Abstract
The Epstein-Barr virus (EBV) immediate-early protein BRLF1 is a transcriptional activator that mediates the switch from latent to lytic viral replication. Many transcriptional activators function, in part, due to an interaction with histone acetylases, such as CREB-binding protein (CBP). Here we demonstrate that BRLF1 interacts with the amino and carboxy termini of CBP and that multiple domains of the BRLF1 protein are necessary for this interaction. Furthermore, we show that the interaction between BRLF1 and CBP is important for BRLF1-induced activation of the early lytic EBV gene SM in Raji cells.
Epstein-Barr virus (EBV) is a human herpesvirus that infects the majority of the world's population. EBV is the causative agent of infectious mononucleosis, and it is associated with a variety of disorders, including nasopharyngeal carcinoma and Burkitt's lymphoma (31, 41). EBV infects both epithelial cells and B lymphocytes. Infection of epithelial cells results in lytic viral replication, with subsequent release of new viral particles (41). Infection of B lymphocytes usually results in viral latency, with only a small fraction of the viral genes being expressed (41). Occasionally, latent EBV infection can be reactivated to enter the viral lytic cycle.
Expression of either one of the EBV immediate-early (IE) proteins, BRLF1 and BZLF1, is sufficient to induce the switch from latency to lytic replication in the infected cell (9, 11, 39, 42, 47, 50, 51). BZLF1 and BRLF1 are both transcriptional activators (12, 18, 21, 22–26, 30, 36, 38, 40, 46). Each IE protein initially activates transcription of the other IE gene (3, 16, 34, 51), and both IE proteins together are required for induction of the full complement of early viral genes necessary for lytic viral replication (3, 16, 40).
Many transcriptional activators function through an interaction with CREB-binding protein (CBP). CBP is a transcriptional activator, regulating transcription via its histone acetylase activity (6). Upon acetylation by CBP, interactions between histones and DNA weaken, thereby altering the conformation and stability of nucleosome core particles and enhancing transcription (32, 37, 49). CBP and its related family member p300 have been shown to interact with a variety of cellular and viral proteins and to enhance their transactivation functions. Cellular transcription factors with which CBP interacts include p53, NF-κB, p65, c-Fos, c-Jun, and c-Myb, among others (5, 7, 10, 13, 19, 33, 44, 45). Several key viral regulatory factors also interact with CBP, including adenovirus E1A, simian virus 40 T antigen, human immunodeficiency virus type 1 Tat, cytomegalovirus IE2-86, and herpes simplex virus VP16 (4, 8, 14, 15, 27, 35, 43, 48).
This laboratory (2) and others (53) have shown that the EBV IE protein BZLF1 interacts with CBP and that this interaction is important for efficient disruption of viral latency by BZLF1. However, BRLF1 expression can also induce lytic EBV infection but must initially activate BZLF1 transcription to do so (51). In addition, certain EBV lytic promoters have been shown to be primarily BRLF1, rather than BZLF1, responsive (16, 40). Therefore, particularly in situations where BRLF1 expression precedes BZLF1 expression, BRLF1 association with CBP could potentially enhance the ability of BRLF1 to induce viral reactivation in the EBV-infected cell.
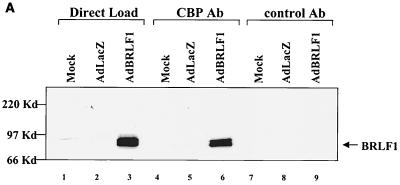
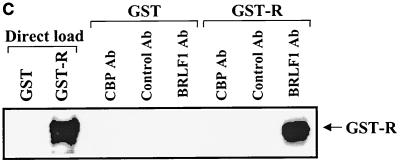
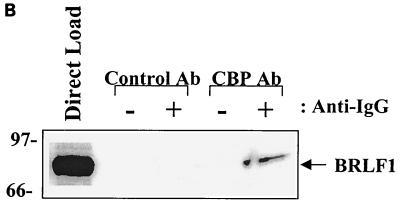
To determine if BRLF1 interacts with CBP, HeLa cells were mock infected or infected with adenovirus vectors (constructed as previously described [50]) expressing the BRLF1 (AdBRLF1) or beta-galactosidase (AdLacZ) genes at a multiplicity of infection of 50 and were harvested at 24 h postinfection. Cell extracts were immunoprecipitated with an antibody directed against CBP (A-22; Santa Cruz) or equal amounts of a control rabbit antibody (anti-ets 1-2, C-275; Santa Cruz). Complexes were separated on a polyacrylamide gel and immunoblotted for the presence of BRLF1 (using a 1:100 dilution of anti-BRLF1 antibody; Argene). As indicated in Fig. 1A, BRLF1 was coimmunoprecipitated with CBP (lane 6) but not with the control antibody (lane 9). Similar results were obtained using extracts from lytically infected, EBV-positive Burkitt lymphoma cells (Akata) (Fig. 1B). The CBP antibody did not immunoprecipitate a glutathione S-transferase (GST)-BRLF1 fusion protein (Fig. 1C), confirming that it does not cross-react with the BRLF1 protein. These results are consistent with a direct interaction between BRLF1 and CBP in the host cell.
FIG. 1.
