Abstract
With increasingly serious environmental pollution problems, the development of efficient photocatalytic materials has become a hotspot in current research. This study focused on phosphorus-doped carbon nitride/titanium dioxide (PCT) Z-type heterojunctions, aiming to deeply investigate their photocatalytic degradation and photosensitive antimicrobial properties. A PCT Z-type heterojunction was successfully fabricated using melamine phosphate, cyanuric acid, and titanium dioxide. The structure, morphology, and optical properties of PCT Z-type heterojunctions were explored by FTIR, XRD, XPS, BET, SEM, UV-Vis DRS, TEM, EIS, and PL. A comprehensive and in-depth analysis of the structure, morphology, and optical properties of PCT Z-type heterojunctions was carried out. The photocatalytic degradation experiments revealed that PC3T Z-type heterojunctions exhibited an excellent degradation capability for methylene blue (MB) under visible light. The effect of PC3T on the adsorption–photocatalytic degradation of MB is more than 1.5 times that of a single titanium dioxide and P-doped carbon nitride. In the photosensitive antimicrobial performance study, PC3T reduced the survival rate of E. coli to 7%, after 120 min. Through free radical trapping experiments, it was shown that the hydroxyl radicals and superoxide radicals exerted an influence on the photocatalytic process. This study offers new ideas and approaches to address environmental pollution problems and holds significant theoretical and applied value.
Keywords: Z-type heterojunction, photocatalytic degradation, photosensitive antibacterial
1. Introduction
As industrialization continues to advance and the scale of urbanization expands rapidly, environmental pollution is becoming increasingly severe, and water pollution has become a key issue that needs to be solved urgently. Traditional methods for environmental treatment have many deficiencies in addressing such problems [1,2]. They are not only inefficient but also consume a large amount of energy. More seriously, new issues like secondary pollution will arise from the treatment process, which fails to meet the high-level environmental protection demands of modern society [3,4,5]. As a green and efficacious emerging technology, photocatalysis has substantial advantages in environmental governance [6]. In the process of pollutant degradation, the reaction conditions of photocatalysis are relatively mild and do not require high pressure, which can realize effective energy saving [7]. In terms of antibacterial research, photocatalytic technology can effectively eliminate pathogenic bacteria in the environment and will not cause secondary pollution [8,9]. Hence, the application prospect of photocatalysis technology in the environmental field is extremely wide, especially in resolving the problem of dye pollution and bacterial transmission.
Among numerous photocatalysts, titanium dioxide (TiO2) has become the research hotspot because of its distinctive properties and excellent performance [10,11]. TiO2 has excellent chemical stability, thermal stability, and photocatalytic activity [12]. Upon the irradiation of TiO2 by a photon with energy exceeding its bandgap width, electrons were stimulated to move from the valence band to the conduction band. This led to the formation of electron (e−) and hole (h+) pairs and subsequently resulted in the generation of reactive oxygen species (ROS) [13]. ROS has a powerful redox capacity and is capable of decomposing organic pollutants into harmless carbon dioxide and water, thus attaining the photocatalytic degradation of pollutants [14]. However, TiO2 has a large forbidden bandwidth and can only absorb ultraviolet light leading to its low utilization of solar energy. What is more, e⁻ and h+ on TiO2 are easily compounded, reducing the photocatalytic efficiency [15]. In order to break through these limitations, researchers are constantly exploring new modification approaches, among which building a heterojunction is an efficient approach.
Carbon nitride (g-C3N4), as a novel type of non-metallic photocatalyst, has become a hot research subject due to its distinctive structure and properties [16,17]. g-C3N4 has a suitable energy band structure and is capable of absorbing visible light, which endows it with an advantage in the utilization of solar energy [18,19]. Additionally, g-C3N4 has excellent thermal and chemical stability, and its preparation method is relatively simple and low in cost [20]. It has been mentioned to be the case that the combination of g-C3N4/TiO2 heterojunctions not only achieves a remarkable broadening of the light absorption range but also effectively facilitates the separation and transfer of photogenerated e− and h+, which results in the enhancement of photocatalytic performance [21]. The band structure of g-C3N4/TiO2 belonged to a type II heterostructure [22,23]. However, some studies suggested that it might be a Z-type heterostructure [24]. Compared with the type II heterostructure, the Z-type heterostructure showed more significant superiority. In the Z-type heterostructure, the transfer paths of photogenerated e− and h+ were more unique, endowing it with stronger redox ability. Ning et al. demonstrated that the creation of a Z-type heterojunction between g-C3N4 and TiO2 was capable of generating hydroxyl and superoxide radicals, while a type II heterojunction could produce only one type of radical [25]. The doping of elemental phosphorus offered new possibilities for the photocatalytic activity of the g-C3N4/TiO2 heterojunction. Based on previous reports, the main raw materials, methods, and applications for the preparation of phosphorus-doped carbon nitride/titanium dioxide composite catalysts are summarized and listed in Table 1. Su et al. prepared the heterogeneous structures of phosphate-doped carbon nitride/titanium dioxide nanotube arrays (P-CN/TiO2 NTs) by electrochemical anodizing, wet dipping, and hot polymerization [26]. P-CN/TiO2 NTs possessed preferable light absorption properties, boosted charge separation and transfer capability, and exhibited good photocatalytic performance for methylene blue (MB). Wadhai et al. prepared phosphorus-doped carbon nitride/titanium dioxide composites (PCN-P25) by the calcination and coupling method using melamine, 1-hydroxyethane-1,1-diphophonic acid, ethylene glycol, and P25 as raw materials [27]. PCN-P25 could be applied for hydrogen production. In addition, Kumar et al. prepared composite catalysts for hydrogen production by the hydrothermal method using various feedstocks [28]; Cako et al. prepared composite photocatalysts for CO2 reduction by the solid sublimation and conversion method [29]. However, their preparation processes were complex or used a wide variety of raw materials. Therefore, it is imperative to formulate a straightforward and practicable approach for preparation.
Table 1.
Summary of materials, methods, and applications of phosphorus-doped carbon nitride/titanium dioxide composites.
| Materials | Preparation Methods | Application Fields | References |
|---|---|---|---|
| Ammonium fluoride, dicyandiamide, 2-aminobenzonitrile, 1-butyl-3-methylimidazolium hexafluorophosphate, ethylene glycol |
Anodizing and wet-soaking method | Degradation and hydrogen production | [26] |
| Melamine, 1-hydroxyethane- 1,1-diphophonic acid, ethylene glycol |
Calcination and coupling method | Hydrogen production | [27] |
| Urea, citric acid, 1-butyl-3 methylimidazolium hexafluorophosphate, hydrofluoric acid, acetic acid |
Hydrothermal method | Hydrogen production | [28] |
| Ammonium fluoride, glycerol, phosphate, urea, sodium sulfate |
Solid sublimation and conversion method | CO2 reduction | [29] |
In this study, a phosphorus-doped carbon nitride/TiO2 (PCT) Z-type heterostructure was synthesized through an in situ method with melamine phosphate, cyanuric acid, and titanium dioxide as raw materials. The objective was to combine the respective advantages of phosphorus doping with carbon nitride and TiO2 to achieve more efficient photocatalytic degradation and antimicrobial effects. FTIR, XRD, XPS, BET, SEM, UV-Vis DRS, TEM, EIS, and PL were employed to conduct a comprehensive and meticulous analysis of its structure, morphology, and optical properties. Through photocatalytic degradation, photosensitive antimicrobial, and free radical capture experiments, the photocatalytic degradation and antimicrobial performance as well as the related mechanisms were investigated thoroughly, with the aim of providing new ideas and effective methods for addressing the environmental pollution problems.
