Abstract
Hepatitis E virus (HEV), a positive-strand RNA virus, is an important causative agent of waterborne hepatitis. Expression of cDNA (encoding amino acids 1 to 979 of HEV nonstructural open reading frame 1) in insect cells resulted in synthesis of a 110-kDa protein (P110), a fraction of which was proteolytically processed to an 80-kDa protein. P110 was tightly bound to cytoplasmic membranes, from which it could be released by detergents. Immunopurified P110 catalyzed transfer of a methyl group from S-adenosylmethionine (AdoMet) to GTP and GDP to yield m7GTP or m7GDP. GMP, GpppG, and GpppA were poor substrates for the P110 methyltransferase. There was no evidence for further methylation of m7GTP when it was used as a substrate for the methyltransferase. P110 was also a guanylyltransferase, which formed a covalent complex, P110-m7GMP, in the presence of AdoMet and GTP, because radioactivity from both [α-32P]GTP and [3H-methyl]AdoMet was found in the covalent guanylate complex. Since both methyltransferase and guanylyltransferase reactions are strictly virus specific, they should offer optimal targets for development of antiviral drugs. Cap analogs such as m7GTP, m7GDP, et2m7GMP, and m2et7GMP inhibited the methyltransferase reaction. HEV P110 capping enzyme has similar properties to the methyltransferase and guanylyltransferase of alphavirus nsP1, tobacco mosaic virus P126, brome mosaic virus replicase protein 1a, and bamboo mosaic virus (a potexvirus) nonstructural protein, indicating there is a common evolutionary origin of these distantly related plant and animal virus families.
Hepatitis E virus (HEV) is an important etiological agent of acute epidemic and sporadic enteric hepatitis affecting millions of people mainly in developing countries. The first confirmed HEV epidemic, due to contamination of drinking water in New Delhi, India, was described in 1955. In addition to large epidemics in India and China, there are annually about 2 million sporadic cases of HEV infections in India alone. The mortality among HEV patients has been 0.5 to 4%, except in the case of pregnant women, for whom the average mortality is 20% (for reviews, see references 22 and 33). Recently, closely related viruses have been isolated from pigs, cows, sheep, goats, and rats, indicating zoonotic HEV infections (13).
HEV is a nonenveloped virus with a diameter of 27 to 34 nm (9, 10), which does not replicate in cell cultures (1). The complete nucleotide sequence of the positive-strand RNA genome has been determined for several isolates from different parts of the world (40). (For references, see reference 8.) The HEV genome consists of a 27-nucleotide (nt)-long 5′ noncoding region followed by an open reading frame (ORF) coding for a nonstructural protein of 1,693 aa residues. ORF2 starts 38 nt downstream of the termination codon of ORF1 and codes for the capsid protein of 660 aa. ORF3 between nt 5105 and 5476 overlaps with ORF2 and codes for a 123-aa-long polypeptide with unknown function. The 3′ noncoding region is 65 nt, ending with a 150- to 200-nt-long poly(A) tail. A recent finding indicates that the HEV genome has an m7G cap structure at the 5′ end of the RNA (15).
Expression of HEV capsid protein in COS-1 cells revealed that it is a glycoprotein with a size of 88 kDa, which is synthesized as a precursor (ggPORF2), processed to gPORF2 by cleavage of a signal sequence of 22 aa, and transported to the plasma membrane (14, 46). A more stable cytosolic form of capsid protein PORF2 with a size of 78 kDa has been detected in HepG2 cells by using a Semliki Forest virus (SFV) expression vector (41, 42). So far, which form of capsid protein is responsible for the production of infectious HEV is unknown. A truncated 50-kDa form of PORF2 produced in insect cells is able to assemble into hollow particles with an icosahedral symmetry of T=1 (25, 44). Expression of ORF3 in eukaryotic cells revealed that it is a phosphoprotein with affinity for the cytoskeleton through a hydrophobic amino-terminal domain of 32 aa residues (45).
Expression of complete ORF1 coding for 1,693 aa residues in vitro, in Escherichia coli and in HepG2 cells, resulted in a large 186-kDa protein that was not autocatalytically processed (7). In another study, prolonged in vivo expression yielded N-terminal 78-kDa and C-terminal 107-kDa fragments (34). Transfection of in vitro-synthesized RNA, consisting of the complete HEV genome, to HepG2 cells resulted in synthesis of ORF1, ORF2, and ORF3 products as well as release of small amounts of infectious virus into the culture medium (29). However, no 186-kDa protein was detected in pulse-chase experiments. Instead, region-specific antisera precipitated smaller 35- to 40-kDa polypeptides.
Computer-assisted assignments for the putative functions of HEV ORF1 suggested that the amino-terminal domain from 60 to 240 aa may be a methyltransferase followed by a Y domain with unknown function, a papain-like protease domain (around 440 to 610 aa), a proline-rich spacer region, an X domain of unknown function, a helicase domain (around 960 to 1,200 aa), and an RNA polymerase domain (around 1,200 to 1,700 aa) (17). None of the predicted functions has been experimentally verified. Indeed, it was recently shown that one of the putative protease active site residues is not conserved in different isolates and that mutation of the predicted active site cysteine did not abrogate P186 processing (34). Thus, the predicted protease appears not to exist or have enzymatic activity.
Based on the ORF1 sequence, HEV belongs to a large alphavirus-like superfamily of positive-strand RNA viruses with putative methyltransferase, protease, helicase, and RNA polymerase domains (18). The distinctive methyltransferase domain is the hallmark of the alphavirus-like superfamily (28, 35). It contains sequence similarity to cellular S-adenosylmethionine (AdoMet)-dependent methyltransferases (5). In addition to guanine-7-methyltransferase activity (20, 36), the amino-terminal part of alphavirus nonstructural polyprotein, termed nsP1, also posesses guanylyltransferase activity (2). Both activities are needed in the capping of viral mRNAs (3, 5), and thus the conserved domain can more appropriately be designated as the capping enzyme domain. Both nsP1-catalyzed reactions are virus specific (i.e., there are no known host cell enzymes with similar specificities). Methyltransferase catalyzes the transfer of a methyl group from AdoMet to GTP, resulting in m7GTP, which forms the covalent complex nsP1-m7GMP. These reactions can be inhibited by cap analogs (22).
