Abstract
During primary varicella-zoster virus (VZV) infection, it is presumed that virus is transmitted from mucosal sites to regional lymph nodes, where T cells become infected. The cell type responsible for VZV transport from the mucosa to the lymph nodes has not been defined. In this study, we assessed the susceptibility of human monocyte-derived dendritic cells to infection with VZV. Dendritic cells were inoculated with the VZV strain Schenke and assessed by flow cytometry for VZV and dendritic cell (CD1a) antigen expression. In five replicate experiments, 34.4% ± 6.6% (mean ± SEM) of CD1a+ cells were also VZV antigen positive. Dendritic cells were also shown to be susceptible to VZV infection by the detection of immediate-early (IE62), early (ORF29), and late (gC) gene products in CD1a+ dendritic cells. Infectious virus was recovered from infected dendritic cells, and cell-to-cell contact was required for transmission of virus to permissive fibroblasts. VZV-infected dendritic cells showed no significant decrease in cell viability or evidence of apoptosis and did not exhibit altered cell surface levels of major histocompatibility complex (MHC) class I, MHC class II, CD86, CD40, or CD1a. Significantly, when autologous T lymphocytes were incubated with VZV-infected dendritic cells, VZV antigens were readily detected in CD3+ T lymphocytes and infectious virus was recovered from these cells. These data provide the first evidence that dendritic cells are permissive to VZV and that dendritic cell infection can lead to transmission of virus to T lymphocytes. These findings have implications for our understanding of how virus may be disseminated during primary VZV infection.
Varicella-zoster virus (VZV) is an alphaherpesvirus that is highly species specific, having a natural host range that is restricted to humans. VZV causes varicella (chicken pox) during primary infection in susceptible individuals, establishes latency in dorsal root ganglia, and may reactivate many years later as herpes zoster (shingles) (3). The initial stage of primary infection involves the inoculation of mucosal sites with virus from respiratory droplets or cutaneous vesicle fluid from an infected individual. After inoculation, the virus remains undetected by the host immune system during a prolonged incubation period (10 to 21 days). During this time the virus is presumed to spread to draining regional lymph nodes, resulting in T-cell infection and subsequent transport to other sites, including cells of the reticuloendothelial system in the liver (3, 18). The lymphotropism of VZV is critical for the dissemination of virus from peripheral blood mononuclear cells (PBMC) to epithelial cells, resulting in infection of the skin and the characteristic varicella rash (4). It remains unclear how VZV is transmitted from mucosal sites of inoculation to T-cell-containing draining regional lymph nodes. However, it is hypothesized that dendritic cells (DCs) of the respiratory mucosa may be the first target cells to encounter VZV during primary infection and subsequently transport virus to the draining lymph nodes to enable T-cell infection as well as initiating a virus-specific immune response (21).
DCs are bone marrow-derived potent antigen-presenting cells that are located in most tissues, including the skin, blood, lymph, and mucosal surfaces (23). DCs function to take up and process antigen in the periphery and transport viral antigens to T-cell-rich areas of the lymphoid organs, where they display major histocompatibility complex (MHC)-peptide complexes, together with costimulatory molecules. This results in the activation of naive and resting antigen-specific T cells and effector-T-cell differentiation (7).
Several human viruses, including human immunodeficiency virus (HIV), measles virus, influenza virus, human herpesvirus 6 (HHV-6), human cytomegalovirus, and herpes simplex virus (HSV), have been shown to infect human DCs (6, 8, 15, 16, 19, 31, 33, 34, 37). Virus infection of DCs has been postulated to enable these viruses to potentially interfere with immune responses as well as to contribute to the transmission of the virus in the host.
In this study, we assessed the susceptibility of human monocyte-derived DCs to VZV infection. We applied a combination of immunofluorescence, flow cytometry, and infectious center assays to demonstrate that VZV can productively infect human DCs and produce infectious virus. VZV infection of DC did not alter the cell surface expression of immune molecules or induce apoptosis. Furthermore, VZV-infected DCs were capable of transferring virus to autologous human T lymphocytes, causing productive infection of these cells. This study provides the first evidence that DCs are permissive to VZV infection and that infected DCs can transfer infectious virus to T lymphocytes.
MATERIALS AND METHODS
Cells and viruses.
Human foreskin fibroblasts (HFFs) were grown in tissue culture medium (Dulbecco's modified Eagle's medium; Gibco, Gaithersburg, Md.) supplemented with heat-inactivated fetal calf serum (FCS) (CSL, Parkville, Victoria, Australia), 2 mM l-glutamine (Gibco), 50 IU of penicillin, and 50 μg of streptomycin (Pen/Strep; ICN Biomedicals, Inc., Costa Mesa, Calif.). The VZV strain used in this study was a low-passage clinical isolate, designated strain Schenke. Virus was propagated in HFFs and stored in tissue culture medium with 10% dimethyl sulfoxide (Sigma).
Isolation of peripheral blood monocytes and T lymphocytes and the propagation of monocyte-derived DCs.
PBMC were separated from human blood by density gradient sedimentation on Ficoll-Paque (Lymphoprep; Nycomed). Monocytes and lymphocytes were then separated by countercurrent elutriation as previously described (27).
Cell fractions containing monocytes were collected and resuspended at 5 × 105 cells/ml in RPMI containing 10% FCS (RPMI-GM) and allowed to adhere to 24-well tissue culture dishes. After 2 h at 37°C in a 5% CO2 atmosphere, nonadherent cells were removed by aspiration and adherent cells were cultured with RPMI-GM containing 500 U of interleukin-4 (IL-4) (Schering Plough)/ml and 400 U of granulocyte-macrophage colony-stimulating factor (GM-CSF) (Schering Plough)/ml. On day 7 of culture, nonadherent cells were collected by gentle aspiration and transferred to fresh 24-well tissue culture plates (5 × 105 cells/well) (11). These cells consisted of >90% immature DCs as determined by their CD1a+ CD40+ CD86+ HLA-DR+ CD14− phenotype following immunostaining and flow cytometry.
Cell fractions containing T lymphocytes following countercurrent elutriation were resuspended at a concentration of 2 × 106 cells/ml in RPMI-GM supplemented with 10% IL-2 (Boehringer, Mannheim, Germany) and incubated in a tissue culture flask at 37°C in a 5% CO2 atmosphere. Cells were resuspended every 2 days with fresh RPMI-GM containing IL-2 and plated at 2 × 106 cells/ml in 12-well culture plates. An aliquot of these cells was immunostained with an anti-CD3 antibody and analyzed by flow cytometry. This fraction of cells contained ∼95% CD3+ T lymphocytes.
Infection of DCs with VZV.
DCs were infected with VZV by adding VZV-infected fibroblasts to DCs at a ratio of 1:2. Prior to mixing with DCs, the degree of VZV infection of the inoculating fibroblasts was scored using a scale from 0 to 4+ , where 0 corresponded to no detectable infection and 4+ corresponded to 100% cytopathic effect. Inoculation of DCs was done using fibroblasts showing a 2 to 3+ infection. By infectious center assay, inoculation of 105 fibroblasts at 2 to 3+ infection typically results in a titer of 104 infectious centers on fibroblast monolayers.
DCs and infected fibroblasts were mixed together in 24-well tissue culture plates and centrifuged at 150 × g for 15 min at room temperature. Mock-infected cultures were set up as described above using uninfected fibroblasts. Twenty-four hours after inoculation, the nonadherent DCs were removed and placed into another 24-well plate with RPMI–10% FCS medium containing GM-CSF and IL-4 cytokines. The medium containing cytokines was replaced every 2 days of culture.
Infection of T lymphocytes with VZV-infected DC.
