Abstract
Fruit rots caused by filamentous fungi such as Monilinia fructicola and Monilinia laxa have a strong impact on crop yield and fruit commercialization, especially as they affect a wide variety of stone fruits. The antifungal efficacy of benzylidene-cycloalkanones has been previously described in in vitro assays against M. fructicola; so, this study aims to evaluate the in vivo inhibitory potential of these hybrids on fruits that have been inoculated with M. fructicola, and use molecular docking to visualize the main interactions of these molecules in the active site of the enzyme succinate dehydrogenase (SDH). The results indicate that compound C achieves the highest inhibition of both Monilinia species (15.7–31.4 µg/mL), spore germination in vitro (<10 µg/mL), and has promising results in vivo, without causing phytotoxicity in fruits. The results from molecular docking suggest that hydroxyl groups play a crucial role in enhancing the binding of compound C to SDH and contribute to the formation of hydrogen bonds with amino acid residues on the enzyme active site.
Keywords: dihydrocarvone, Monilinia spp., postharvest fruits, nectarines
1. Introduction
Fungi diseases that affect fruit cause devastating damage to commercial crop plants pre- and post-harvest, resulting in large economic losses. Among those causing these are the filamentous fungi Monilinia laxa (Aderhold and Ruhland) Honey and Monilinia fructicola (G. Winter) Honey [1]. Its main hosts are stone fruits such as cherries, peaches, and nectarines, among others, in which it causes brown rot. The onset of the disease is characterized by the appearance of a light brown, soft and watery spot that develops rapidly after harvesting, especially if the fruit is wounded [2], reaching losses of up to 10% of post-harvest productivity worldwide [3]. The genus Monilinia belongs to the family Sclerotiniaceae, of the phylum Ascomycota, and its distinctive feature is that the fruiting body originates from pseudosclerotia formed in mummified fruits in soil or their remains, from which spores are produced [4]. Disease, mycelium development and sporulation find their ideal conditions at a relative humidity of more than 80% and at a temperature of approximately 25 °C. M. laxa is most prevalent worldwide in orchards [5]; however, M. fructicola is more aggressive and has been considered a quarantine pest in several stone-fruit-producing countries [6] and is less sensitive to fungicides used for its control [4], especially to Methyl Benzimidazole Carbamates (MBCs); its resistance mechanism is associated with mutations in the binding site of the β2-tubulin proteins, which are part of the mechanism of action of these antimicrobials [7,8].
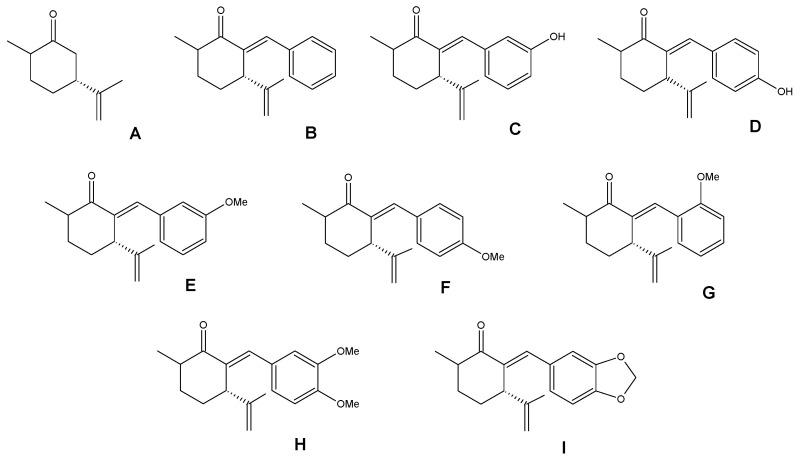
Considering the challenges of controlling Monilinia spp. and its catastrophic effect on fruit productivity, strategies have been sought, such as avoiding post-harvest conditions that favor the disease, the use of biological control agents [9] and the use of natural bioactive compounds [10]. Regarding the latter group, the metabolites used are those produced by plants in interaction with the environment and associated with their own defense mechanisms so that they can have antifungal activity [3]. Among these are molecules with confirmed antifungal capacity, such as terpenic ketones, one of which is dihydrocarvone, a major compound in the essential oil of Poiretia latifolia flowers [11]. Díaz et al. 2021, in a previous publication, demonstrated the antifungal activity of dihydrocarvone hybrids, namely, benzylidene-cycloalkanones (shown in Figure 1), which had remarkable efficacy and low toxicity in Artemia salina models, which is significant considering that their application will be on edible fruits [12]. Therefore, we will extend this study to determine the effect of the dihydrocarvone (A) and its nine benzylidene-cycloalkanones (B–I) on conidial germination and inhibitory effect on nectarine fruits inoculated with M. fructicola; additionally, we will visualize the main interactions with the active site of the enzyme succinate dehydrogenase (SDH) by molecular docking studies.
Figure 1.
Dihydrocarvone (A) and its previously reported benzylidene-cycloalkanones (B–I).
2. Materials and Methods
2.1. General Chemistry
Dihydrocarvone (A), reagents and chemicals were obtained commercially from Sigma-Aldrich Co. (St. Louis, MO, USA) and AK Scientific Inc. (30023 Ahern Ave, Union City, CA, USA) and were used unpurified. The benzylidene-cycloalkanones (B–I) were available in the laboratory due to previous research and their NMR data can be found in the Supplementary Materials.
2.2. Assessment of Antifungal Capacity
2.2.1. Fungal Isolate and Growing Conditions
The isolates of Monilinia fructicola and M. laxa used in these experiments were provided by the collection of the Mycology Unit, Servicio Agrícola y Ganadero (SAG), Chile. Cultures of the isolate were grown and incubated on potato dextrose agar (PDA) medium in 90 mm diameter Petri dishes under aseptic conditions at 23 °C for 7 days, and subsequently stored at 4 °C. The spore suspension applied in the in vitro studies was obtained from a 7–10-day growth culture in Petri dishes, which were filtered through a sterile muslin cloth [13]. Spore concentration was adjusted through serial dilution in sterile distilled water.
