Abstract
Patients with heart failure with reduced ejection fraction who have secondary mitral regurgitation (SMR) have poorer outcomes and quality of life than those without SMR. Guideline-directed medical therapy is the cornerstone of SMR treatment. Careful evaluation of landmark trials using mitral transcatheter edge-to-edge repair in SMR has led to an improved understanding of who will benefit from percutaneous interventions with emphasis on a multidisciplinary approach. The success with mitral transcatheter edge-to-edge repair in SMR has also spurred the evaluation of its role in populations that were not initially studied, such as end-stage heart failure and cardiogenic shock. A spectrum of transcatheter devices in development and clinical trials promise to further provide a growing array of management options for heart failure with reduced ejection fraction patients with symptomatic SMR.
Keywords: guideline-directed medical therapy, mitral transcatheter edge-to-edge repair, secondary mitral regurgitation, MitraClip, transcatheter mitral valve replacement
Mitral regurgitation (MR) is the most common valvular heart disorder, with moderate or greater MR complicating over 50% of all acute heart failure (HF) admissions.1 MR is classified as primary (degenerative) or secondary (functional) and can have either an acute or chronic presentation. Secondary mitral regurgitation (SMR) is an independent predictor of poor outcomes including mortality and hospitalizations in patients with heart failure with reduced ejection fraction (HFrEF).2
Although a fundamentally appreciated mechanism of MR for decades, the SMR phenotype only received separate guideline management considerations within the last decade. Earlier ACC/AHA (American College of Cardiology/American Heart Association) valvular heart disease guidelines had MR dichotomized as either acute or chronic with “no generally accepted medical therapy for asymptomatic chronic MR” and a focus on surgical management.3 In subsequent guidelines, the “degenerative” or “functional” nomenclature was overhauled and replaced with “primary” or “secondary” MR, reflecting the unique pathophysiology, natural history, and response to therapies.4,5
Historically, guideline-directed medical therapy (GDMT) and cardiac resynchronization therapy (CRT) were considered the mainstay of the treatment approach in HFrEF, whereas surgical repair/replacement of SMR was recommended in carefully selected patients. The simultaneous publication of the COAPT (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation) and MITRA-FR (Multicenter Randomized Study of Percutaneous Mitral Valve Repair MitraClip Device in Patients With Severe Secondary Mitral Regurgitation) trials resulted in a major paradigm shift for SMR management.6,7 With the emergence of transcatheter therapies, typified by mitral transcatheter edge-toedge repair (mTEER), therapeutic options in SMR have evolved significantly.6 There is a renewed interest in understanding the interplay between left ventricular (LV) dysfunction and the degree of MR in order to identify a phenotype more responsive to specific interventions. However, without the interventions that are aimed at correcting the severe SMR in appropriately selected candidates as well as the underlying HF, the risk of morbidity and mortality remains unacceptably high.8 This review describes the current landscape of mitral valve (MV) interventions, focusing on SMR, and previews emerging technologies and paradigms.
MV ANATOMY/FUNCTION
ANATOMY.
The MV apparatus is a dynamic structure with 4 key components: the mitral annulus (MA), the leaflets, the chordae tendineae, and the papillary muscles; abnormalities involving any of these components can result in MR9 (Figure 1). The mitral leaflets consist of anterior (larger in size and sail-shaped) and posterior (smaller in size and crescent-shaped) leaflets with variable commissural scallops. The ventricular surface of the leaflets is attached to chordae tendineae (primary and secondary) that are classified based on their insertion points on mitral leaflets and 2 main papillary muscles (lateral and medial).
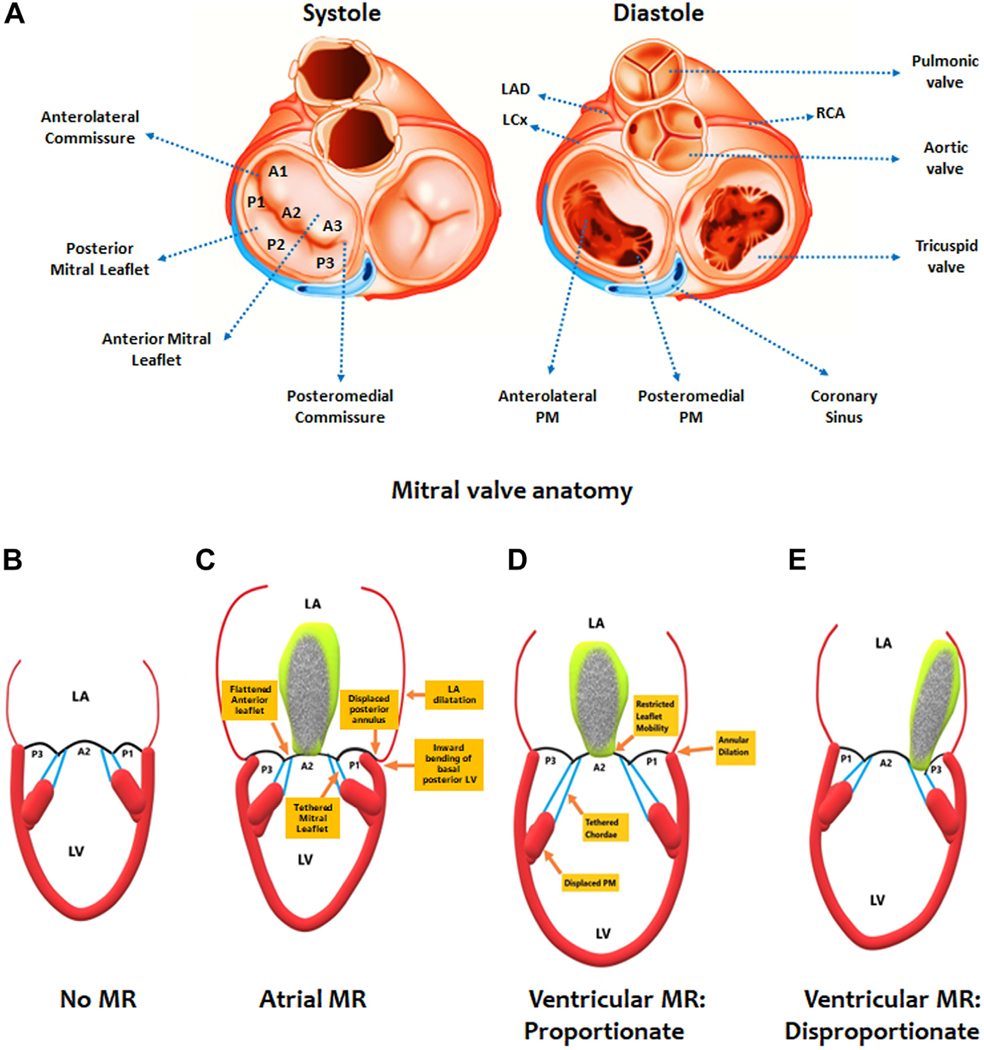
FIGURE 1. Mitral Valve Anatomy and Types of Regurgitation.
(A) The leaflets of the mitral valve (divided into anterior and posterior segments 1–3) during systole on the left while the right side allows for visualization inferiorly into the left ventricle during diastole, permitting the identification of the papillary muscles. (B) A simplified example of normal mitral valve coaptation to contrast to other forms of secondary mitral regurgitation (MR). (C) Atrial MR is characterized by severe left atrial dilatation leading to a displaced posterior annulus and inward bending of the basal posterior left ventricle. Tethering of the mitral leaflets causes the MR. Ventricular MR can be proportionate (D) or disproportionate (E), a distinction dependent on the degree of ventricular dilatation. LA = left atrium; LAD = left anterior descending coronary artery; LCx = left circumflex coronary artery; LV = left ventricle; PM = papillary muscle; RCA = right coronary artery.
MECHANISMS FOR SMR.
The etiologies of SMR can be broadly considered as atrial (ie, related to left atrial [LA] and/or mitral annular dilatation) and ventricular (ie, related to LV dysfunction, focal wall motion abnormalities, and enlargement).4 Abnormalities involving the left atrium can result in malcoaptation of structurally normal mitral leaflets and loss of LA function, which can eventually lead to the development of SMR. Atrial relaxation after the end-diastolic atrial contraction may also exert a “Venturi effect” on mitral leaflets and aid in their tighter approximation, an effect that is lost in atrial fibrillation. In addition, massive LA enlargement may result in flattening of the anterior mitral leaflet along the mitral annular plane, with bending of the posterior mitral leaflet toward the LV cavity.
Abnormalities involving the left ventricle, such as dysfunction and enlargement or focal wall motion abnormalities, can also result in the development of SMR. The MA dilatation resulting from global LV enlargement can cause loss of MA folding and saddle-shape accentuation in early systole—a mechanism that can contribute to the development of early systolic SMR. LV dysfunction and enlargement can also result in abnormal tethering geometry because of abnormal interpapillary muscle approximation and paradoxical movement of the posteromedial PM in midsystole—a mechanism that can contribute to the development of mid-to-late systolic SMR.10
Regardless of the underlying mechanism, chronic volume overload resulting from progressive SMR induces unfavorable neurohormonal and structural changes and causes worsening of HF symptoms. Progressive SMR leads to higher LV end-diastolic pressure, LA pressure, and pulmonary arterial pressure and results in worsening right ventricular (RV) function and tricuspid regurgitation.
