Abstract
Induction of apoptosis by different types of pathogenic retroviruses is an important step in disease development. We have observed that infection of thymic lymphocytes by the mink cell focus-forming murine leukemia virus (MCF MLV) during the preleukemic period resulted in an enhancement of apoptosis of these cells. To further study the ability of MCF MLVs to induce apoptosis and the role of this process in viral pathogenesis, we have developed an in vitro system of virus-induced apoptosis. MCF13 MLV infection of mink epithelial cells resulted in the production of cytopathic foci. In contrast, infection of mink cells with the 4070A amphotropic MLV did not produce any cytopathic effects. Staining of MCF13 MLV-infected cells with propidium iodide and annexin V-fluorescein isothiocyanate indicated that virus-induced cell death was due to apoptosis. At 6 days postinfection, the percentage of apoptotic MCF13 MLV-infected cells was 27% compared with 2 to 3% for mock- or amphotropic MLV-infected cells, representing a 9- to 14-fold difference. Assays for caspase-3 activation confirmed the detection by flow cytometry of apoptosis of MCF13 MLV-infected cells. Large amounts of unintegrated linear viral DNA were detectable by Southern blot analysis during the acute phase of infection, which indicated that MCF13 MLV is able to superinfect mink cells. Unintegrated viral DNA of only the linear form was detectable in thymic lymphocytes isolated from MCF13 MLV-inoculated mice during the preleukemic period. These results indicated that the ability of MCF13 MLV to induce apoptosis is correlated with its ability to superinfect cells and that this occurs as an early step in thymic lymphoma development.
The induction or inhibition of apoptosis by viruses of different families is a critical determinant of their pathogenicity (36, 42). Among retroviruses, the induction of apoptosis has been shown to be important for the generation of immunodeficient diseases as well as various forms of neoplasia. Retroviruses for which apoptosis is thought to play an important role in the generation of immunodeficiencies include the human, simian, and feline immunodeficiency viruses (HIV, SIV, and FeLV-FAIDS, respectively) (1, 15, 28, 34) and a mutant of the Moloney murine leukemia virus (ts-1 Mo MLV) (35). Examples of oncogenic retroviruses which induce cell killing and apoptosis include the avian leukosis virus (ALV) of subgroups B and D (10, 46, 47), avian reticuloendotheliosis viruses (REVs) (22), Abelson MLV (33, 43), and Mo MLV (9). It has recently been shown that thymic lymphocytes isolated from preleukemic mice inoculated with the mink cell focus-forming (MCF) MLV underwent enhanced apoptosis (52). We observed that apoptosis of thymic lymphocytes in these mice resulted in diffuse lymphocyte depletion mainly in the cortical region of the thymus. This region of the thymus consists mainly of the subpopulation of cells that we and others have shown are predominantly infected by MCF MLVs (12, 31, 51). Similar abnormalities of the preleukemic thymus have been detectable by others during the development of spontaneous T-cell lymphomas in the AKR mouse (30).
The relevance of MCF MLV-induced apoptosis of thymic lymphocytes to AKR lymphomagenesis was observed in early studies by D. Metcalf, who noted that cell death in the thymus was a requirement for leukemogenesis in the AKR mouse (27). Moreover, work by Bonzon and Fan (9) on the induction of thymic lymphoma by Mo MLV showed a strong correlation between enhanced apoptosis of thymic lymphocytes from mice inoculated with the wild-type virus and the reduced apoptosis induced by a mutant which is attenuated in pathogenicity. Because of the paradigm that tumorigenesis results from abnormal cellular proliferation, it is not obvious why cell killing should be part of the mechanism used by oncogenic retroviruses to induce tumors. It is possible, however, that cells undergoing apoptosis may be rescued from this initial crisis by genetic changes which may be provided by proviral insertion adjacent to cellular genes involved in the regulation of apoptosis.
It has been shown that retroviral glycoproteins are involved in the cytopathic effects of several different oncogenic retroviruses. A direct involvement in apoptosis has been observed for the interaction of the ALV subgroup B glycoprotein with its cellular receptor CAR1, a member of the tumor necrosis factor (TNF) receptor family (10). Activation of TNF receptor proteins induces apoptosis (3), and a similar effect is observed for the subgroup B ALV glycoprotein. For FeLV-C, genetic and mutagenesis studies have mapped viral cytopathicity to the surface portion of the envelope glycoprotein (34). However, the receptor for FeLV-C, which has recently been identified, does not resemble any known cellular protein that participates in apoptosis (32, 41). Therefore, the mechanism used by FeLV-C to induce apoptosis may be different from that used by ALV.
A close correlation has been observed between the ability of a retrovirus to induce cytopathic effects and its ability to synthesize high levels of unintegrated linear viral DNA in acutely infected cells (22, 29, 46, 47). It has been demonstrated that the large amounts of unintegrated viral DNA are the consequence of virus superinfection for ALV of subgroups B and D (46, 47), REVs (22), and FeLV-FAIDS (29). The relevance of this observation to AKR lymphomagenesis is underscored by the detection of high levels of unintegrated linear MLV DNA in thymuses of AKR mice during the preleukemic period of spontaneous lymphoma development (21). To better understand the role of MCF13 MLV-induced apoptosis in the generation of thymic lymphoma, we have examined whether this retrovirus resembles other cytopathic retroviruses in its ability to superinfect cells and synthesize high levels of unintegrated linear viral DNA. We summarize our studies employing cultured mink epithelial cells in this report.
MATERIALS AND METHODS
Virus infection of cells.
Ten thousand CCL64 mink epithelial cells (American Type Culture Collection) were plated in each well of a 24-well plate 1 day before infection with 1 ml of cell-free supernatant containing MCF13 or 4070A amphotropic MLV and 2 μg of polybrene. Mock-infected cells were treated with 1 ml of Dulbecco's minimal essential medium containing 2 μg of polybrene. Cells were incubated at 37°C for 6 h, after which virus was removed, cells were rinsed once, and fresh Dulbecco's minimal essential medium containing 10% fetal bovine serum was added. Viable cells were enumerated by trypan blue dye exclusion.
Detection of cytopathic foci and virus-infected cells.
Ten thousand CCL64 mink cells were plated on a 6-cm culture dish and infected with either MCF13 or 4070A amphotropic MLV or mock infected as described above. Cytopathic foci were detected by phase microscopy using a Leica DMIRB microscope connected to a digital camera from DIAGNOSTIC Instrument, Inc. Detection of virus-infected cells was made by an indirect immunofluorescence assay, which involved the initial treatment of cells with 0.5 ml of hybridoma supernatant of monoclonal antibody (MAb) 83A25, which recognizes the glycoprotein of MCF and amphotropic MLVs (17), for 30 min at 37°C. This was followed by the addition of 1 ml of a secondary fluorescein isothiocyanate (FITC)-labeled anti-rat antibody (Sigma) for another 30 min. The MAb 83A25 hybridoma was a generous gift of L. H. Evans, Rocky Mountain Laboratories, Hamilton, Mont.
