Abstract
Expression of the Epstein-Barr virus (EBV) immediate-early (IE) protein BRLF1 induces the lytic form of viral replication in most EBV-positive cell lines. BRLF1 is a transcriptional activator that binds directly to a GC-rich motif present in some EBV lytic gene promoters. However, BRLF1 activates transcription of the other IE protein, BZLF1, through an indirect mechanism which we previously showed to require activation of the stress mitogen-activated protein kinases. Here we demonstrate that BRLF1 activates phosphatidylinositol-3 (PI3) kinase signaling in host cells. We show that the specific PI3 kinase inhibitor, LY294002, completely abrogates the ability of a BRLF1 adenovirus vector to induce the lytic form of EBV infection, while not affecting lytic infection induced by a BZLF1 adenovirus vector. Furthermore, we demonstrate that the requirement for PI3 kinase activation in BRLF1-induced transcriptional activation is promoter dependent. BRLF1 activation of the SM early promoter (which occurs through a direct binding mechanism) does not require PI3 kinase activation, whereas activation of the IE BZLF1 and early BMRF1 promoters requires PI3 kinase activation. Thus, there are clearly two separate mechanisms by which BRLF1 induces transcriptional activation.
Epstein-Barr virus (EBV) infects the majority of the world's population and causes infectious mononucleosis (25, 39). EBV infection is also associated with an increasing number of malignancies (39). As is the case for all herpesviruses, EBV can infect cells in either a latent or lytic form. Viral proteins expressed during the latent form of EBV infection are sufficient to immortalize B cells in vitro and contribute to the development of EBV-associated malignancies in vivo (25, 39). However, the virus must periodically convert to the lytic form of infection to promote secretion of viral particles in the saliva and infection of new hosts (25, 39).
Expression of either EBV immediate-early (IE) protein, BZLF1 or BRLF1, is sufficient to convert the latent form of EBV infection into the lytic form in most cell types (5, 7, 38, 41, 44, 50, 51). Both BZLF1 and BRLF1 are transcriptional activators (4, 14, 15, 20–24, 29, 32, 37, 38), and each IE protein activates transcription of the other (1, 2, 11, 28, 37, 42, 51). Mutational analysis in the intact viral genome has recently confirmed that both IE proteins are required for lytic EBV infection (11). However, in certain EBV-positive cell lines (such as the Raji Burkitt's lymphoma line), only BZLF1 expression induces lytic EBV infection (37, 51). BZLF1 binds directly to AP1-like motifs present in many early EBV promoters, as well as the BRLF1 promoter (2, 4, 10, 14, 36, 41, 42). In contrast, BRLF1 binds directly to a GC-rich motif present in certain early promoters (17, 18, 36) but activates other promoters (including the two promoters driving BZLF1 transcription) through an indirect mechanism (1, 20). The inability of BRLF1 to induce the lytic form of EBV infection in Raji cells is associated with its inability to activate BZLF1 transcription from the endogenous viral genome, although BRLF1 is capable of activating the EBV SM early promoter in Raji cells (37, 51).
The ability of BRLF1 to induce transcription of some target genes but not others in Raji cells suggests that it activates genes by more than one mechanism. We recently demonstrated that BRLF1 activates BZLF1 transcription at least partially through an indirect mechanism requiring a CREB motif in the Zp promoter (1). We showed that this CREB site is bound by a c-Jun/ATF2 heterodimer and that BRLF1 induces phosphorylation of the ATF2 transcription factor by activation of the c-Jun and p38 stress kinase pathways (1). However, the exact mechanism(s) by which BRLF1 activates these signal transduction pathways remains unknown.
In this report, we have further examined the effect of BRLF1 on signal transduction pathways in the host cell. We show that BRLF1 induces Akt phosphorylation through a phosphatidylinositol-3 (PI3) kinase-dependent pathway and that PI3 kinase activation is required for BRLF1-induced (but not BZLF1-induced) disruption of viral latency. The requirement for PI3 kinase activation is promoter dependent, in that BRLF1 can efficiently activate the early SM promoter but not the IE BZLF1 and early BMRF1 promoters in the presence of a PI3 kinase inhibitor. In addition, we show that activated RAS is required for both BRLF1- and BZLF1-induced disruption of viral latency, at a stage downstream of BZLF1 and BRLF1 transcription. Our results suggest that BRLF1 activates EBV promoters through at least two different mechanisms. One mechanism is mediated by direct binding of BRLF1 to GC-rich motifs in certain early viral promoters, as previously described (17, 18, 36). The other mechanism (which may be cell type dependent) is mediated through activation of PI3 kinase and the stress mitogen-activated protein (MAP) kinases and is required for activation of the IE BZLF1 promoter.
MATERIALS AND METHODS
Cell culture and reagents.
Raji and Akata cells are EBV-positive Burkitt's lymphoma cell lines. D98/HE-R-1 is an epithelial cell line formed by the fusion of a HeLa subclone (D98) with the EBV-positive Burkitt's lymphoma cell line P3HR/1. Normal human fibroblasts (NHF5-neo), a gift from William Kaufmann at the University of North Carolina at Chapel Hill, were derived from neonatal foreskin. NHF5-neo was maintained in Eagle's minimal essential medium (Gibco BRL) supplemented with 10% fetal bovine serum (FBS) and nonessential amino acids. The AGS-EBV cell line (a gift from Lindsey Hutt-Fletcher) was obtained by G418 selection of AGS cells (a gastric carcinoma line) that were infected with a recombinant Akata virus in which a neomycin resistance cassette had been inserted into the nonessential BDLF3 open reading frame. All lymphoid cell lines were maintained in RPMI 1640 medium (Gibco BRL) supplemented with 10% FBS. Epithelial cell lines were maintained in Dulbecco's modified Eagle's medium H (Sigma) supplemented with 10% FBS or, in the case of the AGS-EBV cells in Ham's F-12 medium, with 10% FBS and 400 μg of G418/ml. All media contained penicillin (100 U/ml) and streptomycin (100 μg/ml).
Adenovirus vectors and infection.
