Abstract
Meroterpenoid-type marine natural compounds have attracted an increasing amount of attention due to their peculiar chemical structures and their potential for the development of therapeutically important probes. Within this group of substances pelorol stands out; it is a natural compound isolated from marine organisms with a unique structure and an interesting biological profile. In this article, we summarize and highlight the most interesting aspects of the synthetic procedures towards this compound, which have two common key steps. The first is the coupling of a drimanyl derivative with a compound derived from an arene. The second is a Friedel–Crafts cyclization which forms the C ring of the natural product. Despite the synthetic advances achieved so far, we consider that a more efficient synthetic procedures could be carried out, since their synthetic routes are difficult to scale up due to numerous reaction steps and the limitations imposed by the use of some reagents. In this article, we present a new and versatile retrosynthetic analysis of (-)-pelorol and analogs, which is highly desirable for their easy preparation and subsequent broad study of their biological activities. This is a retrosynthetic route that could improve those reported in the literature in terms of cost-effectiveness.
Keywords: marine natural products, meroterpenoids, pelorol
1. Introduction
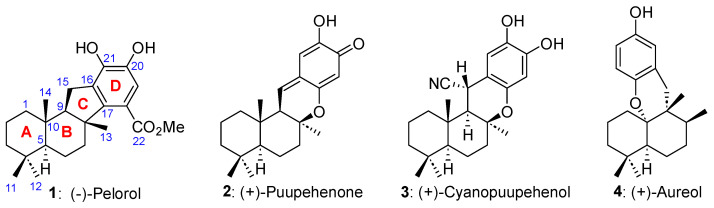
Drimane meroterpenoids are defined as compounds of mixed terpenoid–polyketide origin [1,2,3] (Figure 1). These natural compounds have been isolated from marine organisms, particularly from algae and sponges [4,5,6], and they are excellent examples of natural products with structural diversity having interesting biological activities, such as anti-HIV [7], antibacterial [8], antitumor [9,10,11], antifungal [12], etc. [13,14]. For these reasons, these fascinating compounds have attracted widespread attention from synthetic chemical pharmacologists and biologists. It should be noted that the efforts made in natural products synthesis generates synergistic effects with synthetic methodology, since the results obtained help the general development of chemical synthesis.
Figure 1.
Representative bioactive drimane meroterpenoids.
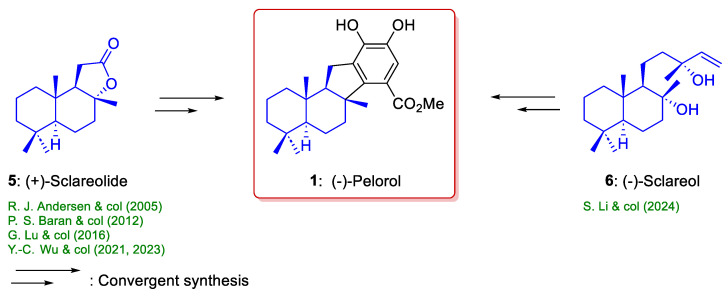
Within the marine meroterpenoid family, (-)-pelorol (1) is a remarkable compound which integrates a sesquiterpenoid unit and a phenolic moiety. This natural marine compound, which was initially isolated from Dactylospongia elegants [15] and later from Petrosaspongia metachromia [16] and Hyrtros erectus [17], is bioactive against Plasmodium and trypanosome, showing significant activity against P. falciparum with an IC50 value of 0.8 μM [15,16], has insecticidal activity [16,17], exhibits cytotoxicity against HeLa cancer cells [18], has anti-inflammatory activity by activating inositol-5-phosphatase (SHIP) [19], and possesses good antifungal activity against Rhizoctonia solani, with EC50 values of 7.7 μM [20]. Of the various biological activities mentioned, the anti-cancer activity deserves special mention. Previous studies demonstrated that (-)-pelorol (1) inhibits the enzyme phosphatidylinositol 3-kinase (PI3) [21] and has anti-inflammatory activity because it can activate inositol-5-phosphatase (SHIP) [19]. Due to the fact that the deactivation of aberrant PI3K signaling in cancer cells, by the activation of SHIP, has been proposed to be a promising approach to treating blood cancers [22,23] (-)-pelorol (1) can be considered as a promoting lead compound for therapeutic development. Several elegant syntheses of (-)-pelorol (1) have employed a convergent synthetic route (Scheme 1), where two fragments are linked to form the desired marine compound, normally by the addition of an aryl-metallic reagent to a sesquiterpenoid carrying a carbonyl group through a Suzuki coupling or a palladium-catalyzed carbene migratory insertion (see Section 2). In all cases, the starting materials are commercially available precursors, such as (+)-sclareolide (5) or (-)-sclareol (6). These starting materials selected for the preparation of 1 exhibit the same absolute configurations at C-5, C-9, and C-10 as those predicted for (-)-pelorol (1). Scheme 1 summarizes the chiral starting material selected in the synthesis of 1 by convergent synthetic routes [22].
Scheme 1.
Chiral starting materials used in previous syntheses of (-)-pelorol (1): (+)-sclareolide [19,21,24,25,26] and (-)-sclareol [20].
This article highlights the previous approaches to (-)-pelorol (1), and finally, a strategy to access to this natural product from a common intermediate that can be employed in the total divergent synthesis of natural products will be discussed.
