Abstract
Infection of quiescent fibroblasts with human cytomegalovirus (HCMV) was found to cause a rapid activation of cellular phosphatidylinositol 3-kinase (PI3-K). Maximum PI3-K activation occurred from 15 to 30 min postinfection. This activation was transient, and by 2 h postinfection (hpi), PI3-K activity had declined to preinfection levels. However, at 4 hpi, a second tier of PI3-K activation was detected, and PI3-K activity remained elevated relative to that of mock-infected cells for the remainder of infection. The cellular kinases Akt and p70S6K and the transcription factor NF-κB were activated in a PI3-K-dependent manner at similar times following HCMV infection. Analysis using UV-irradiated virus indicated that no viral protein synthesis was necessary for the first phase of PI3-K activation, but viral protein expression was required for the second tier of PI3-K activation. Treatment of infected fibroblasts with LY294002, a potent and specific inhibitor of PI3-K kinase activity, caused a 4-log decrease in viral titers. LY294002 did not inhibit viral entry, but it did decrease viral immediate-early gene expression. In addition, the protein levels of two viral early genes required for DNA replication, UL84 and UL44, were significantly lower in the presence of LY294002. Furthermore, viral DNA replication was strongly inhibited by LY294002 treatment. This inhibition of viral DNA replication could be reversed by adding back the products of PI3-K activity (PI-3,4-P2 and PI-3,4,5-P3), demonstrating that the effect of LY294002 on the viral life cycle was specifically due to the inhibition of PI3-K activity. These results are the first to suggest that PI3-K mediates HCMV-induced activation of host cell mitogenic pathways. They also provide strong evidence that PI3-K activation is important for initiation of viral DNA replication and completion of the viral lytic life cycle.
Human cytomegalovirus (HCMV) is a widespread human pathogen that does not cause significant clinical manifestations in healthy individuals (29, 32, 50). On the other hand, it causes severe diseases in immunocompromised individuals that, if left untreated, can be fatal. In addition, it is a leading cause of certain types of birth defects (29, 32, 50). Individuals suffering from diseases caused by HCMV are currently treated with chemical compounds, such as ganciclovir and phosphocarnet, which block the viral lytic life cycle by inhibiting viral DNA replication (48, 51, 66). However, the substantial toxicity of these drugs and the emergence of drug-resistant strains of HCMV indicate that better antiviral compounds are needed (5, 66, 69). Recently, we have begun to identify and characterize signal transduction pathways that are activated following HCMV infection of human fibroblasts. By studying these pathways, we hope not only to better understand HCMV pathogenesis at the molecular level but also to eventually identify unique, virus-specific targets which can be utilized for the development of potent anti-HCMV compounds (33, 34).
Like all herpesviruses, the lytic life cycle of HCMV is a temporally regulated cascade of events which is initiated when the virus binds to host cell receptors (50). Following viral entry and translocation of the viral DNA to the nucleus, viral immediate-early (IE) genes are expressed. Next, early (E) gene expression occurs, followed by viral DNA replication. After initiation of viral DNA replication, late (L) genes are expressed. The viral DNA is then encapsidated and infectious virus is released from the cell, completing the life cycle.
One hallmark of HCMV infection of quiescent cells is the up-regulation of many host cell proteins, including DNA replication enzymes and transcription factors, which are necessary for both viral gene expression and viral DNA replication (2, 8, 21, 30, 32, 84). Recent studies suggest that host cell kinases must also be activated before viral DNA replication can begin (12, 34). For example, the cyclin-dependent kinase 2 (CDK2) and mitogen-activated protein kinases (MAPK) p38 and ERK1/2 are all activated following HCMV infection of quiescent fibroblasts, and inhibiting the kinase activity of any of these proteins significantly inhibits viral DNA replication (12, 14, 15, 33, 34, 35).
Phosphatidylinositol 3-kinases (PI3-K) are a cellular family of heterodimeric enzymes that consist of a regulatory subunit (p85) and a catalytic subunit (p110) (16, 28, 67, 70). When activated by phosphorylation on specific, conserved tyrosine residues, the p85 subunit recruits substrates to the dimer, where they are phosphorylated by the p110 catalytic subunit (23, 54, 70). PI3-K is activated by many different mitogenic signals, such as epidermal growth factor (70). Upon activation, PI3-K phosphorylates inositol phospholipids at the D-3 position of the inositol ring (46, 73). Once phosphorylated at the D-3 position, these lipids serve as second messengers and are able to regulate phosphorylation of a number of kinases, including Akt (also known as protein kinase B [PKB]), cyclic AMP-dependent kinase (PKA), some isoforms of PKC, and the ribosomal S6 kinases p70 and p85 (p70S6K and p85S6K, respectively) (23, 73, 75, 76). Because PI3-K controls the activation of so many different pathways, it is a critical mediator of many different cellular processes, including cell growth, protection from different types of apoptosis, cell migration, and changes in cell morphology (1, 38–40, 44, 49).
The ability of PI3-K to regulate multiple mitogenic pathways, coupled with the need for HCMV to induce an environment favorable for viral DNA synthesis, prompted us to examine PI3-K signaling during HCMV infection. In this study, we show that PI3-K is strongly activated immediately following infection of quiescent fibroblasts with HCMV (15 to 30 min postinfection). A second tier of PI3-K activation was detected beginning at 4 h postinfection (hpi) and continuing throughout the course of infection. PI3-K activity was found to be required for activation of Akt, p70S6K, and the transcription factor NF-κB in HCMV-infected fibroblasts. In addition, inhibition of PI3-K activity dramatically decreased viral titers, at least partly by inhibiting viral DNA replication. Finally, inhibition of PI3-K activity decreases the protein levels of viral IE and E genes required for DNA replication. Collectively, this study demonstrates that PI3-K activity not only mediates virus-induced signaling following infection but also that this activity is essential for completion of the HCMV lytic life cycle.
MATERIALS AND METHODS
Chemical compounds.
The p38 MAPK kinase inhibitor SB202190 [4-(4-fluorophenyl)-2-(4-hydroxyphenyl)-5-(4-pyridyl)1H-imidazole] (45), rapamycin, an inhibitor of p70S6K phosphorylation (63, 71), phosphatidylinositol-3,4-bisphosphate (PI-3,4-P2 or PtDns 3,4), and phosphatidylinositol-3,4,5-triphosphate (PI-3,4,5-P3 or PtDns 3,4,5) were from Calbiochem (La Jolla, Calif.). Ganciclovir (1,3-dihydroxy-2-propoxymethylguanine [DHPG]), a nucleotide analog that specifically inhibits HCMV viral DNA replication (48), was from Syntex Inc. (Palo Alto, Calif.). Bisindolylmaleimide I, a specific inhibitor of PKC (74), verapamil, an inhibitor of calcium flux (4), and the PI3-K kinase inhibitors LY294002 [20(4-morphodinyl)-8-phenyl-1(4H)-benzopyran-4-one] (40, 80) and wortmannin (55, 76) were from Sigma (St. Louis, Mo.). DHPG was dissolved in water, and verapamil was dissolved in ethanol. All other compounds were dissolved in dimethyl sulfoxide. Unless otherwise indicated, the chemical compounds were used at the following final concentrations: SB202190 (10 μM), rapamycin (10 nM), DHPG (20 μM), LY294002 (20 μM), bisindolylmaleimide I (200 nM), verapamil (50 μg/ml), and wortmannin (100 nM). PI-3,4-P2 and PI-3,4,5-P3 were each used at a final concentration of 10 μM.
Cell culture and viral infection.
Human embryonic lung (HEL) fibroblasts were cultured as previously described (33). All experiments were done using HEL fibroblasts that were between passages 15 and 21. HCMV (Towne strain, passages 39 to 42) was also propagated as previously described (31). For infection, cells were grown to confluence and then were serum starved for 48 h in minimal essential media (MEM) plus antibiotics. Cells were infected with HCMV that had been purified through a sucrose cushion to eliminate cytokines and growth factor contamination. Unless otherwise indicated, a multiplicity of infection of 5 PFU per cell was used. Where indicated, virus was UV irradiated as previously described to prevent viral protein synthesis following infection (10, 84). At the indicated time after infection, cells were washed once and then were maintained in MEM plus antibiotics until they were harvested. If the infection was performed in the presence of chemical compounds, cells were pretreated for 1 h with the compound prior to infection. In addition, the compound was present during infection and subsequent incubation periods. Mock-infected samples were treated and harvested in the same manner as the infected samples except that MEM without virus was used during the infection. For all experiments, the time at which virus was first added to the cells is the zero hour.
Immunoprecipitations.
Fibroblasts were harvested in lysis buffer (150 mM NaCl, 20 mM Tris-HCl [pH 7.5], 1.0% Triton X-100, 0.5 mM EDTA, 50 mM NaF, 10% glycerol, 20 μg of leupeptin/ml, 20 μg of phenylmethylsulfonyl fluoride [PMSF]/ml, and 1 mM sodium vanadate) and incubated on ice for 10 min with occasional vortexing. Cells were then frozen and stored at −70°C until the time course was completed. Cells were then thawed, and debris was removed by centrifugation (15,000 × g for 10 min at 4°C). Protein concentration was determined using the Bio-Rad Protein Assay according to the manufacturer's protocol. Five hundred micrograms of whole-cell extract (total volume of 500 μl) was mixed with phosphotyrosine monoclonal PY20 antibody (1:50 dilution) and rocked overnight at 4°C. Twenty milliliters of protein G-Sepharose was then added, and rocking was allowed to continue for another 90 min. Beads were then washed four times with lysis buffer. Twenty microliters of 2× sodium dodecyl sulfate (SDS)-lysis buffer was added to the beads and boiled for 3 min. The insoluble material was removed by centrifugation in a microcentrifuge, and the supernatant was then subjected to Western blot analysis as described below.
