Abstract
Kaposi's sarcoma-associated herpesvirus (KSHV) open reading frame 50 (ORF50) encodes a viral transcriptional activator which stimulates the transcription of viral early and late genes of KSHV. Here we show that ORF50 represses transcriptional activity of p53 and p53-induced apoptosis through interaction with CREB binding protein (CBP). This inhibitory effect of ORF50 on the transcriptional activity of p53 was relieved by the addition of CBP. ORF50 mutants, which are defective in interaction with CBP, lost the inhibitory effects on p53. Our data provide a framework for delineating the regulatory mechanisms used by KSHV to modulate cellular transcription and the cell cycle.
Kaposi's sarcoma-associated herpesvirus (KSHV) has been identified as an important pathogen in Kaposi's sarcoma (2, 4). DNA sequences of KSHV have been determined, and its in vitro culture system was recently developed (15, 16). The most important step in the KSHV life cycle may be the switch from latency to lytic replication. Upon chemical induction, KSHV produces immediate-early viral transcripts. These transcripts encode viral transcriptional activator proteins, such as open reading frame 50 (ORF50) and K8, which are necessary to induce the lytic phase (18). ORF50 is a homolog of the Epstein-Barr virus (EBV) immediate-early gene product Rta. ORF50 is a viral transcriptional activator, which activates the early and late genes in the KSHV lytic cycle (11, 12, 17). It has been reported that ORF50 activates the lytic cycle of KSHV and is expressed earlier than K8, a homolog of the EBV Zta protein, which induces the lytic cycle of EBV (12, 17). Previously we reported that ORF50 binds to the C/H3 domain and the carboxyl-terminal transcriptional activation domain of CREB binding protein (CBP), while CBP binds to the amino-terminal basic domain and the carboxyl-terminal transactivation domain. The LXXLL motif of ORF50 and both of these domains are necessary for the complete activity of binding to CBP in vivo (7). Many viral proteins modify the transactivation function of cellular transcription factors through a CBP-related mechanism. CBP-related transcriptional activation of c-Jun and CREB is inhibited by the adenovirus E1A (9, 13). The E6 protein from human papillomavirus inhibits the intrinsic transcriptional activation activity of CBP, and it decreases the activity of the p300/CBP coactivator complex to activate p53- and NF-κB-responsive promoter elements (14). The Tax protein from human T-cell leukemia virus type 1 not only increases the binding of CREB to the viral CREB-responsive element but also recruits CBP to the site of transcription (8). These results show that the viral proteins modulate the activities of cellular transcription factors that are important for cell cycle progression, cellular differentiation, and cell proliferation through the interaction of viral proteins with p300/CBP.
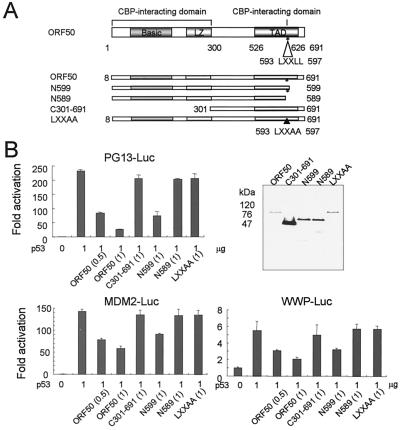
Because p53 uses CBP as a transcription cofactor and binds to the carboxyl-terminal transactivation domain of CBP (6, 10), we investigated whether or not ORF50 could inhibit transcriptional activation by p53 by using transient-transfection assays. PG13-Luc, WWP-Luc, MDM2-Luc, and the p53 expression vectors were provided by B. Vogelstein and R. Roeder. ORF50 and the ORF50 deletion mutants were subcloned into pME18S (the EcoRI and XhoI site; a kind gift of W. M. Yang) by using PCR. Transfection assays were performed in 293T cells by the standard calcium precipitation method. In all assays, the luciferase activity derived from the reporter plasmids was determined after being normalized to β-galactosidase activity from a cotransfected RSV-βgal control plasmid. All experiments were performed at least in triplicate. One microgram of reporter plasmid and 20 ng of RSV-βgal control plasmid were transfected into 293T cells, and the amounts of the expression plasmids are indicated in the figures. The total amount of each expression vector was kept constant by adding an empty expression plasmid. Figure 1A shows the various mutants of ORF50. In our previous work (7) we showed that ORF50 should have both an N-terminal region (amino acids 1 to 300) and a C-terminal LXXLL region for