Abstract
Human T-cell leukemia virus type 1 (HTLV-1) establishes a persistent infection in the host despite a vigorous virus-specific immune response. Here we demonstrate that an HTLV-1-encoded protein, p12I, resides in the endoplasmic reticulum (ER) and Golgi and physically binds to the free human major histocompatibility complex class I heavy chains (MHC-I-Hc) encoded by the HLA-A2, -B7, and -Cw4 alleles. As a result of this interaction, the newly synthesized MHC-I-Hc fails to associate with β2-microglobulin and is retrotranslocated to the cytosol, where it is degraded by the proteasome complex. Targeting of the free MHC-I-Hc, and not the MHC-I-Hc–β2-microglobulin complex, by p12I represents a novel mechanism of viral interference and disrupts the intracellular trafficking of MHC-I, which results in a significant decrease in surface levels of MHC-I on human T-cells. These findings suggest that the interaction of p12I with MHC-1-Hc may interfere with antigen presentation in vivo and facilitate escape of HTLV-1-infected cells from immune recognition.
Human T-cell leukemia virus type 1 (HTLV-1) is the etiologic agent of adult T-cell leukemia/lymphoma (ATLL) (16, 55, 37), as well as the neurologic disorder tropical spastic paraparesis/HTLV-1-associated myelopathy (TSP-HAM) (14, 33, 42). HTLV-1 induces a lifelong chronic infection, which may result in ATLL in 1 to 5% of carriers 20 to 30 years after infection. Despite a vigorous host immune response, HTLV-1 persists in the infected host, suggesting that the virus may have developed strategies to evade the host's immune response, as is the case with other chronic viruses (36, 49).
The major histocompatibility complex class I (MHC-I) molecules, which are essential for presentation of foreign peptides to the host cytotoxic T lymphocytes (CTL), are targets of many pathogens, including viruses (36, 49). CTL recognize virus-infected cells through the specific interaction of their T-cell receptor with an MHC-I molecule presenting a viral peptide. The MHC-I complex consists of a heavy chain (Hc) containing the peptide binding site and β2-microglobulin, which assemble very rapidly in the lumen of the endoplasmic reticulum (ER). Peptides, generated by the proteasome in the cytoplasm, are translocated by TAP (transporter associated with antigen processing) into the ER where they assemble in ternary complexes and are transported to the cell surface for presentation to CTL (54). Interference with the assembly and/or trafficking of the MHC-I complex can contribute to the persistence of a virus, although natural killer (NK) cells can recognize and lyse cells that lack MHC-I antigens (19).
Several viruses that induce chronic infections encode proteins that target or modulate the host's immune system (36, 49). Adenovirus was the first virus shown to affect antigen presentation; the E3/19K adenovirus protein binds to MHC-I in the ER and prevents its transport to the cell surface (49). In addition, the E3/19K protein binds TAP and prevents TAP-class I association, thereby interfering with peptide loading (3). Human cytomegalovirus (HCMV) encodes multiple proteins that target MHC-I synthesis, peptide loading, and transport. Murine CMV glycoprotein, gp34, also interacts with the Hc–β2-microglobulin complex in the ER and has been recently shown to target MHC-I for degradation in the lysosomes (49). ICP47, a protein encoded by herpes simplex virus, inhibits the TAP transporter (36, 49). More recently, it was demonstrated that the K3 and K5 proteins encoded by Kaposi's sarcoma-associated herpesvirus downregulated MHC-I from the cell surface (17).
The human immunodeficiency virus (HIV) or simian deficiency virus (SIV) Nef protein downregulates both CD4 and MHC-I expression at the cell surface by interacting with the intracellular sorting machinery of the cell (1, 6, 27, 46). Binding of Nef to a vacuolar ATPase results in the internalization and degradation of CD4 (26). Nef also misroutes MHC-I complexes to the clathrin-coated vesicles (25, 46).
In the case of HTLV-1, alterations in HLA expression on the cell surface have been demonstrated in peripheral mononuclear lymphocytes isolated from patients with adult T-cell leukemia, as well as in HTLV-1-infected cell lines (28, 47, 51). A loss of HLA antigens on the surface of cells from asymptomatic carriers and a gain in their cell surface expression after the development of ATLL has also been suggested (47). Ectopically expressed Tax, the viral transactivator, has also been shown to increase MHC-I expression on the surface of transfected glial cells (44), an event that could contribute to escape from NK cells (51).
The x-I open reading frame of HTLV-1 encodes a protein termed p12I that exhibits weak oncogenic activity, shares amino acid similarities with the bovine papillomavirus type 1 E5 oncoprotein (13), and binds to the interleukin-2 receptor (IL-2R) β and γc chains (30). Although p12I expression has been difficult to demonstrate in HTLV-1-infected cells, indirect evidence suggests its importance. The spliced mRNA encoding p12I has been detected in vitro and ex vivo HTLV-1-infected T-cell lines and macrophages (5, 21, 22). Sera from rabbits experimentally infected with HTLV-1, or sera from humans infected with HTLV-1, recognize the ORF-1 protein product (9). Moreover, a CTL response to the ORF-1 products can be detected in HTLV-1-infected individuals (35). Two natural variants of the p12I protein have been identified; one carries a lysine at position 88 and is found mainly in HTLV-1 strains from TSP-HAM patients; the second carries an arginine at position 88 and is found in HTLV-1 strains from all ATLL patients and healthy carriers studied (50). The p12IR88 protein has a much greater stability compared to the p12IK88 protein, which is ubiquitinated and rapidly degraded by the proteasome (50), suggesting that this sequence variation might be important. The functional relevance of the natural variants of p12I remains unclear and, while p12I does not appear necessary for HTLV-1 replication in vitro (10, 41), the appropriate splicing and expression of the ORF-1 mRNA is necessary for the establishment of persistent infection in an in vivo rabbit model (8).
MATERIALS AND METHODS
Expression plasmids and antibodies.
The pME18S expression vector was obtained from Atsushi Miyajima (DNAX, Palo Alto, Calif.) and contains a hybrid promoter consisting of the simian virus 40 early region promoter and the R region of the HTLV-1 long terminal repeat (LTR). This plasmid was used to express the p12IK88 and p12IR88 cDNAs, tagged with the HA1 epitope (23).
The lentiviral HIV-based retroviral vector HR′CMV-Luc, the CMV-driven HIV helper virus deleted for the envelope and Nef genes (pDNL6), and the HIV LTR-vesicular stomatitis virus G protein (VSV-G) envelope have been previously reported (31). HA1-tagged p12IR88 was amplified by PCR, sequenced and cloned between BamHI and XhoI restriction sites (HRCMV p12IHA1), as described by Nicot et al. (32a).
The porcine MHC-1-Hc expression vector, PD55, was a generous gift from Dinah Singer (R. Ehlrich and D. Singer, unpublished results). The HLA-A2 and B7 expression plasmids were generous gifts from Olivier Schwartz (25). The HLA-A2 tailless and HLA-Cw4 plasmids were kindly provided by Hidde Ploegh (48).
