Abstract
Lipid nanoparticles (LNPs) have emerged as leading non-viral carriers for messenger RNA (mRNA) delivery in clinical applications. Overcoming challenges in safe and effective mRNA delivery to target tissues and cells, along with controlling release from the delivery vehicle, remains pivotal in mRNA-based therapies. This review elucidates the structure of LNPs, the mechanism for mRNA delivery, and the targeted delivery of LNPs to various cells and tissues, including leukocytes, T-cells, dendritic cells, Kupffer cells, hepatic endothelial cells, and hepatic and extrahepatic tissues. Here, we discuss the applications of mRNA–LNP vaccines for the prevention of infectious diseases and for the treatment of cancer and various genetic diseases. Although challenges remain in terms of delivery efficiency, specific tissue targeting, toxicity, and storage stability, mRNA–LNP technology holds extensive potential for the treatment of diseases.
Keywords: lipid nanoparticles, mRNA vaccine, infectious disease, cancer, genetic diseases
1. Introduction
Lipid nanoparticles (LNPs), based on ionizable lipids, are the most commonly used messenger RNA (mRNA) delivery vectors and play a crucial role in the research and clinical translation of mRNA therapy. By encapsulating mRNA within a lipid core, LNPs safeguard against mRNA degradation, enhance cellular uptake, and facilitate cytoplasmic release upon cellular entry [1]. Notably, LNPs boast an excellent drug-loading capacity, high encapsulation efficiency, sustained release, high stability, low toxicity, and enhanced efficacy. Currently, LNPs are the best choice for delivering nucleic acids. To date, three nucleic acid medicines based on LNPs have been approved. Patisiran, based on DLin-MC3-DMA lipids, is an RNA interference therapeutic drug used for treating hereditary transthyretin-mediated amyloidosis (hATTR amyloidosis) [2]. BNT162b2, which is based on ALC-0315 lipids, and mRNA-1273, which is based on SM-102 lipids, are mRNA vaccines used to prevent COVID-19 [3,4]. These applications demonstrate the success of LNP delivery systems for gene drugs and vaccines.
The momentum of preclinical research and clinical trials for mRNA–LNP drugs is on the rise. However, the current focus predominantly on liver delivery results in a prevalence of liver-targeted delivery among LNPs. DLin-MC3-DMA (MC3) LNPs are the most representative vector for liver-targeted delivery. Both SM-102 and ALC-0315 are MC3 LNPs that primarily deliver mRNA to the liver after intravenous injection [5]. However, LNPs for extrahepatic-targeted mRNA delivery are still in the early stages of development. To fully utilize the advantages of mRNA technology, such as its low cost, high efficiency, and short developmental cycles, the development of an extrahepatic-targeted delivery strategy for mRNA–LNPs is of great significance. Current research is focused on enhancing the accuracy of mRNA–LNP delivery to specific cells, tissues, or organs to improve the therapeutic effect and minimize potential side-effects. In this review, we summarize the latest strategies for the targeted delivery of mRNA–LNPs to cells and tissues, including tissue-specific or cell-specific LNPs, following different injection modes. The review also highlights the preclinical and clinical research progress of mRNA–LNPs in the treatment of cancer, infectious diseases, and hereditary diseases. Finally, the current challenges and potential future research directions are briefly reviewed.
2. General Characteristics of LNPs
2.1. Composition and Structure of LNPs
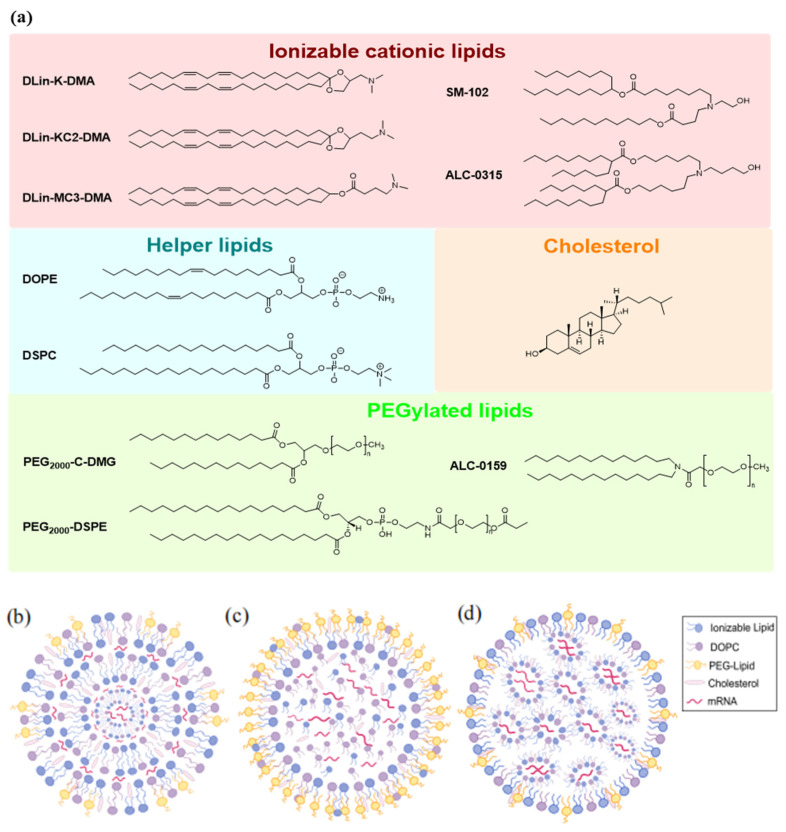
In general, LNPs are composed of ionizable cationic lipids (ICLs), helper lipids, cholesterol, and polyethylene glycol (PEG) lipids (Figure 1a). ICLs are key factors that determine mRNA delivery and transfection efficiency [6]. Their acid dissociation constant (pKa) determines the ionization behavior and surface charge of LNPs, further affecting their stability and toxicity. The pH sensitivity of ICLs enhances in vivo mRNA delivery by reducing interaction with anionic blood cell membranes, thereby enhancing LNP biocompatibility. In acidic endosomal environments, protonated ICLs interact with negatively charged endosomal vesicle membranes, facilitating endosomal membrane disruption and promoting escape [7]. Helper lipids, such as 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) and 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), envelope the lipid–mRNA complex, thereby ensuring LNP stability. They can regulate the fluidity of LNP bilayers and improve the delivery efficiency by enhancing the LNP fusion with biological membranes. Additionally, these helper lipids may contribute to the escape of LNPs from the endosomes [8,9]. Cholesterol, a class of steroid compounds, possesses a distinctive structure that distinguishes it from other components of LNPs, regulating membrane fluidity by filling gaps between phospholipids. When bound to phospholipids at a low phase transition temperature, it can decrease membrane fluidity and increase the thickness of the bilayer membrane. When bound to lipids with a high phase transition temperature, it improves membrane fluidity and narrows the bilayer membrane. This property shields the LNPs from serum proteins, thereby enhancing their stability in biological environments [9,10]. PEG–lipids typically contain hydrophilic PEG chains and two flexible hydrophobic alkyl tails. PEG–lipids improve particle stability, reduce particle binding to plasma proteins in vivo, and prolong the systemic circulation time [11]. Additionally, PEG–lipids protect the LNP shell, inhibit LNP aggregation, and extend the formulation half-life. This influences LNP biodistribution in vivo and shields them from nonspecific endocytosis by immune cells (Table 1).
The core–shell structure of LNPs constitutes an efficient drug delivery platform capable of protecting and delivering various cargoes, including small-molecule drugs, mRNA, proteins, or peptides. The core is formed by the combination of these loads and lipid molecules through electrostatic or hydrophobic interactions. The shell is composed of lipids that encapsulate the core and exhibits good biocompatibility, which can effectively protect the core and enhance its stability. Functionalized auxiliary components are often modified on the surface of the shell to improve the cellular uptake efficiency and targeting of the nanoparticles. For example, PEG–lipids can be modified on the surface of the shell to form a hydrophilic protective layer, reducing recognition and clearance by the immune system and thereby prolonging the circulation time of LNPs in vivo. In addition, the targeting of LNPs to specific cells can be further enhanced by introducing specific targeting ligands (such as antibodies, peptides, and glycans). The design flexibility of LNPs makes them important tools in drug delivery and gene therapy. The pharmacokinetics, cellular uptake efficiency, and therapeutic efficacy of LNPs can be optimized by adjusting the composition of the core and shell layers. Several self-assembled LNP structures have been described in the literature, including multilamellar vesicles (Figure 1b) [12], nanostructured core particles (Figure 1c) [13], and homogeneous core–shell structures (Figure 1d) [14].
Figure 1.
Composition and models of mRNA lipid nanoparticles: (a) Chemical structure of representative lipids used for delivery of therapeutic nucleic acids. (b) Multilamellar vesicle (onion-like). (c) Particle with a nanostructured core. (d) Homogeneous core–shell structure. (b–d) are reprinted with permission from [15]. Copyright 2023 Elsevier Inc.
Table 1.
Typical composition and function of lipids in LNPs for mRNA delivery.
| Component | Examples | Proportion (%) | Function | Modifications and Related Functions | Refs. |
|---|---|---|---|---|---|
| Ionizable lipid | DLin-MC3-DMA, ALC-0315, SM-102 |
30–50 | Encapsulates payload mRNA, improves transfection efficacy, and enables differential delivery |
Introduction of multiple tertiary amine nitrogen atoms: higher delivery efficiency and safety | [1,8,16,17,18,19,20,21,22] |
| Phospholipid | DOPE, DSPC, DOPG | 10–20 | Helper lipids considered to improve the structural stability of formulations | A 3-phosphate group modification: improves transfection efficiency; increases the distribution in vivo | [23,24] |
| Sterol | Cholesterol, cholesterol analogues |
20–50 | Structural stability |
C-24 alkyl derivatives: regulate the integrity of the membrane; impact on delivery efficiency and biodistribution | [8,16,17] |
| PEG–lipid | PEG-DMG, PEG-DSPE |
~1.5 | Stealthiness: provides colloidal stability and enables evasion of the mononuclear phagocytic system | Covalent coupling modification: improving the half-life of LNPs | [1,18,19] |
2.2. Toxicity of Lipids Used in LNP Formulations
Cationic lipids play a key role in LNPs by binding to nucleic acids through electrostatic interactions and facilitating the cellular uptake of LNPs through the cell membrane. However, these lipids may also induce cytotoxicity, especially at high concentrations. The cytotoxicity of cationic liposomes increases with increasing concentrations of cationic lipids [25,26,27]. For example, high concentrations of dimethyl dioctadecyl ammonium chloride (DODAC) in cationic liposomes correlate with enhanced toxicity to mammalian cells [28]. In addition, the toxicity of cationic lipids varies with their chemical properties. For example, lipids with quaternary ammonium head groups are more toxic than those with tertiary amine head groups [29]. Moreover, the composition and structure of liposomes significantly affect their cytotoxicity. For example, liposomes containing 50% DOPE induce approximately 80% mRNA silencing, whereas the degree of reduction in cell viability depends on the N/P ratio, which is the ratio of cationic lipids to phospholipids in the lipid, and the nature of the cationic lipids [30]. PEG–lipid couplers may also cause undesirable toxicity. Nanoparticles containing PEG–lipid couplers interact with immune cells, leading to the production of undesirable antibodies against PEGylated lipids. These toxic effects may affect the safety and efficacy of LNPs in vivo [31].
Therefore, to mitigate the cytotoxicity of LNPs, researchers need to carefully select and optimize the type and concentration of cationic lipids, as well as the composition and structure of liposomes. Such strategies include selecting less toxic cationic lipids, adjusting the ratio of cationic lipids to phospholipids, optimizing the composition and structure of liposomes, and avoiding the use of PEG–lipid couplings that may cause adverse immune responses [32]. These approaches may enhance the safety and therapeutic efficacy of LNPs.
2.3. Mechanism of mRNA–LNP Delivery
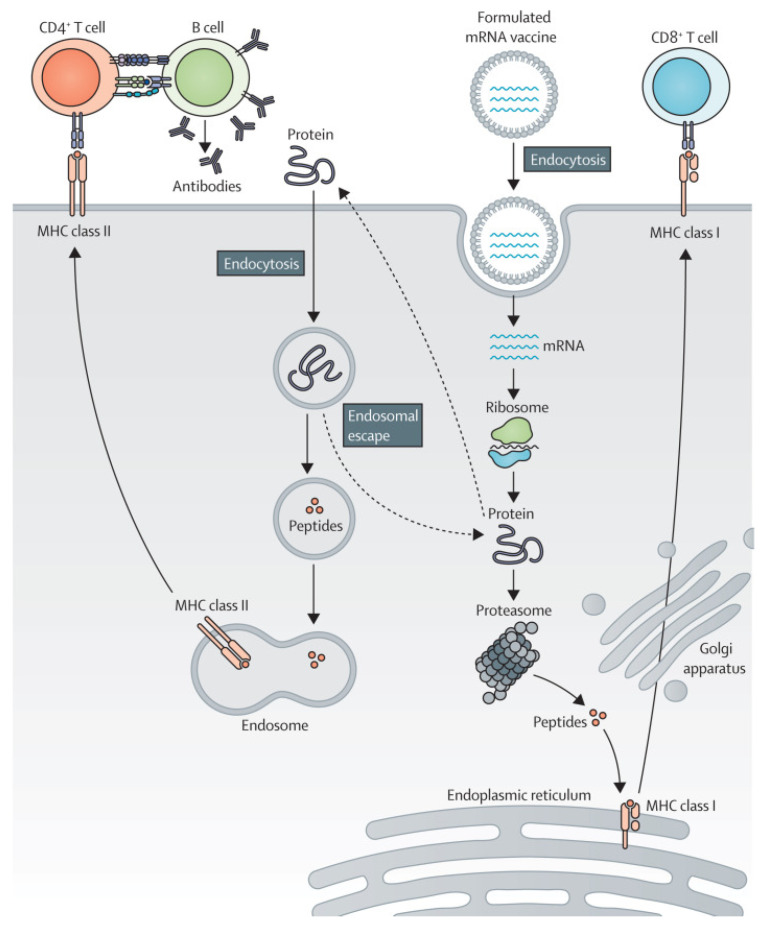
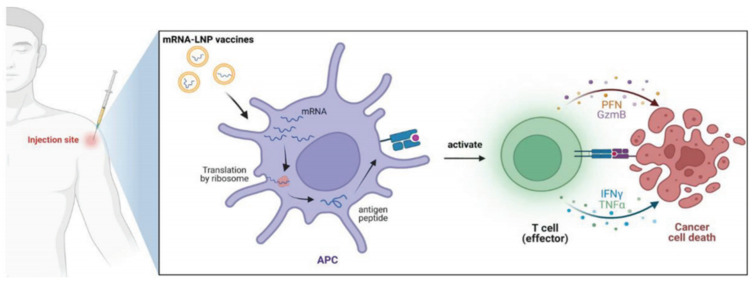
The mRNA–LNP delivery system undergoes essential processes to fulfill its functions in vivo. For example, mRNA–LNPs must be transported to different tissues and cells via the circulatory system [33]. Depending on the characteristics of the lipid molecules on the outer surface of the LNPs, they can interact with proteins on the cell membrane, thereby mediating the binding of mRNA–LNPs to specific cell membranes and initiating the endocytosis of LNPs. For instance, when the LNP surface is coupled with a CD5-specific antibody, the antibody binds to T-cells expressing the CD5 antigen, thereby mediating the endocytosis of mRNA–LNPs by T-cells [34]. This process endows LNPs with a targeting ability, which is of great significance in the development of targeted therapeutic methods for related diseases. Upon cellular internalization, LNPs form endosomes in the cytoplasm. Most LNPs are degraded by enzymes within endosomes; however, only a small fraction of mRNA–LNPs can complete the process of endosomal release, allowing exogenous mRNA molecules to enter the cytoplasm. With the assistance of cellular organelles such as ribosomes, the translation process is initiated, leading to the successful expression of exogenous proteins and the completion of the regulatory process of cellular functions. Most mRNA–LNP vaccines are internalized by cells after systemic administration. Subsequently, mRNA is released into the cytoplasm to guide the synthesis of antigenic proteins. These proteins are presented to T- and B-cells via major histocompatibility complex (MHC) molecules, activating the immune system to recognize and eliminate diseased cells while promoting tumor cell death (Figure 2).