VZV strain Schenke-infected DCs were incubated with autologous T lymphocytes at a ratio of 1:5 in RPMI-GM supplemented with IL-4, GM-CSF, and IL-2 for 4 days. Cells were aliquoted into 24-well tissue culture plates at 5 × 105 T lymphocytes/well and centrifuged at 150 × g for 20 min at room temperature. Mock-infected cultures were set up as described above using uninfected DCs. The medium containing cytokines was replaced every 2 days of culture.
Antibodies.
Monoclonal antibodies specific for human MHC class II DR (clone TU36; R-phycoerythrin [PE] conjugated), human CD14 (clone TUK4; fluorescein isothiocyanate [FITC] conjugated), human CD40 (clone 14G7; unconjugated), human CD71 (clone T56/14), human CD3 (clone S4.1; tricolor [TC] conjugated), human CD4 (clone S3.5; PE conjugated), human CD8 (clone 3B5; TC conjugated), PE-conjugated goat anti-human immunoglobulin G (IgG), FITC-conjugated goat anti-human IgG, TC-conjugated goat anti-human IgG, mouse IgG, mouse IgG2a-PE, mouse IgG2a-TC, and mouse IgG2a-FITC were obtained from Caltag Laboratories (San Francisco, Calif.). Monoclonal antibodies specific for human CD1a (clone NA1/34; unconjugated) [Dako (Australia), Botany, New South Wales], human CD3 (clone UCHT1) (Dako), HLA-DR (clone IQU9; unconjugated) (Novacastra, Peterborough, United Kingdom); CD86 (clone FUN-1; PE conjugated), and CD80 (clone BB1; FITC conjugated) (PharMingen, San Diego, Calif.) were also utilized. VZV-immune or nonimmune (IgG-purified) polyclonal human serum was used for the detection of VZV-infected cells and was kindly provided by A. M. Arvin (Stanford University). Rabbit polyclonal antibodies specific for VZV open reading frames (ORFs) 4, 29, 61, and 62 and glycoprotein C were kindly provided by P. R. Kinchington (University of Pittsburgh).
FACS immunostaining.
Cells were harvested, and aliquots of 5 × 105 cells were washed and resuspended in 100 μl of fluorescence-activated cell sorter (FACS) staining buffer (phosphate-buffered saline [PBS] with 1% FCS, 0.2% sodium azide). Primary antibodies (VZV-immune and nonimmune human polyclonal IgG, anti-human CD1a, and anti-human CD40) were diluted 1:40 in FACS staining buffer. Secondary antibodies, goat anti-human FITC-conjugated F(ab′)2 fragments, and goat anti-mouse PE-conjugated and goat anti-mouse TC-conjugated F(ab)2 fragments were diluted 1:100. Tertiary mouse monoclonal antibodies (anti-HLA-DR-PE, anti-CD71-PE, anti-CD3-PE, anti-CD4-PE, anti-CD8-TC, and anti-CD86-PE) were diluted 1:50. As a negative control, cells were incubated with the appropriate isotype control antibodies to control for nonspecific antibody binding. All antisera were diluted in FACS staining buffer, and all reactions were done in the dark on ice for 30 min. The cells were washed between each antibody step with 2 ml of FACS staining buffer. After the final wash, cells were resuspended in orthofixative (PBS with 1% electron microscopy-grade formaldehyde) and analyzed using FACS Calibur and Cell Quest software (Becton Dickinson, San Jose, Calif.). Positive and negative staining with cell-surface-specific antibodies was determined by the level of fluorescence that exceeded, or did not exceed, levels determined by <98% of the cells from the same starting population when incubated with isotype control fluorochrome-conjugated antibodies.
Immunofluorescence staining and confocal microscopy.
Immunofluorescence staining was performed on cell spots and cells grown on coverslips. Approximately 5 × 104 cells were spotted onto glass slides and air dried. Cells were fixed and permeabilized with acetone at 4°C for 15 min and air dried for 1 h. Slides were washed once in PBS and incubated with blocking buffer (10% normal goat serum in PBS) at 37°C for 30 min, washed three times with PBS, and incubated with the primary antibodies diluted in blocking buffer for 30 min at 37°C. Primary antibodies used were mouse anti-human CD1a or anti-human CD3 (diluted 1:50), rabbit polyclonal antibodies against ORF4, ORF29, ORF61, ORF62, and glycoprotein C (diluted 1:100), and VZV-immune human polyclonal serum (diluted 1:100). Isotype control antibodies were used to control for nonspecific binding. Slides were washed three times in PBS, and the secondary antibodies were added for 30 min in the dark at 37°C. Secondary antibodies included Texas Red-conjugated goat anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, Pa.) (1:100) and FITC-conjugated goat anti-rabbit IgG (Caltag Laboratories) (1:100) diluted in blocking solution. After three washes in PBS, slides were mounted with Syva mounting fluid (Bering Diagnostics Inc.) and examined using an Optiscan laser scanning confocal microscope.
Infectious center assay.
Cells (104) from DC and T-cell infection experiments were added to sterile 24-well plates containing glass coverslips, preseeded with 105 HFFs. The 24-well plates were centrifuged at 150 × g for 15 min at room temperature. The cells were incubated for 7 days at 37°C in 5% CO2. Following aspiration of the supernatant, the coverslips containing adherent cells were fixed in acetone at 4°C for 15 min and viral antigens were detected by indirect immunofluorescence using a VZV immune human polyclonal antibody as described above. The number of infected centers was determined using a fluorescence microscope.
Transwell assay.
Cell-free virus release from VZV-infected DCs was evaluated by a transwell assay. Cells were placed in 1-μm-pore-size transwells over an HFF cell monolayer grown on glass coverslips. As a control, mock-infected DCs and infected and uninfected HFFs were treated in a similiar manner. Seven days later, the coverslips were fixed and stained for VZV antigens as described above.
Cell separation.
CD1a+ DC and CD3+ T lymphocytes were separated from total cells using an indirect cell selection method. Cells were washed in PBS, resuspended in 0.1% EDTA, and incubated at 37°C for 10 min. Cells were washed and resuspended in PBS containing 2 mM EDTA–1% FCS and incubated with the appropriate antibody against the desired cell surface marker for 30 min at 4°C. In these experiments, an anti-human CD1a antibody and an anti-human CD3 antibody were used as selection markers for DCs and T lymphocytes, respectively. Cells were washed twice in PBS containing 2 mM EDTA–1% FCS and then incubated with goat anti-mouse IgG microbeads (Miltenyi Biotech), and selection and release of target cells was done according to the manufacturer's directions (Miltenyi Biotech). Typically, 98% cell purity was obtained, as shown by immunostaining and flow cytometry.
TdT-mediated dUTP-biotin nick end labeling (TUNEL) staining.
Cell spots were fixed in 10% phosphate-buffered formalin for 15 min at room temperature, washed twice with PBS and once with 70% ethanol, and air dried overnight. Cell spots were incubated with 2 μg of proteinase K (GIBCO BRL)/ml in PBS for 15 min at 37°C and then washed three times in PBS at room temperature. Fifty microliters of 3′-OH DNA labeling mix {1× terminal deoxynucleotidyl transferase (TdT) reaction buffer (0.5 M potassium cacodylate [pH 7.2], 10 mM CaCl2, 1 mM dithiothreitol [GIBCO BRL]), 50 μM biotin-14-dCTP (GIBCO BRL), and 0.2 U of TdT (GIBCO BRL)/μl} was added to each slide and incubated at 37°C for 30 min. Positive control slides were incubated with 50 μl of reaction mixture plus 3 U of DNase I (GIBCO BRL) in 0.1 M sodium acetate–5 mM MgCl2 (pH 5.0) for 30 min at 37°C prior to the addition of the labeling mix. The negative control slides were incubated with a 3′-OH DNA labeling mixture containing no TdT. Slides were washed three times with PBS and incubated with 5 μg of streptavidin-fluorescein (GIBCO BRL)/ml in 5% skim milk in PBS for 30 min at 37°C. Finally, slides were washed three times in PBS, one drop of fluorescence Syva antifade mounting fluid (Bering Diagnostics Inc.) was added, and a glass coverslip (Mediglass, Taren Point, New South Wales, Australia) was placed over the cells. In parallel with apoptosis experiments, cell viability was assessed by staining with 0.04% trypan blue for 5 min before counts were done using light microscopy.
