Abstract
Background: Hypertension is a major risk factor for ischemic stroke. An important strategy in controlling hypertension is dietary modification. The present study evaluates the effect of Dietary Approaches to Stop Hypertension (DASH) diet on the risk of ischemic stroke. Methods: A case–control study was carried out, including 214 ischemic stroke cases recruited within the first 48 h of diagnosis and 214 controls, divided equally into hospitalized and non-hospitalized participants. Controls were matched to cases based on age and gender. Socio-demographic characteristics were assessed, in addition to adherence to the DASH diet, which was measured using a preconstructed DASH diet index (ranging from 0 (lowest) to 11 (highest)). For stroke patients, Modified Rankin Score (mRS) was measured to assess disability. Results: Smoking, hypertension, hyperlipidemia, atrial fibrillation, and myocardial infarction were significantly associated with ischemic stroke (p < 0.001). Higher adherence to the DASH diet was correlated to lower rates of stroke, where cases scored 5.042 ± 1.486 compared to 6.654 ± 1.471 for controls (p < 0.001). Eating more grains, vegetables, fruits, dairy products, nuts, seeds, and beans, and lower levels of fat, fewer sweets, and less sodium were associated with lower rates of ischemic stroke (p = 0.038 for sweets and p < 0.001 for all the remaining), while meat, poultry, and fish did not have any significant effect (p = 0.46). A multivariate analysis showed that lower adherence to the DASH diet (p < 0.001, OR: 0.526, CI95% 0.428–0.645) was associated with a higher incidence of ischemic stroke and an increased likelihood of having high disability levels (mRS 5–6) (p = 0.041, OR: 2.49 × 10−8, CI95% 0–2.49 × 10−8). Conclusions: The relation between the DASH diet and risk of stroke highlights the necessity for strict adherence to dietary restrictions, suggesting a protective role for the DASH diet in stroke pathogenesis and prognosis.
Keywords: ischemic stroke, risk factors, DASH diet, hypertension, disability
1. Background
Stroke is one of the main causes of death and functional inability worldwide [1]. In the last decade, the global incidence of stroke increased by 20% in low- and middle-income countries such as Lebanon [2]. Stroke has a great impact on the quality of life of patients and their family carers who provide long-term day-to-day care. Not only the patients but also their caregivers need professional attention and support in order to maintain their own physical and emotional health and well-being [3].
Risk factors for stroke can be categorized as modifiable and nonmodifiable. Age, sex, and race are nonmodifiable, while hypertension, smoking, diet, and physical inactivity are the main reported modifiable risk factors, comprising around 90% of all the stroke-related risk factors [4].
Hypertension is one of the major risk factors causing stroke. It can lead to stroke through several mechanisms, such as causing alterations in and damage to the endothelium, which can lead to local thrombi formation and ischemic lesions. Moreover, degenerative changes in smooth muscle cells and endothelium caused by hypertension predispose for intracerebral hemorrhages. Furthermore, hypertension accelerates the arteriosclerotic process, which increases the risk of cerebral lesions related to stenosis and embolism [5].
An unhealthy diet is one of the most important behavioral risk factors for hypertension [6]. The Dietary Approaches to Stop Hypertension (DASH) diet originated early in the 1990s [7]. It underwent several innovative features, where DASH trials were assessed in the field of nutrition and blood pressure research. By shifting an individual’s diet to follow a certain pattern, the DASH diet achieved impressive results in lowering blood pressure [8]. The DASH diet has been advocated as the first-line pharmacologic therapy, along with lifestyle modifications [7]. This type of diet is easier to follow compared to other types, as it targets major food groups such as vegetables, fruits, carbohydrates, low-fat dairy products, lean meat products, nuts, and grains. Another important point is that the DASH diet promotes a high intake of potassium (K), calcium (Ca), magnesium (Mg), and fibers [9].
In addition to treating hypertension, the DASH diet helps lower blood glucose levels, triglycerides, low-density lipoprotein (LDL-C), and insulin resistance, making it a management strategy in metabolic syndromes. Through these effects, this diet could potentially aid in preventing atherosclerosis, leading to a decrease in the incidence of consequent diseases, such as ischemic stroke [10].
There is evidence that shows that the DASH diet lowers the risk of stroke directly and through acting on risk factors, yet there are limited studies on its effectiveness [11]. Therefore, this study aims to assess the relation between the DASH diet and ischemic stroke, in addition to its effect in the presence of other risk factors. Also, this study aims to assess the effect of DASH diet adherence on disability levels in ischemic stroke patients, confirming its predicted role in decreasing ischemic stroke.
2. Materials and Methods
2.1. Study Design
A case–control study was carried out to assess the effect of DASH diet adherence on ischemic stroke. An informed consent form was given to all participants, outlining the study’s objectives, benefits, and concerns, and assuring the confidentiality of the collected information. Participation was entirely optional. Information was gathered between February 2023 and December 2023 by collecting clinical information from patients’ medical records and a face-to-face interview to assess dietary habits.
2.2. Participants
All included cases were Lebanese people, aged 18 and above, admitted due to an incidence of ischemic stroke to Sahel General Hospitals or Al Rassoul Al Azam Hospital in Beirut.
Inclusion criteria: To be included, patients must have been recruited during the first 3 days following stroke, while the patient is still in the observation period. The diagnosis must be confirmed by computed tomography (CT) and/or magnetic resonance imaging (MRI). Clinical confirmation of the diagnosis was also needed before including each case [12]. Controls were gender- and age-matched, with no clinical indications of stroke, no history of stroke, nor any known risk factors correlated to hypercoagulability or other states linked to cerebrovascular diseases. Controls were recruited from the same hospitals (48%), including patients attending outpatient clinics for illnesses not related to cerebrovascular diseases such as acute kidney injury, bone fractures, COPD exacerbation, pneumonia, urinary tract infection, cataracts, and diabetic ketoacidosis, or from the general population (52%), including visitors or relatives of patients.
Exclusion criteria: The exclusion of cases was based on the absence of consent, the absence of clinical confirmation or a CT/MRI, and the presence of other types of cerebrovascular attack (CVA), such as transient ischemic attack or hemorrhagic stroke [13]. For controls, exclusion was based on the absence of consent and admission for a cause that is a directly related risk of stroke.
2.3. Variables and Data Source Measures
The questionnaire was filled out using patient’s medical file records and via a face-to-face interview that took around 20 min to answer.
The socio-demographic characteristics of each participant were assessed, such as age, gender, marital status, education, and employment, in addition to pre-existing health-related conditions, such as smoking, family history of stroke, hypertension (systolic blood pressure > 130 mm Hg or diastolic blood pressure > 80 mm Hg [14]), hyperlipidemia (elevated lipid levels: cholesterol > 200 mg/dL or triglyceride > 15 mg/dL [15]), deep vein thrombosis (DVT) or pulmonary embolism (PE) (blood clots venous circulation or pulmonary circulation [16]), atrial fibrillation (cardiac electricity disturbance [17]), migraine (type of headache [18]), and myocardial infarction (MI) (decreased blood flow to the myocardium [19]).
For ischemic stroke patients, the level of disability was measured using the Modified Rankin Scale, which classifies each patient based on their disability level, ranging from 0 (no disability) to 6 (death) [20].
