Abstract
Hepatitis C virus (HCV), a major cause of liver disease worldwide, is frequently resistant to the antiviral alpha interferon (IFN). We have recently found that the HCV NS5A protein induces expression of the proinflammatory chemokine IL-8 to partially inhibit the antiviral actions of IFN in vitro. To extend these observations, in the present study we examined the relationship between levels of IL-8 in serum, HCV infection, and biochemical response to IFN therapy. Levels of IL-8 were significantly elevated in 132 HCV-infected patients compared to levels in 32 normal healthy subjects and were also significantly higher in patients who did not respond to IFN therapy than in patients who did respond to therapy. This study suggests that HCV-induced changes in levels of chemokine and cytokine expression may be involved in HCV antiviral resistance, persistence, and pathogenesis.
Chronic hepatitis C virus (HCV) infection is a significant clinical problem throughout the world. About 85% of people infected with HCV develop chronic infection, and approximately 70% of patients develop histological evidence of chronic liver disease (9).
Interferon (IFN) and the guanosine analogue ribavirin are widely used treatments for chronic HCV infection (5, 11, 17). However, as many as 60% of patients with high-titer HCV genotype 1 infections remain nonresponsive to combination therapy.
The HCV NS5A protein has been implicated in the resistance of HCV to antiviral therapy (reviewed in reference 14). We have recently found that NS5A induces the CXC chemokine interleukin 8 (IL-8) to inhibit the antiviral actions of IFN in vitro (15). To investigate the clinical significance of these results, in this study we investigated the relationship among levels of IL-8 and tumor necrosis factor alpha (TNF-α) (a potent inducer of IL-8 [12]) in serum, HCV infection, and response to IFN therapy.
One hundred thirty-two patients from Saudi Arabia with hepatitis C disease were studied. Diagnosis was reached using appropriate serological, virological, biochemical, and histological criteria. All sera from patients diagnosed to have chronic hepatitis C showed elevated liver enzymes, tested positive for anti-HCV antibodies by a second-generation enzyme-linked immunosorbent assay (ELISA), and were confirmed to be reactive with HCV recombinant immunoblot assay-2. Patients with hepatitis B surface antigen positivity, autoimmune disease, alcohol- or drug-induced liver diseases, hepatic failure, decompensated cirrhosis, schistosoma mansoni, or hematological abnormalities were excluded from the study. Genotypes were not determined, although the predominant HCV genotypes in Saudi Arabia are genotype 4 and 1 (2, 19). IFN-α2a was administered intramuscularly three times per week at 3 million U per dose. The study was performed with the approval of the King Faisal Specialist Hospital and Research Centre research advisory council. Response to therapy was assessed biochemically based on normalization of alanine aminotransferase values 6 months after termination of therapy.
To measure IL-8 protein levels in patient serum, the following ELISA protocol was followed. High-level-binding microtiter plates (Lab Systems, Helsinki, Finland) were coated overnight with 2 μg of monoclonal antibodies to IL-8 (R&D Systems) and blocked with 2% Dulbecco's phosphate-buffered saline buffer. Samples or a recombinant IL-8 standard (obtained from K. Matsushima, University of Tokyo), diluted in human serum, was added to the microwells for 2 h, and the plates were then washed with 0.1% Tween 20–bovine serum albumin–Dulbecco's phosphate-buffered saline buffer. Polyclonal antibodies to IL-8 (Genzyme, Boston, Mass.) were added. After extensive washing, horseradish peroxidase-conjugated anti-rabbit immunoglobulin G (Accurate Chemicals, Westbury, N.Y.) was added and the plates were further washed before the substrate TMB-H2O2 (KPL, Gaithersburg, Md.) was added. When color developed, the reaction was stopped with 2 N H2SO4 and absorbance was read at 450 nm in an automated ELISA plate reader. A standard curve of optical densities versus concentrations of IL-8 was generated to determine the concentrations of IL-8 in serum samples. The detection limit of the assay was 2 pg/ml. TNF-α in serum samples was quantitated using a TNF-α high-sensitivity ELISA kit (R&D Systems). The detection limit was 0.5 pg/ml. Patient groups were tested for Gaussian distribution using the Kolmogorov-Smirnov test. The nonparametric Mann-Whitney test was used for unpaired comparisons of levels of TNF-α and IL-8 in patient sera. Data are presented as means ± standard errors of the means (SEM).