Stripping assay procedure.
Acid stripping of cell-surface-bound extraneous membranes was performed in a stripping medium consisting of cold RPMI lacking bicarbonate, supplemented with 1% BSA and adjusted to pH 2.8 with HCl. Cells were washed once in cold PBS, centrifuged at 270 × g for 7 min, and then resuspended in stripping medium for 3 min at 4°C. Stripping was stopped by the addition of neutralizing wash medium RPMI supplemented with 100 mM HEPES, pH 7.2, followed by two consecutive washes in this medium. Cells were resuspended in RPMI-GM. This procedure removes any extraneous cellular membranes bound to cells without significantly altering cell viability or the membrane expression of cell surface molecules (13). A control for this assay involved stripping biotinylated HIV gp120 from the surfaces of human monocyte-derived DCs. The success of the assay was determined by flow cytometry assessment for surface gp120.
RESULTS
VZV infection of human DCs.
VZV is a highly species-specific virus which replicates efficiently in human cells such as fibroblasts and has been shown to infect T lymphocytes and neuronal cells (24, 38). However, susceptibility of DCs to VZV infection has yet to be studied. To determine whether VZV can infect DCs, human DCs were generated from adult PBMC in the presence of GM-CSF and IL-4 as previously described (11). On day 7, nonadherent cells were collected and >90% were shown by immunostaining and flow cytometry to have converted to an immature DC phenotype (i.e., CD1a+ MHC II+ CD14−) (data not shown). VZV is a highly cell-associated virus in experimentally infected cells, and high-titer cell-free virus stocks cannot be generated (3). We therefore inoculated DCs with the VZV strain Schenke (a low-passage clinical isolate) by mixing VZV-infected fibroblasts and DCs at a ratio of 1:2. Twenty-four hours postinoculation, nonadherent cells were collected and cultured in the presence of GM-CSF and IL-4. On day 2 postinfection, cells were stained with antibodies to VZV antigens and CD1a and analyzed by flow cytometry. Negative controls included DCs incubated with uninfected fibroblasts (mock infection) and incubation of both mock- and VZV-infected cells with isotype control antibodies.
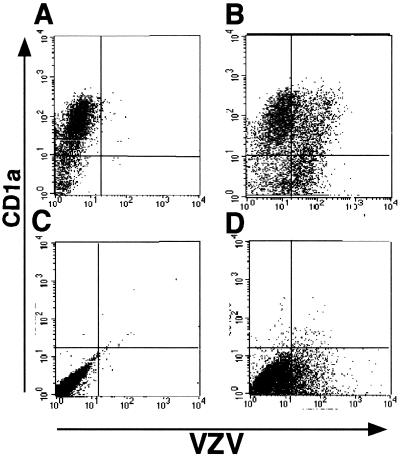
At 2 days postinfection, 42.0% of CD1a+ cells (i.e., DCs) were VZV+ (Fig. 1B). VZV+ cells were not detected in mock-infected CD1a+ DCs (Fig. 1A). In a total of five replicate experiments using five different adult PBMC donors to generate DCs, between 15.4 and 45.0% (mean ± standard error of the mean [SEM] = 34.4% ± 6.6%) of CD1a+ cells expressed VZV antigens on day 2 postinfection. In two of these experiments, we also examined DCs on days 4 and 7 postinfection but saw no significant increase or decrease in the percentage of CD1a+ VZV+ cells compared to results for day 2 (data not shown). We also assessed the expression of CD1a on mock- and VZV-infected fibroblasts to determine whether these cells had the ability to express CD1a. Neither mock- nor VZV-infected fibroblasts expressed CD1a, as determined by flow cytometry (Fig. 1C and D), validating our use of CD1a as a DC-specific marker in these experiments. We concluded that human DCs express VZV antigens after exposure to VZV.
FIG. 1.
Flow cytometry analysis of expression of VZV antigen on the surfaces of human DCs and human fibroblasts infected with VZV. Human DCs were inoculated with uninfected or VZV-infected fibroblasts and collected 2 days postinoculation. Cells from mock-infected DCs (A), VZV-infected DCs (B), uninfected fibroblasts (C), and VZV-infected fibroblasts (D) were stained with antibodies and fluorescent conjugates to CD1a and VZV proteins and analyzed by flow cytometry.
Analysis of viral gene expression in VZV-infected DCs.
Like HSV, VZV is assumed to follow a cascade of immediate-early, early, and late viral gene expression (14). We used a panel of anti-VZV antibodies to assess the expression and subcellular localization of representative viral genes from each kinetic class by immunofluorescence staining and confocal microscopy. DCs were inoculated with VZV strain Schenke-infected fibroblasts or were mock infected as described above. At days 2, 4, and 7 postinfection, cells were spotted onto microscope slides, fixed and permeabilized with acetone, and incubated with rabbit polyclonal antibodies specific to either immediate-early (ORF62, ORF4), early (ORF29, ORF61), or late (gC) viral proteins. Cells were also stained with a mouse monoclonal antibody to CD1a. The bound rabbit polyclonal antibodies and mouse monoclonal antibody were detected with goat anti-rabbit-FITC and goat anti-mouse Texas Red conjugates, respectively. Negative controls were mock-infected DCs and incubation of both mock- and VZV-infected DCs with isotype control antibodies.
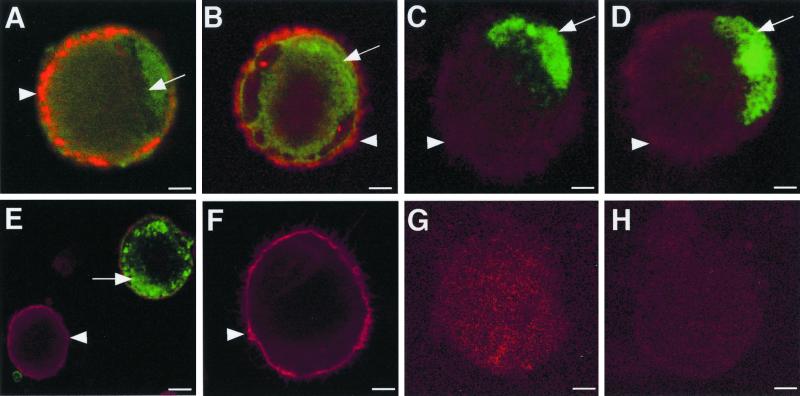
In VZV-infected DC cultures, dual-staining CD1a-positive (red staining) and VZV antigen-positive (green staining) cells were readily detectable at all time points tested (Fig. 2). The immediate-early VZV protein ORF62 localized to the nucleus and cytoplasm of CD1a+ DCs, whereas ORF4 was detected in the cytoplasm (Fig. 2A and B). The early-gene-product ORF29 and ORF61 viral antigens showed nuclear localization (Fig. 2C and D), and the late viral antigen gC localized to the cytoplasm and cell surface of CD1a+ DCs (Fig. 2E). Mock-infected cells stained positive for CD1a but did not stain positive for VZV IE62 antigen (Fig. 2F). In addition, neither VZV-infected nor mock-infected cell populations stained positive when isotype control antibodies for both CD1a and VZV antigens were used (Fig. 2G and H). In a further six experiments using different donor-derived DCs, VZV antigens from all three kinetic classes were observed in CD1a+ DCs. The subcellular localization of the viral gene products we assessed in DCs was consistent with that previously reported for productive infection of permissive cells (22). It was concluded that human DCs are permissive to VZV infection and are likely to support the full virus replicative cycle.
FIG. 2.