The αHA1 antibody (clone 12CA5) utilized for immunoprecipitation was from Roche (Indianapolis, Ind.) and the αAU1 antibody was from Covance (Richmond, Calif.). Antibodies against MHC-I antigens include: PT85A, which recognized both complexed and free porcine MHC-I molecules (VMRD, Pullman, Wash.); the A2 antibody, which recognized predominantly human Hc–β2-microglobulin complexes, was produced by the BB7.2 mouse hybridoma (ATCC, Manassas, Va.) and was kindly provided by Steven Jacobson; W6/32, which recognized predominantly human Hc–β2-microglobulin complexes (Harlan, Loughborough, England); and rabbit αHc serum, which recognized the free Hc of human HLA-A, -B, and -C alleles was kindly provided by Hidde Ploegh (2); the β2-microglobulin antibody was from Dako (Carpinteria, Calif.); and the calnexin antibody (Calbiochem, San Diego, Calif.).
DNA transfection and metabolic labeling.
One million HeLa-Tat cells were plated in a 10-cm dish and transfected the following day with 10 μg of each plasmid using the calcium phosphate method (15). The total DNA transfected was normalized to 20 μg in each transfection. At 24 h after transfection, cells were incubated for 1 h in medium that lacks methionine and cysteine and was supplemented with 2 mM l-glutamine. Cells were then metabolically labeled for 2 to 3 h with 100 μCi of EXPRES35S35 (NEN Life Sciences, Boston, Mass.) per ml. Cells were washed with 1× phosphate-buffered saline (PBS) and lysed in 1 ml of 1× RIPA buffer (1% deoxycholic acid, 0.1% sodium dodecyl sulfate [SDS], 1% Trition X-100, 0.15 M NaCl, 50 mM Tris-Cl [pH 7.5]) containing 20 μg of aprotinin (Sigma, St. Louis, Mo.) per ml, 20 μg of leupeptin (Roche) per ml, 1 mM AEBSF (ICN, Aurora, Ohio), and 0.5 mM dithiothreitol (DTT; Sigma) and sheared through a 25-gauge needle. Lysates were precleared for 2 h at 4°C with 50 μl of protein A-agarose beads (Roche) and 20 μl of normal rabbit serum. The supernatant was incubated overnight at 4°C with 5 μg of specific antibody. Bound immunocomplexes were extensively washed in cold 1× RIPA buffer and boiled in 2× SDS-loading buffer (Novex, San Diego, Calif.) and β-mercaptoethanol. Proteins were analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) on 10 and 15% polyacrylamide gels. Gels were placed for 20 min in Enlightening-Rapid Autography Enhancing solution (NEN Life Sciences), dried under vacuum, and exposed to film.
In pulse-chase experiments, cells were starved for 1 h in medium lacking fetal calf serum (FCS), labeled for 10 min (“pulse”), washed in 1× PBS, and then “chased” for the indicated time points in starvation medium supplemented with 1% FCS, 2 mM l-glutamine, 10× methionine, and 10× cysteine. All subsequent manipulations were performed as described previously.
For Endo-H (endoglycosidase H) treatment, cells were labeled and immunoprecipitated as described above. After the washing of bound immunocomplexes in 1× RIPA buffer, 20 mU of Endo H (Roche) or an equivalent volume of sodium phosphate for control tubes was added to each immunoprecipitate, and the mixture was incubated overnight at 37°C. Each sample was then resuspended in 2× SDS-loading buffer (Novex), boiled, and analyzed by SDS-PAGE.
Lactacystin treatment.
HeLa-Tat cells were transfected as described above with the addition of the following: 10 μM lactacystin (Calbiochem, San Diego, Calif.) or as a control, the equivalent volume of dimethyl sulfoxide (DMSO) was added to the starvation medium for 1 h. Cells were then metabolically pulsed for 10 min in labeling medium supplemented with 10 μM lactacystin or DMSO, which was followed by a chase for the indicated time points. All subsequent manipulations were performed as described previously.
Confocal microscopy.
The dual-staining immunofluorescence studies were carried out using transfected HeLa-Tat cells. At 30 to 40 h after transfection, cells were analyzed by indirect immunofluorescence after being fixed in paraformaldehyde (3.7%) and permeabilized with Nonidet P-40 (0.1%). AU1-tagged p12I was detected using αAU1 antibody. The plasmid pCMV-Go-GFP was generated by fusing the Golgi targeting sequence of sialyl transferase (34) to the Aequorea victoria green fluorescent protein (GFP) open reading frame (38). Expression was driven by the human CMV promoter and the protein was detected using a rabbit polyclonal serum recognizing GFP (a gift of Markus Neumann, GSF, Munich, Germany). Cells were stained with a mixture of rabbit αGFP sera and mouse αAU1 antibody, followed by a mixture of Texas red-conjugated anti-mouse antibody to detect p12I and fluorescein isothiocyanate (FITC)-conjugated anti-rabbit antibody to detect Go-GFP. The sample was then analyzed by laser scanning confocal microscopy using excitation wavelengths of 488 and 543 nm. The ER compartment was visualized using a rabbit polyclonal antiserum specific for calreticulin (Affinity Bioreagents, Inc.) and was visualized with a Texas red-conjugated secondary antibody at an excitation wavelength of 568 nm. For this study, p12I-AU1 was visualized with an FITC-conjugated secondary antibody at an excitation wavelength of 488 nm. FITC- and Texas red-conjugated secondary antibodies were purchased from Sigma and Jackson Immunoresearch Laboratories (West Grove, Pa.), respectively. Laser scanning confocal microscopy was performed using a Leica TCS-SP system equipped with Argon and helium-neon laser sources using a ×60 objective (see Fig. 5A to C) or an Olympus system equipped with an argon-krypton laser source using a ×60 objective (Fig. 5D to F).
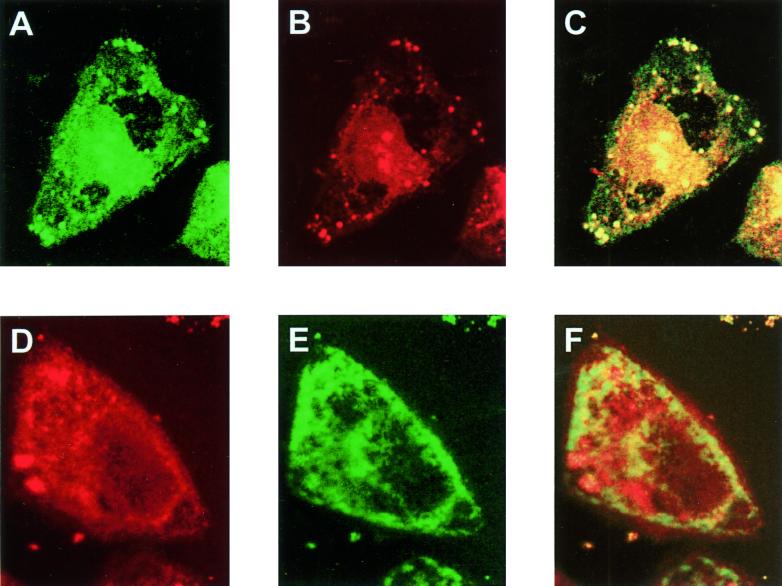
FIG. 5.