2. Results and Discussion
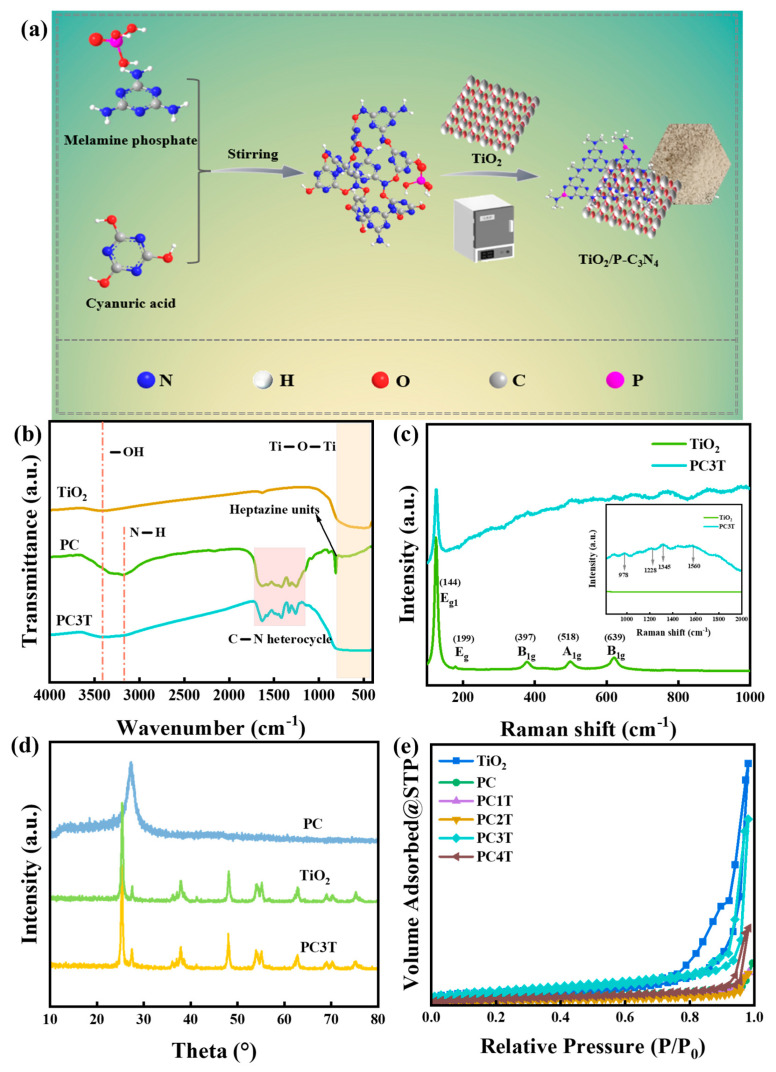
The bonding characteristics were explained through FTIR spectra as shown in Figure 1b. The absorption peak at 607 cm−1 was attributed to the stretching oscillation of the Ti-O bond [30]. Moreover, the absorption peak at 3400 cm−1 was designated to the stretching band of the -OH group, indicating substantial adsorption of water molecules on TiO2 [31]. The FTIR spectrum of the PC disclosed distinctive stretching modes of the C-N heterocycle system in the range of 1200–1700 cm−1, as well as the heptazine units at 810 cm−1 [32]. The multiple bands within the range of 3000–3500 cm⁻1 were ascribed to N-H and -OH [33]. In PC3T, the featured peak of the CN heterocycle was observed. Although no heptazine units were detected, the absorption peak near the Ti-O bond broadened, suggesting that the composite of PC and TiO2 was successful.
Figure 1.
(a) Schematic illustration of PC3T, (b) FTIR spectra, (c) Raman spectra, (d) XRD patterns, and (e) N2 adsorption–desorption isotherms of various samples.
Raman spectroscopy also demonstrated the successful preparation of PC3T, as shown in Figure 1c. The Raman spectra of TiO2 displayed five characteristic Raman bands (2Eg, 2B1g, and A1g), among which the strongest Eg band was detected at approximately 144 cm−1 [27]. All the characteristic peaks belonging to TiO2 were maintained in PC3T. In the Raman spectra of TiO2, there was no hump seen around 1500 cm⁻1. After coupling with PC, the broad peak (978, 1228, 1345, and 1560 cm−1) between 900 cm−1 and 2000 cm−1 and all the peaks of TiO2 (140–800 cm−1) were observed, implying the formation of PC3T (interior illustration) [34,35]. However, the Raman peaks of PC3T exhibited slight shifts to lower wavenumbers in comparison with TiO2, attributed to the interaction of PC with TiO2. This might further confirm the variation in the electronic energy level of TiO2 after being combined with PC.
Figure 1d presents the XRD patterns of TiO2, PC, and PC3T samples illustratively. The crystal structures of TiO2 were mixtures of anatase and rutile crystals. The characteristic peaks were in correspondence with the standard data of JCPDS: 021-1272 and JCPDS: 021-1276 [36]. The significant peak at 27.23° corresponded to the (002) plane and the interlayer stacking structure of the aromatic compound [37]. Additionally, the peak at 13.01° was in connection with the (100) plane and was due to the in-plane repeat period of tri-s-triazine [38]. Due to the low PC doping, no distinct characteristic peaks were observed in PC3T. However, the characteristic peak width at 27.30° was broader than that of TiO2. This might be attributed to the presence of strong peaks for PC.
N2 adsorption–desorption isotherms were measured to conduct an analysis of the surface areas and porosity. As shown in Figure 1e, the specific surface areas of TiO2, PC, PC1T, PC2T, PC3T, and PC4T were 35.43 m2/g, 9.13 m2/g, 12.22 m2/g, 16.43 m2/g, 21.34 m2/g, and 20.15 m2/g. Compared to PC, PC3T had a larger specific surface area, which might be due to the incorporation of TiO2. In addition, PC3T had a large pore width relative to the other samples (Table S1). The large specific surface area and pore width of PC3T facilitated the exposure of more active sites for the adsorption–degradation of dyes and photosensitized antibacterial activity [39,40].
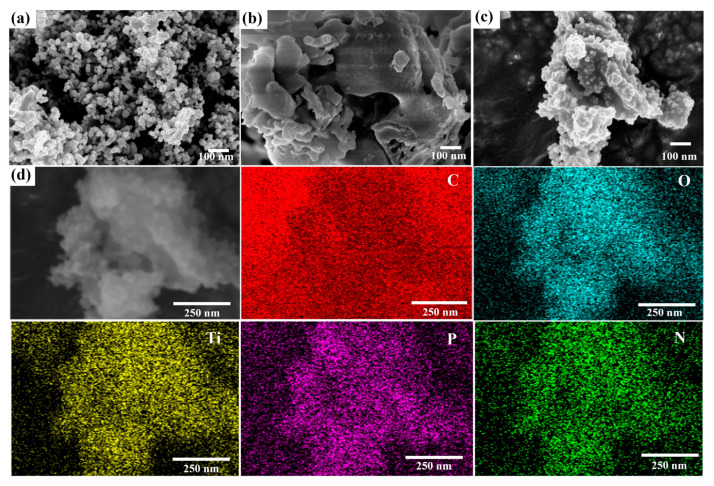
As observed in Figure 2a, TiO2 showed evenly dispersed tiny spherical particles in aggregates, with a uniform diameter [41]. The PC exhibited irregularly stacked morphologies (Figure 2b) [42]. Figure 2c revealed that regular spherical nanoparticles were embedded on the surface of PC. The large number of TiO2 was uniformly dispersed and well-embedded in PC. At the same time, the element mapping indicated that Ti, O, C, N, and P were uniformly present on the surface of PC3T (Figure 2d).