ASSESSMENT OF SMR
Echocardiography remains the screening test of choice for the assessment of MR.11 Transthoracic echocardiography is often the first-line test of choice for its ease and reproducibility; however, transesophageal echocardiography may be necessary depending on the quality of the acoustic windows, the ability to perform quantitative measurements, and SMR jet eccentricity. The echocardiographic definition of SMR severity has evolved over the last decade. Severe SMR is currently defined by ACC/AHA and ESC (European Society of Cardiology) guidelines as an effective regurgitant orifice area (EROA) ≥0.4 cm2, regurgitant fraction ≥50%, and regurgitant volume ≥60 mL/beat.12 A proximal isovelocity surface area (PISA) radius ≥1 cm is also considered a criterion.13
There are several pitfalls to these methods of SMR characterization. The dynamic nature of SMR means that changes to loading conditions, such as with sedation necessary for transesophageal echocardiography, can cause a significant reduction in MR severity.13 The calculation of the EROA uses the PISA method, which requires several assumptions that can be erroneous in SMR. In SMR, the MV often has an elongated or elliptical orifice as opposed to the ideal hemispheric shape in PISA calculation, leading to underestimation of regurgitant severity.14 Similarly, the PISA shape itself is assumed to be planar when it may be more conical, requiring adjustment in the EROA calculation. Eccentric and multiple jets, which are frequently encountered in SMR, can also lead to the underestimation of severity. The timing of the flow and velocity is also critical, and measurement of a single-frame, midsystolic EROA may overestimate SMR that is biphasic with early and late peaks.15 Regardless of these limitations, quantitative assessments with EROA as well as regurgitant volume are closely associated with clinical endpoints.16
Additive imaging modalities should be considered for the most accurate assessment of SMR severity. Three-dimensional transesophageal echocardiography may allow for more accurate PISA measurement despite eccentric jet or elliptical orifice shape.17 If mTEER is being considered, 3-dimensional color Doppler allows for spatial recognition of the ideal repair location. Cardiac magnetic resonance can provide highly accurate regurgitant volume measurements despite the presence of eccentric or multiple MR jets.18
INITIAL APPROACH TO SMR
The current guidelines give mTEER a Class 2a recommendation for the treatment of patients with moderately severe or severe SMR who meet COAPT criteria.11,19 Additional recommendations include an emphasis for patients with SMR to be evaluated by a multidisciplinary team (MDT) as well as optimizing GDMT.
MEDICAL THERAPY FOR SMR.
Optimizing GDMT is the first-line therapy for all patients with HFrEF, including those with SMR.11 The long-term administration of GDMT reverses LV remodeling, which may in turn lead to improved MV leaflet coaptation.20 Nearly 60% of patients with HFrEF and SMR may have a significant improvement in the degree of MR after treatment with GDMT.21 Of note, none of these studies had a substantial number of patients on sodium-glucose cotransporter 2 inhibitors—a drug class that also improves adverse LV remodeling.22 Moreover, continuing GDMT with reassessment of up-titration plays a key role in achieving optimal outcomes after mTEER.6,23
Beta-blockers, angiotensin-converting enzyme inhibitors, and sacubitril/valsartan have established benefits with improving LV remodeling and SMR in patients with HFrEF. In small, nonrandomized studies, carvedilol in patients with HFrEF and SMR led to a significant improvement in left ventricular ejection fraction (LVEF), a reduction in EROA and regurgitant volume, and an improvement in the grade of SMR.24,25 Additionally, metoprolol resulted in a significant improvement in MR in 1 double-blind, placebo-controlled trial of patients with LV systolic dysfunction.26 Captopril has also demonstrated a dose-dependent improvement in SMR.27 Sacubitril/valsartan, an angiotensin receptor–neprilysin inhibitor (ARNI), has demonstrated superiority over angiotensin-converting enzyme inhibitors or angiotensin receptor blocker treatment with respect to clinical outcomes and LV reverse remodeling in patients with HFrEF.28,29 In the PRIME (Pharmacological Reduction of Functional, Ischemic Mitral Regurgitation) trial, the ARNI group had a significantly larger reduction in EROA and lower regurgitant volume compared with the valsartan group in patients with SMR and LV systolic dysfunction.30 In the open-label PROVE-HF (Effects of Sacubitril/Valsartan Therapy on Biomarkers, Myocardial Remodeling and Outcomes) trial, just under 15% had 3 to 4+ MR at baseline, and ARNI led to an improvement to ≤2+ MR in 45% of that subgroup, a majority of whom were previously treated with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers.31 Outside of their long-known acute hemodynamic benefits in afterload reduction and MR improvement, hydralazine and nitrates have unclear long-term benefits, specifically in the SMR population, although they certainly are appropriate to initiate in the context of their Class 1 recommendation in African Americans with symptomatic HFrEF.19,32
The COAPT trial is 1 of the first trials to require HF medication optimization systematically by HF experts. A recent analysis of the trial’s GDMT use by Cox et al33 provides much needed insight. Given the timing of COAPT enrollment, sodium-glucose cotransporter 2 inhibitors were not an approved “pillar” of GDMT for HFrEF, and the use of ARNI was also low (3.2%). Only 2.2% of all patients with HFrEF tolerated target doses of all 3 GDMT medications. These rates of target-dose GDMT use are lower than desired for “optimal titration” and are not far from what has been found in other real-world HF registries, such as CHAMP-HF (Change the Management of Patients with Heart Failure).34 COAPT patients, being a higher-risk group of patients (by virtue of their SMR), may be expected to have more GDMT intolerance. Medication changes during the follow-up period are also low, likely because of both the intense prerandomization screening for optimal up-titration and the suggestion to investigators to limit routine medication changes in the first 2 years postrandomization. As proposed by the editorial35 to the paper by Cox et al,33 future trials may benefit from objective criteria for drug intolerance, prioritization of ARNI over angiotensin-converting enzyme inhibitors and angiotensin receptor blockers, and protocolized “tolerance” of asymptomatic blood pressure or minor changes in renal function.
OTHER THERAPIES.
COAPT trial inclusion also mandated the use of implantable cardiac defibrillators and CRT in patients who met Class 1 guideline recommendations. CRT results in a quantifiable improvement in the LV end-systolic volume index and MR area.36,37 Inversely, the withdrawal of CRT can lead to worsening of SMR.38 Treatment of SMR also includes addressing concurrent conditions such as atherosclerotic coronary artery disease in the presence of LV dysfunction via percutaneous or surgical revascularization.13
MV INTERVENTIONS IN HF PATIENTS
TRANSCATHETER EDGE-TO-EDGE REPAIR (MitraClip).
Based on the results of the COAPT trial, mTEER with MitraClip (Abbott Vascular) was the first percutaneous therapy to be approved for the treatment of SMR in the United States.6 In COAPT, there was a significant reduction in the primary endpoint of HF hospitalizations (35.8% vs 67.9%; HR: 0.53 [95% CI: 0.40–0.70]; P < 0.001) at 2 years of follow-up in the intervention group compared with GDMT alone. Furthermore, all 10 secondary endpoints were improved with mTEER, including all-cause mortality at 2 years (29.1% vs 46.1%; HR: 0.62 [95% CI: 0.46–0.82]; P < 0.001), NYHA functional class I or II at 1 year (72.2% vs 49.6%; P < 0.001), a change in the Kansas City Cardiomyopathy Questionnaire (KCCQ) score (12.5 vs 3.6; P < 0.001), and so on. The 5-year follow-up of the COAPT trial reported that in the intention-to-treat analysis, the annualized HF hospitalization rate (33.1%/y vs 57.2%/y; HR: 0.53 [95% CI: 0.41–0.68]) and all-cause mortality (57.3% vs 67.2%; HR: 0.72 [95% CI: 0.58–0.89]) were significantly lower in the mTEER arm compared with GDMT alone.39 Patients treated with mTEER were more likely to show improvements in health status and exercise capacity than were those treated with GDMT alone.40
On the contrary, the MITRA-FR trial reported no difference in the primary outcome of all-cause mortality or HF hospitalization at 1 year between the MitraClip and GDMT arms (54.6% vs 51.3%; HR: 1.16 [95% CI: 0.73–1.84]; P = 0.53)7 (Figure 2). In addition to the combined endpoint, there was no difference in the individual endpoints of all-cause mortality (24.3% vs 22.4%; HR: 1.11 [95% CI: 0.69–1.77]) or HF hospitalizations (48.7% vs 47.4%; HR: 1.13 [95% CI: 0.811.56]) at 1 year. The 2-year follow-up of the MITRA-FR trial showed comparable results with no difference between the intervention and control arms.41
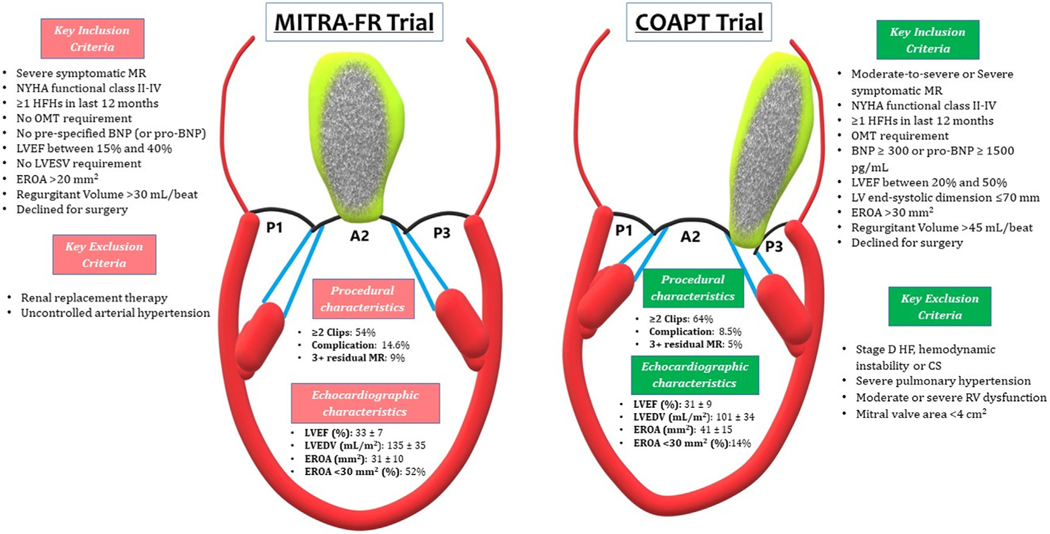
FIGURE 2. Comparison of MITRA-FR and COAPT Trials Including Key Inclusion and Exclusion Criteria and Important Procedural and Echocardiographic Differences.
