Abstract
An earlier report showed that viruses lacking the open reading frames encoding glycoproteins J and D but containing the glycoprotein D in their envelopes (gD−/+ stocks) and viruses lacking both the open reading frames and the glycoproteins in their envelopes (gD−/− stocks) induce apoptosis (G. Zhou, V. Galvan, G. Campadelli-Fiume, and B. Roizman, J. Virol. 74:11782–11791, 2000). Furthermore, apoptosis was blocked by delivery in trans of genes expressing glycoprotein D or J. Whereas gD−/− stocks attach but cannot initiate productive infection, gD−/+ stocks infect cells and produce gD−/− progeny virus. The difference in the infectivity of these two stocks suggested the possibility that the requirements for blocking apoptosis may be different. To test this hypothesis, we cloned into baculoviruses the entire wild-type glycoprotein D (Bac-gD-WT), the ectodomain only (Bac-gD-A), the ectodomain and the transmembrane domain (Bac-gD-B), the ectodomain and the cytoplasmic domain without the transmembrane domain (Bac-gD-C), or the transmembrane domain and the carboxyl-terminal cytoplasmic domain (Bac-gD-D). We report the following. Apoptosis induced by gD−/+ stocks was blocked by delivery in trans of recombinant baculovirus Bac-gD-WT, Bac-gD-A, Bac-gD-B, or Bac-gD-C but not of Bac-gD. Apoptosis induced by gD−/− stocks was blocked by Bac-gD-WT or by a mixture of Bac-gD-B and Bac-gD-D but not by any baculoviruses expressing truncated glycoprotein D alone or by the mixture of Bac-gD-A and Bac-gD-D. We conclude that the requirements to block apoptosis induced by the two virus stocks are different. The gD ectodomain is sufficient to block apoptosis induced by gD, whereas both the ectodomain and the cytoplasmic domain are required to block apoptosis induced by gD−/− stocks. The results indicate that in the case of gD−/− stocks, the transmembrane domain is required either to deliver the ectodomain to the appropriate intracellular compartment or to form multimeric constructs which virtually reconstitute gD through the interaction of transmembrane domains.
In an earlier article this laboratory reported that herpes simplex virus 1 (HSV-1) mutants lacking glycoprotein D (gD) and glycoprotein J (gJ) induced apoptosis in SK-N-SH cells and that either gD or gJ delivered in trans blocked apoptosis (22). Very little is known about gJ, a small glycoprotein shown not to be essential for viral replication (3, 5, 20). gD is one of at least 12 glycoproteins encoded by HSV-1. Following attachment to heparin-sulfated proteoglycans, gD interacts with at least one of two protein receptors on the cell surfaces, and with another viral protein, it causes the fusion of the envelope to the plasma membrane (for recent reviews, see references 4 and 18). The absence of gD enables the virus to attach to the heparin-sulfated proteoglycans, but the sequence of events varies depending on the cells in which the gD− mutants were produced. Specifically, gD− mutants replicating in cells expressing gD pick up the glycoprotein during envelopment. These viruses, designated gD−/+, carry gD in their envelopes and therefore are able to interact with the gD receptors and infect cells (15). Infection of cells which do not carry and express the gD gene produce an entirely different viral progeny. This progeny virus, designated gD−/−, replicates, assembles into virions lacking gD in their envelopes, and cannot egress from the infected cells. The main characteristic of this stock is that it can attach but cannot enter the infected cells in a productive mode. The particles that are taken up in endosome-like vesicles are degraded, and productive infection does not ensue (22). In the earlier study (22) we reported that both gD−/− and gD−/+ stocks induced apoptosis and that gD or gJ delivered in trans blocked apoptosis in cells infected with either stock of gD− virus (22).
The outcomes of infection with gD−/− and gD−/+ virus stocks are diametrically opposed. The cells infected with gD−/− stocks exhibit no evidence of productive infection. In contrast, cells infected with gD−/+ viruses exhibit by all criteria a typical single-cycle productive infection. The question of whether the mechanism by which the absence of gD translates into apoptosis differs for the two virus stocks arose. One way to approach this question is to determine whether the requirements for blocking apoptosis induced by gD delivered in trans differs for the two stocks of gD− viruses. We report that while the apoptosis induced by gD−/+ stocks can be blocked by the delivery in trans of a DNA sequence encoding the ectodomain, apoptosis induced by gD−/+ stocks requires polypeptides encoding all of the gD domains although not necessarily in one molecule.
