Abstract
Natural killer (NK) cells have recently gained popularity as an alternative for cancer immunotherapy. Adoptive cell transfer employing NK cells offers a safer therapeutic option compared to T-cell-based therapies, due to their significantly lower toxicity and the availability of diverse autologous and allogeneic NK cell sources. However, several challenges are associated with NK cell therapies, including limited in vivo persistence, the immunosuppressive and hostile tumor microenvironment (TME), and the lack of effective treatments for solid tumors. To address these limitations, the modification of NK cells to stably produce cytokines has been proposed as a strategy to enhance their persistence and proliferation. Additionally, the overexpression of activating receptors and the blockade of inhibitory receptors can restore the NK cell functions hindered by the TME. To further improve tumor infiltration and the elimination of solid tumors, innovative approaches focusing on the enhancement of NK cell chemotaxis through the overexpression of chemotactic receptors have been introduced. This review highlights the latest advancements in preclinical and clinical studies investigating the engineering of activating, inhibitory, and chemotactic NK cell receptors; discusses recent progress in cytokine manipulation; and explores the potential of combining the chimeric antigen receptor (CAR) technology with NK cell receptors engineering.
Keywords: cancer immunotherapy, adoptive cell transfer, NK cells, tumor microenvironment, receptor engineering, NK cell clinical trials
1. Introduction
The field of cancer immunotherapy has shown exponential growth over the last decades. Several immunotherapy methods have been developed, such as cancer vaccines, oncolytic virus therapies, cytokine therapies, immune checkpoint inhibitors (ICIs), and adoptive cell transfer therapies (ACTs) [1]. In ACT, immune effector cells are ex vivo expanded and later intravenously infused in cancer patients to enhance their immune system [2,3]. Immune T cells used for ACT are mostly autologous, i.e., sourced directly from the patient, but other types of immune cells, such as γδ Τ cells and natural killer (NK) cells, can also be allogeneic, i.e., derived from donors [1,4]. Recent developments in cancer immunotherapy with T cells include T cell receptor (TCR) engineering and the chimeric antigen receptor (CAR) T cell technology [4]. CAR–T cells have already been applied for the treatment of B-cell malignancies, multiple myeloma (MM), acute lymphocytic leukemia (ALL), and other hematological tumors [5]. Nevertheless, CAR–T cell therapies show toxic side effects, such as the possible induction of the cytokine release syndrome (CRS) or the immune effector cell-associated neurotoxicity syndrome [6]. Moreover, the usage of allogeneic T cells poses the risk of inducing graft-versus-host disease (GvHD) [6]. Thus, recent research has shifted its focus to using NK cells as a seemingly safer and more efficient alternative for cancer immunotherapy [2,6].
In contrast to T cells, NK cells offer a broader application for anti-tumor immunotherapy, mainly due to their innate cytotoxic potential [7,8]. NK cells eliminate aberrant cells without the need for pre-activation or major histocompatibility complex class I (MHC-I)-dependent antigen presentation by the targeted cells [3,9,10]. Tumor cell killing is achieved by multiple mechanisms: degranulation, i.e., the release of the cytolytic granules containing perforin and granzyme; antibody-dependent cellular cytotoxicity (ADCC), ignited by the CD16 receptor-mediated identification of antibody-coated target cells; and the induction of tumor cell apoptosis by expressing tumor necrosis factor α (TNF-α), FasL, or TNF-related apoptosis-inducing ligands [3,7,8,11,12,13]. Clinical application of allogeneic NK cell transplantation is safer compared to allogeneic T cells, as they do not induce neurotoxicity, GvHD, or CRS [2,14]. Cytokines secreted by NK cells, like IFN-γ and GM-CSF, are safer than the ones produced by T cells, such as TNF-α and IL-6 [15,16]. Finally, yet importantly, the short life span of allogeneic NK cells limits the possibility of off-target in vivo events in contrast to the longer survival of autologous T cells [8,17].
NK cells are defined as CD56+CD3- large granular lymphoid cells capable of producing IFN-γ [16]. Constituting almost 10% of the peripheral blood mononuclear cells (PBMCs), NK cells are distinguished into two major subpopulations based on the expression of the low-affinity Fc gamma receptor 3A (FcγRIIIa), also known as CD16, and the adhesion molecule CD56 (NCAM); the CD56brightCD16dim and the CD56dimCD16bright cells. The CD56brightCD16dim subpopulation (10% of the total PB-NK cells) consists of immunomodulatory cytokine-producing NK cells, while the CD56dimCD16bright cells (90% of the total PB-NK cells) have stronger cytotoxicity [8,10,13].
NK cell cytotoxicity depends on their capability of identifying malignant or infected cells through their wide variety of activating and inhibitory surface receptors [10,16]. The spontaneous cytolytic killing activity of the NK cells is ignited by their activating receptors, such as NKG2D, CD16, and the natural cytotoxicity receptors (NCRs) that empower the NK cells to recognize cells with increased levels of ligands induced by stress, a state called “induced self” [14,18]. Inhibitory receptors play a pivotal role in regulating NK cell function and serve as checkpoints that control immune responses and inflammation [19]. They are often upregulated on chronically stimulated NK cells, leading to reduced activation and diminished overall response to cancer [20]. NK cells can distinguish healthy cells from malignant cells depending on the deficiency of MHC-I, also known as human leukocyte antigen (HLA) molecules, on the target cell surface. This “missing-self” state is an escape mechanism of tumor cells against T-cell-mediated killing [7]. However, it induces the exact opposite outcome against the HLA-binding NK cell inhibitory receptors, such as killer-cell immunoglobulin-like receptors (KIRs) and NKG2A/CD94, resulting in subsequent NK cell activation and killing of the cancer cells [7,14,18]. Additionally, NK cells are equipped with a variety of chemotactic surface receptors, which are responsible for their migration to chemokine-expressing tumor sites [12,21].
NK cells can be obtained from several sources, either autologous or allogeneic [2,4]. Primary NK cells deriving from peripheral blood (PB-NK), umbilical cord blood (UCB-NK), the bone marrow, or the placenta (obtained by apheresis) are the most studied sources with many applications [4,13,22]. However, due to the possible induction of GvHD by the remaining alloreactive T cells, the dose limitations of allogeneic NK cells indicate the importance of prior thorough purification of the cells [22]. In addition, hematopoietic stem/progenitor cells, human embryonic stem cells, and induced pluripotent stem cells (iPSCs) offer an off-the-shelf solution [3,23,24]. A subcategory of NK cells available for adoptive cell therapy are the allogeneic cytokine-induced memory-like (CIML) NK cells. CIML NK cells are PB-NK cells incubated in vitro with the cytokines IL-12, IL-15, and IL-18 prior to their infusion in the patient to boost their activity and persistence for weeks to months [3,13,21,24]. NK cell lines have also been explored, namely NK-92, NK-YS, KHYG-1, NKG, IMC-1, NKL, and NK3.3 [9,13]. The immortalized NK-92 cell line, deriving from a large granular lymphocyte (LGL) lymphoma patient, is currently used in clinical trials due to its indefinite proliferation, resulting in cell abundancy and easier manipulation for therapeutic purposes [23,25]. The other existing NK cell lines do not appear to have the same cytotoxic potential as the NK-92 cell line [9,13].
The NK-92 cells express only the NKG2A inhibitory receptor and not the KIR receptors, in addition to being CD16- and thus unable to induce the ADCC pathway [14,23]. Furthermore, irradiation of the NK-92 cells is required to avoid oncogenic adverse events in the patients, leading to reduced cell fitness and the need for multiple infusions [8,13,14]. However, their IL-2 dependency raises toxicity issues from the possible repeated IL-2 administration [8]. For this reason, the NK-92MI cell line presents a promising alternative, as it derives from the non-viral transfection of NK-92 cells to enable endogenous production of IL-2, thereby eliminating the need for additional cytokine injections [13].
Prior to infusion, NK cells are primed and stimulated via cytokines such as IL-2, IL-12, IL-15, IL-18, IL-21, and type 1 interferons like IFN-α [26]. However, prolonged exposure to high doses of those cytokines, especially IL-2, can result in toxicity and the expansion of unwanted immunosuppressive cells, such as T regulatory cells (Tregs) [6,8]. Feeder cell lines, like the modified K562 leukemia cells expressing IL-15 or the recent version expressing IL-21 and 4-1BBL, offer an alternative strategy for ex vivo expansion and are considered safer for clinical application [21,27].
The tumor-cell-targeted cytotoxicity of NK cells is dependent on their surface receptor repertoire, other inhibitory molecules, metabolic and transcriptional factors, and the immunosuppressive tumor microenvironment (TME) [1,12,18,21]. These parameters can hinder activating signals, tumor infiltration, and NK cell proliferation. In addition, tumor immune escape mechanisms can disrupt the NK cell receptor balance and diminish the expression of the activating receptors while boosting the expression of inhibitory molecules [28]. To address this, specific monoclonal antibodies (mAbs), also known as ICIs, are employed to block the inhibitory receptors’ functions by obstructing the receptor–ligand inhibitory effect. Several mAbs targeting NK cell inhibitory receptors are currently being evaluated in clinical trials [1]. Moreover, bi- and tri-specific killer-cell engagers (BiKEs and TriKEs), also referred to as NK cell engagers (NKCEs), have been recently introduced to link NK cell receptors with tumor-presenting antigens, thus bringing NK cells and tumors into proximity and offering a cost-effective solution for NK-cell-based therapies [29].
In addition to ICIs and NKCEs, NK cells can be engineered ex vivo, thereby boosting their post-infusion persistence and cytotoxic potential [4]. One example is the recent application of CAR technology on NK cells (CAR–NK), leading to a more potent lysis of tumor cells compared to CAR–T cells, via both CAR-dependent and CAR-independent activation [3,15]. Researchers have successfully genetically manipulated NK cells via viral and non-viral methods, with many studies having already reached the stage of clinical trials [9]. Viral transduction of NK cells is possible with the use of retroviruses or lentiviruses, with safety concerns surrounding the genomic integration of viral DNA [13,30]. On the contrary, transfection of NK cells via mRNA electroporation has shown high transfection efficiency, yet it results in transient gene expression only lasts for a few days [18,31,32]. DNA transposon delivery is a rising alternative for a more persistent DNA expression of longer DNA sequences [2,9,33]. Other non-viral methods of NK cell transfection, such as nucleofection, lipofection, mechanoporation, trogocytosis, and polymer or lipid-based nanoparticles (LNPs), have also been introduced [8,30,32,34]. In particular, LNPs have obtained clinical approval for the production of mRNA vaccines against COVID-19 and have recently shown promising efficiency of RNA delivery to NK cells [35,36]. Genetic engineering of NK cells is also feasible with gene-editing techniques, such as the clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 complex, zinc finger nucleases, or transcriptional activator-like effector nucleases [8]. Figure 1 summarizes and illustrates all preclinical and clinical efforts aimed to enhance NK cell cytotoxicity.
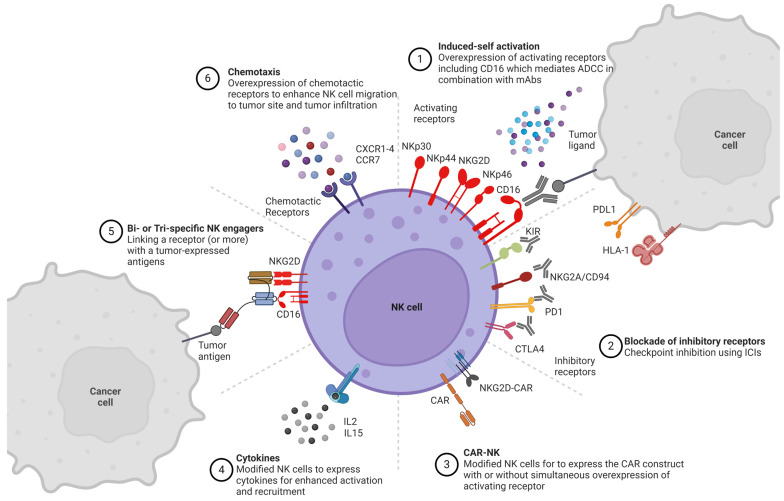
Figure 1.
Schematic representation of the key NK-cell-NK-cell-based strategies for cancer immunotherapy. (1) Overexpression of surface-activating receptors for enhanced cell activation and cancer antigen recognition. CD16 overexpression to boost the antibody-dependent cellular cytotoxicity (ADCC) function in combination with therapeutic monoclonal antibodies (mAbs). (2) Blockade of inhibitory receptor interaction with their ligands to ignite NK cell cytotoxicity using immune checkpoint inhibitors (ICIs). (3) Adoptive cell transfer (ACT) of modified NK cells with chimeric antigen receptor (CAR) constructs with or without the simultaneous overexpression of receptor repertoire. (4) Cytokine administration and modified NK cells to endogenously express cytokines for enhanced stimulation. (5) Use of bi- and tri-specific killer engagers for the simultaneous targeting/linking of NK cell receptors and tumor-associated antigens. (6) NK cell chemotactic receptor activation for tumor site migration and subsequent infiltration. Created with BioRender.
This review provides a comprehensive overview of the NK cell receptors that are mainly used as NK cell immunotherapy targets, with a focus on the ongoing preclinical and clinical trials involving the manipulation of activating receptors, inhibitory receptors, and other pivotal factors of NK cells for monotherapy or combinational approaches in cancer immunotherapy.
2. NK Cell Receptor Engineering
The upcoming sections of this review will focus on the most extensively researched NK cell receptors for cancer immunotherapy, as indicated in Table 1. The expression of activating receptors is often downregulated under the influence of the TME; thus, restoring or enhancing their expression can improve tumor cell targeting [18]. Increased expression is achieved by receptor delivery either in their endogenous form or as CAR molecules. Conversely, inhibitory receptors that are upregulated in tumor sites can be blocked with ICIs or knocked out via receptor engineering. The most recent preclinical and clinical trials with NK cell receptor engineering for the field of cancer immunotherapy are summarized in Table 2 and Table 3, respectively, and are reviewed below. Table 2 does not include combinational studies with multiple antibodies or feeder cell-mediated overexpression of the most studied receptors.
Table 1.
List of the most investigated NK cell receptors for cancer immunotherapy and their ligands.
| Receptor/Molecule | Ligand/Mode of Action | References |
|---|---|---|
| Activating Receptors | ||
| CD16 (FcγRIII) | IgG-ADCC | [25] |
| NKG2D | MHC-I, MICA, MICB, ULBPs | [16,37] |
| NKG2C | HLA-E | [38] |
| NKp46 (NCR1, CD335) | Viral hemagglutinins | [39] |
| NKp44 (NCR2, CD336) | Viral hemagglutinins, Nidogen-1, PCNA, 21spe MML5 | [39,40,41,42,43] |
| NKp30 (NCR3, CD337) | B7–H6, BAT3, pp65 | [39,44] |
| Inhibiting Receptors | ||
| KIR family | HLA-A,B,C | [11,24] |
| NKG2A/CD94 | HLA-E | [45] |
| TIGIT | PVR (CD155), Nectin-2 (CD112) | [13,39,46] |
| TIM3 | Galectin-9 | [27,47,48] |
| PD-1 | PD–L1, PD–L2 | [28,39] |
| CTLA-4 | B7-1 (CD80), B7-2 (CD86) | [49] |
| CD96 | PVR (CD155) | [39,50] |
| Chemotactic Receptors | ||
| CXCR1 | CXCL6, CXCL8 | [51] |
| CXCR2 | CXCL1–CXCL7 | [6,51] |
| CXCR3 | CXCL9, CXCL10, CXCL11 | [23,52] |
| CXCR4 | CXCL12 (or SDF-1a) | [53,54] |
| CCR7 | CCL19, CCL21 | [55] |
| CX3CR1 | CX3CL1 | [56] |
2.1. Activating NK Cell Receptors
2.1.1. CD16
The CD16a isoform of the CD16 receptor (FcRγIII), expressed by NK cells and other immune cells, induces the ADCC pathway by binding multivalently to the Fc portion of IgG, which is bound to tumor-associated antigens on the surface of tumor cells [25,57]. This leads to the intracellular phosphorylation of immunoreceptor tyrosine-based motif (ITAM) molecules of the homo- or heterodimers of CD3ζ and FcεRIγ [58], leading to the release of granzyme B and perforin as well as the generation of inflammatory cytokines directed towards the target cell (Figure 2) [59]. Surface expression of CD16 is regulated by a disintegrin and metalloprotease 17 (ADAM17), which acts as a regulatory checkpoint by causing ectodomain shedding and NK cell detachment from the tumor cell [25,60]. Such a mechanism boosts serial engagement of target cells, improves motility, survival, and targeted-cell engagement, but could limit cytotoxic activity by CD16 downregulation [61]. To overcome ADAM17 cleavage, researchers have developed a high-affinity (F158V) and non-cleavable (S197P) CD16a variant, also known as hnCD16, by introducing point mutations on the extracellular domain [61].
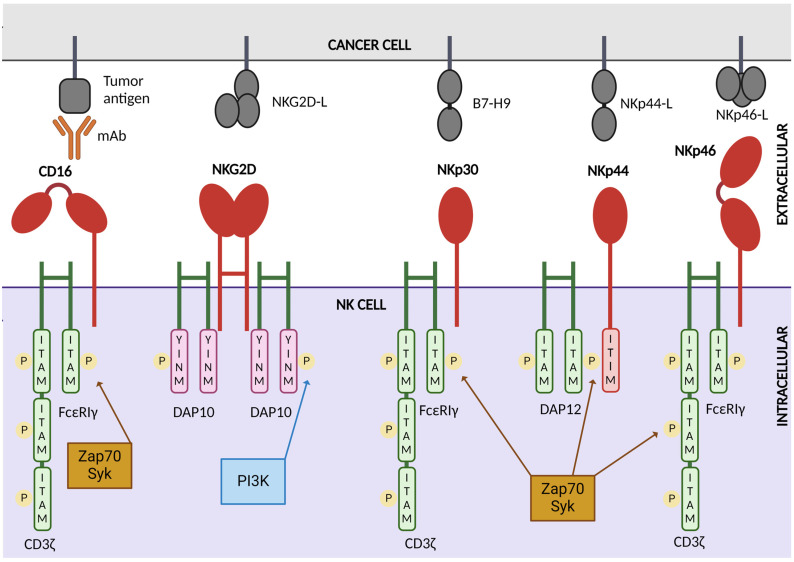
Figure 2.
Schematic representation of key activating receptors of natural killer cells, their intracellular domains, and their respective tumor antigens. CD16 binds to the Fc region of IgG monoclonal antibodies that are bound to tumor antigens, initiating the antibody-dependent cellular cytotoxicity function of NK cells. The receptors CD16, NKp30, and NKp46 signal through the phosphorylation of immunoreceptor tyrosine-based motifs (ITAMs) within their associated intracellular domains, CD3ζ and FcεRIγ, mediated by Zap70 and Syk kinases. Similarly, NKp44 transmits signals through the phosphorylation of ITAMs of DAP12 by Zap70 and Syk kinases. The receptor NKG2D signals via two DAP10 intracellular domains, which employ tyrosine–isoleucine–asparagine–methionine (YINM) that is phosphorylated by phosphoinositide 3-kinase (PI3K). Created with Biorender.
Successful transduction or electroporation of the CD16a gene in NK-92 cells has been achieved by different groups [12,25]. Jochems and colleagues engineered NK-92 cells via electroporation with a plasmid DNA containing both the high-affinity variant of CD16 (CD16-F158V) and IL-2, leading to increased cytotoxicity against breast and lung tumor cell lines when combined with avelumab in vitro [62]. CRISPR/Cas9 technology has been applied by Pomeroy and colleagues to knock out the ADAM17 cleavage sequence in PB-NK cells, which led to upregulation of IFN-γ production and ADCC in a PD-1 knockout combined in vitro study including tumor-targeting mAbs [58,63]. Zhu and colleagues recently engineered iPSCs with lentiviral particles expressing hnCD16. This modification led to the production of human-induced pluripotent-stem-cell-derived NK (hnCD16-iNK) cells which were more effective against both in vivo B-cell lymphoma and ovarian cancer xenograft models when combined with anti-CD20 and anti-HER2 mAbs, respectively [60]. In another study, van Hauten and colleagues successfully transduced NK cells derived from CD34+ hematopoietic stem and progenitor cells with hnCD16, leading to enhanced ADCC in combination with the tumor-targeting antibody rituximab when tested in vitro towards tumor cell lines and primary leukemia cells [64].
Daratumumab is a clinically effective monoclonal antibody that targets CD38 on the surface of multiple myeloma cells, exhibiting efficacy both as monotherapy and in combination with other anticancer treatments [65]. However, CD38 is also highly expressed on the surface of PB-NK cells, playing a crucial role in mediating cell activation [66]. Consequently, treatment with daratumumab has been shown to deplete NK cells in both humans and murine models, thereby diminishing the antibody’s effectiveness in ADCC [23]. To address this, ex vivo-expanded NK cells derived from an iPSC line were genetically modified via CRISPR/Cas9 and mRNA electroporation to knock out the CD38 receptor and simultaneously overexpress hnCD16, to enhance CD38-directed cytotoxicity and to induce robust ADCC in antibody-combined therapies. According to their results, CD38KOCD16+ NK cells exhibited significantly increased cytotoxicity in vitro against NCI-H929 myeloma cells when combined with daratumumab, reaching approximately 90% cell lysis at the highest effector/target (E/T) ratio. Conversely, CD38WTCD16+ NK cells and non-engineered NK cells both resulted in 60% cell lysis in combination with the mAb at the same E/T ratio [65]. A phase 1 clinical study was recently conducted to evaluate the dose tolerability of CD38KOCD16+ NK cells (FT538) in combination with daratumumab in six MM patients [clinicaltrials.gov ID: NCT04614636]. Both doses tested (1 × 108 and 3 × 108 cells) were well-tolerated, with no signs of neurotoxicity, CRS, or GvHD [67]. Additionally, the safety and efficacy of the FT538 and daratumumab combination were assessed in another phase 1 clinical study involving AML patients, with doses ranging from 1 × 108 to 15 × 108 cells [clinicaltrials.gov ID: NCT04714372]. Two out of four patients achieved an objective response rate of 50% and subsequently received an additional hematopoietic stem cell transplant, which further prolonged their survival. Although no neurotoxicity or dose-limiting toxicity were observed, 80% of the patients experienced infection and febrile neutropenia, and one patient exhibited CRS symptoms [68].