Intracellular localization of p12I. HeLa-Tat cells were cotransfected with pCMV-p12I-AU1 and pCMV-Go-GFP, which expresses a Golgi-targeted GFP. (A) The G-GFP protein, detected as a green signal. (B) p12I, detected as a red signal. (C) Overlay of panels A and B. (D and E). Staining of pCMV-p12I-AU1-transfected HeLa-Tat cells with antibodies to calreticulin, an ER-resident protein (red signal) (D), and αAU1 (green signal) (E) to detect p12I. (F) Overlay of panels D and E.
The MHC-p12I confocal microscopy studies were performed on HeLa-Tat cells following transfection with 1 μg of porcine MHC-I-Hc and 5 μg of p12IR88. At 48 h after transfection, cells were fixed in 2% paraformaldehyde, permeabilized in 0.1% saponin (Sigma), and stained with PT85A or αHA1, followed by the addition of a secondary anti-mouse antibody conjugated with Cy2 for detection (Jackson ImmunoResearch). The 63× objective was used for all imaging. The samples were then analyzed by confocal microscopy at the DBS, LRBGE Fluorescence Imaging Facility (NIH, Bethesda, Md.) with the assistance of J. McNally and T. Karpova.
Pseudotype virus production and concentration.
293T cells were seeded at 2 × 106 in a 10-cm dish and transfected the following day with VSV-G (2 μg), CMVHIV (4 μg), and HR′CMV-Luc or HR′CMV p12I (4 μg) using the Effectene reagent kit (Quiagen) according to the manufacturer's instructions. Supernatant from 20 dishes was collected every 12 h from 24 to 72 h, cleared of cellular debris by centrifugation at 8,000 × g for 10 min at room temperature, filtered (0.45 μm [pore size]), and stored at −80°C. Pseudotype viruses were pelleted by ultracentrifugation at 50,000 × g (28,000 rpm using an SW41 rotor) for 1 h and 45 min at 4°C. Virus was resuspended in PBS for 4 h on ice, collected, aliquoted, and stored at −80°C. HIV GAG p24 was measured using an antigen capture assay, and infections were performed using comparable amounts of virus particles. Infectivity and proper expression was further verified by immunofluorescence.
Flow cytometric analysis of infected Jurkat cells.
Jurkat cells were infected with an equivalent concentration of pseudotyped virus carrying either p12I or the luciferase genes in RPMI 1640–2% FCS at 37°C with 5% CO2. After 4 h, the concentration of FCS was increased to 10%. At days 2, 4, and 5 postinfection, cells were removed, washed in PBS, incubated at 4°C with phycoerythrin (PE)-conjugated CD3 or CD4 antibody (PharMingen, San Diego, Calif.) or with anti-human MHC-I antibody, W6/32 (Sigma), followed by a PE-conjugated secondary antibody (PharMingen). Cells were washed with PBS–1% FCS, fixed in 2% paraformaldehyde, and analyzed on a Becton Dickinson FacScan.
RESULTS
p12I binds to newly synthesized MHC-I-Hc before its association with β2-microglobulin.
The fact that HTLV-1 persists in the host despite the vigorous virus-specific host immune response suggests that this pathogen has developed mechanisms to evade immune recognition. We hypothesize that the p12I protein of HTLV-1 may affect the MHC-I-Hc–β2-microglobulin complex on the basis of the observation that, like the HIV/SIV Nef protein, which affects class I-restricted antigen presentation (7), p12I is essential for efficient viral propagation in vivo (8). To investigate whether one or both of the two natural variants of p12I interact with the MHC-I complex, we performed coimmunoprecipitation assays on lysates of HeLa-Tat cells transfected with plasmids expressing HA1-tagged p12I cDNA and the porcine MHC-I-Hc cDNA; porcine MHC-I-Hc was used in these initial experiments because of its ease of detection. As expected, the tagged p12IK88 and its ubiquitinated forms were readily immunoprecipitated with the HA1 antibody, as demonstrated in lanes 1 and 2 of Fig. 1B. Interestingly, the 46-kDa porcine MHC-I-Hc protein coimmunoprecipitated with p12IK88 (Fig. 1A and B, lane 2), indicating that the two proteins interact with each other. Neither p12IK88 nor the porcine Hc immunoprecipitated with an isotype-matched negative control immunoglobulin G (IgG) antibody (Fig. 1A and B, lanes 7 to 9). Surprisingly, endogenous β2-microglobulin did not coimmunoprecipitate with p12IK88 and MHC-I-Hc. This finding, combined with the observation that p12I appeared to bind faster-migrating forms of MHC-I-Hc (data not shown), suggested that p12IK88 may bind to a less-glycosylated form of MHC-I-Hc, perhaps in the ER, before its association with β2-microglobulin. Since the association of the Hc with β2-microglobulin occurs quite rapidly, within 4 min after translation (32), it is possible that p12I competes with β2-microglobulin for binding to newly synthesized Hc. This hypothesis was further supported by the finding that the PT85A antibody, which mainly recognizes the Hc–β2-microglobulin complex, readily precipitated both the MHC-I-Hc and the β2-microglobulin but did not coprecipitate p12IK88 (Fig. 1B, lane 5). In parallel experiments, the other variant of p12I, p12IR88, also was demonstrated to bind to the free MHC-I-Hc (data not shown).
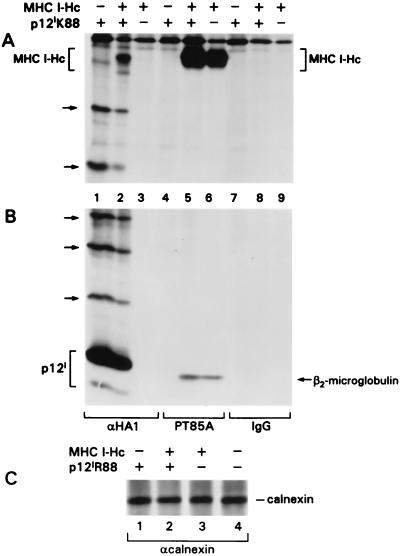
FIG. 1.
The HTLV-1 p12I protein binds to the free MHC-I-Hc but not to the MHC-I-Hc–β2-microglobulin complex. Radioimmunoprecipitation was performed on lysates of transfected HeLa-Tat cells with αHA1, PT85A, or isotype-matched IgG antibodies. Lysates were analyzed on 10% (A) and 15% (B) acrylamide gels. Arrows indicate the expression of p12IK88 (lanes 1 and 2) and MHC-I-Hc and β2-microglobulin (lanes 5 and 6) and the coimmunoprecipitation of free MHC I-Hc and p12IK88 with the HA1 antibody (lane 2). Ubiquitinated forms of p12IK88 are indicated by arrows on the left. (C) Lysates were also precipitated with an αcalnexin antibody (10% acrylamide gel).
To investigate the specificity of the MHC I-Hc–p12I interaction, calnexin, an ER-resident molecular chaperone involved in the folding, assembly, and retention of proteins, including the MHC-I-Hc (39), was analyzed for its ability to bind p12I and MHC-I-Hc in contransfection assays. The levels of calnexin in the transfected cells were equivalent (Fig. 1C) and, while neither β2-microglobulin nor p12IR88 coprecipitated with calnexin, the MHC-I-Hc did (data not shown). In addition to providing evidence for the specificity of the MHC I-Hc–p12I interaction, these data suggest that p12I does not compete with calnexin for binding to MHC-I; calnexin binds to the free Hc soon after Hc translation and before its assembly with β2-microglobulin (39). Altogether, these findings indicate that p12IK88 preferentially binds free porcine MHC-I-Hc and that this binding is specific since p12I did not associate with calnexin, an ER-resident protein, in the same conditions and did not inhibit MHC-I-Hc–calnexin complex formation.