It is useful to put the apoptosis induced by HSV-1 mutants into proper perspective. Viruses with mutations in several classes of viral genes, but not wild-type viruses, have been shown to induce apoptosis at least in some instances in a cell type-dependent manner. These viruses include deletion mutants lacking α4, α27, or gD, and one virus carrying a temperature-sensitive lesion in the UL36 open reading frame (ORF) (1, 2, 7–9, 13, 14, 21). The available data indicate that in the case of the α4− and most likely α27−, the inducer is a gene expressed relatively early, whereas the gene blocking apoptosis is most likely expressed later in infection. Thus, in the case of α4− mutant the blocking gene is US3 encoding a protein kinase (14, 17), whereas in the case of the gD− mutants the blocking gene is gD or gJ (22). It is of interest and significant that wild-type HSV-1 also protects cells from apoptosis induced by exogenous agents and that blocking genes appear to be pathway specific (7–9).
The interest in gD stems from the fact it is involved in the first interaction between a viral and cellular protein. The ultimate objective of these studies is to define the interaction that induces the exposed cell to initiate programmed cell death and the precise site and time at which gD acts to block apoptosis.
MATERIALS AND METHODS
Cells and viruses.
SK-N-SH cells were obtained from the American Type Culture Collection (Rockville, Md.) and maintained in Dulbecco's modified Eagle minimal essential medium containing 10% fetal bovine serum. Insect cell line sf9 (Spodoptera frugiperda) was obtained from PharMingen (San Diego, Calif.). Unless indicated otherwise, cultures were seeded less than 20 h prior to infection and assayed at 60 to 70% confluence. gD−/− and gD−/+ mutant viruses were produced as described in detail elsewhere (22). gD−/− titers were estimated as described in the earlier report (22). Dose-response analyses described earlier (22) have shown that gD−/− stocks required approximately 10-fold-higher titers to induce programmed cell death in HEp-2 cells than those of gD−/+ stocks.
Construction of baculovirus recombinants expressing truncated forms of gD.
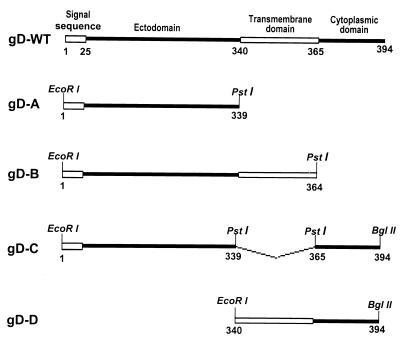
The baculovirus transfer vector pAc-CMV, which contained human cytomegalovirus immediate-early promoter-enhancer sequences in the XhoI-BamHI sites of pAc-SG2, was described elsewhere (1). The structure of the wild-type gD (gD-WT) has been extensively studied and is shown schematically in Fig. 1. We have included the signal sequence in the amino acid count. Depending on the algorithm used, the transmembrane domain was mapped starting with amino acids 340 to 364 (6, 12, 16). To construct the baculovirus recombinant expressing the ectodomain of gD (amino acids 1 to 339; named gD-A [Fig. 1]), an EcoRI-PstI fragment was amplified by PCR from pEA99 with primers CGGAATTCATGGGGGGGGCTGCCGCCAG and AACTGCAGGTTGTTCGGGGTGGCCGGGGG and then inserted into the EcoRI-PstI sites of pAc-CMV transfer vector. To construct the baculovirus recombinant expressing the ectodomain and transmembrane domain of gD (amino acids 1 to 364; named gD-B [Fig. 1]), an EcoRI-PstI fragment was amplified by PCR from pEA99 with primers CGGAATTCATGGGGGGGGCTGCCGCCAG and AACTGCAGCATCCAGTACACAATTCCGCAAATG and then inserted into the EcoRI-PstI sites of pAc-CMV. To construct the baculovirus recombinant lacking the transmembrane domain of gD (amino acids 340 to 364 deleted; named gD-C [Fig. 1]), a PstI-BglII fragment was amplified by PCR from pEA99 with primers AACTGCAGCGCCGCCACACTCAAAAAGCCCC and GAAGATCTCTAGTAAAACAAGGGCTGGTGCG, ligated in frame to the DNA fragment encoding the gD-A PCR fragment, and inserted into the EcoRI-BglII sites of pAc-CMV transfer vector. To construct the baculovirus recombinant expressing the transmembrane and cytoplasmic domains of gD (amino acids 340 to 394; named gD-D [Fig. 1]), an EcoRI-BglII fragment was amplified by PCR from pEA99 with primers GGAATTCATGGGCCTGATCGCCGGCGC and GAAGATCTCTAGTAAA ACAAGGGCTGGTGCG and inserted into the EcoRI-BglII sites of pAc-CMV. All of the constructs were sequenced to ensure fidelity.
FIG. 1.
Schematic diagram of the sequence arrangement of wild-type gD (amino acids 1 to 394) and truncated forms used in this study: gD-A (amino acids 1 to 339), gD-B (amino acids 1 to 364), gD-C (amino acids 340 to 364 deleted), and gD-D (amino acids 340 to 394).
The recombinant baculoviruses were generated, and mammalian cells were infected as described elsewhere (22).
Immunoblot assays.