Bispecific antibodies and innate cell engagers (ICE) bring together the target tumor cells and NK cells by simultaneously binding tumor antigens and engaging with CD16 [69]. In a study conducted by Toffoli and colleagues, a bispecific single domain antibody (VHH) was constructed to target C21 for CD16 and 7D12 for epidermal growth factor receptor (EGFR) [57]. The application of this engager resulted in in vitro and ex vivo augmented activation of patient PBMC NK cells and lysis of EGFR-expressing tumor cell lines and metastatic colorectal patient-derived cancer cells [57]. The construction of another BiKE construct, which acts through CD16 while also targeting the myeloid differentiation antigen CD33, could induce the degranulation and lytic ability of NK cells towards acute myeloid leukemia (AML) cells in vitro [15,70]. In another study by Sarhan and colleagues, a TriKE construct was developed to target myelodysplastic syndrome, which engages CD16 and CD33 on the surface of myelodysplastic cells and simultaneously induces the self-production of IL-15 [71]. The engager contains two distinct scFv domains that recognize CD16 and CD33, respectively, and are connected by a linker encoding for IL-15, which binds to the IL-15 receptor on NK cells, thereby enhancing NK cell activity [72,73]. Finally, Kerbauy and colleagues successfully targeted CD30+ tumor cells with an AFM13 BiKE construct utilized for complex CD30 expressed by lymphoma or leukemia cells with CD16+CB-NK cells while testing the influence of prior NK cell activation with IL-15 in vitro and in vivo [24,74]. The AFM13 BiKE is currently being tested in the clinic in combination with non-engineered allogeneic NK cells and AFM13-pretreated CB-NK cells (modified NK cells) for CD30+ Hodgkin lymphoma and non-Hodgkin lymphoma [clinicaltrials.gov ID: NCT05883449 and NCT04074746]. Previous clinical studies from Affimed GmbH assessing AFM13 safety and tolerability in patients with Hodgkin lymphoma demonstrated activation of NK cells and a reduction in CD30 levels in peripheral blood samples [clinicaltrials.gov ID: NCT01221571]. Moreover, 61.5% of evaluated patients showed overall disease control, including 11.5% who demonstrated partial remission. When a higher AFM13 dose was administrated (≥1.5 mg/kg), the disease control rate was 77% [75].
2.1.2. NKG2D
The natural killer group 2 member D protein (NKG2D) activating receptor belongs to the C-lectin family and can recognize ligands associated with viral- or bacterial-infected cells, but most notably with tumor-transformed cells [16,76]. NKG2D plays an important role in the anti-tumor activity of NK cells due to its ability to bind to MHC-I-chain-related molecules, such as MICA, MICB, and the UL16-binding proteins (ULBPs) [10,16,37]. In mice, the retinoic acid early inducible-1 gene (RAE-1) and the UL16-binding protein-like (MULT)-1 have also been reported as NKG2D ligands [16]. These NKG2D ligands are overexpressed by tumor-transformed cells and infected cells, thus distinguishing them from healthy cells [37,76]. NKG2D induces NK cell cytotoxic activity and cytokine secretion via signaling through the DNAX-activating protein of 10 kDa (DAP10) in humans and DAP12 in mice (Figure 2) [37,76].
Malignant cells often escape immune cell targeting by secreting soluble forms of the NKG2D ligands, decreasing the effectiveness of NKG2D signaling [76]. Analyzing the specific molecular pathways of NKG2D downregulation in tumors, Xing et al. reported that both soluble and surface forms of MICA and MICB cause the desensitization of NK cells [77]. Other studies have shown that histone deacetylases (HDACs) inhibitors can induce higher levels of MICA/MICB expression on tumor cells, thus promoting NK cell tumor activity [77]. MICA-gene-specific transcriptional activation and overexpression by tumor cells using CRISPR/Cas9 technology were studied by Sekiba and colleagues and resulted in increased NKG2D-mediated clearance of the targeted cells in vitro [78,79]. Chitosan-based nanoparticles were employed by Tan and colleagues for the successful in vivo delivery of a plasmid encoding NKG2D and IL-21 (dsNKG2D-IL-21) into CT-26-induced solid tumors in mice, inducing the augmented secretion of the ligand and cytokines [80]. This led not only to NK cell but also to T cell stimulation and migration to the tumor tissue [80,81]. In another study by Youness and colleagues, overexpression of miR-486-5p in primary NK cells through lipofection caused an increase in NKG2D and perforin in vitro [82]. In addition, the NK-92 cell line was lentivirally transduced by Sayitoglu and colleagues to overexpress the NKG2D receptor to target sarcomas. NKG2D+ NK-92 cells showed enhanced degranulation towards all sarcoma explants and all tested tumor cell lines apart from the neuroblastoma cell line SH-SY5Y [83]. Poly ADP-ribose polymerase 1 (PARP1) is known to repress NKG2D ligand expression, thereby promoting immune escape. Its inhibition using either siRNA methodologies or inhibitors like talazoparib, olaparib, and AG-14361 has been shown to significantly increase ligand levels on AML stem cells by 6- to 50-fold [84]. The German Cancer Research Center is now initiating a clinical trial for AML, combining allogeneic NK cell therapy with the PARP1 inhibitor talazoparib to induce re-expression of NKG2D ligands on tumor cells [clinicaltrials.gov ID: NCT05319249].
The knockout of NKG2D has also been studied by several groups, with findings proving its importance in cancer immunotherapy. Inhibition of NKG2D in early NK cell developmental stages has been associated with the hyperactivity of the NKp46 (NCR1)-activating receptor and the targeting of NKp46-ligand-expressing tumors [85]. Wang and colleagues co-incubated the Kasumi-1 AML cancer cell line with NK92MI cells and the anti-NKG2D antibody, resulting in a reduction in the apoptotic ratio of the AML cell line, demonstrating the importance of the NKG2D cytotoxicity potential [76]. In another study, a BiKE construct was produced combining an anti-CS1 scFv domain and an anti-NKG2D scFv domain [15]. Its application in an in vitro human MM model proved the dose-dependency of IL-2-primed PBMC-derived NK cell cytotoxicity and cytokine secretion in the presence of the NKG2D receptor [86]. Similarly, a BiKE construct with Fab fragments for the binding of NKG2D and HER2, a tumor-expressed antigen, could stimulate NK cell cytotoxicity in vitro [87,88]. Novel strategies with bi-specific immunoconjugate constructs are focused on simultaneous targeting of NKG2D ligands, like MICA or ULBP1/2, and tumor-expressed antigens such as BCMA, CD19, and VEGFR2 [87]. In vivo preclinical studies confirmed the efficiency of such molecules in inducing NKG2D activation in NK cells, with subsequent enhancement of NK cell cytotoxic activity towards the respective antigen-presenting tumor cells [87].
2.1.3. NKG2C
The C-type lectin CD94/NKG2C-activating receptor is highly expressed by the adaptive CD56dim NK cells, usually post-stimulation by a cytomegalovirus (CMV) infection, and is very potent for ADCC induction and IFN-γ secretion [38,89]. Incubation of allogeneic NK cells with feeder cells and IL-15 induces NKG2C+ adaptive NK cells with cytotoxic potential [45]. Haroun-Izquierdo and colleagues produced adaptive single self-KIR+NKG2C+ NK cells, named ADAPT-NK cells, which have a higher proliferation rate compared to common adaptive NK cells, in both in vitro and in vivo AML tumor cell models [38]. The improved expansion and activity of these cells were caused by three factors: (1) the expression of single self-KIR, which provided higher alloreactivity; (2) the targeting of HLA-E; and (3) the induction of CD16-mediated ADCC [38]. NKG2C+CD57+ NK cells, deriving from CMV-seropositive donors, highly expressed those molecules after CMV reactivation post-hematopoietic stem cell transplantation (HSCT), depicting a potent activity of cytolysis and a “memory-like” behavior [90]. The same memory NK cell subtype was used on bone marrow-transplanted patients with leukemia to reduce the relapse rate [91,92]. In another study, NKG2C+ NK cells were expanded with an engineered feeder cell line to express HLA-E*spG, an artificial disulfide-stabilized trimeric HLA-E ligand [93]. When tested against K562 and primary glioblastoma multiforme cells, these NKG2C+ cells showed enhanced cytotoxicity in vitro compared to wild-type NK cells. Finally, an anti-NKG2C/IL-15/anti-CD33 TriKE construct was produced by Chiu and colleagues in order to target CD33+ AML cells with NKG2C+ CMV-reactivated patient-derived PB-NK cells but also NKG2C-engineered iPSC-derived NK cells [94]. The outcome of this study was an increase in NKG2C+ NK cell proliferation, cytotoxicity, degranulation, ΙFN-γ production, and efficient AML tumor cell elimination in vitro [94].
2.1.4. NKp46
NKp46 (NCR1) is a crucial activating receptor of the NK cells, responsible for stimulating their cytolytic activity and cytokine secretion [95]. NKp46 signals through phosphorylation of two ITAM-bearing molecules, CD3ζ and FcR-γ (Figure 2) [95]. As mentioned earlier, NK cell TriKEs combining an NKp46 scFv-binding domain, a CD16 Fc binding domain, and a tumor-associated antigen have been manufactured, leading to augmented ADCC and NK cell cytotoxicity against mouse cancer [72,87,95]. Another available multi-specific killer engager called FLEX-NK, developed by Cytovia Therapeutics in 2021, targets NKp46 and GPC3, a glycoprotein expressed by solid tumors, and CD38 to simultaneously eliminate solid tumors and MM. This engager was tested in a preclinical hepatocellular carcinoma mouse model, leading to increased NK cell detection and enhanced tumor inhibition [96].
2.1.5. NKp44
NKp44 (NCR2) is only found in human-activated NK cells and other types of immune cells, and it has been associated with the identification of transformed cells, signaling via DAP12 (Figure 2) [97]. Several NKp44 ligands have been mentioned in the literature, with the most dominant being Nidogen-1, the proliferating cell nuclear antigen (PCNA), the mixed-lineage leukemia-5 protein (21spe MML5), and viral hemagglutinins [40,41,42,43]. Barrow and colleagues found that the platelet-derived growth factor isoform PDGF-DD, produced by tumor cells, is recognized by NKp44 [97]. This results in IFN-γ and TNF-a secretion by the NK cells, as shown in NCR2-transgenic mice experiments [97]. Finally, in another in vitro study on MM cells, the inhibitory binding of PCNA on cancer cells with NKp44 was blocked with the mAb 14-25-9, enhancing NK cell anti-tumor activity, IFN-γ production, and degranulation [98].
2.1.6. NKp30
NKp30 (NCR3, CD337) belongs to the immunoglobulin superfamily and is a type I transmembrane NK cell receptor, signaling via the ITAM-associated molecules CD3ζ and FcεRIγ (Figure 2) [87]. The activation of NK cells through recognition of the NKp30 ligands, such as the surface molecule B7-H6 and the nuclear factor HLA-B-associated transcript 3 (BAT3)/Bcl2-associated athanogene 6 (BAG6), stimulates NK cell cytotoxicity and cytokine secretion [44,87]. NK cell killer engager molecules, also known as immunoligands, have been produced for simultaneous NKp30 and EGFR targeting, with the bi-specific construct consisting of a humanized Fab variant from the Ab cetuximab and affinity-optimized variants of the N-terminal Ig-like V-type domain of B7-H6 [44]. These BiKEs successfully stimulated EGFR-positive tumor cell killing and IFN-γ and TNF-α secretion by NK cells [44,87]. A follow-up study targeting NKp30 on NK cells and EGFR on tumor cells used a BiKE construct combining NKp30-specific VHH of Camelidae origin and the EGFR-specific humanized Fab of cetuximab, leading to a more potent NK-cell-mediated tumor-killing effect in vitro when compared to the previous B7-H6 construct or cetuximab treatment [99]. Finally, Compass Therapeutics developed CTX-8573, a multi-specific construct combining anti-NKp30 Fab fragments and the C-terminus of the B-cell maturation antigen (BCMA) [87]. It has been used to engage with CD16 and NKp30 NK cell receptors in both in vitro and in vivo studies [27,87].
2.2. Inhibitory NK Cell Receptors
Alongside enhancing NK cell cytotoxicity through induction of the activating receptors, downregulating the expression of inhibitory receptors can also shift the NK-signaling balance towards effective anti-tumor activation [18]. These receptors and molecules are presented in Figure 3 and can be divided into two subgroups based on their binding to HLA molecules, namely HLA-specific or non-HLA-specific.
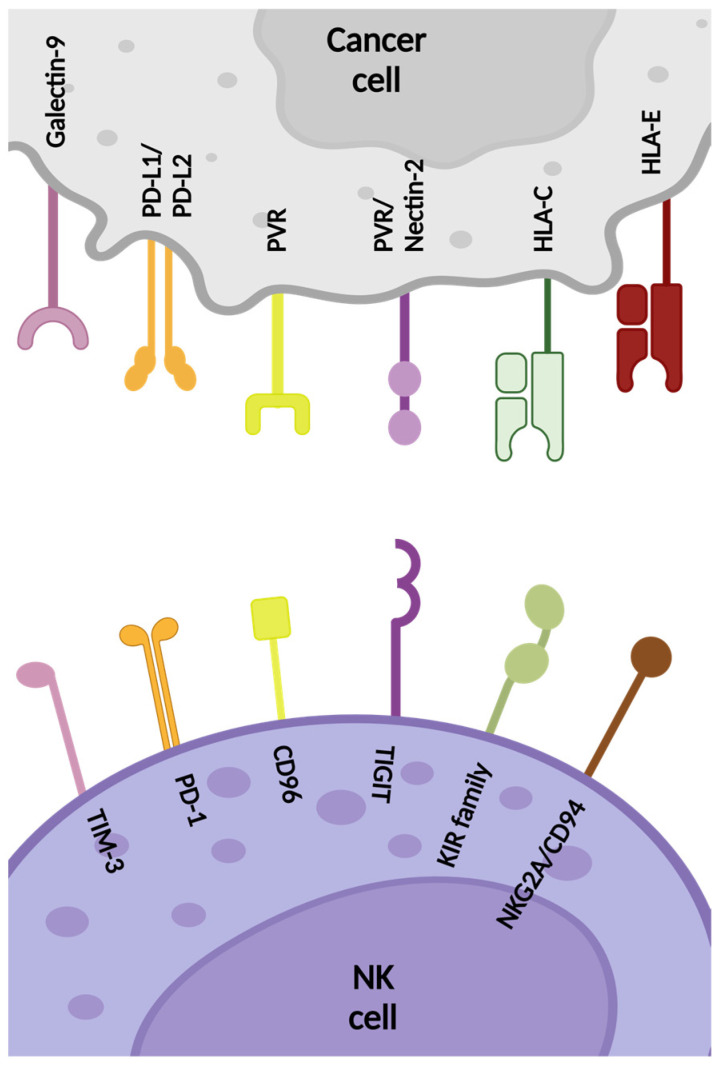
Figure 3.
Schematic representation of key inhibitory receptors expressed on the surface of natural killer (NK) cells with their corresponding tumor antigens. Created with Biorender.
2.2.1. HLA-Specific Inhibitory Receptors
KIR
The killer-cell immunoglobulin-like receptor (KIR) family consists of polymorphic activating and inhibitory transmembrane proteins critical for the interaction between NK cells and major MHC-I molecules, particularly MHCIa [24,45]. Out of the fourteen KIR receptors, seven are inhibitory (KIR2DL1, KIR2DL2, KIR2DL3, KIR2DL5, KIR3DL1, KIR3DL2, and KIR3DL3), and six are activating receptors (KIR2DS1-2DS5, KIR3DS1), depending on the presence of an intracellular tail on the activating domain [21,45]. The remaining KIR2DL4 has both activating and inhibitory features [21,33,100]. KIR inhibitory receptors are important for the “missing-self” state recognition by the NK cells, facilitating the distinction between healthy and malignant cells in the absence of MHC-I on the tumor cell surface [11,45].
Anti-KIR antibodies, which block the KIR association with HLA-C molecules and the subsequent inhibition of NK cell cytotoxic activity, have recently reached the stage of clinical trials reporting limited side effects [101]. The humanized IgG4 mAb 1-7F9 (IPH2101, anti-KIR2D) was produced for the blockage of KIR2DL1/L2/L3 and KIR2DS1/S2 receptors binding to HLA-class I ligands [11,15]. In preclinical studies, this antibody induced longer NK cell survival against AML cells, and when tested in AML and MM patients, successful KIR2D binding also increased NK cell cytotoxicity [11,27,102,103,104]. IPH2101 was co-administered with lenalidomide, an agent that enhances NK cell activity by increasing the ligands of NK-cell-activating receptors, in phase I/II clinical trials with AML and MM patients [50,104,105] [clinicaltrials.gov ID: NCT01248455]. Nevertheless, further studies were suspended since lower NK cell degranulation and cytokine secretion were observed, possibly due to phagocytes removing KIR2D from the NK cell surface via FcγRI-mediated trogocytosis [21]. Lirilumab (IPH2102) is a recombinant version of IPH2101 and is the first fully clinically tested anti-KIR monoclonal antibody, created for the inhibition of the KIR–HLA class I ligand interaction in autologous NK cell applications [11,13]. The effectiveness of this anti-KIR2DL1/2/3 antibody was first confirmed by Sola and colleagues in an HLA-C-expressing B-cell lymphoma xenograft model on RAG-1-deficient mice [106]. Yet, the clinical efficacy of lirilumab monotherapy remains debatable [24,72,103]. Lirilumab has also been used in combination therapy with the immunosuppressing drug lenalidomide to induce ADCC [11].
Using different combinations of anti-PD-1, anti-CD20, anti-SLAMF7, and 5-azacytidine in ongoing clinical trials has shown promising results in KIR inhibition [101,103]. Moreover, clinical studies on advanced or metastatic solid tumor patients are currently ongoing with the combination of lirilumab, nivolumab (targeting PD-1), and ipilimumab (targeting CTLA-4) [21,106,107]. A recent addition to the anti-KIR antibody group is IPH4102 (lacutamab), a humanized IgG1 mAb targeting KIR3DL2 [102,103]. This antibody has already entered the phase of clinical studies, has shown a good safety profile for relapsed/refractory cutaneous T-cell lymphoma, and has been used in combination with chemotherapy [102,103,107]. Finally, in in vivo studies performed by Wei and colleagues, the HHLA2+ human lung cancer cell HCC827 was used to challenge immunodeficient mice, showing how KIR3DL3 inhibition can enhance NK cell activity [108].
NKG2A/CD94
One of the most important inhibitory receptors of NK cells, NKG2A, encoded by the killer-cell lectin receptor C1 (KLRC1) gene, binds to CD94 and forms a heterodimer. This complex recognizes the tumor-cell-expressed non-classical MHCIb molecules HLA-E in humans or the Qa-1 molecule in mice [45,72]. The ligation of NKG2A/CD94–HLA-E induces inhibitory signals via the phosphorylation of the tyrosine residue in the immunoreceptor tyrosine-based inhibitory motif (ITIM) domain [45]. The interaction of NKG2A/CD94 is also important for the “missing-self” recognition ability of the NK cells [45]. Notably, NKG2A/CD94 facilitates NK cell migration and contact with the target, and subsequently, the cytotoxicity and serial-killing capacity [45,109]. Due to their non-polymorphic nature, the literature has mentioned both NKG2A/CD94 and HLA-E as potential therapeutic targets to enhance NK cell anti-tumor-killing efficacy [105].
PB-NK lentiviral vector-driven RNAi knockout of the NKG2A gene was achieved by Figueiredo and colleagues, resulting in enhanced NK cell activity in vitro against an HLA-E-expressing B-lymphoblastoid cell line [12,110]. Similar results were also observed on AML-derived HLA-E-negative K562 cells, possibly due to the stimulation by the increased levels of NKp30 in the NKG2A-deficient cells [110]. Another study targeted NKG2A/CD94 using protein expression blockers (PEBLs) [18,111]. Such constructs include an anti-NKG2A antibody single-chain variable fragment connected with endoplasmic reticulum-retention domains, which block the NKG2A transport from the endoplasm to the cell membrane [18]. NKG2A-deficient NK cells had improved cytotoxic functionality in targeting HLA-E-expressing and HLA-E-deficient cancer cells deriving from Ewing’s sarcoma, osteosarcoma, and AML [12,111]. They also successfully interfered with the de novo expression of NKG2A/CD94, induced via IL-12 incubation, without any side effects on NK cell proliferation [18,111]. The in vitro results were also confirmed in in vivo xenograft models [111]. Gene-editing methods using CRISPR/Cas9 technology to knock out the killer-cell lectin receptor C1 (KLRC1), encoding for NKG2A in primary NK cells, have been applied by Bexte and colleagues. This group successfully generated a 90% NKG2D- NK cell population after cell sorting, which resulted in enhanced cytotoxicity towards an HLA-E-expressing B lymphoblast cell line [112]. Similar results were obtained by Mac Donald and colleagues when tested in solid tumor cell lines in vitro and in a xenogeneic mouse model of metastatic breast cancer in vivo. Interestingly, the expression of the NKG2C-activating receptor was enhanced in KLRC1-knocked-out NK cells, a fact that also contributed to their advanced cytotoxicity [113].
Most recently, the inhibition of NKG2A/CD94 has been achieved using the humanized anti-NKG2A/CD94 mAb monalizumab (IPH2201), which has already been tested both in vitro and in vivo [103]. Monalizumab has been proven to stimulate not only NK cell activity but also CD8+ T effector cell anti-tumor functionality, both in mice and in humans [50,114]. NK cells from chronic lymphoid leukemia (CLL) patients showed recovery of their cytotoxic activity in preclinical studies with monalizumab [105,115]. Monalizumab has also been applied in combination therapies with other therapeutic mAbs [15,27,39]. More specifically, co-administration of monalizumab and cetuximab, an anti-EGFR antibody, in patients with head and neck squamous cell carcinoma (HNSCC) resulted in the induction of anti-tumor memory [27,39]. The co-administration with the anti-programmed death protein 1 (PD-L1) mAb durvalumab showed a similar effect for a variety of tumor types, such as mouse lymphoma and colorectal cancer (CRC) patients [50,73,114].