2. Synthesis of (-)-Pelorol (1)
2.1. Andersen’s Synthesis of (-)-Pelorol (1)
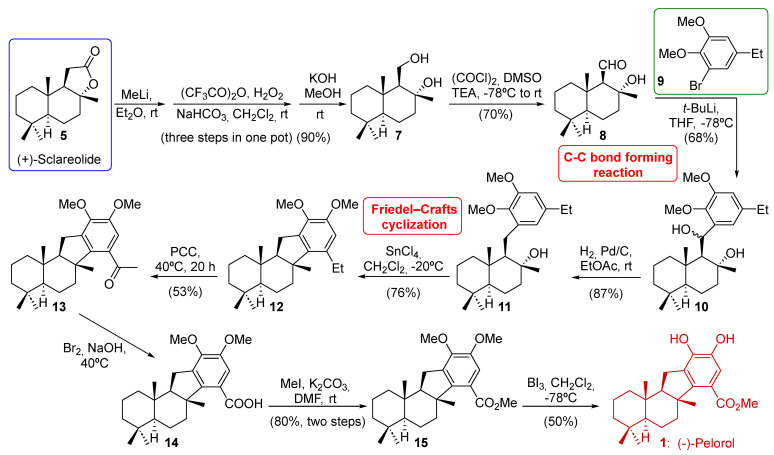
In 2005, Andersen’s group [19] reported the first enantioselective synthesis of natural (-)-pelorol (1) via an addition of an aryl-metallic reagent formed from 9 to the carbonyl compound 8 as a key step (Scheme 2). Andersen’s synthesis of 1 used (+)-sclareolide (5) as the starting material. The diol 7 was synthesized in three steps in one pot from (+)-sclareolide (5) through a degradative sequence of reactions (90% overall yields). Its oxidation under Swern conditions gave the β-hydroxy drimanaldehyde 8 in 70% yield. Then, the coupling reaction between 8 and the organolithium derived from 9 gave, after hydrogenolysis, the tertiary alcohol 11 as key compound. The Friedel–Crafts-type cyclization of 11 with SnCl4 afforded the indane-fused decalin 12 as cyclization product in 76% yield. The oxidation of compound 12 with PCC selectively oxidized the C-22 methylene to form methyl ketone 13 in a moderate yield. The treatment of 13 with Br2 gave the desired benzoic acid 14, which after esterification with MeI gave the compound 15 with an 80% yield (two steps). Finally, the selective cleavage of the phenyl methyl ethers with BI3 at −78 °C completed the synthesis of (-)-pelorol (1) at a 50% yield. This total synthesis was achieved in 11 steps (6% overall yield) from starting material 5. The strategy opens the door to the preparation of an array of different natural products through a modular strategy, through the reaction of 8 with a choice of aryl bromides similar to 9. The major drawbacks of this approach are the moderate yield of the degradative oxidation of 12 and the need to use of protective groups for the phenolic hydroxy groups. Also, this synthetic route is difficult to scale up due to the numerous steps.
Scheme 2.
Andersen’s enantioselective synthesis of (-)-pelorol (1) from (+)-sclareolide (5). DMSO = dimethyl sulfoxide; PCC = pyridinium chlorochromate; DMF = N,N-dimethyl formamide.
2.2. Baran’s Synthesis of (-)-Pelorol (1)
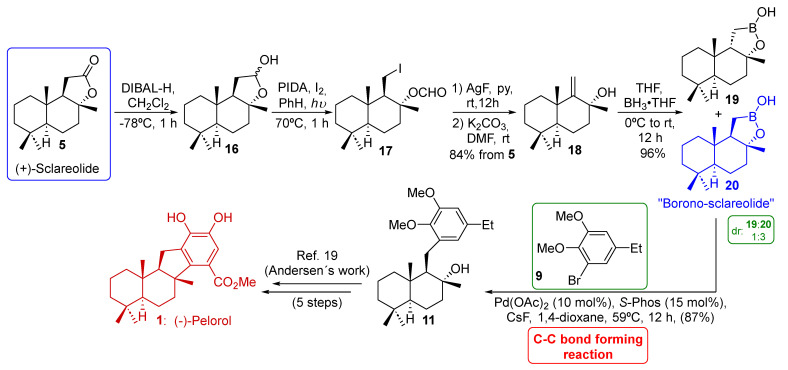
Baran and colleagues [24] reported the formal synthesis of the (-)-enantiomer of pelorol (1) from (+)-sclareolide (5) as a starting material. Their approach was based on the synthesis of a “borono-sclareolide” 20 as a common pluripotent intermediate widely used in the divergent synthesis of meroterpenoids. As shown in Scheme 3, the key intermediate 20 required the excision of carbon monoxide from (+)-sclareolide (5) and the introduction of B-OH in its place. The reduction in starting material 5 using diisobutylaluminium hydride (DIBAL-H) gave sclareal 16. The treatment of 16 under the C-C bond cleavage conditions of Suarez [27] delivered drimanal iodoformate 17. Then, a two-step dehydroiodination/hydrolysis procedure (AgF in pyridine followed by K2CO3 in MeOH) gave the natural exocyclic decalin 18 with an excellent 84% overall yield from 5. Compound 18 was synthesized on the decagram scale and was used without the need for technical chromatographic separation. The hydroboration of 18 with BH3 gave 19 and 20 as a mixture of diastereomers (19:20 = 1:3) at a 96% yield. Borono-sclareolide 20 was synthesized at the gram-scale and purified by column chromatography. This compound is a versatile terpene donor, which can be used as a partner of aryl bromides in Suzuki coupling reactions for the preparation of meroterpenoids. The C-C bond forming reaction between 20 and aryl bromide 9 gave the tertiary alcohol 11 at a 87% yield. As alcohol 11 is an advanced intermediate in Andersen’s synthesis of (-)-pelorol (1) (Scheme 2) [19], this procedure constitutes a formal synthesis of 1. In this way, the synthesis of 1 can be achieved in 11 steps (8.5% overall yield) from commercially available (+)-sclareolide (5).
Scheme 3.
Baran’s enantioselective synthesis of (-)-pelorol (1) from (+)-sclareolide (5). DIBAL-H = diisobutylaluminium hydride; PIDA = phenyliodine (III) diacetate; DMF = N,N-dimethyl formamide.
The key component of this procedure was the invention of “borono-sclareolide” 20 as a common intermediate employed in the design of the divergent, modular, and scalable access of meroterpenoids and analogues [24]. The synthesis of the intermediate 20 represents an excellent example of how synthetic methodology can generate synergistic effects with natural product synthesis, since an efficient and versatile reaction development can also be of use in the preparation of natural products. One of the main limitations of this synthetic route is the use of a palladium catalyst which could be expensive.