Figure 2.
The mRNA–LNP vaccine immune response process. Reprinted with permission from [35]. Copyright 2022 Elsevier Inc.
3. Targeted Delivery of mRNA–LNPs
3.1. Cell-Targeted LNPs
Immune cells are important targets for nucleic acid delivery because they play a key role in many diseases, including cancer, inflammatory conditions, and autoimmune diseases. In addition to the reticuloendothelial system of the liver, immune cells are distributed throughout the body, particularly in the spleen. The delivery of therapeutic nucleic acids to leukocytes, such as macrophages, dendritic cells (DCs), and lymphocytes, provides an immune stimulation strategy to introduce genetic material with anti-inflammatory potential or to induce T-cell regulation. Targeting immune cells can directly affect the immune response, which is particularly important in treating diseases related to immune system dysfunction [36,37].
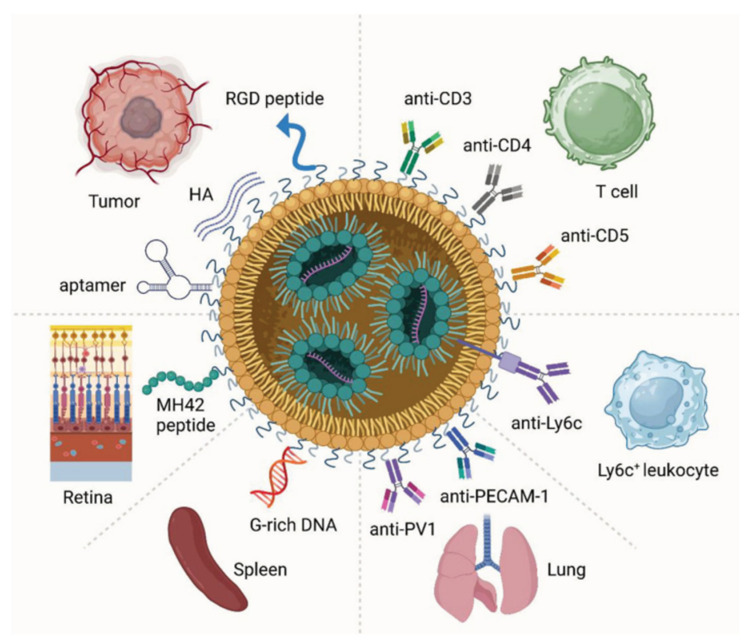
To enhance mRNA delivery efficiency and cell specificity, researchers have developed LNPs that target specific cells. These LNPs are designed for selective uptake by particular cells, facilitating efficient protein expression of the encapsulated mRNA within the target cells. Cell-specific uptake usually relies on ligand–receptor interactions in which the ligand binds to a specific receptor on the cell surface and promotes the internalization of LNPs. Surface modification is an effective strategy for enhancing the targeting ability of LNPs [38]. By attaching antibodies, peptides, carbohydrates, or other molecules to LNP surfaces, these molecules act as recognition signals, guiding LNPs to bind with target cells (Figure 3). For example, coupling antibodies against tumor cells with LNPs can target tumor cells more effectively in vivo, thereby improving the efficacy of the tumor treatment.
Figure 3.
Representative surface modification strategies for LNP targeting. Reprinted with permission from [39]. Copyright 2023 Wiley.
3.1.1. Leukocyte-Targeted LNPs
Leukocytes represent a major therapeutic target because of their pivotal role in the immune system and their involvement in various diseases, including cancer, chronic inflammation, and autoimmune disorders [14]. These cells are ubiquitously distributed throughout the body, predominantly in organs such as the spleen and liver, as well as in the blood and lymphatic system.
Inflammatory bowel disease (IBD), a chronic autoimmune disease, remains incurable and causes chronic inflammation, severely impacting the quality of life of patients. To facilitate the cell-specific mRNA treatment of IBD, Veiga et al. [40] developed an antibody-modified LNP designed to target interleukin-10 (IL-10) mRNA to Ly6c+ inflammatory leukocytes. To elicit sustained IL-10 production, LNPs were initially formulated to encapsulate IL-10 mRNA. These LNPs were then incubated with Anchored Secondary scFv Enabling Targeting (ASSET) micelles at 4 °C for 48 h and subsequently with anti-Ly6c monoclonal antibodies (mAbs) for 30 min. The ASSET platform represents an innovative modular targeting system that facilitates the coupling of LNPs with targeting antibodies under mild conditions. As Ly6c+ leukocytes play a key role in inflammatory diseases, they are potential targets for the treatment of IBD [41]. Studies have demonstrated the inhibitory effect of the immunosuppressive cytokine IL-10 on IBD progression [42]. Various strategies for targeting leukocytes exist alongside this approach. For example, coupling a fusion protein associated with α4β7 integrins expressed on intestinal leukocytes to the surface of LNPs enables the silencing of interferon γ in the intestine and significantly improves the therapeutic efficacy in colitis [43]. Another approach is to design novel ionizable amino lipid libraries based on ethanolamine, hydrazine, or hydroxylamine ligands to efficiently deliver mRNA to leukocytes [44].
3.1.2. DC-Targeted LNPs
DCs and antigen-presenting cells (APCs) play crucial roles in recognizing antigens and activating the immune response after the ingestion of foreign particles [45]. The internalization mechanism of DCs depends on phagocytosis or receptor-mediated endocytosis, which is significantly influenced by the particle size and surface modification. Given their ability to express antigen proteins directly, mRNA vaccines represent a promising strategy for activating the immune response, making DCs an ideal target for vaccine design [41]. Tang et al. [46] developed an mRNA vaccine modified with silicon-based neuraminic acid (SA) to effectively target DCs and escape endocytoses/lysosomes. SA-modified mRNA facilitated a twofold increase in the uptake of LNPs by DCs, with over 90% of the SA-modified LNPs escaping from early endosomes, avoiding lysosomal entry. This facilitated mRNA translation in ribosomes distributed in the cytoplasm and endoplasmic reticulum, significantly enhancing the transfection efficiency of mRNA–LNPs in DCs. Haase et al. [47] demonstrated that lipopeptide carriers are key components of highly efficient mRNA–LNPs. In particular, a series of lipophilic tertiary lipoamino fatty acid vectors with bundle-like topological structures (LAF4-Stp) and enhanced lipophilicity have exhibited excellent performance in effective mRNA encapsulation, cellular uptake, and endosomal escape. These novel LNPs exhibit superior transfection efficiency and faster transfection kinetics than conventional LNP formulations. Even with very low serum mRNA doses, high transfection levels were achieved. Upon intravenous injection of 3 µg of mRNA per mouse, in vivo mRNA expression was highly selective for DCs and macrophages, particularly in the spleen, where expression levels were significantly increased.
Elicitation of cellular responses by mRNA–LNP platforms relies on the activation of pattern recognition receptor (PRR) pathways and the engagement of DCs. Consequently, the incorporation of PRR ligands or mRNA encoding T-cell-polarizing cytokines into LNPs can induce the differentiation of specific T-cell subsets. An important strategy proposed to mitigate the inflammation caused by LNP components in mRNA–LNP vaccines is to target LNPs lacking cationic/ionizable lipids to DC subgroups that can initiate an antibody response in the absence of inflammatory factors [48].
3.1.3. T-Cell-Targeted LNPs
LNP-based therapies targeting T-cells show great potential for the treatment of aggressive diseases, particularly cancer. Such therapies provide a novel strategy for treating various diseases by activating a patient’s immune system. In cancer treatment, mRNA–LNP technology can generate chimeric antigen receptor (CAR) T-cells that target tumors by delivering CAR mRNA, which can recognize and kill tumor cells [49]. Thatte et al. [50] developed a novel platform of ionizable LNPs designed for the efficient delivery of mRNA encoding the Foxp3 protein to CD4+ T-cells, enabling the engineering of Foxp3-T-cells in vitro. These Foxp3-T-cells temporarily exhibit an immunosuppressive phenotype and inhibit effector T-cell proliferation. This finding underscores the therapeutic potential of the LNP platform in modulating T-cell immunosuppression, which could significantly impact the advancement of autoimmune therapies. Tombácz et al. [51] studied a method for targeting human T-cells using Luc mRNA–LNPs coupled with an anti-CD4 antibody. Their findings revealed that anti-CD4/mRNA–LNPs resulted in stronger binding and dose-dependent luciferase expression in human CD4+ T-cells compared to control IgG-coupled LNPs. Yan et al. [52] showed that the subcutaneous administration of lead-induced ovalbumin (OVA) mRNA–LNPs can induce potent and durable CD8+ T-cell responses, substantially inhibiting the growth of aggressive B16-OVA tumors.
Cardiac fibrosis arises from the excessive production of the extracellular matrix by cardiac fibroblasts [53]. The efficacy of antifibrotic therapeutics in limiting fibrotic progression has not met expectations for the effective treatment of cardiac fibrosis [54]. The fibroblast activation protein CAR (FAP-CAR) is encoded by an mRNA [54], which is then encapsulated into LNPs modified with an anti-CD5 antibody to facilitate the specific targeting of CD5+ T-cells [55,56]. Rurik et al. [57] evaluated the therapeutic efficacy of reprogrammed CAR-T cells by injecting LNPs targeting CD5 into a mouse model of heart failure. The results showed that CD5-targeted LNPs effectively delivered therapeutic mRNA to lymphocytes in vivo, thereby forming transient anti-fibrotic CAR-T cells.
3.1.4. Kupffer Cell- and Liver Endothelial Cell-Targeted LNPs
Kupffer cells, as macrophages in the liver, play a key role in immune monitoring and the inflammatory response of the liver. They are mainly responsible for removing particles and pathogens from the blood through phagocytosis [58]. To enhance the uptake of LNPs by Kupffer cells and their immunoregulation ability, research has explored various strategies, including increasing the size of LNPs and modifying the LNP surface with hydrophobic molecules [59]. Liver sinusoidal endothelial cells (LSECs) are located in the sinusoidal space of the liver, where they play important roles in blood filtration, metabolic regulation, antigen presentation, and lipid metabolism. LSECs have a unique structure and function, enabling them to play a crucial role in immune and metabolic processes in the liver [60]. Targeting LNPs to Kupffer cells and LSECs can lead to more effective drug or gene delivery, especially in the treatment of liver diseases. For example, in diseases that require liver-specific treatment, such as hepatitis, liver cirrhosis, and liver cancer, this targeted delivery strategy can improve the therapeutic outcomes and reduce unnecessary side-effects. Paunovska et al. [61] explored the use of different types of cholesterol to modify LNPs and reported that the structure of the cholesterol significantly affects the targeting ability of the LNPs. In addition, by using the Fast Identification Nanoparticle Delivery (FIND) system, they screened LNPs formulated with different oxidized cholesterol and observed that LNPs containing oxidized cholesterol can efficiently deliver mRNA to cells in the liver microenvironment, including Kupffer cells and liver endothelial cells. This contrasts with conventional LNPs, which primarily target hepatocytes, and suggests that the targeting of LNPs to specific cell types can be improved by specific chemical modifications. This improved targeting has significant implications for the treatment of liver diseases and the application of immunotherapy.
3.2. Tissue-Specific Targeting
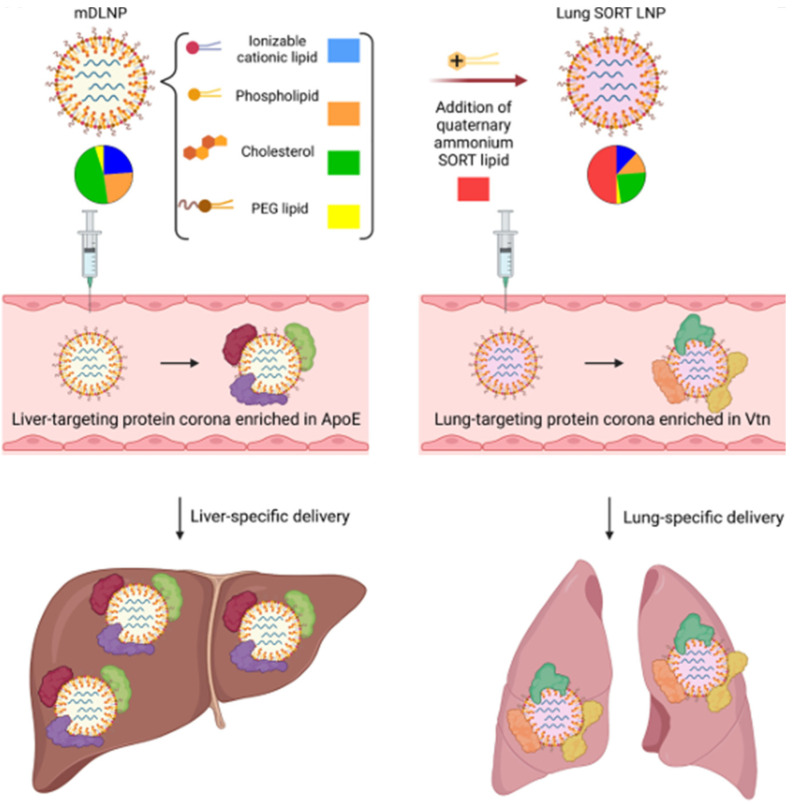
By adjusting the ratio of lipids in LNPs, the targeting ability of LNPs to different organs can be tailored. Siegwart et al. [62] discovered that modifying the composition and components of LNPs allows for the selective targeting of organs. These passively targeted LNPs are termed selective organ targeting (SORT) nanoparticles, facilitating controlled nucleic acid delivery to specific tissues. Lung-targeting SORT LNPs feature a notably different protein corona from liver-targeting LNPs, with a reduced enrichment of ApoE, complement components, and immunoglobulins as well as increased enrichment of vitronectin (Vtn) among other proteins [63] (Figure 4). SORT LNPs contain SORT molecules and can accurately regulate delivery to the mouse liver, lungs, and spleen after intravenous injection [64] (Table 2). Siegwart et al. [65] conducted an exploration of the targeted mechanism of SORT LNPs. They speculated that different SORT molecules may cause LNPs to absorb different plasma proteins, thereby achieving tissue-specific mRNA delivery. Mass spectrometry analysis of the protein corona of different SORT LNPs revealed that the main proteins adsorbed by LNPs from the liver, lungs, and spleen were apolipoprotein E (apoE), vitronectin, and β-2-glycoprotein I, respectively. This suggests the potential to elucidate the targeting mechanism in extrahepatic tissues. Flux screening represents another method for developing tissue-specific LNPs. Dahlman et al. [66] developed a research strategy called the Joint Rapid DNA Analysis of Nanoparticles, utilizing DNA barcodes and sequencing techniques. This strategy enables the high-throughput screening of LNP formulations by encapsulating unique DNA sequences. Each LNP formulation is associated with a unique DNA barcode sequence. Using flow cytometry and next-generation sequencing techniques, hundreds of LNP formulations can be simultaneously analyzed to identify the relationship between LNPs and tissue- or cell-specific delivery [67].
Figure 4.
Selective organ targeting (SORT) technology achieves liver- and lung-specific mRNA delivery. Reprinted with permission from [63]. Copyright 2023 Elsevier Inc.
Table 2.