The p12I protein interacts with the human MHC-I- A2, -B7, and -Cw4 Hcs.
To determine whether p12I interacts with human MHC-I-Hc, immunoprecipitations were performed on lysates of HeLa-Tat cells cotransfected with plasmids expressing HA1-tagged p12I and human MHC-I-Hc encoded by either the A2, the B7, or the Cw4 alleles. In these experiments, the p12IR88 variant was used, since it is more stable and thus easier to detect than p12IK88 (50). Figure 2A to C shows results obtained for MHC-I-Hc-A2, analyzed using αHc serum, which recognizes free Hc, and the HA1 antibody. The αHc serum precipitated both the p12I protein and MHC-I-Hc A2 from cells cotransfected with the p12I- and A2 Hc-expressing plasmids (Fig. 2A and B, lane 8); p12I was not coprecipitated in transfections carried out in the absence of the A2 Hc plasmid, since the HeLa-Tat cell line does not express the A2 Hc and p12I does not appear to bind to the endogenous Hc alleles found in HeLa-Tat cells (Fig. 2A and B, lane 6). It was difficult to confirm the identity of A2 Hc in the αHA1 (i.e., anti-p12I) immunoprecipitate due to the presence of a nonspecific comigrating protein (Fig. 2A, lane 4). Control immunoprecipitations carried out with an antibody directed against β2-microglobulin confirmed that the lysates contained equivalent amounts of the endogenous β2-microglobulin (Fig. 2C).
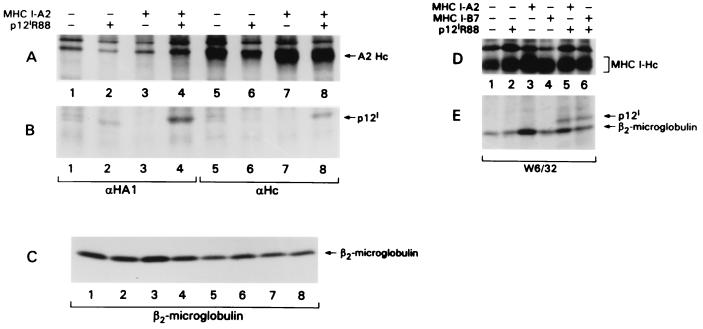
FIG. 2.
p12I binds to the human A2 and B7 MHC-I-Hc alleles. HeLa-Tat cells were transfected with cDNAs encoding the human A2 and B7 Hc genes in the presence or absence of p12I, metabolically labeled, lysed, and immunoprecipitated with αHA1 (A and B, lanes 1 to 4), αHc serum (A and B, lanes 5 to 8), or the W6/32 antibody that recognizes MHC-I A, B, and C complexes (D and E). Lysates were analyzed on 10% (A and D) and 15% (B, C, and E) acrylamide gels. (C) Cell lysates were then immunoprecipitated with antibodies recognizing β2-microglobulin to demonstrate equivalent levels in all transfected cells.
In experiments performed to test whether p12I also interacted with the human HLA-B7 Hc, transfected cell lysates were separately immunoprecipitated with either αHA1 or W6/32 antibodies. The W6/32 antibody recognized predominantly the human Hc–β2-microglobulin complex (HLA-A, -B, and -C), including the endogenous MHC alleles expressed in HeLa-Tat cells, as demonstrated in Fig. 2D. However, p12I was coprecipitated only when the exogenous A2 or B7 Hc alleles were present (Fig. 2E, lanes 5 and 6). β2-Microglobulin coprecipitated with MHC-I-Hc, as expected. However, the fact that β2-microglobulin did not coprecipitate with p12I in the αHA1 immunoprecipitates (data not shown) indicates that p12I predominantly associates with the free Hc. In a similar experiment, p12I also bound to HLA-Cw4 (data not shown). Altogether, these findings demonstrate that p12I interacts with the free chains of at least three MHC-I molecules: A2, B7, and Cw4.
p12I associates with the immature form of the MHC-I-Hc in a pre-Golgi compartment.
The absence of β2-microglobulin in the Hc-p12I complex, together with the fact that p12I binds to a faster-migrating, presumably immature form of the Hc, suggested that this interaction may occur in the ER prior to the association of MHC-I-Hc with β2-microglobulin. To test this hypothesis, pulse-chase experiments were performed following transfection of the either MHC-I-Hc alone or in the presence of p12I. The majority of the MHC-I-Hc protein band immunoprecipitated by the PT85A antibody was shifted to higher-molecular-weight forms within the 6-h chase (Fig. 3A, lanes 6 to 8), as expected, which is consistent with the occurrence of glycosylation. In contrast, the size of the MHC-I-Hc complexed to p12I did not change within 6 h of the chase (Fig. 3A, lane 3) and, interestingly, by 8 h the amount of MHC-I complexed to p12I was significantly reduced (Fig. 3A, lane 4), suggesting its possible degradation. These data indicate that p12I binds to the immature, probably incompletely glycosylated forms of the MHC-I-Hc and that this complex may not progress to the Golgi.
FIG. 3.
p12I binds to the free Endo-H-sensitive MHC-I-Hc. (A) Transfected HeLa-Tat cells were metabolically labeled, and the decay of the MHC-I-Hc was measured at the time intervals indicated. Cell lysates were immunoprecipitated with either αHA1 or PT85A antibodies. (B) The immunoprecipitates were incubated in the absence or presence of Endo-H, with the porcine MHC-I-Hc cleavage product indicated by arrows.
To verify this hypothesis, the status of the N-linked carbohydrates on the MHC-I Hc complexed with p12I was examined. Endo-H removes high-mannose H-linked oligosaccharides, an early stage in oligosaccharide processing typically found on proteins that have not yet passed from the ER through the Golgi; additional processing of the oligosaccharide chain in the Golgi renders the glycoprotein Endo-H resistant. We therefore assessed whether the MHC-I-Hc bound to p12I was sensitive to cleavage by Endo-H. To this end, MHC-I-Hc–p12I complexes were immunoprecipitated from transfected cells with antibodies to HA1-tagged p12I, and the immunoprecipitates were treated with Endo-H. The majority of MHC-I-Hc associated with p12I was sensitive to Endo-H cleavage, as demonstrated in Fig. 3B (compare lanes 4 and 5). As expected, the majority of the MHC-I-Hc associated with β2-microglobulin was resistant to Endo-H cleavage, as demonstrated by its unchanged migration rate (lanes 6 and 7 of Fig. 3B). Similar results were also obtained in experiments carried out using the p12IK88 variant (data not shown). Thus, while these data indicate that both natural variants of p12I bind to the MHC-I-Hc in the ER and prevent its association with β2-microglobulin, they also suggest that the transport of the complex to the Golgi compartment may be impaired.
