Abstract
In a previous study, we demonstrated that infected-cell polypeptide 0 (ICP0) is necessary for the efficient reactivation of herpes simplex virus type 1 (HSV-1) in primary cultures of latently infected trigeminal ganglion (TG) cells (W. P. Halford and P. A. Schaffer, J. Virol. 75:3240–3249, 2001). The present study was undertaken to determine whether ICP0 is sufficient to trigger HSV-1 reactivation in latently infected TG cells. To test this hypothesis, replication-defective adenovirus vectors that express wild-type and mutant forms of ICP0 under the control of a tetracycline response element (TRE) promoter were constructed. Similar adenovirus vectors encoding wild-type ICP4, wild-type and mutant forms of the HSV-1 origin-binding protein (OBP), and wild-type and mutant forms of VP16 were also constructed. The TRE promoter was induced by coinfection of Vero cells with the test vector and an adenovirus vector that expresses the reverse tetracycline-regulated transactivator in the presence of doxycycline. Northern blot analysis demonstrated that transcription of the OBP gene in the adenovirus expression vector increased as a function of doxycycline concentration over a range of 0.1 to 10 μM. Likewise, Western blot analysis demonstrated that addition of 3 μM doxycycline to adenovirus vector-infected Vero cells resulted in a 100-fold increase in OBP expression. Wild-type forms of ICP0, ICP4, OBP, and VP16 expressed from adenovirus vectors were functional based on their ability to complement plaque formation in Vero cells by replication-defective HSV-1 strains with mutations in these genes. Adenovirus vectors that express wild-type forms of ICP0, ICP4, or VP16 induced reactivation of HSV-1 in 86% ± 5%, 86% ± 5%, and 97% ± 5% of TG cell cultures, respectively (means ± standard deviations). In contrast, vectors that express wild-type OBP or mutant forms of ICP0, OBP, or VP16 induced reactivation in 5% ± 5%, 8% ± 0%, 0% ± 0%, and 13% ± 6% of TG cell cultures, respectively. In control infections, an adenovirus vector expressed green fluorescent protein efficiently in TG neurons but did not induce HSV-1 reactivation. Therefore, expression of ICP0, ICP4, or VP16 is sufficient to induce HSV-1 reactivation in latently infected TG cell cultures. We conclude that this system provides a powerful tool for determining which cellular and viral proteins are sufficient to induce HSV-1 reactivation from neuronal latency.
The life cycle of herpes simplex virus type 1 (HSV-1) in humans can be divided into three phases: (i) productive replication of virus at the site of primary infection, (ii) establishment and maintenance of latency in sensory neurons, and (iii) periodic reactivation of viral infection from neuronal latency. The first phase, productive replication, is accurately reproduced in vitro in mammalian cell lines, and thus the molecular events that occur during productive HSV-1 replication have been studied extensively (44). The second and third phases of the HSV-1 life cycle, latency and reactivation, respectively, have been experimentally reproduced in animals such as mice, guinea pigs, and rabbits. These models were instrumental in identifying sensory neurons of the peripheral nervous system as the primary sites of HSV-1 latency (52), identifying and characterizing the latency-associated transcripts (LATs) (43, 53), and investigating the physiological stimuli that induce HSV-1 reactivation (19, 28, 48). Because of the problems associated with conducting molecular studies in animals, however, it has proven difficult for investigators to move beyond descriptive and phenomenological observations. Therefore, the molecular mechanisms that control HSV-1 latency and reactivation remain to be elucidated.
Primary trigeminal ganglion (TG) cell cultures were developed as an alternative model in which to study HSV-1 reactivation (16, 29, 36). Although HSV-1 latency is established in mice by conventional methods in this model (18, 28, 48), reactivation is analyzed ex vivo in dissociated cultures of latently infected TG cells. Monolayer cultures are treated transiently with acyclovir (ACV) or other antiviral drugs to repress reactivation during culture establishment (16, 17, 36), and latently infected, nondividing neurons are randomly distributed among dividing support cells (16). After removal of antiviral drugs, reactivation of latent HSV-1 can be induced in 70 to 95% of TG cell cultures by heat stress, and neurons have been shown to be the site of reactivation (16). Intracellular changes that induce HSV-1 reactivation can also be examined using defined, exogenous stimuli such as pharmacological agonists (16) or replication-defective HSV-1 mutants (17).
This report describes the third in a series of three studies aimed at developing new procedures to facilitate the molecular genetic analysis of HSV-1 latency and reactivation in the TG cell culture model. The first study in the series demonstrated that HSV-1 mutants that fail to express infected-cell polypeptide 0 (ICP0) establish reduced levels of latent ICP0− genomes in mouse TG cells but are capable of establishing wild-type levels of genomes in TG cells if mice are transiently immunosuppressed at the time of infection (18). Using this procedure, it was possible in the second study to measure the effect of the absence of ICP0 function on reactivation efficiency and thus demonstrate that ICP0 is necessary for the efficient reactivation of HSV-1 from neuronal latency (17). Although ICP0 may be required for many steps during the reactivation process, its well-recognized role as a global transcriptional activator suggested a direct role for this protein as an initiator of HSV-1 reactivation (3, 10). Therefore, in this study, the question of whether ICP0 is sufficient to trigger HSV-1 reactivation from neuronal latency was posed.
To address this question, an experimental system that allows specific gene products to be tested for their capacity to trigger HSV-1 reactivation was developed. Although similar in principle to past studies with human embryonic lung (HEL) cells (20, 59), the present study involved the use of replication-defective adenovirus vectors (58) to deliver ICP0 to neurons latently infected with HSV-1. While the in vitro milieu in primary TG cell cultures is decidedly different from the in vivo environment, the target cells are the same terminally differentiated neurons present in mice latently infected with HSV-1. The results of this study demonstrate that adenovirus vectors themselves do not induce reactivation but can deliver proteins to cultured TG neurons efficiently. Using this experimental system, specific HSV-1 proteins of the three major kinetic classes were tested for their capacity to induce reactivation of latent viral genomes in TG cell cultures by measuring the production of new infectious virus. Although the HSV-1 origin-binding protein (OBP; an early protein) did not induce reactivation, immediate-early (IE) proteins ICP0 and ICP4 and late virion protein 16 (VP16) were sufficient, individually, to induce reactivation of latent HSV-1 genomes. Therefore, the combination of latently infected TG cell cultures and adenovirus vectors should prove useful for examination of the molecular mechanisms that control the balance between HSV-1 latency and reactivation.
MATERIALS AND METHODS
Cells and viruses.
Vero and 293 cells (American Type Culture Collection, Manassas, Va.) were propagated in Dulbecco's modified Eagle medium containing 0.375% HCO3−, 10% fetal bovine serum, penicillin G (100 U/ml), streptomycin (100 mg/ml), and 2 mM l-glutamine (complete DMEM). Likewise, ICP0-complementing L7 cells (46), ICP4-complementing E5 cells (8), and OBP-complementing 2B.11 cells (31) were maintained in complete DMEM. TG cell cultures were prepared and maintained in TG cell medium, which is minimal essential medium containing 0.075% HCO3−, 10% fetal bovine serum, penicillin G (100 U/ml), streptomycin (100 mg/ml), 2 mM l-glutamine, and 2.5S nerve growth factor (10 ng/ml) as previously described (17). Wild-type HSV-1 strain KOS (passage 12 from human isolation) was used in this study and propagated in Vero cells in complete DMEM as described previously (50). The ICP0− mutant virus 7134 (1) and the ICP4− mutant virus n12 (9) were propagated as previously described. The OBP− mutant virus hr94 and complementing cell line 2B.11 were kindly provided by Sandra Weller (University of Connecticut Health Sciences Center, Farmington), and hr94 was propagated on 2B.11 cells as previously described (31). The VP16− mutant virus RP5 (54), kindly provided by Rath Pichyangkura and Steve Triezenberg (Michigan State University, East Lansing), was propagated on ICP4-complementing E5 cells in the presence of 5 mM hexamethylenebisacetamide (HMBA), a compound that complements the replication of ICP0− and VP16− mutants in vitro (33, 41). The parental adenovirus vector H5.010CMVEGFP (5), hereafter referred to as Ad.C-GFP, was obtained from the Institute of Human Gene Therapy at the University of Pennsylvania School of Medicine, Philadelphia. Adenovirus vectors were propagated in 293 cells (0.03 PFU/cell) in complete DMEM, and infected cells were incubated at 34°C for 4 to 5 days. Adenovirus vector-infected cells were pelleted by low-speed centrifugation, resuspended in one-fifth the original volume, subjected to freezing and thawing, sonicated, and centrifuged to produce clarified lysates with virus titers ranging from 0.5 × 109 to 1.5 × 109 PFU/ml, as determined by plaque assay on 293 cells.
Construction of recombinant adenovirus vectors.
Recombinant adenovirus vectors were constructed according to the procedure of Davis et al. (5). Briefly, the adenovirus vector Ad.C-GFP was propagated at a multiplicity of infection (MOI) of 0.03 PFU/cell in ∼8 × 108 293 cells, and adenovirus virions were purified in CsCl gradients. DNA was isolated from Ad.C-GFP virions, and ClaI digestion was performed to remove the cytomegalovirus (CMV) promoter-green fluorescent protein (GFP) cassette from the 5′ end of the virus genome (6). Recombinant adenovirus vectors were generated by cotransfecting 293 cells with EcoRI-digested pBHad plasmids and ClaI-digested Ad.C-GFP genomes. Specifically, 293 cells were seeded in collagen-coated six-well plates at a density of 3.5 × 105 per well, and 16 h later, 0.4 μg of ClaI-digested adenovirus DNA and 0.5 μg of EcoRI-digested plasmid DNA were precipitated by the calcium phosphate method. The precipitate was incubated with 293 cells for 8 h. After transfection of the cells, the medium was replaced with complete DMEM containing 0.2% methyl cellulose and the plates were incubated at 37°C for 6 to 9 days. Plaques produced by recombinant adenovirus vectors were identified, using a fluorescence microscope, by green/white plaque selection, and white plaques were plaque purified three times. The identity of each recombinant adenovirus vector was confirmed by PCR and Southern blot analysis of DNA from infected 293 cells.