Figure 2.
SEM images of (a) TiO2, (b) PC, and (c) PC3T; (d) HAADF-STEM image and corresponding EDS elemental (C, O, Ti, P, and N) mapping of PC3T.
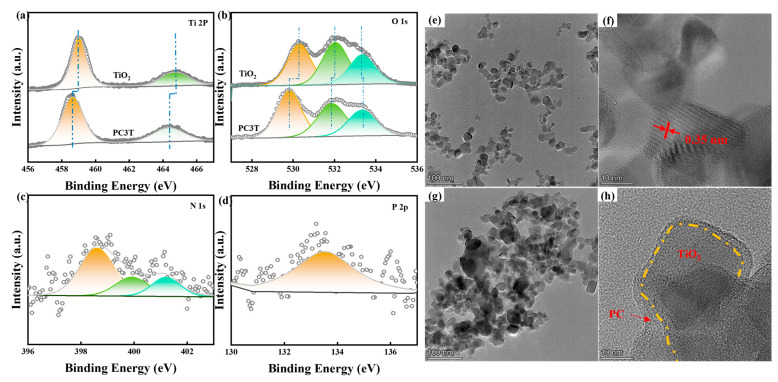
The surface constituents and chemical statuses of the samples were examined through XPS. The survey scan of XPS presented the typical spectra of TiO2, and no other peaks were observed except for Ti, O, and C (Figure S1). PC possessed the peaks of C, N, O, and P. PC3T included all the characteristic peaks of the aforementioned TiO2 and PC. To acquire deeper insights into the molecular architecture and interatomic chemical bonding in the composite, the high-resolution spectra of Ti 2p, O 1s, N 1s, and P 2p of the samples are shown in Figure 3a–d. The bi-peaks of Ti 2p3/2 and Ti 2p1/2 belonging to PC3T were present in the Ti 2p peaks (Figure 3a). The energy interval between the double peaks was 5.66 eV, which was a characteristic of a typical Ti4+ species with a Ti-O structure [43]. Moreover, in contrast to TiO2, the binding energies of Ti 2p shifted negatively from 458.99 and 464.64 eV to 458.63 and 464.32 eV, respectively. In the O 1s spectrum of CP3T, three peaks at 529.80, 531.87, and 533.33 eV, correspond to the lattice oxygen, oxygen vacancy (Ov) of TiO2, and the surface adsorbed oxygen. Likewise, the binding energy of adsorbed oxygen in PC3T had a positive shift compared to that in TiO2, which might be caused by the addition of PC. However, the lattice oxygen and the Ov in TiO2 had a negative shift from 532.04 and 533.35 eV to 531.87 and 533.33 eV, respectively. Binding energy shifts in XPS spectra indicated a strong interaction between TiO2 and PC in PC3T [44]. As depicted in Figure 3c, regarding the XPS spectrum of N 1s, three peaks at 398.61 eV, 398.81 eV, and 399.91 eV were, respectively, ascribed to the C-N-C, N-(C)3, and -NHX bonds [45]. Figure 3d presents the high-resolution XPS of P 2p for PC3T and the peaks at 133.5 eV. As reported, the binding energies of P 2p in P-N and P-C bonds were approximately 133.5 eV and fall within the range of 131.5–132.5 eV, respectively. Therefore, P might tend to enter the g-C3N4 network as P-N in place of the C site, which might promote the redshift of the visible absorption [46].
Figure 3.
(a) Ti 2p and (b) O 1s spectra of TiO2 and PC3T; (c) N 1s and (d) P 2p spectra of PC3T; and TEM images of (e,f) TiO2 and (g,h) PC3T.
TEM images further revealed the successful preparation of PC3T (Figure 3e–h). As illustrated in Figure 3e,f, the particle had a lattice spacing of 0.35 nm, aligning with the (101) crystal planes of anatase TiO2 [47]. After the formation of the composite photocatalysts, the morphology and lattice spacing of TiO2 remained largely unchanged. However, disordered structural features appeared around TiO2, echoing the characteristics of PC [48]. Therefore, PC3T had been successfully prepared.
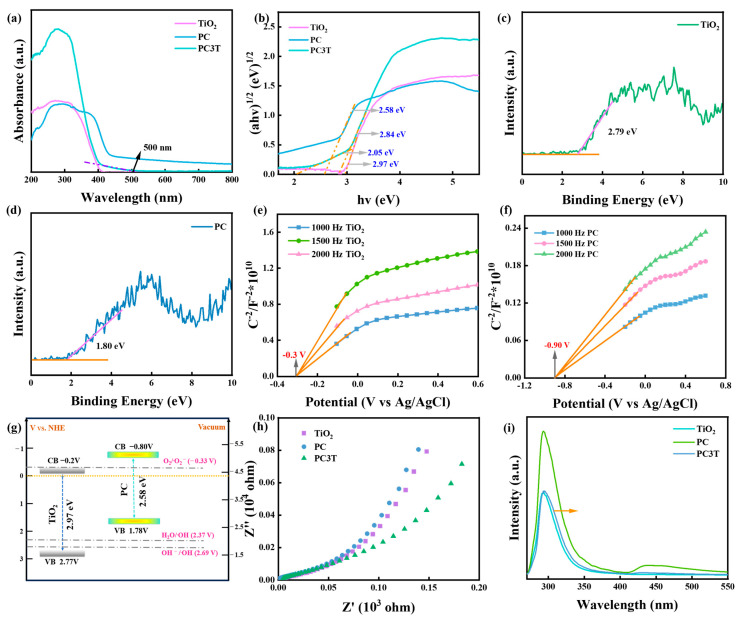
There existed a strong connection between the performance and the photo-absorption of the photocatalysts. As depicted in Figure 4a, the UV-vis DRS absorption spectra of TiO2 merely absorbed within the UV irradiation range of about 410 nm. PC had a steep visible absorption peak around 450 nm [49]. The absorption peak was shifted toward visible wavelengths by the in situ binding of the two materials. Moreover, PC3T had a tail peak with an absorption of about 500 nm. This tail absorption peak could be an intermediate energy level formed between the conduction and valence bands, allowing electrons to be excited more easily and improving photocatalytic performance [23,50]. The bandgaps of the samples were identified using the Kubelka–Munk function method and are shown in Figure 4b. The bandgaps of TiO2, PC, and PC3T were 2.97 eV, 2.58 eV, and 2.84 eV.
Figure 4.
(a) UV-Vis DRS and (b) bandgap energy spectra of the samples; (c,d) valence band spectra of TiO2 and PC3T; Mott–Schottky plots of (e) TiO2 and (f) PC3T; (g) band structures of TiO2 and PC; and (h) EIS plots and (i) PL spectra of samples.
