Abstract
Gliomas, the most common type of primary malignant brain tumors in adults, pose significant challenges in diagnosis and management due to their heterogeneity and potential aggressiveness. This review evaluates the utility of O-(2-[18F]fluoroethyl)-L-tyrosine ([18F]FET) positron emission tomography (PET), a promising imaging modality, to enhance the clinical management of gliomas. We reviewed 82 studies involving 4657 patients, focusing on the application of [18F]FET in several key areas: diagnosis, grading, identification of IDH status and presence of oligodendroglial component, guided resection or biopsy, detection of residual tumor, guided radiotherapy, detection of malignant transformation in low-grade glioma, differentiation of recurrence versus treatment-related changes and prognostic factors, and treatment response evaluation. Our findings confirm that [18F]FET helps delineate tumor tissue, improves diagnostic accuracy, and aids in therapeutic decision-making by providing crucial insights into tumor metabolism. This review underscores the need for standardized parameters and further multicentric studies to solidify the role of [18F]FET PET in routine clinical practice. By offering a comprehensive overview of current research and practical implications, this paper highlights the added value of [18F]FET PET in improving management of glioma patients from diagnosis to follow-up.
Keywords: neuro-oncology, glioma, fluoroethyltyrosine (FET), PET, nuclear medicine
1. Introduction
Gliomas represent the majority of primary malignant brain tumors in adults, with a yearly incidence of approximately 6 per 100,000 in Europe [1]. They are categorized according to the World Health Organization (WHO) classification into grades ranging from 1 to 4 depending on their malignancy [2]. Glioblastoma, the most aggressive and common type of glioma, remains incurable with an almost systematic progression within the year and a median survival of 14.6 months despite optimal treatment [3].
In high-grade tumors, treatment usually consists of maximal resection of the tumor (if feasible) followed by chemotherapy and radiotherapy depending on tumor grade and analysis of molecular markers (i.e., 1p/19q codeletion, IDH mutation, and MGMT promoter methylation) [4]. Treatment of grade 4 gliomas, the same since 2005, is based on the so-called “Stupp protocol”, which includes concomitant radiochemotherapy with Temozolomide [3].
Patients’ monitoring consists of MRI before and after treatment with periodic follow-up. An increase in enhancing areas is considered suspect of recurrence according to the Response Assessment in Neuro-Oncology (RANO) criteria but is not specific [5]. Indeed, frequent post-radiation changes such as pseudoprogression and radionecrosis can cause the same type of suspicious gadolinium-enhancing lesion.
Pseudoprogression typically occurs several weeks up to months (often less than 3 months) after completion of radiotherapy. This phenomenon is responsible for a transitory worsening of MR imaging with an increased contrast enhancement area, resolving without changes in treatment on subsequent MRI scans. There is generally no symptom associated.
Radionecrosis is a severe reaction to radiotherapy, which generally occurs later, months to several years after radiation therapy. MRI findings involve a space-occupying necrotic lesion with a mass effect, which can cause neurological dysfunction.
MRI changes can also be induced by treatments such as corticosteroids, antiangiogenic therapy, or immunotherapy.
For these reasons, there is a need to find other reliable methods to differentiate glioma recurrence from treatment-related changes, given the different managements of these two processes.
Different MRI techniques have been implemented in this indication, such as diffusion weighted imaging (DWI) [6], perfusion-weighted imaging (PWI) [7], and magnetic resonance spectroscopy (MRS) [8].
In nuclear medicine, positron emission tomography using 2-deoxy-2-[18F]fluoro-D-glucose ([18F]FDG) has already proven itself in oncology imaging and has become common practice in numerous pathologies. However, its physiologically high brain metabolism and increased uptake in inflammatory lesions make it difficult to appreciate tumor uptake [9].
Radiolabeled amino acids are preferred in neuro-oncology due to low uptake in normal brain tissue contrasting with increased uptake in neoplastic processes, resulting in a better signal-to-noise ratio [10].
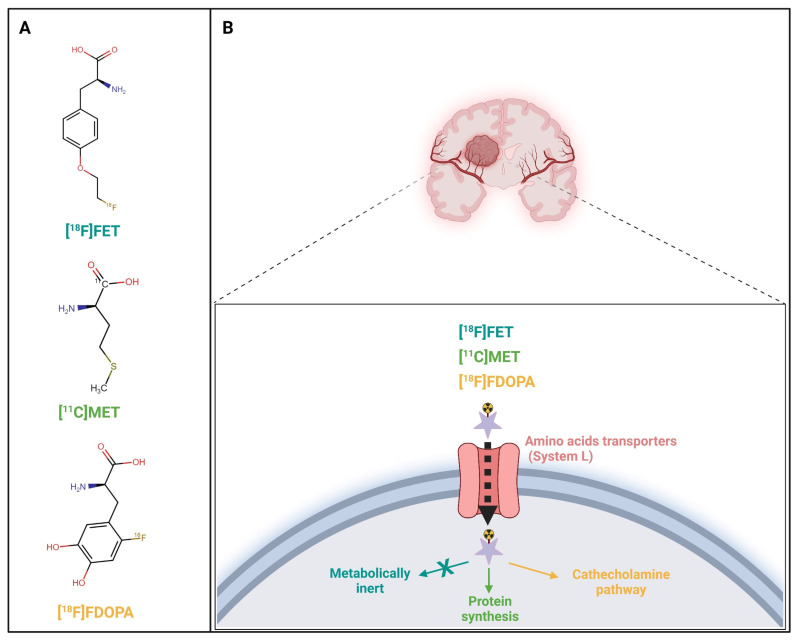
The most widely used amino acid tracers for PET are [11C-methyl]-methionine ([11C]MET), O-(2-[18F]fluoroethyl)-L-tyrosine ([18F]FET), and 3,4dihydroxy-6-[18F]fluoro-L-phenylalanine ([18F]F-DOPA) (Table 1). Their uptake is believed to be driven by an overexpression of the L-type amino-acid transporter (LAT) by brain tumors (Figure 1).
Table 1.
Comparative table of different radiolabeled amino acids.
| Aspect | [11C]MET | [18F]F-DOPA | [18F]FET |
|---|---|---|---|
| Radiotracer Type | Amino acid analog | Amino acid precursor | Amino acid analog |
| Mechanism of Uptake | Uptake via L-type amino acid transporter (LAT) into tumor cells with high protein synthesis. | Uptake via amino acid transport (LAT) is overexpressed in tumor cells. Converted into dopamine in dopaminergic neurons. | Uptake via LAT, reflecting increased amino acid transport correlated to tumor proliferation. |
| Half-Life | 20 min | 110 min | 110 min |
| Production | Requires on-site cyclotron due to short half-life. | Can be produced off-site, longer shelf life. | Can be produced off-site, longer shelf life. |
| Sensitivity in Gliomas | High sensitivity, more effective in detecting high-grade gliomas. | High sensitivity in detecting glioma. | High sensitivity, more effective in detecting high-grade gliomas. |
| Specificity in Gliomas | Moderate specificity, possible uptake in inflammatory lesions. | High specificity, with potential uptake in inflammatory tissues. | High specificity, with less non-specific uptake in inflammatory tissues compared to [11C]MET. |
| Advantages | Rapid uptake, good lesion contrast. | Longer half-life allows broader clinical application. | Longer half-life allows broader clinical application. Dynamic acquisition allows additional information on tracer kinetics, particularly useful for tumor grading. |
| Disadvantages | Short half-life limits use to facilities with a cyclotron, potential uptake in inflammation. | May have false positives in inflamed tissues. High physiologic uptake in the basal ganglia. | Potential uptake in inflammatory lesions but less than [11C]MET. |
| Clinical Application | Primarily used in facilities with a cyclotron, used to detect tumor recurrence and in monitoring the response to therapy. | Mostly used for differentiating tumor recurrence from necrosis, especially in high-grade gliomas. | Widely used for differentiating high-grade glioma early and late progression from radiation effects. |
Figure 1.
Radiolabeled amino acids O-(2-[18F]fluoroethyl)-L-tyrosine ([18F]FET), [11C-methyl]-methionine ([11C]MET), and L-3,4-dihydroxy-6-[18F]fluoro-phenyl-alanine ([18F]FDOPA) metabolic pathways. Molecular structures (A) and associated uptake mechanism (B) of each radiolabeled amino acid. Created with BioRender.com.
Detailed Description of different radiolabeled amino acids
11C-Methionine ([11C]MET)
Mechanism: [11C]MET is an amino acid analog taken up by tumor cells via the L-type amino acid transporter (LAT). It reflects increased protein synthesis, which is often elevated in gliomas.
Advantages: High sensitivity in detecting both low- and high-grade gliomas; more effective in high-grade gliomas [11]. Provides rapid uptake and good contrast between tumor and normal brain tissue. It is particularly effective to detect tumor recurrence [12] and in monitoring therapy response [13].
Disadvantages: The short half-life of 11C (about 20 min) necessitates the use of an on-site cyclotron, limiting its use to specialized centers. [11C]MET may also accumulate in inflammatory tissues, leading to potential false positives [14].
[18F]F-DOPA
Mechanism: [18F]F-DOPA is a precursor to dopamine and is taken up by dopaminergic neurons, with uptake also observed in gliomas due to increased amino acid transport and altered tumor metabolism. It is decarboxylated to dopamine and subsequently trapped in cells.
Advantages: The longer half-life of 1⁸F (about 110 min) allows for broader clinical application as it can be transported from off-site production facilities. It has high sensitivity for gliomas [15] and is particularly useful in differentiating between tumor recurrence and radiation necrosis [16].
Disadvantages: Uptake of [18F]F-DOPA in inflamed tissues can lead to false-positive results [17].
1⁸F-Fluoroethyl-L-tyrosine ([18F]FET)
Mechanism: [18F]FET is an artificial amino acid taken up by glioma cells via LAT, reflecting the increased amino acid transport associated with tumor proliferation.
Advantages: [18F]FET has a longer half-life, like 1⁸F-DOPA, allowing it to be produced off-site. It has high sensitivity for gliomas, especially high-grade gliomas [18], with low uptake in inflammatory lesions, making it particularly effective in distinguishing tumor recurrence from treatment-induced changes. Additionally, dynamic acquisition allows information on tracer kinetics, particularly useful for tumor grading [19].
Disadvantages: Though it offers high specificity. There is also potential, though reduced, for uptake in inflammatory tissues [20].
While recent meta-analyses report high sensitivity and specificity of both 1⁸F-DOPA and [18F]FET to differentiate true progression to treatment-related changes, there are still discrepancies in determining the best radiolabeled amino acid [21,22,23].
[18F]FET market authorizations have been delivered in Europe recently, enabling its widespread use in hospitals.
Its high efficiency production and its half-life of 110 min allow its transportation to other sites. For these reasons, it is being increasingly used in glioma management in Europe.
In the present review, we aimed to summarize its performance in different indications in low- and high-grade gliomas.
2. Materials and Methods
2.1. Search Strategy
The primary literature was searched up to 31 December 2023, using the PubMed database.
A combination of the search terms «PET», «FET» OR «amino acid» OR «fluoroethyltyrosine» OR «fluoroethylltyrosine», «Glioma» OR «brain tumor», «pediatric», and «neuro-oncology» were used. The screening of abstracts and full-text articles was performed by one reviewer (J.A.R.).
Inclusion criteria were studies in English, using FET, and in humans with a full text available.
Exclusion criteria included studies that included less than 20 patients, did not report on diagnostic test parameters or metrics representing impact on clinical management decisions and/or survival outcomes, did not give information about histology or tumor grades, and studies that included other malignancies. We also excluded studies that did not include histological confirmation or follow-up.
2.2. Data Synthesizing
For each study, the indication, principal author, publication year, study design, number of patients, grade, age, sex, type of imaging modality, test parameter, cut-off used, and their performances were recorded.
3. Results
3.1. Literature Search
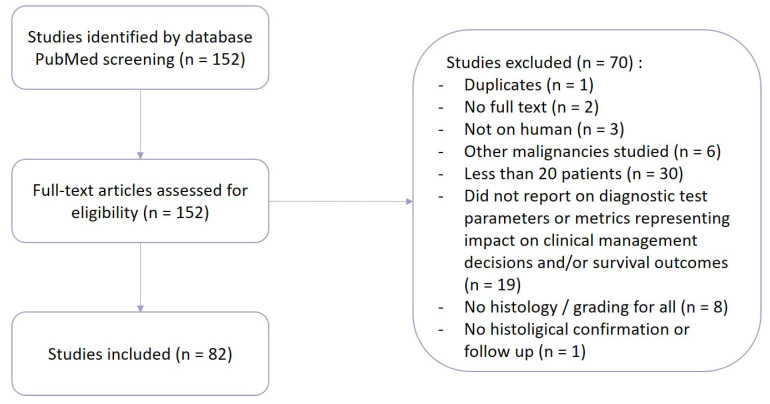
We selected 152 studies according to their title and abstract, but upon full-text review, 70 studies were excluded (Figure 2).
Figure 2.
Flowchart of the literature selection.
The remaining 82 studies [19,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104] were included in this review, with a total of 4657 patients. Details of these study characteristics can be found in Table 2.
Table 2.
Characteristics of the 82 included studies. §: did not reach significance, &: did not reach significance after Bonferroni multiple-test correction, #: significance not available.
