Abstract
This report describes novel baculovirus vectors designed to express mammalian β1,4-galactosyltransferase and α2,6-sialyltransferase genes at early times after infection. Sf9 cells infected with these viral vectors, unlike cells infected with a wild-type baculovirus, produced a sialylated viral glycoprotein during the late phase of infection. Thus, the two mammalian glycosyltransferases encoded by these viral vectors are necessary and sufficient for sialylation of a foreign glycoprotein in insect cells under the conditions used in this study. While some of the new baculovirus vectors described in this study produced less, one produced wild-type levels of infectious budded virus progeny.
Baculovirus expression vectors have been widely used for the past 15 years to produce recombinant proteins, including many different mammalian glycoproteins (10, 22, 27). While the insect cells that serve as hosts for these viral vectors can provide eucaryotic protein modifications, including glycosylation, most recombinant glycoproteins produced by the baculovirus-insect cell system are not sialylated (2, 10, 18, 19). The absence of terminal sialic acids on baculovirus-expressed recombinant glycoproteins can be a significant problem, because these acidic, terminal sugars often have a direct or indirect influence on glycoprotein functions (15, 32).
Sialylation of newly synthesized N-glycoproteins is the last step in an elaborate biosynthetic pathway, which begins with the cotranslational transfer of a glycan precursor to a nascent polypeptide (16). The glycan precursor is subsequently trimmed and elongated by various enzymes in the endoplasmic reticulum and Golgi complex. In mammalian cells, elongation of N-glycan precursors yields products generally known as “hybrid” or “complex” N-glycans. The mammalian enzymes involved in the final elongation steps are the galactosyltransferases and sialyltransferases. Insect cells have an analogous N-glycan processing pathway, but typically fail to produce terminally sialylated N-glycans, as discussed above. One reason for this is that insect cells have extremely low levels of galactosyltransferase activity, if any, and no detectable sialyltransferase activity (1, 4, 9, 26, 28).
Thus, the goal of this study was to produce a new baculovirus expression vector capable of expressing mammalian glycosyltransferase genes during the early phase of infection. The ability of this vector to provide N-glycan processing enzymes that are absent or present at only very low levels in insect cells would fulfill one requirement for sialylation of a foreign glycoprotein expressed later in infection. If the other requirements, including production and transport of UDP-galactose and CMP-sialic acid, were fulfilled by the host, this new vector could be used to produce sialylated foreign glycoproteins in insect cells.
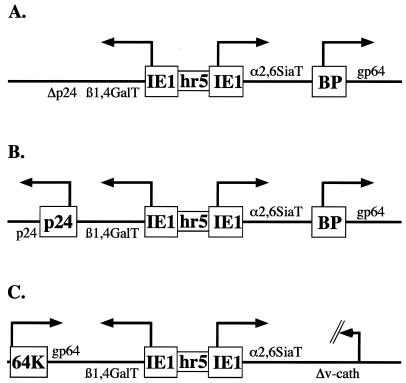
The design of this new vector called for a recombinant baculovirus encoding two mammalian glycosyltransferases, each under the transcriptional control of a baculovirus early promoter, in a new gene cassette located within a region of the viral genome other than that coding for polyhedrin (Fig. 1). This design preserves the polyhedrin region for the subsequent insertion of a foreign gene encoding a recombinant glycoprotein of interest, which is typically placed under the control of the polyhedrin promoter. Although many other transcriptional control elements could have been used to express the mammalian glycosyltransferases, we chose the Autographa californica nuclear polyhedrosis virus (AcMNPV) immediate-early 1 (ie1) promoter (6) and hr5 enhancer (5) because we had previously used this combination for early gene expression in other projects (see 10 and references therein). Similarly, the glycosyltransferase gene cassette could have been targeted to another region of the AcMNPV genome, but we initially chose the p24-gp64 region because we had a plasmid from another project that could be used to target genes to this region (J. Aumiller and D. Jarvis, unpublished data).