Flow cytometric analysis of apoptotic cells.
One hundred thousand mink cells were trypsinized and washed twice with phosphate-buffered saline (PBS) containing 1% bovine serum albumin and 0.02% sodium azide. Cells were stained with 0.5 μg of propidium iodide (PI) (Sigma) per ml and 0.6 μg of annexin V-FITC (Pharmingen) per ml in 10 mM HEPES (pH 7.4), 140 mM NaCl, and 2.5 mM CaCl2 for 20 min at room temperature in the dark. Samples were analyzed within 1 h after staining on a Becton Dickinson FACScan. Data on 2 × 104 cells were analyzed using the CELLQuest software (Becton Dickinson). Regions with populations of cells that were negative for PI (PI−) and for annexin V (annexin V−) (live), PI− and positive for annexin V (annexin V+) (apoptotic), or positive for PI (PI+) and annexin V+ (dead) were selected. Analysis gates were set on single-color controls.
Caspase-3 assay.
One million mink cells which were either infected with virus or mock infected were trypsinized, washed twice with PBS, and lysed in 200 μl of 50 mM Tris-HCl (pH 7.5) containing 0.03% Nonidet P-40 and 1 mM dithiothreitol. Nuclei were removed by centrifugation at 1,200 × g for 5 min at 4°C. Caspase-3 activity was measured by using an EnzChek caspase-3 assay kit (Molecular Probes) in a 96-well plate format. Fluoromethylcoumarin fluorescence, released from the Z-DEVD-AMC peptide substrate by caspase-3 activity, was detected at 450 nm for 30 min with a Spectra MAX Gemini fluorometer (Molecular Devices). A 7-amino-4-methyl coumarin standard curve was prepared in the range of 0.137 to 17.5 μg. Protein amounts were measured using the bicinchoninic acid protein assay (Pierce). Units of caspase-3 activity are expressed as picomoles of substrate hydrolyzed per microgram of protein per half hour.
Hirt extraction of unintegrated viral DNA.
Hirt extractions of mink epithelial cells growing on 10-cm tissue culture plates were performed as described previously (53). Briefly, cells were rinsed twice with PBS and lysed by the addition of 3 ml of lysis buffer (10 mM Tris-HCl [pH 7.5], 10 mM EDTA, 100 mM NaCl, 1% sodium dodecyl sulfate) at 37°C for 20 min. Cell lysates were then made to 1 N NaCl final concentration and incubated overnight at 4°C. The salt precipitate was pelleted at 27,000 × g for 1 h at 4°C. DNA was precipitated from the supernatant, which had been extracted twice with chloroform, with the addition of 2.5 volumes of ethyl alcohol at −20°C overnight. DNA was subsequently pelleted by centrifugation at 1,100 × g at 4°C and dissolved in 200 μl of Tris-EDTA buffer (10 mM Tris-HCl [pH7.5], 1 mM EDTA), after which 20 μl of 3 M sodium acetate, 60 μg of glycogen, and 3 volumes of ethyl alcohol were added with an overnight precipitation at −20°C. DNA was pelleted by centrifugation at 16,000 × g for 30 min at 4°C and dissolved in 15 μl of Tris-EDTA buffer.
Hirt extractions were also performed on 2 × 108 thymic lymphocytes that were isolated as described previously (51) from AKR/J mice that were inoculated neonatally with 106 infectious units of MCF13 MLV. Control mice were inoculated with tissue culture medium.
Southern blot detection of unintegrated viral DNA.
Hirt-extracted DNA was electrophoresed through a 1% agarose gel in Tris-borate EDTA buffer (89 mM Tris-borate [pH 8.0], 2 mM EDTA) at 150 V for 3 to 5 h at 4°C. One-kilobase ladder DNA was electrophoresed in one lane of each agarose gel to provide size markers. Transfer of DNA to a GeneScreen Plus nylon membrane (New England Nuclear Life Sciences) was performed as described before (49). The DNA probe for hybridization was either a 8.2-kb linear plasmid DNA corresponding to the complete MCF13 MLV genome or a 1.6-kb fragment consisting of MCF13 MLV gag sequences. Both DNA probes were 32P-labeled with the DNA Nick Translation Kit (Boehringer Mannheim). Five million counts per minute of labeled probe was used for hybridization of the membrane overnight at 68°C in a roller bottle hybridization oven (Boekel Scientific). After hybridization, nylon filters were rinsed as previously described (49). DNA bands were detected and analyzed with a Molecular Dynamics PhosphorImager and Storm 840 scanner.
RESULTS
In a previous study we observed that thymic lymphocytes isolated during the preleukemic period from AKR/J mice inoculated with MCF13 MLV underwent enhanced apoptosis compared with thymic lymphocytes from uninoculated animals (52). To facilitate studies of the mechanism by which MCF13 MLV induces apoptosis, we have developed an in vitro system employing the CCL64 mink epithelial cell line. The rationale for performing these studies in vitro is that experiments with mice are costly and require long incubation periods to detect apoptotic effects (approximately 4 to 8 weeks postinoculation) and that mink cells are easily grown in culture, unlike primary lymphocytes, which rapidly die when cultured. Moreover, we wished to determine whether MCF13 MLV could induce apoptosis of other cell types besides thymic T cells and to facilitate further studies to elucidate the mechanism of viral cytopathicity.
Cytopathic effects of MCF13 MLV infection of CCL64 mink epithelial cells.
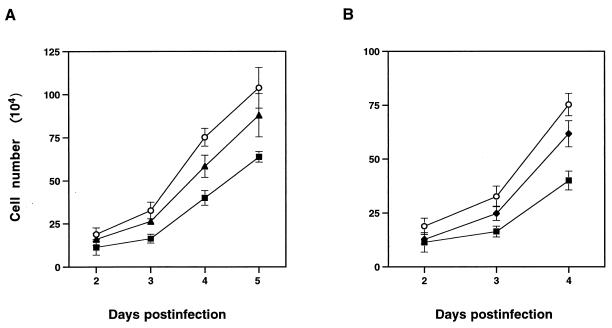
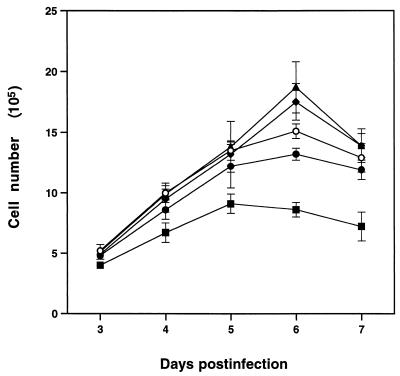
When CCL64 cells were infected with MCF13 MLV at different multiplicities of infection (MOI), we detected a depletion of cells over time. Figure 1A shows that a reduction in cell number was detectable starting at 3 days postinfection (p.i.) and continued through 5 days p.i. at MOI of 1 and 7. Cell depletion level correlated with the amount of virus added. In comparison, we did not detect a similar reduction in the number of mink cells that were infected with the 4070A amphotropic MLV, another MLV that infects CCL64 cells (11), at an MOI of 7 (Fig. 1B). At this MOI, however, a slight decrease in cell number was detectable for the amphotropic MLV.