2.2.2. Effect of the Benzylidene-Cycloalkanones Compounds on the Mycelial Growth on Monilinia laxa
Determination of mycelial growth inhibition of the samples was performed by measuring the radius of growth. The PDA plates contained the compounds (dihydrocarvone A and benzylidene-cycloalkanones B–I) at concentrations of 10, 25, 50, 150 and 250 µg/mL previously dissolved in ethanol and water. The percentage of inhibition was determined according to standard methods [12]. PDA 1% ethanol medium was used as a negative control, while a commercial fungicide, Mystic® 520 SC (BAYER AG, Leverkusen, Germany), was used as a positive control; also, a commercial organic fungicide based on grapefruit extracts, BC-1000® Dust (CHEMIE Research & Manufacturing, Casselberry, FL, USA), was used. The results are expressed as the average effective concentration (EC50), that is, the concentration at which mycelial growth was reduced by 50%. This value was determined by regression of the values of the percentage inhibition of the radial growth versus the concentration values of the compound using Origin ProV. 8 software (OriginLab Corporation, Northampton, MA, USA). Treatments were performed in triplicate.
2.2.3. Effect of the Compounds on Conidial Germination of Monilinia fructicola
To evaluate the effect of the compounds (B–I) on conidial germination following the protocol previously described by Pereira et al., 2017, with modifications [14], M. fructicola 6-day-old colonies grown on PDA at 22 °C were used to prepare spore suspensions at a concentration of 1 × 105 conidia/mL. A 40 µL aliquot of the conidial suspension was spread on the media with different treatments containing compounds at the final concentrations of 10, 25, 50, 50, 150, and 250 µg/mL. Sterile distilled water was used as a negative control. The plates containing the dispersed conidia on media were stored at 22 °C for 9 to 12 h in the dark. Approximately 100 spores were randomly measured under a microscope to determine their percentage of germination (PIG); each treatment consisted of three replicates. Conidia were considered germinated if they produced germ tubes at least twice their width. The results are expressed as the average effective concentration (EC50), that is, the concentration at which inhibition conidial was reduced by 50%.
2.2.4. In Vivo Antifungal Effects of Hybrids Based on Dihydrocarvone (B, C and G) on Inoculated Nectarines Fruits
The assay was performed following the methodology described by Brito et al., 2021, with slight modifications [13] on commercially obtained nectarines (Prunus persica var. nucipersica). The selected fruits were healthy. Their surface was sterilized by a 10 min immersion in a 15% sodium hypochlorite solution, and then washed and dried with sterile absorbent paper to be inoculated with M. fructicola. Artificial inoculation was carried out at room temperature by puncturing the fruit with a sterile needle at four separate points, and 100 µL of a suspension containing 1 × 106 CFU/mL of fungal spores was applied to each puncture. One day before inoculation, the fruits were sprayed with the selected treatments, B, C and G, at a concentration of 250 µg/mL; the selected compounds were defined according to their in vitro inhibition concentrations of low, medium and high efficacy. As a negative control, wounded fruits sprayed with sterile distilled water were used, as well as non-inoculated fruits that were sprayed to observe if there was phytotoxicity of the treatments. Each experiment consisted of three replicates and each replica had four nectarines. Organic fungicide BC1000® Dust was used as a positive control at the same compound concentrations.
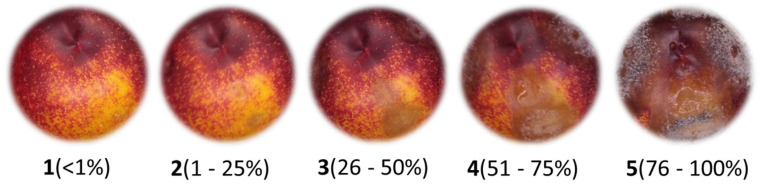
They were kept at room temperature (25 °C), under light, in a humid chamber with a relative humidity of about 95% for 5 days in dark. After 4 days of incubation, the percent disease incidence (PIC) was calculated as PIC = (number of infected lesions)/(number of lesions evaluated) × 100. The disease severity index (DSEI) was calculated using the Townsend–Heuberger equation [15]. This equation uses a visual symptom scale described in Figure 2 [16].
Figure 2.
Visual scale (1–5) of symptoms caused by M. fructicola to quantify the incidence and severity of the disease on nectarines at 5 days post-inoculation: 1 = no sign of disease (not infected); 2 = 1–25% lesion visible but no sporulation (mild infection); 3 = 26–50% sporulating area on lesion smaller than a quarter of the fruit (moderate infection); 4 = 51–75% sporulating area larger than a quarter of the fruit, but less than half of the fruit; (strong infection); 5 = 76–100% sporulating area larger than half of the fruit (severe infection).
The presence of the pathogen was verified by re-isolating it from the edge of the lesions; a slice (1.5 cm) was cut and the tissue was placed in Petri dishes with PDA (applying Koch’s postulates), which were inspected and morphologically identified using a Leica EZ4HD stereo microscope with camera software and DM500 microscope (Leica Microsystems, Wetzlar, Germany).
2.3. Statistical Analysis
Statistical analysis was performed with the program InfoStat v.2020, (Universidad de Cordoba, Córdoba, Argentina) and using the multi-comparison function with a least significance difference test (LSD) with a p ≤ 0.05 of significance.
2.4. Molecular Docking and Prediction of the Physicochemical Properties
The construction of three-dimensional models of the ligands was performed using Avogadro 1.2.0n software. The geometry of the ligands was optimized, and their energy was minimized using the MMFF94 force field. Images were created using the Discovery-Studio visualize free version. The crystallized structure of succinate dehydrogenase (SDH PDB ID: 2FBW, 2.06 Å resolution) [17] was obtained from the Protein Data Bank (http://www.resb.org/pdb). Molecular docking of SDH and the selected ligands was carried out with the AutoDock4 package using the Lamarckian genetic algorithm [18] and assuming rigid ligands in the macromolecule and full flexibility for the inhibitors. The search parameters were 50 runs and a maximum number of 25,000,000 evaluations for each ligand. The RMSD threshold for multiple clustering was set at <0.5 Å. The ligand crystallized in the enzyme 2-methyl-n-phenyl-5,6-dihydro-1,4-oxathiine-3-carboxamide (CBE) was used as a reference point for docking. The result was analyzed by ranked cluster and binding energy ΔG, where the lowest energy and most populated cluster was selected as the best protein–ligand complex for further analysis. To check the docking accuracy, the co-crystallized ligand was docked again under the same conditions and an RMSD of 2.49 Å was obtained. All experiments were performed at physiological pH. Prediction of the physicochemical properties of the compounds was compiled using Swiss ADME software (http://www.swissadme.ch/index.php).