The BZLF1 and BRLF1 cDNAs were cloned under the control of the cytomegalovirus IE promoter into shuttle vectors containing a Lox P site, the left adenovirus terminal repeat, and a packaging signal. Recombination of these shuttle vectors into the Lox P site of an E1- and E3-gene-deficient adenovirus produced adenovirus Z (Ad-Z; vector for BZLF1) and adenovirus R (Ad-R; vector for BRLF1) (50). The control vector, adenovirus Lac Z (Ad-Lac Z), contains the bacterial β-galactosidase gene and was constructed in the same manner.
Normal human fibroblasts were cultured at a cell density of 3 × 106 per 150 mM plate and were infected at a multiplicity of infection (MOI) of 500. D98/HE-R-1 cells were cultured in 100 mM plates at a cell density of 1.5 × 106 and were infected at an MOI of 50. Normal growth media were removed from all cells prior to infection and were replaced with Dulbecco's modified Eagle's medium plus 2% heat-inactivated serum. Cells were infected for 2 h; media were removed and were then replaced with normal growth medium.
DNA plasmids.
The BRLF1 expression plasmid pRTS15 was provided by Diane Hayward and contains a genomic BRLF1 fragment expressed by the simian virus 40 promoter in the pSG5 vector (Stratagene). The dominant-negative RAS plasmid (pZip-HRAS17N, a gift from Channing Der) has been previously described (12, 35) and contains a cysteine-to-serine substitution of the cysteine residue in the C-terminal CXXX prenylation signal sequence present in all RAS proteins.
DNA transfections.
Plasmid DNA was purified using the QIAGEN maxi kit as described by the manufacturer. DNA was transfected by electroporation at 1,500 V with a Zapper electroporation unit (Medical Electronics Shop, University of Wisconsin) using 6 μg of DNA. Epithelial cells were harvested and resuspended in RPMI 1640 medium prior to electroporation.
Immunoblot analysis.
Immunoblot analysis was performed for the detection of total Akt, BMRF1, BZLF1, BRLF1, and SM as follows: equal amounts of protein were loaded in each lane, and sodium dodecyl sulfate-polyacrylamide gel electrophoresis was performed. The proteins were transferred overnight onto nitrocellulose (Osmonics), blocked in 1× phosphate-buffered saline (PBS) containing 5% milk and 0.1% Tween 20, and incubated in primary antibody for 1 h at room temperature or 24 h at 4°C. Antibodies were as follows: total Akt (1:1,000) (catalog no. 9272; from Cell Signaling Technology); BMRF1 (1:100) from Capricorn; BZLF1 (1:100) anti-EBV ZEBRA from Argene; anti-BRLF1 (1:100) from Argene; and anti-SM rabbit polyclonal antibody (1:400), a gift from Sankar Swaminathan. The membrane was washed in PBS–0.1% Tween 20, incubated in secondary antibody for 1 h at room temperature (horseradish peroxidase-conjugated goat anti-mouse [1:10,000] or horseradish peroxidase-conjugated goat anti-rabbit [1:10,000] from Promega), and washed, and the results were visualized with the enhanced-chemiluminescence kit (Amersham) as specified by the manufacturer.
The detection of phosphorylated Akt (Ser 473) differed as follows: membranes were blocked in 1× Tris-buffered saline containing 0.1% Tween 20 and 5% bovine serum albumin (BSA) and were incubated overnight at 4°C (phospho-Akt [1:1,000], catalog no. 9271S; New England Biolabs). The secondary antibody was diluted in blocking buffer and was incubated for 1 h at room temperature.
Protein preparation.
Cells used in the immunoblots with phosphospecific antibodies were washed twice with PBS, resuspended in 150 μl of low-salt buffer (150 mM NaCl, 20 mM Tris-HCl [pH 7.5], 5 mM EDTA, 1% Triton X-100, 50 mM NaF, 10% glycerol, 1 mM Na3VO4, and protease inhibitors), tumbled for 15 min at 4°C, and centrifuged. All other cells were resuspended in 50 μl of buffer (0.25 M NaCl, 0.1% NP-40, 0.05 M HEPES [pH 7.0], 5 mM EDTA, and protease inhibitors), freeze-thawed twice, and centrifuged. The supernatants from centrifuged cells were used for Western blots.
PI3 kinase inhibition.
Cells treated with the PI3 kinase inhibitors LY294002 (Sigma) and wortmannin (Calbiochem) were incubated in medium containing final inhibitor concentrations of 15 μM (LY294002) and 0.1 μM (wortmannin) for 24 to 48 h. Inhibitors were replenished after 24 h.
FACS analysis.
Akata cells were induced into the lytic form of EBV infection using anti-immunoglobulin G (IgG) as previously described (45). Anti-IgG-treated Akata cells (100 μg of anti-human IgG [Sigma]/ml for 24 h) in the absence or presence of the PI3 kinase inhibitor LY294002 were washed twice with PBS, fixed with 60% acetone for 10 min on ice, washed three times with PBS–0.5% BSA, and incubated with a 1:100 dilution of the primary antibody (BMRF1; Capricorn) for 1 h at room temperature. The cells were washed three times and were then incubated in the secondary antibody (donkey anti-mouse–fluorescein isothiocyanate [1:100]; Sigma) for 1 h at room temperature. The cells were washed three times and resuspended in 0.5 ml of PBS. The percentage of immunofluorescent cells was determined with a fluorescence-activated cell sorter (FACS) (Becton-Dickinson). To examine the level of BZLF1 expression, similar experiments were performed using a BZLF1 antibody (1:200 dilution; Argene).
Immunocytochemistry.
Normal human fibroblasts were grown on glass coverslips and infected with Ad-R or Ad-LacZ at an MOI of 250. Two days later, cells were rinsed in PBS and fixed in 100% methanol for 10 min at −20°C. The cells were rehydrated in cold PBS with 0.01% Tween for 5 min, incubated in incubation mix (PBS–0.3% BSA–5% donkey serum) at room temperature for 10 min, and then incubated in primary antibody (monoclonal anti-BRLF1 [1:100] from Argene or equal amounts of an isotype control) diluted in incubation mix. Cells were washed four times with PBS–0.5% BSA for 5 min each and were then incubated in secondary antibody (donkey anti-mouse fluorescein isothiocyanate [1:100] [Jackson Immuno Labs] diluted in incubation mix) for 45 min at 37°C. Cells were washed as done before and mounted on Antifade media (Molecular Probes) and viewed on a Zeiss confocal microsope.