FIG. 1.
Effect of MCF13 MLV infection on cell growth. (A) Ten thousand CCL64 mink epithelial cells were infected with MCF13 MLV at an MOI of 1 (▴) or 7 (■) or mock infected with medium (○). (B) Mink cells were infected with MCF13 MLV (■) or 4070A amphotropic MLV (⧫) at an MOI of 7 or mock infected (○). Cells were trypsinized at different days after infection, and viable cells were enumerated by trypan blue dye exclusion. Values represent the means and standard deviations calculated from counting duplicate samples from four independent experiments.
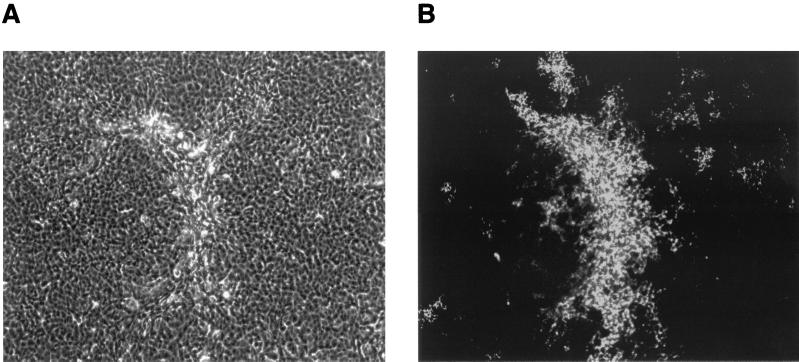
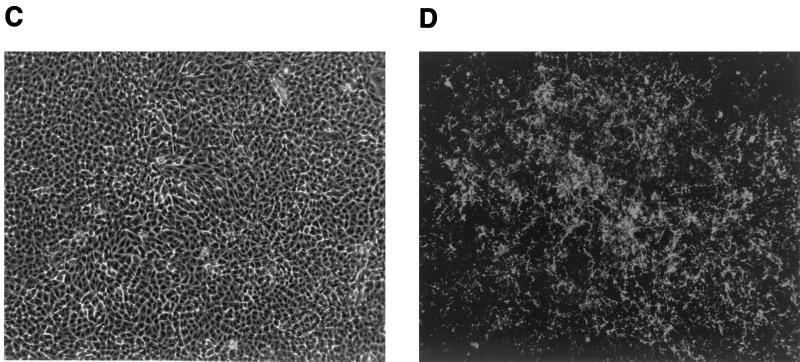
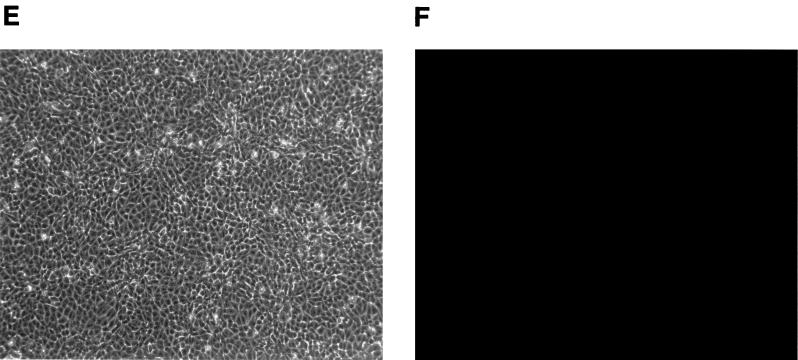
Starting at approximately 5 days after MCF13 MLV infection, cytopathic foci were detectable by phase microscopy throughout the mink cell culture (Fig. 2A). These foci consisted of cells with altered morphology including pycnotic cells and resembled the foci which were described for the initial isolation of MCF MLVs (19). Detection of MCF glycoprotein expression on the cell surface by indirect immunofluorescence staining demonstrated that cells constituting the cytopathic foci were infected with MCF13 virus (Fig. 2B). Cytopathic foci were not detectable with cells that were either infected with the 4070A amphotropic MLV (Fig. 2C and D) or mock infected (Fig. 2E and F). Cells infected with the amphotropic MLV showed positive staining by the indirect immunofluorescence assay, thereby demonstrating that the absence of cell death and cytopathic foci was not due to the inability of this virus to infect mink cells (Fig. 2D).
FIG. 2.
MCF13 MLV infection of mink epithelial cells produces cytopathic foci. Ten thousand CCL64 mink epithelial cells were infected with MCF13 MLV (A and B) or 4070A amphotropic MLV (C and D) at an MOI of 1. Cells were also mock infected with medium (E and F). Six days after virus infection, cells were examined by phase microscopy (A, C, and E) and fluorescence microscopy (B, D, and F). Fluorescence assays were performed by first staining cells with MAb 83A25, a monoclonal antibody that recognizes nearly all MLV glycoproteins, and subsequently with an FITC-labeled secondary anti-rat antibody.
Mink cell killing by MCF13 MLV occurs by apoptosis.
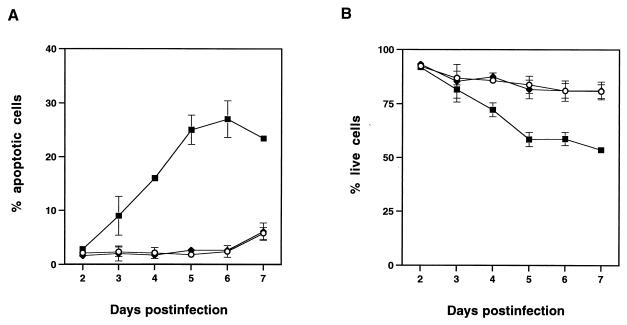
Because we had observed that MCF13 MLV infection of thymic lymphocytes resulted in an enhancement of apoptosis, we investigated whether apoptosis was also the cause of mink cell killing. It has been shown that flow cytometric analysis of cells stained with PI and FITC-labeled annexin V (annexin V-FITC) is an effective method to distinguish between live (PI−, annexin V−), apoptotic (PI−, annexin V+), and dead (PI+, annexin V+) cells (23). Using this method, we observed an increase over time in the percentage of apoptotic mink cells infected with MCF13 MLV (Fig. 3A). The greatest percentage of apoptotic MCF13 MLV-infected cells was detectable at 6 days p.i. when 27% of cells were apoptotic. This represented a 14-fold increase over the percentage of apoptotic cells that were detectable for mock-infected cells or cells infected with the 4070A amphotropic MLV. As a correlate, we also examined the percentage of live cells by flow cytometric analysis and observed that the percentage of live cells that were infected with MCF13 MLV decreased steadily over time compared with that of mock-infected cells (Fig. 3B). In contrast, no difference in the percentage of live cells infected with the amphotropic MLV was detectable.