High-fidelity PCR.
Several DNA sequences used in the construction of pBHad, pBHad-TRE, pBT-OBP, and pBT-VP16 were generated by PCR. The introduction of mutations into these 1- to 3-kb segments of DNA was minimized by choosing PCR conditions that maintained high fidelity, which included (i) the use of 5 U of high-fidelity Expand polymerase mixture (Roche Molecular Biochemicals, Indianapolis, Ind.), 200 μM each deoxynucleoside triphosphate, and 100 ng of each primer; (ii) the presence of a high concentration of DNA template; and (iii) incubation of samples for 20 thermal cycles of 94°C for 1.25 min, 57°C for 1.5 min, and 72°C for 2.5 min.
Construction of adenovirus shuttle plasmids.
The adenovirus shuttle vector pAd-CMV-Link1 was obtained from the Institute of Human Gene Therapy at the University of Pennsylvania School of Medicine. Using this plasmid as a template, we constructed a new set of adenovirus shuttle plasmids in which the low-copy-number pAT153 vector backbone was eliminated and the multicloning site was streamlined such that promoter sequences and open reading frames could be readily introduced into the gene cassette.
(i) pBHad.
The pUC-based adenovirus shuttle vector pBHad was constructed as follows. After deletion of the CMV promoter from pAd-CMV-Link1 by the use of EcoRI and BglII, a 531-bp PCR product (pAd-left) that contained adenovirus map units (m.u.) 0 to 1 and the 5′ end of the multicloning site and a 2,700-bp PCR product (pAd-right) that contained the simian virus 40 polyadenylation signal and adenovirus m.u. 9 to 16 were amplified. After PCR, pAd-left and pAd-right were digested with KpnI, ligated with T4 DNA ligase, and used as a template for high-fidelity PCR with outer primers that amplified a new 3.1-kb adenovirus gene cassette. The resulting 3.1-kb PCR product was cloned into the TA vector (Invitrogen, Carlsbad, Calif.), sequenced, and subcloned into the EcoRI site of a modified pUC 18 vector (i.e., everything but the EcoRI site was deleted with SacI and NarI) to create the new adenovirus shuttle plasmid pBHad.
(ii) pBHad-TRE and pBHad-CMV.
Using pRev-TRE (Clontech Laboratories Inc., Palo Alto, Calif.) as a template, a tetracycline response element (TRE) promoter was amplified by high-fidelity PCR using 5′ and 3′ primers that resulted in the addition of SbfI and PmlI sites, respectively. Using these restriction enzymes, the TRE promoter was cloned into pBHad to generate pBHad-TRE. Using NheI and NotI, the CMV promoter of pAd-CMV-link1 was subcloned into pBHad to generate pBHad-CMV.
(iii) pBC-rtTA.
The reverse tetracycline-regulated transactivator (rtTA; also known as Tet-On 153) coding sequence was subcloned from pRevTet-On (Clontech Laboratories Inc.) by digestion with BamHI, treatment with T4 DNA polymerase to create a blunt end, digestion with ClaI, and ligation of the gel-purified rtTA fragment into pBHad-CMV after it had been digested with SbfI, treated with T4 DNA polymerase, and digested with ClaI. The resulting plasmid was designated pBC-rtTA.
(iv) pBT-ICP0 and pBT-n212.
Wild-type and mutant (n212) alleles of the ICP0 coding sequences from HSV-1 strain KOS were subcloned from plasmids pSH and pn212 (2), respectively, by digestion with DrdI, treatment with T4 DNA polymerase to create a blunt end, digestion with HindIII, and ligation of the gel-purified ICP0 and n212 fragments into EcoRV- and HindIII-digested pBHad-TRE to create pBT-ICP0 and pBT-n212, respectively.
(v) pBT-ICP4.
A wild-type allele of the ICP4 coding sequence was subcloned from pn11 (9) by digestion with HpaI, treatment with T4 DNA polymerase to create a blunt end, digestion with AgeI, and ligation of the gel-purified ICP4 fragment into AgeI- and EcoRV-digested pBHad-TRE to create pBT-ICP4.
(vi) pBT-OBP and pBT-OBPψ.
Wild-type and mutant alleles of the OBP coding sequence of strain KOS were generated by PCR amplification of HSV-1 strain KOS DNA with the primers OBP-clone-a (5′-GTCCGAGATCTGGTCATGCCTTTCGTG) and OBP-clone-b (5′-CCGAACGAAAGCTTTCCCGAGGACTTATAGG). These primers generated a 2,594-bp PCR product with BglII and HindIII sites (italicized in primer sequences) at the 5′ and 3′ ends, respectively. Random mutations were generated in the mutant OBP PCR product by using PCR conditions that favored the introduction of point mutations into the coding sequence (i.e., Taq polymerase, 30 thermal cycles), whereas the wild-type OBP PCR product was amplified by high-fidelity PCR. The wild-type sequence was identical to the OBP gene in HSV-1 strain KOS, as determined by DNA sequencing, and was subcloned into pBHad-TRE to create pBT-OBP. Relative to the HSV-1 genome, the mutant OBP sequence contained point mutations at nucleotide positions 20980, 22078, and 22361, which generated three amino acid changes in OBP (T761I, S395G, and C301R, respectively). This OBP triple point mutant was designated OBPψ, and pBT-OBPψ is the pBHad-TRE plasmid that carries this insert.
(vii) pBT-VP16 and pBT-VP16Δ.
The wild-type VP16 PCR product was amplified from HSV-1 strain KOS using the primers VP16-clone-a (5′-ATCGGATCCACCCAATGGACCT) and VP16-clone-b (5′-GCAGGTTTTGTAATGTATGTGCTCGTG), which generated a 1,584-bp PCR product that contained a BamHI site at the 5′ end of the VP16 coding sequence. A PCR product in which amino acids 417 to 490 of the VP16 acidic transactivation domain (Δ417–490) had been eliminated was amplified using primers VP16-clone-a and VP16-mut-b (5′-GTCCCCCAGGCTATCATCGGTC), which generated a 1,280-bp PCR product that also contained a 5′ BamHI site and introduced two sequential stop codons that followed codon 416 of the VP16 coding sequence. Both PCR products were amplified by high-fidelity PCR as described above, digested with BamHI, and ligated into BglII- and EcoRV-digested pBHad-TRE to create pBT-VP16 and pBT-VP16Δ. DNA sequence analysis confirmed that no mutations were introduced during the construction of these two plasmids.
Southern, Northern, and Western blot analyses of recombinant adenovirus vectors. (i) Southern blot analysis.
293 cells were infected with 2.5 PFU of each adenovirus vector/cell, and total DNA was harvested at 24 h postinfection (p.i.). DNA samples from cells infected with Ad.C-GFP or Ad.C-rtTA (3 μg) were digested with ClaI, and DNA samples from cells infected with all other adenovirus vectors were digested with HindIII. Following electrophoresis on a 0.8% agarose gel, DNA was blotted onto a nylon membrane, using 0.5 M NaOH–0.6 M NaCl as the transfer buffer, and the blot was irradiated with 0.2 J of UV radiation/cm2 in a UV cross-linker (Stratagene, La Jolla, Calif.). An oligonucleotide probe specific for the 5′ end of the adenovirus vector genome (5′-AGGCGGATGTTGTAGTAAATTTGGGCGTAACCGAGTAAGA-3′) was 3′-end labeled with [α-32P]dATP by using terminal transferase (Promega Corp., Madison, Wis.), and the probe was hybridized to the blot at 50°C for 16 h in a hybridization solution containing 2 ng of labeled probe/ml, 7% sodium dodecyl sulfate (SDS), 120 mM NaH2PO4, and 250 mM NaCl. Excess probe was removed from the membrane by rinsing in 0.1× standard saline citrate containing 0.1% SDS. Labeled DNA was visualized with a PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.) and analyzed with ImageQuant 3.3 software (Molecular Dynamics).
(ii) Northern blot analysis.
Vero cells infected at multiplicities of 3 to 300 PFU of Ad.T-OBP or Ad.T-ICP0/cell were incubated in the presence of 0 to 10 μM doxycycline (DOX; Sigma Chemical Co., St. Louis, Mo.), and RNA was harvested with Ultraspec RNA isolation reagent (Biotecx Inc., Houston, Tex.). Equal amounts of total RNA (10 μg) were electrophoretically separated on a formaldehyde-containing, 1.2% agarose gel according to the procedure of Maniatis et al. (32). Following electrophoresis, RNA was vacuum blotted onto a nylon membrane, using 10× standard saline citrate as a transfer buffer, and blots were irradiated with 0.2 J of UV radiation/cm2 in a UV cross-linker (Stratagene). Oligonucleotide probes specific for ICP0 (5′-AGGTCGTCGTCATCCTCGTCCGTGGTGGGCTCCGGGTGGG-3′) and OBP (5′-AATAAACCGCGTGCGTCCCATCAGGCTGTTGAGGTTGCGC-3′) were 3′-end labeled with terminal transferase (Promega Corp.) and [α-32P]dATP. Hybridization, washes, and image acquisition were performed as described above for Southern blot analysis.
(iii) Western blot analysis.