FIG. 1.
Genetic maps of recombinant baculoviruses containing mammalian glycosyltransferase genes. Shown are the key genetic features of the AcSWT series of baculovirus expression vectors described in this study. The boxes depict regulatory elements, including promoters and the hr5 enhancer element, while the lines indicate coding sequences. (A) AcSWT-1. (B) AcSWT-2. (C) AcSWT-2c.
The baculovirus transfer plasmid pAcSWT-TV1 was constructed to meet these design requirements by using standard recombinant DNA technology (23) and used to produce AcSWT-1, the first new baculovirus expression vector described in this study. This virus contains bovine β1,4GalT and rat α2,6SiaT cDNAs positioned under the control of back-to-back copies of the AcMNPV ie1 promoter separated by the hr5 enhancer element (Fig. 1A). The dual ie1-hr5-glycosyltransferase cassette is located between the AcMNPV gp64 and p24 genes, the p24 promoter and the 5′ half of the p24 coding sequence are deleted, and the gp64 gene is expressed under the control of the promoter from the AcMNPV basic protein (p6.9) gene. The polyhedrin region of AcSWT-1 is intact.
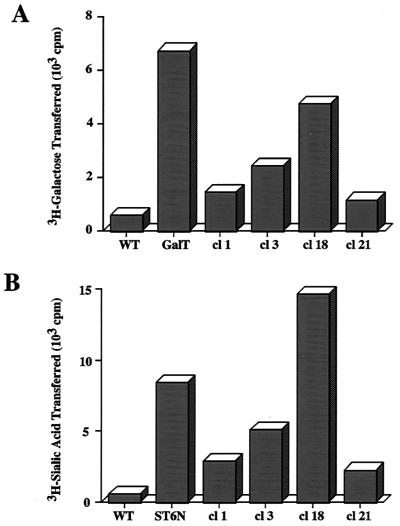
AcSWT-1 was produced by cotransfection of Sf9 cells (30) by a standard calcium phosphate method (27) with pAcSWT-TV1 and Bsu36I-digested viral DNA from a recombinant baculovirus called AcDCW. This parental virus has two Bsu36I sites (one just upstream and one within the gp64 coding sequence) and produces occlusion-positive, white plaques. Bsu36I digestion deletes much of the gp64 gene from AcDCW and effectively inactivates this parental viral DNA. Thus, Bsu36I-digested AcDCW viral DNA can be used to efficiently isolate recombinant viruses with alterations in the gp64 region because homologous recombination with the transfer plasmid is required to restore the essential gp64 gene and rescue the viral DNA. The progeny budded viruses produced by the cotransfected cells were resolved by plaque assay and screened for the presence of the β1,4-GalT and α2,6-SiaT cDNAs by dot blot hybridization, as described previously (27). Four clones that hybridized with both probes (data not shown) were subjected to two additional rounds of plaque purification and amplified in Sf9 cells, and then the titer was determined by plaque assay. Sf9 cells were infected at a multiplicity of infection of 2 PFU/cell with each clone or control viruses, and β1,4-GalT and α2,6-SiaT activities were measured in infected cell lysates prepared at 24 h postinfection, as described previously (3, 8). The results showed that extracts from Sf9 cells infected with each AcSWT-1 clone had at least marginally higher levels of both activities than the negative control extracts from wild-type AcMNPV-infected cells, which had only background levels of activity in these assays (Fig. 2). AcSWT-1 clone 18, which produced the highest levels of both activities, was used for the remainder of this study. The positive control viruses used in these assays encoded just one glycosyltransferase each—either bovine β1,4-GalT (12) or rat α2,6-SiaT (25)—under the control of the i.e.1 promoter, and both chimeric genes were located in the polyhedrin region.
FIG. 2.
