Abstract
This comprehensive literature review explores the involvement of the gastrointestinal (GI) tract in sarcoidosis, a multisystem granulomatous disorder of unknown etiology. GI sarcoidosis presents a diagnostic and therapeutic challenge due to its rarity and nonspecific clinical manifestations, including overlap with other gastrointestinal diseases. We conducted a comprehensive screening of articles addressing the clinical features, diagnostic approaches, and treatment strategies for GI sarcoidosis. Our findings reveal that GI sarcoidosis can affect any part of the gastrointestinal tract, with the stomach and small intestine being the most involved. Clinical presentations range from asymptomatic cases to severe complications such as obstruction and perforation, with reflux being a common symptom. Diagnosis is often delayed due to the nonspecific nature of symptoms and the need for histopathological confirmation. Therapeutic approaches are poorly defined, typically involving corticosteroids as the mainstay of treatment. However, the long-term efficacy and safety of these treatments remain uncertain in this patient group, given the significant risks and complications associated with prolonged glucocorticoid therapy. There is a clear need to develop accurate diagnostic protocols to distinguish GI sarcoidosis from other conditions and to establish standardized therapeutic guidelines to optimize patient outcomes. Further research is essential to enhance our understanding and management of this complex condition.
Keywords: gastric sarcoidosis, hepatic sarcoidosis, spleen sarcoidosis, colon sarcoidosis, gastrointestinal sarcoidosis treatment, GERD
1. Introduction
Sarcoidosis is a rare, multisystemic granulomatous disease characterized by non-caseating granulomas, most commonly in the lungs and intrathoracic lymph nodes. Gastrointestinal involvement is particularly rare and can occur as part of systemic disease or as an isolated condition. In the literature, articles describing isolated gastric sarcoidosis are very limited, highlighting not only the rarity of the condition but also the challenges in achieving an accurate diagnosis. This scarcity of cases underscores the importance of considering it in differential diagnoses despite its infrequency [1,2,3].
The histopathological hallmark of sarcoidosis, whether it affects the lungs or other organs, is the presence of non-necrotizing, or non-caseating, granulomas, consisting of a tightly packed center surrounded by a looser outer layer. Finally, the whole structure is surrounded by lamellar rings of hyaline collagen with scarce cellularity [1]. Sarcoidosis is a multi-organ disease characterized by the formation of non-caseating granulomas, which are clusters of immune cells that form in response to an unknown trigger. While the exact cause of sarcoidosis remains unclear, it is believed that various factors, such as viruses, pollutants, or allergens, could act as irritant triggers. In most cases, however, the specific triggering substance is not identified. The granuloma formation may involve immune responses to potential infective agents, although this has not been definitively established. The irritant, which is then phagocytized and broken down by dendritic cells and macrophages, is presented to the cluster differentiation (CD)4+ T helper cells, which secrete pro-inflammatory interleukins and recruit macrophages, creating the tightly packed center of the granuloma. Subsequently, B lymphocytes and T lymphocytes aggregate around the center, creating the second, looser layer of the granuloma. Finally, fibroblasts settle on the outside of the granuloma and secrete collagen, creating the outer lamellar rings and forming a fibrous matrix [1,3]. Immunohistochemical markers can help in the diagnosis of sarcoidosis by identifying specific immune cells within granulomas, such as CD68 and CD163 for macrophages and the CD4/CD8 ratio for T lymphocytes. These markers also help exclude other granulomatous diseases like tuberculosis and fungal infections. However, they are not specific to sarcoidosis and should be interpreted in conjunction with clinical and radiological assessments [4].
Diagnosis of sarcoidosis is often difficult, as symptoms widely vary depending on the organs involved and the severity of the disease. In 2020, the American Thoracic Society (ATS) released its first-ever statement regarding the diagnosis of pulmonary sarcoidosis, basing it on three criteria: (1) a compatible clinical and radiological manifestation, (2) histological evidence of non-caseating granulomas and (3) the exclusion of any alternative cause [5]. The thoracic radiological manifestation is based on the Scadding scale, which divides sarcoidosis into four classes according to thoracic radiological presentation. These classes are not related to disease severity and progression and only indicate organ involvement within the thorax; although originally used with simple chest radiography, it can be adapted to the more commonly used computerized tomography scan (CT scan) [6].
These guidelines, however, are only viable for diagnosing pulmonary or mediastinal sarcoidosis, making diagnosing any extrathoracic manifestations of the disease less uniform and much more dependent on the individual physician’s experience examining the patient. Consequently, a tool for assessing organ involvement in sarcoidosis, named ACCESS, or A Case Control Etiologic Study of Sarcoidosis, was first suggested in 1999 [6]. Based first and foremost on bioptic findings in 736 enrolled patients, the tool defines the probability of multi-organ involvement in patients with a single tissue finding of sarcoidosis via biopsy. It covers most to least frequently involved organs and systems and relies both on the physician’s pursuit of an accurate diagnosis and routine laboratory and instrumental follow-up such as radiographic imaging, respiratory function tests (RFTs), hemochrome and blood serum analysis, and other organ-specific tests to help determine involvement rate. Results suggested that organ involvement can be classified into three main groups: (1) likely involved (e.g., lungs, skin, liver, eyes, or calcium metabolism), (2) unusual but clinically significant (nervous system, heart, and kidneys), (3) and other sites (e.g., spleen, bones, bone marrow, muscles, upper airways, ears, salivary glands, and extrathoracic lymph nodes) [6,7].
This approach, however, only offered a preliminary assessment and did not allow for a more minute characterization of the condition the involved organ or system were in. In 2007, a method called STAI (sarcoidosis three-dimensional assessment instrument) was introduced for a more accurate and revised vision of the specific organ. The method was based on three axes: organ involvement, disease severity, and disease activity [8].
The first axis in the STAI method expands upon the findings of ACCESS, adding another category dubbed “other organs”, which were not included previously, as well as accounting for alternative diagnostic methods outside of biopsies, such as liver involvement being defined by a threefold elevation of hepatic enzymes, compatible Computerised Tomography (CT) scan, and elevated alkaline phosphatase [8]. The second axis allows for assessing the severity of sarcoidosis, categorizing results into one of four classes I-IV with growing severity of limitation to function. The third axis assesses the disease activity as part of an effort to minimize unnecessary steroid treatment [8].
The first axis specifically offers an important update missing from the ACCESS approach. As mentioned, sarcoidosis is a systemic condition with visible effects beyond just the fifteen organs originally examined. The GI system outside of the liver and spleen is rarely involved, and unfortunately, the STAI system does not offer any benefits for their differential diagnosis over the ACCESS one. Such cases of GI sarcoidosis that do not involve the liver or spleen have been documented in the past mostly as sparse case reports and never explicitly cataloged in a single comprehensive review of the literature [9]. Moreover, the STAI method has not yet been validated for sarcoidosis assessment and diagnosis.
2. Material and Methods
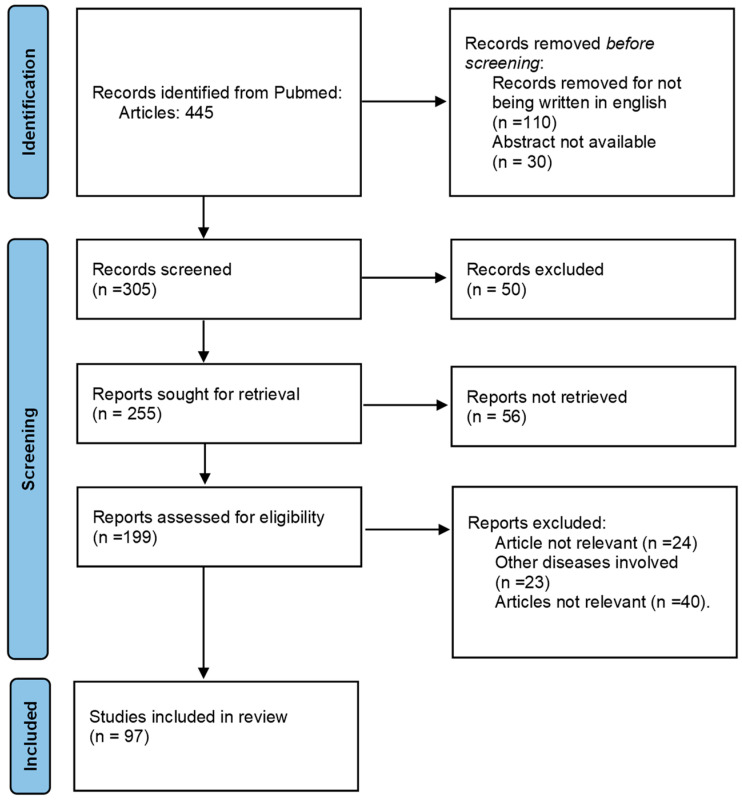
A comprehensive literature review was conducted to investigate sarcoidosis involvement of the whole GI tract. Articles included in PubMed and Google Scholar were searched using the keywords “gastric sarcoidosis”, “digestive tract sarcoidosis”, “stomach sarcoidosis”, and “gastric sarcoid granulomas”. For liver, spleen, and pancreatic sarcoidosis, PubMed was searched for articles published from January 1967 to May 2024 using the terms “hepatic sarcoidosis”, “hepatic sarcoidosis involvement”, “hepatic sarcoidosis treatment”, “pancreatic sarcoidosis”, “pancreatic sarcoidosis treatment”, “spleen sarcoidosis”, and “spleen sarcoidosis treatment”, excluding non-English and irrelevant articles. For bowel involvement, PubMed was searched from January 2000 to May 2024 using “colon sarcoidosis”, “large intestine sarcoidosis”, “colon sarcoidosis involvement”, “large intestine sarcoidosis involvement”, “colon sarcoidosis treatment”, “gastrointestinal sarcoidosis”, “gastrointestinal tract”, and “large intestine sarcoidosis treatment”, excluding non-English articles. Inclusion criteria were publications from 1950 to 2024, adult human subjects, and English language, while abstracts without full-text articles and conference abstracts were excluded. Our research yielded 445 articles, with 97 deemed to be most relevant for our review article (Figure 1).