Vero cells were infected with 10 PFU of KOS or Ad.C-GFP/cell or were coinfected with Ad.C-rtTA at an MOI of 10 and Ad.T-OBP, Ad.T-OBPψ, Ad.T-n212, or Ad.T-ICP0 at an MOI of 40. Cultures were incubated in the absence or presence of 3 μM DOX. Total cell protein was harvested at 24 h p.i. in 500 μl of denaturing buffer (50 mM Tris, 100 mM dithiothreitol, 2% SDS, 10% glycerol, 0.1% bromophenol blue) and boiled for 10 min, and then 100 μg of each protein sample was resolved by electrophoresis in a discontinuous 7.5% polyacrylamide (19:1 acrylamide/bisacrylamide ratio) gel with a 4.5% stacking gel. Proteins were transferred to nitrocellulose, blocked in phosphate-buffered saline containing 0.05% Tween 20 and 5% dry milk (TM-PBS), and incubated overnight with ICP0- or OBP-specific antibodies in TM-PBS. Membranes were washed with TM-PBS, incubated with a 1:5,000 dilution of a horseradish peroxidase-labeled goat anti-rabbit secondary antibody (Pierce Chemical Co., Rockford, Ill.), washed with TM-PBS, and incubated in enhanced chemiluminescence reagent (Pierce Chemical Co.) Chemiluminescence was visualized by exposure to film. J17 rabbit polyclonal antiserum against ICP0, generated by Wendy Sacks (45), was used at a 1:500 dilution in TM-PBS. RH7 rabbit polyclonal antiserum against amino acids 531 of 851 of OBP (35) was generously provided by Deborah Parris (Ohio State University, Columbus) with permission from Daniel Tenney (Bristol-Meyers Squibb, Wallingford, Conn.). RH7 was used at a dilution of 1:1,000 in TM-PBS.
Analysis of reactivation in TG cell cultures.
Male ICR mice (6 to 8 weeks old; mean weight ± standard deviation, 29 ± 2 g) were obtained from Harlan-Sprague Dawley (Indianapolis, Ind.) and handled in accordance with The Guide for the Care and Use of Laboratory Animals (22). Mice were anesthetized by intraperitoneal administration of xylazine (6.6 mg/kg of body weight) and ketamine (100 mg/kg). Mice were infected with HSV-1 by corneal scarification of both eyes with a 26-gauge needle, blotting of tear film from the eyes with a tissue, and application of 3 μl of virus inoculum containing 2 × 105 PFU of KOS on each eye. Primary cultures of TG cells were prepared as previously described (17). In brief, TG were removed from mice 30 days after inoculation and dissociated to form a cell suspension, and the cells from ∼50% of a mouse TG were placed in each well of a 24-well plates in TG cell medium containing 200 μM ACV. After 7 days in culture, by which time TG cells had formed an adherent monolayer, the ACV-containing medium was removed, and cultures were incubated thereafter in TG cell medium lacking ACV. To test for the presence of infectious HSV-1, 100 μl of TG cell culture medium was transferred to freshly seeded monolayers of Vero indicator cells on day 7 as well as every day from days 10 to 20.
Infection of TG cell cultures with adenovirus vectors to assess their ability to induce reactivation was performed on day 10 as follows. In all cultures, a cell density of 3 × 105 per well of a 24-well plate was estimated. TG cell cultures were coinfected with Ad.C-rtTA (MOI = 10) and TRE-regulated adenovirus vectors (MOI = 40) (e.g., 3 × 106 PFU of Ad.C-rtTA and 1.2 × 107 PFU of Ad.T-ICP0). Infections were performed by (i) aspirating the medium from each culture, (ii) infecting each monolayer with viral vectors in a volume of 200 μl, (iii) allowing 1 h for viral adsorption, (iv) aspirating the inoculum from each well, (v) rinsing with 0.5 ml of TG cell medium, and (vi) adding 1.5 ml of TG cell medium containing 3 μM DOX to each culture to induce expression from the TRE promoter. In dose-response experiments, TG cell cultures received medium containing 0, 0.1, or 3 μM DOX.
Nucleotide sequence accession numbers.
The nucleotide sequences of pBHad, pBHad-TRE, and pBHad-CMV have been filed with GenBank under accession no. AF326319, AF326320, and AF326321, respectively.
RESULTS
An adenovirus vector that delivers GFP to cultured TG neurons does not induce HSV-1 reactivation.
Primary TG cell cultures are a heterogeneous mixture of many morphologically distinct cell types and contain 250 to 400 neurons per culture when established in 24-well plates (16). Adenovirus expression vectors would provide a useful means of examining the effects of specific viral and cellular gene products on HSV-1 latency in latently infected TG cell cultures if these vectors were capable of delivering a gene product efficiently to TG neurons and did not themselves induce reactivation of latent HSV-1. Ad.C-GFP, an adenovirus vector that constitutively expresses GFP, was used to test this hypothesis.
Examination of TG cell cultures infected with Ad.C-GFP by fluorescence microscopy demonstrated that this replication-defective viral vector delivered GFP efficiently to TG neurons in culture (Fig. 1A and B). Identification of fluorescing cells as neurons was made on the basis of their characteristic morphology, i.e., large, single cells that contain a darkly pigmented cytoplasm and a prominent nucleus. In a previous study, we found that 100% of cells identified as neurons on the basis of their morphology expressed neuron-specific enolase when tested by immunocytochemistry (16). Interestingly, neurons appeared to express the highest levels of GFP in primary TG cell cultures. In contrast, the fibroblasts and most of the support cells that predominate in these cultures expressed low to undetectable levels of GFP (Fig. 1A and B). The reason for low-level GFP expression in these cells is unknown, but either adenovirus entry or expression of the adenovirus transgene may be inefficient.
FIG. 1.
Expression of GFP from an adenovirus vector in TG cell cultures latently infected with HSV-1. (A and B) Representative photomicrographs of a neuron in a latently infected TG cell culture 72 h after superinfection with 40 PFU of Ad.C-GFP/cell, as seen when illuminated with visible light (A) or the 360- to 400-nm spectrum of light, which excites GFP fluorescence (B). Magnification, ×40. (C) Frequency of HSV-1 reactivation in latently infected TG cell cultures superinfected with 20 or 100 PFU of Ad.C-GFP/cell, with or without heat stress (HS) (n = 12 per group). ACV off, point at which ACV was removed from culture.
To determine whether Ad.C-GFP is able to induce reactivation, two 24-well plates of TG cell cultures latently infected with HSV-1 were established in the presence of ACV. On day 10 (3 days after ACV removal), half the TG cell cultures in each plate were infected with either 20 or 100 PFU of Ad.C-GFP/cell. After infection, one of the two plates was subjected to heat stress at 43°C for 3 h, a known reactivation stimulus, and the presence of infectious virus in cultures in both plates was monitored over the next 10 days. In TG cells infected with 20 or 100 PFU/cell of Ad.C-GFP, reactivation was detected in 8 and 0% of cultures, respectively (Fig. 1C). In contrast, reactivation occurred in 80% of cultures that were heat stressed after infection (Fig. 1C). Therefore, although Ad.C-GFP can deliver GFP efficiently to neurons in TG cell cultures, it is a poor inducer of HSV-1 reactivation.
Construction of adenovirus vectors that express ICP0, ICP4, OBP, or VP16.
The combination of the inducible TRE promoter and rtTA, which was originally developed by Gossen and Bujard (15), has since been integrated into adenovirus vectors by other investigators (4, 34). In the present study, adenovirus vectors that expressed wild-type or mutant forms of HSV-1 proteins of the three major kinetic classes were constructed under the control of the TRE promoter. The coding sequences for wild-type strain KOS alleles of ICP0, ICP4, VP16, and OBP were cloned into the adenovirus shuttle plasmid pBHad-TRE (Fig. 2A), as were mutant alleles of ICP0 (n212) (2), VP16Δ (Δ417–490), and OBPψ (C301R, S395G, T761I). The coding sequence of rtTA was cloned into a second adenovirus shuttle plasmid, pBHad-CMV. Recombinant adenovirus vectors were generated by cotransfecting 293 cells with each gene-containing pBHad plasmid and ClaI-digested Ad.C-GFP DNA (i.e., the recipient adenovirus genome).
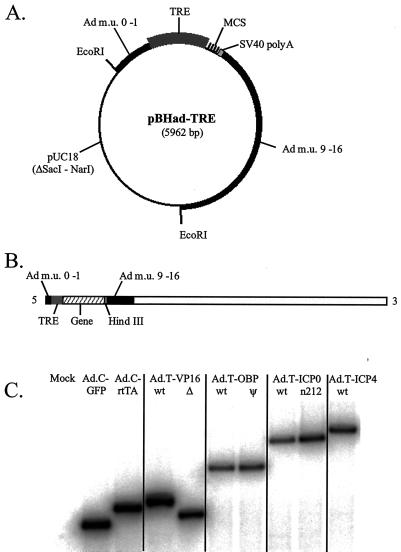
FIG. 2.
Construction of recombinant adenovirus vectors. (A) Map of the adenovirus shuttle plasmid pBHad-TRE. Two EcoRI sites separate the pUC18 vector backbone from the adenovirus gene cassette that contains (from 5′ to 3′) human adenovirus type 5 m.u. 0 to 1, the TRE promoter, a multicloning site (MCS), a simian virus 40 polyadenylation sequence (SV40 polyA), and human adenovirus type 5 DNA including m.u. 9 to 16. (B) Map of a typical recombinant adenovirus vector. This diagram also illustrates the strategy by which the adenovirus gene cassette of pBHad-TRE is introduced into the adenovirus vector genome by homologous recombination as described in the text. (C) Southern blot analysis of recombinant adenovirus vectors. Total DNA (3 μg) from adenovirus vector-infected 293 cells was digested with HindIII, separated on a 0.8% agarose gel, blotted, and hybridized to a 32P-labeled oligonucleotide specific for adenovirus m.u. 0 to 1. DNA samples were isolated from mock-infected 293 cells (Mock) or 293 cells infected with Ad.C-GFP, Ad.C-rtTA, Ad.T-VP16 (wild type [wt]) or Ad.T-VP16Δ, Ad.T-OBP (wild type) or Ad.T-OBPψ, Ad.T-ICP0 (wild type) or Ad.T-n212 (n212), and Ad.T-ICP4 (wild type).