RESULTS
BRLF1, but not BZLF1, induces Akt phosphorylation through a PI3 kinase-dependent pathway.
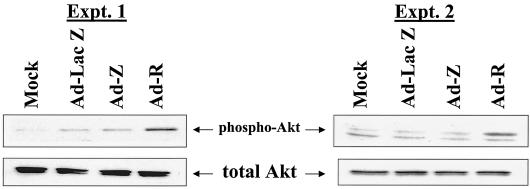
The PI3 kinase pathway is a major signaling pathway involved in the control of cell proliferation and survival (16, 26, 27), and activation of this pathway can lead to activation of a variety of downstream kinases (3, 26). To study the effects of BZLF1 and BRLF1 on the PI3 kinase pathway, we examined their effect on phosphorylation of the Akt protein using an antibody that specifically recognizes only the activated form (phosphorylated at residue Ser 473) of Akt. Phosphorylation and activation of the serine/threonine kinase Akt have been shown to be downstream effects of PI3 kinase activation (reviewed in reference 16). Serum-starved normal human fibroblasts were infected with Ad-Z, Ad-R, or Ad-Lac Z, and the level of Akt phosphorylation was examined by immunoblot analysis 2 days later. As shown in Fig. 1, BRLF1 expression in normal human fibroblasts induced Akt phosphorylation while not increasing the level of total Akt. In contrast, infection with the control or BZLF1 adenovirus had little effect.
FIG. 1.
BRLF1 expression is associated with Akt phosphorylation. Normal human fibroblasts were serum starved for 3 days and then either mock infected or infected with adenovirus vectors encoding β-galactosidase (Ad-LacZ), BZLF1 (Ad-Z), or BRLF1 (Ad-R). Phosphorylation of Akt was quantitated 2 days later by immunoblot analysis using an antibody that recognizes only the activated (phosphorylated on Ser 473 residue) form of Akt (top panels) or an antibody which recognizes total Akt (bottom panels). Two separate experiments are shown.
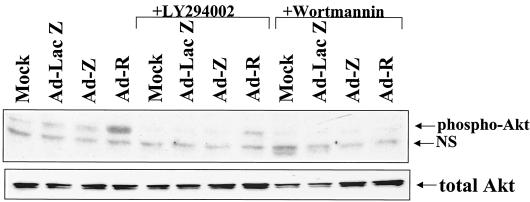
To confirm that BRLF1 activates Akt phosphorylation through a PI3 kinase-dependent pathway, we examined the effect of two different PI3 kinase inhibitors, wortmannin and LY294002, on BRLF1-induced Akt phosphorylation. As shown in Fig. 2, both PI3 kinase inhibitors significantly decreased the ability of BRLF1 to activate Akt phosphorylation while not affecting the level of total Akt. Therefore, BRLF1 expression in cells appears to activate the PI3 kinase signaling pathway.
FIG. 2.
BRLF1 induces Akt phosphorylation through a PI3 kinase-dependent pathway. Serum-starved normal human fibroblasts were infected with the various adenovirus vectors in the presence or absence of the PI3 kinase inhibitors, LY294002 (15 μM) or wortmannin (0.1 μM). The amount of phosphorylated Akt was quantitated 2 days later by immunoblot analysis using an antibody that recognizes only the phosphorylated form of Akt (top panel; NS is a nonspecific band) or an antibody that recognizes total Akt (bottom panel).
PI3 kinase activation is required for BRLF1 induction of lytic EBV infection.
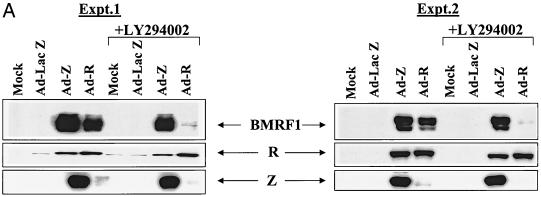
To determine if the ability of BRLF1 to activate the PI3 kinase pathway is in fact important for induction of lytic EBV infection, latently infected EBV-positive D98/HE-R-1 cells were infected with Ad-LacZ, Ad-Z, or Ad-R in the presence or absence of the PI3 kinase inhibitor LY294002. Two days later, induction of the lytic form of EBV infection was quantitated by immunoblot analysis using antibodies directed against the early lytic EBV protein, BMRF1, as well as the two EBV IE proteins.
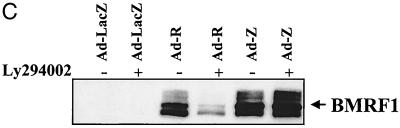
As shown in Fig. 3A, the PI3 kinase inhibitor LY294002 almost completely abrogated the ability of Ad-R to induce expression of the early lytic BMRF1 gene, although the level of BRLF1 expression in cells was not affected. In sharp contrast, induction of lytic EBV infection using Ad-Z did not require the PI3 kinase pathway. Since it is known that activation of the early BMRF1 gene requires both BZLF1 and BRLF1 (2, 37, 51), we also examined the effect of the PI3 kinase inhibitor on BRLF1 induction of BZLF1 expression from the endogenous viral genome. The ability of BRLF1 to activate BZLF1 expression was decreased in the presence of the PI3 kinase inhibitor (Fig. 3A).
FIG. 3.