H170, a well-characterized monoclonal antibody against gD, was purchased from the Goodwin Cancer Research Institute (Plantation, Fla.). Protein concentrations in whole-cell lysates were determined with Bio-Rad protein assay reagent (Bio-Rad Laboratories, Hercules, Calif.). Infected- or uninfected-cell lysates (50 μg of protein per lane) were electrophoretically separated in a 12% denaturing polyacrylamide gel, electrically transferred to a nitrocellulose sheet, blocked for 2 h in 5% milk in phosphate-buffered saline (PBS) at room temperature, and then allowed to react with the primary antibody as indicated in Results. The protein bands were visualized by using an enhanced chemiluminescence detection system (Pierce, Rockford, Ill.) according to the instructions of the manufacturer.
Double infection.
Subconfluent cultures of SK-N-SH cells in 25-cm2 flasks were first exposed to 10 PFU of recombinant baculovirus per cell for 2 h at 37°C and then to either 100 PFU equivalents of gD−/− or 10 PFU of gD−/+ mutant HSV-1 viruses per cell. The cultures were then maintained for an additional 24 h at 37°C in medium containing 2.5 mM sodium butyrate.
Immunofluorescence.
Glass slides were seeded with 5 × 104 SK-N-SH per well and either exposed to baculoviruses only or doubly infected as described above. At the end of the incubation period, the cells were fixed in ice-cold methanol for 20 min at −20°C, then blocked in PBS containing 1% bovine serum albumin at room temperature, rinsed three times with PBS, and allowed to react for 24 h at 4°C with a 1:2,000 dilution of mouse monoclonal antibody against gD in PBS. The cells were rinsed five times in PBS, allowed to react for 1 h with a goat anti-mouse immunoglobulin G (IgG) (diluted 1:64), conjugated to fluorescein isothiocyanate (FITC) (Sigma, St. Louis, Mo.) in PBS, rinsed five times with PBS, and mounted in 90% glycerol. The fluorescent images were captured with the aid of a Zeiss confocal microscope.
DNA fragmentation assay.
The cellular DNA was fragmented as described elsewhere (22).
RESULTS
Expression of truncated gD polypeptides.
As detailed in Materials and Methods and illustrated in Fig. 1, we constructed four gD ORF truncations which expressed the ectodomain (gD-A), the ectodomain and transmembrane region (gD-B), ectodomain and cytoplasmic domain but without the intervening transmembrane domain (gD-C), and transmembrane and cytoplasmic domains (gD-D). For constructs gD-A, gD-B, and gD-C, the integrity and expression of the gene products were analyzed in three different ways shown below.
(i) All constructs were sequenced to ensure that they do not contain amino acid substitutions. The portions of the gD gene retained in the construct were identical to the sequences contained in HSV-1(F) gD.
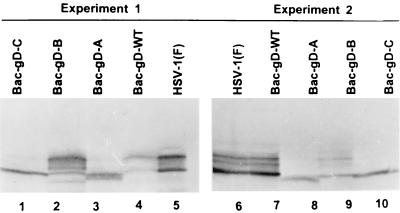
(ii) SK-N-SH cells were infected with 10 PFU of recombinant baculoviruses expressing gD-WT, gD-A, gD-B, or gD-C. The cultures were harvested 24 h after infection and lysed, and the lysates were subjected to electrophoresis in a denaturing polyacrylamide gel, transferred to a nitrocellulose sheet, and allowed to react with an anti-gD monoclonal antibody. The results shown in Fig. 2 indicate that all recombinant baculoviruses expressed a protein that reacted with the antibody to gD. The data show that gD encoded by HSV-1(F) and by the baculoviruses expressing gD-WT or the construct gD-B in which the ectodomain was linked to the transmembrane domain were extensively posttranslationally processed, whereas gD expressed by constructs gD-A and gD-C was not extensively processed.
FIG. 2.
Agarose gel of lysates of SK-N-SH cells infected with wild-type HSV-1 or with recombinant baculoviruses expressing intact or truncated forms of gD and reacted with monoclonal antibody against gD. SK-N-SH cells were infected with 10 PFU of recombinant baculoviruses expressing construct gD-WT, gD-A, gD-B, or gD-C per cell and incubated at 37°C for 24 h. The cells were then harvested, solubilized, electrophoretically separated in a denaturing polyacrylamide gel, transferred to a nitrocellulose sheet, and allowed to react with the anti-gD monoclonal antibody and then with anti-mouse IgG conjugated to alkaline phosphatase. The results of two experiments are shown.
