Abstract
Staphylococcus pseudintermedius is frequently associated with several bacterial infections in dogs, highlighting a One Health concern due to the zoonotic potential. Given the clinical significance of this pathogen, we performed comprehensive genomic analyses of 28 S. pseudintermedius strains isolated from canine infections throughout whole-genome sequencing using Illumina HiSeq, and compared the genetic features between S. pseudintermedius methicillin-resistant (MRSP) and methicillin-susceptible (MSSP) strains. Our analyses determined that MRSP genomes are larger than MSSP strains, with significant changes in antimicrobial resistance genes and virulent markers, suggesting differences in the pathogenicity of MRSP and MSSP strains. In addition, the pangenome analysis of S. pseudintermedius from canine and human origins identified core and accessory genomes with 1847 and 3037 genes, respectively, which indicates that most of the S. pseudintermedius genome is highly variable. Furthermore, phylogenomic analysis clearly separated MRSP from MSSP strains, despite their infection sites, showing phylogenetic differences according to methicillin susceptibility. Altogether our findings underscore the importance of studying the evolutionary dynamics of S. pseudintermedius, which is crucial for the development of effective prevention and control strategies of resistant S. pseudintermedius infections.
Keywords: resistance, virulence, zoonosis, MSSP, MRSP, wgNGS
1. Introduction
Staphylococcus pseudintermedius is a commensal bacteria frequently found in the mucosal tissue and skin of mammals [1]. In dogs, some virulent S. pseudintermedius strains cause opportunistic infections [2], including pyoderma, otitis, cystitis, pyometra, bacteremia and post-surgical infections [1,3,4]. The effective treatment of such infections may be hampered by the high virulence of S. pseudintermedius and the emergence of antimicrobial resistance among the circulating clinical specimens [5,6].
Accordingly, S. pseudintermedius with clinical importance are classified as methicillin-susceptible S. pseudintermedius (MSSP) or methicillin-resistant S. pseudintermedius (MRSP), based on the presence of mecA gene. This gene encodes a penicillin-binding protein 2a (PBP2a), which confers resistance to beta-lactam antibiotics, including methicillin [7]. Beta-lactams are a crucial class of medications frequently used to treat bacterial infections in canines. Although multidrug resistance (MDR) is commonly observed in MRSP, some MSSP also can show a MDR profile [5,8]. Therefore, understanding the differences between MSSP and MRSP is essential for both monitoring their role in the spread of antimicrobial resistance patterns and their impact on animal health.
Furthermore, S. pseudintermedius is also a concern for One Health due to its zoonotic potential; thus, it is necessary that more studies study this issue, regarding the characterization of the pathogenicity profile of canine clinical strains and their dissemination potential to humans. Hence, comparative genomic analyses based on next generation sequencing (NGS) emerge as an impressive tool to enhance the comprehension about the epidemiology, antimicrobial susceptibility, pathogenicity, genetic diversity and evolutive dynamics of S. pseudintermedius isolated from clinical infections [9,10,11].
Therefore, this study performed a comprehensive genomic characterization of 28 S. pseudintermedius strains isolated from canine infections, considering MRSP and MSSP strains. In addition, we performed a comparative genomic analysis with S. pseudintermedius from human infections to assess the relation of canine and human isolates and infer the zoonotic potential of the investigated S. pseudintermedius canine strains.
2. Materials and Methods
2.1. Bacterial Strains and DNA Isolation
For this study, 28 S. pseudintermedius strains previously isolated from canine infections between 2017 and 2018 were investigated, including cases of otitis (n = 5), pyodermatitis (n = 14), pyometra (n = 5), cystitis (n = 2) and sepsis (n = 2). The strains were classified as MRSP (n = 15) or MSSP (n = 13) based on the mecA gene presence [5]. The clonal diversity of the strains was previously assessed using multilocus sequence typing (MLST) [5]. The total DNA was isolated directly from colonies that underwent 24 h culture at 37 °C in Tryptic Soy Agar (TSA; Difco, Franklin Lakes, NJ, USA). For cell lysis, the colonies were suspended in 200 μL of lysis-buffer (25 mM Tris–HCl pH 8, 2,5 mM EDTA, 1% TRITON-100X) containing lysozyme (20 mg/mL) and lysostaphin (1 mg/mL), incubated at 37 °C for 18 h. Then, DNA extraction was performed using a Purelink Genomic DNA Kit (Thermo Fisher Scientifics, MA, USA), according to the manufacturer’s instructions. DNA samples were quantified using Qubit® (Life technologies, Grand Island, NY, USA) and DNA quality was assessed using NanoDrop Lite (Thermo Fischer Scientific, Waltham, MA, USA).
2.2. Whole-Genome Sequencing and Analysis of Canine Staphylococcus pseudintermedius Strains
DNA libraries were prepared and submitted for 2 × 250 bp Illumina HiSeq sequencing (HiSeq® Reagent Kit v2, 500 cycles), according to the manufacturers’ instructions (Illumina, San Diego, CA, USA). The raw reads quality was assessed using FastQC 0.11.9 [12], then adapter, short-length (>150 nt) and low-quality (Phred ≥ 30) sequences were trimmed using Trimmomatic 0.39 [13]. Genome assembly was performed using Edena 3 [14], while plasmid sequences identification and segregation was made using two programs: MOB-suite 3.0.3 [15] and PlasmidFinder 2.1 [16]. The IMAGE 2.4.1 [17] was used to close sequence gaps after assembly. Genome quality was assessed using Quast 5.2 [18]; then, the genomes were aligned against the referential S. pseudintermedius SP_113043A (NZ_CP065921) using Mauve 2.4.0 [19]. Afterward, genome annotation was performed using Prokka 1.14.5. [20].
2.3. Resistance and Virulence Genotypic Characterization
The 28 S. pseudintermedius genomes were submitted to an in silico prediction of several genetic elements. Briefly, CRISPRCasFinder [21], PHASTER [22], IslandViewer 4 [23], Isfinder [24], antiSMASH 7.0 [25] and SCCmecFinder 1.2 [26] were used to identify Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR), prophages, genomic islands (GI), insertion sequences (IS), biosynthetic gene clusters (BGC) and elements of the staphylococcal cassette chromosome mec (SCCmec), respectively.
In addition, antimicrobial resistance genes (ARG) were predicted through the compiling of several research databases: ResFinder 4.1 [27], Resistance Gene Identifier (RGI) [28] with the Comprehensive Antibiotic Resistance Database (CARD) [28], ABRIcate 1.0.0 [29] and Patric [30] in conjunction with the National Database of Antibiotic Resistant Organisms (NDARO) [31]. Alternatively, virulence genes were predicted using the Virulence Factor Database (VFDB) [32] and Patric using the Victors Database [33]. For the data, we only considered predictions with ≥80% coverage, ≥80% identity and an E-value <10−5.