3. Results and Discussion
3.1. Antifungal Activity of Dihydrocarvone and Its Hybrid Derivatives
The antifungal activity of the compounds (Table 1) determined from the mycelial growth inhibition of Monilinia spp. showed that M. laxa was the most sensitive pathogen to the treatments, achieving mean growth inhibition at concentrations within the range of 8.38 to 55.97 µg/mL, with the exception of sample A, corresponding to the starting compound which was inactive for both species, suggesting that the modifications made to obtain the hybrids improved their antifungal activity. Structure–activity relationship studies of synthetic and natural product derivates relate the presence of hydroxyl groups to improved solubility and pharmacokinetic properties, including beneficial effects on the formation of indirect hydrogen bonds through water molecules to the active site [19]. Among the molecules synthesized, C stands out for the presence of a hydroxyl group and its high antifungal capacity in M. fructicola compared to the rest of the treatments.
Table 1.
Lipophilicity and EC50 values of dihydrocarvone (A) and its derivatives (B–I) calculated for inhibition of mycelial growth of Monilinia spp. in vitro and inhibition conidial germination of M. fructicola.
| Compound | EC50 (µg/mL) ± SD a | Lipophilicity Log P | ||
|---|---|---|---|---|
| M. fructicola d | M. laxa | Conidia M. fructicola e |
||
| A | >250 | >250 | >250 | 2.51 |
| B | >250 | 8.38 ± 0.68 | <10 | 4.02 |
| C | 15.7 ± 1.33 | 31.46 ± 2.82 | <10 | 3.61 |
| D | >250 | 27.30 ± 0.04 | 10 | 3.60 |
| E | 39.64 ± 1.13 | 42.49 ± 1.04 | <10 | 4.01 |
| F | 23.17 ± 1.22 | 10.88 ± 2.82 | <10 | 4.01 |
| G | 58.03 ± 0.74 | 55.97 ± 0.98 | <10 | 3.98 |
| H | 47.79 ± 0.88 | 7.57 ± 0.48 | >250 | 3.96 |
| I | 18.13 ± 0.0 | 5.06 ± 1.05 | <10 | 3.84 |
| Control A b | 9.19 ± 0.0 | 10.56 ± 0.0 | <10 | - |
| Control B c | 10.55 ± 1.74 | 13.64 ± 1.38 | 110 ± 1.09 | - |
a EC50: concentration causing 50% mycelial growth inhibition + SD, standard deviation; values are expressed as the mean of three experiments; b Control A: Mystic® 520 SC; c Control B: BC-1000 ® Dust; d data from Díaz et al., 2021 [12]; e conidial germination.
It is worth mentioning that it is evident that samples B and D are inactive in M. fructicola; however, they have an EC50 of 8.38 and 27.30 µg/mL in M. laxa, respectively, and this difference in sensitivity has also been described by other authors [3]. Studies of other monoterpenoids of natural origin also attribute activity to them in M. fructicola [20] and have shown that monoterpenes such as thymol are effective against it, where the compound crystallizes on the surface of the conidia, hyphae and cell wall of the fungus, being absent in the cytoplasm [21]. Likewise, the antifungal capacity of monoterpenes was also attributed to the presence of a phenolic hydroxyl group [22]. These discrepancies in pathogen–compound interaction could be attributed to culture specificity, presence or absence of specific receptors and resistance to death mechanisms.
Regarding the conidia of this species, which can be found in mummified fruits, where the infection can start, they are dispersed by water or wind, and subsequently infect the different tissues of the plant. These conidia are able to germinate at an optimum temperature between 15 and 30 °C; however, they can germinate even in a range of 0 to 35 °C [3]. Although brown rot is a disease that appears more frequently post-harvest at the points of contact between the fruits [23], infection of the fruit usually occurs while they are still on the tree, remaining latent until harvest; therefore, a treatment for this disease must not only control the development of Monilinia spp. but also be able to inhibit the conidia present on the surface of the fruit. Most of the treatments tested in this study were highly effective in inhibiting conidia germination (<10 µg/mL), with the exception of precursor A and hybrid H.
Table 1 also shows the lipophilicity of the compounds expressed as Log P, and these physicochemical parameters were obtained from the SwissADME platform. Compound A is the one that experimentally shows the lowest mycelial inhibition capacity of the two Monilinia species and is the compound with the lowest lipophilicity. The rest of the compounds have Log P values greater than 3, which indicates that they are more lipophilic compounds with moderate water solubility. For a molecule to exert biological action on a cell, it must meet certain physical and chemical requirements, including the ability to diffuse through the cell membrane. The speed at which diffusion occurs depends largely on the lipid solubility of the molecule. Since the cell membrane is lipid in nature, liposoluble molecules diffuse more quickly through it [24]. The balance between lipophilicity and hydrophilicity is of great importance in biological processes, since if the lipophilicity of the compounds is too high, the desired effects are not achieved either; for example, compound B shows a Log P value of 4.02, being the most lipophilic compound of those evaluated. Additional physicochemical properties of the compounds such as the number of rotatable bonds, H-bond acceptors, H-bond donors, molar refractivity and topological polar surface area can be found in Supplementary Materials.
To summarize, the derivatives of dihydrocarvone hybrids described by Díaz et al. [12] are active on Monilinia spp., especially on M. laxa, two compounds (B and I) proved to be more active than the positive controls in inhibiting mycelial growth and germination of conidia of this species. However, due to the relevance of M. fructicola for its devastating effect on commercial crops, difficult control and resistance, compound C was selected as the most effective candidate in the control of Monilinia spp. for the purposes of this study, as it was able to inhibit M. fructicola at a concentration of 15.7 µg/mL, M. laxa at a concentration of 31.46 µg/mL and to achieve an outstanding inhibition on conidia.