Immunofluorescence staining of CD1a and VZV antigens in VZV-infected DCs. Two days postinfection, DCs infected with VZV strain Schenke (A to E, G) and mock infected (F and H) were incubated with a mouse monoclonal antibody to CD1a (A to F) and rabbit polyclonal antibodies to ORF62 (A and F), ORF4 (B), ORF29 (C), ORF61 (D), and glycoprotein C (E). CD1a binding was detected using a Texas Red-conjugated anti-mouse antibody (red fluorescence). Rabbit antibodies generated against viral proteins were detected using a FITC-conjugated anti-rabbit antibody (green fluorescence). Negative controls were VZV-infected and mock-infected DCs incubated with isotype control antibodies (G and H, respectively). All negative control images were obtained by increasing the laser voltage to enable the visualization of cells. The arrowheads indicate CD1a staining, and the arrows indicate VZV-specific staining.
Transfer of infectious virus from DCs to human fibroblasts.
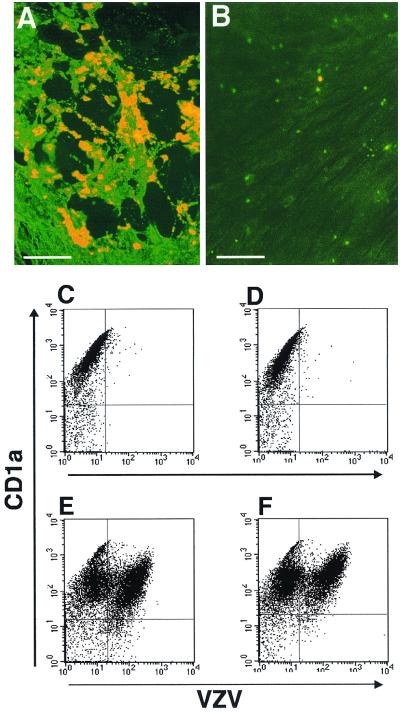
The above-described experiments assessed whether VZV could infect and synthesize viral antigens in human DCs. We next applied an infectious center assay to determine whether VZV-infected DCs could transmit infectious virus to fibroblasts. DCs were infected with the VZV strain Schenke as described earlier. On day 4 after virus inoculation, cells were harvested and incubated with an anti-CD1a monoclonal antibody. CD1a+ cells were isolated using a magnetic bead cell selection method, and aliquots of 10,000 cells were seeded per well onto fibroblast monolayers in 24-well plates. Cells were incubated for 7 days before being fixed with acetone and stained for VZV antigens using polyclonal human VZV immune serum. In a total of three separate experiments, VZV antigen-positive infectious centers (i.e., plaques) were readily detectable (Fig. 3A). The numbers of plaques ranged from approximately 50 to 150 per well. In these three experiments, the percentage of CD1a+ cells which were VZV+ was approximately 20, suggesting that approximately 2,000 CD1a+ VZV+ cells were seeded into each well. Our finding of 50 to 150 plaques per well indicates that 2.5 to 7.5% of CD1a+ VZV+ cells were capable of initiating an infectious center on fibroblasts under these assay conditions.
FIG. 3.
Infectious center assay and flow cytometry analysis of VZV-infected DCs. Dendritic cells inoculated with VZV-infected HFF were harvested at day 4 postinoculation. CD1a+ sorted cells (A) or media from VZV-infected fibroblasts (B) were incubated directly on HFF monolayers. Seven days later, cell monolayers were fixed and incubated with a human polyclonal VZV immune serum, followed by an FITC-conjugated anti-human antibody. In parallel, flow cytometry analysis of VZV antigen expression was performed on DCs stripped of surface antigens. Dendritic cells were inoculated with VZV-infected (E and F) or mock-infected (C and D) fibroblasts. Four days postinoculation, VZV-infected and mock-infected DCs from unstripped (C and E) and low-pH-buffer (stripped) cultures (D and F) were immunostained for CD1a and VZV antigens and analyzed by flow cytometry.
The following controls were included to ensure that the detection of infectious centers was due to the transfer of virus from infected DCs. First, in parallel with our model of DC infection, replicate cultures of VZV-infected fibroblasts, but without the addition of DCs, were also established. The medium from these cultures was collected and used to inoculate fresh fibroblast monolayers, which were then incubated for 7 days and assessed for infection as described above. In these cultures, no evidence of VZV infection or plaque formation was detected (Fig. 3B). Second, no plaques were detected when mock-infected DCs were placed in contact with fibroblast monolayers and incubated for 7 days (data not shown).
To determine whether cell-to-cell contact or release of cell-free virus was required to transmit virus to human fibroblasts, VZV-infected DCs (day 2 and day 4 postinfection) were seeded in the upper well of 1-μm transwells that were placed above fibroblast monolayers. After 7 days, the monolayers were fixed with acetone and analyzed by immunofluorescence staining for VZV antigen expression. No VZV antigen-positive cells or plaques were detected, indicating that cell-free virus was not the source of virus transmitted from DCs to fibroblasts. It was concluded that VZV productively infects DCs and transmits infectious virus to permissive fibroblasts by cell-to-cell contact in cultured cells.
To confirm that the transfer of virus from DCs to fibroblasts was not a result of infectious virus stuck to the outer cell surface membranes of DCs, the above experiments were repeated using a well-characterized acid stripping protocol to remove surface-bound molecules from infected DCs (13). Human DCs were inoculated with VZV-infected and uninfected fibroblasts for a period of 24 h and then washed in a low-pH buffer (stripping buffer) and recultured. As a control, DC-fibroblast cultures were not subjected to low-pH-buffer stripping but were washed in culture media and analyzed in parallel with infected cultures. On days 2, 4, and 7 postinfection, cells were stained for VZV antigens and for the CD1a cell surface marker and analyzed by flow cytometry. There was no significant difference in the number of CD1a+ VZV+ cells in either low-pH-buffer-stripped or unstripped VZV-infected DC cultures (Fig. 3E and F). CD1a+ cells from mock-infected DC cultures (stripped or unstripped) showed no expression of VZV antigens (Fig. 3C and D).
In addition, CD1a+ cells from VZV-infected day 2 DC cultures (stripped and unstripped) were selected using an immunomagnetic bead separation technique, resulting in a purity of ∼98% CD1a+ cells as determined by immunostaining and flow cytometry. These CD1a+ cells were seeded onto monolayers of human fibroblasts grown on glass coverslips in 24-well plates. Coverslips harvested on day 7 postinoculation were fixed and stained for VZV antigens. VZV-antigen-positive infectious centers were readily detectable in monolayers incubated with infected CD1a+ DCs from stripped and unstripped cultures (data not shown). Mock-infected CD1a+ DCs from both stripped and unstripped cultures failed to produce plaques or stain for VZV antigen expression. Taken together with the previous data, these experiments demonstrated that the transmission of infectious virus from DCs to fibroblasts was a result of bona fide VZV infection of DCs and not simply a transfer of infectious virus which had adhered to the surfaces of DCs.
Assessment of cell surface immune molecule expression on VZV-infected DCs.
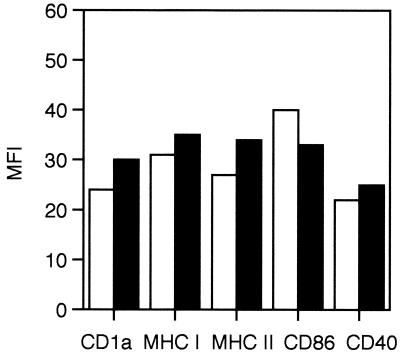
It has been previously shown that VZV can downmodulate cell surface expression of MHC class I and inhibit gamma interferon (IFN-γ)-induced MHC class II expression on the surfaces of VZV-infected fibroblasts (1, 2). To determine whether VZV infection of DCs alters the expression of cell surface immune molecules, VZV-infected and mock-infected DCs were assessed by flow cytometry for the expression of cell surface CD1a, CD86, CD40, MHC class I, and MHC class II. Human DCs were inoculated with VZV-infected and uninfected fibroblasts as previously described and were harvested on days 2, 4, and 7 postinfection. Cells were dual stained with a polyclonal human VZV immune serum to VZV antigens and a mouse monoclonal antibody specific to one of the following cell surface antigens: MHC class I, MHC class II, CD86, CD1a, and CD40. Negative controls included mock-infected cells and incubation of both mock- and VZV-infected cells with isotype control antibodies. In a total of three replicate dual-staining experiments at each of the time points tested, the mean fluorescence intensities of CD1a, CD86, CD40, MHC class I, and MHC class II were not altered significantly when VZV+ and VZV− DC populations were compared (Fig. 4 and data not shown). It was concluded that productive VZV infection of DCs did not affect the expression of these cell surface molecules.