Western blot analysis.
Phosphotyrosine antibody (PY20) was from Santa Cruz (Santa Cruz, Calif.). All other phosphospecific antibodies were from New England Bio-Labs (Beverly, Mass.). The polyclonal PI3-K antibody was from Upstate Biotechnology Inc. (Lake Placid, N.Y.). The UL44 monoclonal antibody was from Fitzgerald Industries International (Concord, Mass.). Monoclonal antibodies to the protein products of the IE1-72, IE2-86, UL84, and UL94 viral genes were prepared in our laboratory and have been described (27, 43, 81). Western blot analysis was performed as previously described. Briefly, confluent cells were infected as described above. At the indicated times, cells were harvested in 2× Laemmli SDS sample buffer, boiled, and loaded onto SDS-polyacrylamide gels. Proteins were separated by electrophoresis and transferred overnight at 14 V to Immobilon-P Transfer Membrane (Millipore, Bedford, Mass.). Blots were blocked for 30 min in 10% (wt/vol) Carnation nonfat dry milk dissolved in phosphate-buffered saline (PBS) plus 0.1% Tween 20 (PBST). Blots were then probed with primary antibody for 2 h at room temperature or overnight at 4°C in PBST. Blots were washed three times with PBST. After washing, the blots were probed with secondary antibody (horseradish peroxidase-conjugated anti-mouse or anti-rabbit immunoglobulin G [Sigma and New England Bio-Labs, respectively]) for 1 h at room temperature. Blots were washed three times in PBST and then developed by enhanced chemiluminescence according to the manufacturer's protocol (New England Bio-Labs).
Nuclear extract isolation.
The nuclear extracts were prepared as previously described (84, 85). Briefly, mock-infected or infected HEL fibroblasts were washed in cold PBS, harvested using a rubber cell scraper, and centrifuged to collect the cell pellet. The cell pellets were then incubated for 5 min on ice with a cytoplasmic isolation buffer (10 mM HEPES [pH 7.6], 60 mM KCl, 1 mM EDTA, 0.1% NP-40, 1 mM dithiothreitol, 1 mM PMSF [Sigma], 2 mM phenanthroline [Sigma], 0.25 mM dichloroisocoumarin [Sigma], 100 μM E-64 [Sigma], and 10 μM pepstatin A [Sigma]). The samples were centrifuged, and the nuclear pellets were collected by removing the supernatant containing the cytoplasmic extract. The nuclear pellets were then washed in cytoplasmic isolation buffer without NP-40, centrifuged, and incubated for 10 min on ice with a nuclear isolation buffer (20 mM Tris-HCl [pH 8.0], 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM PMSF, 25% glycerol, 2 mM phenanthroline [Sigma], 0.25 mM dichloroisocoumarin, 100 μM E-64, and 10 μM pepstatin A). Following centrifugation, the supernatant was collected and stored in aliquots at −70°C.
EMSAs.
The electrophoretic mobility shift assays (EMSAs) were performed as previously described (84, 85). Briefly, collected nuclear extracts were incubated for 15 min in a binding buffer (10 mM Tris-HCl [pH 7.9], 50 mM NaCl, 0.5 mM EDTA, 10% glycerol, 1 mM dithiothreitol), 7.5 mM MgCl2, 0.1 μg poly(dIdC), and a 32P-labeled wild-type major histocompatibility complex κB binding site (5′-CCTTTTTTTTTGGGGATTCCCCA-3′) or a mutant κB binding site (5′-CCTTTTTTTTTGCGGCTTCCCGA-3′) double-stranded oligonucleotide probe for experiments examining NF-κB activity (mutated nucleotides are italicized). The annealed double-stranded oligonucleotide probes with T overhangs and C ends were labeled by filling in the recessed 3′ ends of the oligonucleotide with [α-32P]dATP (ICN, Irvine, Calif.) using Klenow enzyme (Boehringer Mannheim, Indianapolis, Ind.), followed by a chase with cold dATP and dGTP, and then were finally G-25 Sephadex (Boehringer Mannheim) column purified. The samples were electrophoresed on a 5% polyacrylamide gel, dried, and developed with intensifier screens at −70°C. Antibodies were used to supershift the specific complexes of interest by pretreating the extracts for 30 min to 1 h at 4°C with 1 μg of antibody prior to their addition to the binding buffer, MgCl2, dIdC, and labeled probes. Specific antibodies to p50 and p65 of NF-κB (a generous gift from Albert S. Baldwin, Jr. [18, 65]) were used in the supershift experiments.
Titer reduction assay.
Titer reduction assays were performed as previously described (34). Briefly, confluent HEL fibroblasts were infected as described above in the presence of the indicated concentration of inhibitor compound. To maintain a stable concentration of inhibitor, fresh media containing appropriate concentrations of inhibitor were added every 48 h. At day 6 postinfection, the supernatant was harvested and used to perform an HCMV standard plaque assay in the 24-well plate using 1% methyl cellulose overlayer containing 1× MEM and 4% fetal bovine serum. HCMV plaque numbers were scored under an inverted microscope.
Dot blot analysis.
Dot blot analysis was performed as previously described (34). Briefly, cells were grown to confluence, serum starved, and infected with HCMV at a multiplicity of infection of 5 PFU per cell. Cells were harvested at the indicated times postinfection, and dot blot hybridization was performed using 32P-radiolabeled, purified, genomic HCMV DNA as a probe (34). After washing, the radioactivities on the membranes were detected by autoradiography using Kodak X-ray films. The intensity of radioactive exposure is correlated to the amount of viral DNA on the membrane.
RESULTS
Effect of HCMV infection on PI3-K activity.
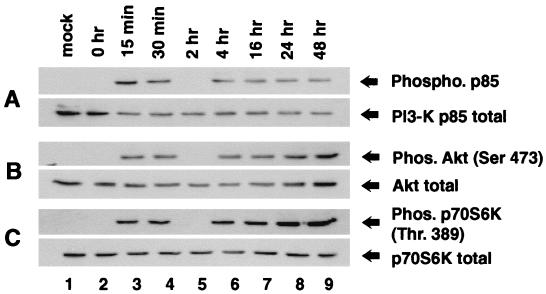
The first step in this study was to determine the effect of HCMV infection on PI3-K activity by examining phosphorylation of the p85 subunit of PI3-K, which correlates with PI3-K kinase activity in vivo (23, 54, 70). Confluent, serum-starved HEL fibroblasts were infected with HCMV and harvested at the indicated times. p85 phosphorylation was determined by immunoprecipitation using a phosphotyrosine antibody (PY20; Santa Cruz), followed by Western blot analysis using an antibody specific for p85. Figure 1A demonstrates that between 15 and 30 min after the addition of HCMV, p85 phosphorylation increased dramatically (lanes 3 and 4). This activation was transient, and by 2 hpi, p85 phosphorylation declined to a level close to that observed in 0-h cells and in mock-infected cells (lanes 2 and 5). However, beginning at 4 hpi, a second tier of PI3-K phosphorylation was observed, and this increase was sustained for the remainder of the infection (lanes 6 to 9). Further Western blot analysis showed that the overall level of p85 remained stable throughout infection, indicating that the changes in phosphorylation were not due to changes in the overall level of p85 (Fig. 1A, bottom).
FIG. 1.
Activation of PI3-K, Akt, and p70S6K following HCMV infection. HEL fibroblasts were grown to confluence, serum starved for 48 h, infected with HCMV, and harvested at the indicated times postinfection. (A) Active p85 was immunoprecipitated from equal amounts of infected whole-cell lysate (500 μg) by using a phosphotyrosine antibody, PY20 (Santa Cruz). The amount of immunoprecipitated protein was determined by Western blot analysis using a primary antibody that recognizes total p85 (Upstate Biotechnology). Western blot analysis of whole-cell extracts using a p85 antibody showed that the overall level of p85 does not fluctuate during infection (bottom). (B) Western blot analysis of whole-cell extracts probed with either phosphospecific (to demonstrate Akt activation) or nonphosphospecific (to demonstrate that overall levels of Akt are equal) Akt antibodies, α-phosphoAkt (Ser 473 [top]) and α-Akt (bottom), respectively. (C) Mock-infected and HCMV-infected whole-cell extracts were studied for p70S6K activation by Western blot analysis using either a phosphospecific p70S6K antibody which recognized only the active form of p70S6K (top) or an antibody which recognized all forms of p70S6K (bottom). Each experiment was performed a minimum of five times, and representative results are shown. Ser 473 and Thr 389 are the phosphorylated residues which are recognized by the phosphospecific antibodies. Mock, mock-infected HEL fibroblasts; Phos, phosphorylated.
Activation of cellular Akt and p70S6 kinases by PI3-K.
To assess whether PI3-K was involved in cellular signaling during HCMV infection, we examined activation of the cellular kinases Akt and p70S6K, both of which can be activated in a PI3-K-dependent manner. First, the effect of HCMV infection on Akt activity was examined by Western blot analysis using an Akt phosphospecific antibody (no. 9275; New England Bio-Labs). This antibody recognizes only Akt that is phosphorylated on Ser 473, which has been shown to correlate extremely well with Akt kinase activity and provides a convenient, reliable method to analyze AKT activity (3, 7). Western blot analysis indicated that Akt was activated in a two-tiered manner following HCMV infection (Fig. 1B). In addition, the time course of Akt activation mirrored that of PI3-K (compare Fig. 1A and B). The amount of total Akt protein remained constant throughout infection, indicating that the changes in Akt phosphorylation were not due to changes in protein levels (Fig. 1B, bottom).