3.2. In Vivo Antifungal Effects of Hybrids Based on Dihydrocarvone (B, C and G) on Inoculated Nectarine Fruits
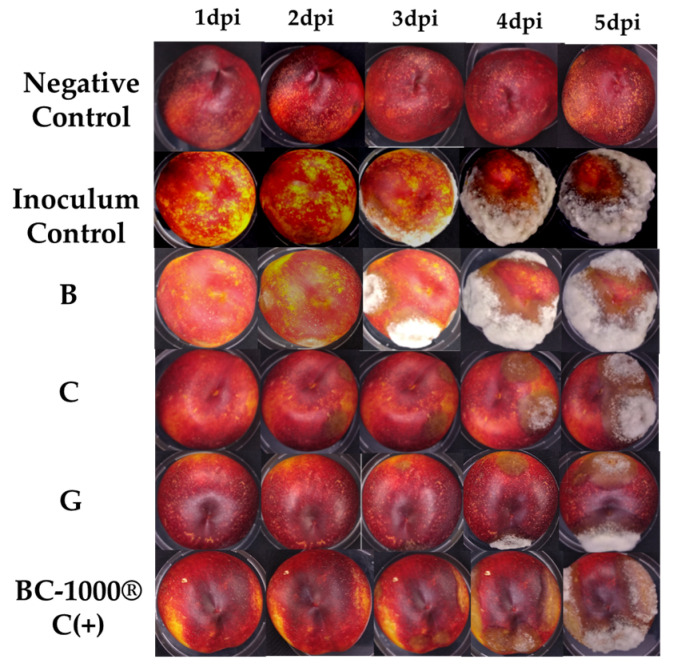
To determine the effects of the hybrids on fruit, commercially obtained nectarines were chosen to be inoculated with M. fructicola (Figure 3); before this, treatments were applied with three derivatives that were selected according to the results obtained in the in vitro tests. Compound C presented activity similar to the positive control, G presented intermediate activity, and B was proven to be a compound of low antifungal activity, thus determining a correlation with the in vivo results. Figure 3 shows the severity of damage in fruits inoculated with the pathogen treated with the different treatments, showing the percentage of disease incidence at 1–5 dpi (Table 2). This trial demonstrates the protective effect that the compounds had on disease development. As expected, according to the results obtained in the previous stage, fruits treated with compound B had a higher disease development. Likewise, C and G had an excellent inhibitory effect on disease incidence up to the fourth day after inoculation, showing the first symptoms on the fifth day with lower incidence in fruits treated with hybrid C. On the other hand, the positive control BC-1000® Dust showed symptoms on the fourth day with low incidence; however, on the fifth day, disease incidence increased to values close to 100%.
Figure 3.
Effects of compounds B, C and G at 250 µg/mL controlling the development of M. fructicola in artificially inoculated nectarines for five days post inoculation (dpi). Negative Control: without inoculum; Inoculum Control: with inoculum of M. fructicola; B: compound of low antifungal activity in vitro; C: compound with antifungal activity similar at positive control in vitro; G: compound of intermediate antifungal activity; BC-1000® Dust.
Table 2.
Effect of different treatments applied in vivo on nectarine fruits on the incidence of peach brown rot disease over five days post-inoculation (dpi).
| Disease Incidence Percentage of Peach Brown Rot Disease (%) ± SD [*] | |||||
|---|---|---|---|---|---|
| Treatments | 1 dpi | 2 dpi | 3 dpi | 4 dpi | 5 dpi |
| Negative Control | 0 ± 0.0 b | 0 ± 0.0 e | 0 ± 0.0 e | 0 ± 0.0 c | 0± 0.0 b |
| Inoculum Control | 6 ± 3.1 a | 31 ± 13 c | 100 ± 0.0 d | 100 ± 0.0 a | 100 ± 0.0 a |
| BC1000® Dust | 0 ± 0.0 b | 44 ± 10 d | 69 ± 13 b | 100 ± 0.0 a | 100 ± 0.0 a |
| B | 0 ± 0.0 b | 38 ± 14 c | 88 ± 14 c | 88 ± 14 b | 100 ± 0.0 a |
| C | 0 ± 0.0 b | 13 ± 3.0 a | 31 ± 13 a | 88 ± 10 b | 94 ± 13 a |
| G | 0 ± 0.0 b | 25 ± 0.0 b | 56 ± 10 b | 100 ± 0.0 a | 100 ± 0.0 a |
[*] Same letters in superscript do not present significant differences (p ≤ 0.05); B: compound of low antifungal activity in vitro; C: compound with antifungal activity similar at positive control in vitro; G: compound of intermediate antifungal activity.
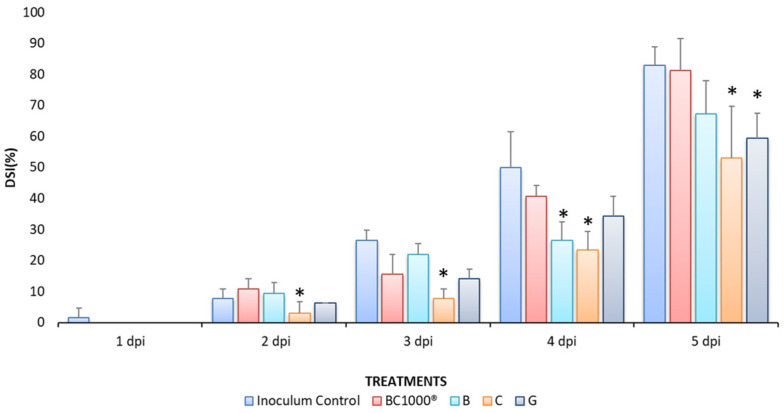
In general, the results obtained in the in vivo trials were consistent with the results obtained in vitro in terms of the structure–activity relationship. The effect on disease inhibition was enhanced when the molecule had a hydroxyl group at the C-3 position compared to when there was a methoxyl group at the C-2 position of the molecule, and decreased disease severity by 6–12%. In contrast, the disease did not decrease when the molecule did not have functional groups attached to the aromatic ring, which accelerated the infection during the bioassay, as in the case of compound B (Figure 4). The disease severity index (DSI) was assessed using a visual scale and calculated with the Townsend–Heuberger equation [15]. The results are presented in Figure 4 as the percentage of damaged fruit being the highest result for the control treatment that was inoculated with the pathogen, and the positive control, which, prior to inoculation, was sprayed with a commercial organic pesticide used in different vegetable crops (BC-1000®), unlike all other treatments that reduce the disease index, and could have maintained the control of the pathogen if it had been inoculated again with these compounds. However, the fruits only received one application. It is expected in the future to conduct new experiments that include a second one to reduce the disease index.