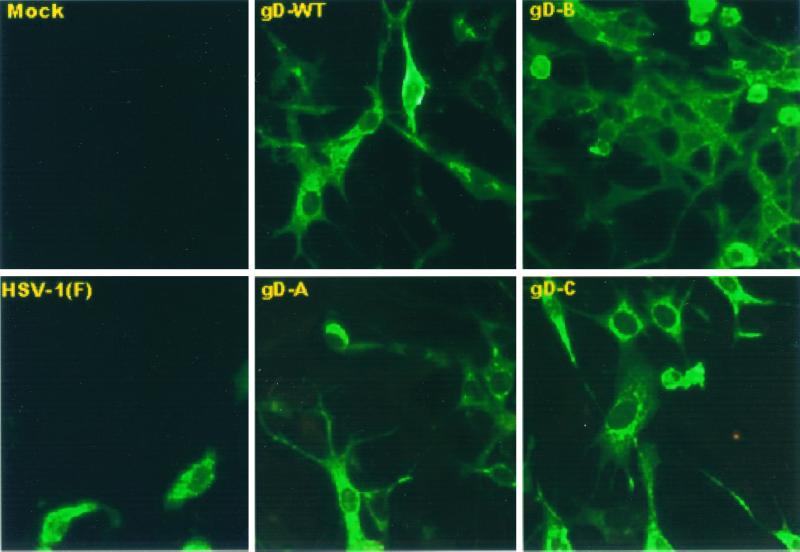
(iii) We verified the expression of gD in SK-N-SH cells infected with baculoviruses encoding gD-WT, gD-A, gD-B, and gD-C by visualization of the fluorescence patterns of infected cells. Cells infected with the baculoviruses encoding these constructs reacted with the anti-gD monoclonal antibody as shown in Fig. 3. Note that in cells infected with individual constructs, the immunofluorescence patterns varied from cell to cell far more than the immunofluorescence patterns in cells infected by various constructs. In general, for each construct, the cells exhibited a diffuse cytoplasmic fluorescence or a pattern suggesting that the antigen was diffusely distributed in the endoplasmic reticulum or a pattern showing the antigens concentrated in structures resembling Golgi apparatus.
FIG. 3.
Digitized images of cells infected with HSV-1(F) and recombinant baculovirus as indicated. The cells infected with HSV-1(F) were fixed 12 h after infection, whereas the cells exposed to recombinant baculoviruses were fixed 24 h after infection. The cells were reacted with anti-gD antibody and then with anti-mouse IgG conjugated to FITC. The images were collected with a Zeiss confocal microscope as described in Materials and Methods.
Wild-type but not truncated forms of gD protect SK-N-SH from apoptosis induced by gD−/− virus.
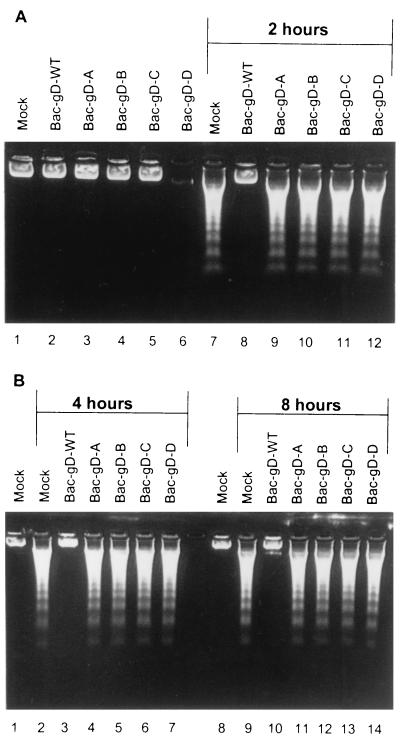
SK-N-SH cells were exposed to 10 PFU of recombinant baculovirus per cell and maintained for 2, 4, or 8 h (Fig. 4) before infection with 100 PFU equivalents of gD−/− virus per cell. The cells were harvested 24 h after gD−/− virus infection and examined for the presence of fragmented DNA. As shown in Fig. 4, baculoviruses expressing intact gD (Bac-gD-WT) blocked DNA fragmentation. In contrast, none of the baculoviruses expressing truncated gD were able to block the DNA fragmentation induced by gD−/− mutant virus stocks (Fig. 4A, lanes 7 and 9 to 12, and B, lanes 2, 4 to 7, 9, and 11 to 14). Baculoviruses expressing gD-WT or truncated gD did not cause fragmentation of cellular DNA (Fig. 4A, lanes 2 to 6).
FIG. 4.
Agarose gels containing electrophoretically separated DNA from mock-infected cells or cells infected first with recombinant baculoviruses and then with the indicated gD− virus stocks. Replicate cultures of subconfluent SK-N-SH cells in 25-cm2 flasks were infected with 10 PFU of the indicated recombinant baculovirus per cell. After the time intervals indicated in the figure, the cells were exposed to 100 PFU equivalents of gD−/− virus stock. The cells were harvested 24 h after HSV infection and processed as described in Materials and Methods.