2.2.2. Non-HLA-Specific Inhibitory Receptors
TIGIT
T cell immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domain (TIGIT) is a non-MHC class I-specific NK cell receptor expressed by both T cells and NK cells. It negatively regulates NK cells by blocking their cytotoxic activity via competing with the DNAM-1 (CD226) receptor for the binding of its ligands, poliovirus receptor PVR (CD155), and Nectin-2 (CD112) [13,39,46,50,116]. TIGIT has a higher binding affinity towards PVR compared to DNAM-1. Therefore, the inhibition of DNAM-1 is linked to NK cell exhaustion and suppression of IFN-γ production [46,50,116]. Its inhibitory signals derive from the phosphorylation of the immunoglobulin tail tyrosine (ITT) and ITIM domains located in the cytoplasmic region [117]. Interestingly, Jia and colleagues proposed in their functional experiment studies in AML patients that TIGIT expression might be correlated with increased secretion of IFN-γ and TNF-α cytokines and granzyme B, supporting the NK anti-tumor activity in AML [105].
Recent studies have mostly focused on the effect of TIGIT inhibition on CD8+ T and Treg cells and its competition with DNAM-1, either with anti-TIGIT mAbs as monotherapy or in combination with anti-PD-1 and anti-PD-L1 [72,118]. Currently, there are multiple clinical trials focusing on TIGIT inhibition [119]. For instance, the TIGIT antibodies vibostolimab, etigilimab, and tiragolumab are being clinically evaluated in phase I and III clinical trials [120]. The blockade of TIGIT could be a promising alternative to enhance anti-tumor immunity. Nevertheless, the exact mechanism of action behind the NK cell interaction with CD8+ T cells upon TIGIT inhibition is yet to be fully defined [116,121].
An anti-TIGIT mouse mAb, called 13G6, was produced and used to block TIGIT activity in a CT26 colon cancer, 4T1 mammary cancer, or methylocholanthrene (MCA)-induced fibrocarcinoma mouse model [116,118,122]. Interestingly, the use of this antibody resulted in tumor growth inhibition via tumor-infiltrating NK cell protection and in the increase in CD8+ T cell responses and elimination of tumor cells [118,121,122]. In a more recent mouse study, B16F10 and LWT1 metastatic melanoma tumor types were eradicated by combining anti-TIGIT mAb with IL-15-induced NK cell cytotoxic activity [118,123]. Similar results were obtained with this combination approach in a B16 melanoma mouse model, where NK cell-specific TIGIT-deficiency resulted in the upregulation of DNAM-1 (CD226) expression by tumor-infiltrating NK cells and in tumor growth inhibition in CD155-deficient mice [124]. Another study with TIGIT-expressing NK cells found that inhibiting this receptor reduced the immunosuppressive effect of myeloid-derived suppressor cells (MDSCs) on NK cytotoxicity [122].
Furthermore, efforts have been made to genetically modify TIGIT in order to eliminate its inhibitory functions and, therefore, promote NK cell activity [6]. The TIGIT knockout effect was studied by Zhang and colleagues, demonstrating positive outcomes regarding NK cell protection and tumor immunity in mouse models [6,15,116]. The knockout of the TIGIT receptor from expanded NK cells using electroporation was also performed by Hasan and colleagues, demonstrating higher cytotoxicity against various cancer cell lines and spheroids in vitro [125]. Finally, an innovative approach of TIGIT exploitation came from Lupo and colleagues, who genetically engineered iPSCs-derived NK cells with lentiviral transduction to express a genetic construct called synNotch to simultaneously target CD155 and CD73 on the surface of glioblastoma cells. In short, this construct contains an extracellular TIGIT domain coupled with a synNotch transmembrane domain and the GAL4-VP64 transcription factor. Post-TIGIT binding to CD155, a conformational change yields the cleavage of a peptide and the subsequent release of the transcription factor, which promotes the generation of an anti-CD73 antibody to bind to CD73. After successful cell engineering and expansion, synNotch-iNK cells were intracranially injected in an orthotopic, patient-derived xenograft mouse model for glioblastoma. Mice treated with engineered iNK cells exhibited a significant decrease in tumor growth and enhanced survival (40% probability after 50 days) compared to those treated with non-engineered iNK cells (0% probability after 50 days). Moreover, studies on isolated brain samples showed increased levels of NKp46+ and granzyme B+ NK cells in mice treated with synNotch iNK cells, indicating an enhanced functional activation of NK cells at tumor sites. Their results also suggest effective reprogramming of the glioblastoma TME via higher CD8+ T cell recruitment [126].
CD96
CD96 is a type I transmembrane Ig glycoprotein, and even though it is mostly expressed by T cells, it has recently been indicated as another inhibitory receptor of NK cells [121]. CD96 interacts with PVR (CD155) expressed by tumor cells, similar to the interaction of TIGIT and DNAM1 [39,50]. This binding leads to a decrease in IFN-γ production by the NK cells [50]. Stimulation of human NK cells with IL-15 or TGF-β led to upregulation of CD96 expression [20]. Higher CD96+ NK cell levels, with distinctive exhaustion and cytokine secretion, have been found in intra-tumoral sites of hepatocellular carcinoma (HCC) patients or ovarian cancer ascites cases [20,39,50]. Additionally, it has been reported that CD96 knockout increased NK cell cytokine production and lung metastasis control in mouse models [50]. In animal models, CD96 blocking with anti-CD96 mAb has demonstrated higher efficacy when combined with anti-CTLA-4 or anti-PD-1 mAbs [127]. The impact of CD96 inhibition was not significant in terms of cytokine production and degranulation [20]. The precise mechanism of action of CD96 on NK cells, either inhibitory or activating, remains controversial [121,128].
CD112R
Recently identified as a lymphocyte inhibitory receptor, CD112R (or PVRIG) can ligate with CD112 with higher affinity compared to TIGIT or DNAM-1 [20,129]. It is expressed on both CD16-positive and CD16-negative NK cells, as well as by intra-tumor NK cells in prostate and endometrial cancer patients [20]. The use of anti-CD112R mAbs in a CD112+ and CD155+ human breast cancer cell line enhanced NK cell cytokine production and degranulation, confirming the inhibitory functions of CD112R [20,130]. In vivo studies performed by Li and colleagues revealed that anti-CD112R mAbs protected mice against subcutaneous MC38 tumors due to increased degranulation and IFN-γ production by tumor-infiltrating NK cells [20,131]. The combination of TIGIT and CD112R inhibition by Xu and colleagues led to enhanced NK-cell-mediated ADCC against breast cancer cells coated with trastuzumab in vitro [20,129,130,132].
CTLA-4
Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4 or CD152) is expressed at very low levels by NK cells compared to its expression by T cells and Treg [47,49]. CTLA-4 binds with high affinity to B7-1 (CD80) and B7-2 (CD86) [49]. The expression of CTLA-4 is mainly observed in murine NK cells after cell treatment with IL-2 [20]. An FDA- and EMA-approved anti-CTLA-4 monoclonal antibody, ipilimumab, has already been characterized for its effect on T cells and is currently being clinically tested in combination with the anti-PD-1 mAb nivolumab [49]. Treatment with ipilimumab stimulated ADCC and TNF-α secretion by NK cells in vitro and resulted in tumor inhibition in a chimeric murine xenograft model [133]. CTLA-4 inhibition via treatment with ipilimumab and tremelimumab demonstrated promising results in NK cell survival rate in melanoma and malignant pleural mesothelioma patients [134]. The combination of ipilimumab with cytokine-induced memory-like (CIML) NK cell infusion and an IL-15 superagonist is currently being tested in a phase 1 clinical trial for advanced head and neck cancer [clinicaltrials.gov ID: NCT04290546]. Despite the encouraging results, the low expression of CTLA-4 in NK cells could limit its therapeutic application [47].
PD-1
The membrane receptor PD-1, also known as CD279, is a T-cell-associated checkpoint molecule that is also expressed by NK cells and upregulates upon CMV infection or interaction of NK cells with tumor cells [28,39]. PD-1 is bound by PD-L1 and PD-L2 to facilitate immune cell inactivation [28,39]. PD-1 is endogenously expressed by NK cells of cancer patients, and its expression can be increased by soluble factors released from the TME [39,135]. Reports have mentioned the presence of PD-1+ NK cells in ovarian carcinoma, Hodgkin lymphoma, intestinal adenocarcinoma, Kaposi sarcoma, bladder carcinoma, lung, breast, and uterine cancer patients [28,39].
In a promising gene-editing approach, CRISPR/Cas9 technology was used by the Moriarity group on PBNK cells to knockout the inhibitory genes encoding for PD-1 and ADAM17, performing the targeted integration through homology-directed repair using a recombinant adeno-associated virus [2,63]. Although these in vitro studies demonstrated augmented cytotoxic killing by the NK cells via non-ADCC-related pathways, in vivo results demonstrated only a modest enhancement in survival [63].
Preclinical studies in MM also showed that blocking PD-1 enhanced NK-cell-killing activity [105]. Hsu and colleagues demonstrated within their in vivo mouse studies that inhibition of PD-1 or PD-L1 can stimulate NK cell activity [39,136]. Moreover, PD-1/PD-L1 blockade seems to be crucial for HLA-negative cancer types [50]. For instance, inhibition of PD-1/PD-L1 in MHC-I-deficient tumor cells, like most Hodgkin’s lymphomas that express higher levels of PD-L1, resulted in a better response and in a higher NK cell activity in patients [50]. Makowska and colleagues have confirmed the role of IFN-β in inducing PD-L1 expression by nasopharyngeal carcinoma cells (NPC) and PD-1 expression by NK cells [137,138]. Most importantly, those studies showed that the stimulation of NK cell anti-tumor cytotoxicity by chemotherapy could be supported by PD-1/PD-L1 inhibition with anti-PD-1 antibody treatment in NPC patients [137,138]. This was confirmed in a phase 2 clinical study involving patients with late-stage non-small cell lung cancer treated with autologous NK cells and the PD-1 antibody sintilimab [clinicaltrials.gov ID: NCT03958097]. This second-line treatment demonstrated promising antitumor activity with no unexpected adverse events. Two out of twenty patients experienced neutropenia, hypertriglyceridemia, and elevated creatine kinase, which were attributed to the ICI rather than the NK cell transfer [139]. In mouse models, the combination of anti-PD-1 and anti-KIR showed the pivotal role of NK cells in targeting lymphoma tumors [140].
Several anti-PD-1 antibodies have been designed, such as nivolumab, pidilizumab, and pembrolizumab, and their effects have been mostly studied at a clinical stage for blocking T cell immunosuppression by PD-1 [15]. However, the anti-PD-1 mAb nivolumab has been used in combination therapies for solid tumors to successfully restore NK cell activity [47]. Lastly, anti-PD-1 and/or anti-LAG-3 mAbs have been used in combination with IL-12 treatment to induce NK cell stimulation in a 4T1 metastatic breast cancer mouse model [141].
TIM-3
T cell immunoglobulin mucin receptor 3 (TIM-3), also known as hepatitis A virus cellular receptor 2, is another inhibitory receptor of CD16-high NK cells that plays an important role in the NK cell maturation process [21,48]. The association of TIM-3 with galectin-9, expressed in various metastatic cancer types, has been reported to induce either IFN-γ production or suppression of NK cell cytotoxic activity [27,47,48]. In an in vitro study performed by Xu and colleagues, the stimulation of CXCR1 and CXCR3 chemotactic NK cell receptors following activation of the TIM-3 pathway was verified [48]. Thus, this NK cell receptor might have both activating and inhibiting effect on the NK cell anti-tumor activity [142]. Other ligands of TIM-3 that have been reported are phosphatidylserine, carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1), and high-mobility group protein B1 (HMGB1) [142].
The expression of TIM-3 has been shown to be upregulated in the presence of TNF-α [50]. TIM-3+ NK cells have been identified in lung cancer and advanced-stage melanoma, showing its suitability as a target for non-small cell lung cancer and other types of anti-tumor treatment [21,134]. The inhibition of TIM-3 in vitro resulted in IFN-γ and TNF-α restoration and revitalization of NK cell cytotoxic activity in liver, lung, and melanoma cancer [20,50,134,143]. Several other preclinical studies on tumor-bearing mouse models have proven the efficacy of TIM-3 knockout or antibody blocking on the NK cell contribution in inhibiting sarcoma, colon carcinoma, or prostate tumor growth [50,105]. The combination of antibodies blocking TIM-3 and PD-1 proved to be very effective in in vivo models of melanoma, fibrosarcoma, colon cancer, and leukemia [50]. In another in vitro study on glioblastoma by Morimoto and colleagues, the knockout of TIM-3 with the use of CRISPR/Cas9 technology was shown to be beneficial in increasing inhibition of glioblastoma tumor cell growth without interfering with the expression of other NK cell inhibitory receptors [142]. Anti-TIM-3 antibodies have reached the stage of clinical trials as monotherapy or in combination therapies with anti-PD-1 and anti-LAG-3 antibodies, mostly focusing on their effect on T cells [134]. In clinical trials of patients with solid tumors, the use of a TIM-3/PD-1 bi-specific antibody is also under examination [134].
2.3. Chemotactic Receptors
Several NK cell receptors control chemotaxis in response to chemokines secreted by tumor cells [12]. The most important chemotactic receptors of NK cells are CXCR4, CCR7, CXCR3, CXCR2, CXCR1, CX3CR1, and CCR3-CCR5 [21,144]. Following association with their respective ligands, these receptors facilitate efficient NK cell migration and infiltration towards the tumor site, an essential process for cancer eradication [12].
2.3.1. CXCR4
The importance of CXCR4 and its interaction with CXCL12 (or SDF-1a) for the homing of NK cells to the bone marrow has previously been proven by inhibiting CXCR4–SDF-1a binding [53]. Similarly, Levy and colleagues successfully transfected NK cells in vitro with CXCR4 mRNA via electroporation, suggesting that CXCR4 overexpression is a promising approach to tackle tumors in the bone marrow, such as myeloma and leukemia [53]. In studies performed by Yang and colleagues, upregulation of IL-18 expression and subsequent NK cell activation were observed after CXCR4 knockout via tissue-specific LysM-Cre-mediated recombination gene editing [54]. This method resulted in total remission and higher survival rate in mouse models [54]. Furthermore, when the NK cell line YTS was lentivirally transduced with an anti-EGFRvIII CAR construct and CXCR4, the chemotaxis towards glioblastoma cells and cancer survival in xenograft mouse models were enhanced [145,146].
2.3.2. CCR7
Other studies have focused on CCR7, a receptor responsible for the lymph node homing of NK cells, aiming to improve tumor targeting by NK cells [147]. K562 feeder cells were used to transfer CCR7 to NK cells via trogocytosis, leading to increased NK cell migration to the lymph nodes in athymic nude mice via binding to the associated cytokines CCL19 and CCL21 [6,148]. Moreover, this specific homing of NK cells has been enhanced by Carlsten and colleagues via CCR7 mRNA electroporation of primary NK cells in combination with CD16 to induce ADCC and NK-cell-mediated killing of lymphoma cells in vitro [149]. Finally, the genetic modification of NK-92 cells via lentiviral transduction to simultaneously overexpress CXCR4 and CCR7 receptors on the cell surface showed increased migration towards human colon cells and ameliorated tumor prognosis in mouse xenograft models [55].
2.3.3. CXCR3
Another important chemotactic NK cell receptor is CXCR3, which binds to the IFN-γ-induced and tumor-secreted CXCL9, CXCL10, and CXCL11 chemokines [23,150]. CXCR3 is crucial for NK cell solid tumor infiltration [150]. In a study using irradiated EBV-LCL feeder cells with IL-2, the increased expression of CXCR3 by NK cells led to improved homing and tumor-killing activity against CXCL10-transfected melanoma xenograft mice [151]. Nevertheless, the observations on the CXCR3-mediated accumulation of NK cells in the blood and the average survival rate of MM patients emphasize the need for further studies to determine the therapeutic role of CXCR3 [150].
2.3.4. CXCR1-CXCR2
CXCR1 and CXCR2 are highly expressed by CD56dim NK cells and facilitate their tumor infiltration [78,144]. Their ligands are the cancer cell-expressed chemokines CXCL6 and CXCL8 for CXCR1, and CXCL1–CXCL7 for CXCR2 [51]. In a renal cell carcinoma in vitro study, primary NK cells were retrovirally transduced with CXCR2, resulting in enhanced migration to the tumor sites [152]. Interestingly, the inhibition of CXCR2 in a melanoma mouse study resulted in a reduction in NK cell tumor infiltration and an enhancement of the survival rate of melanoma-bearing mice [153]. In another study, CRISPR/Cas9 gene editing was applied to simultaneously overexpress CXCR2 and IL-2 in NK-92 cells. These results from Gao and colleagues demonstrated enhanced proliferation and chemotaxis to tumor sites, as well as tumor reduction and an increased survival rate in vivo [154]. Moreover, the importance of CXCR1 was observed in ovarian cancer xenograft models when its overexpression via mRNA electroporation enhanced the migration, infiltration, and efficacy of NKG2D-engineered CAR–NK cells [155].
Finally, numerous studies have shown the importance of the fractalkine (CX3CL1) receptor CX3CR1 for the recruitment of NK cells as well as other immune cells like monocytes and dendritic cells [56]. Interestingly, NK cell migration was substantially decreased via CX3CR1 antagonism in an esophagogastric adenocarcinomas study [156].
2.4. Cytokine Manipulation
The administration of exogenous cytokines is a standard method for expanding infused NK cells in vivo [6]. To moderate cytokine toxicity and the necessity of multiple cell infusions, researchers have directed their attention towards the genetic modulation of cytokine expression in NK cells [6]. To this extent, Nagashima and colleagues retrovirally transduced NK-92 cells to express IL-2, expanding their in vitro persistence for up to 5 months without the need for additional cytokine administration [31,157]. Primary NK and NK-92 cells have been transduced with retroviral vectors for the expression of IL-2 or IL-15, enhancing NK cell expansion and perseverance in vivo in mice [158]. Researchers have also retrovirally transduced CB-NK cells to express IL-15 together with CAR–CD19, then administered them to a xenograft Raji lymphoma murine model. Based on their results, CB-NK cells restored their function, and overall survival was prolonged [78,159]. The IL-15 transgene has been incorporated into a CAR construct by Daher and colleagues, increasing NK cell proliferation and sustainment in vivo [6]. However, it has been shown that IL-15 is negatively regulated by the cytokine-inducible SH2-containing protein, also known as CIS regulatory element, which is encoded by the CISH gene [17,23]. Blocking CIS activity leads to a significant reduction in the NK cell activation threshold [17]. The importance of the CISH gene knockout for the NK cell metabolic activity in murine in vivo studies against several cancer types, such as prostate, melanoma, and breast cancer, has been demonstrated by Delconte and colleagues [18,160,161]. The absence of CISH regulation of IL-15 resulted in increased levels of IL-15, overcoming this inhibitory obstacle against NK cell perseverance and cytotoxicity [25,160]. CRISPR/Cas9 technology was also utilized by the Kaufman group for the CISH gene deletion in iPSC-derived cells before their differentiation into NK cells, providing NK cells with elevated killing potential and survival in AML in vitro and in vivo studies [23,25,162].
Table 2.