Recombinant adenovirus vectors were identified through green/white selection by fluorescence microscopy (5). To confirm that each white-plaque isolate (i.e., recombinant adenovirus vector) contained a gene cassette of the predicted molecular weight, total DNA from infected 293 cells was digested with HindIII to release the cassette from the 5′ end of the adenovirus vector genome (Fig. 2B). Southern blot analysis verified that the rtTA adenovirus vector and TRE-regulated adenovirus vectors Ad.T-VP16, Ad.T-VP16Δ, Ad.T-OBP, Ad.T-OBPψ, Ad.T-ICP0, Ad.T-n212, and Ad.T-ICP4 each contained a gene cassette of the predicted molecular weight (Fig. 2C).
The efficiency of HSV-1 gene expression from TRE-regulated adenovirus vectors is dependent on MOI and DOX concentration. (i) Northern blot analysis.
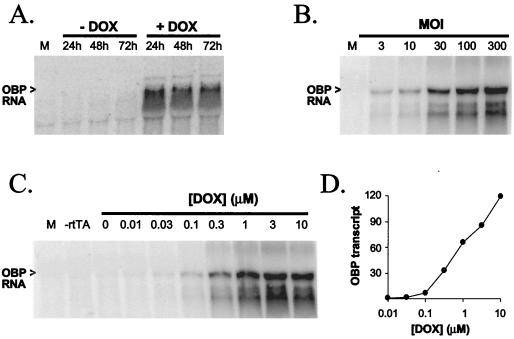
Vero cells infected with Ad.T-OBP or Ad.T-ICP0 were analyzed by Northern blot analysis in a series of experiments to determine (i) the amount of RNA transcribed from representative adenovirus vectors between 24 and 72 h p.i., (ii) the effect of MOI on the amount of RNA transcribed, and (iii) the effect of DOX concentration on rtTA-stimulated transcription from the TRE promoter. In the first series of experiments, when transcription from Ad.T-OBP (MOI = 100) was induced with Ad.C-rtTA (MOI = 10) and 1 μM DOX, OBP transcript levels were similar at 24, 48, and 72 h p.i. and were at least 50-fold higher than in cultures not treated with DOX (Fig. 3A). Similarly, when transcription from Ad.T-ICP0, Ad.T-n212, or Ad.T-OBP (MOI = 30) was induced with Ad.C-rtTA (MOI = 10) and 1 μM DOX, transcript levels were constant at 24, 48, and 72 h p.i. (data not shown). In a second experiment, levels of OBP transcripts increased as a linear function of the MOI of Ad.T-OBP when measured at 24 h p.i. (Fig. 3B). Finally, when Vero cells were infected with a constant amount of Ad.T-OBP (MOI = 100) and Ad.C-rtTA (MOI = 10), OBP transcript levels at 24 h p.i. increased as a function of the concentration of DOX in the culture medium (Fig. 3C). Moreover, OBP transcript levels increased as a linear function of DOX concentration in the range of 0.1 and 10 μM (Fig. 3D). In Vero cells infected with 30 PFU of Ad.T-ICP0 or Ad.T-n212/cell, gene expression was also dependent on DOX concentration (data not shown).
FIG. 3.
Northern blot analysis of transcription from Ad.T-OBP. Total RNA (10 μg) isolated from mock-infected or Ad.T-OBP-infected Vero cells was separated on 1.2% formaldehyde agarose gels, blotted, and hybridized to 32P-labeled oligonucleotides specific for OBP. (A) Kinetics of OBP transcription from Ad.T-OBP. In addition to being isolated from mock-infected Vero cells (M), total RNA was isolated from Vero cells coinfected with Ad.C-rtTA (MOI = 10) and Ad.T-OBP (MOI = 100), which were (+) or were not (−) treated with 1 μM DOX and then were harvested at 24, 48, and 72 h p.i. (B) Effect of multiplicity of the adenovirus vector on OBP transcript levels. From left to right, RNA samples were isolated at 24 h p.i. from mock-infected Vero cells or Vero cells treated with Ad.C-rtTA (MOI = 10) and 1 μM DOX and infected with 3, 10, 30, 100, or 300 PFU of Ad.T-OBP/cell. (C and D) Effect of DOX concentration on OBP transcript levels. (C) From left to right, RNA samples were isolated at 24 h p.i. from mock-infected Vero cells, Vero cells infected with 100 PFU of Ad.T-OBP/cell only (−rtTA), or Vero cells treated with 0 to 10 μM DOX after coinfection with Ad.C-rtTA (MOI = 10) and Ad.T-OBP (MOI = 100). (D) OBP transcript levels plotted as a function of DOX concentration in Vero cells coinfected with Ad.C-rtTA (MOI = 10) and Ad.T-OBP (MOI = 100).
(ii) Western blot analysis.
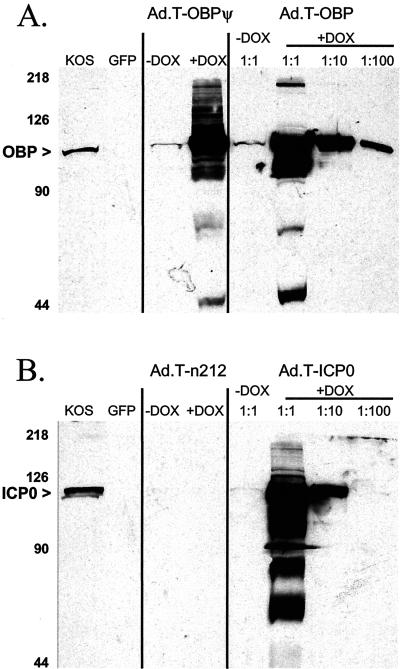
The quantities and molecular weights of OBP and ICP0 expressed from adenovirus vectors in Vero cells were compared with those of OBP and ICP0 produced in cells infected with wild-type HSV-1 strain KOS by Western blot analysis (Fig. 4). OBPψ and wild-type OBP expressed from adenovirus vectors migrated to the same position as OBP expressed in KOS-infected Vero cells (Fig. 4A). No cross-reactivity was observed between the anti-OBP antibody and nonspecific proteins present in Vero cells infected with Ad.C-GFP (Fig. 4A). In the presence of 3 μM DOX and Ad.C-rtTA, high levels of expression of mutant OBPψ and wild-type OBP were achieved in Vero cells (Fig. 4A). In the absence of DOX, the levels of OBPψ and OBP were less than 1% of the levels observed in DOX-treated cultures (Fig. 4A).
FIG. 4.
Western blot analysis of expression of wild-type and mutant OBP and ICP0 from adenovirus vectors. Vero cells were infected with KOS and Ad.C-rtTA, each at an MOI of 10 PFU/cell, whereas infection with all other TRE-regulated adenovirus vectors was carried out at 40 PFU/cell. (A) Western blot analysis of OBP. Total protein was isolated from Vero cells 24 h after infection with (left to right) wild-type HSV-1 (KOS), Ad.C-GFP (GFP), Ad.C-rtTA and Ad.T-OBPψ, or Ad.C-rtTA and Ad.T-OBP in the absence (−) or in the presence (+) of 1 μM DOX. To facilitate quantitative comparison of OBP levels, three dilutions of the protein sample obtained from the culture with DOX were electrophoresed on the gel (1:1, 1:10, and 1:100). (B) Western blot analysis of ICP0. Total protein was isolated from Vero cells 24 h after infection with wild-type HSV-1 (KOS), Ad.C-GFP (GFP), Ad.C-rtTA and Ad.T-n212, or Ad.C-rtTA and Ad.T-ICP0 in the absence or in the presence of 1 μM DOX. To facilitate quantitative comparison of ICP0 levels, three dilutions of the protein sample obtained from the DOX-containing culture were electrophoresed on the gel (1:1, 1:10, and 1:100). The numbers to the left of the gels indicate the positions of molecular size markers (in kilodaltons).
Similar results were obtained with Ad.T-ICP0. In the presence of 3 μM DOX and Ad.C-rtTA, wild-type ICP0 expressed from Ad.T-ICP0 migrated to the same position as ICP0 expressed in KOS-infected Vero cells (Fig. 4B). The predicted 211-amino-acid peptide encoded by Ad.T-n212 was not detected in the Western blot, presumably because proteins of less than ∼35 kDa ran off the gel (Fig. 4B). In the absence of DOX, the level of ICP0 expressed from Ad.T-ICP0 in Vero cells was ∼1 to 3% of that observed in DOX-treated cultures (Fig. 4B).
Complementation of HSV-1 mutants by ICP0, ICP4, OBP, and VP16 adenovirus vectors.