FIG. 4.
Mean fluorescence intensity of CD1a, MHC class I, MHC class II, CD86, and CD40 protein expression on VZV-infected DCs. VZV-infected DCs were harvested at day 2 postinfection and dual-stained with VZV immune polyclonal human serum and monoclonal antibodies specific for CD1a, MHC class I (MHC I), MHC class II (MHC II), CD86, or CD40. The mean fluorescence intensity (MFI) values of VZV− cells (white boxes) and VZV+ cell populations (black boxes) are shown.
Analysis of DC viability and induction of apoptosis after VZV infection.
It has previously been reported that infection of DCs with some viruses can decrease cell viability by cell necrosis or apoptosis (9). VZV is cytopathic in many cell types and has also been show to induce apoptosis in specific cell types (20, 30, 32). We therefore determined whether VZV-infected DCs underwent apoptosis, using the TUNEL assay. The TUNEL assay detects fragmented DNA termini as a marker of apoptosis (17). DCs were infected with VZV as previously described, and on days 2, 4, and 7 postinfection, cells were fixed, treated with proteinase K, and incubated with a TdT reaction buffer containing biotin-14-dCTP which would label 3′ OH termini on fragmented DNA. Streptavidin-FITC was used to detect biotin-14-dCTP incorporation. Positive controls were VZV-infected and mock-infected DCs treated with DNase prior to TdT labeling. Negative controls included mock-infected DCs and VZV-infected and mock-infected DCs incubated with TdT reaction buffer containing no TdT.
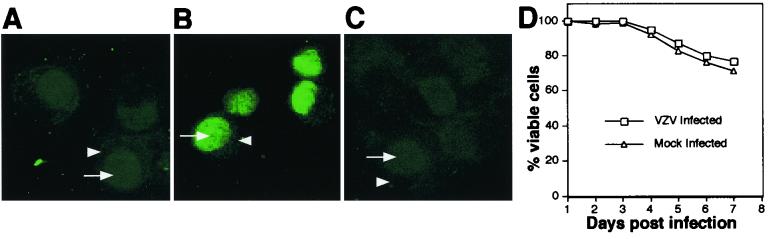
In VZV-infected DC cultures, none of the cells stained for fragmented DNA termini at any of the time points tested (Fig. 5A). In contrast, all the cells from VZV-infected or mock-infected samples treated with DNase stained for fragmented DNA termini (Fig. 5B). No fragmented DNA termini were detected in VZV-infected DC cultures incubated with TdT reaction buffer containing no TdT (Fig. 5C). Cell viability was also assessed by trypan blue exclusion staining. DCs were harvested on days 1 to 7 postinfection, stained with trypan blue, and counted. At all time points tested, there was no significant difference in cell viability between mock- and VZV-infected DC cultures (Fig. 5D). These data demonstrate that VZV infection of DCs does not induce detectable apoptosis by TUNEL staining or decrease cell viability.
FIG. 5.
Assessment of apoptosis and cell viability in VZV-infected DCs. DCs inoculated with uninfected fibroblasts or infected fibroblasts were harvested and stained with trypan blue or spotted onto sides for a TUNEL assay. TUNEL staining on VZV-infected DCs (A), in the presence of DNase (B), and in the absence of TdT (C) is shown. Each arrowhead indicates the cytoplasm, and each arrow indicates the nucleus of the cell. The percentages of viable cells for infected and uninfected DC culture over a 7-day culture period are shown (D).
Transfer of VZV from infected DCs to human T cells.
VZV has previously been shown to infect human T cells by injection of infected fibroblasts into SCID-hu thymus or liver implants (28) or by exposure of umbilical cord blood cells or a T-cell line to infected fibroblasts during mammalian cell culture (35, 39). While infection of T cells is likely to be a critical step in the dissemination of virus in the host, the question of how T cells become infected has not been fully addressed. Results from the present study have demonstrated VZV infection of DCs, a finding consistent with our hypothesis that these cells play a role in VZV pathogenesis. We therefore sought to determine whether infected DCs could transmit infectious virus to T cells. DCs and T cells from the same blood donor were used for these experiments. VZV-infected DCs were incubated with human T cells at a ratio of 1:5. On day 2 postinfection, cells were incubated with antibodies specific for VZV and the T-cell marker CD3 and analyzed by flow cytometry. Negative controls were mock-infected DCs cultured with T cells and cells from all cultures incubated with isotype control antibodies.
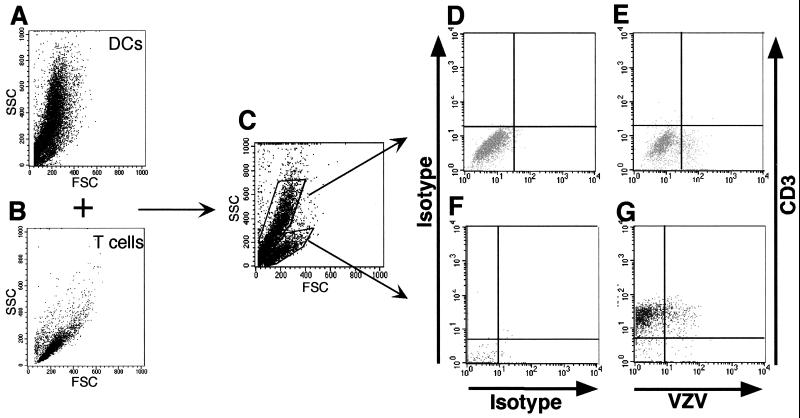
Flow cytometry analysis of VZV-infected DCs and T cells prior to incubation together showed two distinct cell populations as determined by forward scatter (FSC) and side scatter (SSC) profiles (Fig. 6A and B). Once mixed together, the DC and T-cell populations remained readily distinguishable based upon their FSC and SSC profiles (Fig. 6C). Gates were drawn around the DC and T-cell populations, and these cells were assessed for VZV antigen and CD3 expression by flow cytometry. In the T-cell population, 8.0% of CD3+ cells were VZV+ (Fig. 6G). In the DC population, 13.5% of cells were VZV+ (Fig. 6E). Importantly, these DCs (infected or uninfected) did not express CD3, validating the use of CD3 as a T-cell-specific marker. In four separate experiments from four different blood donors, 8.2% ± 1.1% (mean ± SEM) of CD3+ T cells were VZV+. VZV+ cells were not detected in cultures of mock-infected DCs incubated with T cells (data not shown). We also examined DC–T-cell cultures on days 2 and 4 postinfection and found no difference in cell viability between mock-infected and infected populations as assessed by trypan blue exclusion staining (data not shown).
FIG. 6.
Flow cytometry analysis of VZV-infected DCs cultured with T lymphocytes. VZV-infected DCs and autologous T cells were analyzed for their FSC and SSC properties before (A and B) and after (C) coculturing. The cocultured cells were stained 2 days later with antibodies and fluorescent conjugates to CD3 and VZV proteins. The flow cytometry analyses for T lymphocytes (G) and DCs (E) are shown with their appropriate isotype controls (F and D). The same numbers of cells were stained with antigen-specific (VZV and CD3) and isotype control antibodies.