2.3. Lu’s Synthesis of (-)-Pelorol (1)
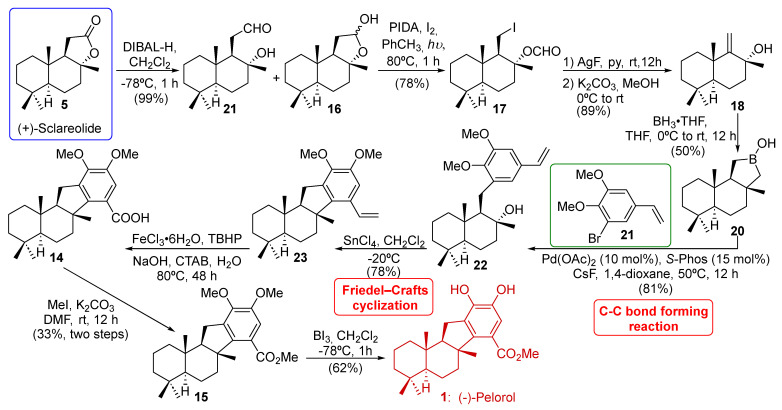
In 2016, Lu and coworkers [21] accomplished another formal synthesis of (-)-pelorol (1) using the procedure previously developed by Baran et at. [24], but choosing a more efficient aryl bromide 21 as coupling partner of “bonoro-sclareolide” 20 (Scheme 4). The synthesis of 1 started with the reduction in available (+)-sclareolide (5) with DIBAL-H yielding a mixture of 21 and 16 with an excellent yield of 99%, which was used without separation in the next step. The iodination of the mixture under Suarez’s conditions [27], but replacing benzene with toluene, resulted in a 78% yield of 17. A sequential dehydroiodination/hydrolysis reaction led to the formation of compound 18 at a 89% yield. Then, hydroboration of 18 gave a mixture of diastereomeric boronates (95% combined yield). Column chromatography allowed the isolation of pure borono-sclareolide 20 at a 50% yield. In a first instance, this research group used arylbromide with ethyl substituent (9) to form the C-C connection with the borono-sclareolide 20 under Suzuki conditions, but the oxidation of the intermediate 12, obtained after the cyclization of the coupling product 11, gave a poor yield (27%). Considering this inconvenience, this research group changed the arylbromide 9 to the vinyl-substituted arylbromide 21, yielding the intermediate 22 (81%) under Suzuki coupling conditions. The subsequent Friedel–Crafts cyclization of 22 afforded the indane-fused decalin 23 with a good yield (78%). The oxidation of the vinyl group in 23 using a tert-butyl hydroperoxide (TBHP)-based procedure gave the acid 14, which after esterification with MeI, generated the compound 15, although at a low yield of 33% (two steps). The deprotection of the OMe group in 15, with the procedure previously reported in Andersen’s synthesis [19], gave the desirable (-)-pelorol (1) at a 62% yield. As product 14 was an advanced intermediate in the Andersen’s synthesis of (-)-pelorol [19], this procedure described by Lu and coworkers constitutes a formal synthesis of the natural marine product 1. This formal synthesis was completed in nine steps (4.4% overall yield) from commercially available (+)-sclareolide (5).
Scheme 4.
Lu’s enantioselective synthesis of (-)-pelorol (1) from (+)-sclareolide (5). DIBAL-H = diisobutylaluminium hydride; PIDA = phenyliodine (III) diacetate; TBHP = tert-Butyl hydroperoxide; CTAB = Cetrimonium bromide; DMF = N,N-dimethyl formamide.
Again, in this synthetic route, the power of “borono-sclareolide” 20 for a divergent and scalable access to (-)-pelorol 1 was demonstrated. Furthermore, a better choice of aryl bromide 21 as coupling partner of 20 resulted in a more effective synthetic route.
2.4. Wu’s Synthesis of (-)-Pelorol (1)
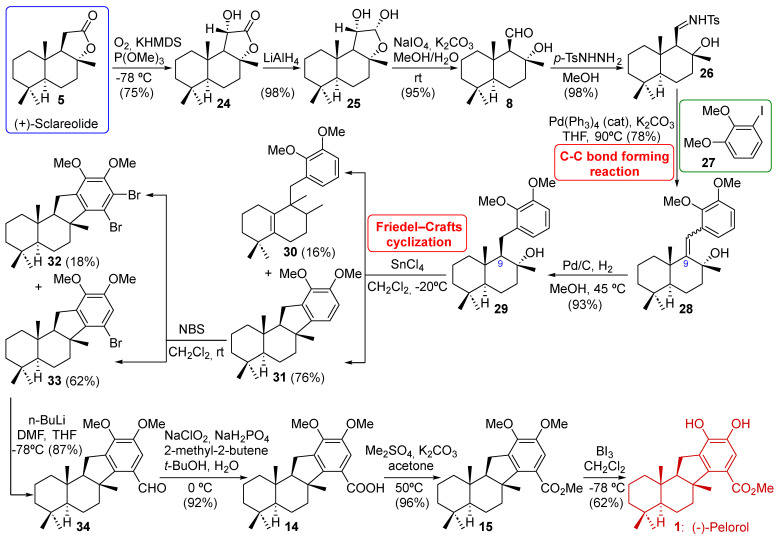
In 2021, Wu’s group [25] achieved the formal synthesis of (-)-pelorol (1) through the development of a modular strategy for the synthesis of meroterpenoid marine compounds, having a palladium-catalyzed coupling of an aryliodide and a tosylhydrazone as key step [28]. As shown in Scheme 5, the key intermediate drimanal hydrazone 26 was prepared from (+)-sclareolide (5) in four steps (68% overall yield). The sequence includes the selective oxidation of 5 with O2 to give 24, a reduction in 24 with LiAlH4 to form 25, the subsequent cleavage of the diol 25 with NaIO4 to generate 8, and finally, the formation of drimanal hydrazone 26 with p-TsNHNH2. A Pd(0)-catalyzed tandem carbene migratory insertion reaction allowed the cross-coupling of 26 and ortho-iodoveratrol (27), previously obtained from commercially available veratrole. Product 28 was formed as a mixture of isomers ((Z)-28a and (E)-28b in 37% and 41% yield, respectively). This methodology can be applied to the preparation of other meroterpenoids by simply choosing a different aryl iodide. The advantages of these couplings between hydrazone 26 and aryl iodides with different substitution patterns are that the reaction conditions are mild and that both partners can be easily synthesized, although the use of a palladium catalyst in this coupling limits the scale-up of synthetic strategy due to its high costs.