BRLF1 interacts with CBP in vivo. (A) HeLa cells were mock infected or infected with adenovirus vectors expressing beta-galactosidase (AdLacZ) or BRLF1 (AdBRLF1). Anti-CBP antibody (Ab) or a control rabbit antibody were used to coimmunoprecipitate BRLF1 (300 μg; lanes 4 to 9). Immunocomplexes were electrophoresed on a 7.5% polyacrylamide gel, transferred to nitrocellulose, and immunoblotted for BRLF1. Proteins were visualized by chemiluminescence and autoradiography. Direct loads (30 μg; lanes 1 to 3) confirmed the presence of BRLF1 in the HeLa cell extracts. (B) Coimmunoprecipitation experiments (as described for panel A) were performed using extracts from Akata cells with or without anti-immunoglobulin G (anti-IgG) treatment (100 μg/ml; Sigma) for 4 h, which induces lytic EBV infection. The direct load lane is lysate from Akata cells treated with an anti-IgG antibody. (C) The same CBP and control rabbit antibodies used for panels A and B as well as a BRLF1-reactive antibody were used to immunoprecipitate GST or GST-BRLF1 fusion protein (GST-R), followed by immunoblot analysis with a BRLF1 antibody.
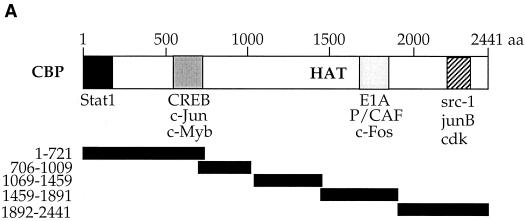
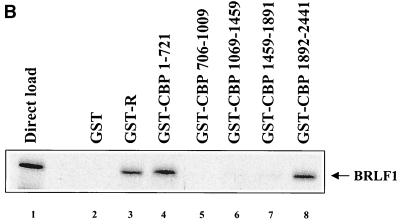
To determine the domain(s) of CBP necessary for interaction with BRLF1, mapping experiments were performed using a series of GST-CBP fusion constructs (a gift from Michael Rosenfeld [29]) as shown in the schematic diagram in Fig. 2A. GST-CBP constructs, GST-BRLF1 (a gift from Alain Sergeant [21]), and a GST control were incubated with in vitro-translated 35S-labeled BRLF1, and glutathione bead spin-down assays were subsequently performed as previously described (2). As expected, in vitro-translated BRLF1 dimerized with GST-BRLF1 but did not interact with control GST (Fig. 2B, lanes 2 and 3). BRLF1 associated with GST-CBP 1-721 and GST-CBP 1892-2441 (lanes 4 and 8) but did not interact with other GST-CBP constructs (lanes 5 to 7). This indicates that both the amino- and carboxy-terminal regions of CBP are independently capable of interaction with BRLF1.
FIG. 2.
BRLF1 interacts with the amino- and carboxy-terminal regions of CBP in vitro. (A) Schematic diagram of GST-CBP fusion constructs. Regions implicated in CBP-protein interactions are indicated. The five CBP fragments fused in frame with GST are indicated below the GST-CBP construct (29). HAT, histone acetyltransferase. (B) Five microliters of [35S]methionine-labeled in vitro-translated BRLF1 was incubated with GST alone (lane 2), GST-BRLF1 (lane 3), or GST-CBP constructs (lanes 4 to 8) bound to glutathione beads. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and visualized by autoradiography. A direct load of in vitro-translated BRLF1 (2.5 μl) is shown in lane 1.
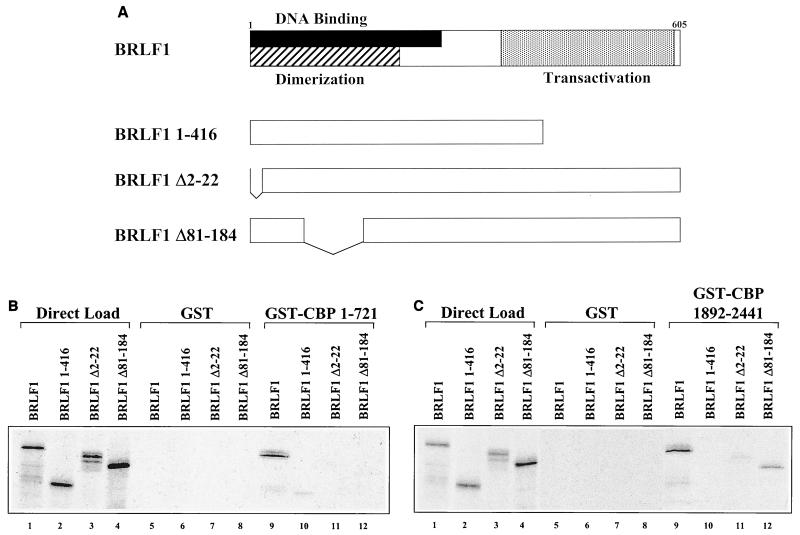
To map the region(s) of BRLF1 necessary for interaction with CBP a series of in vitro-translated BRLF1 mutants (a gift from Alain Sergeant [36]) were examined for the ability to interact with the GST constructs containing either the amino-terminal or carboxy-terminal portions of CBP. A schematic diagram (Fig. 3A) shows the functional domains of full-length BRLF1 protein and the BRLF1 mutants used in these studies. The BRLF1 1-416 mutant is missing most of the transactivator domain (located in the carboxy terminus of BRLF1), whereas both BRLF1 Δ2-22 (amino acids 2 through 22 deleted) and BRLF1 Δ81-184 (amino acids 81 through 184 deleted) contain deletions within the DNA binding and dimerization domains (located in the amino terminus of BRLF1).
