Abstract
SM is an Epstein-Barr virus (EBV) gene expressed during early lytic replication of EBV. SM encodes a nuclear phosphoprotein that functions as a posttranscriptional regulator of gene expression. SM has been implicated in several aspects of gene regulation, including nuclear mRNA stabilization, posttranscriptional processing, and nuclear mRNA export. Activation by SM is promoter independent but gene specific. The mechanism by which SM selectively activates some EBV target genes or heterologous reporter genes remains to be determined. SM binds RNA in vitro, suggesting that sequence- or structure-specific mRNA interactions might mediate SM specificity. We have further analyzed RNA binding by SM and demonstrated that proteolytic cleavage of SM and consequent exposure of an arginine-rich region are necessary to allow RNA binding in vitro. However, SM mutants with deletions of this arginine-rich region localized normally in the nucleus and were fully functional in gene activation. We therefore developed an assay to study in vivo interactions of SM with target mRNAs based on immunoprecipitation of SM from cell lysates followed by RNase protection analysis. Using this assay, we demonstrated that SM forms complexes with specific mRNAs in vivo. SM binds mRNAs from both SM-responsive as well as nonresponsive intronless genes and increases the nuclear accumulation of both types of mRNAs. In addition, SM preferentially associates with newly transcribed mRNAs. These data indicate that SM forms complexes with mRNAs in the nucleus and enhances their nuclear accumulation. However, SM does not enhance cytoplasmic accumulation of all transcripts that it binds to the same degree, suggesting that additional mRNA-specific characteristics, such as nuclear retention motifs or binding sites for cellular proteins, also determine responsiveness to SM.
The Epstein-Barr virus (EBV) nuclear phosphoprotein SM, also referred to as EB2 and Mta, is a posttranscriptional regulator of gene expression (9, 30, 35, 45). The SM protein is expressed from an early gene during lytic EBV replication (10, 46). Although SM has been extensively studied, many aspects of its mechanism of action remain incompletely understood. We and others have shown that SM activates expression of the chloramphenicol acetyltransferase (CAT) gene, when cotransfected, in a variety of cell types (6, 9, 30, 31, 35, 45, 53). Steady-state levels of both cytoplasmic and nuclear CAT mRNAs increase in SM-transfected cells (45). The increase in CAT activity corresponds well with the increased amount of cytoplasmic CAT mRNA seen with SM transfection (12, 45). SM does not affect the rate of CAT mRNA transcription as measured by nuclear run-on transcription assays (45). Activation of CAT by SM is also independent of the promoter used to transcribe CAT mRNA. These data, taken together, demonstrate that SM has a posttranscriptional mechanism of action.
The herpes simplex virus (HSV) homolog of SM, ICP27, is a global inhibitor of host cell splicing and is thought to generally inhibit expression of intron-containing genes (21, 22). The majority of HSV lytic genes are intronless, and it has been suggested that ICP27 may play a role in facilitating selective expression of intronless HSV genes (47). Investigators from our laboratory have reported that introduction of heterologous introns into otherwise identical CAT reporter plasmids results in inhibition rather than activation by SM (45). We also reported that SM inhibits expression of the human growth hormone (hGH) gene, which contains four introns, and also of the spliced EBV BZLF1 gene. SM-mediated inhibition was demonstrated to occur at the posttranscriptional level. However, others have found that SM increases cytoplasmic accumulation of some intron-containing transcripts (4). The effect of SM on intron-containing genes is therefore incompletely characterized, and the effect of SM on a specific intron-containing gene may be dependent on multiple gene-specific factors, such as the nature of the splicing signals and other posttranscriptional processing signals.
A distinct property of SM-mediated activation is its gene specificity. Specifically, SM activates some reporter genes, such as CAT, but not others, such as β-galactosidase or hGH (30, 45). SM transfection also induces increased cytoplasmic accumulation of several lytic EBV transcripts. SM enhances cytoplasmic accumulation of mRNA from the EBV replicative genes BMRF1, BALF2, BALF5, BSLF1, and BBLF4, but not BBLF2/3 (50). The nonresponsiveness of BBLF2/3 to activation by SM persisted even when a potential intron was removed from the BBLF2/3 gene. Homologs of SM are present in several human herpesviruses, including HSV, cytomegalovirus (CMV), and Kaposi's sarcoma-associated herpesvirus (KSHV; also known as human herpesvirus 8) (1, 7, 14, 20, 33, 40, 44, 52). We have shown that the KSHV homolog of SM also demonstrates selective activation of target genes in transfection assays (20). Activation by ICP27 is also target gene dependent, and it has been suggested that its specificity is based on the ability of ICP27 to selectively bind different mRNAs (47). Recently, it has also been shown that ICP27 leads to increased nuclear and cytoplasmic accumulation of intron-containing transcripts of the cellular alpha-globin gene (8, 16). Thus, it appears that there may be gene-specific signals, independent of the presence of introns, that determine the responsiveness of an individual gene to activation by SM and perhaps by other herpesvirus homologs.
Recombinant SM binds several mRNAs in vitro, suggesting that SM interacts with target mRNAs and enhances their stability or processing (45, 50). SM shuttles from nucleus to cytoplasm in a heterokaryon assay and translocates to the cytoplasm in response to overexpression of CRM 1 (exportin 1) (2, 50). Other investigators have also reported SM-mediated effects on nuclear RNA export that are CRM-1 independent (17). These findings indicate that SM could function by binding target mRNAs and enhancing their nucleocytoplasmic export. Physical interaction of SM with pre-mRNA and mRNA of target genes could also explain many of the gene-specific aspects of SM function outlined above. In this context, it is significant that SM has been reported to bind a fragment of RNA transcribed from BMRF1, an SM-responsive EBV gene, but not an unrelated cellular RNA, in vitro (50). We undertook the present study to further characterize SM-mRNA interactions and to determine whether the gene specificity of activation by SM is due to an ability to differentially bind various mRNA targets.
MATERIALS AND METHODS
Cells and plasmids.
BJAB cells (43) were cultured in RPMI 1640 tissue culture medium supplemented with 10% fetal bovine serum. COS-7 cells (19) were cultured in Dulbecco's modification of Eagle's medium supplemented with 10% fetal bovine serum.
SM, aSM, and CMV-CAT have been previously described (45). CMV-hGH was made by inserting a HindIII-to-SmaI fragment containing the entire hGH cDNA (15) into pcDNA 3.1 (Invitrogen, Carlsbad, Calif.). CMV-luc was constructed by inserting a HindIII-to-XbaI fragment containing the luciferase coding sequence from pGL3-Promoter (Promega, Madison, Wis.) into pcDNA 3.1. CMV-cGAPDH was constructed by inserting the PvuI-to-FspI fragment containing the human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA from pHcGAP (51) into pcDNA 3.1. This fragment also contained 126 bp of the β-lactamase gene from pBR322 located 5′ to the GAPDH cDNA in pHcGAP.
Construction of glutathione S-transferase (GST)-SM fusion plasmids in a derivative of pGEX has been previously described (45). Truncations of SM consisting of amino acids (aa) 1 to 149, 1 to 185, and 1 to 281 were constructed by subcloning the fragments of SM from BglII-to-SmaI, -XhoI, or -HincII, respectively, in frame with GST. Deletion mutants (ΔRXP and Δ281) were constructed by removing the internal SmaI-XhoI fragment from the full-length GST-SM and the GST-1-281 fusion plasmid, respectively.
