Abstract
Respiratory Syncytial Virus (RSV) is responsible for a considerable burden of respiratory disease among children and older adults. Several prophylactic strategies have recently been introduced. We review the available evidence on the interplay between RSV infection and HIV, looking at the specific role of RSV prophylactic strategies in individuals affected by or exposed to HIV. We conducted a systematic review on the association between HIV infection and RSV incidence and severity. We searched in PubMed/MEDLINE for clinical epidemiological studies covering outcomes such as RSV-associated illness, severity, and mortality in individuals affected by or exposed to HIV. A total of 36 studies met the inclusion criteria and were included, the majority conducted in sub-Saharan Africa. There was no compelling evidence suggesting a higher incidence of RSV illness among HIV-infected people. A higher risk of severe disease was consistent among both HIV-positive and HIV-exposed but uninfected (HEU) children. Case fatality rates were also higher for these groups. Evidence on a differing risk among adults was scarce. HIV-positive pregnant women should be given priority for recently approved RSV vaccination, for protection of their newborns. HIV-infected and HEU infants should be considered risk groups for nirsevimab prophylaxis in their first year of life and possibly beyond.
Keywords: respiratory syncytial virus, human immunodeficiency virus, vaccination
1. Introduction
Respiratory Syncytial Virus (RSV) is known to be the main etiological agent of lower respiratory tract infections (LRTIs) in infants and the main cause for hospitalization due to respiratory disease in this age group, mainly for bronchiolitis and pneumonia [1,2]. Beyond its pathogenic implications in pediatric ages, this virus is also increasingly recognized as implicated in acute disease among older adults, presenting a considerable burden of hospital admissions and mortality rates, which are close to those of influenza-driven disease [3,4,5]. Disease severity is aggravated by the presence of respiratory and cardiac comorbidities as well as immunosuppression associated with the bone marrow or a lung transplant [6]. Recently, several prophylactic strategies have been approved and are now being implemented. These include nirsevimab, a monoclonal antibody for universal prophylaxis in infants [7], and two vaccines both approved for use in adults, one of which is also licensed for administration during pregnancy with the aim of infants’ protection [8,9,10]. Concerning vaccines, indications may be extended or strengthened among adults with certain conditions that put them at a higher risk of severe RSV disease, including immune compromise [11,12,13]. These indications are not directly based on evidence acquired during drug trials but rather on the extrapolation of observational data on RSV disease among risk groups.
People living with HIV are known to have a higher risk of unfavorable outcomes for respiratory infections caused by influenza [14,15] and SARS-CoV-2 [16], but data on RSV infections are currently very limited.
In this article, we aim to explore the existing literature on the characteristics of RSV infection among people living with and/or exposed to HIV. Specifically, we sought to determine if they are at higher risk of severe disease and ultimately stronger candidates for vaccination or tailored prophylactic strategies.
2. Methods
We carried out a literature search through a systematic review of studies on the RSV infection incidence and severity among HIV patients. A search was conducted in PubMed/MEDLINE combining three different search queries using Boolean operators: “Respiratory Syncytial Virus” OR “RSV” AND “Human Immunodeficiency Virus” OR “HIV”. At the last stage, we searched the reference lists of included studies to look for other potentially relevant studies. Automated duplicate detection was provided by Rayyan software web application [17] and manually confirmed or overruled. After deduplication, title and abstract screening was undertaken against the inclusion/exclusion criteria. The full texts of the selected studies were then reviewed for eligibility.
We included all clinical studies published in peer-reviewed journals that compared the RSV incidence and severity endpoints according to HIV infection and/or exposure. These included ecological, cross-sectional, case–control, and cohort studies, as these are epidemiological frameworks that are equipped to study associations between HIV as an exposure and RSV infection or disease as an outcome. Interventional studies were not expected to be among the retrieved literature as their use would imply a deliberate viral challenge, which carries significant restrictions on ethical grounds. Case reports, case series, and review articles were excluded. Articles based on basic rather than clinical or translational research were excluded. Literature presented in a language other than English was excluded.
Two main reviewers (A. Almeida and R.A.) independently screened all of the literature, selecting, extracting, and excluding reports retrieved during the review process. Conflicts were resolved through collaborative discussion. A data chart was created with a summary of the most relevant aspects present in the included studies. This included the author and year of publication, sampling period, setting, and size, as well as study aims and main findings. Findings of interest were those pertaining to the effect of HIV on RSV outcomes, such as disease incidence, hospitalization, and death. Data charting was carried out primarily by A.A. and checked by all other authors. Data were tabulated separately for hospitalized and community-dwelling patients. Quality appraisal was undertaken using Newcastle–Ottawa Scores. Point estimates of effect measures for studies that used endpoints related to RSV illness severity were graphically represented.
Review items were reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA 2020 [18]), whose checklists are presented in Tables S1 and S2 (Supplementary Materials).
3. Results
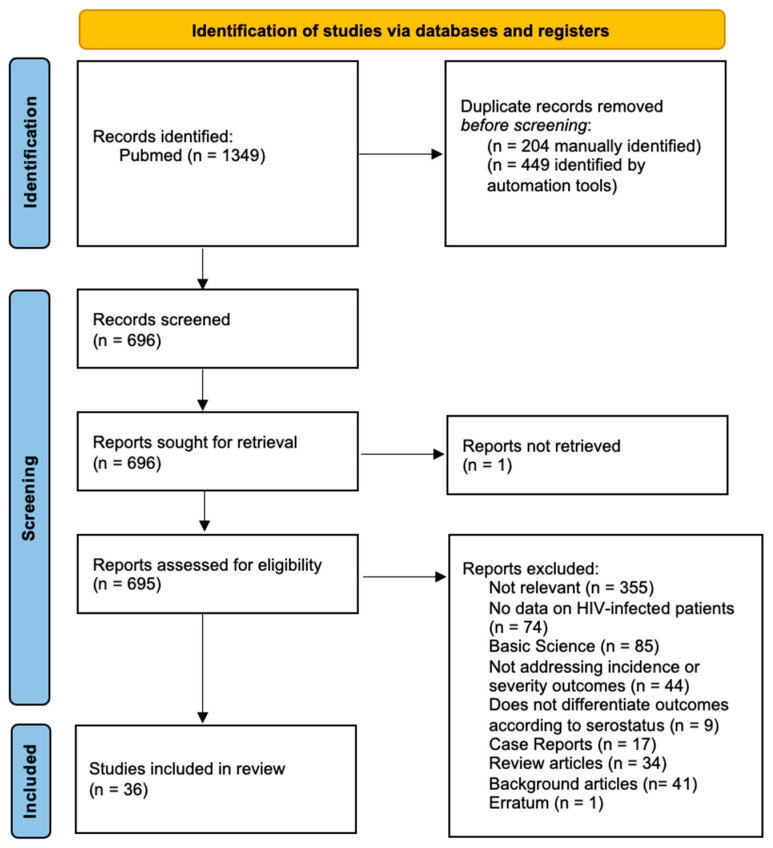
After an initial identification of 1349 articles meeting the search criteria, 36 were finally included to meet the aim of this review (Figure 1).
Figure 1.
PRISMA (Preferred Reporting Items for Systematic review and Meta-Analysis) Flowchart of included studies.
Article data including publication and sampling figures, the study type, summary statistics with effect estimates, and quality scoring are presented in Table 1 and Table 2.
Table 1.
