Abstract
Experiments designed to distinguish virus-specific from non-virus-specific T cells showed that bystander T cells underwent apoptosis and substantial attrition in the wake of a strong T-cell response. Memory CD8 T cells (CD8+ CD44hi) were most affected. During acute viral infection, transgenic T cells that were clearly defined as non-virus specific decreased in number and showed an increase in apoptosis. Also, use of lymphocytic choriomeningitis virus (LCMV) carrier mice, which lack LCMV-specific T cells, showed a significant decline in non-virus-specific memory CD8 T cells that correlated to an increase in apoptosis in response to the proliferation of adoptively transferred virus-specific T cells. Attrition of T cells early during infection correlated with the alpha/beta interferon (IFN-α/β) peak, and the IFN inducer poly(I:C) caused apoptosis and attrition of CD8+ CD44hi T cells in normal mice but not in IFN-α/β receptor-deficient mice. Apoptotic attrition of bystander T cells may make room for the antigen-specific expansion of T cells during infection and may, in part, account for the loss of T-cell memory that occurs when the host undergoes subsequent infections.
Immune responses to viruses and other infectious agents can lead to lymphocyte hyperplasia and enlargements of the spleen and lymph nodes (LN). This occurs as a consequence of a dramatic expansion of antigen-specific T cells associated with a plethora of T-cell and B-cell growth and differentiation factors. A central question has focused on how much of a virus-induced T-cell response is specific for the virus and to what degree there is bystander activation of T cells not specific for the virus. Because viral infections can activate allospecific cytotoxic T lymphocytes (CTL) and memory CTL specific to previously encountered viruses (49, 50) and because relatively low frequencies of T cells had scored as virus specific in limiting dilution assays (1, 2, 34, 56), it was thought at one time that the bulk of the T-cell response to a viral infection may be accounted for by bystander stimulation of T cells not specific for the virus. Supporting this argument are recent publications suggesting that alpha/beta interferon (IFN-α/β) and interleukin 15 (IL-15), which are induced during viral infection, may nonspecifically promote the division of memory (CD44hi) CD8+ T cells (39, 42, 54).
Much evidence, however, challenges the concept that bystander activation accounts for most of the virus-induced T-cell hyperplasia. First, virus infections fail to stimulate the expansion of naive or memory transgenic T cells that do not cross-react with the virus (7, 53). Second, much of the virus-induced allospecific CTL response can be accounted for by T-cell clones cross-reacting between alloantigens and virus-modified self-major histocompatibility complex (MHC) (28). Selective virus-induced activation of T cells with a distinct allospecificity can be shown in mice having comparable frequencies of T-cell precursors to either of two alloantigens (28, 53). Third, the ability of viruses to reactivate memory CTL specific to previously encountered antigens can also be at least partially explained by unexpected T-cell cross-reactivities between putatively unrelated viruses (34). Finally, and most convincingly, new methods to quantify antigen-specific T cells, including MHC tetramer binding (11, 27), immunoglobulin G-MHC dimer binding (13, 33), and peptide-induced intracellular IFN-γ staining (7, 27), have revealed dramatically high percentages of virus-specific cells. In mice infected with lymphocytic choriomeningitis virus (LCMV), over 50% of the CD8 T cells can be accounted for as virus specific.
These experiments do not, however, rule out the possibility that some antigen-nonspecific T cells receive activation signals by the abundance of proliferation-inducing cytokines, nor do they explain the finding that IFN-α/β appears to induce DNA synthesis in memory T cells (42, 54). Here we investigated the fate of antigen-nonspecific T cells during viral infections and under conditions of IFN stimulation. We report that, rather than being the subject of a proliferation-inducing activation, bystander CD8 T cells, particularly of the memory phenotype, are induced into apoptosis and decline considerably in number. We first show that bystander T cells undergo attrition during virus-induced T-cell responses and then demonstrate that one possible mechanism for this centers on the ability of IFN to induce apoptosis in memory T cells. This T-cell attrition may make room in lymphoid organs for the development of a new antigen-specific T-cell response, and it may help to explain the loss in CD8 T-cell memory specific to previously encountered pathogens after a host mounts a T-cell response to another infectious agent (33, 35).
MATERIALS AND METHODS
Mice.
Male C57BL/6 (B6, H-2b) mice, gld mice, and 129 mice were purchased from Jackson Laboratories, Bar Harbor, Maine, at 4 to 5 weeks of age. Animals were used between 6 and 12 weeks of age. IFN-α/β receptor knockout (R KO) mice (also abbreviated as IFN-α/β R−/−, strain 129) were provided by R. Woodland (University of Massachusetts Medical Center, Worcester, Mass.) (14). IFN-γ R−/− mice were derived and kindly supplied by M. Aguet (University of Zurich, Zurich, Switzerland) (14). Perforin−/− mice (strain C57BL/6) were derived and provided by C. M. Walsh and W. R. Clark (University of California, Los Angeles) (46).
Virus stocks and inoculation.
The LCMV Armstrong stain was propagated in baby hamster kidney BHK21 cells. LCMV was titrated by plaque assay on Vero cells, and mice were inoculated intraperitoneally (i.p.) with 4 × 104 PFU of virus in 0.1 ml of phosphate-buffered saline (PBS).
Lymphocyte preparation for FACS analysis.
Spleens from experimental mice were homogenized and depleted of erythrocytes by suspending the cell pellet in a 0.84% NH4Cl solution. Cells were washed in fluorescence-activated cell sorter (FACS) buffer (see below) prior to use for FACS analysis.
Antibodies and staining reagents.
The following monoclonal antibodies (MAbs) and reagents were used for phenotypic analysis of lymphocytes from mice studied herein: anti-CD8-PerCP (clone 2.43), anti-CD44-fluorescein isothiocyanate (FITC) or antigen-presenting cells (clone 7D4), and annexin V-phycoerythrin (PE) or -FITC (all reagents were obtained from PharMingen, San Diego, Calif.). Staining was performed in FACS buffer (PBS–2% fetal calf serum [Sigma]–0.1% [wt/vol] sodium azide [Sigma]) with the exception of annexin staining, which was performed in annexin buffer (PharMingen). Samples were analyzed using a Becton Dickinson FACSCalibur (Becton Dickinson, San Diego, Calif.) and CellQuest software (Becton Dickinson) or FlowJo (Treestar, Inc., San Carlos, Calif.). In these experiments, 30,000 to 80,000 events were routinely examined, and lymphocyte gating was based on forward-scatter versus side-scatter properties. Unless otherwise stated, gating on CD44hi versus CD44lo cells was determined using the appropriate day 0 controls.
MHC tetramer staining.
MHC H-2Db tetramers, labeled with PE and loaded with the LCMV nucleoprotein peptide (NP396–404) (15), were used for analysis of memory CD8 T cells in LCMV-immune mice treated with poly(I:C) (Sigma). LCMV-immune mice were inoculated with LCMV Armstrong 8 to 12 weeks prior to use in experiments.
IFN stimulation and injections.
Poly(I:C) (Sigma) was injected i.p. at a dose of 100 μg/100 μl of either PBS or Hanks balanced salt solution (HBSS) per mouse. Recombinant IFN-α/β (PBL Biomedical Laboratories, New Brunswick, N.J.) was delivered i.p. at 104 U/100 μl of HBSS per mouse.
Detection of HY+ T cells.
Splenocytes, prepared for FACS analysis, were stained on ice with anti-CD8-PE and the MAb T3.70 (a gift from H. Teh, University of British Columbia, Vancouver, Canada) (40). The cells were washed and incubated with anti-mouse immunoglobulin G1-FITC for 30 min on ice. Samples were washed prior to FACS analysis, as described above. CD8+ T3.70+ cells are designated HY transgenic (HY+), and CD8+ T3.70− cells are designated HY nontransgenic (HY−).
Adoptive transfer of splenocytes into LCMV carrier mice.
Splenocytes (3 × 107) from LCMV-immune (4 to 6 weeks post-i.p. inoculation, as described above) or naive B6.PL Thy1a/Cy mice (Thy 1.1+) were injected intravenously via the tail vein into C57BL/6 LCMV carrier mice (Thy 1.2+) in 0.5 ml of HBSS, without phenol red (Gibco BRL, Gaithersburg, Md.). Spleens were harvested 6 days after transfer and prepared for flow cytometry, as described above.
CDR3 length spectratyping of T-cell receptor (TCR) repertoire.
RNA samples, equivalent to 5 × 105 to 10 × 105 cells or 0.12 ml of blood, were amplified by using a GeneAmp RNA PCR kit (Perkin-Elmer Corp., Branchburg, N.J.) with Vβ8.1 and Cβ primers, according to the manufacturer's instructions and as detailed previously (22, 29). The amplification started with a denaturing step of 1 min at 94°C, followed by 40 cycles consisting of 1 min at 94°C, 1 min at 55°C, and 1 min at 72°C and a 5-min incubation at 72°C, to complete the product extension.