Next, the activation of p70S6K in HCMV-infected fibroblasts was examined. Again, Western blot analysis was performed using a phosphospecific antibody that recognizes p70S6K only if it is phosphorylated on Thr 389. This phosphorylation is dependent upon PI3-K activity and is indicative of p70S6K activity (53, 57, 72). Figure 1C demonstrates that, as with Akt, p70S6K activation following HCMV infection was two-tiered and mirrored PI3-K activation.
The first tier of activation of cellular kinases does not require viral protein synthesis.
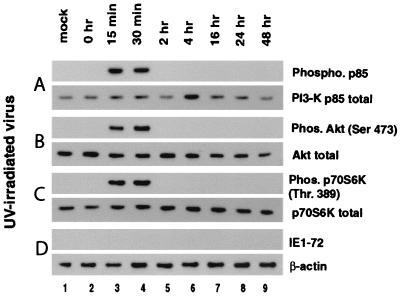
To further characterize HCMV-mediated activation of PI3-K, p70S6K, and Akt, fibroblasts were infected with virus stock that was UV irradiated prior to infection. UV irradiation creates thymidine dimers, which prevent transcription of viral genes without inhibiting the ability of the virus to bind to and enter the host cell. Interestingly, following infection of fibroblasts with UV-irradiated virus, activation of PI3-K, Akt, and p70S6K was detected at 15 to 30 min postinfection (Fig. 2A to C, lanes 1 to 4). However, by 2 hpi, the activity of all three kinases had declined to preinfection levels and no further activation of these kinases was detected (lanes 5 to 9). These data indicate that while viral protein expression is not required for the first tier of activation (15 to 30 min postinfection), it is necessary for the second tier of activation (beginning at 4 hpi).
FIG. 2.
Activation of PI3-K, Akt, and p70S6K following infection with UV-irradiated virus. HEL fibroblasts were grown to confluence, serum starved for 48 h, infected with HCMV, and harvested at the indicated times postinfection. HEL fibroblasts were infected with the HCMV Towne strain, which was UV irradiated prior to infection to prevent viral gene expression. Cells were harvested and analyzed for phosphorylated (top) and total (bottom) PI3-K (A), Akt (B), and p70S6K (C) activations as described for Fig. 1. (D) Western blot analysis was also performed, using an antibody to the HCMV IE1-72 protein to demonstrate that viral proteins were not being expressed following infection with UV-irradiated virus. Representative results from four separate experiments are shown. Ser 473 and Thr 389 are the phosphorylated residues which are recognized by the phosphospecific antibodies. Mock, mock-infected HEL fibroblasts; Phos, phosphorylated.
Activation of p70S6K and Akt is dependent on PI3-K kinase activity.
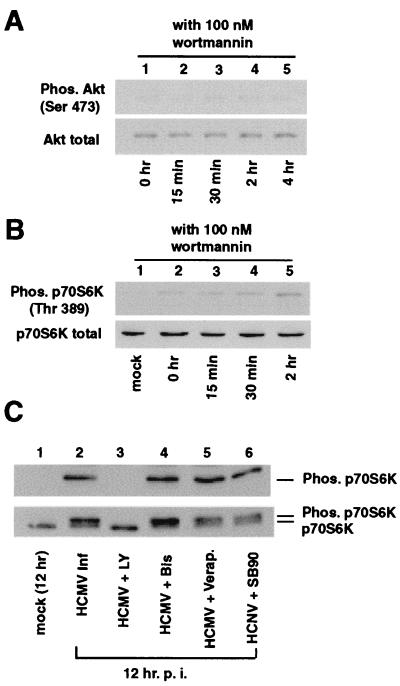
The chemical compound wortmannin is a potent, specific, cell-permeable inhibitor of PI3-K activity (55, 76). To determine if HCMV-mediated activation of Akt and p70S6K was dependent upon PI3-K activity, cells were treated with wortmannin during infection. Figure 3A and B demonstrate that in the presence of wortmannin, no Akt or p70S6K activation was detected at early times of infection. This indicates that PI3-K activity is required for HCMV-mediated activation of Akt and p70S6K.
FIG. 3.
Inhibition of p70S6K and Akt activation by PI3-K inhibitors. HEL fibroblasts were grown to confluence, serum starved for 48 h, infected with HCMV, and harvested at the indicated times postinfection. Shown are inhibitions of Akt (A) and p70S6K (B) activation at early times of HCMV infection by wortmannin. Fibroblasts were pretreated and treated with 100 nM wortmannin prior to and during infection. Cells were harvested and whole-cell extract was probed for activated and total Akt and p70S6K as described for Fig. 1. (C) LY294002 inhibits HCMV-mediated p70S6K activation. HEL fibroblasts were pretreated with inhibitors (LY294002 [LY], bisindolylmaleimide I [Bis], verapamil, and SB202190 [SB90]), infected, and harvested at 12 hpi. p70S6K activation was determined by Western blot analysis (top). Note that only the PI3-K inhibitor LY294002 inhibited p70S6K phosphorylation. The extracts were then probed for total p70S6K to show that the overall levels of p70S6K were not altered by viral infection. Due to the percentage of polyacrylamide in the gel that was used, it is possible to distinguish the unphosphorylated form of p70S6K (lower bands) from the phosphorylated forms (upper bands) in the lower panel. Each experiment was performed a minimum of three times, and representative results are shown. Ser 473 and Thr 389 are the phosphorylated residues which are recognized by the phosphospecific antibodies. Mock, mock-infected cells; Inf, infection.
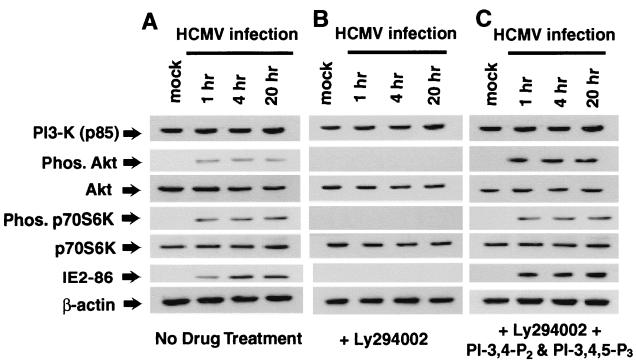
To determine if PI3-K activity was necessary for this second tier of Akt and p70S6K activation, infected fibroblasts were treated with the chemical compound LY294002. Like wortmannin, LY294002 is a potent and specific inhibitor of PI3-K activity, but it is much more stable at 37°C than wortmannin and is therefore better suited for studies that require inhibiting PI3-K activity for an extended time. As Fig. 4A and B illustrate, treatment of infected cells with LY294002 completely inhibits virus-mediated p70S6K and Akt activation. LY294002 has been found to be an extremely potent and specific inhibitor of PI3-K activity (40, 80). However, as with any inhibitor compound, the possibility of nonspecific inhibition of cellular or viral enzymes remains. The two main products of active PI3-K are PI-3,4-P2 and PI-3,4,5-P3. We reasoned that if the effect of LY294002 on HCMV-mediated Akt and p70S6K activation was due to inhibition of PI3-K activity, adding PI-3,4-P2 and PI-3,4,5-P3 to infected fibroblasts treated with LY294002 should permit activation of Akt and p70S6K. This possibility was tested experimentally. As can be seen in Fig. 4B and C, infected cells treated with PI-3,4-P2, PI-3,4,5-P3, and LY294002 had a high level of Akt and p70S6K activation, indicating that the effect of LY294002 on these kinases is mediated by inhibition of PI3-K activity.
FIG. 4.
Both tiers of HCMV-mediated activation of p70S6K and Akt are dependent upon PI3-K kinase activity. HEL fibroblasts were grown to confluence, serum starved for 48 h, infected with HCMV, and harvested at the indicated times postinfection. HEL fibroblasts were infected with only HCMV (A), infected in the presence of the PI3-K inhibitor LY294002 (20 μM) (B), or infected in the presence of LY294002 (20 μM) and the products of activated PI3-K, PI-3,4-P2, and PI-3,4,5-P3 (each at 10 μM) (C). Cells were harvested and were analyzed for Akt and p70S6K activation as shown in Fig. 1. Representative results from three independent experiments are shown. Phos, phosphorylated.
Regulation of p70S6K is very complex and requires kinases in addition to PI3-K. To better characterize p70S6K activation following HCMV infection, cells were infected in the presence of compounds that have been shown to inhibit different p70S6K activation pathways in other systems. Figure 3C illustrates that while LY294002 inhibited p70S6K activation (lane 3), none of the other inhibitors tested—SB202190 (p38 MAPK inhibitor), bisindolymaleimide I (PKC inhibitor), or verapamil (calcium flux inhibitor)—affected p70S6K activation (Fig. 3C, lanes 4, 5, and 6). Thus, these pathways are likely not involved in HCMV-mediated activation of p70S6K.
Effect of PI3-K inhibitors on HCMV-mediated NF-κB activation.