Summary of different tissue-specific LNPs.
| Disease | Target Organ |
LNP Composition | mRNA | Administration | Ref. |
|---|---|---|---|---|---|
| Acute intermittent porphyria | Liver | 2-(dinonylamino)-1-(4-(N-(2-(dinonylamino)ethyl)-N-nonylglycyl)piperazin-1-yl)ethan-1-one, DSPC, cholesterol, PEG-DMG | hPBGD mRNA | Intratumor injection | [68] |
| Hemophilia | Liver | 246C10, DOPE, cholesterol, PEG–ceramide lipids | Cas9 mRNA and mouse AT-targeted sgRNA | Intratumor injection | [69] |
| Liver cancer | Liver | Dlin-MC3-DMA-based lipids, helper lipids, cholesterol, PEG–lipids | BisCCL2/5i mRNA | Intratumor injection | [70] |
| Liver cancer | Liver | G0-C14, PDSA, DSPE-PEG, DMPE-PEG | p53 mRNA | Intratumor injection | [71] |
| Liver cancer | Liver | 5A2-SC8, DOPE, cholesterol, PEG–lipids | Cas9 mRNA, PD-L1 sgRNA, and FAK siRNA | Intratumor injection | [72] |
| SARS-CoV-2 | Liver | MC3 lipids, cholesterol or β-sitosterol, DMG PEG2000, DSPC | hsACE2mRNA | Intratumor injection | [73] |
| Liver fibrosis and cirrhosis | Liver | PEG–lipids, ionizable lipids, structural lipids, cholesterol | HNF4A mRNA | Intratumor injection | [74] |
| Acute respiratory distress syndrome | Lung | DLin-MC3-DMA, DSPC, cholesterol, PEG2000-DMG | sPD-L1 mRNA | IV | [75] |
| H1N1 infection | Lung | Modified PEIcompound7C1, cholesterol, DMG-PEG2000, cationic lipid DOTAP | mRNA encoded with broadly neutralizing antibody targeting hemagglutinin | Inhalation | [76] |
| Cystic fibrosis | Lung | 5A2-SC8, DOPE, cholesterol, DMG-PEG | Cas9 mRNA, sgRNA, and donor ssDNA templates | IV | [77] |
| Idiopathic pulmonary fibrosis | Lung | DLin-MC3-DMA, DPPC, cholesterol, DSPE-PEG | eGFP mRNA | intraperitoneal injection | [78] |
| LAM | Lung | 306-N16B + 113-N16B, cholesterol, DOPE + DSPC, DMG-PEG2000 | Cas9 mRNA, sgRNA, and Tsc2 mRNA | IV | [79] |
| Hematologic cancers and malignancies | Liver and spleen | IC8, DSPC, cholesterol, DMG-PEG | B7H3-CD3 mRNA | Intratumor Injection | [80] |
| NA | Spleen, lung, or liver | 5A2-SC8 SORT lipids, DOTAB, cholesterol, DMG-PEG | Cas9 mRNA and sgRNA | Intratumor Injection | [81] |
| NA | Lymphoid tissues | ssPalm, phosphatidylcholine (PC), cholesterol, DMG-PEG2000 | Cre-mRNA | IV | [82] |
| Skeletal diseases | Bone | Lipids with bisphosphonate head groups | BMP-2 mRNA | IV | [83] |
| Orthotopic glioblastoma | Tumor | DLin-MC3-DMA, DSPC, cholesterol, DMG-PEG, DSPE-PEG | Cas9 mRNA and sgPLK1 | Intracerebral Injection | [84] |
| Melanoma | Tumor | DALs, DOPE, cholesterol, DMG-PEG | IL-12 mRNA | Hypodermic Injection | [85] |
| Melanoma | Tumor | C12-200 + CKK-12, DOPE + DSPC, cholesterol, C14- | TRP2/gp100 mRNA | Hypodermic Injection | [12] |
| Melanoma | Tumor | PEG1000 + C14-PEG350, arachidonic acid A2, DOPE, cholesterol, C14-PEG | OVA RNA | IV | [86] |
| Lymphoma | Tumor | Ionizable cationic lipids, phosphatidylcholine, cholesterol, PEG–lipids | Rituximab mRNA | IV | [87] |
| Breast cancer | Tumor | cKK-E12, DSPC, cholesterol, PEG2000-DMG | HER2 antibody mRNA | IV | [88] |
| Melanoma | T-cells | PL1, DOPE, cholesterol, DMGPEG2000 | OX40 costimulatory receptor mRNA | IV | [84] |
| Cardiac fibrosis | T-cells | Anti-CD5 antibody, ionizable lipids, phosphatidylcholine, cholesterol, PEG–lipids | FAP-CAR mRNA | IV | [57] |
| Inflammatory bowel disease | Leukocyte | Anti-Ly6cmAbs, MC3, DSPC, cholesterol, DMG-PEG, DSPE-PEG | IL-10mRNA | IV | [40] |
| NA | Kupffer cells and liver endothelial cells |
Oxidized cholesterol, cKK-E12, PEG–lipids, DOPE | Cre mRNA | IV | [61] |
| Peanut allergy | LSECs | MC3, DSPC, cholesterol, DSPE-PEG2000-mannose | Ara h2 mRNA | IV | [60] |
| NA | SECs/LSECs | MC3, DSPG, cholesterol, DMG-PEG2000 eGFP mRNA, mCherry | eGFP mRNA or mCherry | IV | [89] |
IV: intravenous injection; hsACE2: human angiotensin-converting enzyme 2; HNF4A: human hepatocyte nuclear factor alpha; hPBGD: human porphobilinogen deaminase; LAM: lymphangioleiomyomatosis; NA: Not applicable; LSECs: Liver sinusoidal endothelial cells; SECs: sinusoidal endothelial cells.
3.2.1. Liver-Targeted LNPs
The liver is an important organ that participates in digestion, excretion, detoxification, and immunity. Liver diseases are clinically common and frequently occurring. Some liver diseases, such as viral hepatitis, cirrhosis, and liver cancer, greatly endanger human health [90,91]. While many drugs are clinically used to treat liver diseases, most have limited clinical use owing to their poor distribution in the liver, significant toxic side-effects on other organs, and instability within the body. Consequently, there is an ongoing need to explore effective treatments for liver disease. Liver-targeted LNPs can effectively deliver drugs to lesion sites in the liver, reduce systemic distribution, decrease the dosage and frequency of medications, improve the therapeutic index of drugs, and reduce adverse reactions.
The success of patisiran (Onpattro®) demonstrates the potential application of liver-targeted LNPs in clinical treatment. This drug achieves efficient therapeutic effects by utilizing the natural uptake of LNPs by the liver and the expression of specific receptors on the surface of liver cells. During patisiran treatment, LNPs bind to apoE, which in turn binds to low-density lipoprotein receptors (LDLRs). Given the high expression of LDLRs on the surface of liver cells, this binding mechanism enables LNPs to effectively deliver small interfering RNA (siRNA) to the liver, thereby silencing the expression of abnormal proteins associated with hereditary thyroxine transporter amyloidosis. The therapeutic strategy of using LNPs for liver-targeted delivery has shown significant advantages in treating hepatocellular carcinoma (HCC), liver fibrosis, and other liver diseases [92]. Xu et al. [60] explored the possibility of delivering epitope mRNA–LNPs by targeting hepatic sinusoidal endothelial cells (LSECs), aimed at inhibiting allergic reactions to peanut crude protein extract. To achieve this goal, they developed mannose-modified LNPs that encapsulated mRNA-expressing peanut epitopes designed to enter the liver via biodistribution and be taken up by LSECs, which in turn induced a tolerance effect that effectively inhibited allergic reactions to crude peanut allergen extracts. This tolerance effect was accompanied by the inhibition of the Th2 immune response, IgE antibody production, and degranulation of mast cells. Kim et al. [93] reported the development of engineered LNPs for the targeted delivery of mRNA to hepatocytes and LSECs. They evaluated the effects of the particle size and PEG–lipid content of LNPs on the specific delivery of mRNA in the liver. Targeted mRNA delivery to the LSECs was further explored by introducing active ligands. Interestingly, Saunders et al. [94] utilized the dimensions of fenestrations in the liver and administered nanoprimers to mice as a pretreatment. This strategy aimed to reduce LNP uptake, consequently enhancing LNP accumulation in cell types other than Kupffer cells and LSECs.
Liver-targeted LNPs show potential for the treatment of hereditary diseases such as hemophilia and acute intermittent porphyria [95]. Studies have identified antithrombin (AT) as a negative regulatory factor that can be targeted to treat hemophilia A and B [96,97]. Restoration of the coagulation system balance has been achieved using the RNA interference drug fitusiran to selectively inhibit AT [98]. However, it is not a long-acting medication and requires repeated injections [99]. To seek long-acting treatments for hemophilia, Han et al. [69] developed LNPs encapsulating Cas9 mRNA and mouse AT-targeted single-guide RNA (sgRNA) for AT gene editing and inhibition. They evaluated therapeutic efficacy in hemophilia A and B mouse models by assessing thrombin generation mediated by mAT gene editing. They demonstrated that delivering CRISPR-Cas9 in vivo using LNPs could facilitate AT gene editing, thereby achieving the sustainable treatment of hemophilia A and B. The organ-specific delivery potential of LNPs was confirmed via the intravenous administration of luciferase-containing LNPs, resulting in luciferase expression primarily in the mouse liver, with no detectable expression in other organs. Recently, Genevant collaborated with Novo Nordisk to develop in vivo gene editing therapies for the treatment of hemophilia A by combining proprietary LNPs with innovative mRNA-based megaTAL technology [100]. Mitchell et al. [101] developed a high-throughput, low-cost synthetic strategy for constructing degradable branching-like lipids (DB-lipidoids), which can significantly improve mRNA delivery efficiency by up to threefold. The DB-LNPs, based on this strategy, exhibit high efficiency in liver mRNA delivery. DB-LNPs were used to deliver Cas9 mRNA/sgRNA for editing the TTR gene and mRNA encoding human fibroblast growth factor 21 (FGF21) to the liver as a strategy to treat the genetic disease transthyretin amyloidosis, and obesity and fatty liver, respectively. The results indicated that DB-LNPs achieved an approximately fivefold TTR gene editing efficiency and therapeutic FGF21 protein expression compared to MC3-LNP. These results suggest that DB-LNPs have great potential as a novel LNP platform for mRNA-based gene editing therapies and protein supplementation therapies.
3.2.2. Extrahepatic-Targeted LNPs
Unlike hepatic targets, there are no specific proteins, such as apoE, that mediate the targeting pathways for extrahepatic targets. Consequently, several approaches have been employed for nonhepatic targeting. In this context, SORT was investigated by Dillard et al., in which a fifth component (or SORT molecule), in addition to the conventional four-lipid composition of LNPs, facilitates extrahepatic targeting [65]. Di et al. [102] have extensively reviewed methods for the extrahepatic targeting of mRNA delivery, which align with the SORT approach. Their findings indicated that positively charged lipids and negatively charged LNPs preferentially accumulate in the lungs and spleen, respectively.
The lungs represent a common target for nucleic acid treatment because of their association with numerous diseases and relatively accessible surface. However, they pose unique challenges due to their intricate biological mechanisms, such as mucociliary clearance and alveolar macrophage engulfment, which clear inhaled particles. Xue et al. [103] synthesized 180 cationic degradable (CAD) lipids and screened them using high-throughput DNA barcode technology to identify those that could effectively deliver mRNA to the lungs. Screening results showed that LNP-CAD9 efficiently delivered mRNA to the lungs, providing a potential novel therapeutic strategy for the treatment of lung diseases. Wei et al. [77] optimized and improved the Lung SORT LNP delivery system, aiming to effectively deliver Cas9 mRNA, sgRNA, and donor single-stranded DNA (ssDNA) templates to lung basal cells, thereby achieving precise homologous directed repair-mediated gene correction in a cystic fibrosis (CF) model. The optimized Lung SORT LNP system efficiently delivered mRNA to basal lung cells in Ai9 mice. Furthermore, LNPs can bypass pulmonary barriers and reach the lung endothelium through the bloodstream by modifying surface charge or functionalization using peptides, antibodies, or small-molecule ligands [81,104]. Massaro et al. [78] demonstrated the potential of the LNP delivery of mRNA therapeutics to lungs undergoing fibrosis. The utilization of small-sized LNPs ensures the efficient encapsulation of mRNA, offering effective protection against degradation and enabling the sustained release of mRNA. Notably, in vitro experiments confirmed the robust mRNA transfection efficiency, particularly at higher doses and longer intervals post-administration.
The inherent properties, composition, pKa, size, and charge modulation of LNPs offer significant potential for tissue-specific targeting. Further enhancement of ligand-based modifications has been recognized as a valuable strategy, effectively evaluated throughout the development of non-viral delivery vectors [40,51,105]. Longze et al. [106] developed a novel spleen-selective mRNA vaccine that can deliver unmodified mRNA and Toll-like receptor agonists to the spleen following systemic administration. This vaccine was designed to generate a sufficient and lasting anti-tumor cellular immune response, thus exerting a powerful tumor immunotherapeutic effect. Wang et al. [107] developed non-cationic thiourea lipid nanoparticles (NC-TNPs) to encapsulate mRNA through strong hydrogen-bonding interactions between the thiourea moiety of the NC-TNPs and the phosphate moiety of mRNA, discarding traditional anionic electrostatic interactions and constructing a non-ionized mRNA delivery system. The NC-TNP preparation technique is simple, convenient, and repeatable, and results in negligible inflammation and cytotoxic side-effects. It achieves a high gene transfection efficiency and can target the spleen for immune induction and disease treatment. Gomi et al. [82] developed a novel type of LNP loaded with phosphatidylserine (PS) to promote the targeted delivery of mRNA to the spleen. Compared with traditional LNPs, PS-LNPs show higher efficiency in delivering mRNA to the spleen. Sub-organ analysis showed that the PS-LNPs were mainly absorbed by macrophages in the red pulp and marginal area of the spleen, providing a promising strategy for the clinical application of mRNA in the spleen. Lymphoid organs contain high concentrations of immune cells and play a pivotal role in adaptive immune responses [108]. Efforts to target immune cells within these organs have been central to the development of vaccines and immunotherapies because they can elicit robust immune activation [22,109,110]. Ensuring the effective delivery and retention of vaccine candidates in the lymphatic system is essential for inducing protective immune responses, particularly for preventing infectious diseases (e.g., new coronaviruses and influenza viruses) [111,112]. Ramishetti et al. [113] showed that siRNAs can be efficiently delivered to CD4+ T lymphocytes by coupling anti-CD4 mAbs to LNPs.
In addition, the targeted delivery of mRNA to organs such as the brain, bone, heart, and eyes is a burgeoning research area. Tylawsky et al. [114] developed a fucoidan nanoparticle targeting P-selectin, which can penetrate the blood–brain barrier and transport anti-tumor drugs to brain tumor tissue. In a mouse model, these LNPs exhibited enhanced cancer treatment efficacy and reduced adverse effects. Mitchell et al. [115] designed 13 unique mRNA–LNPs based on different ionizable lipids. Through strict screening, “LNP A4” was identified as efficiently delivering mRNA to the placenta while avoiding fetal entry. This discovery has the potential to treat pregnancy complications such as pre-eclampsia. They used bisphosphate (BP)-modified LNPs to achieve the specific targeting of the bone microenvironment. A series of novel LNPs were prepared by introducing BP head groups into lipid molecules. These BP lipids can combine with calcium ions in bones through chelation, allowing the LNPs remain in the bone microenvironment for an extended period. This design enables LNPs to effectively accumulate in the bones, thus improving the effectiveness of drugs or gene therapy in the treatment of bone diseases [83].
4. Progress in Therapeutic Aspects
4.1. Treatment of Cancer
Cancer is a significant threat to human health worldwide, with rising incidence rates and high mortality rates presenting ongoing challenges to therapy [116]. Despite decades of research into tumor mechanisms and treatments, traditional approaches such as surgical resection, radiotherapy, and chemotherapy persist in clinical cancer management. However, these methods are not universally effective, and the toxicity and side-effects associated with radiotherapy and chemotherapy underscore the need for improved therapeutic strategies. Advances in science and technology have introduced numerous novel tumor treatment approaches in recent years, including biological therapies, particularly mRNA-based therapies. LNPs have emerged as an effective means of delivering mRNA to tumor cells, taking advantage of the unique characteristics of solid tumors such as high permeability and retention effects. Table 3 summarizes preclinical research on mRNA–LNP vaccines for cancer [117].
Table 3.