Expression of glycosyltransferase activities by AcSWT-1. Sf9 cells were infected with AcMPNV (wild type [WT]), AcP(+)IE1HRGalT (GalT), AcP(+)IE1α26ST (ST), or various AcSWT-1 clones (indicated by their clone numbers [cl 1, 3, 18, or 21]). Cell lysates were prepared at 24 h postinfection and assayed in duplicate for glycosyltransferase activities, which were reported as the average cpm of tritiated galactose or sialic acid transferred to the acceptor substrate/0.25 × 106 cells/h. (A) β1,4GalT assays. (B) α2,6SiaT assays.
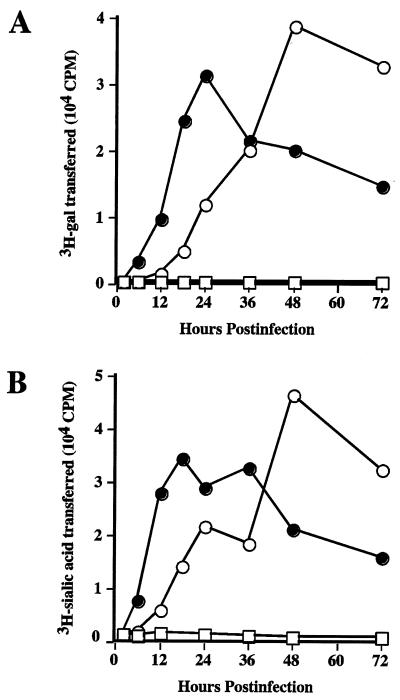
Subsequently, Sf9 cells were infected with AcSWT-1 (clone 18 [cl 18]) at a multiplicity of infection of 2 PFU per cell, extracts were prepared at various times after infection, and enzyme assays were performed to examine the time course of β1,4-GalT and α2,6-SiaT expression (Fig. 3). Both activities peaked at about 48 h postinfection, which was slightly later than we had observed in previous studies of foreign gene expression by immediate-early baculovirus vectors (12, 13). Nonetheless, AcSWT-1 produced active β1,4-GalT and α2,6-SiaT starting at about 12 h after infection, and the levels of both activities remained high until at least 72 h postinfection. Thus, both activities were available before and during the time of infection when they would be needed to process foreign glycoproteins expressed under the control of baculovirus late or very late promoters, including the polyhedrin promoter.
FIG. 3.
Time course of glycosyltransferase expression during AcSWT-1 and AcSWST-2c infection. Sf9 cells were infected with AcMNPV (squares), AcSWT-1 (open circles), or AcSWT-2c (solid circles). Cell lysates were prepared from equivalent numbers of cells at various times after infection, and each was assayed in duplicate for glycosyltransferase activities, which were reported as the average cpm of tritiated galactose or sialic acid transferred to the acceptor substrate/0.25 × 106 cells/h. (A) β1,4GalT assays. (B) α2,6SiaT assays.