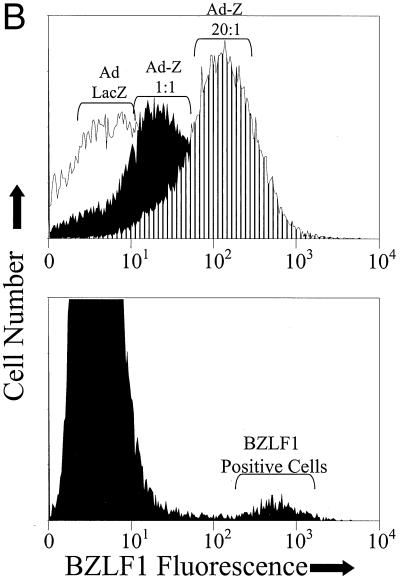
PI3 kinase activation is required for BRLF1-induced, but not BZLF1-induced, lytic EBV infection. (A) EBV-positive D98/HE-R-1 cells were infected with the various adenovirus vectors (MOI of 50) in the presence or absence of the PI3 kinase inhibitor LY294002. Induction of the lytic form of EBV infection was quantitated by immunoblot analysis 1 day later using antibodies directed against the early lytic EBV protein BMRF1 or against the IE proteins BRLF1 (R) and BZLF1 (Z). Two separate experiments are shown. (B) The level of cellular BZLF1 expression was quantitated by FACS analysis in D98/HE-R-1 cells (top panel) infected with Ad-LacZ (MOI of 20) or Ad-Z (MOI of 1 or of 20) and in AGS-EBV cells (bottom panel). In AGS-EBV cells, only about 5% of cells express BZLF1. Isotype control antibodies and EBV-negative AGS cells were also used in the experiments described above to confirm which cells are the BZLF1-expressing cells (data not shown). (C) Procedure given for panel A was followed, except that D98/HE-R-1 cells were infected with adenovirus vectors using an MOI of 1. Abbreviations are the same as those given for panel A. −, absence of LY294002; +, presence of LY294002.
Given that the BRLF1 adenovirus induces only a small amount of BZLF1 expression from the endogenous EBV genome in D98/HE-R-1 cells, we considered the possibility that an inhibitory effect of LY294002 on cells infected with Ad-Z (using an MOI of 50) was missed due to nonphysiologic overexpression of BZLF1. To confirm that this is not the case, we used FACS analysis to compare the level of BZLF1 expression in D98/HE-R-1 cells infected with various amounts of Ad-Z with the level of cellular BZLF1 expression in EBV-positive gastric epithelial cells (AGS-EBV). Although the level of cellular BZLF1 expression in D98/HE-R-1 cells infected with Ad-Z at an MOI of 1 was considerably lower than the level of BZLF1 expression observed in AGS-EBV cells (Fig. 3B), BMRF1 expression was not inhibited by LY294002 in D98/HE-R-1 cells infected with Ad-Z at an MOI of 1 (Fig. 3C). These results confirm that BRLF1-induced but not BZLF1-induced lytic EBV infection requires activation of the PI3 kinase pathway.
BRLF1 activation of the early SM gene does not require PI3 kinase activation.
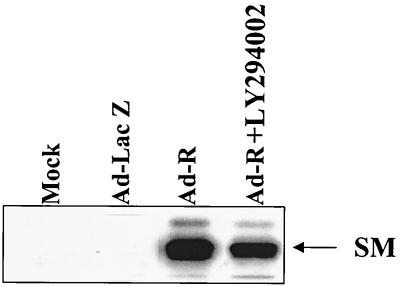
It was recently shown that BRLF1 induces transcription of the early EBV gene SM but not of the BZLF1 or BMRF1 genes in Raji cells (37). Thus, BRLF1 activates the SM early gene even in the absence of any concomitant BZLF1 expression. To determine if PI3 kinase activation is required only for a subset of BRLF1-responsive genes, we examined the effect of the PI3 kinase inhibitor LY294002 on BRLF1 activation of the SM gene in D98-HE-R-1 cells. Immunoblot analysis was performed on the same cell extracts shown in Fig. 3A (experiment 1), using an antibody that recognizes the SM protein. As shown in Fig. 4, inhibition of PI3 kinase activity with LY294002 did not significantly affect the ability of BRLF1 to activate the SM promoter in D98-HE-R-1 cells. Thus, PI3 kinase activation is required for only a subset of BRLF1-responsive promoters.
FIG. 4.
PI3 kinase activation is not required for BRLF1 activation of the early SM promoter. EBV-positive D98/HE-R-1 cells were infected with the various adenovirus vectors in the presence or absence of the PI3 kinase inhibitor LY294002. Induction of the early SM EBV gene was quantitated by immunoblot analysis using an antibody directed against the early lytic EBV protein SM.
BRLF1 is both a nuclear and cytoplasmic protein.
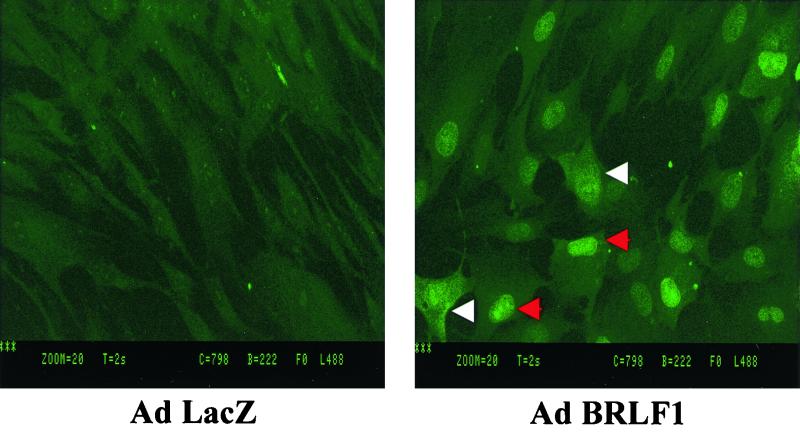
To further investigate the potential mechanism(s) by which BRLF1 induces PI3 kinase activation, we examined the cellular distribution of BRLF1 using confocal microscopy. Although it is generally accepted that BRLF1 is a nuclear protein, the original description of BRLF1 in fact characterized it as both a nuclear and cytoplasmic protein (33). As shown in Fig. 5, although the majority of normal human fibroblasts infected with Ad-R had BRLF1 protein visualized only in the nucleus, in some cells BRLF1 was also clearly observed in the cytoplasm. The diffuse BRLF1 staining observed in some cells did not result from membrane localization, since this type of BRLF1 staining was observed on multiple different cuts of the same cell using confocal microscopy (data not shown). Thus, the ability of BRLF1 to induce PI3 kinase activation could potentially be due to either transcriptional or nontranscriptional mechanisms in either the nucleus or cytoplasm.
FIG. 5.
BRLF1 is both a nuclear and cytoplasmic protein. Normal human fibroblasts were infected with Ad-LacZ or Ad-R. Immunocytochemistry was performed 2 days later using a BRLF1 monoclonal antibody and confocal microscopy. An isotype control primary antibody produced no visible staining (data not shown). Cells containing primarily nuclear BRLF1 (red arrows) versus those containing diffuse BRLF1 (white arrows) are indicated.