Plasmid templates for in vitro RNA probe synthesis were constructed in pBluescript (Stratagene, La Jolla, Calif.). The CAT template pRP1 consisted of a 514-bp SspI fragment derived from pLGU (45), containing the complement to the 3′-terminal 135 residues of the CAT mRNA (49). The luciferase template pVR193 was constructed with a 350-bp DNA fragment from pGL3-Basic (Promega), encoding the 3′-terminal 350 residues of the luciferase mRNA, and a 125-bp fragment from bacteriophage lambda to allow differentiation of full-length probe from the protected fragment. The GAPDH template was constructed with a 1.0-kb HindIII fragment from pHcGAP containing the 5′-terminal 172 bp of the human GAPDH gene (54). The template for probe to specifically detect mRNA transcribed from transfected CMV-cGAPDH consisted of a 355-bp PvuI-HindIII fragment from pHcGAP containing 126 bp of pHcGAP 5′ to the initiator codon of GAPDH and 229 bp of the amino-terminal portion of the GAPDH gene.
Transfections and reporter gene assays.
BJAB cells were transfected by electroporation with 10 μg of each plasmid, as previously described (45). Lysates were prepared 48 h after electroporation. CAT assays and radioimmunoassays for hGH were performed as previously described (45). Luciferase assays were performed with beetle luciferin as a substrate per the manufacturer's protocol (Promega). COS-7 cells were transfected with Lipofectamine Plus (Life Technologies, Gaithersburg, Md.) as previously described (2).
RNA methods.
For RNA isolation, cells were harvested 48 h after transfection and lysed in 0.5% Nonidet P-40 (NP-40) buffer. Nuclei were separated by centrifugation, and RNA was prepared from cytoplasmic and nuclear fractions as described previously (11). Selection of poly(A)+ RNA was performed as described previously (45). Detection of in vitro RNA binding by SM fusion proteins was performed by hybridization of radiolabeled RNA probes to proteins transferred to membranes as previously described (45).
Assay for in vivo RNA binding: IP-RPA.
Confluent monolayers of transfected COS-7 cells were scraped from tissue culture flasks and washed with phosphate-buffered saline. Cell pellets were lysed in ice-cold immunoprecipitation (IP) lysis buffer (25 mM Tris, 150 mM NaCl [pH 7.4], 1% Triton X-100, 1 mM dithiothreitol, 400 U of human placental RNase inhibitor [Amersham Pharmacia, Piscataway, N.J.] per ml, and protease inhibitor cocktail [P2714; Sigma, St. Louis, Mo.]). Cells were incubated in lysis buffer for 15 min and centrifuged for 5 min at 4°C and 13,000 × g. Supernatants were precleared with preimmune rabbit serum and protein A-agarose beads (Life Technologies) for 1 h at 4°C. Lysates were then incubated with gentle rocking at 4°C with polyclonal rabbit anti-SM serum or with preimmune rabbit serum for 30 min. Protein A-agarose beads were then added and the incubation was continued for an additional 90 min. The immunoprecipitates were washed extensively with RNase-free lysis buffer and then treated with 1,430 μg of proteinase K (Roche Molecular Biochemicals, Indianapolis, Ind.) per ml for 1 h at 37°C in a solution containing 10 mM Tris-HCl (pH 7.0), 0.5 M NaCl, 1 mM EDTA, and 0.05% sodium dodecyl sulfate. The resulting material was then extracted with phenol-chloroform (50% [vol/vol]) and ethanol precipitated with the addition of 140 μg of yeast tRNA/ml as a carrier. RNA immunoprecipitated in this manner was then analyzed by RNase protection assay (RPA). 32P-labeled probes for RPA were generated by in vitro transcription from the plasmid templates described above. Plasmids were linearized with the appropriate restriction enzyme, phenol extracted, and transcribed in vitro with either T7 or T3 RNA polymerase (New England Biolabs, Beverly, Mass., and Promega, respectively) and [α-32P]UTP, as per the manufacturer's protocol. Probes were purified by DNase treatment, phenol extraction, and Sephadex G50 gel filtration. Sample RNA (either from IP or directly isolated from transfected cells) was hybridized overnight with 2.5 × 106 cpm of RNA probe at 42°C in a solution containing 80% formamide, 400 mM NaCl, 40 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (pH 6.4). In each hybridization with immunoprecipitated RNA, the RNA sample represented material from 1.25 × 105 transfected cells. Control hybridizations with radiolabeled probe and tRNA instead of sample RNAs were performed in parallel. Hybridization reaction mixtures were diluted 20-fold, and unprotected probe was hydrolyzed by treatment with 30 U of RNase T2 (Life Technologies) per ml for 1 h at 37°C. The reactions were terminated with sodium dodecyl sulfate and precipitated with 130 μg of carrier yeast tRNA/ml and ethanol. Samples were electrophoresed on 6 or 8% polyacrylamide gels containing 8 M urea, dried, and autoradiographed.
RESULTS
Arginine-rich region of SM is required for RNA binding in vitro but is not required for transactivation function or nuclear localization.
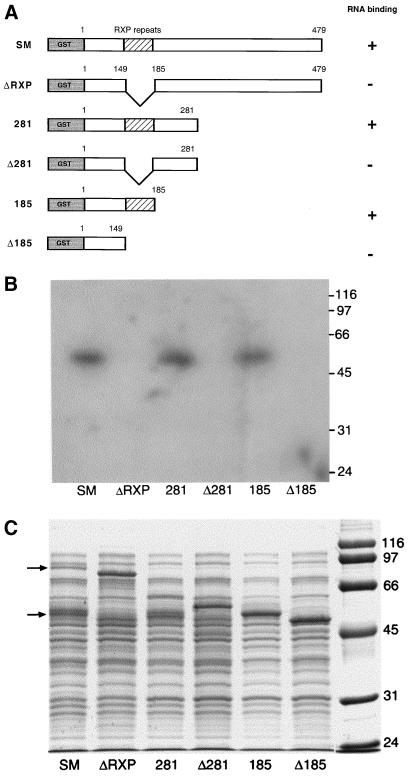
Two arginine-rich regions of ICP27, the HSV homolog of SM, have been implicated in RNA binding in vitro and in vivo (42, 47). Although it does not contain well-defined RNA recognition motifs, the SM protein sequence encodes an arginine-rich region from aa 152 to 182 (18). This region contains 11 arginines, including eight triplet repeats of the pattern RXP, where X is usually alanine. Our investigators have previously shown that bacterially produced SM-GST fusion proteins can bind radiolabeled RNA molecules in vitro (45). Similar findings were also reported by Semmes et al. (50). We found that the RNA-binding capacity was located in the amino-terminal half of the protein. Specifically, a fusion protein consisting of GST and aa 1 to 281 of SM was shown to be capable of binding labeled CAT mRNA when bound to membranes (Northwestern assay) as well as in solution (45). In order to further delineate the region of SM involved in binding RNA in vitro, we performed Northwestern assays with several internally deleted or truncated mutants of SM fused to GST. Three fusion proteins consisting of GST fused with full-length SM, aa 1 to 281, or aa 1 to 185 were synthesized in bacteria. Three corresponding deletion mutants were also produced in which the arginine-rich regions (aa 149 to 185) were removed (Fig. 1A). The results of a Northwestern assay performed with these fusion proteins and a CAT RNA probe are shown in Fig. 1B. All three fusion proteins which contained the arginine-rich region (SM, 281, and 185) bound RNA, whereas all three RXP-deleted fusions (ΔRXP, Δ281, and Δ185) did not. Thus, deletion of aa 149 to 185 completely abolished the ability of any of the SM fusion proteins to bind the probe RNA, indicating that this region was required for RNA binding. Surprisingly, although the length of the different fusion proteins varied, the size of the protein which bound RNA on Northwestern assay was the same in all cases, approximately 55 kDa (Fig. 1B). Examination of the wild-type SM-GST fusion protein on Coomassie-stained gels revealed, in addition to a band at the expected size (90 kDa), an additional prominent doublet band at approximately 55 kDa (Fig. 1C). A similar band of identical mobility at approximately 55 kDa was also seen with the 281 lysate. Taking into account the contribution of GST to the size of the fusion protein, these data indicate that a proteolytic site was present at approximately the center of the arginine-rich region in SM. Consistent with such an interpretation is the finding that when the arginine-rich region was removed (as in ΔRXP and Δ281), only a band of the expected size for the fusion protein was present and no smaller cleavage product was seen (Fig. 1C).