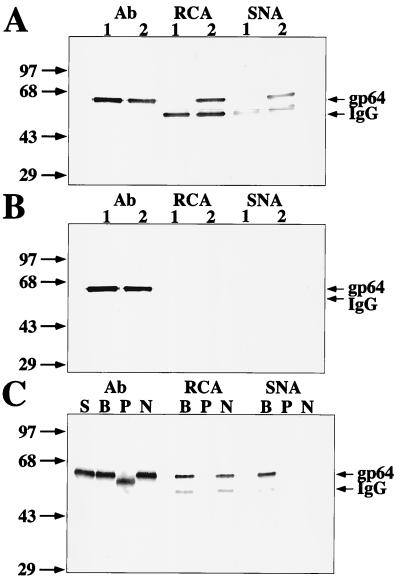
The major structural glycoprotein of AcMNPV is a late gene product called GP64. The N-glycans on GP64 produced by various lepidopteran insect cell lines contain no detectable galactose or sialic acids (11). However, GP64 can acquire galactosylated and terminally sialylated N-glycans when produced by COS cells, which have the requisite glycosyltransferase activities (11). Thus, GP64 was a convenient model that could be used to assess the ability of AcSWT-1-infected insect cells to sialylate a foreign glycoprotein. GP64 was extracted and immunoprecipitated from partially purified budded virus progeny from Sf9 cells infected with wild-type AcMNPV or AcSWT-1 and analyzed by lectin blotting assays, as described previously (3, 8). The results showed that SNA, which is a lectin that specifically recognizes terminal sialic acids, bound strongly to the GP64 produced by AcSWT-1-infected cells, but not to the same protein produced by the wild-type virus (Fig. 4A). Preincubation with a competing sugar blocked or greatly reduced SNA binding to all proteins, including the immunoglobulin G (IgG) heavy-chain internal controls (Fig. 4B), indicating that SNA binding was carbohydrate specific. In addition, pretreatment of the GP64 isolated from AcSWT-1-infected Sf9 cells with either glycoN-sidase F (PNGase-F), which removes N-glycans, or Arthrobacter urefaciens neuraminidase, which removes terminal sialic acid residues, precluded SNA binding (Fig. 4C), confirming that the lectin blotting assay was carbohydrate specific. These results demonstrated that, unlike AcMNPV-infected cells, AcSWT-1-infected Sf9 cells can produce a terminally sialylated form of GP64. Thus, the two mammalian glycosyltransferases encoded by AcSWT-1 are necessary and sufficient for sialylation of a foreign glycoprotein in baculovirus-infected Sf9 cells under the conditions described in this study.
FIG. 4.
Lectin blotting analysis of GP64 produced by various baculoviruses. GP64 was isolated from the progeny budded virus produced by AcMNPV (lanes 1)- or AcSWT-1 (lanes 2)-infected Sf9 cells, and equivalent samples were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to Immobilon filters. The filters were probed with rabbit anti-GP64 antibody (Ab), RCA, or SNA. (A) Lectin blots done in the absence of competing sugars. (B) Lectin blots done in the presence of competing sugars. (C) Lectin blots of GP64 from AcSWT-1-infected Sf9 cells after pretreatment with nothing (S), buffer alone (B), PNGase-F (P), or neuraminidase (N). The arrows on the right indicate the positions of GP64 and the IgG heavy chain. The arrows on the left mark the positions of protein standards, labeled according to their molecular masses in kilodaltons.
The ability of AcSWT-1 to produce a sialylated glycoprotein in Sf9 cells was somewhat surprising. AcSWT-1 encodes mammalian β1,4GalT and α2,6SiaT, both of which are type II membrane glycoproteins of the Golgi complex. These enzymes transfer galactose and sialic acid from UDP-galactose and CMP-sialic acid, respectively, to acceptor glycoproteins in the lumen of the Golgi complex (29). To perform these functions, each enzyme had to achieve its proper intracellular distribution and spatial positioning with respect to the other N-glycan processing enzymes within the Sf9 Golgi complex. This capability was not entirely unexpected, in light of past evidence that recombinant proteins are usually localized to their proper intracellular locations when expressed in the baculovirus-insect cell system (10, 17, 22). However, we made no attempt to engineer additional capabilities into this system, such as the ability to synthesize and transport CMP-sialic acid, which is also required for glycoprotein sialylation. Thus, the ability of AcSWT-1 to produce a sialylated foreign glycoprotein during infection of Sf9 cells implies that these cells were able to produce CMP-sialic acid and transport it into the lumen of the Golgi complex, where it was used as the donor substrate for GP64 sialylation by rat α2,6SiaT. This finding suggests that insect cells have more extensive infrastructure for glycoprotein sialylation than is generally recognized. The presence of this infrastructure was unexpected from most structural data, which indicate that glycoproteins produced by the baculovirus-insect cell system almost never acquire terminally sialylated N-glycans (2, 10, 18, 19). In addition, a direct attempt to detect CMP-sialic acid in Sf9 cells provided only negative results (9). The precise mechanisms by which Sf9 cells produce and transport the CMP-sialic acid required for glycoprotein sialylation by the α2,6SiaT encoded by AcSWT-1 are currently under investigation by our group.