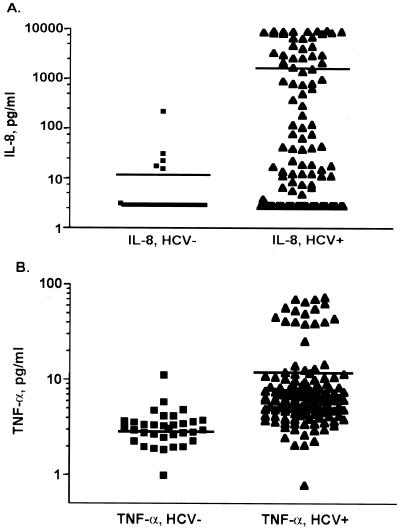
HCV infection and levels of IL-8 and TNF-α in serum.
We first measured the levels of IL-8 in the sera of 132 patients with chronic hepatitis C and 32 healthy control subjects to determine if serum IL-8 levels are associated with HCV infection. Furthermore, since TNF-α is a primary inducer of IL-8, we also examined TNF-α levels in the sera of the same patients. Figure 1 depicts levels in sera of IL-8 (Fig. 1A) and TNF-α (Fig. 1B) as detected by ELISA for the two patient groups. The mean levels of IL-8 were significantly higher in patients with chronic hepatitis C than in normal subjects (1,731 ± 290 pg/ml versus 12.35 ± 7.0 [means ± SEM], P < 0.0001). The mean level of TNF-α was also significantly higher in HCV-infected patients than in normal healthy subjects (12.46 ± 1.4 pg/ml versus 6.11 ± 3.3 [means ± SEM], P < 0.001).
FIG. 1.
Determination of levels of IL-8 and TNF-α in the sera of HCV-infected patients and control patients not infected with HCV. (A) Levels of IL-8 in sera were determined by a specific ELISA of 132 HCV-infected patients (triangles) and 32 healthy subjects (squares). The difference in IL-8 levels between infected patients and healthy control subjects was highly significant (P < 0.0001). (B) Serum TNF-α levels, determined by a specific ELISA with the same group of patients. The levels of TNF-α in HCV-infected patient sera were significantly higher than in healthy control subjects (P < 0.0001). Data are expressed as means ± SEM. P values were derived from a two-tailed probability generated from a Mann-Whitney test.
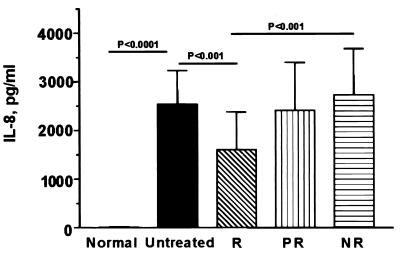
Levels of IL-8 in serum and response to IFN therapy.
Figure 2 depicts the association between levels of IL-8 in serum and the biochemical response to IFN therapy in the HCV-infected patients. Response to therapy was defined by normalization of ALT levels 6 months following termination of therapy. There was a stepwise increase in pretreatment levels of IL-8, with biochemical nonresponders having the highest IL-8 levels (2,727 ± 951 pg/ml), followed by partial responders (2,409 ± 986 pg/ml) and then responders (1,606 ± 773 pg/ml). Nonresponsive patients had significantly higher levels of IL-8 than responsive patients (P < 0.001), and responders also had significantly lower levels of IL-8 than patients who were not treated (P < 0.001). These data indicate that HCV infection is associated with elevated levels of IL-8 and TNF-α in serum, that high levels of IL-8 are associated with the lack of a biochemical response to IFN therapy, and that IFN therapy reduces the expression of IL-8.
FIG. 2.
Pretreatment levels of IL-8 are correlated with the response to IFN therapy. We measured by ELISA IL-8 levels in patients with hepatitis C disease who were not treated (n = 33) or who were complete responders (R; n = 18), partial responders (PR; n = 18), or nonresponders (NR; n = 17) to IFN-α therapy. A complete response to IFN-α therapy was assessed biochemically via normalization of ALT levels to normal levels for more than 6 months after cessation of therapy. Data are expressed as means ± SEM. P values were derived from a two-tailed probability generated from a Mann-Whitney test.
We demonstrate significant increases in levels of IL-8 in HCV-infected patients compared to levels in uninfected patients, and patients who were biochemical nonresponders to IFN therapy had higher pretreatment levels of IL-8. These in vivo data corroborate our recent finding that the HCV NS5A protein induces IL-8 mRNA and protein expression via transcriptional activation of the IL-8 promoter (15). Because NS5A exists as a quasispecies in vivo (13, 16), it is possible that clinical isolates of NS5A may have different IL-8 transactivation activities which may lead to different levels of IL-8 in serum and different responses to IFN therapy. Although HCV genotype was not evaluated in the patients analyzed in this study, the prevalent genotypes in Saudi Arabia are types 4 and 1 (2, 19), and similar to what has been observed in U.S. studies, types 1 and 4 tend to be quite resistant to IFN therapy (1, 3). In future studies, it will be interesting to explore the relationship between levels of IL-8 in serum, NS5A amino acid sequence, HCV genotype, and virological response to therapy.