2.4. Comparative Genomics and Phylogenomic of Staphylococcus pseudintermedius from Canine and Human
To assess the phylogenetic relation and zoonotic potential of the investigated canine S. pseudintermedius strains, we retrieved other S. pseudintermedius strains from the National Center for Biotechnology Information (NCBI): two from dogs (NZ_CP065921 and CP066702.1) and four from humans (NZ_CP045086.1, NZ_CP031561.1, NZ_CP031605.1, and NZ_CP030715.1) (Table S1). Pangenome analysis was performed using Roary 3.11.2 [34] to assess the core and accessory genome from the analyzed dataset (n = 34); Phandango [35] was used for visualization. Then, a phylogenomic tree of the S. pseudintermedius core genome was built with Maximum Likelihood (ML) using the General Time Reversible (GTR; bootstrap = 1000) in the MEGA11 [36].
2.5. Statistical Analyses
The genome length of the MRSP and MSSP strains was compared via Wilcoxon test (p < 0.05) [37,38]. Data visualization and analysis were performed using R 4.3.1. [39]. For comparing the number of islands between MRSP and MSSP groups, a t-test was conducted using WinPEPI 11.65 [40].
3. Results
3.1. Genome Characterization of Staphylococcus pseudintermedius from Canine Infections
The comparative genome analysis demonstrated that the genome length of the investigated canine S. pseudintermedius strains ranged from 2.4 to 2.8 Mb (Table 1), being longer in MRSP than in MSSP (p < 0.05) (Figure S1). The mapping statistics of genomes sequencing and assembly showed a 92 to 236X coverage, the contigs count ranged from 34 to 182, N50 from 29,816 to 190,599 bp, and G + C content from 37.3 to 37.7%. The number of predicted coding sequences (CDS) ranged from 2342 to 2868.
Table 1.
The mapping statistics of the Staphylococcus pseudintermedius strains isolated from canine infections.
| Identification | Contigs | N50 | Total Size (bp) | Coverage (X) | GC (%) | CDS | tRNAs | rRNAs |
|---|---|---|---|---|---|---|---|---|
| 166/18 | 54 | 154,498 | 2,629,757 | 151 | 37.45 | 2502 | 59 | 11 |
| 205/18 | 34 | 190,599 | 2,488,793 | 194 | 37.64 | 2342 | 59 | 14 |
| 072/17 | 65 | 131,476 | 2,709,226 | 230 | 37.31 | 2626 | 59 | 11 |
| 072/18 | 113 | 49,697 | 2,531,306 | 199 | 37.66 | 2371 | 59 | 11 |
| 215/18 | 49 | 122,689 | 2,535,717 | 236 | 37.48 | 2391 | 59 | 12 |
| 269/18 | 65 | 102,455 | 2,712,159 | 215 | 37.34 | 2626 | 59 | 9 |
| 034/18 | 87 | 60,695 | 2,492,124 | 193 | 37.70 | 2367 | 59 | 9 |
| 009/19 | 58 | 93,282 | 2,595,405 | 180 | 37.57 | 2509 | 59 | 10 |
| 187/18 | 40 | 138,227 | 2,547,925 | 227 | 37.53 | 2452 | 59 | 11 |
| 511OD | 53 | 108,93 | 2,576,436 | 163 | 37.54 | 2472 | 59 | 13 |
| 511OE | 79 | 74,309 | 2,580,431 | 226 | 37.54 | 2471 | 59 | 14 |
| 1044 | 79 | 57,906 | 2,623,021 | 228 | 37.44 | 2510 | 59 | 14 |
| 1259 | 82 | 83,218 | 2,846,852 | 153 | 37.33 | 2830 | 59 | 9 |
| 1387 | 39 | 172,802 | 2,567,762 | 167 | 37.48 | 2424 | 59 | 10 |
| 554 | 182 | 29,816 | 2,850,261 | 150 | 37.34 | 2804 | 59 | 9 |
| 559 | 58 | 107,552 | 2,572,158 | 166 | 37.40 | 2644 | 59 | 8 |
| 561 | 47 | 165,974 | 2,605,024 | 158 | 37.45 | 2495 | 59 | 10 |
| 619 | 155 | 37,758 | 2,848,699 | 118 | 37.33 | 2808 | 60 | 9 |
| 651 | 66 | 109,829 | 2,766,829 | 138 | 37.37 | 2716 | 59 | 9 |
| 695 | 100 | 120,157 | 2,686,739 | 128 | 37.40 | 2588 | 59 | 9 |
| 705 | 56 | 87,508 | 2,526,988 | 151 | 37.50 | 2391 | 59 | 10 |
| 843 | 69 | 89,873 | 2,688,725 | 155 | 37.41 | 2587 | 59 | 9 |
| 1037 | 78 | 75,758 | 2,881,635 | 152 | 37.30 | 2868 | 59 | 9 |
| 1346 | 89 | 102,677 | 2,820,424 | 142 | 37.35 | 2783 | 59 | 9 |
| 1378 | 66 | 107,552 | 2,575,532 | 92 | 37.43 | 2443 | 59 | 7 |
| 1379 | 114 | 65,855 | 2,851,013 | 140 | 37.33 | 2812 | 59 | 9 |
| 1382 | 65 | 133,050 | 2,818,793 | 147 | 37.35 | 2793 | 59 | 10 |
| 6848 | 81 | 88,449 | 2,844,513 | 148 | 37.33 | 2820 | 59 | 9 |
Considering the prediction of genetic elements in the S. pseudintermedius strains, we observed a high number of GI, ranging from 3 to 19 (Table 2), with an average of 10.2 GI in MSSP and 16.2 GI in MRSP strains, with the number being higher in MRSP (p < 0.05). Moreover, the discovered number of prophages per genome in the analyzed strains was low, with similar counts between MRSP and MSSP (Table 2).
Table 2.
