Abstract
Lymphocytic choriomeningitis virus (LCMV) induces type I interferon (alpha and beta interferon [IFN-α and IFN-β]) upon infection and yet is sensitive to the addition of type II interferon (gamma interferon [IFN-γ]) to the culture media. This sensitivity is biologically important because it correlates inversely with the ability of certain LCMV strains to persist in mice (D. Moskophidis, M. Battegay, M. A. Bruendler, E. Laine, I. Gresser, and R. M. Zinkernagel, J. Virol. 68:1951-1955, 1994). The cellular oncoprotein PML is induced by both IFN-α/β and IFN-γ, and PML binds the LCMV Z protein and becomes redistributed within cells from nucleus to cytoplasm upon LCMV infection. In the present study, increased PML expression results in diminished LCMV replication, implicating PML in the IFN sensitivity of LCMV. Virus production in PML −/− murine embryonic fibroblasts (MEF) exceeds virus production in PML +/+ MEF, and this difference is exacerbated by IFN treatment that upregulates PML expression. IFN-γ also diminishes LCMV production in PML −/− cells; therefore, viral IFN sensitivity is not entirely due to PML. Both viral mRNA production and viral protein production decrease as PML expression increases. Here we propose that PML reduces LCMV transcription through its interaction with the Z protein.
Arenaviruses can replicate without significantly impacting the host or causing cytopathic effects. The arenavirus replication complex contains the viral genomic single-stranded RNA segments, nucleocapsid protein (NP), an RNA-dependent RNA polymerase (RdRp or L protein), and a small zinc-binding protein (Z) (17). Cellular proteins are also involved in viral replication (3, 4, 12). Here we describe the inhibitory influence of the promyelocytic leukemia protein (PML) that coprecipitates and colocalizes with cell-associated arenavirus complexes (2). PML is an oncoprotein that is expressed primarily in myeloid, epithelial, and endothelial cells, all infectable by arenaviruses and important in the pathogenesis of arenaviral hemorrhagic fevers. PML is induced by the alpha/beta interferons (IFN-α/β) acting on the ISRE and GAS promoter response elements (5, 13, 20). Interferons IFN-α and IFN-β are produced by many cell types upon viral infection, and IFN-γ is produced in T lymphocytes or natural killer cells in response to antigens (16). IFNs are known for their inhibitory effects on cellular proliferation, and PML, as an effector of this function, is capable of suppressing cell proliferation (11, 22, 24).
IFNs are also known for their antiviral effects. There are 50 to 100 IFN-inducible genes and several of them have antiviral activity, e.g., the p68 protein kinase, the 2′,5′-oligoadenylate synthetase (OAS), and certain Mx family proteins (19, 20, 23). The IFN-inducible PML has also recently been shown to have antiviral activity. In the absence of IFN, overexpression of PML diminishes infection by vesicular stomatitis virus (VSV) and influenza A virus, without affecting infection by encephalomyocarditis virus (EMCV), a virus known to be IFN resistant (6).
Coimmunoprecipitation studies show specific interaction between PML and Z proteins of LCMV and Lassa fever virus, a related arenavirus. Genetically engineered mutations in PML were used to show that the Z protein binds the N-terminal region of PML, and this domain of PML, unlike the PML RING or the nuclear localization signal, is essential for colocalization of Z and PML (2). The work presented here demonstrates that PML expression diminishes LCMV expression, possibly through its interaction with the LCMV Z protein.
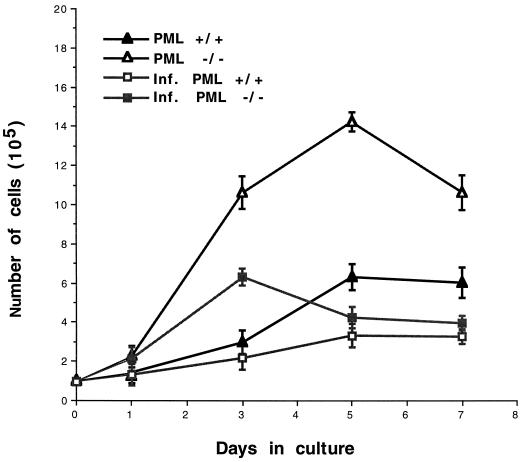
PML and LCMV affect proliferation of MEF.