Figure 1.
Flow chart of the diagnosis and treatment of sarcoidosis involving the gastrointestinal tract articles.
3. Sarcoidosis Involvement of the Gastrointestinal Tract
This comprehensive literature review explores gastrointestinal (GI) involvement in sarcoidosis, a multisystem granulomatous disorder. Despite its rarity, GI sarcoidosis presents significant diagnostic and therapeutic challenges due to nonspecific symptoms and overlap with other GI diseases. The GI system is among the rarest, if not the rarest, to be affected by sarcoidosis [10,11]. Manifestations of GI sarcoidosis are heterogeneous, though many manifest in the form of pain and do not affect the GI tract uniformly. Nearly 80% of cases affect the upper GI tract (oropharynx, esophagus, stomach, and duodenum) [11]. Oral cavity sarcoidosis often manifests in swollen gingiva, submucosal nodules, mucosal lesions, and tooth mobility due to bone erosion [12]. Pharyngeal sarcoidosis is frequently asymptomatic but may manifest with a progressive loss of function, such as dysphonia and dysphagia [13]. Esophageal involvement is characterized by dysphagia but may also manifest symptoms stemming from extrinsic compression of nerves, such as neuropathy [14]. Gastric sarcoidosis often presents itself with vague and subtle symptoms that may coincide with that of duodenal sarcoidosis and are, for the most part, a result of subcontinuous mucosal lesions that reduce nutrient absorption capacity and disrupt the physiologic gastric motility [10,11,12,13,14,15]. Small intestinal sarcoidosis may present as an intestinal obstruction, causing stenosis or blockage of the lumen [16]. Large intestinal sarcoidosis does not often cause intestinal blockage but does, similarly to the duodenal and gastric ones, cause weight loss, bowel pain, diarrhea, and constipation [17]. Finally, rectal sarcoidosis may manifest as a rectal mass in affected patients [17,18].
Sarcoidosis manifestations vary depending on the organ or system involved, but treatment is, first and foremost, that of watchful waiting [19,20,21,22,23,24,25,26,27,28,29]. According to the most recent ATS guidelines, the watchful waiting approach is to be preferred over oral steroid-based therapy in case of asymptomatic patients or patients with mild symptoms that do not affect the quality of life. On the contrary, when symptoms become severe or in cases of disease progression, long-term oral steroid-based treatment is the first choice of treatment, and steroid-sparing agents and biological drugs are the second and third lines of treatment, respectively [7,8,9,10,11,12,13,14,15,16,17,18,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38].
3.1. Gastric Sarcoidosis Involvement
GI involvement in sarcoidosis is rare, occurring in 0.1% to 3.4% of all cases, with symptomatic GI sarcoidosis being even less common, affecting less than 1% of patients. Macroscopic lesions can develop in any part of the digestive tract, with the stomach being the most frequently involved site, seen in approximately 10% of those with gastrointestinal sarcoidosis [19]. Gastric sarcoidosis, whether isolated or as part of systemic sarcoidosis, represents a challenging diagnosis due to its rarity and nonspecific symptoms (Table 1 and Table A1 in Appendix A) [39,40,41,42,43,44,45,46,47,48,49,50,51]. The various symptoms reflect the disease’s patchy mucosal involvement and subtle nature. Thus, the diagnosis requires a combination of high clinical suspicion, imaging, and histopathological confirmation. The association between gastroesophageal reflux disease (GERD) and sarcoidosis likely stems from the inflammatory nature of sarcoidosis, which can cause esophageal motility disorders due to nerve involvement and granuloma formation [14,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73]. Diagnosis is often delayed due to the need for histopathological confirmation. While corticosteroids are the mainstay of treatment, their long-term efficacy and safety relative to their long-term important adverse effects remain uncertain (and presumably may exacerbate GERD symptoms), and steroid-sparing agents (e.g., methotrexate, azathioprine, antimalarials) and ursodeoxycholic acid have shown some success. Effective management of GERD through lifestyle modifications, medications, or surgery is also crucial. This review underscores the need for accurate diagnostic protocols and standardized therapeutic guidelines to optimize patient outcomes, highlighting the importance of further research in this area [14,15,16,17,18,19,20,21,22,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89].
Table 1.
Most common symptoms associated with gastric sarcoidosis.
Patients with known systemic sarcoidosis, particularly those with extrapulmonary manifestations, are more likely to develop GI involvement. Gastric granulomas have been reported in up to 10% of patients with pulmonary sarcoidosis [6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104]. Endoscopy is the primary tool for visualizing gastric mucosal abnormalities and obtaining biopsy samples, avoiding delays in diagnosis and treatment. Biopsy and histopathology remain the gold standard for diagnosis, revealing non-caseating granulomas that contain multinucleate giant cells in the absence of crypt abscesses on histology. However, the patchy distribution of these granulomas can lead to false negatives, especially if superficial biopsies are performed. In association with endoscopy, F-FDG-PET/CT may be helpful in patients with sarcoidosis for determining the intrathoracic and extrathoracic extensity of disease, detecting active disease, and accessing the response to treatment, particularly in atypical, complex, and multisystem forms of sarcoidosis. Gallium-67 scan requires multiple visits for assessment and is less accurate, involving increased radiation exposure (~15 mSv) and necessitating imaging up to 48 h after injection, making it a prolonged procedure [24,25,105,106,107,108,109,110,111,112,113,114].
In developed countries, sarcoidosis is the second most common cause of granulomatous gastritis. The differential diagnosis involves ruling out other granulomatous diseases like Crohn’s disease, Whipple’s disease, infections (e.g., tuberculosis, fungal infections, H. pylori), functional dyspepsia, vasculitis, Langerhans cell histiocytosis and malignancies (lymphomas, hematological malignancies, and gastric adenocarcinoma). Histiocytic markers such as CD68 are usually strongly reactive in sarcoid granulomas. Pan-cytokeratin staining, which is seen in malignancy, should be negative. Staining for infectious agents can aid in this exclusion. Although not always pathognomonic, immunohistochemical markers can be useful. A rare association between sarcoidosis and Crohn’s disease is reported, and additional targeted therapy against tumor necrosis factor-α and integrins is associated with the induction of sarcoidosis when used to treat inflammatory bowel disease [26,27].
Propionibacterium acnes has been associated with granuloma formation, with 92% positive staining using the PAB antibody reported in Japanese cases of sarcoidosis. Inomata et al. also confirmed this association in the gastric lesion. In a study of five cases of gastric sarcoidosis, Helicobacter pylori infection was associated with 40% of cases [113].
The primary treatment for gastric sarcoidosis, like systemic sarcoidosis, involves the use of corticosteroids to reduce inflammation and granuloma formation (to manage active disease and symptoms). In cases where corticosteroids are not effective or cause significant side effects, alternative immunosuppressive agents like methotrexate or azathioprine may be considered [27]. Proton pump inhibitors (PPIs) or Histamine2 (H2) blockers may be used to manage gastric symptoms, such as acid reflux and dyspepsia. Nutritional support is vital, especially in patients experiencing significant weight loss. The prognosis for isolated gastric sarcoidosis is generally good with appropriate treatment. Longitudinal studies have shown that most asymptomatic patients with gastric sarcoidosis typically do not develop GI symptoms over time or exhibit changes in biopsy patterns. However, GI involvement, though rare, is possible in patients with pulmonary and extrapulmonary sarcoidosis. These patients remain susceptible to disease progression even while on high doses of immunosuppressive therapy and may require more prolonged and intensive management. Therefore, patients with sarcoidosis and persistent gastrointestinal symptoms should be evaluated with endoscopy and biopsy, as described in the work of Hassan et al. [23].
The prognosis for isolated gastric sarcoidosis is generally good with appropriate treatment. Longitudinal studies have shown that most of the asymptomatic patients with gastric sarcoidosis typically do not develop GI symptoms over time, nor do they exhibit changes in biopsy patterns; in contrast, patients with pulmonary and extrapulmonary sarcoidosis remain susceptible to disease progression, even when on high dose immunosuppressive therapy, and may require more prolonged and intensive management [32].