BNP = brain natriuretic peptide; COAPT = Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation; CS = cardiogenic shock; EROA = effective regurgitant orifice area; HF = heart failure; HFH = heart failure hospitalization; OMT = optimal medical therapy; LVEF = left ventricular ejection fraction; LVEDV = left ventricular end-diastolic volume; LVESV = left ventricular end-systolic volume; MITRA-FR = Multicenter Randomized Study of Percutaneous Mitral Valve Repair Mitra Clip Device in Patients with Severe Secondary Mitral Regurgitation; RV = right ventricle; LV = left ventricular; other abbreviation as in Figure 1.
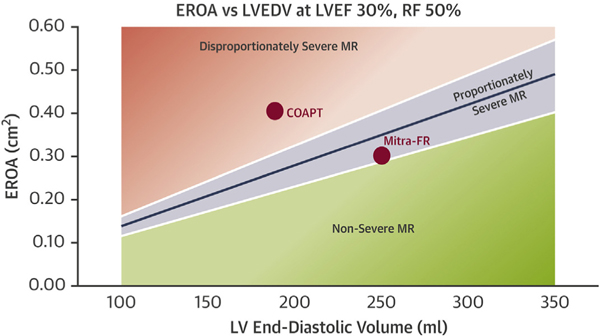
Despite having similar LVEF, patients enrolled in the MITRA-FR trial had lower EROA (0.31 cm2 vs 0.41 cm2) and higher left ventricular end-diastolic volume (LVEDV) (135 mL/m2 vs 101 mL/m2) compared with those in the COAPT trial. Of note, outcomes in the GDMT arms of both trials were similar at 2 years with a composite endpoint of all-cause mortality or HF hospitalization of 67.1% in MITRA-FR and 67.9% in COAPT. Therefore, the differences in outcomes between the trials are secondary to the outcomes in their mTEER arms. These differences could be explained by the proportionality hypothesis, using EROA as a quantitative estimation of MR severity and LVEDV as a quantitative measurement of LV dilatation.42 For a given LVEF and regurgitant fraction, EROA normalized to LVEDV allows for the creation of a proportionate SMR “trend line” (Figure 3). Visualized relative to this trend line, COAPT patients fall in the “disproportionate severe MR” category, whereas the MITRA-FR patients land on the other side (MR proportionate to the degree of LV dilation) of the trend line. The MR in patients with proportionate MR would respond to drugs and devices that reduce LVEDV, whereas those with disproportionate MR would preferentially benefit from interventions directed at the MV. However, significant interobserver variability exists in the measurement of EROA and LVEDV by echocardiography that may limit the general applicability of the proportionality hypothesis.43
FIGURE 3. Proportionate and Disproportionate Mitral Regurgitation.
Relationship between EROA and LVEDV at LVEF 30%, RF 50% and corresponding visualization of average population for the COAPT and MITRA-FR trials. Figure used with permission from Grayburn et al.42 RF = regurgitant fraction; other abbreviations as in Figures 1 and 2.
In a subanalysis of the COAPT trial, there was no benefit of mTEER in terms of HF hospitalizations and/or all-cause mortality at 2 years in patients with proportionate MR (smaller EROA ≤0.30 cm2 and larger LV end-diastolic volume index >96 mL/m2, similar to patients in MITRA-FR). However, mTEER plus GDMT resulted in significant improvements in quality of life (KCCQ score) and 6-minute walk distance at 12 months compared to GDMT alone. The results suggest that the benefits of mTEER may be greatest in those with disproportionate SMR but that some benefits on hospitalization may be present in “proportionate” SMR when the analysis was extended to 24 months.44
The risk for major complications, including death and major stroke, is low after MitraClip placement, with rates much lower compared to open surgical repair45 (Videos 1 to 4). Complications may include access site bleeding, transseptal complications, pericardial effusion, clip detachment from a single leaflet, or very rarely device embolization. It is also important to note that the current generation of the MitraClip system (G4) allows for independent grasping and multiple sizing options. The procedural results with G3 and G4 have been incrementally better than the older-generation devices (G1 and G2) that were used in the COAPT trial, with 97% patients having ≤2+ residual MR at 1 year46 (Table 1).
TABLE 1.
Effectiveness of MitraClip in Reducing MR in Clinical Trials
| Years Enrolled | Type of MR | N | MitraClip Generation System | ≤2+ Residual MR at 1 Year (%) | ≤1+ Residual MR at 1 Year (%) | |
|---|---|---|---|---|---|---|
|
| ||||||
| EVEREST II RCT (PMID: 21463154) | 2005–2008 | Primary (73%) and secondary MR | 184 | NT (G1) | 82 | 43 |
| Everest II Realism registry (PMID: 30586701) | 2005–2013 | Secondary MR | 616 | NT (G1) | 84.5 | 42.9 |
| MITRA-FR | 2013–2017 | Secondary MR | 152 | NT (G2) | 83.0 | 49.5 |
| COAPT trial | 2013–2017 | Secondary MR | 302 | NT (G2) | 94.8 | 69.1 |
| EXPAND prospective registry | 2018–2019 | Primary and secondary MR (~50/50) | 509 | G3 | 96.0 | 83.5 |
| EXPAND Secondary MR | 2018–2019 | Secondary MR | 213 | G3 | 99.1 | 89.5 |
COAPT = Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation; EVEREST = Endovascular Valve Edge-to-Edge Repair Study; EXPAND = A Contemporary, Prospective, Multi-Center Study Evaluating Real-World Experience of Performance and Safety for the Next Generation of MitraClip Devices; MR = mitral regurgitation; PMID = PubMed identifier; RCT = randomized controlled trial.
The COAPT and MITRA-FR trials and the ongoing trials of SMR have primarily focused on ventricular SMR. Recent studies suggest that 5% to 10% of all MR patients and 25% of SMR cases may have atrial SMR.47 mTEER has been evaluated in retrospective studies of atrial SMR and is effective in reducing the grade of MR similar to that seen in ventricular SMR. The hemodynamic impact and symptom relief have varied among studies, which may be related to different definitions used to define atrial SMR.48,49
Other devices.
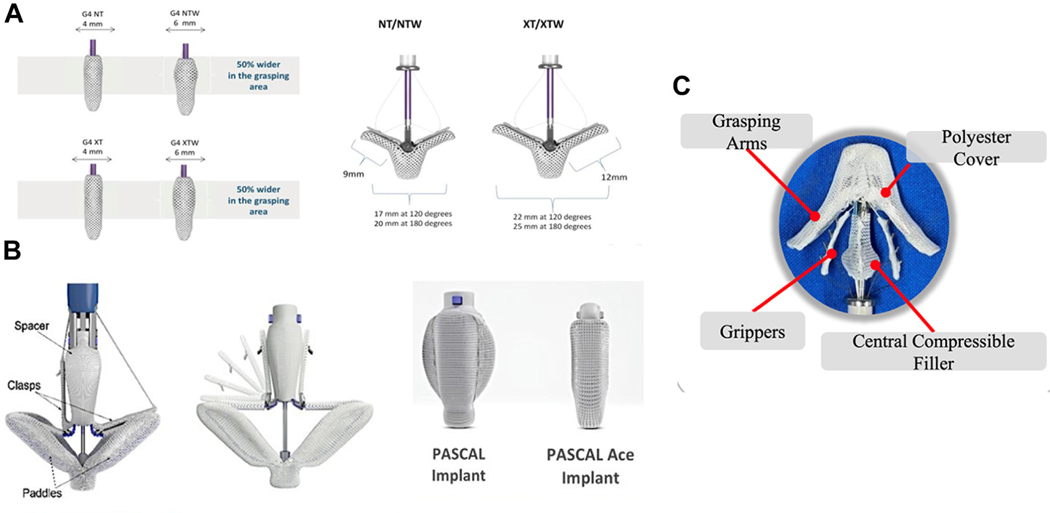
The PASCAL system (Edward Lifesciences) also uses the concept of edge-to-edge repair and received Food and Drug Administration approval for primary MR in 2022. There is limited retrospective experience comparing PASCAL with MitraClip showing similar results in terms of MR reduction and safety.50,51 The CLASP IIF (Edwards PASCAL CLASP IID/IIF Pivotal Clinical Trial; NCT03706833) randomized trial is currently enrolling and is evaluating the safety and effectiveness of PASCAL compared to MitraClip in SMR patients. Another transcatheter repair system, the DragonFly Transcatheter Repair device (Hangzhou Valgen Medtech Co, Ltd), also uses the concept of edge-to-edge degenerative MV repair with a compressible spacer in the center52 (Dragonfly-M Early Feasibility Study; NCT04528576) (Figure 4).
