Abstract
The E7 oncoprotein of human papillomavirus 16 (HPV16) transforms basal and suprabasal cervical epithelial cells and is a tumor-specific antigen in cervical carcinoma, to which immunotherapeutic strategies aimed at cytotoxic T-lymphocyte (CTL) induction are currently directed. By quantifying major histocompatibility complex class I tetramer-binding T cells and CTL in mice expressing an HPV16 E7 transgene from the keratin-14 (K14) promoter in basal and suprabasal keratinocytes and in thymic cortical epithelium, we show that antigen responsiveness of both E7- and non-E7-specific CD8+ cells is down-regulated compared to non-E7 transgenic control mice. We show that the effect is specific for E7, and not another transgene, expressed from the K14 promoter. Down-regulation did not involve deletion of CD8+ T cells of high affinity or high avidity, and T-cell receptor (TCR) Vβ-chain usage and TCR receptor density were similar in antigen-responsive cells from E7 transgenic and non-E7 transgenic mice. These data indicate that E7 expressed chronically from the K14 promoter nonspecifically down-regulates CD8+ T-cell responses. The in vitro data correlated with the failure of immunized E7 transgenic mice to control the growth of an E7-expressing tumor challenge. We have previously shown that E7-directed CTL down-regulation correlates with E7 expression in peripheral but not thymic epithelium (T. Doan et al., J. Virol. 73:6166–6170, 1999). The findings have implications for the immunological consequences of E7-expressing tumor development and E7-directed immunization strategies. Generically, the findings illustrate a T-cell immunomodulatory function for a virally encoded human oncoprotein.
Human papillomavirus type 16 (HPV16) is tropic for human anogenital epithelium and is associated with the development of premalignant and invasive squamous disease. The product of the HPV16 E7 open reading frame transforms basal cells of cervical epithelium. Since persistence of E7 oncoprotein is necessary to maintain the transformed cell phenotype (42), E7 functions as a tumor-specific antigen in cervical carcinoma, to which immunotherapeutic strategies are being directed (6, 32). Patients with HPV16- associated cervical carcinoma make poor E7-directed cytotoxic T-lymphocyte (CTL) responses either to endogenously expressed E7 (34) or following E7 immunization (6), and poor non-E7-directed CTL responses, at least to certain antigens (34), in spite of immunocompetence measured by other criteria.
Mice which express an E7 transgene driven from an epithelium-specific keratin-14 (K14) promoter in basal and suprabasal cells of peripheral epithelium and in thymic cortical (but not medullary) epithelium display hyperplasia and dysplasia, frequently leading to papillomatosis and squamous cell carcinoma (22), and provide a model for E7-mediated epithelial transformation in humans. The mice display down-regulated E7-directed T-cell responses, as evidenced in vitro by diminished CTL activity (14) and in vivo as failure to control E7-associated tumor development following specific immunization (12). We have demonstrated using bone marrow radiation chimeras that E7-directed precursor CTL (pCTL) are functionally down-regulated in reconstituted mice expressing E7 in peripheral epithelium but not thymus but are not down-regulated in mice expressing E7 in thymus but not peripheral epithelium (13). We have interpreted this finding to indicate that E7 expression in the peripheral epithelium is sufficient to down-regulate E7-directed pCTL. In the present study we characterize this E7-induced T-cell down-regulation using the more sensitive tool of major histocompatibility complex (MHC) class I tetramers to quantify CD8+ T cells bearing cognate T-cell receptors (TCR). We report that not only E7-specific CD8+ T-cell responses but also non-E7 CD8+ T-cell responses are down-regulated in immunized E7 transgenic mice which express E7 in peripheral epithelium and thymic cortical epithelium. The down-regulation is manifest in vitro as a failure of CD8+ T cells to respond to restimulation with cognate antigens. We show that down-regulation is E7 dependent since topographically identical expression of a non-E7 transgene did not down-regulate CD8+ T-cell responses. Our data indicate that down-regulated pCTLs from E7-transgenic mice have a range of TCR affinities for peptide-MHC complex similar to that of pCTL from non-E7 transgenic mice and have similar TCR Vβ-chain usage. We interpret these data to indicate that E7 expressed chronically in epithelium nonspecifically down-regulates CD8+ T-cell responses and that T-cell deletion does not play a major role in this tolerizing process.
The observations are of potential relevance to cervical carcinoma patients who may have experienced many years, even decades, of expression of E7 in cervical epithelium during tumor development. The possibility arises that chronic expression of E7 in transformed cervical epithelium serves to functionally down-regulate E7-directed and other pCTL. Thus, E7-induced putative CD8+ T-cell down-regulation may confound E7-based immunotherapy. The results of this study are relevant to the understanding of the immunological consequences of E7 expression in epithelium. Generically, the findings illustrate a T-cell immunomodulatory function for a human oncoprotein.
MATERIALS AND METHODS
Mice.
K14.E7+/+ mice (22) are transgenic for a DNA fragment containing HPV16 positions 79 to 883 engineered to transcribe E7-specific RNA from the human K14 promoter, which is restricted in its activity to the stratum basale of peripheral squamous epithelium and thymic cortical epithelium (26, 29). K14.hgh/B7+/+ mice (43) express a human growth hormone/B7.1 transgene from the K14 promoter. By crossing each line of mice with A2.1Kb+/+ mice (41), the F1 offspring also express a chimeric class 1 transgene comprising the α-1 and α-2 domains of HLA A∗0201 and the α-3 domain of H-2Kb on all nucleated cells. K14.E7 crosses are designated KA(E7+). Parental (FVB) non-E7 transgenic crosses are designated FA(E7−). Mice were housed under specific-pathogen-free conditions, and genetic authenticity was tested at intervals. Mice were used at 6 to 20 weeks of age but within a given experiment were littermates or closely age and sex matched.
Cells.
EL4.A2 cells express α-1 and α-2 chains of HLA A∗0201 and are susceptible to specific CTL lysis through both H-2b and A∗0201 pathways (12). Cells were maintained as described previously (12).
Peptides and epitopes.
Peptides containing (i) HLA A∗0201-restricted CTL epitopes of HPV16 E7 (82LLMGTLGIV90 [33], designated E7/A2) and influenza virus matrix protein (58GILGFVFTL66 [21], designated influenza virus/A2) and (ii) H-2b-restricted epitopes of HPV16 E7 (49RAHYNIVTF57 [15], designated E7/H-2b) and ovalbumin (SIINFEKL [7], designated OVA/H-2b) were synthesized with free ends using 9-fluorenylmethoxy carbonyl chemistry and analyzed by high-pressure liquid chromatography by Chiron Corp. (Melbourne, Victoria, Australia). Peptide stocks were made at 10 mg/ml in dimethyl sulfoxide and diluted into tissue culture medium for assays.
CTL assays.
CTL assays were conducted as previously described (12).
Immunizations and restimulations.
For generation of a primary response, groups of three mice were immunized once with 50 μg of an equimolar peptide mix of CTL epitopes, 10 μg of Quil A adjuvant (18), and 0.25 μg of tetanus toxoid (CSL, Melbourne, Victoria, Australia), as a source of T-helper epitope(s), in 100 μl of phosphate-buffered saline (PBS) subcutaneously at the base of the tail. Ten days later, pooled splenocytes were harvested and restimulated in vitro for 6 days in the presence of 1 μg of each peptide per ml prior to inclusion in tetramer staining or CTL assays. To recall a memory response, the in vitro restimulation was performed 2 months after immunization.
MHC class I tetramers and FACS staining.
Phycoerythrin (PE)-conjugated HLA A∗0201 tetramers of epitopes E7/A2 and influenza virus/A2 and H-2b tetramers of E7/H-2b and OVA/H-2b epitopes were constructed by the National Institutes of Health (NIH) Tetramer Facility (Atlanta, Ga.). For staining, splenocytes or post-in vitro restimulation cells were separated over Ficoll-Paque (Pharmacia, Uppsala, Sweden), and 106 leukocytes were costained with tetramer and fluorescein isothiocyanate (FITC)-anti-mouse CD8a (Sigma Chemicals) at 4°C for 60 min. prior to washing, fixation in 1% paraformaldehyde, and fluorescence-activated cell sorting (FACS) analysis. Tetramer dilutions and staining conditions were optimized in preliminary experiments. Data sets in all experiments included staining with irrelevant MHC-matched tetramer and staining of cognate tetramer on irrelevant CD8+ T cells as negative controls. FACS plots record 60,000 events. Cells positive for both tetramer and CD8 staining are expressed as a percentage of total CD8+ cells.
Antibodies.