3.2. Small Bowel Sarcoidosis Involvement
GI sarcoidosis, particularly in the small bowel, is rare (less than 10 cases reported in the extant literature) but a clinically significant manifestation of sarcoidosis. Patients with small bowel sarcoidosis often present in their fifth or sixth decade of life and usually have multisystem sarcoidosis. Imaging modalities such as computed tomography (CT) are essential for patients presenting with abdominal pain with known sarcoidosis. Endoscopic examination is the preferred initial investigative approach for patients with diarrhea. Diagnosis relies heavily on histopathological confirmation and exclusion of other granulomatous diseases such as tuberculosis, characterized by the absence of necrosis [1], or Whipple’s disease, which may be identified via the periodic acid-Schiff (PAS) coloration [33]. Histological findings may not always be clear-cut, however. Crohn’s disease may present itself with either diffuse nonspecific inflammation, diffuse granulomatous inflammation or focal granulomas, which are present in nearly 50% of patients and are virtually indistinguishable from the sarcoid granuloma [34]. In such cases, differential diagnosis relies on other criteria such as the age of onset or involvement of organs outside the gastrointestinal tract, as well as serological markers such as the presence of anti-neutrophil cytoplasm antibodies (ANCAs) or anti saccharomyces cerevisiae antibodies (ASCAs) [35].
These cases underscore the importance of considering sarcoidosis in the differential diagnoses of patients with sarcoidosis for acute abdominal presentations and the necessity of timely surgical intervention to prevent complications such as perforation or life-threatening hemorrhagic ascites. Treatment is tailored based on symptom severity, with corticosteroids being the cornerstone of therapy [36,37,38,39].
3.3. Hepatic Sarcoidosis
Hepatic sarcoidosis is characterized by non-caseating granulomas in the liver, biliary tract, and gallbladder. There are no formalized diagnostic criteria for hepatic sarcoidosis; diagnosis typically involves a clinical history of systemic sarcoidosis and liver biopsy evidence of non-caseating granulomas. The prevalence of hepatic involvement in patients with systemic sarcoidosis varies widely between studies, ranging from 3.6 to 30% [40,41,42,43,44]. However, autopsy studies have demonstrated a higher prevalence of disease (up to 70%), highlighting that hepatic involvement is a frequent, yet often under-recognized, complication of systemic sarcoidosis [45]. While many patients are asymptomatic despite granulomas on biopsy, abnormal liver enzymes, or radiological findings [41], about 15% experience hepatomegaly, right upper quadrant pain, and systemic symptoms (e.g., fatigue, fever, and arthralgias), which are nonspecific but are present in most patients with active liver sarcoidosis [46]. Liver function test abnormalities primarily show a cholestatic pattern [47]. Hepatic sarcoidosis manifesting solely as biliary sarcoidosis with cholestasis symptoms is documented in a few case reports. In these instances, ERCP with brush cytology was necessary when cholangiocarcinoma was highly suspected [44,45,46,47,48,49,50,51,52]. Despite hepatic sarcoidosis being generally a chronic disease with a benign course, a minority of patients may develop chronic liver disease that advances to portal hypertension associated with risks of variceal bleeding, progression to end-stage cirrhosis, and increased incidence of hepatocellular carcinoma (HCC) [43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58]. Active treatment is not indicated for all patients with hepatic sarcoidosis. Observation is appropriate for those with asymptomatic liver disease, mild elevations in serum liver enzymes, and normal liver function without evidence of cholestasis [59]. An interesting aspect to consider is the connection between sarcoidosis and malignant tumors. As described in the work of Cohen et al. [60], three types of associations exist, the first of which is the association between sarcoidosis and liquid tumors such as lymphomas and bears little relevance to the gastrointestinal system, while the other two pose a direct connection. The second type of association consists of patients with sarcoidosis developing subsequent malignant tumors or vice versa, among which hepatocellular carcinoma is one of the most noted examples [61,62]. The third type of association is not considered a full-blown sarcoidosis in and of itself but rather a sarcoid-like reaction in the case of malignant tumors, in which non-necrotizing granulomas develop and are confined to the regional lymph nodes. Such a reaction, however, lacks the ‘systemic’ aspect of sarcoidosis as it is confined to the regional lymph nodes and thus can only be considered a sarcoid-like reaction [60,61,62,63].
In symptomatic patients, the treatment goal for hepatic sarcoidosis is to control symptoms and prevent progression to cirrhosis, portal hypertension, and liver transplant. However, responses to conventional therapies are variable, and no standardized protocols exist. In our review of the literature, we found three articles (shown in Table 2) that are focused on the treatment of hepatic sarcoidosis outcomes. Corticosteroids, often combined with ursodeoxycholic acid (UDCA) for cholestasis, are the first line of therapy; they reduce hepatic granulomas and alleviate symptoms like fever, fatigue, pruritus, and weight loss, but their long-term benefits are unclear [46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64]. UDCA helps reduce cholestasis symptoms and potentially delays disease progression, benefiting patients unresponsive to or dependent on steroids [41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72]. When patients require a step up in therapy, azathioprine, methotrexate, cyclophosphamide, and infliximab have shown some benefits, but the extant literature lacks strong evidence. Studies indicate varying effectiveness, with some patients responding with changes in the cholestatic enzyme levels and others not. Sedki et al. [42] identified that antimetabolites elicited a statistically significant change in ALP in individuals with preexisting ALP elevation (Table 2). In contrast, patients receiving oral glucocorticoids or biologic agents showed a decrease in their ALP level, which was not statistically significant, and only two patients received orthotopic liver transplants due to complications of hepatic sarcoidosis. Kennedy et al. [41] approximately one-third of the patients had a complete clinical response, one-third had a partial response, and one-third showed no response. Graf et al. [40] found that 69.4% of patients were treated with glucocorticoids and 40.3% with ursodeoxycholic acid (UDCA). ALP levels decreased by 60.8% with glucocorticoids and 59.9% with UDCA. Few patients needed a second-line agent, with eight achieving normalized ALP levels during follow-up. These studies show that corticosteroids, antimetabolites, and immunosuppressants are important in controlling disease. Hepatic sarcoidosis is a rare indication for orthotopic liver transplantation. There are only a few case reports and small series evaluating the outcomes of these recipients. In our review, we found that liver transplantation provides satisfactory long-term patient and graft survival for patients with liver failure due to hepatic sarcoidosis. The incidence of disease recurrence in the liver is not known. However, it appears to have a minimal impact on the long-term outcomes for both patients and grafts [45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75].
Table 2.
Studies showing the major outcomes for hepatic sarcoidosis after drug therapy and number of liver transplantation.
| Reference | Population | Drug Therapy | Outcomes | Liver Transplant |
|---|---|---|---|---|
| Graf et al. [40] | n = 62 (f = 51.6%, m = 80.6%) |
Glucocorticoids (n = 43) Ursodeoxycholic acid (n = 25) MTX (n = 9) MMF (n = 1) CMF (n = 2) Infliximab (n = 1) |
Patients who needed a second-line immunosuppressive (n = 17) Deaths for liver-related complications (n = 3) |
n = 0 |
| Kennedy et al. [41] | n = 180 (f = 89, m = 91) |
Glucocorticoids, MTX, MMF or CMF Infliximab |
Patients who received a second-line immunosuppressive (n = 16) | n = 6 |
| Sedki et al. [42] | n = 286 (f = 223, m = 63 |
Glucocorticoids (n = 17) MTX, MMF or CMF (n = 15) Infliximab (n = 5) |
Patients who responded well to treatment with normalization of liver biochemistries (n = 18) Deaths for liver-related complications (n = 0) |
n = 2 |
f = females. m = males, MMF = mycophenolate, CMF = cyclophosphamide, MTX = methotrexate.
3.4. Splenic Sarcoidosis
Splenic sarcoidosis is characterized by granulomatous involvement of the spleen, observed in 40–80% of systemic sarcoidosis cases, although symptomatic manifestation is infrequent, occurring in less than 5% of cases [76,77,78,79,80,81,82]. Symptomatic presentations include splenomegaly, which may be associated with cytopenia (such as anemia, leukopenia, thrombocytopenia, or pancytopenia), spleen infarction, and abdominal pain localized to the left upper quadrant. The diagnosis is based on clinical evaluation and radiologic imaging, typically ultrasound or computed tomography (CT) scans. Therapeutic intervention with corticosteroids is indicated primarily in instances of hypersplenism or significant splenomegaly. In rare cases, where there is an insufficient response to pharmacological management or the presence of severe complications, splenectomy may be warranted [82,83,84,85].
3.5. Pancreatic Sarcoidosis
Pancreatic involvement in systemic sarcoidosis is rare, with autopsy studies showing a prevalence of 1–5% and even lower rates in clinical series. Pancreatic sarcoidosis can present itself with nodular abnormalities or as a mass [46,85,86,87].
Since their initial description in 1950 in the study of Curran et al. [88], only 25 cases of symptomatic sarcoidosis presenting as a pancreatic mass have been documented. Symptoms of pancreatic sarcoidosis, resulting from tissue infiltration or bile duct obstruction, often resemble those of pancreatitis or pancreatic cancer. Non-surgical biopsy methods, such as CT or endoscopic ultrasound, have proven unreliable for diagnosing pancreatic sarcoidosis. A definitive preoperative diagnosis of sarcoidosis with biopsy is crucial for avoiding unnecessary pancreatic surgery [85,86,87,88,89,90,91].