Figure 4.
Disease severity index (%DSI) on nectarine fruits measured for five days post-inoculation (dpi) with Monilinia fructicola. Mean with asterisk (*) denotes a statistically significant difference between BC-1000® Dust treatment and compounds according to analysis of variance (ANOVA, p < 0.05) and comparison of the groups using Fisher’s LSD. Data are expressed as means ± SD.
It has been previously described that Monilinia spp. are intercellularly lodged [3] and, upon infection of fruits, respond with the accumulation of proteins [25], and activation of the jasmonic acid and ethylene pathways, in addition to phenylpropanoid metabolism, among other responses. In the pathogen, the activation of genes related to transporters, phospholipases, oxidoreductases, and proteases occurs [26], in addition to the secretion of other enzymes characteristic of necrotrophic fungi such as Monilinia spp. The cascade of molecules exhibited by the pathogen and fruits in their interaction [27] is a factor to consider because it could interfere with the mechanism of action of the proposed hybrids, which is why it is necessary to test their activity in in vivo models. In addition, the use of in vivo models allows us to evaluate possible phytotoxicity reactions of the compound on the fruits; the compounds evaluated did not cause changes in the appearance of the fruits or phytotoxicity.
Different varieties of stone fruit can be affected by Monilinia spp., and among these are cherries, plums, peaches, etc.; however, nectarines were chosen for this study since their size allows for the visualization of the effects of the treatments on the disease. The results could be extrapolated to other fruits, considering that the peel of fruits in general is composed of biopolymer cellulose and lignin and of usual compounds of the plant cell wall [28]. Therefore, these dihydrocarvone hybrids could be used as potential organic fungicides in more than one stone fruit crop.
3.3. Molecular Docking of Benzylidene-Cycloalkanones B, G and C
Succinate dehydrogenase, also known as succinate coenzyme Q reductase or mitochondrial complex II, (SDH) is a protein bound to the inner mitochondrial membrane. This protein, present in aerobic cells, participates in the Krebs cycle as well as in the electron transport chain. This enzyme is made up of four protein subunits, subunits A and B of a hydrophilic nature, while subunits C and D of a hydrophobic nature are attached to the mitochondrial membrane [29]. Various investigations have shown that SDH is an ideal objective and one of the preferred targets for the development of new fungicides [30,31,32]. SDH-inhibiting pesticides are an important class of agricultural fungicides with the advantages of high efficiency and have also been shown to act as bactericides on a broad bacterial spectrum [33]. The literature shows that there is a wide structural diversity of potential SDH inhibitors. A previous work reported by Soto et al. [34] indicates that the antifungal activity on Botrytis cinerea caused by hydrated geranylated phenols could be through the inhibition of the SDH enzyme.
The molecular docking of the dihydrocarvone derivatives was performed on the crystal structure of SDH obtained from the PDB Database (PDB: 2FBW). 2-methyl-n-phenyl-5,6-dihydro-1,4-oxathiine-3-carboxamide was used as a reference ligand, which corresponds to the ligand crystallized in the enzyme. The compounds evaluated in vivo were chosen to measure the activity on SDH. The results are shown in Table 3, which indicates the binding energy (Kcal/mol) of compounds B, G and C, which were considered in order of increasing activity, with compound C showing the best activity of the set of molecules evaluated.
Table 3.
Binding energies obtained from molecular docking studies on SDH. CBE: 2-methyl-n-phenyl-5,6-dihydro-1,4-oxathiine-3-carboxamide, ligand crystalized in SDH (2FBW).
| Compound | Binding Energy (Kcal/mol) |
|---|---|
| B | −5.3 |
| C | −7.1 |
| G | −6.6 |
| CBE | −7.2 |
B: compound of low antifungal activity in vitro; C: compound with antifungal activity similar at positive control in vitro; G: compound of intermediate antifungal activity.
We can observe that the binding energy values were −5.3 for B, −6.6 for G and −7.1 for C. As can be seen in Figure 5, the binding site of all dihydrocarvone derivatives to SDH would be in the D subunit of this enzyme. It is worth noting that there is a high correlation between the results obtained theoretically and experimentally, with the R2 value for M. fructicola being 0.90 and for M. laxa being 0.94.
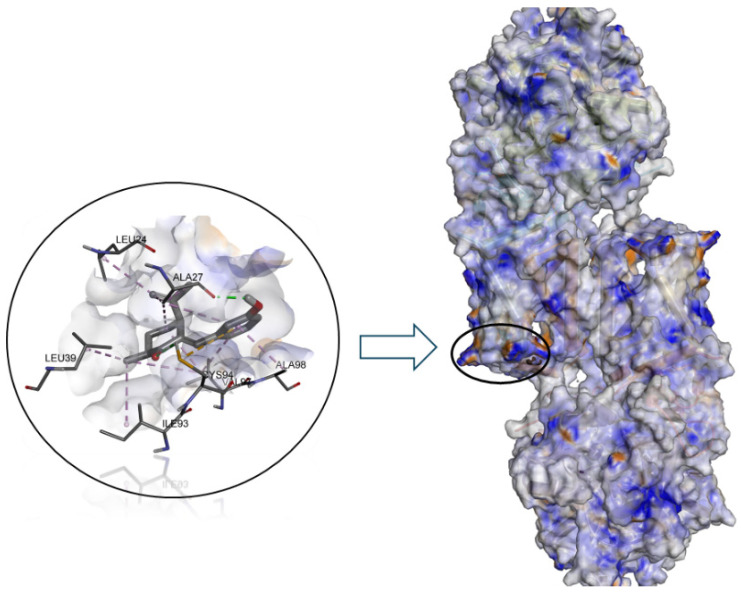
Figure 5.