The valence band (VB) positions of TiO2 and PC were determined by valence band XPS spectra (Figure 4c,d). The VB XPS of TiO2 indicated the VB position at 2.79 eV, whereas the VB of PC was at 1.80 eV. Based on the following formula, EVB, NHE = ϕ + EVB, XPS − 4.44, the work function (ϕ) of the instrument was 4.42 eV. Thus, the actual values of the valence band positions of TiO2 and PC were 2.77 V and 1.78 V, respectively. The determination of the conduction bands (CBs) was achieved by extrapolating Mott–Schottky curves. Figure 4e,f displays the Schottky curves of TiO2 and PC. Through extrapolating the curve to 1/C2 = 0, the Fermi level (EF) values of TiO2 and PC were determined as −0.30 and −0.90 V, respectively (vs. Ag/AgCl) [51,52]. These values were further changed to −0.10 and −0.7 eV (vs. NHE). It was found that the conduction band potential (ECB) of an n-type semiconductor was about 0.1 eV lower than the value of EF. The ECB potentials of TiO2 and PC were, respectively, determined to be −0.20 V and −0.80 V. The bandgap was determined with the following formula: ECB = EVB + Eg. The findings that integrated the analysis of bandgaps and valence band positions were in accordance with the conduction position [53]. Figure 4g displays the band structures of both TiO2 and PC.
The photogenerated charge transfer was intimately connected to the interface resistance, and this connection could be manifested through electrochemical impedance spectroscopy (EIS) [54]. The Nyquist plots of TiO2 and PC are presented in Figure 4h. The EIS results disclosed that PC3T had a smaller impedance arc radius compared to TiO2 and PC, signifying the lower charge transfer resistance.
Furthermore, the PL spectra of TiO2 and PC showed a clear emission peak at around 300 nm (Figure 4i). This was due to the recombination of photogenerated electrons and holes. The PL intensity of TiO2 was lower than that of PC. The PL spectrum of PC3T appeared to be shifted to the right, and the intensity was lower than that of TiO2 and PC. This phenomenon successfully demonstrated that the combination of TiO2 and PC promoted electrons and holes, preventing the recombination of photogenerated carriers, and thereby improving photocatalytic performance [55].
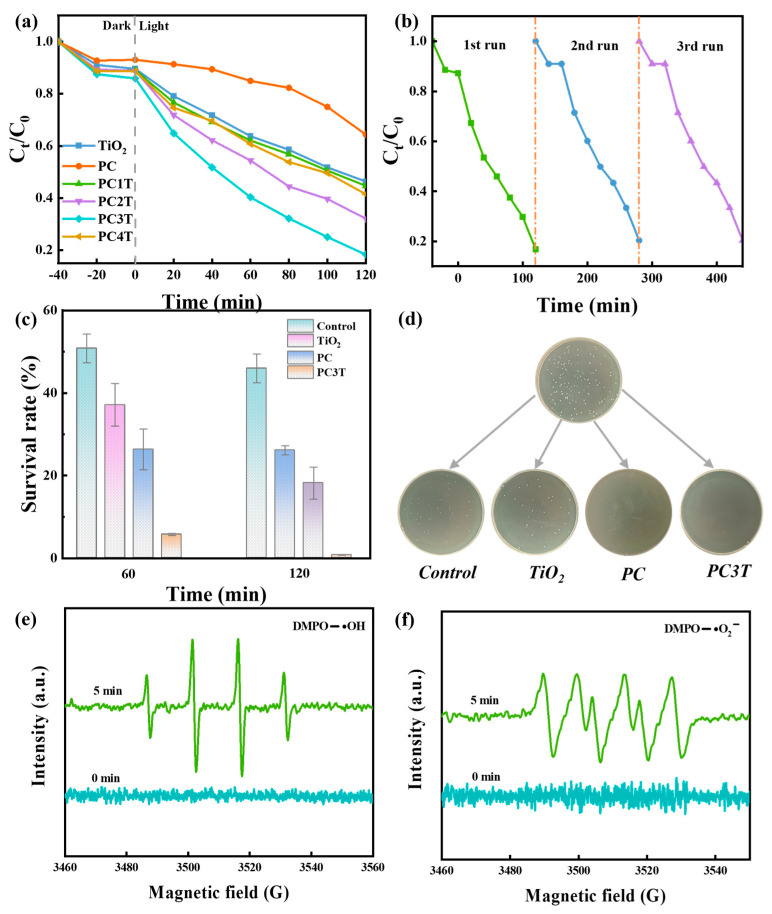
The photocatalytic degradation performance of MB catalyzed by various catalysts is shown in Figure 5a. The respective photocatalytic degradation rates of TiO2, PC, PC1T, PC2T, PC3T, and PC4T were 54%, 36%, 56%, 68%, 82%, and 59%. PC3T demonstrated exceptional adsorption–photocatalytic degradation performance. This could be attributed to the combined effect of a larger specific surface area and the creation of heterogeneous structures. The cycling test was conducted to assess the recycling potential of PC3T (Figure 5b). The result indicated that no significant change occurred in its degradation capacity after three cycles, confirming the outstanding recyclability of PC3T [56]. The photosensitive antibacterial properties of the materials were studied using E. coli as model bacteria. After 120 min, it could be seen from Figure 5c that PC3T showed outstanding antibacterial performance under illumination conditions, and the survival rate of E. coli was reduced to 7%. The related practical images can be found in Figure 5d.
Figure 5.
(a) Photocatalytic degradation of MB for various samples; (b) stability of PC3T for the photocatalytic degradation of MB; (c) photosensitive antimicrobial survival and (d) real images of control, TiO2, PC, and PC3T against E. coli; and ESR spectra of (e) DMPO-•O2− and (f) DMPO-•OH for PC3T.
As illustrated in Figure 5e,f, no signals of hydroxyl (•OH) and superoxide (•O2−) radicals could be observed under dark conditions. Nevertheless, the appearance of the characteristic peaks of DMPO-•O2− and DMPO-•OH under light irradiation indicated the production of •OH and •O2− in the reaction system, which was consistent with the characterization of the Z-type heterojunction for the production of radicals. What is more, the signals of •O2− were weaker compared to those of •OH, suggesting that •OH radicals probably had a greater effect on the photocatalytic process than •O2− radicals. Overall, •OH radicals significantly participated in the photocatalytic process of PC3T.