Two microliters of the amplified PCR products was subjected to five cycles of runoff with fluorophore-labeled Jβ primers (Jβ1.3, Jβ1.4, Jβ1.5, Jβ1.6, Jβ2.5, and Jβ2.7) in a final volume of 10 μl of reaction mixture containing 50 mM KCl, 10 mM Tris HCl (pH 8.3), 1 mM MgCl2, 200 μM dNTP, 0.25 U of Taq polymerase (Perkin-Elmer Corp.), and 0.1 μM concentrations of labeled Jβ primers. One microliter of the fluorescent products was mixed with an equal volume of gel-loading buffer (5 parts 100% formamide and 1 part 2.5% blue dextran–50 mM EDTA) and loaded onto a 4.75% acrylamide sequencing gel. The results were analyzed on an automated DNA sequencer using GeneScan software (Perkin-Elmer Applied Biosystems, Emeryville, Calif.).
Statistical analyses.
Student's t test was used for data analysis where appropriate. Results are expressed as the mean ± standard deviation.
RESULTS
Decrease in the number of non-virus-specific bystander T cells as a result of LCMV infection in HY transgenic mice.
To determine the fate of non-virus-specific T cells in response to a viral infection, HY-specific TCR transgenic mice were inoculated i.p. with LCMV. HY transgenic mice are “leaky” and can produce an LCMV-specific response due to the combination of a transgenic TCR β chain with an endogenously rearranged TCR α chain, producing a proportion of T cells of various specificities (32, 53). Spleens from LCMV-infected mice were harvested and stained using MAbs that allow for the identification of HY+ versus HY− CD8+ T cells, some of which are LCMV specific. Table 1 shows the numbers of HY+ and HY− CD8+ T cells found in the spleens of LCMV-infected HY transgenic mice. During the peak of the LCMV-specific CTL response at day 8, the non-virus-specific (HY+) T cells had significantly decreased by 26 to 44%, and the number of HY− T cells containing the LCMV-specific cells had more than doubled (Table 1). In some experiments HY transgenic mice were intravenously inoculated with 5 × 107 C57BL/6 splenocytes prior to LCMV infection in order to populate the mouse with a more complete T-cell repertoire. The results were similar to the nonreconstituted mice, in that there was a substantial loss in the HY+ T cells. For example, on day 9 post-LCMV infection, reconstituted mice exhibited a 28% reduction (P < 0.05) in HY+ cells, while HY− cells increased by 128%.
TABLE 1.
Attrition of non-virus-specific T cells (HY+) during acute LCMV infection of HY transgenic micea
| Expt | Treatment | n | Results for HY+ cells
|
Results for HY− cells
|
||
|---|---|---|---|---|---|---|
| No. of HY+ cellsb (106) (% change) | % Annexin+ | No. of HY− cellsb (106) (% change) | % Annexin+ | |||
| 1 | None | 4 | 2.6 ± 0.4 | NDc | 5.2 ± 0.7 | ND |
| Day 8 LCMV | 3 | 1.7 ± 0.5 (−35)d | ND | 14.8 ± 2.1 (+183)d | ND | |
| 2 | None | 2 | 2.9 ± 0.8 | ND | 4.2 ± 2.0 | ND |
| Day 8 LCMV | 3 | 1.6 ± 0.4 (−44)e | ND | 14.0 ± 2.5 (+233)d | ND | |
| 3 | None | 4 | 1.9 ± 0.3 | 12 ± 1.0 | 2.5 ± 0.4 | 32 ± 2.8 |
| Day 8 LCMV | 4 | 1.4 ± 0.3 (−26)d | 23 ± 1.7d | 5.8 ± 2.2 (+132)d | 41 ± 1.7d | |
| 4 | None | 2 | 1.1 ± 0.3 | 7 ± 2.7 | 3.3 ± 1.0 | 15 ± 3.4 |
| Day 8 LCMV | 3 | 0.7 ± 0.1 (−31)e | 17 ± 1.7d | 11.1 ± 4.1 (+236)d | 30 ± 4.2d | |
HY transgenic female mice were infected i.p. with LCMV, and spleens were harvested on the days listed. Cells were stained for CD8 and the HY+ TCR (see Materials and Methods) to determine the number of cells in each population. The LCMV-specific fraction is found in the HY− CD8+ T cells.
HY+/− cells are defined as described in Materials and Methods.
ND, not done.
P < 0.05 compared to grouped control.
P < 0.1 compared to grouped control.
The disappearance of cells from the spleen could either be due to migration from the spleen or to death of the cell population. The i.p. inoculation route of LCMV causes a dramatic increase in CD8+ T cells in the peritoneal cavity by day 8. However, HY+ T cells were at low levels in the peritoneal exudate cells (PECs), indicating that they were not recruited into this site of viral infection (data not shown). We therefore assessed whether the loss of bystander cells may be due to apoptosis and analyzed the remaining bystander cells for apoptosis by using annexin V. Annexin V binds preferentially to phosphatidylserine, normally found on the inner leaflet of the cell membrane, and is used as an indicator of membrane inversion, which is a sign of the early stages of apoptosis (43). We prefer this early marker of apoptosis for analyzing apoptotic cells directly ex vivo, as techniques based on DNA fragmentation have generally yielded few apoptotic cells in fresh isolates, unless they were cultivated for several hours in vitro. There was about a twofold increase in the proportion of annexin V+ HY+ T cells as a result of the LCMV infection by 8 to 9 days postinfection (Table 1). This increase was consistently observed in multiple experiments. Also of note were increases in the proportion of apoptotic cells in the expanded HY− population, but apoptosis among virus-induced activated T cells has been demonstrated previously (30); our unique observation is that the bystander cells are also undergoing apoptosis. Flow cytometry analyses indicated that the annexin V+ cells were small and displayed an increased side scatter, properties consistent with apoptotic cells, as will be shown in another system in Fig. 1C.
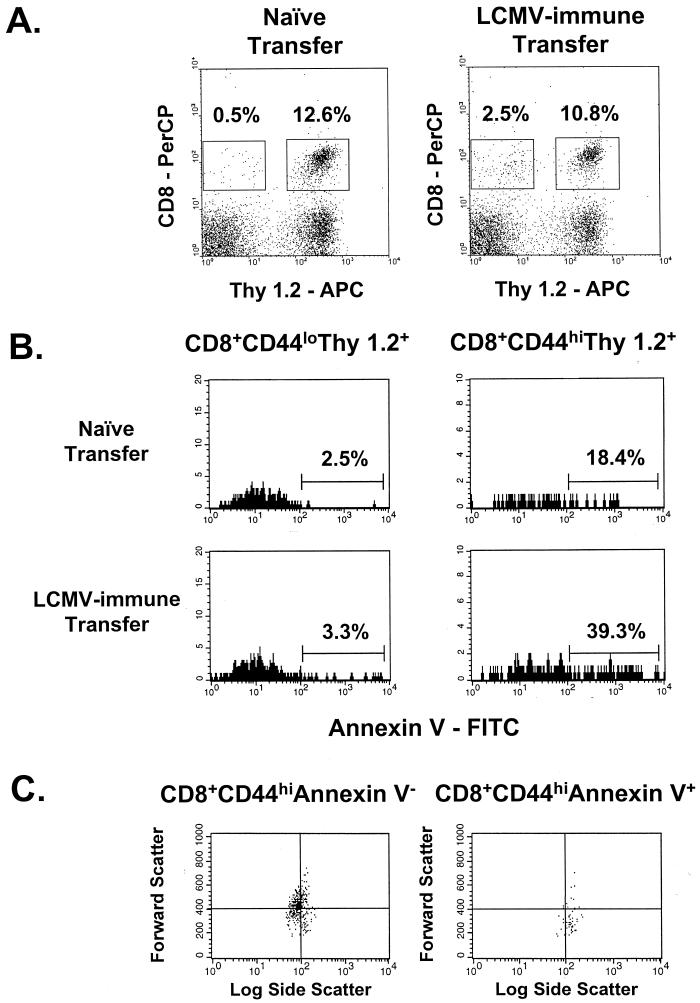
FIG. 1.
Adoptive transfer of LCMV-immune splenocytes into LCMV carrier mice leads to a reduction in the number of host CD8 T cells and an increase in host T-cell apoptosis. (A) Spleens from LCMV carrier mice (Thy 1.2+), which received an adoptive transfer of 3 × 107 naive or LCMV-immune Thy 1.1+ splenocytes (Thy 1.2−), were stained to determine the proportions of donor and host-derived CD8 T cells 6 days after reconstitution. APC, antigen-presenting cells. (B) Host-derived cells were gated on memory (CD44hi) and naive (CD44lo) phenotypes, and apoptosis of these populations was assessed using annexin V. (C) Light scatter properties of annexin V+ or annexin V− host-derived memory T cells is shown, demonstrating that the annexin V+ population is smaller and more granular than the annexin V− T cells. In all cases, data shown are from an individual mouse in a representative experiment (n = 3).
These experiments indicate that bystander cells undergo apoptosis and decline in number rather than increase during a viral infection, but the transgenic T cells examined in these studies were of the naive phenotype, and memory T cells may have behaved differently. In a previous study designed to see if there was an increase in the number of bystander memory cells, we noted that when “memory” HY+ T cells were generated by exposing them to male antigens, the LCMV infection caused even greater reductions in the transgenic memory cells than in transgenic naive cells (53). To address the relative susceptibilities of naive and memory phenotype bystander T cells in the same mice to apoptotic deletion, we turned to another model.