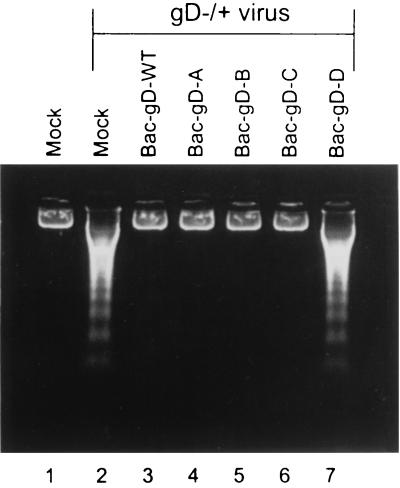
Apoptosis induced by gD−/+ mutant stocks is blocked by the expression of gD-A, gD-B, and gD-C.
SK-N-SH cells were exposed to 10 PFU of recombinant baculovirus per cell for 2 h and then infected with gD−/+ virus stock. The results are shown in Fig. 5. In contrast to the results obtained with the gD−/− stocks, all constructs containing the ectodomain alone or other domains of gD blocked the fragmentation of DNA induced by the gD−/+ mutant virus stock (lanes 3 to 6). DNA fragmentation was induced by the mutant virus stock in mock-infected cells and cells exposed to baculovirus encoding the gD-D construct (lanes 2 and 7, respectively).
FIG. 5.
Agarose gel containing electrophoretically separated DNA from mock-infected cells or cells infected first with recombinant baculoviruses and then with the indicated gD−/+ virus stocks. Replicate cultures of subconfluent SK-N-SH cells in 25-cm2 flasks were infected with 10 PFU of the indicated recombinant baculovirus per cell. The inoculate of doubly infected cells contained 10 PFU of each recombinant baculovirus per cell. After 2 h, the cells were infected with 10 PFU of gD−/+ virus per cell. The cells were harvested 24 h after HSV infection and processed as described in Materials and Methods.
Apoptosis induced by gD−/− mutant stock is blocked by double infection of SK-N-SH cells expressing a mixture of ectodomain plus transmembrane domain and cytoplasmic domain plus transmembrane domain.
Since we demonstrated above that only gD-WT blocked DNA fragmentation induced by gD−/− stock, the question then became whether all of the components of gD had to be present on a single molecule or whether they could be distributed on different molecules.
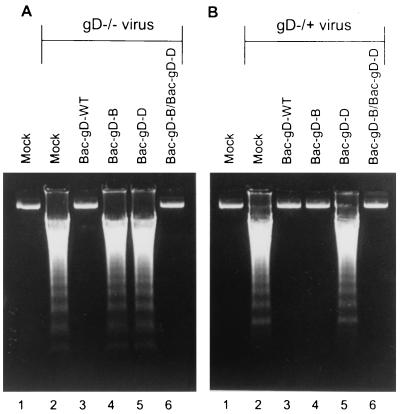
In the first of the experiments designed to answer this question, SK-N-SH cells were infected with baculoviruses expressing gD-WT or construct gD-B or gD-D or a mixture of baculoviruses expressing gD-B or gD-D and then infected with either gD−/− or gD−/+ HSV-1 mutant stocks. The results shown in Fig. 6 were as follows.
FIG. 6.
Agarose gels containing electrophoretically separated DNA from mock-infected cells or cells infected first with recombinant baculoviruses and then with the indicated gD−/− or gD−/+ virus stocks. Replicate cultures of subconfluent SK-N-SH cells in 25-cm2 flasks were infected with 10 PFU of indicated recombinant baculovirus per cell. The inoculum of doubly infected cells contained 10 PFU of each recombinant baculovirus per cell. After 2 h, the cells were infected with 100 PFU equivalents of gD−/− virus or 10 PFU of gD−/+ mutant virus stock per cell. The cells were harvested 24 h after HSV infection and processed as described in Materials and Methods.
(i) As shown above, the gD-B construct protected cells infected with gD−/+ stock but not cells infected with the gD−/− stock, whereas the gD-D construct did not protect cells infected with either virus (Fig. 6A, lanes 4 to 6, and B, lanes 4 to 6).
(ii) SK-N-SH cells infected with baculoviruses expressing gD-B and gD-D constructs blocked DNA fragmentation induced by infection with the stocks of either mutant viruses (Fig. 6A, lane 6, and B, lane 6).