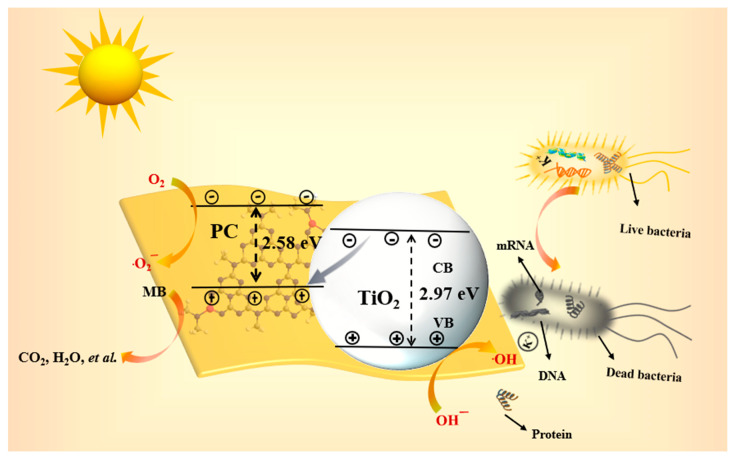
Based on the aforementioned results, Figure 6 presents the diagram of the photocatalytic degradation and antimicrobial mechanism of PC3T. XPS, TEM, etc., demonstrated the existence of better bonding between PC and TiO2 to form a heterogeneous structure. From the perspective of an energy band structure, when PC was combined with TiO2, a Z-type heterojunction was created at their interfaces. Under visible light excitation, both TiO2 and PC generate electrons (e⁻) from the valence band (VB) to the conduction band (CB) and retain holes (h⁺) in the VB. When the two photocatalysts are in close contact, part of the e⁻ of TiO2 can be rapidly transferred to the VB of PC, combining with the h⁺ of PC, and then excited into the CB of PC. The CB of the PC is positioned above the level at which reactive oxygen species are produced, so the e⁻ on the CB of the PC can interact with the O2 adsorbed on the surface to form ·O2−. In addition, the holes in VBs of TiO2 can interact with water or hydroxide ions (OH⁻) to form ·OH [57,58]. Consequently, the produced free radicals could achieve good degradation of MB and antimicrobial effects.
Figure 6.
The diagram of the possible mechanism of photocatalytic degradation and photosensitive antibacterial for PC3T.
3. Conclusions
The above results suggested that it was feasible and effective to prepare PCT Z-type heterojunctions using melamine phosphate, cyanuric acid, and titanium dioxide through an in situ method. The characterization-based in-depth analysis of the structure, morphology, and optical properties of the composite photocatalysts laid the foundation for comprehending their structure and performance. BET tests demonstrated that PC3T had a large specific surface area and pore size. UV-Vis DRS evidenced a significant redshift of PC3T toward visible light. PL and EIS synergistically proved that photogenerated electrons and holes were well separated in PC3T. The photocatalytic degradation experiments confirmed the excellent degradation ability of PC3T Z-type heterojunctions for MB under visible light and the better photosensitive antibacterial activity. In terms of the adsorption–photocatalytic degradation of MB, the effect of PC3T is over 1.5 times greater than that of a single TiO2 and P-doped carbon nitride. Regarding the photosensitive antimicrobial performance, after 120 min, PC3T was able to reduce the survival rate of E. coli to 7%. ·O2− and ·OH were proved to be the active species in the photocatalytic process by the EPR test, which was also in accordance with the characteristics of the Z-type heterojunction for the generation of free radicals. PCT Z-type heterojunctions were promising as effective materials for solving environmental pollution problems.
4. Experimental Sections
4.1. Materials
Melamine phosphate and melamine were acquired from Shanghai Macklin Biochemical Technology Co. (Shanghai, China). Cyanic acid was purchased from Shanghai Aladdin Reagent Co. (Evonik, Germany). TiO2 was obtained from Degussa GmbH in Germany. Beef extract, sodium chloride, peptone, agar powder, and yeast powder were purchased from Sinopharm Chemical Reagent Co. (Shanghai, China). Anhydrous sodium sulfate was provided by Aladdin Chemical Reagents Ltd. (Shanghai, China). Lyophilized powders of E. coli were acquired from the Henan Provincial Engineering and Technology Research Centre for Industrial Microbial Strains in China. Ultrapure water was used in the experiment.
4.2. Sample Preparation
As shown in Figure 1a, the composite photocatalysts were fabricated by an in situ method. Firstly, a certain mass of melamine phosphate was dissolved and then the cyanuric acid solution was added and stirred for 30 min. Secondly, 0.1 g of TiO2 was added and stirred for 3 min, and then centrifuged and dried. Subsequently, the solution was filtered and dried. Eventually, the powders were calcined at 500 °C for 1 h with the aim of obtaining PC1T, PC2T, PC3T, and PC4T, respectively. PC1T, PC2T, PC3T, and PC4T corresponded to different masses of cyanuric acid (0.129 g, 0.193 g, 0.258 g, and 0.323 g), respectively.
4.3. Material Characterization
The functional groups were ascertained by means of a Nicolet IS10 FTIR spectrometer (Gangdong Technology Development Co., Ltd., Tianjin, China) (in the range of 4000–400 cm−1). The crystal structures were identified through X-ray powder diffraction with the DMAX-D8X instrument (Shimadzu Co., Ltd., Kyoto, Japan). The morphologies were characterized via FE-SEM (Carl Zeiss AG Co., Ltd., Oberkochen, Germany) and FE-TEM (Thermo Fisher Scientific, Waltham, MA, USA). The molecular structures of samples were utilized for characterization through X-ray photoelectron spectroscopy (Thermo Fisher Scientific Co., Ltd., Waltham, MA, USA). The specific surface area and pore size distribution of the materials were gauged by a specific surface area analyzer (Quantachrome Instrument Co., Ltd., Boynton Beach, FL, USA). Raman spectroscopy was employed to verify the structural alterations in the samples using the Hrobioa Xplra plus Raman system (HORIBA Instrument Co., Ltd., Oberursel, Germany). UV–visible diffuse reflectance spectroscopy was measured within the range of 200–2000 nm by means of UH4150 (Hitachi Instrument Co., Ltd., Hitachi, Japan). The photoluminescence spectra of the materials were measured by the FLS1000 fluorescence spectrometer (Edinburgh Instruments Co., Ltd., Edinburgh, UK). Electrochemical measurements were performed by means of an electrochemical workstation equipped with a three-electrode setup (Shanghai Chenhua Instrument Co., LTD., Shanghai, China). Pt and Ag/AgCl electrodes were, respectively, employed as the counter and reference electrodes. A 0.5 M sodium sulfate solution was used as an electrolyte to test the samples for electrochemical impedance and Mott–Schottky measurements. The Bruker A300 could be used to obtain electron spin resonance (ESR) signals (Bruker Instrument Co., Ltd., Billerica, MA, USA).
4.4. Photocatalytic Test
The 10 mg samples were placed in 50 mL of the 10 mg L−1 (2.67 × 10−5 mol L−1) MB solution and shaken in the darkness for 40 min to achieve the adsorption–desorption equilibrium. Next, the solution was exposed to radiation from a 500 W Xenon lamp that had a filter (λ = 420 nm), and 4 mL of the suspension was withdrawn every 20 min. The supernatant was obtained by centrifugation. The absorbance of the MB solution at 664 nm was measured by a UV-Vis spectrophotometer (Shanghai Youke Instrument Co., Ltd., Shanghai, China). The degree of MB degradation was determined by absorbance values.
Cyclic stability experiments were conducted to demonstrate the reusability of the sample. The experimental procedure was similar to the photocatalytic degradation experiment described above. After the first photocatalytic degradation experiment, the photocatalyst was centrifuged, washed, and dried, and then the second photo–photocatalytic degradation experiment was carried out. After the same steps, the third photocatalytic experiment was carried out.
4.5. Photosensitive Antibacterial Experiment
The samples (5 mg mL−1) and the E. coli suspension (C ≈ 1.2 × 105 CFU mL−1) were exposed to irradiation beneath a 200 W incandescent lamp. Every 60 min, the bacteria were aspirated and diluted for coating. The bacteria were cultivated for 15 h and then counted.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29184342/s1, Figure S1: XPS spectra of full spectrum of TiO2, PC, and PC3T; Table S1: Surface areas and pore sizes of all the samples.