The effects of PML expression on cell proliferation were examined in early-passage mouse embryonic fibroblasts (MEFs) (22; this study). Fibroblasts lacking PML (PML −/−) grew faster and achieved higher cell densities than wild-type (PML +/+) cells and yet their cultures were morphologically indistinguishable. IFN treatment, which increases PML expression, reduces cell growth rates even more in both PML +/+ and −/− fibroblasts. Infection with LCMV shortens the life of both MEF cultures approximately twofold (P < 0.05) (Fig. 1).
FIG. 1.
PML expression and LCMV infection decrease the proliferation of MEF. MEF from wild-type (PML +/+) or knockout (PML −/−) mouse embryos were supplied by P. P. Pandolfi (22) and were propagated in Dulbecco minimal essential medium (DMEM; GIBCO, Grand Island, N.Y.) supplemented with 20% fetal bovine serum (FBS). To measure cell proliferation rates, infected or uninfected cells were seeded at 105 per 6-cm culture dish, and viable cell counts were determined by trypan blue exclusion. Infections employed LCMV-Armstrong 53b strain at an MOI of 1 PFU per cell. The number of cells per dish represents the average of triplicate measurements ± the standard deviation (SD).
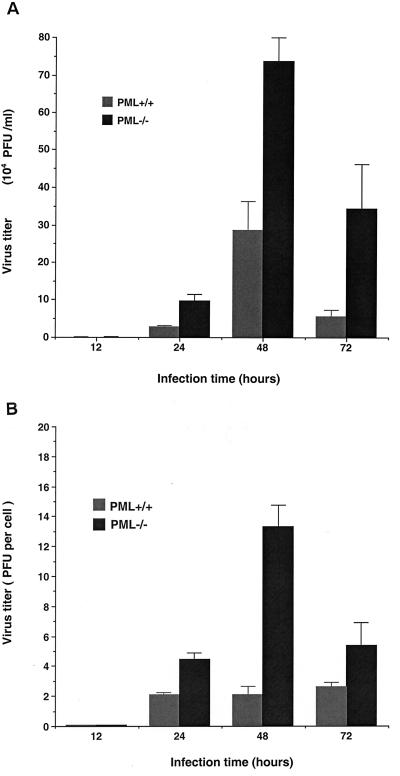
PML-expressing or IFN-treated MEF have reduced virus replication.
Levels of LCMV replication were assessed in MEF with or without PML. Cells were cultured and infected with LCMV for the times indicated. This experiment was performed in two different ways to minimize the effects of differing cell proliferation rates: virus yields are described as the total PFU/milliliter in cultures that were replated to achieve the same densities (Fig. 2A) or as the PFU/cell in cultures that were terminated for cell counts at various intervals (Fig. 2B). The highest virus yield in these cells was obtained at 48 h after infection. At this time point, four- to fivefold increases in virus yield were found in cells lacking PML (PML −/−) compared to PML +/+ cells.
FIG. 2.
Replication of LCMV Armstrong in PML +/+ and PML −/− MEF. Monolayer cultures of MEF (2 × 106 per T25 flask) were infected with LCMV-Armstrong at an MOI of 1 PFU per cell. After a 1 h adsorbtion period, the inoculum was removed, fresh DMEM with 20% FBS was added, and the cells were incubated at 37°C for the different time points indicated. To determine virus yields, culture media were frozen, and virus titers were determined by plaque assay on Vero E6 cells as described earlier (8). Titrations were performed on two separate occasions in duplicate wells of a six-well plate, and the results are presented as the mean viral yields ± the SD. (A) Virus yields (total PFU/milliliter) were determined for the MEF of different PML genotypes. Cells were cultured for 24-h intervals, after which media were collected for titration and cells were trypsinized, counted, and replated to equalize the numbers of PML +/+ and PML −/− cells/well for another 24-h culture period. (B) Virus yields were normalized to cell number (PFU/cell) to overcome the problem of different cell proliferation rates. In these experiments, cell counts were determined after the medium was removed for titration, and the cells were discarded after counting (i.e., separate cultures were used to determine each time point).