Scheme 5.
Wu’s first enantioselective synthesis of (-)-pelorol (1) from (+)-sclareolide (5). KHDMS = Potassium bis (trimethylsilyl)amide; NBS = N-Bromosuccinimide; DMF = N,N-dimethyl formamide.
The next logical step should be cyclization to form ring C. However, to avoid the inversion of configuration at C-9 during the process, the double bond in 28 had to be hydrogenated prior to the cyclization. The reverse procedure (cyclization and subsequent hydrogenation) led to products with the opposite configuration at the C-9 position, and was used by Bisai and coworkers for the synthesis of 9-epi-pelorol [29]. The hydrogenation of the intermediates 28 gave 29 at a 93% yield. The Friedel–Crafts cyclization of 29 in the presence of SnCl4 allowed the formation of the desired C ring in the tetracyclic compound 31 in 76% yield, although a rearranged minor compound 30 was also formed with a 16% yield. The bromination of 31 with NBS gave monobrominated 33 as the major product (62% yield), together with the dibromoderivative 32 as a minor one (18% yield). The formylation of the bromo compound 33 was achieved with DMF in the presence of n-BuLi, which led to the formation of aldehyde 34 at an 87% yield. The Pinnick oxidation [29] of 34 yielded carboxylic acid 14 at a 92% yield, which was then methylated to form the ester 15 in 96% yield. Finally, the selective demethylation of 15, using the conditions previously reported by Andersen in the synthesis of 1 [19], resulted in the marine (-)-pelorol (1) at a 62% yield. The formal synthesis of 1 was completed in 12 steps (9% overall yield) from available starting material (+)-sclareolide (5).
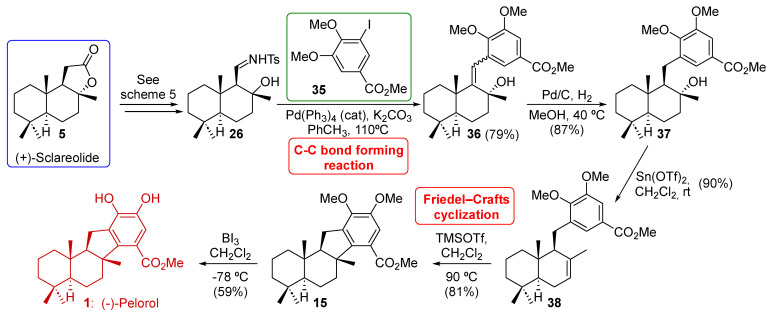
Two years later, the same research group published [26] an improved synthesis of 1 using the same synthetic strategy but varying the aryliodide partner (Scheme 6). In the synthesis discussed above, the methoxycarbonyl group was introduced into the aromatic ring after creating the cyclopentane ring C, since the deactivating effect of the ester group would make rather difficult the Friedel–Crafts cyclization. Wu and colleagues [26] found that TMSOTf is an extremely powerful additive, which can efficiently promote the Friedel–Crafts process even in when the aryl ring has electron-withdrawing groups, such as an ester. For that reason, they chose the aryliodide 35 as cross-coupling partner of the tosylhydrazone 26. The palladium catalyzed reaction afforded 36 as a mixture of diastereomers in a 79% combined yield. The hydrogenation of this mixture with H2 over Pd/C afforded compound 37 at a 87% yield. The stereoselective dehydroxylation of 37 was carried out with Sn(OTf)2, giving the trisubstituted alkene 38 at a 90% yield. The optimization of the cyclization of 38 into 15 in terms of Si4+ reagent, solvent, and temperature led to the conclusion that at high temperatures, TMSOTf was the most efficient promotor in dichloromethane, giving the tetracyclic compound 15 at a 81% yield. With the deprotection of the aryl methyl ethers in 15 by treatment with BI3, the synthesis of (-)-pelorol (1) [19] was completed in nine steps (19.5% overall yield) from (+)-sclareolide (5). The optimization of this modular strategy allows the shortening of the number of reaction steps, considerably improving the global yield, and represents an excellent methodology for the synthesis of multiple natural compounds. However, the high cost of the transition metal used to catalyze the coupling reaction between 26 and 35 limits the use of this synthetic route.
Scheme 6.
Wu’s second enantioselective synthesis of (-)-pelorol (1) from (+)-sclareolide (5).