complete interacting activity with CBP in vivo. ORF50 binds to CBP, while N589, C301-691, and LXXAA mutants, which each had one of the CBP binding regions deleted, lost their binding activity to CBP in vivo. Meanwhile, N599 shows a relatively reduced binding activity to CBP compared to that of wild-type ORF50. ORF50 inhibited p53-mediated activation of PG13-Luc, MDM2-Luc, and WWP-Luc in 293T cells (Fig. 1B). This inhibitory effect of ORF50 was observed in C33A and SAOS2 cells as well (data not shown). C301-691, N589, and LXXAA mutants did not show any inhibitory effects on p53-driven transcriptional activation. N599 showed a moderate inhibitory effect on p53-driven transcriptional activation compared to that of wild-type ORF50. As shown in Fig. 1B, the expressed amounts of the mutants were no smaller than the amount of ORF50, although some variations were detected. Since these ORF50 mutants do not repress the p53-driven transcriptional activation activity that wild-type ORF50 did, we conclude that the decreased inhibitory effects of C301-691, N589, and LXXAA were not due to the smaller amount of expression of the mutated forms.
FIG. 1.
ORF50 represses p53-induced transactivation. (A) The domains within ORF50 and the mutated forms of ORF50 are shown. ORF50 contains the basic domain, the leucine zipper motif (LZ), and the transcriptional activation domain (TAD). The LXXLL motif, which interacts with CBP, is located between amino acids 593 and 597 of ORF50. (B) The fold activation of the luciferase activity was determined with the p53-responsive reporters, and the following reporters were used: PG13-Luc (synthetic p53 binding site), MDM2-Luc (the promoter of MDM2 protein), and WWP-Luc (the promoter of p21). All of the ORF50 mutants were introduced into pME18S, a mammalian expression vector containing the SRα promoter and a Flag tag. 293T cells were transfected with the indicated vectors, and extracts were analyzed by blotting with anti-Flag antibody (upper right panel).
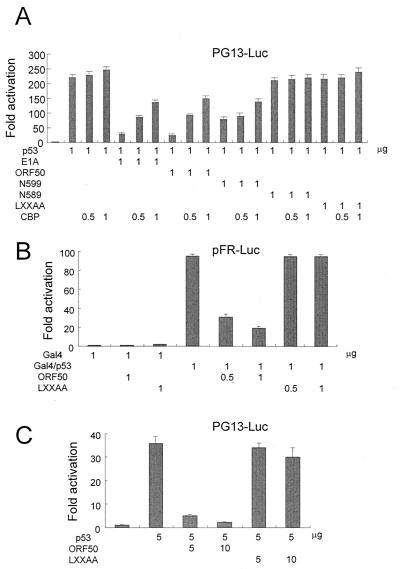
For the following reasons, we hypothesized that this inhibitory effect was mostly derived from the inhibition of interaction between p53 and CBP by ORF50, although we still cannot rule out the possibility of the direct interaction between p53 and ORF50. ORF50 mutants C301-691, N589, and LXXAA, which do not bind to CBP, do not show any p53 inhibitory effect, while ORF50 inhibits p53 activity (Fig. 1B). N599, which has a reduced binding activity to CBP, still shows the inhibitory effects on p53-driven transcriptional activation, although this inhibitory effect was reduced compared to that of wild-type ORF50. Using various techniques, such as the mammalian two-hybrid technique and immunoprecipitation, we did not detect any direct interaction between ORF50 and p53 from the cellular extract cotransfected with the expression vectors containing ORF50 and p53 genes in our conditions (data not shown). To further clarify this result, we performed a comparative analyses (transient-transfection and luciferase assays) of ORF50 and adenovirus E1A, which are known to repress p53 transcriptional activity mediated by CBP (6, 10). Indeed, ORF50 inhibited the p53-responsive promoters and the inhibitory effects were relieved by the addition of CBP, as was observed when adenovirus E1A was used as a control (Fig. 2A). The inhibitory effect of N599 was slightly relieved by the addition of CBP. We thought that this weak relief was related to the weak binding activity of N599 to CBP. The addition of CBP did not affect the p53-driven transcriptional activation activity. This is not surprising, because an earlier group found that a large amount of CBP was necessary to increase the p53-driven transcriptional activation activity (6). They used an amount of CBP a thousand times greater than that of p53 to observe an approximately sixfold activation of p53-induced activation. We used an equal amount of CBP to p53, so it is hard to see p53-driven transcriptional activation by the addition of CBP. In conclusion, the inhibitory effect of ORF50 on p53-driven transcriptional activation was relieved by the addition of CBP, and this relief was not derived from the increase of p53 transcriptional activation by CBP.