To determine DASH diet adherence, a Food Frequency Questionnaire (FFQ), which targets 168 foods, was classified into the 11 different food groups (total grains, whole grain, vegetables, fruits, dairy food, meat—poultry and fish, nuts—seeds and dry beans, %kcal from fat, %kcal from saturated fatty acids, sweets, and sodium [21]. Each of the 11 groups was divided into three levels, where the best adherence was denoted by 1 point, moderate adherence by 0.5 points, and worse adherence by 0 points. The total score ranges from 0 (least adherent) to 33 (most adherent). This method is used and accurately explained in another study [21]. Furthermore, the DASH score was categorized into either three (low, moderate, and high adherence) or four categories (low, low–moderate, high–moderate, and high adherence) by dividing the score into equal categories.
2.4. Ethical Considerations
This study respects confidentiality and anonymity. Ethical approval was granted from the Institutional Review Board (IRB) at the hospitals included in the data collection (ID number: 1/2023).
2.5. Statistical Analysis
Data were analyzed using SPSS software version 25. A descriptive analysis was performed using frequencies and percentages for categorical variables and means and standard deviations for continuous variables. A bivariate analysis was carried out to identify potential risk factors for ischemic stroke, including the adherence to a DASH diet. Student’s test was utilized to compare means between two groups, and the ANOVA test to compare means between more than two groups. The Chi-square and Fisher exact tests were used to compare percentages between two groups. Simple linear regression was used to correlate between continuous variables. A p < 0.05 was considered statistically significant.
A binomial logistic regression model was performed to investigate the odds ratio (OR) with a 95% confidence interval (CI) for marital status, education, smoking, family history, hypertension, hyperlipidemia, atrial fibrillation, myocardial infarction, and DASH diet adherence level among participants with ischemic stroke and the control group. The Hosmer–Lemeshow test was non-significant, demonstrating the test’s adequacy. All covariates with a p < 0.2 in the bivariate analysis were included in the logistic regression model. The CI was set at 95%, and a value of p < 0.05 was considered significant.
Additionally, a multinomial logistic regression was conducted, taking the levels of disability, as measured by the mRS scoring system, as the dependent variable. All variables with a p < 0.2 were included in the final model as independent variables, including marital status, education, employment, age, smoking, hypertension, hyperlipidemia, DVT or PE, atrial fibrillation, migraine, myocardial infarct, and DASH diet adherence level.
3. Results
3.1. Effect of Socio-Demographic Factors on Ischemic Stroke
Table 1 shows the socio-demographic characteristics of cases and controls in this study, where a total of 428 participants, divided into 214 cases and 214 controls, were included. The mean age of cases was 68.589 ± 13.436, and that of controls was 66.841 ± 14.488; p = 0.196. Males were predominant in both groups, having a percentage of 51.86 in the cases and 50.47 in controls (p = 0.772). There was a significant difference in marital status and education between cases and controls (p value < 0.001), while no significance was reported in employment (p value = 0.65).
Table 1.
Bivariate analysis of socio-demographic characteristics associated with ischemic stroke.
| Factor | Category | Cases | Control | p Value | ||
|---|---|---|---|---|---|---|
| Number | Percentage | Number | Percentage | |||
| Gender | Female | 103 | 48.13 | 106 | 49.53 | 0.772 |
| Male | 111 | 51.86 | 108 | 50.47 | ||
| Marital Status | Single | 13 | 6.07 | 20 | 9.34 | <0.001 * |
| Married | 119 | 55.60 | 149 | 69.63 | ||
| Divorced | 5 | 2.34 | 4 | 1.87 | ||
| Widowed | 77 | 35.98 | 41 | 19.16 | ||
| Education | Not educated | 51 | 23.83 | 83 | 38.60 | <0.001 * |
| School education | 99 | 46.26 | 78 | 36.45 | ||
| Non-healthcare education | 60 | 28.04 | 36 | 16.82 | ||
| Healthcare education | 4 | 1.87 | 17 | 7.94 | ||
| Employment | Not employed | 152 | 71.03 | 147 | 68.69 | 0.65 |
| Employed | 31 | 14.48 | 29 | 13.55 | ||
| Free profession | 31 | 14.48 | 38 | 17.76 | ||
| Age | Mean + SD | 68.589 ± 13.436 | 66.841 ± 14.488 | 0.196 | ||
* represents p < 0.05.
3.2. Effect of Socio-Demographic Factors on DASH Diet Adherence
Table 2 shows the level of adherence to the DASH diet in different socio-demographic groups. Females had a significantly higher level of adherence (6.16 ± 1.611) compared to males (5.55 ± 1.7); p < 0.001. Having a non-healthcare-related education and being employed were associated with significantly lower levels of adherence (p = 0.044 and p = 0.026, respectively). There was no significance reported in marital status and age (p value = 0.976 and 0.084, respectively).
Table 2.
Bivariate analysis of socio-demographic characteristics associated with DASH diet adherence score.
| Factor | Category | DASH Score | p Value |
|---|---|---|---|
| Mean ± SD | |||
| Gender | Female | 6.16 ± 1.611 | <0.001 * |
| Male | 5.55 ± 1.7 | ||
| Marital Status | Single | 5.879 ± 1.653 | 0.976 |
| Married | 5.823 ± 1.722 | ||
| Divorced | 5.778 ± 1.563 | ||
| Widowed | 5.903 ± 1.629 | ||
| Education | Not educated | 6.071 ± 1.471 | 0.044 * |
| School education | 5.805 ± 1.631 | ||
| Non-healthcare education | 5.51 ± 1.998 | ||
| Healthcare education | 6.333 ± 1.599 | ||
| Employment | Not employed | 5.992 ± 1.652 | 0.026 * |
| Employed | 5.475 ± 1.812 | ||
| Free profession | 5.551 ± 1.638 | ||
| Age | R (R squared) | 0.084 (0.007) | 0.084 |
* represents p < 0.05.
3.3. Effect of Pre-Existing Health-Related Conditions Associated with Ischemic Stroke
Table 3 shows the relationship between ischemic stroke and several factors. Smoking was significantly associated with a higher risk of ischemic stroke, where 126 (58.88%) of cases were smokers compared to 88 (41.12%) of the controls (p < 0.001). Additionally, 105 (49.06%) of cases had a positive family history of stroke, versus only 29 (13.55%) controls (p < 0.001). Regarding hypertension, more than 90% (194 participants) of the cases were hypertensive while only half of the controls were hypertensive (p < 0.001). When compared to controls, cases had a higher rate of hyperlipidemia compared to controls (151 (70.56%) vs. 81 (37.85%); (p < 0.001)). Patients with atrial fibrillation also had a higher risk of having an ischemic stroke (34.58%) than the controls (6.07%) (p < 0.001). Similarly, the risk of developing stroke in patients with MI was higher in cases (49 cases, 22.90%) compared to controls (17 controls, 7.94%) (p < 0.001). Having DVT/PE or migraine was shown to have no significant difference between cases and controls (p = 0.111 and p = 0.21, respectively).
Table 3.