Induction of PML expression by IFNs has been previously demonstrated by Northern blot and immunofluorescence analysis (22). To assess the capacity of murine IFN-γ to inhibit LCMV replication, both PML +/+ and PML −/− MEF were treated for 48 h with 500, 1,000, or 1500 U of IFN-γ per ml, replated for equivalent densities, and then infected with LCMV at a multiplicity of infection (MOI) of 1. To minimize the contribution of cell proliferation to virus replication, virus titers were compared at the early time point of 24 h. At 12 h postinfection, there would be no significant differences in viral replication, since that is only enough time for one arenavirus replicative cycle (18). In the absence of IFN, we found only a twofold decrease in the virus yields in PML +/+ cells compared to the control PML −/− cells (Table 1) that could be attributable to different rates of cell proliferation. IFN treatment similarly decreased the proliferation of both uninfected and LCMV-infected MEF. In addition to the effects on cell proliferation, IFN affected virus production: significantly, a 16-fold decrease in virus yield was observed in IFN-treated PML +/+ cells compared to untreated PML +/+ cells (P < 0.01). At 1,000 U of IFN, PML +/+ cells produced fivefold less virus compared to PML −/− cells (P < 0.05). Since IFN also inhibited virus production in the PML −/− cells, additional IFN sensitivity mechanisms (besides PML) are involved in LCMV replication. Both PML +/+ and PML −/− MEF express IFN-α/β but, unlike IFN-γ, these were not detectable by Western blot (not shown) and were probably at insufficient levels to affect virus production.
TABLE 1.
Reduced LCMV yields in IFN-treated MEFa
| Treatment (U of IFN/ml) | Mean PFU ± SD/ml of virus from:
|
|
|---|---|---|
| PML +/+ MEF | PML −/− MEF | |
| Untreated | (1.3 ± 0.21) × 106 | (2.7 ± 0.11) × 106 |
| 500 | (1.3 ± 0.53) × 105 | (5.7 ± 0.38) × 105 |
| 1,000 | (0.8 ± 0.53) × 105 | (4.0 ± 0.01) × 105 |
| 1,500 | (1.1 ± 0.60) × 105 | (2.4 ± 0.56) × 105 |
Results are expressed as PFU/milliliter at 24 h postinfection. Although the differences are not dramatic at this early time point (see Fig. 2), data from later time points are corrupted by the IFN effects on cell growth rates. Mean virus yields from two independent experiments are presented. Each titration was done in duplicate and allows plaques to be counted in wells of two to three dilutions to determine a standard deviation for the plaque count.
Viral RNA levels are diminished in PML-expressing fibroblasts.
We examined whether the presence or absence of PML and the addition of IFN in LCMV-infected MEF affects mRNA expression of viral genes. Total cellular RNA was extracted from IFN-treated or untreated and LCMV-infected MEF, reverse transcribed, PCR amplified in the presence of random hexanucleotide primers (7), and subjected to quantitative real-time PCR analysis.
To determine the levels of viral GP and NP cDNA relative to 18S internal control, the following primer pairs were used: GP (5′-TCATCGATGAGGTGATCAAC-3′, 5′-CTTGGTGAACTCTCTAGACT-3′), NP (5′-CAATGGACGCAAGCATTGAG-3′, 5′-GTTCTTCTGCACTGAGCCTCC-3′), and 18S rRNA primers (Ambion, Austin, Tex.). Real-time PCR employed a SYBR Green I PCR Core kit to produce fluorescence-labeled PCR products. Fluorescent NP and GP amplicons were detected during the course of the reaction using a Perkin-Elmer GeneAmp 5700. The PCR cycle at which the amplicon begins exponential amplification is the threshold cycle (CT) which depends on the starting concentration of NP, GP, or 18S templates. The relative levels of NP and GP messages are derived by normalizing the NP and GP CT to the CT of 18S rRNA and comparing the results from PCR −/− cells to results from PCR +/+ cells. Data calculations are described in Table 2, and Fig. 3 shows the final relative quantitation (RQ) values that indicate the excess of GP and NP mRNA in PML −/− cells in comparison to these mRNAs in PML +/+ cells.
TABLE 2.