FIG. 1.
Analysis of in vitro RNA binding by SM mutants. (A) Structure of GST-SM fusion proteins. GST fused to the amino terminus of SM mutants is shown in gray. Amino acids at sites of deletion or truncation are shown above each diagram. The arginine-rich region (RXP repeats) is shown with diagonal lines. Potential cleavage sites in the arginine-rich region are depicted by the wavy line. (B) Northwestern assay. Lysates of bacteria expressing full-length or mutant SM fusion proteins were electrophoresed, transferred to polyvinylidene difluoride membranes, and probed with single-stranded radioactively labeled CAT RNA probe. Molecular masses are shown at right in kilodaltons. (C) Coomassie-stained gel of proteins analyzed by Northwestern assay in panel B. Locations of full-length GST-SM (upper arrow) and its major cleavage product (lower arrow) are shown at left. Molecular masses are shown at right in kilodaltons.
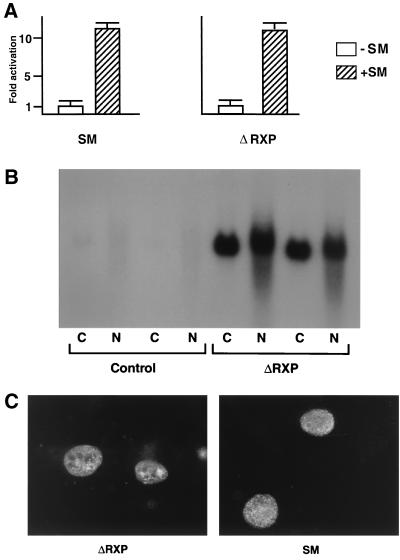
As is clear from the Northwestern assay (Fig. 1B) and although they contain the RXP domain, the intact (uncleaved) forms of SM and 281 do not bind RNA in vitro. Therefore, it appears that cleavage at the proteolytic site, resulting in the exposure of an arginine-rich region at the carboxy terminus of the protein fragment, is required for in vitro RNA binding. It has been previously reported that a deletion mutant of SM, similar to ΔRXP, is capable of mediating RNA export (4). Coupled with these data, our findings suggested that in vitro RNA binding by SM may not be relevant to its in vivo function. In order to determine whether ΔRXP SM, which is completely unable to bind RNA in vitro, nevertheless retains some or all of its activation function, we compared the ability of ΔRXP SM and wild-type (wt) SM to activate CAT in a cotransfection assay. BJAB cells (from an EBV-negative B-cell lymphoma) were transfected with wt or ΔRXP SM expression plasmid and a CMV promoter-driven CAT reporter plasmid by electroporation. As shown in Fig. 2A, ΔRXP was fully functional in its ability to activate CAT expression.
FIG. 2.
Transactivation function and cellular localization of the ΔRXP mutant. (A) Activation of CAT by ΔRXP and SM. BJAB cells were transfected with CMV-CAT reporter plasmid and either ΔRXP, SM, or control plasmid. CAT assays were performed on cellular lysates 36 h after transfection. (B) Measurement of CAT RNA in ΔRXP-transfected cells. BJAB cells were transfected with CMV-CAT reporter plasmid and either ΔRXP or control plasmid. RNA was isolated from cytoplasmic (C) and nuclear (N) fractions and analyzed by Northern blotting with CAT probe. (C) Nuclear localization of SM and ΔRXP. COS-7 cells were transfected with either SM or ΔRXP. Forty-eight hours after transfection, cells were washed, fixed, and stained with polyclonal anti-SM antibodies and Texas Red-labeled secondary antibodies.
We also wished to confirm that ΔRXP enhanced cytoplasmic and nuclear accumulation of target mRNA in a manner similar to wt SM. We therefore isolated cytoplasmic and nuclear RNA from BJAB cells transfected with CMV-CAT plasmid and either ΔRXP or control plasmid and performed Northern blotting using a CAT probe. As shown in Fig. 2B, ΔRXP strongly enhanced accumulation of CAT mRNA in both nucleus and cytoplasm. These data therefore indicate that SM-mediated activation and enhanced RNA accumulation do not require the arginine-rich domain.
We next examined whether the intracellular localization of SM was affected by deletion of the in vitro RNA-binding domain. Arginine-rich motifs are commonly found in regions involved in both RNA binding and nuclear localization. For example, arginine-rich regions of ICP27 are required for nuclear localization, and certain HSV ICP27 mutations that alter RNA binding also affect their nuclear distribution (23, 42). COS-7 cells were transfected with either ΔRXP or wt SM plasmid and examined by immunofluorescence 48 h after transfection. As shown in Fig. 2C, deletion of the RXP motifs does not affect the nuclear staining pattern of SM. Thus, the ability of SM to bind RNA in vitro is completely dispensable for transactivation of CAT and for proper nuclear localization of the SM protein. These findings, although somewhat surprising, do not exclude the possibility that SM binds RNA in vivo, either directly or indirectly, via other proteins.
SM binds mRNA in vivo.