Bivariate analysis of pre-existing health-related conditions on ischemic stroke.
| Factor | Category | Cases | Control | p Value | ||
|---|---|---|---|---|---|---|
| Number | Percentage | Number | Percentage | |||
| Smoking | Yes | 126 | 58.88 | 88 | 41.12 | <0.001 * |
| No | 88 | 41.12 | 126 | 58.88 | ||
| Family history of stroke | Yes | 105 | 49.06 | 29 | 13.55 | <0.001 * |
| No | 109 | 50.94 | 185 | 86.45 | ||
| Hypertension | Yes | 194 | 90.65 | 107 | 50 | <0.001 * |
| No | 20 | 9.35 | 107 | 50 | ||
| Hyperlipidemia | Yes | 151 | 70.56 | 81 | 37.85 | <0.001 * |
| No | 63 | 29.44 | 133 | 62.15 | ||
| DVT or PE | Yes | 27 | 11.21 | 17 | 7.94 | 0.111 |
| No | 187 | 87.39 | 197 | 92.06 | ||
| Atrial fibrillation | Yes | 74 | 34.58 | 13 | 6.07 | <0.001 * |
| No | 140 | 65.42 | 201 | 93.93 | ||
| Migraine | Yes | 28 | 13.08 | 27 | 12.62 | 0.21 |
| No | 186 | 86.92 | 187 | 87.38 | ||
| Myocardial infarction | Yes | 49 | 22.90 | 17 | 7.94 | <0.001 * |
| No | 165 | 77.10 | 197 | 92.06 | ||
* represents p < 0.05.
3.4. Effect of DASH Diet Adherence on Ischemic Stroke
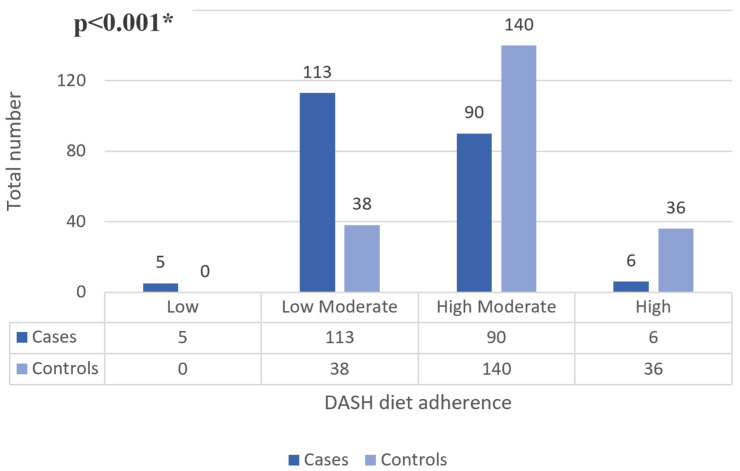
Figure 1 shows the level of adherence to the DASH diet in cases and controls, which was significantly lower in ischemic stroke patients (p < 0.001). The number of cases with a high level of adherence was lower compared to controls (36 vs. 6). Regarding low adherence, 5 cases had low adherence compared to 0 controls. A total of 113 cases had low–moderate adherence compared to 38 controls, and 90 cases had high–moderate adherence compared to 140 controls.
Figure 1.
Descriptive and bivariate analysis of DASH diet adherence level on ischemic stroke. * represents p < 0.05.
Table 4 shows the level of adherence to the DASH diet and each of its factors in cases and controls. The total score for DASH diet adherence was significantly lower in cases (5.042 ± 1.486) compared to controls (6.654 ± 1.471), (p < 0.001). Considering each factor alone, adherence was significantly lower in cases compared to controls for all factors (p = 0.038 for sweets and p < 0.001 for all the remaining), except for meat, poultry, and fish, which did not show any significant difference (p = 0.46).
Table 4.
Bivariate analysis of DASH diet adherence associated with ischemic stroke.
| Factor | Category | Cases | Control | p Value | ||
|---|---|---|---|---|---|---|
| Number | Percentage | Number | Percentage | |||
| Total grain | >7 | 21 | 9.81 | 41 | 19.16 | <0.001 * |
| 2–3 | 128 | 59.81 | 59 | 27.57 | ||
| <5 | 65 | 30.38 | 114 | 53.27 | ||
| Whole grain | >2 | 39 | 18.22 | 56 | 21.17 | <0.001 * |
| 1 | 64 | 29.90 | 98 | 45.79 | ||
| <1 | 111 | 5.14 | 60 | 28.04 | ||
| Vegetables | >4 | 57 | 26.63 | 116 | 54.21 | <0.001 * |
| 2–3 | 135 | 63.09 | 73 | 34.11 | ||
| <2 | 22 | 10.28 | 25 | 11.68 | ||
| Fruits | >4 | 41 | 19.16 | 101 | 47.20 | <0.001 * |
| 2–3 | 100 | 46.73 | 76 | 35.51 | ||
| <2 | 73 | 34.11 | 37 | 17.29 | ||
| Dairy foods | >2 | 82 | 38.32 | 121 | 56.54 | <0.001 * |
| 1 | 104 | 48.60 | 63 | 24.44 | ||
| <1 | 28 | 13.08 | 30 | 14.02 | ||
| Meat, poultry, and fish | >2 | 61 | 28.50 | 69 | 32.24 | 0.46 |
| 3 | 100 | 46.73 | 102 | 47.66 | ||
| >4 | 53 | 24.77 | 43 | 20.09 | ||
| Nuts, seeds, and dry beans | >4 | 60 | 28.04 | 41 | 19.16 | <0.001 * |
| 2–3 | 98 | 45.79 | 76 | 35.51 | ||
| <2 | 56 | 26.17 | 97 | 45.33 | ||
| %Kcal from fat | <30% | 80 | 37.38 | 26 | 12.15 | <0.001 * |
| 31–32 | 110 | 51.41 | 81 | 37.85 | ||
| >33 | 24 | 11.21 | 107 | 50 | ||
| %Kcal from saturated fatty acids | <10 | 84 | 39.25 | 24 | 11.22 | <0.001 * |
| 11–12 | 104 | 48.60 | 76 | 35.52 | ||
| >13 | 26 | 12.15 | 114 | 53.27 | ||
| Sweets | <5 | 36 | 16.82 | 36 | 16.82 | 0.038 * |
| 6–7 | 74 | 34.58 | 51 | 23.83 | ||
| >8 | 104 | 48.60 | 127 | 59.35 | ||
| Sodium | >1500 | 113 | 52.80 | 29 | 13.55 | <0.001 * |
| 1501–2400 | 91 | 42.52 | 63 | 29.44 | ||
| >2400 | 10 | 4.68 | 122 | 57.01 | ||
| Total Score | Mean + SD | 5.042 ± 1.486 | 6.654 ± 1.471 | <0.001 * | ||
* represents p < 0.05.
3.5. Stroke-Related Characteristics in Ischemic Stroke Patients
Table 5 shows the disability level, number of strokes and age at first diagnosis for the included cases. Around one-third of cases (65 cases; 30%) had moderate disability (mRS score = 3), around one-fourth (50 cases; 23.4%) had slight disability (mRS score = 2), and another one-fourth (46 cases; 21.5%) had moderate–severe disability (mRS score = 4). Only two cases (0.9%) had no symptoms and eight cases (3.7%) died. The majority of patients that were enrolled had only one stroke (151 participants, 70.6%), while one-fourth had two strokes (23.8%), and the average age at the first stroke was 67.724 ± 13.284.
Table 5.