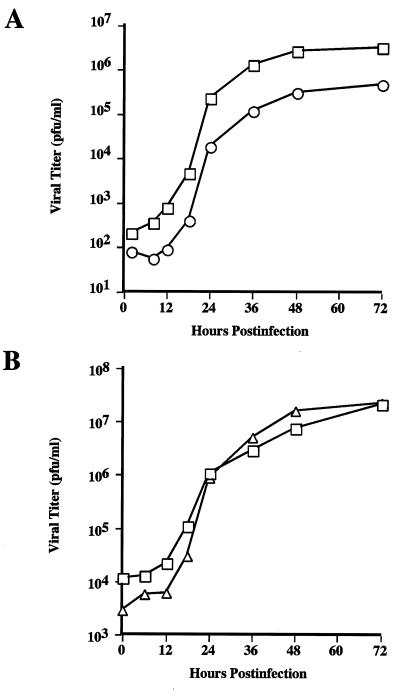
The ability of AcSWT-1 to provide glycoprotein sialylation indicated that it would be a useful tool for recombinant glycoprotein production. Thus, we needed to evaluate the growth properties of this virus and determine if it had a wild-type growth profile. We suspected that AcSWT-1 might be slightly defective, because it produced smaller plaques and working stocks of this virus all had relatively lower titers than those of other baculoviruses produced in our laboratory. This suspicion was confirmed by the results of one-step growth experiments, which demonstrated that AcSWT-1 produced about 1 order of magnitude less infectious budded virus progeny than the wild type (Fig. 5A). A detailed analysis of the genomic structure of AcSWT-1 revealed several possible reasons for this defect.
FIG. 5.
One-step growth curves. Triplicate cultures of Sf9 cells were infected with AcMNPV (squares), AcSWT-1 (circles), or AcSWT-2c (triangles) at a multiplicity of infection of 3 PFU per cell. Samples were taken from each culture at various times after infection, the triplicates were pooled, and infectious progeny were measured with a standard baculovirus plaque assay. (A) AcMNPV versus AcSWT-1. (B) AcMNPV versus AcSWT-2c.
The most obvious potential problem was that the p24 gene was partially deleted from AcSWT-1 (Fig. 1A). Because the p24 gene is not highly conserved among baculoviruses and is functionally inactivated by a transposable element in some strains of AcMNPV (24), we considered it to be nonessential. However, there was no formal proof of this, and because p24 is a minor viral nucleocapsid protein (33), it was possible that the p24 deletion could account for the lower yields of infectious budded virus produced by AcSWT-1. This possibility was addressed in two different ways. First, we produced a second baculovirus expression vector, AcSWT-2, which is identical to AcSWT-1 in all respects, except it has an intact p24 gene (Fig. 1B). Second, we isolated AcCtlHB64Δp24, which is a recombinant baculovirus that has a p24 deletion, but has no other alterations in the p24-gp64 region and no mammalian glycosyltransferase genes. One-step growth experiments showed that AcSWT-1 and AcSWT-2 both produced about 1 order of magnitude less progeny, while AcCtlHB64Δp24 produced wild-type levels of infectious budded virus progeny (data not shown). Together, these results showed that restoration of the p24 gene in AcSWT-1 did not rescue the slight growth defect, and deletion of the p24 gene from AcMNPV did not produce this defect. These results demonstrate that the p24 deletion is not responsible for this growth defect and formally demonstrate that the p24 gene is not essential for AcMNPV replication in Sf9 cells in vitro.