Preclinical studies focused on NK cell receptors and their modification for cancer immunotherapy.
| NK Cell Receptor | Type of Engineering/Antibody | Method of Engineering | Target | NK Cell Source | Key Outcomes | Year | Ref. |
|---|---|---|---|---|---|---|---|
| Activating Receptors | |||||||
| CD16 | Overexpression of hnCD16 and IL-2 | DNA electroporation | Head and neck, breast, lung, colorectal, and pancreatic cancer | NK-92 | In vitro lysis of many tumor cell lines, including lung, breast, head and neck, and colon | 2016 | [62] |
| CD16 | Knockout of ADAM17 and PD-1 | CRISPR/Cas9 electroporation | B-cell lymphoma (Raji cell line) | PB-NK | In vitro enhanced ADCC with rituximab, and elevated IFN-γ levels | 2020 | [63] |
| CD16 | Overexpression of hnCD16 | Lentiviral vectors | B-cell lymphoma, ovarian cancer | iPSC-NK | In vivo enhancement of ADCC with anti-CD20 and anti-HER2 mAbs | 2020 | [60] |
| CD16 | Overexpression of hnCD16 | Retroviral vectors | B-cell leukemia and lymphoma (cell lines and primary patient material) | UCB-NK or PB-NK | Successful expansion and sorting of CD16+ cells and in vitro enhancement of ADCC with rituximab or elotuzumab | 2024 | [64] |
| CD16 CD38 |
Overexpression of CD16 and knockout of CD38 | CRISPR/Cas9 and mRNA Electroporation |
Myeloma (MM.1S, NCI-H929, and EJM cell lines) | iPSC-NK | In vitro and in vivo enhanced ADCC with daratumumab reaching high lysis levels of NCI-H929 myeloma cells and reduction of tumor burden in MM.1S xenograft mouse | 2022 | [65] |
| CD16 | Overexpression of CD16 in combination with CAR construct | Lentiviral vectors | Triple-negative breast cancer | NK92MI | In vitro enhancement of ADCC with L-ICON immunoconjugate agent and in vivo reduction in tumor growth in cell line- and patient-derived xenograft mouse models | 2020 | [163] |
| CD16 | Bispecific single domain antibody (VHH) to engage CD16 and EGFR | - | Colorectal cancer (cell lines) | PB-NK | In vitro and ex vivo enhanced activation of NK cells and lysis of EGFR-expressing tumor cell lines | 2021 | [57] |
| CD16 | BiKE for CD16 and CD33 engagement | - | Leukemia and B-cell lymphoma (HL60 and Raji cell lines) | PB-NK | In vitro activation of NK cells with CD16xCD33 BiKE and ADAM17 inhibition against refractory CD33+ AML cells | 2013 | [70] |
| CD16 | 161533 TriKE for CD16 and CD33 engagement and IL-15 production | - | Myelodysplastic syndrome (MDS) | PB-NK | In vitro enhancement of NK cell proliferation and targeting of primary MDS blasts | 2018 | [71] |
| CD16 | AFM13 bispecific antibody for CD16 and CD30 engagement | - | T-cell lymphoma (Karpas 299 and HuT-78 cell lines and Karpas-NSG mice) | PB-NK or CB-NK | In vitro and in vivo enhancement of NK cell cytotoxicity and cytokine production against CD30+ lymphoma targets | 2021 | [74] |
| NKG2D | Overexpression of NKG2D and IL-21 | DNA delivery via chitosan-based nanoparticles | CT-26-induced solid tumors in Balb/c mice | - | In vivo transfection of tumor cells increased stimulation and migration of NK cells to tumor sites, slowed tumor growth and prolonged the life spam of tumor-expressing mice | 2017 | [80] |
| NKG2D | Overexpression of miR-486-5p | Lipofection (HiPerfect Transfection Reagent) | Hepatocellular carcinoma (Huh7 cell line) |
PB-NK | In vitro activation of NK cells, induction of NKG2D levels, and enhanced lysis of Huh7 cells | 2016 | [82] |
| NKG2D | Overexpression of NKG2D | Lentiviral vectors | Human sarcoma explants and tumor cell lines | NK-92 | In vitro activation of NK-92 cells and enhanced degranulation towards sarcoma explants and tumor cell lines | 2020 | [83] |
| NKG2D | Overexpression of NKG2D in combination with CAR construct | Retroviral vectors | Myeloid-derived suppressor cells (MDSCs) | PB-NK | In vitro and in vivo increased cytotoxicity against MDSCs, pro-inflammatory cytokine and chemokine production, and tumor infiltration. Exogenous NKG2D was not susceptible to TME compared to endogenous NKG2D | 2021 | [164] |
| NKG2D | BiKE for NKG2D and CS1 engagement | - | MM | PB-NK | In vivo activation of NK cells and improved tumor clearance when tested in a xenograft NOD-SCID (NSG) mouse model | 2018 | [86] |
| NKG2D | BiKE for NKG2D and HER2 engagement | - | Breast ductal carcinoma (BT-474 cell line) | Human NK (source not specified) |
In vitro enhancement of unstimulated NK-cell-mediated killing of BT-474 cells but did not promote the secretion of pro-inflammatory cytokines | 2020 | [88] |
| NKG2C | Overexpression of NKG2C and TriKE for NKG2C and CD33 engagement and IL-15 production | Feeder cells | AML (cell lines and blasts) | iPSC-NK | In vitro enhancement of NK cell proliferation, degranulation, and IFN-γ production against AML cell lines and primary AML blasts | 2021 | [94] |
| NKp46 | FLEX-NK engager for NKp46, CD38, and Glypican-3 (GPC3) engagement | - | Hepatocellular carcinoma (cell lines and spheroids) | PB-NK | In vitro enhancement of antibody-dependent cellular phagocytosis and complement-dependent cytotoxicity of NK cells towards GPC3-expressing tumors | 2023 | [96] |
| NKp44 | mAb 14-25-9 for NKp44 and PCNA binding inhibition | - | MM | NK92-44-1 (transduced NK-92 cells to express NKp44) | In vitro enhancement of degranulation and IFN-γ production of NK92-44-1 cells against MM primary cells | 2022 | [98] |
| NKp30 | B7-H6 engagers for NKp30 and EGFR engagement | - | Epidermoid carcinoma and non-small cell lung carcinoma (A431 and A549 cell lines) | PB-NK | In vitro enhancement of IFN-γ and TNF-α production, and cell-mediated cytotoxicity of NK cells against EGFR-expressing tumor cell lines | 2021 | [44] |
| NKp30 | Engagers for NKp30 and EGFR engagement | - | Epidermoid carcinoma and non-small cell lung carcinoma, colorectal and colon cancer (A431, A549, SW-480, HCT116, and H2030 cell lines) | MNC-NK | In vitro enhancement of NK cell activation and cell-mediated killing of NK cells against EGFR-expressing cell lines | 2022 | [99] |
| Inhibitory receptors | |||||||
| KIR | IPH2101 mAb to block KIR and HLA-I engagement | - | MM (U266 and K562 cell lines) | PB-NK | In vitro enhancement of NK cell survival and activity against AML cell lines | 2011 | [104] |
| KIR | Overexpression of KIR2DL1 in combination with a CAR construct (iCARs) | Retroviral vectors | B-cell lymphoma (Raji cell line) | CB-NK | In vitro and in vivo improvement of antitumor-activity when iCARs were administrated in Raji tumor-bearing NSG mice. iCARs prevent trogocytosis-induced self-recognition and fratricide, maintaining tumor recognition and cytotoxicity | 2022 | [165] |
| NKG2A | shRNA knockout of NKG2A | Lentiviral vectors | B lymphoblastoid cells | PB-NK | In vitro induction of target cell lysis by 40% compared to NKG2A-expressing NK cells | 2008 | [110] |
| NKG2A | Blockade of NKG2A | Protein expression blockers with retroviral vectors | HLA-E tumors | PB-NK | In vivo enhancement of NK cell cytotoxicity against HLA-E tumor-expressing immunodeficient mice | 2019 | [111] |
| NKG2A | Knockout of NKG2A | CRISPR/Cas9 nucleofection | MM (primary cells) | PB-NK | In vitro enhancement of cytolytic activity of NKG2A–KO NK cells with no significant difference in cytokine production comparing with NKG2A-expressing NK cells | 2022 | [112] |
| NKG2A | Knock out of NKG2A | CRISPR/Cas9 Lentiviral vectors |
Metastatic breast cancer | PB-NK | In vivo delay of tumor progression and enhancement of survival in an HLA-E+ metastatic breast cancer xenogeneic mouse model | 2023 | [113] |
| NKG2A | Monalizumab for blockade of NKG2A and CD94 engagement | - | Chronic lymphocytic leukemia (K562 cell line) | PB-NK | In vitro restoration of NK cell activity against HLA-E-expressing targets, without impacting ADCC | 2016 | [115] |
| NKG2A | Knockout of NKG2A in combination with CAR construct | CRISPR/Cas9 Lentiviral vectors |
AML | PB-NK | In vivo complete elimination of AML and AML-initiating cells in an AML-xenografted mouse model | 2022 | [166] |
| TIGIT | 13G6 mAb for TIGIT blockade | - | Colon cancer | - | In vivo restoration of NK cell activity and cytokine production, and prolonged CT26 tumor-bearing mice survival | 2018 | [116] |
| TIGIT | Anti-mouse TIGIT (4B1) and aCD266 mAb for the blockade of TIGIT and CD266 | - | Melanoma | - | In vitro and in vivo enhancement of NK-cell mediated cytotoxicity and decrease in tumor metastasis in mouse melanoma models | 2020 | [123] |
| TIGIT | Knockout of TIGIT | CRISPR/Cas9 electroporation | Pediatric and lung cancer (cell lines and spheroids) | PB-NK | In vitro enhancement of NK-cell cytotoxicity against all tumor cell lines and spheroids tested except CHLA90 that expresses less DNAM-1 and H1975 that is generally susceptible to NK-cell-mediated killing | 2023 | [125] |
| TIGIT | CD155 and CD73 targeting | SynNorch Lentiviral vectors |
Glioblastoma | iPSC-NK | In vivo decrease in tumor growth by 40% with iNK cells, enhancement of NKp46 and granzyme B in a xenograft glioblastoma mouse model and isolated brain samples | 2024 | [126] |
| CD96 | anti-CD96 mAb for CD96 blockade | - | Lung cancer | - | In vivo enhancement of survival of mice bearing B16F10 or RM-1 lung metastases | 2016 | [127] |
| CD112R | anti-CD112R mAb for CD112R blockade | - | Colon adenocarcinoma | - | In vivo restoration of NK cell and T-cell function, and prolongation of survival of MC38 tumor-bearing mice | 2021 | [131] |
| CTLA-4 | Ipilimumab for CTLA-4 blockade | - | Melanoma | PB-NK | In vivo inhibition of tumor after treatment of chimeric murine xenograft model with allogeneic NK cells and Ipilimumab | 2013 | [133] |
| PD-1 | Anti-PD-1 and anti-PD-L1 for blockage of PD-1 and PD-L1 | - | HLA-negative cancers | - | In vivo enhancement of NK cell response towards HLA-negative tumor-bearing mice | 2018 | [136] |
| TIM-3 | Knockout of TIM-3 | CRISPR/Cas9 electroporation |
Glioblastoma (cell lines) | PB-NK | In vitro enhancement of NK-cell-mediated growth inhibition of GBM cells | 2021 | [142] |
| Chemotactic receptors | |||||||
| CXCR4 | Overexpression of CXCR4 | mRNA electroporation | - | PB-NK | In vitro enhanced chemotaxis towards SDF-1a, and in vivo increased bone marrow homing in NSG mice | 2019 | [53] |
| CXCR4 | Knockout of CXCR4 in myeloid cells | Knockout mice | Melanoma | - | In vivo reduction in tumor growth and of FasL-expressing myeloid cells, and enhancement of Fas-expressing NK cells | 2018 | [54] |
| CXCR4 | Overexpression of CXCR4 in combination with CAR construct | Lentiviral vectors | Leukemia (NALM-6 cell line) | PB-NK | In vitro enhancement of migration towards SDF-1a, and CD16+ tumor eradication while retaining functional activity | 2020 | [146] |
| CCR7 | Overexpression of CCR7 | Feeder cells trogocytosis | - | PB-NK | In vitro migration of NK cells towards CCL19 and CCL21 and in vivo enhancement of lymph node homing in athymic nude mice | 2012 | [148] |
| CCR7 | Overexpression of CCR7and CD16 | mRNA electroporation | Chronic myelogenous leukemia and melanoma (K562 and MM.1S cell lines) | PB-NK | In vitro promotion of migration towards CCL19 and enhancement of CD16-mediated ADCC with rituximab | 2016 | [149] |
| CCR7 | Overexpression of CCR7 and CXCR4 | Lentiviral vectors | Colon cancer | NK-92 | In vitro and in vivo promotion of migration to colon cells and increased survival of HT-29 tumor-bearing SCID mice | 2020 | [55] |
| CXCR3 | Overexpression of CXCR3 | Feeder cells | Renal cell carcinoma (cell lines) and melanoma (526MEL tumor-expressing mice) | PB-NK | In vitro and in vivo NK cell infiltration to CXCL10-expressing solid tumors, reduction in tumors, and increased survival of CXCL10-positive melanoma xenograft mice | 2014 | [151] |
| CXCR2 | Overexpression of CXCR2 | Retroviral vectors | Renal cell carcinoma (cell lines) | PB-NK | In vitro promotion of NK cell migration to tumor sites with no significant difference in degranulation against K562 cells compared to CXCR2- NK cells | 2017 | [152] |
| CXCR2 | Overexpression of CXCR2 and IL-2 | CRISPR/Cas9 | Colon cancer | NK-92 | In vitro enhancement of NK cell infiltration to tumor sites and in vivo prolongation of survival and reduction in colon tumor growth in tumor-bearing mice | 2021 | [154] |
| CXCR1 | Overexpression of CXCR1 in combination with CAR construct | mRNA electroporation | Peritoneal ovarian cancer | PB-NK | In vitro enhancement of NK-cell migration and in vivo infiltration to tumor sites and tumor shrinking in a intraperitoneal xenograft NSG mouse model | 2020 | [155] |
Table 3.
Recent clinical studies focused on NK cell receptors and their modification for cancer immunotherapy.
| NK Cell Receptor | Product/Study | Malignancy | NK Cell Source | Sponsor | Location | Status | Clinical Phase | Year | Key Outcomes | ClinicalTrials.gov Identifier |
|---|---|---|---|---|---|---|---|---|---|---|
| Activating Receptors | ||||||||||
| CD16 | AFM13 | Hodgkin Lymphoma | Intravenous infusion | Affimed GmbH | Houston TX USA, Würzburg GER | Completed | I | 2010 | In total, 3 of 26 patients achieved partial remission (11.5%) and 13 patients achieved stable disease (50%), with an overall disease control rate of 61.5% | NCT01221571 |
| CD16 | AFM24, SNK01 | Squamous Cell Carcinoma of Head and Neck, Non-Small-Cell Lung Carcinoma, Colorectal Neoplasms, Advanced Solid Tumor, Refractory Tumor, and Metastatic Tumor | Autologous SNK01 | NKGen Biotech, Inc. | Los Angeles CA, Chicago IL USA | Terminated | I/II | 2021 | Patients had a manageable safety profile, and SNK01 monotherapy has also shown to be well-tolerated in patients with rapidly progressive solid tumors | NCT05099549 |
| CD16 | ha-NK, Avelumab, N-803 | Merkel Cell Carcinoma | NK-92 | ImmunityBio, Inc. | San Francisco CA, Miami FL, Saint Louis MO USA | Terminated | II | 2020 | Did not meet recruitment goal | NCT03853317 |
| CD16 | haNK, Avelumab, Bevacizumab | Pancreatic Cancer | NK-92 | ImmunityBio, Inc. | El Segundo CA USA | Active, not recruiting | I/II | 2017 | - | NCT03329248 |
| Terminated | 2017 | The study was terminated early due to low enrollment. Safety data showed a 2/4 (50%) all-cause mortality. | NCT03387098 | |||||||
| Active, not recruiting | 2018 | - | NCT03586869 | |||||||
| CD16 | haNK, Avelumab, Bevacizumab | Squamous Cell Carcinoma | NK-92 | ImmunityBio, Inc. | El Segundo CA USA | Terminated | I/II | 2018 | The study was terminated early due to low enrollment. Safety data showed a 4/4 (50%) all-cause mortality. | NCT03387111 |
| CD16 | FT516 (hnCD16), CD20 Ab/PD-L1 Ab | B-cell Lymphoma and Acute Myeloid Leukemia/Solid Tumors | iPSCs | Fate Therapeutics | Phoenix AZ, San Diego CA, Minneapolis MN USA | Terminated | I | 2019 | The study was terminated by the Sponsor | NCT04023071 |
| 2020 | NCT04551885 | |||||||||
| CD16 | FT516 (hnCD16), Enoblituzumab, IL-2 | Ovarian, Fallopian Tube, and Primary Peritoneal Cancer | iPSCs | Masonic Cancer Center, University of Minnesota | Minneapolis MN USA | Completed | I | 2021 | - | NCT04630769 |
| CD16, CD38 | FT538 (hnCD16, CD38KO), IL15RF, Daratumumab | Multiple Myeloma and Acute Myeloid Leukemia/Solid Tumors | iPSCs | Fate Therapeutics | Denver CO, Minneapolis MN, Saint Louis MO, Hackensack NJ, Nashville TN USA | Terminated | I | 2020 | Interim Outcomes: Administration of FT538 cells in combination with daratumumab was safe and well tolerated without indication of CRS, neurotoxicity, or GvHD | NCT04614636 |
| 2021 | This study was terminated by the Sponsor | NCT05069935 | ||||||||
| CD16, CD38 | FT538 (hnCD16, CD38KO), Daratumumab, Fludarabine, Cyclophosphamide | Acute Myeloid Leukemia | iPSCs | Masonic Cancer Center, University of Minnesota | Minneapolis MN USA | Active, not recruiting | I | 2021 | FT538 combined with daratumumab has been well-tolerated in a heavily pre-treated patient group, showing expected toxicities and some signs of efficacy | NCT04714372 |
| NKG2D | NAKIP-AML, Talazoparib | Acute Myeloid Leukemia | Haploidentical human allogeneic NK cells | German Cancer Research Center | - | Not yet recruiting | I/II | 2024 | - | NCT05319249 |
| NKG2C and PD-1 | Dasatinib | Chronic Myeloid Leukemia | CMV-activated NKG2C+NK | Nanfang Hospital of Southern Medical University | Guangzhou CHI | Unknown | Obs. | 2021 | - | NCT04991532 |
| NY-ESO-1 TCR/IL-15 | NY-ESO-1 TCR/IL-15 NK cells | Multiple Myeloma | CB-NK | M.D. Anderson Cancer Center | Houston TX USA | Recruiting | I/II | 2023 | - | NCT06066359 |
| KIR | IPH2101 | Multiple Myeloma, Myeloma, and Smoldering Multiple Myeloma | Intravenous infusion | National Cancer Institute (NCI) | Bethesda MD USA | Terminated | II | 2010 | Lack of patients meeting the defined primary objectives | NCT01248455 |
| Inhibitory Receptors | ||||||||||
| CTLA4 | Ipilimumab, Cetuximab, CIML NK cells, N-803 | Squamous Cell Carcinoma of the Head and Neck, Recurrent Head and Neck Squamous Cell Carcinoma | CIML NK | Dana-Farber Cancer Institute | Boston MA USA | Recruiting | I | 2020 | Initial Outcomes: Allogeneic CIML NK cells combined with N-803 may promote tumor regression in patients with advanced head-and-neck cancer | NCT04290546 |
| PD-1 | SMT-NK Pembrolizumab | Biliary Tract Cancer | Allogeneic SMT-NK | SMT bio Co., Ltd. | Seoul KOR | Completed | I/II | 2019 | In phase 2a, 126 adverse events (AEs) were observed in 29 patients (85.3%). Severe AEs occurred in 16 patients (47.1%), but no dose-limiting toxicities were reported. The overall response rate (ORR) was 17.4% in the full-analysis set and 50.0% in the per-protocol set | NCT03937895 |
| PD-1 | NK cells Sintilimab | Non-small Cell Lung Cancer | Autologous PBMCs | The First Hospital of Jilin University | Changchun CHI | Completed | II | 2019 | Autologous NK cells combined with sintilimab demonstrated promising antitumor activity and had an acceptable safety profile in advanced NSCLC patients. No unexpected AE were observed | NCT03958097 |
| PD-1 | Pembrolizumab, DC-NK cells | Solid Tumors | Intravenous infusion | Allife Medical Science and Technology Co., Ltd. | - | Unknown | I | 2019 | - | NCT03815084 |
| PD-1 | NK and DC cells, Pembrolizumab, Nivolumab, Sintilimab, Toripalimab, Camrelizumab, Tislelizumab | Digestive Carcinoma, Gastrointestinal Tumors | Autologous NK cells | China Medical University | - | Not yet recruiting | II | 2022 | - | NCT05461235 |
| PD-1 | COH06 Azetolizumab | Several types of Non-Small cell Lung carcinoma | CB-NK | City of Hope Medical Center | Duarte CA USA | Active, not recruiting | I | 2022 | - | NCT05334329 |
| PD-1 | D-CIK cells, Axitinib | Renal Metastatic Cancer | PBMCs | Sun Yat-sen University | Guangzhou CHI | Unknown | II | 2018 | - | NCT03736330 |
| PD-1 | CCICC-002b, CIK cells, Sintilimab | Non-small cell lung cancer | Autologous CIK cells | Tianjin Medical University Cancer Institute and Hospital | Tianjin CHI | Unknown | II | 2021 | - | NCT04836728 |
| PD-1 | D-CIK, anti-PD-1 | Refractory Solid Tumors | PBMCs | Sun Yat-sen University | Guangzhou CHI | Unknown | I/II | 2016 | This study indicated enhanced antitumor immunity following combination treatment, particularly in patients with significant long-term survival benefits. In contrast, those with minimal survival benefit exhibited a higher proportion of peripheral CD8+TIM3+ T cells and a lower serum-level immunostimulatory cytokine profile | NCT02886897 |
| PD-1 | D-CIK and Pembrolizumab | Lung cancer neoplasms | Autologous PBMCs | Capital Medical University | Beijing CHI | Completed | I/II | 2016 | - | NCT03360630 |
| PD-1 | Anti-PD-1 P-GEMOX | High-risk Extranodal NK/T-cell lymphoma | Intravenous infusion | Cancer Institute and Hospital, Chinese Academy of Medical Sciences | Beijing CHI | Recruiting | II | 2021 | - | NCT05254899 |
| PD-1 | Pembrolizumab | NK/T cell lymphoma | Intravenous infusion | The University of Hong Kong | Hong Kong HKG | Unknown | II | 2016 | - | NCT03021057 |
| PD-1 | Merck NK-IIT Pembrolizumab | Melanoma | Intravenous infusion | Nina Bhardwaj | New York NY USA | Terminated | II | 2017 | Did not meet recruitment goal | NCT03241927 |
| PD-1 | SHR-1210 CIK cells | Renal Cell Carcinoma | Autologous CIK cells | Tianjin Medical University Cancer Institute and Hospital | Tianjin CHI | Unknown | II | 2019 | - | NCT03987698 |
| PD-1 | Toripalimab | Extranodal NK/T-cell lymphoma | Intravenous infusion | Beijing Tongren Hospital | - | Unknown | II | 2020 | - | NCT04338282 |
| PD-1 | Anti-PD-1 Chidamide Lenalidomide Etoposide | Relapsed or refractory NK/T-cell lymphoma | Intravenous infusion | Mingzhi Zhang | Zhengzhou CHI | Unknown | IV | 2019 | The 12-month progression-free survival (PFS) rate was 86.8%. All 19 patients experienced treatment-related adverse events (TRAEs), with 4 patients (21.1%) reporting immune-related AEs, including grade 1 hypothyroidism | NCT04038411 |
| PD-1 | Anti-PD-1 Pegaspargase | Extranodal NK/T-cell lymphoma | Intravenous infusion | Ruijin Hospital | Shanghai CHI | Unknown | II | 2019 | The combination of pegaspargase and sintilimab is effective and safe for treating advanced-stage NKTCL, with potential benefits in targeting fatty acid metabolism and CTLA-4 to overcome treatment resistance | NCT04096690 |
| PD-1 | SHR1210 Apatinib | NK/T-cell lymphoma | Intravenous infusion | Peking University | - | Unknown | II | 2018 | The overall response rate (ORR) was 30%, with 10% of patients achieving a complete response. The median progression-free survival (PFS) was 5.6 months, and the median overall survival was 16.7 months | NCT03701022 |
| PD-1 | Toripalimab Chemoradiotherapy | NK/T-cell lymphoma | Intravenous infusion | Sun Yat-sen University | Guangzhou CHI | Recruiting | III | 2020 | A total of 714 NKTCL patients were included. The median overall survival (OS) was 36 months, and cancer-specific survival (CSS) was 57 months | NCT04365036 |
| PD-1 | CAR2BRAIN, NK-92/5.28.z Ezabenlimab | Glioblastoma | NK-92 | Johann Wolfgang Goethe University Hospital | Frankfurt, Mannheim, Mainz GER | Active, not recruiting | I | 2017 | Study Objectives: Assessing for safety and tolerability to establish the maximum tolerated dose | NCT03383978 |
| PD-L1 | QUILT-3.060 NANT haNK | Pancreatic Cancer | NK-92 | ImmunityBio, Inc. | El Segundo CA USA | Active, not recruiting | I/II | 2017 | Initial Outcomes: Beneficial to patients with NSCLC | NCT03329248 |
| PD-L1 | QUILT-3.064, PD-L1 t-haNK | Advanced or metastatic solid tumors | NK-92 | ImmunityBio, Inc. | El Segundo CA USA | Active, not recruiting | I | 2019 | - | NCT04050709 |
| PD-L1 | Sacituzumab, PD-L1 t-haNK, N-803 | Advanced Triple Negative Breast Cancer | NK-92 | ImmunityBio, Inc. | Newport Beach CA USA | Terminated | I/II | 2021 | Low enrollment | NCT04927884 |
| PD-L1 | PD-L1 t-haNK, N-803, Aldoxorubicin | Pancreatic Cancer | NK-92 | ImmunityBio, Inc. | El Segundo CA, Newport beach CA, East Brunswick NJ USA | Active, not recruiting | II | 2020 | - | NCT04390399 |
| PD-L1 | QUILT-3.063 Avelumab haNK | Merkel Cell Carcinoma | NK-92 | ImmunityBio, Inc. | San Francisco CA, Miami FL, Saint Louis MO USA | Terminated | II | 2020 | Did not meet recruitment goal | NCT03853317 |
| PD-1/PD-L1 | QUILT-3.055, Anti-PD-1, Anti-PD-L1, PD-L1 t-haNK | Cancers previously treated with PD-1/PD-L1 Immune Checkpoint Inhibitors | NK-92 | ImmunityBio, Inc. | Anchorage AK, Hot Springs AR, El Segundo CA USA | Active, not recruiting | II | 2018 | Initial Outcomes: N803 shows low toxicity and promising efficacy in halting progression and inducing durable stable disease in patients who had previously progressed on various tumor types and CPI regimens | NCT03228667 |
| TGF-β/NR3C1 | CB-NK-TGF-betaR2-/NR3C1- | Glioblastoma | CB-NK | M.D. Anderson Cancer Center | Houston TX USA | Recruiting | I | 2023 | - | NCT04991870 |
| Chemotactic Receptors | ||||||||||
| CXCR4 | Revolution CXCR4 antagonists in combination with Nivolumab | Metastatic Renal Cell Carcinoma | PBMCs | National Cancer Institute, Naples | Naples ITA | Recruiting | I | 2016 | Baseline NK activity and early detection of CXCR4-dependent reversal of Treg suppressive function at two weeks are significant indicators of response in mRCC patients treated with Nivolumab | NCT03891485 |
2.5. Combining CAR–NK with NK Receptor Engineering
The CAR approach has been established over the past few decades as a breakthrough cell manipulation technology for tumor targeting, with CAR–T constructs already receiving FDA and EMA approval [5,24]. A CAR is a synthetic protein construct, usually derived from the combination of four different domains: an extracellular antigen-binding domain, a hinge region, a transmembrane domain, and an intracellular signaling domain (Figure 4) [167,168,169]. The target-binding specificity is determined by the antigen-binding domain, which in most cases includes a single-chain variable fragment (scFv), deriving from antibodies, and more rarely, a native protein or a peptide [167,170]. CD19 has emerged as the predominant CAR target for hematological malignancies, demonstrating promising results in terms of cell expansion and patient remission rates [22,171,172].