Descriptive analysis of stroke-related characteristics in ischemic stroke patients.
| Factor | Category | Number | Percentage |
|---|---|---|---|
| mRS score | 0 | 2 | 0.9 |
| 1 | 8 | 3.7 | |
| 2 | 50 | 23.4 | |
| 3 | 65 | 30.4 | |
| 4 | 46 | 21.5 | |
| 5 | 35 | 16.4 | |
| 6 | 8 | 3.7 | |
| Number of strokes | 1 | 151 | 70.6 |
| 2 | 51 | 23.8 | |
| 3 | 10 | 4.7 | |
| 4 | 2 | 0.9 | |
| Age | Mean ± SD | 68.589 ± 13.436 | |
| Age at first stroke | Mean + SD | 67.724 ± 13.284 | |
3.6. Effect of DASH Diet Adherence on Disability Level (mRS) in Ischemic Stroke Patients
Table 6 shows the association between socio-demographic characteristics, pre-existing health-related factors, DASH diet adherence level, and disability level in ischemic stroke patients.
Table 6.
Bivariate analysis of socio-demographic and health-related factors, and DASH diet adherence, and their association with disability level (mRS) in ischemic stroke.
| Factor | Category | mRS Category | Total | p Value | ||
|---|---|---|---|---|---|---|
| 0 until 2 | 3 until 4 | 5 until 6 | ||||
| Gender | Female | 27 (26.21%) |
54 (52.43%) |
22 (21.36%) |
103 | 0.816 |
| Male | 33 (29.73%) |
57 (51.35%) |
21 (18.92%) |
111 | ||
| Marital Status | Single | 2 (15.38%) |
8 (61.54%) |
3 (23.08%) |
13 | 0.003 * |
| Married | 38 (31.93%) |
66 (55.46%) |
15 (12.61%) |
119 | ||
| Divorced | 4 (80%) |
1 (20%) |
0 (0%) |
5 | ||
| Widowed | 16 (20.78%) |
36 (46.75%) |
25 (32.47%) |
77 | ||
| Education | Not educated | 6 (11.76%) |
27 (52.94%) |
18 (35.3%) |
51 | 0.001 * |
| School education | 25 (25.25%) |
57 (57.57%) |
17 (17.18%) |
99 | ||
| Non-healthcare education | 27 (45%) |
26 (43.33%) |
7 (11.67%) |
60 | ||
| Healthcare education | 2 (50%) |
1 (25%) |
1 (25%) |
4 | ||
| Employment | Not employed | 32 (21.05%) |
84 (55.26%) |
36 (23.69%) |
152 | 0.004 * |
| Employed | 16 (51.61%) |
13 (41.94%) |
2 (6.45%) |
31 | ||
| Free profession | 12 (38.71%) |
14 (45.16%) |
5 (16.13%) |
31 | ||
| Age | Mean + SD | 63.15 + 15.741 | 69.36 + 11.952 | 74.186 + 10.839 | 68.589 + 13.436 | <0.001 * |
| Smoking | Yes | 37 (29.37%) |
71 (56.35%) |
18 (14.28%) |
126 | 0.038 * |
| No | 23 (26.14%) |
40 (45.45%) |
25 (28.41%) |
88 | ||
| Family history of stroke | Yes | 31 (29.52%) |
53 (50.48%) |
21 (20%) |
105 | 0.887 |
| No | 29 (26.61%) |
58 (53.21%) |
22 (20.18%) |
109 | ||
| Hypertension | Yes | 49 (25.25%) |
104 (53.61%) |
41 (21.14%) |
194 | 0.018 * |
| No | 11 (55%) |
7 (35%) |
2 (10%) |
20 | ||
| Hyperlipidemia | Yes | 31 (20.53%) |
87 (57.61%) |
33 (21.85%) |
151 | 0.001 * |
| No | 29 (46.03%) |
24 (38.1%) |
10 (15.87%) |
63 | ||
| DVT or PE | Yes | 3 (11.11%) |
14 (51.85%) |
10 (37.04%) |
27 | 0.023 * |
| No | 57 (30.48%) |
97 (51.87%) |
33 (17.65%) |
187 | ||
| Atrial fibrillation | Yes | 10 (13.51%) |
41 (55.41%) |
23 (31.08%) |
74 | <0.001 * |
| No | 50 (35.71%) |
70 (50%) |
20 (14.29%) |
140 | ||
| Migraine | Yes | 20 (71.43%) |
8 (28.57%) |
0 | 28 | <0.001 * |
| No | 40 (21.51%) |
103 (55.38%) |
43 (23.11%) |
186 | ||
| Myocardial infarction | Yes | 10 (20.41%) |
25 (51.02%) |
14 (28.57%) |
49 | 0.165 |
| No | 50 (30.3%) |
86 (52.12%) |
29 (17.58%) |
165 | ||
| DASH adherence | Low | 2 (40%) |
0 (0%) | 3 (60%) |
5 | 0.018 * |
| Moderate | 54 (26.60%) |
110 (54.19%) |
39 (19.21%) |
203 | ||
| High | 4 (66.68%) |
1 (16.67%) |
1 (16.67%) |
6 | ||
* represents p < 0.05.
Higher ages were associated with a higher mRS score, corresponding to a higher disability level (p < 0.001). Similarly, the percentage of those with hypertension, hyperlipidemia, DVT or PE, atrial fibrillation, and migraine was higher in patients with high disability levels compared to those with lower disability levels (p = 0.023 for DVT or PE and p < 0.001 for the rest).
DASH diet adherence and the level of disability (mRS score) showed a significant association (p = 0.018). A total of 60% of those with low adherence to the DASH diet had an mRS score between 5 and 6, compared to 0% and 40% for those with scores of 3–4 and 0–2, respectively. Of those with high adherence to the diet, 66.67% had an mRS score of 0–2, 16.67% had an mRS score of 3–4, and 16.67% had an mRS score of 5–6.
3.7. Multivariate Analysis: Logistic Regression
Table 7 shows the bimonial regression regarding the incidence of ischemic stroke. Being divorced (p = 0.036, OR: 3.884, CI95% 1.092–13.818), being non-educated (p = 0.004, OR: 2.713, CI95% 1.38–5.333), having no education following school level (p < 0.001, OR: 5.602, CI95%2.369–13.246), being a smoker (p = 0.035, OR: 1.885, CI95% 1.044–3.402), having a family history of stroke (p < 0.001, OR: 4.707, CI95% 2.48–8.934), having hypertension (p < 0.001, OR: 6.536, CI95% 3.093–13.81), having atrial fibrillation (p < 0.001, OR: 5.828, CI95% 2.647–12.831), and lower adherence to the DASH diet (p < 0.001, OR: 0.526, CI95% 0.428–0.645) were all associated with a higher incidence of ischemic stroke.
Table 7.
Multivariable analysis: Binomial regression regarding the incidence of ischemic stroke.
| Independent Variables | p Value | OR | CI 95% | |
|---|---|---|---|---|
| Lower Bound | Upper Bound | |||
| Marital Status | 0.03 * | |||
| Single | 0.488 | 1.540 | 0.455 | 5.212 |
| Married | 0.293 | 3.087 | 0.378 | 25.212 |
| Divorced | 0.036 * | 3.884 | 1.092 | 13.818 |
| Educational Level | 0.01 * | |||
| Not educated | 0.004 * | 2.713 | 1.38 | 5.333 |
| School education | <0.001 * | 5.602 | 2.369 | 13.246 |
| Non-healthcare-related education | 0.788 | 1.228 | 0.274 | 5.497 |
| Smoking | 0.035 * | 1.885 | 1.044 | 3.402 |
| Family History of Stroke | <0.001 * | 4.707 | 2.48 | 8.934 |
| Hypertension | <0.001 * | 6.536 | 3.093 | 13.81 |
| Hyperlipidemia | 0.285 | 1.397 | 0.757 | 2.578 |
| DVT or PE | 0.692 | 0.822 | 0.311 | 2.17 |
| Atrial fibrillation | <0.001 * | 5.828 | 2.647 | 12.831 |
| Myocardial Infarction | 0.444 | 1.37 | 0.611 | 3.073 |
| DASH score | <0.001 * | 0.526 | 0.428 | 0.645 |
| Constant | 0.659 | 0.656 | ||
Dependent variable: cases vs. controls; * represents p < 0.05.