Predicted binding mode of compound C into the allosteric site of enzyme SDH (PDB 2FBW).
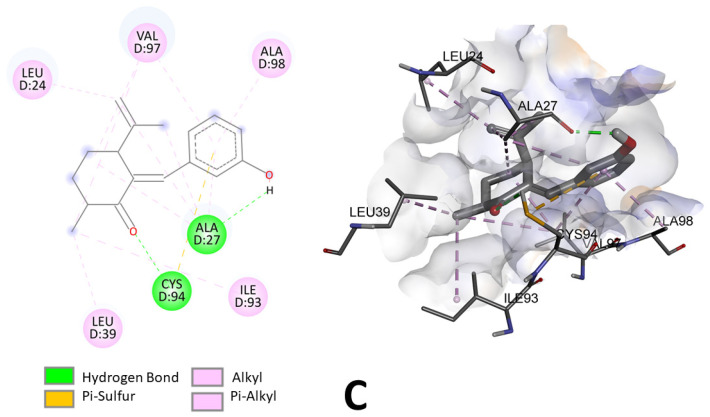
Compound C was the one that had the greatest effect on the inhibition of mycelial growth of Monilinia spp. This compound acts as a ligand binding to the amino acid residues leucine-24, valine-97, alanine-98, alanine-27, cysteine-94 and leucine-39. As can be seen in Figure 6, two hydrogen bonds would be formed between compound C and SDH, one at 2.83 Å distance between the carbonyl group of the compound and the cysteine-94 residue and a second hydrogen bond at 2.12 Å between the hydroxyl group and the alanine-27 residue. This same compound shows a π–sulfur-type bond between the phenolic ring (5.72 Å) and the cysteine-94 residue. Several alkyl and π–alkyl interactions can also be observed, which are considered to be lower-intensity interactions compared to hydrogen and sulfide bonds [35].
Figure 6.
Two-dimensional and three-dimensional predicted binding mode of compound C into the allosteric site of enzyme SDH (PDB 2FBW).
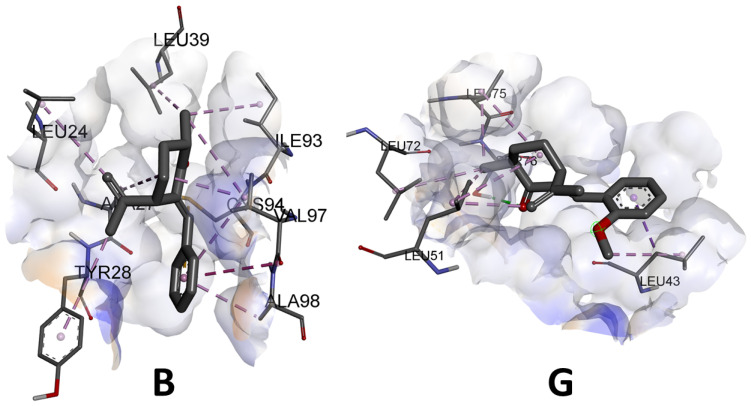
As previously mentioned, the activity of the compounds on SDH is related to the number of hydrogen bonds that can be generated between the ligands and the amino acid residues of the enzyme [19]. Most of the benzylidene-cycloalkanone derivatives form hydrogen bonds and it should be noted that the most active compound (C) forms two hydrogen bonds with the enzyme, whereas, as can be seen in Figure 7, the compound evaluated in vivo with the lowest activity (B) would not generate this type of interaction with the enzyme; rather, weaker alkyl interactions predominate. Compound G forms a single hydrogen bond. It should also be noted that the most active compound generated a sulfur bond with the enzyme, which would explain the greater stability of the complexes obtained.
Figure 7.
Three-dimensional predicted binding mode of compound B and G into the allosteric site of enzyme SDH (PDB 2FBW).
4. Conclusions
The benzylidene-cycloalkanones tested confirmed their antifungal potential against M. fructicola and M. laxa strains, demonstrating the effectiveness of pathogen growth inhibition in vitro and disease control in vivo. The structure of compound C is the most decisive in inhibiting the growth of M. fructicola, owing to the presence of a hydroxyl group on carbon 3 of the aromatic ring, which allows it to bind to the active site of the SDH enzyme.
A molecular docking study shows that binding energies of these compounds to the allosteric binding site of SDH are −7.1 kcal mol−1 similar to CBE. The results suggest that the experimental antifungal activity correlates with the number of H-bonds that can be formed in the binding site.
For future studies, the application of new synthesis methodologies could be explored to improve their performance and reduce reaction times due to their valuable activity. For this reason, it is also necessary to investigate the mechanism of action through which the hybrids cause the inhibition of the pathogen. Considering that the potential application of these compounds would be in the agricultural industry, technologies that could be used as a vehicle for their application could be evaluated with the use of nanoemulsions loaded with the active compounds, a technology that is valuable in this industry due to its advantages in cost and safety. On the other hand, it would also be interesting to explore if its application results in changes in the taste of fruits, or if it represents any risk of oral toxicity.
Acknowledgments
The authors thank the Dirección General de Investigación de la Universidad de Playa Ancha for their support in the hiring of the technicians Valentina Silva Pedreros for the “Apoyos tecnicos para laboratorios y grupos de investigacion UPLA 2024 (SOS Technician)” program, the project Fondecyt Post Doctoral No. 3230296 awarded by the National Research and Development Agency (ANID) Chile and Servicio Agrícola Ganadero Chile (SAG) for their contribution in facilitating the strains of Monilinia spp studied. The APC was funded by Universidad de Playa Ancha, Plan de Fortalecimiento Universidades Estatales-Ministerio de Educación, Convenio UPA 1999.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof10090609/s1. NMR data and additional physicochemical properties (number of rotatable bonds, H-bond acceptors; H-bond donors, molar refractivity and topological polar surface area) for dihydrocarvone derivatives are available in the Supplementary Materials.