FIG. 3.
Multiple domains in the BRLF1 protein are required for efficient interaction with CBP. (A) Schematic diagram of BRLF1 and BRLF1 mutants used in the mapping study. The DNA binding, dimerization, and transactivation domains are indicated. (B) Five microliters of [35S] methionine-labeled in vitro-translated BRLF1 or BRLF1 mutants was incubated with GST alone (lanes 5 to 8) or with GST-CBP 1-721 (lanes 9 to 12) bound to glutathione beads. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and visualized by autoradiography. Direct loads of in vitro-translated protein (2.5 μl) were also included (lanes 1 to 4). (C) Five microliters of in vitro-translated BRLF1 or BRLF1 mutants was incubated with GST alone (lanes 5 to 8) or with GST-CBP 1892-2441 (lanes 9 to 12) bound to glutathione beads. Direct loads of in vitro-translated protein (2.5 μl) were also included (lanes 1 to 4).
In experiments performed using the amino-terminal CBP construct (GST-CBP 1-721), only the full-length BRLF1 protein interacted efficiently with CBP (Fig. 3B, lanes 9 through 12). Thus, the DNA binding, dimerization, and transactivator domains of BRLF1 are all required for efficient interaction with the amino terminus of CBP. In experiments performed with the carboxy-terminal CBP construct, the BRLF1 transactivator domain as well as amino acids 2 through 22 were shown to be necessary for efficient interaction (Fig. 3C, lanes 9 through 11). In contrast to the results seen with the amino terminus of CBP, the BRLF1 Δ81-184 mutant still interacted with GST-CBP 1982-2441 (Fig. 3C, lane 12), although somewhat less efficiently than the intact BRLF1 protein. These results indicate that multiple domains of the BRLF1 protein are necessary for efficient interaction with both the amino and carboxy termini of GST-CBP.
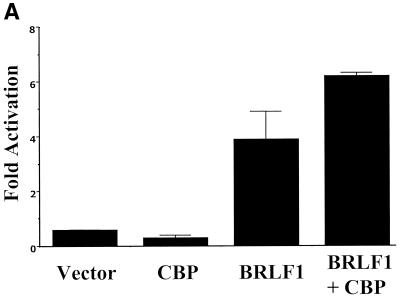
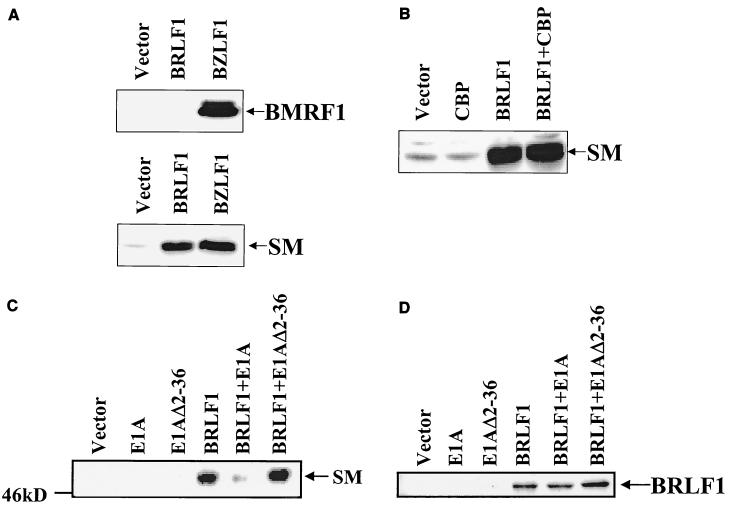
To examine the functional significance of the interaction between BRLF1 and CBP, experiments were performed to determine whether CBP affects BRLF1 transactivation in a transient-reporter-gene assay. HeLa cells were transfected with a chloramphenicol acetyltransferase (CAT) reporter plasmid (S-CAT) (30) driven by the EBV early SM gene promoter in the presence or absence of cotransfected expression plasmids for CBP (a gift from Michael Rosenfeld [29]) alone, BRLF1 alone, or BRLF1 and CBP together. Cells were harvested at 2 days posttransfection and analyzed for CAT activity as previously described (20). CBP enhanced BRLF1-mediated activation of the EBV early gene promoter, SM, in HeLa cells (Fig. 4A).
FIG. 4.