Table 8 shows the multinomial regression regarding the disability level in stroke patients. Having atrial fibrillation versus not having atrial fibrillation (p < 0.001, OR: 10.286, CI95% 3.07–34.465) and low versus high DASH diet adherence (p = 0.041, OR: 44.263, CI95% 1.163–1684.246) were significantly associated with an increased likelihood of having moderate disability levels (mRS 3–4) compared to those with low disability levels (mRS 0–2).
Table 8.
Multivariable analysis: Multinomial regression of the disability level in ischemic stroke patients.
| Independent Variables | p Value | OR | CI 95% | |
|---|---|---|---|---|
| Lower Bound | Upper Bound | |||
| Model 1: mRS 3–4 vs. mRS 0–2 | ||||
| Age | 0.154 | 0.950 | 0.886 | 1.019 |
| Single vs. widowed | 0.586 | 2.224 | 0.126 | 39.344 |
| Married vs. widowed | 0.300 | 0.454 | 0.102 | 2.022 |
| Not educated vs. HC-related education | 0.920 | 0.812 | 0.014 | 47.219 |
| School education vs. HC-related education | 0.391 | 0.182 | 0.004 | 8.879 |
| Non-HC vs. HC education | 0.122 | 0.049 | 0.001 | 2.244 |
| Not employed vs. free profession | 0.784 | 1.273 | 0.226 | 7.169 |
| Employed vs. free profession | 0.369 | 0.353 | 0.036 | 3.419 |
| Smoker | 0.747 | 0.821 | 0.248 | 2.723 |
| Hypertensive | 0.609 | 1.779 | 0.195 | 16.185 |
| Hyperlipidemic | 0.209 | 2.227 | 0.639 | 7.757 |
| History of DVT or PE | 0.401 | 2.072 | 0.367 | 11.707 |
| History of atrial fibrillation | <0.001 * | 10.286 | 3.07 | 34.465 |
| History of myocardial Infarction | 0.314 | 1.875 | 0.551 | 6.373 |
| Low vs. high DASH adherence | 0.041 * | 44.263 | 1.163 | 1684.246 |
| Moderate vs. high DASH adherence | 0.246 | 5.431 | 0.312 | 94.642 |
| Model 2: mRS 5–6 vs. mRS 0–2 | ||||
| Age | 0.115 | 0.96 | 0.912 | 1.01 |
| Single vs. widowed | 0.273 | 3.929 | 0.339 | 45.482 |
| Married vs. widowed | 0.440 | 1.579 | 0.496 | 5.03 |
| Not educated vs. HC education | 0.419 | 3.935 | 0.142 | 108.91 |
| School vs. HC-related education | 0.76 | 1.644 | 0.067 | 40.128 |
| Non-HC vs. HC-related education | 0.807 | 0.679 | 0.03 | 15.16 |
| Not employed vs. Free profession | 0.042 * | 3.692 | 1.051 | 12.977 |
| Employed vs. Free profession | 0.624 | 1.381 | 0.38 | 5.024 |
| Smoker | 0.571 | 1.304 | 0.521 | 3.259 |
| Hypertensive | 0.737 | 1.259 | 0.327 | 4.843 |
| Hyperlipidemic | 0.055 | 2.513 | 0.981 | 6.44 |
| History of DVT or PE | 0.620 | 1.493 | 0.307 | 7.265 |
| History of atrial fibrillation | 0.007 * | 4.075 | 1.468 | 11.307 |
| History of migraine | <0.001 * | 0.069 | 0.019 | 0.252 |
| History of myocardial Infarction | 0.842 | 0.899 | 0.315 | 2.561 |
| Low vs. high DASH adherence | 0.041 * | 2.49 × 10−8 | 0 | 2.49 × 10−8 |
| Moderate vs. high DASH adherence | 0.059 | 12.668 | 0.91 | 176.427 |
Dependent variable: mRS categories; reference category: 0–2; * represents p < 0.05.
Having no employment versus a free profession (non-employed workers) (p < 0.001, OR: 3.692, CI95% 1.051–12.977), having atrial fibrillation versus not having atrial fibrillation (p = 0.007, OR: 4.075, CI95% 1.468–11.307), having migraine versus not having migraine (p < 0.001, OR: 0.069, CI95% 0.019–0.252), and low versus high DASH diet adherence (p = 0.041, OR: 2.49 × 10−8, CI95% 0–2.49 × 10−8) were significantly associated with an increased likelihood of having high disability levels (mRS 5–6) compared to those with low disability levels (mRS 0–2).
4. Discussion
This case–control study assessed the effect of some health-related factors on ischemic stroke incidence and disability levels, focusing on the effect of DASH diet adherence. Smoking, family history of stroke, hypertension, hyperlipidemia, atrial fibrillation, and myocardial infarction were associated with a higher risk of stroke, and hypertension, hyperlipidemia, DVT/PE, atrial fibrillation, and migraine were associated with a higher disability level. Furthermore, the results of this case–control study demonstrated a protective role for the DASH diet regarding the incidence of ischemic stroke, as cases had lower adherence to the DASH diet compared to controls. In addition, higher adherence to the DASH diet was found to be associated with a lower level of disability.
Similar to this study, several studies previously reached similar results regarding the increased risk of ischemic stroke caused by smoking, family history of ischemic stroke, dyslipidemia, atrial fibrillation, and MI (p < 0.001 for all factors) [22]. Smoking has a dose–response relationship with ischemic stroke, where lower smoking levels are associated with lower stroke levels [23]. Smoking is believed to cause this risk by acting on other risk factors, such as inducing atherosclerosis and leading to hypercoagulable states [24,25]. A family history of ischemic stroke is also correlated with higher risk; several studies have confirmed the presence of a genetic component in ischemic stroke [26]. Hyperlipidemia is also considered as one of the major risk factors, as it leads to the formation of plaque inside the blood vessels, leading to an increased risk of MI or stroke [27]. MI was also found to be associated with a higher incidence of stroke; the similarity between risk factors such as smoking and dislipidemia leads to their co-occurrence [28]. Around 9% of ischemic stroke patients have a silent MI, and the highest risk of stroke occurs 5 days after a heart attack [29,30]. In addition to hyperlipidemia, atrial fibrillation is linked to both MI and stroke by causing blood clots, increasing the risk of stroke by 5-fold [31].