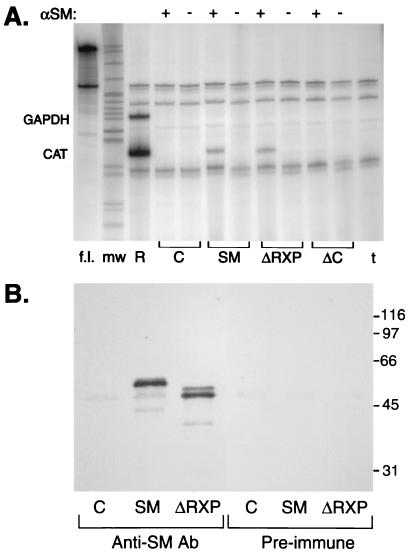
Although the ability of SM to bind RNA in vitro did not correlate with its activation function, many aspects of SM activity suggest that it interacts with the mRNA of its target genes in vivo. We therefore wished to determine whether SM protein could bind RNA in vivo and developed the following assay to measure mRNA binding by SM in transfected cells. First, target RNAs and SM or SM mutant proteins were expressed in COS-7 cells by transfecting plasmids encoding the target CAT gene and CMV promoter-driven SM or mutant-SM expression vectors. Forty-eight hours after transfection, the cells were lysed in the presence of both protease and RNase inhibitors. A fraction of the lysate was reserved for RNA and protein analysis, and the remainder was immunoprecipitated with either anti-SM antibodies or preimmune serum. Any RNA that was coimmunoprecipitated was isolated by protease treatment followed by extraction with phenol and ethanol precipitation. The presence of specific mRNAs in the immunoprecipitates was then quantitated using an RPA. The results of a representative experiment are shown in Fig. 3A. SM immunoprecipitates contained clearly detectable CAT mRNA. Comparison with the amount of CAT mRNA present in RNA isolated directly from the same cell lysate as used for the IP (Fig. 3A, lane R) indicated that approximately 10% of the total CAT mRNA in the cells was present in an immunoprecipitable complex with SM. ΔRXP, which lacks the in vitro RNA binding domain, also bound CAT mRNA in vivo, with an efficiency indistinguishable from that of wt SM. RPA was performed simultaneously with antisense RNA probes specific for CAT and GAPDH, an abundant cellular transcript. Although GAPDH mRNA was easily detectable in the total RNA (as seen in Fig. 3A, lane R), it was not present in the anti-SM immunoprecipitates, demonstrating that the association of CAT mRNA with SM was, to some degree, specific. Controls were performed with preimmune serum and SM-transfected cells or with anti-SM antibodies and control vector-transfected cells. These were both negative for the presence of CAT mRNA (Fig. 3A). A carboxy-terminal deletion mutant of SM (ΔC) which is nonfunctional and poorly soluble (data not shown) also did not form immunoprecipitable complexes with CAT mRNA.
FIG. 3.
In vivo binding of RNA by SM and SM mutants. (A) COS-7 cells were transfected with CMV CAT and either control vector (C), SM, ΔRXP, or ΔC mutants. Lysates from transfected cells were precipitated with either preimmune serum (−) or anti-SM antibodies (+). RNA was prepared from immunoprecipitates and analyzed by RPA to detect CAT and GADPH RNAs. Locations of protected GADPH and CAT fragments are shown at left. RPA was also performed using total RNA from CAT- and SM-transfected cells (R) or tRNA (t) as a control. Full-length probe is shown in lane labeled f.l. mw, molecular size markers. (B) Immunoprecipitates from cells transfected with either vector plasmid (C), SM, or ΔRXP were electrophoresed and immunoblotted with anti-SM antibodies. Lysates were immunoprecipitated with anti-SM antibodies or with preimmune serum.
Although ΔRXP is not cleaved when produced in bacteria and is fully competent to bind CAT mRNA in vivo, as shown above, it was nevertheless possible that a cleaved fragment of wt SM was produced in vivo and was responsible for some of the binding capacity observed in the IP-RPA experiments. We therefore reserved a fraction of the immunoprecipitates used for the RPA and analyzed them by immunoblotting with anti-SM antibodies. As shown in Fig. 3B, full-length SM was present in the immunoprecipitates from SM-transfected cells and a cleavage product was not detected. Therefore, exposure of the arginine-rich region by proteolytic cleavage or truncation during synthesis, as observed in bacteria and required for in vitro RNA binding, does not occur in mammalian cells and is not required for in vivo RNA binding.
Gene-specific activation by SM correlates with ability of SM to enhance cytoplasmic accumulation of target gene mRNAs.
SM activates genes by a promoter-independent mechanism. Expression of reporter genes is activated by SM when they are transcribed from either EBV or other heterologous viral promoters (30). Activation of a variety of bacterial and cellular reporter genes as well as EBV genes has been demonstrated to occur at the posttranscriptional level (5, 30, 45). Nevertheless, activation by SM is gene specific. For example, in cotransfection assays, SM increases the cytoplasmic accumulation of mRNAs for several EBV genes involved in DNA replication but not of that for the BBLF2/3 gene (50). Similarly, in earlier studies it had been reported that expression of CAT, but not β-galactosidase, was activated by SM (30). The basis for these differences has remained unclear. In order to further study the mechanism of selective activation by SM, we compared the effect of SM on three commonly used reporter genes whose expression is easily quantitated at the RNA and protein levels. BJAB cells were cotransfected with either SM or antisense control plasmid and the reporter plasmid. In these experiments, the reporter plasmids encoded hGH cDNA, luciferase, or CAT. SM strongly increased the expression of CAT, as has been demonstrated previously, and the increase in protein activity corresponded well with the increase in cytoplasmic CAT mRNA (approximately 10-fold) (Fig. 4). However, SM did not increase the level of luciferase activity or the amount of secreted hGH (Fig. 4A). As shown in Fig. 4B, the accumulation of luciferase or GH mRNA in the cytoplasm was also not increased by SM. One possible explanation for these findings is that SM is able to bind some RNAs but not others. Such specific binding could potentially result in the stabilization and/or increased nuclear export of some but not all mRNAs. It should be noted that there is a moderate increase in the nuclear accumulation of all three mRNAs when SM is cotransfected (approximately two- to threefold), which we have observed consistently (data not shown).
FIG. 4.
Gene-specific activation by SM. (A) Effect of SM on protein expression. BJAB cells were transfected with either control vector (−SM) or SM expression vector (+SM). CAT lysates were prepared 48 h later, and protein levels were measured. CAT assays were performed with [14C]chloramphenicol. Thin-layer chromatography was performed and quantitated by direct counting of acetylated fractions. Luciferase activity was measured with a luminometer, and hGH was measured by radioimmunoassay. Each value represents the mean of three individual experiments, and error bars represent the standard error of the mean. (B) Effect of SM on nuclear and cytoplasmic RNA levels. BJAB cells were transfected as for panel A and RNA was prepared from nuclear and cytoplasmic fractions. Ten micrograms of each RNA was electrophoresed, transferred to nylon membrane, and hybridized to radiolabeled hGH, luciferase, or CAT DNA probe. Each blot was stripped and rehybridized to GAPDH probe to verify equal loading.
Differential mRNA binding is not the basis for gene specificity of SM-mediated activation.