In the present paper, we show the first enzymatic activity found for the HEV nonstructural protein. Truncated nonstructural protein P110 derived from HEV ORF1 produced in insect cells has virus-specific methyltransferase and guanylyltransferase activities similar to those of alphavirus replicase proteins. This finding provides a novel approach for development of specific inhibitors against hepatitis E infection.
MATERIALS AND METHODS
HEV cDNA constructs.
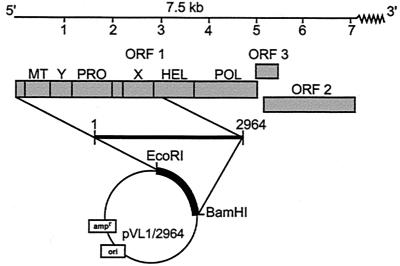
Preparation of a pool of acute-phase stool specimens from patients with sporadic non-A, non-B hepatitis in Myanmar in 1986 and intravenous injection into rhesus monkeys (Macaca mulatta) were described previously (43). After six successive passages in monkeys, bile was collected and used for further study. The total RNA was extracted with RNAzol (Biotecx Laboratories, Inc., Houston, Tex.), and poly(A)-containing RNA was purified with oligotex-dT30 (Super) (Roche Diagnostic Systems, Tokyo, Japan) according to the manufacturer's protocol. RNA was converted into cDNA as described previously (39). The N-terminal 2,964-bp segment of ORF1 was then amplified with primers EP-1 (5′-AGGCAGACCACATATGTGGTCGAT-3′) and U-15 (5′-AGCGGGACTTGCCGGATCCAGGCA-3′). The PCR product was cloned into a TA cloning vector, PCRII (Invitrogen, San Diego, Calif.), and digested with the restriction enzymes EcoRI and BamHI, and the resultant ∼3-kb fragment was ligated into the transfer vector pVL1392 (Pharmingen, San Diego, Calif.) to yield plasmid pVL [1/2964].
Generation of recombinant baculoviruses.
Sf9 cells (Riken Cell Bank, Tsukuba, Japan), derived from Spodoptera frugiperda (31), were cotransfected with linearized wild-type Autographa californica nuclear polyhedrosis virus DNA (Pharmingen) and pVL [1/2964] by the Lipofectin-mediated method as specified by the manufacturer (GIBCO BRL, Gaithersburg, Md.). The cells were incubated at 28°C in TC-100 medium (GIBCO) supplemented with 8% fetal bovine serum and 0.26% Bacto tryptose phosphate broth (Difco Laboratories, Detroit, Mich.). Each recombinant virus was plaque purified three times (38). The baculovirus recombinant thus obtained was designated Ac[1/2964]. In addition to Sf9 cells, we used an insect cell line from Trichoplusia ni, BTL-Tn 5B1–4 (Tn5) (Invitrogen), for protein expression.
Cell fractionation and membrane flotation.
Tn5 cells grown on petri plates (2 × 106 cells per 35-mm-diameter plate) were infected with Ac-[1/2964] containing cDNA encoding aa 1 to 979 of HEV ORF1 (hereafter designated as Ac-P110) at 10 PFU/cell. At 44 h postinfection, the cells were collected as described previously (20). Cells were washed twice with cold phosphate-buffered saline and once with RS buffer (10 mM Tris-HCl [pH 8.0], 10 mM NaCl) containing 0.2 mM phenylmethylsulfonyl fluoride and EDTA-free protease inhibitor cocktail (Boehringer Mannheim GmbH, Mannheim, Germany). After washes, the cell pellet was resuspended in 5 volumes of RS buffer, left on ice for 15 min to swell, and disrupted in a Dounce homogenizer with 40 strokes. Nuclei were removed by centrifugation (500 × g for 10 min). Postnuclear supernatant was subjected to flotation in a sucrose gradient as described previously (21). The membrane fractions that had undergone flotation were collected, mixed with TN buffer (50 mM Tris-HCl [pH 7.5], 100 mM NaCl), and subjected to centrifugation (70,000 × g for 30 min). The pellet was resuspended in RS buffer containing 10% glycerol and stored at −80°C.
Enzyme assays.
Methyltransferase activity was assayed in a final volume of 25 μl containing 100 mM HEPES (pH 7.0), 10 mM GTP, 4 μM AdoMet, 4 mM MnCl2, 2 mM dithiothreitol, and 0.75 μCi of S-adenosyl[methyl-3H]methionine (88 Ci/mmol; Amersham) for 2 h at 37°C. The reaction was stopped by adding EDTA to a final concentration of 10 mM. Labeled product was isolated in small DEAE-Sephadex columns and quantitated by liquid scintillation as described previously (20). For the formation of the covalent guanylate complex, 30-μl reaction mixtures containing 5 μCi of [α-32P]GTP (400 Ci/mmol; Amersham) in 50 mM Tris-HCl (pH 7.5), 10 mM KCl, 2 mM MgCl2, 5 mM dithiothreitol, and 100 μM AdoMet were incubated for 20 min at 30°C, as described previously (2). The reaction was stopped by boiling in the presence of 1% sodium dodecyl sulfate (SDS). The proteins were separated by SDS-polyacrylamide gel electrophoresis (PAGE) in 10% polyacrylamide gels, and the radioactive bands were visualized by autoradiography as described previously (20).
Radioactive labeling.