Decrease of bystander host T cells in LCMV carrier mice in response to the adoptive transfer of LCMV-specific donor cells.
It was our goal to utilize a system in which we could observe bystander events on a subpopulation of definitively non-cross-reactive cells of both naive and memory phenotypes. LCMV carrier mice (Thy 1.2) congenitally infected with LCMV lack LCMV-specific T cells and are persistently infected with the virus throughout their lifetime (25). LCMV does not replicate or present antigens in their CD8+ T cells (25, 41), and these mice are otherwise relatively normal and can mount T-cell responses to antigens other than LCMV. These mice were used as recipients for the adoptive transfer of donor splenocytes from naive or LCMV-immune congenic mice (Thy 1.1). The LCMV-specific T cells, present at a much higher frequency in LCMV-immune mice than in naive mice, will proliferate when adoptively transferred into LCMV carrier mice (17, 52, 53). The effects of this antigen-specific T-cell response on the non-virus-specific host-derived CD8 T cells were determined by using the congenic Thy 1 marker to distinguish host (Thy 1.2) from donor (Thy 1.1) cells (17).
As shown by their numbers in Table 2 and Fig. 1A, donor CD8+ T cells from LCMV-immune mice were successfully implanted into the carrier mice, reflective of their proliferation in response to LCMV antigens (53). Extensive studies have previously shown that carrier mice receiving adoptively transferred splenocytes from naive mice exhibit very little increase in the number of donor cells, perhaps because of antigen excess leading to their death (17, 25, 52, 53); in such mice the number and function of the host cells are relatively normal. In carrier mice receiving LCMV-immune splenocytes, the increase in donor CD8+ T cells was associated with a decrease in the number of host (Thy 1.2) CD8+ T cells by 27 to 29% (Table 2) compared to the number in carrier mice receiving donor splenocytes from LCMV-naive mice. It is important to note that there was no statistically significant decrease in the number of host CD4 T cells as a result of the adoptive transfer (data not shown). Since LCMV is found in CD4 T cells but rarely in CD8 T cells (17, 25), CD4 T cells in the LCMV carrier mice would be likelier to be presenting LCMV antigens. Because the host CD4 T-cell numbers are unaffected, it is unlikely that donor CTL-mediated killing of infected host CD8 T cells is responsible for the decrease in CD8 T-cell number.
TABLE 2.
Attrition of non-virus-specific T cells (Thy 1.2+) in LCMV carrier mice after adoptive transfer of LCMV-immune or naive splenocytesa
| Expt | Donor cell type | n | % Donor CD8+ reconstitution | % Host CD8+ reduction | Results for memory cells
|
Results for naive cells
|
||
|---|---|---|---|---|---|---|---|---|
| No. of CD8+ memoryb host cells (106) (% change) | % Annexin V+ | No. of CD8+ naivec host cells (106) (% change) | % Annexin V+ | |||||
| 1 | Naive | 2 | 0.5 | 5.6 ± 1.0 | 15 ± 3.1 | 10.2 ± 0.5 | 5 ± 0.8 | |
| LCMV-immune | 3 | 3.7 | 28 | 3.2 ± 1.0 (−43)d | 26 ± 5.1 | 8.1 ± 1.9 (−21) | 7 ± 1.2 | |
| 2 | Naive | 2 | 0.6 | 1.1 ± 0.1 | 15 ± 2.0 | 2.2 ± 0.3 | 5 ± 1.7 | |
| LCMV-immune | 2 | 5.8 | 27 | 0.6 ± 0.1 (−45)d | 37 ± 1.0 | 1.8 ± 0.2 (−18) | 7 ± 1.1 | |
| 3 | Naive | 2 | 0.6 | 1.5 ± 0.0 | 11 ± 0.5 | 5.3 ± 1.3 | 4 ± 0.7 | |
| LCMV-immune | 3 | 3.7 | 29 | 0.7 ± 0.3 (−53)d | 21 ± 3.0 | 4.1 ± 1.4 (−23) | 3 ± 1.6 | |
LCMV carrier mice (Thy 1.2+), deficient in LCMV-specific T cells from birth, received 3 × 107 donor splenocytes intravenously from either naive or LCMV-immune Thy 1.1+ mice. After 6 days, recipient mice were sacrificed and host splenocytes were analyzed using MAbs to CD8, CD44, and Thy 1.2. The percentage of donor CD8 T cells was determined using MAbs to CD8 and Thy 1.1.
Host memory cells defined as CD44hi Thy 1.2+
Host naive cells defined as CD44lo Thy 1.2+.
P < 0.05 compared to grouped control.
Bystander memory phenotype T cells are decreased more than bystander naive phenotype T cells.
Since it was previously suggested that memory cells were more susceptible than naive cells to bystander proliferation (20, 42, 54), the memory marker CD44 was used to define non-virus-specific naive (CD44lo) and memory (CD44hi) bystander T cells (Thy 1.2+). The decrease in bystander host Thy 1.2+ T cells was the result of a preferential attrition of memory cells (Table 2). In three experiments, CD8+ Thy 1.2+ CD44hi cells were reduced by 43 to 53% in mice receiving an adoptive transfer of LCMV-immune splenocytes compared to mice receiving splenocytes from naive donors (Table 2). Bystander naive CD8 T cells decreased in number by 18 to 23%.
Annexin V was used to determine whether the non-virus-specific population exhibited early signs of undergoing apoptosis. Figure 1B shows, in a representative experiment, that there was an increase in annexin-positive host CD8+ T cells concomitant with their decrease in number and that the memory phenotype (CD44hi) host T cells contained a higher proportion of apoptotic cells than the naive (CD44lo) fraction. Table 2 also shows that in three separate experiments the memory host CD8+ T cells exhibited approximately a doubling in the proportion staining positively with annexin V compared to only minor changes in the annexin V+ proportion in naive host CD8 T cells. Annexin V+ cells had light scatter properties consistent with those of apoptotic cells, with reduced forward scatter (mean fluorescence intensity of annexin V+, 342 ± 31.8; that of annexin V−, 462 ± 9.4; n = 3; P < 0.05) indicative of smallness and an increase in side scatter (mean fluorescence intensity of annexin V+, 121 ± 4.8; that of annexin V−, 94.5 ± 1.1; n = 3; P < 0.05) as a measure of cell granularity. Similar trends were seen in two other experiments (n = 3 per group). Figure 1C presents an example of these light-scattering properties in this small residual population of annexin V+ cells.
Kinetics of CD8 T-cell attrition during LCMV infection.
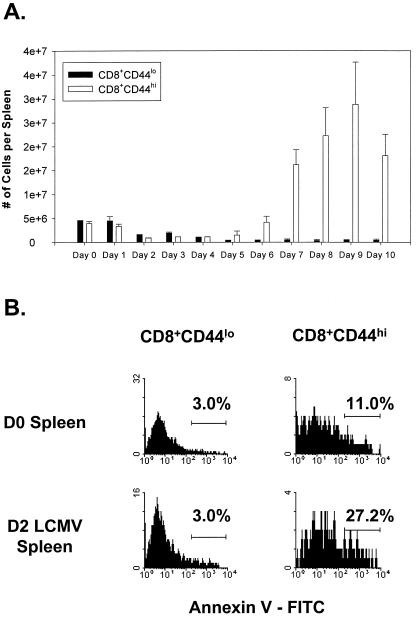
Having shown that bystander T cells undergo apoptotic attrition during virus-induced T-cell responses, we examined the cell number and apoptosis of T cells during an acute LCMV infection of normal C57BL/6 mice. Figure 2 presents the CD44lo and CD44hi spleen CD8 T-cell numbers at different days after LCMV infection; here the CD44 gate was drawn with the activated T cells at the peak of the response and used consistently for each time point, resulting in a slightly wider gate for the CD44hi cells in the naive day 0 T-cell populations. There were two notable declines in CD8 T-cell numbers flanking the peak of the LCMV-specific T-cell response. The first decrease was observed early after infection on days 2 to 4 (Fig. 2A). The second decrease was detected after day 9 postinfection, which is approximately 2 days after the clearance of antigen during the silencing of the LCMV-specific immune response and has previously been well documented (30). The early decrease in the number of CD8 T cells corresponds to the peak of the NK cell response and the peak in production of IFN-α/β (47). It also corresponds to a peak in total splenocyte apoptosis at day 3, as shown previously by terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling staining of spleen sections (30). This early decrease (days 2 to 4) in CD8 T-cell numbers occurred prior to the detection of LCMV-specific T cells, suggesting that non-virus-specific bystander cells may be eliminated during this phase of infection. Consistent with this finding was an increase in the proportion of memory CD8 T cells staining positively for annexin V, which is an early indicator for cells undergoing apoptosis. Figure 2B shows annexin staining from representative mice gated on naive (CD44lo) or memory (CD44hi) phenotype CD8 T cells on days 0 and 2 following i.p. infection with LCMV.