| Indication | Author, Year | Reference | Design | Number of Patients | Grade | Mean Age | Sex | Imaging Modality | Parameters | Optimal Cut-Off | Sensitivity | Specificity | AUC | Accuracy |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis | ||||||||||||||
| Pauleit et al., 2009 | [24] | Prospective | 52 | Not glioma:9 | 46 | 36 M 16 F | PET | Lmean/B # | - | |||||
| Grade 2:22 | Lmax/B # | - | ||||||||||||
| Grade 3:12 | Visual grading system # | - | ||||||||||||
| Grade 4:9 | ||||||||||||||
| Mauler et al., 2023 | [25] | Prospective | 30 | Not glioma:6 | 48 | 16 M 14 F | PET | 18F-FETn uptake | 1.4 x background | 76% | 80% | 0.89 | 78% | |
| Grade 2:7 | MRI | Cho/NAAn | 2.16 | 59% | 83% | 0.81 | 71% | |||||||
| Grade 3:7 | ||||||||||||||
| Grade 4:10 | ||||||||||||||
| Floeth et al., 2005 | [26] | Prospective | 50 | Not glioma:16 | 44 | 21 M 29 F | PET | FET lesion/brain ratio | 1.6 | 88% | 88% | - | ||
| Grade 1:2 | MRI | Gd enhancement | - | 44% | 69% | 68% | ||||||||
| Grade 2:13 | NAA/Cho ratio | 0.7 | 100% | 81% | - | |||||||||
| Grade 3:14 | ||||||||||||||
| Grade 4:5 | ||||||||||||||
| Pauleit et al., 2005 | [27] | Prospective | 28 | Not glioma:5 | 42 | 9 M 19 F | PET | FET ratio | 1.6 | 92% | 81% | - | ||
| Grade 1:2 | MRI | T1 ratio | 1.0 | 85% | 12% | - | ||||||||
| Grade 2:7 | Gd-T1 ratio | 1.0 | 38% | 96% | - | |||||||||
| Grade 3:12 | FLAIR ratio | 1.0 | 96% | 4% | - | |||||||||
| Grade 4:2 | T1/Gd-T1/FLAIR ratio | - | 96% | 53% | 68% | |||||||||
| PET/CT + MRI | FET/T1/Gd-T1/FLAIR ratio | - | 93% | 94% | 94% | |||||||||
| Grading (LGG vs. HGG) | ||||||||||||||
| Jeong and Lim, 2012 | [28] | Prospective | 20 | Grade 2:3 | 52 | 13 M 7 F | PET | SUVmax | - | |||||
| Grade 3:2 | TNR | - | ||||||||||||
| Grade 4:15 | ||||||||||||||
| Verger et al., 2017 | [29] | Retrospective | 72 | Grade 1:1 | 49 | 42 M 30 F | PET | TBRmax | 2.62 | 82% | 68% | 0.83 | 78% | |
| Grade 2:21 | TBRmean | 1.69 | 82% | 68% | 0.80 | 78% | ||||||||
| Grade 3:25 | TTP | 30 min | 54% | 91% | 0.78 | 65% | ||||||||
| Grade 4:25 | Slope | −0.03 SUV/h | 64% | 91% | 0.78 | 72% | ||||||||
| PWI rCBF | TBRmax | 1.51 | 64% | 64% | 0.74 | 64% | ||||||||
| TBRmean | 0.69 | 62% | 59% | 0.66 | 61% | |||||||||
| PWI rCBV | TBRmax | 1.80 | 88% | 72% | 0.81 | 83% | ||||||||
| TBRmean | 1.14 | 72% | 77% | 0.80 | 74% | |||||||||
| PWI MTT | TBRmax § | 1.16 | 64% | 50% | 0.58 | 60% | ||||||||
| TBRmean § | 0.98 | 54% | 36% | 0.43 | 49% | |||||||||
| Lopez et al., 2015 | [30] | Prospective | 23 | No-grade:2 | 56 | 18 M 5 F | PET | UR | 3.0 | |||||
| Grade 1:1 | ||||||||||||||
| Grade 2:7 | ||||||||||||||
| Grade 3:2 | ||||||||||||||
| Grade 4:11 | ||||||||||||||
| Lohmann et al., 2015 | [31] | Prospective | 36 | Grade 2:12 | 49 | 19 M 17 F | PET | TBRmean § | 2 | 83% | 58% | 0.65 | 75% | |
| Grade 3:8 | ∆TBRmean 20–40 min/70–90 min | −8% | 83% | 75% | 0.85 | 81% | ||||||||
| Grade 4:16 | TTP | 35 min | 58% | 92% | 0.76 | 69% | ||||||||
| Kinetic pattern | II/III | 88% | 75% | - | 83% | |||||||||
| Calcagni et al., 2011 | [32] | Prospective | 32 | Grade 1:3 | 41 | 21 M 11 F | PET | TAC # | I/II vs. III | 73% | 100% | 87% | ||
| Grade 2:14 | Early SUV | 2.32 | 73% | 71% | 72% | |||||||||
| Grade 3:11 | Middle SUV § | - | - | - | - | |||||||||
| Grade 4:4 | Late SUV § | - | - | - | - | |||||||||
| e-m ratio | 0.93 | 93% | 94% | 94% | ||||||||||
| e-l ratio | 0.95 | 87% | 88% | 87% | ||||||||||
| Tpeak | 25 min | 87% | 100% | 94% | ||||||||||
| SoD | 0.5 | 93% | 82% | 87% | ||||||||||
| Logistic regression using Early SUV + SoD § | 50% | 93% | 100% | 97% | ||||||||||
| Albert et al., 2016 | [33] | Retrospective | 314 | Grade 1:3 | 49 | 181 M 133 F | PET | TBRmax (20–40 min) | 2.7 | 67% | 78% | 70% | ||
| Grade 2:128 | TBRmax (0–10 min) | 2.8 | 76% | 79% | 76% | |||||||||
| Grade 3:95 | TBRmax (5–15 min) | 2.7 | 78% | 76% | 77% | |||||||||
| Grade 4:88 | TBRmax (5–20 min) | 2.6 | 80% | 74% | 76% | |||||||||
| TBRmax (10–30 min) | 2.5 | 75% | 75% | 74% | ||||||||||
| Kinetic pattern # | Decreasing | 90% | 66% | 80% | ||||||||||
| Pöpperl et al., 2007 | [19] | Prospective | 54 | Grade 2:15 | 49 | 30 M 24 F | PET | SUVmax/BG | 2.58 | 71% | 85% | 0.798 | ||
| Grade 3:21 | SUV90 10–60 min | 0.20 | 94% | 100% | 0.969 | |||||||||
| Grade 4:18 | SUV90 15–60 min | −0.41 | 94% | 100% | 0.965 | |||||||||
| Grade 2/3 vs. grade 4 | Hua et al., 2021 | [34] | Retrospective | 58 | Grade 2:33 | 42 | 37 M 21 F | PET | TBRmax | 2.67 | 92% | 61% | 0.824 | 67% |
| Grade 3:13 | TBRpeak | 2.35 | 92% | 61% | 0.832 | 67% | ||||||||
| Grade 4:12 | TBRmean | 2.31 | 58% | 93% | 0.791 | 86% | ||||||||
| COV | 27.21 | 58% | 91% | 0.808 | 84% | |||||||||
| HI | 1.77 | 67% | 87% | 0.826 | 83% | |||||||||
| MTV | 20.13 | 75% | 80% | 0.801 | 79% | |||||||||
| TLU | 50.93 | 75% | 83% | 0.841 | 81% | |||||||||
| SUVsd | 0.45 | 67% | 87% | 0.816 | 83% | |||||||||
| TBRmax + SUVsd + TBRmean | - | 75% | 85% | 0.850 | 83% | |||||||||
| HI + SUVsd + MTV | - | 75% | 83% | 0.848 | 81% | |||||||||
| HI + SUVsd + TLU | - | 75% | 84% | 0.848 | 81% | |||||||||
| Kunz et al., 2011 | [35] | Prospective | 55 | Grade 2:31 | 44 | 33 M 22 F | PET | TAC | Increasing vs. decreasing | 96% | 94% | |||
| Grade 3:22 | MRI | Tumor volume § | - | - | - | |||||||||
| Grade 4:2 | ||||||||||||||
| Grade 2/3 vs. grade 4 | Röhrich et al., 2018 | [36] | Retrospective | 44 | Grade 2:10 | 53 | - | PET | TAC # | LGG-like vs. mixed vs. HGG-like | - | - | - | |
| Grade 3:13 | SUVmax/BG | - | - | - | - | |||||||||
| Grade 4:21 | TTP § | - | - | - | - | |||||||||
| Relative K1 | - | 85% | 60% | 0.766 | ||||||||||
| Relative K2 § | - | - | - | - | ||||||||||
| Relative K3 § | - | - | - | - | ||||||||||
| Relative FD | - | 67% | 78% | 0.716 | ||||||||||
| SUVmax/BG + TTP | - | - | - | 0.745 | ||||||||||
| SUVmax/BG + TTP + relative K1 + relative FD | - | - | - | 0.799 | ||||||||||
| Jansen et al., 2012 | [37] | Retrospective | 127 | No tumor:7 | 46 | 72 M 55 F | PET | TAC # | Increasing vs. decreasing | 95% | 72% | |||
| Grade 1:4 | FET uptake # | Reduced vs. normal vs. increased | - | - | ||||||||||
| Grade 2:69 | FET uptake pattern § | Inhomogeneous vs. diffuse vs. focal | - | - | ||||||||||
| Grade 3:42 | SUVmax/BG § | - | - | - | ||||||||||
| Grade 4:5 | SUVmean/BG § | - | - | - | ||||||||||
| BTV § | - | - | - | |||||||||||
| grade 2 vs. 3 | Jansen et al., 2012 | [38] | Prospective | 144 | Grade 2:79 | 45 | 84 M 60 F | PET | TAC # | Decreasing | 88% | 63% | ||
| Grade 3:65 | SUVmax/BG § | - | - | - | ||||||||||
| BTV § | - | - | - | |||||||||||
| SUVtotal/BG § | - | - | - | |||||||||||
| SUVmean/BG § | - | - | - | |||||||||||
| grade 3 vs. 4 | Pyka et al., 2016 | [39] | Retrospective | 113 | Grade 3:26 | 59 | 43 M 70 F | PET | TBRmax § | 2.74 | 0.614 | |||
| Grade 4:87 | TBRmean | 1.68 | 0.644 | |||||||||||
| MTV | 19.7 | 0.710 | ||||||||||||
| TLU | 46.2 | 0.704 | ||||||||||||
| Textural parameters: | ||||||||||||||
| Coarseness | 0.607 | 0.757 | ||||||||||||
| Contrast | 0.203 | 0.775 | ||||||||||||
| Busyness | 1.12 | 0.737 | ||||||||||||
| Complexity | 0.069 | 0.633 | ||||||||||||
| Combined | 2.05 | 0.830 | ||||||||||||
| IDH status determination | ||||||||||||||
| Hua et al., 2021 | [34] | Retrospective | 58 | Grade 2:33 | 42 | 37 M 21 F | PET | TBRmax | 2.21 | 48% | 87% | 0.658 | 72% | |
| Grade 3:13 | TBRpeak § | 2.15 | 57% | 73% | 0.638 | 67% | ||||||||
| Grade 4:12 | TBRmean § | 1.84 | 62% | 68% | 0.633 | 66% | ||||||||
| COV | 8.85 | 52% | 76% | 0.650 | 67% | |||||||||
| HI | 1.26 | 48% | 87% | 0.676 | 72% | |||||||||
| MTV | 19.48 | 90% | 46% | 0.660 | 62% | |||||||||
| TLU | 28.95 | 81% | 57% | 0.698 | 66% | |||||||||
| SUVsd | 0.11 | 47% | 57% | 0.710 | 66% | |||||||||
| TBRmax + SUVsd + TBRmean | - | 76% | 84% | 0.821 | 81% | |||||||||
| HI + SUVsd + MTV | - | 86% | 81% | 0.804 | 83% | |||||||||
| HI + SUVsd + TLU | - | 76% | 84% | 0.799 | 81% | |||||||||
| Zhou et al., 2021 | [40] | Retrospective | 58 | Grade 2:31 | - | 26 M 22 F | PET | SUVSD | 0.23 | - | - | - | - | |
| Grade 3:14 | TLU § | - | - | - | - | - | ||||||||
| Grade 4:13 | MTV § | - | - | - | - | - | ||||||||
| TBRmax § | - | - | - | - | - | |||||||||
| TBRmean § | - | - | - | - | - | |||||||||
| TBRpeak § | - | - | - | - | - | |||||||||
| Midline involvement | Yes vs. no | - | - | - | - | |||||||||
| Simple predictive model | - | 85% | 71% | 0.786 | 76% | |||||||||
| Radiomics models: | ||||||||||||||
| PET-Rad model | - | 80% | 74% | 0.812 | 76% | |||||||||
| CT | CT-Rad model | - | 85% | 76% | 0.883 | 79% | ||||||||
| PET/CT | PET/CT-Rad model | - | 85% | 87% | 0.912 | 86% | ||||||||
| Lohmann et al., 2018 | [41] | Retrospective | 84 | Grade 2:7 | 54 | 50 M 34 F | PET | TBRmean | 1.68 | 12% | 100% | 0.66 | 73% | |
| Grade 3:26 | TBRmax § | 2.07 | 8% | 100% | 0.59 | 71% | ||||||||
| Grade 4:51 | TTP | 45 min | 27% | 93% | 0.75 | 73% | ||||||||
| Slope | 0.30 SUV/h | 58% | 90% | 0.79 | 80% | |||||||||
| Slope + Radiomic feature SZHGE | - | 54% | 93% | - | 81% | |||||||||
| Radiomic features: | ||||||||||||||
| SkewnessH § | - | 31% | 90% | 0.53 | 71% | |||||||||
| LRHGE § | - | 8% | 100% | 0.52 | 71% | |||||||||
| Verger et al., 2018 | [42] | Retrospective | 90 | Grade 2:16 | 51 | 55 M 35 F | PET | TBRmean | 1.85 | 44% | 92% | 0.73 | 69% | |
| Grade 3:27 | TBRmax | 2.15 | 56% | 77% | 0.68 | 67% | ||||||||
| Grade 4:47 | TTP | 25 min | 86% | 60% | 0.75 | 72% | ||||||||
| Slope | −0.26 SUV/h | 81% | 60% | 0.75 | 70% | |||||||||
| TBRmean + TBRmax | 1.85 and 2.15 | 44% | 91% | - | 69% | |||||||||
| TTP + Slope | 25 min and −0.26 SUV/h | 77% | 70% | - | 73% | |||||||||
| TBRmean + TTP | 1.85 and 25 min | 40% | 96% | - | 69% | |||||||||
| TBRmax + TTP | 2.15 and 25 min | 51% | 94% | - | 73% | |||||||||
| TBRmean + Slope | 1.85 and −0.26 SUV/h | 40% | 94% | - | 68% | |||||||||
| TBRmax + Slope | 2.15 and −0.26 SUV/h | 47% | 91% | - | 70% | |||||||||
| Blanc-Durand et al., 2018 | [43] | Retrospective | 37 | Grade 1:3 | 45 | 23 M 14 F | PET | TBRmax | - | - | ||||
| Grade 2:15 | TBRmean | - | - | |||||||||||
| Grade 3:14 | TTP | - | - | |||||||||||
| Grade 4:5 | Slope | - | - | |||||||||||
| TAC | Centroid #1 vs. centroid #3 | - | - | |||||||||||
| Bette et al., 2016 | [44] | Retrospective | 65 | Grade 1:11 | 38 | 36 M 29 F | PET | TBR # | 1.3 | 89% | 36% | |||
| Grade 2:54 | TBR # | 1.6 | 71% | 53% | ||||||||||
| TBR # | 2.0 | 57% | 68% | |||||||||||
| TBRmax § | - | - | - | |||||||||||
| Prediction of oligodendroglial components | ||||||||||||||
| Jansen et al., 2012 | [38] | Prospective | 144 | Grade 2:79 | 45 | 84 M 60 F | PET | SUVmax/BG | 2.6 | 70% | 72% | |||
| Grade 3:65 | BTV | 4.0 | 71% | 69% | ||||||||||
| SUVmean/BG | 2.1 | 61% | 59% | |||||||||||
| SUVtotal/BG | 6.9 | 75% | 66% | |||||||||||
| Bette et al., 2016 | [44] | Retrospective | 65 | Grade 1:11 | 38 | 36 M 29 F | PET | TBR # | 1.3 | 100% | 23% | |||
| Grade 2:54 | TBR # | 1.6 | 93% | 48% | ||||||||||
| TBR # | 2.0 | 86% | 65% | |||||||||||
| TBRmax | - | - | - | |||||||||||
| Guided resection/biopsy | ||||||||||||||
| Ort et al., 2021 | [45] | Retrospective | 30 | Grade 3:5 | 59 | 19 M 11 F | PET | BTV | 1 cm3 | |||||
| Grade 4:25 | ||||||||||||||
| Floeth et al., 2011 | [46] | Prospective | 30 patients/38 biopsies | Grade 2:17 | 43 | 20 M 10 F | PET | TBR | 1.6 | |||||
| Grade 3:19 | MRI | Gd-DTPA enhancement | - | |||||||||||
| Grade 4:2 | 5-ALA-fluorescence | Fluorescent areas | - | |||||||||||
| Ewelt et al., 2011 | [47] | Prospective | 30 | Grade 2:13 | 42 | 20 M 10 F | LGG subgroup: | |||||||
| Grade 3:15 | PET | Tumor/brain tissue ratio | 1.6 | 54% | 12% | |||||||||
| Grade 4:2 | MRI | Gd enhancement | - | 8% | 36% | |||||||||
| 5-ALA-fluorescence | Fluorescent areas | - | 8% | 29% | ||||||||||
| PET/MRI | - | - | 8% | 35% | ||||||||||
| MRI/5-ALA | - | - | 8% | 41% | ||||||||||
| PET/5-ALA | - | - | 8% | 29% | ||||||||||
| PET/MRI/5-ALA | - | - | 8% | 41% | ||||||||||
| HGG subgroup: | ||||||||||||||
| PET | Tumor/brain tissue ratio | 1.6 | 88% | 46% | ||||||||||
| MRI | Gd enhancement | - | 65% | 92% | ||||||||||
| 5-ALA-fluorescence | Fluorescent areas | - | 71% | 92% | ||||||||||
| PET/MRI | - | - | 65% | 92% | ||||||||||