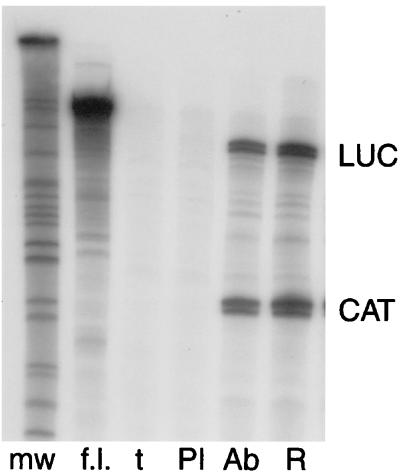
Because SM had clearly distinguishable effects on the accumulation of different mRNAs, we wished to determine whether the efficiency of mRNA binding by SM in vivo depended on the sequence of the specific target mRNA. Such an ability to discriminate between various mRNA targets could allow SM to specifically activate some but not all EBV genes. Intronless genes are known to be inherently poorly expressed, and the expression of many viral genes lacking introns is known to be facilitated by the presence of specific RNA sequences that permit the binding of cellular or viral proteins that increase their cytoplasmic accumulation. Examples include human immunodeficiency virus (HIV) RNAs with rev-responsive elements, the HSV thymidine kinase gene, which contains an hnRNP L-binding motif, and the constitutive transport element of type D retroviruses (3, 36, 38). Importantly, ICP27, the HSV homolog of SM, has been reported to bind some lytic HSV mRNAs with greater affinity than others (47). We therefore applied our assay to ask whether SM bound the mRNAs of a responsive target gene (CAT) with greater avidity than it bound the mRNA of a nonresponsive target (luciferase). As in the previous experiments, COS-7 cells were transfected with SM plasmid and either CMV-luciferase or CMV-CAT. Equal numbers of cells from SM-luciferase and SM-CAT transfections were pooled and lysed together. Aliquots were reserved for direct preparation of RNA, and the remainder of the lysates was immunoprecipitated with either anti-SM antibodies or preimmune serum. RNA was isolated from the immunoprecipitates and subjected to an RPA using probes for the luciferase and CAT genes simultaneously. RNA prepared directly from the transfected cells was analyzed in parallel as a measure of the relative amounts of luciferase and CAT mRNAs present in the cells (Fig. 5). As in the previous experiments, CAT mRNA was precipitated by anti-SM antibodies but not by preimmune serum. Surprisingly, luciferase mRNA was also immunoprecipitable in association with SM, with an efficiency indistinguishable from that of CAT mRNA. These data indicate that the presence or absence of a specific SM-response element in the mRNA sequence is not a likely explanation for the gene specificity displayed by SM. We initially chose to pool luciferase plus SM- and CAT plus SM-transfected cells in an effort to avoid potential complications introduced by competition for SM in the same cell by the two target mRNA species and the variables introduced by the unknown frequencies of cotransfection of the three individual plasmids. However, we have subsequently repeated the experiment by cotransfecting the three plasmids simultaneously and we obtained a similar result (data not shown). These results therefore indicate that although SM binds luciferase mRNA with considerable affinity, similar to that which it displays for CAT mRNA, it nevertheless does not significantly increase the cytoplasmic accumulation of luciferase transcripts. This difference may reflect intrinsic differences between the two mRNAs, such as the presence or absence of binding motifs capable of mediating interaction with other host cell proteins (see Discussion).
FIG. 5.
SM binds to transcripts of SM-responsive and SM-unresponsive genes. COS-7 cells were transfected with SM and CMV-CAT or CMV-luc plasmids. Lysates were prepared 48 h after transfection, pooled, and immunoprecipitated with either preimmune serum (PI) or anti-SM antibodies (Ab). RNA was prepared from immunoprecipitates, and RPA was performed to detect CAT and luciferase mRNA. Total RNA was also prepared from pooled CMV-luc- and CMV-CAT- transfected cells and analyzed in parallel (R). Control RPAs were also performed with tRNA (t). Unhydrolyzed full-length luciferase and CAT probes (which are approximately the same length) are also shown (f.l.). mw, molecular size markers.
SM binds newly transcribed RNA.
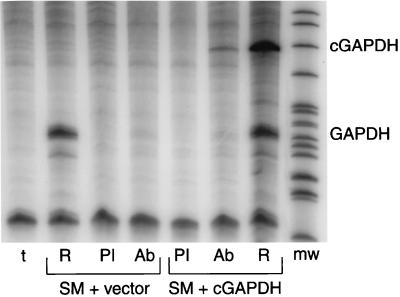
The finding that SM was capable of binding luciferase mRNA also raised the question of why SM was apparently incapable of binding host GAPDH mRNA in the IP-RPAs shown in Fig. 3. In fact, it was this apparently specific association of SM with CAT mRNA, but not with GAPDH mRNA, that led to the hypothesis that SM specifically associates with certain mRNAs. However, GAPDH mRNA has a relatively low turnover rate, and the majority of the steady-state amount of GAPDH mRNAs had been synthesized and processed prior to the 48 h during which mRNA was synthesized from transfected genes before RNA isolation. Thus, most of the GAPDH mRNA present in the cells transfected with SM plasmid was not undergoing nuclear posttranscriptional processing and was perhaps unavailable for interaction with SM. Consistent with such an analysis is the fact that unlike the transfected reporter mRNAs, the majority of GAPDH mRNA detected by Northern blotting was found in the cytoplasm (Fig. 4B). We therefore performed the following experiment to ask whether SM could bind newly transcribed GAPDH mRNA. We first cloned the human GAPDH cDNA in the same CMV promoter-driven expression vector as had been used for our other reporter genes. In order to be able to differentiate newly synthesized GAPDH mRNA from host cell GAPDH mRNA, we inserted a small DNA fragment from the β-lactamase gene upstream of the initiator codon for GAPDH in the GAPDH expression vector, as described in Materials and Methods. This addition allowed the differentiation of transcripts derived from the transfected GAPDH gene from those derived from the endogenous host cell GAPDH gene. We transfected COS-7 cells with the marked GAPDH expression vector and either SM or control plasmid. Forty-eight hours after transfection, lysates were prepared as described previously and immunoprecipitated with either anti-SM antibodies or preimmune serum. RNA was prepared from the immunoprecipitates and analyzed for the presence of GAPDH transcripts by RPA. As shown in Fig. 6, when RNA isolated directly from the transfected cells was simultaneously measured by RPA, the transfected GAPDH gene was seen to be expressed at levels comparable to those of the host cell GAPDH gene. As was the case in previous experiments, such as the one shown in Fig. 3, host cell-derived GAPDH mRNA was not detectable in association with SM. However, anti-SM antibodies did immunoprecipitate GAPDH mRNA newly transcribed from the transfected GAPDH gene. It should also be noted that SM associated only with the latter RNA species (derived from transfected GAPDH gene) despite the fact that the endogenous cellular GAPDH mRNA was present in similar amounts (Fig. 6). Approximately 10% of the total RNA derived from the transfected GAPDH gene was recovered in association with SM, an efficiency comparable to that seen with luciferase and CAT mRNA. These data suggest that SM-RNA association in vivo may be sequence independent but occurs primarily with newly transcribed RNA, perhaps in association with one or more of the multiple steps in posttranscriptional RNA processing.
FIG. 6.
SM binds to newly synthesized RNA. COS-7 cells were transfected with SM and either GAPDH cDNA plasmid (cGAPDH) or empty vector. Cell lysates were immunoprecipitated with preimmune serum (PI) or anti-SM antibodies (Ab). RNA was prepared from immunoprecipitates and RPA was performed to detect GAPDH RNAs. Total RNA from each set of transfected cells was prepared and analyzed by RPA in parallel (R). The sizes of fragments protected by RNA from transfected GAPDH (cGADPH) or endogenous cellular GAPDH (GAPDH) are shown at the right. Probe incubated with tRNA was also analyzed in parallel as a specificity control (t).