For palmitate labeling, Tn5 cells infected with Ac-P110 (10 PFU/cell) were labeled at 44 h postinfection with 300 to 400 μCi (per 60-mm-diameter plate) of [9,10(n)-3H]palmitic acid (52 Ci/mmol, Amersham) in Sf-900 II serum-free medium for 8 h at 28°C. For labeling with [35S]methionine, infected cells were incubated in methionine-free Grace medium for 30 min at 28oC and pulse-labeled with 500 μCi (per 100-mm-diameter plate) of [35S]methionine (1,000 Ci/mmol; Amersham) in methionine-free Grace medium for 2 h at 28°C. The cells were chased with 20-fold excess unlabeled methionine for 1 h at 28oC.
Immunological techniques.
For preparation of immune sera, cDNA encoding aa 1 to 464 (P50) of HEV ORF1 was inserted in plasmid pBAT (31), which was expressed in E. coli BL21 after induction with 10 μM isopropyl-β-d-thiogalactopyranoside overnight at 15°C. After cell breakage with a French press and sedimentation, the inclusion bodies were washed successively with PBS, 2 M urea, and PBS followed by solubilization in 0.0625 M Tris-HCl (pH 6.6), 2% SDS, 10% glycerol, and 5% 2-β-mercaptoethanol. After SDS-PAGE, the band migrating at 50 kDa was eluted in a buffer consisting of 0.1% SDS, 200 mM NaCl, and 50 mM Tris-HCl (pH 7.2). Immunization of two rabbits and two guinea pigs was carried out exactly as described previously (19). E. coli-specific antibodies were removed by passing the sera through a Sepharose column containing covalently linked bacterial proteins. Furthermore, the antisera were absorbed with HeLa cells as described previously (19). Immunoprecipitation of protein samples under nondenaturing conditions was done as described previously (32). The immunoprecipitates were analyzed by SDS-PAGE in 10% polyacrylamide gels. Western blotting was done as described previously (20).
RESULTS
Expression of HEV ORF1 protein P110 in insect cells.
A cDNA fragment encoding HEV ORF1 aa 1 to 979 (Fig. 1) was cloned under the polyhedrin promoter of the baculovirus genome as described in Materials and Methods. Tn5 insect cells were infected with the recombinant baculovirus at 10 PFU/cell. The expression of HEV-specific ORF1 proteins was monitored at different times after infection in cell lysates solubilized with SDS. Coomassie blue staining of protein gels showed a clear band with an apparent molecular mass of about 110 kDa, which first appeared 30 h after infection and increased in intensity during further incubation (Fig. 2A, lanes 3 to 5). Western blotting with antiserum against ORF1 aa 1 to 464 (P50), identified the 110-kDa band as an HEV-specific protein, which was designated P110. Another band with an apparent molecular mass of 80 kDa (P80) was also detected by the antiserum (Fig. 2B). The intensity of P80 band was variable, suggesting that it was a proteolytic cleavage product of P110. This result resembles that of Ropp et al. (34), who occasionally observed an N-terminal ORF1 cleavage product with a size of 78 kDa.
FIG. 1.
Organization of the HEV RNA genome with three overlapping ORFs (ORF1, -2, and -3). ORF1 encodes a large nonstructural protein, P186, with predicted functional domains of methyltransferase (MT), protease (PRO), helicase (HEL), and polymerase (POL) plus sequence motifs (Y and X) resembling those found in other alphavirus-like RNA virus genomes (19, 20). Below is shown the P110 construct used in this study.
FIG. 2.
Synthesis of HEV P110 protein in Tn5 insect cells at different times after infection with Ac-P110 baculovirus. (A) Coomassie blue staining of cell lysates after SDS-PAGE. (B) Same samples detected by immunoblotting with anti-P50 antiserum. (C) Membrane association of HEV P110. Postnuclear supernatant was subjected to flotation in a discontinuous sucrose gradient. Fractions (0.5 ml each) were collected from the top and subjected to SDS-PAGE followed by immunoblotting with anti-P50 antiserum. The top of the gradient is on the left. Percentages of sucrose are shown below panel C.
Crude fractionation of the postnuclear supernatant from infected cells at 44 h after infection revealed that P110 was mostly associated with a membrane fraction sedimenting at 15,000 × g. To establish its membrane association, we used a flotation assay, which separates membranes from soluble and aggregated components during centrifugation in a discontinuous sucrose gradient (6, 11, 20, 21, 24). By this criterion, P110 and P80 were both associated with cytoplasmic membranes floating to the top of the gradient (Fig. 2C, fractions 2 and 3). To assess the mode of membrane association of P110, the membranes from the top fractions were pooled, sedimented, and resuspended in buffers containing 1 M KCl, 50 mM EDTA, or alkaline sodium carbonate at pH 11.5 or 12, conditions used to dissociate peripherally associated proteins from membranes (11). Membranes were resedimented and analyzed together with the respective supernatants by SDS-PAGE and Western blotting. The HEV proteins were not released by high-salt or EDTA treatments and only partially released by alkaline solutions (data not shown), indicating tight binding similar to that of integral membrane proteins or the palmitoylated form of nsP1 methyltransferase or guanylyltransferase of SFV (21).
Next we studied whether P110 was acylated by producing it in Tn5 cells in the presence of [3H]palmitic acid. As a control, we used recombinant baculovirus expressing SFV nsP1. All our attempts to label P110 with palmitate failed (data not shown), while nsP1 was readily labeled as described previously (20). Thus, the tight membrane binding was apparently not due to acylation of P110 with palmitate.
Methyltransferase and guanylyltransferase activities of P110.