Main features of studies of hospitalized patients included in the systematic review.
| Author and Publication Year | Sampling Period | Country | Population | Study Type | Sample Size (n) | Outcome/Aim | Main Findings | Effect of HIV Exposure | Quality |
|---|---|---|---|---|---|---|---|---|---|
| Owusu 2024 [19] | 2017–2020 | Ghana | Hospitalized children 3–12 months | Case–control | 404 | Pneumonia | Non-HIV cases had higher odds of having RSV (7.49, 95% CI: 2.55–21.98) | Decreased risk | high |
| Liu 2020 [20] | 2015–2017 | South Africa | Hospitalized children < 3 months with sepsis or LRTI; 3–12 months with LRTI | Cohort | 147 | Characterize circulating RSV strains | No differences between HIV-exposed and HUU | None | high |
| Madhi 2000 [21] |
1997–1998 | South Africa | Hospitalized children 2 months–5 years | Cohort | 1434 | Viral isolation, disease presentation and LRTI disease burden | Estimated incidence of RSV SLRTI higher among HIV-infected vs. HIV-uninfected (RR 1.92 [1.29–2.83]) RSV isolated less frequently (p < 0.001) Higher case fatality rate among all viral LRTIs (p < 0.001) |
Increased risk | high |
| Madhi 2001 [22] |
1997–1999 | South Africa | Hospitalized children < 5 years | Cohort | 113 | Presentation of RSV-associated LRTI | HIV-infected more likely to present with pneumonia (p < 0.002), bacteremia (p = 0.003), T > 38 °C, leukocytes > 15,000 (p = 0.04), and higher case fatality ratio (RR 4.4) [1.02–18.92] | Increased risk | high |
| Moyes 2023 [23] |
2012–2016 | South Africa | Hospitalized (cases) and community-dwelling (controls) children and adults | Case–control | 12,048 | Non-hospitalized ILI or hospitalized SARI | RSV detection significantly associated with ILI in HIV-positive among 5–24 and 25–44-year age groups (78.1%, 95% CI, 36.5–92.5% and 76.6%, 95% CI 13.6–93.7%, respectively); not significant among SARI cases | Increased risk | high |
| Madhi 2004 [24] | 2001 | South Africa | Hospitalized children < 5 years | Cohort | 222 | Outcomes of nosocomial and community-acquired RSV infection | Risk factors for severe RSV infection more prevalent among HIV-negative (OR 1.92 [95% CI 1.92–3.61]). Non-significant trend for HIV-positive to present with pneumonia; no differences in mortality or length of stay | Increased risk | high |
| Moyes 2013 [25] |
2010–2011 | South Africa | Hospitalized children < 5 years | Cohort | 2992 | ALRTIs requiring hospitalization; hospitalization outcomes | Among all age groups of children, < 5 years higher incidence of RSV-related ALRTIs (RR 3.1–5.6 [2.6–6.4]) HIV-positive more likely to receive oxygen therapy (OR 1.1 [1.0–3.2]), endure a prolonged hospitalization (4.2 [2.0–7.5]), or die (22.2 [4.8–102]) |
Increased risk | high |
| Famoroti 2018 [26] |
2018 | South Africa | Hospitalized children < 5 years | Cross-sectional | 2172 | Detection of viral respiratory pathogens | Nasopharyngeal swab specimens from HIV-negative patients more frequently associated with RSV detection (p = 0.001) | Decreased risk | low |
| McMorrow 2019 Feb [27] |
2011–2016 | South Africa | Hospitalized children < 5 years | Cohort | 3650 | Influenza and RSV-related SARI | HIV exposure associated with increased incidence of RSV-related hospitalization in ages 0–5 months (RR 1.4 [95% CI, 1.3–1.6]); HIV infection associated with RSV-related hospitalization in all age groups | Increased risk | high |
| Annamalay 2016 Sep [28] |
2010–2013 | Mozambique | Hospital presenting children < 10 years | Cohort | 277 | Rhinovirus species among pneumonia inpatients | No significant difference in RSV detection rates between HIV-positive and HIV-negative patients | None | high |
| Annamalay 2016 Aug [29] |
2011–2012 | South Africa | Hospitalized children < 2 years | Case–control | 208 | Describe viral agents among ALRTIs and their interactions with HIV | RSV more frequently isolated from HIV-uninfected patients with ALRTI (p = 0.013) | Increased risk | low |
| Cohen 2016 [30] |
2016 | South Africa | Hospitalized infants < 6 months | Cohort | 3537 | Study the epidemiology of LRTI hospitalization in HEU vs. HUU infants | Higher incidence of RSV-related LRTI hospitalization in HEU vs. HUU (IRR 1.4; 95% CI 1.3–1.6); higher case fatality rate of RSV infection (OR 2.1, 95% CI 1.1–3.8) | Increased risk | high |
| Cohen 2015 [14] |
2009–2012 | South Africa | Hospitalized children < 5 years | Cohort | 8723 | Describe LRTI hospitalizations | HIV-infected admitted for ALRTI were less likely to test positive for RSV (p < 0.001) | None | moderate |
| Madhi 2000 [21] |
1997–1998 | South Africa | Hospitalized children 2 months–5 years | Cohort | 990 | Estimate the burden of disease and clinical course of viral-associated SLRTI | HIV-infected were less likely to test positive for RSV (p < 0.001); estimated incidence higher (RR 1.92 (1.29–2.83) | Increased risk | moderate |
| Elabbadi 2020 [31] |
2011–2017 | France | ICU-admitted adults | Cohort | 123 | Prevalence of respiratory viruses according to the CD4 cell count among patients with ARF | RSV detection not associated with CD4 counts |
None | moderate |
| Chandwani 1990 [32] |
1986–1987 | USA | Hospital admitted-children (age not specified) | Cohort | 28 | Describe the clinical course of RSV-related admission | HIV-infected children were more prone to pneumonia and prolonged viral shedding (no statistical testing) |
Increased risk | low |
| O’Callaghan-Gordo 2010 [33] |
2006–2007 | Mozambique | Hospitalized children < 5 years | Cohort | 835 | Present surveillance data on the epidemiology of several respiratory viruses associated with clinical pneumonia | Risk of RSV-related pneumonia higher among HIV-infected patients (IRR 2.2–6.5 [no confidence intervals provided]) | Increased risk | moderate |
| Miller 1996 [34] |
1994–1995 | UK | Adults undergoing bronchoscopy for LRTI work-up | Cohort | 44 | Viral detection in bronchoalveolar lavage of HIV+-positive patients with respiratory infections | No viruses detected | None | low |
| Moyes 2017 [24] |
2009–2013 | South Africa | Hospitalized adults | Cohort | 7796 | Study the epidemiology of RSV-associated SARI hospitalizations | Among HIV-positive patient age groups, higher risk among 18–64 years (OR 26.3 [6.2–112.1] and 11.4 [2.6–50.0]) compared to older patients and for female sex (OR 2.7 [1.4–5.4]); compared to HIV-uninfected, HIV-infected had longer symptom duration before hospitalization (2 to <5 days aOR 4.4 (95% CI 1.7–11.0) and 5–7 days aOR 4.2 (95% CI 1.6–10.4) compared to <2 days) and higher incidence of RSV-related SARI (RR 14 [11,12,13,14,15,16,17,18,25] | Increased risk | high |
| Rha 2019 [35] |
2009–2014 | South Africa | Hospitalized children < 5 years | Cohort | 7179 | Assess the performance of various case definitions in detecting severe RSV disease | HIV infection reported less commonly in RSV-related SARI cases (aOR 0.4 [0.3–0.5]) | None | high |
| McMorrow 2019 Nov [36] | 2011–2016 | South Africa | Hospitalized infants < 12 months | Cohort | 2243 | Magnitude and duration of HIV exposure on RSV hospitalization | HEU had a higher incidence of RSV-related hospitalization compared to HUU until 5 months of age (1.4 [1.4–1.6]); at 6–11 months of age, the difference was not significant | Increased risk | High |
| Patel 2019 [37] |
2012–2016 | Botswana | Hospitalized children 1–23 months | Cohort | 123 | Risk factors for poor outcomes among the subset of children with RSV-ALRI | Longer duration of oxygen support among HE vs. HUU (IRR: 1.63; 95% CI: 1.02–2.61; p = 0.04); lack of clinical response did not differ |
Increased risk | high |
Table 2.