FITC-pan-TCRβ (PharMingen), FITC-anti-CD8a (Sigma), and PE-anti-CD4 (Cappel) were used according to manufacturers' instructions. A panel of anti-mouse TCR Vβ-chain specific monoclonal antibodies was obtained from Larry Palmer, NIH, Bethesda, Md. Specific anti-Vβ-chain binding was detected with FITC anti-rat, anti-mouse, or anti-hamster immunoglobulin, as appropriate.
Tumor challenge.
Groups of five mice were immunized on days 0 and 21 with E7/H-2b or PBS as described above and challenged 14 days later with the E7-expressing epithelial tumor line TC-1 (28), 2 × 105 cells per mouse subcutaneously in the flank. Tumor growth was quantified as percent tumor-free mice over a 21-day period.
RESULTS
Immunization-induced E7-and non-E7-CD8+ T-cell responses are down-regulated in mice expressing E7 in peripheral epithelium and thymic cortical epithelium.
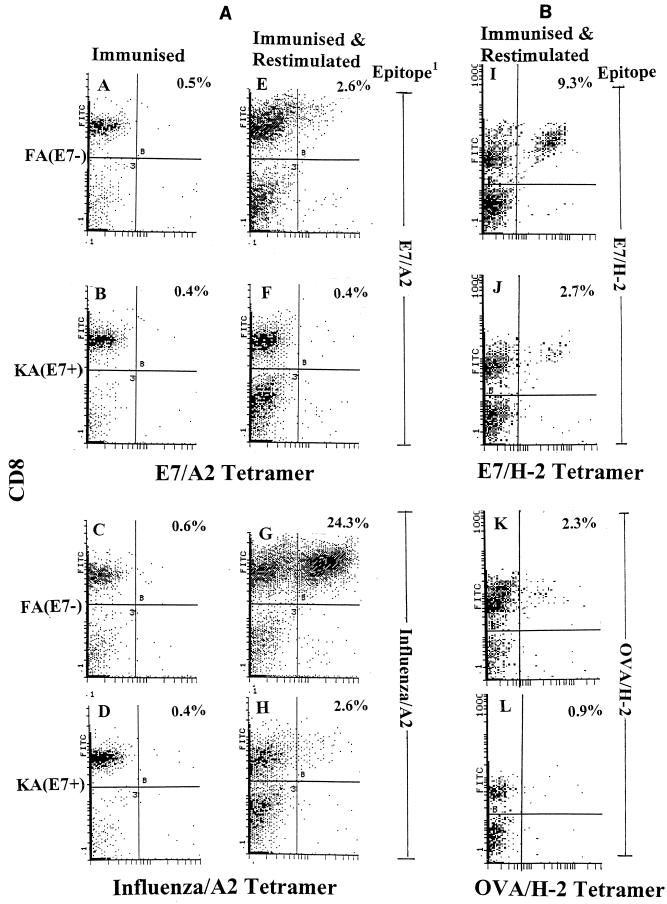
We used HLA A∗0201 class I tetramers of CTL epitopes E7/A2 and influenza virus/A2 and H-2b class I tetramers of CTL epitopes E7/H-2b and OVA/H-2b to examine the CD8+ T-cell response in immunized KA(E7+) and FA(E7−) mice, before and after restimulation of spleen cells in vitro with cognate peptide. Few E7/A2 and influenza virus/A2 tetramer+ CD8+ cells were found in ex vivo splenocytes from E7-immunized and influenza virus-immunized FA(E7−) and KA(E7+) mice, respectively (Fig. 1A to D). Following restimulation and expansion of splenocytes in vitro with cognate peptide, ca. sixfold fewer E7/A2 tetramer+ CD8+ T cells were found in E7/A2-immunized KA(E7+) mice than identically immunized FA(E7−) mice (Fig. 1E and F). A similar fold reduction in the numbers of influenza virus/A2 tetramer+ CD8+ cells was found in restimulated spleen cells from influenza virus/A2-immunized KA(E7+) mice compared with identically immunized FA(E7−) mice (Fig. 1G and H). Comparable results were observed when draining lymph node cells were used in place of splenocytes (data not shown). Similarly, fewer E7/H-2b tetramer+ CD8+ cells were seen in restimulated splenocytes from E7/H-2b-immunized KA(E7+) mice than from identically immunized FA(E7−) mice (Fig. 1I and J), and fewer OVA/H-2b tetramer+ CD8+ cells were seen in restimulated splenocytes from OVA/H-2b immunized KA(E7+) mice than from identically immunized FA(E7−) mice (Fig. 1K and L). Comparable results were obtained when whole E7 protein and ovalbumin were used for immunization in place of E7 and OVA peptides, respectively (not shown).
FIG. 1.
Specific tetramer staining of splenic CD8+ T cells from E7/A2-, influenza virus A2-, E7/H-2b, or OVA/H-2b-immunized FA(E7−) and KA(E7+) mice. Stainings were performed on fresh ex vivo spleen cells (Immunised) or on spleen cells after 6 days restimulation in vitro with cognate epitope as indicated (Immunised and Restimulated). CD8+ tetramer+ cells appear in top right quadrant of each plot. 1, epitope used for immunization of mice and in vitro restimulation of splenocytes.
Thus, as demonstrated by responses to two E7 epitopes (E7/A2 and E7/H-2b) and two non-E7 epitopes (influenza virus/A2 and OVA/H-2b) restricted through MHC class I haplotypes HLA A∗0201 or H-2b, these data indicate that expression of E7 as a transgene from the K14 promoter is associated with down-regulation of the numbers of antigen-responsive E7- and non-E7-specific CD8+ T cells. The down-regulation could not be reversed by provision of interleukin-2 (50 U/ml) during in vitro restimulation (not shown).
Numbers of A2 tetramer+ CD8+ cells were monitored throughout the 6-day period of restimulation. An increase in numbers was first recorded after 4 days of restimulation with E7/A2 epitope or influenza virus/A2 epitope from immunized KA(E7+) and FA(E7−) mice (not shown), accumulating at 6 days to maximal levels shown in Fig. 1E to H. This indicates that the kinetics of accumulation of tetramer+ CD8+ cells over the 6-day period of restimulation was the same in specifically restimulated splenocytes from both KA(E7+) and FA(E7−) mice.
Impairment of maturation of immunization-induced E7 and non-E7 CD8+ T-cell responses is associated with expression of E7.
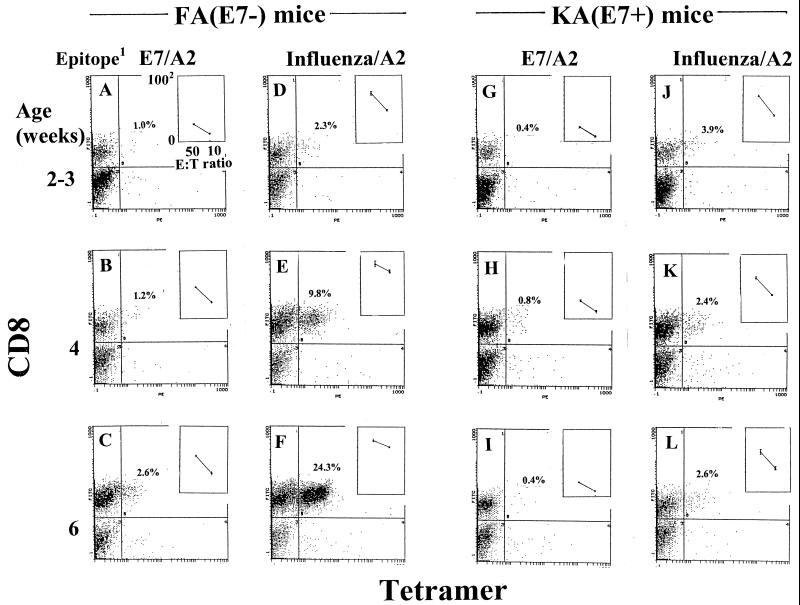
We examined whether the capacity to mount TCR-specific responses during immune system maturation was associated with K14-driven expression of E7. KA(E7+) and FA(E7−) mice 2 to 3, 4, and 6 weeks old were immunized with E7/A2 or influenza virus/A2, and spleen cells subsequently were restimulated in vitro with cognate peptide. One half of the restimulated cells was stained with specific tetramer and anti-CD8, and the remaining half was reacted in a 51Cr release CTL assay against peptide-pulsed target cells at various effector/target cell (E:T) ratios. Restimulated spleen cells from FA(E7−) mice immunized with E7/A2 or influenza virus/A2 showed increasing staining with E7/A2 tetramer (Fig. 2A to C) and influenza virus/A2 tetramer (Fig. 2D to F), respectively, with age of the mice. In contrast, restimulated spleen cells from KA(E7+) mice immunized with E7/A2 or influenza virus/A2 failed to show increasing tetramer staining with either E7/A2 tetramer (Fig. 2G to I) or influenza virus/A2 tetramer (Fig. 2J to L) with increasing age of the mice.