Preclinical research of mRNA–LNP vaccines for cancer treatment.
| Disease | Vaccine | Lipid Nanoparticle Components | Molar Lipid Ratios |
Target Antigen |
Route | Ref. |
|---|---|---|---|---|---|---|
| Lymphoma | Rituximab mRNA-LNP |
ALC-0315 lipids, phosphatidylcholine, cholesterol, PEG–lipids |
50:10:38.5:1.5 | Rituximab | ID | [87] |
| Melanoma | TRP2/gp100- mRNA-LNP |
C12-200 + CKK-12, DOPE + DSPC, cholesterol, C14-PEG1000 + C14-PEG350, arachidonic acid |
31.5:10:36:2.5:20 | Tumor-associated antigens GP100 and TRP2 | IH | [12] |
| Melanoma | IL-12-mRNA-LNP | DALs, DOPE, cholesterol, DMG-PEG | 20:30:40:0.75 | Interleukin-12 | IH | [85] |
| Melanoma | A2-mOVA-LNP | A2, DOPE, cholesterol, C14-PEG | 45:10:42.5:2.5 | OVA | ID | [86] |
ID: intradermal injection; IH: hypodermic injection; OVA: ovalbumin.
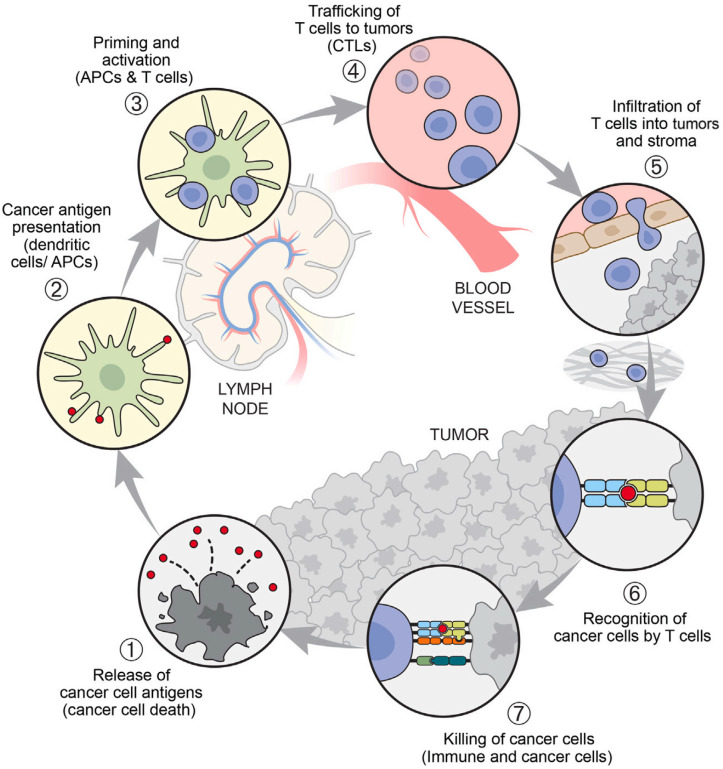
In cancer therapy, antibodies play a dual role: they directly modulate cancer cells and interact with the immune system to elicit both innate and adaptive immune responses [118]. It is assumed that all steps of the cancer immunity cycle function harmoniously to generate an effective immune response (Figure 5) [119]. In patients with cancer, the cancer immunity cycle may be impaired, resulting in an ineffective sequence and consequent lack of anti-tumor immunity, allowing cancer development and progression [120]. Although antibody therapies have demonstrated promising clinical efficacy, they have certain limitations. Challenges such as the stability, complexity of large-scale production, and substantial production and treatment costs may hinder their widespread application [88]. A promising approach involves producing antibodies in vivo by delivering the encoded mRNA, which can result in the effective in vivo expression of the desired antibodies [80,121]. Through sequence design, mRNA can encode therapeutic antibodies. Antibodies produced by mRNA have longer half-lives and lower costs. Various types of antibodies, including monoclonal antibodies (mAbs), bispecific antibodies (bsAbs), and their derivatives, can be expressed using mRNA. Wu et al. [122] showed that mRNA prepared by in vitro transcription (IVT) and formulated into LNPs could efficiently express endogenous therapeutic antibodies in hepatocytes, including intact pembrolizumab (an immune checkpoint inhibitor). This finding offers an alternative approach to antibody therapy for cancer treatment and highlights avenues for further research to optimize mRNA translation and secretion efficiency. This study also lays the foundation for the development of mRNA-coding therapies based on mAbs. Wang et al. [70] developed a bispecific antibody, BisCCL2/5i, which binds to the chemokines CCL2 and CCL5, thereby inhibiting immune cell chemotaxis. Using an mRNA nanoplatform, BisCCL2/5i can significantly induce the polarization of tumor-associated macrophages to the anti-tumor M1 phenotype and alleviate immunosuppression in the tumor microenvironment. BisCCL2/5i combined with a PD-1 ligand inhibitor (PD-Li) resulted in long-term survival in mouse models of primary liver cancer, colorectal cancer, and pancreatic cancer with liver metastasis. This study provides an effective bispecific targeting strategy for the treatment of malignant liver tumors and is expected to extend PD-Li therapy to many types of human liver malignant tumors.
Figure 5.
Key steps in the cancer immune cycle. Reprinted with permission from [123]. Copyright 2023 Elsevier Inc.
The mRNA vaccine is first delivered to APCs, where tumor-associated antigens or neoantigens are produced. Subsequently, these expressed antigens bind to the MHC on the surface of APCs and are presented to T lymphocytes, activating CD4+ and CD8+ T lymphocytes, which then proceed to eliminate tumor cells (Figure 6). Compared with conventional vaccines, mRNA vaccines can stimulate stronger interferon type I responses and effectively activate human CD8+ T-cells [124]. These immune responses play a key role in the eradication of tumors. In a prior study, Oberli et al. [12] developed a library of LNPs to deliver an mRNA vaccine encoding a model antigen OVA to induce a cytotoxic CD8+ T-cell response. The efficacy of this vaccine was tested using an invasive B16F10 melanoma model. They observed robust CD8+ T-cell activation after a single immunization. Bevers et al. [125] optimized an mRNA–LNP formulation to enhance the delivery efficiency of an mRNA vaccine encoding human papillomavirus type 16 antigen. In a homozygous mouse TC-1 tumor model, the optimal LNP composition triggered a strong CD8+ T-cell response. This response could be further enhanced by repeated administration, resulting in notable anti-tumor effects. In addition, type I interferons and phagocytes play key roles in the T-cell response. These findings indicate the feasibility of using mRNA–LNPs as cancer vaccines.
Figure 6.
Schematic illustration of the mRNA–LNP cancer vaccines. Reprinted with permission from [39]. Copyright 2023 Wiley.
In many types of tumor cells, tumor suppressor genes such as PTEN and TP53 often exhibit dysfunction. Restoring the functions of these genes and their expressed proteins by mRNA delivery can regulate the anti-tumor response of cells and induce apoptosis. Approximately 36% of patients with HCC and non-small-cell lung cancer (NSCLC) carry TP53 mutations [126]. Therefore, the restoration of TP53 function may be a potential strategy for cancer treatment. Kong et al. [71] used LNP polymers consisting of G0-C14, PDSA, DMPE-PEG, and DSPE-PEG mixed with the mRNA encoding TP53 for cancer therapy. They found that a TP53 inhibitor not only inhibited the growth of tumor cells by inducing apoptosis and cell cycle arrest, but also increased the sensitivity of tumor cells to everolimus (an mTOR inhibitor). Kamath et al. [127] reported a redox-responsive LNP loaded with TP53-coding mRNA, and studied the therapeutic effects of this LNP in TP53-deficient Hep3B HCC and H1299 NSCLC cells. Apoptosis was significantly increased in both in vitro cellular experiments and an in vivo animal model.
mRNA-4157 is a novel individualized mRNA-based cancer vaccine that encodes 34 patient-specific tumor neoantigens. Currently, its efficacy in the treatment of melanoma is being evaluated. According to recent clinical trial data, the combination of mRNA-4157 and pembrolizumab has shown remarkable efficacy in the treatment of high-risk melanoma. Weber et al. [128] evaluated whether mRNA-4157 in combination with PD-1 could improve recurrence-free and distant metastasis-free survival in patients with high-risk melanoma. The results indicated that combination therapy resulted in longer recurrence-free survival and a lower rate of recurrence or death than monotherapy. This combination is currently being assessed in phase III clinical trials.
The delivery efficiency and biosafety of mRNA can be further improved by modifying the chemical structures of LNP components. Zhang et al. [129] described a fluorinated modification of DSPE-PEG2000, termed FPD. Despite constituting only 1.5% of the lipid components in LNPs, FPD significantly improved the mRNA expression efficiency in B16F10 tumor cells and primary DCs by fivefold and nearly twofold, respectively. Moreover, a mechanistic study showed that FPD facilitates the internalization and endosome escape of LNPs. Shin et al. [130] developed a novel delivery platform based on polyethyleneimine-modified porous Si nanoparticles (PPSNs) for local immunotherapy in vivo. This platform can effectively carry cytokine mRNA and translate it locally, thereby activating the immune response in the tumor microenvironment and avoiding the toxicity of systemic administration. The direct injection of PPSNs with cytokine mRNA into tumors can lead to high-level protein expression in tumors and promote the death of immunogenic cancer cells.
These findings underscore the potential of IVT-mRNA–LNPs as a novel approach for protein delivery, offering an effective strategy for cancer therapy. Compared to vaccines that stimulate active immunity, passive-immunity mRNA–LNP vaccines can rapidly and directly induce immune responses. Upon administration, the antibodies produced from the mRNA provide immediate, specific, albeit transient, immunity. Therefore, these vaccines require continuous administration to effectively treat tumors. Despite extensive research on the application of mRNA–LNPs in tumor therapy, their clinical applications remain limited. Thus, mRNA–LNP vaccines have broad research prospects in tumor therapy.
4.2. Treatment of Infectious Diseases
The mRNA vaccines represent an innovative immunization strategy for combating infectious diseases, offering technical advantages such as high flexibility and rapid response. Because the mRNA production process does not rely on live or inactivated viruses, the scale of production can be rapidly expanded without sacrificing safety. In addition, mRNA vaccines can be designed to rapidly adapt to viral mutations that are critical for responding to pathogens with high genetic variability such as influenza viruses and SARS-CoV-2 (COVID-19) [131]. During the COVID-19 pandemic, mRNA vaccines, including Moderna’s mRNA-1273 and Pfizer/BioNTech’s BNT162b2, encapsulated by LNPs, demonstrated exceptional preventive efficacy and safety in clinical trials. Both vaccines received emergency-use authorization from the FDA by the end of 2020 and have since been widely used worldwide, playing a key role in controlling the COVID-19 epidemic [132]. Besides the COVID-19 vaccine, mRNA technology is also expected to be used to develop vaccines against other infectious diseases such as influenza, AIDS, rabies, and Zika virus. Progress in this field offers a new platform for future vaccine research and development, potentially transforming our approach to managing infectious diseases. Table 4 summarizes the preclinical research for mRNA–LNP vaccines against infectious diseases.
Table 4.
Preclinical research for mRNA–LNP vaccines against infectious diseases.
| Virus | Vaccine | Lipid Nanoparticle Components | Molar Lipid Ratios | Target Antigen | Route | Ref. |
|---|---|---|---|---|---|---|
| HIV-1 | Env-mRNA-LNP | L319, DSPC, cholesterol, PEG-DMG | 55:10:32.5:2.5 | HIV-1 Env gp160 | IM | [133] |
| HIV-1 | VCR01- mRNA-LNP |
L319, DSPC, cholesterol, PEG-DMG | 50:10:38.5:1.5 | Light- and heavy-chain broadly neutralizing anti-HIV-1 antibody VRC01 |
IV | [134] |
| Lyme | OspA-mRNA-LNP | ALC-0315 lipids, DSPC, cholesterol, and PEG-DAG | NR | Outer surface protein A (OspA) | Subcutaneous injection | [135] |
| Rabis | RABV-G-mRNA | Ionizable amino lipids, phospholipids, cholesterol, PEGylated lipids |
NR | Glycoprotein of the Pasteur strain | ID/IM | [136] |
| Ebola | GP-mRNA-LNP | DMAP-BLP, DSPC, cholesterol and PEG–lipids | 50:10:38.5:1.5 | EBOV envelope GP | IM | [137] |
| CMV | PC mRNA-LNP | DMAP-BLP, DSPC, cholesterol, PEG-DSG | 50:10:38.5:1.5 | CMV glycoprotein gB and pentameric complex |
IM | [138] |
| RSV | F-mRNA-LNP | Asymmetric ionizable amino lipids, DSPC, cholesterol, PEG2000-DMG | 58:30:10:2 | F protein of RSV and its related variants | IM | [139] |
| IBV | HA/NA/NP/M2- mRNA-LNP |
ALC-0315 lipids, phosphatidylcholine, cholesterol, PEGylated lipids | NR | HAs from two lineages | ID | [140] |
| ZIKV | ZIKV prM-E mRNA-LNP | Ionizable amino lipids, phospholipids, cholesterol, PEGylated lipids |
NR | prM and E glycoproteins of ZIKV strain Brazil SPH2015 |
IP | [141] |
| DENV-2 | PrME, E80, NS1-mRNA-LNP | D-Lin-MC3-DMA, DSPC, cholesterol, PEGylated lipids |
50:10:38.5:1.5 | Structural proteins prME and E80 and nonstructural protein NS1 from DENV-2 |
IM | [142] |
| Powassan | POWV prM-E-mRNA-LNP | ALC-0315 lipids, structural lipids, sterol, PEGylated lipids |
50:10:38.5:1.5 | POWV prM and E proteins | IP | [143] |
IM: intramuscular injection; ID: intradermal injection; IV: intravenous injection; NR: no report; IP: intraperitoneal injection; CMV: Cytomegalovirus; RSV: Respiratory Syncytial Virus; IBV: Infectious Bronchitis Virus; ZIKV: Zika Virus; DENV-2: Dengue virus.
Vaccines can effectively induce CD4+ and CD8+ T-cell responses, which are crucial in the fight against acute SARS-CoV-2 infection and the development of long-term immunity [144]. Notably, mRNA vaccination elicits significant T-cell responses, particularly after the second dose. For instance, one study found that, although circulating CD4+ T-cells and neutralizing antibodies were weakly detected one week after BNT162b2 vaccination, a robust CD8+ T-cell response was elicited, further enhanced after the booster vaccination [145]. Additionally, memory spike-specific CD8+ T-cells exhibited lower CD8+ expression during the early post-vaccination period compared to the natural infection period, potentially influencing long-term maintenance characteristics. Research and development of mRNA–LNP vaccine candidates is currently in the preclinical and clinical stages, covering a wide range of viruses, including HIV, seasonal influenza, Zika virus, respiratory syncytial virus (RSV), and Epstein–Barr virus. These vaccine candidates elicit strong protective immune responses against various viruses [146]. For example, mRNA-based influenza vaccines can stimulate immune responses against both heterologous and homologous influenza viruses [147]. The research team of Norbert Pardi cooperated with Professor Drew Weissman, the pioneer of mRNA vaccines, to develop an mRNA–LNP vaccine against Borrelia burgdorferi, the pathogen of Lyme disease. This vaccine used LNPs to deliver mRNA expressing outer surface protein A (OspA) of B. burgdorferi. In preclinical animal experiments, mice produced strong antigen-specific antibodies and T-cell responses following a single inoculation of this mRNA–LNP vaccine targeting OspA, thus preventing B. burgdorferi infection. In addition, this vaccine caused a strong memory B-cell response that could be activated after a long time to help prevent subsequent B. burgdorferi infection [135].