2.5. Li’s Synthesis of (-)-Pelorol (1)
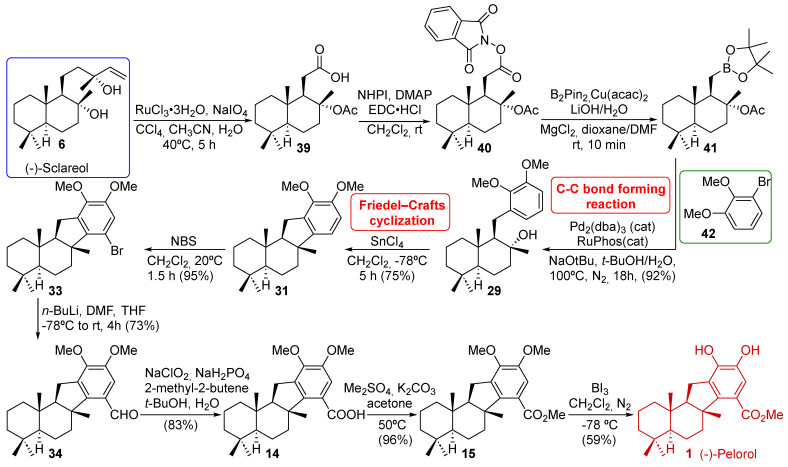
More recently, in 2024, Li and colleagues [20] reported an efficient synthesis of marine (-)-pelorol (1) from the inexpensive starting material (-)-sclareol (6). The key step was a Suzuki coupling of drimanyl Bpin 41 and arylbromide 42. As shown in Scheme 7, drimanyl Bpin 41 can be generated in three steps from 6 through a degradative sequence of reactions, which includes a cascade oxidative process of (-)-sclareol (6) with RuCl3·3H2O to yield homodrimanic acid 39. This acid can be converted to redox-active homodrimanyl-N-hydroxyphalimide ester 40 with N-hydroxyphthalimide (NHPT), and finally Cu-catalyzed decarboxylative borylation of 40 gives the desirable drimanyl Bpin 41. This intermediate was prepared at a 27.5% overall yield, after the flash-column chromatography purification of the intermediates, or at a 25% overall yield, after the solvent partitioning/aqueous wash workup of the crude residues of the three steps. The C-C bond formation reaction by Suzuki coupling between drimanyl Bpin 41 and 1-bromo-2,3-dimethoxybenzene 42 gave the key intermediate 29 at a 92% yield. The aromatic derivative 42 was obtained from 3-bromocathecol at a 91% yield. Subsequent Friedel–Crafts-type cyclization of the key intermediate 29 with SnCl4 at −78 °C gave the tetracyclic indane-fused decalin drimane 31 at a 75% yield with the desired diastereoselectivity. The introduction of formaldehyde in the aromatic ring was achieved through a two-step bromination- and lithium–halogen exchange-promoted formylation. Thus, when compound 31 was treated with NBS, the bromine derivative 32 was formed (95% yield). The reaction of 32 with n-BuLi and N,N-dimethyl formamide gave aldehyde 34 at a 73% yield. The subsequent Pinnick oxidation [29] of 34 followed by esterification gave methylated pelorol 14 in two steps (80% overall yield). Finally, the demethylation of product 15, previously reported by Andersen and colleagues [19], formed (-)-pelorol (1) with a 59% yield. The formal synthesis of (-)-pelorol (1) was completed in 10 steps, with a 5.6% overall yield.
Scheme 7.
Li’s enantioselective synthesis of (-)-pelorol (1) from (-)-sclareol (6). NHPI = N-Hydroxyphthalimide; DMAP = 4-Dimethylaminopyridine; EDC.HCl = N-Ethyl-N′-(3-dimethylaminopropyl)carbodiimide hydrochloride; B2Pin2 = Bis (pinacolato)diboron; NBS = N-Bromosuccinimide; DMF = N,N-dimethyl formamide.
The main drawback of this easy synthesis is the low yield of the three-step sequence required to prepare the drimanyl Bpin 41 from (-)-sclareol, even though it is scalable, and that the palladium catalyst employed in the coupling reaction between 41 and 42 has a high cost.
3. Future Perspectives
In light of the comprehensive review of the different synthesis of the marine (-)-pelorol (1) from the inexpensive and commercially available (+)-sclareolide (5) and (-)-sclareol (6) presented in Section 2, it is clear that the carbon skeleton of (-)-pelorol (1) can been successfully prepared through processes that include two key steps. The first one is the coupling of two partners (a drimanic derivative and an aromatic derivative) by different synthetic approaches that might include the addition of aryl-metallic reagents to a carbonyl group, the Suzuki coupling of the boron derivatives of the drimane portion, or the palladium-catalyzed tandem carbene migratory insertion of tosylhydrazone derivatives. The second key step is a Lewis acid-promoted C-C bond formation on the aromatic ring through a Friedel–Crafts reaction, with concomitant cyclization to form the C ring present in the tetracyclic indane-fused decalin drimane. The success of the first key step is due to the efforts made in the development of appropriate procedures for the preparation of the intermediates (20, 37, 41) that have been used in the divergent syntheses of this and other marine natural products. It is also worth mentioning that the introduction of the ester group in the aromatic ring before the cyclopentane ring formation by Friedel–Crafts cyclization (Scheme 6) can improve the overall yield of (-)-pelorol (1). The creation of the cyclopentane ring by the Friedel–Crafts reaction from an aromatic derivative with a deactivating substituent is possible thanks to the use of TMSOTf as an extremely effective additive.
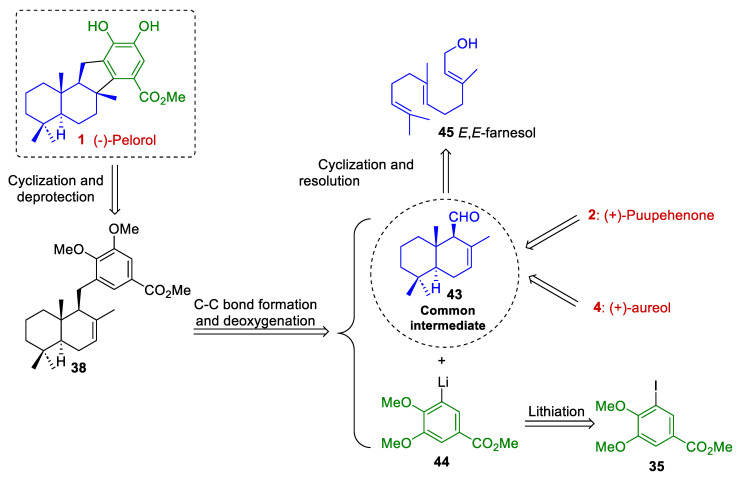
However, despite all the advances made, we think that a general strategy for (-)-pelorol (1) and analogues in a concise and divergent way is still desirable. In this section, based on our personal experience in the synthesis of natural compounds [30,31,32,33,34,35,36,37] and on the antecedents discussed in Section 2, we propose a retrosynthetic analysis of 1 in six steps, using the common synthetic intermediate 43 as the starting material. This aldehyde 43 could allow the divergent synthesis of meroterpenoids such as (-)-pelorol (1), (+)-puupehenone (2) [38], and (+)-aureol (4) using procedures previously reported by us (Scheme 8) [36,37,38,39,40].