FIG. 2.
Tetramer and CD8 staining and specific CTL lysis (insets) of restimulated splenocytes from E7/A2- and influenza virus/A2-immunized FA(E7−) and KA(E7+) mice of various ages. Cells were stained with E7/A2 tetramer or with influenza virus/A2 tetramer, as indicated. In CTL assays, effector cells were reacted with EL4.A2 target cells pulsed with cognate peptide or with irrelevant peptide (background) at E:T ratios of 50:1 and 10:1. Results are shown for cognate peptide. Background lysis was <15% at an E:T ratio of 50:1. 1, epitope used for immunization of mice and in vitro restimulation of splenocytes. 2, percent cytotoxicity.
Tetramer binding correlated with CTL killing. The CTL activity of restimulated splenocytes from immunized FA(E7−) mice on E7/A2-pulsed targets (Fig. 2A to C, insets) and influenza virus/A2-pulsed targets (Fig. 2D to F, insets) increased in mice 2 to 4 weeks of age (but note that a maximal level of CTL killing could be reached, upon which further increase in specific tetramer-binding cells did not occur [Fig. 2E and F]). The CTL activity of restimulated spleen cells from immunized KA(E7+) mice on E7/A2-pulsed targets (Fig. 2G to I, insets) and on influenza virus/A2-pulsed targets (Fig. 2J to L, insets) was down-regulated compared to corresponding responses of FA(E7−) mice, and no augmentation of the KA(E7+) responses was seen with increasing age of mice, representing increasing cumulative lifetime experience of E7.
These results demonstrate that expression of transgene E7 is associated with the lack of maturation of the ability to mount cognate CD8+ T-cell responses to both E7- and non-E7 antigen following specific immunization, as measured by accumulation of TCR-specific CD8+ T cells and by functional (CTL) assay.
Expression of a non-E7 transgene from the K14 promoter does not down-regulate immunization-induced T-cell responses.
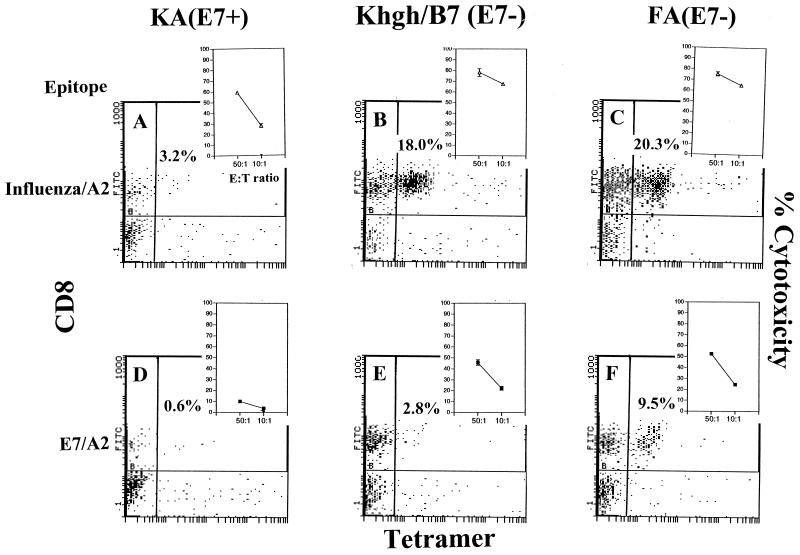
We used K14.hgh/B7 mice expressing a human growth hormone/B7 transgene driven from a K14 promoter to confirm that down-regulated T-cell responses in mice expressing E7 from the K14 promoter were due to E7 and not to some other transgene(s) expressed with an identical tissue distribution and in similar amounts. Restimulated splenocytes from influenza virus/A2- and E7/A2-immunized K14.hgh/B7 mice exhibited comparable CTL killing of target cells pulsed with cognate peptide to FA(E7−) mice (Fig. 3B, C, E, and F, insets), in contrast to restimulated splenocytes from immunized KA(E7+) mice, which exhibited characteristic down-regulated CTL responses against target cells pulsed with either influenza virus/A2- or E7/A2 epitope (Fig. 3A and D, insets). Similarly, specifically restimulated splenocytes from K14.hgh/B7 mice immunized with influenza virus/A2 or E7/A2 displayed high levels of E7/A2 tetramer or influenza virus/A2 tetramer binding, as in restimulated splenocytes from FA(E7−) mice (Fig. 3B, C, E, and F) in contrast to restimulated splenocytes from immunized KA(E7+) mice, which showed characteristic low levels of influenza virus/A2 and E7/A2 tetramer staining (Fig. 3A and D).
FIG. 3.
Tetramer and CD8 staining and specific CTL lysis (insets) of restimulated splenocytes from influenza virus/A2 or E7/A2-immunized KA(E7+), K14.hgh/B7 (E7−), and FA(E7−) mice. Cells were stained with influenza virus A2-tetramer (A to C) or E7/A2 tetramer (D to F). In CTL assays, effector cells were pulsed with EL4.A2 target cells pulsed with influenza virus/A2 peptide (A to C), E7/A2 peptide (D to F), or irrelevant peptide (background). Results are shown for influenza virus/A2 peptide and E7/A2 peptide. Background lysis was <20% at an E:T ratio of 50:1.
These results show that expression of E7, but not another gene, from the K14 promoter is associated with down-regulation of immunization-induced T-cell responses to E7 and non-E7 immunogens.
E7 expression down-regulates memory as well as primary T-cell responses to immunization with E7 and non-E7 antigens.
To examine whether E7 expressed as a transgene down-regulates memory as well as primary T-cell responses, we compared specific CTL activity of restimulated splenocytes from KA(E7+) and FA(E7−) mice which had been immunized 2 months previously. Restimulated splenocytes from both E7/A2-immunized and influenza virus/A2-immunized KA(E7+) mice displayed lower CTL responses against target cells pulsed with specific peptide than restimulated splenocytes from correspondingly immunized FA(E7−) mice (Table 1). These data are consistent with the notion that E7 expression down-regulates memory CTL responses to E7 and non-E7 immunogens.
TABLE 1.
CTL lysis of peptide-pulsed target cells by restimulated splenocytes from FA(E7−) and KA(E7+) mice 2 months after immunization
| Epitopea | % CTL lysis (mean ± SD) at E:T ratio of:
|
|
|---|---|---|
| 50:1 | 10:1 | |
| E7/A2 | ||
| FA(E7−) | 11.6 ± 1.7 | 3.5 ± 1.5 |
| KA(E7+) | −0.2 ± 0.3 | 0.1 ± 0.4 |
| Influenza virus/A2 | ||
| FA(E7−) | 51.2 ± 1.6 | 60.7 ± 2.3 |
| KA(E7+) | 13.7 ± 0.9 | 5.7 ± 0.1 |
Epitope used for immunizing mice, restimulating splenocytes, and sensitizing target cells.
Avidity and affinity of CTL cells in KA(E7+) mice do not differ from those of CTL cells in FA(E7−) mice.
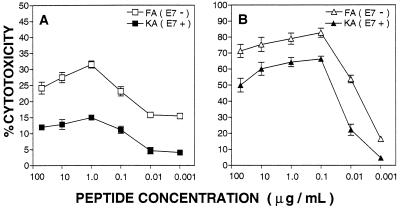
A number of animal models demonstrate that peripheral tolerization involves deletion of high-affinity and -avidity pCTL which recognize cognate peripherally expressed antigen, leaving a residual population of low-avidity and -affinity pCTL (4, 25, 30). To examine the avidity of E7/A2- and influenza virus/A2-specific T cells in KA(E7+) and FA(E7−) mice, equal numbers of restimulated splenocytes from E7/A2- and influenza virus/A2-immunized mice were reacted in 51Cr release assays with target cells pulsed with a range of cognate peptide concentrations. The data in Fig. 4 show a uniformly lower level of killing by specifically restimulated splenocytes from KA(E7+) mice than from FA(E7−) mice on E7/A2-pulsed targets (Fig. 4A) and on influenza virus/A2-pulsed targets (Fig. 4B). However, specific tetramer labeling indicated the number of tetramer-binding cells in restimulated splenocytes from KA(E7+) mice to be 6- to 10-fold lower than in FA(E7−) mice (Fig. 1), confirming earlier findings of lower pCTL frequency in limiting-dilution CTL assays (12). Were the data in Fig. 4A and B normalized to the number of E7/A2- and influenza virus/A2 tetramer-binding cells, respectively, then the difference in cytotoxic efficacy between effector populations from KA(E7+) and FA(E7−) mice would vanish. Thus, the data in Fig. 4 suggest that E7/A2- and influenza virus/A2-specific CTL populations in KA(E7+) mice have a spread of avidities for target cells displaying E7/A2 and influenza virus/A2 epitopes, respectively, similar to the spread of avidities seen in corresponding CTL populations from FA(E7−) mice.