Based on tests with Ad.C-GFP, it was evident that adenovirus vectors delivered GFP to over 95% of Vero cells infected at an MOI of 30 or greater (data not shown). Therefore, each adenovirus vector was tested for its ability to complement plaque formation by HSV-1 ICP0−, ICP4−, OBP−, and VP16− mutants on Vero cells. Vero cells were infected with ∼100 to 200 PFU of KOS or KOS-derived virus strains with a mutation in the gene encoding ICP0, ICP4, OBP, or VP16 and then superinfected with 10 PFU of adenovirus vector expressing wild-type or mutant forms of the corresponding proteins/cell. Wild-type KOS produced an average of 128 plaques on Vero cell monolayers, and similar numbers of plaques were produced on Vero cell monolayers superinfected with vectors expressing ICP0, n212, OBP, OBPψ, or VP16 (Table 1). In contrast, superinfection of KOS-infected Vero cells with vectors expressing ICP4 or VP16Δ produced a dominant-negative effect, because the numbers of KOS plaques were reduced by ∼50% relative to nonsuperinfected controls (Table 1). The ICP0− mutant, 7134, produced an average of 62 plaques on monolayers of ICP0-complementing L7 cells but only 1 plaque on Vero cell monolayers. Although the adenovirus vectors expressing n212, ICP4, OBP, OBPψ, VP16, or VP16Δ did not complement 7134 plaque formation, superinfection of 7134-infected Vero cells with Ad.T-ICP0 allowed the formation of an average of 90 plaques on Vero cell monolayers. Likewise, the ICP4− and OBP− mutants (n12 and hr94, respectively) produced plaques only on Vero cells superinfected with Ad.T-ICP4 and Ad.T-OBP, respectively (Table 1). The VP16− mutant, RP5, produced on average 148 plaques on HMBA-treated E5 cells but only on average 3 plaques on nonsuperinfected Vero cells. In Vero cells superinfected with Ad.T-ICP0 or Ad.T-VP16, however, RP5 produced averages of 186 and 108 plaques, respectively (Table 1). Thus, both ICP0 and VP16 complemented plaque formation by the VP16− mutant in Vero cell monolayers. In contrast, the mutant proteins encoded by Ad.T-n212, Ad.T-OBPψ, and Ad.T-VP16Δ did not complement plaque formation by any of the HSV-1 mutants in Vero cells (Table 1). Therefore, we conclude that the HSV-1 wild-type proteins encoded by Ad.T-ICP0, Ad.T-ICP4, Ad.T-OBP, and Ad.T-VP16 are functional.
TABLE 1.
Complementation of HSV-1 viruses with mutations in ICP0, ICP4, OBP, or VP16 by adenovirus expression vectors
| Complementing vector | No. of plaques produced bya:
|
||||
|---|---|---|---|---|---|
| KOS | 7134 | n12 | hr94 | RP5 | |
| Controls | |||||
| None (Vero cells) | 128 ± 2 | 1 ± 0 | 0 ± 0 | 0 ± 0 | 3 ± 1 |
| None (complementing cells)b | 62 ± 6∗ | 114 ± 6∗ | 104 ± 5∗ | 148 ± 5∗ | |
| Test vectorsc | |||||
| Ad.T-ICP0 | 110 ± 2 | 90 ± 5∗ | 0 ± 0 | 0 ± 0 | 186 ± 8∗ |
| Ad.T-n212 | 122 ± 10 | 1 ± 1 | 0 ± 0 | 0 ± 0 | 9 ± 1 |
| Ad.T-ICP4 | 66 ± 11 | 1 ± 1 | 115 ± 7∗ | 0 ± 0 | 22 ± 1 |
| Ad.T-OBP | 99 ± 7 | 1 ± 0 | 0 ± 0 | 454 ± 14∗ | 8 ± 0 |
| Ad.T-OBPΨ | 97 ± 8 | 1 ± 1 | 0 ± 0 | 0 ± 0 | 6 ± 1 |
| Ad.T-VP16 | 111 ± 2 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 108 ± 6∗ |
| Ad.T-VP16Δ | 57 ± 1 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 6 ± 1 |
| Ad.C-rtTA only | 112 ± 12 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 4 ± 1 |
Vero cells in six-well plates were infected with 100 to 200 PFU of wild-type HSV-1 strain KOS or KOS-derived mutant 7134 (ICP0−), n12 (ICP4−), hr94 (OBP−), or RP5 (VP16−). Mean numbers of HSV plaques (± standard deviations) observed on untreated Vero cells (n = 2 per group), on complementing cells (n = 3 per group), or on Vero cells superinfected with one of eight adenovirus vectors (n = 2 per group) are given. The values for primary tests in which HSV-1 mutants were complemented with adenovirus vectors that provided the missing gene products in trans are shown in boldface type. Values with asterisks represent a more than 30-fold increase in the number of plaques formed on Vero cells relative to no-vector controls.
Mutant viral inocula were plated on the following complementing cell lines: 7134, on L7 cells; n12, on E5 cells; hr94, on 2B.11 cells; and RP5, on E5 cells treated with 5 mM HMBA.
Vero cells were coinfected with 10 PFU of Ad.C-rtTA/cell and 10 PFU of the indicated TRE adenovirus vector/cell and were overlaid with complete DMEM containing 1% methyl cellulose and 3 μM DOX.
Adenovirus vectors expressing ICP0, ICP4, or VP16 induce HSV-1 reactivation in latently infected TG cell cultures.
Having shown that adenovirus vectors expressed functional (wild-type) and nonfunctional (mutant) proteins, we tested the ability of individual HSV-1 proteins to induce reactivation. Primary TG cell cultures were established in the presence of ACV by using TG from mice latently infected with wild-type HSV-1 strain KOS. On day 10 (3 days after ACV removal), cultures were infected with adenovirus vectors, and culture supernatants were monitored daily through day 20 p.i. for the presence of infectious HSV-1. In three independent tests, infectious HSV-1 was detected in 11% of mock-superinfected TG cell cultures by day 20 (Table 2). Likewise, in other control superinfections, virus was detected in 13 and 17% of cultures superinfected with Ad.C-GFP and Ad.C-rtTA, respectively (Table 2). Collectively, the results from these tests, involving a total of 84 TG cell cultures, defined the background rate (mean ± standard deviation) of reactivation in TG cell cultures as 12% ± 3% for wild-type HSV-1 strain KOS (Table 2).
TABLE 2.
Adenovirus vector-induced reactivation of HSV-1 in TG cell cultures
| Treatment | Reactivation frequency in expt:
|
Total no. reactivated/ total culturese (mean % ± SD) | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | |||
| Negative controls | |||||
| Mock | 1/12 | 2/12 | 1/12 | 4/36 (11 ± 5) | |
| Ad.C-GFPa | 2/12 | 1/12 | NDb | 3/24 (13 ± 6) | |
| Ad.C-rtTA onlya | 2/12 | 2/12 | ND | 4/24 (17 ± 0) | |
| Σ Backgroundc | 5/36 | 5/36 | 1/12 | 11/84 (12 ± 3) | |
| Test vectorsd | |||||
| Ad.T-ICP0 | 10/12 | 11/12 | 10/12 | 31/36∗∗ (86 ± 5) | |
| Ad.T-n212 | 1/12 | 1/12 | ND | 2/24 (8 ± 0) | |
| Ad.T-ICP4 | 10/12 | 10/12 | 11/12 | 31/36∗∗ (86 ± 5) | |
| Ad.T-OBP | 1/12 | 0/12 | ND | 1/24 (5 ± 5) | |
| Ad.T-OBPψ | 0/12 | 0/12 | ND | 0/24 (0 ± 0) | |
| Ad.T-VP16 | 11/12 | 12/12 | 12/12 | 35/36∗∗ (97 ± 5) | |
| Ad.T-VP16Δ | 2/12 | 1/12 | ND | 3/24 (13 ± 6) | |
Cultures were superinfected with 10 PFU of Ad.C-GFP or Ad.C-rtTA/cell and incubated in TG cell culture medium containing 3 μM DOX.
ND, not determined.
Σ Background, the sum of the frequencies of reactivation observed in negative-control cultures that were either mock-superinfected or superinfected with Ad.C-GFP or Ad.C-rtTA only.
Cultures were cosuperinfected with 10 PFU of Ad.C-rtTA/cell and 40 PFU of TRE adenovirus vector/cell and incubated in TG cell medium containing 3 μM DOX.
∗∗, P < 0.00001, H0: reactivation frequency in treatment group is equal to the background, based on comparison of reactivation frequencies by Fisher's exact test.
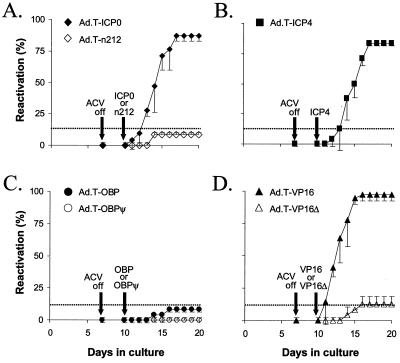
In tests of adenovirus vectors expressing HSV-1 proteins, latently infected TG cell cultures were superinfected with Ad.C-rtTA (MOI = 10) and TRE-regulated adenovirus vectors (MOI = 40) and treated with 3 μM DOX to induce expression of the transgene. Although reactivation had occurred in 86% of TG cell cultures superinfected with Ad.T-ICP0 by day 17, Ad.T-n212 had no effect on HSV-1 latency above the background (Table 2 and Fig. 5A). Likewise, HSV-1 reactivation occurred in 86% of TG cell cultures superinfected with Ad.T-ICP4 by day 17 (Table 2 and Fig. 5B). In contrast, neither Ad.T-OBP nor Ad.T-OBPψ induced HSV-1 reactivation above background levels (Table 2 and Fig. 5C). Finally, expression of wild-type VP16 from Ad.T-VP16 triggered HSV-1 reactivation in 97% of cultures by day 16, whereas expression of a protein containing the first 416 of the 490 amino acids of VP16 from Ad.T-VP16Δ had no effect on HSV-1 latency above the background in TG cell cultures (Table 2 and Fig. 5D). Therefore, the C-terminal transactivation domain of VP16 (56) is essential for VP16 to induce reactivation of latent HSV-1 in TG cell cultures.
FIG. 5.
Abilities of wild-type and mutant forms of ICP0, ICP4, OBP, and VP16 to induce reactivation of HSV-1 in latently infected TG cell cultures. HSV-1 reactivation frequencies in TG cell cultures superinfected with 40 PFU of Ad.T-ICP0 or Ad.T-n212 (A), Ad.T-ICP4 (B), Ad.T-OBP or Ad.T-OBPψ (C), or Ad.T-VP16 or Ad.T-VP16Δ (D) per cell and coinfected with Ad.C-rtTA (MOI = 10) were determined. After infection, all cultures were treated with 3 μM DOX. HSV-1 reactivation was detected by testing for the presence of infectious virus in the culture medium on day 7 as well as days 10 to 20 post-culture establishment. For Ad.T-ICP0 and Ad.T-VP16, each point represents the mean reactivation frequency ± the standard deviation of values for three independent experiments (n = 12 cultures per group per experiment). For all other groups, reactivation frequencies are based on two independent experiments (n = 12 cultures per group per experiment). The dashed lines indicate the background reactivation frequency of HSV-1 observed in these experiments, as defined in Table 2. ACV off, point at which ACV was removed from the culture.