Author Contributions
Conceptualization, Y.Z.; Methodology, J.Y. and S.Z.; Software, Y.Z. and D.T.; Formal analysis, K.L.; Resources, D.T.; Data curation, K.L.; Writing—original draft, J.Y.; Writing—review & editing, Y.Z. and Y.L. (Yi Liu); Supervision, X.Y., Y.L. (Yuesheng Li) and Y.L. (Yi Liu); Project administration, S.Z., X.Y. and Y.L. (Yi Liu); Funding acquisition, Y.L. (Yuesheng Li). All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the National Natural Science Foundation of China (No. 11405050), the Hubei Provincial Colleges and Universities Outstanding Young and Middle-aged Technological Innovation Team Project (No. T2020022), Xianning City Key Program of Science & Technology (No. 2021GXYF021), and the Science Development Foundation of Hubei University of Science & Technology (Nos. 2020TD01 and 2021ZX01).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Zhang F., Wang X., Liu H., Liu C., Wan Y., Long Y., Cai Z. Recent Advances and Applications of Semiconductor Photocatalytic Technology. Appl. Sci. 2019;9:2489. doi: 10.3390/app9122489. [DOI] [Google Scholar]
- 2.Saravanan A., Senthil Kumar P., Jeevanantham S., Karishma S., Tajsabreen B., Yaashikaa P.R., Reshma B. Effective water/wastewater treatment methodologies for toxic pollutants removal: Processes and applications towards sustainable development. Chemosphere. 2021;280:130595. doi: 10.1016/j.chemosphere.2021.130595. [DOI] [PubMed] [Google Scholar]
- 3.Noureen L., Wang Q., Humayun M., Shah W.A., Xu Q., Wang X. Recent advances in structural engineering of photocatalysts for environmental remediation. Environ. Res. 2023;219:115084. doi: 10.1016/j.envres.2022.115084. [DOI] [PubMed] [Google Scholar]
- 4.Hussaina A., Kumaria R., Sachana S.G., Sachanb A. Biological Wastewater Treatment Technology: Advancement and Drawbacks. Elsevier; Amsterdam, The Netherlands: 2021. pp. 175–192. [Google Scholar]
- 5.Zhou Y., Lu J., Zhou Y., Liu Y. Recent advances for dyes removal using novel adsorbents: A review. Pt AEnviron. Pollut. 2019;252:352–365. doi: 10.1016/j.envpol.2019.05.072. [DOI] [PubMed] [Google Scholar]
- 6.Prakruthi K., Ujwal M.P., Yashas S.R., Mahesh B., Kumara Swamy N., Shivaraju H.P. Recent advances in photocatalytic remediation of emerging organic pollutants using semiconducting metal oxides: An overview. Environ. Sci. Pollut. Res. 2022;29:4930–4957. doi: 10.1007/s11356-021-17361-1. [DOI] [PubMed] [Google Scholar]
- 7.Mohamadpour F., Amani A.M. Photocatalytic systems: Reactions, mechanism, and applications. RSC Adv. 2024;14:20609–20645. doi: 10.1039/D4RA03259D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vevers R., Kulkarni A., Seifert A., Poschel K., Schlenstedt K., Meier-Haack J., Mezule L. Photocatalytic Zinc Oxide Nanoparticles in Antibacterial Ultrafiltration Membranes for Biofouling Control. Molecules. 2024;29:1274. doi: 10.3390/molecules29061274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang J.Y., Tang D.X., Liu D.L., Liu K., Yang X.J., Li Y.S., Liu Y. Excellent Dark/Light Dual-Mode Photoresponsive Activities Based on g-C3N4/CMCh/PVA Nanocomposite Hydrogel Using Electron Beam Radiation Method. Molecules. 2023;28:7544. doi: 10.3390/molecules28227544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang J.Y., Liu D.L., Li Y.S., Yang X.J., Liu Y. Radiation construction and excellent performances of Ag NPs/TiO2/PEG/PVP multifunctional aerogel: Adsorption-photocatalytic degradation, photosensitive antibacterial and cytotoxicity. J. Mater. 2024;10:585–593. doi: 10.1016/j.jmat.2023.08.008. [DOI] [Google Scholar]
- 11.Junior E.S., La Porta F.A., Liu M.S., Andrés J.J., Varelaa A., Longoa E. A relationship between structural and electronic order–disorder effects and optical properties in crystalline TiO2 nanomaterials. Dalton. Trans. 2015;44:3159–3175. doi: 10.1039/C4DT03254C. [DOI] [PubMed] [Google Scholar]
- 12.Crişan M., Ianculescu A.-C., Crișan D., Drăgan N., Todan L., Niţoi I., Oancea P. Nanotechnology in the Beverage Industry. Elsevier; Amsterdam, The Netherlands: 2020. Fe-doped TiO2 nanomaterials for water depollution; pp. 265–313. [Google Scholar]
- 13.Dong H., Zeng G., Tang L., Fan C., Zhang C., He X., He Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015;79:128–146. doi: 10.1016/j.watres.2015.04.038. [DOI] [PubMed] [Google Scholar]
- 14.Nabi I., Bacha A.-U.-R., Ahmad F., Zhang L. Application of titanium dioxide for the photocatalytic degradation of macro- and micro-plastics: A review. J. Environ. Chem. Eng. 2021;9:105964. doi: 10.1016/j.jece.2021.105964. [DOI] [Google Scholar]
- 15.Han L., Yue X., Wen L., Zhang M., Wang S. A Novel Vermiculite/TiO2 Composite: Synergistic Mechanism of Enhanced Photocatalysis towards Organic Pollutant Removal. Molecules. 2023;28:6398. doi: 10.3390/molecules28176398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin H., Xiao Y., Geng A., Bi H., Xu X., Xu X., Zhu J. Research Progress on Graphitic Carbon Nitride/Metal Oxide Composites: Synthesis and Photocatalytic Applications. Int. J. Mol. Sci. 2022;23:12979. doi: 10.3390/ijms232112979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amorin L.H., Suzuki V.Y., de Paula N.H., Duarte J.L., da Silva M.A.T., Taft C.A., La Porta F.D.A. Electronic, structural, optical, and photocatalytic properties of graphitic carbon nitride. New J. Chem. 2019;43:13647–13653. doi: 10.1039/C9NJ02702E. [DOI] [Google Scholar]
- 18.Suzuki V.Y., Amorin L.H., Fabris G.S., Dey S., Sambrano J.R., Cohen H., Oron D., La Porta F.A. Enhanced photocatalytic and photoluminescence properties resulting from type-I band alignment in the Zn2GeO4/g-C3N4 nanocomposites. Catalysts. 2022;12:692. doi: 10.3390/catal12070692. [DOI] [Google Scholar]