Scheme 8.
Retrosynthetic plan of (-)-pelorol (1), (+)-puupehenone (2) [38], and (+)-aureol (4) [36] from a common intermediate 43 [40,41].
We propose that a convergent preparation of compound 1 is the best strategy for the obtention of this marine compound, since it can improve the overall yield and the efficiency of the multistep synthesis. Therefore, the key merosesquiterpene 38 could be prepared via addition of an aryllithium reagent 44 to the carbonyl group of 43. The aldehyde 43 can be prepared in an optically pure form from E,E-farnesol 45 by a cationic cyclization reaction and the subsequent resolution of the racemic mixture via the chromatographic techniques of the diastereomeric camphanoate derivatives [41,42].
The aryllithium 44 can be synthesized through the lithiation of 35. We consider the aromatic ring 44 to be the second partner in the coupling reaction with 43, since it has been shown to be useful in the Friedel–Crafts cyclization, and it shortened the synthetic route to (-)-pelorol (1) (Scheme 6) [26]. The deoxygenation of the C15-OH group of the coupling product between 43 and 44 would yield the key intermediate 38. The subsequent Friedel–Crafts cyclization and deprotection of the OMe groups would afford marine pelorol (1). Considering the retrosynthetic Scheme 8, it could be stated that the intermediate 43 would be a very valuable candidate in divergent synthesis. Its coupling with an appropriate aromatic partner would generate valuable intermediates for the synthesis of marine products with indane-fused decalin (e.g., (-)-pelorol (1)), the tetracyclic system with benzopyran-fused-naphthane (e.g., (+)-puupehenone (2) [38]), or the tetracyclic system with a substituted benzopyran moiety (e.g., (+)-aureol (4) [36,40].
The general retrosynthetic analysis reported in Scheme 8 represents a low-cost and powerful tool the synthesis of (-)-pelorol (1), which could be used to address its scarcely explored biological activities. This retrosynthetic analysis can also be easily used in the synthesis of an array of analogues of (-)-pelorol (1), simply by modifying the choice of the aromatic ring. Some derivatives with simple modifications of functional groups in their aromatic ring have been shown to be potential lead products for cancer therapy [21]. The quick and facile access to (-)-pelorol (1) and analogues through Scheme 8 would enable the establishment of a preliminary structure and activity relationships for further optimization.
4. Conclusions
A review of the synthesis of natural product (-)-pelorol (1) has been reported in terms of the synthetic procedure employed. Despite the successful synthesis of pelorol in a reasonable number of synthetic operations (9–12 steps; see Section 2) more practical access to this marine product is still desirable. Taking into consideration the best results from each strategy, we propose a concise synthesis of pelorol. The synthetic strategy is designed to require the minimum number of steps and is supported in mild conditions and operational easiness, which could be employed to address future biological activities.
Acknowledgments
Antonio Rosales Martínez acknowledges the University of the Sevilla for his position. The support by the Campus de Excelencia Internacional Agroalimentario (ceiA3) and the Centro de Investigación en Agrosistemas Intensivos Mediterráneos y biotecnología Agroalimentaria (CIAIMBITAL) is acknowledged.
Author Contributions
A.R.M.: conceptualization, design and coordination of the project, writing—original draft, writing—review and editing. I.R.-G.: writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
No new data were created.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This work was supported by the University of Seville, through the Vicerrectorado de Investigación (projects 2020/00001014 and 2021/00000422: Ayudas a Consolidación de Grupos de la Junta de Andalucía and Project Politec-Biomat: Red de Biomateriales en la Universidad de Sevilla); Vicerrectorado de Investigación e Innovación of University of Almería (Project: Fortalecimiento de Grupos 2023/88); and the Horizon 2020—Research and Innovation Framework Program of the European Commission, project 101022507 LAURELIN.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Geris R., Simpson T.J. Meroterpenoids produced by fungi. Nat. Prod. Rep. 2009;26:1063–1094. doi: 10.1039/b820413f. [DOI] [PubMed] [Google Scholar]
- 2.Gomm A., Nelson A. A radical approach to diverse meroterpenoids. Nat. Chem. 2020;12:109–111. doi: 10.1038/s41557-019-0414-7. [DOI] [PubMed] [Google Scholar]
- 3.Nazir M., Saleem M., Tousif M.I., Anwar M.A., Surup F., Ali I., Wang D., Mamadalieva N.Z., Alshammari E., Ashour M.L., et al. Meroterpenoids: A Comprehensive Update Insight on Structural Diversity and Biology. Biomolecules. 2021;11:957. doi: 10.3390/biom11070957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marcos I.S., Conde A., Moro R.F., Basabe P., Diez D., Urones J.G. Quinone/Hydroquinone Sesquiterpenes. Mini-Rev. Org. Chem. 2010;7:230–254. doi: 10.2174/157019310791384128. [DOI] [Google Scholar]