FIG. 4.
Cytotoxicity (51Cr release) of specifically restimulated splenocytes from E7/A2 (A)- and influenza virus/A2 (B)-immunized FA(E7−) and KA(E7+) mice against EL4.A2 target cells pulsed with a range of concentrations of cognate peptide. The E:T ratio was 50:1. Controls included target cells without peptide or pulsed with irrelevant peptide (background). Background lysis was <16% for FA(E7−) assays or 5% for KA(E7+) assays.
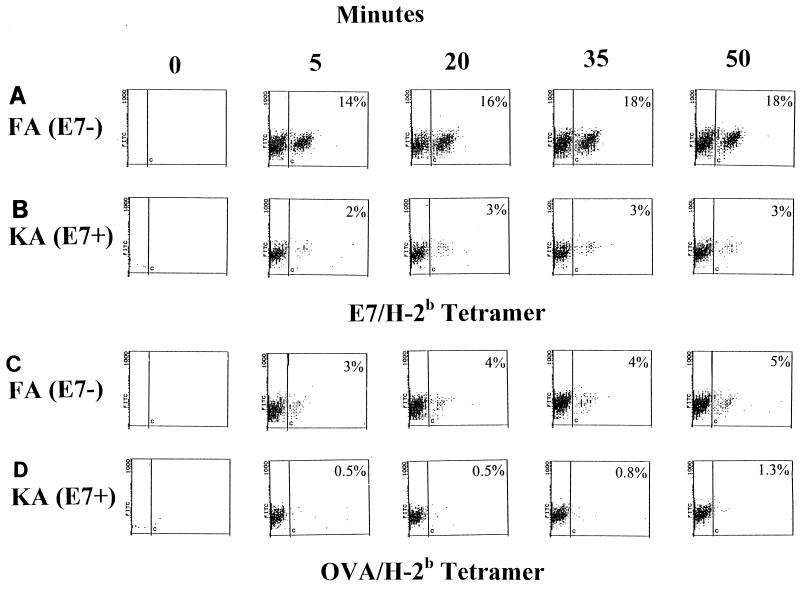
The kinetics of binding of epitope tetramer to cognate TCR provide a measure of affinity of the TCR for the epitope-MHC class I complex (44). To monitor the affinities of the E7/H-2b-specific and OVA/H-2b-specific T cells of KA(E7+) mice compared with those of FA(E7−) mice, specifically restimulated splenocytes from E7/H-2b and OVA/H-2b-immunized KA(E7+) and FA(E7−) mice were reacted with cognate tetramer and examined at intervals over a 50-min period. (Preliminary experiments indicated that maximal staining occurred by 50 min). More than 65% of maximal staining with E7/H-2b tetramer occurred after 5 min and >85% occurred after 20 min in restimulated splenocytes from E7/H-2b-immunized FA(E7−) and KA(E7+) mice (Fig. 5A and B, respectively). [A similar kinetics of staining with OVA/H-2b tetramer was seen in restimulated splenocytes from OVA/H-2b-immunized FA(E7−) and KA(E7+) mice (Fig. 5C and D, respectively).] Comparable kinetics of tetramer binding in KA(E7+) mice and FA(E7−) mice indicate a similar range of affinities of E7/H-2b- (and OVA/H-2b-) CD8+ T cells for cognate epitope-MHC complex in mice which express E7 from the K14 promoter [KA(E7+)] and those which do not [FA(E7−)]. A similar conclusion regarding affinities was inferred from the binding kinetics of E7/A2 and influenza virus/A2 tetramers to restimulated splenocytes from appropriately immunized KA(E7+) and FA(E7−) mice (data not shown).
FIG. 5.
Binding kinetics of E7/H-2b tetramer and OVA/H-2b tetramer to specifically restimulated splenocytes from E7/H-2b-immunized FA(E7−) mice, OVA/H-2b-immunized FA(E7−) mice, E7/H-2b-immunized KA(E7+) mice, and OVA/H-2b-immunized KA(E7+) mice. Cells were reacted at time zero with anti-CD8 antibody and tetramer and then sampled for specific staining at the time intervals shown. Data are gated on CD8+ cells.
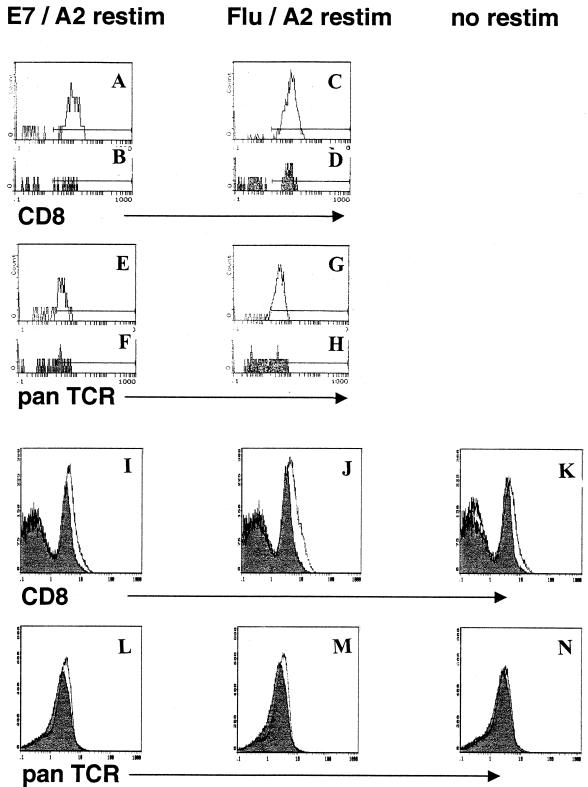
Down-regulation of tetramer-binding and CTL activities in specifically restimulated splenocytes from KA(E7+) mice is not associated with TCR down-regulation.
It has been demonstrated at least in some models that a way to achieve a low-avidity phenotype and tolerance is to decrease the density of specific TCR on the cell surface (16, 20). We examined whether the down-regulated tetramer-binding and CTL activities observed in restimulated splenocytes from KA(E7+) mice were associated with TCR down-regulation in specific tetramer-binding cells. Data showed that the intensity of TCR staining in E7/A2 tetramer+ CD8+ cells did not differ appreciably between restimulated splenocytes from E7/A2-immunized FA(E7−) and KA(E7+) mice (Fig. 6E and F). Similarly, the intensity of TCR staining in influenza virus/A2 tetramer+ CD8+ T cells did not differ appreciably between restimulated splenocytes from influenza virus/A2-immunized FA(E7−) and KA(E7+) mice (Fig. 6G and H). CD8 staining is included as a control (Fig. 6A to D). Note that CD8 and TCR antibody binding levels were unaffected by cobinding of tetramer, indicating that no competition for binding occurred (data not shown). Since we hypothesize that E7 expression in epithelium is associated with a general down-regulation of responsiveness in CD8+ T cells of all specificities, we also examined levels of TCR expression in total T cells from KA(E7+) and FA(E7−) mice. No difference in intensity of TCR (Fig. 6L to N) or CD8 (Fig. 6I to K) staining was observed between splenic T cells from immunized FA(E7−) and KA(E7+) mice either with or without in vitro restimulation. These data indicate that tolerance in CD8+ T cells from mice expressing E7 in epithelium is not associated with TCR (or with CD8) down-regulation on activated or nonactivated T cells.
FIG. 6.
CD8 and pan-TCR staining of T cells from E7/A2-immunized and influenza virus/A2-immunized FA(E7−) and KA(E7+) mice gated on E7/A2 tetramer + cells (A, B, E, and F) or influenza virus/A2 tetramer+ cells (C, D, G, and H) or total splenic T cells (I to N). Shaded, KA(E7+) splenocytes; unshaded, FA(E7−) splenocytes. [Functional tolerization of KA(E7+) splenocytes compared with FA(E7−) splenocytes was confirmed in this experiment by down-regulation of specific cytolysis in a concomitantly conducted CTL assay (not shown).]
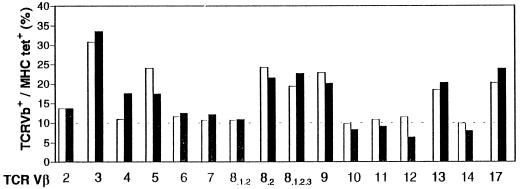
Down-regulation of tetramer-binding and CTL activities in specifically restimulated splenocytes from immunized KA(E7+) mice is not associated with overall alteration in TCR Vβ-chain usage.