FIGURE 4. mTEER Devices.
Specifications of mitral transcatheter edge-to-edge repair devices for the generations of MitraClip (A), PASCAL (B), and Dragonfly (C).
TRANSCATHETER MITRAL VALVE REPLACEMENT.
Demand exists for alternative device-based therapies such as transcatheter mitral valve replacement (TMVR) considering that up to one-third of individuals with significant (all-cause) MR have anatomy that is not ideal for mTEER.53 The initial experience with TMVR began with transapically implanted prostheses, namely the Tendyne (Abbott Vascular) and Intrepid (Medtronic) valves. Two-year data from the multicenter, international single-arm early feasibility study enrolling 100 participants with 3+ or 4+ MR demonstrated very high rates of successful implantation (97%), no residual MR, and a reduction in HF hospitalization.54 The ongoing SUMMIT (Clinical Trial to Evaluate the Safety and Effectiveness of Using the Tendyne Transcatheter Mitral Valve System for the Treatment of Symptomatic Mitral Regurgitation; NCT03433274) trial is currently enrolling a target of 958 people with symptomatic 3 to 4+ MR and randomizing them to mTEER with the MitraClip device vs Tendyne with a primary endpoint of all-cause mortality at 1 year. The Intrepid valve, a bovine pericardial trileaflet valve, is now deployed transeptally and is being evaluated in the APOLLO (Transcatheter Mitral Valve Replacement with the Medtronic Intrepid[TM] TMVR System in Patients with Severe Symptomatic Mitral Regurgitation) trial.55 The number of transfemoral TMVR devices is rapidly expanding and includes devices like the Sapien M3 (Edwards Lifesciences), EVOQUE (Edwards Lifesciences), Altavalve (4C Medical), Clarity (HighLife), Cephea (Abbott Vascular), Cardiovalve (Cardiovalve), Innovalve (Innovalve), and Saturn (InnovHeart) valves56–61 (Table 2). Alternative mechanisms for replicating the effects of annuloplasty have also been explored with devices that externally remodel the annulus via the coronary sinus.62,63
TABLE 2.
Transcatheter Mitral Valve Devices
| Mechanism | Device | Manufacturer | Implant Method | Sheath Size | Device Details | Study Details | EFS | Pivotal | CE Mark | FDA |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| mTEER | MitraClip | Abbott Structural | TFV, TS | 24-F | Recapitulates the surgical Alfieri stitch, creating a biorifice MV that reduces MR. 4 clip sizes. | COAPT (NCT01626079) | X | X | X | |
| Pascal | Edwards Lifesciences | TFV, TS | 22-F | 1 clip size. Central spacer reduces central MR. | CLASP IIF (NCT03706833) | X | X | X | ||
| Dragonfly | Hangzhou Valgen | TFV, TS | 24-F | 4 clip sizes: 4- to 6-mm width, 9- to 12-mm length | NCT04528576 | X | ||||
| TMVR | Tendyne | Abbott Structural | TA | 34-F | Valve seated in an inner frame, which rests within a conformable outer frame. Device is secured by an apical tether. | SUMMIT (NCT03433274) | X | X | ||
| Intrepid | Medtronic | TA, TFV, TS | 35-F | Dual construction with a conformable outer stent with flexible brim and a circular inner stent housing the valve | APOLLO (NCT03242642) | X | ||||
| Sapien M3 | Edwards Lifesciences | TFV, TS | 20-F | 2-component device: valve implanted within a subannular dock | ENCIRCLE (NCT04153292) | X | ||||
| Evoque Eos | Edwards Lifesciences | TFV, TS | 28-F | Bovine pericardial valve secured by subannular anchors | MISCEND (NCT02718001) | X | ||||
| AltaValve | 4C Medical | TA | 32-F | Valve secured within a supra-annular spherical cage | NCT03997305 | X | ||||
| Clarity | Highlife Medical | TFV, TS | 39-F | 2-component device; valve implanted within a subannular ring | HighFLO (NCT04888247) | X | ||||
| Cephea | Abbott Structural | TFV, TS | 38-F | Valve anchored by axial compression created by ventricular and atrial disks | NCT05061004 | X | ||||
| Cardiovalve | Cardiovalve | TFV, TS | 28-F | Low-profile design featuring 3 scalloped bovine pericardial leaflets | AHEAD (NCT03339115) | X | ||||
| Saturn | Innovheart | TA | Central valve component anchored in an annular ring | NCT04464876 | X | |||||
| Innostay | Innovalve | TFV, TS | 32-F | Circumferential arm design seals annulus using a rotational maneuver during deployment | TWIST-EFS (NCT04919980) | X | ||||
| Annuloplasty | Carillon | Cardiac Dimensions | CS | 9-F | Device with proximal and distal helical anchors that plicate mitral periannular tissue | EMPOWER (NCT03142152) | X | X | ||
| ARTO | MVRx | CS | 12-F | Anchors implanted in the lateral wall via the CS and the septum are bridged to reduce the MV minor axis dimensions. | MAVERICK (NCT03311295) | X | ||||
| Other | NeoChord | NeoChord | TA (DS1000) TFV, TS (NeXuS) |
28-F | Neochordae created using a device that features a helical papillary muscle anchor and a suture that grasps the MV leaflet. | ReChord (NCT02803957) (TA) |
X | X (TA) | ||
| Half Moon | Half Moon | TFV, TS | 29-F | Improved native leaflet coaptation by filling the posterior aspect of the regurgitant orifice with an ePTFE-coated device implanted in a circular frame | NCT04343313 | X | ||||
CE = Conformite Europeenne; CS = coronary sinus; EFS = early feasibility study; ePTFE = expanded polytetrafluoroethylene; FDA = Food and Drug Administration; mTEER = mitral transcatheter edge-to-edge repair; MV = mitral valve; TA = transapical; TFV = transfemoral vein; TMVR = transcatheter mitral valve replacement; TS = transseptal; other abbreviation as in Table 1.
Of note, most of these potential interventions are still in initial trials, with little to no evidence for SMR application. TMVR has unique challenges that hinder its widespread application such as left ventricular outflow tract obstruction, annular sizing, leaflet morphology, and valve shape. A recently published study reported the outcomes of patients undergoing TMVR for SMR and compared them to patients in the GDMT arm of the COAPT trial.64 The propensity-matched comparison reported that the rate of HF hospitalizations was significantly lower (32.8% vs 54.4%) in the TMVR group compared to GDMT alone, whereas all-cause mortality at 2 years was similar. Future clinical trials need to compare TMVR to mTEER-based strategies in SMR patients.
SURGICAL MV INTERVENTIONS.
Although surgical interventions to re-establish mitral competence in SMR have been performed since the 1990s, there are no convincing data for survival benefit in SMR associated with HFrEF.65 The RIME (Randomized Ischemic Mitral Evaluation) randomized trial reported that MV repair combined with surgical revascularization improved function capacity and promoted reverse LV remodeling.66 A notable MR recurrence rate of 58% at the 2-year follow-up after mitral annuloplasty alone in the Cardiothoracic Surgical Trials Network has led the surgical community to favor MV replacement instead in this setting.67 The 2021 European Society of Cardiology/European Association for Cardio-Thoracic Surgery and American Association for Thoracic Surgery consensus guidelines currently recommend (Class 1) concomitant mitral surgery for severe MR when cardiac surgery is performed for other indications.68,69 Isolated MV surgery may be considered in symptomatic patients with HFrEF and severe MR secondary to nonischemic cardiomyopathy if judged appropriate by the MDT (Class 2b).68,69 Although there are no data on the superiority of surgical mitral repair vs replacement in the nonischemic setting, mitral replacement may be preferred in advanced LV remodeling in which valve repair is not feasible.70 In fact, a chordal-sparing mitral replacement is favored over a downsizing annuloplasty approach (Class 2b) given the high rate of SMR recurrence with the latter.11 Features associated with subsequent surgical failure include LV enddiastolic diameter >65 mm, MV tenting height >10 mm, posterior leaflet–annulus angle >45°, anterior leaflet–annulus angle >25°, end-systolic interpapillary distance >20 mm, systolic sphericity index >0.7, and a spherical LV shape.65,71,72
CONSIDERATIONS FOR THERAPEUTIC OPTIONS.