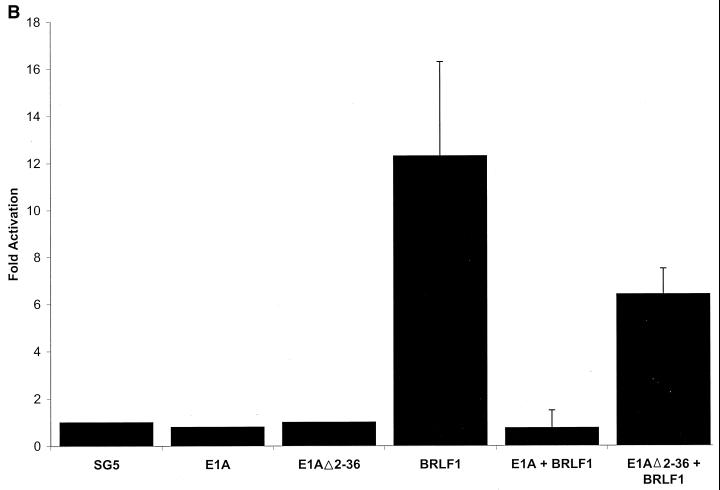
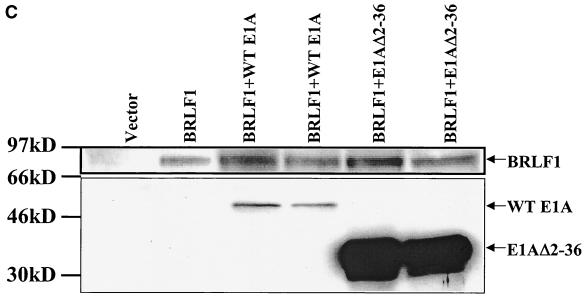
BRLF1-CBP interaction affects BRLF1 transactivator function. (A) Transient reporter assays were performed in which HeLa cells were transfected with 4 μg of promoter construct (S-CAT, containing SM gene promoter sequences), 0.3 μg of BRLF1 expression vector (CMV-RIE) (26), or control vector (pHD1013), with or without 1 μg of the CMV-CBP expression vector (29). CAT activity was determined as previously described (20). The results represent data from two separate experiments. (B) The S-CAT plasmid (1 μg) was transfected into HeLa cells with either vector DNA (8.5 μg) (SG5), wild-type E1A vector (8 μg), an E1A mutant (Δ2-36) which is unable to bind CBP (8 μg), the BRLF1 expression vector (pRTS15; a gift from Diane Hayward) (0.5 μg) and control vector DNA (8 μg), the BRLF1 expression vector (0.5 μg) and wild-type E1A (8 μg), or the BRLF1 expression vector (0.5 μg) and the E1A mutant (Δ2-36) (8 μg). The E1A vectors were gifts from David Livingston (8). (C) Immunoblot analysis was performed using extracts from the experiment shown in panel 4B to assess the level of transfected wild-type versus mutant E1A proteins and of BRLF1.
To further confirm that an interaction between CBP and BRLF1 is required for efficient BRLF1 transactivator function, we examined the effect of the adenovirus protein E1A. It has previously been shown that the amino-terminal portion of E1A interacts very strongly with CBP and competitively inhibits the interaction of CBP with other transcription factors (5, 8). As shown in Fig. 4B, wild-type E1A completely inhibited BRLF1 transactivation of the SM reporter construct, whereas an E1A mutant that is unable to bind CBP (E1AΔ2-36) (8) had reduced inhibition. To ensure that the mutant E1A and BRLF1 proteins were expressed at an adequate level in these experiments, immunoblot analysis was performed on the same extracts used in the CAT assays in Fig. 4B. The mutant E1A protein was expressed at a higher level than the wild-type E1A, and BRLF1 expression was not inhibited by wild-type E1A (Fig. 4C).
The major role of BRLF1 in the EBV life cycle is to mediate the switch between latent and lytic viral replication. However, BZLF1 and BRLF1 activate each other's promoters in most EBV-positive cell lines, and both the BZLF1 and BRLF1 transcriptional functions are required for activation of at least a portion of the viral early genes, such as BMRF1 (3, 40). Because the BZLF1 transactivator function has already been shown to require CBP (2, 53), it is difficult to examine the specific effect of CBP on BRLF1 (versus BZLF1) transactivator function in most EBV-positive cell lines. However, even though we and others have shown that BRLF1 cannot activate BZLF1 transcription in the Raji cell line (40, 51), the Miller group recently demonstrated that BRLF1 can activate the SM (but not BMRF1) early promoter in Raji cells (40).
We therefore examined the effect of CBP and E1A on the ability of BRLF1 to activate the early SM promoter from the endogenous EBV genome in Raji cells. As shown in Fig. 5A, only BZLF1 (but not BRLF1) induced expression of the early viral BMRF1 protein in Raji cells, whereas the BZLF1 and BRLF1 IE proteins induced equivalent expression of the early SM protein. Cotransfected CBP did not significantly enhance the ability of BRLF1 to induce expression of the SM early gene from the endogenous viral genome in Raji cells (Fig. 5B). Nevertheless, wild-type E1A, but not the mutant E1A (Δ2-36) defective in CBP binding, significantly inhibited the ability of BRLF1 to activate SM expression (Fig. 5C), while not significantly affecting the level of transfected BRLF1 protein (Fig. 5D). These results suggest that while the level of constitutive CBP expression in Raji cells is apparently already sufficient for maximal BRLF1 transactivator function, the interaction between BRLF1 and CBP is nevertheless essential.
FIG. 5.