Deletional models of T-cell tolerance indicate that clones of T cells whose TCRs recognize self antigen plus MHC with high affinity are deleted, leaving holes in the T-cell repertoire detectable at the population level by skewed TCR Vβ-chain usage (25, 30). We examined Vβ-chain usage in restimulated splenocytes from E7/A2-immunized KA(E7+) and FA(E7−) mice. Restimulated KA(E7+) splenocytes which stained with E7/A2 tetramer displayed an oligoclonal response, utilizing predominantly TCR Vβ3 but also Vβ4, Vβ5, Vβ8.2, Vβ8.1.2.3, Vβ9, Vβ13, and Vβ17 (Fig. 7). Restimulated FA(E7−) splenocytes which stained with E7/A2 tetramer had a similar pattern of TCR Vβ-chain usage. (Note from Fig. 7 that positive staining with the above Vβ antibodies accounts for about 100% of the E7/A2 tetramer+ cells. Thus, we consider it unlikely that we have missed any significant TCR Vβ usage.) These data lend support to the premise that down-regulation of T-cell responses in mice expressing E7 in epithelium is not associated with deletion of E7-specific T-cell clones from the T-cell repertoire.
FIG. 7.
Restimulated splenocytes from E7/A2-immunized KA(E7+) mice (shaded bars) and FA(E7−) mice (unshaded bars) have similar patterns of TCR Vβ-chain usage. Specific Vβ-staining cells are shown as a percentage of total E7/A2 tetramer-binding cells. The level of background staining is indicated by the dotted line. [Functional tolerization of KA(E7+) splenocytes compared with FA(E7−) splenocytes was confirmed in this experiment by down-regulation of specific cytolysis in a concomitantly conducted CTL assay (not shown).]
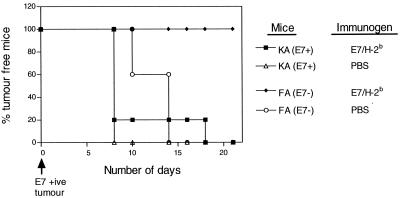
T-cell functional down-regulation is associated with inability to control E7-expressing tumor.
We have previously shown that control of E7-expressing tumor is effected by CD8+ T cells (17). To determine whether lack of CD8+ T-cell responsiveness in KA(E7+) mice, as measured in vitro, translated into a lack of responsiveness in vivo, we challenged E7/H-2b-immunized KA(E7+) and FA(E7−) mice with the E7-expressing epithelial tumor TC-1. E7/H-2b-immunized FA(E7−) mice developed no tumors, indicating that specific immunization can confer tumor protection in these mice. However, E7/H-2b-immunized KA(E7+) mice developed tumors almost as readily as PBS-immunized (control) KA(E7+) mice (Fig. 8). Note also that KA(E7+) mice developed tumors more readily than control FA(E7−) mice, suggesting that they were unable to respond to endogenous E7 expressed by the tumor. These data are consistent with the notion that lack of CD8+ T-cell responsiveness associated with E7 expression in epithelium precludes effective control of E7-expressing tumor.
FIG. 8.
Growth of E7-expressing tumor (TC-1) in immunized KA(E7+) and FA(E7−) mice.
T-cell development in mice expressing E7 in cortical thymic epithelium.
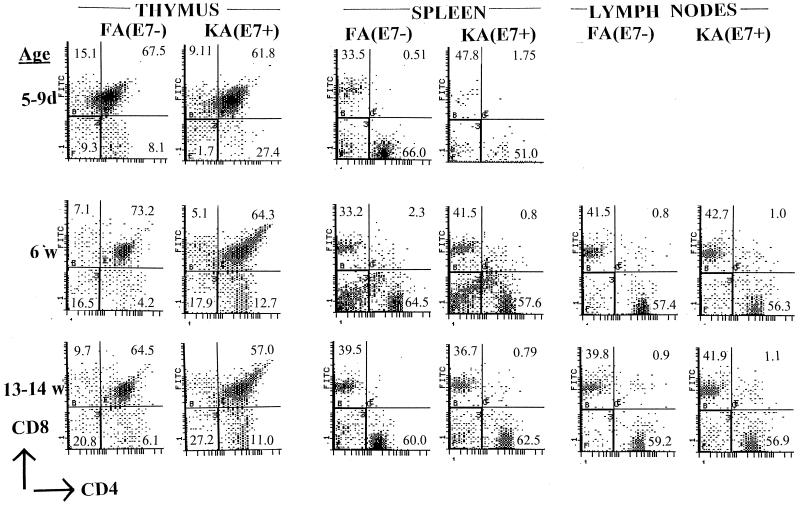
Our previous experiments indicated that E7 expression in thymic cortical epithelium is not necessary for the development of down-regulated E7-directed CTL responses in KA(E7+) mice, consistent with a lack of negative selection of E7-directed precursors during T-cell maturation in the thymus. However, we wished to inquire whether E7 expression in thymic cortical epithelium otherwise perturbed gross T-cell development which might influence the outcome of CTL responses in the periphery. Groups of three KA(E7+) and FA(E7−) mice were examined at various ages. Thymuses of all and spleens and lymph nodes of some KA(E7+) mice at 6 and 13 to 14 weeks, but not 5 to 9 days, were larger than those of age-matched FA(E7−) mice (not shown). We examined CD4 and CD8 expression in thymic, splenic, and lymph node cells of KA(E7+) and FA(E7−) mice (Fig. 9). Overall proportions of CD4/CD8 double-negative, CD4/CD8 double-positive, CD4 single-positive, and CD8 single-positive T cells were similar in thymuses from age-matched FA(E7−) and KA(E7+) mice. However, thymuses from KA(E7+) mice at 6 weeks and 13 to 14 weeks contained CD4/CD8 double-positive cell populations with a wider spread of staining intensities, and somewhat higher numbers of CD4 single-positive cells, than thymuses from correspondingly aged FA(E7−) mice (Fig. 9). Absolute numbers of CD8 single-positive T cells and proportions of CD4 single-positive and CD8 single-positive T cells in peripheral lymphoid organs were comparable between KA(E7+) and FA(E7−) mice at all ages tested (Fig. 9).
FIG. 9.
CD4 and CD8 expression on cells of thymus, pooled inguinal, and popliteal lymph nodes and spleens from KA(E7+) and FA(E7−) mice of various ages, measured by FACS analysis. Numbers in quadrants are percentages. For thymus, percentages are given as percent total cells (i.e., all four quadrants). For spleens and lymph nodes, percentages are given as percent total CD4+ plus CD8+ plus CD4/CD8 double-positive cells (i.e., not including the CD4/CD8 double-negative quadrant, which contains B cells, etc.).
DISCUSSION
There is no natural animal model of HPV-associated cervical cancer, since (i) HPV infection is species-specific and (ii) animal papillomaviruses produce dissimilar diseases. Mice which express an E7 transgene driven from an epithelium-specific human K14 promoter display hyperplasia and dysplasia, frequently leading to papillomatosis and squamous cell carcinoma (22), and provide a model for E7-mediated epithelial transformation in humans.
The human K14 promoter drives gene expression in skin, tongue, and esophagus epithelium and in thymic epithelium, where it is specifically active in cortical epithelial cells, the cell type involved in positive selection of T cells (26, 29). Mice which express HPV16 E7 oncoprotein from a K14-driven transgene show down-regulation of E7-directed CTL responses as measured by classical 51Cr release assays (12, 19). Using bone marrow-reconstituted, thymus-grafted radiation chimeric mice, we have shown that (i) T cells made to mature through a non-E7-expressing thymus into a mouse expressing E7 in peripheral epithelium show down-regulated CTL responses compared to T cells from non-E7 transgenic mice, and (ii) in contrast, T cells made to mature through an E7-expressing thymus into a mouse which does not express E7 in peripheral epithelium do not show down-regulated CTL responses compared to T cells from non-E7 transgenic mice (13). In further experiments (14), we have shown that precursor CTLs in E7-naive adult splenocytes transferred into E7 transgenic (but not non-E7 transgenic mice) showed down-regulated CTL responses. We interpreted these data to indicate that expression of E7 in peripheral epithelium is sufficient to cause down-regulation of E7-directed CTL responses.
In the present study we use the more sensitive tool of MHC class I tetramer binding to quantify CD8+T cells bearing cognate TCR in specifically immunized mice expressing E7 in peripheral epithelium and thymic cortical epithelium and in controls. We show that E7 epithelial expression down-regulates the expansion of not only E7-directed CD8+ T cells but also, surprisingly, non-E7-directed CD8+ T cells in response to restimulation with specific antigen.
