham A L, Turner R R, Miller A C, Para M F, Merigan T C. Evolution of recurrent herpes simplex lesions. An immunohistologic study. J Clin Investig. 1985;75:226–233. doi: 10.1172/JCI111678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Bruijn M L, Nieland J D, Schumacher T N, Ploegh H L, Kast W M, Melief C J. Mechanisms of induction of primary virus-specific cytotoxic T lymphocyte responses. Eur J Immunol. 1992;22:3013–3020. doi: 10.1002/eji.1830221137. [DOI] [PubMed] [Google Scholar]
- 11.Drillien R, Spehner D, Bohbot A, Hanau D. Vaccinia virus-related events and phenotypic changes after infection of dendritic cells derived from human monocytes. Virology. 2000;268:471–481. doi: 10.1006/viro.2000.0203. [DOI] [PubMed] [Google Scholar]
- 12.Engelmayer J, Larsson M, Subklewe M, Chahroudi A, Cox W I, Steinman R M, Bhardwaj N. Vaccinia virus inhibits the maturation of human dendritic cells: a novel mechanism of immune evasion. J Immunol. 1999;163:6762–6768. [PubMed] [Google Scholar]
- 13.Fruh K, Ahn K, Djaballah H, Sempe P, van Endert P M, Tampe R, Peterson P A, Yang Y. A viral inhibitor of peptide transporters for antigen presentation. Nature. 1995;375:415–418. doi: 10.1038/375415a0. [DOI] [PubMed] [Google Scholar]
- 14.Fugier-Vivier I, Servet-Delprat C, Rivailler P, Rissoan M C, Liu Y J, Rabourdin-Combe C. Measles virus suppresses cell-mediated immunity by interfering with the survival and functions of dendritic and T cells. J Exp Med. 1997;186:813–823. doi: 10.1084/jem.186.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galvan V, Roizman B. Herpes simplex virus 1 induces and blocks apoptosis at multiple steps during infection and protects cells from exogenous inducers in a cell-type-dependent manner. Proc Natl Acad Sci USA. 1998;95:3931–3936. doi: 10.1073/pnas.95.7.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grosjean I, Caux C, Bella C, Berger I, Wild F, Banchereau J, Kaiserlian D. Measles virus infects human dendritic cells and blocks their allostimulatory properties for CD4+ T cells. J Exp Med. 1997;186:801–812. doi: 10.1084/jem.186.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hart D N. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood. 1997;90:3245–3287. [PubMed] [Google Scholar]
- 18.Hill A, Jugovic P, York I, Russ G, Bennink J, Yewdell J, Ploegh H, Johnson D. Herpes simplex virus turns off the TAP to evade host immunity. Nature. 1995;375:411–415. doi: 10.1038/375411a0. [DOI] [PubMed] [Google Scholar]
- 19.Honess R W, Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974;14:8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Honess R W, Roizman B. Regulation of herpesvirus macromolecular synthesis: sequential transition of polypeptide synthesis requires functional viral polypeptides. Proc Natl Acad Sci USA. 1975;72:1276–1280. doi: 10.1073/pnas.72.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jerome K R, Tait J F, Koelle D M, Corey L. Herpes simplex virus type 1 renders infected cells resistant to cytotoxic T-lymphocyte-induced apoptosis. J Virol. 1998;72:436–441. doi: 10.1128/jvi.72.1.436-441.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jugovic P, Hill A M, Tomazin R, Ploegh H, Johnson D C. Inhibition of major histocompatibility complex class I antigen presentation in pig and primate cells by herpes simplex virus type 1 and 2 ICP47. J Virol. 1998;72:5076–5084. doi: 10.1128/jvi.72.6.5076-5084.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kazazi F, Mathijs J M, Chang J, Malafiej P, Lopez A, Dowton D, Sorrell T C, Vadas M A, Cunningham A L. Recombinant interleukin 4 stimulates human immunodeficiency virus production by infected monocytes and macrophages. J Gen Virol. 1992;73:941–949. doi: 10.1099/0022-1317-73-4-941. [DOI] [PubMed] [Google Scholar]