Author Contributions
Conceptualization, A.M.; methodology, K.D., X.B. and E.M.; software, K.D. and E.M.; validation, A.M., I.M. and E.W.; formal analysis, A.M., V.S., K.D. and E.M.; investigation, V.S., C.R., K.D., X.B., I.M., E.M. and E.W.; resources, A.M.; data curation, K.D.; writing—original draft preparation, V.S.; writing—review and editing, A.M., V.S. and K.D.; visualization, A.M.; supervision, A.M.; project administration, A.M.; funding acquisition, A.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in this article/Supplementary Materials; further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was funded by Agencia Nacional de Desarrollo y investigación (ANID), Fondo Nacional de Desarrollo Científico y Tecnológico FONDECYT grant number 1230311 and 1230368.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Byrde R.J.W., Willetts H.J. The Brown Rot Fungi of Fruit: Their Biology and Control. Elsevier; Amsterdam, The Netherlands: 2013. pp. 15–31. [DOI] [Google Scholar]
- 2.Dini M., Raseira M.d.C.B., Corre M.-N., Signoret V., Quilot-Turion B. Influence of Fruit Wounding on Subsequent Monilinia laxa Infection of Nectarines. Agronomy. 2023;13:1235. doi: 10.3390/agronomy13051235. [DOI] [Google Scholar]
- 3.Martini C., Mari M. Monilinia fructicola, Monilinia laxa (Monilinia Rot, Brown Rot) In: Bautista-Baños S., editor. Postharvest Decay. Academic Press; Cambridge, MA, USA: 2014. [DOI] [Google Scholar]
- 4.Mustafa M.H., Bassi D., Corre M.-N., Lino L.O., Signoret V., Quilot-Turion B., Cirilli M. Phenotyping Brown Rot Susceptibility in Stone Fruit: A Literature Review with Emphasis on Peach. Horticulturae. 2021;7:115. doi: 10.3390/horticulturae7050115. [DOI] [Google Scholar]
- 5.Rungjindamai N., Jeffries P., Xu X.-M. Epidemiology and management of brown rot on stone fruit caused by Monilinia laxa. Eur. J. Plant Pathol. 2014;140:1–17. doi: 10.1007/s10658-014-0452-3. [DOI] [Google Scholar]
- 6.Oliveira L., Pacheco I., Mercier V., Faoro F., Bassi D., Bornard I., Quilot-Turion B. Brown Rot Strikes Prunus Fruit: An Ancient Fight Almost Always Lost. J. Agric. Food Chem. 2016;64:4029–4047. doi: 10.1021/acs.jafc.6b00104. [DOI] [PubMed] [Google Scholar]
- 7.Bai Y., Hou Y., Wang Q., Lu C., Ma X., Wang Z., Xu H. Analysis of the binding modes and resistance mechanism of four methyl benzimidazole carbamates inhibitors fungicides with Monilinia fructicola β2-tubulin protein. J. Mol. Struct. 2023;1291:136057. doi: 10.1016/j.molstruc.2023.136057. [DOI] [Google Scholar]
- 8.Ma Z., Yoshimura M.A., Michailides T.J. Identification and characterization of benzimidazole resistance in Monilinia fructicola from stone fruit orchards in California. Appl. Environ. Microbiol. 2003;69:7145–7152. doi: 10.1128/AEM.69.12.7145-7152.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hrustić J., Medić O., Berić T., Mihajlović M., Milijašević-Marčić S., Stanković S., Pešić B. Suppression of Monilinia Brown Rot by Bacillus spp. Strains. Agronomy. 2023;13:2839. doi: 10.3390/agronomy13112839. [DOI] [Google Scholar]
- 10.Álvarez-García S., Moumni M., Romanazzi G. Antifungal activity of volatile organic compounds from essential oils against the postharvest pathogens Botrytis cinerea, Monilinia fructicola, Monilinia fructigena, and Monilinia laxa. Front. Plant Sci. 2023;4:1274770. doi: 10.3389/fpls.2023.1274770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Porto C., Stüker C.Z., Mallmann A.S., Simionatto E., Flach A., Canto-Dorow T.D., Morel A.F. (R)-(-)-carvone and (1R, 4R)-trans-(+)-dihydrocarvone from Poiretia latifolia Vogel. J. Braz. Chem. Soc. 2010;21:782–786. doi: 10.1590/S0103-50532010000500003. [DOI] [Google Scholar]
- 12.Díaz K., Werner E., Besoain X., Flores S., Donoso V., Said B., Caro N., Vega E., Montenegro I., Madrid A. In Vitro Antifungal Activity and Toxicity of Dihydrocarvone-Hybrid Derivatives against Monilinia fructicola. Antibiotics. 2021;10:818. doi: 10.3390/antibiotics10070818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brito C., Hansen H., Espinoza L., Faúndez M., Olea A.F., Pino S., Díaz K. Assessing the Control of Postharvest Gray Mold Disease on Tomato Fruit Using Mixtures of Essential Oils and Their Respective Hydrolates. Plants. 2021;10:1719. doi: 10.3390/plants10081719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pereira W.V., Primiano I.V., Morales R.G.F., Peres N.A., Amorim L., May De Mio L.L. Reduced Sensitivity to Azoxystrobin of Monilinia fructicola Isolates from Brazilian Stone Fruits is Not Associated with Previously Described Mutations in the Cytochrome b Gene. Plant Dis. 2017;101:766–773. doi: 10.1094/PDIS-09-16-1247-RE. [DOI] [PubMed] [Google Scholar]
- 15.Townsend G.R., Heuberger J.W. Methods for estimating losses caused by diseases in fungicides experiments. Plant Dis. Report. 1943;27:340–343. [Google Scholar]
- 16.Di Liberto M.G., Stegmayer M.I., Fernández L.N., Quiroga A.D., Svetaz L.A., Derita M.G. Control of Brown Rot Produced by Monilinia fructicola in Peaches Using a Full-Spectrum Extract of Zuccagnia punctata Cav. Horticulturae. 2023;9:1141. doi: 10.3390/horticulturae9101141. [DOI] [Google Scholar]