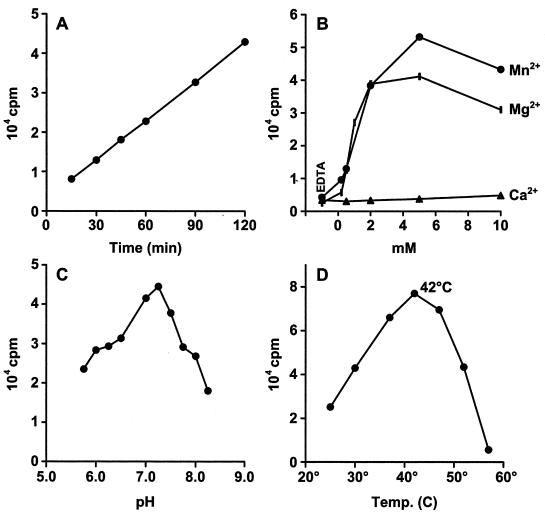
Guanine-7-methyltransferase activity was assayed from postnuclear supernatant (15,000 × g pellet) and supernatant fractions of Tn5 cells expressing P110. As a substrate, we used GTP, and as a methyl donor, we used AdoMet labeled with tritium in the methyl group. Most experiments were carried out with flotation-treated membranes from Tn5 cells. There was linear production of m7GTP with respect to time (Fig. 3A). Divalent cations Mn2+, Mg2+ (Fig. 3B), and Co2+ (not shown) were essential for the reaction, but the optimum concentration range was wide, whereas Ca2+ did not stimulate the reaction. The optimum pH was 7.25 (Fig. 3C). The methyltransferase of HEV P110 was rather heat resistant, with a temperature optimum of 42°C (Fig. 3D).
FIG. 3.
Properties of flotation-treated membrane preparations of HEV P110 methyltransferase. Incorporation of methyl-3H from AdoMet as function of time of incubation (A), in the presence of EDTA or different amounts of divalent cations (B), at different pHs (C), and at different temperatures (D) is shown.
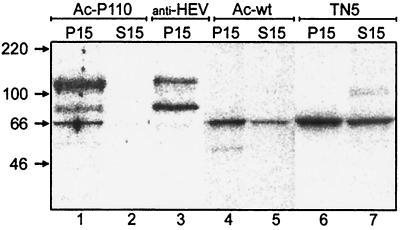
The HEV P110 preparation that had undergone flotation was exposed to [α-32P]GTP in the presence and absence of AdoMet followed by immunoprecipitation by anti-HEV (P50) antiserum. The same reactions were carried out for mock-infected and wild-type baculovirus-infected Tn5 cell preparations. As a further control, we used a membrane preparation from recombinant baculovirus-infected cells expressing SFV capping enzyme nsP1 (data not shown). Before immunoprecipitation, in preparations containing P110, three radioactive bands with apparent molecular masses of 110, 80, and 66 kDa were detected (Fig. 4, lane 1). Of these, the 110- and 80-kDa proteins were detected after immunoprecipitation (Fig. 4, lane 3), indicating that they represented HEV-specific proteins P110 and P80. Labeling of these proteins took place only if AdoMet served as a methyl donor (Fig. 5), whereas a 66-kDa protein was labeled in the absence of AdoMet in mock-infected (Fig. 4, lane 5) and in wild-type baculovirus-infected cells (Fig. 4, lane 4). This protein is evidently the guanylyltransferase of the host cells (37). The additional band of about 50 kDa, seen in the baculovirus wild-type-infected cells (Fig. 4, lane 4), most probably represents the baculovirus-specific guanylyltransferase LEF-4 (12).
FIG. 4.
Formation of enzyme-guanylate complexes in Tn5 insect cells infected with Ac-P110 (lanes 1 to 3) or Ac-wt (wild type; lanes 4 and 5) and in mock-infected cells (lanes 6 and 7). P15 and S15 fractions were incubated with [α-32P]GTP under standard conditions (see Materials and Methods). From 15 to 30 μg of total protein was applied per lane, and the proteins were separated by SDS-PAGE, followed by phosphoimaging. Part of the reaction mixture of Ac-P110 P15 material was subjected to immunoprecipitation prior to SDS-PAGE analysis (lane 3).
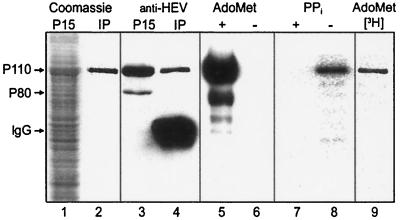
FIG. 5.
Immunoprecipitated HEV P110. Coomassie blue staining before (lane 1) and after (lane 2) immunoprecipitation of P15 fraction from Ac-P110-infected Tn5 insect cells. Immunoblotting by anti-P50 antiserum of lanes 3 and 4 respective to that in lanes 1 and 2 is shown. Also shown is the covalent complex formed after incubation of immunoprecipitated P110 with [α-32P]GTP in the presence (lane 5) and absence (lane 6) of 100 μM AdoMet. The same reaction was carried out in the presence (lane 7) or absence (lane 8) of 800 μM pyrophosphate (PPi). Covalent guanylate complex formation in the presence of unlabeled GTP and Ado[methyl-3H]Met is shown in lane 9.
Immunopurified HEV P110.
Treatment of the flotation-treated membrane fraction with 1% sodium deoxycholate or 1% Triton X-100 did not inhibit the HEV methyltransferase or guanylyltransferase activities (data not shown). It was therefore possible to use immunoprecipitation to obtain enzymatically active P110 preparations. When the P15 fraction of P110-expressing Tn5 cells was subjected to immunoprecipitation with anti-P50 antiserum, substantial purification of the HEV-specific protein was achieved (Fig. 5, lanes 1 and 2). The purified P110 formed a covalent complex with [α-32P]GTP, but only in the presence of AdoMet (Fig. 5, lanes 5 and 6). The recovery of methyltransferase activity was almost quantitatively and linearly dependent on the amount of P15 membranes used for immunoprecipitation (not shown). The finding that a covalent complex between guanosine and P110 was formed only in the presence of AdoMet suggested that the reaction took place between P110 and m7GTP rather than between P110 and GTP. This would imply that the methyl group from AdoMet should be found in the covalent guanylate-P110 complex. To test this, we carried out the reaction in the presence of S-adenosyl-l-[methyl-3H]methionine. P110 was resolved by SDS-PAGE and visualized by phosphoimaging. There was a clear band migrating at the position of P110 (Fig. 5, lane 9). The result was confirmed by quantitation of radioactivity from several parallel gel slices at the position of P110. To obtain evidence that in synthesis of the covalent complex pyrophosphate was released, we added PPi in excess to revert the reaction. No covalent complex was formed in the presence of 800 μM pyrophosphate (Fig. 5, lanes 7 and 8).