DOX-induced expression of ICP0, ICP4, and VP16 increases the rate of HSV-1 reactivation in latently infected TG cell cultures.
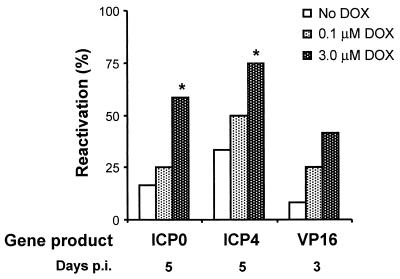
If the wild-type HSV-1 transgenes were in fact responsible for inducing reactivation, then DOX induction of the TRE promoter should increase the rate of HSV-1 reactivation in TG cell cultures following superinfection with Ad.T-ICP0, Ad.T-ICP4, or Ad.T-VP16. To test this possibility, latently infected TG cell cultures were superinfected with 40 PFU of TRE-regulated adenovirus vector/cell and 10 PFU of Ad.C-rtTA/cell, and transgene expression was induced with 0, 0.1, or 3 μM DOX. By day 10 p.i., HSV-1 had reactivated with similar efficiencies in all TG cell cultures regardless of the dose of DOX, presumably because of the high MOI (i.e., 40 PFU/cell and 1.2 × 107 PFU/culture). In all treatment groups, however, HSV-1 reactivation occurred most rapidly in TG cell cultures treated with 3 μM DOX (Fig. 6). In latently infected TG cell cultures superinfected with Ad.T-ICP0 or Ad.T-ICP4, the frequency at which reactivation was detected on day 5 p.i. was dependent on the concentration of DOX (Fig. 6). Consistent with earlier experiments (Fig. 5), Ad.T-VP16 induced reactivation more rapidly than Ad.T-ICP0 or Ad.T-ICP4, and DOX-dependent differences were observed at an earlier time point. On day 3 p.i., reactivation was detected in 8, 25, and 42% of Ad.T-VP16-infected cultures treated with 0, 0.1, and 3 μM DOX, respectively (Fig. 6). Thus, in each case, DOX-dependent induction of ICP0, ICP4, or VP16 increased the rate of HSV-1 reactivation in latently infected TG cell cultures.
FIG. 6.
DOX-induced expression of ICP0, ICP4, and VP16 from adenovirus vectors increases the efficiency of HSV-1 reactivation in TG cell cultures. TG cell cultures superinfected with 40 PFU of Ad.T-ICP0, Ad.T-ICP4, or Ad.T-VP16/cell and 10 PFU of Ad.C-rtTA/cell were either not treated with DOX or treated with 0.1 or 3.0 μM DOX (n = 12 cultures per group). The reactivation efficiencies in TG cells superinfected with Ad.T-ICP0 or Ad.T-ICP4 and treated with different concentrations of DOX were compared on day 5 p.i. Likewise, the reactivation efficiencies in TG cells superinfected with Ad.T-VP16 and treated with different concentrations of DOX were compared on day 3 p.i. A significant increase in reactivation frequency relative to cultures that received no DOX is indicated by an asterisk (P < 0.05, Fisher's exact test).
DISCUSSION
Three viral transcriptional activators induce reactivation of latent HSV-1 in TG cell cultures.
In the present study, adenovirus vectors were used to determine whether ICP0, ICP4, OBP, or VP16 is capable of inducing reactivation of HSV-1 in latently infected TG cell cultures. Expression of each of the three major HSV-1 transcriptional activators tested induced viral reactivation efficiently. In contrast, OBP, an essential component of the HSV-1 origin-dependent DNA replication complex, exhibited no reactivation-inducing ability above the background level.
(i) ICP0.
ICP0 is a ring finger protein that modifies the cellular milieu by activating the ubiquitin-proteasome pathway (10, 12). This modification results in a global increase in transcriptional activity (3, 25), thus relieving the transcriptional repression of the HSV-1 genome that occurs in quiescent cells (37, 39, 40). ICP0 also appears to block the cell cycle in late G1/S, thereby resetting the cell cycle clock, presumably to favor viral gene expression (21, 30). In past studies, ICP0-expressing adenovirus vectors induced reactivation of quiescent HSV-2 genomes in HEL cells (20) and the ICP0 ring finger motif was shown to be essential for this activity (59).
In the present study, complementation tests with adenovirus vectors demonstrated that ICP0 provided in trans fully complemented plaque formation by both VP16− and ICP0− mutants in Vero cells (Table 1). Therefore, VP16 is apparently not needed to initiate virus replication when ICP0 is provided in trans. The converse is not true, however, because VP16 does not complement ICP0− mutant plaque formation in Vero cells (Table 1). Therefore, VP16's capacity to initiate productive-phase HSV-1 gene expression is highly dependent on ICP0. Given ICP0's ability to initiate viral gene expression in the absence of VP16, one would predict that expression of ICP0 should be sufficient to reactivate latent HSV-1 genomes. The results of the present study demonstrate that ICP0 is indeed sufficient to induce HSV-1 reactivation in primary TG cell cultures. Given that ICP0 is also necessary for efficient HSV-1 reactivation in TG cell cultures (17), transactivation of the ICP0 promoter by stress-induced cellular factors may be a critical event in the initiation of reactivation of HSV-1 from latency. Further investigation will be required to test this hypothesis.
(ii) ICP4.
In previous studies in the HEL in vitro model of latency, an ICP4-expressing adenovirus vector did not trigger reactivation of quiescent HSV-2 genomes (59). In TG cells, however, ICP4 triggered HSV-1 reactivation as efficiently as ICP0. Moreover, the kinetics of HSV-1 reactivation in cultures treated with Ad.T-ICP0 was virtually identical to that in cultures treated with Ad.T-ICP4. Because ICP4 is essential for HSV-1 replication (8), it follows that ICP4 is also necessary and sufficient to induce efficient reactivation of HSV-1 from neuronal latency.
Given the recognized role of ICP5 as a repressor of IE gene expression (44), the underlying mechanism(s) that accounts for its ability to induce HSV-1 reactivation in TG cell cultures is unclear. It is possible that the form of ICP4 expressed from Ad.T-ICP4 is able to activate early- and late-gene expression but not suppress IE gene expression. Alternatively, one intriguing possibility is that ICP4 serves as a negative regulator of the LATs, the only viral gene products present in abundance in latently infected neurons (53). The LAT promoter contains a well-characterized ICP4-binding site that, when bound by ICP4, represses LAT transcription in vitro and in vivo (11, 13). Although the mechanism by which the LATs achieve their biological effects is not known, they may facilitate the establishment of latency (47, 55) by serving as negative regulators of productive-phase gene expression in neurons (13, 53). Based on this hypothesis, ICP4 may induce HSV-1 reactivation in TG cell cultures by decreasing steady-state levels of LATs in latently infected neurons (11, 42) and/or by increasing expression of productive-phase genes. Further investigation to test these intriguing hypotheses is currently under way.
(iii) OBP.
Initiation of viral DNA replication and amplification of the viral genome are critical events in the reactivation process, and available evidence indicates that viral DNA replication is a central regulatory checkpoint in the switch from latency to productive infection (38). If OBP-mediated initiation of viral DNA replication is rate limiting in reactivation, then transcriptional (7) or posttranslational (23) regulation of OBP function could be a critical event(s) in the switch from latency to reactivation. The failure of OBP to induce reactivation in the present study does not disprove the hypothesis that viral DNA replication is a regulatory checkpoint in HSV-1 reactivation. Rather, this finding indicates that expression of OBP alone is not sufficient to trigger reactivation of latent HSV-1 genomes in TG cell cultures, as measured by the appearance of infectious virus in culture supernatants, or that OBP expressed from Ad.T-OBP is not appropriately modified.
(iv) VP16.
VP16 is a potent transactivator of all five HSV-1 IE genes (56). The results of the present study demonstrate that VP16 is sufficient to trigger HSV-1 reactivation from latency. In three independent experiments, Ad.T-VP16 induced reactivation with slightly higher efficiency (Table 2) and somewhat faster kinetics (Fig. 5) than did Ad.T-ICP0 or Ad.T-ICP4. If one assumes that the IE promoters of latent HSV-1 genomes are available in TG cell cultures, a simple explanation for this result is that VP16 induces expression of all five IE genes and thereby initiates HSV-1 reactivation more efficiently than is possible with either ICP0 or ICP4 alone. It should be noted that despite the presence of VP16 in the virion tegument, UV-inactivated HSV virions do not trigger HSV-1 reactivation in TG cell cultures (17). Reasonable explanations for this discrepancy are that UV-inactivated virions deliver much lower levels of VP16 to latently infected TG neurons than does Ad.T-VP16 and that no active synthesis of VP16 occurs from UV-inactivated virions.
Relevance of ICP0, ICP4, and VP16 to HSV-1 reactivation from neuronal latency.
In the present study, we have shown that wild-type forms of ICP0, ICP4, and VP16 are sufficient to induce HSV-1 reactivation in primary TG cell cultures when these proteins are delivered by replication-defective adenovirus vectors. These findings demonstrate that all three proteins are expressed in a functional form in TG cells and that the viral and cellular targets of their respective activities are available to be acted upon. Although the natural processes by which latent HSV-1 genomes are stimulated to reactivate in vivo are unknown, several inferences can be made from the results of the present study in light of the available literature on HSV-1 latency and reactivation.