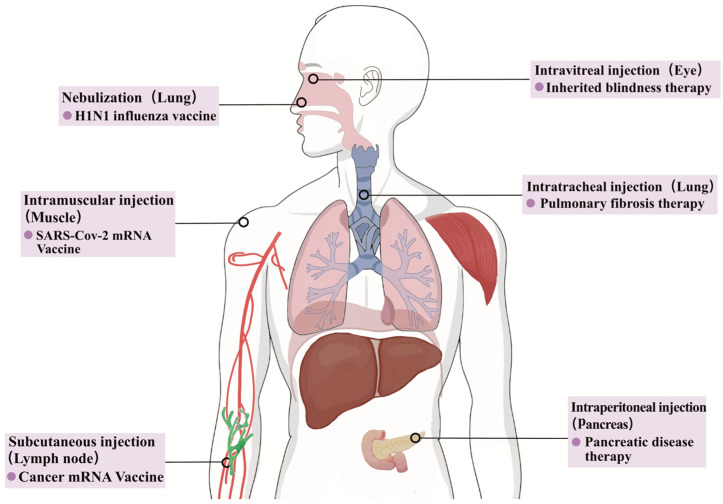
Furthermore, mRNA technology extends beyond vaccine development and is promising for therapeutic applications. For example, Pardi et al. [134] demonstrated that the administration of mRNA encoding an anti-HIV antibody encapsulated in LNPs can be used as passive immunotherapy against HIV-1. The importance of this finding is that the delivery system and route of administration of mRNAs have significant impacts on their biodistribution and efficacy in vivo. This study demonstrates the potential of mRNA vaccines in the fight against HIV-1 and provides a new perspective for future vaccine research and treatment strategies. In preclinical or clinical trials, LNPs are primarily administered via intradermal, subcutaneous, or intramuscular injections, facilitating the targeting of nearby draining lymph nodes and enabling the presentation of antigens to T-cells and exposure to B-cells, thereby activating the immune response [76,148,149]. The two most widely used COVID-19 mRNA vaccines are primarily administered via intramuscular injection, a method that allows the vaccine to be rapidly absorbed into the bloodstream, thereby inducing a systemic immune response. Regarding tumor vaccines, in addition to the above three pathways, alternative methods such as colon administration, nasal drip, or intratumoral delivery can also enhance the targeting and efficacy of LNPs [150,151]. The selection of an appropriate route of administration is critical for accelerating the development of targeted mRNA–LNP vaccines. Different routes of administration may affect the distribution, stability, and induction of immune responses against mRNA–LNPs. For example, for certain diseases such as respiratory infections or certain types of cancer, a specific route of administration may be required to ensure that the drug can effectively reach the target tissue or cell [152]. Figure 7 provides a summary of the representative administration routes and their applications in specific diseases.
Figure 7.
Representative administration routes and their applications of mRNA–LNP.
4.3. Treatment of Inherited Diseases
Hereditary diseases arise from complex and challenging pathologies, often involving pathological DNA sequences in various organelles containing the genome. Treatments for hereditary diseases can be divided into three main categories: exogenous gene introduction and replacement of damaged sequences, non-targeted exogenous supplementation of deficient genetic material, and gene editing techniques to directly rectify genetic mutations [153]. Currently, there are no effective treatments for many hereditary diseases. The advent of mRNA–LNP technology has provided innovative ideas for the treatment of these diseases. For genetic diseases caused by single-gene mutations, such as cystic fibrosis (CF) and thalassemia, mRNA–LNP technology offers the possibility of disease treatment by repairing or replacing abnormal genes [154]. This targeted approach offers fewer potential side-effects and is considered a valuable addition to traditional therapeutic strategies for hereditary diseases. For example, in the treatment of CF, an inherited lung disease, mRNA–LNP technology is used to introduce an mRNA sequence that can repair mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. This therapy has shown remarkable results in clinical trials, helping to improve lung function and quality of life in patients with CF. Table 5 summarizes the preclinical research on mRNA–LNP vaccines for genetic diseases.
Table 5.
Preclinical research of mRNA–LNP vaccines for genetic disease treatment.
| Disease | Vaccine | Lipid Nanoparticle Components | Molar Lipid Ratios | Target Antigen | Route | Ref. |
|---|---|---|---|---|---|---|
| Hemophilia A | hFVIII-mRNA-LNP | F8-N6, DSPC, cholesterol, PEGylated lipids |
50:10:38.5:1.5 | Antihemophilic globulin (Factor VIII) | IV | [155] |
| MMA | hMUT-mRNA-LNP | Ionizable lipids, DSPC, cholesterol, PEGylated lipids | 50:10:38.5:1.5 | Human methylmalonyl-coenzyme A mutase (hMUT) | IV | [156] |
| Cystic fibrosis | MRT5005 | DMG-PEG2000, DOPE, ETI, imidazole cholesterol ester | NR | Fully functional CF transmembrane conductance regulator protein | Nebulzatin | [157] |
| Propionic acidemia | mRNA-3927 | SM-102, DSPC, cholesterol, PEGylated lipids | NR | α and β subunits of propionyl-CoA carboxylase | IV | [158] |
IV: intravenous injection; NR: no report; MMA: Methylmalonic acidemia.
Currently, clinical trials of protein replacement therapies using LNP-mRNA formulations are focused on inherited metabolic disorders, including argininosuccinate lyase deficiency (ASLD), propionic acidemia, and methylmalonic acidemia propionic acidemia. The common feature of these disorders is the absence of key enzymes, leading to the accumulation of specific metabolites, which, in turn, triggers clinical symptoms. Supplementation of the required enzymes with LNP-mRNA preparations retards the progression of the disease and may improve the symptoms and quality of life of patients [156,159,160]. Argininosuccinate lyase deficiency (ASLD) is the second most common urea cycle disorder, accounting for 15–20% of all urea production disorders. Under normal conditions, arginine breaks down arginine into alanine and glutamate. However, in patients with ASLD, because of the absence of ASL, argininosuccinate cannot be effectively decomposed, leading to the excessive accumulation of nitrogen in the blood in the form of ammonia, resulting in hyperammonemia [161]. This condition can cause neurotoxicity, affecting central nervous system function. Jalil et al. [162] have proposed a potential therapeutic strategy targeting the c.1153C>T variant of ASL using a CRISPR base editor delivered by LNPs. Unlike viral vectors, this method offers liver targeting and can effectively address the metabolic phenotype of ASLD, thereby improving patient health. Propionic acidemia, a rare inherited metabolic disorder caused by the accumulation of toxic metabolites owing to defects in propionyl-CoA carboxylase (PCC), poses a threat to the lives of infants. Early screening, diagnosis, and timely treatment are critical for saving the lives of affected infants and improving their prognoses. However, therapeutic drugs targeting the etiology of PCC have not yet been approved. Koeberl et al. [163] first attempted to use the mRNA expression of intracellular proteins as a protein replacement therapy in patients with rare diseases. Participants receiving various doses of mRNA-3927 demonstrated good safety and tolerability, with no observed dose-limiting toxicity. In addition, this therapy continuously reduced the levels of harmful metabolites related to propionate in the body, thereby significantly reducing the probability of metabolic compensatory events in children.
Previous studies have shown that conventional LNP-mediated mRNA delivery is limited to the retinal pigment epithelium (RPE) and Müller glial cells, leaving photoreceptor cells, crucial for visual phototransduction, untargeted due to their inability to penetrate the neural retina. Herrera et al. [164] successfully delivered mRNA to photoreceptor cells by modifying LNPs with peptide ligands. Peptides are composed of amino acid sequences of different lengths, which can be synthesized naturally or artificially. The structure and bioactivity of peptide chains can be tailored based on factors such as the charge density, hydrophobicity, hydrophilicity, structural conformation, and chemical modification, particularly to facilitate crossing biological barriers. Peptides found wide-ranging applications in medicine, including enhanced drug delivery, imaging agents, and nanoparticle drug targeting. Translating mouse model experiments into more clinically relevant non-human primate experiments, researchers observed significant expression of target proteins in photoreceptors, Müller glial cells, and RPE, the potential effectiveness of LNP–mRNA therapeutics in treating inherited blindness.
5. Summary and Outlook
mRNA–LNP therapy is an innovative nucleic acid therapy method that uses lipid nanoparticles as carriers to deliver mRNA into cells, enabling the regulation of specific genes. This approach is characterized by strong targeting, high delivery efficiency, and relatively good safety, making it a promising treatment for certain diseases. The successful application of mRNA vaccines during the COVID-19 pandemic significantly advanced the development of mRNA–LNP technology, offering new possibilities for treating a wide range of diseases, such as cancer, infectious diseases, and genetic diseases. Given that the physical properties of LNPs, such as particle size, morphology, and surface properties, are significantly affected by the structure and composition of lipids, the efficacy, safety, and drug distribution of LNPs can be improved by optimizing the lipid composition. In addition, the development of targeted mRNA–LNP delivery technologies for specific tissues and cells could further improve the therapeutic efficacy and safety of mRNA–LNPs, expand the application of mRNA technology, and promote advancement of the field.
Non-viral vectors such as LNPs can efficiently load mRNA, improve the transfection efficiency, and trigger an immune response, thereby increasing antibody titers. Biodegradable LNPs can be rapidly eliminated from plasma and tissues, improving their safety and tolerability. Cationic lipids play a key role in determining the delivery efficiency and transfection efficiency of mRNA–LNP preparations, and the toxicity of the vector is related to the structure of the cationic lipids. To improve mRNA delivery efficiency, lipid head groups and hydrophobic tails can be modulated to increase the cellular uptake and endosomal escape of LNPs. In addition, the modulation of the lipid structure enables the cell-specific and tissue-specific delivery of LNPs. As the expression of mRNA in cells is transient, repeated injections of LNPs loaded with mRNA are often required to maintain the therapeutic effects. However, repeated injections may elicit an immune response, such as the production of antibodies against PEG, which may reduce the efficacy of the drug. Therefore, the search for disease-specific mRNA or the development of strategies that can prolong mRNA expression are important for improving drug efficacy and reducing the frequency of injections.
Overall, mRNA–LNPs represent a powerful and versatile platform for vaccine and drug development and are expected to emerge as a cornerstone in future tumor vaccine and drug development. While screening novel ICLs offers chemical innovation, predicting their delivery efficiency and targeting remains uncertain, posing challenges. Flux screening of LNP formulations, although beneficial, has limitations such as a heavy workload and poor organ specificity. Moreover, while SORT LNPs provide a clear research direction, the potential safety risks associated with the addition of cationic and anionic lipids require further data. Although antibody-modified LNPs offer significant advantages in cellular targeted delivery, addressing their shortcomings, such as poor tissue specificity and complex preparation processes, is imperative. In addition, attention should be directed toward designing the mRNA molecules themselves. Furthermore, in the era of rapid advancements in big data and artificial intelligence, their application in the development of targeted mRNA–LNPs could significantly enhance the research efficiency and success rates.
In summary, mRNA–LNP technology holds significant scientific and economic value. By employing various LNP development strategies, coordinating mRNA sequence research, and integrating advanced artificial intelligence technologies, novel targeted technologies are anticipated to emerge. The authors of this review anticipate successful delivery to more critical organs/cells and addressing unmet clinical needs in mRNA therapeutics.
Author Contributions
Y.L. and Y.H. wrote the review article, and L.W., J.D., C.G., and G.H. critically revised all versions of the article. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was funded by the National Key R&D Program of China (No. 2023YFF0724200), Guangdong Pearl River Talents Program (No. 2017GC010411), Science and Technology Projects in Guangzhou (No. 2024A04J3912), and the Scientific Research Project of the Sixth Affiliated Hospital of Sun Yat-Sen University (No. 2022JBGS07).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Singh M.S., Ramishetti S., Landesman-Milo D., Goldsmith M., Chatterjee S., Palakuri R., Peer D. Therapeutic gene silencing using targeted lipid nanoparticles in metastatic ovarian cancer. Small. 2021;17:2100287. doi: 10.1002/smll.202100287. [DOI] [PubMed] [Google Scholar]
- 2.Zhang X., Goel V., Robbie G.J. Pharmacokinetics of patisiran, the first approved RNA interference therapy in patients with hereditary transthyretin-mediated amyloidosis. J. Clin. Pharmacol. 2020;60:573–585. doi: 10.1002/jcph.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saadati F., Cammarone S., Ciufolini M.A. A Route to Lipid ALC-0315: A Key component of a COVID-19 mRNA Vaccine. Chemistry. 2022;28:e202200906. doi: 10.1002/chem.202200906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schreckenberg R., Woitasky N., Itani N., Czech L., Ferdinandy P., Schulz R. Cardiac side effects of RNA-based SARS-CoV-2 vaccines: Hidden cardiotoxic effects of mRNA-1273 and BNT162b2 on ventricular myocyte function and structure. Br. J. Pharmacol. 2024;181:345–361. doi: 10.1111/bph.16262. [DOI] [PubMed] [Google Scholar]
- 5.Ferraresso F., Strilchuk A.W., Juang L.J., Poole L.G., Luyendyk J.P., Kastrup C.J. Comparison of DLin-MC3-DMA and ALC-0315 for siRNA delivery to hepatocytes and hepatic stellate cells. Mol. Pharm. 2022;19:2175–2182. doi: 10.1021/acs.molpharmaceut.2c00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Semple S.C., Akinc A., Chen J., Sandhu A.P., Mui B.L., Cho C.K., Sah D.W.Y., Stebbing D., Crosley E.J., Yaworski E., et al. Rational design of cationic lipids for siRNA delivery. Nat. Biotechnol. 2010;28:172-U118. doi: 10.1038/nbt.1602. [DOI] [PubMed] [Google Scholar]
- 7.Schlich M., Palomba R., Costabile G., Mizrahy S., Pannuzzo M., Peer D., Decuzzi P. Cytosolic delivery of nucleic acids: The case of ionizable lipid nanoparticles. Bioeng. Transl. Med. 2021;6:e10213. doi: 10.1002/btm2.10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hajj K.A., Whitehead K.A. Tools for translation: Non-viral materials for therapeutic mRNA delivery. Nat. Rev. Mater. 2017;2:17056. doi: 10.1038/natrevmats.2017.56. [DOI] [Google Scholar]