On the other hand, this study found no significant correlation between DVT/PE, migraine and stroke incidence (p = 0.111 and p = 0.21, respectively). These is some contradiction in the literature, where some studies have found a positive correlation, while others did not [32]. Several studies suggested that DVT/PE are complications commonly seen in ischemic stroke patients [33], while the risk of ischemic stroke occurring after a DVT or PE remains low, ranging between 1 and 10 percent [34]. As for migraine, despite its possible co-occurrence with ischemic stroke, there is no strong evidence considering migraine as a risk factor. When ischemic stroke occurs during migraine attacks, it is differentiated from other strokes and called Migraneous Infarction, with a low incidence of 0.8/100,000/year [35]. Further investigation is needed to better understand this relationship.
As for hypertension, this study confirms its correlation with a higher rate of ischemic stroke (90.65% of cases; p < 0.001) and higher disability levels (95.35% of those with severe disability; p < 0.018). The results also showed that better adherence to the DASH diet was associated with a lower rate of severe disability (mRS 5–6). Only 16.67% of those with high adherence to the DASH diet had severe disability, compared to 19.21% of those with moderate adherence, and 60% for those with low adherence (p = 0.018).
Previous studies classified hypertension as the number one risk factor for stroke, being present in around 84% of cases [4]. Therefore, preventing and controlling hypertension are the main approaches to prevent stroke and minimize any resulting disability [36]. Of the multiple ways to control blood pressure, diet remains one of the most important factors, acting acutely and in the long-term [37]. Thus, those susceptible to ischemic stroke could benefit from antihypertensive diets.
The DASH diet consists of consuming vegetables, fruits, carbohydrates, low-fat dairy products, lean meat products, nuts, and grains, with a significant reduction in sodium intake [7]. It is designed to lower high blood pressure by decreasing the amount of saturated fat and salt intake [9]. It also has a natriuretic effect by interacting with the renin–angiotensin–aldosterone system (RAAS). This interaction has a hypotensive effect by stimulating hormonal and vascular responses [38]. Based on these effects, the DASH diet could also be of value in pharmacological interventions through managing hypertension [37].
Several mechanisms are believed to interact, leading to the prevention of ischemic stroke, following a high level of adherence to the DASH diet. The decrease in blood pressure is considered the main reason for the reduction in stroke incidence, but the DASH diet was also found to reduce lipid levels and body weight, which also contribute to this reduction [39,40]. Furthermore, metabolic syndrome and diabetes mellitus 2, which are linked to the pathology of ischemic stroke, were decreased following high adherence to the DASH diet [41,42,43]. Moreover, the DASH diet was found to decrease inflammation and to have an antioxidant role, both of which are believed to contribute to the pathophysiology of ischemic stroke [44,45].
Other studies assessed the effect of the DASH diet on stroke incidence. For instance, a study on a Chinese cohort found that adherence to the DASH diet was beneficial for reducing BP and stroke rates in the long term [46]. Similarly, a case–control study in Iran found that the rate of stroke was lower in those with higher levels of DASH diet adherence [47]. The results of these studies align with our findings, which favor a protective role for the DASH diet, with no studies to this day, to our knowledge, disagreeing with the above-mentioned role of DASH.
Compared to other diets, an advantage of the DASH diet is its conventional guidelines on the size and number of servings [48]. The DASH diet led to a significantly lower BP compared to those following a regular diet with restricted sodium [49]. In addition to its function in lowering blood pressure, it appeared to play a role in preventing the development of hypertension [9]. Several studies demonstrated that better blood pressure was recorded after following the DASH diet compared to a regular diet in patients at risk of hypertension [7]. The modification of the DASH diet’s macronutrients by increasing proteins and removing saturated fat had a favorable effect on maintaining an optimal BP and lowering systolic and diastolic blood pressure [50]. Furthermore, the 10-year Framingham score used for assessing cardiovascular risks was also reduced by approximately 13% in patients following a DASH diet [51]. Consequently, the 2013 AHA/ACC Guidelines considered the DASH diet as a dietary pattern for reducing BP and LDL-C [52].
A commitment to a healthy lifestyle based on a healthy diet, such as the DASH diet, reduces the risk of stroke in patients at risk and modifies the impact of comorbidity by ameliorating the disability score in stroke patients [47,53]. These findings support our results, which showed milder disability in stroke patients with a high adherence to the DASH diet. Although the DASH diet meets the global recommendations and demonstrated promising results in adherent patients, there are no clear studies on its long-term effectiveness and further studies should focus on the dietary adherence rate, in addition to providing a clear pathway to explaining how the DASH diet exerts its effect.
This study has some limitations that are worth mentioning. Due to the case–control nature of this study, the potential for recall bias is present, as participants may have recalled their dietary habits with a lack of precision. The cognitive impairment that might result from stroke may also affect the recall process. In addition, this study lacked a timing assessment when considering DASH diet adherence. Moreover, the matching between cases and controls was based on age and gender, disregarding other factors. For instance, educational level was significantly different between cases and controls, which might affect the credibility of the self-reported information. Furthermore, the majority of the included sample had moderate adherence to the DASH diet, with only a few having high or low adherence. This could limit the understanding of the effect of the DASH diet on disability rates. Further studies with other study designs are required to overcome such limitations, such as prospective cohort studies.
5. Conclusions
This study demonstrated a protective role for the DASH diet regarding ischemic stroke, and links adherence to this diet to a lower level of disability. Furthermore, this study confirmed that smoking, family history of ischemic stroke, hypertension, hyperlipidemia, atrial fibrillation, and myocardial increase the risk of stroke, and hypertension, hyperlipidemia, DVT/PE, atrial fibrillation, and migraine lead to a higher disability level. This role highlights the necessity for strict adherence to the DASH diet, especially in patients with higher susceptibility to ischemic stroke. Further studies are needed to better understand the mechanistic role of the DASH diet in preventing ischemic stroke, and to reach evidence-based guidelines regarding dietary approaches in patients with specific risk factors for ischemic stroke. The correlation between DASH diet adherence and specific treatments could also be of great benefit in managing ischemic stroke cases.
Author Contributions
All authors contributed to the study conception and design. Material preparation was performed by J.E.M. and M.G., data collection was performed by H.F., T.B., N.A. and M.H., and analyses were performed by J.E.M. The first draft of the manuscript was written by J.E.M. and M.G., and all authors commented on previous versions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was approved on January by the IRB committee at Sahel General Hospital, Beirut, Lebanon (IRB# 1/2023) approval: Jan 2023.
Informed Consent Statement
All participants consented verbally to participation in this study.