FIG. 2.
Kinetic analysis of CD8+ T cells found in the spleens of mice undergoing an acute LCMV infection. (A) Spleens from LCMV-infected B6 mice were analyzed using flow cytometry to determine the number of memory (CD44hi) or naive (CD44lo) CD8 T cells during the course of the viral infection. Results are expressed as the mean number of cells per spleen ± standard deviation (n = 3 per time point). (B) Annexin staining of memory and naive CD8 T cells from a representative mouse 2 days following LCMV infection. In both panels, gating on CD44hi versus CD44lo cells was determined using the day 9 postinfection time point to include the majority of activated cells. This resulted in a broader definition of CD44hi cells than would occur if day 0 control mice were used for determining the gates. D0, day 0; D2, day 2.
Attrition of memory CD8 T cells under conditions of IFN-α/β induction.
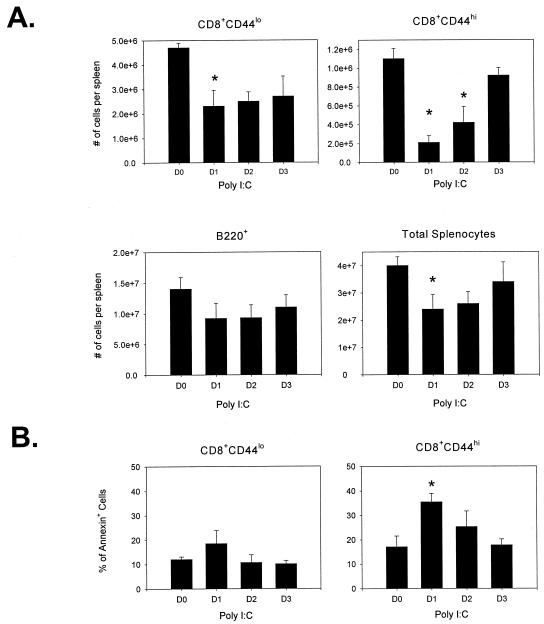
To determine whether IFN-α/β could be partly responsible for the loss of CD8 T cells, mice were treated with the IFN-α/β inducer poly(I:C). Mice receiving 100 μg of poly(I:C) i.p. were assessed for the number of naive (CD44lo) and memory (CD44hi) CD8 T-cell populations. Day 1 following poly(I:C) treatment resulted in a greater than 50% reduction in the number of memory CD8 T cells (Fig. 3A). A smaller, though significant, reduction in naive CD8 T cells (CD8+ CD44lo) was also observed. The number of memory CD8 T cells began to recover to control levels on day 3 (Fig. 3A). It is interesting that at no time point studied did the number of CD8 T cells in the spleens of poly(I:C)-treated mice ever significantly exceed the number found in the untreated controls. Annexin V staining revealed that there was a significant increase in the proportion of CD8+ CD44hi T cells that exhibited the early signs of apoptosis (Fig. 3B). The percentage of CD8+ CD44hi cells that were annexin V+ peaked on day 1 following poly(I:C) treatment and then decreased during the “recovery phase.” Also, in preliminary experiments using bromodeoxyuridine (BrdU) to determine cell division, we observed no increase in labeling with BrdU by the CD8+ CD44hi population on day 1 following poly(I:C) treatment (data not shown). This suggests that there was no cell division immediately following IFN-α/β induction by poly(I:C) in these cells.
FIG. 3.
Poly(I:C) injection leads to a significant decrease in the number of CD8+ CD44hi T cells in the spleen and an increase in their apoptosis. (A) Splenocytes from B6 mice injected with poly(I:C) were stained for CD8, CD44, and B220 expression to determine the number of CD8 T cells (memory [CD44hi] or naive [CD44lo]) and B cells present on days 1 to 3 postinjection. The proportion of annexin V+ cells for these mice is shown in panel B. Data shown are from a representative experiment (n = 5). D0, day 0; D1, day 1; etc. *, P < 0.05, compared to day 0 control.
We questioned whether the mechanism for the decrease in the number of memory CD8 T cells could be credited to the migration of memory phenotype CD8 T cells to the peritoneal cavity as a result of the i.p. injection of poly(I:C), but this was not the case, as there was also a reduction in CD8 T-cell number in that compartment (recovery from PECs: CD8+ CD44hi, day 0, 6.0 × 104; day 1 [poly(I:C)], 3.6 × 103; n = 3, pooled). Two separate LN were studied and exhibited losses in CD8 T-cell numbers similar to those found in the spleen (inguinal LN, CD8+ CD44hi cells, day 0, 2.8 × 105; day 1 [poly(I:C)], 1.3 × 105) (aortic lumbar LN, CD8+ CD44hi cells, day 0, 4.2 × 105; day 1 [poly(I:C)], 3.2 × 105; n = 3) as a result of poly(I:C) injection. We also examined the liver and bone marrow and found reductions in CD8 T-cell number (liver, CD8+ CD44hi cells, day 0, 8.5 × 103; day 1 [poly(I:C)], 7.8 × 103) (bone marrow, CD8+ CD44hi cells, day 0, 4.6 × 105; day 1 [poly(I:C)], 8.2 × 104; n = 3, pooled). B-cell numbers (B220+) were not significantly reduced (Fig. 3A). Also, B cells had no significant increases in the proportion of cells that were annexin V+ at the times tested (data not shown).
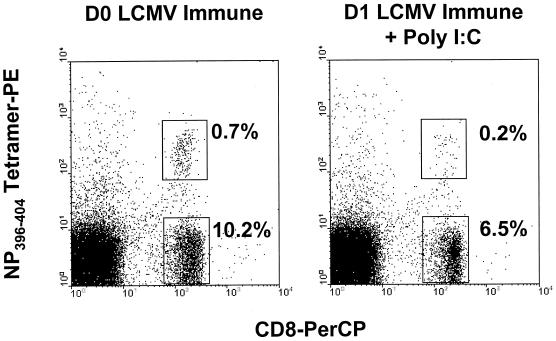
Thus far, we have defined memory CD8 T cells based on their surface expression of CD44. To assess the attrition of memory CD8 T cells using a well-defined memory CD8 T-cell population of a known antigenic specificity, we stained T cells from LCMV-immune mice with an H-2Db tetramer loaded with the LCMV NP396–404 immunodominant peptide. Figure 4 shows representative staining from LCMV-immune mice on days 0 and 1 after poly(I:C) injection. The tetramer-positive cells were reduced in frequency as a result of the poly(I:C) injection, similar to what was observed for the memory CD8 T-cell fraction defined as CD8+ CD44hi (Table 3). Also, annexin staining increased on the tetramer-positive cells, similar to the increases seen in the CD8+ CD44hi population (Table 3). This indicates that poly(I:C) induces apoptosis and attrition in a population of memory cells with a defined antigen specificity.
FIG. 4.
Poly(I:C) induces attrition of LCMV-specific memory CD8 T cells. Splenocytes from LCMV-immune mice (see Materials and Methods) were analyzed for expression of CD8 and an MHC tetramer (H-2Db) specific for the LCMV peptide NP396–404. LCMV-immune mice were injected with poly(I:C) and analyzed, as previously described. Representative histograms from an experiment performed twice are shown. D0, day 0; D1, day 1.
TABLE 3.
Attrition of LCMV-specific memory cells following poly(I:C) injection
| Expt | Cell type and treatment receivedd | n | Population | No. of cells (104) (% change) | % Annexin+ |
|---|---|---|---|---|---|
| 1 | LCMV-immune (day 0) | 2 | CD8+ | 589 ± 69.8 | NDa |
| CD8+ CD44hi | 323 ± 123 | ND | |||
| CD8+ CD44lo | 266 ± 53.4 | ND | |||
| CD8+ NP398–404 tetramer+ | 37.1 ± 13.7 | ND | |||
| LCMV-immune + day 1 poly(I:C) | 3 | CD8+ | 262 ± 20.6 (−55)b | ND | |
| CD8+ CD44hi | 109 ± 10.5 (−66)c | ND | |||
| CD8+ CD44lo | 154 ± 25.3 (−42)b | ND | |||
| CD8+ NP396–404 tetramer+ | 7.9 ± 0.6 (−79)b | ND | |||
| 2 | LCMV-immune (day 0) | 3 | CD8+ | 966 ± 273 | 19 ± 10 |
| CD8+ CD44hi | 458 ± 102 | 15 ± 3.7 | |||
| CD8+ CD44lo | 508 ± 180 | 21 ± 15 | |||
| CD8+ NP396–404 tetramer+ | 53.2 ± 2.1 | 63 ± 11 | |||
| LCMV-immune + day 1 poly(I:C) | 4 | CD8+ | 543 ± 189 (−44)c | 14 ± 5.6 | |
| CD8+ CD44hi | 152 ± 28.5 (−66)b | 25 ± 0.5b | |||
| CD8+ CD44lo | 391 ± 163 (−23) | 15 ± 4.3 | |||
| CD8+ NP396–404 tetramer+ | 25.5 ± 6.4 (−52)b | 78 ± 3.1b |
ND, not done.