Main features of studies of non-hospitalized patients included in the systematic review.
| Author and Publication Year | Sampling Period | Country | Population | Study Type | Sample Size (n) | Outcome/Aim | Main Findings | Effect of HIV Exposure | Quality |
|---|---|---|---|---|---|---|---|---|---|
| Smith 2021 [38] |
2002–2013 | USA | Infants < 56 weeks | Cohort | 556 | Assess the association between seroconversion and hospitalization | Seroconversion to RSV associated with hospitalization among HEU infants (aRR 1.95 [1.21–3.15]) 4.3% of HEU infants hospitalized due to respiratory infection during first year of life |
Increased risk | high |
| King Jr 1993 [39] |
1990–1993 | USA | Children < 12 months | Cohort | 131 | Clinical presentation, viral shedding | More prolonged viral shedding in the HIV-infected vs. the HIV-uninfected (30 vs. 6 days, p = 0.038) Attack rate of 49/100 vs. 19/100 child-years and more frequent pneumonia (no statistical testing) |
Increased risk | moderate |
| Madhi 2006 [36] |
1998–2004 | South Africa | Children > 28 days enrolled in an RCT | Cohort | 39,836 | Hospitalization and death due to RSV-LRTI | Incidence of hospitalization for RSV-LRTI greater among HIV-positive (OR 2.5 [2.04–3.03]) Case fatality rate also higher (OR 12.7 [3.9–31.4]) No difference in re-admissions |
Increased risk | moderate |
| Wedderburn 2024 [40] |
2012–2015 | South Africa | Infants < 2 years | Cohort | 1136 | Hospitalization rates | HEU had higher odds of hospitalization, even though difference not significant for RSV-LRTI | None | high |
| Cohen 2021 [41] | 2016–2018 | South Africa | Household members of all ages | Cohort | 1116 | RSV incidence and household transmission | There were no differences in symptomatic fraction, shedding duration, or probability of transmission or acquisition of infection when comparing HIV-infected with HIV-uninfected | None | high |
| De Souza Luna 2023 [42] |
2005–2013 | Brazil | Community-dwelling and hospitalized adults and children | Cohort | 1380 | Compare RSV detection rates and viral loads | Viral loads and frequency rates not higher among HIV-infected patients, though no statistical tests conducted | None | moderate |
| Weinberg 2017 [43] |
2011–2013 | Brazil | Infants < 2 years and their mothers | Cohort | 335 | Incidence of common childhood respiratory tract infections | No significant difference in RSV seroconversion between HIV-exposed and HUU children | None | high |
| Peterson 2016 [44] |
2011–2014 | Malawi | Children 3 months–14 years presenting to the Emergency Department | Cohort | 2363 | Identify factors associated with clinical severity in LRTIs and coviral clustering | No difference in incidence of RSV-positive SARI between HIV-infected and HIV-uninfected | None | moderate |
| Mendoza Sanchez 2006 [45] |
1989–2003 | Spain | Children < 15 years attending an Immunodeficiency Clinic | Cohort | 26 | Evaluate the occurrence and clinical significance of infections produced by respiratory viruses | RSV was the most commonly isolated virus responsible for LRTIs | None | low |
| Feikin 2012 [46] |
2007–2010 | Kenya | Community-dwelling and hospitalized adults and children > 5 years | Case–control | 1039 | Health-seeking acute respiratory infection (ARI) | Incidence rate of RSV-ARI higher among HIV-positive than HIV-negative adults (0.98 [0.51–1.45] vs. 0.13 [0.59–2.01]), but no significance testing done | Increased risk | moderate |
| Madhi 2018 [47] |
2011–2012 | South Africa | Women from midpregnancy until 24 weeks postpartum | Cohort | 2410 | Incidence of RSV illness | No significant differences in RSV incidence rates among pregnant or post-partum HIV-infected women | None | moderate |
| Nyawanda 2022 [48] |
2015–2019 | Kenya | Pregnant and postpartum women and their infants < 24 weeks | Cohort | 2877 | RSV detection and presentation in mothers and infants | RSV-related ARI incidence was higher among HIV-positive compared to HIV-negative women, both pregnant (p = 0.01) and postpartum (p = 0.03); no difference in RSV-related signs and symptoms; no difference in incidence among infants up to 12 weeks (HEU vs. HUU) | Increased risk | high |
| Tempia 2015 [49] |
1998–2009 | South Africa | Countrywide adults and children > 5 years | Ecological | 7536 | Estimate deaths attributable to influenza and RSV | RSV-associated mortality rate for all causes of death higher for HIV-positive than HIV-negative persons (aRR 66.1, 95% CI 26.0–167.8) | Increased risk | NA |
Quality assessment through Newcastle–Ottawa Scores is presented in Table S3. Among the included studies, 27 (75%) were conducted in sub-Saharan Africa (n = 21 in South Africa), 3 in the USA, 2 in Brazil, and 3 in Europe. Moreover, 30 studies included children, 23 of which (64% of the total) were focused exclusively on children. Among these, HIV exposure (to a mother living with HIV) and/or living with HIV were used to differentiate between exposure groups. Nine studies (25%) sampled non-pregnant adults and two pregnant women. The majority of the studies (n = 27; 75%) were conducted in contexts where highly active antiretroviral therapy (HAART) was already made available by local healthcare structures even though this did not translate into a majority effectively taking it [25,30].
There was considerable heterogeneity regarding outcomes, which ranged from the RSV incidence measured by either seroconversion or viral detection [14,21,26,28,29,30,31,34,42,43,45,47,48,50], viral shedding/loads [39,42], death [36,49], hospitalization [3,27,30,35,36,40], or other proxies for disease severity such as the need for oxygen support and length of hospital stay [19,21,22,24,32,33,37,39,44,46].
Looking at the incidence of RSV-related illness, one high-quality study conducted in Kenya found a higher risk among HIV-positive pregnant and postpartum women [48], while a post hoc moderate-quality analysis of influenza vaccine trial data found none [47]. One high-quality study conducted in Brazil where 48% of pregnant women were under HAART did not find a significant difference in RSV seroconversion rates between HIV-exposed and HIV-unexposed infants [43]. HIV-infected children had a higher attack rate of RSV illness in a pre-HAART American cohort during the early 1990s [39]. RSV detection was significantly associated with influenza-like illness in people living with HIV among the 5–24- and 25–44-years age groups in a high-quality study [50]. In a community study looking at the RSV incidence and household transmission, Cohen et al. found no differences in the symptomatic fraction, probability of transmission, or acquisition of infection when comparing HIV-infected with HIV-uninfected [14]. Acute respiratory infection incidence rates were found to be higher among HIV-positive adults in a moderate-quality study in Kenya where no significance testing was performed [46].
One moderate-quality pre-HAART study showed more prolonged RSV viral shedding among HIV-positive children [39], whilst one post-HAART observed no differences among an all-age cohort [14].