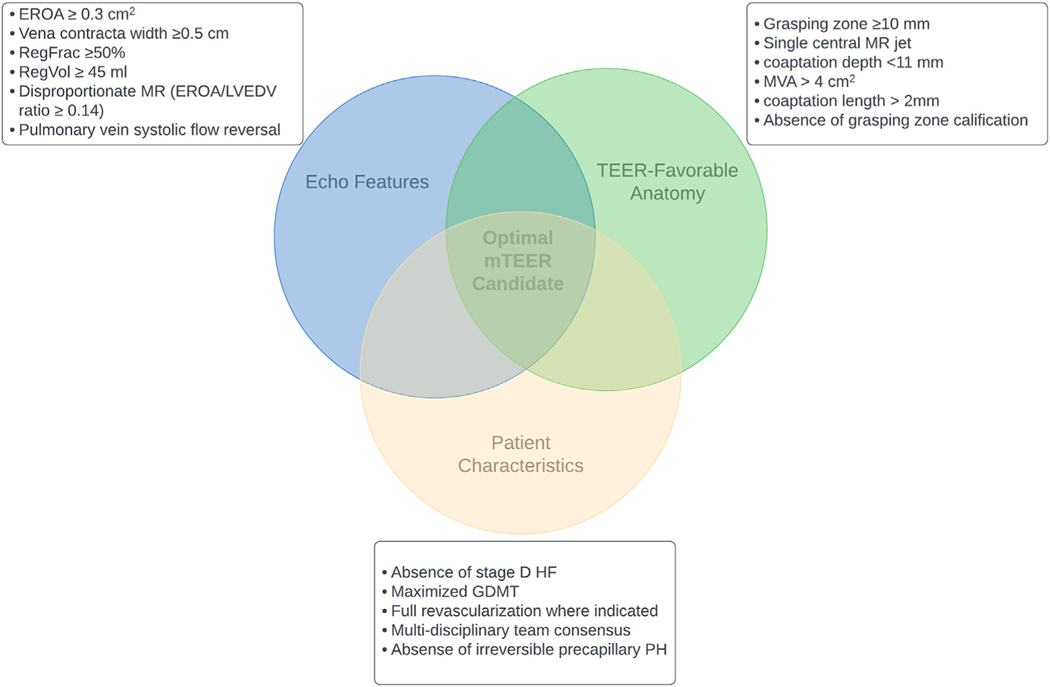
Various clinical, echocardiographic, and anatomical criteria have been described to screen symptomatic HFrEF patients with SMR (Figure 5). Patients referred with symptomatic SMR benefit from HF clinic–led rapid GDMT and volume optimization. After a period of 1 to 6 months (a duration that requires further data to support), repeat clinical and imaging assessments may reaffirm or defer the need for mTEER. Similarly, reassessment may reveal prior barriers to mTEER such as pulmonary hypertension or RV dysfunction have improved with optimization and no longer are an additive risk.
FIGURE 5. Imaging and Patient-Specific Characteristics Pertinent to mTEER Candidate Consideration.
GDMT = guideline-directed medical therapy; mTEER = mitral transcatheter edge-to-edge repair; MVA = mitral valve area; PH = pulmonary hypertension; RegFrac = regurgitant fraction; RegVol = regurgitant volume; TEER = transcatheter edge-to-edge repair; other abbreviations as in Figures 1 and 2.
The COAPT trial criteria have been widely adopted as a guide for SMR assessment and intervention candidacy for mTEER. Key inclusion criteria in the trial include NYHA functional class II, III, or ambulatory IV; LVEF of 20% to 50%; LV end-systolic diameter ≤70 mm; estimated pulmonary artery systolic pressure ≤70 mm Hg; and absence of end-stage HF.6 When combined with the trial’s novel hierarchical approach to screen for severe SMR, incrementally lower EROA cutoffs can be used with the other parameters such as pulmonary vein systolic flow reversal (should the EROA not be consistent with severe SMR).73 Over time, transcatheter experience has allowed for further identification of characteristics that are less favorable for TEER, including MV area <4 cm2, severe mitral annular calcification (MAC), rheumatic MV disease, MV clefts, commissural MR, flail gap >10 mm, flail width >15 mm, and coaptation length <2 mm and depth >11 mm.13
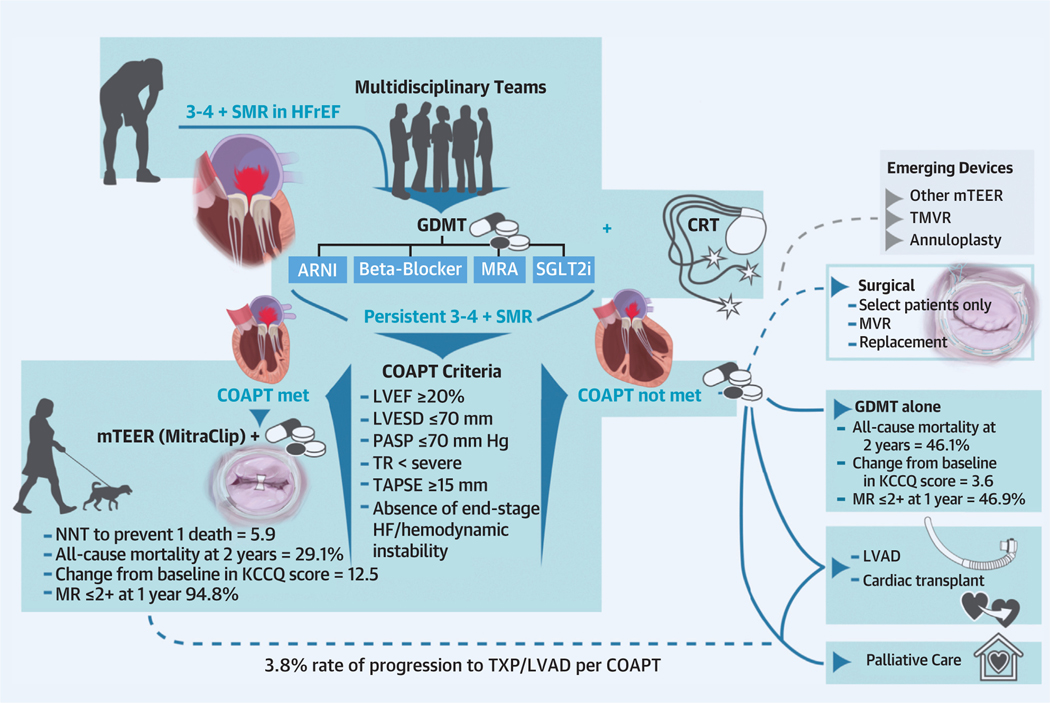
For symptomatic HFrEF patients with SMR who do not meet the COAPT trial criteria by echocardiography, options include conservative management with GDMT or, for a subset, heart replacement therapies (Central Illustration). The impact of mTEER in patient populations excluded in COAPT because of other comorbidities such as concomitant chronic obstructive pulmonary disease, severe tricuspid regurgitation, severe pulmonary hypertension, and RV dysfunction is being evaluated in multiple registry-based studies. Recent real-world data from the TVT and EuroSMR (European Registry of Transcatheter Repair for Secondary Mitral Regurgitation) registry shows that mTEER with MitraClip was associated with a significant improvement in quality of life and NYHA functional class, a durable reduction in MR, and a low adverse event rate.74
CENTRAL ILLUSTRATION. Considerations in Patient Selection for Secondary Mitral Regurgitation Intervention in HFrEF Patients.
ARNI = angiotensin receptor–neprilysin inhibitor; COAPT = Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation; CRT = cardiac resynchronization therapy; GDMT = guideline-directed medical therapy; HF = heart failure; HFrEF = heart failure with reduced ejection fraction; KCCQ = Kansas City Cardiomyopathy Questionnaire; LVAD = left ventricular assist device; LVEF = left ventricular ejection fraction; LVESD = left ventricular end-systolic dimension; MR = mitral regurgitation; MRA = mineralocorticoid receptor antagonist; mTEER = mitral transcatheter edge-to-edge repair; MVR = mitral valve replacement; NNT = number needed to treat; PASP = pulmonary artery systolic pressure; SGLT2i = sodium-glucose cotransporter 2 inhibitor; SMR = secondary mitral regurgitation; TAPSE = tricuspid annular plane systolic excursion; TMVR = transcatheter mitral valve replacement; TR = tricuspid regurgitation; TXP = transplant.
SMR IN UNIQUE PATIENT POPULATIONS.
Although there is enthusiasm for the expansion of mTEER candidacy outside of COAPT criteria, there remains a lack of data to support widespread adoption of this strategy.12 Future studies should evaluate the role of mTEER in patients with atrial functional MR, those with borderline elevated mitral gradients, and those on inotropic support, among other populations. The RESHAPE-HF2 (A Clinical Evaluation of the Safety and Effectiveness of the MitraClip System in the Treatment of Clinically Significant Functional Mitral Regurgitation; NCT02444338) trial is ongoing and evaluating the impact of MitraClip in patients with SMR and LVEF ≥15% to ≤35% (if in NYHA functional class II) or ≥15% to ≤45% (if in NYHA functional class III or IV).
Cardiogenic shock.
About 5% to 10% of patients with acute myocardial infarction–associated coronary sinus present with severe MR, which portends additional poor prognosis.75 Recent reports have suggested that mTEER may improve in-hospital and 30-day survival in patients with cardiogenic shock and MR (both functional and degenerative).76–78 Carefully designed clinical trials and predefined subgroup analysis are required to identify patient and procedural characteristics, hemodynamic parameters, and the optimal time for intervention to ultimately address this benefit.79
End-stage HF.