| MRI/5-ALA | - | - | 59% | 92% | ||||||||||
| PET/5-ALA | - | - | 71% | 92% | ||||||||||
| PET/MRI/5-ALA | - | - | 59% | 92% | ||||||||||
| Verburg et al., 2020 | [48] | Prospective | 20 | Grade 2:8 | - | 12 M 8 F | PET | TBR | - | - | - | 0.76 | ||
| Grade 4:12 | T1G-MRI | - | - | - | - | 0.56 | ||||||||
| PET/MRI | ADC + TBR | - | - | - | 0.89 | |||||||||
| Detection of residual tumor | ||||||||||||||
| Buchmann et al., 2016 | [49] | Retrospective | 62 | Grade 4:62 | 61 | 37 M 25 F | PET | TBR | 1.6 | |||||
| MRI | Contrast-enhanced tissue areas | - | ||||||||||||
| Kläsner et al., 2015 | [50] | Prospective | 25 | Grade 2:4 | 62 | 16 M 9 F | PET | Visual uptake | >Background | |||||
| Grade 3:3 | MRI | Contrast-enhancement volume | 0.175 cm2 | |||||||||||
| Grade 4:18 | ||||||||||||||
| Guided radiotherapy | ||||||||||||||
| Allard et al., 2022 | [51] | Prospective | 23 | Grade 3:3 | 59 | 14 M 9 F | PET | TBRmax # | 1.6 | |||||
| Grade 4:20 | SUVmax # | 30% | ||||||||||||
| SUVmax # | 40% | |||||||||||||
| SUVmax # | 50% | |||||||||||||
| SUVmax # | 60% | |||||||||||||
| SUVmax # | 70% | |||||||||||||
| SUVmax # | 80% | |||||||||||||
| SUVmax # | 90% | |||||||||||||
| CE-MRI | Visual analysis # | - | ||||||||||||
| Munck af Rosenschold et al., 2015 | [52] | Prospective | 54 | Grade 3:19 | 55 | - | PET | TBR # | 1.6 | |||||
| Grade 4:35 | CE-MRI | Visual analysis # | - | |||||||||||
| Fleischmann et al., 2020 | [53] | Retrospective | 36 | Grade 4:36 | 66 | 20 M 16 F | PET | TBRmax # | 1.6 | |||||
| MRI | Visual analysis # | |||||||||||||
| Harat et al., 2016 | [54] | Prospective | 34 | Grade 4:34 | - | - | PET | FET uptake # | 1.6 x SUVmean | |||||
| MRI | Visual analysis # | - | ||||||||||||
| Dissaux et al., 2020 | [55] | Prospective | 30 | Grade 3:5 | 63 | 20 M 10 F | PET | TBR# | 1.6 | |||||
| Grade 4:25 | MRI | Visual analysis # | - | |||||||||||
| Hayes et al., 2018 | [56] | Retrospective | 26 | Grade 3:5 | 61 | 17 M 9 F | PET | TBR # | 1.6 | |||||
| Grade 4:21 | CE-MRI | Visual analysis # | - | |||||||||||
| FLAIR-MRI | Visual analysis # | - | ||||||||||||
| Detection of malignant transformation in LGG | ||||||||||||||
| Galldiks et al., 2013 | [57] | Prospective | 27 | Grade 2:27 | 44 | 18 M 9 F | PET | TBRmax | ∆33% | 72% | 89% | 0.87 | 78% | |
| TBRmean | ∆13% | 72% | 78% | 0.80 | 74% | |||||||||
| TTP | ∆-6 min | 72% | 89% | 0.78 | 78% | |||||||||
| Kinetic pattern change | I to II/III | 72% | 89% | - | 78% | |||||||||
| TBRmax + TTP + Kinetic pattern change | ∆ + 33% or ∆-6 min or I to II/III | 83% | 78% | - | 81% | |||||||||
| MRI | Contrast enhancement change | - | 44% | 100% | - | 63% | ||||||||
| Unterrainer et al., 2016 | [58] | Retrospective | 31 | Grade 2:26 | 38 | 18 M 13 F | PET | TBRmax | 2.46 | 82% | 89% | 0.92 | 85% | |
| Grade 3:5 | TTPmin | 17.5 min | 73% | 67% | - | 70% | ||||||||
| Bashir et al., 2018 | [59] | Retrospective | 42 patients/47 PET | Inconclusive:2 | 41 | 18 M 24 F | PET | TBRmax § | - | 57% | 41% | 0.476 | ||
| Grade 1:1 | TAC § | - | 71% | 41% | 0.549 | |||||||||
| Grade 1/2:1 | TTP § | 25 min | 57% | 47% | 0.511 | |||||||||
| Grade 2:43 | TBRmax + TAC + TTP § | 1.6 + II/III + 25 min | 65% | 58% | 0.634 | |||||||||
| TBRmax + TAC§ | 1.6 + II/III | 65% | 58% | 0.639 | ||||||||||
| TBRmax + TTP § | 1.6 + 25 min | 96% | 25% | 0.591 | ||||||||||
| MRI | Contrast enhancement § (CE) | new area | 43% | 77% | 0.597 | |||||||||
| PET/MRI | TBRmax + TAC + TTP + CE § | - | 70% | 50% | 0.643 | |||||||||
| TBRmax + TAC + CE § | - | 52% | 75% | 0.656 | ||||||||||
| TBRmax + TTP + CE § | - | 57% | 58% | 0.620 | ||||||||||
| Recurrence vs. treatment-related changes | ||||||||||||||
| Jeong et al., 2010 | [60] | Retrospective | 32 | Grade 2:10 | 47 | 12 M 20 F | PET | SUVmax | 1.66 | 87% | 100% | 0.978 | ||
| Grade 3:8 | LNR | 2.18 | 86% | 88% | 0.940 | |||||||||
| Grade 4:14 | LGG subgroup: | |||||||||||||
| SUVmax | 1.48 | 88% | 89% | 0.951 | ||||||||||
| LNR | 1.64 | 100% | 75% | 0.893 | ||||||||||
| HGG subgroup: | ||||||||||||||
| SUVmax | 1.66 | 93% | 100% | 0.993 | ||||||||||
| LNR | 2.46 | 86% | 100% | 0.964 | ||||||||||
| Jansen et al., 2013 | [61] | Prospective | 33 | Grade 3:20 | - | - | PET | BTV after 6 months | - | |||||
| Grade 4:13 | SUVmax/BG after 6 months | - | ||||||||||||
| Puranik et al., 2021 | [62] | Retrospective | 72 | Grade 3:13 | - | 47 M 25 F | PET | T/Wm | 2.65 | 80% | 88% | |||
| Grade 4:59 | ||||||||||||||
| Kertels et al., 2019 | [63] | Retrospective | 36 | Grade 4:36 | 54 | 22 M 14 F | PET | TBRmax | 3.69 | 79% | 88% | 0.86 | ||
| TBRmax | 3.58 | 64% | 100% | 0.84 | ||||||||||
| TBRmax | 3.44 | 86% | 88% | 0.86 | ||||||||||
| TBRmean | 2.31 | 61% | 100% | 0.83 | ||||||||||
| TBRmean | 2.19 | 71% | 88% | 0.80 | ||||||||||
| TBR16 mm | 2.44 | 82% | 75% | 0.82 | ||||||||||
| TBR10 mm | 2.86 | 86% | 75% | 0.81 | ||||||||||
| TBR90% | 3.23 | 71% | 100% | 0.85 | ||||||||||
| TBR80% | 3.08 | 82% | 88% | 0.88 | ||||||||||
| TBR70% | 2.72 | 86% | 88% | 0.87 | ||||||||||
| Verger et al., 2018 | [64] | Retrospective | 31 patients/32 tumors | Grade 2:2 | 52 | 16 M 15 F | PET | TBRmax | 2.61 | 80% | 86% | 0.78 | 81% | |
| Grade 3:3 | TBRmean § | - | - | - | 0.74 | - | ||||||||
| Grade 4:27 | TTP § | - | - | - | 0.71 | - | ||||||||
| Slope § | - | - | - | 0.70 | - | |||||||||
| PWI rCBF | TBRmax § | - | - | - | 0.65 | - | ||||||||
| TBRmean § | - | - | - | 0.55 | - | |||||||||
| PWI rCBV | TBRmax § | - | - | - | 0.58 | - | ||||||||
| TBRmean § | - | - | - | 0.64 | - | |||||||||
| PWI MTT | TBRmax § | - | - | - | 0.59 | - | ||||||||
| TBRmean § | - | - | - | 0.59 | - | |||||||||
| Pyka et al., 2018 | [65] | Retrospective | 47 patients/63 lesions | Grade 2:5 | 54 | 22 M 25 F | PET | TBR30–40 min | 2.07 | 80% | 85% | 0.863 | ||
| Grade 3:20 | TBR10–20 min | 1.71 | 76% | 85% | 0.848 | |||||||||
| Grade 4:38 | TTP | 20 min | 64% | 79% | 0.728 | |||||||||
| PWI MRI | rCBVuncor | 4.32 | 62% | 77% | 0.726 | |||||||||
| rCBVcor | 3.35 | 66% | 77% | 0.708 | ||||||||||
| DWI MRI | ADC | 1610 × 10−6 mm2/s | 50% | 77% | 0.688 | |||||||||
| nADC | 1.22 | 62% | 77% | 0.697 | ||||||||||
| FA § | 98.9 | 65% | 62% | 0.593 | ||||||||||
| PET/MRI | TBR30–40 min + TTP + rCBVcor + nADC | - | 78% | 92% | 0.891 | |||||||||
| Werner et al., 2021 | [66] | Retrospective | 23 | Grade 4:23 | 58 | 13 M 10 F | PET | TBRmax | 2.85 | 64% | 92% | 0.75 | 78% | |
| TBRmean | 1.95 | 82% | 92% | 0.77 | 87% | |||||||||
| Slope § | 0.02 SUV/h | 73% | 75% | 0.72 | 74% | |||||||||
| TTP | 35 min | 64% | 83% | 0.82 | 74% | |||||||||
| TBRmax + TTP | 2.85 and 35 min | 36% | 100% | - | 70% | |||||||||
| TBRmean + TTP | 1.95 and 35 min | 55% | 100% | - | 78% | |||||||||
| MRI | RANO criteria § | - | 30% | 79% | - | 58% | ||||||||
| Galldiks et al., 2015 | [67] | Retrospective | 22 | Grade 4:22 | 56 | 14 M 8 F | PET | TBRmax | 2.3 | 100% | 91% | 0.94 | 96% | |
| TBRmean | 2.0 | 82% | 82% | 0.91 | 82% | |||||||||
| Kinetic pattern | II/III | - | - | - | - | |||||||||
| TBRmax+ Kinetic pattern | 2.3 and II/III | 80% | 91% | - | 86% | |||||||||
| TBRmean+ Kinetic pattern | 2.0 and II/III | 60% | 91% | - | 76% | |||||||||
| Werner et al., 2019 | [68] | Retrospective | 48 | Grade 3:8 | 50 | 29 M 19 F | PET | TBRmax | 1.95 | 100% | 79% | 0.89 | 83% | |
| Grade 4:40 | TBRmean | 1.95 | 100% | 79% | 0.89 | 83% | ||||||||
| TTP | 32.5 min | 80% | 69% | 0.79 | 72% | |||||||||
| Slope | 0.32 SUV/h | 70% | 75% | 0.82 | 74% | |||||||||
| TBRmax/mean + TTP | 1.95 and 32.5 min | 89% | 91% | - | 90% | |||||||||
| TBRmax/mean + Slope | 1.95 and 0.32 SUV/h | 78% | 97% | - | 93% | |||||||||
| DWI-MRI | Visual assessment § | - | 70% | 66% | - | 67% | ||||||||
| ADC § | 1.09×10−3 mm2/s | 60% | 71% | 0.73 | 69% | |||||||||
| PET/MRI | TBRmax/mean + ADC | - | 67% | 94% | - | 89% | ||||||||
| Lohmann et al., 2020 | [69] | Retrospective | 34 | Grade 3:1 | 57 | 21 M 13 F | PET | TBRmax | 2.25 | 81% | 67% | 0.79 | 74% | |
| Grade 4:33 | TBRmean | 1.95 | 75% | 61% | 0.73 | 68% | ||||||||
| TTP § | 25 min | 75% | 44% | 0.61 | 59% | |||||||||
| Slope § | 0.3 SUV/h | 56% | 61% | 0.55 | 59% | |||||||||
| TBRmean + TBRmax | - | 75% | 72% | - | 74% | |||||||||
| TBRmean + TTP | - | 69% | 78% | - | 74% | |||||||||
| TBRmean + Slope § | - | 50% | 78% | - | 65% | |||||||||
| TBRmax + TTP | - | 69% | 83% | - | 76% | |||||||||
| TBRmax + Slope | - | 50% | 89% | - | 71% | |||||||||
| TTP + Slope § | - | 56% | 61% | - | 59% | |||||||||
| TBRmax + TBRmean + TTP | - | 69% | 89% | - | 79% | |||||||||
| Radiomics features | - | 100% | 40% | 0.74 | 70% | |||||||||
| Kebir et al., 2016 | [70] | Retrospective | 26 | Grade 4:26 | 58 | 21 M 5 F | PET | TBRmax | 1.9 | 84% | 86% | 0.88 | 85% | |
| TBRmean | 1.9 | 74% | 86% | 0.86 | 77% | |||||||||
| TAC | II/III | 84% | 100% | - | 89% | |||||||||
| TTP | - | - | - | 0.86 | - | |||||||||
| Rachinger et al., 2005 | [71] | Retrospective | 45 | Grade 1:1 | 45 | 23 M 22 F | PET | SUVmax | 2.2 | 100% | 93% | |||
| Grade 2:10 | MRI | Volume/Gd-enhancing area | ∆25%/new area | 94% | 50% | |||||||||
| Grade 3:12 | ||||||||||||||
| Grade 4:22 | ||||||||||||||
| Lohmeier et al., 2019 | [72] | Retrospective | 42 | Grade 1–2:2 | 47 | 32 M 10 F | PET | SUVmax § | - | - | - | - | ||
| Grade 3–4:40 | SUV80mean § | - | - | - | - | |||||||||
| SUV-BG § | - | - | - | - | ||||||||||
| TBR80mean | - | - | - | - | ||||||||||
| TBRmax | 2.0 | 81% | 60% | 0.81 | ||||||||||
| DWI-MRI | ADCmean | 1254 × 10−6 mm2/s | 62% | 100% | 0.82 | |||||||||
| ADC-BG § | - | - | - | - | ||||||||||
| rADCmean | - | - | - | - | ||||||||||
| PET/MRI | TBRmax + ADCmean | - | 97% | 60% | 0.90 | |||||||||
| Bashir et al., 2019 | [73] | Retrospective | 146 | Grade 4:146 | 60 | 96 M 50 F | PET | TBRmax | 2.0 | 99% | 94% | 0.970 | 99% | |
| TBRmean | 1.8 | 96% | 94% | 0.977 | 96% | |||||||||
| BTV | 0.55 cm3 | 98% | 94% | 0.955 | 98% | |||||||||
| Steidl et al., 2020 | [74] | Retrospective | 104 | Grade 2:9 | 52 | 68 M 36 F | PET | TBRmax | 1.95 | 70% | 60% | 0.72 | 68% | |
| Grade 3:24 | TBRmean | - | - | - | 0.72 | - | ||||||||
| Grade 4:71 | TTP § | - | - | - | 0.60 | - | ||||||||
| Slope | 0.69 SUV/h | 84% | 62% | 0.69 | 80% | |||||||||
| TBRmax + Slope # | 1.95 and/or 0.69 SUV/h | 96% | 43% | - | 86% | |||||||||
| MRI | rCBVmax | 2.85 | 54% | 100% | 0.75 | 63% | ||||||||
| PET/MRI | rCBVmax + TBRmax + Slope # | - | 98% | 43% | - | 87% | ||||||||
| Pöpperl et al., 2006 | [75] | Prospective | 24 | Grade 3:5 | 49 | 15 M 9 F | PET | Tumax/BG # | 2.0 | 100% | 78% | |||
| Grade 4:19 | Tumax/BG # | 2.1 | 97% | 91% | ||||||||||
| Tumax/BG # | 2.2 | 82% | 95% | |||||||||||
| Tumax/BG # | 2.3 | 74% | 98% | |||||||||||
| Tumax/BG # | 2.4 | 74% | 100% | |||||||||||
| Tumax/BG # | 2.5 | 62% | 100% | |||||||||||
| Visual analysis # | Nodular vs. non-nodular | 94% | 94% | |||||||||||
| Müller et al., 2022 | [76] | Retrospective | 151 | Grade 2:28 | 52 | 97 M 54 F | PET | TBRmax | - | - | - | - | ||
| Grade 3:40 | TBRmean | - | - | - | - | |||||||||
| Grade 4:83 | TBRmax + TBRmean # | - | 66% | 80% | 0.78 | |||||||||
| Radiomics features # | - | 73% | 80% | 0.85 | ||||||||||
| TBRmax + TBRmean + radiomics features # | - | 81% | 70% | 0.85 | ||||||||||
| Mehrkens et al., 2008 | [77] | Prospective | 31 | Grade 2:17 | 46 | 17 M 14 F | PET | SUVmax/BG § | 2.0 | |||||
| Grade 3:6 | ||||||||||||||
| Grade 4:8 | ||||||||||||||
| Galldiks et al., 2015 | [78] | Retrospective | 124 | Grade 2:55 | 52 | 81 M 43 F | PET | TBRmax | 2.3 | 68% | 100% | 0.85 | 71% | |
| Grade 3:19 | TBRmean | 2.0 | 74% | 91% | 0.91 | 75% | ||||||||
| Grade 4:50 | TTP | 45 min | 82% | 73% | 0.81 | 81% | ||||||||
| Curve pattern | II/III | 78% | 73% | - | 77% | |||||||||
| TBRmax + Curve pattern | 2.3 and/or II/III | 93% | 73% | - | 91% | |||||||||
| TBRmean + Curve pattern | 2.0 and/or II/III | 93% | 73% | - | 91% | |||||||||
| TBRmax + TTP | 2.3 and/or 45 min | 92% | 73% | - | 90% | |||||||||
| TBRmean + TTP | 2.0 and/or 45 min | 93% | 100% | - | 93% | |||||||||
| MRI | RANO criteria § | - | 92% | 9% | - | 85% | ||||||||
| Pöpperl et al., 2004 | [79] | Prospective | 53 | Grade 1:1 | - | 28 M 25 F | PET | SUVmax | 2.2 | |||||
| Grade 2:9 | SUVmax/BG | 2.0 | ||||||||||||