3.6. Large Intestinal Sarcoidosis
Large intestinal sarcoidosis is often nonspecific in its presentation, with weight loss, diarrhea, constipation, and abdominal pain being the most encountered symptoms [92]. In one case, a patient’s large intestinal sarcoidosis presented as an endoluminal mass. Diagnosis, as per the ACCESS system, requires a compatible biopsy presenting non-caseating granulomas and exclusion of any other similar possible causes of the disease [93]. In five of the articles, sarcoidosis was either considered to be a tumor initially or was concomitant to one, usually large intestine adenocarcinoma. A possible novel approach to differential diagnosis between adenocarcinoma of the large intestine and sarcoidosis might lie in a comparison between Fluorodeoxyglucose-18 (FDG-18) and 68Galluim-citrate Positron Emission Tomography-Computerized Tomography (PET-TC), seeing how the two entities present discrepancies when examining uptake rate, with sarcoidosis resulting positive exclusively in the FDG-18 scan. In several cases, the localization of the disease activity was not limited to the large intestine, which existed in other districts as well, with cutaneous sarcoidosis being the most frequently associated [94,95].
Large intestine involvement in sarcoidosis remains an extremely rare occurrence, and as such, it is difficult to estimate its prevalence within society and the population of patients affected by it. Treatment of large intestinal sarcoidosis remains like that of most other organs involved, with high-dose oral steroids being the first line of treatment, methotrexate second, and finally, monoclonal antibodies such as adalimumab third [9]. The initial approach is conservative, with surgical intervention being reserved for refractory cases, like the indication for organ transplant in pulmonary sarcoidosis in the most recent ERS and ATS guidelines [96]. In certain instances, dedicated, supportive treatment is required, such as in cases of excessive bleeding and consequent anemia, where blood transfusions may be considered [97].
4. Discussion
GI involvement is rare, typically asymptomatic, and reported in only 0.1–0.9% of sarcoidosis cases. When present, GI sarcoidosis can significantly impact patient health, particularly due to diagnostic challenges. Reflux is a frequent manifestation, often accompanied by symptoms such as epigastric pain, nausea, vomiting, and weight loss. The stomach is the most affected GI organ, noted in approximately 10% of cases. Gastric sarcoidosis primarily presents as either gastric ulcer formation due to localized mucosal infiltration or diffuse granulomatous infiltration leading to reduced lumen size secondary to fibrosis [3,98,99].
Diagnosis is complicated by the similarity of GI sarcoidosis symptoms to other GI conditions, including irritable bowel syndrome (IBS), GERD, gastroparesis, and gastric cancer. This reinforces the need to consider an accurate differential diagnosis to establish appropriate treatment [10,50,98,99,100]. The prognosis for patients with gastric sarcoidosis varies, with complications such as strictures, fistulas, or chronic malabsorption potentially occurring, especially if the disease is not adequately controlled. Additionally, cases have been reported in which patients with gastric sarcoid developed mucosa-associated lymphoid tissue lymphoma, hematological malignancies, and gastric adenocarcinoma, all of which negatively impact the patient’s prognosis [3].
Hepatic sarcoidosis, a more common extrathoracic manifestation, is often asymptomatic but can present with signs of cholestasis. Due to the lack of specific serological markers, diagnosis relies upon a high index of clinical suspicion, physical examination, laboratory abnormalities, and histological confirmation. In rare cases involving the small bowel and large intestine, histological diagnosis is essential to exclude more common diseases. Increased clinical awareness and suspicion of hepatic sarcoidosis are crucial for the timely diagnosis and introduction of effective treatment [17,40,41,42,43,94,101,102,103].
The monitoring of GI sarcoidosis is achieved clinically and radiographically, but there is no evidence of the role of serum angiotensin-converting enzymes or serum interleukin-2 receptors in monitoring. Asymptomatic sarcoidosis does not require treatment. For symptomatic patients, glucocorticoids (GCs) can reduce liver and spleen size, decrease granulomas, and improve organ function to some extent. However, their effect on disease progression and complications like portal hypertension or hepatic fibrosis is limited. Steroid-sparing agents, defined previously as ursodeoxycholic acid, have shown some success. Ursodeoxycholic acid is particularly used for cholestatic jaundice. Despite preferences for AZA over MTX due to hepatotoxicity concerns, both drugs pose known risks [44,45,46,47,48,49,50,51,104,105]. In end-stage liver disease, orthotopic liver transplantation has been successfully reported, with recurrence rates in the allograft comparable to other diseases [43,67,68,69,70,71,72,73]. Splenectomy is considered only in rare cases of huge splenomegaly, severe hypersplenism, suspected malignancy, or to prevent splenic rupture when medical treatment fails.
Today, developing new therapeutic and diagnostic protocols for GI sarcoidosis is necessary to improve patient outcomes and address the complexities of this rare but impactful disease manifestation.
5. Conclusions
Patients with known systemic sarcoidosis, particularly those with extrapulmonary manifestations, are more likely to develop GI involvement. Despite this, GI involvement in sarcoidosis remains an under-recognized complication with significant implications for patient morbidity and mortality. To date, there is a paucity of dedicated clinical trials that establish the efficacy of anti-inflammatory agents in this context. Future research endeavors must develop diagnostic (including novel biomarkers) and therapeutic protocols aimed at early detection and managing the disease burden and activity, thereby mitigating its progression.
Appendix A
Table A1.
Case reports describing symptoms, treatment, and outcomes of gastric sarcoidosis.
| Case | Symptoms | Diagnosis | Treatment | Outcomes |
|---|---|---|---|---|
| El Sharu H. et al. [106] | Abdominal pain, nausea, vomiting, diarrhea | CT of abdomen and pelvis, MR angiography, EUS, chest CT, head CT, head MRI | Prednisone 40 mg | Symptomatic improvement, persistence of symptoms following prednisone tapering |
| Tao J et al. [107] | Chest pain, shortness of breath, abdominal cramping | Chest CT, PET, left thoracotomy, EGD, colonoscopy | Prednisone | Complete resolution of symptoms in five months |
| Hassan D. et al. [23] | Abdominal pain, nausea, vomiting, weight loss | CT of abdomen and pelvis, barium swallow series, EGDS | Adalimumab and methotrexate | Continued hospital and clinic presentations with nausea, vomiting, and abdominal pain |
| Basida B et al. [108] | Abdominal pain | Chest X-ray, CT abdomen, CT-guided pigtail catheter placement, EGD, colonoscopy | Followed by outpatient lung biopsy | - |
| Gala K et al. [32] | Dyspepsia, nausea | EGDS | Pantoprazole 40 mg | Slight improvement |
| Sameh S et al. [26] | Abdominal pain | EGDS, chest CT, bronchoscopy | Corticosteroids | Favorable clinical course |
| Mota C et al. [109] | Abdominal pain, vomiting, abdominal distension, asthenia, anorexia | Body CT scan, EGD, colonoscopy, bronchoscopy, laparoscopy | Noteworthy corticosteroid therapy in intensive care unit | Regression of lesions, pleural effusion, ascites, no distension of intestinal loops |
| Deepa A et al. [110] | Dysphagia, nausea, vomiting | CT abdomen, CT chest, EGDS, bronchoscopy | Steroids for about 6 months | Regression of lymph nodes and decreased GI symptoms, relapse after 5 months of treatment interruption |
| Stemboroski L et al. [27] | Chronic diarrhea and abdominal pain | CT abdomen and pelvis, EGDS, colonoscopy | Prednisone 40 mg daily | Resolution of diarrhea and abdominal pain within 3 weeks |
| Kariyanna PT et al. [31] | Epigastric pain, dysphagia, vomiting, weight loss | Chest X-ray, EGDS | Prednisone 50 mg daily, omeprazole 20 mg, cosyntropin 80 UI (once every 3 days) | Improvement of symptoms |
| Ceylan E et al. [25] | Chest tightness, inability to take a deep breath | Chest and abdomen CT, PET/CT, EGDS | Partial pleural decortication, wedge resection for lung nodule, pantoprazole 40 mg, metoclopramide hydrochloride 10 mg, sucralfate 2 g | Occasional GI symptoms in 1 year of FU. Persistence of non-caseating epithelial antrum granulomas with normal EGDS |
| Tokala H et al. [111] | Epigastric pain, nausea, vomiting, weight loss | CT abdomen and pelvis, EGDS | Prednisone 60 mg | Alleviation of symptoms within four days, no recurrence of symptoms in 2 years after corticosteroid tapering |
| Shkolnik LE et al. [112] | Abdominal pain, vomiting | Chest radiograph, CT abdomen and pelvis, hepatobiliary scan, abdomen MRI, EGDS | 6-month course of systemic corticosteroid | Complete resolution of symptoms |
| Case 1. (Inomata M et al.) [113] | Epigastric discomfort, weight loss | Chest X-ray, CT, MRCP, EGDS, liver biopsy, barium scintigraphy | Prednisolone 30 mg | Improved symptoms and laboratory values, died 11 months later from MOF and DIC due to infected AAA |
| Case 2. (Inomata M et al.) [113] | None | Chest X-ray, CT, EGDS, barium scintigraphy | Conservative | No symptoms for 5 years after, follow-up EGDS 1 year later showed no remarkable changes |
| Case 3. (Inomata M et al.) [113] | - | Chest X-ray, CT scan, EGDS, barium scintigraphy | PPIs | Slight epigastralgia continued for two and a half years, follow-up EGDS 1 year later revealed a scar on the greater curvature |
| Chaudhary P et al. [114] | Generalized fatigue, early satiety, weight loss | Bone marrow biopsy, CT abdomen, EGDS | Prednisone, B12, and iron supplementation | Gained weight, normalization of neutrophil and white blood cell count |
Table A2.