In silico prediction of genetic elements in Staphylococcus pseudintermedius from canine infection.
| Identification | MLST | Group | SCCmec | CRISPR | IS | GI | BGC | Prophage |
|---|---|---|---|---|---|---|---|---|
| 166/18 | ST2375 | MSSP | - | Cas cluster, CRISPR | 54 | 12 | 9 | 1 |
| 205/18 | ST2377 | MSSP | - | Cas cluster, CRISPR | 18 | 6 | 7 | 0 |
| 072/17 | ST2373 | MSSP | - | CRISPR | 22 | 14 | 8 | 0 |
| 072/18 | ST2373 | MSSP | - | - | 38 | 7 | 5 | 1 |
| 215/18 | ST2381 | MSSP | - | - | 28 | 12 | 6 | 0 |
| 269/18 | ST2378 | MSSP | - | CRISPR | 48 | 14 | 10 | 3 |
| 034/18 | ST2371 | MSSP | - | Cas cluster, CRISPR | 16 | 3 | 8 | 0 |
| 009/19 | ST2374 | MSSP | - | - | 47 | 13 | 8 | 1 |
| 187/18 | ST2376 | MSSP | - | Cas cluster, CRISPR | 17 | 9 | 9 | 0 |
| 511OD | ST2382 | MSSP | - | CRISPR | 28 | 10 | 8 | 2 |
| 511OE | ST2382 | MSSP | - | CRISPR | 28 | 10 | 8 | 1 |
| 1044 | ST919 | MRSP | V | Cas cluster, CRISPR | 34 | 11 | 8 | 1 |
| 1259 | ST71 | MRSP | III | CRISPR | 36 | 18 | 9 | 3 |
| 1387 | ST2380 | MSSP | - | CRISPR | 15 | 9 | 9 | 0 |
| 554 | ST71 | MRSP | III | Cas cluster, CRISPR | 36 | 17 | 9 | 2 |
| 559 | ST71 | MRSP | III | - | 35 | 18 | 9 | 3 |
| 561 | ST649 | MRSP | - | CRISPR | 43 | 12 | 7 | 2 |
| 619 | ST71 | MRSP | III | CRISPR | 36 | 18 | 11 | 2 |
| 651 | ST71 | MRSP | III | - | 36 | 17 | 9 | 3 |
| 695 | ST71 | MRSP | III | - | 36 | 17 | 9 | 1 |
| 705 | ST2379 | MRSP | - | CRISPR | 36 | 13 | 8 | 0 |
| 843 | ST71 | MRSP | III | CRISPR | 36 | 19 | 9 | 1 |
| 1037 | ST71 | MRSP | III | CRISPR | 36 | 16 | 9 | 3 |
| 1346 | ST72 | MRSP | III | CRISPR | 36 | 17 | 9 | 1 |
| 1378 | ST2383 | MSSP | - | - | 36 | 14 | 7 | 1 |
| 1379 | ST73 | MRSP | III | CRISPR | 33 | 14 | 8 | 2 |
| 1382 | ST71 | MRSP | III | CRISPR | 36 | 18 | 9 | 2 |
| 6848 | ST71 | MRSP | III | CRISPR | 36 | 18 | 9 | 3 |
IS: Insertion Sequences; GI: Genomic Islands; BGC: Biosynthetic Gene Clusters; “-” indicates absence.
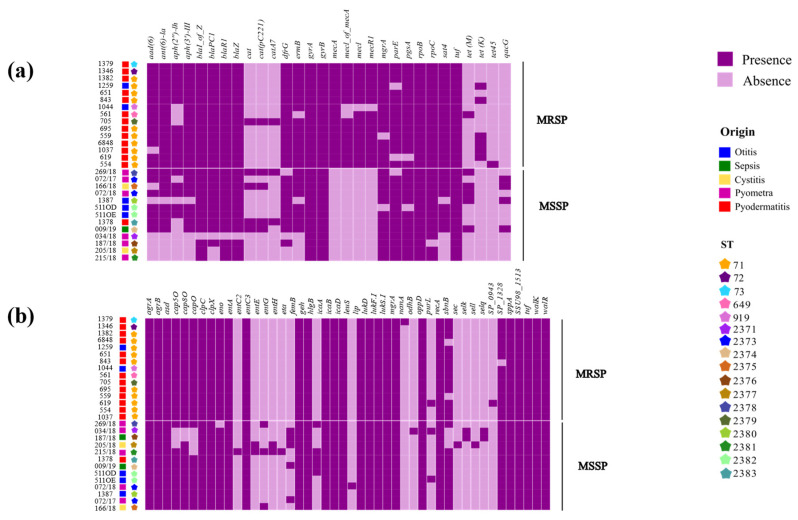
The SCCmec typing showed that the SCCmec type III was the most common among the investigated strains, whereas only the S. pseudintermedius 1044, a MRSP strain, belonged to type V (Table 2). In total, 18% (5/28) of the S. pseudintermedius strains showed a complete CRISPR/Cas system, with four being MSSP and one being MRSP (Table 2). The prediction of BGC ranged from 5 to 11 among the investigated genomes (Table 2), being identified clusters related to non-ribosomal peptide synthetases (NRPS), ribosomally synthesized and post-translationally modified peptides (RiPPs), linear azol(in)e-containing peptides (LAPs) and an opine-like metallophore. In addition, we observed a highly variable number of IS: 52 were predicted in MRSP strains, with 5 being exclusives from this group (IS1272, ISLac1, ISSau3, ISSau5, and ISSep1); 81 IS were predicted for the MSSP strains, with 34 being exclusives for this MSSP group, such as ISSag12, ISS1X, ISLmo14, and IS1469 (Table S2).
The plasmid search using PlasmidFinder and MOB-suite identified plasmids in 6 MSSP strains and 13 MRSP strains, all of which have been previously described in other Staphylococcus spp. (Table S3). In detail, in both MSSP and MRSP groups, we identified the plasmid p222, which harbors the bcrA gene and whose product confers resistance to bacitracin (Table S3).
3.2. Pathogenicity Profile of the Staphylococcus pseudintermedius Strains from Canine Infections
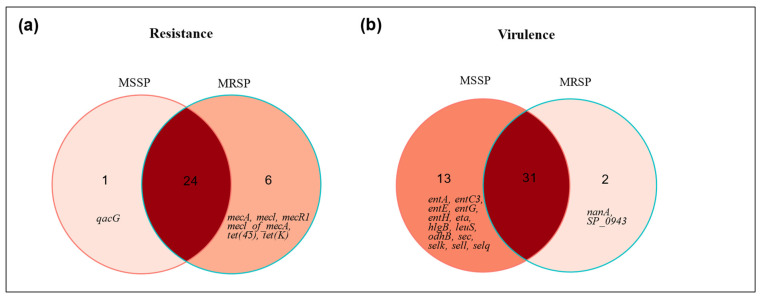
The in silico prediction of ARG suggested that all S. pseudintermedius strains were multidrug resistant, as 100% of the genomes harbored genes from at least three antimicrobial drug classes (Table S4). We observed that MRSP have more genes involved in antimicrobial resistance than MSSP (Figure 1a; Table S4). A total of 24 ARGs were shared between MRSP and MSSP, and both groups have exclusive ARGs. In detail, only one exclusive ARG was predicted in MSSP (qacG), whereas MRSP harbored six exclusive ARGs (mecA, mecl, mecR1, mecI of mecA, tet(45), and tet(K)). Accordingly, the general ARGs distribution indicates differences between the MSSP and MRSP groups (Figure 2a), with a more consistent and homogeneous ARG profile in the MRSP strains, whereas the MSSP group exhibits greater ARG diversity.