DISCUSSION
SM protein, like its homologs in other herpesviruses, is a posttranscriptional regulator of gene expression. Although many of these proteins have been extensively studied, many aspects of their interaction with mRNA remain incompletely understood. In this report, we have demonstrated that SM protein associates with newly transcribed mRNA molecules in vivo and we have further characterized several aspects of its interaction with RNA. SM had previously been shown to bind RNA in vitro, and it had been suggested that such binding was target-RNA specific. SM contains a highly arginine-rich region, and arginine-containing motifs of ICP27 have been linked to its ability to bind RNA (41, 42). Although the arginine-rich domain of the SM protein was absolutely required for the ability of SM to bind RNA in vitro, we found that this domain and the ability to bind RNA in vitro were dispensable for its gene activation function. The ability of the RXP triplet domain to mediate in vitro RNA binding was in fact dependent on cleavage or truncation of the full-length SM protein such that the RXP domain became the 3′-terminal portion of the protein. Further, mutant SM protein lacking the RXP domain was stable in vivo and localized normally in the nucleus of transfected cells. Buisson et al. have previously shown by reverse transcription-PCR that SM mutants lacking this region are nevertheless capable of facilitating the cytoplasmic accumulation of reporter mRNAs (4). These data indicate that while the RXP domain may play some other role in native SM function, its ability to bind RNA in vitro is not relevant to SM-mediated gene activation or intracellular localization.
Although the RXP domain was dispensable for SM function, the question as to whether SM physically associates with mRNA in vivo remained to be answered. Based on several lines of evidence, it appeared likely that SM interacts with RNA directly or in concert with other proteins. SM does not directly increase the rate of transcript initiation of activated target genes as measured in nuclear run-on assays (45). However, the amounts of nuclear and cytoplasmic CAT mRNA, in both the total and polyadenylated fractions, are increased in cells expressing SM (45). SM, like the herpesvirus saimiri ORF57 and HSV ICP27 gene products, colocalizes with splicing components in cell nuclei (13, 48, 50). SM has been reported to associate with proteins involved in pre-mRNA processing and nuclear export, including hnRNP C, exportin 1 (CRM 1), and the nucleoporin Nup 214 (Can) (2, 32). SM also enhances pre-mRNA processing of the EBV DNA polymerase transcript (32). Using the methods described in this report, we have shown that specific mRNAs can be detected in complexes with SM protein. There are several notable aspects to these SM-RNA interactions. First, a significant proportion of the target mRNA present in the cell is found in association with SM. Based on the amount of total CAT and luciferase mRNA present in the cell lysate, at least 10% was precipitated by anti-SM antibodies. Second, the association appears to be confined to newly transcribed mRNAs. This conclusion is based on the fact that mRNA transcribed from a transfected GAPDH gene was associated with SM but cellular GAPDH mRNA was not. It should be noted that the association of SM detected in these experiments may be indirect and mediated by one or more RNA-binding proteins, since the experimental conditions were designed to maintain protein-protein interactions.
It has been previously shown that SM activates some but not all genes, whether of EBV, bacterial, or eukaryotic origin (39, 45, 50). We have demonstrated in this report that gene specificity of SM activation correlates with the ability of SM to increase the cytoplasmic accumulation of the target mRNA. It was therefore an attractive hypothesis that SM specificity might depend on the presence of SM binding elements in SM-responsive mRNAs. Such response elements are the basis for nuclear export of unspliced viral RNAs of HIV, Mason-Pfizer monkey virus, and related type D retroviruses (3, 38). In both cases, the viral RNA is bound by a viral protein (HIV rev) or host cell proteins that facilitate nuclear RNA export. Similarly, a positive processing element in the HSV thymidine kinase mRNA is specifically bound by hnRNP L and enhances expression of thymidine kinase and also other intronless transcripts to which it is linked (36). Somewhat surprisingly, we found that SM bound luciferase mRNA and CAT mRNA with equal affinity, although luciferase expression was completely unresponsive to SM. These data therefore strongly suggest that SM specificity is not based solely on the ability of SM to interact with the target mRNA.
Interestingly, a moderate but consistent increase in nuclear accumulation of target mRNA was observed when SM was coexpressed, regardless of whether the target gene was SM responsive. Thus, nuclear CAT, luciferase, and hGH RNA levels were all increased by SM, although only cytoplasmic CAT mRNA amounts and CAT protein activity were significantly increased by SM. These data suggest that SM may generally enhance the nuclear accumulation of nascent RNA transcripts. However, it appears that other factors determine whether SM is also capable of enhancing the cytoplasmic accumulation of mRNA to which it is bound. There are several possible mechanisms by which such selectivity might operate. First, SM-nonresponsive genes, such as luciferase and EBV BBLF2/3, may contain nuclear restriction elements that SM cannot overcome. Such elements may consist of binding sites for cellular proteins that limit the rate of nuclear export. Alternatively, the mRNAs of responsive genes such as CAT and EBV BMRF1 may contain binding sites for one or more cellular proteins that SM cooperates with to facilitate cytoplasmic accumulation. Intronless genes, in particular, may contain distinctive protein-binding mRNA sequence elements that compensate for the intrinsic inefficiency with which they are exported to the cytoplasm (24, 26–28, 36). The presence of such elements may therefore lead to the assembly of a unique set of proteins on individual mRNPs. Such differences in the proteins which decorate each mRNP would be expected to have various effects on its nuclear export.
It has been well established that the presence of introns in a gene facilitates cytoplasmic accumulation of the final spliced mRNA (25). Injection of synthesized intronless mRNA into Xenopus oocyte nuclei leads to inefficient cytoplasmic accumulation of the mRNA (37). It has recently been shown that in the process of splicing, certain cell proteins remain attached after detachment of spliceosome components (29, 34, 55). These proteins, such as Aly and Y14, are predominantly nuclear nucleocytoplasmic shuttling proteins and do not associate with mRNAs from intronless cDNAs (29, 55). It is likely that SM plays a role similar to these cell proteins in enhancing nuclear export of viral intronless cDNAs, which would otherwise be at a disadvantage in export to the cytoplasm. The net effect of SM on a specific mRNA is therefore likely to be dependent on the other RNA-binding proteins which comprise its mRNP particle. Further dissection of SM-responsive and nonresponsive mRNAs as well as identification of host cell proteins which bind to SM should yield further insight into the mechanism of posttranscriptional, gene-specific activation by SM.
ACKNOWLEDGMENT
This work was supported by Public Health Service grant CA 81133 from the National Cancer Institute.