| Grade 3:16 | SUV80/BG | - | ||||||||||||
| Grade 4:27 | SUV70/BG | - | ||||||||||||
| Prognosis/Treatment response evaluation | ||||||||||||||
| Müther et al., 2019 | [80] | Prospective | 31 | Grade 4:31 | 67 | 13 M 18 F | PET | Volume | 4.3 cm3 | |||||
| Jansen et al., 2013 | [61] | Prospective | 33 | Grade 3:20 | - | - | PET | Uptake kinetics | Increasing | |||||
| Grade 4:13 | ||||||||||||||
| Suchorska et al., 2018 | [81] | Retrospective | 61 | Grade 2:44 | 46 | 31 M 30 F | PET | Initial BTV § | - | |||||
| Grade 3:17 | Initial TBRmax § | - | ||||||||||||
| Initial TAC § | Increasing vs. decreasing | |||||||||||||
| BTV after 6 months | - | |||||||||||||
| TBRmax after 6 months § | - | |||||||||||||
| TAC after 6 months § | Increasing vs. decreasing | |||||||||||||
| BTV response | ∆ ± 25% | |||||||||||||
| TBRmax response | ∆ ± 10% | |||||||||||||
| TAC response § | Stable increasing vs. Decreasing to increasing vs. Increasing to decreasing vs. Stable decreasing | |||||||||||||
| FET-PET response | Yes vs. no | |||||||||||||
| MRI | Initial T2 volume | - | ||||||||||||
| T2 volume after 6 months | - | |||||||||||||
| T2 volume response § | RD vs. SD vs. PD | |||||||||||||
| Galldiks et al., 2012 | [82] | Prospective | 25 | Grade 4:25 | 54 | 15 M 10 F | PET | TBRmax change | ∆-10% (PFS)/∆-20% (OS) | 83% (OS) | 67% (OS) | 0.75 (OS) | ||
| TBRmean change | ∆-5% | 67% | 75% | 0.72 | ||||||||||
| Tvol 1.6 change | ∆0% (PFS) | - | - | - | ||||||||||
| MRI | Gd-volume § | ∆0%/∆-25% | - | - | - | |||||||||
| Suchorska et al., 2015 | [83] | Prospective | 79 | Grade 4:79 | - | - | PET | BTVpreRCx | 9.5 cm3 | 64% | 70% | |||
| LBRmax-preRCx | 2.9 (OS) | 68% | 73% | |||||||||||
| Initial TAC | Increasing vs. decreasing (OS) | - | - | |||||||||||
| MRI | Gd+ volume | - | - | - | ||||||||||
| Jansen et al., 2014 | [84] | Retrospective | 59 | Grade 2:59 | 43 | 32 M 27 F | PET | TAC | Increasing vs. decreasing | |||||
| Uptake § | Positive vs. negative | |||||||||||||
| SUVmax/BG § | - | |||||||||||||
| SUVmean/BG § | - | |||||||||||||
| SUVtotal/BG § | - | |||||||||||||
| BTV § | - | |||||||||||||
| MRI | Contrast enhancement § | Yes vs. no | ||||||||||||
| Largest diameter | 6 cm (PFS) | |||||||||||||
| Tumor crossing midline § | Yes vs. no | |||||||||||||
| Thon et al., 2015 | [85] | Prospective | 98 | Grade 2:54 | - | 56 M 42 F | PET | TAC | Homogeneous decreasing vs. focal decreasing vs. homogeneous increasing | |||||
| Grade 3:40 | SUVmax § | 2.3 | ||||||||||||
| Grade 4:4 | MRI | Tumor volume § | 35 mL | |||||||||||
| Kunz et al., 2018 | [86] | Prospective | 98 | Grade 2:59 | - | - | PET | TAC | Homogeneous increasing vs. mixed vs. homogeneous decreasing | |||||
| Grade 3:35 | TTPmin | >25 min vs. 12.5 < t ≤ 25 min vs. ≤12.5 min | ||||||||||||
| Grade 4:4 | SUVmax § | 2.3 | ||||||||||||
| MRI | Tumor volume § | 35 mL | ||||||||||||
| Ceccon et al., 2021 | [87] | Prospective | 41 | Grade 2:1 | 52 | 22 M 19 F | PET | TBRmax baseline | 2.0 (PFS)/1.9 § (OS) | |||||
| Grade 3:2 | TBRmean baseline § | 1.9 (PFS)/1.8 (OS) | ||||||||||||
| Grade 4:38 | MTV baseline | 28.2 mL (PFS)/13.8 mL (OS) | ||||||||||||
| TBRmax change | 0% | |||||||||||||
| TBRmean change § | 0% | |||||||||||||
| MTV change | 0% | |||||||||||||
| MRI | RANO criteria § | SD/PR/CR vs. PD | ||||||||||||
| Galldiks et al., 2018 | [88] | Prospective | 21 | Grade 4:21 | 55 | 13 M 8 F | PET | TBRmax relative reduction § | 27% | 92% | 63% | 0.78 | ||
| TBRmean relative reduction § | 16% | 92% | 63% | 0.81 | ||||||||||
| MTV relative reduction § | 27% | 77% | 63% | 0.82 | ||||||||||
| Absolute MTV at follow-up | 5 mL | 85% | 88% | 0.92 | ||||||||||
| MRI | RANO criteria § | PR or SD | 63% | 69% | - | |||||||||
| Carles et al., 2021 | [89] | Prospective | 32 | Grade 4:32 | 52 | 17 M 15 F | PET | Radiomic features: | ||||||
| SUVmin & | - | |||||||||||||
| SUVmean & | - | |||||||||||||
| GLV & | - | |||||||||||||
| GLV2 & | - | |||||||||||||
| WF_GLV & | - | |||||||||||||
| Qacor & | - | |||||||||||||
| QHGZE & | - | |||||||||||||
| QSZHGE & | - | |||||||||||||
| QGLN2 & | - | |||||||||||||
| QHGRE & | - | |||||||||||||
| QSRHGE & | - | |||||||||||||
| QLRHGE & | - | |||||||||||||
| SZLGE | - | |||||||||||||
| Busyness & | - | |||||||||||||
| WF_TS & | - | |||||||||||||
| QvarianceCM & | - | |||||||||||||
| Eccentricity & | - | |||||||||||||
| SUVmean + WF_GLV + QLRHGE + SUVmin | - | |||||||||||||
| SZLGE + Busyness + QVarianceCM + Eccentricity | - | |||||||||||||
| Suchorska et al., 2018 | [90] | Retrospective | 300 | Grade 2:121 | 48 | 166 M 134 F | PET | TBRmax § | 1.6 | |||||
| Grade 3:106 | TBRmax § | 2.6 | ||||||||||||
| Grade 4:73 | TTPmin | 17.5 min (OS) | ||||||||||||
| MRI | Contrast enhancement § | Yes vs. no | ||||||||||||
| T2 volume § | 49 mL | |||||||||||||
| Wirsching et al., 2021 | [91] | Retrospective | 31 | Grade 4:31 | - | - | PET | TBR in non-contrast enhancing tumor portions at follow-up | High vs. low | |||||
| MRI | Contrast enhancement at baseline | - | ||||||||||||
| ADC at baseline | - | |||||||||||||
| Contrast enhancement at follow-up | - | |||||||||||||
| Sweeney et al., 2013 | [92] | Retrospective | 28 | Grade 2:5 | - | 21 M 7 F | PET | SUVmax | 2.6 | |||||
| Grade 3:12 | TBRmax § | - | ||||||||||||
| Grade 4:11 | TBRmean§ | - | ||||||||||||
| Tumor volume § | ||||||||||||||
| VolSUVmax ≥ 2.2 | - | |||||||||||||
| Vol ≥ 40%SUVmax | - | |||||||||||||
| MRI | VolMRI | - | ||||||||||||
| PET/MRI | VolMRI + VolSUVmax ≥ 2.2 | - | ||||||||||||
| VolMRI + Vol≥ 40%SUVmax | - | |||||||||||||
| Non-overlap, VolMRI + VolSUVmax ≥ 2.2 | - | |||||||||||||
| Non-overlap, VolMRI + Vol ≥ 40%SUVmax | - | |||||||||||||
| Pyka et al., 2014 | [93] | Retrospective | 34 | Grade 1:2 | 41 | 22 M 12 F | PET | TBRmax | 2.5 | 0.696 | ||||
| Grade 2:19 | TBRmean | 2.3 | 0.696 | |||||||||||
| Grade 3:3 | TTP | 20 min | 0.848 | |||||||||||
| Grade 4:10 | Peak TBR | 2.2 | 0.704 | |||||||||||
| Slope-to-peak | 7 × 10−5/s | 0.711 | ||||||||||||
| Wollring et al., 2022 | [94] | Retrospective | 36 | Grade 3:8 | 54 | 20 M 16 F | PET | New distant FET hotspot | Yes vs. no | |||||
| Grade 4:28 | TBRmax change | 0% | ||||||||||||
| TBRmean change § | 0% | |||||||||||||
| MTV change | 0% | |||||||||||||
| TTP change § | 0% | |||||||||||||
| MRI | RANO criteria | SD/PR/CR vs. PD | ||||||||||||
| Bauer et al., 2020 | [95] | Retrospective | 60 | Grade 3:15 | 55 | 35 M 25 F | PET | TBRmax § | 2.55 | 70% | 57% | 0.63 | ||
| Grade 4:45 | TBRmean § | 2.05 | 60% | 70% | 0.69 | |||||||||
| MTV § | 11.15 mL | 72% | 54% | 0.56 | ||||||||||
| TTP | 25 min | 90% | 87% | 0.90 | ||||||||||
| Slope § | −0.103 SUV/h | 70% | 90% | 0.77 | ||||||||||
| Piroth et al., 2011 | [96] | Prospective | 44 | Grade 4:44 | 57 | 16 M 28 F | PET | VolTBR ≥ 1.6 | 25 mL | |||||
| VolTBR ≥ 2.0 | 10 mL | |||||||||||||
| TBRmax | 2.4 | |||||||||||||
| TBRmean | 2.0 | |||||||||||||
| MRI | Gd-volume § | 10 mL | ||||||||||||
| Jansen et al., 2015 | [97] | Retrospective | 121 | Grade 3:51 | 54 | 73 M 48 F | PET | TTPmin | 12.5 min | |||||
| Grade 4:70 | SUVmax/BG § | - | ||||||||||||
| SUVmean/BG § | - | |||||||||||||
| BTV § | - | |||||||||||||
| MRI | contrast enhancement § | Yes vs. no | ||||||||||||
| Moller et al., 2016 | [98] | Prospective | 31 | Grade 3:6 | 54 | - | PET | BTV baseline | - | |||||
| Grade 4:25 | Tmax/B baseline # | - | ||||||||||||
| ∆BTV scan 2 § | - | |||||||||||||
| ∆BTV scan 3 § | - | |||||||||||||
| ∆Tmax/B scan 2 # | - | |||||||||||||
| ∆Tmax/B scan 3 # | - | |||||||||||||
| MRI | Volume (+necrosis) § | - | ||||||||||||
| Volume (−necrosis) | - | |||||||||||||
| Dissaux et al., 2020 | [99] | Prospective | 29 | Grade 3:3 | 60 | 17 M 12 F | PET | TBRmax | Median (5.03) | |||||
| Grade 4:26 | TBRmean § | Median | ||||||||||||
| SUVmax § | Median | |||||||||||||
| SUVmean § | Median | |||||||||||||
| SUVpeak § | Median | |||||||||||||
| TLG § | Median | |||||||||||||
| Volume § | Median | |||||||||||||
| Piroth et al., 2011 | [100] | Prospective | 22 | Grade 4:22 | 56 | 13 M 9 F | PET | Volume | 20 mL | |||||
| TBRmax § | 3.0 | |||||||||||||
| TBRmean § | 2.0 | |||||||||||||
| TBRmean | 2.4 | |||||||||||||
| Early TBRmax response | ∆-10% | |||||||||||||
| Early TBRmean response | ∆-10% | |||||||||||||
| MRI | Diameter of contrast-enhanced area | 4 cm | ||||||||||||
| Schneider et al., 2020 | [101] | Retrospective | 42 | Grade 2:19 | 46 | 26 M 16 F | PET | SUVmax | 3.4 | |||||
| Grade 3:23 | TBRmax | 3.03 | ||||||||||||
| BTV | 10 cm3 | |||||||||||||
| Kertels et al., 2019 | [102] | Retrospective | 35 | Grade 2:14 | 48 | 20 M 15 F | PET | FET positivity | Yes vs. no | |||||
| Grade 3:21 | ||||||||||||||
| Floeth et al., 2007 | [103] | Prospective | 33 | Grade 2:33 | - | 20 M 13 F | PET | Mean FET uptake | 1.1 | |||||
| Maximum FET uptake § | 2.0 | |||||||||||||
| MRI | Hemisphere§ | Right vs. left | ||||||||||||
| Brain lobe location § | - | |||||||||||||
| Extension § | Deep vs. superficial | |||||||||||||
| Size § | 3 cm | |||||||||||||
| Mass shift § | Yes vs. no | |||||||||||||
| Appearance | Circumscribed vs. diffuse | |||||||||||||
| PET/MRI | Mean FET uptake + MRI appearance | - | ||||||||||||
| Niyazi et al., 2012 | [104] | Retrospective | 56 | Grade 3:13 | 50 | 34 M 22 F | PET | Kinetics pre re-RT | G1–2 vs. G3 vs. G4–5 | |||||
| Grade 4:43 | Kinetics post re-RT § | G1–2 vs. G3 vs. G4–5 | ||||||||||||
| SUVmax/BG pre re-RT § | 3.3 | |||||||||||||
| SUVmax/BG post re-RT § | 2.6 | |||||||||||||
| SUVmean/BG pre re-RT § | 2.2 | |||||||||||||
| SUVmean/BG post re-RT § | 2.3 | |||||||||||||
| BTV pre re-RT § | 13.7 cc | |||||||||||||
| BTV post re-RT § | 7.3 cc | |||||||||||||
| Pyka et al., 2016 | [39] | Retrospective | 113 | Grade 3:26 | 59 | 43 M 70 F | PET | TBRmax § | 2.5 | |||||
| Grade 4:87 | TBRmean § | 1.56 (PFS)/1.57 (OS) | ||||||||||||
| MTV | 19.4 (PFS) §/18.9 (OS) | |||||||||||||
| TLU | 35.0 (PFS) §/17.1 (OS) | |||||||||||||
| Textural parameters: | ||||||||||||||
| Coarseness | 5.96 × 10−3 (PFS)/6.88 × 10−3 (OS) | |||||||||||||
| Contrast | 0.427 | |||||||||||||
| Busyness | 1.366 (PFS)/0.984 (OS) | |||||||||||||
| Complexity | 0.085 (PFS)/0.094 (OS) | |||||||||||||
| Blanc-Durand et al., 2018 | [43] | Retrospective | 37 | Grade 1:3 | 45 | 23 M 14 F | PET | TBRmax § | - | |||||
| Grade 2:15 | TBRmean § | - | ||||||||||||
| Grade 3:14 | TTP | - | ||||||||||||
| Grade 4:5 | Slope | - | ||||||||||||
| TAC | - |
Regarding PET parameters, we noticed a high variability in the determination of tumor region of interest (ROI) with an impact on the subsequent calculation of tumor-to-brain ratios (TBRs). We consequently sorted different TBRs according to the methodology used to obtain them (Table 3) in order to be able to compare their performances and then grouped every PET parameter in Table 4. We signified the change of parameters in the legend of Table 4 by writing the name of the parameter used in the table and the name of the original parameter(s) corresponding to this approach.
Table 3.
Different tumor-to-brain ratios and the methodology used to obtain them.
| Parameter | Definition |
|---|---|
| TBRmean | Mean uptake in the tumor area with a TBR ≥ 1.6 divided by mean uptake in the normal brain |
| TBRmax | Maximal uptake in the tumor area divided by mean uptake in the normal brain |
| TBR10/16mm | Mean uptake in a ROI/VOI with a diameter of 10/16 mm centered on the tumor area with the highest uptake divided by mean uptake in the normal brain |
| TBR25mm2 | Mean uptake in a standardized ROI/VOI with a size of 25 mm2 placed manually at the biopsy sites centered to the titanium pellets on postoperative images divided by mean uptake in the normal brain |
| TBR3SD | Mean uptake in an isocontour region around the lesion maximum using a cutoff of three standard deviations above average activity in the reference region divided by mean uptake in the normal brain |
| TBR70/80% | Mean in a 70/80% isocontour region divided by mean uptake in the normal brain |
| TBR | Uptake in the tumor area (unspecified) divided by mean uptake in the normal brain |
| SUVmax/mean/BG | SUVmax/mean of the tumor area divided by maximal uptake in the normal brain |
Table 4.