BRLF1 interaction with CBP is important for activation of the early SM gene in Raji cells. (A) Latently infected, EBV-positive Raji cells were transfected with a control vector or expression vectors for BZLF1 or BRLF1. Cell extracts were harvested 1 day later and immunoblotted with antibodies directed against the BMRF1 or SM early EBV proteins. BMRF1 protein was detected using a monoclonal antibody (Capricorn; 1:100 dilution) and SM protein was detected using a rabbit polyclonal antibody (1:400 dilution) provided by Sankar Swaminathan. (B) Raji cells were transfected with 2 μg of the BRLF1 expression vector (pRTS15) or control vector (SG5) in the presence or absence of the CBP expression vector (5 μg) (keeping the total DNA level constant). Cell extracts were harvested at 24 h posttransfection and immunoblotted for SM early protein. (C) Raji cells were transfected with the BRLF1 expression vector (2 μg), control vector (2 μg), wild-type E1A (10 μg), or mutant E1A (Δ2-36) (10 μg), keeping the total DNA level constant. Cell extracts were harvested at 24 h posttransfection and immunoblotted for SM early protein. (D) The same extracts shown in panel C were immunoblotted using an antibody which recognizes BRLF1.
In this report we have demonstrated that BRLF1 directly interacts with the histone acetylase CBP and that this interaction is important for the ability of BRLF1 to activate transcription of at least one EBV early lytic gene, SM. BRLF1 induces lytic EBV infection in most (but not all) cell types (16, 39, 40, 50, 51) by transcriptionally activating lytic EBV genes through both direct binding and indirect mechanisms. The critical role of BRLF1 in lytic EBV infection was recently confirmed by the demonstration that an EBV mutant lacking BRLF1 is unable to undergo the lytic form of EBV infection (16).
The direct interaction between cellular and viral transcription factors with histone acetylases such as CBP and p300 is an increasingly well-recognized phenomenon. As shown in Fig. 2A, multiple different domains of CBP are bound by various transcription factors. In the case of BRLF1, we demonstrate in this report that BRLF1 can interact independently with both the amino and carboxy termini of CBP. Several other regulatory proteins, including CREB, NF-κB, c-Jun, and c-Myb, have been found to interact with the amino-terminal region of CBP (amino acids 1 to 721) (7, 10, 13, 19, 33). Viral proteins adenovirus E1A, simian virus 40 T antigen, and human immunodeficiency virus Tat, as well as cellular proteins Src-1, JunB, and Cdk, have likewise been shown to bind to the carboxy terminus of CBP (amino acids 1892 to 2441) (4, 14, 15, 17, 27, 29, 33, 35). Interaction with both the amino- and carboxy-terminal regions of CBP is not unique to BRLF1 in that the EBV BZLF1 protein and NF-κB (19, 53) can likewise bind to both the amino and carboxy termini of CBP. The ability of BRLF1 to interact with both the amino and carboxy termini of CBP may serve to strengthen the interaction between BRLF1 and CBP. In any event, the BRLF1-CBP interaction appears to be quite strong in that this interaction was easily observed in coimmunoprecipitation experiments in vivo, and BRLF1 was retained by the amino- and carboxy-terminal GST-CBP fusion proteins as efficiently as by the GST-BRLF1 fusion protein.
We demonstrate in this report that the carboxy terminus of BRLF1, which contains the transcriptional activation domain, is required for efficient interaction with both the amino and carboxy termini of CBP. However, as can be seen in Fig. 3, deletion of the BRLF1 transactivator domain, while completely abolishing interactions with the carboxy terminus of CBP, reduces but does not completely abolish interactions between BRLF1 and the amino terminus of CBP. The amino terminus of BRLF1, which contains the DNA binding and dimerization domains, is also required for efficient interaction with both the amino and carboxy termini of CBP. Nevertheless, there are also differences in the requirements for the BRLF1 amino-terminal domain in that deletion of BRLF1 amino acid residues 81 through 184 completely abolishes the interaction between BRLF1 and the amino terminus of CBP, while only reducing the efficiency of the interaction between BRLF1 and the carboxy terminus of CBP (Fig. 3).
The direct interaction between transcription factors and CBP is thought to enhance the function of these factors. In the case of BRLF1, it appears that the constitutive level of CBP in Raji cells is already sufficient for maximal BRLF1 transactivator function, since increasing the level of CBP did not augment BRLF1-dependent transactivation. Nevertheless, our finding that E1A inhibits BRLF1 transactivator function through a CBP binding-dependent mechanism suggests that BRLF1 does indeed require CBP to activate the SM promoter.
Histone acetylation of the BZLF1 IE EBV gene promoter was recently shown to be important during the reactivation of latent EBV (28). However, the mechanism by which BRLF1 enhances BZLF1 transcription remains somewhat obscure, since BRLF1 does not bind directly to either BZLF1 promoter (Zp and Rp) (21, 52) but instead induces BZLF1 transcription through an indirect mechanism involving activation of the stress mitogen-activated protein kinase pathways (1). The SM promoter is one of the few EBV promoters known to be directly bound by BRLF1 (22, 30, 38), and it thus remains possible that the ability of BRLF1 to activate a variety of other promoters through indirect mechanisms is not necessarily CBP dependent.
Interestingly, both EBV IE transactivators, BRLF1 and BZLF1, have now been shown to interact with CBP (2, 53; the present study). Similar to the effect of CBP on BRLF1, the interaction between BZLF1 and CBP likewise enhances BZLF1 transactivator activity and increases the ability of BZLF1 to induce the lytic form of EBV infection in latently infected cells (2, 53). CBP is thought to be limiting in cells, and we previously showed that BZLF1 is able to inhibit CREB transactivator function by interacting with CBP, thereby competing for limiting amounts of CBP (2). In the context of lytic EBV infection, BRLF1 (which appears to interact with CBP at least as strongly as BZLF1) and BZLF1 may thus regulate one another's function by competing for limiting quantities of CBP in the host cell.