Specific tetramer binding was unable to distinguish between the numbers of precursors in spleen or lymph nodes of specifically immunized KA(E7+) mice and FA(E7−) mice ex vivo after specific immunization (Fig. 1). This may reflect the limits of sensitivity of detection of the assay (ca. 1 in 104). However, the capacity of primed E7/A2- and influenza virus/A2 precursors to proliferate in vitro (measured by tetramer binding) in response to restimulation with cognate antigen was severely compromised in KA(E7+) mice, and this was reflected in diminished CTL responses against target cells coated with relevant epitope (Fig. 1). Topographically identical expression of a non-E7 transgene did not appreciably compromise cognate antigen restimulation or the capacity of restimulated cells to kill relevant targets (Fig. 3), indicating that the CD8+ T-cell functional down-regulation is associated with expression of E7.
Tetramer binding represents interaction between TCR and cognate MHC complex, and the kinetics of binding provides a measure of affinity of TCR for its peptide-MHC ligand. The kinetics of tetramer binding of E7/H-2b-restricted precursors of immunized KA(E7+) mice was similar to the corresponding precursors of immunized FA(E7−) mice (Fig. 5). A similar finding was obtained with E7/A2-restricted precursors. A recent suggestion that interaction of the CD8 molecule with the class I domain may supplement TCR binding (9) is unlikely to be relevant at least in the latter result since murine CD8 has limited capacity for binding the human α-3 domain (23) present in our tetramers. We interpret the tetramer-binding kinetics data to indicate that the range of affinities displayed by E7-directed (and OVA- and influenza virus-directed) CD8+ T cells in KA(E7+) mice is similar to the range of affinities displayed by CD8+ T cells from FA(E7−) mice.
Likewise, the avidity of pCTL from KA(E7+) mice is similar to that seen in FA(E7−) mice, as evidenced by similar sensitivity to E7/A2 peptide concentration used for sensitizing target cells (Fig. 4). Additionally, we note that overall levels of CD8 and TCR expression (which contribute to T-cell avidity) are similar on E7/A2 and influenza virus/A2 tetramer-binding CD8+ T cells from KA(E7+) and FA(E7−) mice (Fig. 6). We have not excluded the possibility that the expression of a second α chain, which can compete with the same β chain to produce another TCR with a different specificity, may have reduced the actual density of TCR specific for cognate antigen. This phenomenon was not observed, however, in tolerized CTL in p53 self-tolerant mice (23, 39) or influenza virus nucleoprotein transgenic mice (10).
A mechanism of CD8+ T-cell tolerance induction by peripheral expression of neo-antigen, at least in some other transgenic mouse systems, is specific deletion of high-affinity pCTL by apoptosis, leaving a residual population of low affinity pCTL (see reference 30 for a review). We argue from the above data that CD8+ T-cell functional down-regulation induced by expression of E7 transgene in epithelium does not involve appreciable deletion of high-affinity/avidity E7-directed CD8+ pCTL. The argument for lack of deletion is supported by our observation of similar TCR Vβ-chain usage in E7/A2 tetramer+ CD8+ T cells from KA(E7+) and FA(E7−) mice (Fig. 7). (While variation in Vβ-chain usage may occur among individual mice [10], this has been minimized in our experiment by pooling splenocytes from five mice per group.) Our data suggest the absence of holes in the repertoire which may be observed among T-cell populations in which clonotypic deletion of self-reactive T cells has occurred (4), in accord with recent findings in tolerized T-cell populations in other transgenic models where autoreactive T cells have escaped deletion (e.g., reference 10).
A further outcome of our study is the demonstration of the limitations of bulk CTL readouts as a measure of immune status (31). Figure 2D to F, Fig. 2J to L, and Fig. 3A to C clearly demonstrate that percent CTL lysis does not accurately reflect numbers of antigen-specific CD8+ cells, and indeed it is only when these cells are quantified using tetramers that the magnitude of the down-regulation of CD8+ T-cell responsiveness to E7- and non-E7 antigens in KA(E7+) mice becomes apparent. We cannot exclude the possibility that CD8+ T cells of too low an affinity to bind tetramer, yet still capable of specific target cell lysis (23), may have been present in our system. In six of seven assays documented here and elsewhere (13, 14) and in many others from our laboratory, influenza virus/A2 CTL responses were consistently ca. 5 to 15% lower in KA(E7+) mice than in FA(E7−) mice. In the absence of tetramer data prior to this study, we had previously overlooked this difference (13, 14). In the light of our present results, we now interpret these CTL data to indicate that the KA(E7+) mice display down-regulated CD8+ T-cell responses to non-E7 (i.e., influenza virus/A2) as well as E7 epitopes.
The data presented in this study do not formally distinguish whether down-regulated CD8+ T-cell responses to E7 and non-E7 antigens occur as a result of E7 expression in peripheral epithelium, thymic cortical epithelium, or both. However, we have previously demonstrated that down-regulated E7 CTL responses occur in mice expressing E7 in peripheral epithelium in the absence of E7 in the thymus but not in mice expressing E7 in thymic cortical epithelium in the absence of E7 in peripheral epithelium (i.e., peripheral rather than central down-regulation). An additional possibility with respect to the KA(E7+) mice used in the present study is that while negative selection of cognate T cells on E7 expressed in thymus cortical epithelium may not have occurred, nonetheless E7 expression in cortical epithelium may have nonspecifically perturbed T-cell maturation and/or function. The observation of thymomegaly, frequent splenomegaly, and lymph node enlargement and a somewhat different pattern of CD4/CD8 T-cell subsets in thymuses (but not spleen or lymph nodes) of adult KA(E7+) mice compared to their FA(E7−) counterparts (Fig. 9) suggests that T-cell maturation and homeostasis are not identical in the two lines of mice. However, the marked down-regulation of CD4 and CD8 coreceptors in thymic T cells in mice expressing a K14-driven non-E7 transgene (ovalbumin peptide) (29) and in K14.E7 mice, which unlike the KA(E7+) mice in the present study are homozygous for the E7 gene (and are on a solely H-2q background) (K. Malcolm, personal communication), was not seen in the present study.
In support of a peripheral mechanism for generalized down-regulation of CD8+ T cells responses, down-regulated influenza virus/A2 responses as well as E7/A2 responses were seen in chimeric mice expressing E7 in skin but not thymus which were reconstituted with KA(E7+) bone marrow (high influenza virus/A2 responses were recorded in the absence of E7 in skin, with or without E7 expression in thymus) (13). While this result was less clear when FA(E7−) bone marrow was used for reconstitution, in a further experiment adult splenocytes from FA(E7−) mice adoptively transferred to irradiated adult KA(E7+) recipients showed down-regulated reconstitution of E7 and influenza virus CTL responses compared to FA(E7−) recipients 2 weeks after transfer (14).
These observations, and our previous results demonstrating cross presentation of E7 (14) are consistent with a model in which E7 in skin is accessed by professional antigen-presenting cells (dendritic cells, macrophages) which deliver a nonspecific down-regulatory signal to CD8+ cells in primary and secondary lymphoid organs. The concept of a distinct set of dendritic cells which transports antigen from the periphery to T-cell areas of draining lymph nodes for induction and maintenance of peripheral T-cell tolerance is supported by other observations (36, 37, 38). Additionally, T-cell functional down-regulation in KA(E7+) mice may result from expression of E7 in thymic cortical epithelium which may serve to perturb the development of and/or the ability of mature CD8+ T cells to respond to cognate antigen. Antigen expression in thymic cortical epithelium is classically thought to induce positive selection (26) or at the very least, activation, but not deletion (29) of cognate T cells. Our data on similar Vβ-chain usage in KA(E7+) and FA(E7−) mice might argue against the differential expansion of E7-specific T-cell clones in the former mice. Nonetheless, recently reported cognate TCR editing occurring on thymic cortical epithelium (29), in which autoreactive (in our case E7) TCR may be internalized and new secondary TCR gene rearrangements may allow the expression of a TCR of modified specificity, could have perturbed the T-cell repertoire in our system.
Dendritic cells and macrophages pretreated with E7 (but not other HPV proteins) in vitro produce high levels of immunosuppressive alpha interferon (27) and inhibit T-cell responsiveness as measured by allogeneic mixed lymphocyte reaction. Furthermore, E7 added directly to peripheral blood mononuclear cell cultures inhibits the T-cell response to unrelated recall antigens (27), probably by the same mechanism. These in vitro observations suggest a possible mechanism for the nonspecifically down-regulated CD8+ T-cell responses in vivo observed in the present study. Dendritic cells have been shown to secrete a number of other cytokines which may inhibit T-cell activation under various circumstances, e.g., NO (1), interleukin-10 (35), and transforming growth factor β (5). Our data are also consistent with observations that anergic T cells inhibit responsive T cells by perturbation of antigen presentation by dendritic cells (40).