Figure 1.
Venn diagram of antimicrobial resistance genes and virulence markers in MRSP and MSSP strains isolated from canine. (a) Comparison of antimicrobial resistance genes between MSSP and MRSP; (b) comparison of virulence genes between MSSP and MRSP.
Figure 2.
Heatmap representation indicating the presence (in purple) and absence (in lilac) of virulence genes and antimicrobial resistance marker genes across all 28 genomes of Staphylococcus pseudintermedius. (a) The distribution of antimicrobial resistance marker genes; (b) the distribution of virulence genes.
On the other hand, comparing the number of virulence genes in the investigated S. pseudintermedius strains, we determined a higher number of virulence genes in MSSP than in MRSP (Figure 1b; Table S4). A total of 31 virulence genes were shared between the analyzed groups; 13 genes were found only in MSSP strains (entA, entC3, entE, entG, entH, sec, sell, selq, selk, eta, hlgB, leuS, and odhB), and only 2 exclusive predicted virulence factors were found in the MRSP strains (SP_0943 and nanA). In addition, distinct virulence profiles were observed when comparing MSSP and MRSP groups; despite the fact that most virulence genes are shared between MRSP and MSSP strains, MSSP strains showed a more diverse profile (Figure 2b).
3.3. Comparative Genomics and Phylogenomic of Staphylococcus pseudintermedius from Canine and Human Hosts
The genomes of S. pseudintermedius from multiples sources and hosts were analyzed, including the 28 from canine infections from this study, and 6 were retrieved from NCBI, representing isolates from human and canine origins (Table S1). In detail, canine MRSP strains were associated with otitis and pyoderma cases, whereas MSSP strains were isolated from cases of pyometra, sepsis, cystitis and otitis; among the S. pseudintermedius strains from humans, there were four MRSP strains previously isolated from skin samples and mixed cultures. The MLST analyses showed that sequence types (STs) varied considerably among the analyzed strains, regardless of their origin. ST71 was the most frequent type, circulating predominantly among the canine hosts in the analyzed genomes (Table S1).
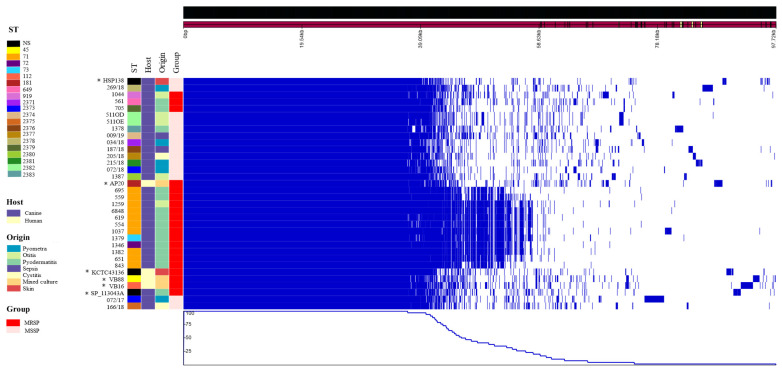
We identified a total of 4884 genes in the analyzed S. pseudintermedius genomes, with 1847 genes as part of the core genome and 3037 belonging to the accessory genome. The overall genomic analysis highlighted the conserved and non-conserved regions among the investigated genomes (Figure 3). We observed that the ST71 of S. pseudintermedius, associated with pyoderma, exhibited a more conserved region compared to other strains, grouping together cohesively. Overall, there was generally a closer alignment among isolates according to the MSSP or MRSP groups, except for isolates 072/17, 166/18, 1044, 561 and 705 (Figure 3).
Figure 3.
Genomic analysis of 34 isolates of Staphylococcus pseudintermedius related to presence and absence of genes by homology. ST: Sequencing Type based on MLST. NS: Not specified. “*” = Genomes retrieved from NCBI database.
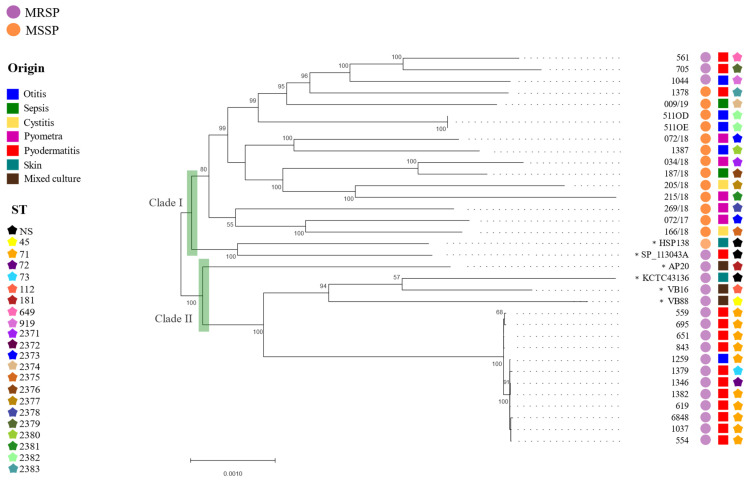
The phylogenomic analysis of S. pseudintermedius based on 1847 genes from the core genome grouped the analyzed genomes in two separate clades: (i) clade I clustered 18 S. pseudintermedius, mostly belonging to MSSP group except for four strains (561, 705, 1044 and SP_113043A), and (ii) clade II that exclusively comprised MRSP strains (Figure 4). Moreover, no phylogenetic clusterization was observed related to the isolation source. In detail, in clade I, the four MRSP strains were isolated from pyoderma cases and show diverse sequence types (ST649, ST919 and ST2379). In clade II, most of the S. pseudintermedius strains from pyoderma were identified as ST71, except the canine strains 1346 and 1379, which were classified as ST72 and ST73, respectively. Among the human strains in clade II, S. pseudintermedius AP20 (ST181) stands out by clearly separating from the others (Figure 4). Figure 4 shows that the selected human S. pseudintermedius were classified into different STs.