- 24.Koelle D M, Posavad C M, Barnum G R, Johnson M L, Frank J M, Corey L. Clearance of HSV-2 from recurrent genital lesions correlates with infiltration of HSV-specific cytotoxic T lymphocytes. J Clin Investig. 1998;101:1500–1508. doi: 10.1172/JCI1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kruse M, Rosorius O, Kratzer F, Stelz G, Kuhnt C, Schuler G, Hauber J, Steinkasserer A. Mature dendritic cells infected with herpes simplex virus type 1 exhibit inhibited T-cell stimulatory capacity. J Virol. 2000;74:7127–7136. doi: 10.1128/jvi.74.15.7127-7136.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwong A D, Frenkel N. Herpes simplex virus-infected cells contain a function(s) that destabilizes both host and viral mRNAs. Proc Natl Acad Sci USA. 1987;84:1926–1930. doi: 10.1073/pnas.84.7.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwong A D, Kruper J A, Frenkel N. Herpes simplex virus virion host shutoff function. J Virol. 1988;62:912–921. doi: 10.1128/jvi.62.3.912-921.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ludewig B, Ehl S, Karrer U, Odermatt B, Hengartner H, Zinkernagel R M. Dendritic cells efficiently induce protective antiviral immunity. J Virol. 1998;72:3812–3818. doi: 10.1128/jvi.72.5.3812-3818.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ludewig B, Oehen S, Barchiesi F, Schwendener R A, Hengartner H, Zinkernagel R M. Protective antiviral cytotoxic T cell memory is most efficiently maintained by restimulation via dendritic cells. J Immunol. 1999;163:1839–1844. [PubMed] [Google Scholar]
- 30.Luksch C R, Winqvist O, Ozaki M E, Karlsson L, Jackson M R, Peterson P A, Webb S R. Intercellular adhesion molecule-1 inhibits interleukin 4 production by naive T cells. Proc Natl Acad Sci USA. 1999;96:3023–3028. doi: 10.1073/pnas.96.6.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macatonia S E, Taylor P M, Knight S C, Askonas B A. Primary stimulation by dendritic cells induces antiviral proliferative and cytotoxic T cell responses in vitro. J Exp Med. 1989;169:1255–1264. doi: 10.1084/jem.169.4.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mason R R, Weiner R S. Application of the Beckman JE6-B Elutriator System in the isolation of human monocyte subpopulations. Scand J Haematol. 1985;34:5–8. doi: 10.1111/j.1600-0609.1985.tb00735.x. [DOI] [PubMed] [Google Scholar]
- 33.Mikloska Z, Danis V A, Adams S, Lloyd A R, Adrian D L, Cunningham A L. In vivo production of cytokines and beta (C-C) chemokines in human recurrent herpes simplex lesions–do herpes simplex virus-infected keratinocytes contribute to their production? J Infect Dis. 1998;177:827–838. doi: 10.1086/515236. [DOI] [PubMed] [Google Scholar]
- 34.Mikloska Z, Kesson A M, Penfold M E, Cunningham A L. Herpes simplex virus protein targets for CD4 and CD8 lymphocyte cytotoxicity in cultured epidermal keratinocytes treated with interferon-gamma. J Infect Dis. 1996;173:7–17. doi: 10.1093/infdis/173.1.7. [DOI] [PubMed] [Google Scholar]
- 35.Posavad C M, Huang M L, Barcy S, Koelle D M, Corey L. Long term persistence of herpes simplex virus-specific CD8+ CTL in persons with frequently recurring genital herpes. J Immunol. 2000;165:1146–1152. doi: 10.4049/jimmunol.165.2.1146. [DOI] [PubMed] [Google Scholar]
- 36.Posavad C M, Koelle D M, Corey L. High frequency of CD8+ cytotoxic T-lymphocyte precursors specific for herpes simplex viruses in persons with genital herpes. J Virol. 1996;70:8165–8168. doi: 10.1128/jvi.70.11.8165-8168.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Posavad C M, Koelle D M, Corey L. Tipping the scales of herpes simplex virus reactivation: the important responses are local. Nat Med. 1998;4:381–382. doi: 10.1038/nm0498-381. [DOI] [PubMed] [Google Scholar]