NS5A induction of IL-8 expression is associated with inhibition of the antiviral actions of IFN in vitro (15). This may represent a distinct mechanism by which the NS5A protein circumvents the IFN-induced antiviral response. It was recently demonstrated that the HCV core protein could also transactivate the IL-8 promoter (8). However, in this study, only truncated IL-8 promoters were used and the effect of core protein expression on IL-8 mRNA and protein levels was not investigated. Nonetheless, it is possible that other HCV proteins contribute to induction of IL-8 and perhaps that other cytokines affect responses to antiviral therapy and influence HCV persistence and pathogenesis. In other clinical studies, it has been demonstrated that chronic hepatitis C patients with high histologic activities have increased levels of IL-8 mRNA expression (6, 18). In one study, levels of intrahepatic IL-8 mRNA were higher in IFN nonresponders than in responders, although the difference was not statistically significant (6). In agreement with the present study, one previous study also found that serum IL-8 protein levels were elevated in HCV-infected patients (7). To our knowledge, this is the first study to examine serum IL-8 levels and the biochemical response to IFN therapy. In alcoholic hepatitis, serum IL-8 levels are elevated (10), and several studies suggest a relationship between hepatic IL-8 and neutrophil infiltration (reviewed in reference 4). Thus, the association of IL-8 with viral and nonviral hepatitis suggests that this chemokine may play a role in the pathogenesis of liver disease.
Acknowledgments
This research was partially supported by an NIH Hepatitis C Cooperative Center Pilot Feasibility Grant and Schering-Plough (S.J.P.), the University of Washington Royalty Research Fund and NIH grants AI41320-02 and AI39049-02 (D.R.G.), and the King Faisal Specialist Hospital and Research Centre (K.S.A.K.). S.J.P. is a Liver Scholar of the American Liver Foundation.
REFERENCES
- 1.al-Faleh F Z, Aljumah A, Rezeig M, Al-Otaibi M, Alahdal M, Al-Humayed S, Mayet I, Al-Juhani M, Al-Karawi M, George K, Sbeih F. Treatment of chronic hepatitis C genotype IV with interferon-ribavirin combination in Saudi Arabia: a multicentre study. J Viral Hepatol. 2000;7:287–291. doi: 10.1046/j.1365-2893.2000.00213.x. [DOI] [PubMed] [Google Scholar]
- 2.al-Faleh F Z, Huraib S, Sbeih F, al-Karawi M, al-Rashed R, al-Mofleh I A, Sougiyyah M, Shaheen M, Ramia S. Hepatitis C virus genotypes in patients with chronic liver disease and haemodialysis patients from Saudi Arabia. J Viral Hepatol. 1995;2:293–296. doi: 10.1111/j.1365-2893.1995.tb00044.x. [DOI] [PubMed] [Google Scholar]
- 3.al-Faleh F Z, Sbeih F, al-Karawi M, al-Mofleh I A, al-Rashed R S, Ayoola A, al-Amri S, Mayet I, al-Habbal T M, al-Omair A, al-Sohaibani M, Abdullah O, Mohamed S A, el-Sheikh M A. Treatment of chronic hepatitis C genotype 4 with alpha-interferon in Saudi Arabia: a multicenter study. Hepatogastroenterology. 1998;45:488–491. [PubMed] [Google Scholar]
- 4.Bird G. Interleukin-8 in alcoholic liver disease. Acta Gastroenterol Belgium. 1994;57:255–259. [PubMed] [Google Scholar]
- 5.Davis G L, Esteban-Mur R, Rustgi V, Hoefs J, Gordon S C, Trepo C, Shiffman M L, Zeuzem S, Craxi A, Ling M H, Albrecht J. Interferon alpha-2b alone or in combination with ribavirin for the treatment of relapse of chronic hepatitis C. N Engl J Med. 1998;339:1493–1499. doi: 10.1056/NEJM199811193392102. [DOI] [PubMed] [Google Scholar]
- 6.Fukuda R, Ishimura N, Ishihara S, Chowdhury A, Morlyama N, Nogami C, Miyake T, Niigake M, Tokuda A, Satoh S, Sakai S, Akagi S, Watanabe M, Fukomoto S. Intrahepatic expression of pro-inflammatory cytokine mRNAs and interferon efficacy in chronic hepatitis C. Liver. 1996;16:390–399. doi: 10.1111/j.1600-0676.1996.tb00768.x. [DOI] [PubMed] [Google Scholar]