FIG. 3.
Flow cytometric analysis of mink epithelial cells stained with PI and annexin V-FITC after virus infection. One hundred thousand mink epithelial cells were infected with either MCF13 MLV (■) or 4070A amphotropic MLV (⧫) or mock infected (○). Cells were trypsinized at different days after virus infection at an MOI of 4 and stained with PI and annexin V-FITC. Twenty thousand cells were analyzed on a FACScan flow cytometer. Values are the means and standard deviations calculated from cell numbers from four independent experiments. (A) Percentage of apoptotic cells (PI−, annexin V+). (B) Percentage of live cells (PI−, annexin V−).
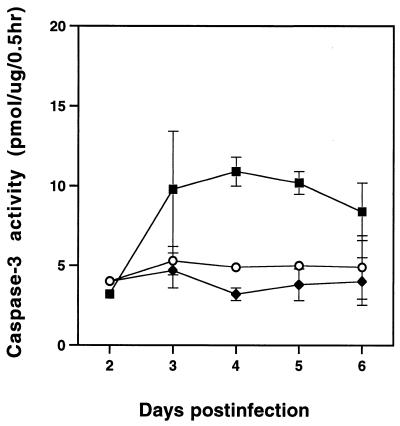
As an independent assay for apoptosis, we monitored caspase-3 activity in mink cells at various times after infection with MCF13 MLV. Activation of caspase-3 occurs in apoptotic cells by specific cleavage of procaspase-3 by other caspases, such as caspase-8 and caspase-9, which themselves are activated by more upstream events in apoptotic signaling (44, 45). For this assay, we monitored the production of fluoromethylcoumarin fluorescence, which is released from the Z-DEVD-AMC peptide substrate by caspase-3 cleavage. As shown in Fig. 4, we detected greater caspase-3 activity in MCF13 MLV-infected cells than in mock-infected and amphotropic MLV-infected cells. The peak of caspase-3 activity occurred at 4 days p.i., which is slightly before the peak of apoptotic cell number that was detectable by flow cytometry analysis (Fig. 3A). This result is consistent with the observation that caspase-3 activation is a relatively early event in apoptosis and occurs in some cell types before the detection of phosphatidylserine on the outer plasma membrane of the cell (6, 16).
FIG. 4.
Caspase-3 activity is increased in cells infected with MCF13 MLV. Cytosolic extracts were prepared from MCF13 MLV-infected (■), 4070A amphotropic MLV-infected (⧫), or mock-infected (○) cells on different days p.i. Caspase-3 activity was measured by using the EnzChek kit (Molecular Probes), which uses the Z-DEVD-AMC peptide as a substrate. Fluoromethylcoumarin fluorescence was detected with a SpectraMAX Gemini fluorometer (Molecular Devices). Units of caspase-3 activity are expressed as picomoles of substrate hydrolyzed per microgram of protein per half hour.
Detection of unintegrated linear viral DNA in MCF13 MLV-infected cells.
It has been shown that there is a strong correlation between cell killing by a retrovirus and its ability to superinfect cells (22, 29, 46, 47). A consequence of superinfection by these retroviruses is the accumulation predominantly of the linear form of unintegrated viral DNA during the acute phase of infection when cytopathic effects are most prominent (22, 29, 46, 47). We therefore wished to determine whether MCF13 MLV infection also produced high levels of unintegrated linear viral DNA.
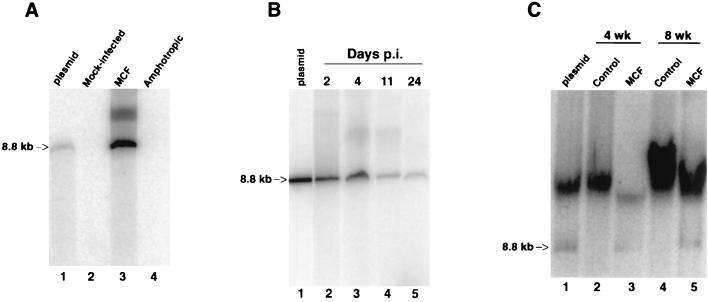
After mink epithelial cells were infected with MCF13 for 6 days, we performed Hirt extractions to isolate unintegrated viral DNA. By Southern blot analysis of Hirt-extracted DNA from cells infected with MCF13 MLV, we detected an intense band of unintegrated viral DNA (Fig. 5A, lane 3) that comigrated with a linear DNA plasmid fragment corresponding to the cloned genomic length of MCF13 MLV (lane 1) (50). The smear also detectable with this probe and migrating above the unintegrated linear viral DNA corresponds to genomic DNA, which often is present in Hirt-extracted DNA preparations and was detectable by ethidium bromide staining of the agarose gel. Hybridization to this high-molecular-weight DNA could be due to the presence of either integrated proviral DNA or unintegrated viral DNA, which can copurify with genomic DNA during Hirt extraction (reference 47 and our personal observation). It does not appear to be due to nonspecific hybridization, since both the Hirt DNA extractions from mock-infected and amphotropic MLV-infected cells contained comparable amounts of genomic DNA as shown by ethidium bromide staining. Cells that were infected with the amphotropic MLV lacked detectable unintegrated viral DNA (lane 4) as did mock-infected cells (lane 2). The hybridization probe used to detect amphotropic MLV DNA consisted of highly conserved gag sequences of MCF13 MLV, which should detect the two viral DNAs equally well. However, to ensure that no amphotropic MLV unintegrated DNA was present, we also hybridized the same filter with an amphotropic-specific env probe and obtained no signal in lane 4 (data not shown). Cleavage of unintegrated viral DNA with EcoRI, which makes a single cut in the MCF13 MLV genome, produced fragments with sizes that were also consistent with a linear DNA molecule (data not shown).
FIG. 5.