Several nucleoside triphosphates (NTPs), guanine nucleotides, and cap analogs were used as substrates for the purified P110. No incorporation of methyl group to ATP, CTP, or UTP was found (Table 1). The best substrate was GDP, followed by GTP, whereas GMP was poorly methylated by the enzyme. Two 5′- 5′ dinucleotide triphosphates (GpppG and GpppA) could serve as substrates, whereas m7GTP was unable to accept the methyl group, suggesting that only position 7 of the guanosine was methylated by P110.
TABLE 1.
Substrate specificity of HEV P110 methyltransferase
| Nucleotide | Methyltransferase activity (% of GTP)a |
|---|---|
| GTP | 100 |
| GDP | 182 |
| GMP | 4 |
| dGTP | 64 |
| GpppG | 20 |
| GppppG | 18 |
| GpppA | 18 |
| m7GTP | 5 |
| ATP | <1 |
| CTP | <1 |
| UTP | <1 |
The mean value of three experiments is shown.
Inhibition of the capping enzyme P110.
The inhibitory activity of some cap analogs on P110 was tested with 3 mM GTP as the methyl acceptor and the analogs at concentrations of 3 and 0.3 mM (Table 2). As expected, m7GTP inhibited incorporation of the methyl group from AdoMet to GTP at both concentrations. m7GDP was a somewhat better inhibitor, whereas m7GMP was less efficient. This was in accordance with the properties of GDP and GMP as substrates for the methyltransferase (Table 1). The double-substituted guanylate analogs et2m7GMP and m2et7GMP had an inhibitory effect similar to that of m7GMP, but clearly less than that of m7GDP, suggesting that the phosphorylation status of the analogs was more important than the substitutions of the guanylate moiety.
TABLE 2.
Inhibition of HEV P110 methyltransferase activity by nucleotide analogs
| Nucleotide analog | % Methyltransferase activity remaininga
|
|
|---|---|---|
| 3 mM | 0.3 mM | |
| GTP | 100 | |
| m7GTP | 23 | 44 |
| m7GDP | 9 | 36 |
| m7GMP | 50 | 80 |
| m2,7GMP | 44 | 92 |
| m2et7GMP | 46 | 60 |
| et2m7GMP | 39 | 77 |
Methyltransferase activity in the presence of a nucleotide analog as a percentage of methyltransferase activity in the absence of analog (mean value of three parallel assays of three different experiments) is shown.
DISCUSSION
Very little is known about the functions of HEV ORF1-encoded nonstructural protein(s). Since it was shown recently that the virus RNA has a 5′ cap structure (15), we expected that the cap might be synthesized by virus-encoded enzymes. HEV ORF1 has features in the amino-terminal region that resemble methyltransferases (5, 17, 35) and guanine-7N-methyltransferase, which is one of the activities needed in RNA capping (37). The first reaction in the capping of cellular mRNAs is the removal of γ-phosphate from the 5′ end of nascent RNA molecules by RNA 5′ triphosphatase. In the second reaction, guanylyltransferase reacts with GTP to make a covalent enzyme-GMP complex releasing pyrophosphate, followed by a third reaction in which the enzyme-bound GMP is transferred to the 5′ end of the RNA molecule. This unmethylated, G5′ppp5′N-capped RNA is the substrate in the fourth reaction for the methyltransferase, which transfers the methyl group from AdoMet to position 7 of the guanylate, resulting in m7G5′ppp5′N cap 0 structure (37).
Here we have shown that the HEV ORF1 fragment encoding P110 catalyzes capping reactions that are different from those described above for eukaryotic cells. Like alphaviruses (2), tobacco mosaic virus (TMV) (27), brome mosaic virus (BMV) (1, 16), and bamboo mosaic virus (a potexvirus) (26), HEV P110 instead of methylating unmethylated capped mRNA methylates GTP to form m7GTP, which then reacts to form a covalent enzyme-m7GMP complex. In alphavirus-, TMV-, and BMV-infected cells, both methyltransferase and guanylyltransferase activities are associated with nonstructural proteins nsP1, P126, and 1a, respectively. In the case of HEV, methyltransferase and guanylyltransferase activities were associated with an amino-terminal fragment of the nonstructural ORF1 (979 residues). We failed in our attempts to demonstrate these enzymatic activities for fragments of 470 and 527 residues, suggesting a rather large translational product may be required for enzymatic activity. Therefore, the capping enzyme domain may extend to what was initially predicted to be a protease domain (17), but which appears not to act as a protease (34).
Comparison of the enzymatic properties of HEV P110 with those previously reported for SFV nsP1 (2, 20, 23) and BMV 1a (1) shows that they all are unable to methylate ATP, CTP, UTP, or substrates with a methyl group at position 7 of the guanylate (m7GTP, m7GpppG, m7GpppA, or m7GppppG). In the case of BMV 1a, dinucleotides GpppA and GpppG are severalfold better substrates than GTP, whereas for both SFV nsP1 and HEV P110 methyltransferases, these two dinucleotides are poor substrates. All of these viral enzymes are specific for small acceptor substrates, whereas host methyltransferase involved in the capping of mRNAs methylates only longer GpppN-capped oligonucleotides (37). It is not surprising that the members of this virus-encoded enzyme family show some differences in their in vitro substrate specificity, given that their protein sequences have diverged considerably during evolution (35). The significant in vivo substrate is likely to be GTP, because this is the only nucleotide that after its methylation can form a covalent complex with the enzyme (1, 2) and thereafter be transferred to the RNA acceptor.