Real-time PCR detection of NP mRNA in IFN-γ-treated and LCMV-infected MEFa
| Treatment (U of IFN/ml) | MEF phenotype | Mean CT ± SDb
|
ΔCTc | ΔΔCTd | RQe (fold) | |
|---|---|---|---|---|---|---|
| 18S RNA | NP mRNA | |||||
| Untreated | PML +/+ | 34.40 ± 0.58 | 22.15 ± 0.17 | 12.30 | 8 | |
| PML −/− | 34.38 ± 0.40 | 25.19 ± 0.12 | 9.19 | 3.11 | ||
| 500 | PML +/+ | 34.99 ± 0.50 | 23.61 ± 0.44 | 11.38 | 6.9 | |
| PML −/− | 34.19 ± 0.24 | 25.60 ± 0.21 | 8.59 | 2.79 | ||
| 1,000 | PML +/+ | 36.24 ± 1.76 | 24.86 ± 0.03 | 11.38 | 11 | |
| PML −/− | 34.50 ± 0.93 | 26.72 ± 0.19 | 7.78 | 3.60 | ||
MEF were either PML +/+ or PML −/− as indicated. At 24 h postinfection, cDNA was made from MEF and amplified with 18S and LCMV NP primers as described for Fig. 3.
CT is the threshold PCR cycle.
ΔCT is the difference between the mean CT values of NP and the endogenous control (18S). ΔCT values were normalized to those for the 18S internal control.
ΔΔCT is the difference between the mean ΔCT values from PML +/+ and PML −/− cDNA.
RQ value = 2−ΔΔCT.
FIG. 3.
Effect of IFN on viral mRNA transcription. MEF were IFN treated, trypsinised 48 h later, counted, and then divided into equivalent pools for LCMV infection. Recombinant mouse IFN-γ was from R&D Systems, Minneapolis, Minn. Cellular RNA was extracted using TRIzol (Life Technologies, Gaithersburg, Md.). The PCR cycle at which the amplicon begins exponential amplification is the threshold cycle (CT), which depends on the starting concentration of the NP, GP, or 18S templates. The relative levels of the NP and GP messages are derived by normalizing the NP and GP CT values to the CT of 18S rRNA and then comparing results from PCR −/− cells to results from PCR +/+ cells. In the exponential phase, a CT difference (ΔCT) of 1 means that one template is twice as abundant as the other. (Table 2 gives an example of the actual data and calculations.) RQ values depend upon ΔΔCT (or the difference between the amplicon and the standard in PML −/− cells and between the amplicon and the standard in PML +/+ cells) such that RQ = 2−ΔΔCT. Here the RQ value indicates the relative excess of GP and NP mRNA in PML −/− cells in comparison to the amounts of these mRNAs in PML +/+ cells.
The analysis confirmed that the PML −/− cells had seven- to elevenfold more NP mRNA than PML +/+ cells (P < 0.05), whereas the PML −/− cells had only fourfold more GP mRNA than the wild-type cells (P < 0.05) (Fig. 3, Table 2). A total of 500 U of IFN per ml showed no effect on LCMV RNA levels in PML-expressing MEF compared to PML −/− cells, but 1,000 U of IFN per ml showed a mean 30% decrease in both GP and NP mRNA in PML +/+ cells compared to PML −/− cells. We conclude that IFN-γ upregulates PML and that this upregulation affects LCMV RNA production.
PML expression in MEF results in decreased expression of viral proteins.
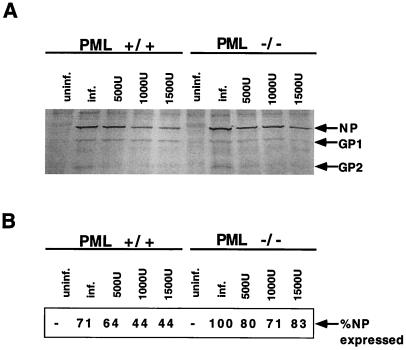
IFN treatment is known to upregulate PML expression at both mRNA and protein levels (5, 13, 20, 22). To investigate whether PML upregulation modulates the expression of viral proteins, cells were grown in the presence or absence of IFN-γ and infected with LCMV. Protein extracts from equal numbers of PML +/+ and PML −/− cells, treated or untreated with IFN, were compared on Western blots with respect to viral protein expression. The induction of PML resulted in the inhibition of LCMV antigen expression and was confirmed by Western blot analysis using anti-LCMV antibodies (Fig. 4).