Figure 4.
Phylogenomic analysis of 34 Staphylococcus pseudintermedius strains based on core genome. The phylogenetic tree was built using Maximum Likelihood method (bootstrap = 1000; General Time Reversible model) in MEGA11. ST: Sequencing Type based on MLST. “*” = Genomes obtained from NCBI database.
4. Discussion
The high occurrence of S. pseudintermedius infections with the MDR profile in dogs, associated with the zoonotic potential of some clinical strains, encourages studies regarding the genotypic characterization of MSSP and MRSP strains with epidemiological importance. Hence, in this study, we performed a comprehensive genomic characterization of S. pseudintermedius strains isolated from canine infections that provided insights into genetic variations and the potential zoonotic transmission of the pathogen, based on the pangenomic comparison of strains from human and canine origins.
The phenotypical characterization and genomic virulence potential of the canine S. pseudintermedius strains used in this study were described in a previous study [5]. Among the analyzed canine isolates, S. pseudintermedius ST71 was the most predominant, which is often associated with pyoderma and has a wide geographic distribution [41], being the most prevalent clone in Europe [42] and commonly found in Brazil [3,5,43]. Nevertheless, recent evidence suggests a decline in ST71 prevalence, accompanied by the emergence of ST258 [44]. In addition, our findings also identified S. pseudintermedius ST68, ST69 and ST90, reflecting a diversity of sequence types currently in circulation in dogs in Brazil.
Genome analysis determined that the genome size of the 28 S. pseudintermedius strains analyzed in this study are within the expected amount for this species [10,45,46]. Moreover, the MRSP genomes were significantly larger than the MSSP ones, which is in accordance with previous data [10]. From the search for genetic features, we highlight the prediction of IS1272 exclusively in the MRSP group in accordance with previous data that associated this IS to MRSP strains, usually located downstream of the mecR gene [47], suggesting that IS1272 may serve as a distinctive marker for the MRSP group. In MSSP, ISSag12 was exclusively found in these strains, being associated with the truncation of sat4 that encodes streptomycin adenyltransferase related to streptomycin resistance [48].
In terms of pathogenicity, we predicted ARGs and virulence genes and compared pathogenicity patterns between MSSP and MRSP, which enable us to identify exclusive genetic elements involved in the pathogenesis of such strains. In MSSP strains, qacG was the only exclusive ARG, which confers resistance to disinfectants, whereas in MRSP, exclusive ARG included genes involved in methicillin resistance (mecA, mecl and mecI of mecA), as expected, and genes related to tetracycline resistance (tet(45) and tet(K)). In agreement with our findings, a previous study assessing the phenotypic antimicrobial susceptibility of MRSP strains isolated from dogs with otitis has also identified a high occurrence of MRSP strains resistant to tetracycline (61.4%) [49]. Even though MRSP strains are commonly resistant genes for several non-beta-lactam drugs, including tetracycline, this antimicrobial drug is still a choice for treating S. pseudintermedius infections in small animals [50]. Altogether, the presence of tet(45) and tet(K) in MRSP genomes might suggest that these isolates were subjected to more intense selective pressure, potentially due to the prolonged or excessive use of such antimicrobials.
Additionally, the in silico prediction of ARGs determined that all analyzed S. pseudintermedius strains harbored antimicrobial resistance genes associated with at least three different classes of antibiotics, suggesting a potential MDR profile; however, in the previous phenotypic analysis, not all strains exhibited an MDR in vitro profile, with 25 strains being classified as MDR in the phenotypic analysis [5].
Overall, we observed genotypic homogeneity among MRSP isolates when considering ARGs distribution, which suggests that MRSP isolates share a common set of resistance genes, potentially associated with specific mechanisms of antimicrobial resistance. In contrast, the MSSP group exhibits greater genotypic diversity, evidenced by the variable presence of antimicrobial resistance genes among the isolates, reflecting a more diverse adaptation to the environments in which they were found.
Considering exclusive virulence genes, MSSP harbored genes associated with enterotoxins production (entA, entC3, entE, entG, entH, sell, selk, selq), exfoliative toxins (sec, eta), citotoxins (leuS), a gene related to metabolism (odhB), and a gene in immune system evasion (hlgB), whereas MRSP’s exclusive virulence genes encode N-acetylneuraminate lyase (SP_0943) and methyltransferase (nanA), which are involved in immune system evasion. In comparison, the main difference between MSSP and MRSP virulence profile lies in toxin genes. MSSP produces a wide variety of toxins, including enterotoxins and exfoliative toxins that are associated with tissue damage and infection dissemination [51]. In contrast, MRSP employs genes for immune evasion, such as N-acetylneuraminate lyase and methyltransferase, which facilitate its persistence in the host [51]. These differences in the range of exclusive virulence genes between MSSP and MRSP may reflect distinct strategies for pathogenicity and immune system evasion, highlighting the adaptive diversity of the two isolate groups.
The pangenome analysis of S. pseudintermedius revealed significant differences between isolates of human and canine origin, highlighting the complexity of zoonotic interactions. The core genome phylogenomic analysis revealed that human isolates clustered together with canine MRSP strains, which is particularly concerning from a One Health perspective, as it suggests a close phylogenetic relationship and a similar genome content among these strains that might facilitate interspecies transmission [52,53]. Moreover, to assess the zoonotic potential, the epidemiological distribution of S. pseudintermedius must be taken into account. As already mentioned, S. pseudintermedius ST71, which has a worldwide distribution and great epidemiological importance [43,54], was reported in both humans and dogs hosts analyzed in this study. Altogether, these findings highlight the ability of S. pseudintermedius clones to adapt to different hosts; therefore, there is a need for ongoing monitoring and control measures to address the zoonotic transmission and spread of resistant bacterial strains.
5. Conclusions
Overall, the genomic characterization of S. pseudintermedius strains isolated from infections in dogs revealed significant differences between MRSP and MSSP strains in terms of genetic characteristics, including the identification of potential antimicrobial resistance and virulence markers that improve the understanding of the pathogenicity of S. pseudintermedius. In addition, the comparison with strains from human infections provides insights into genetic variations and the possible zoonotic transmission of the pathogen. Our results emphasize the need for further studies on the zoonotic potential of S. pseudintermedius from canine infections and its ability to spread to humans in order to understand the evolutionary dynamics of this pathogen and to prevent the spread of resistant strains involved in canine infections.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/pathogens13090760/s1. Figure S1: Box-plot distribution of genome length from MSSP and MRSP strains isolated from canine infections. Statistical differences were calculated using Wilcoxon test, with significance indicated by * (p < 0.05); Table S1: Complete genomes of Staphylococcus pseudintermedius strains from canine and human hosts for comparative genomic and phylogenomic analyses; Table S2: Diversity of insertion sequences found in the 28 genomes of Staphylococcus pseudintermedius from this study, according to the ISFinder program; Table S3: Diversity of plasmids identified in the 28 genomes of Staphylococcus pseudintermedius, according to the PlasmidFinder program; Table S4: Comprehensive characterization of resistance and virulence gene profiles in 28 Staphylococcus pseudintermedius genomes.