Another notable feature of this RNA virus capping enzyme family is that all of its members are membrane associated (1, 21, 26, 27; this study). It has been suggested that the capping enzyme domain might be responsible for the membrane association and targeting of the entire viral RNA replication complex (6). In the case of SFV nsP1, the membrane-binding mechanism has been studied in some detail. First, we found that the tight binding of the capping enzyme to membranes was correlated with the palmitoylation of cysteine residues 418 to 420 of nsP1 (20, 21, 30). However, mutation of cysteines 418 to 420 to alanines did not convert nsP1 to a soluble protein or prevent virus replication in cell cultures (4, 21). Weaker membrane binding of nsP1 was shown to be due to a 20-amino-acid-long amphipathic peptide in the central region of the protein (6, 24).
HEV P110 was tightly membrane-bound, mimicking integral membrane proteins as it is not released by treatments with high salt concentrations, EDTA, or even sodium carbonate at pH 11, all of which are known to release peripheral membrane proteins (11). P110 lacks continuous apolar sequences typical for transmembrane segments of integral membrane proteins. Our attempts to show that P110 is palmitoylated gave negative results. Thus, the mechanism of membrane binding of HEV P110 has to be solved by more extensive analysis, such as that used for SFV nsP1 (6).
Interactions with lipids and detergents in vitro also reveal differences between the viral capping enzymes. Interaction with anionic lipids is essential for the methyltransferase and guanylyltransferase activities of nonpalmitoylated nsP1, suggesting that membrane binding affects the conformation of the protein. All detergents interfere with the enzymatic activities of nonpalmitoylated nsP1 (6). In contrast, the activities of BMV 1a and bamboo mosaic virus capping enzyme have been studied in the presence of detergents (1, 26), and HEV P110 is active in the presence of sodium deoxycholate and Triton X-100 (this study). The precise mechanism of membrane association and its effects on the enzyme activities of these proteins need to be established.
Although the sequence homologies between alphavirus, BMV, and HEV methyl- and guanylyltransferases could be revealed only by advanced bioinformatic methods, they have highly similar enzymatic properties. The demonstration that five distantly related virus families (tobamoviruses, bromoviruses, potexviruses, alphaviruses, and HEV) within the alphavirus-like superfamily have functionally identical RNA capping steps suggests strongly that these reactions are to be found within all members of the superfamily. This would imply that these viruses have a common evolutionary origin, as predicted by sequence comparisons by Koonin and Dolja (18). The biochemical evidence presented in this study establishes HEV as a member of the alphavirus-like superfamily.
Most importantly, both the methyltransferase and guanylyltransferase reactions are virus specific and therefore offer targets for designing novel inhibitors of virus replication. This is especially significant in the case of HEV, because it is a major human pathogen. Due to the similarity of the methyltransferase and guanylyltransferase reactions within the alphavirus-like superfamily, it may even be possible to design compounds with broad-spectrum antiviral properties.
ACKNOWLEDGMENTS
We thank Airi Sinkko and Tarja Välimäki for excellent technical assistance and Anja Lampio for valuable advice.
This study was supported by The Academy of Finland (grant no. 8397) and by the Technology Development Centre (TEKES). It was also supported in part by a grant for study of non-A, non-B hepatitis, Research on Emerging and Re-emerging Infectious Diseases, Health Sciences Research grants, and the Ministry of Health and Welfare, Japan. L.K. is a Biocentrum Helsinki fellow, and T.L. is an expert of the Scientific Research on Priority Areas of the Science and Technology Agency, Japan.
REFERENCES
- 1.Ahola T, Ahlquist P. Putative RNA capping activities encoded by brome mosaic virus: methylation and covalent binding of guanylate by replicase protein 1a. J Virol. 1999;73:10061–10069. doi: 10.1128/jvi.73.12.10061-10069.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahola T, Kääriäinen L. A unique reaction in alphavirus mRNA capping: formation of a covalent complex of nonstructural protein nsP1 with 7-methyl-GMP. Proc Natl Acad Sci USA. 1995;92:507–511. doi: 10.1073/pnas.92.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahola T, den Boon J A, Ahlquist P. Helicase and capping enzyme active site mutations in brome mosaic virus protein 1a cause defects in template recruitment, negative-strand synthesis, and viral mRNA capping. J Virol. 2000;74:8803–8811. doi: 10.1128/jvi.74.19.8803-8811.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahola T, Kujala P, Tuittila M, Blom T, Laakkonen P, Hinkkanen A, Auvinen P. Effects of palmitoylation of replicase protein nsP1 on alphavirus infection. J Virol. 2000;74:6725–6733. doi: 10.1128/jvi.74.15.6725-6733.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahola T, Laakkonen P, Vihinen H, Kääriäinen L. Critical residues of Semliki Forest virus RNA capping enzyme involved in methyltransferase and guanylyltransferase-like activities. J Virol. 1997;71:392–397. doi: 10.1128/jvi.71.1.392-397.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahola T, Lampio A, Auvinen P, Kääriäinen L. Semliki Forest virus mRNA capping enzyme requires association with anionic membrane phospholipids for activity. EMBO J. 1999;18:3164–3172. doi: 10.1093/emboj/18.11.3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ansari I H, Nanda S K, Durgapal H, Agrawal S, Mohanty S K, Gupta D, Jameel S, Panda S K. Cloning, sequencing, and expression of the hepatitis E virus (HEV) nonstructural open reading frame 1 (ORF1) J Med Virol. 2000;60:275–283. [PubMed] [Google Scholar]