- 7.Kaplanski G, Farnarier C, Payan M J, Bongrand P, Durand J M. Increased levels of soluble adhesion molecules in the serum of patients with hepatitis C: correlation with cytokine concentrations and liver inflammation and fibrosis. Dig Dis Sci. 1997;42:2277–2284. doi: 10.1023/a:1018818801824. [DOI] [PubMed] [Google Scholar]
- 8.Kato N, Yoshida H, Ono N-S K, Kato J, Goto T, Otsuka M, Lan K H, Matsushima K, Shiratori Y, Omata M. Activation of intracellular signaling by hepatitis B and C viruses: C-viral core is the most potent signal inducer. Hepatology. 2000;32:405–412. doi: 10.1053/jhep.2000.9198. [DOI] [PubMed] [Google Scholar]
- 9.Liang T J, Rehermann B, Seeff L B, Hoofnagle J H. Pathogenesis, natural history, treatment, and prevention of hepatitis C. Ann Intern Med. 2000;132:296–305. doi: 10.7326/0003-4819-132-4-200002150-00008. [DOI] [PubMed] [Google Scholar]
- 10.McClain C J, Barve S, Deaciuc I, Kugelmas L M, Hill D. Cytokines in alcoholic liver disease. Semin Liver Dis. 1999;19:205–219. doi: 10.1055/s-2007-1007110. [DOI] [PubMed] [Google Scholar]
- 11.McHutchison J G, Gordon S C, Schiff E R, Shiffman M L, Lee W M, Rustgi V K, Goodman Z D, Ling M H, Cort S, Albrecht J K. Interferon alpha-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. N Engl J Med. 1998;339:1485–1492. doi: 10.1056/NEJM199811193392101. [DOI] [PubMed] [Google Scholar]
- 12.Mukaida N, Harada A, Yasumoto K, Matsushima K. Properties of pro-inflammatory cell type-specific leukocyte chemotactic cytokines, interleukin 8 (IL-8) and monocyte chemotactic and activating factor (MCAF) Microbiol Immunol. 1992;36:773–789. doi: 10.1111/j.1348-0421.1992.tb02080.x. [DOI] [PubMed] [Google Scholar]
- 13.Pawlotsky J M, Germanidis G, Neumann A U, Pellerin M, Frainais P O, Dhumeaux D. Interferon resistance of hepatitis C virus genotype 1b: relationship to nonstructural 5A gene quasispecies mutations. J Virol. 1998;72:2795–2805. doi: 10.1128/jvi.72.4.2795-2805.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polyak S J, Gerotto M. Molecular basis of responsiveness to interferon in hepatitis C. Trends Exp Clin Med Forum. 2000;10:46–58. [PubMed] [Google Scholar]
- 15.Polyak S J, Khabar K S A, Paschal D M, Ezelle H J, Duverlie G, Barber G N, Levy D E, Mukaida N, Gretch D R. Hepatitis C virus nonstructural 5A protein induces interleukin-8, leading to partial inhibition of the interferon-induced antiviral response. J Virol. 2001;75:6095–6106. doi: 10.1128/JVI.75.13.6095-6106.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polyak S J, McArdle S, Liu S L, Sullivan D G, Chung M J, Hofgartner W T, Carithers R L, McMahon B J, Mullins J I, Corey L, Gretch D R. Evolution of hepatitis C virus quasispecies in hypervariable region 1 and the putative interferon sensitivity-determining region during interferon therapy and natural infection. J Virol. 1998;72:4288–4296. doi: 10.1128/jvi.72.5.4288-4296.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poynard T, Marcellin P, Lee S S, Niederau C, Minuk G S, Ideo G, Bain V, Heathcote J, Zeuzem S, Trepo C, Albrecht J. Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group. Lancet. 1998;352:1426–1432. doi: 10.1016/s0140-6736(98)07124-4. [DOI] [PubMed] [Google Scholar]
- 18.Shimoda K, Begum M A, Shibuta K, Mori M, Bonkovsky H L, Banner B F, Barnard G F. Interleukin-8 and hIRH (SDF1-alpha/PBSF) mRNA expression and histological activity index in patients with chronic hepatitis C. Hepatology. 1998;28:108–115. doi: 10.1002/hep.510280116. [DOI] [PubMed] [Google Scholar]
- 19.Shobokshi O A, Serebour F E, Skakni L, Saffy Y H AI-, Ahdal M N. Hepatitis C genotypes and subtypes in Saudi Arabia. J Med Virol. 1999;58:44–48. [PubMed] [Google Scholar]