Figure 4.
Illustration of the different generations of chimeric antigen receptor (CAR)-NK cells. (A) First-generation CAR–NKs containing an extracellular antigen-binding domain, a hinge domain, a transmembrane domain of either CD16, NKp46, NKp44, DNAM1, or NKG2D, and an intracellular signaling domain of either CD3ζ, DAP10, or DAP12, (B) Second-generation CAR–NKs with the addition of co-stimulatory domains of either CD28, 2B4, or 4-1BB, (C) Third-generation CAR–NKs with the addition of a second intracellular stimulatory domain of either CD28, 2B4, or 4-1BB, (D) Fourth-generation CAR–NKs with the inclusion of a transgenic response modulator for the expression of cytokines, and (E) NKG2D CAR–NK cells, with NKG2D expressed in the extracellular domain, and CD3ζ and DAP10 expressed intracellularly [34,169]. Created with Biorender.
As previously discussed in this review, NK cells present a significant advantage in addressing the toxicity challenges associated with T cell therapies. Similarly, CAR–NK cells have demonstrated the ability to overcome CRS, GvHD, and neurotoxicity, which are commonly linked to CAR–T cells, as CAR–NK express a distinct cytokine profile and, due to MHC-I recognition, do not attack self-tissues [172]. Moreover, while CAR–T cells primarily rely on CAR-mediated recognition of specific antigens, CAR–NK cells target tumors through both CAR-dependent and endogenous receptor-mediated methods, including tumors that lack HLA expression [173]. The absence of HLA-matching requirements enables the development of “off-the-shelf” CAR–NK products, which could reduce the substantial logistical costs associated with CAR–T cell therapies [174]. The diverse tumor recognition mechanisms of CAR–NK cells offer the potential for targeting both hematological malignancies, such as lymphomas, and solid tumors, including colorectal, breast, and pancreatic cancer [175].
This is why CAR–NK cells have gained a lot of attention recently as clinical trials for hematological and solid tumors are growing rapidly [176]. CAR–NKs have been developed for solid tumor elimination targeting EGFRvIII, HER2, and mesothelin, as these molecules are present in various cancer types, such as glioblastoma, colorectal, ovarian, and breast cancer [22]. Other types of tumor antigens have been targeted as well with recent CAR–NK applications, such as CD20, CD22, CD33, CD123, and GD2 [169,177]. The hinge region of the CAR–NK construct, usually derived from CD8, CD28, or IgG4, is responsible for exposing the antigen-binding domain on the cell surface [167,178]. The transmembrane domain anchors the CAR construct on the cell membrane and links it to the intracellular signaling domain [167,170].
The intracellular signaling domain stimulates NK cell cytotoxic activity and is the most thoroughly studied domain in the CAR-engineering field [24,167,169]. CARs have evolved through four different generations, based on variations in the design of the intracellular domain [167]. Conventional first-generation CAR–NKs, similarly to CAR–Ts, include only CD3ζ as a signaling domain, while second- and third-generation CAR–NKs include one or two co-stimulatory domains, mostly CD28 and 4-1BB (CD137), derived from CAR–T cells [167,170]. Recently, novel CAR–NKs have been developed with the inclusion of DNAX-activation proteins DAP12 and DAP10, which are responsible for the stimulation of NKp44, activating KIR receptors (KIR2DS and KIR3DS), and NKG2C [24,173,179]. Finally, fourth-generation CARs consist of engineered cells to self-produce cytokines such as IL-2, IL-15, or IL-12, thereby enhancing their proliferation, migration, and activation towards tumor cells [168,170,176,180]. In this case, a transgenic response modulator is integrated into the CAR construct for the simultaneous production of immune modulators such as cytokines, chemokines, and suicide genes, which recruit other components of the immune system, leading to an enhanced antitumor response [176]. Although fourth-generation CARs are less investigated in NK cells compared to T cells, recent studies employing CD19-CAR/IL-15 NK-92 cells have shown increased cytotoxicity in vitro and in a xenograft mouse model [181,182].
NK cell receptor engineering, parallel to CAR technology, represents an alternative approach to augment CAR-mediated cytotoxicity while simultaneously modulating the regulatory mechanisms of activating and inhibitory receptors. NKG2D and PD-1 are the only NK receptors currently involved in CAR–NK clinical trials [178]. NKG2D ligands are expressed by several tumor types, making this activating receptor a promising tool for applications targeting hematological malignancies and solid tumors [168,173,178,183]. Besides inducing CAR–NK cytotoxicity towards antigens expressed by various tumor types, NKG2D–CAR–NKs have been designed by Parihar and colleagues to ameliorate NK cell persistence within the immunosuppressive TME by eradicating MDSCs [164]. In addition, PD-L1 CAR–NK cells have been shown to successfully target and eliminate tumors and are currently being evaluated in a phase II clinical trial for HNSCC and gastroesophageal junction cancer [184]. However, researchers are exploring alternative NK receptors in recent preclinical studies. For instance, Li and colleagues developed KIR-based inhibitory CAR–NK cells to overcome the negative effect of trogocytosis in activating CAR–NK cells [165]. In contrast, another group knocked out the inhibitory receptor NKG2A by means of CRISPR/Cas9 technology to potentially enhance the efficiency of CD33-CAR–NK cells in AML [166]. Moreover, Zhiwei Hu’s research has shown interesting results in targeting triple-negative breast cancer using CAR-engineered NK cells co-expressing CD16 for simultaneous surface antigen targeting and ADCC in combination with L-ICON. The efficacy of CAR–NK cells was significantly enhanced in combination with L-ICON-mediated ADCC both in vitro and in vivo using patient’ tumor-derived xenograft mouse models [163]. All the aforementioned methods to introduce new genetic material into the NK cells for the expression of the fusion CAR–NK receptor have been utilized by various research groups in preclinical studies [22]. Table 4 summarizes the CAR–NK studies in combination with NK cell receptor engineering, which are currently in the stage of clinical trials.
Table 4.
Recent CAR–NK clinical trials including NK cell receptors in the CAR construct.
| NK Cell Receptor | Product Name | Malignancy | NK Cell Source | Sponsor | Location | Status | Clinical Phase | Year | Key Outcomes | ClinicalTrials.gov Identifier |
|---|---|---|---|---|---|---|---|---|---|---|
| NKG2DL | NKG2D-CAR–NK92 cells | Relapsed/Refractory Solid Tumors | NK-92 | Xinxiang medical university | Xinxiang CHI | Recruiting | I | 2023 | - | NCT05528341 |
| NKG2DL | NKG2D CAR–NK Cell Therapy | Relapsed or Refractory Acute Myeloid Leukemia | Intravenous infusion | Hangzhou Cheetah Cell Therapeutics Co., Ltd. | Sanhe CHI | Terminated | Unknown | 2021 | - | NCT05247957 |
| NKG2DL | CAR–NK cells targeting NKG2D ligands | Metastatic Solid Tumors | PBMCs | The Third Affiliated Hospital of Guangzhou Medical University | Guangzhou CHI | Unknown | I | 2018 | In total, 2 out of 3 patients showed reduced ascites and fewer tumor cells. 1 out of 3 patients experienced rapid tumor regression and complete metabolic liver response. | NCT03415100 |
| NKG2DL | NKG2D CAR–NK | Refractory Metastatic Colorectal Cancer | - | Zhejiang University | Hangzhou CHI | Recruiting | I | 2021 | - | NCT05213195 |
| NKG2DL | NKX101 | Relapsed/Refractory AML and MDS | PB-NK | Nkarta Inc. | Denver CO, Jacksonville FL, Atlanta GA USA | Active, not recruiting | I | 2020 | Initial Outcomes: NKX101 shows encouraging early responses in relapsed/refractory AML, even in high-risk cases. The toxicity profile matches expectations, with no CRS, ICANS, or treatment-related deaths. | NCT04623944 |
| NKG2DL | NKG2D CAR–NK | AML | Unknown | Zhejiang University | Hangzhou CHI | Recruiting | Unknown | 2023 | - | NCT05734898 |
| NKG2DL | NKG2D CAR–NK | Ovarian Cancer | Unknown | Hangzhou Cheetah Cell Therapeutics Co., Ltd. | Hangzhou CHI | Recruiting | NA | 2023 | - | NCT05776355 |
| NKG2DL and PD-L1 | PD-1/NKG2D CAR–NK | Non-small Cell Lung Carcinoma | NK-92 | Xinxiang medical university | Xinxiang CHI | Completed | I | 2018 | A previously unreported instance of cytokine release syndrome (CRS) occurred, marking the first known case during CAR–NK therapy | NCT03656705 |
| PD-L1 | PD-L1 CAR–NK, Pembrolizumab, N-803 | Gastroesophageal Junction (GEJ) Cancers, Advanced HNSCC | t-haNK | National Cancer Institute (NCI) | Bethesda MD USA | Recruiting | II | 2021 | - | NCT04847466 |
3. Conclusions
Although NK cells have emerged as alternative source of effector cells addressing the toxicity challenges linked to T cell therapies and CAR–T cells, significant limitations remain, namely their low proliferation and persistence in the body, their low infiltration and efficacy in solid tumors, the immunosuppressive TME, and off-target toxicities. This review provides an in-depth look at the preclinical and clinical advancements in endogenous NK receptor engineering and blocking, indicating their potential impact on cancer immunotherapy. These studies highlight the versatile anti-tumor capability of NK cells while underscoring the necessity of these modifications to overcome the barriers typically associated with NK cell therapeutics.
Following ACT, the in vivo proliferation and persistence of NK cells are restricted and highly dependent on the selected source. To date, NK-92 remains the only immortalized NK cell line that has been used in clinical settings due to its enhanced proliferation rate. However, NK-92 lack critical activating receptors, such as CD16 which mediates ADCC and enhances cytotoxicity [23]. On the other hand, primary NK cells can be obtained as allogeneic products by several sources, including peripheral blood, cord blood, or iPSCs, and have been widely applied in most clinical studies [22]. Ex vivo expansion of NK cells is challenging and often results in diminished cytotoxicity. Therefore, repeated injections are needed to maximize the response, leading to elevated costs and inconvenience for patients [185]. Although the low persistence of infused cells can serve as a safety mechanism in case of life-threatening side effects, it also restricts the efficacy of such treatments, as infused NK cells are typically detectable for a few weeks after ACT [186]. Independent injections of cytokine analogs are known to enhance NK cell in vivo proliferation; therefore, current research is focusing on ex vivo genetic modification of NK cells to stably produce such cytokines [159]. For example, engineered NK cells to overexpress IL-2 or IL-15 have significantly enhanced in vivo perseverance in murine models [158]. Additionally, the deletion of the CISH gene, coding the CIS regulatory element of IL-15, is an alternative approach of preclinical studies to promote in vivo persistence and cytotoxicity of NK cells [160,162]. Moreover, cytokine expression has been integrated as an engineering strategy for CAR–NK cells, as fourth-generation CARs enclose a transgenic response modulator to stably produce endogenous cytokines. Studies have shown that IL-15 expression in combination with a CD19-CAR construct can support NK cell function in vivo [159,176]. Promising preclinical studies from MD Anderson Cancer Center using CD19–CAR/IL-15 (CAR19/IL-15) from CB-NK cells have led to the initiation of a phase I/II clinical trial targeting CD19+ B lymphoid malignancies, reporting overall response rates ranging from 32% to 68% [clinicaltrials.gov ID: NCT03056339]. Notably, CAR19/IL-15 cells derived from a CB unit with nucleated red blood cells and less than 24 h collection-to-cryopreservation time exhibited superior antitumor activity without treatment-related toxicity, underscoring the importance of NK cell expansion and priming conditions [171].
NK cell therapeutics have demonstrated significant clinical responses in patients with hematological malignancies [187,188,189]. However, their effectiveness in solid tumors, such as non-small cell lung carcinoma, renal cell carcinoma, and colorectal cancer, remains limited [190]. This is primarily attributed to insufficient tumor infiltration and the immunosuppressive effects of the TME on NK cell cytotoxicity [191]. NK cells express a wide array of chemokine receptors which allow for cell migration and infiltration into tumors. However, the processes of expansion or cryopreservation of primary NK cells often result in the downregulation of these receptors, leading to insufficient chemotaxis and reduced efficacy in targeting solid tumors [191]. Engineering of NK cells to overexpress chemokine receptors, including CXCR3, CXCR4, and CCR7, has been investigated in preclinical studies. Noteworthy examples include the overexpression of CXCR2 in NK cells targeting renal cell carcinoma and the combined overexpression of CXCR4 and CCR7 for colon cancer. Both approaches demonstrated enhanced migration to tumor sites and prolonged survival of tumor-bearing mice [55,152]. The genetic modification of NK cell to overexpress chemotactic receptors has not yet been explored in clinical settings. However, the promising outcomes observed in the preclinical studies discussed in this review offer valuable insights that can benefit the design of future clinical trials.
The immunosuppressive and hypoxic TME is another bottleneck of NK cell therapies in both hematological malignancies and solid tumors. The TME exerts a dual inhibitory effect on NK cell function by hindering activating signals and concurrently upregulating the expression of inhibitory receptors and regulatory molecules [185]. To overcome the inhibitory influence of the TME and restore NK cell functionality, current research focus on engineering strategies aims at enhancing the expression of key activating receptors and downregulating inhibitory receptors that impair NK cell activation and cytotoxicity. Among the activating receptors, CD16 and NKG2D appear to be the most preclinically and clinically studied. ADCC is undoubtedly one of the most crucial activating mechanisms of NK cells, and the discovery of therapeutic mAbs has significantly advanced the field. The upregulation of CD16 has already confirmed clinical efficacy in combination therapies with tumor-targeting mAbs. While CD16-engineered NK cells have been well-tolerated in patients, mortality is also observed in some clinical trials from ImmunityBio, Inc, potentially deriving from the combination with tumor-targeting antibodies. Moreover, overexpression of NKG2D, which is also included in many CAR–NK constructs, exhibit promising preclinical results. Recently designed NKG2D–CAR–NKs have been shown to enhance NK cell persistence within the immunosuppressive TME of MDSCs [164].
The blockade of inhibitory receptors has also been shown to enhance NK cell cytotoxicity counteracting the suppressive effects of the TME. Specifically, KIR and PD-1 inhibitory receptors have undergone extensive research and have been targeted with both ICIs (lirilumab and nivolumab, respectively) and knockout techniques. Several other inhibitory receptors were targeted with specific mAbs, such as monalizumab for NKG2A/CD94 or anti-TIM-3 antibodies, as monotherapy or in combination therapies, although their clinical efficacy could be re-evaluated. Other receptors, such as CTLA-4, TIM-3, TIGIT, and CD96, should be studied more thoroughly for their potential clinical targeting for cancer immunotherapies. Moreover, combination therapies have already been applied in preclinical studies, but their clinical translation has mostly focused on their effect on T cells. Finally, the novel breakthrough of CRISPR/Cas9 technology has been successfully employed in several preclinical studies but its application in clinical settings remains limited. Nonetheless, this technology holds significant potential for future clinical studies, particularly for the ex vivo and in vivo genetic manipulation of NK cells.
Overall, the preclinical studies discussed in this review have highlighted the potential of NK cell engineering in addressing the current limitations of NK cell therapeutics in cancer immunotherapy. However, as most clinical trials employing these methods are still in early phases, a comprehensive understanding of the benefits of receptor engineering has yet to be fully established. Future advancement in this field should prioritize optimizing the conditions for isolating, expanding, culturing, and cryopreserving NK cells to preserve their functionalities and to prolong their persistence on clinical applications. The careful selection of donors and phenotypes for “off-the-shelf” NK cell products could contribute to prolonged NK cell persistence in patients [192]. Moreover, harnessing NK chemotactic receptors is pivotal for improving NK cell migration and tumor infiltration, especially in the context of solid tumors. However, this approach has only been explored preclinically in vitro and in vivo. Investigating the potential of NK cells with enhanced anti-tumor chemotaxis represents a promising approach for future clinical studies. Additionally, the co-expression of chemotactic NK cell receptors alongside four-generation CAR constructs presents an intriguing direction, potentially increasing both the cytotoxicity and chemotaxis of NK cells against solid tumors.
As the field of CAR–NK cells continues to advance rapidly, further exploration of NK cell receptors, either as extracellular binding domains or as intracellular signaling domains, offers a promising alternative to strategies derived from CAR–T. To date, only NKG2D and PD-1 have been clinically investigated in combination with CAR constructs. Nevertheless, preclinical studies that combine CAR–NK cells with modified NK cell receptors, such as CD16, NKG2A, and KIR, pave the way for future clinical development [163,165,166]. Additionally, the already applied concept of dual CAR–Ts, which targets two different tumor-expressed antigens simultaneously, resulting in increased resistance to immunosuppression and overcoming tumor immune escape, should also be considered for CAR–NKs [24].
Although NK cell therapy is generally considered safer than T cell therapies, off-target toxicities have been observed in some clinical trials involving infused NK cells. These toxicities are commonly reported in studies where NK cell transfers are combined with other therapeutic agents, such as mAb, ICIs, engagers, or other drugs. Consequently, it remains unclear whether the adverse events derive from NK cell transfers or from the combination with other therapeutic agents. However, in a recent clinical study on advanced non-small cell lung carcinoma, one patient developed CRS after receiving three cycles of CAR–NK92 cells [clinicaltrials.gov ID: NCT03656705], with a reported 10-fold increase in IFN-γ and TNF-α levels. The clinicians attributed the CRS to a potential overactivation of NK cells or to the recognition of CAR–NK92 cells as foreign substances [193]. Future evaluation of the reasons behind the occurrence of NK-cell-mediated toxicities is crucial for the development of safe NK-cell-NK-cell-based therapies.
In addition to safety and risk evaluation, further ethical considerations are essential to the development and application of NK cell therapies. Similar to T cells therapeutics and CAR–T cells, NK cell products are often costly and not widely accessible to all populations. Although addressing the limitations of safety and efficacy of effector cells is important, it is also crucial for scientists to ensure that these therapies can and will be available globally. Moreover, ethical concerns arise from the genetic engineering of NK cells, particularly when using transduction or CRISPR/Cas9 technologies, which often result in irreversible modifications. The exploration of transient engineering methods could hold an ethical alternative for modified NK cell products.
Regarding the methods of engineering, viral DNA transduction and RNA electroporation are the most well-established delivery methods for NK cell receptor modification that have reached clinical development. However, to avoid the toxicity and potential increased cancer risk of long-persistent engineered cell therapies, there has been a recent shift in the field’s interest towards the promising use of LNPs for the transfection of immune cells. Although there are currently no NK cell receptor-related clinical trials reporting the use of LNPs, there is an ongoing trend towards their application through several preclinical studies [35,36,194]. It is anticipated that this delivery method will be widely applied in the field of cancer immunotherapy in the near future [195,196,197,198].
Overall, the engineering and modification of NK cell receptors represents a powerful and promising strategy for addressing the current limitations encountered in NK-cell-NK-cell-based immunotherapies targeting both solid and hematological malignancies. Continued clinical evaluation of these approaches is anticipated, offering the potential for significant advancements in the field of cancer immunotherapy.