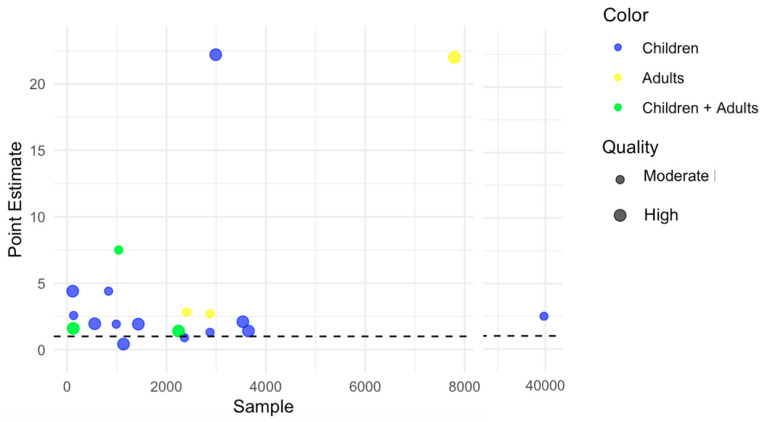
Studies analyzing the effect of HIV on RSV illness severity with reported point estimates of association measures are presented in Figure 2. When looking at the severe disease incidence among children, there was high-quality evidence for a higher risk of pneumonia, severe LRTI, and/or hospitalization both among HIV-positive [21,22,25,33,36,51] as well as HIV-exposed but uninfected children (HEU) [22,25,27,30,51]. One study found more frequent bacteremia [22] among those HIV-positive while another found a longer duration of oxygen support among those HIV-exposed or infected [37]. Only one study covering the incidence of severe disease among adults was found (high quality), reporting higher rates among all age groups > 18 years old [23]. One moderate-quality study conducted on an ICU HIV-positive cohort (n = 123) in France did not find differences in RSV detection according to CD4 counts [31].
Figure 2.
Bubble plot representing point estimates of endpoints used in studies covering RSV severity outcomes.
There was mostly low-quality evidence that among children hospitalized for LRTI, RSV is isolated more frequently from HIV-negative hospitalized children compared to their HIV-positive counterparts [19,21,26,28,29].
Case fatality rates were higher among HIV-exposed children [21,30,36,49]. One countrywide ecological study in South Africa revealed a higher RSV-associated mortality rate for all causes of death among people living with HIV at between 5 and 44 years old [49].
4. Discussion
Our review found scarce literature on the interplay between chronic HIV infection and the risk of severe RSV disease among adults, precluding robust conclusions on the use of prophylactic measures in this age group. Nevertheless, as the little evidence available suggests an increased risk, extending the inoculation of approved vaccines may be considered in this population in line with scientific society statements on immunocompromised populations [11,12,13].
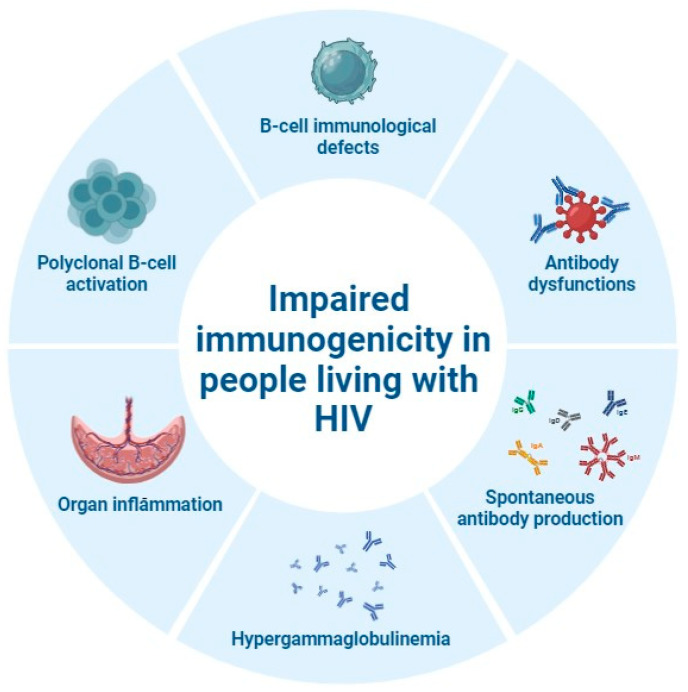
As for immunogenicity in people living with HIV, several factors including HIV-related immunological alterations and peculiarities of the target population should be considered. HIV infection is associated with major B-cell immunological defects, including impairments of antibody functions, polyclonal B-cell activation leading to hypergammaglobulinemia, and spontaneous antibody production (Figure 3) [52].
Figure 3.
Immunological alterations leading to impaired immunogenicity in people living with HIV (figure created on BioRender.com).
One of the most studied immunological models is that of pregnant women living with HIV and their HEU children, given the attractive opportunity to actively immunize the mother to protect both. A systematic review and meta-analysis, including nine studies from low-and-middle-income countries and three from the USA, showed that HEU children have impaired growth and higher infection-related morbidity/mortality rates, especially in the first six months of life [53]. Women living with HIV have been reported to present with lower immunogenicity for different vaccines, especially in the case of a low CD4+ T-cell count or detectable viral load [54,55]. On the other hand, HIV infection especially in the absence of viral control has been reported to be associated with modifications of the biophysical antibody structure, including glycosylation, also lowering the efficiency of transplacental transfer in pregnant women [56]. This impairment can be further compounded by placental inflammation, induction of maternal hyperglobulinemia through polyclonal B-cell activation, and impairment of the maternal immune response, leading to lower maternal titers of specific antibodies [57]. Both maternal hypergammaglobulinemia and HIV infection have been reported to contribute to low antibody titers at birth and early infections in HEU neonates as shown for tetanus, measles, and Streptococcus pneumoniae [58] or lower specific antibody responses to Haemophilus influenzae type b, Streptococcus pneumoniae, Bordetella pertussis antigens, tetanus toxoid, and hepatitis B surface antigen [59].
Finally, a remarkable reduction in placental transfer of natural RSV-specific antibodies has been reported among pregnant women living with HIV as compared with HIV-uninfected pregnant women [60]. This might explain the considerable evidence elicited in our review that both HIV-infected but also HEU have a higher risk of severe RSV infection, a risk that was consistently observed with several surrogates of severity as endpoints such as hospitalization for severe LRTI [30,40] or death [27].
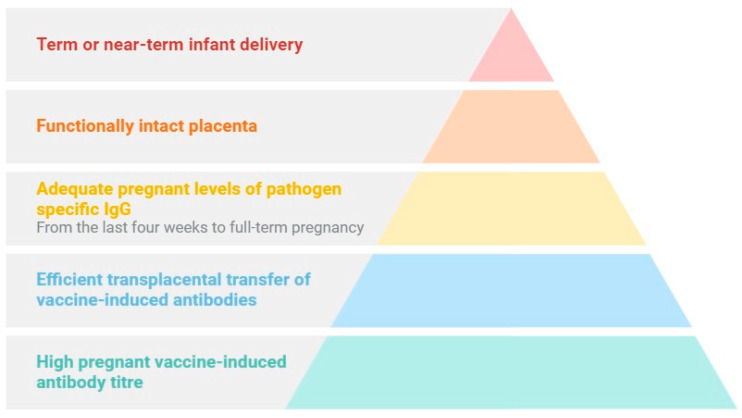
Therefore, protection of infants following maternal active immunization has been reported to depend on (a) high vaccine-induced antibody titers among pregnant women, (b) efficient transplacental transfer of vaccine-induced antibodies; (c) adequate maternal levels of pathogen-specific IgG when the transfer is most significant—from the last four weeks to full-term pregnancy; (d) a functionally intact placenta; and (e) a term or near-term delivery (Figure 4) [11,61].
Figure 4.
Factors associated with protection of infants following maternal active immunization (figure created on BioRender.com).
Several vaccines administered during pregnancy showed safety and efficacy in the prevention of adverse maternal and neonatal outcomes, although most of the evidence was generated in non-HIV populations [8,54] and limited data on maternal vaccine outcomes are available so far [53].