PI3 kinase activation is required for anti-Ig-induced lytic EBV infection in Akata cells.
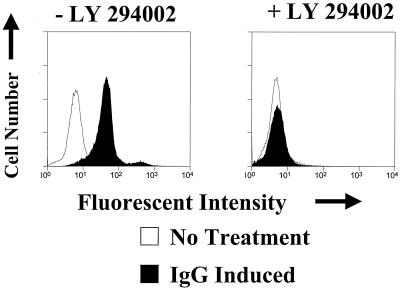
Engagement of the B-cell receptor activates PI3 kinase signaling (8) and also induces the lytic form of EBV infection in some cell lines (45). To determine if activation of the PI3 kinase pathway is required for induction of lytic EBV infection by B-cell receptor activation, we treated Akata cells (an EBV-positive Burkitt's lymphoma line) with anti-IgG either in the absence or presence of the PI3 kinase inhibitor LY294002. The number of cells expressing the lytic BMRF1 viral protein before and after engagement of the B-cell receptor was quantitated using a BMRF1 antibody and FACS analysis; only viable cells were included in the analysis (Fig. 6). In the absence of the PI3 kinase inhibitor, anti-IgG treatment of Akata cells induced BMRF1 expression in essentially all cells. In the presence of the PI3 kinase inhibitor, BMRF1 induction by anti-IgG was completely abolished. However, LY294002 treatment of Akata cells also completely inhibited the ability of anti-IgG treatment to induce expression of both the BZLF1 and BRLF1 IE proteins (data not shown). Similar results were obtained using immunoblot analysis (data not shown). Therefore, the disruption of EBV viral latency by B-cell receptor activation requires the PI3 kinase signaling pathway for the activation of both BZLF1 and BRLF1 transcription.
FIG. 6.
PI3 kinase activation is required for anti-Ig-induced lytic EBV infection in Akata cells. Akata cells were treated with 100 μg of anti-IgG/ml for 24 h in the absence (−) or presence (+) of the PI3 kinase inhibitor LY294002. The level of BMRF1 expression in cells was determined by FACS analysis using a BMRF1-specific antibody. Similar results were obtained in two separate experiments.
Activated RAS is required for both BRLF1- and BZLF1-induced lytic EBV infection.
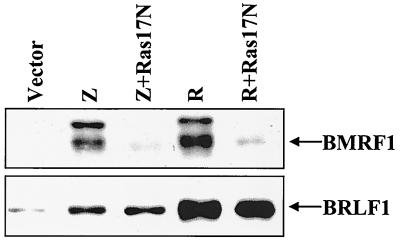
Our previous results (1), in combination with the data presented above, indicate that BRLF1 can activate PI3 kinase signaling, as well as that of the c-Jun and p38 stress MAP kinases. Since RAS can mediate activation of PI3 kinase as well as of the stress MAP kinases (6, 40, 46), we examined whether a dominant-negative RAS mutant (17N) can inhibit the ability of transfected BRLF1 or BZLF1 to induce the lytic form of EBV infection in D98/HE-R-1 cells. As shown in Fig. 7, the dominant-negative RAS mutant significantly reduced the ability of both BZLF1 and BRLF1 to activate BMRF1 expression while not affecting the level of transfected BRLF1 protein or the ability of BZLF1 to induce BRLF1 transcription from the endogenous viral genome. In addition, using reverse transcriptase PCR analysis, we did not find that the ability of transfected BRLF1 to activate BZLF1 transcription from the endogenous viral genome was reduced by the dominant-negative RAS mutant (data not shown). Thus, activated RAS may be required for induction of lytic EBV infection at a step downstream of IE gene transcription.
FIG. 7.
Activated RAS is required for BRLF1- and BZLF1-induced lytic EBV infection. D98/HE-R-1 cells were transfected with a control vector (vector) or BRLF1 (R) and BZLF1 (Z) expression vectors in the presence or absence of a dominant-negative RAS mutant (RAS17N). The level of BMRF1 induction (top panel) was analyzed 2 days after transfection by immunoblot analysis. The level of transfected BRLF1 (R) (or BRLF1 induced by BZLF1 from the endogenous viral genome) is shown in the bottom panel.
DISCUSSION
The EBV IE protein, BZLF1, was shown to mediate the switch from latent to lytic EBV infection some time ago, but it has only recently been recognized that the other EBV IE protein, BRLF1, can also mediate this switch (38, 50, 51). Interestingly, only the BRLF1 homologue (ORF 50), not the BZLF1 homologue, can induce the lytic form of viral infection in human herpesvirus 8 (the closest human herpesvirus relative of EBV) (31, 43). Thus, BRLF1 and its homologues encode a more universal mechanism for inducing the lytic form of viral infection in the gammaherpesviruses, whereas BZLF1 activation of lytic viral infection may be specific to EBV. As yet, however, the mechanism by which BRLF1 induces lytic viral infection has remained somewhat elusive, since the first essential step in this cascade (activation of BZLF1 transcription) occurs through an indirect mechanism (1). Our results here suggest that BRLF1 induces lytic EBV infection by activating a signal transduction cascade that culminates in BZLF1 transcription.
There are a number of interesting differences between the induction of lytic EBV infection by BZLF1 and that by BRLF1, suggesting that the two IE proteins accomplish the same task through quite different mechanisms. We previously demonstrated that BRLF1, but not BZLF1, requires activation of the p38 and c-Jun stress MAP kinase pathways for induction of lytic EBV infection (1), and here we have shown that BRLF1, but not BZLF1, also requires PI3 kinase activation. In addition, the ability of BRLF1 to induce lytic EBV infection appears to be more cell type dependent than that of BZLF1 (37, 51). For example, transfected BZLF1 efficiently induces lytic EBV infection in Raji cells, whereas transfected BRLF1 is incapable of inducing BZLF1 (or BMRF1) expression in this cell line (37, 51). However, since BRLF1 can activate expression of certain lytic viral promoters (such as SM) in Raji cells and since the BZLF1 promoter is not mutated in this line (37), there appear to be at least two different mechanisms by which BRLF1 activates transcription (only one of which is defective in Raji cells).