End-stage HF patients were excluded from the mTEER trials, leaving a gap in the literature on best practices for this group. The MitraBridge (Transcatheter Mitral Valve Repair as Bridge Therapy to Heart Transplantation) registry included patients with end-stage HF with transplant eligibility with a long wait time or potentially reversible transplant contraindications.80 This nonrandomized trial totaled 119 patients across 17 centers on maximal GDMT who underwent mTEER, of whom nearly one-fourth were later removed from transplant consideration because of clinical improvement. This suggests that mTEER may provide a safe bridge to improvement or eventual transplant candidacy in the right patients, although further data are necessary for any firm recommendations.
There are emerging tools to assist the clinician faced with concerns of “missing the window” for a patient in between heart replacement candidacy and mTEER. Several risk stratification scores have been developed (MITRALITY, MitraScore, and COAPT risk score) that all consider various preprocedural variables to predict postprocedural outcomes with mTEER.81–83 However, these scores have their own deficiencies, such as a lack of external validation, modest discrimination, and for the end user a lack of real cutoffs or guidance as to what score should impact decision making. Clinicians who want to follow high-risk patients post-mTEER, and perhaps identify “nonresponders,” may be best served by tools already widely used such as the KCCQ, as demonstrated in a COAPT substudy by Arnold et al.84 For example, a 10-point or greater improvement from baseline to month 1 in the KCCQ overall summary score was associated with a significant difference in death or HF hospitalization incidence at 2 years from those with no change (40.2% vs 58.2%; P < 0.001).
Mitral annular calcification.
MAC is increasingly prevalent with an aging population with associated risk of MV dysfunction and mortality. Contemporary transcatheter trials have often excluded patients with severe MAC, limiting uniform understanding of these interventions for this population. Coincident severe MAC and SMR (although uncommon) are typically found in elderly patients with comorbidities and prohibitive surgical risk and are best addressed using a multidisciplinary heart team approach with careful attention to anatomical compatibility, which may elicit candidacy for TMVR.85 Although there is a paucity of data on TEER in MAC and SMR, a recent real-world cohort with ~60% SMR suggested high technical success and a low rate of complications with similar improvements in HF readmissions and mortality.86
Left ventricular assist device therapy.
Left ventricular assist device (LVAD) placement frequently leads to a reduction in the severity of functional MR. Although a notable number of LVAD-supported patients have residual MR, its impact on outcomes and hence the role of MV interventions/concomitant MV repair at the time of LVAD implant is controversial. Patients with moderate or severe RV dysfunction are particularly susceptible to the afterload exerted by significant residual MR and have a much higher incidence of postoperative RV and renal failure.87,88 In contrast, a MOMENTUM 3 (Multicenter Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy with HeartMate) analysis showed that although nearly half (43.5%) of the patients undergoing LVAD had clinically significant MR at baseline, residual MR was present in only 6.2% of patients with HeartMate 3 (Abbott Vascular) implant at 1 month.89 Moreover, residual MR at 1 month postimplant did not impact 2-year mortality (HR: 1.41 [95% CI: 0.52–3.89]; P = 0.50). The risk of performing concomitant MV repair must be weighed for individual patients. It is important to note that mitral stenosis, especially with TEER, may affect LVAD outcomes significantly.
CONCLUSIONS
Technical and technological advances in the field of valvular heart disease, especially SMR, have amplified the role of an MDT approach and GDMT. A variety of percutaneous or transcatheter valve repair/replacement systems are now available for the management of SMR. Avoiding the need for cardiopulmonary bypass, these MV interventions offer a remarkable safety profile and broadening clinical applications. The growing experience and evidence regarding the safety and efficacy of MitraClip has made mTEER the first-line therapy in patients with HF and significant SMR despite maximal GDMT. Moreover, results from COAPT have set the benchmark for future trials in the field of percutaneous mitral repair. We continue to witness expansion in minimally invasive transcatheter techniques with better safety and efficacy profiles over time.
Supplementary Material
HIGHLIGHTS.
Significant SMR has major clinical consequences for the already vulnerable HFrEF population.
GDMT in HFrEF with SMR remains the first step in patient management.
Persistent SMR despite GDMT requires multidisciplinary evaluation with consideration of transcatheter treatment.
Current and emerging transcatheter devices will further challenge the conventional approach to SMR in HF patients.
FUNDING SUPPORT AND AUTHOR DISCLOSURES
Dr Lander has served as an advisory board member for Abiomed; and has received speaker fees from Abbott. Dr Brener has received consulting fees from Artract and Osprey Medical. Dr Goel has received consulting and proctoring fees from Edwards Lifesciences and Abbott. Dr Zalawadiya has received consulting fees from Vectorius, ADI, and Endotronix. Dr Lindenfeld has received consulting fees from Abbott, Alleviant, AstraZeneca, Boston Scientific, CVRx, Edwards Lifesciences, Merck, Medtronic, VWave, Biotronik, Vascular Dynamics, Cordio, Vectorius, and Whiteswell; and has received grant funding from AstraZeneca. Dr Kanwar has served as an advisory board member for Abiomed, CareDx, and Corwave; and has served as a speaker for Abiomed. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS
- ARNI
angiotensin receptor-neprilysin inhibitor
- CRT
cardiac resynchronization therapy
- EROA
effective regurgitant orifice area
- GDMT
guideline-directed medical therapy
- HFrEF
heart failure with reduced ejection fraction
- mTEER
mitral transcatheter edge-to-edge repair
- PISA
proximal isovelocity surface area
- SMR
secondary mitral regurgitation
- SGLT2
sodium-glucose transporter 2
- TMVR
transcatheter mitral valve replacement
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
REFERENCES
- 1.Kataria R, Castagna F, Madan S, et al. Severity of functional mitral regurgitation on admission for acute decompensated heart failure predicts long-term risk of rehospitalization and death. J Am Heart Assoc. 2022;11(1):e022908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bursi F, Barbieri A, Grigioni F, et al. Prognostic implications of functional mitral regurgitation according to the severity of the underlying chronic heart failure: a long-term outcome study. Eur J Heart Fail. 2010;12:382–388. [DOI] [PubMed] [Google Scholar]
- 3.Bonow RO, Carabello BA, Chatterjee K, et al. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2008;52(13):e1–e142. [DOI] [PubMed] [Google Scholar]
- 4.Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:e521–643. [DOI] [PubMed] [Google Scholar]
- 5.Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–2791. [DOI] [PubMed] [Google Scholar]
- 6.Stone GW, Lindenfeld J, Abraham WT, et al. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med. 2018;379:2307–2318. [DOI] [PubMed] [Google Scholar]
- 7.Obadia JF, Messika-Zeitoun D, Leurent G, et al. Percutaneous repair or medical treatment for secondary mitral regurgitation. N Engl J Med. 2018;379:2297–2306. [DOI] [PubMed] [Google Scholar]
- 8.Sannino A, Smith RL 2nd, Schiattarella GG, Trimarco B, Esposito G, Grayburn PA. Survival and cardiovascular outcomes of patients with secondary mitral regurgitation: a systematic review and meta-analysis. JAMA Cardiol. 2017;2:1130–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCarthy KP, Ring L, Rana BS. Anatomy of the mitral valve: understanding the mitral valve complex in mitral regurgitation. Eur J Echocardiogr. 2010;11:i3–i9. [DOI] [PubMed] [Google Scholar]
- 10.Gaasch WH, Meyer TE. Left ventricular response to mitral regurgitation: implications for management. Circulation. 2008;118:2298–2303. [DOI] [PubMed] [Google Scholar]
- 11.Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;77(4):e25–e197. [DOI] [PubMed] [Google Scholar]
- 12.Coats AJS, Anker SD, Baumbach A, et al. The management of secondary mitral regurgitation in patients with heart failure: a joint position statement from the Heart Failure Association (HFA), European Association of Cardiovascular Imaging (EACVI), European Heart Rhythm Association (EHRA), and European Association of Percutaneous Cardiovascular Interventions (EAPCI) of the ESC. Eur Heart J. 2021;42:1254–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonow RO, O’Gara PT, Adams DH, et al. 2020 focused update of the 2017 ACC Expert Consensus Decision Pathway on the Management of Mitral Regurgitation: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;75:2236–2270. [DOI] [PubMed] [Google Scholar]