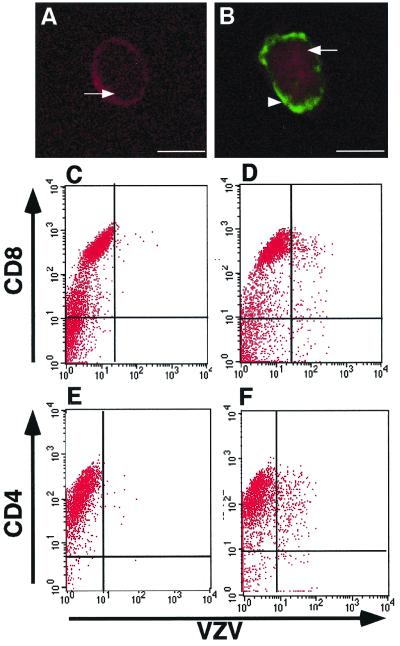
To visualize infection of T cells, magnetic bead separation was used to enrich for CD3+ cells from infected DC–T-cell cultures. These cells were then analyzed by immunofluorescence staining and confocal microscopy for the expression of CD3 and VZV antigens. Dual-staining CD3+ VZV+ cells were detected among cells isolated from infected DC–T-cell cultures (Fig. 7A) but not when cells were isolated from mock-infected DC–T-cell cultures (Fig. 7B) or when supernatants from infected DC cultures were incubated with T cells. These data demonstrated that infected DCs could directly transmit VZV to human T cells. In addition, acid stripping of infected DCs (as described above) did not alter the ability of DCs to transfer virus to human T cells (data not shown).
FIG. 7.
Immunofluorescence staining and flow cytometry analysis of human T lymphocytes inoculated with VZV-infected DCs. T lymphocytes were inoculated with uninfected or VZV-infected DCs. Two days later, cell preparations from mock-infected T cells (A, C, and E) and VZV-infected T cells (B, D, and F) were stained with antibodies and fluorescent conjugates to CD3, CD4, CD8, and VZV proteins and analyzed by confocal microscopy (A and B) or flow cytometry (C to F). The arrowhead indicates VZV-antigen-specific staining, and the arrows indicate CD3-specific staining.
We also sought to assess infection of CD4+ and CD8+ T-cell subsets by VZV-infected DCs. T cells were incubated with VZV-infected DC cultures for 2 days. Cells were harvested, stained with antibodies to VZV and either CD8 or CD4, and subjected to flow cytometry. The T-cell population was gated on the basis of FSC and SSC profiles, and this gated population was then analyzed for CD8-VZV or CD4-VZV expression. In VZV-infected cultures, 14.6% of CD8+ T cells and 11.3% of CD4+ T cells were VZV+ (Fig. 7D and F). In mock-infected cultures, VZV+ cells were not detected (Fig. 7C and E). It was concluded that VZV-infected DCs could transmit virus to both CD8+ and CD4+ T-cell subsets.
Transfer of infectious virus from infected T cells to human fibroblasts.
Given our finding that VZV-infected DCs could infect T cells, we used an infectious center assay to determine whether infected T cells produced infectious virus. VZV-infected DC cultures were used to inoculate T cells as described earlier. On day 2 after inoculation, cells were incubated with anti-human CD3 antibody and CD3+ T cells were isolated by passing cells through two magnetic bead separation columns. This method resulted in a purity of >98% CD3+ T cells as determined by immunostaining and flow cytometry analysis. These cells were then incubated with monolayers of human fibroblasts for 7 days before being fixed with acetone and stained for VZV antigens using polyclonal human VZV immune serum. In a total of two separate experiments, VZV antigen-positive infectious centers (i.e., plaques) were readily detectable. No plaques were detected when mock-infected T cells were placed in contact with fibroblast monolayers and incubated for 7 days (data not shown). It was concluded that VZV-infected DCs can productively infect human T cells, which can produce infectious virus.
DISCUSSION
This study shows for the first time that human monocyte-derived DCs are permissive to productive VZV infection. In addition, VZV-infected DCs can transfer infectious virus to autologous human T cells, resulting in a productive infection. These data support the hypothesis that DCs may be a major target for VZV infection and that Langerhans cells play a pivotal role in the transport of VZV from the site of initial entry (mucosal sites) to draining lymph nodes where the virus then infects T cells. The tropism of VZV for human T cells has been shown to play an important role in virus dissemination (3, 28), but there have been no previous studies examining the permissiveness of DCs to VZV.
We did not observe a decrease in DC viability or the induction of apoptosis when DCs were productively infected with VZV. T-cell viability also remained unaltered following DC-mediated VZV infection. These results imply that the virus has evolved a strategy to limit or prevent the onset of apoptosis. Such a strategy would provide a transient advantage to the virus, allowing it to successfully disseminate during the first critical days after primary infection. In addition, it remains possible that VZV may also infect DCs during the reactivation phase.
Several other viruses have also been shown to infect DCs and transmit virus to human T-cells with various outcomes. Our findings are similar to those of Asada et al. (6), who showed that HHV-6 infection of DCs and the subsequent transfer of virus to T cells did not affect the viability of either of these cell types. In contrast, HIV, which also infects DCs and rapidly transmits virus to T cells, causes rapid cell death of CD4+ T cells (12). Measles virus-infected DCs can also transfer virus to T cells, and this infection also leads to induction of apoptosis in both cell types (16). Infection of DCs by human cytomegalovirus is also cytopathic and results in cell lysis and death (31).
Despite the resistance of VZV-infected DCs and T cells to cell death, infectious center assays demonstrated that infectious virus was readily produced by both T cells and DCs infected with VZV, but virus transmission required cell-to-cell contact. The lack of cell-free virus released from infected T cells and DCs is in concordance with the highly cell-associated nature of VZV in vitro. The SCID-hu thymus-liver model of VZV infection is the only model to date that has demonstrated the release of cell-free virus from T cells and skin into the surrounding environment (29).
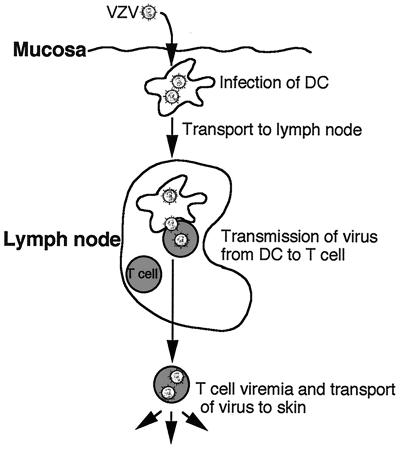
There are two major outcomes resulting from the interaction of VZV with DCs which could account for transfer of infectious virus to other cell types. First, VZV may productively infect DCs and transfer new progeny virus to other cells (e.g., T cells). Alternatively, because VZV envelope glycoproteins are known to bind mannose receptors found on immature DCs, the virus may be captured by DCs, internalized into trypsin-resistant compartments, and subsequently transmitted to other cells. Our data clearly support the former, as we were able to readily detect (by immunofluorescent staining and confocal microscopy) viral antigen expression from all three kinetic gene classes in subcellular locations consistent with those reported during productive infection of fully permissive fibroblasts (22). We have therefore proposed a model of VZV dissemination following primary inoculation (Fig. 8). In this model, upon entering the host at respiratory mucosa, VZV infects DCs (Langerhans cells), which are then triggered to mobilize and migrate to the T-cell-rich areas of regional lymph nodes. Direct interaction of productively infected DCs with T cells would then result in transmission of virus and productive infection of these T cells. Once infection of T cells occurs, the virus would continue to replicate and be disseminated to other sites of the body, infecting cutaneous epithelial cells with the formation of the characteristic vesicular rash of varicella. However, it remains possible that another mechanism exists by which VZV is only captured by DCs. Interestingly, it has been reported that HIV can either productively infect or be captured by DCs and that these two outcomes are mediated by separate pathways (10). Further analysis of VZV-DC interactions, including the potential to isolate infected DCs from patients undergoing primary (varicella) or recurrent (herpes zoster) infection, are likely to provide additional information on these outcomes.