Case reports describing symptoms, treatment, and outcomes for small bowel sarcoidosis.
| Case | Other Disorders | Symptoms | Diagnosis | Treatment | Outcome |
|---|---|---|---|---|---|
| Paone G et al. [39] | - | Nausea, vomiting, abdominal pain, constipation, fever | Abdominal X-ray, CT of abdomen and pelvis, Total body CT | Laparotomy, Prednisone 25 mg/daily (slowly tapered) | Complete response to therapy in 3 months |
| Pendela VS et al. [38] | Kidney stones | Acute right iliac fossa pain, vomiting, chronic low back pain | Abdominal ultrasound, CT scan of the abdomen, MRI of the spine, 18 FDG PET, Bronchoscopic ultrasound-guided biopsy | Laparoscopic appendectomy: steroid 30 mg daily (slowly tapered), added mycophenolate mofetil after six months | Pain resolved postoperatively, and decrease in the size of the vertebral lesions |
| Zakaria A et al. [37] | Inactive pulmonary sarcoidosis | Nausea, vomiting, abdominal pain, constipation | Abdominal X-ray, CT scan of the abdomen and pelvis, Gastrografin small-bowel follow-through, Diagnostic laparoscopy | Conservative treatment (bowel rest, nasogastric tube, IV fluids) followed by prednisone taper dose | Good response after corticosteroid therapy |
| Esmadi M et al. [36] | Diabetes, previous Potus, and mediastinal lymphadenopathy, Liver biopsy | Abdominal pain, severe peripheral arthralgias, chronic diarrhea | X-ray and CT abdomen and pelvis, Abdominal ultrasound, Bone marrow biopsy, Colonoscopy | Prednisone | Improvement in his symptoms |
Author Contributions
Conceptualization, B.R., P.C., S.N., M.C. (Maria Chernovsky), D.A. and Z.M.; methodology, B.R., M.M., F.S., L.M., M.B., D.A., G.S., M.C. (Marco Confalonieri) and E.B.; writing—original draft preparation, B.R., M.H., G.B. and Z.M.; writing—review and editing, B.R., M.H., G.B. and Z.M.; supervision, B.R. and M.C. (Marco Confalonieri). All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Soler P., Basset F., Bernaudin J.F., Chretien J. Morphology and distribution of the cells of a sarcoid granuloma: Ultrastructural study of serial sections. Ann. N. Y Acad. Sci. 1976;278:147–160. doi: 10.1111/j.1749-6632.1976.tb47026.x. [DOI] [PubMed] [Google Scholar]
- 2.Polverino F., Balestro E., Spagnolo P. Clinical Medicine Clinical Presentations, Pathogenesis, and Therapy of Sarcoidosis: State of the Art. J. Clin. Med. 2020;9:2363. doi: 10.3390/jcm9082363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afshar K., BoydKing A., Sharma O.P., Shigemitsu H. Gastric sarcoidosis and review of the literature. J. Natl. Med. Assoc. 2010;102:419–422. doi: 10.1016/S0027-9684(15)30577-0. [DOI] [PubMed] [Google Scholar]
- 4.Ahmadzai H., Thomas P.S., Wakefield D., Ahmadzai H., Thomas P.S., Wakefield D. Sarcoidosis. IntechOpen; Rijeka, Croatia: 2013. Laboratory Investigations and Immunological Testing in Sarcoidosis. [DOI] [Google Scholar]
- 5.Knipe H., Maller V. Thoracic sarcoidosis (staging) Radiopaedia. 2008 doi: 10.53347/RID-4997. [DOI] [Google Scholar]
- 6.Freemer M., King J. The ACCESS study: Characterization of sarcoidosis in the United States. Am. J. Respir. Crit. Care Med. 2001;164:1754–1755. doi: 10.1164/ajrccm.164.10.2109111b. [DOI] [PubMed] [Google Scholar]
- 7.Newman L.S., Rose C.S., Bresnitz E.A., Rossman M.D., Barnard J., Frederick M., Terrin M.L., Weinberger S.E., Moller D.R., McLennan G., et al. A case control etiologic study of sarcoidosis: Environmental and occupational risk factors. Am. J. Respir. Crit. Care Med. 2004;170:1324–1330. doi: 10.1164/rccm.200402-249OC. [DOI] [PubMed] [Google Scholar]
- 8.Judson M.A. A proposed solution to the clinical assessment of sarcoidosis: The sarcoidosis three-dimensional assessment instrument (STAI) Med. Hypotheses. 2007;68:1080–1087. doi: 10.1016/j.mehy.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 9.Rao D.A., Dellaripa P.F. Extrapulmonary Manifestations of Sarcoidosis. Rheum. Dis. Clin. N. Am. 2013;39:277. doi: 10.1016/j.rdc.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brito-Zerón P., Bari K., Baughman R.P., Ramos-Casals M. Sarcoidosis Involving the Gastrointestinal Tract: Diagnostic and Therapeutic Management. Am. J. Gastroenterol. 2019;114:1238–1247. doi: 10.14309/ajg.0000000000000171. [DOI] [PubMed] [Google Scholar]
- 11.Albaba I., Feustel P.J., Kenneth M.F., Judson M.A. Rare organ manifestations of sarcoidosis. Respir. Med. 2022;201:106945. doi: 10.1016/j.rmed.2022.106945. [DOI] [PubMed] [Google Scholar]
- 12.Borges G.S.V., Ferreira G.T., Henrique P.R., de Araujo M.S., Silva Servato J.P. Upper Lip Nodule as the First Manifestation of Sarcoidosis: A Case Report. J. Ski. Stem Cell. 2023;10:139072. doi: 10.5812/jssc-139072. [DOI] [Google Scholar]
- 13.Hilal F., Mahdi E., Nada A. Atypical and uncommon presentation of sarcoidosis with long segment involvement of the pharynx and larynx: Case report and review of literature. Radiol. Case Rep. 2022;17:2878. doi: 10.1016/j.radcr.2022.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abraham A., Hajar R., Virdi R., Singh J., Mustacchia P. Esophageal Sarcoidosis: A Review of Cases and an Update. ISRN Gastroenterol. 2013;2013:836203. doi: 10.1155/2013/836203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naumann A.A., Rodriguez V.I., Shychuk A. Recurrent small bowel obstruction as a rare presentation of undiagnosed sarcoidosis. BMJ Case Rep. CP. 2022;15:e252486. doi: 10.1136/bcr-2022-252486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leroy C., Girard C., Girard-Madoux M.-H., Coppéré B., Desmurs-Clavel H., Pérard L., Hot A., Ninet J. Sarcoïdose duodénale: À propos d’un cas. An unusual case of duodenal sarcoidosis. Rev. Med. Interne. 2015;36:773–776. doi: 10.1016/j.revmed.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 17.Erra P., Crusco S., Nugnes L., Pollio A.M., Di Pilla G., Biondi G., Vigliardi G. Colonic sarcoidosis: Unusual onset of a systemic disease. World J. Gastroenterol. WJG. 2015;21:3380. doi: 10.3748/wjg.v21.i11.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ungprasert P., Crowson C.S., Simonetto D.A., Matteson E.L. Clinical Characteristics and Outcome of Hepatic Sarcoidosis: A Population-Based Study 1976–2013. Am. J. Gastroenterol. 2017;112:1556–1563. doi: 10.1038/ajg.2017.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Namsrai T., Phillips C., Parkinson A., Gregory D., Kelly E., Cook M., Desborough J. Diagnostic delay of sarcoidosis: An integrated systematic review. Orphanet J. Rare Dis. 2024;19:156. doi: 10.1186/s13023-024-03152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nadpara N., Greenwald H.S., Parkman H.P. Treatment of a Gastrointestinal Sarcoidosis Flare: A Multidisciplinary Approach for a Multisystem Disease. Am. J. Case Rep. 2021;22:e932494-1. doi: 10.12659/AJCR.932494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harrison N.K. Cough, sarcoidosis and idiopathic pulmonary fibrosis: Raw nerves and bad vibrations. Cough. 2013;9:9. doi: 10.1186/1745-9974-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Serag H.B., Sweet S., Winchester C.C., Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: A systematic review. Gut. 2014;63:871–880. doi: 10.1136/gutjnl-2012-304269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hassan D., Weit N., Patel N. Symptomatic Gastric Sarcoidosis in a Patient with Pulmonary and Neurosarcoidosis: A Case Report. Am. J. Case Rep. 2022;23:e936578-1. doi: 10.12659/AJCR.936578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kouranos V., Wells A.U., Sharma R., Underwood S.R., Wechalekar K. Advances in radionuclide imaging of cardiac sarcoidosis. Br. Med. Bull. 2015;115:151–163. doi: 10.1093/bmb/ldv033. [DOI] [PubMed] [Google Scholar]