- 9.Cheng X., Lee R.J. The role of helper lipids in lipid nanoparticles (LNPs) designed for oligonucleotide delivery. Adv. Drug Deliv. Rev. 2016;99:129–137. doi: 10.1016/j.addr.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 10.Tenchov B.G., MacDonald R.C., Siegel D.P. Cubic phases in phosphatidylcholine-cholesterol mixtures: Cholesterol as membrane “fusogen”. Biophys. J. 2006;91:2508–2516. doi: 10.1529/biophysj.106.083766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suk J.S., Xu Q., Kim N., Hanes J., Ensign L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016;99:28–51. doi: 10.1016/j.addr.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oberli M.A., Reichmuth A.M., Dorkin J.R., Mitchell M.J., Fenton O.S., Jaklenec A., Anderson D.G., Langer R., Blankschtein D. Lipid nanoparticle assisted mRNA delivery for potent cancer immunotherapy. Nano Lett. 2017;17:1326–1335. doi: 10.1021/acs.nanolett.6b03329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eygeris Y., Gupta M., Kim J., Sahay G. Chemistry of lipid nanoparticles for RNA Delivery. Acc. Chem. Res. 2022;55:2–12. doi: 10.1021/acs.accounts.1c00544. [DOI] [PubMed] [Google Scholar]
- 14.Sabnis S., Kumarasinghe E.S., Salerno T., Mihai C., Ketova T., Senn J.J., Lynn A., Bulychev A., McFadyen I., Chan J., et al. A novel amino lipid series for mRNA delivery: Improved endosomal escape and sustained pharmacology and safety in non-human primates. Mol. Ther. 2018;26:1509–1519. doi: 10.1016/j.ymthe.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Z., Ma W., Fu X., Qi Y., Zhao Y., Zhang S. Development and applications of mRNA treatment based on lipid nanoparticles. Biotechnol. Adv. 2023;65:108130. doi: 10.1016/j.biotechadv.2023.108130. [DOI] [PubMed] [Google Scholar]
- 16.Meng C., Chen Z., Li G., Welte T., Shen H. Nanoplatforms for mRNA Therapeutics. Adv. Ther. 2021;4:2000099. doi: 10.1002/adtp.202000099. [DOI] [Google Scholar]
- 17.Patel S., Ashwanikumar N., Robinson E., Xia Y., Mihai C., Griffith J.P., III, Hou S., Esposito A.A., Ketova T., Welsher K., et al. Naturally-occurring cholesterol analogues in lipid nanoparticles induce polymorphic shape and enhance intracellular delivery of mRNA. Nat. Commun. 2020;11:983. doi: 10.1038/s41467-020-14527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heyes J., Hall K., Tailor V., Lenz R., MacLachlan I. Synthesis and characterization of novel poly(ethylene glycol)-lipid conjugates suitable for use in drug delivery. J. Control. Release. 2006;112:280–290. doi: 10.1016/j.jconrel.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Jokerst J.V., Lobovkina T., Zare R.N., Gambhir S.S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine. 2011;6:715–728. doi: 10.2217/nnm.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kowalski P.S., Rudra A., Miao L., Anderson D.G. Delivering the messenger: Advances in technologies for therapeutic mRNA delivery. Mol. Ther. 2019;27:710–728. doi: 10.1016/j.ymthe.2019.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alameh M.-G., Tombacz I., Bettini E., Lederer K., Ndeupen S., Sittplangkoon C., Wilmore J.R., Gaudette B.T., Soliman O.Y., Pine M., et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity. 2022;55:1136–1138. doi: 10.1016/j.immuni.2022.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen K., Fan N., Huang H., Jiang X., Qin S., Xiao W., Zheng Q., Zhang Y., Duan X., Qin Z., et al. MRNA vaccines against SARS-CoV-2 variants delivered by lipid nanoparticles based on novel ionizable lipids. Adv. Funct. Mater. 2022;32:2204692. doi: 10.1002/adfm.202204692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kauffman K.J., Dorkin J.R., Yang J.H., Heartlein M.W., DeRosa F., Mir F.F., Fenton O.S., Anderson D.G. Optimization of lipid nanoparticle formulations for mRNA delivery in vivo with fractional factorial and definitive screening designs. Nano Lett. 2015;15:7300–7306. doi: 10.1021/acs.nanolett.5b02497. [DOI] [PubMed] [Google Scholar]
- 24.Zhang R., El-Mayta R., Murdoch T.J., Warzecha C.C., Billingsley M.M., Shepherd S.J., Gong N., Wang L., Wilson J.M., Lee D., et al. Helper lipid structure influences protein adsorption and delivery of lipid nanoparticles to spleen and liver. Biomater. Sci. 2021;9:1449–1463. doi: 10.1039/D0BM01609H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inglut C.T., Sorrin A.J., Kuruppu T., Vig S., Cicalo J., Ahmad H., Huang H.C. Immunological and toxicological considerations for the design of liposomes. Nanomaterials. 2020;10:190. doi: 10.3390/nano10020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strachan J.B., Dyett B.P., Nasa Z., Valery C., Conn C.E. Toxicity and cellular uptake of lipid nanoparticles of different structure and composition. J. Colloid Interface Sci. 2020;576:241–251. doi: 10.1016/j.jcis.2020.05.002. [DOI] [PubMed] [Google Scholar]
- 27.McClements D.J. Edible lipid nanoparticles: Digestion, absorption, and potential toxicity. Prog. Lipid Res. 2013;52:409–423. doi: 10.1016/j.plipres.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 28.Dass C.R. Lipoplex-mediated delivery of nucleic acids: Factors affecting in vivo transfection. J. Mol. Med. 2004;82:579–591. doi: 10.1007/s00109-004-0558-8. [DOI] [PubMed] [Google Scholar]
- 29.Lv H., Zhang S., Wang B., Cui S., Yan J. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control. Release. 2006;114:100–109. doi: 10.1016/j.jconrel.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 30.Biscans A., Ly S., McHugh N., Cooper D.A., Khvorova A. Engineered ionizable lipid siRNA conjugates enhance endosomal escape but induce toxicity in vivo. J. Control. Release. 2022;349:831–843. doi: 10.1016/j.jconrel.2022.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Q., Lai S.K. Anti-PEG immunity: Emergence, characteristics, and unaddressed questions. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015;7:655–677. doi: 10.1002/wnan.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tenchov R., Bird R., Curtze A.E., Zhou Q. Lipid nanoparticles—From liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement. ACS Nano. 2021;15:16982–17015. doi: 10.1021/acsnano.1c04996. [DOI] [PubMed] [Google Scholar]
- 33.Nawaz M., Heydarkhan-Hagvall S., Tangruksa B., Garibotti H.G.-K., Jing Y., Maugeri M., Kohl F., Hultin L., Reyahi A., Camponeschi A., et al. Lipid nanoparticles deliver the therapeutic VEGFA mRNA in vitro and in vivo and transform extracellular vesicles for their functional extensions. Adv. Sci. 2023;10:2206187. doi: 10.1002/advs.202206187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Billingsley M.M. Ph.D. Thesis. University of Pennsylvania; Philadelphia, PA, USA: 2023. Ionizable Lipid Nanoparticles for mRNA CAR T Cell Engineering. [Google Scholar]
- 35.Lorentzen C.L., Haanen J.B., Met O., Svane I.M. Clinical advances and ongoing trials of mRNA vaccines for cancer treatment. Lancet Oncol. 2022;23:E450–E458. doi: 10.1016/S1470-2045(22)00372-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Freeley M., Long A. Advances in siRNA delivery to T-cells: Potential clinical applications for inflammatory disease, cancer and infection. Biochem. J. 2013;455:133–147. doi: 10.1042/BJ20130950. [DOI] [PubMed] [Google Scholar]
- 37.Granot-Matok Y., Kon E., Dammes N., Mechtinger G., Peer D. Therapeutic mRNA delivery to leukocytes. J. Control. Release. 2019;305:165–175. doi: 10.1016/j.jconrel.2019.05.032. [DOI] [PubMed] [Google Scholar]
- 38.Moulahoum H., Ghorbanizamani F., Zihnioglu F., Timur S. Surface biomodification of liposomes and polymersomes for efficient targeted drug delivery. Bioconjugate Chem. 2021;32:1491–1502. doi: 10.1021/acs.bioconjchem.1c00285. [DOI] [PubMed] [Google Scholar]
- 39.Zong Y., Lin Y., Wei T., Cheng Q. Lipid nanoparticle (LNP) enables mRNA delivery for cancer therapy. Adv. Mater. 2023;35:e2303261. doi: 10.1002/adma.202303261. [DOI] [PubMed] [Google Scholar]
- 40.Veiga N., Goldsmith M., Granot Y., Rosenblum D., Dammes N., Kedmi R., Ramishetti S., Peer D. Cell specific delivery of modified mRNA expressing therapeutic proteins to leukocytes. Nat. Commun. 2018;9:4493. doi: 10.1038/s41467-018-06936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Veiga N., Goldsmith M., Diesendruck Y., Ramishetti S., Rosenblum D., Elinav E., Behlke M.A., Benhar I., Peer D. Leukocyte-specific siRNA delivery revealing IRF8 as a potential anti-inflammatory target. J. Control. Release. 2019;313:33–41. doi: 10.1016/j.jconrel.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 42.Veenbergen S., Li P., Raatgeep H.C., Lindenbergh-Kortleve D.J., Simons-Oosterhuis Y., Farrel A., Costes L.M.M., Joosse M.E., van Berkel L.A., de Ruiter L.F., et al. IL-10 signaling in dendritic cells controls IL-1β-mediated IFNγ secretion by human CD4 T cells: Relevance to inflammatory bowel disease. Mucosal Immunol. 2019;12:1201–1211. doi: 10.1038/s41385-019-0194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dammes N., Goldsmith M., Ramishetti S., Dearling J.L.J., Veiga N., Packard A.B., Peer D. Conformation-sensitive targeting of lipid nanoparticles for RNA therapeutics. Nat. Nanotechnol. 2021;16:1030–1038. doi: 10.1038/s41565-021-00928-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramishetti S., Hazan-Halevy I., Palakuri R., Chatterjee S., Gonna S.N., Dammes N., Freilich I., Shmuel L.K., Danino D., Peer D. A combinatorial library of lipid nanoparticles for RNA delivery to leukocytes. Adv. Mater. 2020;32:1906128. doi: 10.1002/adma.201906128. [DOI] [PubMed] [Google Scholar]
- 45.Mayordomo J.I., Zorina T., Storkus W.J., Zitvogel L., GarciaPrats M.D., DeLeo A.B., Lotze M.T. Bone marrow-derived dendritic cells serve as potent adjuvants for peptide-based antitumor vaccines. Stem Cells. 1997;15:94–103. doi: 10.1002/stem.150094. [DOI] [PubMed] [Google Scholar]
- 46.Tang X., Zhang J., Sui D., Yang Q., Wang T., Xu Z., Li X., Gao X., Yan X., Liu X., et al. Simultaneous dendritic cells targeting and effective endosomal escape enhance sialic acid-modified mRNA vaccine efficacy and reduce side effects. J. Control. Release. 2023;364:529–545. doi: 10.1016/j.jconrel.2023.11.008. [DOI] [PubMed] [Google Scholar]
- 47.Haase F., Pohmerer J., Yazdi M., Grau M., Zeyn Y., Wilk U., Burghardt T., Hohn M., Hieber C., Bros M., et al. Lipoamino bundle LNPs for efficient mRNA transfection of dendritic cells and macrophages show high spleen selectivity. Eur. J. Pharm. Biopharm. 2024;194:95–109. doi: 10.1016/j.ejpb.2023.11.025. [DOI] [PubMed] [Google Scholar]
- 48.Igyarto B.Z., Jacobsen S., Ndeupen S. Future considerations for the mRNA-lipid nanoparticle vaccine platform. Curr. Opin. Virol. 2021;48:65–72. doi: 10.1016/j.coviro.2021.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yong S.-B., Ramishetti S., Goldsmith M., Diesendruck Y., Hazan-Halevy I., Chatterjee S., Naidu G.S., Ezra A., Peer D. Dual-targeted lipid nanotherapeutic boost for chemo-immunotherapy of cancer. Adv. Mater. 2022;34:2106350. doi: 10.1002/adma.202106350. [DOI] [PubMed] [Google Scholar]
- 50.Thatte A.S., Hamilton A.G., Nachod B.E., Mukalel A.J., Billingsley M.M., Palanki R., Swingle K.L., Mitchell M.J. mRNA lipid nanoparticles for ex vivo engineering of immunosuppressive T cells for autoimmunity therapies. Nano Lett. 2023;23:10179–10188. doi: 10.1021/acs.nanolett.3c02573. [DOI] [PubMed] [Google Scholar]
- 51.Tombacz I., Laczko D., Shahnawaz H., Muramatsu H., Natesan A., Yadegari A., Papp T.E., Alameh M.-G., Shuvaev V., Mui B.L., et al. Highly efficient CD4+ T cell targeting and genetic recombination using engineered CD4+ cell-homing mRNA-LNPs. Mol. Ther. 2021;29:3293–3304. doi: 10.1016/j.ymthe.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yan Y., Liu X., Wang L., Wu C., Shuai Q., Zhang Y., Liu S. Branched hydrophobic tails in lipid nanoparticles enhance mRNA delivery for cancer immunotherapy. Biomaterials. 2023;301:122279. doi: 10.1016/j.biomaterials.2023.122279. [DOI] [PubMed] [Google Scholar]
- 53.Henderson N.C., Rieder F., Wynn T.A. Fibrosis: From mechanisms to medicines. Nature. 2020;587:555–566. doi: 10.1038/s41586-020-2938-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rurik J.G., Aghajanian H., Epstein J.A. Immune cells and immunotherapy for cardiac injury and repair. Circ. Res. 2021;128:1766–1779. doi: 10.1161/CIRCRESAHA.121.318005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Voynova E., Hawk N., Flomerfelt F.A., Telford W.G., Gress R.E., Kanakry J.A., Kovalovsky D. Increased activity of a NK-specific CAR-NK framework targeting CD3 and CD5 for T-cell leukemias. Cancers. 2022;14:524. doi: 10.3390/cancers14030524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu H., Yu Y., Zhao Y., Liu W., Liu Z., Zhang G., Chen Z. A hinge region enhances the cytotoxicity of anti-CD5 CAR-T cells targeting T cell acute lymphoblastic leukemia. Int. Immunopharmacol. 2023;124:110904. doi: 10.1016/j.intimp.2023.110904. [DOI] [PubMed] [Google Scholar]
- 57.Rurik J.G., Tombacz I., Yadegari A., Fernandez P.O.M., Shewale S.V., Li L., Kimura T., Soliman O.Y., Papp T.E., Tam Y.K., et al. CAR T cells produced in vivo to treat cardiac injury. Science. 2022;375:91–96. doi: 10.1126/science.abm0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu X., Xia T. Recent advances in site-specific lipid nanoparticles for mRNA delivery. ACS Nanosci. Au. 2023;3:192–203. doi: 10.1021/acsnanoscienceau.2c00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Colino C.I., Lanao J.M., Gutierrez-Millan C. Targeting of hepatic macrophages by therapeutic nanoparticles. Front. Immunol. 2020;11:218. doi: 10.3389/fimmu.2020.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu X., Wang X., Liao Y.P., Luo L., Xia T., Nel A.E. Use of a liver-targeting immune-tolerogenic mRNA lipid nanoparticle platform to treat peanut-induced anaphylaxis by single- and multiple-epitope nucleotide sequence delivery. ACS Nano. 2023;17:4942–4957. doi: 10.1021/acsnano.2c12420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paunovska K., Da Silva Sanchez A.J., Sago C.D., Gan Z., Lokugamage M.P., Islam F.Z., Kalathoor S., Krupczak B.R., Dahlman J.E. Nanoparticles containing oxidized cholesterol deliver mRNA to the liver microenvironment at clinically relevant doses. Adv. Mater. 2019;31:e1807748. doi: 10.1002/adma.201807748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Siegwart L., Piton G., Jourdan C., Sauze J., Sugihara S., Bertrand I. Carbon and nutrient colimitations control the microbial response to fresh organic carbon inputs in soil at different depths. Geoderma. 2023;440:116729. doi: 10.1016/j.geoderma.2023.116729. [DOI] [Google Scholar]