MCF13 MLV infection produces high levels of unintegrated linear viral DNA. Southern blot analysis was performed on Hirt-extracted DNA from mink epithelial cells (A and B) and thymic lymphocytes from virus-inoculated mice (C). (A) Hirt-extracted DNA from mink cells that were either mock infected (lane 2) or infected with MCF13 MLV (lane 3) or with 4070A amphotropic MLV (lane 4) after 6 days p.i. Plasmid DNA (2.5 ng) corresponding to the genomic-length MCF13 MLV was electrophoresed in lane 1. DNA was hybridized with a 32P-labeled 1.6-kb DNA fragment corresponding to MCF13 MLV gag sequences. (B) Mink cells were infected with MCF13 MLV at an MOI of 1 for 2 days (lane 2), 4 days (lane 3), 11 days (lane 4), and 24 days (lane 5). Lane 1 contains 10 ng of linear plasmid MCF13 MLV genomic-length DNA. A 32P-labeled probe consisting of 8.2 kb of MCF13 MLV genomic DNA was used for hybridization. (C) Hirt DNA was prepared from thymic lymphocytes which were isolated from thymuses of either uninoculated age-matched control AKR mice (lanes 2 and 4) or MCF13 MLV-inoculated mice (lanes 3 and 5) at 4 and 8 weeks postinoculation of virus. Lane 1 contains 2.5 ng of linear plasmid MCF13 MLV DNA and Hirt DNA from a control mouse. DNA was hybridized with a 32P-labeled probe consisting of 8.2-kb MCF13 MLV DNA. Arrows, 8.8-kb linear MCF13 MLV DNA.
A time course analysis of the appearance of unintegrated viral DNA indicated that synthesis peaked at 2 to 4 days p.i. (Fig. 5B). After that time, we observed a decrease in the amount of unintegrated viral DNA at 11 and 24 days p.i. At 2 days p.i., there were 55 copies of unintegrated viral DNA per cell, and this number decreased to 10 copies per cell at 24 days p.i. Copy numbers of unintegrated viral DNA were derived from a standard curve generated by plotting different amounts of a purified plasmid DNA fragment comprising the complete MCF13 genome against their band intensities measured from the Southern blots using the ImageQuant software (Molecular Dynamics). These values were divided by the number of cells used to obtain the Hirt-extracted DNA to obtain the copy number per cell. These results indicated that large amounts of unintegrated viral DNA are synthesized primarily during the acute phase of virus infection. Thus, similar to what has been observed for other cytopathic retroviruses, MCF13 MLV produces large amounts of unintegrated viral DNA, which is predominantly linear in form, during the acute phase of virus infection of mink cells. We did not detect any covalently closed circular DNA molecules even upon longer exposure of the Southern blots.
To determine whether MCF13 MLV infection of thymic lymphocytes in virus-inoculated mice also produced unintegrated linear viral DNA, we performed Hirt extractions of thymic lymphocytes at 4 and 8 weeks postinoculation. These two time points during the preleukemic period were those at which a previous study had detected enhanced apoptosis of thymic cells isolated from MCF13 MLV-inoculated mice (52). Southern blotting analysis of Hirt DNA extracted from thymic lymphocytes isolated from either MCF13 MLV-inoculated or uninoculated control mice produced the results shown in Fig. 5C. A band corresponding to MCF13 MLV unintegrated DNA (shown by the arrow) was detectable only for virus-inoculated mice. The more slowly migrating thick bands detectable in all lanes corresponded to the positions of high-molecular-weight cellular DNA. Hybridization of cellular DNA with our probe was due to the presence of multiple copies of endogenous MCF MLV-related proviruses in the genome of the AKR mouse (2, 14, 39). Similar to what was seen in mink cells, we observed that the unintegrated viral DNA corresponded to the linear form since it comigrated with the genome-length linear plasmid MCF13 MLV DNA present in lane 1. Hirt-extracted DNA from lymphocytes of control mice was added to the lane containing the plasmid DNA because of the effect that different amounts of DNA have on its electrophoretic mobility. Unintegrated linear viral DNA was detectable as early as 4 weeks postinoculation (lane 3) and increased slightly in amount at 8 weeks after virus inoculation (lane 5).
Inhibition of superinfection resulted in a decrease in cell killing and lower levels of unintegrated viral DNA.
It has been proposed that cell killing and the presence of high levels of unintegrated linear viral DNA are due to the ability of some retroviruses to reinfect the same cell (22, 29, 46, 47). We wished to determine whether a similar mechanism was responsible for the cytopathic effects of MCF13 MLV. To inhibit superinfection of mink cells, we added neutralizing antibody (MAb 83A25) (reference 17 and unpublished data) at 6 h after the addition of virus to cells, which is the time when medium is normally changed after virus infection. The numbers of virus-infected mink cells grown either in the absence or in the presence of different amounts of neutralizing antibody were monitored over time (Fig. 6). We observed that inhibition of superinfection by MCF13 MLV resulted in a decrease in cell killing and that the level of cell killing decreased with increasing amounts of MAb 83A25 in the supernatant.
FIG. 6.
Neutralizing antibody inhibits MCF13 MLV-induced cell killing. Mink cells were infected with MCF13 MLV at an MOI of 7 for 6 h, after which fresh medium containing no (■), 1% (●), 5% (▴), or 10% (⧫) of hybridoma supernatant containing neutralizing MAb 83A25 was added to cells. Mock-infected cells (○) were also included. Cells were trypsinized at different days after infection, and viable cells were enumerated by trypan blue dye exclusion. Values represent the means and standard deviations calculated from two independent experiments.
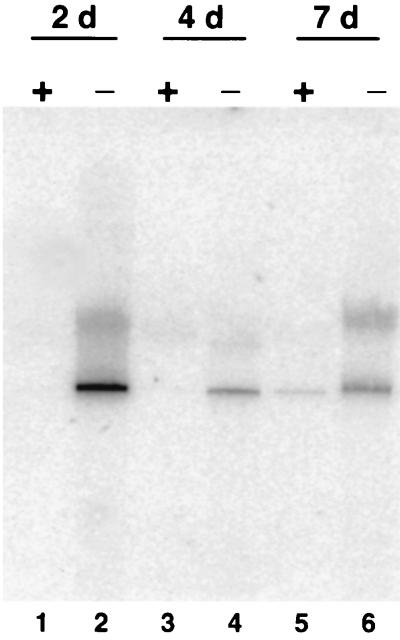
To examine the effect of superinfection inhibition on the synthesis of unintegrated viral DNA, we performed Southern blotting analysis on Hirt-extracted DNA from cells that were similarly treated with neutralizing antibody. Our results showed that unintegrated linear viral DNA was undetectable in virus-infected mink cells grown in the presence of MAb 83A25 at 2 and 4 days p.i. but was present in untreated cells (Fig. 7). At 7 days p.i. we did detect a small amount of unintegrated viral DNA for antibody-treated cells (lane 5), but it was much less than the amount detectable in untreated cells (lane 6). Again, some hybridization was detectable for high-molecular-weight DNA from cells that also had detectable unintegrated viral DNA. Taken together, these results indicated that mink cell killing by MCF13 MLV and the presence of high levels of unintegrated viral DNA were the results of viral superinfection.