While VP16 is sufficient to induce HSV-1 reactivation in TG cell cultures, it is improbable that this protein normally contributes to the initiation of viral reactivation in vivo. The VP16 gene is a late gene whose expression is dependent on IE and early-protein functions, as well as the onset of HSV-1 DNA replication (24, 49). Moreover, VP16 is not necessary for the efficient reactivation of HSV-1 from TG explants (51). In contrast to late genes, because IE promoters have much higher levels of intrinsic activity (14, 27), it is more likely that cellular factors activate ICP0 or ICP4 gene expression during latency (26). If ICP0 and ICP4 are both necessary and sufficient to induce HSV-1 reactivation in vivo, then repression of IE genes may be necessary for the maintenance of HSV-1 latency. Although a repressor role has been proposed for the LATs (13, 53), the mechanism by which the LATs might inhibit IE gene expression and maintain neuronal latency has not been resolved.
Use of adenovirus vectors in TG cell culture studies of HSV-1 latency and reactivation.
No definitive conclusions can yet be drawn regarding the relevance of the findings of the present study to the series of events by which HSV-1 reactivates from latency in humans. Four obvious variables that differ between the experimental system and the natural history of HSV-1 infection are (i) the use of mice as a host for a human virus, (ii) the analysis of latently infected TG neurons in vitro, (iii) the use of adenovirus vectors to deliver proteins to TG neurons, and (iv) the expression of unnaturally high levels of ICP0, ICP4, and VP16 in TG neurons. With regard to points i and ii, the limitations of mouse TG cell culture as a reactivation model for HSV-1 are widely acknowledged and have been discussed previously (16). With regard to the use of adenovirus vectors to deliver proteins to TG neurons, E1- and E3-deleted adenovirus vectors are replication defective but express sufficient E4 protein to achieve biological effects (57), and other viral activities cannot be ruled out. In the present study, infection of TG cell cultures with adenovirus vectors that express GFP, rtTA, OBP, or mutant forms of ICP0, OBP, or VP16 did not increase the frequency of HSV-1 reactivation. Nonetheless, it is possible that E4 or some other adenovirus activity provides a second signal that, in combination with ICP0, ICP4, or VP16, is essential for induction of reactivation of latent HSV-1. It should be noted, however, that the second-signal hypothesis can be applied to any manipulation used to introduce gene products into latently infected neurons. Regarding the fourth point, the amounts of ICP0, ICP4, and VP16 expressed in TG neurons are likely much larger than are physiologically relevant in vivo. The lack of a means of readily quantifying the magnitude of protein overexpression in individual TG neurons is clearly an inherent limitation of the system.
Despite these drawbacks, the ability of adenovirus vectors to deliver specific gene products to cultures of TG cells latently infected with HSV-1 provides an opportunity to identify viral and cellular proteins that are able to induce reactivation. Likewise, this approach may prove useful in identifying repressors that facilitate the maintenance of latency. Thus, despite its drawbacks, the usefulness of the TG cell culture model shows much promise. As for any experimental model, the ultimate usefulness of the TG cell culture model is dependent on its sensitivity and the reproducibility of the resulting data. Comparison of the reactivation frequencies and kinetics of reactivation induced by ICP0, ICP4, and VP16 (Fig. 5 and 6) indicates that the reproducibility of reactivation induced by proteins expressed from adenovirus vectors is high. An additional criterion for assessing the suitability of the TG cell culture as a model of HSV-1 latency and reactivation in vivo is whether the same proteins that induce HSV-1 reactivation in vitro will do so in vivo. Clearly, much additional work will be required to evaluate this final criterion.
ACKNOWLEDGMENTS
This investigation was supported by Public Health Service Program Project grant P01 NS 35138 from the National Institute of Neurological Disorders and Stroke. W.P.H. was the recipient of individual National Research Service award F32 AI 10147 from the National Institute of Allergy and Infectious Diseases. J.A.I. was supported by NIH training grant T32 AI 00732S, and D.J.D. was supported by postdoctoral fellowship PF-00-021001-MBC from the American Cancer Society.
We thank Rath Pichyangkura and Steven Triezenberg (Michigan State University, East Lansing) for generously providing the VP16− mutant RP5, Sandra Weller (University of Connecticut Health Sciences Center, Farmington) for providing the OBP− mutant virus hr94 and the OBP-complementing cell line 2B.11, and Deborah Parris (Ohio State University, Columbus) and Daniel Tenney (Bristol-Meyers Squibb, Wallingford, Conn.) for providing the RH7 rabbit polyclonal antiserum against OBP.
REFERENCES
- 1.Cai W, Astor T L, Liptak L M, Cho C, Coen D M, Schaffer P A. The herpes simplex virus type 1 regulatory protein ICP0 enhances virus replication during acute infection and reactivation from latency. J Virol. 1993;67:7501–7512. doi: 10.1128/jvi.67.12.7501-7512.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cai W, Schaffer P A. Herpes simplex virus type 1 ICP0 plays a critical role in the de novo synthesis of infectious virus following transfection of viral DNA. J Virol. 1989;63:4579–4589. doi: 10.1128/jvi.63.11.4579-4589.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai W, Schaffer P A. Herpes simplex virus type 1 ICP0 regulates expression of immediate-early, early, and late genes in productively infected cells. J Virol. 1992;66:2904–2915. doi: 10.1128/jvi.66.5.2904-2915.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corti O, Sabate O, Horellou P, Colin P, Dumas S, Buchet D, Buc-Caron M H, Mallet J. A single adenovirus vector mediates doxycycline-controlled expression of tyrosine hydroxylase in brain grafts of human neural progenitors. Nat Biotechnol. 1999;17:349–354. doi: 10.1038/7901. [DOI] [PubMed] [Google Scholar]
- 5.Davis A R, Meyers K, Wilson J M. High throughput method for creating and screening recombinant adenoviruses. Gene Ther. 1998;5:1148–1152. doi: 10.1038/sj.gt.3300705. [DOI] [PubMed] [Google Scholar]
- 6.Davis A R, Wivel N A, Palladino J L, Tao L, Wilson J M. Construction of adenoviral vectors. Methods Mol Biol. 2000;135:515–523. doi: 10.1385/1-59259-685-1:515. [DOI] [PubMed] [Google Scholar]
- 7.Deb S P, Deb S, Brown D R. Analysis of the promoter sequences of the UL9 gene of herpes simplex virus type 1. Biochem Biophys Res Commun. 1993;193:617–623. doi: 10.1006/bbrc.1993.1669. [DOI] [PubMed] [Google Scholar]
- 8.DeLuca N A, McCarthy A M, Schaffer P A. Isolation and characterization of deletion mutants of herpes simplex virus type 1 in the gene encoding immediate-early regulatory protein ICP4. J Virol. 1985;56:558–570. doi: 10.1128/jvi.56.2.558-570.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeLuca N A, Schaffer P A. Activities of herpes simplex virus type 1 (HSV-1) ICP4 genes specifying nonsense peptides. Nucleic Acids Res. 1987;15:4491–4511. doi: 10.1093/nar/15.11.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Everett R D, Orr A, Preston C M. A viral activator of gene expression functions via the ubiquitin-proteasome pathway. EMBO J. 1998;17:7161–7169. doi: 10.1093/emboj/17.24.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farrell M J, Margolis T P, Gomes W A, Feldman L T. Effect of the transcription start region of the herpes simplex virus type 1 latency-associated transcript promoter on expression of productively infected neurons in vivo. J Virol. 1994;68:5337–5343. doi: 10.1128/jvi.68.9.5337-5343.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freemont P S. RING for destruction? Curr Biol. 2000;10:R84–R87. doi: 10.1016/s0960-9822(00)00287-6. [DOI] [PubMed] [Google Scholar]
- 13.Garber D A, Schaffer P A, Knipe D M. A LAT-associated function reduces productive-cycle gene expression during acute infection of murine sensory neurons with herpes simplex virus type 1. J Virol. 1997;71:5885–5893. doi: 10.1128/jvi.71.8.5885-5893.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gelman I H, Silverstein S. Herpes simplex virus immediate-early promoters are responsive to virus and cell trans-acting factors. J Virol. 1987;61:2286–2296. doi: 10.1128/jvi.61.7.2286-2296.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halford W P, Gebhardt B M, Carr D J J. Mechanisms of herpes simplex virus type 1 reactivation. J Virol. 1996;70:5051–5060. doi: 10.1128/jvi.70.8.5051-5060.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halford W P, Schaffer P A. ICP0 is required for efficient reactivation of herpes simplex virus type 1 from neuronal latency. J Virol. 2001;75:3240–3249. doi: 10.1128/JVI.75.7.3240-3249.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halford W P, Schaffer P A. Optimized viral dose and transient immunosuppression enable herpes simplex virus ICP0-null mutants to establish wild-type levels of latency in vivo. J Virol. 2000;74:5957–5967. doi: 10.1128/jvi.74.13.5957-5967.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harbour D A, Hill T J, Blyth W A. Recurrent herpes simplex in the mouse: inflammation in the skin and activation of virus in the ganglia following peripheral stimulation. J Gen Virol. 1983;64:1491–1498. doi: 10.1099/0022-1317-64-7-1491. [DOI] [PubMed] [Google Scholar]