Regarding RSV in particular, protection against its associated infection is primarily antibody-mediated, with passively acquired neutralizing antibodies that can protect infants in the first months of life [61,62]. Transplacental transfer of RSV antibodies has been defined as highly efficient in mother–infant pairs in rural Nepal, though higher antibody titers showed no protection against earlier or more severe RSV disease in infants [63]. Similar results have been shown in a study on American Indian infants, even if the high rates of RSV severe disease could not be attributed to a failure of protection by the maternal RSV-neutralizing antibodies [64].
Several candidate vaccines have completed phase 3 trials evaluating their safety and efficacy in preventing RSV illness in infants following administration to their pregnant mothers. The recently approved bivalent prefusion RSV vaccine (RSVpreF) demonstrated in its phase 3 trial an efficacy of 81.8% (99.5% CI 40.6 to 96.3) in reducing medically attended severe LRTI and 57.1% (99.5% CI 14.7 to 79.8) in reducing medically attended RSV-associated LRTI within 90 days after birth, though with the latter result not meeting statistical success criteria [8]. The monovalent RSVpreF3-Mat phase 3 trial was stopped early due to safety concerns, as a higher number of preterm births occurred in the intervention arm (relative risk 1.37 95% CI [1.09–1.74]), possibly contributing to a non-significant imbalance of neonatal fatalities (relative risk 2.16; 95% CI, 0.62 to 7.56; p = 0.23) [65]. However, accurate measurements of gestational age were lacking for many of the trial participants, possibly contributing to misclassification of age at delivery. Furthermore, no possible mechanisms for an increased risk of preterm delivery were elicited after several post hoc analyses [66]. Efficacy data were nevertheless encouraging, showing a vaccine efficacy of 69.0% (95% credible interval, 33.0 to 87.6) in reducing medically assessed severe RSV-associated lower respiratory tract disease through 6 months of age. For the RSVpreF bivalent vaccine, pregnant persons at an increased risk of preterm delivery were excluded from the phase 2b and phase 3 trials and a small non-significant trend for pre-eclampsia and preterm birth was found. It is worth noting that 72% of preterm births occurred at 36 weeks of gestation. As the benefits seem to substantially outweigh the risks, vaccine recommendations for pregnancy hold, albeit for the period spanning from 32 to 36 weeks of gestational age. The American Center for Disease Control and Prevention reports that post-marketing studies covering the aforementioned adverse events are to be conducted by the manufacturer [67].
Furthermore, a heightened risk of severe disease seemed to persist among HIV-infected children over one year of age in several of the studies covered in this review, rendering the waning effect of maternal immunization less protective over time. Nirsevimab is currently indicated for universal administration in the first year of life at the beginning of the RSV season for children whose mothers were not RSV vaccinated [68,69,70]. This monoclonal antibody, besides being a priority for HIV-exposed infants in their first year of life, could provide an extension of active immunization to HIV-infected children if administered after that period in the same fashion as it is to be considered for other special high-risk populations.
The finding that HIV-negative hospitalized children are more likely to test positive for RSV than their HIV-positive counterparts may seem out of line with the other evidence elicited. The fraction of RSV disease among the overall causes of hospitalization among HIV-positive children is probably lower than that of HIV-negative children, possibly due to the burden of opportunistic disease, which is far less likely among the latter [19,26].
5. Conclusions
There is ample evidence, mostly stemming from studies conducted in sub-Saharan Africa, that children who were exposed to HIV-positive mothers carry a significantly higher risk of severe RSV infection. This makes the case for considering prioritizing and extending existing prophylactic strategies to this population. Further data are needed to support a review of the existing recommendations for adult people living with HIV.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/pathogens13090802/s1, Table S1. PRISMA 2020 Checklist. Table S2. PRISMA 2020 Abstract Checklist. Table S3. Quality assessment for included studies according to the Newcastle–Ottawa Score.
Author Contributions
Conceptualization: A.A. (André Almeida) and M.B.; methodology, A.A. (André Almeida) and M.B.; validation, A.A. (André Almeida) and R.A.; formal analysis, all authors; data curation, A.A. (André Almeida) and R.A.; writing—original draft preparation, A.A. (André Almeida) and M.B.; writing—review and editing, A.A. (André Almeida), M.B. and A.A. (Arianna Aceti). All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
No new data were generated.
Conflicts of Interest
A. Almeida has received honoraria for teaching at a post-graduate course that had Pfizer among its sponsors. All other authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Scheltema N.M., Gentile A., Lucion F., Nokes D.J., Munywoki P.K., Madhi S., Groome M., Cohen C., Moyes J., Thorburn K., et al. Global respiratory syncytial virus-associated mortality in young children (RSV GOLD): A retrospective case series. Lancet Glob. Health. 2017;5:e984–e991. doi: 10.1016/S2214-109X(17)30344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi T., McAllister D.A., O’Brien K.L., Simoes E.A.F., Madhi S.A., Gessner B.D., Polack F.P., Balsells E., Acacio S., Aguayo C., et al. RSV Global Epidemiology Network. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: A systematic review and modelling study. Lancet. 2017;390:946–958. doi: 10.1016/S0140-6736(17)30938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nam H.H., Ison M.G. Respiratory syncytial virus infection in adults. BMJ. 2019;2019:366. doi: 10.1136/bmj.l5021. [DOI] [PubMed] [Google Scholar]
- 4.Almeida A., Boattini M., Christaki E., Marques T.M., Moreira I., Cruz L., Tosatto V., Antão D., Bianco G., Iannaccone M., et al. Comparative virulence of seasonal viruses responsible for lower respiratory tract infections: A southern European multi-centre cohort study of hospital admissions. Infection. 2021;49:483–490. doi: 10.1007/s15010-020-01569-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi T., Denouel A., Tietjen A.K., Campbell I., Moran E., Li X., Campbell H., Demont C., Nyawanda B.O., Chu H.Y., et al. Global Disease Burden Estimates of Respiratory Syncytial Virus–Associated Acute Respiratory Infection in Older Adults in 2015: A Systematic Review and Meta-Analysis. J. Infect. Dis. 2020;222((Suppl. 7)):S577–S583. doi: 10.1093/infdis/jiz059. [DOI] [PubMed] [Google Scholar]
- 6.Boattini M., Almeida A., Comini S., Bianco G., Cavallo R., Costa C. From Forgotten Pathogen to Target for New Vaccines: What Clinicians Need to Know about Respiratory Syncytial Virus Infection in Older Adults. Viruses. 2024;16:531. doi: 10.3390/v16040531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammitt L.L., Dagan R., Yuan Y., Cots M.B., Bosheva M., Madhi S.A., Muller W.J., Zar H.J., Brooks D., Grenham A., et al. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N. Engl. J. Med. 2022;386:837–846. doi: 10.1056/NEJMoa2110275. [DOI] [PubMed] [Google Scholar]
- 8.Kampmann B., Madhi S.A., Munjal I., Simões E.A., Pahud B.A., Llapur C., Baker J., Marc G.P., Radley D., Shittu E., et al. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. N. Engl. J. Med. 2023;388:1451–1464. doi: 10.1056/NEJMoa2216480. [DOI] [PubMed] [Google Scholar]
- 9.Walsh E.E., Pérez Marc G., Zareba A.M., Falsey A.R., Jiang Q., Patton M., Polack F.P., Llapur C., Doreski P.A., Ilangovan K., et al. Efficacy and Safety of a Bivalent RSV Prefusion F Vaccine in Older Adults. N. Engl. J. Med. 2023;388:1465–1477. doi: 10.1056/NEJMoa2213836. [DOI] [PubMed] [Google Scholar]
- 10.Papi A., Ison M.G., Langley J.M., Lee D.-G., Leroux-Roels I., Martinon-Torres F., Schwarz T.F., van Zyl-Smit R.N., Campora L., Dezutter N., et al. Respiratory Syncytial Virus Prefusion F Protein Vaccine in Older Adults. N. Engl. J. Med. 2023;388:595–608. doi: 10.1056/NEJMoa2209604. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention RSV Vaccination for Adults 60 Years of Age and Over. [Internet] [(accessed on 1 July 2024)]; Available online: https://www.cdc.gov/vaccines/vpd/rsv/hcp/older-adults.html.