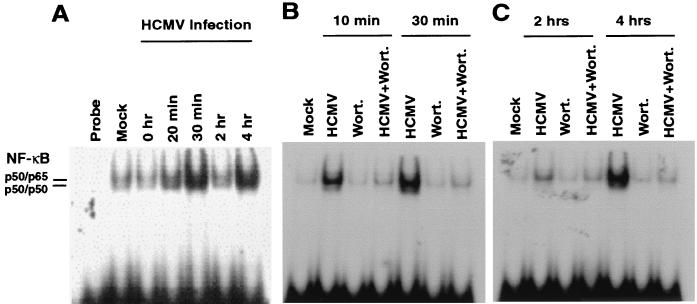
There have been several reports that the transcription factor NF-κB can be activated in a PI3-K-dependent manner (9, 37, 47, 58, 62). Since NF-κB is activated during HCMV infection and this activation is thought to be an important signaling event during HCMV infection, the effect of wortmannin and LY294002 on HCMV-mediated NF-κB activation was examined (42, 64, 85). Quiescent fibroblasts were infected in the presence or absence of PI3-K inhibitors. At the indicated times, cells were harvested, nuclear extracts were isolated, and NF-κB activation was assessed by EMSA analysis. As has been previously reported, HCMV induced a two-tiered activation of NF-κB (Fig. 5A) (84, 85). Interestingly, as with p70S6K and Akt, inhibition of PI3-K activity by either wortmannin or LY294002 completely inhibited both the first tier and the second tier of NF-κB activation (Fig. 5B and C and data not shown). This series of experiments suggest that PI3-K is a major mediator of HCMV-induced signaling during infection.
FIG. 5.
Inhibition of HCMV-mediated NF-κB activation by wortmannin. HEL fibroblasts were grown to confluence, serum starved for 48 h, infected with HCMV, and harvested at the indicated times postinfection. Fibroblasts were infected in the presence or absence of wortmannin (100 nM), and nuclear extracts were harvested at the indicated times as described in Materials and Methods. NF-κB activation was measured by EMSA. Note that wortmannin inhibits both the first tier (10 to 30 min postinfection) and the second tier (4 hpi) of NF-κB activation. Locations of p50-p50 and p50-p65 homodimers and heterodimers were confirmed by supershift analysis using specific antibodies (data not shown). Each experiment was performed three times, and representative results are shown.
Effect of LY294002 on HCMV lytic life cycle.
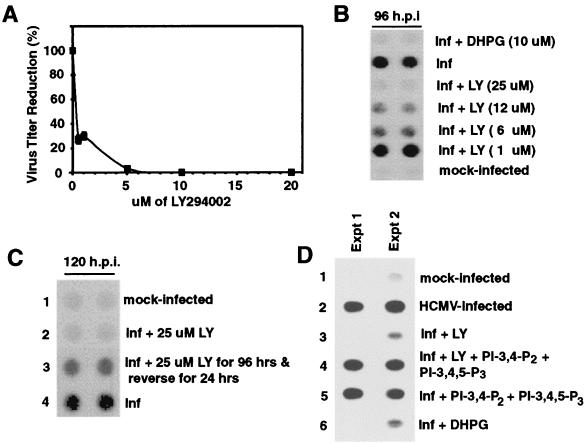
To determine if PI3-K signaling was important for completion of the viral life cycle, a titer reduction assay was performed in the presence of LY294002. Figure 6A illustrates the effect of different concentrations of LY294002 on viral titers at day 6 postinfection. A significant decrease in viral titers was observed with concentrations of inhibitor as low as 1 μM. At a concentration of 5 μM, viral titers were decreased by more than 95% compared to those of control titers, and at 20 μM, viral titers were decreased by greater than 4 logs. Next, viral DNA replication in the presence of LY294002 was assessed by dot blot analysis. Figure 6B demonstrates that treatment with LY294002 significantly inhibited viral DNA replication.
FIG. 6.
Antiviral effects of LY294002 on HCMV lytic life cycle. HEL fibroblasts were grown to confluence, serum starved for 48 h, infected with HCMV, and harvested at the indicated times postinfection. (A) Viral titers are reduced in the presence of LY294002. Confluent, serum-starved fibroblasts were pretreated with LY294002 and then were infected with HCMV in the presence of LY294002. Six days after infection, supernatant was harvested and a standard HCMV plaque assay was performed to determine the infectious virus titer. The graph shows the relative number of plaques in each sample, where 100% is the infectivity of supernatant harvested from cells infected in the absence of LY294002. Each sample was done in triplicate, and the entire assay was performed three times. Error bars represent the standard deviation for the experiment. (B to D) Effect of LY294002 on viral DNA replication. Confluent, serum-starved fibroblasts were infected with HCMV in the presence of the indicated chemical compounds. Where indicated, LY294002 was removed from the culture media at 96 hpi, and the infection was allowed to continue for an additional 24 h. Cells were harvested at 96 hpi (B and D) or 120 hpi (C) and were analyzed for total viral DNA by dot blot hybridization. DHPG was used as a positive control. PI-3,4-P2 and PI-3,4,5-P3 are the lipid products of active PI3-K and were used to demonstrate that the inhibition of viral DNA replication by LY294002 is due to inhibition of PI3-K activity. (A to C) Each experiment was performed a minimum of three times, and representative results are shown. (D) Results from two separate experiments are shown. Dot blot analysis was viewed on X-ray film. DHPG, ganciclovir; inf, infection; mock, mock-infected cells.
PI3-K regulates many important cellular processes, and in certain cell types, inhibition of PI3-K activity causes cell death (68, 82). While infected fibroblasts treated with LY294002 had altered morphology, no apoptosis was detected by propidium iodide staining and flow cytometry analysis (data not shown). To demonstrate that these cells were capable of supporting viral DNA replication, fibroblasts were infected in the presence of LY294002 for 96 h, after which the inhibitor was removed and the infection was allowed to proceed for an additional 24 h. Cells were then harvested and analyzed for viral DNA replication by dot blot analysis. Figure 6C shows that after LY294002 was removed, high levels of viral replication were detected. In addition to demonstrating that LY294002-treated fibroblasts can still support viral DNA replication, this also indicates that the effect of inhibiting PI3-K kinase activity on viral DNA replication is reversible.
Again, PI-3,4-P2 and PI-3,4,5-P3 were utilized to demonstrate the specificity of LY294002. As can be seen in Fig. 6D, while LY294002 treatment inhibited viral DNA replication (lane 3), treatment with PI-3,4-P2, PI-3,4,5-P3, and LY294002 yielded high levels of viral DNA replication which were comparable to those of control infections (lane 4). This indicates that the effect of LY294002 on viral DNA replication is indeed due to inhibition of PI3-K kinase activity.
Effect of rapamycin on viral life cycle.
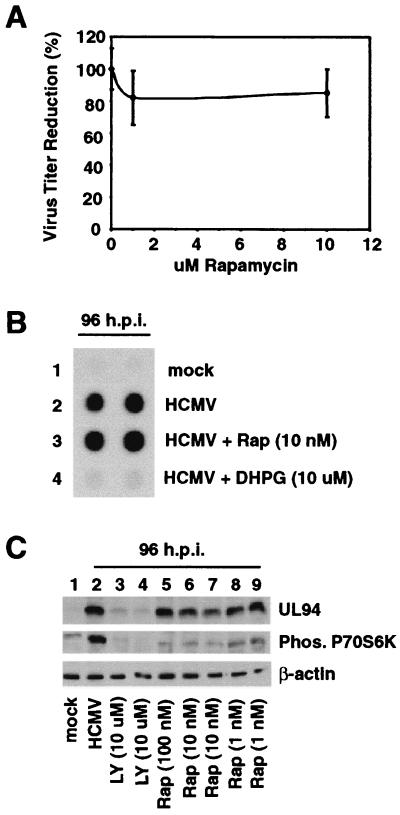
p70S6K regulates translation of mRNAs by phosphorylating, and hence activating, the ribosomal S6K subunit (56, 57). We hypothesized that the function of PI3-K during HCMV infection is to ensure activation of p70S6K, which in turn would ensure translation of various viral and cellular mRNAs necessary for completion of the viral life cycle. To test this hypothesis, the small molecule rapamycin, which inhibits p70S6K activation or phosphorylation without affecting PI3-K activity, was utilized in a titer reduction assay (13, 17, 20, 26, 71). As can be seen in Fig. 7A, rapamycin had no significant effect on viral titers. Furthermore, no effect on viral DNA replication was observed with rapamycin (Fig. 7B). Viral L gene expression does not occur until after viral DNA replication. To provide further evidence that viral DNA replication was not inhibited by rapamycin treatment, cells were infected for 96 h in the presence of rapamycin, harvested, and assayed for expression of the true L gene UL94 by Western blot analysis. Figure 7C demonstrates that, even with 100 nM rapamycin, no significant decrease in UL94 expression was observed (top). Further Western blot analysis shows that 10 nM rapamycin still inhibited HCMV-mediated p70S6K activation, indicating that the results obtained in Fig. 7A and B were not due to a loss of rapamycin activity (Fig. 7C, middle, compare lanes 2 with lanes 5 to 7). These data suggest that under these experimental conditions, HCMV-mediated p70S6K activation is not required for HCMV to complete its viral life cycle in a timely manner. Also, the antiviral effects of LY294002 are not due to inhibition of p70S6K activation.
FIG. 7.
Rapamycin does not affect viral titers, viral DNA replication, or viral late gene expression. HEL fibroblasts were grown to confluence, serum starved for 48 h, infected with HCMV, and harvested at the indicated times postinfection. Fibroblasts were infected in the presence of the indicated concentrations of rapamycin, harvested, and assayed for titer reduction (A) or dot blot analysis (B) as described for Fig. 6. Dot blot analysis was viewed by X-ray film. (C) Rapamycin inhibits HCMV-mediated p70S6K activation but does not inhibit UL94 expression. Cells were infected in the presence or absence of rapamycin or LY294002 (positive control). At 96 hpi, cells were harvested and Western blot analysis was performed using a monoclonal antibody to the viral true late protein UL94 (top). Western blot analysis for p70S6K activation was performed on the same lysate described for Fig. 1. Western blot analysis of β-actin levels demonstrated equal protein loading between samples. Phos, phosphorylated.