- 25.Ceylan E., Şen S., Coşkun A., Meteoğlu İ., Demirtaş N., Çildağ O. Gastric involvement of sarcoidosis in a patient with multiple lung nodules. J. Res. Med. Sci. 2015;20:525. doi: 10.4103/1735-1995.163981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sameh S., Mohamed Salah H., Mohamed G., Issam M., Najah B., Rim A., Nadia B.A., Faida A., Bassam L. Gastric sarcoidosis: Rare revealing feature of systemic sarcoidosis. Arab. J. Gastroenterol. 2020;21:62–64. doi: 10.1016/j.ajg.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Stemboroski L., Gaye B., Makary R., Monteiro C., Eid E. Isolated Gastrointestinal Sarcoidosis Involving Multiple Gastrointestinal Sites Presenting as Chronic Diarrhea. ACG Case Rep. J. 2016;3:e198. doi: 10.14309/crj.2016.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farman J., Ramirez G., Rybak B., Lebwohl O., Semrad C., Rotterdam H. Gastric sarcoidosis. Abdom. Imaging. 1997;22:248–252. doi: 10.1007/s002619900182. [DOI] [PubMed] [Google Scholar]
- 29.Espinel J., Jorquera F., Fernández-Gundín M.J., Muñoz F., Herrera A., Olcoz J.L. Endoscopic management in symptomatic gastric sarcoidosis. Endoscopy. 1999;31:S35. [PubMed] [Google Scholar]
- 30.Leeds J.S., McAlindon M.E., Lorenz E., Dube A.K., Sanders D.S. Gastric sarcoidosis mimicking irritable bowel syndrome-Cause not association? [(accessed on 7 July 2024)];World J. Gastroenterol. 2006 12:4754–4756. doi: 10.3748/wjg.v12.i29.4754. Available online: www.wjgnet.com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kariyanna P.T., Jayarangaiah A., Adrah R., Yi J., Majumder M. Gastric Sarcoidosis: A Difficult to Diagnose Rare Disease. Am. J. Med. Case Rep. 2016;4:58–61. doi: 10.12691/AJMCR-4-2-7. [DOI] [Google Scholar]
- 32.Gala K., Luckett R.T., Shah N. Gastric Sarcoidosis Presenting As Dyspepsia. Cureus. 2020;12:e7139. doi: 10.7759/cureus.7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lepidi H., Fenollar F., Gerolami R., Mege J.L., Bonzi M.F., Chappuis M., Sahel J., Raoult D. Whipple’s disease: Immunospecific and quantitative immunohistochemical study of intestinal biopsy specimens. Hum. Pathol. 2003;34:589–596. doi: 10.1016/S0046-8177(03)00126-6. [DOI] [PubMed] [Google Scholar]
- 34.Williams W.J. Histology of Crohn’s syndrome. Gut. 1964;5:510. doi: 10.1136/gut.5.6.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Villanacci V., Reggiani-Bonetti L., Salviato T., Leoncini G., Cadei M., Albarello L., Caputo A., Aquilano M.C., Battista S., Parente P. Histopathology of IBD Colitis. A practical approach from the pathologists of the Italian Group for the study of the gastrointestinal tract (GIPAD) Pathologica. 2021;113:39–53. doi: 10.32074/1591-951X-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Esmadi M., Ahmad D.S., Odum B., Diaz-Arias A., Hammad H. Sarcoidosis: An extremely rare cause of granulomatous enterocolitis. J. Gastrointestin Liver Dis. 2012;21:423–425. [PubMed] [Google Scholar]
- 37.Zakaria A., Al Share B., Turk I., Ahsan S., Farra W. An Uncommon Cause of a Small-Bowel Obstruction. Case Rep. Gastrointest. Med. 2017;2017:16. doi: 10.1155/2017/1628215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pendela V.S., Munoz A., Chhabria M., Kudaravalli P., Soliman M., Soliman Y. Appendiceal sarcoidosis presenting as acute appendicitis. Bayl. Univ. Med. Cent. Proc. 2020;33:384. doi: 10.1080/08998280.2020.1744794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paone G., Steffanina A., De Rose G., Leonardo G., Colombo D., Ricci P., Sabetta F., Vaccaro F., Rosato E., Palange P. A life–threatening small bowel obstruction as onset of an unknown sarcoidosis: A case report. Respir. Med. Case Rep. 2021;33:101379. doi: 10.1016/j.rmcr.2021.101379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Graf C., Arncken J., Lange C.M., Willuweit K., Schattenberg J.M., Seessle J., Lang-Meli J., Böttler T., Dietz J., Wetzstein N., et al. Hepatic sarcoidosis: Clinical characteristics and outcome. JHEP Rep. 2021;3:100360. doi: 10.1016/j.jhepr.2021.100360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kennedy P.T., Zakaria N., Modawi S.B., Papadopoulou A.M., Murray-Lyon I., du Bois R.M., Jervoise N Andreyev H., Devlin J. Natural history of hepatic sarcoidosis and its response to treatment. Eur. J. Gastroenterol. Hepatol. 2006;18:721–726. doi: 10.1097/01.meg.0000223911.85739.38. [DOI] [PubMed] [Google Scholar]
- 42.Sedki M., Fonseca N., Santiago P., Diaz L., Garcia-Buitrago M., Mirsaeidi M., Levy C. Hepatic Sarcoidosis: Natural History and Management Implications. Front. Med. 2019;6:232. doi: 10.3389/fmed.2019.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deutsch-Link S., Fortuna D., Weinberg E.M. A Comprehensive Review of Hepatic Sarcoid. Semin. Liver Dis. 2018;38:284–297. doi: 10.1055/s-0038-1666853. [DOI] [PubMed] [Google Scholar]
- 44.Rossi G., Ziol M., Roulot D., Valeyre D., Mahévas M. Hepatic Sarcoidosis: Current Concepts and Treatments. Semin. Respir. Crit. Care Med. 2020;41:652–658. doi: 10.1055/s-0040-1713799. [DOI] [PubMed] [Google Scholar]
- 45.Lipson E.J., Fiel M.I., Florman S.S., Korenblat K.M. Patient and graft outcomes following liver transplantation for sarcoidosis. Clin. Transplant. 2005;19:487–491. doi: 10.1111/j.1399-0012.2005.00372.x. [DOI] [PubMed] [Google Scholar]
- 46.Shukla M., Hassan M.F., Toor V., Kaur J., Solomon C., Cohen H. Symptomatic pancreatic sarcoidosis. Case report and review of literature. J. Pancreas. 2007;8:770–774. [PubMed] [Google Scholar]
- 47.Tadros M., Forouhar F., Wu G.Y. Hepatic Sarcoidosis. J. Clin. Transl. Hepatol. 2013;1:87. doi: 10.14218/JCTH.2013.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alam I., Levenson S.D., Ferrell L.D., Bass N.M. Diffuse intrahepatic biliary strictures in sarcoidosis resembling sclerosing cholangitis. Case report and review of the literature. Dig. Dis. Sci. 1997;42:1295–1301. doi: 10.1023/A:1018874612166. [DOI] [PubMed] [Google Scholar]
- 49.Ebert E.C., Kierson M., Hagspiel K.D. Gastrointestinal and hepatic manifestations of sarcoidosis. Am. J. Gastroenterol. 2008;103:3184–3192. doi: 10.1111/j.1572-0241.2008.02202.x. [DOI] [PubMed] [Google Scholar]
- 50.Cutolo M., Trombetta A.C., Melsens K., Pizzorni C., Sulli A., Ruaro B., Paolino S., Deschepper E., Smith V. Automated assessment of absolute nailfold capillary number on videocapillaroscopic images: Proof of principle and validation in systemic sclerosis. Microcirculation. 2018;25:e12447. doi: 10.1111/micc.12447. [DOI] [PubMed] [Google Scholar]
- 51.Park Y.J., Woo H.Y., Kim M.B., Ahn J., Heo J. Primary hepatic sarcoidosis presenting with cholestatic liver disease and mimicking primary biliary cholangitis: A case report. J. Yeungnam Med. Sci. 2022;39:256–261. doi: 10.12701/yujm.2021.01151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muhanna A., Al Momani L., Likhitsup A. Sarcoidosis Manifesting as Liver Granuloma with Asteroid Bodies. Cureus. 2021;13:e17915. doi: 10.7759/cureus.17915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tombazzi C., Waters B., Ismail M.K., Sylvestre P.B., Martinez-Hernandez A., Riely C.A. Sarcoidosis mimicking primary sclerosing cholangitis requiring liver transplantation. Ann. Hepatol. 2008;7:83–86. doi: 10.1016/S1665-2681(19)31894-0. [DOI] [PubMed] [Google Scholar]
- 54.Buxbaum J., Papademetriou M., Klipfel N., Selby R., Fong T.L., Sharma O. Biliary sarcoidosis: Early diagnosis minimizes the need for surgery. Am. J. Respir. Crit. Care Med. 2013;187:556–559. doi: 10.1164/ajrccm.187.5.556. [DOI] [PubMed] [Google Scholar]