Data Availability Statement
Data are available upon request from corresponding author due to privacy.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Lackland D.T., Roccella E.J., Deutsch A.F., Fornage M., George M.G., Howard G., Kissela B.M., Kittner S.J., Lichtman J.H., Lisabeth L.D., et al. Factors Influencing the Decline in Stroke Mortality: A Statement from the American Heart Association/American Stroke Association. Stroke. 2014;45:315–353. doi: 10.1161/01.str.0000437068.30550.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khalid W., Rozi S., Ali T.S., Azam I., Mullen M.T., Illyas S., Un-Nisa Q., Soomro N., Kamal A.K. Quality of Life after Stroke in Pakistan. BMC Neurol. 2016;16:250. doi: 10.1186/s12883-016-0774-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Opara J., Jaracz K. Quality of Life of Post–Stroke Patients and Their Caregivers. J. Med. Life. 2010;3:216–220. [PMC free article] [PubMed] [Google Scholar]
- 4.Boehme A.K., Esenwa C., Elkind M.S.V. Stroke Risk Factors, Genetics, and Prevention. Circ. Res. 2017;120:472–495. doi: 10.1161/CIRCRESAHA.116.308398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johansson B.B. Hypertension Mechanisms Causing Stroke. Clin. Exp. Pharmacol. Physiol. 1999;26:563–565. doi: 10.1046/j.1440-1681.1999.03081.x. [DOI] [PubMed] [Google Scholar]
- 6.Feigin V.L., Roth G.A., Naghavi M., Parmar P., Krishnamurthi R., Chugh S., Mensah G.A., Norrving B., Shiue I., Ng M., et al. Global Burden of Stroke and Risk Factors in 188 Countries, during 1990-2013: A Systematic Analysis for the Global Burden of Disease Study 2013. Lancet Neurol. 2016;15:913–924. doi: 10.1016/S1474-4422(16)30073-4. [DOI] [PubMed] [Google Scholar]
- 7.Challa H.J., Ameer M.A., Uppaluri K.R. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2022. DASH Diet To Stop Hypertension. [PubMed] [Google Scholar]
- 8.The DASH Diet and Blood Pressure: EBSCOhost. [(accessed on 3 March 2024)]. Available online: https://web-p-ebscohost-com.ezproxy.aub.edu.lb/ehost/pdfviewer/pdfviewer?vid=0&sid=029f0a5d-f5eb-43a0-85ae-7ad2859184aa%40redis.
- 9.DASH Diet: Healthy Eating to Lower Your Blood Pressure—Mayo Clinic. [(accessed on 2 March 2024)]. Available online: https://www.mayoclinic.org/healthy-lifestyle/nutrition-and-healthy-eating/in-depth/dash-diet/art-20048456.
- 10.Tuttolomondo A., Puleo M.G., Velardo M.C., Corpora F., Daidone M., Pinto A. Molecular Biology of Atherosclerotic Ischemic Strokes. Int. J. Mol. Sci. 2020;21:9372. doi: 10.3390/ijms21249372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang T., Heianza Y., Sun D., Huang T., Ma W., Rimm E.B., Manson J.E., Hu F.B., Willett W.C., Qi L. Improving Adherence to Healthy Dietary Patterns, Genetic Risk, and Long Term Weight Gain: Gene-Diet Interaction Analysis in Two Prospective Cohort Studies. BMJ. 2018;360:j5644. doi: 10.1136/bmj.j5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hui C., Tadi P., Patti L. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2024. Ischemic Stroke. [PubMed] [Google Scholar]
- 13.Khaku A.S., Tadi P. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2024. Cerebrovascular Disease. [PubMed] [Google Scholar]
- 14.Iqbal A.M., Jamal S.F. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2024. Essential Hypertension. [PubMed] [Google Scholar]
- 15.Hill M.F., Bordoni B. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2024. Hyperlipidemia. [PubMed] [Google Scholar]
- 16.Office of the Surgeon General (US) National Heart, Lung, and Blood Institute (US) The Surgeon General’s Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. Office of the Surgeon General (US); Rockville, MD, USA: 2008. INTRODUCTION: Definitions of Deep Vein Thrombosis and Pulmonary Embolism. [PubMed] [Google Scholar]
- 17.Nesheiwat Z., Goyal A., Jagtap M. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2024. Atrial Fibrillation. [PubMed] [Google Scholar]
- 18.Pescador Ruschel M.A., De Jesus O. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2024. Migraine Headache. [PubMed] [Google Scholar]
- 19.Ojha N., Dhamoon A.S. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2024. Myocardial Infarction. [PubMed] [Google Scholar]
- 20.Pożarowszczyk N., Kurkowska-Jastrzębska I., Sarzyńska-Długosz I., Nowak M., Karliński M. Reliability of the Modified Rankin Scale in Clinical Practice of Stroke Units and Rehabilitation Wards. Front. Neurol. 2023;14:1064642. doi: 10.3389/fneur.2023.1064642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Folsom A.R., Parker E.D., Harnack L.J. Degree of Concordance with DASH Diet Guidelines and Incidence of Hypertension and Fatal Cardiovascular Disease. Am. J. Hypertens. 2007;20:225–232. doi: 10.1016/j.amjhyper.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alawneh K.Z., Al Qawasmeh M., Raffee L.A., Abuzayed B., Bani Hani D.A., Abdalla K.M., Al-Mnayyis A.M., Fataftah J. A Snapshot of Ischemic Stroke Risk Factors, Sub-Types, and Its Epidemiology: Cohort Study. Ann. Med. Surg. 2020;59:101–105. doi: 10.1016/j.amsu.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Markidan J., Cole J.W., Cronin C.A., Merino J.G., Phipps M.S., Wozniak M.A., Kittner S.J. Smoking and Risk of Ischemic Stroke in Young Men. Stroke. 2018;49:1276–1278. doi: 10.1161/STROKEAHA.117.018859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klein L.W. Pathophysiologic Mechanisms of Tobacco Smoke Producing Atherosclerosis. Curr. Cardiol. Rev. 2022;18:e110422203389. doi: 10.2174/1573403X18666220411113112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li C.-J., Liu Y., Chen Y., Yu D., Williams K.J., Liu M.-L. Novel Proteolytic Microvesicles Released from Human Macrophages after Exposure to Tobacco Smoke. Am. J. Pathol. 2013;182:1552–1562. doi: 10.1016/j.ajpath.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu S., Su Z., Miao J., Yu Y., Zhang S., Wu J., Zheng H., Zhang X., Zhong S., Li H., et al. Different Types of Family History of Stroke and Stroke Risk: Results Based on 655,552 Individuals. J. Stroke Cerebrovasc. Dis. 2019;28:587–594. doi: 10.1016/j.jstrokecerebrovasdis.2018.10.038. [DOI] [PubMed] [Google Scholar]
- 27.Alloubani A., Nimer R., Samara R. Relationship between Hyperlipidemia, Cardiovascular Disease and Stroke: A Systematic Review. Curr. Cardiol. Rev. 2021;17:e051121189015. doi: 10.2174/1573403X16999201210200342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muhammad I.F., Borné Y., Zaigham S., Söderholm M., Johnson L., Persson M., Melander O., Engström G. Comparison of Risk Factors for Ischemic Stroke and Coronary Events in a Population-Based Cohort. BMC Cardiovasc. Disord. 2021;21:536. doi: 10.1186/s12872-021-02344-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merkler A.E., Bartz T.M., Kamel H., Soliman E.Z., Howard V., Psaty B.M., Okin P.M., Safford M.M., Elkind M.S.V., Longstreth W.T. Silent Myocardial Infarction and Subsequent Ischemic Stroke in the Cardiovascular Health Study. Neurology. 2021;97:e436–e443. doi: 10.1212/WNL.0000000000012249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mooe T., Eriksson P., Stegmayr B. Ischemic Stroke after Acute Myocardial Infarction. A Population-Based Study. Stroke. 1997;28:762–767. doi: 10.1161/01.STR.28.4.762. [DOI] [PubMed] [Google Scholar]