FIG. 7.
Neutralizing antibody inhibits the production of MCF13 MLV unintegrated viral DNA. Mink cells were infected with MCF13 MLV at an MOI of 1 and treated with medium with 1% neutralizing antibody or without antibody. Hirt-extracted DNA was prepared from antibody-treated (+) and untreated (−) cells at days 2, 4, and 7 after virus infection.
DISCUSSION
We observed previously that during the preleukemic period of thymic lymphoma development by MCF13 MLV inoculation in AKR mice, thymus cellularity was reduced mainly by lymphocyte depletion of the cortical region (52). The role of MCF13 MLV infection in lymphocyte depletion was supported by our observations that this virus predominantly infected the subpopulation of cells that reside in the thymic cortex and that thymic lymphocyte depletion in animals correlated with enhanced apoptosis of virus-infected lymphocytes in culture. Depletion of cortical cells in the thymus has also been detected in AKR mice undergoing spontaneous thymic lymphoma development, arguing for the relevance of this process to tumorigenesis (30). A strong correlation of thymic cell killing and the development of spontaneous tumors in AKR mice has been noted (27). Although cell killing appears to be a characteristic of other oncogenic retroviruses, such as the ALV of subgroups B and D, the REVs, and Mo MLV (9, 10, 22, 46, 47), it is not understood what role this phenomenon plays in tumor development. Therefore, we have established an in vitro system to better understand the role of apoptosis in thymic lymphoma development.
Mink epithelial cells were first used to isolate MCF MLVs, which produced cytopathic foci of infected cells (19). We therefore used a mink epithelial cell line (CCL64) to further study the apoptotic effects of MCF13 MLV. By different independent assays we demonstrated that the cytopathic effects of this retrovirus were due to cells undergoing apoptosis and were a direct consequence of virus infection. We also showed that similar to other cytopathic retroviruses, MCF13 MLV produces high levels of unintegrated linear DNA during the acute phase of infection and that this appears to be the result of virus superinfection. It is curious that only the linear form of the unintegrated viral DNA is detectable in cells infected by cytopathic retroviruses. Acute infection by retroviruses normally produces unintegrated closed circular DNA molecules as well as linear molecules (13), as has been observed for Mo MLV infection of mouse fibroblasts (53). Whether the explanation for this observation resides with the infected cell type, which may be missing cellular enzymes thought to be responsible for the generation of the closed circles from the linear DNA, or with a peculiarity of the viral DNA remains to be determined.
The ability of MCF13 MLV to superinfect cells was suggested by viral interference studies of the receptor used by both MCF and xenotropic MLVs (26), variously referred to as X-receptor (40), XPR1 (5), and Rmc1 protein (48). This proposal was based on results which showed that coexpression of the MCF glycoprotein and the MCF and xenotropic receptor isolated from mink cells did not cause substantial interference of infection by either a xenotropic MLV or MCF MLV (26). In the reciprocal experiment, expression of the xenotropic glycoprotein produced efficient interference of infection by both MLVs. Thus, as noted in this report, these observations suggest a strong correlation between the ability of a retrovirus to superinfect cells and its oncogenic potential since xenotropic MLVs are not associated with neoplastic diseases (25).
We propose two different mechanisms by which MCF13 MLV can induce apoptosis. A mechanism that involves glycoprotein-receptor interactions is supported by work on the ALV subgroup B glycoprotein, which induces apoptosis upon binding to its cellular receptor CAR1, a protein that belongs to the TNF receptor family (10). The possibility that the MCF and xenotropic receptor may also signal apoptosis is based on its amino acid similarity with a yeast protein that induces cell cycle arrest in a G protein-coupled manner in response to pheromone signaling (5, 37, 40, 48). Thus, this receptor is a good candidate for the signaling of cell cycle arrest, which often leads to apoptosis, by MCF glycoprotein binding. This mechanism, however, does not adequately explain why superinfection is required for cell death. A possible explanation for this requirement is taken into account by a second mechanism, which is based on the strong correlation between cell killing and the presence of high levels of unintegrated linear viral DNA. Other cytopathic retroviruses also produce large amounts of unintegrated linear DNA (22, 29, 46, 47). We, in this report, and other investigators previously have shown that these large amounts of unintegrated linear viral DNA are due to the ability of cytopathic retroviruses to superinfect susceptible cells. We propose that linear molecules of unintegrated viral DNA may resemble damaged DNA with double-strand breaks and thus initiate apoptotic signaling through the intrinsic pathway involving mitochondrial damage (18). Another potential consequence of the synthesis of large amounts of unintegrated viral DNA is increased provirus integration, which also may be lethal to a cell. It is also possible that both of our proposed mechanisms may be relevant to MCF MLV-induced apoptosis, similar to what has been observed for the subgroup B ALV (10, 46, 47).
The role of unintegrated viral DNA in cell killing has been examined for HIV-1, for which it was concluded that cell killing could occur in the absence of large amounts of unintegrated viral DNA synthesis (7, 24). In these studies, however, HIV-1 produced predominantly unintegrated closed circular molecules and hence, differs from other cytopathic retroviruses described above. Therefore, the mechanism of HIV-induced apoptosis may be more dependent upon viral proteins, such as gp120, Vpr, and Tat (4, 8, 20, 38). Further studies of MCF MLV-induced apoptosis are required to understand the role of the unintegrated linear viral DNA and glycoprotein-receptor interactions in this process and the role that apoptosis plays in tumorigenesis.
ACKNOWLEDGMENTS
We are grateful to R. Skoff for use of the Leica DMIRB microscope and camera and to members of the WSU Flow Cytometry Core Facility for processing our samples. We also thank H.-R. Kim for help in doing the caspase-3 assays and the Center for Molecular Medicine and Genetics for use of the phosphorimager. Comments by T. R. Reddy and Chris Roberts on the manuscript were much appreciated.
This work was supported by Public Health Service grant CA44166 to F.K.Y. from the National Institutes of Health.