- 55.Scherr B., Tromm A., Voigt E., Müller K.M., Griga T. Association of primary sclerosing cholangitis and sarcoidosis. Med. Klin. 2001;96:550–554. doi: 10.1007/PL00002240. [DOI] [PubMed] [Google Scholar]
- 56.Deliwala S.S., Hussain M., Ponnapalli A., Khanal R., Goyal H., Abdalla A., Elbedawi M.M. Sarcoidosis Masquerading as Long-Standing Cholestasis. Gastroenterol. Res. 2021;14:112–115. doi: 10.14740/gr1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Valla D.C., Benhamou J.P. Hepatic granulomas and hepatic sarcoidosis. Clin. Liver Dis. 2000;4:269–285. doi: 10.1016/S1089-3261(05)70108-2. [DOI] [PubMed] [Google Scholar]
- 58.Delfosse V., de Leval L., De Roover A., Delwaide J., Honoré P., Boniver J., Detry O. Budd-Chiari syndrome complicating hepatic sarcoidosis: Definitive treatment by liver transplantation: A case report. Transplant. Proc. 2009;41:3432–3434. doi: 10.1016/j.transproceed.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 59.Israël-Biet D., Bernardinello N., Pastré J., Tana C., Spagnolo P. High-Risk Sarcoidosis: A Focus on Pulmonary, Cardiac, Hepatic and Renal Advanced Diseases, as Well as on Calcium Metabolism Abnormalities. Diagnostics. 2024;14:395. doi: 10.3390/diagnostics14040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cohen P.R., Kurzrock R. Sarcoidosis and malignancy. Clin. Dermatol. 2007;25:326–333. doi: 10.1016/j.clindermatol.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 61.Chalasani P., Vohra M., Sheagren J.N. An association of sarcoidosis with hepatocellular carcinoma. Ann. Oncol. 2005;16:1714–1715. doi: 10.1093/annonc/mdi306. [DOI] [PubMed] [Google Scholar]
- 62.Wong V.S., Adab N., Youngs G.R., Sturgess R. Hepatic sarcoidosis complicated by hepatocellular carcinoma. Eur. J. Gastroenterol. Hepatol. 1999;11:353–355. doi: 10.1097/00042737-199903000-00023. [DOI] [PubMed] [Google Scholar]
- 63.Huh J.Y., Moon D.S., Song J.W. Sarcoid-like reaction in patients with malignant tumors: Long-term clinical course and outcomes. Front. Med. 2022;9:884386. doi: 10.3389/fmed.2022.884386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shah N., Mitra A. Gastrointestinal and Hepatic Sarcoidosis: A Review Article. Clin. Liver Dis. 2021;17:301–307. doi: 10.1002/cld.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cengiz C., Rodriguez-Davalos M., deBoccardo G., Fiel M.I., Rodriguez-Laiz G., Kovacevic M., Emre S., Schiano T. Recurrent hepatic sarcoidosis post-liver transplantation manifesting with severe hypercalcemia: A case report and review of the literature. Liver Transpl. 2005;11:1611–1614. doi: 10.1002/lt.20626. [DOI] [PubMed] [Google Scholar]
- 66.Gavilán F., Pereda T., Sousa J.M., Serrano J., Gómez M.A., García I., Tamayo M.J., Martin C., Reig M., Hinojosa R., et al. Hepatic cirrhosis with sarcoid granulomas. Differential diagnosis and liver transplantation: A case report. Transplant. Proc. 2003;35:713–714. doi: 10.1016/S0041-1345(03)00060-5. [DOI] [PubMed] [Google Scholar]
- 67.Shibolet O., Kalish Y., Wolf D., Pappo O., Laxer U., Berkman N., Shaham D., Ashur Y., Ilan Y. Exacerbation of pulmonary sarcoidosis after liver transplantation. J. Clin. Gastroenterol. 2002;35:356–358. doi: 10.1097/00004836-200210000-00015. [DOI] [PubMed] [Google Scholar]
- 68.Fidler H.M., Hadziyannis S.J., Dhillon A.P., Sherlock S., Burroughs A.K. Recurrent hepatic sarcoidosis following liver transplantation. Transplant. Proc. 1997;29:2509–2510. doi: 10.1016/S0041-1345(97)00488-0. [DOI] [PubMed] [Google Scholar]
- 69.Smith V., Pizzorni C., Riccieri V., Decuman S., Brusselle G., DE Pauw M., Deschepper E., Piette Y., Ruaro B., Sulli A., et al. Stabilization of Microcirculation in Patients with Early Systemic Sclerosis with Diffuse Skin Involvement following Rituximab Treatment: An Open-label Study. J. Rheumatol. 2016;43:995–996. doi: 10.3899/jrheum.151018. [DOI] [PubMed] [Google Scholar]
- 70.Casavilla F.A., Gordon R., Wright H.I., Gavaler J.S., Starzl T.E., Van Thiel D.H. Clinical course after liver transplantation in patients with sarcoidosis. Ann. Intern. Med. 1993;118:865–866. doi: 10.7326/0003-4819-118-11-199306010-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pescovitz M.D., Jones H.M., Cummings O.W., Lumeng L., Leapman S.B., Filo R.S. Diffuse retroperitoneal lymphadenopathy following liver transplantation--a case of recurrent sarcoidosis. Transplantation. 1995;60:393–396. doi: 10.1097/00007890-199508270-00017. [DOI] [PubMed] [Google Scholar]
- 72.Kikuchi M., Koizumi A., Namisaki T., Asada S., Oyama M., Tomooka F., Fujimoto Y., Kitagawa K., Kawaratani H., Yoshiji H. Improvement of liver histology in hepatic sarcoidosis due to treatment with corticosteroids and ursodeoxycholic acid: A case report. Clin. J. Gastroenterol. 2024;17:327–333. doi: 10.1007/s12328-023-01918-3. [DOI] [PubMed] [Google Scholar]
- 73.Papo T., Piette J.-C., Valla D. Sarcoidosis, liver transplantation, and cyclosporine. Ann. Intern. Med. 1993;119:1148–1149. doi: 10.7326/0003-4819-119-11-199312010-00016. [DOI] [PubMed] [Google Scholar]
- 74.Vanatta J.M., Modanlou K.A., Dean A.G., Nezakatgoo N., Campos L., Nair S., Eason J.D. Outcomes of orthotopic liver transplantation for hepatic sarcoidosis: An analysis of the United Network for Organ Sharing/Organ Procurement and Transplantation Network data files for a comparative study with cholestatic liver diseases. Liver Transpl. 2011;17:1027–1034. doi: 10.1002/lt.22339. [DOI] [PubMed] [Google Scholar]
- 75.Bilal M., Satapathy S.K., Ismail M.K., Vanatta J.M. Long-Term Outcomes of Liver Transplantation for Hepatic Sarcoidosis: A Single Center Experience. J. Clin. Exp. Hepatol. 2016;6:94–99. doi: 10.1016/j.jceh.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kruithoff K.L., Gyetko M.R., Scheiman J.M. Giant splenomegaly and refractory hypercalcemia due to extrapulmonary sarcoidosis: Successful treatment by splenectomy. Arch. Intern. Med. 1993;153:2793–2796. doi: 10.1001/archinte.1993.00410240105013. [DOI] [PubMed] [Google Scholar]
- 77.Okabe Y., Ushijima T., Yasunaga M., Mihara Y., Torimura T. A rare case of sarcoidosis in accessory spleen. Gastrointest. Endosc. 2017;86:918–919. doi: 10.1016/j.gie.2017.05.025. [DOI] [PubMed] [Google Scholar]
- 78.Warshauer D.M., Molina P.L., Hamman S.M., Koehler R.E., Paulson E.K., Bechtold R.E., Perlmutter M.L., Hiken J.N., Francis I.R., Cooper C.J. Nodular sarcoidosis of the liver and spleen: Analysis of 32 cases. Radiology. 1995;195:757–762. doi: 10.1148/radiology.195.3.7754007. [DOI] [PubMed] [Google Scholar]
- 79.Saito S., Kodama K., Kogiso T., Yamanashi Y., Taniai M., Ariizumi S., Yamamoto M., Tokushige K. Atypical sarcoidosis diagnosed by massive splenomegaly. Intern. Med. 2020;59:641–648. doi: 10.2169/internalmedicine.3646-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Madaule S., Lauque D., Sailler L., Arlet P., Carles P. Les splénomégalies sarcoïdosiques: Caracté ristiques cliniques et évolutives. À propos de 17 observations. Rev. Med. Interne. 2004;25:348–356. doi: 10.1016/j.revmed.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 81.Ali Y., Popescu N.A., Woodlock T.J. Extrapulmonary sarcoidosis: Rapid spontaneous remission of marked splenomegaly. J. Natl. Med. Assoc. 1996;88:714–716. [PMC free article] [PubMed] [Google Scholar]
- 82.Salazar A., Mana J., Corbella X., Albareda J.M., Pujol R. Splenomegaly in sarcoidosis: A report of 16 cases. Sarcoidosis. 1995;12:131–134. [PubMed] [Google Scholar]
- 83.Pomianowska L. Dramatic improvement after steroid treatment in a case of sarcoidosis with splenomegaly (Polish) Reumatologia. 1974;12:413–416. [PubMed] [Google Scholar]