FIG. 2.
ORF50 repressed transactivation of p53 through interaction with CBP. (A) To determine whether inhibition by ORF50 could be relieved by the addition of CBP, the fold activation in luciferase activity was determined. (B) ORF50 inhibited the transcriptional activation of Gal4/p53. (C) ORF50 repressed p53 transactivation, while the ORF50 mutant LXXAA, which does not bind to CBP, could not inhibit p53 transactivation in the B-cell lymphoma cell line, BJAB.
To prove that the inhibitory effect of ORF50 was not derived from the modulation of the DNA binding activity of p53, we determined the luciferase activity of p53 fused to the Gal4 DNA binding domain (Gal4/p53) (Fig. 2B). Gal4/p53-activated transcription of Gal4-Luc was greater than that of the Gal4 DNA binding domain alone by about 95-fold. This activity was repressed to 20-fold of that of the Gal4 binding domain alone in the presence of ORF50. The LXXAA mutant could not repress this activity of Gal4/p53 at all. Cotransfection of ORF50 expression vectors reduced transcriptional activation by p53 to 0.1-fold in the B-cell lymphoma BJAB cell line (Fig. 2C). In contrast, increasing concentrations of LXXAA did not affect the transcriptional activity of p53 in the BJAB cells.
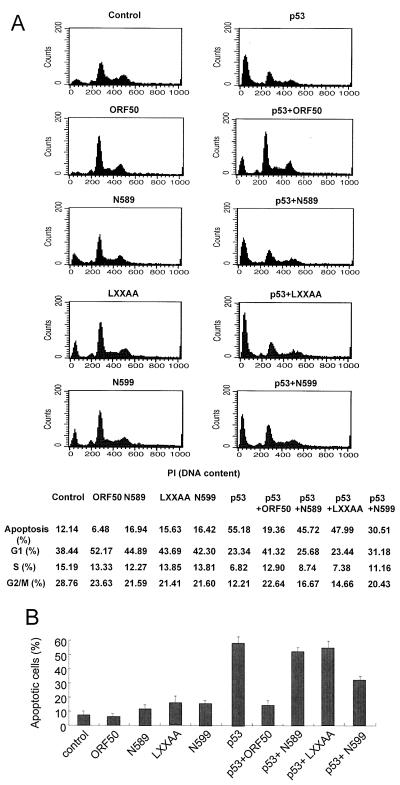
To determine the biological effects of ORF50 on p53 function, we examined ORF50 regulation of p53-induced apoptosis. Overexpression of p53 induces apoptosis in SAOS2 cells. ORF50 and the mutated forms alone did not show any cytotoxic effect when transfected into these cells (Fig. 3A and B). SAOS2 cells (106) were cotransfected with pCMV-p53 (1 μg) and pcDNA3/ORF50 (3 μg), and after 48 h of incubation the cells were fixed with 70% ethanol on ice for 1 h. Cells were stained with propidium iodide (50 μg/ml), and the hypoploid cell fraction was analyzed by a fluorescence-activated cell sorter (FacsCalibur; Becton-Dickinson, Mountain View, Calif.). The p53-transfected cells showed altered DNA contents, which is indicative of apoptosis (Fig. 3A). ORF50 severely repressed p53-induced apoptosis when cotransfected with p53, while N589 and LXXAA mutants did not show any inhibitory effect. N599 showed a moderate inhibitory effect on the p53-induced apoptosis, and these results showed a pattern similar to that of the result of effects on p53-driven transcriptional activation. These observations were confirmed by counting the apoptotic cells cotransfected with green fluorescent protein (GFP). SAOS2 cells (106) were transfected with pEGFP-C1 (0.5 μg) and with a variety of vector constructs. Forty-two hours after transfection the cells were fixed with a 4% formaldehyde–phosphate-buffered saline solution for 30 min, and then they were stained with 10 μg of 4′,6′-diamidino-2-phenylindole (DAPI)/ml for 10 min. The level of apoptosis was determined by using a fluorescent microscope to count DAPI-stained condensed nuclei and then dividing this number by the number of GFP-expressing cells. Transfection of cells with p53 dramatically increased the apoptotic cell population compared to that of GFP-transfected cells (Fig. 3B). The apoptotic population was reduced to the negative control level when it was cotransfected with wild-type ORF50 but not with N589 or LXXAA. N599 shows a reduced inhibitory effect on p53-induced apoptosis.