REFERENCES
- 1.Bello L J, Davison A J, Glenn M A, Whitehouse A, Rethmeier N, Schulz T F, Clements J B. The human herpesvirus-8 ORF 57 gene and its properties. J Gen Virol. 1999;80:3207–3215. doi: 10.1099/0022-1317-80-12-3207. [DOI] [PubMed] [Google Scholar]
- 2.Boyle S M, Ruvolo V, Gupta A K, Swaminathan S. Association with the cellular export receptor CRM 1 mediates function and intracellular localization of Epstein-Barr virus SM protein, a regulator of gene expression. J Virol. 1999;73:6872–6881. doi: 10.1128/jvi.73.8.6872-6881.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bray M, Prasad S, Dubay J W, Hunter E, Jeang K T, Rekosh D, Hammarskjold M L. A small element from the Mason-Pfizer monkey virus genome makes human immunodeficiency virus type 1 expression and replication Rev-independent. Proc Natl Acad Sci USA. 1994;91:1256–1260. doi: 10.1073/pnas.91.4.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buisson M, Hans F, Kusters I, Duran N, Sergeant A. The C-terminal region but not the Arg-X-Pro repeat of Epstein-Barr virus protein EB2 is required for its effect on RNA splicing and transport. J Virol. 1999;73:4090–4100. doi: 10.1128/jvi.73.5.4090-4100.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buisson M, Manet E, Trescol-Biemont M C, Gruffat H, Durand B, Sergeant A. The Epstein-Barr Virus (EBV) early protein EB2 is a posttranscriptional activator expressed under the control of EBV transcription factors EB1 and R. J Virol. 1989;63:5276–5284. doi: 10.1128/jvi.63.12.5276-5284.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chavrier P, Gruffat H, Chevalier-Greco A, Buisson M, Sergeant A. The Epstein-Barr Virus (EBV) early promoter DR contains a cis-acting element responsive to the EBV transactivator EB1 and an enhancer with constitutive and inducible activities. J Virol. 1989;63:607–614. doi: 10.1128/jvi.63.2.607-614.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chee M, Barrell B. Herpesviruses: a study of parts. Trends Genet. 1990;6:86–91. doi: 10.1016/0168-9525(90)90099-r. [DOI] [PubMed] [Google Scholar]
- 8.Cheung P, Ellison K S, Verity R, Smiley J R. Herpes simplex virus ICP27 induces cytoplasmic accumulation of unspliced polyadenylated alpha-globin pre-mRNA in infected HeLa cells. J Virol. 2000;74:2913–2919. doi: 10.1128/jvi.74.6.2913-2919.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chevalier-Greco A, Manet E, Chavrier P, Mosnier C, Daillie J, Sergeant A. Both Epstein-Barr virus (EBV)-encoded trans-acting factors, EB1 and EB2, are required to activate transcription from an EBV early promoter. EMBO J. 1986;5:3243–3249. doi: 10.1002/j.1460-2075.1986.tb04635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho M S, Jeang K T, Hayward S D. Localization of the coding region for an Epstein-Barr virus early antigen and inducible expression of this 60-kilodalton nuclear protein in transfected fibroblast cell lines. J Virol. 1985;56:852–859. doi: 10.1128/jvi.56.3.852-859.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 12.Cook I D, Shanahan F, Farrell P J. Epstein-Barr virus SM protein. Virology. 1994;205:217–227. doi: 10.1006/viro.1994.1637. [DOI] [PubMed] [Google Scholar]
- 13.Cooper M, Goodwin D J, Hall K T, Stevenson A J, Meredith D M, Markham A F, Whitehouse A. The gene product encoded by ORF 57 of herpesvirus saimiri regulates the redistribution of the splicing factor SC-35. J Gen Virol. 1999;80:1311–1316. doi: 10.1099/0022-1317-80-5-1311. [DOI] [PubMed] [Google Scholar]
- 14.Davison A J, Taylor P. Genetic relations between varicella-zoster virus and Epstein-Barr virus. J Gen Virol. 1987;68:1067–1079. doi: 10.1099/0022-1317-68-4-1067. [DOI] [PubMed] [Google Scholar]
- 15.DeNoto F M, Moore D D, Goodman H M. Human growth hormone DNA sequence and mRNA structure: possible alternative splicing. Nucleic Acids Res. 1981;9:3719–3730. doi: 10.1093/nar/9.15.3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellison K S, Rice S A, Verity R, Smiley J R. Processing of alpha-globin and ICP0 mRNA in cells infected with herpes simplex virus type 1 ICP27 mutants. J Virol. 2000;74:7307–7319. doi: 10.1128/jvi.74.16.7307-7319.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farjot G, Buisson M, Duc Dodon M, Gazzolo L, Sergeant A, Mikaelian I. Epstein-Barr virus EB2 protein exports unspliced RNA via a Crm-1-independent pathway. J Virol. 2000;74:6068–6076. doi: 10.1128/jvi.74.13.6068-6076.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farrell P. Epstein-Barr virus genome. Adv Viral Oncol. 1989;8:103–132. [Google Scholar]
- 19.Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981;23:175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- 20.Gupta A K, Ruvolo V, Patterson C, Swaminathan S. The human herpesvirus 8 homolog of Epstein-Barr virus SM protein (KS-SM) is a posttranscriptional activator of gene expression. J Virol. 2000;74:1038–1044. doi: 10.1128/jvi.74.2.1038-1044.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardwicke M A, Sandri-Goldin R M. The herpes simplex virus regulatory protein ICP27 contributes to the decrease in cellular mRNA levels during infection. J Virol. 1994;68:4797–4810. doi: 10.1128/jvi.68.8.4797-4810.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hardy W R, Sandri-Goldin R M. Herpes simplex virus inhibits host cell splicing, and regulatory protein ICP27 is required for this effect. J Virol. 1994;68:7790–7799. doi: 10.1128/jvi.68.12.7790-7799.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hibbard M K, Sandri-Goldin R M. Arginine-rich regions succeeding the nuclear localization region of the herpes simplex virus type 1 regulatory protein ICP27 are required for efficient nuclear localization and late gene expression. J Virol. 1995;69:4656–4667. doi: 10.1128/jvi.69.8.4656-4667.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang J, Liang T J. A novel hepatitis B virus (HBV) genetic element with Rev response element-like properties that is essential for expression of HBV gene products. Mol Cell Biol. 1993;13:7476–7486. doi: 10.1128/mcb.13.12.7476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang M T F, Gorman C M. Intervening sequences increase efficiency of RNA 3′ processing and accumulation of cytoplasmic RNA. Nucleic Acids Res. 1990;18:937–947. doi: 10.1093/nar/18.4.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang Y, Carmichael G G. The mouse histone H2a gene contains a small element that facilitates cytoplasmic accumulation of intronless gene transcripts and of unspliced HIV-1-related mRNAs. Proc Natl Acad Sci USA. 1997;94:10104–10109. doi: 10.1073/pnas.94.19.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang Y, Wimler K M, Carmichael G G. Intronless mRNA transport elements may affect multiple steps of pre-mRNA processing. EMBO J. 1999;18:1642–1652. doi: 10.1093/emboj/18.6.