P < 0.05 compared to grouped control.
P < 0.1 compared to grouped control.
Day 0, untreated at that time.
Memory T-cell loss is a consequence of IFN-α/β.
Poly(I:C) treatment results in an increase in the production of IFN-α/β and other cytokines, such as tumor necrosis factor (TNF) and IL-15 (54). It has been suggested that IFN-α/β and IL-15 may increase the proliferation of bystander memory CD8 T cells (39, 42), but our poly(I:C) results suggested instead that IFN-α/β may induce apoptosis in memory CD8+ T cells, leading to their initial decrease in number. To further clarify the role of IFN-α/β in this process, IFN-α/β R KO mice were treated with poly(I:C), and the number of CD8 T cells was determined. Table 4, experiment A, which is representative of four experiments, shows that the numbers of both naive and memory CD8 T cells increased rather than decreased in IFN-α/β R KO mice, whereas control 129 mice showed significant reductions in memory CD8 T-cell numbers, consistent with our experiments using C57BL/6 mice. These results indicate that in the absence of the signal delivered by IFN-α/β, poly(I:C) treatment does not lead to a reduction in T-cell numbers. In two experiments, i.p. injection of 104 U of recombinant IFN-α/β resulted in a 54 to 55% loss of CD8+ memory T cells on day 1 following injection (experiment 1: CD8+ CD44hi, day 0, [1.3 ± 0.0] × 106, n = 3; day 1 [recombinant IFN-α], [7.2 ± 1.9] × 105, n = 2, P = 0.013) (experiment 2: CD8+ CD44hi, day 0, [8.6 ± 0.7] × 105, n = 3; day 1 [recombinant IFN-α], [4.9 ± 0.7] × 105, n = 3, P < 0.05). These data derived by using purified IFN were consistent with data derived by using poly(I:C), but the experiments were not continued because of the limited availability of the reagent. These reductions in T-cell numbers in IFN-injected mice are significant, especially considering the relatively low dose delivered, compared to the high and sustained systemic levels of IFN-α/β detected as a result of poly(I:C) treatment (48).
TABLE 4.
Parameters of IFN-induced T-cell attritiona
| Expt | Mouse type | Time and treatmente | n | Results for memory cells
|
Results for naive cells
|
||
|---|---|---|---|---|---|---|---|
| No. of memory CD8 cellsb (105) (% change) | % Annexin V+ | No. of naive CD8 cellsc (105) (% change) | % Annexin V+ | ||||
| A | 129 | Day 0 | 2 | 7.4 ± 1.4 | 7 ± 0.1 | 33.0 ± 7.4 | 2 ± 0.2 |
| Day 1 poly(I:C) | 2 | 4.0 ± 1.2 (−46)d | 30 ± 3.3d | 26.0 ± 9.4 (−21) | 5 ± 0.2d | ||
| IFN-α/β R−/− | Day 0 | 3 | 5.7 ± 0.4 | 20 ± 1.7 | 34.0 ± 1.2 | 8 ± 3.7 | |
| Day 1 poly(I:C) | 3 | 9.2 ± 5.4 (+60)d | 14 ± 4.9 | 50.0 ± 3.5 (+47)d | 6 ± 3.4 | ||
| B | C57BL6/J | Day 0 | 2 | 5.6 ± 0.1 | 17 ± 1.2 | 19.5 ± 1.4 | 4 ± 0.8 |
| Day 1 poly(I:C) | 3 | 1.8 ± 0.1 (−68)d | 35 ± 4.5d | 9.0 ± 0.5 (−55)d | 7 ± 0.2d | ||
| Perforin−/− | Day 0 | 2 | 4.7 ± 1.5 | 35 ± 3.9 | 14.6 ± 0.7 | 17 ± 6.8 | |
| Day 1 poly(I:C) | 3 | 1.8 ± 0.8 (−62)d | 45 ± 2.9d | 8.0 ± 2.9 (−45)d | 21 ± 6.3 | ||
| C | C57BL6/J | Day 1 NK1.1 depletion | 2 | 4.7 ± 0.4 | 13 ± 1.9 | 12.8 ± 1.7 | 4 ± 1.4 |
| Day 1 poly(I:C) NK1.1 depletion | 3 | 2.2 ± 0.2 (−53)d | 23 ± 2.5d | 9.7 ± 0.7 (−24) | 4 ± 0.6 | ||
| D | 129 | Day 0 | 2 | 7.6 ± 1.1 | 11 ± 3.2 | 68.0 ± 0.5 | 2 ± 0.2 |
| Day 1 poly(I:C) | 2 | 2.4 ± 2.3 (−69)d | 38 ± 0.7d | 32.0 ± 3.1 (−53) | 4 ± 0.2d | ||
| IFN-γ R−/− | Day 0 | 2 | 9.9 ± 2.4 | 19 ± 0.8 | 64.0 ± 9.9 | 4 ± 1.3 | |
| Day 1 poly(I:C) | 3 | 4.6 ± 1.2 (−54)d | 26 ± 1.7d | 43.0 ± 5.8 (−33) | 1.3 ± 0.4 | ||
| E | C57BL6/J | Day 0 | 3 | 23.0 ±0.6 | 9 ± 1.4 | 56.0 ± 12.0 | 1 ± 0.6 |
| Day 1 poly(I:C) | 3 | 5.4 ± 1.2 (−77)d | 31 ± 9.5d | 42.0 ± 0.6 (−25) | 5 ± 2.9 | ||
| gld (FasL mutant) | Day 0 | 2 | 34.0 ± 2.7 | 12 ± 4.3 | 44.0 ± 2.7 | 4 ± 1.2 | |
| Day 1 poly(I:C) | 3 | 10.0 ± 2.7 (−71)d | 31 ± 3.4d | 31.0 ± 10.0 (−30) | 9 ± 5.5 | ||
Experiments shown are representative experiments performed at least three times independently. Mice received 100 μg of poly(I:C) i.p. and were sacrificed after 1 day. Spleens were harvested and analyzed using flow cytometry (see Materials and Methods).
Host memory cells defined as CD44hi.
Host naive cells defined as CD44lo.
P < 0.05 compared to grouped control.
Day 0, untreated at that time.
Several experiments with IFN-α/β R KO mice were also done to assess the role of IFN-α/β in the LCMV-induced attrition of memory CD8 T cells, and these experiments showed that these mice resist the LCMV-induced T-cell attrition. By day 3 postinfection a dose of 106 PFU of LCMV reduced the control 129 mouse spleen CD8 T-cell number from (10 ± 2.8) × 105 to (4.4 ± 1.8) × 105 and the CD44+ CD8+ T-cell number from (2.2 ± 0.63) × 105 to (0.4 ± 0.07) × 105; in contrast, the CD8 T-cell number in IFN-α/β R KO mice went from (29 ± 3.5) × 105 to (32 ± 9.9) × 105 and the CD44+ CD8+ T-cell number went from (6.0 ± 0.68) × 105 to (7.0 ± 2.8) × 105. These experiments with LCMV [in contrast to those with poly(I:C)] are somewhat difficult to interpret because the LCMV antigen load is much higher in the IFN R KO mice and many other cytokines will be induced (14), yet they nevertheless support the concept that IFN-α/β plays a role in the T-cell attrition seen during viral infections.
Poly(I:C)-induced CD8 T-cell loss is not dependent on NK cells, perforin, or IFN-γ.
To determine whether the lytic activity of NK cells, activated as a result of poly(I:C) treatment, was responsible for part of the CD8 T-cell loss, perforin−/− mice (Table 4, experiment B) or mice depleted of NK cells with a MAb to NK1.1 (Table 4, experiment C) were studied. In these representative experiments, reductions in the number of CD8+ CD44hi cells were similar to those from normal, poly(I:C)-treated mice. Also, perforin−/− mice and mice depleted of NK1.1+ cells showed similar increases in the proportion of annexin V+ CD8+ CD44hi cells, suggesting that neither plays a role in the increase in apoptosis of memory CD8 T cells after poly(I:C) treatment. We also questioned whether IFN-γ, which mediates some effects on T cells similar to those of IFN-α/β (44), played a role in the poly(I:C)-induced apoptotic attrition of memory CD8 T cells. IFN-γ R−/− mice treated with poly(I:C) showed decreases in the number of memory CD8 T cells and an increase in their annexin staining similar to that of the normal 129 controls and consistent with our results in C57BL6 mice (Table 4, experiment D), indicating that IFN-γ was not essential.
Poly(I:C)-induced CD8 T-cell loss is not dependent on Fas-FasL interactions.
The increases observed in annexin V staining indicated that a proapoptotic pathway may be responsible for the loss of CD8 T cells. In three preliminary experiments using flow cytometry to analyze Fas expression, we saw that surface levels of Fas moderately increased on T cells as a result of the poly(I:C) treatment (data not shown). Since Fas-Fas ligand (FasL) interactions could be a potential mechanism for the increases in apoptosis, gld mice, deficient in FasL, were utilized. Table 4, experiment E, shows, however, that gld mice had a decrease in memory CD8 T cells and an increase in apoptosis similar to those observed in normal mice treated with poly(I:C). This suggests that Fas-FasL-mediated mechanisms were not required for either the reduction of CD8 cell number or the increase in apoptosis (Table 4, experiment E).