- 63.Chen J., Ye Z., Huang C., Qiu M., Song D., Li Y., Xu Q. Lipid nanoparticle-mediated lymph node-targeting delivery of mRNA cancer vaccine elicits robust CD8 T cell response. Proc. Natl. Acad. Sci. USA. 2022;119:e2207841119. doi: 10.1073/pnas.2207841119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dilliard S.A., Cheng Q., Siegwart D.J. On the mechanism of tissue-specific mRNA delivery by selective organ targeting nanoparticles. Proc. Natl. Acad. Sci. USA. 2021;118:e2109256118. doi: 10.1073/pnas.2109256118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paunovska K., Sago C.D., Monaco C.M., Hudson W.H., Castro M.G., Rudoltz T.G., Kalathoor S., Vanover D.A., Santangelo P.J., Ahmed R., et al. A direct comparison of in vitro and in vivo nucleic acid delivery mediated by hundreds of nanoparticles reveals a weak correlation. Nano Lett. 2018;18:2148–2157. doi: 10.1021/acs.nanolett.8b00432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huayamares S.G., Lokugamage M.P., Rab R., Sanchez A.J.D.S., Kim H., Radmand A., Loughrey D., Lian L., Hou Y., Achyut B.R., et al. High-throughput screens identify a lipid nanoparticle that preferentially delivers mRNA to human tumors in vivo. J. Control. Release. 2023;357:394–403. doi: 10.1016/j.jconrel.2023.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dilliard S.A., Sun Y., Brown M.O., Sung Y.-C., Chatterjee S., Farbiak L., Vaidya A., Lian X., Wang X., Lemoff A., et al. The interplay of quaternary ammonium lipid structure and protein corona on lung-specific mRNA delivery by selective organ targeting (SORT) nanoparticles. J. Control. Release. 2023;361:361–372. doi: 10.1016/j.jconrel.2023.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jiang L., Berraondo P., Jerico D., Guey L.T., Sampedro A., Frassetto A., Benenato K.E., Burke K., Santamaria E., Alegre M., et al. Systemic messenger RNA as an etiological treatment for acute intermittent porphyria. Nat. Med. 2018;24:1899–1909. doi: 10.1038/s41591-018-0199-z. [DOI] [PubMed] [Google Scholar]
- 69.Han J.P., Kim M., Choi B.S., Lee J.H., Lee G.S., Jeong M., Lee Y., Kim E.-A., Oh H.-K., Go N., et al. In vivo delivery of CRISPR-Cas9 using lipid nanoparticles enables antithrombin gene editing for sustainable hemophilia A and B therapy. Sci. Adv. 2022;8:eabj6901. doi: 10.1126/sciadv.abj6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Y., Tiruthani K., Li S., Hu M., Zhong G., Tang Y., Roy S., Zhang L., Tan J., Liao C., et al. mRNA delivery of a bispecific single-domain antibody to polarize tumor-associated macrophages and synergize immunotherapy against liver malignancies. Adv. Mater. 2021;33:e2007603. doi: 10.1002/adma.202007603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kong N., Tao W., Ling X., Wang J., Xiao Y., Shi S., Ji X., Shajii A., Gan S.T., Kim N.Y., et al. Synthetic mRNA nanoparticle-mediated restoration of p53 tumor suppressor sensitizes p53 deficient cancers to mTOR inhibition. Sci. Transl. Med. 2019;11:eaaw1565. doi: 10.1126/scitranslmed.aaw1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang D., Wang G., Yu X., Wei T., Farbiak L., Johnson L.T., Taylor A.M., Xu J., Hong Y., Zhu H., et al. Enhancing CRISPR/Cas gene editing through modulating cellular mechanical properties for cancer therapy. Nat. Nanotechnol. 2022;17:777–787. doi: 10.1038/s41565-022-01122-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim J., Mukherjee A., Nelson D., Jozic A., Sahay G. Rapid generation of circulating and mucosal decoy ACE2 using mRNA nanotherapeutics for the potential treatment of SARS-CoV-2. bioRxiv. 2020 doi: 10.1002/advs.202202556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang T., Poenisch M., Khanal R., Hu Q., Dai Z., Li R., Song G., Yuan Q., Yao Q., Shen X., et al. Therapeutic mRNA attenuates liver fibrosis in a preclinical model. J. Hepatol. 2022;77:270. doi: 10.1016/j.jhep.2022.03.023. [DOI] [PubMed] [Google Scholar]
- 75.Sun H., Zhang Y., Wang J., Su J., Zhou D., Yu X., Xu Y., Yang W. Application of lung-targeted lipid nanoparticle-delivered mRNA of soluble PD-L1 via SORT technology in acute respiratory distress syndrome. Theranostics. 2023;13:4974–4992. doi: 10.7150/thno.86466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lokugamage M.P., Vanover D., Beyersdorf J., Hatit M.Z.C., Rotolo L., Echeverri E.S., Peck H.E., Ni H., Yoon J.-K., Kim Y., et al. Optimization of lipid nanoparticles for the delivery of nebulized therapeutic mRNA to the lungs. Nat. Biomed. Eng. 2021;5:1059–1068. doi: 10.1038/s41551-021-00786-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wei T., Sun Y., Cheng Q., Chatterjee S., Traylor Z., Johnson L.T., Coquelin M.L., Wang J., Torres M.J., Lian X., et al. Lung SORT LNPs enable precise homology-directed repair mediated CRISPR/Cas genome correction in cystic fibrosis models. Nat. Commun. 2023;14:7322. doi: 10.1038/s41467-023-42948-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Massaro M., Wu S., Baudo G., Liu H., Collum S., Lee H., Stigliano C., Segura-Ibarra V., Karmouty-Quintana H., Blanco E. Lipid nanoparticle-mediated mRNA delivery in lung fibrosis. Eur. J. Pharm. Sci. 2023;183:106370. doi: 10.1016/j.ejps.2023.106370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Qiu M., Tang Y., Chen J., Muriph R., Ye Z., Huang C., Evans J., Henske E.P., Xu Q. Lung-selective mRNA delivery of synthetic lipid nanoparticles for the treatment of pulmonary lymphangioleiomyomatosis. Proc. Natl. Acad. Sci. USA. 2022;119:e2116271119. doi: 10.1073/pnas.2116271119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huang C., Duan X., Wang J., Tian Q., Ren Y., Chen K., Zhang Z., Li Y., Feng Y., Zhong K., et al. Lipid nanoparticle delivery system for mRNA encoding B7H3-redirected bispecific antibody displays potent antitumor effects on malignant tumors. Adv. Sci. 2023;10:2205532. doi: 10.1002/advs.202205532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cheng Q., Wei T., Farbiak L., Johnson L.T., Dilliard S.A., Siegwart D.J. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat. Nanotechnol. 2020;15:313–320. doi: 10.1038/s41565-020-0669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gomi M., Sakurai Y., Sato M., Tanaka H., Miyatake Y., Fujiwara K., Watanabe M., Shuto S., Nakai Y., Tange K., et al. Delivering mRNA to secondary lymphoid tissues by phosphatidylserine-loaded lipid nanoparticles. Adv. Healthc. Mater. 2023;12:e2202528. doi: 10.1002/adhm.202202528. [DOI] [PubMed] [Google Scholar]
- 83.Xue L., Gong N., Shepherd S.J., Xiong X., Liao X., Han X., Zhao G., Song C., Huang X., Zhang H., et al. Rational design of bisphosphonate lipid-like materials for mRNA delivery to the bone microenvironment. J. Am. Chem. Soc. 2022;144:9926–9937. doi: 10.1021/jacs.2c02706. [DOI] [PubMed] [Google Scholar]
- 84.Rosenblum D., Gutkin A., Kedmi R., Ramishetti S., Veiga N., Jacobi A.M., Schubert M.S., Friedmann-Morvinski D., Cohen Z.R., Behlke M.A., et al. CRISPR-Cas9 genome editing using targeted lipid nanoparticles for cancer therapy. Sci. Adv. 2020;6:eabc9450. doi: 10.1126/sciadv.abc9450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu J.-Q., Zhang C., Zhang X., Yan J., Zeng C., Talebian F., Lynch K., Zhao W., Hou X., Du S., et al. Intratumoral delivery of IL-12 and IL-27 mRNA using lipid nanoparticles for cancer immunotherapy. J. Control. Release. 2022;345:306–313. doi: 10.1016/j.jconrel.2022.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Miao L., Li L., Huang Y., Delcassian D., Chahal J., Han J., Shi Y., Sadtler K., Gao W., Lin J., et al. Delivery of mRNA vaccines with heterocyclic lipids increases anti-tumor efficacy by STING-mediated immune cell activation. Nat. Biotechnol. 2019;37:1174–1185. doi: 10.1038/s41587-019-0247-3. [DOI] [PubMed] [Google Scholar]
- 87.Thran M., Mukherjee J., Poenisch M., Fiedler K., Thess A., Mui B.L., Hope M.J., Tam Y.K., Horscroft N., Heidenreich R., et al. mRNA mediates passive vaccination against infectious agents, toxins, and tumors. EMBO Mol. Med. 2017;9:1434–1447. doi: 10.15252/emmm.201707678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rybakova Y., Kowalski P.S., Huang Y., Gonzalez J.T., Heartlein M.W., DeRosa F., Delcassian D., Anderson D.G. mRNA delivery for therapeutic anti-HER2 antibody expression in vivo. Mol. Ther. 2019;27:1415–1423. doi: 10.1016/j.ymthe.2019.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pattipeiluhu R., Arias-Alpizar G., Basha G., Chan K.Y.T., Bussmann J., Sharp T.H., Moradi M.A., Sommerdijk N., Harris E.N., Cullis P.R., et al. Anionic lipid nanoparticles preferentially deliver mRNA to the hepatic reticuloendothelial system. Adv. Mater. 2022;34:e2201095. doi: 10.1002/adma.202201095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang R., Tang R., Li B., Ma X., Schnabl B., Tilg H. Gut microbiome, liver immunology, and liver diseases. Cell. Mol. Immunol. 2021;18:4–17. doi: 10.1038/s41423-020-00592-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zabaleta N., Unzu C., Weber N.D., Gonzalez-Aseguinolaza G. Gene therapy for liver diseases—Progress and challenges. Nat. Rev. Gastroenterol. Hepatol. 2023;20:288–305. doi: 10.1038/s41575-022-00729-0. [DOI] [PubMed] [Google Scholar]
- 92.Chen S., Tam Y.Y.C., Lin P.J.C., Sung M.M.H., Tam Y.K., Cullis P.R. Influence of particle size on the potency of lipid nanoparticle formulations of siRNA. J. Control. Release. 2016;235:236–244. doi: 10.1016/j.jconrel.2016.05.059. [DOI] [PubMed] [Google Scholar]
- 93.Kim M., Jeong M., Hur S., Cho Y., Park J., Jung H., Seo Y., Woo H.A., Nam K.T., Lee K., et al. Engineered ionizable lipid nanoparticles for targeted delivery of RNA therapeutics into different types of cells in the liver. Sci. Adv. 2021;7:eabf4398. doi: 10.1126/sciadv.abf4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Saunders N.R.M., Paolini M.S., Fenton O.S., Poul L., Devalliere J., Mpambani F., Darmon A., Bergere M., Jibault O., Germain M., et al. A nanoprimer to improve the systemic delivery of siRNA and mRNA. Nano Lett. 2020;20:4264–4269. doi: 10.1021/acs.nanolett.0c00752. [DOI] [PubMed] [Google Scholar]
- 95.Truong B., Allegri G., Liu X.-B., Burke K.E., Zhu X., Cederbaum S.D., Haberle J., Martini P.G.V., Lipshutz G.S. Lipid nanoparticle-targeted mRNA therapy as a treatment for the inherited metabolic liver disorder arginase deficiency. Proc. Natl. Acad. Sci. USA. 2019;116:21150–21159. doi: 10.1073/pnas.1906182116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Peters R., Harris T. Advances and innovations in haemophilia treatment. Nat. Rev. Drug Discov. 2018;17:493–508. doi: 10.1038/nrd.2018.70. [DOI] [PubMed] [Google Scholar]
- 97.Bulcha J.T., Wang Y., Ma H., Tai P.W., Gao G. Viral vector platforms within the gene therapy landscape. Signal Transduct. Target. Ther. 2021;6:664–687. doi: 10.1038/s41392-021-00487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pasi K.J., Rangarajan S., Georgiev P., Mant T., Creagh M.D., Lissitchkov T., Bevan D., Austin S., Hay C.R., Hegemann I., et al. Targeting of antithrombin in hemophilia A or B with RNAi therapy. N. Engl. J. Med. 2017;377:819–828. doi: 10.1056/NEJMoa1616569. [DOI] [PubMed] [Google Scholar]
- 99.Machin N., Ragni M.V. An investigational RNAi therapeutic targeting antithrombin for the treatment of hemophilia A and B. J. Blood Med. 2018;9:135–140. doi: 10.2147/JBM.S159297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nguyen N.H., Jarvia N.L., Balu-Iyera S.V. Immunogenicity of therapeutic biological modalities—Lessons from hemophilia a therapies. J. Pharm. Sci. 2023;112:2347–2370. doi: 10.1016/j.xphs.2023.05.014. [DOI] [PubMed] [Google Scholar]
- 101.Han X., Xu J., Xu Y., Alameh M.G., Xue L., Gong N., El-Mayta R., Palanki R., Warzecha C.C., Zhao G., et al. In situ combinatorial synthesis of degradable branched lipidoids for systemic delivery of mRNA therapeutics and gene editors. Nat. Commun. 2024;15:1762. doi: 10.1038/s41467-024-45537-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Di J., Huang P., Chen X. Targeting strategies for site-specific mRNA delivery. Bioconjugate Chem. 2024;35:453–456. doi: 10.1021/acs.bioconjchem.4c00038. [DOI] [PubMed] [Google Scholar]
- 103.Xue L., Hamilton A.G., Zhao G., Xiao Z., El-Mayta R., Han X., Gong N., Xiong X., Xu J., Figueroa-Espada C.G., et al. High-throughput barcoding of nanoparticles identifies cationic, degradable lipid-like materials for mRNA delivery to the lungs in female preclinical models. Nat. Commun. 2024;15:1884. doi: 10.1038/s41467-024-45422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Parhiz H., Shuvaev V.V., Pardi N., Khoshnejad M., Kiseleva R.Y., Brenner J.S., Uhler T., Tuyishime S., Mui B.L., Tam Y.K., et al. PECAM-1 directed re-targeting of exogenous mRNA providing two orders of magnitude enhancement of vascular delivery and expression in lungs independent of apolipoprote in mediated uptake. J. Control. Release. 2018;291:106–115. doi: 10.1016/j.jconrel.2018.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li Q., Chan C., Peterson N., Hanna R.N., Alfaro A., Allen K.L., Wu H., Dall’Acqua W.F., Borrok M.J., Santos J.L. Engineering Caveolae-Targeted Lipid Nanoparticles To Deliver mRNA to the Lungs. ACS Chem. Biol. 2020;15:830–836. doi: 10.1021/acschembio.0c00003. [DOI] [PubMed] [Google Scholar]
- 106.Pan L., Zhang L., Deng W., Lou J., Gao X., Lou X., Liu Y., Yao X., Sheng Y., Yan Y., et al. Spleen-selective co-delivery of mRNA and TLR4 agonist-loaded LNPs for synergistic immunostimulation and Th1 immune responses. J. Control. Release. 2023;357:133–148. doi: 10.1016/j.jconrel.2023.03.041. [DOI] [PubMed] [Google Scholar]
- 107.Wang C., Zhao C., Wang W., Liu X., Deng H. Biomimetic noncationic lipid nanoparticles for mRNA delivery. Proc. Natl. Acad. Sci. USA. 2023;120:e2311276120. doi: 10.1073/pnas.2311276120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Drayton D.L., Liao S., Mounzer R.H., Ruddle N.H. Lymphoid organ development: From ontogeny to neogenesis. Nat. Immunol. 2006;7:344–353. doi: 10.1038/ni1330. [DOI] [PubMed] [Google Scholar]
- 109.Kon E., Elia U., Peer D. Principles for designing an optimal mRNA lipid nanoparticle vaccine. Curr. Opin. Biotechnol. 2022;73:329–336. doi: 10.1016/j.copbio.2021.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nguyen D.N., Mahon K.P., Chikh G., Kim P., Chung H., Vicari A.P., Love K.T., Goldberg M., Chen S., Krieg A.M., et al. Lipid-derived nanoparticles for immunostimulatory RNA adjuvant delivery. Proc. Natl. Acad. Sci. USA. 2012;109:E797–E803. doi: 10.1073/pnas.1121423109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Graeff S. The function of the lymphocytes and the lymphatic system. Monatsschrift Kinderheilkd. 1960;108:301–306. [PubMed] [Google Scholar]
- 112.Thomas S.N., Rohner N.A., Edwards E.E. Implications of lymphatic transport to lymph nodes in immunity and immunotherapy. Annu. Rev. Biomed. Eng. 2016;18:207–233. doi: 10.1146/annurev-bioeng-101515-014413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ramishetti S., Kedmi R., Goldsmith M., Leonard F., Sprague A.G., Godin B., Gozin M., Cullis P.R., Dykxhoorn D.M., Peer D. Systemic gene silencing in primary T lymphocytes using targeted lipid nanoparticles. ACS Nano. 2015;9:6706–6716. doi: 10.1021/acsnano.5b02796. [DOI] [PubMed] [Google Scholar]