Acknowledgments
This work was supported by Public Health Service grant RO1-CA58853 from the National Institutes of Health.
We thank the UNC Gene Therapy Center for construction of AdBRLF1 and AdLacZ, Amy Mauser for preparation of figures, Sankar Swaminathan for SM antibody, Alain Sergeant and Diane Hayward for BZLF1 and BRLF1 expression vectors, and Mary Paula Beckett for technical assistance.
REFERENCES
- 1.Adamson A L, Darr D, Holley-Guthrie E, Johnson R, Mauser A, Swenson J, Kenney S. Epstein-Barr virus immediate-early proteins BZLF1 and BRLF1 activate the ATF2 transcription factor by increasing levels of phosphorylated p38 and c-Jun N-terminal kinases. J Virol. 2000;74:1224–1233. doi: 10.1128/jvi.74.3.1224-1233.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adamson A L, Kenney S. The Epstein-Barr virus BZLF1 protein interacts physically and functionally with the histone acetylase CREB-binding protein. J Virol. 1999;73:6551–6558. doi: 10.1128/jvi.73.8.6551-6558.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adamson A L, Kenney S. Rescue of the Epstein-Barr virus BZLF1 mutant Z(S186A) early gene activation defect by the BRLF1 gene product. Virology. 1998;251:187–197. doi: 10.1006/viro.1998.9396. [DOI] [PubMed] [Google Scholar]
- 4.Arany Z, Newsome D, Oldread E, Livingston D, Eckner R. A family of transcriptional adaptor proteins targeted by E1A oncoprotein. Nature. 1995;374:81–84. doi: 10.1038/374081a0. [DOI] [PubMed] [Google Scholar]
- 5.Bannister A I, Kouzarides T. CBP-induced stimulation of c-fos activity is abrogated by E1A. EMBO J. 1995;14:4758–4762. doi: 10.1002/j.1460-2075.1995.tb00157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bannister A I, Kouzarides T. The CBP co-activator is a histone acetyltransferase. Nature. 1996;384:641–643. doi: 10.1038/384641a0. [DOI] [PubMed] [Google Scholar]
- 7.Bannister A J, Oehler T, Wilhelm D, Angel P, Kouzarides T. Stimulation of c-jun activity by CBP: c-jun residues Ser63/73 are required for CBP induced stimulation in vivo and CBP binding in vitro. Oncogene. 1995;11:2509–2514. [PubMed] [Google Scholar]
- 8.Bhattacharya S, Eckner R, Grossman S, Oldread E, Arany Z, D'Andrea A, Livingston D. Cooperation of Stat2 and p300/CBP in signaling induced by interferon-alpha. Nature. 1996;383:344–347. doi: 10.1038/383344a0. [DOI] [PubMed] [Google Scholar]
- 9.Chevallier-Greco A, Manet E, Chavrier P, Mosnier C, Dallie J, Sergeant A. Both Epstein-Barr virus (EBV)-encoded trans-acting factors, EB1 and EB2, are required to activate transcription from an EBV early promoter. EMBO J. 1986;5:3243–3249. doi: 10.1002/j.1460-2075.1986.tb04635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chrivia J C, Kwok R P, Lamb N, Hagiwara M, Montminy M R, Goodman R H. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- 11.Countryman J, Miller G. Activation of expression of latent Epstein-Barr virus after gene transfer with a small cloned fragment of heterogeneous viral DNA. Proc Natl Acad Sci USA. 1985;82:4085–4089. doi: 10.1073/pnas.82.12.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cox M, Leahy J, Hardwick J M. An enhancer within the divergent promoter of Epstein-Barr virus responds synergistically to the R and Z transactivators. J Virol. 1990;64:313–321. doi: 10.1128/jvi.64.1.313-321.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dai P, Akimaru H, Tanaka Y, Hou D K, Yasukawa T, Kanei-Ishii C, Takahashi T, Ishii S. CBP as a transcriptional coactivator of c-myb. Genes Dev. 1996;10:528–540. doi: 10.1101/gad.10.5.528. [DOI] [PubMed] [Google Scholar]
- 14.Dorsman J C, Teunisse A F, Zantema A, van der Eb A J. The adenovirus 12 E1A proteins can bind directly to proteins of the p300 transcription co-activator family, including the CREB-binding protein CBP and p300. J Gen Virol. 1997;78:423–426. doi: 10.1099/0022-1317-78-2-423. [DOI] [PubMed] [Google Scholar]
- 15.Eckner R, Ludlow J W, Lill N L, Oldread E, Arany Z, Modjtahedi N, DeCaprio J A, Livingston D M, Morgan J A. Association of p300 and CBP with simian virus 40 large T antigen. Mol Cell Biol. 1996;16:3454–3464. doi: 10.1128/mcb.16.7.3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feederle R, Kost M, Baumann M, Janz A, Drouet E, Hammerschmidt W, Delecluse H J. The Epstein-Barr virus lytic program is controlled by the cooperative functions of two transactivators. EMBO J. 2000;19:3080–3089. doi: 10.1093/emboj/19.12.3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Felzien L K, Farrell S, Betts J C, Mosavin R, Nabel G J. Specificity of cyclin E-cdk2, TFIIB, and E1A interactions with a common domain of the p300 coactivator. Mol Cell Biol. 1999;19:4241–4246. doi: 10.1128/mcb.19.6.