Author Contributions
Conceptualization, S.D., V.P. and M.C.; investigation, S.D. and V.P.; writing—original draft preparation, S.D. and V.P.; writing—review and editing, S.D., M.R., E.M. and M.C.; visualization, S.D. and V.P.; supervision, M.R., E.M. and M.C.; funding acquisition, M.C. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This work was funded by the Health Holland Organization [grant number LSHM19131].
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Zhang Y., Zhang Z. The History and Advances in Cancer Immunotherapy: Understanding the Characteristics of Tumor-Infiltrating Immune Cells and Their Therapeutic Implications. Cell. Mol. Immunol. 2020;17:807–821. doi: 10.1038/s41423-020-0488-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robbins G.M., Wang M., Pomeroy E.J., Moriarity B.S. Nonviral Genome Engineering of Natural Killer Cells. Stem Cell Res. Ther. 2021;12:350. doi: 10.1186/s13287-021-02406-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang C., Hu Y., Xiao W., Tian Z. Chimeric Antigen Receptor- and Natural Killer Cell Receptor-Engineered Innate Killer Cells in Cancer Immunotherapy. Cell. Mol. Immunol. 2021;18:2083–2100. doi: 10.1038/s41423-021-00732-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morandi F., Yazdanifar M., Cocco C., Bertaina A., Airoldi I. Engineering the Bridge between Innate and Adaptive Immunity for Cancer Immunotherapy: Focus on Γδ T and NK Cells. Cells. 2020;9:1757. doi: 10.3390/cells9081757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolf B., Zimmermann S., Arber C., Irving M., Trueb L., Coukos G. Safety and Tolerability of Adoptive Cell Therapy in Cancer. Drug Saf. 2019;42:315–334. doi: 10.1007/s40264-018-0779-3. [DOI] [PubMed] [Google Scholar]
- 6.Daher M., Rezvani K. Outlook for New Car-Based Therapies with a Focus on Car Nk Cells: What Lies beyond Car-Engineered t Cells in the Race against Cancer. Cancer Discov. 2021;11:45–58. doi: 10.1158/2159-8290.CD-20-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wendel P., Reindl L.M., Bexte T., Künnemeyer L., Särchen V., Albinger N., Mackensen A., Rettinger E., Bopp T., Ullrich E. Arming Immune Cells for Battle: A Brief Journey through the Advancements of t and Nk Cell Immunotherapy. Cancers. 2021;13:1481. doi: 10.3390/cancers13061481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reindl L.M., Albinger N., Bexte T., Müller S., Hartmann J., Ullrich E. Immunotherapy with NK Cells: Recent Developments in Gene Modification Open up New Avenues. Oncoimmunology. 2020;9:1777651. doi: 10.1080/2162402X.2020.1777651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin C.Y., Gobius I., Souza-Fonseca-Guimaraes F. Natural Killer Cell Engineering—A New Hope for Cancer Immunotherapy. Semin. Hematol. 2020;57:194–200. doi: 10.1053/j.seminhematol.2020.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Zamai L., Ponti C., Mirandola P., Gobbi G., Papa S., Galeotti L., Cocco L., Marco V. NK Cells and Cancer. J. Immunol. 2007;178:4011–4016. doi: 10.4049/jimmunol.178.7.4011. [DOI] [PubMed] [Google Scholar]
- 11.Rezvani K., Rouce R.H. The Application of Natural Killer Cell Immunotherapy for the Treatment of Cancer. Front. Immunol. 2015;6:578. doi: 10.3389/fimmu.2015.00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mantesso S., Geerts D., Spanholtz J., Kučerová L. Genetic Engineering of Natural Killer Cells for Enhanced Antitumor Function. Front. Immunol. 2020;11:607131. doi: 10.3389/fimmu.2020.607131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michel T., Ollert M., Zimmer J. A Hot Topic: Cancer Immunotherapy and Natural Killer Cells. Int. J. Mol. Sci. 2022;23:797. doi: 10.3390/ijms23020797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Müller S., Bexte T., Gebel V., Kalensee F., Stolzenberg E., Hartmann J., Koehl U., Schambach A., Wels W.S., Modlich U., et al. High Cytotoxic Efficiency of Lentivirally and Alpharetrovirally Engineered CD19-Specific Chimeric Antigen Receptor Natural Killer Cells Against Acute Lymphoblastic Leukemia. Front. Immunol. 2020;10:3123. doi: 10.3389/fimmu.2019.03123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu W., Wang G., Huang D., Sui M., Xu Y. Cancer Immunotherapy Based on Natural Killer Cells: Current Progress and New Opportunities. Front. Immunol. 2019;10:1205. doi: 10.3389/fimmu.2019.01205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paolini R., Bernardini G., Molfetta R., Santoni A. NK Cells and Interferons. Cytokine Growth Factor Rev. 2015;26:113–120. doi: 10.1016/j.cytogfr.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Maddineni S., Silberstein J.L., Sunwoo J.B. Emerging NK Cell Therapies for Cancer and the Promise of next Generation Engineering of IPSC-Derived NK Cells. J. Immunother. Cancer. 2022;10:e004693. doi: 10.1136/jitc-2022-004693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimasaki N., Jain A., Campana D. NK Cells for Cancer Immunotherapy. Nat. Rev. Drug Discov. 2020;19:200–218. doi: 10.1038/s41573-019-0052-1. [DOI] [PubMed] [Google Scholar]
- 19.Sivori S., Della Chiesa M., Carlomagno S., Quatrini L., Munari E., Vacca P., Tumino N., Mariotti F.R., Mingari M.C., Pende D., et al. Inhibitory Receptors and Checkpoints in Human NK Cells, Implications for the Immunotherapy of Cancer. Front. Immunol. 2020;11:2156. doi: 10.3389/fimmu.2020.02156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buckle I., Guillerey C. Inhibitory Receptors and Immune Checkpoints Regulating Natural Killer Cell Responses to Cancer. Cancers. 2021;13:4263. doi: 10.3390/cancers13174263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Myers J.A., Miller J.S. Exploring the NK Cell Platform for Cancer Immunotherapy. Nat. Rev. Clin. Oncol. 2021;18:85–100. doi: 10.1038/s41571-020-0426-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu S., Galat V., Galat Y., Lee Y.K.A., Wainwright D., Wu J. NK Cell-Based Cancer Immunotherapy: From Basic Biology to Clinical Development. J. Hematol. Oncol. 2021;14:7. doi: 10.1186/s13045-020-01014-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shankar K., Capitini C.M., Saha K. Genome Engineering of Induced Pluripotent Stem Cells to Manufacture Natural Killer Cell Therapies. Stem Cell Res. Ther. 2020;11:234. doi: 10.1186/s13287-020-01741-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laskowski T.J., Biederstädt A., Rezvani K. Natural Killer Cells in Antitumour Adoptive Cell Immunotherapy. Nat. Rev. Cancer. 2022;22:557–575. doi: 10.1038/s41568-022-00491-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karagiannis P., Kim S.I. IPSC-Derived Natural Killer Cells for Cancer Immunotherapy. Mol. Cells. 2021;44:541–548. doi: 10.14348/molcells.2021.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang S., Gao X., Zhang L., Yang E., Li Y., Yu L. The Advances and Challenges of Nk Cell-Based Cancer Immunotherapy. Curr. Oncol. 2021;28:1077–1093. doi: 10.3390/curroncol28020105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phung S.K., Miller J.S., Felices M. Bi-Specific and Tri-Specific NK Cell Engagers: The New Avenue of Targeted NK Cell Immunotherapy. Mol. Diagn. Ther. 2021;25:577–592. doi: 10.1007/s40291-021-00550-6. [DOI] [PubMed] [Google Scholar]
- 28.Cao Y., Wang X., Jin T., Tian Y., Dai C., Widarma C., Song R., Xu F. Immune Checkpoint Molecules in Natural Killer Cells as Potential Targets for Cancer Immunotherapy. Signal Transduct. Target. Ther. 2020;5:250. doi: 10.1038/s41392-020-00348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang M., Lam K.P., Xu S. Natural Killer Cell Engagers (NKCEs): A New Frontier in Cancer Immunotherapy. Front. Immunol. 2023;14:1207276. doi: 10.3389/fimmu.2023.1207276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El-Mayta R., Zhang Z., Hamilton A.G., Mitchell M.J. Delivery Technologies to Engineer Natural Killer Cells for Cancer Immunotherapy. Cancer Gene Ther. 2021;28:947–959. doi: 10.1038/s41417-021-00336-2. [DOI] [PubMed] [Google Scholar]
- 31.Carlsten M., Childs R.W. Genetic Manipulation of NK Cells for Cancer Immunotherapy: Techniques and Clinical Implications. Front. Immunol. 2015;6:266. doi: 10.3389/fimmu.2015.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loo J., Sicher I., Goff A., Kim O., Clary N., Alexeev A., Sulchek T., Zamarayeva A., Han S., Calero-Garcia M. Microfluidic Transfection of MRNA into Human Primary Lymphocytes and Hematopoietic Stem and Progenitor Cells Using Ultra-Fast Physical Deformations. Sci. Rep. 2021;11:21407. doi: 10.1038/s41598-021-00893-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yilmaz A., Cui H., Caligiuri M.A., Yu J. Chimeric Antigen Receptor-Engineered Natural Killer Cells for Cancer Immunotherapy. J. Hematol. Oncol. 2020;13:168. doi: 10.1186/s13045-020-00998-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hosseini M., Habibi Z., Hosseini N., Abdoli S., Rezaei N. Preclinical Studies of Chimeric Antigen Receptor-Modified Natural Killer Cells in Cancer Immunotherapy: A Review. Expert Opin. Biol. Ther. 2022;22:349–366. doi: 10.1080/14712598.2021.1983539. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura T., Nakade T., Sato Y., Harashima H. Delivering MRNA to a Human NK Cell Line, NK-92 Cells, by Lipid Nanoparticles. Int. J. Pharm. 2023;636:122810. doi: 10.1016/j.ijpharm.2023.122810. [DOI] [PubMed] [Google Scholar]
- 36.Douka S., Brandenburg L.E., Casadidio C., Walther J., Garcia B.B.M., Spanholtz J., Raimo M., Hennink W.E., Mastrobattista E., Caiazzo M. Lipid Nanoparticle-Mediated Messenger RNA Delivery for Ex Vivo Engineering of Natural Killer Cells. J. Control. Release. 2023;361:455–469. doi: 10.1016/j.jconrel.2023.08.014. [DOI] [PubMed] [Google Scholar]
- 37.Bryceson Y.T., March M.E., Ljunggren H.G., Long E.O. Synergy among Receptors on Resting NK Cells for the Activation of Natural Cytotoxicity and Cytokine Secretion. Blood. 2006;107:159–166. doi: 10.1182/blood-2005-04-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haroun-Izquierdo A., Vincenti M., Netskar H., Van Ooijen H., Zhang B., Bendzick L., Kanaya M., Momayyezi P., Li S., Wiiger M.T., et al. Adaptive Single-KIR + NKG2C + NK Cells Expanded from Select Superdonors Show Potent Missing-Self Reactivity and Efficiently Control HLA-Mismatched Acute Myeloid Leukemia. J. Immunother. Cancer. 2022;10:168. doi: 10.1136/jitc-2022-005577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sivori S., Pende D., Quatrini L., Pietra G., Della Chiesa M., Vacca P., Tumino N., Moretta F., Mingari M.C., Locatelli F., et al. NK Cells and ILCs in Tumor Immunotherapy. Mol. Asp. Med. 2021;80:100870. doi: 10.1016/j.mam.2020.100870. [DOI] [PubMed] [Google Scholar]
- 40.Ho J.W., Hershkovitz O., Peiris M., Zilka A., Bar-Ilan A., Nal B., Chu K., Kudelko M., Kam Y.W., Achdout H., et al. H5-Type Influenza Virus Hemagglutinin Is Functionally Recognized by the Natural Killer-Activating Receptor NKp44. J. Virol. 2008;82:2028–2032. doi: 10.1128/JVI.02065-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gaggero S., Bruschi M., Petretto A., Parodi M., Del Zotto G., Lavarello C., Prato C., Santucci L., Barbuto A., Bottino C., et al. Nidogen-1 Is a Novel Extracellular Ligand for the NKp44 Activating Receptor. Oncoimmunology. 2018;7:e1470730. doi: 10.1080/2162402X.2018.1470730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosental B., Brusilovsky M., Hadad U., Oz D., Appel M.Y., Afergan F., Yossef R., Rosenberg L.A., Aharoni A., Cerwenka A., et al. Proliferating Cell Nuclear Antigen Is a Novel Inhibitory Ligand for the Natural Cytotoxicity Receptor NKp44. J. Immunol. 2011;187:5693–5702. doi: 10.4049/jimmunol.1102267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baychelier F., Sennepin A., Ermonval M., Dorgham K., Debre P., Vieillard V. Identification of a Cellular Ligand for the Natural Cytotoxicity Receptor NKp44. Blood. 2013;122:2935–2942. doi: 10.1182/blood-2013-03-489054. [DOI] [PubMed] [Google Scholar]
- 44.Pekar L., Klausz K., Busch M., Valldorf B., Kolmar H., Wesch D., Oberg H.-H., Krohn S., Boje A.S., Gehlert C.L., et al. Affinity Maturation of B7-H6 Translates into Enhanced NK Cell–Mediated Tumor Cell Lysis and Improved Proinflammatory Cytokine Release of Bispecific Immunoligands via NKp30 Engagement. J. Immunol. 2021;206:225–236. doi: 10.4049/jimmunol.2001004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shin M.H., Kim J., Lim S.A., Kim J., Kim S.J., Lee K.M. NK Cell-Based Immunotherapies in Cancer. Immune Netw. 2020;20:e14. doi: 10.4110/in.2020.20.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stanietsky N., Rovis T.L., Glasner A., Seidel E., Tsukerman P., Yamin R., Enk J., Jonjic S., Mandelboim O. Mouse TIGIT Inhibits NK-Cell Cytotoxicity upon Interaction with PVR. Eur. J. Immunol. 2013;43:2138–2150. doi: 10.1002/eji.201243072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Islam R., Pupovac A., Evtimov V., Boyd N., Shu R., Boyd R., Trounson A. Enhancing a Natural Killer: Modification of Nk Cells for Cancer Immunotherapy. Cells. 2021;10:1058. doi: 10.3390/cells10051058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu J., Fu H., Yang Y., Yu H., Ai X., Lei Y., Bao W., Tang Y. Modulation of CXCR1 and CXCR3 Expression on NK Cells via Tim-3 in a Murine Model of Primary Biliary Cholangitis. Mol. Immunol. 2021;135:342–350. doi: 10.1016/j.molimm.2021.04.014. [DOI] [PubMed] [Google Scholar]
- 49.Lisi L., Lacal P.M., Martire M., Navarra P., Graziani G. Clinical Experience with CTLA-4 Blockade for Cancer Immunotherapy: From the Monospecific Monoclonal Antibody Ipilimumab to Probodies and Bispecific Molecules Targeting the Tumor Microenvironment. Pharmacol. Res. 2022;175:105997. doi: 10.1016/j.phrs.2021.105997. [DOI] [PubMed] [Google Scholar]
- 50.Zhang C., Liu Y. Targeting NK Cell Checkpoint Receptors or Molecules for Cancer Immunotherapy. Front. Immunol. 2020;11:1295. doi: 10.3389/fimmu.2020.01295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Teijeira Á., Garasa S., Gato M., Alfaro C., Migueliz I., Cirella A., de Andrea C., Ochoa M.C., Otano I., Etxeberria I., et al. CXCR1 and CXCR2 Chemokine Receptor Agonists Produced by Tumors Induce Neutrophil Extracellular Traps That Interfere with Immune Cytotoxicity. Immunity. 2020;52:856–871.e8. doi: 10.1016/j.immuni.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 52.Fitzgerald A.A., Wang S., Agarwal V., Marcisak E.F., Zuo A., Jablonski S.A., Loth M., Fertig E.J., Macdougall J., Zhukovsky E., et al. DPP Inhibition Alters the CXCR3 Axis and Enhances NK and CD8+ T Cell Infiltration to Improve Anti-PD1 Efficacy in Murine Models of Pancreatic Ductal Adenocarcinoma. J. Immunother. Cancer. 2021;9:e002837. doi: 10.1136/jitc-2021-002837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Levy E., Reger R., Segerberg F., Lambert M., Leijonhufvud C., Baumer Y., Carlsten M., Childs R. Enhanced Bone Marrow Homing of Natural Killer Cells Following MRNA Transfection with Gain-of-Function Variant CXCR4R334X. Front. Immunol. 2019;10:1262. doi: 10.3389/fimmu.2019.01262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang J., Kumar A., Vilgelm A.E., Chen S., Gregory D., Novitskiy S.V., Joyce S., Richmond A. Loss of CXCR4 in Myeloid Cells Enhances Antitumor Immunity and Reduces Melanoma Growth through NK Cell and FASL Mechanisms. Cancer Immunol. Res. 2019;6:1186–1198. doi: 10.1158/2326-6066.CIR-18-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang L., Huang C., Wang C., Zhang S., Li Z., Zhu Y., Li D., Gao L., Ge Z., Su M., et al. Overexpressed CXCR4 and CCR7 on the Surface of NK92 Cell Have Improved Migration and Anti-Tumor Activity in Human Colon Tumor Model. Anticancer Drugs. 2020;31:333–344. doi: 10.1097/CAD.0000000000000868. [DOI] [PubMed] [Google Scholar]
- 56.Korbecki J., Siminska D., Kojder K., Grochans S., Gutowska I. Fractalkine/CX3CL1 in Neoplastic Processes. Int. J. Mol. Sci. 2020;21:3723. doi: 10.3390/ijms21103723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Toffoli E.C., Sheikhi A., Lameris R., King L.A., van Vliet A., Walcheck B., Verheul H.M.W., Spanholtz J., Tuynman J., de Gruijl T.D., et al. Enhancement of Nk Cell Antitumor Effector Functions Using a Bispecific Single Domain Antibody Targeting Cd16 and the Epidermal Growth Factor Receptor. Cancers. 2021;13:5446. doi: 10.3390/cancers13215446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dixon K.J., Wu J., Walcheck B. Engineering Anti-Tumor Monoclonal Antibodies and Fc Receptors to Enhance ADCC by Human NK Cells. Cancers. 2021;13:312. doi: 10.3390/cancers13020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dexiu C., Xianying L., Yingchun H., Jiafu L. Advances in CD247. Scand. J. Immunol. 2022;96:e13170. doi: 10.1111/sji.13170. [DOI] [PubMed] [Google Scholar]
- 60.Zhu H., Blum R.H., Bjordahl R., Gaidarova S., Rogers P., Lee T.T., Abujarour R., Bonello G.B., Wu J., Tsai P.F., et al. Pluripotent Stem Cell-Derived NK Cells with High-Affinity Noncleavable CD16a Mediate Improved Antitumor Activity. Blood. 2020;135:399–410. doi: 10.1182/blood.2019000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Srpan K., Ambrose A., Karampatzakis A., Saeed M., Cartwright A.N.R., Guldevall K., De Matos G.D.S.C., Önfelt B., Davis D.M. Shedding of CD16 Disassembles the NK Cell Immune Synapse and Boosts Serial Engagement of Target Cells. J. Cell Biol. 2018;217:3267–3283. doi: 10.1083/jcb.201712085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jochems C., Hodge J.W., Fantini M., Fujii R., Morillon Y.M., Greiner J.W., Padget M.R., Tritsch S.R., Tsang K.Y., Campbell K.S., et al. An NK Cell Line (HaNK) Expressing High Levels of Granzyme and Engineered to Express the High Affinity CD16 Allele. Oncotarget. 2016;7:86359–86373. doi: 10.18632/oncotarget.13411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pomeroy E.J., Hunzeker J.T., Kluesner M.G., Lahr W.S., Smeester B.A., Crosby M.R., Lonetree C.L., Yamamoto K., Bendzick L., Miller J.S., et al. A Genetically Engineered Primary Human Natural Killer Cell Platform for Cancer Immunotherapy. Mol. Ther. 2020;28:52–63. doi: 10.1016/j.ymthe.2019.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Hauten P.M.M., Hooijmaijers L., Vidal-Manrique M., van der Waart A.B., Hobo W., Wu J., Blijlevens N.M.A., Jansen J.H., Walcheck B., Schaap N.P.M., et al. Engineering of CD34+ Progenitor-Derived Natural Killer Cells with Higher-Affinity CD16a for Enhanced Antibody-Dependent Cellular Cytotoxicity. Cytotherapy. 2024;26:252–260. doi: 10.1016/j.jcyt.2023.11.009. [DOI] [PubMed] [Google Scholar]
- 65.Clara J.A., Levy E.R., Reger R., Barisic S., Chen L., Cherkasova E., Chakraborty M., Allan D.S.J., Childs R. High-Affinity CD16 Integration into a CRISPR/Cas9-Edited CD38 Locus Augments CD38-Directed Antitumor Activity of Primary Human Natural Killer Cells. J. Immunother. Cancer. 2022;10:e003804. doi: 10.1136/jitc-2021-003804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Piedra-Quintero Z.L., Wilson Z., Nava P., Guerau-de-Arellano M. CD38: An Immunomodulatory Molecule in Inflammation and Autoimmunity. Front. Immunol. 2020;11:597959. doi: 10.3389/fimmu.2020.597959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Juckett M., Mailankody S., Eghtedar A., Bickers C., Zong X., Wong L., Ly T., Byon J., Cooley S., Valamehr B., et al. A Phase I Study of FT538, an Off-the-Shelf, Multiplexed-Engineered, IPSC-Derived NK Cell Therapy in Combination with Daratumumab in Relapsed/Refractory Multiple Myeloma. Blood. 2022;140:10327–10328. doi: 10.1182/blood-2022-166728. [DOI] [Google Scholar]