- 114.Tylawsky D.E., Kiguchi H., Vaynshteyn J., Gerwin J., Shah J., Islam T., Boyer J.A., Boue D.R., Snuderl M., Greenblatt M.B., et al. P-selectin-targeted nanocarriers induce active crossing of the blood-brain barrier via caveolin-1-dependent transcytosis. Nat. Mater. 2023;22:391–399. doi: 10.1038/s41563-023-01481-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Swingle K.L., Safford H.C., Geisler H.C., Hamilton A.G., Thatte A.S., Billingsley M.M., Joseph R.A., Mrksich K., Padilla M.S., Ghalsasi A.A., et al. Ionizable lipid nanoparticles for in vivo mRNA delivery to the placenta during pregnancy. J. Am. Chem. Soc. 2023;145:4691–4706. doi: 10.1021/jacs.2c12893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bray F.F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A.J. Erratum: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2020;70:313. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 117.Alasvand Javadi A., Jafarzadeh M., Yazdizadeh M., Askari Hasanvand N., Nikoo Nejad S., Amiri A. Comparison of dentinal defects formation in straight, moderate and severely curved canals by three distinctive nickel titanium instruments: An in vitro study. J. Dent. 2023;24:312–319. doi: 10.30476/dentjods.2022.94475.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McBride A., MacDonald K., Fuentes-Alburo A., Abraham I. Cost-efficiency and expanded access modeling of conversion to biosimilar trastuzumab-dkst with or without pertuzumab in metastatic breast cancer. J. Med. Econ. 2021;24:743–756. doi: 10.1080/13696998.2021.1928515. [DOI] [PubMed] [Google Scholar]
- 119.Chen D.S., Mellman I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 120.Kim J.M., Chen D.S. Immune escape to PD-L1/PD-1 blockade: Seven steps to success (or failure) Ann. Oncol. 2016;27:1492–1504. doi: 10.1093/annonc/mdw217. [DOI] [PubMed] [Google Scholar]
- 121.Wu L., Wang W., Tian J., Qi C., Cai Z., Yan W., Xuan S., Shang A. Engineered mRNA-expressed bispecific antibody prevent intestinal cancer via lipid nanoparticle delivery. Bioengineered. 2021;12:12383–12393. doi: 10.1080/21655979.2021.2003666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wu L., Wang W., Tian J., Qi C., Cai Z., Yan W., Xuan S., Shang A. Intravenous delivery of RNA encoding Anti-PD-1 human monoclonal antibody for treating intestinal cancer. J. Cancer. 2022;13:579–588. doi: 10.7150/jca.63991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mellman I., Chen D.S., Powles T., Turley S.J. The cancer-immunity cycle: Indication, genotype, and immunotype. Immunity. 2023;56:2188–2205. doi: 10.1016/j.immuni.2023.09.011. [DOI] [PubMed] [Google Scholar]
- 124.Zhang C., Maruggi G., Shan H., Li J. Advances in mRNA vaccines for infectious diseases. Front. Immunol. 2019;10:594. doi: 10.3389/fimmu.2019.00594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bevers S., Kooijmans S.A.A., Van de Velde E., Evers M.J.W., Seghers S., Gitz-Francois J.J.J.M., van Kronenburg N.C.H., Fens M.H.A.M., Mastrobattista E., Hassler L., et al. mRNA-LNP vaccines tuned for systemic immunization induce strong antitumor immunity by engaging splenic immune cells. Mol. Ther. 2022;30:3078–3094. doi: 10.1016/j.ymthe.2022.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen J., Lu X., Sun P. Preparation Method and Application thereof of a DNA Electrochemical Sensor. CN116953055-A. 2023 October 27;
- 127.Kamath D., Iwakuma T., Bossmann S.H. Therapeutic potential of combating cancer by restoring wild-type p53 through mRNA nanodelivery. Nanomed.-Nanotechnol. Biol. Med. 2024;56:102732. doi: 10.1016/j.nano.2024.102732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Weber J.S., Carlino M.S., Khattak A., Meniawy T., Ansstas G., Taylor M.H., Kim K.B., McKean M., Long G.V., Sullivan R.J., et al. Individualised neoantigen therapy mRNA-4157 (V940) plus pembrolizumab versus pembrolizumab monotherapy in resected melanoma (KEYNOTE-942): A randomised, phase 2b study. Lancet. 2024;403:632–644. doi: 10.1016/S0140-6736(23)02268-7. [DOI] [PubMed] [Google Scholar]
- 129.Zhang H., Meng C., Yi X., Han J., Wang J., Liu F., Ling Q., Li H., Gu Z. Fluorinated lipid nanoparticles for enhancing mRNA delivery efficiency. ACS Nano. 2024;18:7825–7836. doi: 10.1021/acsnano.3c04507. [DOI] [PubMed] [Google Scholar]
- 130.Shin H., Kang S., Won C., Min D.-H. Enhanced local delivery of engineered IL-2 mRNA by porous silica nanoparticles to promote effective antitumor immunity. ACS Nano. 2023;17:17554–17567. doi: 10.1021/acsnano.3c06733. [DOI] [PubMed] [Google Scholar]
- 131.Hou X., Zaks T., Langer R., Dong Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021;6:1078–1094. doi: 10.1038/s41578-021-00358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cui L., Pereira S., Sonzini S., van Pelt S., Romanelli S.M., Liang L., Ulkoski D., Krishnamurthy V.R., Brannigan E., Brankin C., et al. Development of a high-throughput platform for screening lipid nanoparticles for mRNA delivery. Nanoscale. 2022;14:1480–1491. doi: 10.1039/D1NR06858J. [DOI] [PubMed] [Google Scholar]
- 133.Saunders K.O., Pardi N., Parks R., Santra S., Mu Z., Sutherland L., Scearce R., Barr M., Eaton A., Hernandez G., et al. Lipid nanoparticle encapsulated nucleoside-modified mRNA vaccines elicit polyfunctional HIV-1 antibodies comparable to proteins in nonhuman primates (Dec, 10.1038/s41541-02100307-6, 2020) npj Vaccines. 2021;6:50. doi: 10.1038/s41541-021-00307-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pardi N., Secreto A.J., Shan X., Debonera F., Glover J., Yi Y., Muramatsu H., Ni H., Mui B.L., Tam Y.K., et al. Administration of nucleoside-modified mRNA encoding broadly neutralizing antibody protects humanized mice from HIV-1 challenge. Nat. Commun. 2017;8:14630. doi: 10.1038/ncomms14630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pine M., Arora G., Hart T.M., Bettini E., Gaudette B.T., Muramatsu H., Tombacz I., Kambayashi T., Tam Y.K., Brisson D., et al. Development of an mRNA-lipid nanoparticle vaccine against Lyme disease. Mol. Ther. 2023;31:2702–2714. doi: 10.1016/j.ymthe.2023.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lutz J., Lazzaro S., Habbeddine M., Schmidt K.E., Baumhof P., Mui B.L., Tam Y.K., Madden T.D., Hope M.J., Heidenreich R., et al. Unmodified mRNA in LNPs constitutes a competitive technology for prophylactic vaccines. npj Vaccines. 2017;2:29. doi: 10.1038/s41541-017-0032-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Meyer M., Huang E., Yuzhakov O., Ramanathan P., Ciaramella G., Bukreyev A. Modified mRNA-based vaccines elicit robust immune responses and protect guinea pigs from ebola virus disease. J. Infect. Dis. 2018;217:451–455. doi: 10.1093/infdis/jix592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.John S., Yuzhakov O., Woods A., Deterling J., Hassett K., Shaw C.A., Ciaramella G. Multi-antigenic human cytomegalovirus mRNA vaccines that elicit potent humoral and cell-mediated immunity. Vaccine. 2018;36:1689–1699. doi: 10.1016/j.vaccine.2018.01.029. [DOI] [PubMed] [Google Scholar]
- 139.Espeseth A.S., Cejas P.J., Citron M.P., Wang D., DiStefano D.J., Callahan C., Donnell G.O., Galli J.D., Swoyer R., Touch S., et al. Modified mRNA/lipid nanoparticle-based vaccines expressing respiratory syncytial virus F protein variants are immunogenic and protective in rodent models of RSV infection. npj Vaccines. 2020;5:16. doi: 10.1038/s41541-020-0163-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pardi N., Carreno J.M., O’Dell G., Tan J., Bajusz C., Muramatsu H., Rijnink W., Strohmeier S., Loganathan M., Bielak D., et al. Development of a pentavalent broadly protective nucleoside-modified mRNA vaccine against influenza B viruses. Nat. Commun. 2022;13:4677. doi: 10.1038/s41467-022-32149-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Medina-Maguees L.G., Gergen J., Jasny E., Petsch B., Lopera-Madrid J., Medina-Maguees E.S., Salas-Quinchucua C., Osorio J.E. mRNA vaccine protects against zika virus. Vaccines. 2021;9:1464. doi: 10.3390/vaccines9121464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang M., Sun J., Li M., Jin X. Modified mRNA-LNP vaccines confer protection against experimental DENV-2 infection in mice. Mol. Ther.-Methods Clin. Dev. 2020;18:702–712. doi: 10.1016/j.omtm.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.VanBlargan L.A., Himansu S., Foreman B.M., Ebel G.D., Pierson T.C., Diamond M.S. An mRNA vaccine protects mice against multiple tick-transmitted flavivirus infections. Cell Rep. 2018;25:3382–3392. doi: 10.1016/j.celrep.2018.11.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Maringer Y., Nelde A., Schroeder S.M., Schuhmacher J., Hörber S., Peter A., Karbach J., Jäger E., Walz J.S. Durable spike-specific T-cell responses after different COVID-19 vaccination regimens are not further enhanced by booster vaccination. Sci. Immunol. 2022;7:eadd3899. doi: 10.1126/sciimmunol.add3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang Z., Mateus J., Coelho C.H., Dan J.M., Moderbacher C.R., Gálvez R.I., Cortes F.H., Grifoni A., Tarke A., Chang J., et al. Humoral and cellular immune memory to four COVID-19 vaccines. Cell. 2022;185:2434–2451.e17. doi: 10.1016/j.cell.2022.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pilkington E.H., Suys E.J.A., Trevaskis N.L., Wheatley A.K., Zukancic D., Algarni A., Al-Wassiti H., Davis T.P., Pouton C.W., Kent S.J., et al. From influenza to COVID-19: Lipid nanoparticle mRNA vaccines at the frontiers of infectious diseases. Acta Biomater. 2021;131:16–40. doi: 10.1016/j.actbio.2021.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.A Feldman R., Fuhr R., Smolenov I., Ribeiro A., Panther L., Watson M., Senn J.J., Smith M., Almarsson O., Pujar H.S., et al. mRNA vaccines against H10N8 and H7N9 influenza viruses of pandemic potential are immunogenic and well tolerated in healthy adults in phase 1 randomized clinical trials. Vaccine. 2019;37:3326–3334. doi: 10.1016/j.vaccine.2019.04.074. [DOI] [PubMed] [Google Scholar]
- 148.Sago C.D., Lokugamage M.P., Loughrey D., Lindsay K.E., Hincapie R., Krupczak B.R., Kalathoor S., Sato M., Echeverri E.S., Fitzgerald J.P., et al. Augmented lipid-nanoparticle-mediated in vivo genome editing in the lungs and spleen by disrupting Cas9 activity in the liver. Nat. Biomed. Eng. 2022;6:157–167. doi: 10.1038/s41551-022-00847-9. [DOI] [PubMed] [Google Scholar]
- 149.Kubiatowicz L.J., Mohapatra A., Krishnan N., Fang R.H., Zhang L. mRNA nanomedicine: Design and recent applications. Exploration. 2022;2:20210217. doi: 10.1002/EXP.20210217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Teng Z., Meng L.-Y., Yang J.-K., He Z., Chen X.-G., Liu Y. Bridging nanoplatform and vaccine delivery, a landscape of strategy to enhance nasal immunity. J. Control. Release. 2022;351:456–475. doi: 10.1016/j.jconrel.2022.09.044. [DOI] [PubMed] [Google Scholar]
- 151.Nicolai L., Leunig A., Pekayvaz K., Esefeld M., Anjum A., Rath J., Riedlinger E., Ehreiser V., Mader M., Eivers L., et al. Thrombocytopenia and splenic platelet-directed immune responses after IV ChAdOx1 nCov-19 administration. Blood. 2022;140:478–490. doi: 10.1182/blood.2021014712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Li X., Qi J., Wang J., Hu W., Zhou W., Wang Y., Li T. Nanoparticle technology for mRNA: Delivery strategy, clinical application and developmental landscape. Theranostics. 2024;14:738–760. doi: 10.7150/thno.84291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Blaeschke F., Chen Y.Y., Apathy R., Daniel B., Chen A.Y., Chen P.A., Sandor K., Zhang W., Li Z., Mowery C.T., et al. Modular pooled discovery of synthetic knockin sequences to program durable cell therapies. Cell. 2023;186:4216–4234. doi: 10.1016/j.cell.2023.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Maag D., Lebwohl D., Butler J., Maitland M., Phillips J., Xu Y., Abdelhady Abozeid A. Methods for In Vivo Editting of KLKB1. WO2024011206-A1. 2024 January 11;
- 155.Chen C.-Y., Tran D.M., Cavedon A., Cai X., Rajendran R., Lyle M.J., Martini P.G.V., Miao C.H. Treatment of hemophilia a using factor VIII messenger RNA lipid nanoparticles. Mol. Ther.-Nucleic Acids. 2020;20:534–544. doi: 10.1016/j.omtn.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.An D., Schneller J.L., Frassetto A., Liang S., Zhu X., Park J.-S., Theisen M., Hong S.-J., Zhou J., Rajendran R., et al. Systemic messenger RNA therapy as a treatment for methylmalonic acidemia. Cell Rep. 2017;21:3548–3558. doi: 10.1016/j.celrep.2018.08.049. Correction in Cell Rep. 2018, 24, 2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rowe S.M., Zuckerman J.B., Dorgan D., Lascano J., McCoy K., Jain M., Schechter M.S., Lommatzsch S., Indihar V., Lechtzin N., et al. Inhaled mRNA therapy for treatment of cystic fibrosis: Interim results of a randomized, double-blind, placebo-controlled phase 1/2 clinical study. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2023;22:656–664. doi: 10.1016/j.jcf.2023.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Attarwala H., Lumley M., Liang M., Ivaturi V., Senn J. Translational pharmacokinetic/pharmacodynamic model for mRNA-3927, an investigational therapeutic for the treatment of propionic acidemia. Nucleic Acid Ther. 2023;33:141–147. doi: 10.1089/nat.2022.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhao W., Hou X., Vick O.G., Dong Y. RNA delivery biomaterials for the treatment of genetic and rare diseases. Biomaterials. 2019;217:119291. doi: 10.1016/j.biomaterials.2019.119291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Prieve M.G., Harvie P., Monahan S.D., Roy D., Li A.G., Blevins T.L., Paschal A.E., Waldheim M., Bell E.C., Galperin A., et al. Targeted mRNA therapy for ornithine transcarbamylase deficiency. Mol. Ther. 2018;26:801–813. doi: 10.1016/j.ymthe.2017.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Daly O., Mahiny A.J., Majeski S., McClintock K., Reichert J., Boros G., Szabo G.T., Reinholz J., Schreiner P., Reid S., et al. ASL mRNA-LNP therapeutic for the treatment of argininosuccinic aciduria enables survival benefit in a mouse model. Biomedicines. 2023;11:1735. doi: 10.3390/biomedicines11061735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Jalil S., Keskinen T., Juutila J., Sartori Maldonado R., Euro L., Suomalainen A., Lapatto R., Kuuluvainen E., Hietakangas V., Otonkoski T., et al. Genetic and functional correction of argininosuccinate lyase deficiency using CRISPR adenine base editors. Am. J. Hum. Genet. 2024;111:714–728. doi: 10.1016/j.ajhg.2024.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Koeberl D., Schulze A., Sondheimer N., Lipshutz G.S., Geberhiwot T., Li L., Saini R., Luo J., Sikirica V., Jin L., et al. Interim analyses of a first-in-human phase 1/2 mRNA trial for propionic acidaemia. Nature. 2024;628:872–877. doi: 10.1038/s41586-024-07266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Herrera-Barrera M., Ryals R.C., Gautam M., Jozic A., Landry M., Korzun T., Gupta M., Acosta C., Stoddard J., Reynaga R., et al. Peptide-guided lipid nanoparticles deliver mRNA to the neural retina of rodents and nonhuman primates. Sci. Adv. 2023;9:eadd4623. doi: 10.1126/sciadv.add4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article.