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flemington E K, Lytle J P, Cayrol C, Borras A M, Speck S H. DNA-binding-defective mutants of the Epstein-Barr virus lytic switch activator Zta transactivate with altered specificities. Mol Cell Biol. 1994;14:3041–3052. doi: 10.1128/mcb.14.5.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerritsen M E, Williams A J, Neish A S, Moore S, Shi Y. CREB-binding protein/p300 are transcriptional coactivators of p65. Proc Natl Acad Sci USA. 1997;94:2927–2932. doi: 10.1073/pnas.94.7.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorman C, Moffat L, Howard B. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982;2:1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gruffat H, Sergeant A. Characterization of the DNA-binding site repertoire for the Epstein-Barr virus transcription factor R. Nucleic Acids Res. 1994;22:1172–1178. doi: 10.1093/nar/22.7.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gruffat H, Duran N, Buisson M, Wild F, Buckland R, Sergeant A. Characterization of an R-binding site mediating the R-induced activation of the Epstein-Barr virus BMLF1 promoter. J Virol. 1992;66:46–52. doi: 10.1128/jvi.66.1.46-52.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gutsch D, Marcu K B, Kenney S. The Epstein-Barr virus BRLF1 gene product transactivates the murine and human c-myc promoters. Mol Cell Biol. 1994;40:747–760. [PubMed] [Google Scholar]
- 24.Hardwick J M, Lieberman P M, Hayward S D. A new Epstein-Barr virus transactivator, R, induces expression of a cytoplasmic early antigen. J Virol. 1988;62:2274–2284. doi: 10.1128/jvi.62.7.2274-2284.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hardwick J M, Tse L, Applegren N, Nicholas J, Veliuona M A. The Epstein-Barr virus R transactivator (Rta) contains a complex, potent activation domain with properties different from those of VP16. J Virol. 1992;66:5500–5508. doi: 10.1128/jvi.66.9.5500-5508.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holley-Guthrie E A, Quinlivan E B, Mar E C, Kenney S. The Epstein-Barr virus (EBV) BMRF1 promoter for early antigen (EA-D) is regulated by the EBV transactivators, BRLF1 and BZLF1, in a cell-specific manner. J Virol. 1990;64:3753–3759. doi: 10.1128/jvi.64.8.3753-3759.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hottinger M O, Nabel G J. Interaction of human immunodeficiency virus type 1 Tat with the transcriptional coactivators p300 and CREB binding protein. J Virol. 1998;72:8252–8256. doi: 10.1128/jvi.72.10.8252-8256.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jenkins P E, Binne U K, Farrell P J. Histone acetylation and reactivation of Epstein-Barr virus from latency. J Virol. 2000;74:710–720. doi: 10.1128/jvi.74.2.710-720.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamei Y, Xu L, Heinzel T, Torchia J, Kurokawa R, Gloss B, Lin S C, Heyman R A, Rose D W, Glass C K, Rosenfeld M G. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell. 1996;85:403–414. doi: 10.1016/s0092-8674(00)81118-6. [DOI] [PubMed] [Google Scholar]
- 30.Kenney S, Holley-Guthrie E, Mar E C, Smith M. The Epstein-Barr virus BMLF1 promoter contains an enhancer element that is responsive to the BZLF1 and BRLF1 transactivators. J Virol. 1989;63:3878–3883. doi: 10.1128/jvi.63.9.3878-3883.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kieff E. Epstein-Barr virus and its replication. In: Fields B, Knipes D, Howley P M, Hirsch M, Chanock R, Melnick J, Monath T, Roizman B, Streib J E, editors. Fields virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2343–2395. [Google Scholar]
- 32.Lee D Y, Hayes J J, Pruss D, Wolffe A P. A positive role for histone acetylation in transcription factor access to nucleosomal DNA. Cell. 1993;72:73–84. doi: 10.1016/0092-8674(93)90051-q. [DOI] [PubMed] [Google Scholar]
- 33.Lee J S, See R H, Deng T, Shi Y. Adenovirus E1A downregulates c-jun- and junB-mediated transcription by targeting the coactivator p300. Mol Cell Biol. 1996;16:4312–4326. doi: 10.1128/mcb.16.8.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Roux F, Sergeant A, Corbo L. Epstein-Barr virus (EBV) EB1/Zta protein provided in trans and competent for activation of productive cycle genes does not activate the BZLF1 gene in the EBV genome. J Gen Virol. 1996;77:501–509. doi: 10.1099/0022-1317-77-3-501. [DOI] [PubMed] [Google Scholar]
- 35.Lill N L, Tevethia M J, Eckner R, Livingston D M, Modjtahedi N. p300 family members associate with the carboxyl terminus of simian virus 40 large tumor antigen. J Virol. 1997;71:129–137. doi: 10.1128/jvi.71.1.129-137.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manet E, Rigolet A, Gruffat H, Giot J F, Sergeant A. Domains of the Epstein-Barr virus (EBV) transcription factor R are required for dimerization, DNA binding and activation. Nucleic Acids Res. 1991;19:2661–2667. doi: 10.1093/nar/19.10.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Norton V G, Imai B S, Yau R, Bradbury E M. Histone acetylation reduces nucleosome core particle linking number change. Cell. 1989;57:449–457. doi: 10.1016/0092-8674(89)90920-3. [DOI] [PubMed] [Google Scholar]