- 17.Huang L.S., Sun G., Cobessi D., Wang A.C., Shen J.T., Tung E.Y., Berry E.A. 3-nitropropionic acid is a suicide inhibitor of mitochondrial respiration that, upon oxidation by complex II, forms a covalent adduct with a catalytic base arginine in the active site of the enzyme. J. Biol. Chem. 2006;281:5965–5972. doi: 10.1074/jbc.M511270200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris G.M., Goodsell D.S., Halliday R.S., Huey R., Hart W.E., Belew R.K., Olson A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998;19:1639–1662. doi: 10.1002/(SICI)1096-987X(19981115)19:14<1639::AID-JCC10>3.0.CO;2-B. [DOI] [Google Scholar]
- 19.Cramer J., Sager C.P., Ernst B. Hydroxyl Groups in Synthetic and Natural-Product-Derived Therapeutics: A Perspective on a Common Functional Group. J. Med. Chem. 2019;62:8915–8930. doi: 10.1021/acs.jmedchem.9b00179. [DOI] [PubMed] [Google Scholar]
- 20.Tsao R., Zhou T. Antifungal Activity of Monoterpenoids against Postharvest Pathogens Botrytis cinerea and Monilinia fructicola. J. Essent. Oil Res. 2000;12:113–121. doi: 10.1080/10412905.2000.9712057. [DOI] [Google Scholar]
- 21.Svircev A.M., Smith R.J., Zhou T., Hernade M., Liu W., Chud C.L. Effects of thymol fumigation on survival and ultrastracture of Monilinia fructicola. Postharvest Biol. Technol. 2007;45:228–233. doi: 10.1016/j.postharvbio.2006.12.021. [DOI] [Google Scholar]
- 22.Ben Arfa A., Combes S., Preziosi-Belloy L., Gontard N., Chalier P. Antimicrobial activity of carvacrol related to its chemical structure. Lett. Appl. Microbiol. 2006;43:149–154. doi: 10.1111/j.1472-765X.2006.01938.x. [DOI] [PubMed] [Google Scholar]
- 23.Michailides T.J., Morgan D.P. Influence of Fruit-to-Fruit Contact on the Susceptibility of French Prune to Infection by Monilinia fructicola. Plant Dis. 1997;81:1416–1424. doi: 10.1094/PDIS.1997.81.12.1416. [DOI] [PubMed] [Google Scholar]
- 24.Ramos-Martín F., D’Amelio N. Biomembrane lipids: When physics and chemistry join to shape biological activity. Biochimie. 2022;203:118–138. doi: 10.1016/j.biochi.2022.07.011. [DOI] [PubMed] [Google Scholar]
- 25.Papavasileiou A., Tanou G., Samaras A. Proteomic analysis upon peach fruit infection with Monilinia fructicola and M. laxa identify responses contributing to brown rot resistance. Sci. Rep. 2020;10:7807. doi: 10.1038/s41598-020-64864-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balsells-Llauradó M., Silva C.J., Usuall J., Vall-Llaura N., Serrano-Prieto S., Teixidó N., Mesquida-Pesci S.D., de Cal A., Blanco-Ulate B., Torres R. Depicting the battle between nectarine and Monilinia laxa: The fruit developmental stage dictates the effectiveness of the host defenses and the pathogen’s infection strategies. Hortic. Res. 2020;17:167. doi: 10.1038/s41438-020-00387-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lo Presti L., Lanver D., Schweizer G., Tanaka S., Liang L., Tollot M., Zuccaro A., Reissmann S., Kahmann R. Fungal effectors and plant susceptibility. Annu. Rev. Plant Biol. 2015;66:513–545. doi: 10.1146/annurev-arplant-043014-114623. [DOI] [PubMed] [Google Scholar]
- 28.Stojanovic B.T., Mitic S.S., Stojanovic G.S., Mitic M.N., Kostic D.A., Paunovic D.D., Arsic B.B. Phenolic Profile and Antioxidant Activity of Pulp and Peel from Peach and Nectarine Fruits. Not. Bot. Horti Agrobot. Cluj-Napoca. 2016;44:175–182. doi: 10.15835/nbha44110192. [DOI] [Google Scholar]
- 29.Huang S., Millar A.H. Succinate dehydrogenase: The complex roles of a simple enzyme. Curr. Opin. Plant Biol. 2013;16:344–349. doi: 10.1016/j.pbi.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 30.Zhao Y., Zhang A., Wang X., Tao K., Jin H., Hou T. Novel pyrazole carboxamide containing a diarylamine scaffold potentially targeting fungal succinate dehydrogenase: Antifungal activity and mechanism of action. J. Agric. Food Chem. 2022;70:13464–13472. doi: 10.1021/acs.jafc.2c00748. [DOI] [PubMed] [Google Scholar]
- 31.Luo X., Chen Y., Wang Y., Xing Z., Peng J., Chen J. Design, synthesis and antifungal activity of novel amide derivatives containing a pyrrolidine moiety as potential succinate dehydrogenase inhibitors. Mol. Divers. 2024;28:805–816. doi: 10.1007/s11030-023-10622-w. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y.H., Yang S.S., Zhang Q., Zhang T.T., Zhang T.Y., Zhou B.H., Zhou L. Discovery of N-phenylpropiolamide as a novel succinate dehydrogenase inhibitor scaffold with broad-spectrum antifungal activity on phytopathogenic fungi. J. Agric. Food Chem. 2023;71:3681–3693. doi: 10.1021/acs.jafc.2c07712. [DOI] [PubMed] [Google Scholar]
- 33.Ma Z., Qiu S., Zhang D., Guo X., Lu Y., Fan Y., Chen X. Design, synthesis, and antifungal activity of novel dithiin tetracarboximide derivatives as potential succinate dehydrogenase inhibitors. Pest Manag. Sci. 2023;79:1922–1930. doi: 10.1002/ps.7369. [DOI] [PubMed] [Google Scholar]
- 34.Soto M., Estevez-Braun A., Amesty Á., Kluepfel J., Restrepo S., Diaz K., Taborga L. Synthesis and fungicidal activity of hydrated geranylated phenols against Botrytis cinerea. Molecules. 2021;26:6815. doi: 10.3390/molecules26226815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dai S., Lian Z., Qi W., Chen Y., Tong X., Tian T., Jiang L. Non-covalent interaction of soy protein isolate and catechin: Mechanism and effects on protein conformation. Food Chem. 2022;384:132507. doi: 10.1016/j.foodchem.2022.132507. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in this study are included in this article/Supplementary Materials; further inquiries can be directed to the corresponding authors.