Another possible explanation for the defect in AcSWT-1 was its ability to express enzymatically active glycosyltransferases during infection. As demonstrated above, glycosyltransferase expression by AcSWT-1 led to differential glycosylation of GP64 and, presumably, other viral and cellular glycoproteins produced during AcSWT-1 infection. GP64 has been implicated as a baculovirus attachment protein (7, 14) and is required for baculovirus penetration (20, 31) and efficient release of budded virus from infected cells (21). Thus, there were many reasons to think that differential glycosylation of GP64 might adversely influence infectious budded virus progeny production by AcSWT-1. Alternatively, this defect might reflect the position of the ie1-hr5-glycosyltransferase cassette immediately upstream of the gp64 gene and/or the use of the p6.9 promoter to drive gp64 expression in AcSWT-1. Either of these latter two factors could subtly influence expression of the gp64 gene, which could decrease infectious budded virus progeny production by this virus. These possibilities were addressed, in part, by producing a third baculovirus expression vector, AcSWT-2c, in which the ie1-hr5-glycosyltransferase cassette was inserted within the v-cath gene instead of the p24-gp64 region and the rest of the viral genome is wild type (Fig. 1C). One-step growth experiments showed that AcSWT-2c and wild-type AcMNPV had very similar growth curves (Fig. 5B). Thus, expression of the glycosyltransferase activities was not responsible for the slight defect in infectious budded virus progeny production by AcSWT-1. We concluded that this defect might reflect the position of the ie1-hr5-glycosyltransferase cassette or the use of the basic protein promoter to express the gp64 gene in those viruses. However, considering the wild-type growth phenotype of AcSWT-2c, we made no further effort to determine the reason for the defect in AcSWT-1 and focused the remainder of our efforts on characterizing AcSWT-2c.
Glycosyltransferase expression was first detected in AcSWT-2c-infected Sf9 cells at 8 h postinfection and was sustained at high levels until at least 72 h postinfection (Fig. 3). Thus, the time course of β1,4Gal and α2,6SiaT expression by AcSWT-2c was clearly different from that observed with AcSWT-1, and in this regard, AcSWT-2c was identical to other immediate-early baculovirus expression vectors characterized in previous studies (12, 13). Most importantly, AcSWT-2c produced a galactosylated and sialylated form of GP64 during infection of Sf9 cells, as demonstrated by controlled lectin blotting assays identical to those shown in Fig. 4 for AcSWT-1 (data not shown). Therefore, AcSWT-2c is the most useful new baculovirus expression vector described in this study, because it can provide glycoprotein sialylation and has in vitro growth properties similar to those of wild-type AcMNPV. This virus should be useful to any investigator interested in producing terminally sialylated recombinant glycoproteins in the baculovirus-insect cell expression system.
Acknowledgments
We thank Carla Weinkauf and Jason Hollister for constructing some of the plasmids used for this study, as well as Joel and Nancy Shaper (Johns Hopkins University) and Jim Paulson (The Scripps Research Institute) for contributing the mammalian glycosyltransferase cDNAs. We thank HyClone for donating Sfx-Insect serum-free medium and Invitrogen for donating PCR-TOPO cloning kits.
This work was supported by NIH grant GM49734 and NSF grants BES-9814157 and BES-9818001.