The MHC-I-Hc–121 complex is degraded in the proteasome.
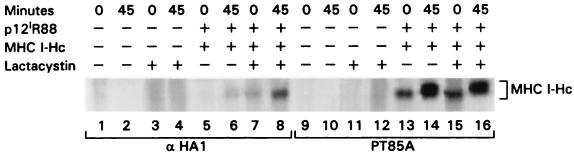
Misfolded, inappropriately glycosylated, or improperly assembled proteins are removed from the ER using the ER-associated protein degradation (ERAD) pathway and are targeted to the proteasome for degradation (4). In the pulse-chase experiment presented in Fig. 3A, the amount of Hc bound to p12I at 8 h was greatly diminished. To investigate whether, like misfolded free MHC-I-Hc molecules, the MHC-I-Hc–p12I complex was targeted for degradation via the proteasome, transfected cells were treated with lactacystin, a specific inhibitor of the proteasome (11). The fate of the Hc-p12I complex was then analyzed in a pulse-chase experiment. At time zero, the MHC-I-Hc bound to p12I was detectable only in the presence of the specific proteasome inhibitor (Fig. 4, compare lanes 5 and 7). Similarly, the amount of MHC-I-Hc bound to p12I was increased in the presence of lactacystin at 45 min (Fig. 4, compare lanes 6 and 8), suggesting that most of the immature MHC-I-Hc bound to p12I is indeed degraded by the proteasome. Altogether, these results suggest that p12I interacts with the free Hc in the ER early after Hc synthesis and before its association with the β2-microglobulin and that most of the MHC-I bound to p12I is rerouted to the cytosol and targeted to the proteasome for degradation.
FIG. 4.
The proteosome inhibitor lactacystin increases the stability of the Hc-p12I complex. Transfected HeLa-Tat cells were metabolically labeled, and the stability of the MHC-I-Hc–p12I complex was assessed by immunoprecipitation with αHA1 and PT85A in the presence or absence of lactacystin.
p12I resides in the ER and/or Golgi compartments.
p12I's association with the free MHC-I suggested that p12I might reside in the ER and/or Golgi as well. Although previous indirect immunofluorescence studies had demonstrated that p12I is membrane associated (24), its possible targeting to the membranes of specific organelles had not been investigated. To assess this, HeLa-Tat cells were therefore cotransfected with pCMV-p12I-AU1 and pCMV-Go-GFP, which expresses a Golgi-targeted GFP (34), and then subjected to immunofluorescence confocal microscopy to detect the two proteins. The G-GFP protein, green signal (Fig. 5A), and p12I, red signal (Fig. 5B), were found in similar cellular locations, as demonstrated by the almost complete overlap, which generated a yellow signal in the overlay (Fig. 5C), a finding indicative of colocalization in the Golgi.
In contrast, staining of pCMV-p12I-AU1-transfected HeLa-Tat cells with a mixture of αAU1 antibody (Fig. 5E) and antibodies to the ER-resident protein calreticulin (29) (Fig. 5D) revealed incomplete colocalization of the two proteins (Fig. 5F), suggesting that p12I transits through the ER but accumulates preferentially in the Golgi compartment.
p12I interferes with the intracellular trafficking of MHC-I and decreases the level of MHC-I from the surface of human T cells.
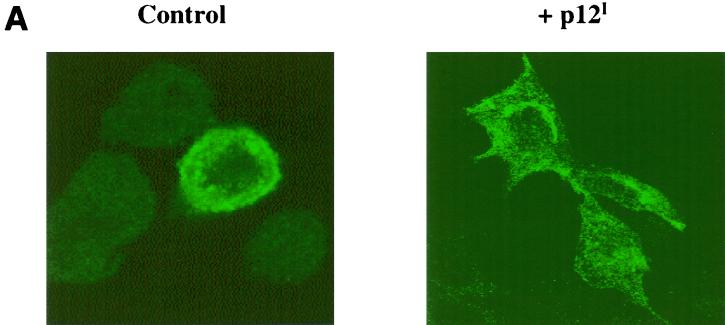
Next we investigated whether the association of MHC-I-Hc with p12I affects the trafficking of the MHC-I molecule to the cell surface. HeLa-Tat cells were therefore transfected with either the porcine MHC-I-Hc alone or together with p12I. The intracellular distribution of the MHC-I-Hc, as well as the expression of p12I, was assessed by confocal microscopy. The MHC-I staining was strongest at or near the cell surface in cells that received a control plasmid, whereas in cells cotransfected with p12I, the level of MHC-I at or near the cell surface was decreased and MHC-I appeared to be distributed predominantly in the cytoplasm or perinuclear area of these cells (Fig. 6A). These results indicate that the interaction of p12I with MHC-I-Hc in the ER interferes with normal trafficking of MHC-I to the plasma membrane.
FIG. 6.
p12I alters the trafficking of MHC-I-Hc to the plasma membrane. (A) HeLa-Tat cells were transfected with a plasmid expressing porcine MHC-I-Hc in the absence or presence of a p12I plasmid and then subjected to immunofluorescence microscopy to examine the effect of p12I on the intracellular distribution and trafficking of the MHC-I-Hc. Control, the MHC I complex is detected at or near the plasma membrane in most cells; p12I cells cotransfected with MHC-I-Hc and p12I have decreased staining at the plasma membrane and increased staining in the cytoplasm, particularly in the perinuclear region. (B) Jurkat T cells were infected with the pseudotyped viruses HR′CMV p12I or HRICMV-Luc, and the surface expression of MHC-I, CD3, and CD4 was examined by flow cytometry over time. There was an approximate 50% reduction in the level of MHC-I on the cell surface in cells expressing p12I at day 5, represented by the heavy lines, while the levels of CD3 and CD4 remain constant throughout. There was no change in MHC-I, CD3, or CD4 levels in control cells, which are represented by the thin lines.
To assess whether p12I affects the level of endogenous MHC-I, we examined human T cells, a relevant target for HTLV-1. p12I was cloned into an HIV-based retrovirus vector (31) and transduction of VSV-pseudotyped virions in human peripheral blood mononuclear cells demonstrated the appropriate localization of p12I to the ER and/or Golgi compartment (data not shown). The human T-cell line, Jurkat, was infected with VSV-pseudotyped HR′CMV p12I virus or a pseudotyped control HR′ CMV-Luc virus expressing the luciferase protein. The levels of CD3, CD4, and MHC-I at the cell surface were examined over time in the transduced culture by flow cytometry. At days 2 and 4 following infection, the level of CD3, CD4, and MHC-I surface expression did not significantly change in cells transduced with p12I or the luciferase gene (Fig. 6B, left and middle panels). In contrast, by day 5 the mean intensity of the endogenous MHC-I on the cell surface of T cells transduced with HR′ CMV p12I was decreased by approximately 50% (mean channel fluorescence of 98 versus 188 in the control), whereas no change was observed in the levels of CD3 and CD4 molecules (Fig. 6B, right panel). These results are consistent with the fact that normal turnover of the MHC-I present at the cell surface is necessary before changes induced by p12I on MHC-I become evident. Thus, p12I's interference with newly synthesized MHC trafficking to the cell surface may require time to become apparent. The lack of effect on the level of CD3 and CD4, which remain constant throughout the course of the experiment (Fig. 6B), demonstrates the specificity of the MHC-I downregulation by p12I.