Effect of LY294002 and rapamycin on viral gene expression.
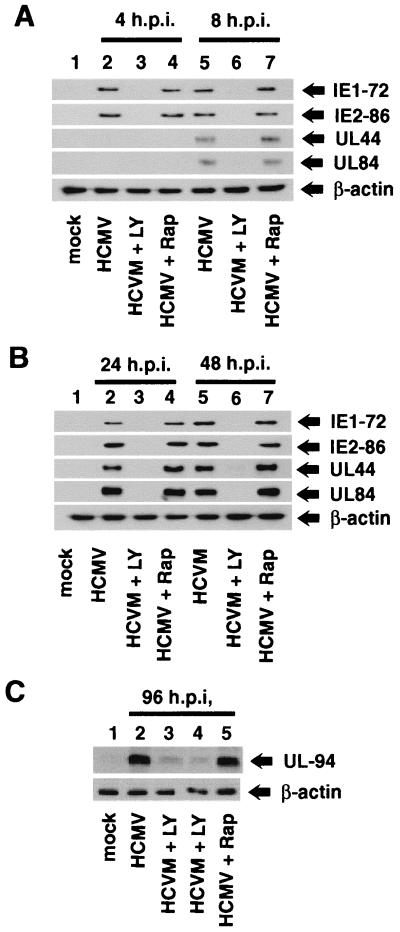
To further characterize the role of PI3-K in the viral life cycle, the effect of LY294002 and rapamycin on protein levels of viral IE, E, and L genes was examined by Western blot analysis. Figure 8A and B show that treatment of fibroblasts with LY294002 decreased the levels of the two major IE proteins, IE1-72 and IE2-86, at early (4 and 8 hpi) and late (24 to 48 hpi) times of virus infection, compared to cells infected in the absence of the compound. Ly294002 also strongly inhibited expression of the E genes UL44 (DNA polymerase processing factor) and UL84, both of which are required for initiation of viral DNA replication (27, 52, 79). Interestingly, rapamycin did not affect the level of viral IE or E proteins at early or late times of infections.
FIG. 8.
Expression of viral proteins in the presence of LY294002 and rapamycin. HEL fibroblasts were grown to confluence, serum starved for 48 h, infected with HCMV, and harvested at the indicated times postinfection. Serum-starved HEL fibroblasts were infected in the presence or absence of 20 μM LY294002 or 10 nM rapamycin. Cells were harvested and analyzed for viral protein expression by Western blot analysis. Western blot analyses were performed (i) with antibodies to the IE proteins (IE1-72 and IE2-86) and early proteins (UL44 and UL84) at 4 and 8 hpi (A) and at 24 and 48 hpi (B) and (ii) on late protein UL94 synthesis at 96 hpi (C). Each experiment was performed at least three times, and representative results are shown. In each case, β-actin levels were determined by Western blot analysis to demonstrate equal protein concentrations between samples.
Finally, expression of the true L gene UL94 was examined (Fig. 8C). High levels of UL94 protein were detected by 96 h in infected control cells and in cells infected in the presence of rapamycin (lanes 2 and 5). In contrast, UL94 protein was barely detected in cells treated with LY294002 (lanes 3 and 4). Since late gene expression cannot occur until after initiation of viral DNA replication, this result supports the data presented in Fig. 6 and 7B, which show that LY294002, but not rapamycin, inhibits viral DNA replication.
DISCUSSION
PI3-K activation following HCMV infection.
It is well documented that following infection, HCMV induces activation of many host cell transcription factors and proteins involved in DNA replication (21, 30, 50, 77, 86). However, the upstream cellular signaling pathways involved in this activation remains for the most part unknown. Initially, we found that PI3-K is activated immediately after the addition of HCMV to resting fibroblasts (Fig. 1A). This finding raises many questions. First, what viral processes are responsible for PI3-K activation? The results obtained with the UV-irradiated virus indicates that viral protein expression is not required to obtain the first tier of HCMV-mediated PI3-K activation (Fig. 2A and D). Other studies have shown that binding of viral glycoproteins located in the virion to host cell receptors activates cellular signaling pathways (12, 86). This receptor-ligand interaction results in activation of a number of cellular proteins, including the NF-κB and Sp-1 transcription factors, the ERK1/2 MAPK pathway, and several genes in the interferon response pathway (12, 35, 84, 86). The fact that this receptor-ligand interaction is sufficient to obtain the first tier of NF-κB activation, that PI3-K activation is required for NF-κB activation, and that UV-irradiated virus is sufficient to activate PI3-K (Fig. 2A and 5B) all suggest that binding of virion glycoprotein to host cell receptors may be sufficient to obtain the first tier of PI3-K activation. We are currently performing experiments to specifically address this possibility.
The fact that the appearance of the second tier of PI3-K activation requires viral protein expression (Fig. 2 and 4) indicates that different viral processes are involved in the two tiers of HCMV-mediated PI3-K activation. The observation that the second tier of PI3-K activation correlates with the increase in IE protein levels suggests that IE proteins may be involved in the second tier of PI3-K activation. Hopefully, future experiments will determine if this is indeed the case.
Another question raised by this initial finding is what cellular kinases or regulators are upstream of HCMV-mediated PI3-K activation. In most of the cases, PI3-K activation is triggered when activated cell surface receptors and associated tyrosine kinases recruit heterodimeric PI3-Ks, which consists of a p110 catalytic subunit and a p85 adapter molecule that contains Src-homology 2 (SH2) and SH3 domains (23, 24, 70). The SH2 domains are essential for mediating the interaction between p85 and the specific phosphotyrosine (pTyr) residues that are located on PI3-K substrate proteins (24, 87). This SH2-pTyr interaction brings the substrate (which is now bound to p85) into close proximity to the p110 catalytic subunit of PI3-K, which in turn increases the rate of substrate phosphorylation (16, 24).
The kinase activity of PI3-K can also be directly activated by the small G protein Ras (49). Ras binds to the p110 subunit of PI3-K in a GTP-dependent manner, which results in an increase in PI3-K activity (60, 61). Thus, the full activation of PI3-K requires concurrent association of the catalytic subunit (p110) with Ras and the adapter unit (p85) with a pTyr-containing protein. Furthermore, in antigen-receptor complex pathways, PI3-K can be also activated by Src family kinases through the SH3 domain of the p85 subunit (54). The data presented in this study suggest that both tiers of PI3-K activation observed following HCMV infection are mediated at least in part by increased phosphorylation of the p85 subunit (Fig. 1A). Currently, we are utilizing specific inhibitors to determine if other events are required for each tier of PI3-K activation following HCMV infection. These ongoing studies should provide new insight into the mechanism of virus-mediated PI3-K activation.
PI3-K signaling in infected fibroblasts.
While activation of NF-κB following HCMV infection is well documented, this is the first report of Akt and p70S6K activation following viral infection (11, 42, 83, 85). Furthermore, this study is the first to show that Akt, p70S6K, and NF-κB are all activated in a PI3-K-dependent manner during HCMV infection (Fig. 1 to 5). Akt was originally identified as a downstream target of PI3-K by overexpression of dominant-negative proteins and by the use of PI3-K inhibitors, such as LY294002 and wortmannin (22, 40, 41). Recent reports have demonstrated that PI3-K-mediated activation of Akt can inhibit apoptosis induced by a wide range of apoptotic stimuli (36). Based on these reports, we speculated that Akt was serving an antiapoptotic role in HCMV-infected fibroblasts. However, this proved not to be the case, as no apoptosis was detected in infected cells treated with LY294002 (Fig. 6C and data not shown). Several HCMV-encoded proteins have been shown to block apoptosis induced by various stimuli, such as overexpression of adenovirus E1A or activation of cell death receptors (25, 88). It is possible that HCMV-mediated activation of Akt is important for one or more of these antiapoptotic functions. This, in turn, would allow HCMV to complete its lytic life cycle and release infectious particles before cell death occurred.
PI3-K-mediated activation of p70S6K has also been well studied, so the finding that p70S6K was activated in a PI3-K-dependent manner was not unexpected (19, 23, 75, 76). The results obtained by treating infected cells with rapamycin, which inhibits p70S6K activation independent of PI3-K, suggests that under our conditions, p70S6K activity is not required for completion of the viral lytic life cycle (Fig. 7 and 8).
Several laboratories have reported that PI3-K can regulate activation of NF-κB (9, 47, 58, 59, 62). The exact mechanism of this activation is still somewhat uncertain, due in part to the fact that NF-κB activity is regulated by multiple mechanisms. In unstimulated cells, the classic NF-κB heterodimer, composed of a p65 subunit and a p50 subunit, is found in the cytoplasm bound to an inhibitor molecule, termed IκB (6). IκB is bound to the nuclear localization signal of NF-κB, which prevents NF-κB from translocating to the nucleus. Upon stimulation, a kinase pathway is activated, which results in phosphorylation and subsequent ubiquitin-mediated degradation of IκB (6). This allows NF-κB to translocate to the nucleus, where it binds DNA and activates expression of cellular genes, many of which are involved in inhibition of apoptosis (62). It was recently discovered that the transactivation function of the p65 subunit can also be regulated by phosphorylation, which is independent of p65 DNA binding (47, 78). Depending upon the stimuli and cell type, PI3-K can activate NF-κB through either mechanism. In addition, activation of Akt by PI3-K is thought to be a critical event in both types of activation. How does this relate to HCMV-mediated NF-κB activation? It has been demonstrated that HCMV infection enhances NF-κB transactivation function by both promoting homodimer and heterodimer formation and increasing the overall level of subunit protein by increasing mRNA levels (42, 85). Both the first and second tiers of NF-κB activation correlate with a decrease in IκB protein levels (data not shown). To date, however, we have been unable to detect p65 phosphorylation following HCMV infection. Based on the fact that PI3-K has been shown to induce IκB degradation, we hypothesize that at least one function of PI3-K activity in NF-κB activation is to induce phosphorylation and degradation of IκB. Studies in progress will show if this is indeed the case and also whether PI3-K activity alters the ability of HMCV to increase transcription of NF-κB. Furthermore, studies using the dominant-negative Akt should provide evidence as to the role of Akt activity, if any, in PI3-K-mediated NF-κB activation during HCMV infection.