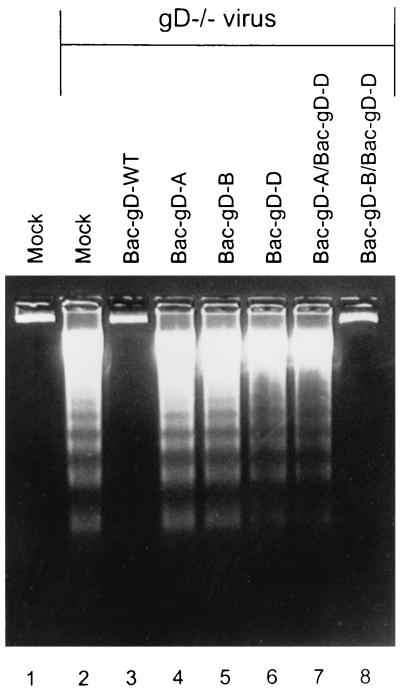
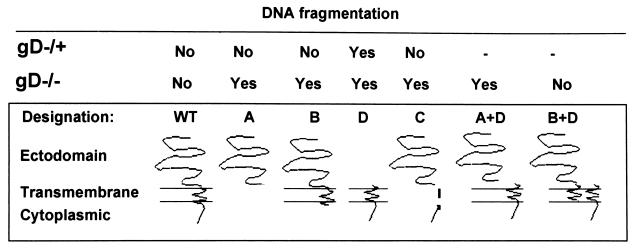
The experiment summarized above indicated that all three domains, i.e., the ectodomain, transmembrane domain, and cytoplasmic domain, were required to block the fragmentation of cellular DNA induced by the gD−/− mutant virus stock and that the three domains need not be present in a single molecule. The results also raised the possibility that the two gD-B and gD-D constructs interact by way of their transmembrane domains to form a heterodimer, and therefore, the question of whether a transmembrane domain was required to flank both the cytoplasmic domain and the ecodomain in order to block apoptosis arose. In the experiment shown in Fig. 7, SK-N-SH cells were infected with baculoviruses expressing gD-A, gD-B, or gD-D or with a mixture of baculoviruses expressing gD-A and gD-D or gD-B and gD-D. Two hours after exposure of cells to the recombinant baculoviruses, the cells were infected with gD−/− mutant virus stocks. The results were as follows. Again as shown in Fig. 4 and 6, gD, gD-A, gD-B, or gD-D did not block DNA fragmentation induced by gD−/− HSV-1 mutant stocks (Fig. 7, lanes 4 to 6). The baculovirus expressing gD-WT and the mixture of baculoviruses expressing gD-D and gD-B blocked DNA fragmentation, whereas the mixture expressing gD-A and gD-D was ineffective (Fig. 7, lanes 2, 7, and 8). A schematic representation of the results presented in this report is shown in Fig. 8.
FIG. 7.
Agarose gels containing electrophoretically separated DNA from mock-infected cells or cells infected first with recombinant baculoviruses and then with the indicated gD− virus stocks. Replicate cultures of subconfluent SK-N-SH cells in 25-cm2 flasks were infected with 10 PFU of indicated baculovirus per cell. The inoculum of doubly infected cells contained 10 PFU of each recombinant baculovirus per cell. After 2 h, the cells were infected with 100 PFU equivalents of gD−/− virus stock per cell. The cells were harvested 24 h after HSV infection and processed as described in Materials and Methods.
FIG. 8.
Schematic representation of the results presented in this report. The designations of the gD constructs cloned and expressed by the recombinant baculoviruses are the same as those shown in Fig. 1. The dashes in the top (gD−/+) row indicate that the experiments were not done.
DISCUSSION
The key feature of the results presented in this report is that the requirements for blocking apoptosis induced by gD−/+ virus stocks differed from those required to block apoptosis induced by gD−/− virus stocks. The following observations were relevant to this conclusion.
(i) All truncated forms of gD which contained an ectodomain blocked apoptosis induced by gD−/+ stocks. The smallest effective construct consisted of the ectodomain only. Notwithstanding differences in structure, it would be expected that gD-WT or all of the truncated forms containing an ectodomain would be present in the endoplasmic reticulum and in the lumen of transport vesicles to the Golgi apparatus and beyond. Our interpretation of the data is that in gD−/+ virus-infected cells, gD targets and interacts with a macromolecular structure accessible to all of the forms of gD that block apoptosis. Since gD interacts with its receptor both in trans, that is when they are in different but opposing membranes, and in cis, when they are in the same membrane, the interaction of gD with the macromolecular structures that blocks apoptosis could be similar (4).
(ii) In the initial series of experiments, only the intact gD-WT ORF delivered in trans blocked apoptosis induced by gD−/− stocks. The hypothesis that gD−/− stocks require in addition to the ectodomain both the transmembrane and cytoplasmic domains was verified in two experiments. In the first, a mixture of Bac-gD-B and Bac-gD-D blocked apoptosis. In the second, a mixture of Bac-gD-A and Bac-gD-D failed to block apoptosis. The data lend themselves to three conclusions. The first conclusion is that all three domains of gD, the ectodomain, transmembrane domain, and cytoplasmic domain, had to be present in order to block apoptosis. Thus, the presence of the cytoplasmic domain and ectodomain without the transmembrane domain (Bac-gD-C) was not sufficient to block apoptosis. The second conclusion is that the cytoplasmic domain and ectodomain could be in separate molecules, but each must be flanked by the transmembrane domain. This is evident from the observation that the Bac-gD-B and Bac-gD-D mixture was effective, whereas the Bac-gD-A and Bac-gD-D mixture was not. Last, the data indicate that the function performed by the ectodomain in the case of gD−/+ stock was not sufficient and that a second function involving the transmembrane domains and the cytoplasmic domains must play a role.