DISCUSSION
Several viruses have evolved mechanisms to escape immune recognition by affecting the expression of MHC-I on the cell surface (36, 49). The data presented here suggest that p12I interferes with the assembly of the free MHC-I-Hc with β2-microglobulin and affects its trafficking to the cell membrane. It may do so by taking advantage of a pathway termed ERAD, whose purpose is to remove misfolded, inappropriately glycosylated or improperly assembled proteins from the ER (4). Proteins are imported into the ER by co- or posttranslational passage through the ER membrane, a process that is mediated by the translocon, a protein channel consisting of the Sec61p protein complex (40). Evidence suggests that the translocon can also function in reverse, retranslocating proteins to the cytosolic face of the ER membrane, where they lose their N-linked oligosaccharides, undergo ubiquitination, and are targeted to the proteasome for degradation (4). It has been demonstrated that misfolded MHC-I-Hc are targeted for destruction through a pathway involving the Sec61p complex and the proteasome (53). In addition, several other proteins have been demonstrated to be translocated from the ER and degraded in a proteasome-dependent manner: improperly assembled mammalian T-cell receptors, yeast carboxypeptidase Y proteins, the cystic fibrosis transmembrane conductance receptor (CFTR), α-antitrypsin, and apolipoprotein B100 (43).
In this study, we demonstrate that p12I binds to both porcine MHC-I-Hc and the products of the human A2, B7, and Cw4 MHC-I-Hc alleles. This interaction prevents association of MHC-I-Hc with β2-microglobulin and reroutes newly synthesized MHC-I-Hc's to the cytosol, where they are degraded by the proteasome, in a similar manner to the HCMV proteins, US2 and US11 (52, 53). However, because both US2 and US11 target the MHC-I-Hc–β2-microglobulin complex for proteosomal degradation, whereas p12I targets the free Hc, the HTLV p12I protein appears to use a novel mechanism for MHC-I downregulation, whereby this viral protein competes with β2-microglobulin for binding to the Hc. Studies of US2-expressing cells demonstrated that the protein induces formation of a deglycosylated breakdown intermediate of MHC-I-Hc that associates with the Sec61 complex (53). The Hc-p12I complex remains in the cis-Golgi compartment, as evidenced by its sensitivity to Endo-H, and most of the complex is retrotranslocated to the cytoplasm and degraded by the proteasome.
Binding of p12I to MHC-I results in redistribution of MHC-I in the perinuclear area of the cell and consequent decrease in its expression at the plasma membrane. The mechanism of retranslocation of MHC-I bound to p12I remains to be investigated, as well as the specific fates of the various Hcs encoded by the HLA-A, -B, and -C alleles; the US2 and US11 proteins selectively target HLA-A and -B, but not HLA-C alleles (45), which is also the case with Nef (6, 25). Furthermore, as demonstrated by US2 and US11, binding does not necessarily result in retranslocation and degradation. US2 and US11 are able to bind to “tailless” MHC-I molecules (48), as well as p12I (data not shown). However, this “tailless” MHC is not retrotranslocated and degraded by US2 and US11 (48). It would be a disadvantage for a virus to downregulate all of the MHC-I at the cell surface, since these cells would become a target for lysis by NK cells (19), and it is interesting to speculate that p12I may downregulate specific alleles in order to allow the virus to escape detection by the immune system but not to become targets for NK cells. Furthermore, the HTLV-1 Tax protein has been shown to upregulate MHC-I on the cell surface (44); thus, a balance of p12I and Tax protein levels may allow modulation of MHC-I levels to serve this purpose. Future work will also examine the effect of p12I on antigen presentation, utilizing the lentiviral vector carrying p12I to determine whether the decrease in MHC levels at the cell surface induced by p12I may be sufficient to effect recognition by CTL.
Interestingly, the HTLV-1 p12I and Nef proteins of HIV and SIV appear to have common features. Nef is dispensible in vitro but is required for in vivo replication and pathogenicity (20), as is p12I (8). In vitro, p12I has been shown to bind the 16-kDa subunit of the vacuolar ATPase (13), and Nef has been shown to bind to the catalytic subunit of the same enzyme (26). Since Nef has been shown to affect MHC-I levels at the cell surface (6, 46), here we demonstrate that p12I interferes with MHC-I–β2-microglobulin assembly and trafficking to the cell membrane using a novel mechanism. In contrast to Nef, however, p12I interacts with the free MHC-I-Hc, prevents its association with β2-microglobulin in the ER, and targets the MHC-I-Hc for proteasome degradation, which results in a decrease of MHC-I at the cell surface. Nevertheless, because p12I also targets the vacuolar ATPase (12, 13), as demonstrated in the case of Nef, it remains to be investigated whether p12I trafficks to the cell membrane and affects also the endocytosis of MHC-I on the cell surface. Lastly, the two natural alleles of p12I, one of which (p12IK88) is ubiquitinated, may differ in their abilities to affect antigen presentation and therefore modulate the host-specific immune response. In this regard, the finding that the ubiquitinated form of p12I (p12IK88) is found mainly in TSP-HAM (50), an immune-mediated disease (18, 33), indicates that dissecting the functional consequence of the two natural alleles of p12I may further our understanding of HTLV-1 pathogenesis.
ACKNOWLEDGMENTS
We thank Donna D'Agostino for critical reading of the manuscript and Rosario Rizzuto, Dinah Singer, Olivier Schwartz, Hidde Ploegh, and Markus Neumann for reagents. We also thank Steven Snodgrass for his editorial assistance and Pierantonio Gallo for artwork.
Part of this work was supported by grants from the Istituto Superiore di Sanitá, the Associazione Italiana per la Ricerca sul Cancro (AIRC), and the Fondazione Italiana per la Ricerca sul Cancro (FIRC).