Summary of PET parameters. *: reached significance, X: did not reach significance, &: did not stay significant after Bonferroni multiple-test correction, NA: not available. TBRmax: Lmax/B, SUVmax/BG, LNR, TNR, LBRmax, T/Wm, TBRmax(20–40min), Tmax/B, maximum FET uptake, Tumax/BG; TBR3SD: Lmean/B, mean FET uptake; TBR25mm2: TBR, FET ratio; TBR10mm: TBRmean; TBR16mm: TBRmean, TBRmax; TBR70%: SUV70/BG; TBR80%: SUV80/BG; TBR: UR, FET lesion/brain ratio, FET uptake, tumor/brain tissue ratio, TBRmean, TBRmax; TAC: kinetic pattern, curve pattern; TTP: Tpeak; BTV: volume, MTV, Vol, Tvol 1.6; radiomic features: textural parameters.
| Indication | Number of Studies | Grade | Parameters | Threshold | Sensitivity | Specificity | AUC | Accuracy | Significance |
|---|---|---|---|---|---|---|---|---|---|
| Diagnosis | |||||||||
| 1 | LGG and HGG | Visual grading system | - | - | - | - | - | NA | |
| 1 | LGG and HGG | TBRmax | - | - | - | NA | |||
| 1 | LGG and HGG | TBR25mm2 | 1.6 | 92% | 81% | - | * | ||
| 1 | LGG and HGG | TBR3SD | - | - | - | NA | |||
| 1 | LGG and HGG | TBR | 1.6 | 88% | 88% | - | * | ||
| 1 | LGG and HGG | 18F-FETn uptake | 1.4 x background | 76% | 80% | 0.89 | 78% | * | |
| Grading (LGG vs. HGG) | |||||||||
| 1 | LGG and HGG | FET uptake | Reduced vs. normal vs. increased | - | - | NA | |||
| 1 | LGG and HGG | FET uptake pattern | Inhomogeneous vs. diffuse vs. focal | - | - | X | |||
| 1 | LGG and HGG | Early SUV | 2.32 | 73% | 71% | 72% | * | ||
| 1 | LGG and HGG | Middle SUV | - | - | - | - | - | X | |
| 1 | LGG and HGG | Late SUV | - | - | - | - | - | X | |
| 1 | LGG and HGG | e-m Ratio | 0.93 | 93% | 94% | 94% | * | ||
| 1 | LGG and HGG | e-l Ratio | 0.95 | 87% | 88% | 87% | * | ||
| 1 | LGG and HGG | SoD | 0.5 | 93% | 82% | 87% | * | ||
| 1 | LGG and HGG | SUVmax | - | - | - | * | |||
| Grade 2/3 vs. Grade 4 | 1 | LGG and HGG | SUVsd | 0.45 | 67% | 87% | 0.816 | 83% | * |
| Grade 2/3 vs. Grade 4 | 1 | LGG and HGG | SUVmax/BG | - | - | - | * | ||
| 2 | LGG and HGG | SUVmean/BG | - | - | - | X | |||
| Grade 2 vs. 3 | LGG and HGG | - | - | - | X | ||||
| Grade 2 vs. 3 | 1 | LGG and HGG | SUVtotal/BG | - | - | - | X | ||
| 1 | LGG and HGG | SUV90 10–60 min | 0.2 | 94% | 100% | 0.969 | * | ||
| 1 | LGG and HGG | SUV90 15–60 min | −0.41 | 94% | 100% | 0.965 | * | ||
| 1 | LGG and HGG | TBRmax(0–10min) | 2.8 | 76% | 79% | 76% | * | ||
| 1 | LGG and HGG | TBRmax(5–15min) | 2.7 | 78% | 76% | 77% | * | ||
| 1 | LGG and HGG | TBRmax(5–20min) | 2.6 | 80% | 74% | 76% | * | ||
| 1 | LGG and HGG | TBRmax(10–30min) | 2.5 | 75% | 75% | 74% | * | ||
| 7 | LGG and HGG | TBRmax | 2.58 | 71% | 85% | 0.798 | * | ||
| LGG and HGG | 2.62 | 82% | 68% | 0.83 | 78% | * | |||
| Grade 2/3 vs. Grade 4 | LGG and HGG | 2.67 | 92% | 61% | 0.824 | 67% | * | ||
| LGG and HGG | 2.7 | 67% | 78% | 70% | * | ||||
| LGG and HGG | - | - | - | * | |||||
| LGG and HGG | - | - | - | X | |||||
| Grade 2 vs. 3 | LGG and HGG | - | - | - | X | ||||
| Grade 2/3 vs. Grade 4 | 1 | LGG and HGG | TBRpeak | 2.35 | 92% | 61% | 0.832 | 67% | * |
| 2 | LGG and HGG | TBRmean | 2 | 83% | 58% | 0.65 | 75% | X | |
| Grade 2/3 vs. Grade 4 | LGG and HGG | 2.31 | 58% | 93% | 0.791 | 86% | * | ||
| 1 | LGG and HGG | ∆TBRmean 20–40 min/70–90 min | −8% | 83% | 75% | 0.85 | 81% | * | |
| 1 | LGG and HGG | TBR16mm | 1.69 | 82% | 68% | 0.8 | 78% | * | |
| Grade 3 vs. 4 | 3 | HGG | TBR | 1.68 | - | - | 0.644 | * | |
| Grade 3 vs. 4 | HGG | 2.74 | - | - | 0.614 | X | |||
| LGG and HGG | 3 | - | - | * | |||||
| 4 | LGG and HGG | TTP | 25 min | 87% | 100% | 94% | * | ||
| LGG and HGG | 30 min | 54% | 91% | 0.78 | 65% | * | |||
| LGG and HGG | 35 min | 58% | 92% | 0.76 | 69% | * | |||
| Grade 2/3 vs. Grade 4 | LGG and HGG | - | - | - | - | X | |||
| 1 | LGG and HGG | Slope | −0.03 SUV/h | 64% | 91% | 0.78 | 72% | * | |
| 7 | LGG and HGG | TAC | II/III | 88% | 75% | 83% | * | ||
| LGG and HGG | I/II vs. III | 73% | 100% | 87% | NA | ||||
| LGG and HGG | Decreasing | 90% | 66% | 80% | NA | ||||
| Grade 2 vs. 3 | LGG and HGG | 88% | 63% | NA | |||||
| LGG and HGG | Increasing vs. Decreasing | 95% | 72% | NA | |||||
| LGG and HGG | 96% | 94% | * | ||||||
| Grade 2/3 vs. Grade 4 | LGG and HGG | LGG-like vs. mixed vs. HGG-like | - | - | - | NA | |||
| Grade 2/3 vs. Grade 4 | 1 | LGG and HGG | COV | 27.21 | 58% | 91% | 0.808 | 84% | * |
| Grade 2/3 vs. Grade 4 | 1 | LGG and HGG | HI | 1.77 | 67% | 87% | 0.826 | 83% | * |
| Grade 3 vs. 4 | 4 | HGG | BTV | 19.7 | - | - | 0.71 | * | |
| Grade 2/3 vs. Grade 4 | LGG and HGG | 20.13 | 75% | 80% | 0.801 | 79% | * | ||
| LGG and HGG | - | - | - | X | |||||
| Grade 2 vs. 3 | LGG and HGG | - | - | - | X | ||||
| Grade 3 vs. 4 | 2 | HGG | TLU | 46.2 | - | - | 0.704 | * | |
| Grade 2/3 vs. Grade 4 | LGG and HGG | 50.93 | 75% | 83% | 0.841 | 81% | * | ||
| Grade 2/3 vs. Grade 4 | 1 | LGG and HGG | Relative K1 | - | 85% | 60% | 0.766 | * | |
| Grade 2/3 vs. Grade 4 | 1 | LGG and HGG | Relative K2 | - | - | - | - | X | |
| Grade 2/3 vs. Grade 4 | 1 | LGG and HGG | Relative K3 | - | - | - | - | X | |
| Grade 2/3 vs. Grade 4 | 1 | LGG and HGG | Relative FD | - | 67% | 78% | 0.716 | * | |
| Grade 2/3 vs. Grade 4 | 1 | LGG and HGG | TBRmax + SUVsd + TBRmean | - | 75% | 85% | 0.850 | 83% | * |
| Grade 2/3 vs. Grade 4 | 1 | LGG and HGG | HI + SUVsd + MTV | - | 75% | 83% | 0.848 | 81% | * |
| Grade 2/3 vs. Grade 4 | 1 | LGG and HGG | HI + SUVsd + TLU | - | 75% | 84% | 0.848 | 81% | * |
| Grade 2/3 vs. Grade 4 | 1 | LGG and HGG | SUVmax/BG + TTP | - | - | - | 0.745 | * | |
| Grade 2/3 vs. Grade 4 | 1 | LGG and HGG | SUVmax/BG + TTP + relative K1 + relative FD | - | - | - | 0.799 | * | |
| 1 | LGG and HGG | Logistic regression using early SUV + SoD | 50% | 93% | 100% | 97% | X | ||
| Radiomic features: | * | ||||||||
| Grade 3 vs. 4 | 1 | HGG | Coarseness | 0.607 | - | - | 0.757 | * | |
| Grade 3 vs. 4 | 1 | HGG | Contrast | 0.203 | - | - | 0.775 | * | |
| Grade 3 vs. 4 | 1 | HGG | Busyness | 1.12 | - | - | 0.737 | * | |
| Grade 3 vs. 4 | 1 | HGG | Complexity | 0.069 | - | - | 0.633 | * | |
| Grade 3 vs. 4 | 1 | HGG | Combined | 2.05 | - | - | 0.830 | * | |
| IDH status determination | |||||||||
| 2 | LGG and HGG | SUVsd | 0.11 | 47% | 57% | 0.710 | 66% | * | |
| LGG and HGG | 0.23 | - | - | - | - | * | |||
| 5 | LGG and HGG | TBRmax | 2.07 | 8% | 100% | 0.59 | 71% | X | |
| LGG and HGG | 2.21 | 48% | 87% | 0.658 | 72% | * | |||
| LGG | - | - | - | - | - | X | |||
| LGG and HGG | - | - | - | - | - | X | |||
| LGG and HGG | - | - | - | - | - | * | |||
| 2 | LGG and HGG | TBRpeak | 2.15 | 57% | 73% | 0.638 | 67% | X | |
| LGG and HGG | - | - | - | - | - | X | |||
| 5 | LGG and HGG | TBRmean | 1.68 | 12% | 100% | 0.66 | 73% | * | |
| LGG and HGG | 1.84 | 62% | 68% | 0.633 | 66% | X | |||
| LGG and HGG | 1.85 | 44% | 92% | 0.73 | 69% | * | |||
| LGG and HGG | - | - | - | - | - | X | |||
| LGG and HGG | - | - | - | - | - | * | |||
| 1 | LGG and HGG | TBR16mm | 2.15 | 56% | 77% | 0.68 | 67% | * | |
| 3 | LGG | TBR | 1.3 | 89% | 36% | - | - | NA | |
| LGG | 1.6 | 71% | 53% | - | - | NA | |||
| LGG | 2.0 | 57% | 68% | - | - | NA | |||
| 3 | LGG and HGG | TTP | 25 min | 86% | 60% | 0.75 | 72% | * | |
| LGG and HGG | 45 min | 27% | 93% | 0.75 | 73% | * | |||
| LGG and HGG | - | - | - | - | - | * | |||
| 3 | LGG and HGG | Slope | −0.26 SUV/h | 81% | 60% | 0.75 | 70% | * | |
| LGG and HGG | 0.30 SUV/h | 58% | 90% | 0.79 | 80% | * | |||
| LGG and HGG | - | - | - | - | - | * | |||
| 1 | LGG and HGG | TAC | centroid #1 vs. centroid #3 | - | - | - | - | * | |
| 1 | LGG and HGG | COV | 8.85 | 52% | 76% | 0.65 | 67% | * | |
| 1 | LGG and HGG | HI | 1.26 | 48% | 87% | 0.676 | 72% | * | |
| 2 | LGG and HGG | BTV | 19.48 | 90% | 46% | 0.66 | 62% | * | |
| LGG and HGG | - | - | - | - | - | X | |||
| 2 | LGG and HGG | TLU | 28.95 | 81% | 57% | 0.698 | 66% | * | |
| LGG and HGG | - | - | - | - | - | X | |||
| 1 | LGG and HGG | TBRmean + TBR16mm | 1.85 and 2.15 | 44% | 91% | - | 69% | * | |
| 1 | LGG and HGG | TTP + Slope | 25 min and −0.26 SUV/h | 77% | 70% | - | 73% | * | |
| 1 | LGG and HGG | TBRmean + TTP | 1.85 and 25 min | 40% | 96% | - | 69% | * | |
| 1 | LGG and HGG | TBR16mm + TTP | 2.15 and 25 min | 51% | 94% | - | 73% | * | |
| 1 | LGG and HGG | TBRmean + Slope | 1.85 and −0.26 SUV/h | 40% | 94% | - | 68% | * | |
| 1 | LGG and HGG | TBR16mm + Slope | 2.15 and −0.26 SUV/h | 47% | 91% | - | 70% | * | |
| 1 | LGG and HGG | TBRmax + SUVsd + TBRmean | - | 76% | 84% | 0.821 | 81% | * | |
| 1 | LGG and HGG | HI + SUVsd + MTV | - | 86% | 81% | 0.804 | 83% | * | |
| 1 | LGG and HGG | HI + SUVsd + TLU | - | 76% | 84% | 0.799 | 81% | * | |
| 1 | LGG and HGG | Midline involvement | Yes vs. no | - | - | - | - | * | |
| 1 | LGG and HGG | Simple predictive model | - | 85% | 71% | 0.786 | 76% | * | |
| 1 | LGG and HGG | PET-Radiomics model | - | 80% | 74% | 0.812 | 76% | * | |
| 1 | LGG and HGG | Slope + Radiomic feature SZHGE | - | 54% | 93% | - | 81% | * | |
| Radiomic features: | * | ||||||||
| 1 | LGG and HGG | SkewnessH | - | 31% | 90% | 0.53 | 71% | * | |
| 1 | LGG and HGG | LRHGE | - | 8% | 100% | 0.52 | 71% | * | |
| Prediction of oligodendroglial components | |||||||||
| 1 | LGG and HGG | SUVmean/BG | 2.1 | 61% | 59% | * | |||
| 1 | LGG and HGG | SUVtotal/BG | 6.9 | 75% | 66% | * | |||
| 2 | LGG and HGG | TBRmax | 2.6 | 70% | 72% | * | |||
| LGG | - | - | - | * | |||||
| 3 | LGG | TBR | 1.3 | 100% | 23% | NA | |||
| LGG | 1.6 | 93% | 48% | NA | |||||
| LGG | 2 | 86% | 65% | NA | |||||
| 1 | LGG and HGG | BTV | 4 mL | 71% | 69% | * | |||
| Guided resection/biopsy | |||||||||
| 1 | HGG | BTV | 1 cm3 | * | |||||
| 1 | LGG and HGG | TBR25mm2 | 1.6 | - | - | * | |||
| 3 | LGG | TBR | 1.6 | 54% | 12% | * | |||
| HGG | 88% | 46% | * | ||||||
| LGG and HGG | - | - | - | 0.76 | * | ||||
| Detection of residual tumor | |||||||||
| 1 | HGG | TBR | 1.6 | - | - | * | |||
| 1 | LGG and HGG | Visual uptake | >Background | - | - | * | |||
| Guided radiotherapy | |||||||||
| 7 | HGG | SUVmax | 30% | - | - | NA | |||
| HGG | 40% | - | - | NA | |||||
| HGG | 50% | - | - | NA | |||||
| HGG | 60% | - | - | NA | |||||
| HGG | 70% | - | - | NA | |||||
| HGG | 80% | - | - | NA | |||||
| HGG | 90% | - | - | NA | |||||
| 1 | HGG | TBRmax | 1.6 | - | - | NA | |||
| 5 | HGG | TBR | 1.6 | - | - | NA | |||
| HGG | - | - | NA | ||||||
| HGG | - | - | NA | ||||||
| HGG | - | - | NA | ||||||
| HGG | - | - | NA | ||||||
| Detection of malignant transformation in LGG | |||||||||
| 3 | LGG | TBRmax | ∆ + 33% | 72% | 89% | 0.87 | 78% | * | |
| LGG and HGG | 2.46 | 82% | 89% | 0.92 | 85% | * | |||
| LGG | - | 57% | 41% | 0.476 | X | ||||
| 1 | LGG | TBRmean | ∆ + 13% | 72% | 78% | 0.8 | 74% | * | |
| 2 | LGG | TTP | ∆-6 min | 72% | 89% | 0.78 | 78% | * | |
| LGG | 25 min | 57% | 47% | 0.511 | X | ||||
| 1 | LGG and HGG | TTPmin | 17.5 min | 73% | 67% | - | 70% | * | |
| 1 | LGG | TAC | - | 71% | 41% | 0.549 | X | ||
| 1 | LGG | TAC change | I to II/III | 72% | 89% | - | 78% | * | |
| 1 | LGG | TBRmax + TTP + TAC change | ∆ + 33% or ∆-6 min or I to II/III | 83% | 78% | - | 81% | * | |
| 1 | LGG | TBRmax + TAC + TTP | 1.6 + II/III + 25 min | 65% | 58% | 0.634 | X | ||
| 1 | LGG | TBRmax + TAC | 1.6 + II/III | 65% | 58% | 0.639 | X | ||
| 1 | LGG | TBRmax + TTP | 1.6 + 25 min | 96% | 25% | 0.591 | X | ||
| Recurrence vs. treatment-related changes | |||||||||
| 1 | HGG | Visual analysis | Nodular vs. non-nodular | 94% | 94% | NA | |||
| 6 | LGG | SUVmax | 1.48 | 88% | 89% | 0.951 | * | ||
| LGG and HGG | 1.66 | 87% | 100% | 0.978 | * | ||||
| HGG | 93% | 100% | 0.993 | * | |||||
| LGG and HGG | 2.2 | 100% | 93% | * | |||||
| LGG and HGG | - | - | * | ||||||
| LGG and HGG | - | - | - | X | |||||
| 1 | LGG and HGG | SUV80mean | - | - | - | X | |||
| 1 | LGG and HGG | SUV-BG | - | - | - | X | |||
| 20 | LGG | TBRmax | 1.64 | 100% | 75% | 0.893 | * | ||
| LGG and HGG | 2 | 81% | 60% | 0.81 | * | ||||
| LGG and HGG | - | - | X | ||||||
| LGG and HGG | - | - | * | ||||||