Effects of poly(I:C) on the T-cell repertoire.
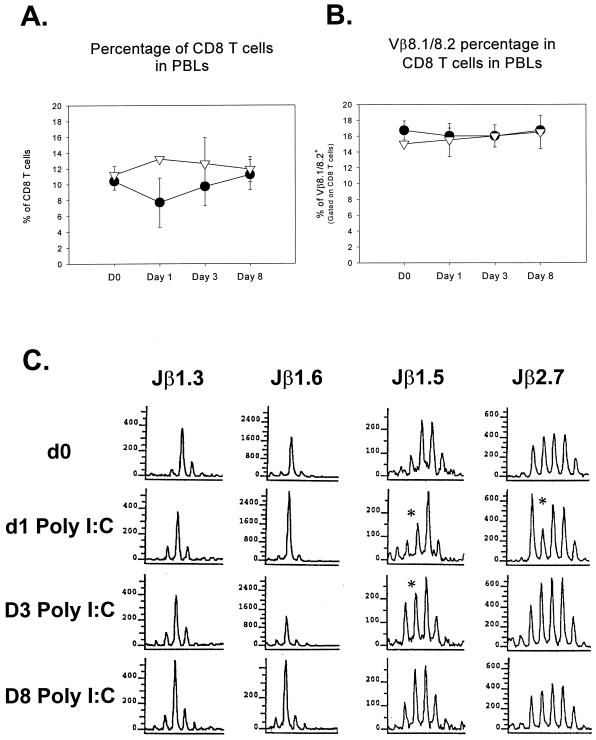
Our data indicate that IFN-α/β significantly decreases the number of CD44hi CD8+ T cells, but eventually they repopulate to normal levels. What remains unknown is the lasting effect that this clearance has on the T-cell repertoire as a whole. The TCR repertoire in the peripheral blood of sequentially analyzed LCMV-immune mice was assessed for significant alterations as a result of poly(I:C) treatment. As seen in the spleen, there is initially a decline in CD8 T-cell percentage in the peripheral blood of LCMV-immune mice following poly(I:C) injection (Fig. 5A). Despite this decrease, analysis of their Vβ8.1/8.2 utilization based on surface expression revealed no significant reduction in the proportion of CD8 T cells staining positively (Fig. 5B). To further analyze CD8 T cells for alterations in the TCR repertoire, CDR3 length spectratyping of the Vβ8.1 subset was performed. T cells from immunologically naive mice have a Gaussian distribution of CDR3 lengths, but the LCMV infection can skew this distribution by eliciting dominant clones that survive in the memory pool. Our examinations of six different Jβ's in each of 11 LCMV-immune mice, on different days following poly(I:C) injection, showed that there were no appreciable, lasting changes in their CDR3 spectratypes in the peripheral blood of LCMV-immune mice by 8 days after poly(I:C) injection. In most cases, virtually no change in the T-cell spectratype was seen at any time point, as presented here with Jβ1.3 and Jβ1.6 (Fig. 5C). Presented here in the Jβ1.5 and Jβ2.7 windows of the T-cell repertoire are the rare examples where any changes were noted, but these changes were transient, occurred in subdominant peaks, and returned to the preexisting levels by day 8 post-poly(I:C) injection. These results suggest that although there is a systemic decrease in the number of CD8+ T cells, this decrease is relatively nonspecific and does not result in major alterations in the TCR repertoire in the absence of antigenic challenge.
FIG. 5.
The T-cell repertoire is not permanently altered by poly(I:C) treatment, as determined by FACS analysis and CDR3 length spectratyping. (A and B) Sequentially bled LCMV-immune mice were analyzed following poly(I:C) treatment to determine if any significant changes in the T-cell repertoire took place as a result of the attrition of memory T cells. Although the percentage of CD8 T cells is reduced on day 1 following poly(I:C) injection (A), the proportion of those cells that are Vβ 8.1/8.2+ was not altered (B). ▿, control mice; ●, poly(I:C)-treated mice. (C) The Vβ8.1 T cells were further analyzed for changes in their repertoire using CDR3 length spectratyping. Mice exhibiting the most significant changes in T-cell repertoire for Jβ1.3, Jβ1.6, Jβ1.5, and Jβ2.7 are shown. Even though these mice exhibited the most significant changes of all mice and Jβ's tested, the changes were transient and the mice returned to their original distribution by 8 days after the poly(I:C) treatment. *, change in spectratype. PBLs, peripheral blood lymphocytes; d0 and D0, day 0; d1, day 1; D3, day 3; D8, day 8.
DISCUSSION
We show here that CD8 T cells undergo apoptosis and decline in number in response to IFN and that non-virus-specific bystander CD8 T cells are driven to apoptosis during the T-cell response to virus infections. Apoptosis and cell loss in each of these systems were most pronounced in CD8 T cells of the memory phenotype (CD44hi). IFN-induced attrition and apoptosis of memory cells were confirmed using MHC tetramer analysis of a well-defined LCMV memory population from poly(I:C)-injected LCMV-immune mice (Fig. 4 and Table 3). An early virus-induced peak in T-cell apoptosis and attrition correlated with the peak in virus-induced IFN-α/β, and the IFN-α/β inducer poly(I:C) dramatically induced apoptosis and attrition in the memory CD8 T-cell compartment. Poly(I:C) is a very potent IFN-α/β inducer, but it also induces potentially cytotoxic cytokines like TNF alpha (44). IFN-α/β, in turn, can stimulate the synthesis of a number of other cytokines, such as IL-15 (20, 54), which is known to act on memory CD8 T cells, and IFN-γ, which can duplicate many of the activities of the IFN-α/β (37). The observed effect of poly(I:C) on T-cell apoptosis and attrition appears, however, to be dependent at least partially on the effects of IFN-α/β, as T cells from mice lacking IFN-α/β receptors did not exhibit increases in apoptosis or cell loss (Table 4, experiment A). This conclusion was reinforced by two experiments showing that purified IFN induced a loss in the number of memory CD8 T cells. Of note is the observation that poly(I:C) did induce apoptosis and attrition of T cells in mice lacking IFN-γ receptors, indicating that IFN-γ is not required for poly(I:C)-induced apoptosis (Table 4, experiment D). This does not exclude the possibility of IFN-γ being a cause of apoptosis under other conditions, such as during potent T-cell responses that release high levels of IFN-γ late in infection. In fact, TCR-driven apoptosis, otherwise known as activation-induced cell death, is impaired in mice lacking IFN-γ receptors (23). Of interest is that in four experiments there was on average a 30% increase in the number of CD8 T cells in poly(I:C)-treated mice lacking IFN-α/β receptors (Table 4, experiment A; data not shown), suggesting that CD8 T-cell growth factors may be induced by poly(I:C) but are normally counterbalanced by the negative effect of IFN-α/β.
How IFN induces apoptosis in memory T cells is not well understood, but a number of studies have shown that IFN induces protein kinase R, whose overexpression can kill cells by apoptosis (51). We have noticed small increases in the staining of the poly(I:C)-exposed CD8 T cells with a MAb to Fas (data not shown), but poly(I:C) induced apoptosis in FasL-deficient mice (Table 4, experiment E), suggesting that there was not a requirement for Fas in this system. Perhaps a partial activation of bystander cells by IFN makes them vulnerable to apoptosis by a number of mechanisms, as might be expected in T cells receiving an incomplete and inadequate signal. IFN can also activate NK cells, which might have the capacity to lyse memory T cells; such a mechanism seems unlikely here, however, as apoptosis and attrition in CD8 T cells were still seen in perforin−/− mice or mice depleted of NK cells (Table 4, experiments B and C).
Our results clearly show that bystander cells have elevated apoptosis during viral infections and poly(I:C) treatments, but enhanced apoptosis can also be seen in activated virus-specific T cells (Table 1) during infection, making the correlation between apoptosis and loss in cell number more complex. Although we hypothesize that the apoptosis of bystander cells accounted for their loss in number, it was possible that their loss in the spleen could be attributed to migration to other parts of the body. Poly(I:C) has been shown to alter migration patterns of T lymphocytes within the spleen and to induce changes in the splenic architecture (16). We therefore attempted to find these cells in as many different compartments as possible, but in every site, including LN, bone marrow, peripheral blood, PECs, and the liver, we found either no significant change in the number of memory CD8 T cells or, more commonly, significant decreases comparable to or greater than those observed in the spleen. In no compartment studied did we find a statistically significant increase in the number of CD8 T cells as a result of poly(I:C) treatment. It is possible that increased adherence properties of activated T cells may have made them more difficult to isolate from tissue, but we find that explanation unlikely, as we saw dramatic increases in the number of NK cells between days 2 and 4 in the PECs as a result of i.p. poly(I:C) injection, demonstrating our ability to recover activated cells in an appropriate site (data not shown). It is possible that some of the activated T cells may have migrated to the gut epithelium or into the gut, as suggested by studies using other forms of T-cell activation (18, 38); however, we know of no evidence that these cells would circulate back into the lymphoid organs, so they could still be considered lost.