- 5.Menna M., Imperatore C., D’Aniello F., Aiello A. Meroterpenes from Marine Invertebrates: Structures, Occurrence, and Ecological Implications. Mar. Drugs. 2013;11:1602–1643. doi: 10.3390/md11051602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.García P.A., Hernández Á.P., San Feliciano A., Castro M.Á. Bioactive Prenyl- and Terpenyl-Quinones/Hydroquinones of Marine Origin. Mar. Drugs. 2018;16:292. doi: 10.3390/md16090292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El Sayed K.A., Bartyzel P., Shen X., Perry T.L., Zjawiony J.K., Hamann M.T. Marine Natural Products as Antituberculosis Agents. Tetrahedron. 2000;56:949–953. doi: 10.1016/S0040-4020(99)01093-5. [DOI] [Google Scholar]
- 8.Nasu S.S., Yeung B.K.S., Hamann M.T., Scheuer P.J., Kelly-Borges M., Goins K. Puupehenone-related metabolites from two Hawaiian sponges, Hyrtios spp. J. Org. Chem. 1995;60:7290–7292. doi: 10.1021/jo00127a039. [DOI] [Google Scholar]
- 9.Piña I.C., Sanders M.L., Crews P. Puupehenone Congeners from an Indo-Pacific Hyrtios Sponge. J. Nat. Prod. 2003;66:2–6. doi: 10.1021/np020279s. [DOI] [PubMed] [Google Scholar]
- 10.Longley R.E., McConnell O.J., Essich E., Harmody D. Evaluation of Marine Sponge Metabolites for Cytotoxicity and Signal Transduction Activity. J. Nat. Prod. 1993;56:915–920. doi: 10.1021/np50096a015. [DOI] [PubMed] [Google Scholar]
- 11.Castro M.E., Gonzalez-Iriarte M., Barrero A.F., Salvador-Tormo N., Munoz-Chapuli R., Medina M.A., Quesada A.R. Study of puupehenone and related compounds as inhibitors of angiogenesis. Int. J. Cancer. 2004;110:31–38. doi: 10.1002/ijc.20068. [DOI] [PubMed] [Google Scholar]
- 12.Faulkner D.J. Marine natural products. Nat. Prod. Rep. 1998;15:113–158. doi: 10.1039/a815113y. [DOI] [PubMed] [Google Scholar]
- 13.Singh M., Pal M., Sharma R.P. Biological Activity of the Labdane Diterpenes. Planta Med. 1999;65:2–8. doi: 10.1055/s-1999-13952. [DOI] [PubMed] [Google Scholar]
- 14.Lin Y.-S., Chen C.-H., Liaw C.-C., Chen Y.-C., Kuo Y.-H., Shen Y.-C. Cembrane diterpenoids from the Taiwanese soft coral Sinularia flexibilis. Tetrahedron. 2009;65:9157–9164. doi: 10.1016/j.tet.2009.09.031. [DOI] [Google Scholar]
- 15.Goclik E., König G.M., Wright A.D., Kaminsky R. Pelorol from the Tropical Marine Sponge Dactylospongia elegans. J. Nat. Prod. 2000;63:1150–1152. doi: 10.1021/np990502u. [DOI] [PubMed] [Google Scholar]
- 16.Kwak J.H., Schmitz F.J., Kelly M. Sesquiterpene Quinols/Quinones from the Micronesian Sponge Petrosaspongia metachromia. J. Nat. Prod. 2000;63:1153–1156. doi: 10.1021/np000079l. [DOI] [PubMed] [Google Scholar]
- 17.Ju E., Latif A., Kong C.-S., Seo Y., Lee Y.-J., Dalal S.R., Cassera M.B., Kingston D.G.I. Antimalarial activity of the isolates from the marine sponge Hyrtios erectus against the chloroquine-resistant Dd2 strain of Plasmodium falciparum. Z. Naturforsch. C Biosci. 2018;73:397–400. doi: 10.1515/znc-2018-0025. [DOI] [PubMed] [Google Scholar]
- 18.Carpi S., Scoditti E., Polini B., Brogi S., Calderone V., Proksch P., Ebada S.S., Nieri P. Pro-Apoptotic Activity of the Marine Sponge Dactylospongia elegans Metabolites Pelorol and 5-epi-Ilimaquinone on Human 501Mel Melanoma Cells. Mar. Drugs. 2022;20:427. doi: 10.3390/md20070427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang L., Williams D.E., Mui A., Ong C., Krystal G., van Soest R., Andersen R.J. Synthesis of Pelorol and Analogues: Activators of the Inositol 5-Phosphatase SHIP. Org. Lett. 2005;7:1073–1076. doi: 10.1021/ol047316m. [DOI] [PubMed] [Google Scholar]
- 20.Sun S., He X., Yang J., Wang X., Li S. Facile Synthesis and First Antifungal Exploration of Tetracyclic Meroterpenoids: (+)-Aureol, (−)-Pelorol, and Its Analogs. J. Nat. Prod. 2024;87:1092–1102. doi: 10.1021/acs.jnatprod.4c00037. [DOI] [PubMed] [Google Scholar]
- 21.Luo Y., Chen H., Weng J., Lu G. Synthesis of Pelorol and Its Analogs and Their Inhibitory Effects on Phosphatidylinositol 3-Kinase. Mar. Drugs. 2016;14:118. doi: 10.3390/md14060118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosales Martínez A., Rodríguez-García I., López-Martínez J.L. Divergent Strategy in Marine Tetracyclic Meroterpenoids Synthesis. Mar. Drugs. 2021;19:273. doi: 10.3390/md19050273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meimetis L.G., Nodwell M., Wang C., Yang L., Mui A.L., Ong C., Krystal G., Andersen R.J. Development of SHIP activators: Potentially selective cancer therapy; Proceedings of the 240th ACS National Meeting; Boston, MA, USA. 22–26 August 2010. [Google Scholar]
- 24.Dixon D.D., Lockner J.W., Zhou Q., Baran P.S. Scalable, Divergent Synthesis of Meroterpenoids via “Borono-sclareolide”. J. Am. Chem. Soc. 2012;134:8432–8435. doi: 10.1021/ja303937y. [DOI] [PubMed] [Google Scholar]