- 38.Raftery M J, Behrens C K, Muller A, Krammer P H, Walczak H, Schonrich G. Herpes simplex virus type 1 infection of activated cytotoxic T cells: induction of fratricide as a mechanism of viral immune evasion. J Exp Med. 1999;190:1103–1114. doi: 10.1084/jem.190.8.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rea D, Schagen F H, Hoeben R C, Mehtali M, Havenga M J, Toes R E, Melief C J, Offringa R. Adenoviruses activate human dendritic cells without polarization toward a T-helper type 1-inducing subset. J Virol. 1999;73:10245–10253. doi: 10.1128/jvi.73.12.10245-10253.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rescigno M, Winzler C, Delia D, Mutini C, Lutz M, Ricciardi-Castagnoli P. Dendritic cell maturation is required for initiation of the immune response. J Leukoc Biol. 1997;61:415–421. [PubMed] [Google Scholar]
- 41.Roizman B, Sears A E. Herpes simplex viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2231–2295. [Google Scholar]
- 42.Salio M, Cella M, Suter M, Lanzavecchia A. Inhibition of dendritic cell maturation by herpes simplex virus. Eur J Immunol. 1999;29:3245–3253. doi: 10.1002/(SICI)1521-4141(199910)29:10<3245::AID-IMMU3245>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 43.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sarmiento M, Kleinerman E S. Innate resistance to herpes simplex virus infection. Human lymphocyte and monocyte inhibition of viral replication. J Immunol. 1990;144:1942–1953. [PubMed] [Google Scholar]
- 45.Schmid D S, Rouse B T. The role of T cell immunity in control of herpes simplex virus. Curr Top Microbiol Immunol. 1992;179:57–74. doi: 10.1007/978-3-642-77247-4_4. [DOI] [PubMed] [Google Scholar]
- 46.Schnorr J J, Xanthakos S, Keikavoussi P, Kampgen E, ter Meulen V, Schneider-Schaulies S. Induction of maturation of human blood dendritic cell precursors by measles virus is associated with immunosuppression. Proc Natl Acad Sci USA. 1997;94:5326–5331. doi: 10.1073/pnas.94.10.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Snijders A, Kalinski P, Hilkens C M, Kapsenberg M L. High-level IL-12 production by human dendritic cells requires two signals. Int Immunol. 1998;10:1593–1598. doi: 10.1093/intimm/10.11.1593. [DOI] [PubMed] [Google Scholar]
- 48.Teute H, Braun R, Kirchner H, Becker H, Munk K. Replication of herpes simplex virus in human T lymphocytes. Intervirology. 1983;20:32–41. doi: 10.1159/000149371. [DOI] [PubMed] [Google Scholar]
- 49.Tomazin R, Hill A B, Jugovic P, York I, van Endert P, Ploegh H L, Andrews D W, Johnson D C. Stable binding of the herpes simplex virus ICP47 protein to the peptide binding site of TAP. EMBO J. 1996;15:3256–3266. [PMC free article] [PubMed] [Google Scholar]
- 50.Whitley R J. Herpes simplex viruses. In: Fields B N, Knipe D M, Howley P M, editors. Virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2297–2342. [Google Scholar]
- 51.Wu S J, Grouard-Vogel G, Sun W, Mascola J R, Brachtel E, Putvatana R, Louder M K, Filgueira L, Marovich M A, Wong H K, Blauvelt A, Murphy G S, Robb M L, Innes B L, Birx D L, Hayes C G, Frankel S S. Human skin Langerhans cells are targets of dengue virus infection. Nat Med. 2000;6:816–820. doi: 10.1038/77553. [DOI] [PubMed] [Google Scholar]
- 52.York I A, Roop C, Andrews D W, Riddell S R, Graham F L, Johnson D C. A cytosolic herpes simplex virus protein inhibits antigen presentation to CD8+ T lymphocytes. Cell. 1994;77:525–535. doi: 10.1016/0092-8674(94)90215-1. [DOI] [PubMed] [Google Scholar]
- 53.Zhou L J, Tedder T F. CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc Natl Acad Sci USA. 1996;93:2588–2592. doi: 10.1073/pnas.93.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou L J, Tedder T F. Human blood dendritic cells selectively express CD83, a member of the immunoglobulin superfamily. J Immunol. 1995;154:3821–3835. [PubMed] [Google Scholar]