Author Contributions
Conceptualization, M.E.R.J.d.S., G.M.B., M.R.d.I.C. and F.M.S.; formal analysis, M.E.R.J.d.S.; investigation, M.E.R.J.d.S., G.M.B. and F.M.S.; writing—original draft preparation, M.E.R.J.d.S.; writing—review and editing, G.M.B. and F.M.S.; resources, F.M.S., M.M.d.C., B.B. and V.A.d.C.A.; funding acquisition, F.M.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data underlying this article are available in the National Center for Biotechnology Information under BioProject PRJNA918652 and can be accessed with Accession Numbers JAQIAZ000000000, JAQIAY000000000, JAQIAX000000000, JAQIAW000000000, JAQIAV000000000, JAQIAU000000000, JAQIAT000000000, JAQIAS000000000, JAQIAR000000000, JAQIAQ000000000, JAQIAP000000000, JAQIAO000000000, JAQIAN000000000, JAQIAM000000000, JAQIAL000000000, JAQIAK000000000, JAQIAJ000000000, JAQIAI000000000, JAQIAH000000000, JAQIAG000000000, JAQIAF000000000, JAQIAE000000000, JAQIAD000000000, JAQIAC000000000, JAQIAB000000000, JAQIAA000000000, JAQHZZ000000000 and JAQHZY000000000.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)—Finance Code 001 and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/MCTI/CT-Saúde)—Grant Number 52/2022, Process: 408693/2022-3.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Bannoehr J., Guardabassi L. Staphylococcus pseudintermedius in the Dog: Taxonomy, Diagnostics, Ecology, Epidemiology and Pathogenicity. Vet. Dermatol. 2012;23:253-e52. doi: 10.1111/j.1365-3164.2012.01046.x. [DOI] [PubMed] [Google Scholar]
- 2.Dos Santos T.P., Damborg P., Moodley A., Guardabassi L. Systematic Review on Global Epidemiology of Methicillin-Resistant Staphylococcus pseudintermedius: Inference of Population Structure from Multilocus Sequence Typing Data. Front. Microbiol. 2016;7:1599. doi: 10.3389/fmicb.2016.01599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Penna B., Silva M.B., Botelho A.M.N., Ferreira F.A., Ramundo M.S., Silva-Carvalho M.C., Rabello R.F., Vieira-da-Motta O., Figueiredo A.M.S. Detection of the International Lineage ST71 of Methicillin-Resistant Staphylococcus pseudintermedius in Two Cities in Rio de Janeiro State. Braz. J. Microbiol. 2022;53:2335–2341. doi: 10.1007/s42770-022-00852-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopes C.E., De Carli S., Riboldi C.I., De Lorenzo C., Panziera W., Driemeier D., Siqueira F.M. Pet Pyometra: Correlating Bacteria Pathogenicity to Endometrial Histological Changes. Pathogens. 2021;10:833. doi: 10.3390/pathogens10070833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breyer G.M., Saggin B.F., de Carli S., da Silva M.E.R.J., da Costa M.M., Brenig B., Azevedo V.A.d.C., Cardoso M.R.d.I., Siqueira F.M. Virulent Potential of Methicillin-Resistant and Methicillin-Susceptible Staphylococcus pseudintermedius in Dogs. Acta Trop. 2023;242:106911. doi: 10.1016/j.actatropica.2023.106911. [DOI] [PubMed] [Google Scholar]
- 6.McCarthy A.J., Harrison E.M., Stanczak-Mrozek K., Leggett B., Waller A., Holmes M.A., Lloyd D.H., Lindsay J.A., Loeffler A. Genomic Insights into the Rapid Emergence and Evolution of MDR in Staphylococcus pseudintermedius. J. Antimicrob. Chemother. 2014;70:997–1007. doi: 10.1093/jac/dku496. [DOI] [PubMed] [Google Scholar]
- 7.Peacock S.J., Paterson G.K. Mechanisms of Methicillin Resistance in Staphylococcus aureus. Annu. Rev. Biochem. 2015;84:577–601. doi: 10.1146/annurev-biochem-060614-034516. [DOI] [PubMed] [Google Scholar]
- 8.Couto N., Belas A., Couto I., Perreten V., Pomba C. Genetic Relatedness, Antimicrobial and Biocide Susceptibility Comparative Analysis of Methicillin-Resistant and -Susceptible Staphylococcus pseudintermedius from Portugal. Microb. Drug Resist. 2014;20:364–371. doi: 10.1089/mdr.2013.0043. [DOI] [PubMed] [Google Scholar]
- 9.Fàbregas N., Pérez D., Viñes J., Cuscó A., Migura-García L., Ferrer L., Francino O. Diverse Populations of Staphylococcus pseudintermedius Colonize the Skin of Healthy Dogs. Microbiol. Spectr. 2023;11:e03393-22. doi: 10.1128/spectrum.03393-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrer L., García-Fonticoba R., Pérez D., Viñes J., Fàbregas N., Madroñero S., Meroni G., Martino P.A., Martínez S., Maté M.L., et al. Whole Genome Sequencing and de Novo Assembly of Staphylococcus pseudintermedius: A Pangenome Approach to Unravelling Pathogenesis of Canine Pyoderma. Vet. Dermatol. 2021;32:654–663. doi: 10.1111/vde.13040. [DOI] [PubMed] [Google Scholar]
- 11.Zakour N.L.B., Bannoehr J., van den Broek A.H.M., Thoday K.L., Fitzgerald J.R. Complete Genome Sequence of the Canine Pathogen Staphylococcus pseudintermedius. J. Bacteriol. 2011;193:2363–2364. doi: 10.1128/JB.00137-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andrews FastQC: A Quality Control Tool for High Throughput Sequence Data. [(accessed on 30 July 2023)]. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc.