- 8.Arankalle V A, Paranjape S, Emerson S U, Purcell R H, Walimbe A M. Phylogenetic analysis of hepatitis E virus isolates from India (1976–1993) J Gen Virol. 1999;80:1691–1700. doi: 10.1099/0022-1317-80-7-1691. [DOI] [PubMed] [Google Scholar]
- 9.Balayan M S, Andjaparidze A G, Savinskaya S S, Ketiladze E S, Braginsky D M, Savinov A P, Poleschuk V F. Evidence for a virus in non-A, non-B hepatitis transmitted via the fecal-oral route. Intervirology. 1983;20:23–31. doi: 10.1159/000149370. [DOI] [PubMed] [Google Scholar]
- 10.Bradley D, Andjaparidze A, Cook E H, McCaustland K, Jr, Balayan M, Stetler H, Velazquez O, Robertson B, Humphrey C, Kane M. Aetiological agent of enterically transmitted non-A, non-B hepatitis. J Gen Virol. 1988;69:731–738. doi: 10.1099/0022-1317-69-3-731. [DOI] [PubMed] [Google Scholar]
- 11.Chong L D, Rose J K. Membrane association of functional vesicular stomatitis virus matrix protein in vivo. J Virol. 1993;67:407–414. doi: 10.1128/jvi.67.1.407-414.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gross C H, Shuman S. RNA 5′ triphosphatase, nucleoside triphosphatase, and guanylyltransferase activities of baculovirus LEF-4 protein. J Virol. 1998;72:10020–10028. doi: 10.1128/jvi.72.12.10020-10028.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Favorov M, Drobeniuc J, Kosoy M, Childs J, Shapiro C, Mast E, Robertson B, Margolis H. Hepatitis E—an emerging zoonotic disease. Antivir Ther. 2000;5:19. [Google Scholar]
- 14.Jameel S, Zafrullah M, Ozdener M H, Panda S K. Expression in animal cells and characterization of the hepatitis E virus structural proteins. J Virol. 1996;70:207–216. doi: 10.1128/jvi.70.1.207-216.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kabrane-Lazizi Y, Meng X-J, Purcell R H, Emerson S. Evidence that the genomic RNA of hepatitis E virus is capped. J Virol. 1999;73:8848–8850. doi: 10.1128/jvi.73.10.8848-8850.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kong F, Sivakumara K, Kao C. The N-terminal half of the Brome mosaic virus 1a protein has RNA capping-associated activities: specificity for GTP and S-adenosylmethionine. Virology. 1999;259:200–210. doi: 10.1006/viro.1999.9763. [DOI] [PubMed] [Google Scholar]
- 17.Koonin E V, Gorbalenya A E, Purdy M A, Rozanov M N, Reyes G R, Bradley D W. Computer-assisted assignment of functional domains in the nonstructural polyprotein of hepatitis E virus: delineation of an additional group of positive-strand RNA plant and animal viruses. Proc Natl Acad Sci USA. 1992;89:8259–8263. doi: 10.1073/pnas.89.17.8259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koonin E V, Dolja V V. Evolution and taxonomy of positive-strand RNA virus: implication of comparative analysis of amino acid sequences. Crit Rev Biochem Mol Biol. 1993;28:375–430. doi: 10.3109/10409239309078440. [DOI] [PubMed] [Google Scholar]
- 19.Kujala P, Ahola T, Ehsani N, Auvinen P, Vihinen H, Kääriäinen L. Intracellular distribution of rubella virus nonstructural protein P150. J Virol. 1999;73:7805–7811. doi: 10.1128/jvi.73.9.7805-7811.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laakkonen P, Hyvönen M, Peränen J, Kääriäinen L. Expression of Semliki Forest virus nsP1-methyltransferase in insect cells and in Escherichia coli. J Virol. 1994;68:7417–7425. doi: 10.1128/jvi.68.11.7418-7425.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laakkonen P, Ahola T, Kääriäinen L. The effects of palmitoylation on membrane association of Semliki Forest virus capping enzyme nsP1. J Biol Chem. 1996;271:7418–7425. doi: 10.1074/jbc.271.45.28567. [DOI] [PubMed] [Google Scholar]
- 22.Labrique A B, Thomas D L, Stoszek S K, Nelson K E. Hepatitis E: an emerging infectious disease. Epidemiol Rev. 1999;21:162–179. doi: 10.1093/oxfordjournals.epirev.a017994. [DOI] [PubMed] [Google Scholar]
- 23.Lampio A, Ahola T, Darzynkiewicz E, Stepinski J, Jankowska-Anyzska M, Kääriäinen L. Guanosine nucleotide analogs as inhibitors of alphavirus mRNA capping enzyme. Antivir Res. 1999;42:35–46. doi: 10.1016/s0166-3542(99)00011-x. [DOI] [PubMed] [Google Scholar]
- 24.Lampio A, Kilpeläinen I, Pesonen S, Karhi K, Auvinen P, Somerharju P, Kääriäinen L. Membrane-binding mechanism of an RNA virus capping enzyme. J Biol Chem. 2000;275:37853–37859. doi: 10.1074/jbc.M004865200. [DOI] [PubMed] [Google Scholar]
