Abstract
In a population-based birth cohort study of respiratory syncytial virus surveillance in the United States, 897/1,680 (53.4%) children were infected during infancy; 25 (2.8%) of those were hospitalized. Among symptomatic infants, 143/324 (44.1%) had lower respiratory tract infections. These data provide benchmarks to monitor effects of maternal vaccines and extended half-life monoclonal antibodies.
Keywords: respiratory syncytial virus, respiratory infections, RSV, risk factors, infant, United States, viruses
Respiratory syncytial virus (RSV) is a leading cause of illness in infants (1). Previous epidemiologic studies of RSV infection during infancy have focused on symptomatic illness, predominantly lower respiratory tract infections (LRTI) and hospitalizations (2). However, population-based surveillance studies to determine prevalence of and risk factors for RSV infection among healthy infants in the United States are lacking.
We determined prevalence of RSV infection by 1 year of age in a population-based birth cohort of healthy term infants in the United States. We excluded infants from the parent study, Infant Susceptibility to Pulmonary Infections and Asthma Following RSV Exposure (INSPIRE) (3,4), if they were not enrolled during well-child visits from 1 of 11 participating regional pediatric practices. We ascertained RSV infections by active surveillance using quantitative reverse transcription PCR (qRT-PCR) testing of nasal samples collected based on symptom surveys every 2 weeks and by passive surveillance by serum RSV antibody testing of all infants at 1 year of age during 2 RSV seasons, 2012–13 and 2013–14. If an infant met specified criteria for an acute respiratory infection, we conducted an in-person respiratory illness assessment and collected a nasal wash sample, which we used for the molecular detection of RSV by qRT-PCR. We also collected blood samples from all participating infants at 1 year of age and measured RSV serum antibody titers by ELISA using published protocols (5). We calculated 1-year prevalence of RSV infections, upper respiratory tract infections, LRTI, and healthcare utilization. We estimated the adjusted association and relative contribution of risk factors for RSV infection in the first year of life using multivariable logistic regression. The Institutional Review Board of Vanderbilt University approved INSPIRE, and 1 parent of each child provided written informed consent. INSPIRE methods have been published, and full study methods are available (3,4) (Appendix).
Among 1,680 infants who met inclusion criteria for our study, 897 (53.4%) were infected with RSV in the first year of life and 783 (46.6%) were not (Figure 1; Appendix Figure). Active surveillance detected 36.1% of RSV infections in symptomatic infants, whereas 63.9% were ascertained by serology alone. In all study infants, 1.5% (95% CI 0.96%–2.1%; n = 25) were hospitalized for RSV and 8.5% (95% CI 7.2%–9.9%; n = 143) had RSV LRTI. Among the subset with RSV infection, 2.8% (95% CI 1.9%–4.1%; n = 25) were hospitalized for RSV and 15.9% (95% CI 13.6%–18.4%; n = 143) had RSV LRTI. Restricted to the subset with symptomatic RSV infections meeting study criteria for a respiratory illness visit (n = 324), 55.9% had upper respiratory tract infections and 44.1% LRTIs. There were no infant deaths from RSV infection. We found that more than half of infants were RSV-infected in the first year of life. The rates of symptomatic disease, LRTI, and healthcare utilization demonstrate the considerable burden of respiratory illness in otherwise healthy infants; 1.5% of the cohort, or 2.8% of those infected, experienced RSV-associated hospitalizations.
Figure 1.
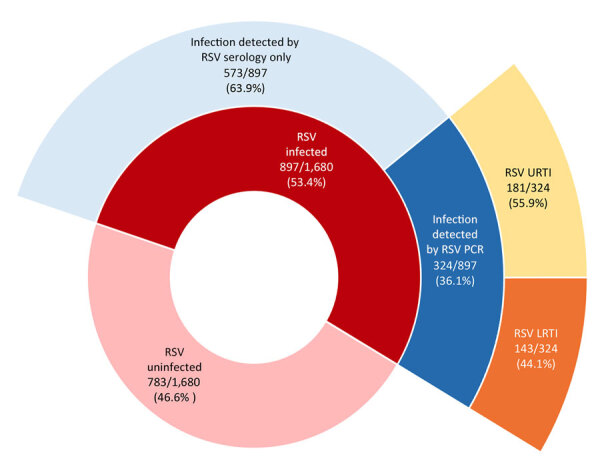
Percentages of RSV infection, symptomatic disease, LRTI, and URTI in the first year of life among healthy term infants, United States. Each ring represents the subset of the inner ring and adds to 100%. LRTI, lower respiratory tract infection; RSV, respiratory syncytial virus; URTI, upper respiratory tract infections.