REFERENCES
- 1.Aiken C, Konner J, Landau N R, Lenburg M E, Trono D. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell. 1994;76:853–864. doi: 10.1016/0092-8674(94)90360-3. [DOI] [PubMed] [Google Scholar]
- 2.Beersma M F C, Bijlmakes M J E, Ploegh H L. Human cytomegalovirus down-regulates HLA class I expression by reducing the stability of class I H chains. J Immunol. 1993;151:4455–4464. [PubMed] [Google Scholar]
- 3.Bennett E M, Bennink J R, Yewdell J W, Brodsky F M. Cutting edge: adenovirus E19 has two mechanisms for affecting class I MHC expression. J Immunol. 1999;162:5049–5052. [PubMed] [Google Scholar]
- 4.Bonifacino J S, Klausner R D. Degradation of proteins retained in the endoplasmic reticulum. In: Ciechanover A, Schwartz A L, editors. Cellular proteolytic systems. New York, N.Y: Wiley-Liss; 1994. p. 137. [Google Scholar]
- 5.Ciminale V, Pavlakis G N, Derse D, Cunningham C P, Felber B K. Complex splicing in the human T-cell leukemia virus (HTLV) family of retroviruses: novel mRNAs and proteins produced by HTLV-I. J Virol. 1992;66:1737–1745. doi: 10.1128/jvi.66.3.1737-1745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen G B, Gandhi R T, Davis D M, Mandelboim O, Chen B K, Strominger J L, Baltimore D. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity. 1999;10:661–671. doi: 10.1016/s1074-7613(00)80065-5. [DOI] [PubMed] [Google Scholar]
- 7.Collins K, Chen B, Kalams S, Walker B, Baltimore D. HIV-I Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature. 1998;391:397–401. doi: 10.1038/34929. [DOI] [PubMed] [Google Scholar]
- 8.Collins N D, Newbound G C, Albrecht B, Beard J L, Ratner L, Lairmore M D. Selective ablation of human T-cell lymphotropic virus type 1 p12I reduces viral infectivity in vivo. Blood. 1998;91:4701–4707. [PubMed] [Google Scholar]
- 9.Dekaban G A, Peters A A, Mulloy J C, Johnson J M, Trovato R, Rivadeneira E, Franchini G. The HTLV-I orf I protein is recognized by serum antibodies from naturally infected humans and experimentally infected rabbits. Virology. 2000;274:86–93. doi: 10.1006/viro.2000.0406. [DOI] [PubMed] [Google Scholar]
- 10.Derse D, Mikovits J, Ruscetti F. X-I and X-II open reading frames of HTLV-I are not required for virus replication or for immortalization of primary T-cells in vitro. Virology. 1997;237:123–128. doi: 10.1006/viro.1997.8781. [DOI] [PubMed] [Google Scholar]
- 11.Fenteany G, Schreiber S L. Lactacystin, proteasome function, and cell fate. J Biol Chem. 1998;273:8545–8548. doi: 10.1074/jbc.273.15.8545. [DOI] [PubMed] [Google Scholar]
- 12.Forgac M. Structure, function and regulation of the coated vesicle V-ATPase. J Exp Biol. 1992;172:155–169. doi: 10.1242/jeb.172.1.155. [DOI] [PubMed] [Google Scholar]
- 13.Franchini G, Mulloy J C, Koralnik I J, Lo Monico A, Sparkowski J J, Andresson T, Goldstein D J, Schlegel R. The human T-cell leukemia/lymphotropic virus type I p12I protein cooperates with the E5 oncoprotein of bovine papillomavirus in cell transformation and binds the 16-kilodalton subunit of the vacuolar H+ ATPase. J Virol. 1993;67:7701–7704. doi: 10.1128/jvi.67.12.7701-7704.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gessain A, Barin F, Vernant J-C, Gout O, Maurs L, Calendar A, de The G. Antibodies to human T-lymphotropic virus type I in patients with tropical spastic paraparesis. Lancet. 1985;ii:407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- 15.Graham F L, van der Eb A J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973;52:456–460. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 16.Hinuma Y, Nagata K, Hanaoka M, Nakai M, Matsumoto T, Kinoshita K I, Shirakawa S, Miyoshi I. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci USA. 1981;78:6476–6480. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishido S, Wang C, Lee B S, Cohen G B, Jung J U. Downregulation of major histocompatibility complex class I molecules by Kaposi's sarcoma-associated herpesvirus K3 and K5 proteins. J Virol. 2000;74:5300–5309. doi: 10.1128/jvi.74.11.5300-5309.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobson S, Raine C S, Mingioli E S, McFarlin D E. Isolation of an HTLV-I-like retrovirus from patients with tropical spastic paraparesis. Nature. 1988;331:540–543. doi: 10.1038/331540a0. [DOI] [PubMed] [Google Scholar]
- 19.Karre K. MHC gene control of the natural killer system at the level of the target and the host. Semin Cancer Biol. 1991;2:295–309. [PubMed] [Google Scholar]
- 20.Kestler H W, III, Ringleer D J, Mori K, Panicali D L, Sehgal P K, Daniel M D, Desrosiers R C. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 21.Koralnik I, Gessain A, Klotman M E, Lo Monico A, Berneman Z N, Franchini G. Protein isoforms encoded by the pX region of the human T-cell leukemia/lymphotropic virus type I. Proc Natl Acad Sci USA. 1992;89:8813–8817. doi: 10.1073/pnas.89.18.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koralnik I, Lemp J F, Jr, Gallo R C, Franchini G. In vitro infection of human macrophages by human T-cell leukemia/lymphotropic virus type 1 (HTLV-1) AIDS Res Hum Retrovir. 1992;8:1845–1849. doi: 10.1089/aid.1992.8.1845. [DOI] [PubMed] [Google Scholar]
- 23.Koralnik I, Mulloy J C, Andresson T, Fullen J, Franchini G. Mapping of the intermolecular association of the human T-cell leukemia/lymphotropic virus type 1 p12I and the vacuolar H+ATPase 16 kDa subunit protein. J Gen Virol. 1995;76:1909–1916. doi: 10.1099/0022-1317-76-8-1909. [DOI] [PubMed] [Google Scholar]
- 24.Koralnik I J, Fullen J, Franchini G. The p12I, p13II, and p30II proteins encoded by human T-cell leukemia/lymphotropic virus type I open reading frames I and II are localized in three different cellular compartments. J Virol. 1993;67:2360–2366. doi: 10.1128/jvi.67.4.2360-2366.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Gall S, Erdtmann L, Benichou S, Berlioz-Torrent C, Liu L, Benarous R, Heard J, Schwartz O. Nef interacts with the μ subunit of clathrin adaptor complexes and reveals a cryptic sorting signal in MHC I molecules. Immunity. 1998;8:483–495. doi: 10.1016/s1074-7613(00)80553-1. [DOI] [PubMed] [Google Scholar]
- 26.Lu X, Yu H, Liu S, Brodsky F, Peterlin B. Interactions between HIV-1 Nef and Vacuolar ATPase facilitate the internalization of CD4. Immunity. 1998;8:647–656. doi: 10.1016/s1074-7613(00)80569-5. [DOI] [PubMed] [Google Scholar]
- 27.Mangasarian A, Foti M, Aiken C, Chin D, Carpentier J, Trono D. The HIV-1 Nef protein acts as a connector with sorting pathways in the Golgi and at the plasma membrane. Immunity. 1997;6:67–77. doi: 10.1016/s1074-7613(00)80243-5. [DOI] [PubMed] [Google Scholar]
- 28.Mann D L, Popovic M, Sarin P, Murray C, Reitz M S, Jr, Strong D M, Haynes B F, Gallo R C, Blattner W A. Cell lines producing human T-cell lymphoma virus show altered HLA expression. Nature. 1983;305:58–60. doi: 10.1038/305058a0. [DOI] [PubMed] [Google Scholar]