- 25.Li T-C, Yamakawa Y, Suzuki K, Tatsumi M, Razak M A, Uchida T, Takeda N, Miyamura T. Expression and self-assembly of empty virus-like particles of hepatitis E virus. J Virol. 1997;71:7207–7213. doi: 10.1128/jvi.71.10.7207-7213.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y I, Chen Y-J, Hsu Y-H, Meng M. Characterization of the AdoMet-dependent guanylyltransferase activity that is associated with the N terminus of bamboo mosaic virus replicase. J Virol. 2001;75:782–788. doi: 10.1128/JVI.75.2.782-788.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merits A, Kettunen R, Mäkinen K, Lampio A, Auvinen P, Kääriäinen L, Ahola T. Virus-specific capping of tobacco mosaic virus RNA: methylation of GTP prior to formation of covalent complex p126–m7GMP. FEBS Lett. 1999;455:45–48. doi: 10.1016/s0014-5793(99)00856-x. [DOI] [PubMed] [Google Scholar]
- 28.Mi S, Durbin R, Huang H V, Rice C M, Stollar V. Association of the Sindbis virus RNA methyltransferase activity with the nonstructural protein nsP1. Virology. 1989;170:385–391. doi: 10.1016/0042-6822(89)90429-7. [DOI] [PubMed] [Google Scholar]
- 29.Panda S K, Ansari I H, Durgapal H, Agrawal S, Jameel S. The in vitro-synthesized RNA from a cDNA clone of hepatitis E virus is infectious. J Virol. 2000;74:2430–2437. doi: 10.1128/jvi.74.5.2430-2437.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peränen J, Laakkonen P, Hyvönen M, Kääriäinen L. The alphavirus replicase protein nsP1 is membrane-associated and has affinity to endocytic organelles. Virology. 1995;208:601–620. doi: 10.1006/viro.1995.1192. [DOI] [PubMed] [Google Scholar]
- 31.Peränen J, Rikkonen M, Hyvönen M, Kääriäinen L. T7 vectors with a modified T7 lac promoter for expression of proteins in Escherichia coli. Anal Biochem. 1996;236:371–373. doi: 10.1006/abio.1996.0187. [DOI] [PubMed] [Google Scholar]
- 32.Peränen J, Takkinen K, Kalkkinen N, Kääriäinen L. Semliki Forest virus-specific nonstructural protein nsP3 is a phosphoprotein. J Gen Virol. 1988;69:2165–2178. doi: 10.1099/0022-1317-69-9-2165. [DOI] [PubMed] [Google Scholar]
- 33.Purcell R H. Hepatitis E virus. In: Fields B, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2831–2843. [Google Scholar]
- 34.Ropp S L, Tam A W, Beames B, Purdy M, Frey T. Expression of the hepatitis E ORF1. Arch Virol. 2000;145:1321–1337. doi: 10.1007/s007050070093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rozanov M N, Koonin E V, Gorbalenya A E. Conservation of the putative methyltransferase domain: a hallmark of the “Sindbis-like” supergroup of positive-strand RNA viruses. J Gen Virol. 1992;73:2129–2134. doi: 10.1099/0022-1317-73-8-2129. [DOI] [PubMed] [Google Scholar]
- 36.Scheidel L, Durbin R K, Stollar V. SVLM21, a Sindbis virus mutant resistant to methionine deprivation, encodes an altered methyltransferase. Virology. 1989;173:408–414. doi: 10.1016/0042-6822(89)90553-9. [DOI] [PubMed] [Google Scholar]
- 37.Shuman S. Capping enzyme in eukaryotic mRNA synthesis. Prog Nucleic Acid Res Mol Biol. 1995;50:101–129. doi: 10.1016/s0079-6603(08)60812-0. [DOI] [PubMed] [Google Scholar]
- 38.Stewart L M D, Possee R D. Baculovirus expression vectors. In: Davison A J, Elliotts R M, editors. Molecular virology: a practical approach. Oxford, United Kingdom: IRL Press; 1993. pp. 227–256. [Google Scholar]
- 39.Supanaranond K, Takeda N, Yamazaki S. The complete nucleotide sequence of a variant of coxsackievirus A24. Virus Genes. 1992;6:149–158. doi: 10.1007/BF01703064. [DOI] [PubMed] [Google Scholar]
- 40.Tam A W, Yarbough P O, Bradley D W. Hepatits E virus. In: Granoff A, Webster R G, editors. Encyclopedia of virology. 2nd ed. New York, N.Y: Academic Press; 1999. pp. 669–676. [Google Scholar]
- 41.Torresi J, Meanger J, Lambert P, Li F, Locarnini S A, Anderson D A. High-level expression of the capsid protein of hepatitis E virus in diverse eukaryotic cells using the Semliki Forest virus replicon. J Virol Methods. 1997;69:81–91. doi: 10.1016/s0166-0934(97)00142-0. [DOI] [PubMed] [Google Scholar]
- 42.Torresi J, Li F, Locarnini S A, Anderson D A. Only the non-glycosylated fraction of hepatitis E virus capsid (open reading frame 2) protein is stable in mammalian cells. J Gen Virol. 1999;80:1185–1188. doi: 10.1099/0022-1317-80-5-1185. [DOI] [PubMed] [Google Scholar]
- 43.Uchida T, Win K M, Suzuki K, Komatsu K, Iida F, Shikata T, Mizuno K, Soe S, Myint H, Tin K M, Nakane K. Serial transmission of a putative causative virus of enterically transmitted non-A, non-B hepatitis to Macaca fascicularis and Macaca mulatta. Jpn J Exp Med. 1990;40:13–21. [PubMed] [Google Scholar]
- 44.Xing L, Kato K, Li T, Takeda N, Miuamura T, Hammar L, Cheng R H. Recombinant hepatitis E capsid protein self-assembles into dual-domain T=1 particles presenting native virus isotopes. Virology. 1999;265:35–45. doi: 10.1006/viro.1999.0005. [DOI] [PubMed] [Google Scholar]
- 45.Zafrullah M, Ozdener M H, Panda S K, Jameel S. The ORF3 protein of hepatitis E virus is a phosphoprotein that associates with the cytoskeleton. J Virol. 1997;71:9045–9053. doi: 10.1128/jvi.71.12.9045-9053.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zafrullah M, Ozdener M H, Kumar R, Panda S K, Jameel S. Mutational analysis of glycosylation, membrane translocation, and cell surface expression of the hepatitis E virus ORF2 protein. J Virol. 1999;73:4074–4082. doi: 10.1128/jvi.73.5.4074-4082.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]