These results would superficially appear to conflict with the work of others showing that virus infections and cytokines, including IFN-α/β, can cause memory phenotype CD8 T cells to incorporate the DNA label BrdU (42, 54). We do not believe that these observations are necessarily contradictory. It is possible that limited cellular proliferation may take place but that this proliferation may coincide with the opening of apoptotic pathways that can lead to cell death. Our preliminary data on BrdU uptake, however, indicate that substantial cell loss and apoptosis occur before proliferation takes place. Previous work has shown that memory CD8+ T cells undergo a low level of proliferation throughout their life span (20, 26, 31, 36, 55), and we show here that even in untreated mice, some CD8+ CD44hi cells react with annexin V. It is reasonable to suggest that there is also a low level of apoptosis that maintains homeostasis of these cells. We propose that under “resting” conditions there is a balance between cell proliferation and cell death that maintains a relatively constant number and proportion of the lymphocyte populations. A viral infection would, by virtue of inducing IFN and exposing the host to foreign antigens, alter this balance for both virus-specific and non-virus-specific cells. For virus-specific cells that encounter their antigen, the balance is shifted toward the proliferative state, resulting in an increase in their frequency. However, bystander cells which do not encounter their antigen may favor death over a productive continued proliferation.
The role of IFN-α/β in T-cell proliferation and activation has been studied for nearly 30 years with often conflicting results. Depending on experimental conditions and IFN concentrations, IFN-α/β has been shown to cause either enhanced antigen-specific T-cell proliferation and CTL activity or T-cell loss and inhibition of CTL activity (6, 9, 10, 19, 45). High levels of IFN in vivo can lead to diminished bone marrow function and severe leukopenia (3). The ability of IFN-α/β and IFN inducers, such as viruses and poly(I:C), to both activate and expand the number of NK cells in vivo was established many years ago (4, 5). Recent studies have renewed interest in the possibility of IFN being either a T-cell survival or growth factor, perhaps by inducing other cytokines, such as IL-15 (24, 54). We would argue that IFN may serve, directly or indirectly, as either a growth factor or an apoptosis factor, similar to that demonstrated with other cytokines, such as IL-2, TNF alpha (21), and transforming growth factor β (8, 12, 47). We do find that after poly(I:C) induces an initial period of cell loss and apoptosis, CD8 T cells gradually repopulate. Whether this repopulation is due to IFN-induced proliferation or to a return to homeostasis independent of IFN remains unknown. CDR3 spectratype analyses suggested that both the bystander T-cell attrition and the subsequent repopulation may be independent of TCR usage (Fig. 5C).
We suggest that both antigen-driven and bystander T cells receive signals from proliferative cytokines but that expansions in cell number occur only if the TCR is stimulated with antigen. In the HY transgenic system, the nontransgenic T cells, containing potential LCMV-specific T cells, underwent a two- to threefold increase in number as a result of an acute LCMV infection, while the HY-specific transgenic T cells were consistently lower in number from day 5 to 11 post-LCMV infection (Table 1; data not shown). This attrition of HY+ cells took place in the presence of the same cytokines and growth factors that drove the proliferation of the virus-specific cells in the spleen during the course of the LCMV infection, but the missing element for the HY+ cells was their cognate antigen. Without the TCR stimulus, the non-virus-specific cells shifted towards apoptosis while the virus-specific cells favored proliferation.
Attrition of bystander T cells early during infection may be a mechanism to prepare the lymphoid organs for the dramatic expansion of antigen-specific T cells. The four- to fivefold increase in the number of CD8 T cells during LCMV infection (Fig. 2A) may initially require a clearing out of the spleen to make room for the expanding population. The selective loss of bystander memory CD8 T cells occurring during infections may also help to explain the observation that multiple heterologous viral infections lead to reductions in the frequency of CTL specific to viruses earlier in the infection sequence (33, 35). It is likely that the process begins with the production of cytokines that lead to the loss of memory CD8 T cells specific for other viruses. Viral antigens and cytokine-derived signals then drive the proliferation and expansion of virus-specific T cells. The final steps in this process are the apoptotic silencing of the immune response after virus is cleared and the seeding of the memory pool with T cells specific for the more recent virus infection. This process would lead to changes in the frequency of memory T cells specific to previously encountered viruses as a result of their loss during the early phase and replacement by new memory cells during the silencing phase.
ACKNOWLEDGMENTS
We thank S. Tevethia (Pennsylvania State University at Hershey) for providing the NP396–404 H-2Db tetramer.
This work was supported by National Institutes of Health training grants AI07272 to J.M.M. and AI07349 to C.C.Z. and M.A.B., research grants AI17672 and AR35506 to R.M.W., and the Diabetes and Endocrinology Research Core P30DK 32520.
REFERENCES
- 1.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relationship. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 2.Askonas B A, Mullbacher A, Ashman R B. Cytotoxic T-memory cells in virus infection and the specificity of helper T cells. Immunology. 1982;45:79–84. [PMC free article] [PubMed] [Google Scholar]
- 3.Binder D, Fehr J, Hengartner H, Zinkernagel R M. Virus-induced transient bone marrow aplasia: major role of interferon-alpha/beta during acute infection with the noncytopathic lymphocytic choriomeningitis virus. J Exp Med. 1997;185:517–530. doi: 10.1084/jem.185.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biron C A, Turgiss L R, Welsh R M. Increase in NK cell number and turnover rate during acute viral infection. J Immunol. 1983;131:1539–1545. [PubMed] [Google Scholar]
- 5.Biron C A, Welsh R M. Blastogenesis of natural killer cells during viral infection in vivo. J Immunol. 1982;129:2788–2795. [PubMed] [Google Scholar]
- 6.Blank K J, McKernan L N, Murasko D M. Poly I:C or IFN-alpha/beta treatment inhibits macrophage induced T cell proliferation. J Interferon Res. 1985;5:215–221. doi: 10.1089/jir.1985.5.215. [DOI] [PubMed] [Google Scholar]
- 7.Butz E A, Bevan M J. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity. 1998;8:167–175. doi: 10.1016/s1074-7613(00)80469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cerwenka A, Kovar H, Majdic O, Holter W. Fas- and activation-induced apoptosis are reduced in human T cells preactivated in the presence of TGF-beta 1. J Immunol. 1996;156:459–464. [PubMed] [Google Scholar]
- 9.Chen L K, Mathieu-Mahul D, Bach F H, Dausset J, Bensussan A, Sasportes M. Recombinant interferon alpha can induce rearrangement of T-cell antigen receptor alpha-chain genes and maturation to cytotoxicity in T-lymphocyte clones in vitro. Proc Natl Acad Sci USA. 1986;83:4887–4889. doi: 10.1073/pnas.83.13.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dumont F J. Treatment of resting T lymphocytes with interferon-alpha/beta augments their proliferative response to activation signals delivered through their surface Ly-6 antigen. Cell Immunol. 1986;101:625–632. doi: 10.1016/0008-8749(86)90172-3. [DOI] [PubMed] [Google Scholar]
- 11.Gallimore A, Glithero A, Godkin A, Tissot A C, Pluckthun A, Elliott T, Hengartner H, Zinkernagel R. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J Exp Med. 1998;187:1383–1393. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Genestier L, Kasibhatla S, Brunner T, Green D R. Transforming growth factor beta1 inhibits Fas ligand expression and subsequent activation-induced cell death in T cells via downregulation of c-Myc. J Exp Med. 1999;189:231–239. doi: 10.1084/jem.189.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greten T F, Slansky J E, Kubota R, Soldan S S, Jaffee E M, Leist T P, Pardoll D M, Jacobson S, Schneck J P. Direct visualization of antigen-specific T cells: HTLV-1 Tax11–19-specific CD8+ T cells are activated in peripheral blood and accumulate in cerebrospinal fluid from HAM/TSP patients. Proc Natl Acad Sci USA. 1998;95:7568–7573. doi: 10.1073/pnas.95.13.7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel R M, Aguet M. Immune response in mice that lack the interferon-gamma receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 15.Hudrisier D, Mazarguil H, Laval F, Oldstone M B A, Gairin J E. Binding of viral antigens to major histocompatibility complex class I H-2Db molecules is controlled by dominant negative elements at peptide non-anchor residues. Implications for peptide selection and presentation. J Biol Chem. 1996;271:17829–17836. doi: 10.1074/jbc.271.30.17829. [DOI] [PubMed] [Google Scholar]