- 14.Matsumura Y, Fukuda S, Tran H, et al. Geometry of the proximal isovelocity surface area in mitral regurgitation by 3-dimensional color Doppler echocardiography: difference between functional mitral regurgitation and prolapse regurgitation. Am Heart J. 2008;155:231–238. [DOI] [PubMed] [Google Scholar]
- 15.Buck T, Plicht B, Kahlert P, Schenk IM, Hunold P, Erbel R. Effect of dynamic flow rate and orifice area on mitral regurgitant stroke volume quantification using the proximal isovelocity surface area method. J Am Coll Cardiol. 2008;52:767–778. [DOI] [PubMed] [Google Scholar]
- 16.Zoghbi WA, Adams D, Bonow RO, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017;30:303–371. [DOI] [PubMed] [Google Scholar]
- 17.Iwakura K, Ito H, Kawano S, et al. Comparison of orifice area by transthoracic three-dimensional Doppler echocardiography versus proximal isovelocity surface area (PISA) method for assessment of mitral regurgitation. Am J Cardiol. 2006;97:1630–1637. [DOI] [PubMed] [Google Scholar]
- 18.Lopez-Mattei JC, Ibrahim H, Shaikh KA, et al. Comparative assessment of mitral regurgitation severity by transthoracic echocardiography and cardiac magnetic resonance using an integrative and quantitative approach. Am J Cardiol. 2016;117: 264–270. [DOI] [PubMed] [Google Scholar]
- 19.Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;79(17):e263–e421. [DOI] [PubMed] [Google Scholar]
- 20.Sannino A, Sudhakaran S, Milligan G, et al. Effectiveness of medical therapy for functional mitral regurgitation in heart failure with reduced ejection fraction. J Am Coll Cardiol. 2020;76:883–884. [DOI] [PubMed] [Google Scholar]
- 21.Spinka G, Bartko PE, Heitzinger G, et al. Guideline directed medical therapy and reduction of secondary mitral regurgitation. Eur Heart J Cardiovasc Imaging. 2022;23:755–764. [DOI] [PubMed] [Google Scholar]
- 22.Santos-Gallego CG, Vargas-Delgado AP, Requena-Ibanez JA, et al. Randomized trial of empagliflozin in nondiabetic patients with heart failure and reduced ejection fraction. J Am Coll Cardiol. 2021;77:243–255. [DOI] [PubMed] [Google Scholar]
- 23.Stolfo D, Castrichini M, Biagini E, et al. Modifications of medical treatment and outcome after percutaneous correction of secondary mitral regurgitation. ESC Heart Fail. 2020;7:1753–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Comin-Colet J, Sanchez-Corral MA, Manito N, et al. Effect of carvedilol therapy on functional mitral regurgitation, ventricular remodeling, and contractility in patients with heart failure due to left ventricular systolic dysfunction. Transplant Proc. 2002;34:177–178. [DOI] [PubMed] [Google Scholar]
- 25.Kotlyar E, Hayward CS, Keogh AM, Feneley M, Macdonald PS. The impact of baseline left ventricular size and mitral regurgitation on reverse left ventricular remodelling in response to carvedilol: size doesn’t matter. Heart. 2004;90(7):800–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waagstein F, Stromblad O, Andersson B, et al. Increased exercise ejection fraction and reversed remodeling after long-term treatment with metoprolol in congestive heart failure: a randomized, stratified, double-blind, placebo-controlled trial in mild to moderate heart failure due to ischemic or idiopathic dilated cardiomyopathy. Eur J Heart Fail. 2003;5:679–691. [DOI] [PubMed] [Google Scholar]
- 27.Seneviratne B, Moore GA, West PD. Effect of captopril on functional mitral regurgitation in dilated heart failure: a randomised double blind placebo controlled trial. Br Heart J. 1994;72:63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Zhou R, Lu C, Chen Q, Xu T, Li D. Effects of the angiotensin-receptor neprilysin inhibitor on cardiac reverse remodeling: meta-analysis. J Am Heart Assoc. 2019;8:e012272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 30.Kang DH, Park SJ, Shin SH, et al. Angiotensin receptor neprilysin inhibitor for functional mitral regurgitation. Circulation. 2019;139:1354–1365. [DOI] [PubMed] [Google Scholar]
- 31.Januzzi JL, Omar AMS, Liu Y, et al. Association between sacubitril/valsartan initiation and mitral regurgitation severity in heart failure with reduced ejection fraction: the PROVE-HF study. Circulation. 2022;146:1638–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levine HJ, Gaasch WH. Vasoactive drugs in chronic regurgitant lesions of the mitral and aortic valves. J Am Coll Cardiol. 1996;28:1083–1091. [DOI] [PubMed] [Google Scholar]
- 33.Cox ZL, Zalawadiya SK, Simonato M, et al. Guideline-directed medical therapy tolerability in patients with heart failure and mitral regurgitation: the COAPT trial. J Am Coll Cardiol HF. 2023;11(7):791–805. [DOI] [PubMed] [Google Scholar]
- 34.Greene SJ, Butler J, Albert NM, et al. Medical therapy for heart failure with reduced ejection fraction. J Am Coll Cardiol. 2018;72:351–366. [DOI] [PubMed] [Google Scholar]
- 35.Desai AS, Deswal A, McMurray JJV, Pinney SP, Vaduganathan M. Optimizing background therapy for heart failure in clinical trials. J Am Coll Cardiol HF. 2023;11:806–809. [DOI] [PubMed] [Google Scholar]
- 36.Breithardt OA, Sinha AM, Schwammenthal E, et al. Acute effects of cardiac resynchronization therapy on functional mitral regurgitation in advanced systolic heart failure. J Am Coll Cardiol. 2003;41:765–770. [DOI] [PubMed] [Google Scholar]
- 37.Di Biase L, Auricchio A, Mohanty P, et al. Impact of cardiac resynchronization therapy on the severity of mitral regurgitation. Europace. 2011;13: 829–838. [DOI] [PubMed] [Google Scholar]
- 38.Ypenburg C, Lancellotti P, Tops LF, et al. Mechanism of improvement in mitral regurgitation after cardiac resynchronization therapy. Eur Heart J. 2008;29:757–765. [DOI] [PubMed] [Google Scholar]
- 39.Stone GW, Abraham WT, Lindenfeld J, et al. Five-year follow-up after transcatheter repair of secondary mitral regurgitation. N Engl J Med. 2023;388(22):2037–2048. [DOI] [PubMed] [Google Scholar]
- 40.Arnold SV, Stone GW, Jain SS, et al. prognostic importance of health status versus functional status in heart failure and secondary mitral regurgitation. J Am Coll Cardiol HF. 2021;9:684–692. [DOI] [PubMed] [Google Scholar]
- 41.Iung B, Armoiry X, Vahanian A, et al. Percutaneous repair or medical treatment for secondary mitral regurgitation: outcomes at 2 years. Eur J Heart Fail. 2019;21:1619–1627. [DOI] [PubMed] [Google Scholar]
- 42.Grayburn PA, Sannino A, Packer M. Proportionate and disproportionate functional mitral regurgitation: a new conceptual framework that reconciles the results of the MITRA-FR and COAPT trials. J Am Coll Cardiol Img. 2019;12:353–362. [DOI] [PubMed] [Google Scholar]
- 43.Ooms JF, Bouwmeester S, Debonnaire P, et al. Transcatheter edge-to-edge repair in proportionate versus disproportionate functional mitral regurgitation. J Am Soc Echocardiogr. 2022;35: 105–115.e8. [DOI] [PubMed] [Google Scholar]
- 44.Lindenfeld J, Abraham WT, Grayburn PA, et al. Association of effective regurgitation orifice area to left ventricular end-diastolic volume ratio with transcatheter mitral valve repair outcomes: a secondary analysis of the COAPT trial. JAMA Cardiol. 2021;6:427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feldman T, Foster E, Glower DD, et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med. 2011;364:1395–1406. [DOI] [PubMed] [Google Scholar]
- 46.Kar S, von Bardeleben RS, Rottbauer W, et al. Contemporary outcomes following transcatheter edge-to-edge repair: 1-year results from the EXPAND study. J Am Coll Cardiol Intv. 2023;16: 589–602. [DOI] [PubMed] [Google Scholar]
- 47.Alkhouli M, Hahn RT, Petronio AS. Transcatheter edge-to-edge repair for atrial functional mitral regurgitation: effective therapy or elusive target? J Am Coll Cardiol Intv. 2022;15:1741–1747. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka T, Sugiura A, Öztürk C, et al. Transcatheter edge-to-edge repair for atrial secondary mitral regurgitation. J Am Coll Cardiol Intv. 2022;15:1731–1740. [DOI] [PubMed] [Google Scholar]
- 49.Sodhi N, Asch FM, Ruf T, et al. Clinical outcomes with transcatheter edge-to-edge repair in atrial functional MR from the EXPAND study. J Am Coll Cardiol Intv. 2022;15:1723–1730. [DOI] [PubMed] [Google Scholar]
- 50.Schneider L, Markovic S, Mueller K, et al. Mitral valve transcatheter edge-to-edge repair using MitraClip or PASCAL: a multicenter propensity score-matched comparison. J Am Coll Cardiol Intv. 2022;15:2554–2567. [DOI] [PubMed] [Google Scholar]
- 51.Mauri V, Sugiura A, Spieker M, et al. Early outcomes of 2 mitral valve transcatheter leaflet approximation devices: a propensity score-matched multicenter comparison. J Am Coll Cardiol Intv. 2022;15:2541–2551. [DOI] [PubMed] [Google Scholar]