- 38.Quinlivan E B, Holley-Guthrie E A, Norris M, Gutsch D, Bachenheimer S L, Kenney S C. Direct BRLF1 binding is required for cooperative BZLF1/BRLF1 activation of the Epstein-Barr virus early promoter, BMRF1. Nucleic Acids Res. 1993;25:1999–2007. doi: 10.1093/nar/21.8.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ragoczy T, Heston L, Miller G. The Epstein-Barr virus Rta protein activates lytic cycle genes and can disrupt latency in B lymphocytes. J Virol. 1998;72:7978–7984. doi: 10.1128/jvi.72.10.7978-7984.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ragoczy T, Miller G. The role of the Epstein-Barr virus Rta protein in activation of distinct classes of viral lytic cycle genes. J Virol. 1999;73:9858–9866. doi: 10.1128/jvi.73.12.9858-9866.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rickinson A B, Kieff E. Epstein-Barr virus. In: Fields B, Knipes D, Howley P M, Chanock R, Melnick J, Monath T, Roizman B, Straus S, editors. Fields virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2397–2446. [Google Scholar]
- 42.Rooney C M, Rowe D T, Ragot T, Farrell P J. The spliced BZLF1 gene of Epstein-Barr virus (EBV) transactivates an early EBV promoter and induces the virus productive cycle. J Virol. 1989;63:3109–3116. doi: 10.1128/jvi.63.7.3109-3116.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwartz R, Helmich B, Spector D H. CREB and CREB-binding proteins play an important role in the IE2 86-kilodalton protein-mediated transactivation of the human cytomegalovirus 2.2-kilobase RNA promoter. J Virol. 1996;70:6955–6966. doi: 10.1128/jvi.70.10.6955-6966.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scolnick D M, Chehab N H, Stavridi E S, Lien M C, Caruso L, Moran E, Berger S L, Halazonetis T D. CREB-binding protein and p300/CBP-associated factor are transcriptional coactivators of the p53 tumor suppressor protein. Cancer Res. 1997;57:3693–3696. [PubMed] [Google Scholar]
- 45.Somasundaram K, el-Deiry W S. Inhibition of p53-mediated transactivation and cell cycle arrest by E1A through its p300/CBP-interacting region. Oncogene. 1997;14:1047–1057. doi: 10.1038/sj.onc.1201002. [DOI] [PubMed] [Google Scholar]
- 46.Swenson J J, Mauser A, Kaufmann W K, Kenney S. The Epstein-Barr virus protein BRLF1 activates S phase entry through E2F1 induction. J Virol. 1999;73:6540–6550. doi: 10.1128/jvi.73.8.6540-6550.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takada K, Shimizu N, Sakuma S, Ono Y. trans activation of the latent Epstein-Barr virus (EBV) genome after transfection of the EBV DNA fragment. J Virol. 1986;57:1016–1022. doi: 10.1128/jvi.57.3.1016-1022.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Utley R T, Ikeda K, Grant P A, Cote J, Steger D J, Eberharter A, John S, Workman J L. Transcriptional activators direct histone acetyltransferase complexes to nucleosomes. Nature. 1998;394:498–502. doi: 10.1038/28886. [DOI] [PubMed] [Google Scholar]
- 49.Vettese-Dadey M, Grant P A, Hebbes T R, Crane-Robinson C, Allis C D, Workman J L. Acetylation of histone H4 plays a primary role in enhancing transcription factor binding to nucleosomal DNA in vitro. EMBO J. 1996;15:2508–2518. [PMC free article] [PubMed] [Google Scholar]
- 50.Westphal E-M, Mauser A, Swenson J, Davis M G, Talarico C L, Kenney S C. Induction of lytic Epstein-Barr virus (EBV) infection in EBV-associated malignancies using adenovirus vectors in vitro and in vivo. Cancer Res. 1999;59:1485–1491. [PubMed] [Google Scholar]
- 51.Zalani S, Holley-Guthrie E, Kenney S. Epstein-Barr viral latency is disrupted by the immediate-early BRLF1 protein through a cell-specific mechanism. Proc Natl Acad Sci USA. 1996;93:9194–9199. doi: 10.1073/pnas.93.17.9194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zalani S, Holley-Guthrie E, Gutsch D, Kenney S. The Epstein-Barr virus immediate-early promoter, BRLF1, can be activated by the cellular Sp1 transcription factor. J Virol. 1992;66:7282–7292. doi: 10.1128/jvi.66.12.7282-7292.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zerby D, Chen C J, Poon E, Lee D, Shiekhattar R, Lieberman P M. The amino-terminal C/H1 domain of CREB binding protein mediates Zta transcriptional activation of latent Epstein-Barr virus. Mol Cell Biol. 1999;19:1617–1626. doi: 10.1128/mcb.19.3.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]