REFERENCES
- 1.Altmann F, Kornfeld G, Dalik T, Staudacher E, Glossl J. Processing of asparagine-linked oligosaccharides in insect cells. N-acetylglucosaminyltransferase I and II activities in cultured lepidopteran cells. Glycobiology. 1993;3:619–625. doi: 10.1093/glycob/3.6.619. [DOI] [PubMed] [Google Scholar]
- 2.Altmann F, Staudacher E, Wilson I B, Marz L. Insect cells as hosts for the expression of recombinant glycoproteins. Glycoconj J. 1999;16:109–23. doi: 10.1023/a:1026488408951. [DOI] [PubMed] [Google Scholar]
- 3.Breitbach, K., and D. L. Jarvis. Improved glycosylation of a foreign protein by Tn-5B1–4 cells engineered to express mammalian glycosyltransferase genes. Biotechnol. Bioeng., in press. [DOI] [PMC free article] [PubMed]
- 4.Butters T D, Hughes R C, Vischer P. Steps in the biosynthesis of mosquito cell membrane glycoproteins and the effects of tunicamycin. Biochim Biophys Acta. 1981;640:672–686. doi: 10.1016/0005-2736(81)90097-3. [DOI] [PubMed] [Google Scholar]
- 5.Guarino L A, Gonzalez M A, Summers M D. Complete sequence and enhancer function of the homologous DNA regions of Autographa californica nuclear polyhedrosis virus. J Virol. 1986;60:224–229. doi: 10.1128/jvi.60.1.224-229.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guarino L A, Summers M D. Nucleotide sequence and temporal expression of a baculovirus regulatory gene. J Virol. 1987;61:2091–2099. doi: 10.1128/jvi.61.7.2091-2099.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hefferon K L, Oomens A G, Monsma S A, Finnerty C M, Blissard G W. Host cell receptor binding by baculovirus GP64 and kinetics of virion entry. Virology. 1999;258:455–468. doi: 10.1006/viro.1999.9758. [DOI] [PubMed] [Google Scholar]
- 8.Hollister J, Jarvis D L. Engineering lepidopteran insect cells for sialoglycoprotein production by genetic transformation with mammalian β1,4-galactosyltransferase and a2,6-sialyltransferase genes. Glycobiology. 2001;11:1–9. doi: 10.1093/glycob/11.1.1. [DOI] [PubMed] [Google Scholar]
- 9.Hooker A D, Green N H, Baines A J, Bull A T, Jenkins N, Strange P G, James D C. Constraints on the transport and glycosylation of recombinant IFN-gamma in Chinese hamster ovary and insect cells. Biotechnol Bioeng. 1999;63:559–572. doi: 10.1002/(sici)1097-0290(19990605)63:5<559::aid-bit6>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 10.Jarvis D L. Baculovirus expression vectors. In: Miller L K, editor. The baculoviruses. New York, N.Y: Plenum Press; 1997. pp. 389–431. [Google Scholar]
- 11.Jarvis D L, Finn E E. Biochemical analysis of the N-glycosylation pathway in baculovirus-infected lepidopteran insect cells. Virology. 1995;212:500–511. doi: 10.1006/viro.1995.1508. [DOI] [PubMed] [Google Scholar]
- 12.Jarvis D L, Finn E E. Modifying the insect cell N-glycosylation pathway with immediate early baculovirus expression vectors. Nat Biotechnol. 1996;14:1288–1292. doi: 10.1038/nbt1096-1288. [DOI] [PubMed] [Google Scholar]
- 13.Jarvis D L, Weinkauf C, Guarino L A. Immediate early baculovirus vectors for foreign gene expression in transformed or infected insect cells. Prot Expr Purif. 1996;8:191–203. doi: 10.1006/prep.1996.0092. [DOI] [PubMed] [Google Scholar]
- 14.Jarvis D L, Wills L, Burow G, Bohlmeyer D A. Mutational analysis of the N-linked glycans on Autographa californica nucleopolyhedrovirus gp64. J Virol. 1998;72:9459–9469. doi: 10.1128/jvi.72.12.9459-9469.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jenkins N, Curling E M A. Glycosylation of recombinant proteins: problems and prospects. Enzyme Microb Technol. 1994;16:354–364. doi: 10.1016/0141-0229(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 16.Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- 17.Luckow V L, Summers M D. Trends in the development of baculovirus expression vectors. Bio/Technology. 1988;6:47–55. [Google Scholar]