- 29.Michalak M, Corbett E F, Mesaeli N, Nakamura K, Opas M. Calreticulin: one protein, one gene, many functions. Biochem J. 1999;344:281–292. [PMC free article] [PubMed] [Google Scholar]
- 30.Mulloy J C, Crowley R W, Fullen J, Leonard W J, Franchini G. The human T-cell leukemia/lymphotropic virus type 1 p12I protein binds the interleukin-2 receptor β and γc chains and affects their expression on the cell surface. J Virol. 1996;70:3599–3605. doi: 10.1128/jvi.70.6.3599-3605.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage F H, Verma I M, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 32.Neefjes J J, Hammerling G J, Momburg F. Folding and assembly of major histocompatibility complex class 1 heterodimers in the endoplasmic reticulum of intact cells precedes the binding of peptide. J Exp Med. 1993;178:1971–1980. doi: 10.1084/jem.178.6.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32a.Nicot, C., J. C. Mulloy, M. G. Ferrari, J. M. Johnson, K. Fu, R. Fukumoto, R. Trovato, J. Fullen, W. J. Leonard, and G. Franchini. The HTLV-1 p12I protein enhances STAT5 activation and decreases the IL-2 requirement for proliferation of primary human PBMC. Blood, in press. [DOI] [PubMed]
- 33.Osame M, Usuku K, Izumo S, Ijichi N, Amitani H, Igata A. HTLV-1 associated myelopathy, a new clinical entity. Lancet. 1986;i:1031–1032. doi: 10.1016/s0140-6736(86)91298-5. [DOI] [PubMed] [Google Scholar]
- 34.Pinton P, Pozzan T, Rizzuto R. The Golgi appartus is an inositol 1,4,5-trisphosphate-sensitive Ca2+ store, with functional properties distinct from those of the endoplasmic reticulum. EMBO J. 1998;17:5298–5308. doi: 10.1093/emboj/17.18.5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pique C, Ureta-Vidal A, Gessain A, Chancerel B, Gout O, Tamouza R, Agis F, Dokhelar M C. Evidence for the chronic in vivo production of human T cell leukemia virus type 1 Rof and Tof proteins from cytotoxic T lymphocytes directed against viral peptides. J Exp Med. 2000;191:567–572. doi: 10.1084/jem.191.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ploegh H L. Viral strategies of immune evasion. Science. 1998;280:248–253. doi: 10.1126/science.280.5361.248. [DOI] [PubMed] [Google Scholar]
- 37.Poiesz B J, Ruscetti F W, Gazdar A F, Bunn P A, Minna J D, Gallo R C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prasher D C, Eckenrode V K, Ward W W, Prendergast F G, Cormier M J. Primary structure of the Aequorea victoria green-fluorescent protein. Gene. 1992;111:229–233. doi: 10.1016/0378-1119(92)90691-h. [DOI] [PubMed] [Google Scholar]
- 39.Rajagopalan S, Brenner M B. Calnexin retains unassembled major histocompatibility complex class I free heavy chains in the endoplasmic reticulum. J Exp Med. 1994;180:407–412. doi: 10.1084/jem.180.1.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rapoport T A, Jungnickel B, Kutay U. Protein transport across the eukaryotic endoplasmic reticulum and bacterial inner membranes. Annu Rev Biochem. 1996;65:271–303. doi: 10.1146/annurev.bi.65.070196.001415. [DOI] [PubMed] [Google Scholar]
- 41.Robek M D, Wong F-H, Ratner L. Human T-cell leukemia virus type 1 pX-I and pX-II open reading frames are dispensable for the immortalization of primary lymphocytes. J Virol. 1998;72:4458–4462. doi: 10.1128/jvi.72.5.4458-4462.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodgers-Johnson P, Gajdusek D C, Morgan O S, Zaninavic V, Sarin P S, Graham D S. HTLV-I and HTLV-III antibodies and tropical spastic paraparesis. Lancet. 1985;ii:1247–1249. doi: 10.1016/s0140-6736(85)90778-0. [DOI] [PubMed] [Google Scholar]
- 43.Romisch K. Surfing the Sec61 channel: bidirectional protein translocation across the ER membrane. J Cell Sci. 1999;112:4185–4191. doi: 10.1242/jcs.112.23.4185. [DOI] [PubMed] [Google Scholar]
- 44.Sawada M, Suzumura A, Yoshida M, Marunochi T. Human T-cell leukemia virus type I transactivator induces class I major histocompatibility antigen expression in glial cells. J Virol. 1987;64:4002–4006. doi: 10.1128/jvi.64.8.4002-4006.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schust D J, Tortorella D, Seebach J, Phan C, Ploegh H L. Trophoblast class I major histocompatibility complex (MHC) products are resistant to rapid degradation imposed by the human cytomegalovirus (HCMV) gene products US2 and US11. J Exp Med. 1998;188:497–503. doi: 10.1084/jem.188.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwartz O, Marechal V, Le Gall S, Lemonnier F, Heard J. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 nef protein. Nat Med. 1996;2:338–342. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- 47.Sonoda S, Yashiki S, Takahashi K, Arima N, Daitoku Y, Matsumoto M, Matsumoto T, Tara M, Shinmyozu K, Sato K. Altered HLA antigens expressed on T and B lymphocytes of adult T-cell leukemia/lymphoma patients and their relatives. Int J Cancer. 1987;40:629–634. doi: 10.1002/ijc.2910400510. [DOI] [PubMed] [Google Scholar]
- 48.Story C M, Furman M H, Ploegh H L. The cytosolic tail of class 1 MHC heavy chain is required for its dislocation by the human cytomegalovirus US2 and US11 gene products. Proc Natl Acad Sci USA. 1999;96:8516–8521. doi: 10.1073/pnas.96.15.8516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tortorella D, Gewurz B, Furman M, Schust D, Ploegh H. Viral subversion of the immune system. Annu Rev Immunol. 2000;18:861–926. doi: 10.1146/annurev.immunol.18.1.861. [DOI] [PubMed] [Google Scholar]
- 50.Trovato R, Mulloy J C, Johnson J M, Takemoto S, de Oliveira M P, Franchini G. A lysine-to-arginine change found in natural alleles of the HTLV-1 p12I protein greatly influences its stability. J Virol. 1999;73:6460–6467. doi: 10.1128/jvi.73.8.6460-6467.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uno H, Matsuoka H, Suzuki M, Tsuda K, Tsubouchi H. Altered expression of class 1 HLA antigen on peripheral mononuclear cells in patients with adult T-cell leukemia: inverse relationship with natural killer susceptibility. Cancer Epidemiol Biomarkers Prev. 1995;4:367–372. [PubMed] [Google Scholar]
- 52.Wiertz E J H J, Jones T R, Sun L, Bogyo M, Geuze H J, Ploegh H L. The human cytomegalovirus US11 gene product dislocates MHC class I heavy chains from the endoplasmic reticulum to the cytosol. Cell. 1996;84:769–779. doi: 10.1016/s0092-8674(00)81054-5. [DOI] [PubMed] [Google Scholar]
- 53.Wiertz E J H J, Tortorella D, Bogyo M, Yu J, Mothes W, Jones T R, Rapaport T A, Ploegh H L. Sec61-mediated transfer of membrane protein from the endoplasmic reticulum to the proteasome for destruction. Nature. 1996;384:432–438. doi: 10.1038/384432a0. [DOI] [PubMed] [Google Scholar]
- 54.York I A, Rock K L. Antigen processing and presentation by the class I major histocompatibility complex. Annu Rev Immunol. 1996;14:369–396. doi: 10.1146/annurev.immunol.14.1.369. [DOI] [PubMed] [Google Scholar]
- 55.Yoshida M, Miyoshi I, Hinuma Y. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc Natl Acad Sci USA. 1982;79:2031–2035. doi: 10.1073/pnas.79.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]