Of the various hypotheses that could explain our data, three are worthy of consideration. The trivial hypothesis is that the two ORFs recombine to regenerate a complete gD. The shared sequence, approximately 75 nucleotides, is too small to generate the huge amounts of recombinants necessary to block apoptosis in so large a number of cells as to fail to detect fragmentation of cellular DNA.
The second hypothesis more relevant to the data at hand is that the two active components, the ectodomain plus transmembrane domain and transmembrane domain plus cytoplasmic domain, each perform a separate function necessary to block apoptosis. A necessary conclusion of this hypothesis is that it predicts that the ectodomain plus transmembrane domain in gD−/− virus-infected cells performs a function that is different from that of ectodomain alone in gD−/+ virus stocks. If this hypothesis were true, it would imply that under different circumstances gD performs three different functions to block apoptosis, one encoded in the cytoplasmic domain, one encoded in the ectodomain linked to the transmembrane domain, and one which would be executed by the ectodomain bereft of the rest of the molecule.
Last, the third and more attractive hypothesis is that the transmembrane domain allows the formation of a multimeric protein that comes close to resembling gD-WT and could function as such for the purpose of blocking apoptosis—functions that may be distinct from those enabling entry of virus into cells. There is support for this hypothesis. HSV-1 gD forms dimers, whereas HSV-2 gD does not (10, 11). HSV-1 gD has an unpaired cysteine (Cys7) located in the transmembrane domain that is absent from HSV-2-gD (19). Substitution of the unpaired Cys7 precludes dimerization (21). Hence, Cys7 in the genes encoded by Bac-gD-D and Bac-gD-B could form a heterodimer that potentially retains some of the properties of gD.
The key conclusion enunciated above is that the requirements for blocking apoptosis induced by gD−/+ virus stocks differed from those required to block apoptosis induced by gD−/− virus stocks, paralleling the differences in the outcome of infection with the two viruses. gD−/+ stocks initiate infection and yield progeny virus, and newly formed viral proteins are translocated to the compartments they normally occupy in a productive infection. In contrast, gD−/− virus stocks do not initiate infection; at best, they are taken up in endosome-like vesicles and degraded (22). Therefore, we are faced with three possibilities. The first is that in the absence of gD, blocking apoptosis requires two different functions and that in gD−/+ virus-infected cells one is blocked by the gD ectodomain, whereas the other is blocked by another viral function. In the case of gD−/− virus, both functions must be blocked by gD. A less-complex postulate is that only one cellular function must be blocked by gD in cells exposed to either gD−/− or gD−/+ virus stock but that the gD ligand which gD must interact with to block apoptosis is in a different compartment. In gD−/+ virus-infected cells, the ligand could be accessible to the ectodomain, even in the absence of the other domains of gD. In the case of gD−/− virus-infected cells, the ligand would be in a compartment accessible only by the reconstituted multimer and not by the truncated species. This compartment would require the cytoplasmic domain, since the Bac-gD-B construct which in theory could also multimerize is not effective. Last, we cannot exclude the possibility that gD performs functions expressed separately by the ectodomain and cytoplasmic domains but that the function performed by the cytoplasmic domain requires that the components be linked.
An interesting property of HSV proteins is that they perform multiple functions. Elucidating the functions of gD may shed light on the selective forces generated by host defenses in the evolution of these viruses.
ACKNOWLEDGMENTS
We thank Gabriella Campadelli-Fiume for invaluable advice.
These studies were aided in part by grants from the National Cancer Institute (CA47451, CA71933, and CA78766) of the United States Public Health Service.