The function of NF-κB in HCMV infection remains to be determined. Activation of NF-κB can protect cells from a variety of different types of apoptosis (47, 49). However, since HCMV-infected fibroblasts did not undergo apoptosis in the presence of LY294002, this is not a function of NF-κB in these cells. As is the case with Akt, it is also very possible that under other environmental conditions, HCMV-mediated activation of NF-κB may protect infected cells from apoptosis. Previously, our laboratory and others have speculated that NF-κB activation is important for expression of the major IE promoter, which encodes IE1-72 and IE2-86 (32, 42, 64, 85). Under the experimental conditions used in this study, inhibition of PI3-K resulted in very significant reductions in IE1-72 and IE2-86 protein levels. By inhibiting NF-κB activation in HCMV-infected cells, we hope to determine if this impact of LY294002 on IE gene expression is due to its effect on NF-κB activation. In addition, these studies will also demonstrate if NF-κB has other roles in viral infection which have not been identified.
Role of PI3-K in viral life cycle.
Treatment of cells with LY294002, but not rapamycin, inhibited viral IE1-72 and IE2-86 expression, as well as viral DNA replication (Fig. 6 to 8). These results imply that PI3-K signaling is important for the initiation of viral DNA replication and subsequent completion of the viral lytic life cycle. Treatment of cells with LY294002 or rapamycin also resulted in a very significant decrease in UL44 and UL84 protein levels. Preliminary results from our laboratory indicate that while LY294002 has a significant effect on UL44 and UL84 mRNA levels, rapamycin has almost no effect (data not shown). Based on these findings, we hypothesize that PI3-K activity is required for optimal transcription and translation of at least IE genes and perhaps some E genes.
Viral IE proteins are required for expression of viral E genes and initiation of viral DNA replication (50). Therefore, the inhibition of viral DNA replication and E gene expression observed with LY294002 is due at least in part to the significant loss of IE gene expression. Since PI3-K activates many proteins that are involved in DNA synthesis, we believe it likely that PI3-K has other roles in regulating the initiation of viral DNA replication besides regulating IE and E protein levels. For example, perhaps in the presence of LY294002, cellular proteins required for viral DNA replication are not being activated. We will try to determine if PI3-K has other functions in HCMV infection by specifically inhibiting individual pathways and proteins that are activated in a PI3-K-dependent manner during HCMV infection, such as Akt and NF-κB, and examining the effect of this inhibition of viral DNA replication. Using this approach, we have already demonstrated that PI3-K-mediated activation of p70S6K does not affect viral DNA replication or viral gene expression (Fig. 7 and 8).
Though many questions remain, this study has identified a cellular kinase that both is an important mediator of viral signaling and is required for viral DNA replication. By focusing on PI3-K signaling in the future, we hope to more clearly define the complex interaction between viral infection and the cellular signaling pathway that is required for successful completion of the lytic life cycle.
ACKNOWLEDGMENTS
Robert A. Johnson and Xin Wang contributed equally toward this study.
We thank M. Mayo and S. Nevada for helpful discussion and M. Hiremath for critical review of the manuscript. R.A.J. was supported in part by a virology training grant (2T32AI07419). This work was supported by grants AI47468 and CA19014 from the National Institutes of Health (to E.-S.H.).
REFERENCES
- 1.Ahmed N N, Grimes H L, Bellacosa A, Chan T O, Tsichlis P N. Transduction of interleukin-2 antiapoptotic and proliferative signals via Akt protein kinase. Proc Natl Acad Sci USA. 1997;94:3627–3632. doi: 10.1073/pnas.94.8.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albrecht T, Boldogh I, Fons M P, Valyi-Nagy T. Activation of proto-oncogenes and cell activation signals in the initiation and progression of human cytomegalovirus infection. In: Becker Y, Darai G, Huang E S, editors. Molecular aspects of human cytomegalovirus diseases. Berlin, Germany: Springer-Verlag; 1993. pp. 384–411. [Google Scholar]
- 3.Alessi D R, Caudwell F B, Andjelkovic M, Hemmings B A, Cohen P. Molecular basis for the substrate specificity of protein kinase B: comparison with MAPKAP kinase-1 and p70 S6 kinase. FEBS Lett. 1996;399:333–338. doi: 10.1016/s0014-5793(96)01370-1. [DOI] [PubMed] [Google Scholar]
- 4.Atlas D, Adler M. Alpha-adrenergic antagonists as possible calcium channel inhibitors. Proc Natl Acad Sci USA. 1981;78:1237–1241. doi: 10.1073/pnas.78.2.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baldanti F, Bloeckh M, Chou S, Crumpacker C, Danner S, Drew W L, Emanuel D, Erice A, Hardy W D, Spector S. Drug resistance in cytomegalovirus: current knowledge and implications for patient management. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;12(Suppl. 1):S1–S22. [PubMed] [Google Scholar]
- 6.Baldwin A S. The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–681. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 7.Bellacosa A, Chan T O, Ahmed N N, Datta K, Malstrom S, Stokoe D, McCormick F, Feng J, Tsichlis P. Akt activation by growth factors is a multiple-step process: the role of the PH domain. Oncogene. 1998;17:313–325. doi: 10.1038/sj.onc.1201947. [DOI] [PubMed] [Google Scholar]
- 8.Benson J D, Huang E S. Human cytomegalovirus induces expression of cellular topoisomerase II. J Virol. 1990;64:9–15. doi: 10.1128/jvi.64.1.9-15.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beraud C, Henzel W J, Baeuerle P A. Involvement of regulatory and catalytic subunits of phosphoinositide 3-kinase in NF-kappaB activation. Proc Natl Acad Sci USA. 1999;96:429–434. doi: 10.1073/pnas.96.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boldogh I, Abubakar S, Albrecht T. Activation of proto-oncogenes: an immediate early event in human cytomegalovirus. Science. 1990;247:561–564. doi: 10.1126/science.1689075. [DOI] [PubMed] [Google Scholar]
- 11.Boldogh I, Fons M P, Albrecht T. Increased levels of sequence-specific DNA-binding proteins in human cytomegalovirus-infected cells. Biochem Biophys Res Commun. 1993;197:1505–1510. doi: 10.1006/bbrc.1993.2647. [DOI] [PubMed] [Google Scholar]
- 12.Boyle K A, Pietropaola R L, Compton T. Engagement of the cellular receptor for glycoprotein B of human cytomegalovirus activates the interferon-responsive pathway. Mol Cell Biol. 1999;19:3607–3613. doi: 10.1128/mcb.19.5.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brennan P, Babbage J W, Thomas G, Cantrell D. p70(s6k) integrates phosphatidylinositol 3-kinase and rapamycin-regulated signals for E2F regulation in T lymphocytes. Mol Cell Biol. 1999;19:4729–4738. doi: 10.1128/mcb.19.7.4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bresnahan W A, Thompson E A, Albrecht T. Human cytomegalovirus infection results in altered Cdk2 subcellular localization. J Gen Virol. 1997;78:1993–1997. doi: 10.1099/0022-1317-78-8-1993. [DOI] [PubMed] [Google Scholar]
- 15.Bresnahan W A, Boldogh I, Chi P, Thompson E A, Albrecht T. Inhibition of cellular cdk2 activity blocks human cytomegalovirus replication. Virology. 1997;231:239–247. doi: 10.1006/viro.1997.8489. [DOI] [PubMed] [Google Scholar]
- 16.Carpenter C L, Auger K R, Chanudhuri M, Yoakim M, Schaffhausen B, Shoelson S, Cantley L C. Phosphoinositide 3-kinase is activated by phosphopeptides that bind to the SH2 domains of the 85-kDa subunit. J Biol Chem. 1993;268:9478–9483. [PubMed] [Google Scholar]
- 17.Chung J, Kuo C J, Crabtree G R, Blenis J. Rapamycin-KFBP specifically blocks growth-dependent activation of and signaling by the 70 kd S6 protein kinases. Cell. 1992;69:1227–1236. doi: 10.1016/0092-8674(92)90643-q. [DOI] [PubMed] [Google Scholar]
- 18.Cogswell P C, Scheinman R I, Baldwin A S., Jr Promoter of the human NF-κB p50/p105 gene. Regulation by NF-κB subunits and by c-REL. J Immunol. 1993;150:2794–2804. [PubMed] [Google Scholar]
- 19.Coulonval K, Vandeput F, Stein R C, Kozma S C, Lamy F, Dumont J E. Phosphatidylinositol 3-kinase, protein kinase B and ribosomal S6 kinases in the stimulation of thyroid epithelial cell proliferation by cAMP and growth factors in the presence of insulin. Biochem J. 2000;348:351–358. [PMC free article] [PubMed] [Google Scholar]