- 19.Wang Q., Li Y., Huang F., Song S., Ai G., Xin X., Zhao B., Zheng Y., Zhang Z. Recent Advances in g-C3N4-Based Materials and Their Application in Energy and Environmental Sustainability. Molecules. 2023;28:432. doi: 10.3390/molecules28010432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venkatesh N., Murugadoss G., Mohamed A.A.A., Kumar M.R., Peera S.G., Sakthivel P. A Novel Nanocomposite Based on Triazine Based Covalent Organic Polymer Blended with Porous g-C3N4 for Photo Catalytic Dye Degradation of Rose Bengal and Fast Green. Molecules. 2022;27:7168. doi: 10.3390/molecules27217168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang W., Fang J., Shao S., Lai M., Lu C. Compact and uniform TiO2@g-C3N4 core-shell quantum heterojunction for photocatalytic degradation of tetracycline antibiotics. Appl. Catal. B Environ. Energy. 2017;217:57–64. doi: 10.1016/j.apcatb.2017.05.037. [DOI] [Google Scholar]
- 22.Yan J., Li P., Bian H., Wu H., Liu S. Synthesis of a nano-sized hybrid C3N4/TiO2 sample for enhanced and steady solar energy absorption and utilization. Sustain. Energy Fuels. 2017;1:95–102. doi: 10.1039/C6SE00048G. [DOI] [Google Scholar]
- 23.Silva I.F., Pulignani C., Odutola J., Galushchinskiy A., Texeira I.F., Isaacs M., Mesa C.A., Scoppola E., These A., Badamdorj B., et al. One-pot Synthesis of a Visible-light Responsive Carbon Nitride/TiO2 Heterointerface Material for Photoelectrocatalytic Applications. ChemRxiv. 2023 [Google Scholar]
- 24.Moradi S., Isari A.A., Hayati F., Rezaei Kalantary R., Kakavandi B. Co-implanting of TiO2 and liquid-phase-delaminated g-C3N4 on multi-functional graphene nanobridges for enhancing photocatalytic degradation of acetaminophen. Chem. Eng. J. 2021;414:128618. doi: 10.1016/j.cej.2021.128618. [DOI] [Google Scholar]
- 25.Ning P., Chen H., Pan J., Liang J., Qin L., Chen D., Huang Y. Surface defect-rich g-C3N4/TiO2 Z-scheme heterojunction for efficient photocatalytic antibiotic removal: Rational regulation of free radicals and photocatalytic mechanism. Catal. Sci. Technol. 2020;10:8295–8304. doi: 10.1039/D0CY01564D. [DOI] [Google Scholar]
- 26.Su J., Geng P., Li X., Zhao Q., Quan X., Chen G. Novel phosphorus doped carbon nitride modified TiO2 nanotube arrays with improved photoelectrochemical performance. Nanoscale. 2015;7:16282–16289. doi: 10.1039/C5NR04562B. [DOI] [PubMed] [Google Scholar]
- 27.Wadhai S., Jadhav Y., Thakur P. Synthesis of metal-free phosphorus doped graphitic carbon nitride-P25 (TiO2) composite: Characterization, cyclic voltammetry and photocatalytic hydrogen evolution. Sol. Energy Mater. Sol. Cells. 2021;223:110958. doi: 10.1016/j.solmat.2021.110958. [DOI] [Google Scholar]
- 28.Kumar P., Kar P., Manuel A.P., Zeng S., Thakur U.K., Alam K.M., Zhang Y., Kisslinger R., Cui K., Bernard G.M., et al. Noble metal free, visible light driven photocatalysis using TiO2 nanotube arrays sensitized by P-doped C3N4 quantum dots. Adv. Opt. Mater. 2020;8:1901275. doi: 10.1002/adom.201901275. [DOI] [Google Scholar]
- 29.Cako E., Dudziak S., Głuchowski P., Trykowski G., Pisarek M., Borzyszkowska A.F., Zielińska-Jurek A., Sikora K. Heterojunction of (P, S) co-doped g-C3N4 and 2D TiO2 for improved carbamazepine and acetaminophen photocatalytic degradation. Sep. Purif. Technol. 2023;311:123320. doi: 10.1016/j.seppur.2023.123320. [DOI] [Google Scholar]
- 30.Li Y., Qin J., Han Y., Du J., Dong Z., Sun S., Liu Y. Controlled preparation and highly photocatalytic activity of portable MCC-g-GMA@TiO2 photocatalyst by pre-radiation grafting-embedding method. Appl. Catal. B Environ. Energy. 2017;218:101–110. doi: 10.1016/j.apcatb.2017.03.083. [DOI] [Google Scholar]
- 31.Wang L., Xie G., Mi X., Zhang B., Du Y., Zhu Q., Yu Z. Surface-Modified TiO2@SiO2 Nanocomposites for Enhanced Dispersibility and Optical Performance to Apply in the Printing Process as a Pigment. ACS Omega. 2023;8:20116–20124. doi: 10.1021/acsomega.3c02679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar R., Thangappan R. 2D g-C3N4 decorated with 1D Bi2S3 nanocomposite as a high performance electrode material for asymmetric supercapacitors. Mater. Chem. Phys. 2023;304:127844. doi: 10.1016/j.matchemphys.2023.127844. [DOI] [Google Scholar]
- 33.Dong L., Chu H., Xu S., Li Y., Zhao S., Li D. Band structure tuning of g-C3N4 via sulfur doping for broadband near-infrared ultrafast photonic applications. Nanophotonics. 2021;11:139–151. doi: 10.1515/nanoph-2021-0549. [DOI] [Google Scholar]
- 34.Wang J., Ma J., Zhang Q., Chen Y., Hong L., Wang B., Chen J., Jing H. New heterojunctions of CN/TiO2 with different band structure as highly efficient catalysts for artificial photosynthesis. Appl. Catal. B Environ. Energy. 2021;285:119781. doi: 10.1016/j.apcatb.2020.119781. [DOI] [Google Scholar]
- 35.Gündoğmuş P., Park J., Öztürk A. Preparation and photocatalytic activity of g-C3N4/TiO2 heterojunctions under solar light illumination. Ceram. Int. 2020;46:21431–21438. doi: 10.1016/j.ceramint.2020.05.241. [DOI] [Google Scholar]
- 36.Liao Y., Qian J., Xie G., Han Q., Dang W., Wang Y., Lv L., Zhao S., Luo L., Zhang W., et al. 2D-layered Ti3C2 MXenes for promoted synthesis of NH3 on P25 photocatalysts. Appl. Catal. B Environ. Energy. 2020;273:119054. doi: 10.1016/j.apcatb.2020.119054. [DOI] [Google Scholar]
- 37.Cao S., Huang Q., Zhu B., Yu J. Trace-level phosphorus and sodium co-doping of g-C3N4 for enhanced photocatalytic H2 production. J. Power Sources. 2017;351:151–159. doi: 10.1016/j.jpowsour.2017.03.089. [DOI] [Google Scholar]
- 38.Chen X., Jin Y., Huang P., Zheng Z., Li L.-P., Lin C.-Y., Chen X., Ding R., Liu J., Chen R. Solar driven photocatalytic disinfection by Z-scheme heterojunction of In2O3/g-C3N4: Performance, mechanism and application. Appl. Catal. B Environ. Energy. 2024;340:123235. doi: 10.1016/j.apcatb.2023.123235. [DOI] [Google Scholar]