- 13.Bolger A.M., Lohse M., Usadel B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hernandez D., François P., Farinelli L., Østerås M., Schrenzel J. De Novo Bacterial Genome Sequencing: Millions of Very Short Reads Assembled on a Desktop Computer. Genome Res. 2008;18:802–809. doi: 10.1101/gr.072033.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robertson J., Nash J.H.E. MOB-Suite: Software Tools for Clustering, Reconstruction and Typing of Plasmids from Draft Assemblies. Microb. Genom. 2018;4:e000206. doi: 10.1099/mgen.0.000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carattoli A., Zankari E., Garciá-Fernández A., Larsen M.V., Lund O., Villa L., Aarestrup F.M., Hasman H. In Silico Detection and Typing of Plasmids Using Plasmidfinder and Plasmid Multilocus Sequence Typing. Antimicrob. Agents Chemother. 2014;58:3895–3903. doi: 10.1128/AAC.02412-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsai I.J., Otto T.D., Berriman M. Open Access METHOD IMAGE Gap Closer IMAGE Generates Local Assemblies, Closing Gaps in Genomes Assembled from Paired-End next Generation Sequencing Data, Often with-out the Need for New Data. Genome Biol. 2010;11:41 [Google Scholar]
- 18.Gurevich A., Saveliev V., Vyahhi N., Tesler G. QUAST: Quality Assessment Tool for Genome Assemblies. Bioinformatics. 2013;29:1072–1075. doi: 10.1093/bioinformatics/btt086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darling A.C.E., Mau B., Blattner F.R., Perna N.T. Implicitfunction.Pdf. Genome Res. 2004;14:1394–1403. doi: 10.1101/gr.2289704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seemann T. Prokka: Rapid Prokaryotic Genome Annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 21.Couvin D., Bernheim A., Toffano-Nioche C., Touchon M., Michalik J., Néron B., Rocha E.P.C., Vergnaud G., Gautheret D., Pourcel C. CRISPRCasFinder, an Update of CRISRFinder, Includes a Portable Version, Enhanced Performance and Integrates Search for Cas Proteins. Nucleic Acids Res. 2018;46:W246–W251. doi: 10.1093/nar/gky425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arndt D., Grant J.R., Marcu A., Sajed T., Pon A., Liang Y., Wishart D.S. PHASTER: A Better, Faster Version of the PHAST Phage Search Tool. Nucleic Acids Res. 2016;44:W16–W21. doi: 10.1093/nar/gkw387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bertelli C., Laird M.R., Williams K.P., Lau B.Y., Hoad G., Winsor G.L., Brinkman F.S.L. IslandViewer 4: Expanded Prediction of Genomic Islands for Larger-Scale Datasets. Nucleic Acids Res. 2017;45:W30–W35. doi: 10.1093/nar/gkx343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siguier P., Perochon J., Lestrade L., Mahillon J., Chandler M. ISfinder: The Reference Centre for Bacterial Insertion Sequences. Nucleic Acids Res. 2006;34:D32–D36. doi: 10.1093/nar/gkj014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blin K., Shaw S., Augustijn H.E., Reitz Z.L., Biermann F., Alanjary M., Fetter A., Terlouw B.R., Metcalf W.W., Helfrich E.J.N., et al. AntiSMASH 7.0: New and Improved Predictions for Detection, Regulation, Chemical Structures and Visualisation. Nucleic Acids Res. 2023;51:W46–W50. doi: 10.1093/nar/gkad344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaya H., Hasman H., Larsen J., Stegger M., Johannesen B., Allesøe L. SCC mec Finder, a Web-Based Tool for Typing of Staphylococcal Cassette Chromosome mec in Staphylococcus aureus Using Whole-Genome Sequence Data. mSphere. 2018;3:e00612-17. doi: 10.1128/mSphere.00612-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Florensa A.F., Kaas R.S., Clausen P.T.L.C., Aytan-Aktug D., Aarestrup F.M. ResFinder—An Open Online Resource for Identification of Antimicrobial Resistance Genes in next-Generation Sequencing Data and Prediction of Phenotypes from Genotypes. Microb. Genom. 2022;8:000748. doi: 10.1099/mgen.0.000748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alcock B.P., Raphenya A.R., Lau T.T.Y., Tsang K.K., Bouchard M., Edalatmand A., Huynh W., Nguyen A.L.V., Cheng A.A., Liu S., et al. CARD 2020: Antibiotic Resistome Surveillance with the Comprehensive Antibiotic Resistance Database. Nucleic Acids Res. 2020;48:D517–D525. doi: 10.1093/nar/gkz935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seemann T. ABRIcate: Mass Screening of Contigs for Antimicrobial Resistance or Virulence Genes. [(accessed on 30 July 2023)]. Available online: https://github.com/tseemann/abricate.
- 30.Wattam A.R., Abraham D., Dalay O., Disz T.L., Driscoll T., Gabbard J.L., Gillespie J.J., Gough R., Hix D., Kenyon R., et al. PATRIC, the Bacterial Bioinformatics Database and Analysis Resource. Nucleic Acids Res. 2014;42:581–591. doi: 10.1093/nar/gkt1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Center for Biotechnology Information (NCBI) National Database of Antibiotic Resistant Organisms (NDARO)—Pathogen Detection—NCBI. [(accessed on 30 July 2023)]; Available online: https://www.ncbi.nlm.nih.gov/pathogens/antimicrobial-resistance/
- 32.Chen L., Yang J., Yu J., Yao Z., Sun L., Shen Y., Jin Q. VFDB: A Reference Database for Bacterial Virulence Factors. Nucleic Acids Res. 2005;33:325–328. doi: 10.1093/nar/gki008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sayers S., Li L., Ong E., Deng S., Fu G., Lin Y., Yang B., Zhang S., Fa Z., Zhao B., et al. Victors: A Web-Based Knowledge Base of Virulence Factors in Human and Animal Pathogens. Nucleic Acids Res. 2019;47:D693–D700. doi: 10.1093/nar/gky999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Page A.J., Cummins C.A., Hunt M., Wong V.K., Reuter S., Holden M.T.G., Fookes M., Falush D., Keane J.A., Parkhill J. Roary: Rapid Large-Scale Prokaryote Pan Genome Analysis. Bioinformatics. 2015;31:3691–3693. doi: 10.1093/bioinformatics/btv421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hadfield J., Croucher N.J., Goater R.J., Abudahab K., Aanensen D.M., Harris S.R. Phandango: An Interactive Viewer for Bacterial Population Genomics. Bioinformatics. 2018;34:292–293. doi: 10.1093/bioinformatics/btx610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tamura K., Stecher G., Kumar S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021;38:3022–3027. doi: 10.1093/molbev/msab120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilcoxon F. Individual Comparison By Ranking Methods. Author(s): Frank Wilcoxon Published by: International Biometric Society Stable. Biom. Bull. 1945;1:80–83. doi: 10.2307/3001968. [DOI] [Google Scholar]