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang Z M, Yen T S. Role of the hepatitis B virus posttranscriptional regulatory element in export of intronless transcripts. Mol Cell Biol. 1995;15:3864–3869. doi: 10.1128/mcb.15.7.3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kataoka N, Yong J, Kim V N, Velazquez F, Perkinson R A, Wang F, Dreyfuss G. Pre-mRNA splicing imprints mRNA in the nucleus with a novel RNA-binding protein that persists in the cytoplasm. Mol Cell. 2000;6:673–682. doi: 10.1016/s1097-2765(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 30.Kenney S, Kamine J, Holley-Guthrie E, Mar E C, Lin J C, Markovitz D, Pagano J. The Epstein-Barr virus immediate-early gene product, BMLF1, acts in trans by a posttranscriptional mechanism which is reporter gene dependent. J Virol. 1989;63:3870–3877. doi: 10.1128/jvi.63.9.3870-3877.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kenney S, Kamine J, Markovitz D, Fenrick R, Pagano J. An Epstein-Barr virus immediate-early gene product trans-activates gene expression from the human immunodeficiency virus long terminal repeat. Proc Natl Acad Sci USA. 1988;85:1652–1656. doi: 10.1073/pnas.85.5.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Key S C, Yoshizaki T, Pagano J S. The Epstein-Barr virus (EBV) SM protein enhances pre-mRNA processing of the EBV DNA polymerase transcript. J Virol. 1998;72:8485–8492. doi: 10.1128/jvi.72.11.8485-8492.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirshner J R, Lukac D M, Chang J, Ganem D. Kaposi's sarcoma-associated herpesvirus open reading frame 57 encodes a posttranscriptional regulator with multiple distinct activities. J Virol. 2000;74:3586–3597. doi: 10.1128/jvi.74.8.3586-3597.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Hir H, Moore M J, Maquat L E. Pre-mRNA splicing alters mRNP composition: evidence for stable association of proteins at exon-exon junctions. Genes Dev. 2000;14:1098–1108. [PMC free article] [PubMed] [Google Scholar]
- 35.Lieberman P M, O'Hare P, Hayward G S, Hayward S D. Promiscuous trans activation of gene expression by an Epstein-Barr virus-encoded early nuclear protein. J Virol. 1986;60:140–148. doi: 10.1128/jvi.60.1.140-148.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu X, Mertz J E. HnRNP L binds a cis-acting RNA sequence element that enables intron-dependent gene expression. Genes Dev. 1995;9:1766–1780. doi: 10.1101/gad.9.14.1766. [DOI] [PubMed] [Google Scholar]
- 37.Luo M J, Reed R. Splicing is required for rapid and efficient mRNA export in metazoans. Proc Natl Acad Sci USA. 1999;96:14937–14942. doi: 10.1073/pnas.96.26.14937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malim M H, Hauber J, Le S Y, Maizel J V, Cullen B R. The HIV-1 rev trans-activator acts through a structured target sequence to activate nuclear export of unspliced viral mRNA. Nature. 1989;338:254–257. doi: 10.1038/338254a0. [DOI] [PubMed] [Google Scholar]
- 39.Markovitz D M, Kenney S, Kamine J, Smith M S, Davis M, Huang E-S. Disparate effects of two herpesviruses immediate-early gene trans-activators on the HIV LTR. Virology. 1989;173:750–754. doi: 10.1016/0042-6822(89)90591-6. [DOI] [PubMed] [Google Scholar]
- 40.McGeoch D J, Dalrymple M A, Davison A J, Dolan A, Frame M C, McNab D, Perry L J, Scott J E, Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988;69:1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- 41.Mears E, Rice S A. The RGG box motif of the herpes simplex virus ICP27 protein mediates an RNA-binding activity and determines in vivo methylation. J Virol. 1996;70:7445–7453. doi: 10.1128/jvi.70.11.7445-7453.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mears W E, Lam V, Rice S A. Identification of nuclear and nucleolar localization signals in the herpes simplex virus regulatory protein ICP27. J Virol. 1995;69:935–947. doi: 10.1128/jvi.69.2.935-947.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Menezes J, Liebold W, Klein G, Clements G. Establishment and characterization of an Epstein-Barr virus (EBV)-negative lymphoblastoid B cell line (BJA-B) from an exceptional EBV-genome-negative African Burkitt's lymphoma. Biomedicine. 1975;22:276–284. [PubMed] [Google Scholar]
- 44.Perera L P, Kaushal S, Kinchington P R, Mosca J D, Hayward G S, Straus S E. Varicella-zoster virus open reading frame 4 encodes a transcriptional activator that is functionally distinct from that of herpes simplex virus homolog ICP27. J Virol. 1994;68:2468–2477. doi: 10.1128/jvi.68.4.2468-2477.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruvolo V, Wang E, Boyle S, Swaminathan S. The Epstein-Barr virus nuclear protein SM is both a post-transcriptional inhibitor and activator of gene expression. Proc Natl Acad Sci USA. 1998;95:8852–8857. doi: 10.1073/pnas.95.15.8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sample J, Lancz G, Nonoyama M. Mapping of genes in BamHI fragment M of Epstein-Barr virus DNA that may determine the fate of viral infection. J Virol. 1986;57:145–154. doi: 10.1128/jvi.57.1.145-154.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sandri-Goldin R M. ICP27 mediates HSV RNA export by shuttling through a leucine-rich nuclear export signal and binding viral intronless RNAs through an RGG motif. Genes Dev. 1998;12:868–879. doi: 10.1101/gad.12.6.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sandri-Goldin R M, Hibbard M K, Hardwicke M A. The C-terminal repressor region of herpes simplex virus type 1 ICP27 is required for the redistribution of small nuclear ribonucleoprotein particles and splicing factor SC35; however, these alterations are not sufficient to inhibit host cell splicing. J Virol. 1995;69:6063–6076. doi: 10.1128/jvi.69.10.6063-6076.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sandri-Goldin R M, Mendoza G E. A herpesvirus regulatory protein appears to act post-transcriptionally by affecting mRNA processing. Genes Dev. 1992;63:848–863. doi: 10.1101/gad.6.5.848. [DOI] [PubMed] [Google Scholar]
- 50.Semmes O J, Chen L, Sarisky R T, Gao Z, Zhong L, Hayward S D. Mta has properties of an RNA export protein and increases cytoplasmic accumulation of Epstein-Barr virus replication gene mRNA. J Virol. 1998;72:9526–9534. doi: 10.1128/jvi.72.12.9526-9534.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tso J Y, Sun X H, Kao T H, Reece K S, Wu R. Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNAs: genomic complexity and molecular evolution of the gene. Nucleic Acids Res. 1985;13:2485–2502. doi: 10.1093/nar/13.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Winkler M, Rice S A, Stamminger T. UL69 of human cytomegalovirus, an open reading frame with homology to ICP27 of herpes simplex virus, encodes a transactivator of gene expression. J Virol. 1994;68:3943–3954. doi: 10.1128/jvi.68.6.3943-3954.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wong K-M, Levine A J. Identification and mapping of Epstein-Barr virus early antigens and demonstration of a viral gene activator that functions in trans. J Virol. 1986;60:149–156. doi: 10.1128/jvi.60.1.149-156.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xiao L, Casero R A., Jr Differential transcription of the human spermidine/spermine N3-acetyltransferase (SSAT) gene in human lung carcinoma cells. Biochem J. 1996;313:691–696. doi: 10.1042/bj3130691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou Z, Luo M J, Straesser K, Katahira J, Hurt E, Reed R. The protein Aly links pre-messenger-RNA splicing to nuclear export in metazoans. Nature. 2000;407:401–405. doi: 10.1038/35030160. [DOI] [PubMed] [Google Scholar]