- 39.Fan X., Liu X., Wang Y. Low-cost and resource-efficient monolithic photocatalyst with enhanced solar light utilization for the photocatalytic treatment of organic wastewater. Pt 1Chemosphere. 2023;312:137052. doi: 10.1016/j.chemosphere.2022.137052. [DOI] [PubMed] [Google Scholar]
- 40.Li W., Ma Q., Wang X., Chu X.-S., Wang F., Wang X.-C., Wang C.-Y. Enhanced photoresponse and fast charge transfer: Three-dimensional macroporous g-C3N4/GO-TiO2 nanostructure for hydrogen evolution. J. Mater. Chem. A. 2020;8:19533–19543. doi: 10.1039/D0TA07178A. [DOI] [Google Scholar]
- 41.Tang Z., Li Y., Zhang K., Wang X., Wang S., Sun Y., Zhang H., Li S., Wang J., Gao X., et al. Interfacial Hydrogen Spillover on Pd-TiO2 with Oxygen Vacancies Promotes Formate Electrooxidation. ACS Energy Lett. 2023;8:3945. doi: 10.1021/acsenergylett.3c01426. [DOI] [Google Scholar]
- 42.Jia Y., Tong X., Zhang J., Zhang R., Yang Y., Zhang L., Ji X. A facile synthesis of coral tubular g-C3N4 for photocatalytic degradation RhB and CO2 reduction. J. Alloys Compd. 2023;965:171432. doi: 10.1016/j.jallcom.2023.171432. [DOI] [Google Scholar]
- 43.Li J., Wang Y., Song H., Guo Y., Hu S., Zheng H., Zhang S., Li X., Gao Q., Li C., et al. Photocatalytic hydrogen under visible light by nitrogen-doped rutile titania graphitic carbon nitride composites: An experimental and theoretical study. Adv. Compos. Hybrid Mater. 2023;6:83. doi: 10.1007/s42114-023-00659-8. [DOI] [Google Scholar]
- 44.Liu J., Yuan X., Sun J., Ke J., Liu B., Wang L. Creating triazine units to bridge carbon nitride with titania for enhanced hydrogen evolution performance. Pt 3J. Colloid Interface Sci. 2022;608:2768–2778. doi: 10.1016/j.jcis.2021.11.004. [DOI] [PubMed] [Google Scholar]
- 45.Li J., Su Q., Yuan H., Zhang L., Hou L., Wang Y., Liu B., Wang B., Li Y. Photocatalytic degradation of sulfamonomethoxine by mesoporous phosphorus-doped titania under simulated solar light irradiation. Chemosphere. 2021;285:131553. doi: 10.1016/j.chemosphere.2021.131553. [DOI] [PubMed] [Google Scholar]
- 46.Tian Y., Tang W., Xiong H., Chen T., Li B., Jing X., Zhang J., Xu S. Luminescence and structure regulation of graphitic carbon nitride by electron rich P ions doping. J. Lumin. 2020;228:117616. doi: 10.1016/j.jlumin.2020.117616. [DOI] [Google Scholar]
- 47.Zhang R., Yu Y., Wang H., Du J. Mesoporous TiO2/g-C3N4 composites with O-Ti-N bridge for improved visible-light photodegradation of enrofloxacin. Sci. Total Environ. 2020;724:138280. doi: 10.1016/j.scitotenv.2020.138280. [DOI] [PubMed] [Google Scholar]
- 48.Liu S.-H., Lin W.-X. Heterostructured graphitic carbon nitride/titanium dioxide for enhanced photodegradation of low-concentration formaldehyde under visible light. J. Photochem. Photobiol. A Chem. 2019;378:66–73. doi: 10.1016/j.jphotochem.2019.04.025. [DOI] [Google Scholar]
- 49.Yang H., Zhou Y., Wang Y., Hu S., Wang B., Liao Q., Li H., Bao J., Ge G., Jia S. Three-dimensional flower-like phosphorus-doped g-C3N4 with a high surface area for visible-light photocatalytic hydrogen evolution. J. Mater. Chem. A. 2018;6:16485–16494. doi: 10.1039/C8TA05723K. [DOI] [Google Scholar]
- 50.Wang H., Ma H., Li Y., Cheng L., Yang J., Liu J. Facile Synthesis of TiO2 with a Narrow Bandgap and Low Valence Band for Efficient Visible-Light Photocatalytic Degradation of Various Phenols. Ind. Eng. Chem. Res. 2023;62:14320–14334. doi: 10.1021/acs.iecr.3c01727. [DOI] [Google Scholar]
- 51.Gong Y., Wu Y., Xu Y., Li L., Li C., Liu X., Niu L. All-solid-state Z-scheme CdTe/TiO2 heterostructure photocatalysts with enhanced visible-light photocatalytic degradation of antibiotic waste water. Chem. Eng. J. 2018;350:257–267. doi: 10.1016/j.cej.2018.05.186. [DOI] [Google Scholar]
- 52.Lv T., Xiao B., Zhou S., Zhao J., Wu T., Zhang J., Zhang Y., Liu Q. Rich oxygen vacancies, mesoporous TiO2 derived from MIL-125 for highly efficient photocatalytic hydrogen evolution. Chem. Commun. 2021;57:9704–9707. doi: 10.1039/D1CC01669E. [DOI] [PubMed] [Google Scholar]
- 53.Lei Z., Ma X., Hu X., Fan J., Liu E. Enhancement of Photocatalytic H2-evolution Kinetics through the Dual Cocatalyst Activity of Ni2P-NiS-decorated g-C3N4 Heterojunctions. Acta Pharm. Sin. B. 2021;38:2110049. [Google Scholar]
- 54.Zhao P., Huang L., Wang H., Wang C., Chen J., Yang P., Ni M., Chen C., Li C., Xie Y., et al. An ultrasensitive high-performance baicalin sensor based on C3N4-SWCNTs/reduced graphene oxide/cyclodextrin metal-organic framework nanocomposite. Sens. Actuators B. Chem. 2022;350:130853. doi: 10.1016/j.snb.2021.130853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eskandari P., Amarloo E., Zangeneh H., Rezakazemi M., Aminabhavi T.M. Photocatalytic degradation of metronidazole and oxytetracycline by novel l-Arginine (C, N codoped)-TiO2/g-C3N4: RSM optimization, photodegradation mechanism, biodegradability evaluation. Chemosphere. 2023;337:139282. doi: 10.1016/j.chemosphere.2023.139282. [DOI] [PubMed] [Google Scholar]
- 56.Wei Y., Wu Q., Meng H., Zhang Y., Cao C. Recent advances in photocatalytic self-cleaning performances of TiO2-based building materials. RSC Adv. 2023;13:20584–20597. doi: 10.1039/D2RA07839B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang J., Li D., Li R., Chen P., Zhang Q., Liu H., Lv W., Liu G., Feng Y. One-step synthesis of phosphorus/oxygen co-doped g-C3N4/anatase TiO2 Z-scheme photocatalyst for significantly enhanced visible-light photocatalysis degradation of enrofloxacin. J. Hazard. Mater. 2020;386:121634. doi: 10.1016/j.jhazmat.2019.121634. [DOI] [PubMed] [Google Scholar]
- 58.Pak S., Ri K., Xu C., Ji Q., Sun D., Qi C., Yang S., He H., Pak M. Fabrication of g-C3N4/Y-TiO2 Z-scheme heterojunction photocatalysts for enhanced photocatalytic activity. New J. Chem. 2021;45:19903–19916. doi: 10.1039/D1NJ03691B. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are contained within the article and Supplementary Materials.