FIG. 4.
Inhibition of LCMV gene expression in IFN-treated PML +/+ and PML −/− MEF. (A) Cells were treated for 48 h with 500, 1,000, or 1,500 U of murine IFN-γ per ml, trypsinized, replated into equivalent pools, and then infected with LCMV at an MOI of 1 PFU for 24 h. Western blot analysis of the MEF extracts was done as described in the text. Blots were probed with our guinea pig anti-LCMV antibodies and revealed by BCIP-NBT, an alkaline phosphatase substrate. (B) The experiment in panel A was repeated; however, Western blots were developed with a chemiluminescent probe and scanned with a PhosphorImager (see the text). Scanning allowed us to attribute values (the AQR) to the band intensities of the viral NP and to normalize these values to an internal standard, murine actin. We considered the amount of NP expressed by untreated PML −/− cells to be 100%, and we presented the NP of all other cells as a percentage of this value.
For Western blots, approximately 3 × 106 cells were lysed in 0.5 ml of lysis buffer, and 30 μg of protein (8 × 104 cell equivalents) were loaded per lane as described elsewhere (3, 10). The primary antibody was hyperimmune guinea pig polyclonal anti-LCMV serum (1:1,000), and the secondary antibody (1:10,000) was anti-guinea pig immunoglobulin conjugated to alkaline phosphatase or horseradish peroxidase (Sigma, St. Louis, Mo.). Blots were developed using BCIP-NBT (Sigma) or enhanced chemiluminescence (Pierce, Rockford, Ill.). Chemiluminescence was detected by scanning with a PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.) and using ImageQuant Software (Molecular Dynamics) to compare the scans of viral proteins to internal standards such as murine actin (Sigma). The area quantitation report (AQR) derived from these scans was used to compare the relative amounts of one protein and another.
Only the most abundant viral proteins, NP and GP, were detected by our polyclonal anti-LCMV sera. IFN treatment resulted in a decrease of NP and GP protein expression levels in both PML +/+ and PML −/− MEF (Fig. 4A). To determine the relative reduction in NP expression, scans of viral proteins (AQR) were normalized to an internal standard, murine actin, and the percent decrease from NP expression in PML −/− cells was expressed (Fig. 4B). Untreated PML +/+ cells expressed 30% less NP than untreated PML −/− cells. Upon the addition of 1,000 U of IFN-γ per ml, the highest inhibition (>50% decrease) of LCMV protein expression was observed in PML +/+ cells. IFN treatment also caused a slight drop in the level of NP and GP proteins in PML −/− cells, indicating that other IFN-inducible genes are involved in antiviral activity.
We conclude that LCMV replication is sensitive to the antiviral activities of IFN, in part through the expression of the IFN-inducible PML gene. IFN upregulates PML, and PML expression leads to reduced production of LCMV. It has been shown that the overexpression of PML reduces production of VSV and influenza A virus but not of an IFN-resistant virus, encephalomyocarditis virus (6). The PML inhibition of LCMV production was similar to the PML inhibition of VSV observed in infected CHO or MEF (6, 13). Modest (fivefold) decreases in LCMV production were attributable to PML expression. Similarly modest (maximum of 16-fold) decreases were attributable to IFN (which includes PML effects). These effects are biologically significant because the IFN sensitivity of LCMV strains in cell culture correlates inversely with their ability to persist in mice (15).
How PML inhibits LCMV replication is not known. In influenza virus and VSV infections, it has been shown that PML expression inhibits viral antigen production and decreases viral titers, but it was not determined whether PML affects the transcription of viral genes (6). We show here that PML expression causes a decrease in the steady-state levels of viral RNA, which we known from previous studies is approximately 1:100 (replicative RNA:mRNA). The decrease in mRNA levels could explain the observed decreases in viral antigen expression and in virus production, though it is impossible to rule out primary PML affects on virus translation or assembly. Previously, we showed that PML protein binds to the LCMV Z protein (2), and we developed the working hypothesis that PML sequesters Z from some essential function in replication. We also showed that PML and the Z protein, separately, can have translation-inhibitory effects (4). However, at this point, there is no evidence to distinguish whether the decreased levels of viral RNA are due to increased turnover or decreased de novo synthesis.