- 31.Wolf P.A., Abbott R.D., Kannel W.B. Atrial Fibrillation as an Independent Risk Factor for Stroke: The Framingham Study. Stroke. 1991;22:983–988. doi: 10.1161/01.STR.22.8.983. [DOI] [PubMed] [Google Scholar]
- 32.Pongmoragot J., Rabinstein A.A., Nilanont Y., Swartz R.H., Zhou L., Saposnik G. Pulmonary Embolism in Ischemic Stroke: Clinical Presentation, Risk Factors, and Outcome. J. Am. Heart Assoc. 2013;2:e000372. doi: 10.1161/JAHA.113.000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rinde L.B., Småbrekke B., Mathiesen E.B., Løchen M., Njølstad I., Hald E.M., Wilsgaard T., Brækkan S.K., Hansen J. Ischemic Stroke and Risk of Venous Thromboembolism in the General Population: The Tromsø Study. J. Am. Heart Assoc. 2016;5:e004311. doi: 10.1161/JAHA.116.004311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Velez F.G.S., Garcia J.G.O. Management Dilemmas in Acute Ischemic Stroke and Concomitant Acute Pulmonary Embolism: Case Series and Literature Review. eNeurologicalSci. 2021;23:100341. doi: 10.1016/j.ensci.2021.100341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiang C.-C., Chen S.-P. Migrainous Infarction. Handb. Clin. Neurol. 2024;199:465–474. doi: 10.1016/B978-0-12-823357-3.00021-5. [DOI] [PubMed] [Google Scholar]
- 36.Carey R.M., Muntner P., Bosworth H.B., Whelton P.K. Prevention and Control of Hypertension: JACC Health Promotion Series. J. Am. Coll. Cardiol. 2018;72:1278–1293. doi: 10.1016/j.jacc.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Appel L.J., Brands M.W., Daniels S.R., Karanja N., Elmer P.J., Sacks F.M. Dietary Approaches to Prevent and Treat Hypertension. Hypertension. 2006;47:296–308. doi: 10.1161/01.HYP.0000202568.01167.B6. [DOI] [PubMed] [Google Scholar]
- 38.Ames M.K., Atkins C.E., Pitt B. The Renin-angiotensin-aldosterone System and Its Suppression. J. Vet. Intern. Med. 2019;33:363–382. doi: 10.1111/jvim.15454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soltani S., Shirani F., Chitsazi M.J., Salehi-Abargouei A. The Effect of Dietary Approaches to Stop Hypertension (DASH) Diet on Weight and Body Composition in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Clinical Trials. Obes. Rev. 2016;17:442–454. doi: 10.1111/obr.12391. [DOI] [PubMed] [Google Scholar]
- 40.Obarzanek E., Sacks F.M., Vollmer W.M., Bray G.A., Miller E.R., Lin P.H., Karanja N.M., Most-Windhauser M.M., Moore T.J., Swain J.F., et al. Effects on Blood Lipids of a Blood Pressure-Lowering Diet: The Dietary Approaches to Stop Hypertension (DASH) Trial. Am. J. Clin. Nutr. 2001;74:80–89. doi: 10.1093/ajcn/74.1.80. [DOI] [PubMed] [Google Scholar]
- 41.Gupta A.K., Dahlof B., Sever P.S., Poulter N.R., Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm Investigators Metabolic Syndrome, Independent of Its Components, Is a Risk Factor for Stroke and Death but Not for Coronary Heart Disease among Hypertensive Patients in the ASCOT-BPLA. Diabetes Care. 2010;33:1647–1651. doi: 10.2337/dc09-2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pimenta A.M., Toledo E., Rodriguez-Diez M.C., Gea A., Lopez-Iracheta R., Shivappa N., Hébert J.R., Martinez-Gonzalez M.A. Dietary Indexes, Food Patterns and Incidence of Metabolic Syndrome in a Mediterranean Cohort: The SUN Project. Clin. Nutr. 2015;34:508–514. doi: 10.1016/j.clnu.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwingshackl L., Bogensberger B., Hoffmann G. Diet Quality as Assessed by the Healthy Eating Index, Alternate Healthy Eating Index, Dietary Approaches to Stop Hypertension Score, and Health Outcomes: An Updated Systematic Review and Meta-Analysis of Cohort Studies. J. Acad. Nutr. Diet. 2018;118:74–100.e11. doi: 10.1016/j.jand.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 44.Yu J.-G., Zhou R.-R., Cai G.-J. From Hypertension to Stroke: Mechanisms and Potential Prevention Strategies. CNS Neurosci Ther. 2011;17:577–584. doi: 10.1111/j.1755-5949.2011.00264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Asemi Z., Samimi M., Tabassi Z., Shakeri H., Sabihi S.-S., Esmaillzadeh A. Effects of DASH Diet on Lipid Profiles and Biomarkers of Oxidative Stress in Overweight and Obese Women with Polycystic Ovary Syndrome: A Randomized Clinical Trial. Nutrition. 2014;30:1287–1293. doi: 10.1016/j.nut.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 46.Lin P.-H., Yeh W.-T., Svetkey L.P., Chuang S.-Y., Chang Y.-C., Wang C., Pan W.-H. Dietary Intakes Consistent with the DASH Dietary Pattern Reduce Blood Pressure Increase with Age and Risk for Stroke in a Chinese Population. Asia Pac. J. Clin. Nutr. 2013;22:482–491. [PubMed] [Google Scholar]
- 47.Niknam M., Saadatnia M., Shakeri F., Keshteli A.H., Saneei P., Esmaillzadeh A. Adherence to a DASH-Style Diet in Relation to Stroke: A Case-Control Study. J. Am. Coll. Nutr. 2015;34:408–415. doi: 10.1080/07315724.2014.943851. [DOI] [PubMed] [Google Scholar]
- 48.Diets For Healthy Heart—DASH vs. Other Diets—Blog—HealthifyMe. [(accessed on 2 March 2024)]. Available online: https://www.healthifyme.com/blog/dash-vs-other-diets/
- 49.Juraschek S.P., Miller E.R., Weaver C.M., Appel L.J. Effects of Sodium Reduction and the DASH Diet in Relation to Baseline Blood Pressure. J. Am. Coll. Cardiol. 2017;70:2841–2848. doi: 10.1016/j.jacc.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin P.-H., Allen J.D., Li Y.-J., Yu M., Lien L.F., Svetkey L.P. Blood Pressure-Lowering Mechanisms of the DASH Dietary Pattern. J. Nutr. Metab. 2012;2012:472396. doi: 10.1155/2012/472396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Y., Lu C., Li X., Fan Y., Li J., Liu Y., Yu Y., Zhou L. Healthy Eating Index-2015 and Predicted 10-Year Cardiovascular Disease Risk, as Well as Heart Age. Front. Nutr. 2022;9:888966. doi: 10.3389/fnut.2022.888966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eckel R.H., Jakicic J.M., Ard J.D., de Jesus J.M., Miller N.H., Hubbard V.S., Lee I.-M., Lichtenstein A.H., Loria C.M., Millen B.E., et al. 2013 AHA/ACC Guideline on Lifestyle Management to Reduce Cardiovascular Risk. Circulation. 2014;129:S76–S99. doi: 10.1161/01.cir.0000437740.48606.d1. [DOI] [PubMed] [Google Scholar]
- 53.Nguyen L.T.K., Do B.N., Vu D.N., Pham K.M., Vu M.-T., Nguyen H.C., Tran T.V., Le H.P., Nguyen T.T.P., Nguyen Q.M., et al. Physical Activity and Diet Quality Modify the Association between Comorbidity and Disability among Stroke Patients. Nutrients. 2021;13:1641. doi: 10.3390/nu13051641. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon request from corresponding author due to privacy.