- 25.Wang H.-S., Nan X., Li H.-J., Cao Z.-Y., Wu Y.-C. A modular strategy for the synthesis of marine originated meroterpenoid-type natural products. Org. Biomol. Chem. 2021;19:9439–9447. doi: 10.1039/D1OB01598B. [DOI] [PubMed] [Google Scholar]
- 26.Cheng Y.-F., Li H.-J., Wang X.-B., Wu Y.-C. Concise synthesis of marine natural products smenodiol and (−)-pelorol. Nat. Prod. Res. 2023;37:1505–1510. doi: 10.1080/14786419.2021.2023871. [DOI] [PubMed] [Google Scholar]
- 27.Concepción J.I., Francisco C.G., Freire R., Hernandez R., Salazar J.A., Suarez E. Iodosobenzene diacetate, an efficient reagent for the oxidative decarboxylation of carboxylic acids. J. Org. Chem. 1986;51:402–404. doi: 10.1021/jo00353a026. [DOI] [Google Scholar]
- 28.Barluenga J., Moriel P., Valdés C., Aznar F. N-Tosylhydrazones as Reagents for Cross-Coupling Reactions: A Route to Polysubstituted Olefins. Angew. Chem. Int. Ed. 2007;46:5587–5590. doi: 10.1002/anie.200701815. [DOI] [PubMed] [Google Scholar]
- 29.Kakde B.N., Kumar N., Mondal P.K., Bisai A. Approach to Merosesquiterpenes via Lewis Acid Catalyzed Nazarov-Type Cyclization: Total Synthesis of Akaol A. Org. Lett. 2016;18:1752–1755. doi: 10.1021/acs.orglett.6b00446. [DOI] [PubMed] [Google Scholar]
- 30.Gansäuer A., Justicia J., Rosales A., Worgull D., Rinker B., Cuerva J.M., Oltra J.E. Transition-Metal-Catalyzed Allylic Substitution and Titanocene-Catalyzed Epoxypolyene Cyclization as a Powerful Tool for the Preparation of Terpenoids. Eur. J. Org. Chem. 2006;2006:4115–4127. doi: 10.1002/ejoc.200600389. [DOI] [Google Scholar]
- 31.Rosales A., López-Sánchez C., Alvarez-Corral M., Muñoz-Dorado M., Rodríguez-García I. Total Synthesis of (±)-Euryfuran Through Ti(III) Catalyzed Radical Cyclization. Lett. Org. Chem. 2007;4:553–555. doi: 10.2174/157017807782795457. [DOI] [Google Scholar]
- 32.Rosales A., Muñoz-Bascón J., Morales-Alcázar V.M., Castilla-Alcalá J.A., Oltra J.E. Ti(III)-Catalyzed, concise synthesis of marine furanospongian diterpenes. RSC Adv. 2012;2:12922–12925. doi: 10.1039/c2ra22281g. [DOI] [Google Scholar]
- 33.Rosales A., Muñoz-Bascón J., López-Sánchez C., Álvarez-Corral M., Muñoz-Dorado M., Rodríguez-García I., Oltra J.E. Ti-Catalyzed Homolytic Opening of Ozonides: A Sustainable C–C Bond-Forming Reaction. J. Org. Chem. 2012;77:4171–4176. doi: 10.1021/jo300344a. [DOI] [PubMed] [Google Scholar]
- 34.Rosales A., Foley L.A.R., Padial N.M., Muñoz-Bascón J., Sancho-Sanz I., Roldan-Molina E., Pozo-Morales L., Irías-Álvarez A., Rodríguez-Maecker R., Rodríguez-García I., et al. Diastereoselective Synthesis of (±)-Ambrox by Titanium(III)-Catalyzed Radical Tandem Cyclization. Synlett. 2016;27:369–374. doi: 10.1055/s-0035-1560594. [DOI] [Google Scholar]
- 35.Rosales Martínez A., Pozo Morales L., Díaz Ojeda E. Cp2TiCl-catalyzed, concise synthetic approach to marine natural product (±)-cyclozonarone. Synth. Commun. 2019;49:2554–2560. doi: 10.1080/00397911.2019.1633671. [DOI] [Google Scholar]
- 36.Rosales Martínez A., Enríquez L., Jaraíz M., Pozo Morales L., Rodríguez-García I., Díaz Ojeda E. A Concise Route for the Synthesis of Tetracyclic Meroterpenoids: (±)-Aureol Preparation and Mechanistic Interpretation. Mar. Drugs. 2020;18:441. doi: 10.3390/md18090441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torres-García I., López-Martínez J.L., López-Domene R., Muñoz-Dorado M., Rodríguez-García I., Álvarez-Corral M. Enantioselective total synthesis of putative dihydrorosefuran, a monoterpene with an unique 2,5-dihydrofuran structure. Beilstein J. Org. Chem. 2022;18:1264–1269. doi: 10.3762/bjoc.18.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosales Martínez A., Rodríguez-García I. Marine Puupehenone and Puupehedione: Synthesis and Future Perspectives. Mar. Drugs. 2023;21:322. doi: 10.3390/md21060322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.López-Martínez J.L., Torres-García I., Moreno-Gutiérrez I., Oña-Burgos P., Rosales Martínez A., Muñoz-Dorado M., Álvarez-Corral M., Rodríguez-García I. A Concise Diastereoselective Total Synthesis of α-Ambrinol. Mar. Drugs. 2023;21:230. doi: 10.3390/md21040230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosales Martínez A., Rodríguez-Maecker R.N., Rodríguez-García I. Unifying the Synthesis of a Whole Family of Marine Meroterpenoids through a Biosynthetically Inspired Sequence of 1,2-Hydride and Methyl Shifts as Key Step. Mar. Drugs. 2023;21:118. doi: 10.3390/md21020118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arjona O., Garranzo M., Mahugo J., Maroto E., Plumet J., Sáez B. Total synthesis of both enantiomers of 15-oxopuupehenol methylendioxy derivatives. Tetrahedron Lett. 1997;38:7249–7252. doi: 10.1016/S0040-4039(97)01683-3. [DOI] [Google Scholar]
- 42.Jordine G., Bick S., Möller U., Welzel P., Daucher B., Maas G. Studies on forskolin ring C forming reactions. Tetrahedron. 1994;50:139–160. doi: 10.1016/S0040-4020(01)80741-9. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created.