FIG. 3.
ORF50 represses p53-induced apoptosis. (A) Apoptosis assays in SAOS2 cells were performed. The hypoploid cell fraction was analyzed by using a fluorescence-activated cell sorter. PI, propidium iodide. (B) To verify the results of fluorescence-activated cell sorting, the level of apoptosis was determined by counting DAPI-stained condensed nuclei in GFP-expressing cells by using a fluorescence microscope.
ORF50 stimulates the viral promoters to promote the lytic phase of KSHV (11). In contrast, ORF50 represses p53-driven transcriptional activation through a CBP-related mechanism. KSHV encodes many cellular homologs related to cell proliferation and survival. ORF72/v-cyclin inactivates the retinoblastoma protein (3). KSHV latency-associated nuclear antigen binds to p53 and inhibits p53-induced apoptosis (5). We demonstrated here that ORF50 inactivated p53-induced transcriptional activation and apoptosis. In fact, many viral transactivators, such as E1A and Tax, inhibit p53-induced apoptosis as well (1, 6, 10). Our results suggest that ORF50 not only acts as a transcriptional activator to induce the lytic phase of KSHV but also acts as a regulator for the cell cycle to sustain viral persistence. ORF50 can either activate or repress cellular transcription factors to benefit viral persistence. Previously we reported that CBP-interacting proteins such as c-Jun and E1A modulate the function of ORF50 (7). In this study, we showed that ORF50 represses p53-driven transcriptional activation through a CBP-related mechanism. CBP seems to be a key molecule in the interaction of ORF50 with transcriptional factors such as c-Jun, E1A, and p53. An experiment to define the effects of ORF50 on other CBP-related promoters is under progress.
Acknowledgments
This work was supported in part by grants from the National Research Laboratory Program of the Korea Institute of Science and Technology Evaluation and Planning (KISTEP), the Korea Science and Engineering Foundation (KOSEF) through the Protein Network Research Center at Yonsei University, and the BK21 Program of the Ministry of Education, Korea. E.-J.C. was supported by the Creative Research Initiatives Program of the Korea Ministry of Science and Technology.