| HGG | 99% | 94% | 0.970 | 99% | * | ||||
| HGG | 100% | 78% | NA | ||||||
| HGG | 2.1 | 97% | 91% | NA | |||||
| LGG and HGG | 2.18 | 86% | 88% | 0.940 | * | ||||
| HGG | 2.2 | 82% | 95% | NA | |||||
| HGG | 2.3 | 74% | 98% | NA | |||||
| HGG | 2.4 | 74% | 100% | NA | |||||
| HGG | 2.46 | 86% | 100% | 0.964 | * | ||||
| HGG | 2.5 | 62% | 100% | NA | |||||
| LGG and HGG | 2.61 | 80% | 86% | 0.78 | 81% | * | |||
| HGG | 2.65 | 80% | 88% | * | |||||
| HGG | 2.85 | 64% | 92% | 0.75 | 78% | * | |||
| HGG | 3.44 | 86% | 88% | 0.86 | * | ||||
| HGG | 3.58 | 64% | 100% | 0.84 | * | ||||
| HGG | 3.69 | 79% | 88% | 0.86 | * | ||||
| LGG and HGG | - | - | - | - | * | ||||
| 1 | HGG | TBRmax after 6 months | - | - | - | * | |||
| 11 | HGG | TBRmean | 1.8 | 96% | 94% | 0.977 | 96% | * | |
| HGG | 1.9 | 74% | 86% | 0.86 | 77% | * | |||
| HGG | 1.95 | 82% | 92% | 0.77 | 87% | * | |||
| HGG | 100% | 79% | 0.89 | 83% | * | ||||
| HGG | 75% | 61% | 0.73 | 68% | * | ||||
| LGG and HGG | 2.0 | 74% | 91% | 0.91 | 75% | * | |||
| HGG | 82% | 82% | 0.91 | 82% | * | ||||
| HGG | 2.19 | 71% | 88% | 0.80 | * | ||||
| HGG | 2.31 | 61% | 100% | 0.83 | * | ||||
| LGG and HGG | - | - | - | 0.72 | * | ||||
| LGG and HGG | - | - | - | - | * | ||||
| 1 | LGG and HGG | TBR30–40min | 2.07 | 80% | 85% | 0.863 | * | ||
| 1 | LGG and HGG | TBR10–20min | 1.71 | 76% | 85% | 0.848 | * | ||
| 1 | HGG | TBR10mm | 2.86 | 86% | 75% | 0.81 | * | ||
| 8 | HGG | TBR16mm | 1.9 | 84% | 86% | 0.88 | 85% | * | |
| LGG and HGG | 1.95 | 70% | 60% | 0.72 | 68% | * | |||
| HGG | 100% | 79% | 0.89 | 83% | * | ||||
| HGG | 2.25 | 81% | 67% | 0.79 | 74% | * | |||
| LGG and HGG | 2.3 | 68% | 100% | 0.85 | 71% | * | |||
| HGG | 100% | 91% | 0.94 | 96% | * | ||||
| HGG | 2.44 | 82% | 75% | 0.82 | * | ||||
| LGG and HGG | - | - | - | 0.74 | X | ||||
| 2 | HGG | TBR70% | 2.72 | 86% | 88% | 0.87 | * | ||
| LGG and HGG | - | - | - | * | |||||
| 2 | HGG | TBR80% | 3.08 | 82% | 88% | 0.88 | * | ||
| LGG and HGG | - | - | - | * | |||||
| 1 | HGG | TBR90% | 3.23 | 71% | 100% | 0.85 | * | ||
| 1 | LGG and HGG | TBR80mean | - | - | - | * | |||
| 8 | LGG and HGG | TTP | 20 min | 64% | 79% | 0.728 | * | ||
| HGG | 25 min | 75% | 44% | 0.61 | 59% | X | |||
| HGG | 32.5 min | 80% | 69% | 0.79 | 72% | * | |||
| HGG | 35 min | 64% | 83% | 0.82 | 74% | * | |||
| LGG and HGG | 45 min | 82% | 73% | 0.81 | 81% | * | |||
| LGG and HGG | - | - | - | 0.60 | X | ||||
| LGG and HGG | - | - | - | 0.71 | * | ||||
| HGG | - | - | - | 0.86 | - | * | |||
| 5 | HGG | Slope | 0.02 SUV/h | 73% | 75% | 0.72 | 74% | X | |
| HGG | 0.3 SUV/h | 56% | 61% | 0.55 | 59% | X | |||
| HGG | 0.32 SUV/h | 70% | 75% | 0.82 | 74% | * | |||
| LGG and HGG | 0.69 SUV/h | 84% | 62% | 0.69 | 80% | * | |||
| LGG and HGG | - | - | - | 0.70 | * | ||||
| 3 | LGG and HGG | TAC | II/III | 78% | 73% | - | 77% | * | |
| HGG | 84% | 100% | - | 89% | * | ||||
| HGG | - | - | - | - | * | ||||
| 1 | HGG | BTV | 0.55 cm3 | 98% | 94% | 0.955 | 98% | * | |
| 1 | HGG | BTV after 6 months | - | * | |||||
| 1 | LGG and HGG | TBRmean + TBRmax | - | 66% | 80% | 0.78 | NA | ||
| 1 | HGG | TBRmean + TBR16mm | - | 75% | 72% | - | 74% | * | |
| 1 | HGG | TBRmax + TTP | 2.85 and 35 min | 36% | 100% | 70% | * | ||
| 3 | LGG and HGG | TBRmean + TTP | 2.0 and/or 45 min | 93% | 100% | 93% | * | ||
| HGG | 1.95 and 35 min | 55% | 100% | 78% | * | ||||
| HGG | - | 69% | 78% | - | 74% | * | |||
| 2 | LGG and HGG | TBR16mm + TTP | 2.3 and/or 45 min | 92% | 73% | 90% | * | ||
| HGG | - | 69% | 83% | 76% | * | ||||
| 1 | HGG | TBR16mm/mean + TTP | 1.95 and 32.5 min | 89% | 91% | 90% | * | ||
| 1 | HGG | TBRmax+ TAC | 2.3 and II/III | 80% | 91% | 86% | * | ||
| 2 | LGG and HGG | TBRmean + TAC | 2.0 and/or II/III | 93% | 73% | 91% | * | ||
| HGG | 2.0 and II/III | 60% | 91% | 76% | * | ||||
| 1 | LGG and HGG | TBR16mm + TAC | 2.3 and/or II/III | 93% | 73% | 91% | * | ||
| 1 | HGG | TBRmean + Slope | - | 50% | 78% | 65% | X | ||
| 2 | LGG and HGG | TBR16mm + Slope | 1.95 and/or 0.69 SUV/h | 96% | 43% | 86% | NA | ||
| HGG | - | 50% | 89% | 71% | * | ||||
| 1 | HGG | TBR16mm/mean + Slope | 1.95 and 0.32 SUV/h | 78% | 97% | 93% | * | ||
| 1 | HGG | TTP + Slope | - | 56% | 61% | 59% | X | ||
| 1 | HGG | TBR16mm + TBRmean + TTP | - | 69% | 89% | 79% | * | ||
| 2 | LGG and HGG | Radiomics features | - | 73% | 80% | 0.85 | NA | ||
| HGG | - | 100% | 40% | 0.74 | 70% | * | |||
| 1 | LGG and HGG | TBRmax + TBRmean + radiomics features | - | 81% | 70% | 0.85 | NA | ||
| Prognosis/Treatment response evaluation | |||||||||
| 1 | LGG | Uptake | Positive vs. negative | - | - | X | |||
| 1 | LGG and HGG | FET positivity | Yes vs. no | - | - | * | |||
| 1 | HGG | New distant FET hotspot | Yes vs. no | * | |||||
| 1 | LGG and HGG | FET-PET response | Yes vs. no | - | - | * | |||
| 3 | LGG and HGG | SUVmax/BG | - | - | - | X | |||
| LGG and HGG | - | - | - | X | |||||
| LGG and HGG | - | - | - | X | |||||
| 1 | LGG and HGG | Initial SUVmax/BG | - | - | - | X | |||
| 2 | LGG | SUVmean/BG | - | - | - | X | |||
| HGG | - | - | - | X | |||||
| 1 | HGG | SUVmean/BG pre re-RT | 2.2 | - | - | X | |||
| 1 | HGG | SUVmean/BG post re-RT | 2.3 | - | - | X | |||
| 1 | LGG | SUVtotal/BG | - | - | - | X | |||
| 5 | LGG and HGG | SUVmax | 2.3 | - | - | X | |||
| LGG and HGG | - | - | X | ||||||
| LGG and HGG | 2.6 | - | - | * | |||||
| LGG and HGG | 3.4 | - | - | * | |||||
| HGG | Median | - | - | X | |||||
| 1 | HGG | SUVmean | Median | - | - | X | |||
| 1 | HGG | SUVpeak | Median | - | - | X | |||
| 12 | LGG and HGG | TBRmax | 1.6 | - | - | X | |||
| LGG | 2 | - | - | X | |||||
| HGG | 2.4 | - | - | * | |||||
| LGG and HGG | 2.5 | - | - | 0.696 | * | ||||
| LGG and HGG | 2.6 | - | - | X | |||||
| HGG | 3 | - | - | X | |||||
| LGG and HGG | 3.03 | - | - | * | |||||
| HGG | Median (5.03) | * | |||||||
| LGG | - | - | - | X | |||||
| LGG and HGG | - | - | - | X | |||||
| LGG and HGG | - | - | - | X | |||||
| HGG | - | - | - | X | |||||
| 1 | HGG | TBRmax-preRCx | 2.9 (OS) | 68% | 73% | * | |||
| 2 | LGG and HGG | TBRmax baseline | 2.0 (PFS)/1.9 (OS) | - | - | * (PFS) | |||
| HGG | - | - | - | NA | |||||
| 1 | LGG and HGG | TBRmax after 6 months | - | - | - | X | |||
| 1 | HGG | Early TBRmax response | ∆-10% | - | - | * | |||
| 1 | LGG and HGG | TBRmax response | ∆ ± 10% | - | - | * | |||
| 3 | LGG and HGG | TBRmax change | 0% | - | - | * | |||
| HGG | - | - | * | ||||||
| HGG | ∆-10% (PFS)/∆-20% (OS) | 83% (OS) | 67% (OS) | 0.75 (OS) | * | ||||
| 1 | HGG | TBRmax pre re-RT | 3.3 | - | - | X | |||
| 1 | HGG | TBRmax post re-RT | 2.6 | - | - | X | |||
| 1 | HGG | TBR16mm relative reduction | 27% | 92% | 63% | 0.78 | NA | ||
| 1 | HGG | ∆TBRmax scan 2 | - | - | - | NA | |||
| 1 | HGG | ∆TBRmax scan 3 | - | - | - | NA | |||
| 2 | HGG | TBRmean | 2 | - | - | * | |||
| HGG | 2.05 | 60% | 70% | 0.69 | X | ||||
| 1 | HGG | TBRmean relative reduction | 16% | 92% | 63% | 0.81 | NA | ||
| 1 | LGG and HGG | TBR16mm baseline | 1.9 (PFS)/1.8 (OS) | - | - | X | |||
| 3 | LGG and HGG | TBR16mm change | 0% | - | - | X | |||
| HGG | - | - | X | ||||||
| HGG | ∆-5% | 67% | 75% | 0.72 | * | ||||
| 1 | HGG | TBR in non-contrast enhancing tumor portions at follow-up | High vs. low | - | - | * | |||
| 1 | LGG | TBR3SD | 1.1 | - | - | * | |||
| 1 | LGG and HGG | TBR10mm | 2.3 | - | - | 0.696 | * | ||
| 1 | HGG | TBR16mm | 2.55 | 70% | 57% | 0.63 | X | ||
| 5 | HGG | TBR | 1.56 (PFS)/1.57 (OS) | - | - | X | |||
| HGG | 2 | - | - | X | |||||
| HGG | 2.4 | - | - | * | |||||
| HGG | 2.5 | - | - | X | |||||
| HGG | Median | - | - | X | |||||
| 1 | HGG | Early TBR response | ∆-10% | * | |||||
| 1 | HGG | TLG | Median | X | |||||
| 1 | HGG | TLU | 35.0 (PFS)/17.1 (OS) | - | - | * (OS) | |||
| 1 | LGG and HGG | TTP | 20 min | - | - | 0.848 | * | ||
| 1 | HGG | 25 min | 90% | 87% | 0.90 | * | |||
| 1 | LGG and HGG | - | - | - | * | ||||
| 1 | HGG | TTP change | 0% | - | - | X | |||
| 1 | HGG | TTPmin | 12.5 min | - | - | * | |||
| 1 | LGG and HGG | >25 min vs. 12.5 < t ≤ 25 min vs. ≤12.5 min | - | - | * | ||||
| 1 | LGG and HGG | 17.5 min | - | - | * | ||||
| 1 | HGG | Slope | −0.103 SUV/h | 70% | 90% | 0.77 | X | ||
| 1 | LGG and HGG | - | - | - | * | ||||
| 1 | LGG and HGG | Slope-to-peak | 7 × 10−5/s | - | - | 0.711 | * | ||
| 5 | LGG | TAC | Increasing vs. decreasing | - | - | * | |||
| LGG and HGG | Homogeneous increasing vs. mixed vs. homogeneous decreasing | - | - | * | |||||
| LGG and HGG | Homogeneous decreasing vs. focal decreasing vs. homogeneous increasing | - | - | * | |||||
| HGG | Increasing | - | - | * | |||||
| LGG and HGG | - | - | - | * | |||||
| 1 | HGG | TAC pre re-RT | G1–2 vs. G3 vs. G4–5 | * | |||||
| 1 | HGG | TAC post re-RT | G1–2 vs. G3 vs. G4–5 | X | |||||
| 1 | LGG and HGG | Initial TAC | Increasing vs. decreasing | - | - | X | |||
| 1 | HGG | Increasing vs. decreasing (OS) | - | - | * | ||||
| 1 | LGG and HGG | TAC after 6 months | Increasing vs. decreasing | - | - | X | |||
| 1 | LGG and HGG | TAC response | Stable increasing vs. decreasing to increasing vs. Increasing to decreasing vs. Stable decreasing | - | - | X | |||
| 1 | LGG and HGG | Peak TBR | 2.2 | - | - | 0.704 | * | ||
| 8 | HGG | BTV | 4.3 cm3 | - | - | * | |||
| LGG and HGG | 10 cm3 | * | |||||||
| HGG | 11.15 mL | 72% | 54% | 0.56 | X | ||||
| HGG | 19.4 (PFS)/18.9 (OS) | - | - | * (OS) | |||||
| HGG | 20 mL | - | - | * | |||||
| HGG | Median | X | |||||||
| LGG | - | - | - | X | |||||
| HGG | - | - | - | X | |||||
| 1 | HGG | BTVpreRCx | 9.5 cm3 | 64% | 70% | * | |||
| 1 | LGG and HGG | Initial BTV | - | - | - | X | |||
| 1 | LGG and HGG | BTV baseline | 28.2 mL (PFS)/13.8 mL (OS) | - | - | * | |||
| 1 | HGG | - | - | - | * | ||||
| 1 | LGG and HGG | BTV after 6 months | - | - | - | * | |||
| 1 | HGG | Absolute BTV at follow-up | 5 mL | 85% | 88% | 0.92 | * | ||
| 1 | LGG and HGG | BTV response | ∆ ± 25% | - | - | * | |||
| 3 | LGG and HGG | BTV change | 0% | - | - | * | |||
| HGG | 0% | - | - | * | |||||
| HGG | 0% (PFS) | - | - | - | * | ||||
| 1 | HGG | BTV relative reduction | 27% | 77% | 63% | 0.82 | NA | ||
| 1 | HGG | ∆BTV scan 2 | - | - | - | X | |||
| 1 | HGG | ∆BTV scan 3 | - | - | - | X | |||
| 1 | LGG and HGG | BTVSUVmax≥2.2 | - | - | - | X | |||
| 1 | LGG and HGG | BTV≥40%SUVmax | - | - | - | X | |||
| 1 | HGG | BTVTBR≥ 1.6 | 25 mL | - | - | * | |||
| 1 | HGG | BTVTBR≥ 2.0 | 10 mL | - | - | * | |||
| 1 | HGG | BTV pre re-RT | 13.7 cc | - | - | X | |||
| 1 | HGG | BTV post re-RT | 7.3 cc | - | - | X | |||
| Radiomic features: | * | ||||||||
| 1 | HGG | SUVmin | - | - | - | *, & | |||
| 1 | HGG | SUVmean | - | - | - | *, & | |||
| 1 | HGG | GLV | - | - | - | *, & | |||
| 1 | HGG | GLV2 | - | - | - | *, & | |||
| 1 | HGG | WF_GLV | - | - | - | *, & | |||
| 1 | HGG | Qacor | - | - | - | *, & | |||
| 1 | HGG | QHGZE | - | - | - | *, & | |||
| 1 | HGG | QSZHGE | - | - | - | *, & | |||
| 1 | HGG | QGLN2 | - | - | - | *, & | |||
| 1 | HGG | QHGRE | - | - | - | *, & | |||
| 1 | HGG | QSRHGE | - | - | - | *, & | |||
| 1 | HGG | QLRHGE | - | - | - | *, & | |||
| 1 | HGG | SZLGE | - | - | - | * | |||
| 1 | HGG | Busyness | 1.366 (PFS)/0.984 (OS) | - | - | * | |||
| 1 | HGG | - | - | - | *, & | ||||
| 1 | HGG | WF_TS | - | - | - | *, & | |||
| 1 | HGG | QvarianceCM | - | - | - | *, & | |||
| 1 | HGG | Eccentricity | - | - | - | *, & | |||
| 1 | HGG | Coarseness | 5.96 × 10−3 (PFS)/6.88 × 10−3 (OS) | - | - | * | |||
| 1 | HGG | Contrast | 0.427 | - | - | * | |||
| 1 | HGG | Complexity | 0.085 (PFS)/0.094 (OS) | - | - | * | |||
| 1 | HGG | SUVmean + WF_GLV + QLRHGE + SUVmin | - | - | - | * | |||
| 1 | HGG | SZLGE + Busyness + QVarianceCM + Eccentricity | - | - | - | * |
3.2. Diagnosis
Four prospective studies [24,25,26,27] evaluated the performance of [18F]FET PET in patients with cerebral lesions suspicious of glioma. Each study chose a different method of TBR determination to detect glioma tissue with a threshold of 1.6 in two of them [26,27], resulting in a sensitivity of 88 to 92% and a specificity of 81 to 88%.