Risk factors for RSV infection during infancy in order of contribution were infant birth month (June vs. referent October, OR 2.42 [95% CI 1.78–3.29]), presence of siblings (OR 1.50 [95% CI 1.22–1.84]), daycare attendance (OR 1.54 [95% CI 1.24–1.93]), increasing percentage below the poverty level in the residential neighborhood (21% vs. 8%; OR 1.19 [95% CI 1.05–1.36]), and public insurance (OR 1.28, 95% CI 1.02–1.62) (Figure 2). Secondhand smoke exposure, sex, ever being breastfed, maternal asthma, and study year were not significantly associated with likelihood of infant RSV infection.
Figure 2.
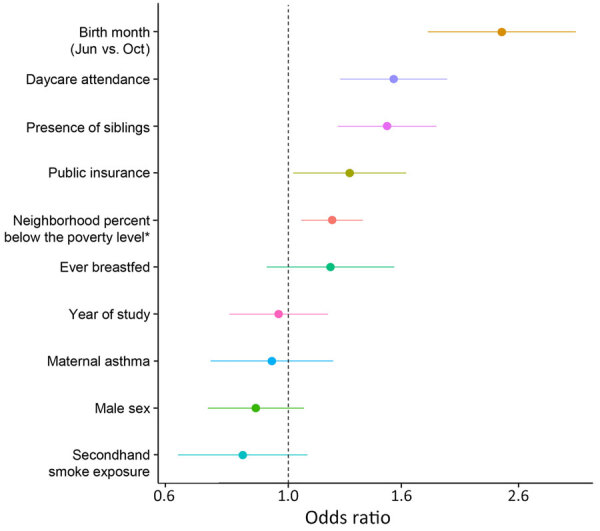
Respiratory syncytial virus infection risk factors in the first year of life in study of RSV among healthy term infants, United States. Adjusted odds ratios were estimated using multivariable logistic regression, referent is October birth month. Dots indicate odds ratio and horizontal line 95% CI. Dashed vertical line indicates the null effect. Asterisk indicates residence in a census tract with increasing percentage of people below the poverty level (interquartile range difference).
The risk factors we identified increase viral exposure and underscore the potential for nonpharmacologic interventions to prevent infection (6). Earlier birth month was the strongest risk factor; we speculate that parental behaviors based on infant age affect exposure intensity (e.g., age at which infants are started in daycare). The risk factors of neighborhood percentage poverty and public insurance indicate the need to address socioeconomic determinants of RSV prevention.
The first limitation of our study is that eligibility criteria and sociodemographic characteristics might not be generalizable to other populations. In addition, our cohort represents a population that may be healthier, because they were term infants; however, this group represents half of RSV hospitalizations who are eligible for RSV prevention products. There is also potential for misclassification of infants categorized as uninfected with RSV in infancy (4). However, the proportions of both those with symptomatic respiratory illness and overall RSV serologic positivity are very similar to estimates from other studies (7–9). Although this cohort represents 2 RSV seasons during 2012–2014, we do not expect that time period to significantly affect the rates or risk factors for infection, which were similar across both RSV seasons.
In conclusion, our data are important estimates of the burden of RSV disease and risk factors for infection in healthy term infants. Our findings provide a benchmark to monitor the effects in the United States of recently available maternal vaccines and extended half-life monoclonal antibodies for severe RSV illness prevention in early life (10).
Additional information about respiratory syncytial virus prevalence and risk factors among healthy term infants, United States.
Acknowledgments
We thank the middle Tennessee pediatric practice sites and all the INSPIRE participants and their families for their involvement in and dedication to this study.
This work was supported with funds from the US National Institute of Allergy and Infectious Diseases (award nos. U19AI095227, UG3OD023282, and K24AI77930); the Vanderbilt Institute for Clinical and Translational Research (grant support from the National Center for Advancing Translational Sciences, award no. UL1TR000445); the US National Heart, Lung, and Blood Institute (award no. K23HL148638); the Department of Pediatrics at Vanderbilt University Medical Center (grant support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, award no. K12HD087023); and the Vanderbilt University Training Program in Environmental Toxicology (NIH T32 ES007028). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies. The National Institutes of Health had no role in the design and conduct of the study.