- 84.Ogiwara Y., Mori S., Iwama M., Sawabe M., Takemoto M., Kanazawa N., Furuta K., Fukuda I., Kondo Y., Kimbara Y., et al. Hypoglycemia due to ectopic secretion of insulin-like growth factor-I in a patient with an isolated sarcoidosis of the spleen. Endocr. J. 2010;57:325–330. doi: 10.1507/endocrj.K09E-370. [DOI] [PubMed] [Google Scholar]
- 85.Takeda S., Kawaratani H., Takami M., Inoue Y., Matsuda T., Kubo T., Fujinaga M., Ozutsumi T., Furukawa M., Kitagawa K., et al. Isolated pancreatic sarcoidosis diagnosed by endoscopic ultrasound-guided fine-needle aspiration. Intern. Med. 2020;59:1407–1412. doi: 10.2169/internalmedicine.4034-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mony S., Patil P.D., English R., Das A., Culver D.A., Panchabhai T.S. A Rare Presentation of Sarcoidosis as a Pancreatic Head Mass. Case Rep. Pulmonol. 2017;2017:7037162. doi: 10.1155/2017/7037162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Snook B., Duggan M., Birkett D. Sarcoidosis of the pancreas: A case report. Am. Surg. 1996;62:947–948. [PubMed] [Google Scholar]
- 88.Curran J.F., Curran J.F. Boeck’s sarcoid of the pancreas. Surgery. 1950;28:574–578. [PubMed] [Google Scholar]
- 89.Matsuura S., Mochizuka Y., Oishi K., Miyashita K., Naoi H., Mochizuki E., Mikura S., Tsukui M., Koshimizu N., Ohata A., et al. Sarcoidosis with pancreatic mass, endobronchial nodules, and miliary opacities in the lung. Intern. Med. 2017;56:3083–3087. doi: 10.2169/internalmedicine.8916-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cook J., Spees T., Telefus P., Ranaudo J.M., Carryl S., Xiao P. Pancreatic sarcoidosis discovered during Whipple procedure. J. Surg. Case Rep. 2013;2013:rjt016. doi: 10.1093/jscr/rjt016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Frank J.L., Goldman M., Nathanson I., Pierangelo D., Kaufman J.L., Freeman J.K., Reed W.P. Surgical management of pancreatic sarcoid. Eur. J. Surg. 2001;167:68–72. doi: 10.1080/110241501750069864. [DOI] [PubMed] [Google Scholar]
- 92.Ghrenassia E., Mekinian A., Chapelon-Albric C., Levy P., Cosnes J., Sève P., Lefèvre G., Dhôte R., Launay D., Prendki V., et al. Digestive-tract sarcoidosis: French nationwide case–control study of 25 cases. Medicine. 2016;95:e4279. doi: 10.1097/MD.0000000000004279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tetikkurt C., Sayman H., Dedeoglu S.E., Kubat B., Tetikkurt S. Simultaneous use of FDG-18 and 68Ga-citrate PET/CT for the differential diagnosis of sarcoidosis and malignant disease. Monaldi Arch. Chest Dis. 2020;90:470–473. doi: 10.4081/monaldi.2020.1320. [DOI] [PubMed] [Google Scholar]
- 94.Mei R., Prediletto I., Nava S., Fanti S., Ambrosini V. Colon Sarcoidosis Mimicking Cancer at 18F-FDG PET/CT. Clin. Nucl. Med. 2020;45:387–388. doi: 10.1097/RLU.0000000000002996. [DOI] [PubMed] [Google Scholar]
- 95.Dopazo M.S., Laso C.J.Á., Arenas M.P., Fernández J.C.F. Colon adenocarcinoma associated with intestinal sarcoidosis. Rev. Esp. Enfermedades Dig. 2023;115:398–399. doi: 10.17235/reed.2022.9279/2022. [DOI] [PubMed] [Google Scholar]
- 96.Crouser E.D., Maier L.A., Wilson K.C., Bonham C.A., Morgenthau A.S., Patterson K.C., Abston E., Bernstein R.C., Blankstein R., Chen E.S., et al. Diagnosis and Detection of Sarcoidosis. An Official American Thoracic Society Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2020;201:e26–e51. doi: 10.1164/rccm.202002-0251ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mahmood R., Al Banaa K., Ibrahim I., Hashim A., Torregrosa L. Sarcoidosis vs. Colon Cancer Metastasis: Diagnostic Dilemma and the Role of PET Scan in Monitoring Disease Activity. Case Rep. Rheumatol. 2021;2021:5529523. doi: 10.1155/2021/5529523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Friedman M., Ali M.A., Borum M.L. Gastric sarcoidosis: A case report and review of the literature. South. Med. J. 2007;100:301–303. doi: 10.1097/SMJ.0b013e318030ed94. [DOI] [PubMed] [Google Scholar]
- 99.Sharma A.M., Kadaki J., Sharma O.P. Gastrointestinal Sarcoidosis. Semin. Respir. Crit. Care Med. 1992;13:442–449. doi: 10.1055/s-2007-1006293. [DOI] [Google Scholar]
- 100.Okumus G., Musellim B., Cetinkaya E., Turker H., Uzaslan E., Yenturk E., Uzun O., Saglam L., Kumbasar O.O., Celik G., et al. Extrapulmonary involvement in patients with sarcoidosis in Turkey. Respirology. 2011;16:446–450. doi: 10.1111/j.1440-1843.2010.01878.x. [DOI] [PubMed] [Google Scholar]
- 101.El Rhaoussi F.Z., Banani S., Bouamama S., Bennani N., Badre W. Multisystemic Sarcoidosis Revealed by Hepatosplenomegaly: A Case Report. Cureus. 2022;14:e23967. doi: 10.7759/cureus.23967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Karagiannidis A., Karavalaki M., Koulaouzidis A. Hepatic sarcoidosis. Ann. Hepatol. 2006;5:251–256. doi: 10.1016/S1665-2681(19)31983-0. [DOI] [PubMed] [Google Scholar]
- 103.Osipenko M.F., Voloshina N.B. Hepatic Sarcoidosis. Eksp. Klin. Gastroenterol. 2016;9:86–90. [PubMed] [Google Scholar]
- 104.Ryland K.L. Hepatic Sarcoidosis: Incidence, Monitoring, and Treatment. Clin. Liver Dis. 2020;16:208–211. doi: 10.1002/cld.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Esfeh J.M., Culver D., Plesec T., John B. Clinical presentation and protocol for management of hepatic sarcoidosis. Expert. Rev. Gastroenterol. Hepatol. 2015;9:349–358. doi: 10.1586/17474124.2015.958468. [DOI] [PubMed] [Google Scholar]
- 106.El Sharu H., Ibarra S., Chaudhary A., Hidri S., Khan Z., Hoo-Fatt D. Gastric sarcoidosis diagnosed with endoscopic ultrasound. Clin. Case Rep. 2024;12:e8623. doi: 10.1002/ccr3.8623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tao J., Generette G.S., Khan M., Khan N. Severe Symptomatic Anemia in Gastrointestinal Tract Sarcoidosis. Cureus. 2023;15:e44867. doi: 10.7759/cureus.44867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Basida B., Haider M.B., Bapatla A., Zalavadiya N., Iqbal S. Subhepatic Abscess Unmasking the Silent Gastric and Pulmonary Sarcoidosis. Cureus. 2021;13:e16957. doi: 10.7759/cureus.16957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mota C., Ferreira C., Oliveira M.E., Santos J.M., Victorino R.M.M. Multisystemic Sarcoidosis with Early Gastrointestinal Symptoms. GE Port. J. Gastroenterol. 2017;24:137. doi: 10.1159/000450899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Deepa A.S., Padegal V.A., Chandra K.S.P., Santhosh H.K. Gastric and pulmonary sarcoidosis complicated by hypercalcemia and acute renal failure: Case report and literature review. Lung India. 2017;34:380. doi: 10.4103/lungindia.lungindia_276_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tokala H., Polsani K., Kalavakunta J.K. Gastric Sarcoidosis: A Rare Clinical Presentation. Case Rep. Gastrointest. Med. 2013;2013:260704. doi: 10.1155/2013/260704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shkolnik L.E., Shin R.D., Brabeck D.M., Rothman R.D. Symptomatic gastric sarcoidosis in a patient with pulmonary sarcoidosis in remission. BMJ Case Rep. 2012;2012:bcr2012006559. doi: 10.1136/bcr-2012-006559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Inomata M., Ikushima S., Awano N., Kondoh K., Satake K., Masuo M., Moriya A., Kamiya H., Ando T., Azuma A., et al. Upper gastrointestinal sarcoidosis: Report of three cases. Intern. Med. 2012;51:1689–1694. doi: 10.2169/internalmedicine.51.7367. [DOI] [PubMed] [Google Scholar]
- 114.Chaudhary P., Gopaluni S., Sanyal S., Shah C. Atypical sarcoidosis masquerading as neutropenia. Sarcoidosis Vasc. Diffus. Lung Dis. 2010;27:160–163. [PubMed] [Google Scholar]