- 20.Harris R A, Everett R D, Zhu X, Silverstein S, Preston C M. Herpes simplex virus type 1 immediate-early protein Vmw110 reactivates latent herpes simplex virus type 2 in an in vitro latency system. J Virol. 1989;63:3513–3515. doi: 10.1128/jvi.63.8.3513-3515.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hobbs W E, II, DeLuca N A. Perturbation of cell cycle progression and cellular gene expression as a function of herpes simplex virus ICP0. J Virol. 1999;73:8245–8255. doi: 10.1128/jvi.73.10.8245-8255.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Institute of Laboratory Animal Resources. Guide for the care and use of laboratory animals. Washington, D.C.: National Academy Press; 1996. [Google Scholar]
- 23.Isler J A, Schaffer P A. Phosphorylation of the herpes simplex virus type 1 origin binding protein. J Virol. 2001;75:628–637. doi: 10.1128/JVI.75.2.628-637.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson P A, Everett R D. The control of herpes simplex virus type-1 late gene transcription: a ‘TATA-box’/cap site region is sufficient for fully efficient regulated activity. Nucleic Acids Res. 1986;14:8247–8254. doi: 10.1093/nar/14.21.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jordan R, Schaffer P A. Activation of gene expression by herpes simplex virus type 1 ICP0 occurs at the level of mRNA synthesis. J Virol. 1997;71:6850–6862. doi: 10.1128/jvi.71.9.6850-6862.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kramer M F, Coen D M. Quantification of transcripts from the ICP4 and thymidine kinase genes in mouse ganglia latently infected with herpes simplex virus. J Virol. 1995;69:1389–1399. doi: 10.1128/jvi.69.3.1389-1399.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kristie T M, Roizman B. Separation of sequences defining basal expression from those conferring alpha gene recognition within the regulatory domains of herpes simplex virus 1 alpha genes. Proc Natl Acad Sci USA. 1984;81:4065–4069. doi: 10.1073/pnas.81.13.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laycock K A, Lee S F, Brady R H, Pepose J S. Characterization of a murine model of recurrent herpes simplex viral keratitis induced by ultraviolet B radiation. Investig Ophthalmol Vis Sci. 1991;32:2741–2746. [PubMed] [Google Scholar]
- 29.Leib D A, Nadeau K C, Rundle S A, Schaffer P A. The promoter of the latency-associated transcripts of herpes simplex virus type 1 contains a functional cAMP-response element: role of the latency-associated transcripts and cAMP in reactivation of viral latency. Proc Natl Acad Sci USA. 1991;88:48–52. doi: 10.1073/pnas.88.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lomonte P, Everett R D. Herpes simplex virus type 1 immediate-early protein Vmw110 inhibits progression of cells through mitosis and from G1 into S phase of the cell cycle. J Virol. 1999;73:9456–9467. doi: 10.1128/jvi.73.11.9456-9467.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malik A K, Martinez R, Muncy L, Carmichael E P, Weller S K. Genetic analysis of the herpes simplex virus type 1 UL9 gene: isolation of a LacZ insertion mutant and expression in eukaryotic cells. Virology. 1992;190:702–715. doi: 10.1016/0042-6822(92)90908-8. [DOI] [PubMed] [Google Scholar]
- 32.Maniatis T, Fritsch E, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. pp. 202–203. [Google Scholar]
- 33.McFarlane M, Daksis J I, Preston C M. Hexamethylene bisacetamide stimulates herpes simplex virus immediate early gene expression in the absence of trans-induction by Vmw65. J Gen Virol. 1992;73:285–292. doi: 10.1099/0022-1317-73-2-285. [DOI] [PubMed] [Google Scholar]
- 34.Molin M, Shoshan M C, Öhman-Forslund K, Linder S, Akusjärvi G. Two novel adenovirus vector systems permitting regulated protein expression in gene transfer experiments. J Virol. 1998;72:8358–8361. doi: 10.1128/jvi.72.10.8358-8361.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monahan S J, Grinstead L A, Olivieri W, Parris D S. Interaction between the herpes simplex virus type 1 origin-binding and DNA polymerase accessory proteins. Virology. 1998;241:122–130. doi: 10.1006/viro.1997.8953. [DOI] [PubMed] [Google Scholar]
- 36.Moriya A, Yoshiki A, Kita M, Fushiki S, Imanishi J. Heat shock-induced reactivation of herpes simplex virus type 1 in latently infected mouse trigeminal ganglion cells in dissociated culture. Arch Virol. 1994;135:419–425. doi: 10.1007/BF01310025. [DOI] [PubMed] [Google Scholar]
- 37.Mossman K L, Saffran H A, Smiley J R. Herpes simplex virus ICP0 mutants are hypersensitive to interferon. J Virol. 2000;74:2052–2056. doi: 10.1128/jvi.74.4.2052-2056.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nichol P F, Chang J Y, Johnson E M, Jr, Olivo P D. Herpes simplex virus gene expression in neurons: viral DNA synthesis is a critical regulatory event in the branch point between the lytic and latent pathways. J Virol. 1996;70:5476–5486. doi: 10.1128/jvi.70.8.5476-5486.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Preston C M. Repression of viral transcription during herpes simplex virus latency. J Gen Virol. 2000;81:1–19. doi: 10.1099/0022-1317-81-1-1. [DOI] [PubMed] [Google Scholar]
- 40.Preston C M, Mabbs R, Nicholl M J. Construction and characterization of herpes simplex virus type 1 mutants with conditional defects in immediate early gene expression. Virology. 1997;229:228–239. doi: 10.1006/viro.1996.8424. [DOI] [PubMed] [Google Scholar]
- 41.Preston C M, McFarlane M. Cytodifferentiating agents affect the replication of herpes simplex virus type 1 in the absence of functional VP16. Virology. 1998;249:418–426. doi: 10.1006/viro.1998.9314. [DOI] [PubMed] [Google Scholar]
- 42.Rock D, Lokensgard J, Lewis T, Kutish G. Characterization of dexamethasone-induced reactivation of latent bovine herpesvirus 1. J Virol. 1992;66:2484–2490. doi: 10.1128/jvi.66.4.2484-2490.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rock D L, Fraser N W. Detection of HSV-1 genome in the central nervous system of latently infected mice. Nature (London) 1983;302:523–525. doi: 10.1038/302523a0. [DOI] [PubMed] [Google Scholar]
- 44.Roizman B, Sears A E. Herpes simplex viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1043–1108. [Google Scholar]
- 45.Sacks W R, Schaffer P A. Deletion mutants in the gene encoding the herpes simplex virus type 1 immediate-early protein ICP0 exhibit impaired growth in cell culture. J Virol. 1987;61:829–839. doi: 10.1128/jvi.61.3.829-839.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Samaniego L A, Wu N, DeLuca N A. The herpes simplex virus immediate-early protein ICP0 affects transcription from the viral genome and infected-cell survival in the absence of ICP4 and ICP27. J Virol. 1997;71:4614–4625. doi: 10.1128/jvi.71.6.4614-4625.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sawtell N M, Thompson R L. Herpes simplex virus type 1 latency-associated transcription unit promotes anatomical site-dependent establishment and reactivation from latency. J Virol. 1992;66:2157–2169. doi: 10.1128/jvi.66.4.2157-2169.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sawtell N M, Thompson R L. Rapid in vivo reactivation of herpes simplex virus in latently infected murine ganglionic neurons after transient hyperthermia. J Virol. 1992;66:2150–2156. doi: 10.1128/jvi.66.4.2150-2156.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smiley J R, Smibert C, Everett R D. Expression of a cellular gene cloned in herpes simplex virus: rabbit beta-globin is regulated as an early viral gene in infected fibroblasts. J Virol. 1987;61:2368–2377. doi: 10.1128/jvi.61.8.2368-2377.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith K O. Relationship between the envelope and the infectivity of herpes simplex virus. Proc Soc Exp Biol Med. 1964;115:814–816. doi: 10.3181/00379727-115-29045. [DOI] [PubMed] [Google Scholar]
- 51.Steiner I, Spivack J G, Deshmane S L, Ace C I, Preston C M, Fraser N W. A herpes simplex virus type 1 mutant containing a nontransinducing Vmw65 protein establishes latent infection in vivo in the absence of viral replication and reactivates efficiently from explanted trigeminal ganglia. J Virol. 1990;64:1630–1638. doi: 10.1128/jvi.64.4.1630-1638.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stevens J G, Cook M L. Latent herpes simplex virus in spinal ganglia of mice. Science. 1971;173:843–845. doi: 10.1126/science.173.3999.843. [DOI] [PubMed] [Google Scholar]
- 53.Stevens J G, Wagner E K, Devi-Rao G B, Cook M L, Feldman L T. RNA complementary to a herpesvirus α gene mRNA is prominent in latently infected neurons. Science. 1987;235:1056–1059. doi: 10.1126/science.2434993. [DOI] [PubMed] [Google Scholar]
- 54.Tal-Singer R, Pichyangkura R, Chung E, Lasner T M, Randazzo B P, Trojanowski J Q, Fraser N W, Triezenberg S J. The transcriptional activation domain of VP16 is required for efficient infection and establishment of latency by HSV-1 in the murine peripheral and central nervous systems. Virology. 1999;259:20–33. doi: 10.1006/viro.1999.9756. [DOI] [PubMed] [Google Scholar]
- 55.Thompson R L, Sawtell N M. The herpes simplex virus type 1 latency-associated transcript gene regulates the establishment of latency. J Virol. 1997;71:5432–5440. doi: 10.1128/jvi.71.7.5432-5440.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Triezenberg S J, Kingsbury R C, McKnight S L. Functional dissection of VP16, the trans-activator of herpes simplex virus immediate early gene expression. Genes Dev. 1988;2:718–729. doi: 10.1101/gad.2.6.718. [DOI] [PubMed] [Google Scholar]
- 57.Wersto R P, Rosenthal E R, Seth P K, Eissa N T, Donahue R E. Recombinant, replication-defective adenovirus gene transfer vectors induce cell cycle dysregulation and inappropriate expression of cyclin proteins. J Virol. 1998;72:9491–9502. doi: 10.1128/jvi.72.12.9491-9502.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yeh P, Perricaudet M. Advances in adenoviral vectors: from genetic engineering to their biology. FASEB J. 1997;11:615–623. doi: 10.1096/fasebj.11.8.9240963. [DOI] [PubMed] [Google Scholar]
- 59.Zhu X, Chen J, Young C S H, Silverstein S. Reactivation of latent herpes simplex virus by adenovirus recombinants encoding mutant IE-0 gene products. J Virol. 1990;64:4489–4498. doi: 10.1128/jvi.64.9.4489-4498.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]