- 12.National Advisory Committee on Immunization (NACI) Summary: Statement on the Prevention of Respiratory Syncytial virus Disease in Older Adults. Public Health Agency of Canada; Ottawa, ON, Canada: 2024. [(accessed on 1 July 2024)]. Available online: https://www.canada.ca/en/public-health/services/publications/vaccines-immunization/national-advisory-committee-immunization-summary-statement-prevention-rsv-disease-older-adults.html. [Google Scholar]
- 13.Australian Technical Advisory Group on Immunisation (ATAGI) Australian Immunisation Handbook. Australian Government Department of Health and Aged Care; Woden, Australia: 2022. [(accessed on 1 July 2024)]. Available online: https://immunisationhandbook.health.gov.au/contents/vaccine-preventable-diseases/respiratory-syncytial-virus-rsv#recommendations. [Google Scholar]
- 14.Cohen C., Moyes J., Tempia S., Groom M., Walaza S., Pretorius M., Dawood H., Chhagan M., Haffejee S., Variava E., et al. Severe influenza-associated respiratory infection in high HIV prevalence setting South Africa 2009–2011. Emerg. Infect. Dis. 2013;19:1766–1774. doi: 10.3201/eid1911.130546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Almeida A.R., Branco S.C., Vesza Z., Pereira R. CURB-65 and other markers of illness severity in community-acquired pneumonia among HIV-positive patients. Int. J. STD AIDS. 2016;27:998–1004. doi: 10.1177/0956462415605232. [DOI] [PubMed] [Google Scholar]
- 16.Ssentongo P., Heilbrunn E.S., Ssentongo A.E., Advani S., Chinchilli V.M., Nunez J.J., Du P. Epidemiology and outcomes of COVID-19 in HIV-infected individuals: A systematic review and meta-analysis. Sci. Rep. 2021;11:628. doi: 10.1038/s41598-021-85359-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Owusu M., Adu E., Kalu L.E., Martey E., Acheampong G., Enimil A., Appiah J.A., Badu-Peprah A., Sylverken J., Sylverken A.A., et al. Aetiological agents of pneumonia among HIV and non-HIV infected children in Ghana: A case-control study. PLoS ONE. 2024;19:e0299222. doi: 10.1371/journal.pone.0299222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu H., Lu B., Tabor D.E., Tovchigrechko A., Wilkins D., Jin H., Madhi S.A., Soofie N., Esser M.T., Nunes M.C. Characterization of human respiratory syncytial virus (RSV) isolated from HIV-exposed-uninfected and HIV-unexposed infants in South Africa during 2015-2017. Influ. Other Respir. Viruses. 2020;14:403–411. doi: 10.1111/irv.12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madhi S.A., Schoub B., Simmank K., Blackburn N., Klugman K.P. Increased burden of respiratory viral associated severe lower respiratory tract infections in children infected with human immunodeficiency virus type-1. J. Pediatr. 2000;137:78–84. doi: 10.1067/mpd.2000.105350. [DOI] [PubMed] [Google Scholar]
- 22.Madhi S.A., Venter M., Madhi A., Petersen M.K., Klugman K.P. Differing manifestations of respiratory syncytial virus-associated severe lower respiratory tract infections in human immunodeficiency virus type 1-infected and uninfected children. Pediatr. Infect. Dis. J. 2001;2:164–170. doi: 10.1097/00006454-200102000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Moyes J., Walaza S., Pretorius M., Groome M., von Gottberg A., Wolter N., Haffejee S., Variava E., Cohen A.L., Tempia S., et al. Respiratory syncytial virus in adults with severe acute respiratory illness in a high HIV prevalence setting. J. Infect. 2017;75:346–355. doi: 10.1016/j.jinf.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madhi S.A., Ismail K., O’Reilly C., Cutland C. Importance of nosocomial respiratory syncytial virus infections in an African setting. Trop. Med. Int. Health. 2004;4:491–498. doi: 10.1111/j.1365-3156.2004.01221.x. [DOI] [PubMed] [Google Scholar]
- 25.Moyes J., Cohen C., Pretorius M., Groome M., von Gottberg A., Wolter N., Walaza S., Haffejee S., Chhagan M., Naby F., et al. Epidemiology of respiratory syncytial virus-associated acute lower respiratory tract infection hospitalizations among HIV-infected and HIV-uninfected South African children 2010–2011. J. Infect. Dis. 2013;208((Suppl. 3)):S217–S226. doi: 10.1093/infdis/jit479. [DOI] [PubMed] [Google Scholar]
- 26.Famoroti T., Sibanda W., Ndung’u T. Prevalence and seasonality of common viral respiratory pathogens including Cytomegalovirus in children between 0-5 years of age in KwaZulu-Natal an HIV endemic province in South Africa. BMC Pediatr. 2018;18:240. doi: 10.1186/s12887-018-1222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMorrow M.L., Tempia S., Walaza S., Treurnicht F.K., Moyes J., Cohen A.L., Pretorius M., Hellferscee O., Wolter N., von Gottberg A., et al. The Role of Human Immunodeficiency Virus in Influenza- and Respiratory Syncytial Virus–associated Hospitalizations in South African Children, 2011–2016. Clin. Infect. Dis. 2019;68:773–780. doi: 10.1093/cid/ciy532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Annamalay A.A., Lanaspa M., Khoo S., Madrid L., Acácio S., Zhang G., Laing I.A., Gern J., Goldblatt J., Bizzintino J., et al. Rhinovirus species and clinical features in children hospitalized with pneumonia from Mozambique. Trop. Med. Int. Health. 2016;21:1171–1180. doi: 10.1111/tmi.12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Annamalay A.A., Abbott S., Sikazwe C., Khoo S.-K., Bizzintino J., Zhang G., Laing I., Chidlow G.R., Smith D.W., Gern J., et al. Respiratory viruses in young South African children with acute lower respiratory infections and interactions with HIV. J. Clin. Virol. 2016;81:58–63. doi: 10.1016/j.jcv.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen C., Moyes J., Tempia S., Groome M., Walaza S., Pretorius M., Naby F., Mekgoe O., Kahn K., von Gottberg A., et al. Epidemiology of Acute Lower Respiratory Tract Infection in HIV-Exposed Uninfected Infants. Pediatrics. 2016;137:e20153272. doi: 10.1542/peds.2015-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elabbadi A., Pichon J., Visseaux B., Schnuriger A., Bouadma L., Philippot Q., Patrier J., Labbé V., Ruckly S., Fartoukh M., et al. Respiratory virus-associated infections in HIV-infected adults admitted to the intensive care unit for acute respiratory failure: A 6-year bicenter retrospective study (HIV-VIR study) Ann. Intensive Care. 2020;10:123. doi: 10.1186/s13613-020-00738-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chandwani S., Borkowsky W., Krasinski K., Lawrence R., Welliver R. Respiratory syncytial virus infection in human immunodeficiency virus-infected children. Pt 1J. Pediatr. 1990;117:251–254. doi: 10.1016/S0022-3476(05)80539-6. [DOI] [PubMed] [Google Scholar]