- 68.Maakaron J.E., Seichter C., Wangen R., Hoeschen A., Kolaseri Krishnadas D., Kent K., Shanley R., O’Leary D., El Jurdi N.H., Holtan S.G., et al. Phase I Study of FT538 + Daratumumab for Treatment of r/r AML. Blood. 2023;142:4842. doi: 10.1182/blood-2023-189132. [DOI] [Google Scholar]
- 69.Shin H.G., Yang H.R., Yoon A., Lee S. Bispecific Antibody-Based Immune-Cell Engagers and Their Emerging Therapeutic Targets in Cancer Immunotherapy. Int. J. Mol. Sci. 2022;23:5686. doi: 10.3390/ijms23105686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wiernik A., Foley B., Zhang B., Verneris M.R., Warlick E., Gleason M.K., Ross J.A., Luo X., Weisdorf D.J., Walcheck B., et al. Targeting Natural Killer Cells to Acute Myeloid Leukemia in Vitro with a CD16×33 Bispecific Killer Cell Engager and ADAM17 Inhibition. Clin. Cancer Res. 2013;19:3844–3855. doi: 10.1158/1078-0432.CCR-13-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sarhan D., Brandt L., Felices M., Guldevall K., Lenvik T., Hinderlie P., Curtsinger J., Warlick E., Spellman S.R., Blazar B.R., et al. 161533 TriKE Stimulates NK-Cell Function to Overcome Myeloid-Derived Suppressor Cells in MDS. Blood Adv. 2018;2:1459–1469. doi: 10.1182/bloodadvances.2017012369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wolf N.K., Kissiov D.U., Raulet D.H. Roles of Natural Killer Cells in Immunity to Cancer, and Applications to Immunotherapy. Nat. Rev. Immunol. 2022;3:90–105. doi: 10.1038/s41577-022-00732-1. [DOI] [PubMed] [Google Scholar]
- 73.Souza-Fonseca-Guimaraes F., Cursons J., Huntington N.D. The Emergence of Natural Killer Cells as a Major Target in Cancer Immunotherapy. Trends Immunol. 2019;40:142–158. doi: 10.1016/j.it.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 74.Kerbauy L.N., Marin N.D., Kaplan M., Banerjee P.P., Berrien-Elliott M.M., Becker-Hapak M., Basar R., Foster M., Melo L.G., Neal C.C., et al. Combining AFM13, a Bispecific CD30/CD16 Antibody, with Cytokine-Activated Blood and Cord Blood-Derived NK Cells Facilitates CAR-like Responses Against CD30+ Malignancies. Clin. Cancer Res. 2021;27:3744–3756. doi: 10.1158/1078-0432.CCR-21-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rothe A., Sasse S., Topp M.S., Eichenauer D.A., Hummel H., Reiners K.S., Dietlein M., Kuhnert G., Kessler J., Buerkle C., et al. A Phase 1 Study of the Bispecific Anti-CD30/CD16A Antibody Construct AFM13 in Patients with Relapsed or Refractory Hodgkin Lymphoma. Blood. 2015;125:4024–4031. doi: 10.1182/blood-2014-12-614636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Siemaszko J., Marzec-przyszlak A., Bogunia-kubik K. Nkg2d Natural Killer Cell Receptor—A Short Description and Potential Clinical Applications. Cells. 2021;10:1420. doi: 10.3390/cells10061420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xing S., Ferrari de Andrade L. NKG2D and MICA/B Shedding: A ‘Tag Game’ between NK Cells and Malignant Cells. Clin. Transl. Immunol. 2020;9:e1230. doi: 10.1002/cti2.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Afolabi L.O., Adeshakin A.O., Sani M.M., Bi J., Wan X. Genetic Reprogramming for NK Cell Cancer Immunotherapy with CRISPR/Cas9. Immunology. 2019;158:63–69. doi: 10.1111/imm.13094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sekiba K., Yamagami M., Otsuka M., Suzuki T., Kishikawa T., Ishibashi R., Ohno M., Sato M., Koike K. Transcriptional Activation of the MICA Gene with an Engineered CRISPR-Cas9 System. Biochem. Biophys. Res. Commun. 2017;486:521–525. doi: 10.1016/j.bbrc.2017.03.076. [DOI] [PubMed] [Google Scholar]
- 80.Tan L., Han S., Ding S., Xiao W., Ding Y., Qian L., Wang C., Gong W. Chitosan Nanoparticle-Based Delivery of Fused NKG2D-IL-21 Gene Suppresses Colon Cancer Growth in Mice. Int. J. Nanomed. 2017;12:3095–3107. doi: 10.2147/IJN.S128032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Raza A., Rossi G.R., Janjua T.I., Souza-Fonseca-Guimaraes F., Popat A. Nanobiomaterials to Modulate Natural Killer Cell Responses for Effective Cancer Immunotherapy. Trends Biotechnol. 2023;41:77–92. doi: 10.1016/j.tibtech.2022.06.011. [DOI] [PubMed] [Google Scholar]
- 82.Youness R.A., Rahmoon M.A., Assal R.A., Gomaa A.I., Hamza M.T., Waked I., El Tayebi H.M., Abdelaziz A.I. Contradicting Interplay between Insulin-like Growth Factor-1 and MiR-486-5p in Primary NK Cells and Hepatoma Cell Lines with a Contemporary Inhibitory Impact on HCC Tumor Progression. Growth Factors. 2016;34:128–140. doi: 10.1080/08977194.2016.1200571. [DOI] [PubMed] [Google Scholar]
- 83.Sayitoglu E.C., Georgoudaki A.M., Chrobok M., Ozkazanc D., Josey B.J., Arif M., Kusser K., Hartman M., Chinn T.M., Potens R., et al. Boosting Natural Killer Cell-Mediated Targeting of Sarcoma Through DNAM-1 and NKG2D. Front. Immunol. 2020;11:40. doi: 10.3389/fimmu.2020.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Paczulla A.M., Rothfelder K., Raffel S., Konantz M., Steinbacher J., Wang H., Ndoh Mbarga M., Schaefer T., Dörfel D., Falcone M., et al. Absence of NKG2D Ligands Defines Human Acute Myeloid Leukaemia Stem Cells and Mediates Their Immune Evasion. Blood. 2018;132:769. doi: 10.1182/blood-2018-99-118047. [DOI] [Google Scholar]
- 85.Jelenčić V., Šestan M., Kavazović I., Lenartić M., Marinović S., Holmes T.D., Prchal-Murphy M., Lisnić B., Sexl V., Bryceson Y.T., et al. NK Cell Receptor NKG2D Sets Activation Threshold for the NCR1 Receptor Early in NK Cell Development. Nat. Immunol. 2018;19:1083–1092. doi: 10.1038/s41590-018-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chan W.K., Kang S., Youssef Y., Glankler E.N., Barrett E.R., Carter A.M., Ahmed E.H., Prasad A., Chen L., Zhang J., et al. A CS1-NKG2D Bispecific Antibody Collectivel Activates Cytolytic Immune Cells against Multiple Myeloma. Cancer Immunol. Res. 2018;6:776–787. doi: 10.1158/2326-6066.CIR-17-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Demaria O., Gauthier L., Debroas G., Vivier E. Natural Killer Cell Engagers in Cancer Immunotherapy: Next Generation of Immuno-Oncology Treatments. Eur. J. Immunol. 2021;51:1934–1942. doi: 10.1002/eji.202048953. [DOI] [PubMed] [Google Scholar]
- 88.Raynaud A., Desrumeaux K., Vidard L., Termine E., Baty D., Chames P., Vigne E., Kerfelec B. Anti-NKG2D Single Domain-Based Antibodies for the Modulation of Anti-Tumor Immune Response. Oncoimmunology. 2021;10:e1854529. doi: 10.1080/2162402X.2020.1854529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gumá M., Budt M., Sáez A., Brckalo T., Hengel H., Angulo A., López-Botet M. Expansion of CD94/NKG2C+ NK Cells in Response to Human Cytomegalovirus-Infected Fibroblasts. Blood. 2006;107:3624–3631. doi: 10.1182/blood-2005-09-3682. [DOI] [PubMed] [Google Scholar]
- 90.Vacca P., Pietra G., Tumino N., Munari E., Mingari M.C., Moretta L. Exploiting Human NK Cells in Tumor Therapy. Front. Immunol. 2020;10:3013. doi: 10.3389/fimmu.2019.03013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Capuano C., Pighi C., Battella S., De Federicis D., Galandrini R., Palmieri G. Harnessing Cd16-Mediated Nk Cell Functions to Enhance Therapeutic Efficacy of Tumor-Targeting Mabs. Cancers. 2021;13:2500. doi: 10.3390/cancers13102500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cichocki F., Cooley S., Davis Z., Defor T.E., Schlums H., Brunstein C.G., Blazar B.R., Wagner J., Diamond D.J., Verneris R., et al. CD56dimCD57+ NKG2C+ NK Cell Expansion Is Associated with Reduced Leukemia Relapse after Reduced Intensity HCT. Leukemia. 2016;30:456–463. doi: 10.1038/leu.2015.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Murad S., Michen S., Becker A., Füssel M., Schackert G., Tonn T., Momburg F., Temme A. NKG2C+ NK Cells for Immunotherapy of Glioblastoma Multiforme. Int. J. Mol. Sci. 2022;23:5857. doi: 10.3390/ijms23105857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chiu E., Felices M., Cichocki F., Davis Z., Wang H., Tuninga K., Vallera D.A., Lee T., Bjordahl R., Malmberg K.J., et al. Anti-NKG2C/IL-15/Anti-CD33 Killer Engager Directs Primary and IPSC-Derived NKG2C+ NK Cells to Target Myeloid Leukemia. Mol. Ther. 2021;29:3410–3421. doi: 10.1016/j.ymthe.2021.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gauthier L., Morel A., Anceriz N., Rossi B., Blanchard-Alvarez A., Grondin G., Trichard S., Cesari C., Sapet M., Bosco F., et al. Multifunctional Natural Killer Cell Engagers Targeting NKp46 Trigger Protective Tumor Immunity. Cell. 2019;177:1701–1713.e16. doi: 10.1016/j.cell.2019.04.041. [DOI] [PubMed] [Google Scholar]
- 96.Arulanandam A., Lin L., Chang H.M., Cerutti M., Choblet S., Gao P., Rath A., Bensussan A., Kadouche J., Teper D., et al. Derivation and Preclinical Characterization of CYT-303, a Novel NKp46-NK Cell Engager Targeting GPC3. Cells. 2023;12:996. doi: 10.3390/cells12070996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Barrow A.D., Edeling M.A., Trifonov V., Luo J., Goyal P., Bohl B., Bando J.K., Kim A.H., Walker J., Andahazy M., et al. Natural Killer Cells Control Tumor Growth by Sensing a Growth Factor. Cell. 2018;172:534–548.e19. doi: 10.1016/j.cell.2017.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Iraqi M., Edri A., Greenshpan Y., Goldstein O., Ofir N., Bolel P., Ahmad M.A., Zektser M., Campbell K.S., Rouvio O., et al. Blocking the PCNA/NKp44 Checkpoint to Stimulate NK Cell Responses to Multiple Myeloma. Int. J. Mol. Sci. 2022;23:4717. doi: 10.3390/ijms23094717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Klausz K., Pekar L., Boje A.S., Gehlert C.L., Krohn S., Gupta T., Xiao Y., Krah S., Zaynagetdinov R., Lipinski B., et al. Multifunctional NK Cell–Engaging Antibodies Targeting EGFR and NKp30 Elicit Efficient Tumor Cell Killing and Proinflammatory Cytokine Release. J. Immunol. 2022;209:1724–1735. doi: 10.4049/jimmunol.2100970. [DOI] [PubMed] [Google Scholar]
- 100.Rajalingam R. The Impact of HLA Class I-Specific Killer Cell Immunoglobulin-like Receptors on Antibody-Dependent Natural Killer Cell-Mediated Cytotoxicity and Organ Allograft Rejection. Front. Immunol. 2016;7:585. doi: 10.3389/fimmu.2016.00585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bi J., Tian Z. NK Cell Dysfunction and Checkpoint Immunotherapy. Front. Immunol. 2019;10:1999. doi: 10.3389/fimmu.2019.01999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Khan M., Arooj S., Wang H. NK Cell-Based Immune Checkpoint Inhibition. Front. Immunol. 2020;11:167. doi: 10.3389/fimmu.2020.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chu J., Gao F., Yan M., Zhao S., Yan Z., Shi B., Liu Y. Natural Killer Cells: A Promising Immunotherapy for Cancer. J. Transl. Med. 2022;20:240. doi: 10.1186/s12967-022-03437-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Benson D.M., Bakan C.E., Zhang S., Collins S.M., Liang J., Srivastava S., Hofmeister C.C., Efebera Y., Andre P., Romagne F., et al. IPH2101, a Novel Anti-Inhibitory KIR Antibody, and Lenalidomide Combine to Enhance the Natural Killer Cell versus Multiple Myeloma Effect. Blood. 2011;118:6387–6391. doi: 10.1182/blood-2011-06-360255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Alfarra H., Weir J., Grieve S., Reiman T. Targeting NK Cell Inhibitory Receptors for Precision Multiple Myeloma Immunotherapy. Front. Immunol. 2020;11:575609. doi: 10.3389/fimmu.2020.575609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Navin I., Lam M.T., Parihar R. Design and Implementation of NK Cell-Based Immunotherapy to Overcome the Solid Tumor Microenvironment. Cancers. 2020;12:3871. doi: 10.3390/cancers12123871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Debska-Zielkowska J., Moszkowska G., Zielinski M., Zielinska H., Dukat-Mazurek A., Piotr T., Stefanska K. KIR Receptors as Key Regulators of NK Cells Activity in Health and Disease. Cells. 2021;10:1777. doi: 10.3390/cells10071777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wei Y., Ren X., Galbo P.M., Moerdler S., Wang H., Alejandro Sica R., Etemad-Gilbertson B., Shi L., Zhu L., Tang X., et al. KIR3DL3-HHLA2 Is a Human Immunosuppressive Pathway and a Therapeutic Target. Sci. Immunol. 2021;6:9792. doi: 10.1126/sciimmunol.abf9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Forslund E., Sohlberg E., Enqvist M., Olofsson P.E., Malmberg K.-J., Önfelt B. Microchip-Based Single-Cell Imaging Reveals That CD56dimCD57-KIR-NKG2A+ NK Cells Have More Dynamic Migration Associated with Increased Target Cell Conjugation and Probability of Killing Compared to CD56dimCD57-KIR-NKG2A- NK Cells. J. Immunol. 2015;195:3374–3381. doi: 10.4049/jimmunol.1500171. [DOI] [PubMed] [Google Scholar]
- 110.Figueiredo C., Seltsam A., Blasczyk R. Permanent Silencing of NKG2A Expression for Cell-Based Therapeutics. J. Mol. Med. 2009;87:199–210. doi: 10.1007/s00109-008-0417-0. [DOI] [PubMed] [Google Scholar]
- 111.Kamiya T., Seow S.V., Wong D., Robinson M., Campana D. Blocking Expression of Inhibitory Receptor NKG2A Overcomes Tumor Resistance to NK Cells. J. Clin. Investig. 2019;129:2094–2106. doi: 10.1172/JCI123955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bexte T., Alzubi J., Reindl L.M., Wendel P., Schubert R., Salzmann-Manrique E., von Metzler I., Cathomen T., Ullrich E. CRISPR-Cas9 Based Gene Editing of the Immune Checkpoint NKG2A Enhances NK Cell Mediated Cytotoxicity against Multiple Myeloma. Oncoimmunology. 2022;11:e2081415. doi: 10.1080/2162402X.2022.2081415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mac Donald A., Guipouy D., Lemieux W., Harvey M., Bordeleau L.J., Guay D., Roméro H., Li Y., Dion R., Béland K., et al. KLRC1 Knockout Overcomes HLA-E-Mediated Inhibition and Improves NK Cell Antitumor Activity against Solid Tumors. Front. Immunol. 2023;14:1231916. doi: 10.3389/fimmu.2023.1231916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.André P., Denis C., Soulas C., Bourbon-Caillet C., Lopez J., Arnoux T., Bléry M., Bonnafous C., Gauthier L., Morel A., et al. Anti-NKG2A MAb Is a Checkpoint Inhibitor That Promotes Anti-Tumor Immunity by Unleashing Both T and NK Cells. Cell. 2018;175:1731–1743.e13. doi: 10.1016/j.cell.2018.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McWilliams E.M., Mele J.M., Cheney C., Timmerman E.A., Fiazuddin F., Strattan E.J., Mo X., Byrd J.C., Muthusamy N., Awan F.T. Therapeutic CD94/NKG2A Blockade Improves Natural Killer Cell Dysfunction in Chronic Lymphocytic Leukemia. Oncoimmunology. 2016;5:e1226720. doi: 10.1080/2162402X.2016.1226720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang Q., Bi J., Zheng X., Chen Y., Wang H., Wu W., Wang Z., Wu Q., Peng H., Wei H., et al. Blockade of the Checkpoint Receptor TIGIT Prevents NK Cell Exhaustion and Elicits Potent Anti-Tumor Immunity. Nat. Immunol. 2018;19:723–732. doi: 10.1038/s41590-018-0132-0. [DOI] [PubMed] [Google Scholar]
- 117.Blake S.J., Dougall W.C., Miles J.J., Teng M.W.L., Smyth M.J. Molecular Pathways: Targeting CD96 and TIGIT for Cancer Immunotherapy. Clin. Cancer Res. 2016;22:5183–5188. doi: 10.1158/1078-0432.CCR-16-0933. [DOI] [PubMed] [Google Scholar]
- 118.Yeo J., Ko M., Lee D.H., Park Y., Jin H.S. Tigit/Cd226 Axis Regulates Anti-Tumor Immunity. Pharmaceuticals. 2021;14:200. doi: 10.3390/ph14030200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chu X., Tian W., Wang Z., Zhang J., Zhou R. Co-Inhibition of TIGIT and PD-1/PD-L1 in Cancer Immunotherapy: Mechanisms and Clinical Trials. Mol. Cancer. 2023;22:93. doi: 10.1186/s12943-023-01800-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rousseau A., Parisi C., Barlesi F. Anti-TIGIT Therapies for Solid Tumors: A Systematic Review. ESMO Open. 2023;8:101184. doi: 10.1016/j.esmoop.2023.101184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jin H., Park Y. Hitting the Complexity of the TIGIT-CD96-CD112R-CD226 Axis for next-Generation Cancer Immunotherapy. BMB Rep. 2021;54:2–11. doi: 10.5483/BMBRep.2021.54.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chiang E.Y., Mellman I. TIGIT-CD226-PVR Axis: Advancing Immune Checkpoint Blockade for Cancer Immunotherapy. J. Immunother. Cancer. 2022;10:e004711. doi: 10.1136/jitc-2022-004711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chauvin J.M., Ka M., Pagliano O., Menna C., Ding Q., DeBlasio R., Sanders C., Hou J., Li X.Y., Ferrone S., et al. IL15 Stimulation with TIGIT Blockade Reverses CD155-Mediated NK-Cell Dysfunction in Melanoma. Clin. Cancer Res. 2020;26:5520–5533. doi: 10.1158/1078-0432.CCR-20-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ge Z., Peppelenbosch M.P., Sprengers D., Kwekkeboom J. TIGIT, the Next Step Towards Successful Combination Immune Checkpoint Therapy in Cancer. Front. Immunol. 2021;12:699895. doi: 10.3389/fimmu.2021.699895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hasan M.F., Campbell A.R., Croom-Perez T.J., Oyer J.L., Dieffenthaller T.A., Robles-Carrillo L.D., Cash C.A., Eloriaga J.E., Kumar S., Andersen B.W., et al. Knockout of the Inhibitory Receptor TIGIT Enhances the Antitumor Response of Ex Vivo Expanded NK Cells and Prevents Fratricide with Therapeutic Fc-Active TIGIT Antibodies. J. Immunother. Cancer. 2023;11:e007502. doi: 10.1136/jitc-2023-007502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lupo K.B., Yao X., Borde S., Wang J., Torregrosa-Allen S., Elzey B.D., Utturkar S., Lanman N.A., McIntosh M., Matosevic S. SynNotch-Programmed IPSC-Derived NK Cells Usurp TIGIT and CD73 Activities for Glioblastoma Therapy. Nat. Commun. 2024;15:1909. doi: 10.1038/s41467-024-46343-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Blake S.J., Stannard K., Liu J., Allen S., Yong M.C.R., Mittal D., Aguilera A.R., Miles J.J., Lutzky V.P., de Andrade L.F., et al. Suppression of Metastases Using a New Lymphocyte Checkpoint Target for Cancer Immunotherapy. Cancer Discov. 2016;6:446–459. doi: 10.1158/2159-8290.CD-15-0944. [DOI] [PubMed] [Google Scholar]