- 20.Dufner A, Thomas G. Ribosomal S5 kinase signaling and the control of translation. Exp Cell Res. 1999;253:100–109. doi: 10.1006/excr.1999.4683. [DOI] [PubMed] [Google Scholar]
- 21.Estes J, Huang E-S. Stimulation of cellular thymidine kinase by human cytomegalovirus. J Virol. 1977;24:13–21. doi: 10.1128/jvi.24.1.13-21.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franke T F, Yang S I, Chan T O, Datta K, Kazlauskas A, Morrison D K, Kaplan D R, Tsichlis P N. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 23.Fruman D A, Meyers R E, Cantley L C. Phosphoinositide kinases. Annu Rev Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- 24.Fruman D A, Cantley L C. PI3-kinases. In: Gutkind J S, editor. Signaling networks and cell cycle control: the molecular basis of cancer and other diseases. Totowa, N.J: Humana Press; 2000. pp. 247–266. [Google Scholar]
- 25.Goldmacher V S, Bartle L M, Skaletskaya A, Dionne C A, Kedersha N L, Vater C A, Han J-W, Lutz R J, Watanabe S, Cahir McFarlan E D, Kieff E D, Mocarski E S, Chittenden T. A cytomegalovirus-encoded mitochondria-localized inhibitor of apoptosis structurally unrelated to Bcl-2. Proc Natl Acad Sci USA. 1999;96:12536–12541. doi: 10.1073/pnas.96.22.12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han J W, Pearson R B, Dennis P B, Thomas G. Rapamycin, wortmannin, and the methylxanthine SQ20006 inactivate p70s6k by inducing dephosphorylation of the same subset of sites. J Biol Chem. 1995;270:21396–21403. doi: 10.1074/jbc.270.36.21396. [DOI] [PubMed] [Google Scholar]
- 27.He Y S, Xu L, Huang E S. Characterization of human cytomegalovirus UL84 early gene and identification of its putative protein product. J Virol. 1992;66:1098–1108. doi: 10.1128/jvi.66.2.1098-1108.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hiles I D, Otsu M, Volinia S, Fry M J, Gout I, Dhand R, Panayotou G, Ruiz L F, Thomson A, Totty N F, Hsuan J J, Courtneidge S A, Parker J P, Waterfield M. Phosphatidylinosital 3-kinase: structure and expression of the 110 kd catalytic subunit. Cell. 1992;70:419–429. doi: 10.1016/0092-8674(92)90166-a. [DOI] [PubMed] [Google Scholar]
- 29.Ho M. Human cytomegalovirus: biology and infection. 2nd ed. New York, N.Y: Plenum; 1991. [Google Scholar]
- 30.Huang E-S. Human cytomegalovirus. III. Virus-induced DNA polymerase. J Virol. 1975;16:298–310. doi: 10.1128/jvi.16.2.298-310.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang E-S, Chen S-T, Pagano J S. Human cytomegalovirus. I. Purification and characterization of viral DNA. J Virol. 1973;12:1473–1481. doi: 10.1128/jvi.12.6.1473-1481.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang E-S, Kowalik T F. The pathogenicity of human cytomegalovirus: an overview. In: Becker Y, Darai G, Huang E S, editors. Molecular aspects of human cytomegalovirus diseases. Berlin, Germany: Springer-Verlag; 1993. pp. 1–45. [Google Scholar]
- 33.Johnson R A, Huong S-M, Huang E-S. Activation of the mitogen-activated protein kinase p38 by human cytomegalovirus infection through two distinct pathways: a novel mechanism for activation of p38. J Virol. 2000;74:1158–1167. doi: 10.1128/jvi.74.3.1158-1167.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson R A, Huong S-M, Huang E-S. Inhibitory effect of 4-(4-fluorophenyl)-2-(4-hydroxyphenyl)-5-(4-pyridyl)1H-imidazole on HCMV DNA replication and permissive infection. Antivir Res. 1999;41:101–111. doi: 10.1016/s0166-3542(99)00002-9. [DOI] [PubMed] [Google Scholar]
- 35.Johnson R A, Ma X-L, Yurochko A D, Huang E-S. The role of MKK1/2 kinase activity in human cytomegalovirus infection. J Gen Virol. 2001;82:493–497. doi: 10.1099/0022-1317-82-3-493. [DOI] [PubMed] [Google Scholar]
- 36.Kandel E S, Hay N. The regulation and activities of the multifunctional serine/threonine kinase Akt/PKB. Exp Cell Res. 1999;253:210–229. doi: 10.1006/excr.1999.4690. [DOI] [PubMed] [Google Scholar]
- 37.Kane L P, Shapiro V S, Stokoe D, Weiss A. Induction of NF-kappaB by the Akt/PKB kinase. Curr Biol. 1999;9:601–604. doi: 10.1016/s0960-9822(99)80265-6. [DOI] [PubMed] [Google Scholar]
- 38.Kauffmann-Zeh A, Rodriguez-Viciana P, Ulrich E, Gilbert C, Coffer P, Downward J, Evan G. Suppression of c-Myc-induced apoptosis by Ras signaling through PI(3)K and PKB. Nature. 1997;385:544–548. doi: 10.1038/385544a0. [DOI] [PubMed] [Google Scholar]
- 39.Khwaja A, Rodriguez-Viciana P, Wennstrom S, Warne P H, Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt survival pathway. EMBO J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.King W G, Mattaliano M D, Chan T O, Tsichlis P N, Bruggee J S. Phosphatidylinositol 3-kinase is required for integrin-stimulated Akt and Raf-1/mitogen-activated protein kinase pathway activation. Mol Cell Biol. 1997;17:4406–4418. doi: 10.1128/mcb.17.8.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klippel A, Reinhard C, Kavanaugh W M, Apell G, Escobedo M A, Williams L T. Membrane localization of phosphatidylinositol 3-kinase is sufficient to activate multiple signal transducing pathways. Mol Cell Biol. 1996;16:4117–4127. doi: 10.1128/mcb.16.8.4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kowalik T F, Wing B, Haskill J S, Azizkhan J C, Baldwin A S, Jr, Huang E-S. Multiple mechanisms are implicated in the regulation of NF-κB activity during human cytomegalovirus infection. Proc Natl Acad Sci USA. 1993;90:1107–1111. doi: 10.1073/pnas.90.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kowalik T F, Yurochko A D, Rinehart C A, Lee C Y, Huang E S. Productive infection of human endometrial stromal cells by human cytomegalovirus. Virology. 1994;202:247–257. doi: 10.1006/viro.1994.1340. [DOI] [PubMed] [Google Scholar]
- 44.Kulik G, Klippel A, Weber M J. Antiapoptotic signaling by the insulin-like growth factor 1 receptor, phosphatidylinositol 3-kinase via Akt protein kinase. Mol Cell Biol. 1997;17:1595–1606. doi: 10.1128/mcb.17.3.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee J C, Laydon J T, McDonnell P C, Gallagher T F, Kumar S, Green D, McNulty D, Blumenthal M J, Heys J R, Landvatter S W, Strickler J E, McLaughlin M M, Siemens I R, Fisher S M, Livi G P, White J R, Adams J L, Young P R. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 46.Lemmon M A, Ferguson K M, Schlessinger J. PH domains: diverse sequences with a common fold recruit signaling molecules to the cell surface. Cell. 1996;85:621–624. doi: 10.1016/s0092-8674(00)81022-3. [DOI] [PubMed] [Google Scholar]
- 47.Madrid L V, Wang C-Y, Guttridge D C, Schottelius A J G, Baldwin A S, Jr, Mayo M W. Akt suppresses apoptosis by stimulating the transactivation potential of the RelA/p65 subunit of NF-κB. Mol Cell Biol. 2000;20:1626–1638. doi: 10.1128/mcb.20.5.1626-1638.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mar E-C, Cheng Y C, Huang E-S. Effect of 9-(1,3-dihydroxy-2 propoxymethyl) guanine on human cytomegalovirus replication in vitro. Antimicrob Agents Chemother. 1983;24:518–522. doi: 10.1128/aac.24.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marte B M, Downward J. PKB/Akt: connecting phosphoinocitide 3-kinase to cell survival and beyond. Trends Biochem Sci. 1997;22:355–358. doi: 10.1016/s0968-0004(97)01097-9. [DOI] [PubMed] [Google Scholar]
- 50.Mocarski E S. Cytomegalovirus biology and replication. In: Roizman B, Whitley R, Lopez C, editors. The human herpesviruses. New York, N.Y: Raven Press; 1993. pp. 173–226. [Google Scholar]
- 51.Oberg B, Behrnetz S, Eriksson B, Jozwiak H, Larsson A, Lernestedt J D, Aberg V L. Clinical use of phoscarnet (phosphonoformate) In: De Clercq E, editor. Clinical use of antiviral drugs. Boston, Mass: Martinus Nijhoff; 1988. pp. 223–240. [Google Scholar]
- 52.Pari G S, Anders D G. Eleven loci encoding trans-acting factors are required for transient complementation of human cytomegalovirus ori-Lyt-dependent DNA replication. J Virol. 1993;67:6979–6988. doi: 10.1128/jvi.67.12.6979-6988.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Person R B, Dennis P B, Han J W, Williamson N A, Kozma S C, Wettenhall R E, Thomas G. The principal traget of rapamycin-induced p70s6k inactivation is a novel phosphorylation site within a conserved hydrophobic domain. EMBO J. 1995;14:5279–5287. doi: 10.1002/j.1460-2075.1995.tb00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pleiman C M, Hertz W M, Cambier J C. Activation of phosphatidyl inositol-3′ kinase by Src-family kinase SH3 binding to the p85 subunit. Science. 1994;263:1609–1612. doi: 10.1126/science.8128248. [DOI] [PubMed] [Google Scholar]