FIG. 8.
Proposed model of virus transport from the site of mucosal inoculation to the T cells in lymph nodes during primary VZV infection.
DCs function to present antigenic peptides on the cell surface and to stimulate T-cells. Jenkins et al. (21) demonstrated that a naïve T-cell response can be induced in vitro by VZV antigenic peptides, suggesting that DCs may be involved in the initiation of the primary immune response in vivo. The induction of the primary T-cell response involves not only the recognition of antigenic peptides in association with cell surface MHC molecules but also the interaction of costimulatory molecules (26, 36). Several molecules are involved in this process, including CD86, CD80, CD40, CD54, and CD83, and the absence or decreased expression of these immune molecules can render a DC less capable of inducing a T-cell response (23).
It has been postulated that interference with DC function following viral infection may enable viruses to avoid immune recognition. In this respect, measles virus and HSV have been shown to interfere with the antigen-presenting capability of infected DCs by a variety of mechanisms. Measles virus can productively infect DCs and interfere with the cells' ability to induce the proliferation of CD4+ naïve T cells (19). Infection of mature DCs by HSV results in a decreased T-cell-stimulatory capacity and the specific degradation of the CD83 cell surface molecule (25). However, not all viruses which infect DCs interfere with DC antigen-presenting function. For example, HHV-6 productively infects DCs, but these cells can still function as antigen-presenting cells (6). We have previously demonstrated that VZV encodes the ability to specifically downmodulate cell surface MHC class I and IFN-γ-induced MHC class II expression during productive infection of primary human fibroblasts (1, 2). Interestingly, in the present study the immature DCs which we infected with VZV showed little or no change in the level of cell surface expression of MHC class I, MHC class II, CD40, and CD86. Further studies are required to determine whether the expression of other immune molecules is downregulated or inhibited or whether VZV infection of immature DCs inhibits DC maturation and hence function. Such studies may ultimately explain the apparent ability of VZV to evade the immune response during the extended 10- to 21-day incubation period following primary infection (5). In this respect, HSV has been shown to inhibit the maturation of immature DCs by preventing upregulation and expression of the costimulatory molecules CD80 and CD86, the maturation marker CD83, and the adhesion molecule ICAM-1 on the cell surface (25, 33).
While it was not the primary focus of the present study, we were able to successfully develop a model to study infection of human T cells. In this respect, there has been considerable effort from a number of groups aimed at developing models to study the interaction of VZV with T cells. The SCID-hu thymus-liver model has been used extensively for this purpose and was the first model to demonstrate VZV tropism for T cells (1, 28, 29). Using this model, typically 10 to 25% of human T cells (CD4+ and CD8+ T cells) can be productively infected with VZV following inoculation of human fetal thymus implants with VZV-infected fibroblasts. In a recent study using a CD4-positive human T-cell hybridoma (II-23) cell line, up to 30% of cells were shown to be green fluorescent protein positive after mixing II-23 cells together with human fibroblasts infected with a green fluorescent protein-tagged VZV (39). However, recovery of infectious virus was limited, with only 1 to 3 in 106 cells able to produce infectious virus as determined by plaque assay. Soong et al. (35) reported that approximately 3 to 4% of human umbilical cord blood-derived T cells could be infected with VZV. These infected T cells were able to transfer infectious virus to melanoma cells by cell-to-cell contact. All of the above models of T-cell infection utilized VZV-infected fibroblasts to inoculate T cells. In our study using infected DCs as the inoculum, we routinely infected >8% of autologous T cells and in some experiments achieved infection rates of up to 15%. In addition, we also demonstrated that VZV-infected DCs could transmit virus equally to both CD4+ and CD8+ T- cell subsets. Thus, the use of VZV-infected DCs as a T-cell inoculum may prove to be another useful and convenient alternative method to generate and study productively infected primary human T cells without the need for a specialized animal model.
In addition to the study of T-cell infection, the experiments described in this study have provided an ideal setting for rapidly testing the growth of VZV mutants in primary human DCs as well as assessing viral and cellular functions necessary for transfer of virus between these specialized cell types. This model would also be useful for testing viral recombinants with the aim of identifying and characterizing viral genes which play roles in cell tropism or spread of virus between these cell types. The infection model described here could also be used to test future candidate VZV vaccines for their ability to replicate and spread in DCs and T cells.
In conclusion, the demonstration that DCs can be productively infected with VZV and transmit infectious virus to T cells has significant implications for our understanding of VZV pathogenesis. This study provides a solid basis for the further assessment of VZV infection of DCs, with the ultimate goal of providing new information for the development of a second-generation live, attenuated vaccine.
ACKNOWLEDGMENTS
We thank Stuart Turville for invaluable assistance with dendritic cell isolation and propagation.
A.A. was supported by a University of Sydney Medical Foundation grant awarded to A.L.C., and B.S. was the holder of a Rolf Edgar Lake Fellowship. This study was supported in part by a grant from the Westmead Hospital Charitable Trust Fund.
REFERENCES
- 1.Abendroth A, Arvin A M. Varicella zoster virus immune evasion. Immunol Rev. 1999;168:143–156. doi: 10.1111/j.1600-065x.1999.tb01289.x. [DOI] [PubMed] [Google Scholar]
- 2.Abendroth A, Slobedman B, Eunice L, Mellins E, Wallace M, Arvin A M. Modulation of MHC class II protein expression by varicella-zoster virus. J Virol. 2000;74:1900–1907. doi: 10.1128/jvi.74.4.1900-1907.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arvin A M. Varicella zoster virus. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. New York, N.Y: Raven; 1996. pp. 2547–2585. [Google Scholar]
- 4.Arvin A M, Moffat J F, Redman R. Varicella-zoster virus: aspects of pathogenesis and host response to natural infection and varicella vaccine. Adv Virus Res. 1996;46:263–309. doi: 10.1016/s0065-3527(08)60074-3. [DOI] [PubMed] [Google Scholar]
- 5.Arvin A M. Varicella zoster virus: virologic and immunologic aspects of persistent infection. In: Ahmed R, Chen I, editors. Persistent viral infections. New York, N.Y: John Wiley and Sons Ltd.; 1998. pp. 183–208. [Google Scholar]
- 6.Asada H, Klaus-Kovtun V, Golding H, Katz S I, Blauvelt A. Human herpesvirus 6 infects dendritic cells and suppresses human immunodeficiency virus type 1 replication in coinfected cultures. J Virol. 1999;73:4019–4028. doi: 10.1128/jvi.73.5.4019-4028.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banchereau J, Steinman R M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 8.Bender A, Albert M, Reddy A, Feldman M, Sauter B, Kaplan G, Hellman W, Bhardwaj N. The distinctive features of influenza virus infection of dendritic cells. Immunobiology. 1998;198:552–567. doi: 10.1016/S0171-2985(98)80078-8. [DOI] [PubMed] [Google Scholar]