In this study, we have further explored the effects of BRLF1 on signal transduction pathways in the host cell. We demonstrate that BRLF1 activates the PI3 kinase pathway and that induction of the PI3 kinase pathway is required for activation of a subset of BRLF1-responsive promoters, as well as disruption of viral latency. These results, together with our previous work showing that activation of the stress MAP kinase pathway is required for BRLF1 to induce lytic EBV infection (1), suggest that BRLF1 must activate a signal transduction cascade(s) in order to induce lytic EBV infection. Although a subset of early EBV promoters (such as SM) remains BRLF1 responsive even in the absence of BRLF1-induced signal transduction, efficient activation of the BZLF1 IE promoter by BRLF1 requires activation of the PI3 kinase and stress MAP kinases. Since the early lytic gene BMRF1 encodes the essential viral polymerase processivity function and since BMRF1 cannot be activated in the intact EBV genome unless both BZLF1 and BRLF1 are present (2, 37), blocking the ability of BRLF1 to transcriptionally activate the BZLF1 IE promoter also blocks its ability to induce the lytic form of EBV replication.
We have previously shown that BRLF1 activation of the BZLF1 promoter is at least partially mediated through a CREB binding motif (1). This motif is bound by the CREB, ATF-2, ATF-1, and c-Jun transcription factors (1, 13, 30, 49). BRLF1 induces an activating phosphorylation of ATF-2 by activating the p38 and c-Jun stress MAP kinases (1, 19), and these same kinases have also been shown to induce activating phosphorylation of the c-Jun transcription factor (9, 19, 47).
Our finding here that BRLF1 activates the PI3 kinase pathway and that activation of this pathway is required for BRLF1-induced lytic EBV infection suggests that BRLF1 activates the stress MAP kinases through a PI3 kinase-dependent mechanism. However, we have found that inhibition of BRLF1-induced PI3 kinase activation does not affect its ability to activate p38 kinase (unpublished observations). Thus, it appears that BRLF1 must activate at least two parallel signal transduction pathways, one leading to downstream stress MAP kinase activation and the other leading to PI3 kinase activation. BRLF1 activation of both pathways appears to be required for activation of the BZLF1 promoter.
We also observed that both BZLF1 and BRLF1 require RAS activation for induction of lytic EBV infection. However, in comparison to the requirement for PI3 kinase and p38 kinase activation, our data suggest that activated RAS is required at a later step during the induction of lytic infection that is downstream of IE gene transcription. It is possible that either BRLF1 or BZLF1 expression in cells activates RAS (although we have as yet been unable to demonstrate this) and that RAS activation then leads to activation of both PI3 kinase and the stress MAP kinases.
At this point, we are uncertain why PI3 kinase activation, in addition to stress MAP kinase activation, is required for BRLF1 activation of the BZLF1 promoter. Activation of the PI3 kinase pathway has been previously shown to activate at least two other promoters through CREB binding motifs (34, 48), and the CREB transcription factor has been shown to bind to and activate the BZLF1 promoter (1, 13, 30, 49). It is possible that efficient BZLF1 transcription requires the combination of the CREB, ATF-2, and c-Jun transcription factors. The requirement for activation of multiple, different signal transduction pathways in order to induce BZLF1 transcription may serve as a protective mechanism that prevents spurious reactivation of the lytic form of EBV infection in B cells.
A key unresolved issue is the exact mechanism by which BRLF1 activates the PI3 kinase and stress MAP kinase signal transduction pathways. Although BRLF1 is primarily a nuclear protein, it is clear from the results shown here as well as from previously published results (33) that there is also a cytoplasmic component of BRLF1. Thus, it is possible that BRLF1 regulates signal transduction pathways through protein-protein interactions in the cytoplasm. Alternatively, BRLF1 may transcriptionally activate expression of a cellular receptor that activates the PI3 kinase and/or RAS signaling pathways or induce expression of a ligand that activates a cellular receptor. We are currently employing both differential gene array experiments, as well as yeast two-hybrid studies, to identify the mechanism(s) for the BRLF1-associated signal transduction effects. In addition, the Raji cell line may prove useful for identifying the mechanism by which BRLF1 induces signal transduction, since this cell line appears to be specifically deficient in its ability to support BRLF1-dependent activation of the BZLF1 promoter. We have shown that many of the same signal transduction pathways required for BRLF1-induced lytic infection (PI3 kinase, c-Jun N-terminal kinase, and p38 kinase) are also required for the induction of lytic EBV infection which occurs following activation of the B-cell receptor (1). Thus, BRLF1 expression may somehow mimic the effect of B-cell receptor engagement.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant 2-RO1-CA58853 from the National Institutes of Health.
We thank Channing Der for the RAS (17N) mutant, Diane Hayward for the pRTS15 plasmid, Sankar Swaminathan for the SM antibody, and Young Whang for helpful discussions and review of the manuscript.