Down-regulation of CD8+ T-cell responses to peripherally expressed antigens depends, at least in some cases, on the generation of regulatory CD4+ T cells (25). It is unlikely that the CD8+ T-cell functional down-regulation that we report here depends on impairment of cognate CD4+ help, since we have previously shown enhanced E7-directed CD4+ T-helper responses in E7 CTL-tolerant mice expressing E7 in peripheral epithelium and thymic cortical epithelium (19). Similarly, patients with E7-associated cervical carcinoma make adequate E7-directed T-proliferative (24) and antibody (11) responses suggesting intact CD4+ helper function.
While we have shown that mice expressing an E7 transgene in epithelium make no measurable CTL responses to the transgene E7 (16), our present data indicate the existence of high-avidity E7-directed pCTL in such mice. Lack of E7-directed autoimmunity in these mice (3) is in agreement with models in which pCTL to self (39) or transgene (10) antigens may be demonstrated without concomitant autoimmune consequences.
Cervical carcinoma patients make poor CTL responses, including responses to both the E7/A2 epitope and influenza virus/A2 epitope used in our study (33), even after specific immunization. This suggests that E7 expression in transformed cervical epithelium may serve to functionally down-regulate T-cell responsiveness, with the result that specific immunization fails to control tumor growth, as it does in our mouse model (Fig. 8). Thus, means to circumvent down-regulation of CTL function may be necessary for optimizing immunotherapy for cervical cancer. That E7-mediated CD8+ T-cell functional down-regulation does not delete high-avidity E7-specific CTL gives optimism for overcoming putative CTL tolerance in cervical cancer, since high-affinity CTL are likely to be most functionally effective in reducing tumor load (3, 44, 45).
There are parallels with mice in which residual CTL responses (reflecting substantive, but incomplete tolerance) to nonmutated self antigens e.g., p53 (23) and melanoma antigens (8, 45) can be enhanced with appropriate immunization. While CTL in such models are predominantly low affinity (because, unlike the situation with E7 expression in epithelium in our mice, tolerization in these models involves deletion of most antigen-specific T cells of high avidity), selective expansion of high-avidity CTL could be achieved with low concentrations of restimulating peptide (2, 45) or peptide tetramers (44).
Overall, our data are consistent with a hypothesis in which expression of HPV16 E7 in epithelium serves to down-regulate E7-specific CD8+ T-cell-mediated responses necessary for control of E7-expressing tumor, and also non-E7-specific CD8+ T-cell responses. The mechanism(s) of this down-regulation is the subject of ongoing investigation in our laboratory.
ACKNOWLEDGMENTS
PE-conjugated HLA A∗0201 tetramers of epitopes E7/A2 and influenza virus/A2 and H-2b tetramers of epitopes E7/H-2b and OVA/H-2b were constructed and supplied by the NIAID Tetramer Facility (Atlanta, Ga.) and the NIH AIDS Research and Reference Reagent Program. Linda Sherman gave permission to use the A2.1Kb+/+ mice. I. R. Williams provided K14.hgh/B7 mice. Donna West and her staff provided excellent animal husbandry.
The work was supported by grants from the National Health and Medical Research Council and Queensland Cancer Fund (Australia).
Footnotes
Contribution no. 131 of the Sir Albert Sakzewski Virus Research Centre.
REFERENCES
- 1.Aiello S, Noris M, Piccinini G, Tomasoni S, Casiraghi F, Bonazzola S, Mister M, Sayegh M H, Remuzzi G. Thymic dendritic cells express inducible nitric oxide synthase and generate nitric oxide in response to self- and alloantigens. J Immunol. 2000;164:4649–4658. doi: 10.4049/jimmunol.164.9.4649. [DOI] [PubMed] [Google Scholar]
- 2.Alexander-Miller M A, Leggatt G R, Berzovsky J A. Selective expansion of high- and low-avidity cytotoxic T lymphocytes and efficacy for adoptive immunotherapy. Proc Natl Acad Sci USA. 1996;93:4102–4107. doi: 10.1073/pnas.93.9.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexander-Miller M A. Differential expansion and survival of high and low avidity cytotoxic T cell populations during the immune response to a viral infection. Cell Immunol. 2000;201:58–62. doi: 10.1006/cimm.1999.1632. [DOI] [PubMed] [Google Scholar]
- 4.Blish C A, Gallay B J, Turk G L, Kline K M, Wheat W, Fink P J. Chronic modulation of the TCR repertoire in the lymphoid periphery. J Immunol. 1999;162:3131–3140. [PubMed] [Google Scholar]
- 5.Borkowski T A, Letterio J J, Farr A G, Udey M C. A role for endogenous transforming growth factor beta 1 in Langerhans cell biology: the skin of transforming growth factor beta 1 null mice is devoid of epidermal Langerhans cells. J Exp Med. 1996;184:2417–2422. doi: 10.1084/jem.184.6.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borysiewicz L K, Fiander A, Nimako M, Man S, Wilkinson G W, Westmoreland D, Evans A S, Adams M, Stacey S N, Boursnell M E, Rutherford E, Hickling J K, Inglis S C. A recombinant vaccinia virus encoding human papillomavirus types 16 and 18, E6 and E7 proteins as immunotherapy for cervical cancer. Lancet. 1996;347:1523–1527. doi: 10.1016/s0140-6736(96)90674-1. [DOI] [PubMed] [Google Scholar]
- 7.Carbone F R, Bevan M J. Induction of ovalbumin-specific cytotoxic T cells by in vivo peptide immunization. J Exp Med. 1989;169:603–612. doi: 10.1084/jem.169.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colella T A, Bullock T N, Russell L B, Mullins D W, Overwijk W W, Luckey C J, Pierce R A, Restifo N P, Engelhard V H. Self-tolerance to the murine homologue of a tyrosinase-derived melanoma antigen: implications for tumor immunotherapy. J Exp Med. 2000;191:1221–1232. doi: 10.1084/jem.191.7.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daniels M A, Jameson S C. Critical role for CD8 in T cell receptor binding and activation by peptide/major histocompatibility complex multimers. J Exp Med. 2000;191:335–346. doi: 10.1084/jem.191.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Visser K E, Cordaro T A, Kioussis D, Haanen J B, Schumacher T N, Kruisbeek A M. Tracing and characterization of the low-avidity self-specific T cell repertoire. Eur J Immunol. 2000;30:1458–1468. doi: 10.1002/(SICI)1521-4141(200005)30:5<1458::AID-IMMU1458>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 11.Di Lonardo A, Marcante M L, Poggiali F, Venuti A. HPV 16 E7 antibody levels in cervical cancer patients: before and after treatment. J Med Virol. 1998;54:192–195. [PubMed] [Google Scholar]
- 12.Doan T, Chambers M, Street M, Fernando G J, Herd K, Lambert P, Tindle R. Mice expressing the E7 oncogene of HPV16 in epithelium show central tolerance, and evidence of peripheral anergising tolerance, to E7-encoded cytotoxic T-lymphocyte epitopes. Virology. 1998;244:352–364. doi: 10.1006/viro.1998.9128. [DOI] [PubMed] [Google Scholar]
- 13.Doan T, Herd K, Street M, Bryson G, Fernando G, Lambert P, Tindle R. Human papillomavirus type 16 E7 oncoprotein expressed in peripheral epithelium tolerizes E7-directed cytotoxic T-lymphocyte precursors restricted through human (and mouse) major histocompatibility complex class I alleles. J Virol. 1999;73:6166–6170. doi: 10.1128/jvi.73.7.6166-6170.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doan T, Herd K A, Lambert P F, Fernando G J, Street M D, Tindle R W. Peripheral tolerance to human papillomavirus E7 oncoprotein occurs by cross-tolerization, is largely Th-2-independent, and is broken by dendritic cell immunization. Cancer Res. 2000;60:2810–2815. [PubMed] [Google Scholar]
- 15.Feltkamp M C, Smits H L, Vierboom M P, Minnaar R P, de Jongh B M, Drijfhout J W, ter Schegget J, Melief C J, Kast W M. Vaccination with cytotoxic T lymphocyte epitope-containing peptide protects against a tumor induced by human papillomavirus type 16-transformed cells. Eur J Immunol. 1993;23:2242–2249. doi: 10.1002/eji.1830230929. [DOI] [PubMed] [Google Scholar]