The well-known antiproliferative effects of IFN-γ treatment are partially attributable to its induction of PML, which is proapoptotic and antiproliferative (1). PML dysfunction leads to proliferation of undifferentiated myeloid cells, which is a hallmark of acute myelocytic leukemia (21). PML expression causes PML +/+ MEF to proliferate more slowly than PML −/− MEF (22). In a previous publication we noted that LCMV infection of a serum-starved culture prolonged the life of the culture, and we speculated that arenavirus infection interferes with PML function and prevents its normal involvement in cell death (1). However, here we show, with different fibroblastic cells, that LCMV infection has an almost twofold antiproliferative effect (Fig. 1). Cell proliferation rates are controlled by both apoptotic pathways and cell cycle progression, and LCMV infection has separate effects on molecules directing these processes (M.S.S. and K.L.B.B., unpublished). As yet, neither the pro- nor the antiproliferative effects of LCMV infection have been connected with PML function or with the function of any particular viral gene, i.e., these effects are not attributable to expression of the Z gene alone, since the Z gene alone promotes cell death (1). Ultimately, to determine the biological impact of PML on LCMV replication, it will be necessary to look at the ability of LCMV to persist or cause disease in PML-negative animals.
In summary, our findings demonstrate that PML expression reduces cell proliferation, that IFN exacerbates this reduction, and that PML expression downregulates the production of virus particles, interfering with both viral RNA and protein expression. Thus, PML contributes to the antiviral effect of IFN.
Acknowledgments
We thank Dave Pauza and Tracy Ruckwardt for helpful comments and discussions.
This work was supported by National Institutes of Health grant AI 32107 (to M.S.S.).
REFERENCES
- 1.Borden K L B, Campbell Dwyer E J, Salvato M S. The promyelocytic leukemia protein PML has a pro-apoptotic activity mediated through its RING domain. FEBS Lett. 1997;418:30–34. doi: 10.1016/s0014-5793(97)01344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borden K L B, Campbell Dwyer E J, Salvato M S. An arenavirus RING (zinc-binding) protein binds the oncoprotein promyelocyte leukemia protein (PML) and relocates PML nuclear bodies to the cytoplasm. J Virol. 1998;72:758–766. doi: 10.1128/jvi.72.1.758-766.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borden K L B, Campbell Dwyer E J, Carlile G W, Djavani M, Salvato M S. Two RING finger proteins, the oncoprotein PML and the arenavirus Z protein, colocalize with the nuclear fraction of the ribosomal P proteins J. Virol. 1998;72:3819–3826. doi: 10.1128/jvi.72.5.3819-3826.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell Dwyer E J, Lai H K, MacDonald R C, Salvato M S, Borden K L B. The lymphocytic choriomeningitis virus RING protein Z associates with eukaryotic initiation factor 4E and represses translation in a RING-dependent manner. J Virol. 2000;74:3293–3300. doi: 10.1128/jvi.74.7.3293-3300.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chelbi-Alix M K, Pelicano L, Quignon F, Koken M H, Venturini L, Stadler M, Pavlovic J, Degos L, de The H. Induction of the PML protein by interferons in normal and APL cells. Leukemia. 1995;9:2027–2033. [PubMed] [Google Scholar]
- 6.Chelbi-Alix M K, Quignon F, Pelicano L, Koken M H, de The H. Resistance to virus infection conferred by the interferon-induced promyelocytic leukemia protein. J Virol. 1998;72:1043–1051. doi: 10.1128/jvi.72.2.1043-1051.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Djavani M, Lukashevich I S, Sanchez A, Nichol S T, Salvato M S. Completion of the Lassa fever virus sequence and identification of a RING finger open reading frame at the L RNA 5′ end. Virology. 1997;235:414–418. doi: 10.1006/viro.1997.8722. [DOI] [PubMed] [Google Scholar]