REFERENCES
- 1.Ariumi Y, Kaida A, Lin J Y, Hirota M, Masui O, Yamaoka S, Taya Y, Shimotohno K. HTLV-1 tax oncoprotein represses the p53-mediated trans-activation function through coactivator CBP sequestration. Oncogene. 2000;19:1491–1499. doi: 10.1038/sj.onc.1203450. [DOI] [PubMed] [Google Scholar]
- 2.Boshoff C, Weiss R A. Kaposi's sarcoma-associated herpesvirus. Adv Cancer Res. 1998;75:57–86. doi: 10.1016/s0065-230x(08)60739-3. [DOI] [PubMed] [Google Scholar]
- 3.Chang Y, Moore P S, Talbot S J, Boshoff C H, Zarkowska T, Godden-Kent D, Paterson H, Weiss R A, Mittnacht S. Cyclin encoded by KS herpesvirus. Nature. 1996;382:410. doi: 10.1038/382410a0. [DOI] [PubMed] [Google Scholar]
- 4.Chang Y, Cesarman E, Pessin M S, Lee F, Culpepper J, Knowles D M, Moore P S. Identification of herpes-like DNA sequences in AIDS-associated Kaposis's sarcoma. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 5.Friborg J, Jr, Kong W, Hottiger M O, Nabel G J. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature. 1999;402:889–894. doi: 10.1038/47266. [DOI] [PubMed] [Google Scholar]
- 6.Gu W, Shi X-L, Roeder R G. Synergistic activation of transcription by CBP and p53. Nature. 1997;387:819–822. doi: 10.1038/42972. [DOI] [PubMed] [Google Scholar]
- 7.Gwack Y, Byun H, Hwang S, Lim C, Choe J. CREB-binding protein and histone deacetylase regulate the transcriptional activity of Kaposi's sarcoma-associated herpesvirus open reading frame 50. J Virol. 2001;75:1909–1917. doi: 10.1128/JVI.75.4.1909-1917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwok R P, Laurance M E, Lundblad J R, Goldman P S, Shih H, Connor L M, Marriott S J, Goodman R H. Control of cAMP-regulated enhancers by the viral transactivator Tax through CREB and the co-activator CBP. Nature. 1996;380:642–646. doi: 10.1038/380642a0. [DOI] [PubMed] [Google Scholar]
- 9.Lee J S, See R H, Deng T, Shi Y. Adenovirus E1A downregulates c-Jun- and JunB-mediated transcription by targeting their coactivator p300. Mol Cell Biol. 1996;16:4312–4326. doi: 10.1128/mcb.16.8.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lill N L, Grossman S R, Ginsberg D, DeCaprio J, Livingston D M. Binding and modulation of p53 by p300/CBP coactivators. Nature. 1997;387:823–827. doi: 10.1038/42981. [DOI] [PubMed] [Google Scholar]
- 11.Lukac D M, Kirshner J R, Ganem D. Transcriptional activation by the product of open reading frame 50 of Kaposi's sarcoma-associated herpesvirus is required for lytic viral reactivation in B cells. J Virol. 1999;73:9348–9361. doi: 10.1128/jvi.73.11.9348-9361.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lukac D M, Renne R, Kirshner J R, Ganem D. Reactivation of Kaposi's sarcoma-associated herpesvirus infection from latency by expression of the ORF 50 transactivator, a homolog of the EBV R protein. Virology. 1998;252:304–312. doi: 10.1006/viro.1998.9486. [DOI] [PubMed] [Google Scholar]
- 13.Nakajima T, Uchida C, Anderson S F, Parvin J D, Montminy M. Analysis of a cAMP-responsive activator reveals a two-component mechanism for transcriptional induction via signal-dependent factors. Genes Dev. 1997;11:738–747. doi: 10.1101/gad.11.6.738. [DOI] [PubMed] [Google Scholar]
- 14.Patel D, Huang S M, Baglia L A, McCance D J. The E6 protein of human papillomavirus type 16 binds to and inhibits co-activation by CBP and p300. EMBO J. 1999;18:5061–5072. doi: 10.1093/emboj/18.18.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Renne R, Zhong W, Herndier B, McGrath M, Abbey N, Kedes D, Ganem D. Lytic growth of Kaposis's sarcoma associated herpesvirus (HHV-8) in culture. Nat Med. 1996;2:342–346. doi: 10.1038/nm0396-342. [DOI] [PubMed] [Google Scholar]
- 16.Russo J J, Bohenzky R A, Chien M C, Chen J, Yan M, Maddalena D, Parry J P, Peruzzi D, Edelman I S, Chang Y, Moore P S. Nucleotide sequence of the Kaposi's sarcoma associated herpesvirus (HHV8) Proc Natl Acad Sci USA. 1997;93:14862–14867. doi: 10.1073/pnas.93.25.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun R, Lin S F, Gradoville L, Yuan Y, Zhu F, Miller G. A viral gene that activates lytic cycle expression of Kaposi's sarcoma-associated herpesvirus. Proc Natl Acad Sci USA. 1998;95:10866–10871. doi: 10.1073/pnas.95.18.10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu F X, Cusano T, Yuan Y. Identification of the immediate-early transcripts of Kaposi's sarcoma-associated herpesvirus. J Virol. 1999;73:5556–5567. doi: 10.1128/jvi.73.7.5556-5567.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]