- 16.Ferber I, Schonrich G, Schenkel J, Mellor A L, Hammerling G J, Arnold B. Levels of peripheral T cell tolerance induced by different doses of tolerogen. Science. 1994;263:674. doi: 10.1126/science.8303275. [DOI] [PubMed] [Google Scholar]
- 17.Fernando G J, Stewart T J, Tindle R W, Frazer I H. Th2-type CD4+ cells neither enhance nor suppress antitumor CTL activity in a mouse tumor model. J Immunol. 1998;161:2421–2427. [PubMed] [Google Scholar]
- 18.Fossum C, Bergstrom M, Lovgren K, Watson D L, Morein B. Effect of iscoms and their adjuvant moiety (matrix) on the initial proliferation and IL-2 responses: comparison of spleen cells from mice inoculated with iscoms and/or matrix. Cell Immunol. 1990;129:414–425. doi: 10.1016/0008-8749(90)90217-f. [DOI] [PubMed] [Google Scholar]
- 19.Frazer I H, Fernando G J, Fowler N, Leggatt G R, Lambert P F, Liem A, Malcolm K, Tindle R W. Split tolerance to a viral antigen expressed in thymic epithelium and keratinocytes. Eur J Immunol. 1998;28:2791–2800. doi: 10.1002/(SICI)1521-4141(199809)28:09<2791::AID-IMMU2791>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 20.Girgis L, Davis M M, de St Groth B F. The avidity spectrum of T cell receptor interactions accounts for T cell energy in a double transgenic model. J Exp Med. 1999;189:265–278. doi: 10.1084/jem.189.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gotch F, Rothbard J, Howland K, Townsend A, McMichael A. Cytotoxic T lymphocytes recognize a fragment of influenza virus matrix protein in association with HLA-A2. Nature. 1987;326:881–882. doi: 10.1038/326881a0. [DOI] [PubMed] [Google Scholar]
- 22.Herber R, Liem A, Pitot H, Lambert P F. Squamous epithelial hyperplasia and carcinoma in mice transgenic for the human papillomavirus type 16 E7 oncogene. J Virol. 1996;70:1873–1881. doi: 10.1128/jvi.70.3.1873-1881.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hernandez J, Lee P P, Davis M M, Sherman L A. The use of HLA A2.1/p53 peptide tetramers to visualize the impact of self tolerance on the TCR repertoire. J Immunol. 2000;164:596–602. doi: 10.4049/jimmunol.164.2.596. [DOI] [PubMed] [Google Scholar]
- 24.Kadish A S, Ho G Y, Burk R D, Wang Y, Romney S L, Ledwidge R, Angeletti R H. Lymphoproliferative responses to human papillomavirus (HPV) type 16 proteins E6 and E7: outcome of HPV infection and associated neoplasia. J Natl Cancer Inst. 1997;89:1285–1293. doi: 10.1093/jnci/89.17.1285. [DOI] [PubMed] [Google Scholar]
- 25.Kurts C, Carbone F R, Barnden M, Blanas E, Allison J, Heath W R, Miller J F. CD4+ T cell help impairs CD8+ T cell deletion induced by cross-presentation of self-antigens and favors autoimmunity. J Exp Med. 1997;186:2057–2062. doi: 10.1084/jem.186.12.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laufer T M, DeKoning J, Markowitz J S, Lo D, Glimcher L H. Unopposed positive selection and autoreactivity in mice expressing class II MHC only on thymic cortex. Nature. 1996;383:81–85. doi: 10.1038/383081a0. [DOI] [PubMed] [Google Scholar]
- 27.Le Buanec H, D'Anna R, Lachgar A, Zagury J F, Bernard J, Ittele D, d'Alessio P, Hallez S, Giannouli C, Burny A, Bizzini B, Gallo R C, Zagury D. HPV-16 E7 but not E6 oncogenic protein triggers both cellular immunosuppression and angiogenic processes. Biomed Pharmacother. 1999;53:424–431. doi: 10.1016/S0753-3322(99)80122-X. [DOI] [PubMed] [Google Scholar]
- 28.Lin K Y, Guarnieri F G, Staveley O F, Levitsky H I, August J T, Pardoll D M, Wu T C. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56:21–26. [PubMed] [Google Scholar]
- 29.McCargill M, Derbinski J, Hogquist K. Receptor editing in developing T cells. Nat Immunol. 2000;1:336–341. doi: 10.1038/79790. [DOI] [PubMed] [Google Scholar]
- 30.Miller J, Morahan G. Peripheral T-cell tolerance. Annu Rev Immunol. 1992;10:51–61. doi: 10.1146/annurev.iy.10.040192.000411. [DOI] [PubMed] [Google Scholar]
- 31.Murali Krishna K, Altman J D, Suresh M, Sourdive D J, Zajac A J, Miller J D, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 32.Ressing M, Toes R, Schoenberger S, Brandt R, de Jong J, Kast M, Offringa R, Melief C. Anti-tumour immunotherapy with synthetic peptides representing tumour associated T-cell epitopes: implications for peptide based vaccination of cervical cancer. In: Tindle R, editor. Vaccines for human papillomavirus infection and anogenital disease. Austin, Tex: Landes Bioscience; 1999. pp. 53–68. [Google Scholar]
- 33.Ressing M E, Sette A, Brandt R M, Ruppert J, Wentworth P A, Hartman M, Oseroff C, Grey H M, Melief C J, Kast W M. Human CTL epitopes encoded by human papillomavirus type 16 E6 and E7 identified through in vivo and in vitro immunogenicity studies of HLA-A∗0201-binding peptides. J Immunol. 1995;154:5934–5943. [PubMed] [Google Scholar]
- 34.Ressing M E, van Driel W J, Celis E, Sette A, Brandt M P, Hartman M, Anholts J D, Schreuder G M, ter Harmsel W B, Fleuren G J, Trimbos B J, Kast W M, Melief C J. Occasional memory cytotoxic T-cell responses of patients with human papillomavirus type 16-positive cervical lesions against a human leukocyte antigen-A∗0201-restricted E7-encoded epitope. Cancer Res. 1996;56:582–588. [PubMed] [Google Scholar]
- 35.Serot J M, Bene M C, Foliguet B, Faure G C. Monocyte-derived IL-10-secreting dendritic cells in choroid plexus epithelium. J Neuroimmunol. 2000;105:115–119. doi: 10.1016/s0165-5728(99)00240-4. [DOI] [PubMed] [Google Scholar]
- 36.Spencer J V, Braciale T J. Incomplete CD8(+) T lymphocyte differentiation as a mechanism for subdominant cytotoxic T lymphocyte responses to a viral antigen. J Exp Med. 2000;191:1687–1698. doi: 10.1084/jem.191.10.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steinman R M, Turley S, Mellman I, Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J Exp Med. 2000;191:411–416. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suss G, Shortman K. A subclass of dendritic cells kills CD4 T cells via Fas/Fas-ligand-induced apoptosis. J Exp Med. 1996;183:1789–1796. doi: 10.1084/jem.183.4.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Theobald M, Biggs J, Hernandez J, Lustgarten J, Labadie C, Sherman L A. Tolerance to p53 by A2.1-restricted cytotoxic T lymphocytes. J Exp Med. 1997;185:833–841. doi: 10.1084/jem.185.5.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vendetti S, Chai J G, Dyson J, Simpson E, Lombardi G, Lechler R. Anergic T cells inhibit the antigen-presenting function of dendritic cells. J Immunol. 2000;165:1175–1181. doi: 10.4049/jimmunol.165.3.1175. [DOI] [PubMed] [Google Scholar]
- 41.Vitiello A, Marchesini D, Furze J, Sherman L A, Chesnut R W. Analysis of the HLA-restricted influenza-specific cytotoxic T lymphocyte response in transgenic mice carrying a chimeric human-mouse class I major histocompatibility complex. J Exp Med. 1991;173:1007–1015. doi: 10.1084/jem.173.4.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.von Knebel Doeberitz M, Rittmuller C, Aengeneyndt F, Jansen Durr P, Spitkovsky D. Reversible repression of papillomavirus oncogene expression in cervical carcinoma cells: consequences for the phenotype and E6–p53 and E7-pRB interactions. J Virol. 1994;68:2811–2821. doi: 10.1128/jvi.68.5.2811-2821.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams I R, Ort R J, Daley D, Manning L, Karaoli T, Barnhill R L, Kupper T S. Constitutive expression of B7–1 (CD80) on mouse keratinocytes does not prevent development of chemically induced skin papillomas and carcinomas. J Immunol. 1996;156:3382–3388. [PubMed] [Google Scholar]
- 44.Yee C, Savage P A, Lee P P, Davis M M, Greenberg P D. Isolation of high avidity melanoma-reactive CTL from heterogeneous populations using peptide-MHC tetramers. J Immunol. 1999;162:2227–2234. [PubMed] [Google Scholar]
- 45.Zeh H J, III, Perry Lalley D, Dudley M E, Rosenberg S A, Yang J C. High avidity CTLs for two self-antigens demonstrate superior in vitro and in vivo antitumor efficacy. J Immunol. 1999;162:989–994. [PubMed] [Google Scholar]