- 52.Liu X, Pu Z, Lim DS, Wang J. Transcatheter mitral valve repair in a high-surgical risk patient with severe degenerative mitral regurgitation using the novel DragonFly Transcatheter Repair device-first in man implantation in China. Catheter Cardiovasc Interv. 2022;99:518–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Forrestal BJ, Khan JM, Torguson R, et al. Reasons for screen failure for transcatheter mitral valve repair and replacement. Am J Cardiol. 2021;148:130–137. [DOI] [PubMed] [Google Scholar]
- 54.Muller DWM, Sorajja P, Duncan A, et al. 2-year outcomes of transcatheter mitral valve replacement in patients with severe symptomatic mitral regurgitation. J Am Coll Cardiol. 2021;78:1847–1859. [DOI] [PubMed] [Google Scholar]
- 55.Bapat V, Rajagopal V, Meduri C, et al. Early experience with new transcatheter mitral valve replacement. J Am Coll Cardiol. 2018;71:12–21. [DOI] [PubMed] [Google Scholar]
- 56.Webb J, Hensey M, Fam N, et al. Transcatheter mitral valve replacement with the transseptal EVOQUE system. J Am Coll Cardiol Intv. 2020;13: 2418–2426. [DOI] [PubMed] [Google Scholar]
- 57.Goel SS, Zuck V, Christy J, et al. Transcatheter mitral valve therapy with novel supra-annular AltaValve: first experience in the United States. J Am Coll Cardiol Case Rep. 2019;1:761–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barbanti M, Piazza N, Mangiafico S, et al. Transcatheter mitral valve implantation using the HighLife system. J Am Coll Cardiol Intv. 2017;10: 1662–1670. [DOI] [PubMed] [Google Scholar]
- 59.Alperi A, Dagenais F, Del Val D, et al. Early experience with a novel transfemoral mitral valve implantation system in complex degenerative mitral regurgitation. J Am Coll Cardiol Intv. 2020;13:2427–2437. [DOI] [PubMed] [Google Scholar]
- 60.Maisano F, Benetis R, Rumbinaite E, et al. 2-year follow-up after transseptal transcatheter mitral valve replacement with the Cardiovalve. J Am Coll Cardiol Intv. 2020;13:e163–e164. [DOI] [PubMed] [Google Scholar]
- 61.Mangieri A, Cannata F, Cozzi O, et al. A fully percutaneous transeptal transcatheter mitral valve replacement with a novel device. J Am Coll Cardiol Intv. Published online May 9, 2023. 10.1016/j.jcin.2023.04.040 [DOI] [PubMed] [Google Scholar]
- 62.Siminiak T, Wu JC, Haude M, et al. Treatment of functional mitral regurgitation by percutaneous annuloplasty: results of the TITAN trial. Eur J Heart Fail. 2012;14:931–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lipiecki J, Siminiak T, Sievert H, et al. Coronary sinus-based percutaneous annuloplasty as treatment for functional mitral regurgitation: the TITAN II trial. Open Heart. 2016;3:e000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ludwig S, Conradi L, Cohen DJ, et al. Transcatheter mitral valve replacement versus medical therapy for secondary mitral regurgitation: a propensity score-matched comparison. Circ Cardiovasc Interv. 2023;16:e013045. [DOI] [PubMed] [Google Scholar]
- 65.Nappi F, Avatar Singh SS, Santana O, Mihos CG. Functional mitral regurgitation: an overview for surgical management framework. J Thorac Dis. 2018;10:4540–4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chan KM, Punjabi PP, Flather M, et al. Coronary artery bypass surgery with or without mitral valve annuloplasty in moderate functional ischemic mitral regurgitation: final results of the Randomized Ischemic Mitral Evaluation (RIME) trial. Circulation. 2012;126:2502–2510. [DOI] [PubMed] [Google Scholar]
- 67.Goldstein D, Moskowitz AJ, Gelijns AC, et al. Two-year outcomes of surgical treatment of severe ischemic mitral regurgitation. N Engl J Med. 2016;374:344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease: developed by the Task Force for the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Rev Esp Cardiol (Engl Ed). 2022;75:524. [DOI] [PubMed] [Google Scholar]
- 69.Bakaeen FG, Gaudino M, Whitman G, et al. 2021: the American Association for Thoracic Surgery Expert Consensus Document: coronary artery bypass grafting in patients with ischemic cardiomyopathy and heart failure. J Thorac Cardiovasc Surg. 2021;162:829–850.e1. [DOI] [PubMed] [Google Scholar]
- 70.Kashiyama N, Toda K, Miyagawa S, et al. Left ventricular stroke work index associated with outcome after mitral valve surgery for functional regurgitation in nonischemic dilated cardiomyopathy. Semin Thorac Cardiovasc Surg. 2020;32:698–709. [DOI] [PubMed] [Google Scholar]
- 71.Magne J, Pibarot P, Dagenais F, Hachicha Z, Dumesnil JG, Senechal M. Preoperative posterior leaflet angle accurately predicts outcome after restrictive mitral valve annuloplasty for ischemic mitral regurgitation. Circulation. 2007;115:782–791. [DOI] [PubMed] [Google Scholar]
- 72.Enriquez-Sarano M, Avierinos JF, Messika-Zeitoun D, et al. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N Engl J Med. 2005;352:875–883. [DOI] [PubMed] [Google Scholar]
- 73.Asch FM, Grayburn PA, Siegel RJ, et al. Echocardiographic outcomes after transcatheter leaflet approximation in patients with secondary mitral regurgitation: the COAPT trial. J Am Coll Cardiol. 2019;74:2969–2979. [DOI] [PubMed] [Google Scholar]
- 74.Koell B, Orban M, Weimann J, et al. Outcomes stratified by adapted inclusion criteria after mitral edge-to-edge repair. J Am Coll Cardiol. 2021;78: 2408–2421. [DOI] [PubMed] [Google Scholar]
- 75.Thompson CR, Buller CE, Sleeper LA, et al. Cardiogenic shock due to acute severe mitral regurgitation complicating acute myocardial infarction: a report from the SHOCK Trial Registry. J Am Coll Cardiol. 2000;36:1104–1109. [DOI] [PubMed] [Google Scholar]
- 76.Jung RG, Simard T, Di Santo P, Hibbert B. Transcatheter edge-to-edge repair in patients with mitral regurgitation and cardiogenic shock: a new therapeutic target. Curr Opin Crit Care. 2022;28: 426–433. [DOI] [PubMed] [Google Scholar]
- 77.Lurz P, Besler C. Transcatheter treatment of mitral regurgitation in cardiogenic shock. J Am Coll Cardiol. 2022;80:2085–2088. [DOI] [PubMed] [Google Scholar]
- 78.Simard T, Vemulapalli S, Jung RG, et al. Transcatheter edge-to-edge mitral valve repair in patients with severe mitral regurgitation and cardiogenic shock. J Am Coll Cardiol. 2022;80: 2072–2084. [DOI] [PubMed] [Google Scholar]
- 79.Parlow S, Di Santo P, Jung RG, et al. Transcatheter mitral valve repair for inotrope dependent cardiogenic shock–design and rationale of the CAPITAL MINOS trial. Am Heart J. 2022;254:81–87. [DOI] [PubMed] [Google Scholar]
- 80.Godino C, Munafò A, Scotti A, et al. MitraClip in secondary mitral regurgitation as a bridge to heart transplantation: 1-year outcomes from the International MitraBridge Registry. J Heart Lung Transplant. 2020;39:1353–1362. [DOI] [PubMed] [Google Scholar]
- 81.Zweck E, Spieker M, Horn P, et al. Machine learning identifies clinical parameters to predict mortality in patients undergoing transcatheter mitral valve repair. J Am Coll Cardiol Intv. 2021;14: 2027–2036. [DOI] [PubMed] [Google Scholar]
- 82.Raposeiras-Roubin S, Adamo M, Freixa X, et al. A score to assess mortality after percutaneous mitral valve repair. J Am Coll Cardiol. 2022;79:562–573. [DOI] [PubMed] [Google Scholar]
- 83.Shah N, Madhavan MV, Gray WA, et al. Prediction of death or HF hospitalization in patients with severe FMR: the COAPT risk score. J Am Coll Cardiol Intv. 2022;15:1893–1905. [DOI] [PubMed] [Google Scholar]
- 84.Arnold SV, Stone GW, Mack MJ, et al. Health status changes and outcomes in patients with heart failure and mitral regurgitation: COAPT trial. J Am Coll Cardiol. 2020;75:2099–2106. [DOI] [PubMed] [Google Scholar]
- 85.Chehab O, Roberts-Thomson R, Bivona A, et al. Management of patients with severe mitral annular calcification: JACC state-of-the-art review. J Am Coll Cardiol. 2022;80:722–738. [DOI] [PubMed] [Google Scholar]
- 86.Fernández-Peregrina E, Pascual I, Freixa X, et al. Transcatheter edge-to-edge mitral valve repair in patients with mitral annulus calcification. EuroIntervention. 2022;17:1300–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tang PC, Haft JW, Romano MA, et al. Right ventricular function and residual mitral regurgitation after left ventricular assist device implantation determines the incidence of right heart failure. J Thorac Cardiovasc Surg. 2020;159(3):897–905.e4. [DOI] [PubMed] [Google Scholar]
- 88.Jain R, Truby LK, Topkara VK. Residual mitral regurgitation in patients with left ventricular assist device support - an INTERMACS analysis. J Heart Lung Transplant. 2022;41:1638–1645. [DOI] [PubMed] [Google Scholar]
- 89.Kanwar MK, Rajagopal K, Itoh A, et al. Impact of left ventricular assist device implantation on mitral regurgitation: an analysis from the MOMENTUM 3 trial. J Heart Lung Transplant. 2020;39:529–537. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.