- 38.Shapiro S.S., Wilk M.B. An Analysis of Variance Test for Normality (Complete Samples) Biometrika. 1965;52:591. doi: 10.1093/biomet/52.3-4.591. [DOI] [Google Scholar]
- 39.Team R.C. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna. [(accessed on 30 July 2023)]. Available online: https://www.r-project.org/
- 40.Abramson J.H. Age-Standardization in Epidemiological Data. Int. J. Epidemiol. 1995;24:238–239. doi: 10.1093/ije/24.1.238. [DOI] [PubMed] [Google Scholar]
- 41.Papić B., Golob M., Zdovc I., Kušar D., Avberšek J. Genomic Insights into the Emergence and Spread of Methicillin-Resistant Staphylococcus pseudintermedius in Veterinary Clinics. Vet. Microbiol. 2021;258:109119. doi: 10.1016/j.vetmic.2021.109119. [DOI] [PubMed] [Google Scholar]
- 42.Haenni M., De Moraes N.A., Châtre P., Médaille C., Moodley A., Madec J.Y. Characterisation of Clinical Canine Meticillin-Resistant and Meticillin-Susceptible Staphylococcus pseudintermedius in France. J. Glob. Antimicrob. Resist. 2014;2:119–123. doi: 10.1016/j.jgar.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 43.Teixeira I.M., de Moraes Assumpção Y., Paletta A.C.C., Aguiar L., Guimarães L., da Silva I.T., Côrtes M.F., Botelho A.M.N., Jaeger L.H., Ferreira R.F., et al. Investigation of Antimicrobial Susceptibility and Genetic Diversity among Staphylococcus pseudintermedius Isolated from Dogs in Rio de Janeiro. Sci. Rep. 2023;13:20219. doi: 10.1038/s41598-023-47549-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bergot M., Martins-Simoes P., Kilian H., Châtre P., Worthing K.A., Norris J.M., Madec J.Y., Laurent F., Haenni M. Evolution of the Population Structure of Staphylococcus pseudintermedius in France. Front. Microbiol. 2018;9:3055. doi: 10.3389/fmicb.2018.03055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Byukusenge M., Banovic F., Li L., Kuchipudi S.V., Jayarao B.M., Watson C.K., Naikare H.K. Complete Genome Sequences of Six Staphylococcus pseudintermedius Strains from Dogs with Superficial Pyoderma in Georgia, USA. Microbiol. Resour. Announc. 2021;10:4–6. doi: 10.1128/MRA.00378-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roozitalab A., Elsakhawy O., Phophi L., Kania S.A., Abouelkhair M.A. Complete Genome Sequences of 11 Staphylococcus pseudintermedius Isolates from Dogs in the United States. Microbiol. Resour. Announc. 2023;12:22–24. doi: 10.1128/mra.00002-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kobayashi N., Urasawa S., Uehara N., Watanabe N. Distribution of Insertion Sequence-like Element IS1272 and Its Position Relative to Methicillin Resistance Genes in Clinically Important Staphylococci. Antimicrob. Agents Chemother. 1999;43:2780–2782. doi: 10.1128/AAC.43.11.2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou K., Xie L., Han L., Guo X., Wang Y., Sun J. ICESag37, a Novel Integrative and Conjugative Element Carrying Antimicrobial Resistance Genes and Potential Virulence Factors in Streptococcus agalactiae. Front. Microbiol. 2017;8:1921. doi: 10.3389/fmicb.2017.01921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scherer C.B., Botoni L.S., Coura F.M., Silva R.O., Santos R.D., Heinemann M.B., Costa-Val A.P. Frequency and Antimicrobial Susceptibility of Staphylococcus pseudintermedius in Dogs with Otitis Externa. Ciência Rural. 2018;48:e20170738. doi: 10.1590/0103-8478cr20170738. [DOI] [Google Scholar]
- 50.Papich M.G. Selection of Antibiotics for Meticillin-resistant Staphylococcus pseudintermedius: Time to Revisit Some Old Drugs? Vet. Dermatol. 2012;23:352. doi: 10.1111/j.1365-3164.2011.01030.x. [DOI] [PubMed] [Google Scholar]
- 51.Cheung G.Y.C., Otto M. Virulence Mechanisms of Staphylococcal Animal Pathogens. Int. J. Mol. Sci. 2023;24:14587. doi: 10.3390/ijms241914587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guimarães L., Teixeira I.M., da Silva I.T., Antunes M., Pesset C., Fonseca C., Santos A.L., Côrtes M.F., Penna B. Epidemiologic Case Investigation on the Zoonotic Transmission of Methicillin-Resistant Staphylococcus pseudintermedius among Dogs and Their Owners. J. Infect. Public Health. 2023;16:183–189. doi: 10.1016/j.jiph.2023.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lozano C., Rezusta A., Ferrer I., Pérez-Laguna V., Zarazaga M., Ruiz-Ripa L., Revillo M.J., Torres C. Staphylococcus pseudintermedius Human Infection Cases in Spain: Dog-to-Human Transmission. Vector-Borne Zoonotic Dis. 2017;17:268–270. doi: 10.1089/vbz.2016.2048. [DOI] [PubMed] [Google Scholar]
- 54.Abdullahi I.N., Zarazaga M., Campaña-Burguet A., Eguizábal P., Lozano C., Torres C. Nasal Staphylococcus aureus and S. pseudintermedius Carriage in Healthy Dogs and Cats: A Systematic Review of Their Antibiotic Resistance, Virulence and Genetic Lineages of Zoonotic Relevance. J. Appl. Microbiol. 2022;133:3368–3390. doi: 10.1111/jam.15803. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the National Center for Biotechnology Information under BioProject PRJNA918652 and can be accessed with Accession Numbers JAQIAZ000000000, JAQIAY000000000, JAQIAX000000000, JAQIAW000000000, JAQIAV000000000, JAQIAU000000000, JAQIAT000000000, JAQIAS000000000, JAQIAR000000000, JAQIAQ000000000, JAQIAP000000000, JAQIAO000000000, JAQIAN000000000, JAQIAM000000000, JAQIAL000000000, JAQIAK000000000, JAQIAJ000000000, JAQIAI000000000, JAQIAH000000000, JAQIAG000000000, JAQIAF000000000, JAQIAE000000000, JAQIAD000000000, JAQIAC000000000, JAQIAB000000000, JAQIAA000000000, JAQHZZ000000000 and JAQHZY000000000.