REFERENCES
- 1.Adamson D C, Dawson T M, Zink M C, Clements J E, Dawson V L. Neurovirulent simian immunodeficiency virus infection induces neuronal, endothelial, and glial apoptosis. Mol Med. 1996;2:417–428. [PMC free article] [PubMed] [Google Scholar]
- 2.Amanuma H, Laigret F, Nishi M, Ikawa Y, Khan A S. Identification of putative endogenous proviral templates for progenitor mink cell focus-forming (MCF) MuLV-related RNAs. Virology. 1988;164:556–561. doi: 10.1016/0042-6822(88)90573-9. [DOI] [PubMed] [Google Scholar]
- 3.Ashkenazi A, Dixit V M. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 4.Bartz S R, Emerman M. Human immunodeficiency virus type 1 Tat induces apoptosis and increases sensitivity to apoptotic signals by up-regulating FLICE/caspase-8. J Virol. 1999;73:1956–1963. doi: 10.1128/jvi.73.3.1956-1963.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Battini J L, Rasko J E, Miller A D. A human cell-surface receptor for xenotropic and polytropic murine leukemia viruses: possible role in G protein-coupled signal transduction. Proc Natl Acad Sci USA. 1999;96:1385–1390. doi: 10.1073/pnas.96.4.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belloc F, Belaud-Rotureau M A, Lavignolle V, Bascans E, Braz-Pereira E, Durrieu F, Lacombe F. Flow cytometry detection of caspase 3 activation in preapoptotic leukemic cells. Cytometry. 2000;40:151–160. doi: 10.1002/(sici)1097-0320(20000601)40:2<151::aid-cyto9>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 7.Bergeron L, Sodroski J. Dissociation of unintegrated viral DNA accumulation from single-cell lysis induced by human immunodeficiency virus type 1. J Virol. 1992;66:5777–5787. doi: 10.1128/jvi.66.10.5777-5787.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biard-Piechaczyk M, Robert-Hebmann V, Richard V, Roland J, Hipskind R A, Devaux C. Caspase-dependent apoptosis of cells expressing the chemokine receptor CXCR4 is induced by cell membrane-associated human immunodeficiency virus type 1 envelope glycoprotein (gp120) Virology. 2000;268:329–344. doi: 10.1006/viro.1999.0151. [DOI] [PubMed] [Google Scholar]
- 9.Bonzon C, Fan H. Moloney murine leukemia virus-induced preleukemic thymic atrophy and enhanced thymocyte apoptosis correlate with disease pathogenicity. J Virol. 1999;73:2434–2441. doi: 10.1128/jvi.73.3.2434-2441.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brojatsch J, Naughton J, Rolls M M, Zingler K, Young J A. CARI, a TNFR-related protein, is a cellular receptor for cytopathic avian leukosis-sarcoma viruses and mediates apoptosis. Cell. 1996;87:845–855. doi: 10.1016/s0092-8674(00)81992-3. [DOI] [PubMed] [Google Scholar]
- 11.Chesebro B, Britt W, Evans L, Wehrly K, Nishio J, Cloyd M. Characterization of monoclonal antibodies reactive with murine leukemia viruses: use in analysis of strains of friend MCF and Friend ecotropic murine leukemia virus. Virology. 1983;127:134–148. doi: 10.1016/0042-6822(83)90378-1. [DOI] [PubMed] [Google Scholar]
- 12.Cloyd M W. Characterization of target cells for MCF viruses in AKR mice. Cell. 1983;32:217–225. doi: 10.1016/0092-8674(83)90512-3. [DOI] [PubMed] [Google Scholar]
- 13.Coffin J M, Hughes S H, Varmus H E. Retroviruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1997. [PubMed] [Google Scholar]
- 14.Coffin J M, Stoye J P, Frankel W N. Genetics of endogenous murine leukemia viruses. Ann N Y Acad Sci. 1989;567:39–49. doi: 10.1111/j.1749-6632.1989.tb16457.x. [DOI] [PubMed] [Google Scholar]
- 15.Donahue P R, Quackenbush S L, Gallo M V, deNoronha C M, Overbaugh J, Hoover E A, Mullins J I. Viral genetic determinants of T-cell killing and immunodeficiency disease induction by the feline leukemia virus FeLV-FAIDS. J Virol. 1991;65:4461–4469. doi: 10.1128/jvi.65.8.4461-4469.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Durrieu F, Belloc F, Lacoste L, Dumain P, Chabrol J, Dachary-Prigent J, Morjani H, Boisseau M R, Reiffers J, Bernard P, Lacombe F. Caspase activation is an early event in anthracycline-induced apoptosis and allows detection of apoptotic cells before they are ingested by phagocytes. Exp Cell Res. 1998;240:165–175. doi: 10.1006/excr.1997.3918. [DOI] [PubMed] [Google Scholar]
- 17.Evans L H, Morrison R P, Malik F G, Portis J, Britt W J. A neutralizable epitope common to the envelope glycoproteins of ecotropic, polytropic, xenotropic, and amphotropic murine leukemia viruses. J Virol. 1990;64:6176–6183. doi: 10.1128/jvi.64.12.6176-6183.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green D R, Reed J C. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 19.Hartley J W, Wolford N K, Old L J, Rowe W P. A new class of murine leukemia virus associated with development of spontaneous lymphomas. Proc Natl Acad Sci USA. 1977;74:789–792. doi: 10.1073/pnas.74.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herbein G, Mahlknecht U, Batliwalla F, Gregersen P, Pappas T, Butler J, O'Brien W A, Verdin E. Apoptosis of CD8+ T cells is mediated by macrophages through interaction of HIV gp120 with chemokine receptor CXCR4. Nature. 1998;395:189–194. doi: 10.1038/26026. [DOI] [PubMed] [Google Scholar]
- 21.Herr W, Gilbert W. Free and integrated recombinant murine leukemia virus DNAs appear in preleukemic thymuses of AKR/J mice. J Virol. 1984;50:155–162. doi: 10.1128/jvi.50.1.155-162.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keshet E, Temin H M. Cell killing by spleen necrosis virus is correlated with a transient accumulation of spleen necrosis virus DNA. J Virol. 1979;31:376–388. doi: 10.1128/jvi.31.2.376-388.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koopman G, Reutelingsperger C P, Kuijten G A, Keehnen R M, Pals S T, van Oers M H. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994;84:1415–1420. [PubMed] [Google Scholar]
- 24.Laurent-Crawford A G, Hovanessian A G. The cytopathic effect of human immunodeficiency virus is independent of high levels of unintegrated viral DNA accumulated in response to superinfection of cells. J Gen Virol. 1993;74:2619–2628. doi: 10.1099/0022-1317-74-12-2619. [DOI] [PubMed] [Google Scholar]
- 25.Levy J A. Xenotropic type C viruses. Curr Top Microbiol Immunol. 1978;79:111–213. doi: 10.1007/978-3-642-66853-1_4. [DOI] [PubMed] [Google Scholar]