REFERENCES
- 1.Auber M, Blaho J A. The herpes simplex virus type 1 regulatory protein ICP27 is required for the prevention of apoptosis in infected human cells. J Virol. 1999;73:2803–2813. doi: 10.1128/jvi.73.4.2803-2813.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aubert M, O'Toole J, Blaho J A. Induction and prevention of apoptosis in human HEp-2 cells by herpes simplex virus type 1. J Virol. 1999;73:10359–10370. doi: 10.1128/jvi.73.12.10359-10370.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balan P, Davis-Poynter N, Bell S, Atkinson H, Browne H, Minson T. An analysis of the in vitro and in vivo phenotypes of mutants of herpes simplex virus type 1 lacking glycoproteins gG, gE, gI or the putative gJ. J Gen Virol. 1994;75:1245–1258. doi: 10.1099/0022-1317-75-6-1245. [DOI] [PubMed] [Google Scholar]
- 4.Campadelli-Fiume G, Cocchi F, Menotti L, Lopez M. The novel receptors that mediate the entry of herpes simplex viruses and animal alphaherpesviruses into cells. Rev Med Virol. 2000;10:305–319. doi: 10.1002/1099-1654(200009/10)10:5<305::aid-rmv286>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 5.Chang Y-E, Menotti L, Filatov F, Campadelli-Fiume G, Roizman B. UL27.5 is a novel γ2 gene antisense to the herpes simplex virus 1 gene encoding glycoprotein B. J Virol. 1998;72:6056–6064. doi: 10.1128/jvi.72.7.6056-6064.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisenberg R J, Long D, Sodora D L, Chiang H-Y, Wilcox W C, Abrams W R, Muggeridge M L, Cohen G H. Structure and function of glycoprotein D of herpes simplex virus. In: Becker Y, Darai G, editors. Frontiers in virology. Vol. 3. Heidelberg, Germany: Springer-Verlag; 1994. pp. 43–46. [Google Scholar]
- 7.Galvan V, Roizman B. Herpes simplex virus 1 induces and blocks apoptosis at multiple steps during infection and protects cells from exogenous inducers in a cell-type-dependent manner. Proc Natl Acad Sci USA. 1998;95:3931–3936. doi: 10.1073/pnas.95.7.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galvan V, Brandimarti R, Roizman B. Herpes simplex virus 1 blocks caspase-3-independent and caspase-dependent pathways to cell death. J Virol. 1999;73:3219–3226. doi: 10.1128/jvi.73.4.3219-3226.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galvan V, Brandimarti R, Munger J, Roizman B. Bcl-2 blocks a caspase-dependent pathway of apoptosis activated by herpes simplex virus 1 infection in HEp-2 cells. J Virol. 2000;74:1931–1938. doi: 10.1128/jvi.74.4.1931-1938.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibson M G, Spear P G. Insertion mutants of herpes simplex virus have a duplication of the glycoprotein D gene and express two different forms of glycoprotein D. J Virol. 1983;48:396–404. doi: 10.1128/jvi.48.2.396-404.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Handler C G, Eisenberg R J, Cohen G H. Oligomeric structure of glycoproteins in herpes simplex virus type 1. J Virol. 1996;70:6067–6070. doi: 10.1128/jvi.70.9.6067-6070.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lasky L A, Dowbenko D J. DNA sequence analysis of the type-common glycoprotein D genes of herpes simplex virus types 1 and 2. DNA. 1984;3:23–29. doi: 10.1089/dna.1.1984.3.23. [DOI] [PubMed] [Google Scholar]
- 13.Leopardi R, Roizman B. The herpes simplex virus major regulatory protein ICP4 blocks apoptosis induced by the virus or by hyperthermia. Proc Natl Acad Sci USA. 1996;93:9583–9587. doi: 10.1073/pnas.93.18.9583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leopardi R, Van Sant C, Roizman B. The herpes simplex virus 1 protein kinase US3 is required for protection from apoptosis induced by the virus. Proc Natl Acad Sci USA. 1997;94:7891–7896. doi: 10.1073/pnas.94.15.7891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ligas M W, Johnson D C. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by β-galactosidase sequences binds to but is unable to penetrate into cells. J Virol. 1988;62:1486–1494. doi: 10.1128/jvi.62.5.1486-1494.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGeoch D J, Dalrymple M A, Davison A J, Dolan A, Frame M C, McNab D, Perry L J, Scott J E, Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988;69:1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- 17.Munger J, Chee A, Roizman B. The US3 protein kinase blocks apoptosis induced by the d120 mutant of herpes simplex virus 1 at a premitochondrial stage. J Virol. 2001;75:5491–5497. doi: 10.1128/JVI.75.12.5491-5497.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spear P G, Eisenberg R J, Cohen G H. Three classes of cell surface receptors for alphaherpesvirus entry. Virology. 2000;275:1–9. doi: 10.1006/viro.2000.0529. [DOI] [PubMed] [Google Scholar]
- 19.Watson R J. DNA sequence of the herpes simplex virus type 2 glycoprotein D gene. Gene. 1983;26:307–312. doi: 10.1016/0378-1119(83)90203-2. [DOI] [PubMed] [Google Scholar]
- 20.Weber P C, Levine M, Glorioso J C. Rapid identification of nonessential genes of herpes simplex virus type 1 by Tn5 mutagenesis. Science. 1987;236:576–579. doi: 10.1126/science.3033824. [DOI] [PubMed] [Google Scholar]
- 21.Wilcox W C, Long D, Sodora D L, Eisenberg R J, Cohen G H. The contribution of cysteine residues to antigenicity and extent of processing of herpes simplex virus type 1 glycoprotein D. J Virol. 1998;62:1941–1947. doi: 10.1128/jvi.62.6.1941-1947.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou G, Galvan V, Campadelli-Fiume G, Roizman B. Glycoprotein D or J delivered in trans blocks apoptosis in SK-N-SH cells induced by a herpes simplex virus 1 mutant lacking intact genes expressing both glycoproteins. J Virol. 2000;74:11782–11791. doi: 10.1128/jvi.74.24.11782-11791.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]