- 33.O’Callaghan-Gordo C., Bassat Q., Morais L., Díez-Padrisa N., Machevo S., Nhampossa T., Nhalungo D., Sanz S., Quintó L., Alonso P.L., et al. Etiology and epidemiology of viral pneumonia among hospitalized children in rural Mozambique: A malaria endemic area with high prevalence of human immunodeficiency virus. Pediatr. Infect. Dis. J. 2011;30:39–44. doi: 10.1097/INF.0b013e3181f232fe. [DOI] [PubMed] [Google Scholar]
- 34.Miller R.F., Loveday C., Holton J., Sharvell Y., Patel G., Brink N.S. Community-based respiratory viral infections in HIV positive patients with lower respiratory tract disease: A prospective bronchoscopic study. Sex. Transm. Infect. 1996;72:9–11. doi: 10.1136/sti.72.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rha B., Dahl R.M., Moyes J., Binder A.M., Tempia S., Walaza S., Bi D., Groome M.J., Variava E., Naby F., et al. Performance of Surveillance Case Definitions in Detecting Respiratory Syncytial Virus Infection Among Young Children Hospitalized with Severe Respiratory Illness—South Africa, 2009–2014. J. Pediatr. Infect. Dis. Soc. 2019;8:325–333. doi: 10.1093/jpids/piy055. [DOI] [PubMed] [Google Scholar]
- 36.Madhi S.A., Kuwanda L., Cutland C., Klugman K.P. Five-year cohort study of hospitalization for respiratory syncytial virus associated lower respiratory tract infection in African children. J. Clin. Virol. 2006;36:215–221. doi: 10.1016/j.jcv.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 37.Patel S.M., Spees L., Smieja M., Luinstra K., Steenhoff A.P., Feemster K.A., Arscott-Mills T., Boiditswe S., Patel M.Z., Shah S.S., et al. Predictors of Poor Outcomes Among Infants With Respiratory Syncytial Virus–associated Acute Lower Respiratory Infection in Botswana. Pediatr. Infect. Dis. J. 2019;38:525–527. doi: 10.1097/INF.0000000000002168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith C., Huo Y., Patel K., Fetters K., Hegemann S., Burchett S., Van Dyke R., Weinberg A. Immunologic and Virologic Factors Associated With Hospitalization in Human Immunodeficiency Virus-Exposed, Uninfected Infants in the United States. Clin. Infect. Dis. 2021;73:1089–1096. doi: 10.1093/cid/ciab272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.King J.C., Jr., Burke A.R., Clemens J.D., Nair P., Farley J.J., Vink P.E., Batlas S.R., Rao M., Johnson J.P. Respiratory syncytial virus illnesses in human immunodeficiency virus- and noninfected children. Pediatr. Infect. Dis. J. 1993;12:733–739. doi: 10.1097/00006454-199309000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Wedderburn C.J., Bondar J., Lake M.T., Nhapi R., Barnett W., Nicol M.P., Goddard L., Zar H.J. Risk and rates of hospitalisation in young children: A prospective study of a South African birth cohort. PLOS Glob. Public Health. 2024;4:e0002754. doi: 10.1371/journal.pgph.0002754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cohen C., McMorrow M.L., Martinson N.A., Kahn K., Treurnicht F.K., Moyes J., Mkhencele T., Hellferscee O., Lebina L., Moroe M., et al. Cohort profile: A Prospective Household cohort study of Influenza, Respiratory syncytial virus and other respiratory pathogens community burden and Transmission dynamics in South Africa, 2016–2018. Influenza Other Respir. Viruses. 2021;15:789–803. doi: 10.1111/irv.12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Souza Luna L.K., Cruz J.S., Chaves T.D.S.S., Bellei N. Comparative analysis of Respiratory Syncytial Virus frequency rates and viral load in different patient cohorts in a University Hospital in São Paulo Brazil over an eight-year period (2005–2013) Braz. J. Infect. Dis. 2023;27:103702. doi: 10.1016/j.bjid.2023.103702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weinberg A., Mussi-Pinhata M.M., Yu Q., Cohen R.A., Almeida V.C., Amaral F., Pinto J., Teixeira M.L., Succi R.C., Freimanis L., et al. Excess respiratory viral infections and low antibody responses among HIV-exposed, uninfected infants. AIDS. 2017;31:669–679. doi: 10.1097/QAD.0000000000001393. [DOI] [PubMed] [Google Scholar]
- 44.Peterson I., Bar-Zeev N., Kennedy N., Ho A., Newberry L., SanJoaquin M.A., Menyere M., Alaerts M., Mapurisa G., Chilombe M., et al. Respiratory Virus–Associated Severe Acute Respiratory Illness and Viral Clustering in Malawian Children in a Setting With a High Prevalence of HIV Infection, Malaria, and Malnutrition. J. Infect. Dis. 2016;214:1700–1711. doi: 10.1093/infdis/jiw426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mendoza Sánchez M.C., Ruiz-Contreras J., Vivanco J.L., Fernández-Carrión F., Baro Fernández M., Ramos J.T., Otero J.R., Folgueira D. Respiratory virus infections in children with cancer or HIV infection. J. Pediatr. Hematol. Oncol. 2006;28:154–159. doi: 10.1097/01.mph.0000210061.96075.8e. [DOI] [PubMed] [Google Scholar]
- 46.Feikin D.R., Njenga M.K., Bigogo G., Aura B., Aol G., Audi A., Jagero G., Muluare P.O., Gikunju S., Nderitu L., et al. Etiology and Incidence of viral and bacterial acute respiratory illness among older children and adults in rural western Kenya 2007–2010. PLoS ONE. 2012;7:e43656. doi: 10.1371/journal.pone.0043656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Madhi S.A., Cutland C.L., Downs S., Jones S., van Niekerk N., Simoes E.A.F., Nunes M.C. Burden of Respiratory Syncytial Virus Infection in South African Human Immunodeficiency Virus (HIV)-Infected and HIV-Uninfected Pregnant and Postpartum Women: A Longitudinal Cohort Study. Clin. Infect. Dis. 2018;66:1658–1665. doi: 10.1093/cid/cix1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nyawanda B.O., Otieno N.A., Otieno M.O., Emukule G.O., Bigogo G., Onyango C.O., Lidechi S., Nyaundi J., Langley G.E., Widdowson M.A., et al. The Impact of Maternal Human Immunodeficiency Virus Infection on the Burden of Respiratory Syncytial Virus Among Pregnant Women and Their Infants, Western Kenya. J. Infect. Dis. 2022;225:2097–2105. doi: 10.1093/infdis/jiaa490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tempia S., Walaza S., Viboud C., Cohen A.L., Madhi S.A., Venter M., von Mollendorf C., Moyes J., McAnerney J.M., Cohen C. Deaths associated with respiratory syncytial and influenza viruses among persons ≥5 years of age in HIV-prevalent area South Africa 1998–2009. Emerg. Infect. Dis. 2015;21:600–608. doi: 10.3201/eid2104.141033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moyes J., Tempia S., Walaza S., McMorrow M.L., Treurnicht F., Wolter N., von Gottberg A., Kahn K., Cohen A.L., Dawood H., et al. The burden of RSV-associated illness in children aged < 5 years, South Africa, 2011 to 2016. BMC Med. 2023;21:139. doi: 10.1186/s12916-023-02853-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McMorrow M.L., Tempia S., Walaza S., Treurnicht F.K., Moyes J., Cohen A.L., Pretorius M., Hellferscee O., Wolter N., von Gottberg A., et al. The Impact of Human Immunodeficiency Virus Exposure on Respiratory Syncytial Virus–associated Severe Respiratory Illness in South African Infants, 2011–2016. Clin. Infect. Dis. 2019;69:2208–2211. doi: 10.1093/cid/ciz288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Milito A., Nilsson A., Titanji K., Thorstensson R., Reizenstein E., Narita M., Grutzmeier S., Sönnerborg A., Chiodi F. Mechanisms of hypergammaglobulinemia and impaired antigen-specific humoral immunity in HIV-1 infection. Blood. 2004;103:2180–2186. doi: 10.1182/blood-2003-07-2375. [DOI] [PubMed] [Google Scholar]