3.3. Grading
Thirteen studies [19,28,29,30,31,32,33,34,35,36,37,38,39] evaluated the performance of [18F]FET PET in glioma grading. Most studies aimed at differentiating low-grade gliomas (LGGs) from high-grade gliomas (HGGs). Multiple TBR methods were used, with a predominance of maximum tumor-to-brain ratio (TBRmax) with sensitivity and specificity ranging from 67 to 92% and 61 to 85%, respectively. Dynamic parameters and notably tumor-activity curves (TAC) had better performance, with a sensitivity of 73 to 96% and a specificity of 63 to 100%.
Notably, one study by Lohmann et al. [31] chose to supplement dynamic imaging from 0 to 50 min post-injection (p.i.) with an additional acquisition from 70 to 90 min p.i. The goal was to compare conventional dynamic imaging to dual-time-point imaging: one acquisition from 20 to 40 min p.i. and a delayed second acquisition from 70 to 90 min p.i. Mean tumor-to-brain ratio (TBRmean) change and TAC achieved similar accuracy of 81% and 83%, respectively.
3.4. IDH Status Determination
Six retrospective studies [34,40,41,42,43,44] evaluated the performance of [18F]FET PET in IDH status determination. Static parameters’ significancy was variable depending on the studies, whereas dynamic ones (Slope, Time-to-peak (TTP), TAC) always showed significant differences between IDH mutated and IDH wild-type groups with an accuracy of around 73%.
3.5. Prediction of Oligodendroglial Components
Two studies [38,44] reported on the performance of [18F]FET PET to determine the presence of oligodendroglial tumor components. Every static parameter tested was significant. Tumor-to-brain ratios showed good sensitivity, but specificity did not exceed 65%.
There were no dynamic parameters studied.
3.6. Guided Resection or Biopsy
Four studies [45,46,47,48] tested the addition of [18F]FET PET to better detect tumor tissue for resection or biopsy. In a study by Ewelt et al. [47], results were separated according to glioma grades (LGG vs. HGG), showing better tissue detection in high-grade glioma with sensitivity and specificity of 88% and 46%. Sensitivity was higher than those of MRI and 5-ALA-fluorescence, with a specificity being the lowest. Combining different modalities did not improve results compared to those of 5-ALA-fluorescence alone (sensitivity of 71% and specificity of 92%).
3.7. Detection of Residual Tumor
Two studies [49,50] aimed at detecting residual tumor tissue after surgery.
Buchmann et al. [49] also aimed to assess whether performing [18F]FET PET after 72 h after neurosurgery had an influence, as it is the case with MRI. Indeed, postoperative MRI after 72 h can lead to falsification of results because of inflammatory reactions. This study found higher sensitivity of PET using a TBR > 1.6 compared to MRI and no influence of timing of [18F]FET PET imaging.
3.8. Guided Radiotherapy
Studies [51,52,53,54,55,56] used the TBR threshold of 1.6 to define the tumor volume to be irradiated. This PET-based volume was increased compared to the MRI-based volume commonly used.
One study (Harat et al. [54]) reported 74% of failures inside primary gross tumor volume (GTV) PET volumes, with no solitary progressions inside the MRI-defined margin +20 mm but outside the GTV PET detected.
3.9. Detection of Malignant Transformation in Low-Grade Gliomas
Three studies [57,58,59] evaluated the use of [18F]FET PET to detect differences between non-transformed LGGs and LGGs that had transformed to high-grade gliomas. Two studies found a good detection value of both static and dynamic parameters in this indication, whether by comparing to baseline or by using parameter thresholds.
The remaining study (Bashir et al. [59]) did not find significant differences when considering all patients. After excluding the oligodendroglial subgroup, however, a significant difference was observed between non-transformed and transformed LGGs when combining [18F]FET parameters. The best result was observed with a combined analysis of TBRmax > 1.6 and TAC with a plateau or decreasing pattern (sensitivity of 75% and specificity of 83%).
3.10. Recurrence vs. Treatment-Related Changes
Twenty studies [60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79] evaluated the performance of [18F]FET PET in the differentiation of recurrence from treatment-related changes.
The majority of studies included patients treated with multiple modalities (such as operation, chemotherapy, and radiotherapy) who had a suspected tumor recurrence or progression as revealed by follow-up MRI. High-grade gliomas represented 87% (992/1141) of tumors.
Most studies used static parameters TBRmax and TBRmean along with dynamic parameters TTP and Slope.
TBRmax was significant in 13 studies with thresholds between 1.64 and 3.69. TBRmean significantly differentiated recurrence from pseudoprogression in 11 studies. The thresholds used varied from 1.8 to 2.31. Accuracy of TBRmax and TBRmean was comparable.
Dynamic parameters, when combined with static ones, allowed to increase diagnostic accuracy in some studies such as Werner et al. [68] and Galldiks et al. [78]. In Werner et al., TBRs alone had a diagnostic accuracy of 83%, which increased to 90% and 93% when combined with TTP and Slope, respectively. This finding was not supported by other studies, such as Werner et al. [66] and Galldiks et al. [67].
3.11. Prognosis and Treatment Response Evaluation
Twenty-eight studies [39,43,61,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104] evaluated the performance of [18F]FET PET in prognosis and treatment response evaluation.
Prognostic parameters can be extracted before, during, and after treatment. For example, Pyka et al. [93] studied patients with untreated, first-diagnosed gliomas and were able to predict tumor recurrence, with dynamic parameters showing better results than static ones, especially in the low-grade subgroup.
Overall, static parameters tended to not reach significance, whereas dynamic ones such as TTP and TAC demonstrated better results. TTP was the best parameter in two studies (Pyka et al. [93] and Bauer et al. [95]) with AUCs of 0.848 and 0.90, respectively.
Many studies also decided to use biological tumor volume (BTV), often determined by an autocontouring process using a TBR threshold of 1.6. Every study used a different cut-off when considering absolute values, and half of them did not reach significance. Three studies [82,87,94] opted for a BTV change after the initiation of chemotherapy to separate responders (relative change 0%) from non-responders (relative change > 0%). Two of them examined patients at first diagnosis and the third one at recurrence. These studies found a decreasing BTV to predict a significantly longer progression-free survival and to be associated with prolonged overall survival.
3.12. Radiomics
Radiomic parameters were used by 1 study, for grading [39] (grade 3 vs. 4), 2 studies in IDH status determination [40,41], 2 studies in the differentiation of recurrence vs. pseudoprogression [69,76], and 2 studies for prognosis [39,89].
Different textural features showed good performance in each study, and the combination of standard PET parameters with textural features could improve results, for example in IDH genotype determination, as shown by Lohmann et al. [41]. Combination of the dynamic parameter Slope with the radiomic feature SZHGE slightly increased diagnostic accuracy to 81% vs. 80% with Slope alone.
4. Discussion/Conclusions
This review proposes an up-to-date summary of PET performance in glioma management using O-(2-[18F]fluoroethyl)-L-tyrosine. The homogenization of PET tumor-to-brain ratios according to the determination of the different regions of interest allowed to truly compare their sensibility, specificity, AUC, and accuracy.
[18F]FET can be useful in every step of glioma management, from diagnosis to suspicion of recurrence.
The ability to discriminate tumor tissue from healthy brain tissue is helpful in diagnosis, to guide a surgical procedure or radiotherapy, and to detect the presence of a residue after surgery. Most studies agree on a TBR threshold > 1.6 to delineate tumor extent.
Different thresholds of tumor-to-brain ratio are also useful to predict histological characteristics (low vs. high grade, malignant transformation of a low-grade glioma, and oligodendroglial components), to differentiate post-treatment changes from a true recurrence, and to extract prognostic parameters and assess treatment response.
It is important to note that while many studies used static parameters TBRmax and TBRmean, the definition of these ratios differs depending on the article. For example, the ratio between the mean standard uptake value (SUVmean) of a 16 mm ROI centered on the maximal tumor uptake and the SUVmean of a contralateral background ROI, named TBR16mm in this review, can be called TBRmean in a study (Verger et al. [64]) and TBRmax in another (Galldiks et al. [78]).
Kertels et al. [63] expressed the need to use comparable approaches to be able to obtain relevant and reliable results. Despite the absence of a significant difference between methods chosen, approaches focusing on voxels with the highest uptake tended to perform superior.
Dynamic acquisition also adds valuable information with parameters such as TTP, TAC, or Slope and should be preferred. An interesting alternative proposed by Lohmann et al. [31] is dual-time point imaging, allowing to reduce costs due to higher patient throughput and imaging time.
Relatively new tools are also available, such as radiomics and hybrid PET/MR imaging, and could be of great interest in the future. The use of hybrid PET/MR is set to increase in neuro-oncology and could improve performance, as suggested by Lohmann et al. [41] concerning radiomics.
Joint EANM/EANO/RANO practice guidelines [9] published in 2018 summarized methods and cut-off values in different clinical situations concerning radiolabeled amino acids and [18F]FDG. It is of importance to note that the studies used to extract these guidelines are often retrospective and/or based on small effectives.
At the beginning of the year, Albert et al. [105] published the first version of PET RANO criteria in an effort to facilitate the structured implementation of PET imaging into clinical research and, ultimately, clinical routine.
The principal limitation of this review is the methodology used and the fact that many of the included studies are also retrospective and do not reflect clinical practice. Additionally, none of the studies included focused on pediatric gliomas, probably because of the limited number of patients in the available research.
While [18F]FET is becoming an important tracer in neuro-oncology, [18F]F-DOPA also showed good results and should not be overlooked. A recent meta-analysis and systematic review compared [18F]F-DOPA and [18F]FET for differentiating treatment-related change from true progression (Yu et al. [21]) and found that [18F]F-DOPA seems to demonstrate superior sensitivity and similar specificity to [18F]FET. Nevertheless, [18F]F-DOPA PET results were obtained from studies with limited sample sizes.
There is a need to pursue research with prospective, multicentric studies to be able to standardize imaging analysis and define the use of technological advancements such as hybrid PET/MRI imaging and radiomics and to compare [18F]FET with existing radiopharmaceuticals such as [18F]F-DOPA head-to-head comparisons.
Author Contributions
Conceptualization, J.V.; methodology, J.A.R. and J.V.; validation, A.L. and J.V.; investigation, J.A.R.; resources, J.A.R.; data curation, J.A.R.; writing—original draft preparation, J.A.R. and J.V.; writing—review and editing, J.A.R., A.L., E.E., M.D., D.A. and J.V.; supervision, J.V.; project administration, J.A.R., A.L. and J.V. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Darlix A., Zouaoui S., Rigau V., Bessaoud F., Figarella-Branger D., Mathieu-Daudé H., Trétarre B., Bauchet F., Duffau H., Taillandier L., et al. Epidemiology for primary brain tumors: A nationwide population-based study. J. Neuro Oncol. 2017;131:525–546. doi: 10.1007/s11060-016-2318-3. [DOI] [PubMed] [Google Scholar]
- 2.Louis D.N., Perry A., Wesseling P., Brat D.J., Cree I.A., Figarella-Branger D., Hawkins C., Ng H.K., Pfister S.M., Reifenberger G., et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021;23:1231–1251. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stupp R., Mason W.P., Van Den Bent M.J., Weller M., Fisher B., Taphoorn M.J.B., Belanger K., Brandes A.A., Marosi C., Bogdahn U., et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 4.Stupp R., Brada M., Van Den Bent M.J., Tonn J.-C., Pentheroudakis G. High-grade glioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2014;25:iii93–iii101. doi: 10.1093/annonc/mdu050. [DOI] [PubMed] [Google Scholar]
- 5.Wen P.Y., Macdonald D.R., Reardon D.A., Cloughesy T.F., Sorensen A.G., Galanis E., DeGroot J., Wick W., Gilbert M.R., Lassman A.B., et al. Updated Response Assessment Criteria for High-Grade Gliomas: Response Assessment in Neuro-Oncology Working Group. J. Clin. Oncol. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 6.Du X., He Q., Zhang B., Li N., Zeng X., Li W. Diagnostic accuracy of diffusion-weighted imaging in differentiating glioma recurrence from posttreatment-related changes: A meta-analysis. Expert. Rev. Anticancer Ther. 2022;22:123–130. doi: 10.1080/14737140.2022.2000396. [DOI] [PubMed] [Google Scholar]
- 7.Zhang J., Wang Y., Wang Y., Xiao H., Chen X., Lei Y., Feng Z., Ma X., Ma L. Perfusion magnetic resonance imaging in the differentiation between glioma recurrence and pseudoprogression: A systematic review, meta-analysis and meta-regression. Quant. Imaging Med. Surg. 2022;12:4805–4822. doi: 10.21037/qims-22-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Abtah M.E., Talati P., Fu M., Chun B., Clark P., Peters A., Ranasinghe A., He J., Rapalino O., Batchelor T.T., et al. Magnetic resonance spectroscopy outperforms perfusion in distinguishing between pseudoprogression and disease progression in patients with glioblastoma. Neuro Oncol. Adv. 2022;4:vdac128. doi: 10.1093/noajnl/vdac128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Law I., Albert N.L., Arbizu J., Boellaard R., Drzezga A., Galldiks N., la Fougère C., Langen K.-J., Lopci E., Lowe V., et al. Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [18F]FDG: Version 1.0. Eur. J. Nucl. Med. Mol. Imaging. 2019;46:540–557. doi: 10.1007/s00259-018-4207-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galldiks N., Lohmann P., Fink G.R., Langen K.-J. Amino Acid PET in Neurooncology. J. Nucl. Med. 2023;64:693–700. doi: 10.2967/jnumed.122.264859. [DOI] [PubMed] [Google Scholar]
- 11.He Q., Zhang L., Zhang B., Shi X., Yi C., Zhang X. Diagnostic accuracy of 13N-ammonia PET, 11C-methionine PET and 18F-fluorodeoxyglucose PET: A comparative study in patients with suspected cerebral glioma. BMC Cancer. 2019;19:332. doi: 10.1186/s12885-019-5560-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deuschl C., Kirchner J., Poeppel T.D., Schaarschmidt B., Kebir S., El Hindy N., Hense J., Quick H.H., Glas M., Herrmann K., et al. 11C–MET PET/MRI for detection of recurrent glioma. Eur. J. Nucl. Med. Mol. Imaging. 2018;45:593–601. doi: 10.1007/s00259-017-3916-9. [DOI] [PubMed] [Google Scholar]
- 13.Galldiks N., Kracht L.W., Burghaus L., Thomas A., Jacobs A.H., Heiss W., Herholz K. Use of 11C-methionine PET to monitor the effects of temozolomide chemotherapy in malignant gliomas. Eur. J. Nucl. Med. Mol. Imaging. 2006;33:516–524. doi: 10.1007/s00259-005-0002-5. [DOI] [PubMed] [Google Scholar]