Author contributions: F.C. conceptualized and designed the study, created the figures, drafted the initial manuscript, and critically reviewed and revised the manuscript. T.G. performed the statistical analysis, created the figures, and critically reviewed and revised the manuscript. L.J.A. and J.D.C. contributed to acquisition of data and biospecimen analysis and critically reviewed and revised the manuscript. C.R.-S. and J.R.O. contributed to analysis and interpretation of data and critically reviewed and revised the manuscript. T.H. designed the cohort and acquired study funding, coordinated and supervised study conduct, conceptualized and designed the current analysis, and critically reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
Biography
Dr. Cacho is a pediatric pulmonology fellow at Vanderbilt University Medical Center and a graduate student in the MPH program at Vanderbilt University School of Medicine. His research interests are in the impact of early-life respiratory infections and the characterization of childhood asthma phenotypes.
Footnotes
Suggested citation for this article: Cacho F, Gebretsadik T, Anderson LJ, Chappell JD, Rosas-Salazar C, Ortiz JR, et al. Respiratory syncytial virus prevalence and risk factors among healthy term infants, United States. Emerg Infect Dis. 2024 Oct [date cited]. https://doi.org/10.3201/eid3010.240609
References
- 1.Li Y, Wang X, Blau DM, Caballero MT, Feikin DR, Gill CJ, et al. ; Respiratory Virus Global Epidemiology Network; RESCEU investigators. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet. 2022;399:2047–64. 10.1016/S0140-6736(22)00478-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rha B, Curns AT, Lively JY, Campbell AP, Englund JA, Boom JA, et al. Respiratory syncytial virus–associated hospitalizations among young children: 2015–2016. Pediatrics. 2020;146:e20193611. 10.1542/peds.2019-3611 [DOI] [PubMed] [Google Scholar]
- 3.Larkin EK, Gebretsadik T, Moore ML, Anderson LJ, Dupont WD, Chappell JD, et al. ; INSPIRE Study. Objectives, design, and enrollment results from the Infant Susceptibility to Pulmonary Infections and Asthma Following RSV Exposure Study (INSPIRE). BMC Pulm Med. 2015;15:45. 10.1186/s12890-015-0040-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosas-Salazar C, Chirkova T, Gebretsadik T, Chappell JD, Peebles RS Jr, Dupont WD, et al. Respiratory syncytial virus infection during infancy and asthma during childhood in the USA (INSPIRE): a population-based, prospective birth cohort study. Lancet. 2023;401:1669–80. 10.1016/S0140-6736(23)00811-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jadhao SJ, Ha B, McCracken C, Gebretsadik T, Rosas-Salazar C, Chappell J, et al. Performance evaluation of antibody tests for detecting infant respiratory syncytial virus infection. J Med Virol. 2021;93:3439–45. 10.1002/jmv.26736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Binns E, Koenraads M, Hristeva L, Flamant A, Baier-Grabner S, Loi M, et al. Influenza and respiratory syncytial virus during the COVID-19 pandemic: time for a new paradigm? Pediatr Pulmonol. 2022;57:38–42. 10.1002/ppul.25719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wildenbeest JG, Billard MN, Zuurbier RP, Korsten K, Langedijk AC, van de Ven PM, et al. ; RESCEU Investigators. The burden of respiratory syncytial virus in healthy term-born infants in Europe: a prospective birth cohort study. Lancet Respir Med. 2023;11:341–53. 10.1016/S2213-2600(22)00414-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munywoki PK, Koech DC, Agoti CN, Cane PA, Medley GF, Nokes DJ. Continuous invasion by respiratory viruses observed in rural households during a respiratory syncytial virus seasonal outbreak in coastal Kenya. Clin Infect Dis. 2018;67:1559–67. 10.1093/cid/ciy313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zylbersztejn A, Pembrey L, Goldstein H, Berbers G, Schepp R, van der Klis F, et al. Respiratory syncytial virus in young children: community cohort study integrating serological surveys, questionnaire and electronic health records, Born in Bradford cohort, England, 2008 to 2013. Euro Surveill. 2021;26:2000023. 10.2807/1560-7917.ES.2021.26.6.2000023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langedijk AC, Bont LJ. Respiratory syncytial virus infection and novel interventions. Nat Rev Microbiol. 2023;21:734–49. 10.1038/s41579-023-00919-w [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional information about respiratory syncytial virus prevalence and risk factors among healthy term infants, United States.