- 53.Nakabembe E., Cooper J., Amaral K., Tusubira V., Hsia Y., Abu-Raya B., Sekikubo M., Nakimuli A., Sadarangani M., Le Doare K. The safety and immunogenicity of vaccines administered to pregnant women living with HIV: A systematic review and meta-analysis. eClinicalMedicine. 2024;69:102448. doi: 10.1016/j.eclinm.2024.102448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dauby N., Gagneux-Brunon A., Martin C., Mussi-Pinhata M.M., Goetghebuer T. Maternal immunization in women living with HIV. AIDS. 2023;38:137–144. doi: 10.1097/QAD.0000000000003758. [DOI] [PubMed] [Google Scholar]
- 55.Goetghebuer T., Smolen K.K., Adler C., Das J., McBride T., Smits G., Lecomte S., Haelterman E., Barlow P., Piedra P.A., et al. Initiation of Antiretroviral Therapy Before Pregnancy Reduces the Risk of Infection-related Hospitalization in Human Immunodeficiency Virus–exposed Uninfected Infants Born in a High-income Country. Clin. Infect. Dis. 2018;68:1193–1203. doi: 10.1093/cid/ciy673. [DOI] [PubMed] [Google Scholar]
- 56.Taylor S.A., Sharma S., Remmel C.A.L., Holder B., Jones C.E., Marchant A., Ackerman M.E. HIV-Associated Alterations of the Biophysical Features of Maternal Antibodies Correlate With Their Reduced Transfer Across the Placenta. J. Infect. Dis. 2022;226:1441–1450. doi: 10.1093/infdis/jiac222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alonso S., Vidal M., Ruiz-Olalla G., González R., Manaca M.N., Jairoce C., Vázquez-Santiago M., Balcells R., Vala A., Rupérez M., et al. Reduced Placental Transfer of Antibodies Against a Wide Range of Microbial and Vaccine Antigens in HIV-Infected Women in Mozambique. Front. Immunol. 2021;12:614246. doi: 10.3389/fimmu.2021.614246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Moraes-Pinto M.I., Almeida A.C.M., Kenj G., Filgueiras T.E., Tobias W., Santos A.M.N., Carneiro-Sampaio M.M.S., Farhat C.K., Milligan P.J.M., Johnson P.M., et al. Placental transfer and maternally acquired neonatal IgG immunity in human immunodeficiency virus infection. J. Infect. Dis. 1996;173:1077–1084. doi: 10.1093/infdis/173.5.1077. [DOI] [PubMed] [Google Scholar]
- 59.Jones C.E., Naidoo S., De Beer C., Esser M., Kampmann B., Hesseling A.C. Maternal HIV infection and antibody responses against vaccine-preventable diseases in uninfected infants. JAMA. 2011;305:576–584. doi: 10.1001/jama.2011.100. [DOI] [PubMed] [Google Scholar]
- 60.Nyiro J.U., Bukusi E., Mwaengo D., Nyaguara A., Nyawanda B., Otieno N., Bigogo G., Murunga N., Widdowson M.A., Verani J.R., et al. Efficiency of transplacental transfer of respiratory syncytial virus (RSV) specific antibodies among pregnant women in Kenya. Wellcome Open Res. 2022;7:43. doi: 10.12688/wellcomeopenres.17636.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Atwell J.E., Lutz C.S., Sparrow E.G., Feikin D.R. Biological factors that may impair transplacental transfer of RSV antibodies: Implications for maternal immunization policy and research priorities for low- and middle-income countries. Vaccine. 2022;40:4361–4370. doi: 10.1016/j.vaccine.2022.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Capella C., Chaiwatpongsakorn S., Gorrell E., Risch Z.A., Ye F., Mertz S.E., Johnson S.M., Moore-Clingenpeel M., Ramilo O., Mejias A., et al. Prefusion F, Postfusion F, G Antibodies, and Disease Severity in Infants and Young Children With Acute Respiratory Syncytial Virus Infection. J. Infect. Dis. 2017;216:1398–1406. doi: 10.1093/infdis/jix489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chu H.Y., Tielsch J., Katz J., Magaret A.S., Khatry S., LeClerq S.C., Shrestha L., Kuypers J., Steinhoff M.C., Englund J.A. Transplacental transfer of maternal respiratory syncytial virus (RSV) antibody and protection against RSV disease in infants in rural Nepal. J. Clin. Virol. 2017;95:90–95. doi: 10.1016/j.jcv.2017.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eick A., Karron R., Shaw J., Thumar B., Reid R., Santosham M., O’Brien K.L. The role of neutralizing antibodies in protection of American Indian infants against respiratory syncytial virus disease. Pediatr. Infect. Dis. J. 2008;27:207–212. doi: 10.1097/INF.0b013e31815ac585. [DOI] [PubMed] [Google Scholar]
- 65.Dieussaert I., Hyung Kim J., Luik S., Seidl C., Pu W., Stegmann J.U., Swamy G.K., Webster P., Dormitzer P.R. RSV Prefusion F Protein-Based Maternal Vaccine—Preterm Birth and Other Outcomes. N. Engl. J. Med. 2024;390:1009–1021. doi: 10.1056/NEJMoa2305478. [DOI] [PubMed] [Google Scholar]
- 66.Rasmussen S.A., Jamieson D.J. Maternal RSV Vaccine—Weighing Benefits and Risks. N. Engl. J. Med. 2024;390:1050–1051. doi: 10.1056/NEJMe2401072. [DOI] [PubMed] [Google Scholar]
- 67.Fleming-Dutra K.E., Jones J.M., Roper L.E., Prill M.M., Ortega-Sanchez I.R., Moulia D.L., Wallace M., Godfrey M., Broder K.R., Tepper N.K., et al. Use of the Pfizer Respiratory Syncytial Virus Vaccine During Pregnancy for the Prevention of Respiratory Syncytial Virus–Associated Lower Respiratory Tract Disease in Infants: Recommendations of the Advisory Committee on Immunization Practices—United States, 2023. Mmwr. Morb. Mortal. Wkly. Rep. 2023;72:1115–1122. doi: 10.15585/mmwr.mm7241e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.American Academy of Pediatrics AAP Recommendations for the Prevention of RSV. [(accessed on 10 July 2024)]. Available online: https://publications.aap.org/redbook/resources/25379/AAP-Recommendations-for-the-Prevention-of-RSV?autologincheck=redirected.
- 69.Centers for Disease Control and Prevention RSV Vaccination for Infants and Children. [(accessed on 10 July 2024)]; Available online: https://www.cdc.gov/vaccines/vpd/rsv/hcp/child.html.
- 70.Swiss Federal Office of Public Health (BAG) Consensus Statement: Recommendation on the Prevention of Respiratory Syncytial Virus (RSV) Infections with the Monoclonal Antibody Nirsevimab (Beyfortus®) [(accessed on 10 August 2024)]; Available online: https://www.bag.admin.ch/dam/bag/en/dokumente/mt/i-und-b/richtlinien-empfehlungen/empfehlungen-spezifische-erreger-krankheiten/rsv/rsv-consensus-statement-nirsevimab.pdf.download.pdf/Consensus-statement-recommendation-on-the-prevention-of-respiratory-syncytial-virus-infections-with-the-monoclonal-antibody-Nirsevimab_Aug_2024.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new data were generated.