- 128.Ye W., Luo C., Liu F., Liu Z., Chen F. CD96 Correlates with Immune Infiltration and Impacts Patient Prognosis: A Pan-Cancer Analysis. Front. Oncol. 2021;11:634617. doi: 10.3389/fonc.2021.634617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zeng T., Cao Y., Jin T., Tian Y., Dai C., Xu F. The CD112R/CD112 Axis: A Breakthrough in Cancer Immunotherapy. J. Exp. Clin. Cancer Res. 2021;40:285. doi: 10.1186/s13046-021-02053-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Xu F., Sunderland A., Zhou Y., Schulick R.D., Edil B.H., Zhu Y. Blockade of CD112R and TIGIT Signaling Sensitizes Human Natural Killer Cell Functions. Cancer Immunol. Immunother. 2017;66:1367–1375. doi: 10.1007/s00262-017-2031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li Y., Zhang Y., Cao G., Zheng X., Sun C., Wei H., Tian Z., Xiao W., Sun R., Sun H. Blockade of Checkpoint Receptor PVRIG Unleashes Anti-Tumor Immunity of NK Cells in Murine and Human Solid Tumors. J. Hematol. Oncol. 2021;14:100. doi: 10.1186/s13045-021-01112-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wu B., Zhong C., Lang Q., Liang Z., Zhang Y., Zhao X., Yu Y., Zhang H., Xu F., Tian Y. Poliovirus Receptor (PVR)-like Protein Cosignaling Network: New Opportunities for Cancer Immunotherapy. J. Exp. Clin. Cancer Res. 2021;40:267. doi: 10.1186/s13046-021-02068-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Laurent S., Queirolo P., Boero S., Salvi S., Piccioli P., Boccardo S., Minghelli S., Morabito A., Fontana V., Pietra G., et al. The Engagement of CTLA-4 on Primary Melanoma Cell Lines Induces Antibody-Dependent Cellular Cytotoxicity and TNF-α Production. J. Transl. Med. 2013;11:108. doi: 10.1186/1479-5876-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gemelli M., Noonan D.M., Carlini V., Pelosi G., Barberis M., Ricotta R., Albini A. Overcoming Resistance to Checkpoint Inhibitors: Natural Killer Cells in Non-Small Cell Lung Cancer. Front. Oncol. 2022;12:886440. doi: 10.3389/fonc.2022.886440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hasim M.S., Marotel M., Hodgins J.J., Vulpis E., Makinson O.J., Asif S., Shih H.Y., Scheer A.K., MacMillan O., Alonso F.G., et al. When Killers Become Thieves: Trogocytosed PD-1 Inhibits NK Cells in Cancer. Sci. Adv. 2022;8:eabj3286. doi: 10.1126/sciadv.abj3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hsu J., Hodgins J.J., Marathe M., Nicolai C.J., Bourgeois-Daigneault M.C., Trevino T.N., Azimi C.S., Scheer A.K., Randolph H.E., Thompson T.W., et al. Contribution of NK Cells to Immunotherapy Mediated by PD-1/PD-L1 Blockade. J. Clin. Investig. 2018;128:4654–4668. doi: 10.1172/JCI99317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Makowska A., Braunschweig T., Denecke B., Shen L., Baloche V., Busson P., Kontny U. Interferon β and Anti-PD-1/PD-L1 Checkpoint Blockade Cooperate in NK Cell-Mediated Killing of Nasopharyngeal Carcinoma Cells. Transl. Oncol. 2019;12:1237–1256. doi: 10.1016/j.tranon.2019.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Makowska A., Meier S., Shen L., Busson P., Baloche V., Kontny U. Anti-PD-1 Antibody Increases NK Cell Cytotoxicity towards Nasopharyngeal Carcinoma Cells in the Context of Chemotherapy-Induced Upregulation of PD-1 and PD-L1. Cancer Immunol. Immunother. 2021;70:323–336. doi: 10.1007/s00262-020-02681-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jia L., Chen N., Chen X., Niu C., Liu Z., Ma K., Wang N., Yang L., Zhao Y., Song W., et al. Sintilimab plus Autologous NK Cells as Second-Line Treatment for Advanced Non-Small-Cell Lung Cancer Previous Treated with Platinum-Containing Chemotherapy. Front. Immunol. 2022;13:1074906. doi: 10.3389/fimmu.2022.1074906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Armand P., Lesokhin A., Borrello I., Timmerman J., Gutierrez M., Zhu L., Popa McKiver M., Ansell S.M. A Phase 1b Study of Dual PD-1 and CTLA-4 or KIR Blockade in Patients with Relapsed/Refractory Lymphoid Malignancies. Leukemia. 2021;35:777–786. doi: 10.1038/s41375-020-0939-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ohs I., Ducimetière L., Marinho J., Kulig P., Becher B., Tugues S. Restoration of Natural Killer Cell Antimetastatic Activity by IL12 and Checkpoint Blockade. Cancer Res. 2017;77:7059–7071. doi: 10.1158/0008-5472.CAN-17-1032. [DOI] [PubMed] [Google Scholar]
- 142.Morimoto T., Nakazawa T., Matsuda R., Nishimura F., Nakamura M., Yamada S., Nakagawa I., Park Y.S., Tsujimura T., Nakase H. CRISPR-Cas9–Mediated TIM3 Knockout in Human Natural Killer Cells Enhances Growth Inhibitory Effects on Human Glioma Cells. Int. J. Mol. Sci. 2021;22:3489. doi: 10.3390/ijms22073489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xu L., Huang Y., Tan L., Yu W., Chen D., Lu C., He J., Wu G., Liu X., Zhang Y. Increased Tim-3 Expression in Peripheral NK Cells Predicts a Poorer Prognosis and Tim-3 Blockade Improves NK Cell-Mediated Cytotoxicity in Human Lung Adenocarcinoma. Int. Immunopharmacol. 2015;29:635–641. doi: 10.1016/j.intimp.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 144.Susek K.H., Karvouni M., Alici E., Lundqvist A. The Role of CXC Chemokine Receptors 1–4 on Immune Cells in the Tumor Microenvironment. Front. Immunol. 2018;9:2159. doi: 10.3389/fimmu.2018.02159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Müller N., Michen S., Tietze S., Töpfer K., Schulte A., Lamszus K., Schmitz M., Schackert G., Pastan I., Temme A. Engineering NK Cells Modified with an EGFRvIII-Specific Chimeric Antigen Receptor to Overexpress CXCR4 Improves Immunotherapy of CXCL12/SDF-1α-Secreting Glioblastoma. J. Immunother. 2015;38:197–210. doi: 10.1097/CJI.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jamali A., Hadjati J., Madjd Z., Mirzaei H.R., Thalheimer F.B., Agarwal S., Bonig H., Ullrich E., Hartmann J. Highly Efficient Generation of Transgenically Augmented CAR NK Cells Overexpressing CXCR4. Front. Immunol. 2020;11:2028. doi: 10.3389/fimmu.2020.02028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Levy E.R., Carlsten M., Childs R.W. MRNA Transfection to Improve NK Cell Homing to Tumors. Methods Mol. Biol. 2016;1441:231–240. doi: 10.1007/978-1-4939-3684-7_19. [DOI] [PubMed] [Google Scholar]
- 148.Somanchi S.S., Somanchi A., Cooper L.J.N., Lee D.A. Engineering Lymph Node Homing of Ex Vivo-Expanded Human Natural Killer Cells via Trogocytosis of the Chemokine Receptor CCR7. Blood. 2012;119:5164–5172. doi: 10.1182/blood-2011-11-389924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Carlsten M., Levy E., Karambelkar A., Li L., Reger R., Berg M., Peshwa M.V., Childs R.W. Efficient MRNA-Based Genetic Engineering of Human NK Cells with High-Affinity CD16 and CCR7 Augments Rituximab-Induced ADCC against Lymphoma and Targets NK Cell Migration toward the Lymph Node-Associated Chemokine CCL19. Front. Immunol. 2016;7:105. doi: 10.3389/fimmu.2016.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bonanni V., Antonangeli F., Santoni A., Bernardini G. Targeting of CXCR3 Improves Anti-Myeloma Efficacy of Adoptively Transferred Activated Natural Killer Cells. J. Immunother. Cancer. 2019;7:290. doi: 10.1186/s40425-019-0751-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wennerberg E., Kremer V., Childs R., Lundqvist A. CXCL10-Induced Migration of Adoptively Transferred Human Natural Killer Cells toward Solid Tumors Causes Regression of Tumor Growth in Vivo. Cancer Immunol. Immunother. 2015;64:225–235. doi: 10.1007/s00262-014-1629-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kremer V., Ligtenberg M., Zendehdel R., Seitz C., Duivenvoorden A., Wennerberg E., Colón E., Scherman-Plogell A.H., Lundqvist A. Genetic Engineering of Human NK Cells to Express CXCR2 Improves Migration to Renal Cell Carcinoma. J. Immunother. Cancer. 2017;5:73. doi: 10.1186/s40425-017-0275-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Groth C., Arpinati L., Shaul M.E., Winkler N., Diester K., Gengenbacher N., Weber R., Arkhypov I., Lasser S., Petrova V., et al. Blocking Migration of Polymorphonuclear Myeloid-Derived Suppressor Cells Inhibits Mouse Melanoma Progression. Cancers. 2021;13:726. doi: 10.3390/cancers13040726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gao L., Yang L., Zhang S., Ge Z., Su M., Shi Y., Wang X., Huang C. Engineering NK-92 Cell by Upregulating CXCR2 and IL-2 Via CRISPR-Cas9 Improves Its Antitumor Effects as Cellular Immunotherapy for Human Colon Cancer. J. Interferon Cytokine Res. 2021;41:450–460. doi: 10.1089/jir.2021.0078. [DOI] [PubMed] [Google Scholar]
- 155.Ng Y.Y., Tay J.C.K., Wang S. CXCR1 Expression to Improve Anti-Cancer Efficacy of Intravenously Injected CAR-NK Cells in Mice with Peritoneal Xenografts. Mol. Ther.—Oncolytics. 2020;16:75–85. doi: 10.1016/j.omto.2019.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mylod E., Melo A.M., Donlon N.E., Davern M., Bhardwaj A., Reynolds J.V., Lysaght J., Conroy M.J. Fractalkine Elicits Chemotactic, Phenotypic, and Functional Effects on CX3CR1+CD27− NK Cells in Obesity-Associated Cancer. J. Immunol. 2021;207:1200–1210. doi: 10.4049/jimmunol.2000987. [DOI] [PubMed] [Google Scholar]
- 157.Konstantinidis K.V., Alici E., Aints A., Christensson B., Ljunggren H.G., Dilber M.S. Targeting IL-2 to the Endoplasmic Reticulum Confines Autocrine Growth Stimulation to NK-92 Cells. Exp. Hematol. 2005;33:159–164. doi: 10.1016/j.exphem.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 158.Matosevic S. Viral and Nonviral Engineering of Natural Killer Cells as Emerging Adoptive Cancer Immunotherapies. J. Immunol. Res. 2018;2018:4054815. doi: 10.1155/2018/4054815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Liu E., Tong Y., Dotti G., Shaim H., Savoldo B., Mukherjee M., Orange J., Wan X., Lu X., Reynolds A., et al. Cord Blood NK Cells Engineered to Express IL-15 and a CD19-Targeted CAR Show Long-Term Persistence and Potent Antitumor Activity. Leukemia. 2018;32:520–531. doi: 10.1038/leu.2017.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Delconte R.B., Kolesnik T.B., Dagley L.F., Rautela J., Shi W., Putz E.M., Stannard K., Zhang J.G., Teh C., Firth M., et al. CIS Is a Potent Checkpoint in NK Cell-Mediated Tumor Immunity. Nat. Immunol. 2016;17:816–824. doi: 10.1038/ni.3470. [DOI] [PubMed] [Google Scholar]
- 161.Zhou Y., Li M., Zhou K., Brown J., Tsao T., Cen X., Husman T., Bajpai A., Dunn Z.S., Yang L. Engineering-Induced Pluripotent Stem Cells for Cancer Immunotherapy. Cancers. 2022;14:2266. doi: 10.3390/cancers14092266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhu H., Blum R.H., Bernareggi D., Ask E.H., Wu Z., Hoel H.J., Meng Z., Wu C., Guan K.L., Malmberg K.J., et al. Metabolic Reprograming via Deletion of CISH in Human IPSC-Derived NK Cells Promotes In Vivo Persistence and Enhances Anti-Tumor Activity. Cell Stem Cell. 2020;27:224–237.e6. doi: 10.1016/j.stem.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hu Z. Tissue Factor as a New Target for CAR-NK Cell Immunotherapy of Triple-Negative Breast Cancer. Sci. Rep. 2020;10:2815. doi: 10.1038/s41598-020-59736-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Parihar R., Rivas C., Huynh M., Omer B., Lapteva N., Metelitsa L.S., Gottschalk S.M., Rooney C.M. NK Cells Expressing a Chimeric Activating Receptor Eliminate MDSCs and Rescue Impaired CAR-T Cell Activity against Solid Tumors. Cancer Immunol. Res. 2019;7:363–375. doi: 10.1158/2326-6066.CIR-18-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Li Y., Basar R., Wang G., Liu E., Moyes J.S., Li L., Kerbauy L.N., Uprety N., Fathi M., Rezvan A., et al. KIR-Based Inhibitory CARs Overcome CAR-NK Cell Trogocytosis-Mediated Fratricide and Tumor Escape. Nat. Med. 2022;28:2133–2144. doi: 10.1038/s41591-022-02003-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Albinger N., Bexte T., Buchinger L., Wendel P., Al-Ajami A., Gessner A., Särchen V., Alzubi J., Mertlitz S., Penack O., et al. CRISPR/Cas9 Gene Editing of Immune Checkpoint Receptor NKG2A Improves the Efficacy of Primary CD33-CAR-NK Cells Against AML. Blood. 2022;140:4558–4559. doi: 10.1182/blood-2022-169758. [DOI] [Google Scholar]
- 167.Pan K., Farrukh H., Chittepu V.C.S.R., Xu H., Pan C., Zhu Z. CAR Race to Cancer Immunotherapy: From CAR T, CAR NK to CAR Macrophage Therapy. J. Exp. Clin. Cancer Res. 2022;41:119. doi: 10.1186/s13046-022-02327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Li H., Song W., Li Z., Zhang M. Preclinical and Clinical Studies of CAR-NK-Cell Therapies for Malignancies. Front. Immunol. 2022;13:992232. doi: 10.3389/fimmu.2022.992232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhang L., Meng Y., Feng X., Han Z. CAR-NK Cells for Cancer Immunotherapy: From Bench to Bedside. Biomark. Res. 2022;10:12. doi: 10.1186/s40364-022-00364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gong Y., Klein Wolterink R.G.J., Wang J., Bos G.M.J., Germeraad W.T.V. Chimeric Antigen Receptor Natural Killer (CAR-NK) Cell Design and Engineering for Cancer Therapy. J. Hematol. Oncol. 2021;14:73. doi: 10.1186/s13045-021-01083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Marin D., Li Y., Basar R., Rafei H., Daher M., Dou J., Mohanty V., Dede M., Nieto Y., Uprety N., et al. Safety, Efficacy and Determinants of Response of Allogeneic CD19-Specific CAR-NK Cells in CD19+ B Cell Tumors: A Phase 1/2 Trial. Nat. Med. 2024;30:772–784. doi: 10.1038/s41591-023-02785-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Liu E., Marin D., Banerjee P., Macapinlac H.A., Thompson P., Basar R., Nassif Kerbauy L., Overman B., Thall P., Kaplan M., et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N. Engl. J. Med. 2020;382:545–553. doi: 10.1056/NEJMoa1910607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wang W., Jiang J., Wu C. CAR-NK for Tumor Immunotherapy: Clinical Transformation and Future Prospects. Cancer Lett. 2020;472:175–180. doi: 10.1016/j.canlet.2019.11.033. [DOI] [PubMed] [Google Scholar]
- 174.Lu H., Zhao X., Li Z., Hu Y., Wang H. From CAR-T Cells to CAR-NK Cells: A Developing Immunotherapy Method for Hematological Malignancies. Front. Oncol. 2021;11:720501. doi: 10.3389/fonc.2021.720501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chan L.Y., Dass S.A., Tye G.J., Imran S.A.M., Wan Kamarul Zaman W.S., Nordin F. CAR-T Cells/-NK Cells in Cancer Immunotherapy and the Potential of MSC to Enhance Its Efficacy: A Review. Biomedicines. 2022;10:804. doi: 10.3390/biomedicines10040804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Li J., Hu H., Lian K., Zhang D., Hu P., He Z., Zhang Z., Wang Y. CAR-NK Cells in Combination Therapy against Cancer: A Potential Paradigm. Heliyon. 2024;10:e27196. doi: 10.1016/j.heliyon.2024.e27196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Acharya U.H., Walter R.B. Chimeric Antigen Receptor (CAR)-Modified Immune Effector Cell Therapy for Acute Myeloid Leukemia (AML) Cancers. 2020;12:3617. doi: 10.3390/cancers12123617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Khawar M.B., Sun H. CAR-NK Cells: From Natural Basis to Design for Kill. Front. Immunol. 2021;12:707542. doi: 10.3389/fimmu.2021.707542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Xie G., Dong H., Liang Y., Ham J.D., Rizwan R., Chen J. CAR-NK Cells: A Promising Cellular Immunotherapy for Cancer. EBioMedicine. 2020;59:102975. doi: 10.1016/j.ebiom.2020.102975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lu T., Ma R., Dong W., Teng K.Y., Kollath D.S., Li Z., Yi J., Bustillos C., Ma S., Tian L., et al. Off-the-Shelf CAR Natural Killer Cells Secreting IL-15 Target Spike in Treating COVID-19. Nat. Commun. 2022;13:2576. doi: 10.1038/s41467-022-30216-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Li L., Mohanty V., Dou J., Huang Y., Banerjee P.P., Miao Q., Lohr J.G., Vijaykumar T., Frede J., Knoechel B., et al. Loss of Metabolic Fitness Drives Tumor Resistance after CAR-NK Cell Therapy and Can Be Overcome by Cytokine Engineering. Sci. Adv. 2023;9:eadd6997. doi: 10.1126/sciadv.add6997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Silvestre R.N., Eitler J., de Azevedo J.T.C., Tirapelle M.C., Fantacini D.M.C., de Souza L.E.B., Swiech K., Covas D.T., Calado R.T., Montero P.O., et al. Engineering NK-CAR.19 Cells with the IL-15/IL-15Rα Complex Improved Proliferation and Anti-Tumor Effect in Vivo. Front. Immunol. 2023;14:1226518. doi: 10.3389/fimmu.2023.1226518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Frazao A., Rethacker L., Messaoudene M., Avril M.F., Toubert A., Dulphy N., Caignard A. NKG2D/NKG2-Ligand Pathway Offers New Opportunities in Cancer Treatment. Front. Immunol. 2019;10:661. doi: 10.3389/fimmu.2019.00661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wrona E., Borowiec M., Potemski P. CAR-NK Cells in the Treatment of Solid Tumors. Int. J. Mol. Sci. 2021;22:5899. doi: 10.3390/ijms22115899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Page A., Chuvin N., Valladeau-Guilemond J., Depil S. Development of NK Cell-Based Cancer Immunotherapies through Receptor Engineering. Cell. Mol. Immunol. 2024;21:315–331. doi: 10.1038/s41423-024-01145-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kennedy P.R., Felices M., Miller J.S. Challenges to the Broad Application of Allogeneic Natural Killer Cell Immunotherapy of Cancer. Stem Cell Res. Ther. 2022;13:165. doi: 10.1186/s13287-022-02769-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Heuser M., Tschan-Plessl A., Thol F., Schwarzer A., Kloos A., Kattre N., Passweg J., Pinkernell K., Ganser A. Allogeneic, CD34 +, Umbilical Cordblood-Derived NK Cell Adoptive Immunotherapy for the Treatment of Acute Myeloid Leukemia Patients with Measurable Residual Disease. Blood. 2021;138:1745–1746. doi: 10.1182/blood-2021-150138. [DOI] [Google Scholar]
- 188.Choi I., Yoon S.R., Park S.Y., Kim H., Jung S.J., Jang Y.J., Kang M., Yeom Y., Lee J.L., Kim D.Y., et al. Donor-Derived Natural Killer Cells Infused after Human Leukocyte Antigen-Haploidentical Hematopoietic Cell Transplantation: A Dose-Escalation Study. Biol. Blood Marrow Transplant. 2014;20:696–704. doi: 10.1016/j.bbmt.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 189.Bachanova V., Maakaron J.E., Cichocki F., McKenna D.H., Cao Q., DeFor T.E., Janakiram M., Wangen R., Cayci Z., Grzywacz B., et al. Gda-201, a Novel Metabolically Enhanced Allogeneic Natural Killer (NK) Cell Product Yields High Remission Rates in Patients with Relapsed/Refractory Non-Hodgkin Lymphoma (NHL): 2-Year Survival and Correlation with Cytokine IL7. Blood. 2021;138:3854. doi: 10.1182/blood-2021-149989. [DOI] [Google Scholar]
- 190.Lian G.Y., Mak T.S.K., Yu X.Q., Lan H.Y. Challenges and Recent Advances in NK Cell-Targeted Immunotherapies in Solid Tumors. Int. J. Mol. Sci. 2022;23:164. doi: 10.3390/ijms23010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tong L., Jiménez-Cortegana C., Tay A.H.M., Wickström S., Galluzzi L., Lundqvist A. NK Cells and Solid Tumors: Therapeutic Potential and Persisting Obstacles. Mol. Cancer. 2022;21:206. doi: 10.1186/s12943-022-01672-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Woan K.V., Kim H., Bjordahl R., Davis Z.B., Gaidarova S., Goulding J., Hancock B., Mahmood S., Abujarour R., Wang H., et al. Harnessing Features of Adaptive NK Cells to Generate IPSC-Derived NK Cells for Enhanced Immunotherapy. Cell Stem Cell. 2021;28:2062–2075.e5. doi: 10.1016/j.stem.2021.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhang X., Guo Y., Ji Y., Gao Y., Zhang M., Liu Y., Zhu W., Lu P. Cytokine Release Syndrome after Modified CAR-NK Therapy in an Advanced Non-Small Cell Lung Cancer Patient: A Case Report. Cell Transplant. 2022;31:9636897221094244. doi: 10.1177/09636897221094244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Delehedde C., Ciganek I., Bernard P.L., Laroui N., Da Silva C.C., Gonçalves C., Nunes J., Bennaceur-Griscelli A.L., Imeri J., Huyghe M., et al. Enhancing Natural Killer Cells Proliferation and Cytotoxicity Using Imidazole-Based Lipid Nanoparticles Encapsulating Interleukin-2 MRNA. Mol. Ther.—Nucleic Acids. 2024;35:102263. doi: 10.1016/j.omtn.2024.102263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wang L., Wu L., Zhu Z., Zhang Q., Li W., Gonzalez G.M., Wang Y., Rana T.M. Role of PCIF1-mediated 5′-cap N6-methyladeonsine MRNA Methylation in Colorectal Cancer and Anti-PD-1 Immunotherapy. EMBO J. 2023;42:e111673. doi: 10.15252/embj.2022111673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhang Z., Huang Y., Li J., Su F., Kuo J.C.T., Hu Y., Zhao X., Lee R.J. Antitumor Activity of Anti-MiR-21 Delivered through Lipid Nanoparticles. Adv. Healthc. Mater. 2023;12:2202412. doi: 10.1002/adhm.202202412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Huang C., Duan X., Wang J., Tian Q., Ren Y., Chen K., Zhang Z., Li Y., Feng Y., Zhong K., et al. Lipid Nanoparticle Delivery System for MRNA Encoding B7H3-Redirected Bispecific Antibody Displays Potent Antitumor Effects on Malignant Tumors. Adv. Sci. 2023;10:2205532. doi: 10.1002/advs.202205532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhang Y., Xie F., Yin Y., Zhang Q., Jin H., Wu Y., Pang L., Li J., Gao J. Immunotherapy of Tumor RNA-Loaded Lipid Nanoparticles against Hepatocellular Carcinoma. Int. J. Nanomed. 2021;16:1553–1564. doi: 10.2147/IJN.S291421. [DOI] [PMC free article] [PubMed] [Google Scholar]