- 16.Ishikawa R, Biron C A. IFN induction and associated changes in splenic leukocyte distribution. J Immunol. 1993;150:3713–3727. [PubMed] [Google Scholar]
- 17.Jamieson B D, Somasundaram T, Ahmed R. Abrogation of tolerance to a chronic viral infection. J Immunol. 1991;147:3521–3529. [PubMed] [Google Scholar]
- 18.Kim S K, Reed D S, Heath W R, Carbone F, Lefrancois L. Activation and migration of CD8 T cells in the intestinal mucosa. J Immunol. 1997;159:4295–4306. [PubMed] [Google Scholar]
- 19.Klimpel G R, Infante A J, Patterson J, Hess C B, Asuncion M. Virus-induced interferon alpha/beta (IFN-alpha/beta) production by T cells and by Th1 and Th2 helper T cell clones: a study of the immunoregulatory actions of IFN-gamma versus IFN-alpha/beta on functions of different T cell populations. Cell Immunol. 1990;128:603–618. doi: 10.1016/0008-8749(90)90052-s. [DOI] [PubMed] [Google Scholar]
- 20.Ku C C, Murakami M, Sakamoto A, Kappler J, Marrack P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 2000;288:675–678. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- 21.Lenardo M, Chan K M, Hornung F, McFarland H, Siegel R, Wang J, Zheng L. Mature T lymphocyte apoptosis—immune regulation in a dynamic and unpredictable antigenic environment. Annu Rev Immunol. 1999;17:221–253. doi: 10.1146/annurev.immunol.17.1.221. [DOI] [PubMed] [Google Scholar]
- 22.Lin M Y, Welsh R M. Stability and diversity of T cell receptor repertoire usage during lymphocytic choriomeningitis virus infection of mice. J Exp Med. 1998;188:1993–2005. doi: 10.1084/jem.188.11.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lohman B L, Welsh R M. Apoptotic regulation of T cells and absence of immune deficiency in virus-infected gamma interferon receptor knockout mice. J Virol. 1998;72:7815–7821. doi: 10.1128/jvi.72.10.7815-7821.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marrack P, Kappler J, Mitchell T. Type I interferons keep activated T cells alive. J Exp Med. 1999;189:521–530. doi: 10.1084/jem.189.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moskophidis D, Lechner F, Pircher H, Zinkernagel R M. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 26.Mullbacher A. The long-term maintenance of cytotoxic T cell memory does not require persistence of antigen. J Exp Med. 1994;179:317–321. doi: 10.1084/jem.179.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murali-Krishna K, Altman J D, Suresh M, Sourdive D J, Zajac A J, Miller J D, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 28.Nahill S R, Welsh R M. High frequency of cross-reactive cytotoxic T lymphocytes elicited during the virus-induced polyclonal cytotoxic T lymphocyte response. J Exp Med. 1993;177:317–327. doi: 10.1084/jem.177.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pannetier C, Cochet M, Darche S, Casrouge A, Zoller M, Kourilsky P. The sizes of the CDR3 hypervariable regions of the murine T-cell receptor beta chains vary as a function of the recombined germ-line segments. Proc Natl Acad Sci USA. 1993;90:4319–4323. doi: 10.1073/pnas.90.9.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Razvi E S, Jiang Z, Woda B A, Welsh R M. Lymphocyte apoptosis during the silencing of the immune response to acute viral infections in normal, lpr, and Bcl-2-transgenic mice. Am J Pathol. 1995;147:79–91. [PMC free article] [PubMed] [Google Scholar]
- 31.Razvi E S, Welsh R M, McFarland H I. In vivo state of antiviral CTL precursors. Characterization of a cycling cell population containing CTL precursors in immune mice. J Immunol. 1995;154:620–632. [PubMed] [Google Scholar]
- 32.Rocha B, von Boehmer H. Peripheral selection of the T cell repertoire. Science. 1991;251:1225–1228. doi: 10.1126/science.1900951. [DOI] [PubMed] [Google Scholar]
- 33.Selin L K, Lin M Y, Kraemer K A, Pardoll D M, Schneck J P, Varga S M, Santolucito P A, Pinto A K, Welsh R M. Attrition of T cell memory: selective loss of LCMV epitope-specific memory CD8 T cells following infections with heterologous viruses. Immunity. 1999;11:733–742. doi: 10.1016/s1074-7613(00)80147-8. [DOI] [PubMed] [Google Scholar]
- 34.Selin L K, Nahill S R, Welsh R M. Cross-reactivities in memory cytotoxic T lymphocyte recognition of heterologous viruses. J Exp Med. 1994;179:1933–1943. doi: 10.1084/jem.179.6.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selin L K, Vergilis K, Welsh R M, Nahill S R. Reduction of otherwise remarkably stable virus-specific cytotoxic T lymphocyte memory by heterologous viral infections. J Exp Med. 1996;183:2489–2499. doi: 10.1084/jem.183.6.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Selin L K, Welsh R M. Cytolytically active memory CTL present in lymphocytic choriomeningitis virus-immune mice after clearance of virus infection. J Immunol. 1997;158:5366–5373. [PubMed] [Google Scholar]
- 37.Sen G C, Ransohoff R M. Interferon-induced antiviral actions and their regulation. Adv Virus Res. 1993;42:57–102. doi: 10.1016/s0065-3527(08)60083-4. [DOI] [PubMed] [Google Scholar]
- 38.Sprent J. Fate of H2-activated T lymphocytes in syngeneic hosts. I. Fate in lymphoid tissues and intestines traced with 3H-thymidine, 125I-deoxyuridine and 51chromium. Cell Immunol. 1976;21:278–302. doi: 10.1016/0008-8749(76)90057-5. [DOI] [PubMed] [Google Scholar]
- 39.Sun S, Zhang X, Tough D F, Sprent J. Type I interferon-mediated stimulation of T cells by CpG DNA. J Exp Med. 1998;188:2335–2342. doi: 10.1084/jem.188.12.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan R, Teh S J, Ledbetter J A, Linsley P S, Teh H S. B7 costimulates proliferation of CD4−8+ T lymphocytes but is not required for the deletion of immature CD4+8+ thymocytes. J Immunol. 1992;149:3217–3224. [PubMed] [Google Scholar]
- 41.Tishon A, Southern P J, Oldstone M B. Virus-lymphocyte interactions. II. Expression of viral sequences during the course of persistent lymphocytic choriomeningitis virus infection and their localization to the L3T4 lymphocyte subset. J Immunol. 1988;140:1280–1284. [PubMed] [Google Scholar]
- 42.Tough D F, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science. 1996;272:1947–1950. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- 43.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 44.Vilcek J, Sen G C. Interferons and other cytokines. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 375–399. [Google Scholar]
- 45.von Hoegen P. Synergistic role of type I interferons in the induction of protective cytotoxic T lymphocytes. Immunol Lett. 1995;47:157–162. doi: 10.1016/0165-2478(95)00065-4. [DOI] [PubMed] [Google Scholar]
- 46.Walsh C M, Matloubian M, Liu C C, Ueda R, Kurahara C G, Christensen J L, Huang M T, Young J D, Ahmed R, Clark W R. Immune function in mice lacking the perforin gene. Proc Natl Acad Sci USA. 1994;91:10854–10858. doi: 10.1073/pnas.91.23.10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weller M, Constam D B, Malipiero U, Fontana A. Transforming growth factor-beta 2 induces apoptosis of murine T cell clones without down-regulating bcl-2 mRNA expression. Eur J Immunol. 1994;24:1293–1300. doi: 10.1002/eji.1830240608. [DOI] [PubMed] [Google Scholar]
- 48.Welsh R M. Cytotoxic cells induced during lymphocytic choriomeningitis virus infection of mice. I. Characterization of natural killer cell induction. J Exp Med. 1978;148:163–181. doi: 10.1084/jem.148.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang H, Welsh R M. Induction of alloreactive cytotoxic T cells by acute virus infection of mice. J Immunol. 1986;136:1186–1193. [PubMed] [Google Scholar]
- 50.Yang H Y, Dundon P L, Nahill S R, Welsh R M. Virus-induced polyclonal cytotoxic T lymphocyte stimulation. J Immunol. 1989;142:1710–1718. [PubMed] [Google Scholar]
- 51.Yeung M C, Lau A S. Tumor suppressor p53 as a component of the tumor necrosis factor-induced, protein kinase PKR-mediated apoptotic pathway in human promonocytic U937 cells. J Biol Chem. 1998;273:25198–25202. doi: 10.1074/jbc.273.39.25198. [DOI] [PubMed] [Google Scholar]
- 52.Zarozinski C C, McNally J M, Lohman B L, Daniels K A, Welsh R M. Bystander sensitization to activation-induced cell death as a mechanism of virus-induced immune suppression. J Virol. 2000;74:3650–3658. doi: 10.1128/jvi.74.8.3650-3658.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zarozinski C C, Welsh R M. Minimal bystander activation of CD8 T cells during the virus-induced polyclonal T cell response. J Exp Med. 1997;185:1629–1639. doi: 10.1084/jem.185.9.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang X, Sun S, Hwang I, Tough D F, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 55.Zimmerman C, Brduscha-Riem K, Blaser C, Zinkernagel R M, Pircher H. Visualization, characterization, and turnover of CD8+ memory T cells in virus-infected hosts. J Exp Med. 1996;183:1367–1375. doi: 10.1084/jem.183.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zinkernagel R M, Bachmann M F, Kundig T M, Oehen S, Pirchet H, Hengartner H. On immunological memory. Annu Rev Immunol. 1996;14:333–367. doi: 10.1146/annurev.immunol.14.1.333. [DOI] [PubMed] [Google Scholar]