- 9.Bhardwaj N. Interactions of viruses with dendritic cells: a double-edged sword. J Exp Med. 1997;186:795–799. doi: 10.1084/jem.186.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blauvelt A, Asada H, Saville W M, Klaus-Kovtun V, Altman D J, Yarchoan R, Katz S I. Productive infection of dendritic cells by HIV-1 and their ability to capture virus are mediated through separate pathways. J Clin Investig. 1997;100:2043–2053. doi: 10.1172/JCI119737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brossart P, Bevan M J. Presentation of exogenous protein antigens on major histocompatibility complex class I molecules by dendritic cells: pathway of presentation and regulation by cytokines. Blood. 1997;90:1594–1599. [PMC free article] [PubMed] [Google Scholar]
- 12.Cameron P U, Freudenthal P S, Barker J M, Gezelter S, Inaba K, Steinman R M. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ cells. Science. 1992;257:383–387. doi: 10.1126/science.1352913. [DOI] [PubMed] [Google Scholar]
- 13.Cefai D, Ferrer M, Serpente N, Idziorek T, Dautry-Varsat A, Debre P, Bismuth G. Internalization of HIV glycoprotein gp120 is associated with down-modulation of membrane CD4 and p56lck together with impairment of T cell activation. J Immunol. 1992;149:285–294. [PubMed] [Google Scholar]
- 14.Cohen J I, Straus S E. Varicella zoster virus and its replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippencott-Raven Publishers; 1996. pp. 2525–2545. [Google Scholar]
- 15.Compton C C, Kupper T S, Nadir K B. HIV infected Langerhans cells constitute a significant proportion of the epidermal Langerhans cell population throughout the course of HIV disease. J Investig Dermatol. 1996;107:822–826. doi: 10.1111/1523-1747.ep12330574. [DOI] [PubMed] [Google Scholar]
- 16.Fugier-Vivier I, Servat-Delprat C, Rivailler P, Rissoan M C, Liu Y J, Rabourdin-Combr C. Measles virus suppresses cell mediated immunity by interfering with the survival and function of dendritic and T-cells. J Exp Med. 1997;186:813–823. doi: 10.1084/jem.186.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gavrieli D, Sherman Y, Ben-Basson S A. Identification of programmed cell death in situ using specific labelling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grose C. Variation on a theme by Fenner: the pathogenesis of chicken pox. Pediatrics. 1981;68:735–737. [PubMed] [Google Scholar]
- 19.Grosjean I, Caux C, Bella C, Berger I, Wild F, Banchereau J, Kaiserlian D. Measles virus infects human dendritic cells and blocks their allostimulatory properties for CD4+ T-cells. J Exp Med. 1997;186:801–812. doi: 10.1084/jem.186.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito M, Watanabe M, Ihara T, Kamiya H, Sakurai M. Fas antigen and bcl-2 expression of lymphocytes cultured with cytomegalovirus and varicella-zoster virus antigen. Cell Immunol. 1995;160:173–177. doi: 10.1016/0008-8749(95)80024-d. [DOI] [PubMed] [Google Scholar]
- 21.Jenkins D E, Yasukawa L L, Bergen R, Benike C, Engleman E G, Arvin A M. Comparison of primary sensitization of naive human T cells to varicella zoster virus peptides by dendritic cells in vitro with responses elicited in vivo by varicella vaccination. J Immunol. 1999;162:550–567. [PubMed] [Google Scholar]
- 22.Kinchington P R, Cohen J I. Varicella zoster virus proteins. In: Arvin A M, Gershon A A, editors. Varicella zoster virus. Virology and clinical management. Cambridge, England: Cambridge University Press; 2000. pp. 74–104. [Google Scholar]
- 23.Klagge I M, Schneider-Schaulies S. Virus interactions with dendritic cells. J Gen Virol. 1999;80:823–833. doi: 10.1099/0022-1317-80-4-823. [DOI] [PubMed] [Google Scholar]
- 24.Koropchak C M, Solem S M, Diaz P S, Arvin A M. Investigation of varicella-zoster virus infection of lymphocytes by in situ hybridization. J Virol. 1989;63:2392–2395. doi: 10.1128/jvi.63.5.2392-2395.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kruse M, Rosorius O, Kratzer F, Stelz G, Kuhnt C, Schuler G, Hauber J, Steinkasserer A. Mature dendritic cells infected with herpes simplex virus type 1 exhibit inhibited T-cell stimulatory capacity. J Virol. 2000;74:7127–7136. doi: 10.1128/jvi.74.15.7127-7136.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marland G, Bakker B, Adema G J, Figdor C G. Dendritic cells in immune response induction. Stem Cells. 1996;14:501. doi: 10.1002/stem.140501. [DOI] [PubMed] [Google Scholar]
- 27.Mason R R, Weiner R S. Application of the Beckman JE6-B Elutriator System® in the isolation of human monocyte subpopulations. Scand J Haem. 1985;34:5–8. doi: 10.1111/j.1600-0609.1985.tb00735.x. [DOI] [PubMed] [Google Scholar]
- 28.Moffat J F, Stein M D, Kaneshima H, Arvin A M. Tropism of varicella-zoster virus for human CD4+ and CD8+ T lymphocytes and epidermal cells in SCID-hu mice. J Virol. 1995;69:5236–5242. doi: 10.1128/jvi.69.9.5236-5242.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moffat J F, Zerboni L, Sommer M H, Heineman T C, Cohen J I, Kaneshima H, Arvin A M. The ORF47 and ORF66 putative protein kinases of varicella-zoster virus determine tropism for human T cells and skin in the SCID-hu mouse. Proc Natl Acad Sci USA. 1998;95:11969–11974. doi: 10.1073/pnas.95.20.11969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pignata C, Fiore M, De Filippo S, Cavalcanti M, Gaetaniello L, Scotese I. Apoptosis as a mechanism of peripheral blood mononuclear cell death after measles and varicella-zoster virus infections in children. Pediatr Res. 1998;45:77–83. doi: 10.1203/00006450-199801000-00012. [DOI] [PubMed] [Google Scholar]
- 31.Riegler S, Hebart H, Einsele H, Brossart P, Jahn G, Sinzger C. Monocyte-derived dendritic cells are permissive to the complete replicative cycle of human cytomegalovirus. J Gen Virol. 2000;81:393–399. doi: 10.1099/0022-1317-81-2-393. [DOI] [PubMed] [Google Scholar]
- 32.Sadzot-Delvaux C, Thonard P, Schoonbroodt S, Piette J, Rentier B. Varicella-zoster virus induces apoptosis in cell culture. J Gen Virol. 1995;76:2875–2879. doi: 10.1099/0022-1317-76-11-2875. [DOI] [PubMed] [Google Scholar]
- 33.Salio M, Cella M, Suter M, Lanzavecchia A. Inhibition of dendritic cell maturation by herpes simplex virus. Eur J Immunol. 1999;29:3245–3253. doi: 10.1002/(SICI)1521-4141(199910)29:10<3245::AID-IMMU3245>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 34.Schnorr J J, Xanthakos S, Keikavoussi P, Kampgen E, ter Meulen V, Schneider-Schaulies S. Induction of maturation of human blood dendritic cell precursors by measles virus in association with immunosuppression. Proc Natl Acad Sci USA. 1997;92:5326–5331. doi: 10.1073/pnas.94.10.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soong W, Schultz J C, Patera A C, Sommer M H, Cohen J I. Infection of human T lymphocytes with varicella-zoster virus: an analysis with viral mutants and clinical isolates. J Virol. 2000;74:1864–1870. doi: 10.1128/jvi.74.4.1864-1870.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steinman R M, Pack M, Inaba K. Dendritic cell development and maturation. Adv Exp Med Biol. 1997;417:1. doi: 10.1007/978-1-4757-9966-8_1. [DOI] [PubMed] [Google Scholar]
- 37.Warren M K, Rose W L, Cone J L, Rice W G, Turpin J A. Differential infection of CD34+ cell-derived dendritic cells and monocytes with lymphocyte-tropic and monocyte-tropic HIV strains. J Immunol. 1997;158:5035–5042. [PubMed] [Google Scholar]
- 38.Wigdahl B, Rong B L, Kinney-Thomas E. Varicella-zoster virus infection of human sensory neurons. Virology. 1986;152:384–399. doi: 10.1016/0042-6822(86)90141-8. [DOI] [PubMed] [Google Scholar]
- 39.Zerboni L, Sommer M, Ware C F, Arvin A M. Varicella-zoster virus infection of a human CD4-positive T-cell line. Virology. 2000;270:278–285. doi: 10.1006/viro.2000.0304. [DOI] [PubMed] [Google Scholar]