REFERENCES
- 1.Adamson A L, Darr D, Holley-Guthrie E, Johnson R, Mauser A, Swenson J, Kenney S. Epstein-Barr virus immediate-early proteins BZLF1 and BRLF1 activate the ATF2 transcription factor by increasing levels of phosphorylated p38 and c-Jun N-terminal kinases. J Virol. 2000;74:1224–1233. doi: 10.1128/jvi.74.3.1224-1233.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adamson A L, Kenney S. Rescue of the Epstein-Barr virus BZLF1 mutant Z (S186A) early gene activation defect by the BRLF1 gene product. Virology. 1998;251:187–197. doi: 10.1006/viro.1998.9396. [DOI] [PubMed] [Google Scholar]
- 3.Bondeva T, Pirola L, Bulgarelli-Leva G, Rubio I, Wetzker R, Wymann M P. Bifurcation of lipid and protein kinase signals of P13K to the protein kinases PKB and MAPK. Science. 1998;282:293–296. doi: 10.1126/science.282.5387.293. [DOI] [PubMed] [Google Scholar]
- 4.Chang Y, Dong D, Hayward G, Hayward S D. The Epstein-Barr virus Zta transactivator: a member of the bZip family with unique DNA-binding specificity and dimerization domain that lacks the characteristic heptad leucine zipper motif. J Virol. 1990;64:3358–3369. doi: 10.1128/jvi.64.7.3358-3369.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chevallier-Greco A, Manet E, Chavrier P, Mosnier C, Dallie J, Sergeant A. Both Epstein-Barr virus (EBV)-encoded trans-acting factors, EB1 and EB2, are required to activate transcription from an EBV early promoter. EMBO J. 1986;5:3243–3249. doi: 10.1002/j.1460-2075.1986.tb04635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cobb M H. MAP kinase pathways. Prog Biophys Mol Biol. 1999;71:479–500. doi: 10.1016/s0079-6107(98)00056-x. [DOI] [PubMed] [Google Scholar]
- 7.Countryman J, Miller G. Activation of expression of latent Epstein-Barr virus after gene transfer with a small cloned fragment of heterogeneous viral DNA. Proc Natl Acad Sci USA. 1985;82:4085–4089. doi: 10.1073/pnas.82.12.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeFranco A. The complexity of signaling pathways activated by the BCR. Curr Opin Immunol. 1997;9:296–308. doi: 10.1016/s0952-7915(97)80074-x. [DOI] [PubMed] [Google Scholar]
- 9.Derijard B, Hibi M, Wu I, Barrett T, Su B, Deng T, Karin M, Davis R. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 10.Farrell P, Rowe D, Rooney C, Kouozarides T. Epstein-Barr virus BZLF1 transactivator specifically binds to consensus Ap1 sites and is related to c-fos. EMBO J. 1989;8:127–132. doi: 10.1002/j.1460-2075.1989.tb03356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feederle R, Kost M, Baumann M, Janz A, Drouet E, Hammerschmidt W, Delecluse H J. The Epstein-Barr virus lytic program is controlled by the cooperative functions of two transactivators. EMBO J. 2000;19:3080–3089. doi: 10.1093/emboj/19.12.3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feig L A, Cooper G M. Inhibition of NIH 3T3 cell proliferation by a mutant ras protein with preferential affinity for GDP. Mol Cell Biol. 1988;8:3235–3243. doi: 10.1128/mcb.8.8.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flamand L, Menezes J. Cyclic AMP-responsive element-dependent activation of Epstein-Barr virus Zebra promoter by human herpesvirus 6. J Virol. 1996;70:1784–1791. doi: 10.1128/jvi.70.3.1784-1791.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flemington E, Speck S. Autoregulation of Epstein-Barr virus putative lytic switch gene BZLF1. J Virol. 1990;64:1227–1232. doi: 10.1128/jvi.64.3.1227-1232.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flemington E, Borras K, Lytle J, Speck S. Characterization of the Epstein-Barr virus BZLF1 protein transactivator domain. J Virol. 1992;66:922–929. doi: 10.1128/jvi.66.2.922-929.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franke T, Kaplan D, Cantley L. PI3K: downstream AKTion blocks apoptosis. Cell. 1997;88:435–437. doi: 10.1016/s0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- 17.Gruffat H, Sergeant A. Characterization of the DNA-binding site repertoire for the Epstein-Barr virus transcription factor R. Nucleic Acids Res. 1994;22:1172–1178. doi: 10.1093/nar/22.7.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gruffat H, Duran N, Buisson M, Wild F, Buckland R, Sergeant A. Characterization of an R-binding site mediating the R-induced activation of the Epstein-Barr virus BMLF1 promoter. J Virol. 1992;66:46–52. doi: 10.1128/jvi.66.1.46-52.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta S, Campbell D, Derijard B, Davis R. Transcription factor ATF2 regulation by the JNK signal transduction pathway. Science. 1995;267:389–393. doi: 10.1126/science.7824938. [DOI] [PubMed] [Google Scholar]
- 20.Gutsch D E, Marcu K B, Kenney S C. The Epstein-Barr virus BRLF1 gene product transactivates the murine and human c-myc promoters. Cell Mol Biol. 1994;40:747–760. [PubMed] [Google Scholar]
- 21.Hardwick J M, Tse L, Applegren N, Nicholas J, Veliuona M A. The Epstein-Barr virus R transactivator (Rta) contains a complex, potent activation domain with properties different from those of VP16. J Virol. 1992;66:5500–5508. doi: 10.1128/jvi.66.9.5500-5508.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hardwick J M, Lieberman P, Hayward S D. A new Epstein-Barr virus transactivator, R, induces expression of a cytoplasmic early antigen. J Virol. 1988;62:2274–2284. doi: 10.1128/jvi.62.7.2274-2284.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holley-Guthrie E A, Quinlivan E B, Mar E C, Kenney S. The Epstein-Barr virus (EBV) BMRF1 promoter for early antigen (EA-D) is regulated by the EBV transactivators, BRLF1 and BZLF1, in a cell-specific manner. J Virol. 1990;64:3753–3759. doi: 10.1128/jvi.64.8.3753-3759.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kenney S, Holley-Guthrie E, Mar E C, Smith M. The Epstein-Barr virus BMLF1 promoter contains an enhancer element that is responsive to the BZLF1 and BRLF1 transactivators. J Virol. 1989;63:3878–3883. doi: 10.1128/jvi.63.9.3878-3883.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kieff E. Epstein-Barr virus and its replication. In: Fields B N, Knipe D N, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2343–2395. [Google Scholar]
- 26.Klippel A, Reinhard C, Kavanaugh W M, Apell G, Escobedo M-A, Williams L T. Membrane localization of phosphatidylinositol 3-kinase is sufficient to activate multiple signal-transducing kinase pathways. Mol Cell Biol. 1996;16:4117–4127. doi: 10.1128/mcb.16.8.4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leevers S, Vanhaesebroeck B, Waterfield M. Signaling through phosphoinositide 3-kinases: the lipids take center stage. Curr Opin Cell Biol. 1999;11:219–225. doi: 10.1016/s0955-0674(99)80029-5. [DOI] [PubMed] [Google Scholar]