- 18.Marchal I, Jarvis D L, Cacan R, Verbert A. Glycoproteins from insect cells: sialylated or not? Biol Chem. 2001;382:151–159. doi: 10.1515/BC.2001.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marz L, Altmann F, Staudacher E, Kubelka V. Protein glycosylation in insects. In: Montreuil J, Vliegenthart J F G, Schachter H, editors. Glycoproteins. 29a. Amsterdam, The Netherlands: Elsevier; 1995. pp. 543–563. [Google Scholar]
- 20.Monsma S A, Oomens A G P, Blissard G W. The GP64 envelope fusion protein is an essential baculovirus protein required for cell-to-cell transmission of infection. J Virol. 1996;70:4607–4616. doi: 10.1128/jvi.70.7.4607-4616.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oomens A G, Blissard G W. Requirement for GP64 to drive efficient budding of Autographa californica multicapsid nucleopolyhedrovirus. Virology. 1999;254:297–314. doi: 10.1006/viro.1998.9523. [DOI] [PubMed] [Google Scholar]
- 22.O'Reilly D R, Miller L K, Luckow V A. Baculovirus expression vectors. W. H. New York, N.Y: Freeman and Company; 1992. [Google Scholar]
- 23.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 24.Schetter C, Oellig C, Doerfler W. An insertion of insect cell DNA in the 81-map-unit segment of Autographa californica nuclear polyhedrosis virus DNA. J Virol. 1990;64:1844–1850. doi: 10.1128/jvi.64.4.1844-1850.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seo, N.-S., J. Hollister, and D. L. Jarvis. Mammalian glycosyltransferase expression allows sialoglycoprotein production by baculovirus-infected insect cells. Prot. Expr. Purif., in press. [DOI] [PubMed]
- 26.Stollar V, Stollar B D, Koo R, Harrap K A, Schlesinger R W. Sialic acid contents of sindbis virus from vertebrate and mosquito cells. Equivalence of biological and immunological viral properties. Virology. 1976;69:104–115. doi: 10.1016/0042-6822(76)90198-7. [DOI] [PubMed] [Google Scholar]
- 27.Summers M D, Smith G E. A manual of methods for baculovirus vectors and insect cell culture procedures. Tex Agric Exp Stn Bull. 1987;1555:1–57. [Google Scholar]
- 28.van Die I, van Tetering A, Bakker H D, van den Eijnden H, Joziasse D H. Glycosylation in lepidopteran insect cells: identification of a β1,4-N-acetylgalactosaminyltransferase involved in the synthesis of complex-type oligosaccharide chains. Glycobiology. 1996;6:157–164. doi: 10.1093/glycob/6.2.157. [DOI] [PubMed] [Google Scholar]
- 29.Varki A, Cummings R, Esko J, Freeze H, Hart G, Marth J. Essentials of glycobiology. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1999. [PubMed] [Google Scholar]
- 30.Vaughn J L, Goodwin R H, Thompkins G J, McCawley P. The establishment of two insect cell lines from the insect Spodoptera frugiperda (Lepidoptera:Noctuidae) In Vitro. 1977;13:213–217. doi: 10.1007/BF02615077. [DOI] [PubMed] [Google Scholar]
- 31.Volkman L E, Goldsmith P A. Mechanism of neutralization of budded Autographa californica nuclear polyhedrosis virus by a monoclonal antibody: inhibition of entry by adsorptive endocytosis. Virology. 1985;143:185–195. doi: 10.1016/0042-6822(85)90107-2. [DOI] [PubMed] [Google Scholar]
- 32.Weigel P H. Galactosyl and N-acetylgalactosaminyl homeostasis: a function for mammalian asialoglycoprotein receptors. Bioessays. 1994;16:519–524. doi: 10.1002/bies.950160713. [DOI] [PubMed] [Google Scholar]
- 33.Wolgamot G M, Gross C H, Russell R L, Rohrmann G F. Immunocytochemical characterization of p24, a baculovirus capsid-associated protein. J Gen Virol. 1993;74:103–107. doi: 10.1099/0022-1317-74-1-103. [DOI] [PubMed] [Google Scholar]