- 26.Marin M, Tailor C S, Nouri A, Kozak S L, Kabat D. Polymorphisms of the cell surface receptor control mouse susceptibilities to xenotropic and polytropic leukemia viruses. J Virol. 1999;73:9362–9368. doi: 10.1128/jvi.73.11.9362-9368.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Metcalf D. The thymus. Its role in immune responses, leukemia development and carcinogenesis. Recent Results Cancer Res. 1966;5:1–144. [Google Scholar]
- 28.Meyaard L, Otto S A, Jonker R R, Mijnster M J, Keet R P, Miedema F. Programmed death of T cells in HIV-1 infection. Science. 1992;257:217–219. doi: 10.1126/science.1352911. [DOI] [PubMed] [Google Scholar]
- 29.Mullins J I, Chen C S, Hoover E A. Disease-specific and tissue-specific production of unintegrated feline leukaemia virus variant DNA in feline AIDS. Nature. 1986;319:333–336. doi: 10.1038/319333a0. [DOI] [PubMed] [Google Scholar]
- 30.Nowinski R C, Doyle T. Cellular changes in the thymuses of preleukemic AKR mice: correlation with changes in the expression of murine leukemia viruses. Cell. 1977;12:341–353. doi: 10.1016/0092-8674(77)90110-6. [DOI] [PubMed] [Google Scholar]
- 31.O'Donnell P V, Woller R, Chu A. Stages in development of mink cell focus-inducing (MCF) virus-accelerated leukemia in AKR mice. J Exp Med. 1984;160:914–934. doi: 10.1084/jem.160.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quigley J G, Burns C C, Anderson M M, Lynch E D, Sabo K M, Overbaugh J, Abkowitz J L. Cloning of the cellular receptor for feline leukemia virus subgroup C (FeLV-C), a retrovirus that induces red cell aplasia. Blood. 2000;95:1093–1099. . (Erratum, 96:8.) [PubMed] [Google Scholar]
- 33.Radfar A, Unnikrishnan I, Lee H W, DePinho R A, Rosenberg N. p19(Arf) induces p53-dependent apoptosis during abelson virus-mediated pre-B cell transformation. Proc Natl Acad Sci USA. 1998;95:13194–13199. doi: 10.1073/pnas.95.22.13194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rojko J L, Hartke J R, Cheney C M, Phipps A J, Neil J C. Cytopathic feline leukemia viruses cause apoptosis in hemolymphatic cells. Prog Mol Subcell Biol. 1996;16:13–43. doi: 10.1007/978-3-642-79850-4_2. [DOI] [PubMed] [Google Scholar]
- 35.Saha K, Yuen P H, Wong P K. Murine retrovirus-induced depletion of T cells is mediated through activation-induced death by apoptosis. J Virol. 1994;68:2735–2740. doi: 10.1128/jvi.68.4.2735-2740.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen Y, Shenk T E. Viruses and apoptosis. Curr Opin Genet Dev. 1995;5:105–111. doi: 10.1016/s0959-437x(95)90061-6. [DOI] [PubMed] [Google Scholar]
- 37.Spain B H, Koo D, Ramakrishnan M, Dzudzor B, Colicelli J. Truncated forms of a novel yeast protein suppress the lethality of a G protein alpha subunit deficiency by interacting with the beta subunit. J Biol Chem. 1995;270:25435–25444. doi: 10.1074/jbc.270.43.25435. [DOI] [PubMed] [Google Scholar]
- 38.Stewart S A, Poon B, Song J Y, Chen I S. Human immunodeficiency virus type 1 vpr induces apoptosis through caspase activation. J Virol. 2000;74:3105–3111. doi: 10.1128/jvi.74.7.3105-3111.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stoye J P, Moroni C, Coffin J M. Virological events leading to spontaneous AKR thymomas. J Virol. 1991;65:1273–1285. doi: 10.1128/jvi.65.3.1273-1285.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tailor C S, Nouri A, Lee C G, Kozak C, Kabat D. Cloning and characterization of a cell surface receptor for xenotropic and polytropic murine leukemia viruses. Proc Natl Acad Sci USA. 1999;96:927–932. doi: 10.1073/pnas.96.3.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tailor C S, Willett B J, Kabat D. A putative cell surface receptor for anemia-inducing feline leukemia virus subgroup C is a member of a transporter superfamily. J Virol. 1999;73:6500–6505. doi: 10.1128/jvi.73.8.6500-6505.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teodoro J G, Branton P E. Regulation of apoptosis by viral gene products. J Virol. 1997;71:1739–1746. doi: 10.1128/jvi.71.3.1739-1746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thome K C, Radfar A, Rosenberg N. Mutation of Tp53 contributes to the malignant phenotype of Abelson virus-transformed lymphoid cells. J Virol. 1997;71:8149–8156. doi: 10.1128/jvi.71.11.8149-8156.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thornberry N A, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 45.Villa P, Kaufmann S H, Earnshaw W C. Caspases and caspase inhibitors. Trends Biochem Sci. 1997;22:388–393. doi: 10.1016/s0968-0004(97)01107-9. [DOI] [PubMed] [Google Scholar]
- 46.Weller S K, Joy A E, Temin H M. Correlation between cell killing and massive second-round superinfection by members of some subgroups of avian leukosis virus. J Virol. 1980;33:494–506. doi: 10.1128/jvi.33.1.494-506.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weller S K, Temin H M. Cell killing by avian leukosis viruses. J Virol. 1981;39:713–721. doi: 10.1128/jvi.39.3.713-721.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang Y L, Guo L, Xu S, Holland C A, Kitamura T, Hunter K, Cunningham J M. Receptors for polytropic and xenotropic mouse leukaemia viruses encoded by a single gene at Rmcl. Nat Genet. 1999;21:216–219. doi: 10.1038/6005. [DOI] [PubMed] [Google Scholar]
- 49.Yoshimura F K, Breda M. Lack of AKR ecotropic provirus amplification in AKR leukemic thymuses. J Virol. 1981;39:808–815. doi: 10.1128/jvi.39.3.808-815.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoshimura F K, Davison B, Chaffin K. Murine leukemia virus long terminal repeat sequences can enhance gene activity in a cell-type-specific manner. Mol Cell Biol. 1985;5:2832–2835. doi: 10.1128/mcb.5.10.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoshimura F K, Wang T, Cankovic M. Sequences between the enhancer and promoter in the long terminal repeat affect murine leukemia virus pathogenicity and replication in the thymus. J Virol. 1999;73:4890–4898. doi: 10.1128/jvi.73.6.4890-4898.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoshimura F K, Wang T, Yu F, Kim H R, Turner J R. Mink cell focus-forming murine leukemia virus infection induces apoptosis of thymic lymphocytes. J Virol. 2000;74:8119–8126. doi: 10.1128/jvi.74.17.8119-8126.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoshimura F K, Weinberg R A. Restriction endonuclease cleavage of linear and closed circular murine leukemia viral DNAs: discovery of a smaller circular form. Cell. 1979;16:323–332. doi: 10.1016/0092-8674(79)90009-6. [DOI] [PubMed] [Google Scholar]