- 8.Doyle M V, Oldstone M B A. Interactions between viruses and lymphocytes. I. In vivo replication of lymphocytic choriomeningitis virus in mononuclear cells during both chronic and acute viral infections. J Immunol. 1987;121:1262–1269. [PubMed] [Google Scholar]
- 9.Garcin D, Rochat S, Kolakofsky D. The Tacaribe arenavirus small zinc finger protein is required for both mRNA synthesis and genome replication. J Virol. 1993;67:807–812. doi: 10.1128/jvi.67.2.807-812.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 11.Koken M H, Linares-Cruz G, Quignon F, Viron A, Chelbi-Alix M K, Sobczak-Thepot J, Juhlin L, Degos L, Calvo F, de The H. The PML growth-suppressor has an altered expression in human oncogenesis. Oncogene. 1995;10:1315–1324. [PubMed] [Google Scholar]
- 12.Lai M M C. Cellular factors in the transcription and replication of viral RNA genomes: a parallel to DNA-dependent RNA transcription. Virology. 1998;244:1–12. doi: 10.1006/viro.1998.9098. [DOI] [PubMed] [Google Scholar]
- 13.Lavau C, Marchio A, Fagioli M, Jansen J, Falini B, Lebon P, Grosveld F, Pandolfi P P, Pelicci P G, Dejean A. The acute promyelocytic leukaemia-associated PML gene is induced by interferon. Oncogene. 1995;11:871–876. [PubMed] [Google Scholar]
- 14.Leung W C, Leung M F, Rawls W E. Distinctive RNA transcriptase, polyadenylic acid polymerase, and polyuridylic acid polymerase activities associated with Pichinde virus. J Virol. 1979;30:98–107. doi: 10.1128/jvi.30.1.98-107.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moskophidis D, Battegay M, Bruendler M A, Laine E, Gresser I, Zinkernagel R M. Resistance of lymphocytic choriomeningitis virus to alpha/beta interferon and to gamma interferon. J Virol. 1994;68:1951–1955. doi: 10.1128/jvi.68.3.1951-1955.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pestka S, Langer J A, Zoon K C, Samuel C E. Interferons and their actions. Annu Rev Biochem. 1987;56:727–777. doi: 10.1146/annurev.bi.56.070187.003455. [DOI] [PubMed] [Google Scholar]
- 17.Salvato M S. Molecular biology of the prototype arenavirus, lymphocytic choriomeningitis virus. In: Salvato M S, editor. The arenaviridae. New York, N.Y: Plenum Press Inc.; 1993. pp. 133–156. [Google Scholar]
- 18.Salvato M S, Rai S K. Arenaviruses. In: Mahy B, Collier L, editors. Topley and Wilson's microbiology and microbial infections. Vol. 1. London, England: Arnold; 1998. pp. 629–650. [Google Scholar]
- 19.Samuel C E. Antiviral actions of interferon: interferon-regulated cellular proteins and their surprisingly selective antiviral activities. Virology. 1991;183:1–11. doi: 10.1016/0042-6822(91)90112-o. [DOI] [PubMed] [Google Scholar]
- 20.Stadler M, Chelbi-Alix M K, Koken M H, Venturini L, Lee C, Saib A, Quignon F, Pelicano L, Guillemin M C, Schindler C, de The H. Transcriptional induction of the PML growth suppressor gene by interferons is mediated through an ISRE and a GAS element. Oncogene. 1995;11:2565–2573. [PubMed] [Google Scholar]
- 21.Tallman M S, Kwann H C. Reassessing the hemostatic disorder associated with acute promyelocytic leukemia. Blood. 1992;79:543–553. [PubMed] [Google Scholar]
- 22.Wang Z G, Delva L, Gaboli M, Rivi R, Giorgio M, Cordon-Cardo C, Grosveld F, Pandolfi P P. Role of PML in cell growth and the retinoic acid pathway. Science. 1998;279:1547–1551. doi: 10.1126/science.279.5356.1547. [DOI] [PubMed] [Google Scholar]
- 23.Welsh R M, Sen G C. Nonspecific host responses to viral infections. In: Nathanson N, editor. Viral pathogenesis. Philadelphia, Pa: Lippincott-Raven Publishers; 1997. pp. 109–141. [Google Scholar]
- 24.Zhong S, Hu P, Ye T Z, Stan R, Ellis N A, Pandolfi P P. A role for PML and the nuclear body in genomic stability. Oncogene. 1999;18:7941–7947. doi: 10.1038/sj.onc.1203367. [DOI] [PubMed] [Google Scholar]