- 55.Powis G, Bonjouklian R, Berggren M M, Gallegos A, Abraham R, Ashendel C, Zalkow L, Matter W F, Dodge J, Grindey G, Vlahos C J. Wortmannin, a potent and selective inhibitor of phosphotidylinositol-3-kinase. Cancer Res. 1994;54:2419–2423. [PubMed] [Google Scholar]
- 56.Proud C G. p70 S6 kinase: an enigma with variations. Trends Biochem Sci. 1996;21:181–185. [PubMed] [Google Scholar]
- 57.Pullen N, Thomas G. The modular phosphorylation and activation of p70s6k. FEBS Lett. 1997;410:78–82. doi: 10.1016/s0014-5793(97)00323-2. [DOI] [PubMed] [Google Scholar]
- 58.Reddy S A, Huang J H, Liao W S. Phospatidylinositol 3-kinase in interleukin signaling: physical interaction with the interleukin 1 receptor and requirement in NFkappaB and AP-1 activation. J Biol Chem. 1997;272:29167–29173. doi: 10.1074/jbc.272.46.29167. [DOI] [PubMed] [Google Scholar]
- 59.Reddy S A G, Huang J H, Liao W S-L. Phosphatidylinositol 3-kinase as a mediator of TNF-induced NF-κB activation. J Immunol. 2000;164:1355–1363. doi: 10.4049/jimmunol.164.3.1355. [DOI] [PubMed] [Google Scholar]
- 60.Rodriguez-Viciana P, Warne P H, Dhand R, Vanhaesebroeck B, Gout I, Fry M J, Waterfield M D, Downward J. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 61.Rodriguez-Viciana P, Warne P H, Vanhaesebroeck B, Waterfield M D, Downward J. Activation of phosphoinositide 3-kinase by interaction with Ras and by point mutation. EMBO J. 1996;15:2442–2451. [PMC free article] [PubMed] [Google Scholar]
- 62.Romashkova J A, Makarov S S. NF-kappaB is a target of AKT in anti-apoptotic PDGF signaling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 63.Sabers C J, Martin M M, Brunn G J, Williams J M, Dumont F J, Wiederrecht G, Abraham R T. Isolation of a protein target of the FKB12-rapamycin complex in mammalian cells. J Biol Chem. 1995;270:815–822. doi: 10.1074/jbc.270.2.815. [DOI] [PubMed] [Google Scholar]
- 64.Sambucetti L C, Cherrington J M, Wilkinson G W, Mocarski E S. NF-κB activation of the cytomegalovirus enhancer is mediated by a viral transactivator and by T cell stimulation. EMBO J. 1989;8:4251–4258. doi: 10.1002/j.1460-2075.1989.tb08610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scheinman R I, Beg A A, Baldwin A S., Jr NF-κB p100 (Lyt-10) is a component of H2TF1 and can function as an IκB-like molecule. Mol Cell Biol. 1993;13:6089–6101. doi: 10.1128/mcb.13.10.6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Serody J S, van der Horst C M. The control of cytomegalovirus infection: chemotherapy. In: Becker Y, Darai G, Huang E-S, editors. Molecular aspects of human cytomegalovirus diseases. Berlin, Germany: Springer-Verlag; 1993. pp. 256–282. [Google Scholar]
- 67.Skolnik E Y, Margolis B, Mohammadi M, Lowenstein E, Fisher R, Drepps A, Ullrich A, Schlessonger J. Cloning of PI3 kinase-associated p85 utilizing a novel method for expression/cloning of target protein for receptor tyrosine kinases. Cell. 1991;65:83–90. doi: 10.1016/0092-8674(91)90410-z. [DOI] [PubMed] [Google Scholar]
- 68.Stambolic V, Mak T W, Woodgett J R. Modulation of cellular apoptotic potential: contributions to oncogenesis. Oncogene. 1999;18:6094–6103. doi: 10.1038/sj.onc.1203126. [DOI] [PubMed] [Google Scholar]
- 69.Stanat S C, Reardon J E, Erice A, Jordan M C, Drew W L, Boiron K K. Ganciclovir-resistant cytomegalovirus clinical isolate: model of resistance to ganciclovir and phosphonylmethoxy-alkyl derivatives. Antimicrob Agents Chemother. 1991;35:2191–2197. doi: 10.1128/aac.35.11.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stoyanov B, Volinia S, Hanck T, Rubio I, Loubtchenkov M, Malek D, Stoyanova S, Vanhaesebroeck B, Dhand R, Nurnberg B, Gierschik P, Seedorf K, Hsuan J J, Waterfield M D, Downward J. Cloning and characterization of a G-protein-activated human phosphoinositide-3 kinase. Science. 1995;269:690–693. doi: 10.1126/science.7624799. [DOI] [PubMed] [Google Scholar]
- 71.Terada N, Lucas J J, Szepesi A, Franklin R A, Takase K, Gelfand E W. Rapamycin inhibits the phosphorylation of p70 S6 kinase in IL-2 and mitogen-activated human T cells. Biochem Biophys Res Commun. 1992;14:1315–1321. doi: 10.1016/s0006-291x(05)81549-9. [DOI] [PubMed] [Google Scholar]
- 72.Tibbetts R S, Abraham R T. PI3K-related kinases: roles in cell-cycle regulation and DNA damage response. In: Gutkind J S, editor. Signaling networks and cell cycle control—the molecular basis of cancer and other diseases. Totowa, N.J: Humana Press; 2000. pp. 267–301. [Google Scholar]
- 73.Toker A, Cantley L C. Signaling through the lipid products of phosphoinositide-3-OH kinase. Nature. 1997;387:673–676. doi: 10.1038/42648. [DOI] [PubMed] [Google Scholar]
- 74.Toullec D, Pianettic P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F, et al. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem. 1994;226:15771–15781. [PubMed] [Google Scholar]
- 75.Vanhaesebroeck B, Alessi D R. The PI3K-PDK1 connection: more than just a road to PKB. Biochem J. 2000;346:561–576. [PMC free article] [PubMed] [Google Scholar]
- 76.Vanhaesebroeck B, Waterfield M D. Signaling by distinct classes of phosphoinositide 3-kinases. Exp Cell Res. 1999;253:239–254. doi: 10.1006/excr.1999.4701. [DOI] [PubMed] [Google Scholar]
- 77.Wade M, Kowalik T F, Mudryj M, Huang E-S, Azizkhan J C. E2F mediates dihydrofolate reductase promoter activation and multiprotein complex formation in human cytomegalovirus infection. Mol Cell Biol. 1992;12:4364–4374. doi: 10.1128/mcb.12.10.4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang D, Baldwin A S. Activation of nuclear factor-kB-dependent transcription by tumor necrosis factor-a is mediated through phosphorylation of RelA/p65 on serine 529. J Biol Chem. 1998;273:29411–29416. doi: 10.1074/jbc.273.45.29411. [DOI] [PubMed] [Google Scholar]
- 79.Weiland K L, Oien N L, Homa F, Wathen M W. Functional analysis of human cytomegalovirus polymerase accesory protein. Virus Res. 1994;34:191–206. doi: 10.1016/0168-1702(94)90124-4. [DOI] [PubMed] [Google Scholar]
- 80.Wennstrom S, Downward J. Role of phosphoinositide 3-kinase in activation of Ras and mitogen-activated protein kinase by epidermal growth factor. Mol Cell Biol. 1999;19:4279–4288. doi: 10.1128/mcb.19.6.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wing B A, Lee G C Y, Huang E-S. The human cytomegalovirus UL94 open reading frame encodes a conserved herpesvirus capsid/tegument-associated virion protein that is expressed with true late kinetics. J Virol. 1996;70:3339–3345. doi: 10.1128/jvi.70.6.3339-3345.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yao R, Cooper G M. Growth factor-dependent survival of rodent fibroblasts requires phosphatidylinositol 3-kinase but is independent of pp70S6K. Oncogene. 1996;13:343–351. [PubMed] [Google Scholar]
- 83.Yurochko A D, Huang E-S. Human cytomegalovirus binding to human monocytes induces immunoregulatory gene expression. J Immunol. 1999;162:4806–4816. [PubMed] [Google Scholar]
- 84.Yurochko A D, Hwang E-S, Rasmussen L, Keay S, Pereira L, Huang E-S. The human cytomegalovirus UL55 (gB) and UL75(gH) glycoprotein ligands initiate the rapid activation of Sp1 and NF-κB during infection. J Virol. 1997;71:5051–5059. doi: 10.1128/jvi.71.7.5051-5059.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yurochko A D, Kowalik T F, Huong S-M, Huang E-S. Human cytomegalovirus upregulates NF-κB activity by transactivating the NF-κB p105/p50 and p65 promoters. J Virol. 1995;69:5391–5400. doi: 10.1128/jvi.69.9.5391-5400.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yurochko A D, Mayo M W, Poma E E, Baldwin A S, Jr, Huang E-S. Induction of the transcription factor Sp1 during human cytomegalovirus infection mediates upregulation of the p65 and p105/p50 NF-κB promoters. J Virol. 1997;71:4638–4648. doi: 10.1128/jvi.71.6.4638-4648.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou S, Shoelson S E, Chaudhuri M, Gish G, Pawson T, Haser W G, King F, Roberts T, Ratnofsky S, Lechleider R J, Nabelm G B, Birge R B, Fajardo J E, Chou M M, Hanafusa H, Schaffhausen B, Cantley L C. SH2 domains recognize specific phosphopeptide sequence. Cell. 1993;72:767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- 88.Zhu H, Shen Y, Shenk T. Human cytomegalovirus IE1 and IE2 proteins block apoptosis. J Virol. 1995;69:7960–7970. doi: 10.1128/jvi.69.12.7960-7970.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]