- 28.Le Roux F, Sergeant A, Corbo L. Epstein-Barr virus (EBV) EB1/Zta protein provided in trans and competent for activation of productive cycle genes does not activate the BZLF1 gene in the EBV genome. J Gen Virol. 1996;77:501–509. doi: 10.1099/0022-1317-77-3-501. [DOI] [PubMed] [Google Scholar]
- 29.Lieberman P, Hardwick J M, Hayward S D. Responsiveness of the Epstein-Barr virus NotI repeat promoter to the Z transactivator is mediated in a cell-type-specific manner by two independent signal regions. J Virol. 1989;63:3040–3050. doi: 10.1128/jvi.63.7.3040-3050.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu P, Liu S, Speck S. Identification of a negative cis element within the ZII domain of the Epstein-Barr virus lytic switch BZLF1 gene promoter. J Virol. 1998;72:8230–8239. doi: 10.1128/jvi.72.10.8230-8239.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lukac D M, Renne R, Kirshner J R, Ganem D. Reactivation of Kaposi's sarcoma-associated herpesvirus infection from latency by expression of the ORF 50 transactivator, a homolog of the EBV R protein. Virology. 1998;252:304–312. doi: 10.1006/viro.1998.9486. [DOI] [PubMed] [Google Scholar]
- 32.Manet E, Rigolet A, Gruffat H, Giot J F, Sergeant A. Domains of the Epstein-Barr virus (EBV) transcription factor R are required for dimerization, DNA binding and activation. Nucleic Acids Res. 1991;19:2661–2667. doi: 10.1093/nar/19.10.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marschall M, Leser U, Seibl R, Wolf H. Identification of proteins encoded by Epstein-Barr virus trans-activator genes. J Virol. 1989;63:938–942. doi: 10.1128/jvi.63.2.938-942.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pugazhenthi S, Nesterova A, Sable C, Heidenreich K, Boxer L, Heasley L, Reusch J. Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. J Biol Chem. 2000;275:10761–10766. doi: 10.1074/jbc.275.15.10761. [DOI] [PubMed] [Google Scholar]
- 35.Quilliam L A, Kato K, Rabun K M, Hisaka M M, Huff S Y, Campbell-Burk S, Der C J. Identification of residues critical for Ras (17N) growth-inhibitory phenotype and for Ras interaction with guanine nucleotide exchange factors. Mol Cell Biol. 1994;14:1113–1121. doi: 10.1128/mcb.14.2.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quinlivan E B, Holley-Guthrie E A, Norris M, Gutsch D, Bachenheimer S L, Kenney S C. Direct BRLF1 binding is required for cooperative BZLF1/BRLF1 activation of the Epstein-Barr virus early promoter, BMRF1. Nucleic Acids Res. 1993;25:1999–2007. doi: 10.1093/nar/21.8.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ragoczy T, Miller G. The role of the Epstein-Barr virus Rta protein in activation of distinct classes of viral lytic cycle genes. J Virol. 1999;73:9858–9866. doi: 10.1128/jvi.73.12.9858-9866.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ragoczy T, Heston L, Miller G. The Epstein-Barr virus Rta protein activates lytic cycle genes and can disrupt latency in B lymphocytes. J Virol. 1998;72:7978–7984. doi: 10.1128/jvi.72.10.7978-7984.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rickinson A B, Kieff E. Epstein-Barr virus. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2397–2446. [Google Scholar]
- 40.Rodriguez-Viciana P, Warne P H, Khwaja A, Marte B M, Pappin D, Das P, Waterfield M D, Ridley A, Downward J. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- 41.Rooney C M, Rowe D T, Ragot T, Farrell P J. The spliced BZLF1 gene of Epstein-Barr virus (EBV) transactivates an early EBV promoter and induces the virus productive cycle. J Virol. 1989;63:3109–3116. doi: 10.1128/jvi.63.7.3109-3116.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sinclair A, Brimmell M, Shanahan F, Farrell P. Pathways of activation of the Epstein-Barr virus productive cycle. J Virol. 1991;65:2237–2244. doi: 10.1128/jvi.65.5.2237-2244.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun R, Lin S F, Gradoville L, Yuan Y, Zhu F, Miller G. A viral gene that activates lytic cycle expression of Kaposi's sarcoma-associated herpesvirus. Proc Natl Acad Sci USA. 1998;95:10866–10871. doi: 10.1073/pnas.95.18.10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takada K, Shimizu N, Sakuma S, Ono Y. trans activation of the latent Epstein-Barr virus (EBV) genome after transfection of the EBV DNA fragment. J Virol. 1986;57:1016–1022. doi: 10.1128/jvi.57.3.1016-1022.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takada K, Ono Y. Synchronous and sequential activation of latently infected Epstein-Barr virus genomes. J Virol. 1989;63:445–449. doi: 10.1128/jvi.63.1.445-449.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taylor S, Shalloway D. Cell cycle-dependent activation of Ras. Curr Biol. 1996;12:1621–1627. doi: 10.1016/s0960-9822(02)70785-9. [DOI] [PubMed] [Google Scholar]
- 47.van Dam H, Wilhelm D, Herr I, Steffen A, Herrlich P, Angel P. ATF2 is preferentially activated by stress-activated protein kinases to mediate c-Jun induction in response to genotoxic agents. EMBO J. 1995;14:1798–1811. doi: 10.1002/j.1460-2075.1995.tb07168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang J-M, Chao J-R, Chen W, Kuo M-L, Yen J J-Y, Yang-Yen H-F. The antiapoptotic gene mcl-1 is up-regulated by the phosphatidylinositol 3-kinase/Akt signaling pathway through a transcription factor complex containing CREB. Mol Cell Biol. 1999;19:6195–6206. doi: 10.1128/mcb.19.9.6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Y, Huang J, Montalvo E. Characterization of proteins binding to the ZII element in the Epstein-Barr virus BZLF1 promoter: transactivation by ATF1. Virology. 1997;227:323–330. doi: 10.1006/viro.1996.8326. [DOI] [PubMed] [Google Scholar]
- 50.Westphal E-M, Mauser A, Swenson J, Davis M G, Talarico C L, Kenney S C. Induction of lytic Epstein-Barr virus infection in EBV-associated malignancies using adenovirus vectors in vitro and in vivo. Cancer Res. 1999;59:1485–1491. [PubMed] [Google Scholar]
- 51.Zalani S, Holley-Guthrie E, Kenney S. Epstein-Barr viral latency is disrupted by the immediate-early BRLF1 protein through a cell-specific mechanism. Proc Natl Acad Sci USA. 1996;93:9194–9199. doi: 10.1073/pnas.93.17.9194. [DOI] [PMC free article] [PubMed] [Google Scholar]