- 14.Nakajima R., Kimura K., Abe K., Sakai S. 11C-methionine PET/CT findings in benign brain disease. Jpn. J. Radiol. 2017;35:279–288. doi: 10.1007/s11604-017-0638-7. [DOI] [PubMed] [Google Scholar]
- 15.Karunanithi S., Sharma P., Kumar A., Khangembam B.C., Bandopadhyaya G.P., Kumar R., Goenka A., Gupta D.K., Malhotra A., Bal C. Comparative diagnostic accuracy of contrast-enhanced MRI and 18F-FDOPA PET-CT in recurrent glioma. Eur. Radiol. 2013;23:2628–2635. doi: 10.1007/s00330-013-2838-6. [DOI] [PubMed] [Google Scholar]
- 16.Youland R.S., Pafundi D.H., Brinkmann D.H., Lowe V.J., Morris J.M., Kemp B.J., Hunt C.H., Giannini C., Parney I.F., Laack N.N. Prospective trial evaluating the sensitivity and specificity of 3,4-dihydroxy-6-[18F]-fluoro-L-phenylalanine (18F-DOPA) PET and MRI in patients with recurrent gliomas. J. Neuro Oncol. 2018;137:583–591. doi: 10.1007/s11060-018-2750-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sala Q., Metellus P., Taieb D., Kaphan E., Figarella-Branger D., Guedj E. 18F-DOPA, a Clinically Available PET Tracer to Study Brain Inflammation? Clin. Nucl. Med. 2014;39:e283–e285. doi: 10.1097/RLU.0000000000000383. [DOI] [PubMed] [Google Scholar]
- 18.Hutterer M., Nowosielski M., Putzer D., Jansen N.L., Seiz M., Schocke M., McCoy M., Göbel G., la Fougère C., Virgolini I.J., et al. [18F]-fluoro-ethyl-l-tyrosine PET: A valuable diagnostic tool in neuro-oncology, but not all that glitters is glioma. Neuro Oncol. 2013;15:341–351. doi: 10.1093/neuonc/nos300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pöpperl G., Kreth F.W., Mehrkens J.H., Herms J., Seelos K., Koch W., Gildehaus F.J., Kretzschmar H.A., Tonn J.C., Tatsch K. FET PET for the evaluation of untreated gliomas: Correlation of FET uptake and uptake kinetics with tumour grading. Eur. J. Nucl. Med. Mol. Imaging. 2007;34:1933–1942. doi: 10.1007/s00259-007-0534-y. [DOI] [PubMed] [Google Scholar]
- 20.Langen K.-J., Stoffels G., Filss C., Heinzel A., Stegmayr C., Lohmann P., Willuweit A., Neumaier B., Mottaghy F.M., Galldiks N. Imaging of amino acid transport in brain tumours: Positron emission tomography with O-(2-[18F]fluoroethyl)- L -tyrosine (FET) Methods. 2017;130:124–134. doi: 10.1016/j.ymeth.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 21.Yu P., Wang Y., Su F., Chen Y. Comparing [18F]FET PET and [18F]FDOPA PET for glioma recurrence diagnosis: A systematic review and meta-analysis. Front. Oncol. 2024;13:1346951. doi: 10.3389/fonc.2023.1346951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cui M., Zorrilla-Veloz R.I., Hu J., Guan B., Ma X. Diagnostic Accuracy of PET for Differentiating True Glioma Progression From Post Treatment-Related Changes: A Systematic Review and Meta-Analysis. Front. Neurol. 2021;12:671867. doi: 10.3389/fneur.2021.671867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ouyang Z.-Q., Zheng G.-R., Duan X.-R., Zhang X.-R., Ke T.-F., Bao S.-S., Yang J., He B., Liao C.-D. Diagnostic accuracy of glioma pseudoprogression identification with positron emission tomography imaging: A systematic review and meta-analysis. Quant. Imaging Med. Surg. 2023;13:4943–4959. doi: 10.21037/qims-22-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pauleit D., Stoffels G., Bachofner A., Floeth F.W., Sabel M., Herzog H., Tellmann L., Jansen P., Reifenberger G., Hamacher K., et al. Comparison of 18F-FET and 18F-FDG PET in brain tumors. Nucl. Med. Biol. 2009;36:779–787. doi: 10.1016/j.nucmedbio.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Mauler J., Lohmann P., Maudsley A.A., Sheriff S., Hoevels M., Meissner A.-K., Hamisch C., Brunn A., Deckert M., Filss C.P., et al. Diagnostic Accuracy of MR Spectroscopic Imaging and 18F-FET PET for Identifying Glioma: A Biopsy-Controlled Hybrid PET/MRI Study. J. Nucl. Med. 2024;65:16–21. doi: 10.2967/jnumed.123.265868. [DOI] [PubMed] [Google Scholar]
- 26.Floeth F.W., Pauleit D., Wittsack H.-J., Langen K.J., Reifenberger G., Hamacher K., Messing-Jünger M., Zilles K., Weber F., Stummer W., et al. Multimodal metabolic imaging of cerebral gliomas: Positron emission tomography with [18F]fluoroethyl-l-tyrosine and magnetic resonance spectroscopy. J. Neurosurg. 2005;102:318–327. doi: 10.3171/jns.2005.102.2.0318. [DOI] [PubMed] [Google Scholar]
- 27.Pauleit D. O-(2-[18F]fluoroethyl)-L-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain. 2005;128:678–687. doi: 10.1093/brain/awh399. [DOI] [PubMed] [Google Scholar]
- 28.Jeong S.Y., Lim S.M. Comparison of 3′-deoxy-3′-[18F]fluorothymidine PET and O-(2-[18F]fluoroethyl)-L-tyrosine PET in patients with newly diagnosed glioma. Nucl. Med. Biol. 2012;39:977–981. doi: 10.1016/j.nucmedbio.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 29.Verger A., Filss C.P., Lohmann P., Stoffels G., Sabel M., Wittsack H.J., Kops E.R., Galldiks N., Fink G.R., Shah N.J., et al. Comparison of 18F-FET PET and perfusion-weighted MRI for glioma grading: A hybrid PET/MR study. Eur. J. Nucl. Med. Mol. Imaging. 2017;44:2257–2265. doi: 10.1007/s00259-017-3812-3. [DOI] [PubMed] [Google Scholar]
- 30.Lopez W.O.C., Cordeiro J.G., Albicker U., Doostkam S., Nikkhah G., Kirch R.D., Trippel M., Reithmeier T. Correlation of 18F-fluoroethyl tyrosine positron-emission tomography uptake values and histomorphological findings by stereotactic serial biopsy in newly diagnosed brain tumors using a refined software tool. Onco Targets Ther. 2015;8:3803–3815. doi: 10.2147/OTT.S87126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lohmann P., Herzog H., Rota Kops E., Stoffels G., Judov N., Filss C., Galldiks N., Tellmann L., Weiss C., Sabel M., et al. Dual-time-point O-(2-[18F]fluoroethyl)-L-tyrosine PET for grading of cerebral gliomas. Eur. Radiol. 2015;25:3017–3024. doi: 10.1007/s00330-015-3691-6. [DOI] [PubMed] [Google Scholar]
- 32.Calcagni M.L., Galli G., Giordano A., Taralli S., Anile C., Niesen A., Baum R.P. Dynamic O-(2-[18F]fluoroethyl)-L-tyrosine (F-18 FET) PET for Glioma Grading: Assessment of Individual Probability of Malignancy. Clin. Nucl. Med. 2011;36:841–847. doi: 10.1097/RLU.0b013e3182291b40. [DOI] [PubMed] [Google Scholar]
- 33.Albert N.L., Winkelmann I., Suchorska B., Wenter V., Schmid-Tannwald C., Mille E., Todica A., Brendel M., Tonn J.-C., Bartenstein P., et al. Early static 18F-FET-PET scans have a higher accuracy for glioma grading than the standard 20–40 min scans. Eur. J. Nucl. Med. Mol. Imaging. 2016;43:1105–1114. doi: 10.1007/s00259-015-3276-2. [DOI] [PubMed] [Google Scholar]
- 34.Hua T., Zhou W., Zhou Z., Guan Y., Li M. Heterogeneous parameters based on 18F-FET PET imaging can non-invasively predict tumor grade and isocitrate dehydrogenase gene 1 mutation in untreated gliomas. Quant. Imaging Med. Surg. 2021;11:317–327. doi: 10.21037/qims-20-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kunz M., Thon N., Eigenbrod S., Hartmann C., Egensperger R., Herms J., Geisler J., la Fougere C., Lutz J., Linn J., et al. Hot spots in dynamic18FET-PET delineate malignant tumor parts within suspected WHO grade II gliomas. Neuro Oncol. 2011;13:307–316. doi: 10.1093/neuonc/noq196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Röhrich M., Huang K., Schrimpf D., Albert N.L., Hielscher T., Von Deimling A., Schüller U., Dimitrakopoulou-Strauss A., Haberkorn U. Integrated analysis of dynamic FET PET/CT parameters, histology, and methylation profiling of 44 gliomas. Eur. J. Nucl. Med. Mol. Imaging. 2018;45:1573–1584. doi: 10.1007/s00259-018-4009-0. [DOI] [PubMed] [Google Scholar]
- 37.Jansen N.L., Graute V., Armbruster L., Suchorska B., Lutz J., Eigenbrod S., Cumming P., Bartenstein P., Tonn J.-C., Kreth F.W., et al. MRI-suspected low-grade glioma: Is there a need to perform dynamic FET PET? Eur. J. Nucl. Med. Mol. Imaging. 2012;39:1021–1029. doi: 10.1007/s00259-012-2109-9. [DOI] [PubMed] [Google Scholar]
- 38.Jansen N.L., Schwartz C., Graute V., Eigenbrod S., Lutz J., Egensperger R., Pöpperl G., Kretzschmar H.A., Cumming P., Bartenstein P., et al. Prediction of oligodendroglial histology and LOH 1p/19q using dynamic [18F]FET-PET imaging in intracranial WHO grade II and III gliomas. Neuro Oncol. 2012;14:1473–1480. doi: 10.1093/neuonc/nos259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pyka T., Gempt J., Hiob D., Ringel F., Schlegel J., Bette S., Wester H.-J., Meyer B., Förster S. Textural analysis of pre-therapeutic [18F]-FET-PET and its correlation with tumor grade and patient survival in high-grade gliomas. Eur. J. Nucl. Med. Mol. Imaging. 2016;43:133–141. doi: 10.1007/s00259-015-3140-4. [DOI] [PubMed] [Google Scholar]
- 40.Zhou W., Huang Q., Wen J., Li M., Zhu Y., Liu Y., Dai Y., Guan Y., Zhou Z., Hua T. Integrated CT Radiomics Features Could Enhance the Efficacy of 18F-FET PET for Non-Invasive Isocitrate Dehydrogenase Genotype Prediction in Adult Untreated Gliomas: A Retrospective Cohort Study. Front Oncol. 2021;11:772703. doi: 10.3389/fonc.2021.772703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lohmann P., Lerche C., Bauer E.K., Steger J., Stoffels G., Blau T., Dunkl V., Kocher M., Viswanathan S., Filss C.P., et al. Predicting IDH genotype in gliomas using FET PET radiomics. Sci. Rep. 2018;8:13328. doi: 10.1038/s41598-018-31806-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verger A., Stoffels G., Bauer E.K., Lohmann P., Blau T., Fink G.R., Neumaier B., Shah N.J., Langen K.-J., Galldiks N. Static and dynamic 18F–FET PET for the characterization of gliomas defined by IDH and 1p/19q status. Eur. J. Nucl. Med. Mol. Imaging. 2018;45:443–451. doi: 10.1007/s00259-017-3846-6. [DOI] [PubMed] [Google Scholar]
- 43.Blanc-Durand P., Van Der Gucht A., Verger A., Langen K.-J., Dunet V., Bloch J., Brouland J.-P., Nicod-Lalonde M., Schaefer N., Prior J.O. Voxel-based 18F-FET PET segmentation and automatic clustering of tumor voxels: A significant association with IDH1 mutation status and survival in patients with gliomas. PLoS ONE. 2018;13:e0199379. doi: 10.1371/journal.pone.0199379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bette S., Gempt J., Delbridge C., Kirschke J.S., Schlegel J., Foerster S., Huber T., Pyka T., Zimmer C., Meyer B., et al. Prognostic Value of O-(2-[18F]-Fluoroethyl)-L-Tyrosine-Positron Emission Tomography Imaging for Histopathologic Characteristics and Progression-Free Survival in Patients with Low-Grade Glioma. World Neurosurg. 2016;89:230–239. doi: 10.1016/j.wneu.2016.01.085. [DOI] [PubMed] [Google Scholar]
- 45.Ort J., Hamou H.A., Kernbach J.M., Hakvoort K., Blume C., Lohmann P., Galldiks N., Heiland D.H., Mottaghy F.M., Clusmann H., et al. 18F-FET-PET-guided gross total resection improves overall survival in patients with WHO grade III/IV glioma: Moving towards a multimodal imaging-guided resection. J. Neurooncol. 2021;155:71–80. doi: 10.1007/s11060-021-03844-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Floeth F.W., Sabel M., Ewelt C., Stummer W., Felsberg J., Reifenberger G., Steiger H.J., Stoffels G., Coenen H.H., Langen K.-J. Comparison of 18F-FET PET and 5-ALA fluorescence in cerebral gliomas. Eur. J. Nucl. Med. Mol. Imaging. 2011;38:731–741. doi: 10.1007/s00259-010-1690-z. [DOI] [PubMed] [Google Scholar]
- 47.Ewelt C., Floeth F.W., Felsberg J., Steiger H.J., Sabel M., Langen K.-J., Stoffels G., Stummer W. Finding the anaplastic focus in diffuse gliomas: The value of Gd-DTPA enhanced MRI, FET-PET, and intraoperative, ALA-derived tissue fluorescence. Clin. Neurol. Neurosurg. 2011;113:541–547. doi: 10.1016/j.clineuro.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 48.Verburg N., Koopman T., Yaqub M.M., Hoekstra O.S., Lammertsma A.A., Barkhof F., Pouwels P.J.W., Reijneveld J.C., Heimans J.J., Rozemuller A.J.M., et al. Improved detection of diffuse glioma infiltration with imaging combinations: A diagnostic accuracy study. Neuro Oncol. 2020;22:412–422. doi: 10.1093/neuonc/noz180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buchmann N., Kläsner B., Gempt J., Bauer J.S., Pyka T., Delbridge C., Meyer B., Krause B.J., Ringel F. 18F-Fluoroethyl-l-Thyrosine Positron Emission Tomography to Delineate Tumor Residuals After Glioblastoma Resection: A Comparison with Standard Postoperative Magnetic Resonance Imaging. World Neurosurg. 2016;89:420–426. doi: 10.1016/j.wneu.2016.02.032. [DOI] [PubMed] [Google Scholar]
- 50.Kläsner B., Buchmann N., Gempt J., Ringel F., Lapa C., Krause B.J. Early [18F]FET-PET in Gliomas after Surgical Resection: Comparison with MRI and Histopathology. PLoS ONE. 2015;10:e0141153. doi: 10.1371/journal.pone.0141153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Allard B., Dissaux B., Bourhis D., Dissaux G., Schick U., Salaün P.-Y., Abgral R., Querellou S. Hotspot on 18F-FET PET/CT to Predict Aggressive Tumor Areas for Radiotherapy Dose Escalation Guiding in High-Grade Glioma. Cancers. 2022;15:98. doi: 10.3390/cancers15010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Munck af Rosenschold P., Costa J., Engelholm S.A., Lundemann M.J., Law I., Ohlhues L., Engelholm S. Impact of [18F]-fluoro-ethyl-tyrosine PET imaging on target definition for radiation therapy of high-grade glioma. Neuro Oncol. 2015;17:757–763. doi: 10.1093/neuonc/nou316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fleischmann D.F., Unterrainer M., Schön R., Corradini S., Maihöfer C., Bartenstein P., Belka C., Albert N.L., Niyazi M. Margin reduction in radiotherapy for glioblastoma through 18F-fluoroethyltyrosine PET?—A recurrence pattern analysis. Radiother. Oncol. 2020;145:49–55. doi: 10.1016/j.radonc.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 54.Harat M., Małkowski B., Makarewicz R. Pre-irradiation tumour volumes defined by MRI and dual time-point FET-PET for the prediction of glioblastoma multiforme recurrence: A prospective study. Radiother. Oncol. 2016;120:241–247. doi: 10.1016/j.radonc.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 55.Dissaux G., Dissaux B., Kabbaj O.E., Gujral D.M., Pradier O., Salaün P.-Y., Seizeur R., Bourhis D., Ben Salem D., Querellou S., et al. Radiotherapy target volume definition in newly diagnosed high grade glioma using 18F-FET PET imaging and multiparametric perfusion MRI: A prospective study (IMAGG) Radiother. Oncol. 2020;150:164–171. doi: 10.1016/j.radonc.2020.06.025. [DOI] [PubMed] [Google Scholar]
- 56.Hayes A.R., Jayamanne D., Hsiao E., Schembri G.P., Bailey D.L., Roach P.J., Khasraw M., Newey A., Wheeler H.R., Back M. Utilizing 18F-fluoroethyltyrosine (FET) positron emission tomography (PET) to define suspected nonenhancing tumor for radiation therapy planning of glioblastoma. Pract. Radiat. Oncol. 2018;8:230–238. doi: 10.1016/j.prro.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 57.Galldiks N., Stoffels G., Ruge M.I., Rapp M., Sabel M., Reifenberger G., Erdem Z., Shah N.J., Fink G.R., Coenen H.H., et al. Role of O-(2-18F-Fluoroethyl)-l-Tyrosine PET as a Diagnostic Tool for Detection of Malignant Progression in Patients with Low-Grade Glioma. J. Nucl. Med. 2013;54:2046–2054. doi: 10.2967/jnumed.113.123836. [DOI] [PubMed] [Google Scholar]
- 58.Unterrainer M., Schweisthal F., Suchorska B., Wenter V., Schmid-Tannwald C., Fendler W.P., Schüller U., Bartenstein P., Tonn J.-C., Albert N.L. Serial 18 F-FET PET Imaging of Primarily 18 F-FET–Negative Glioma: Does It Make Sense? J. Nucl. Med. 2016;57:1177–1182. doi: 10.2967/jnumed.115.171033. [DOI] [PubMed] [Google Scholar]
- 59.Bashir A., Brennum J., Broholm H., Law I. The diagnostic accuracy of detecting malignant transformation of low-grade glioma using O-(2-[18F]fluoroethyl)-l-tyrosine positron emission tomography: A retrospective study. J. Neurosurg. 2018;130:451–464. doi: 10.3171/2017.8.JNS171577. [DOI] [PubMed] [Google Scholar]
- 60.Jeong S.Y., Lee T.H., Rhee C.H., Cho A.R., Il Kim B., Cheon G.J., Choi C.W., Lim S.M. 3′-Deoxy-3′-[18F]fluorothymidine and O-(2-[18F]fluoroethyl)-L-tyrosine PET in Patients with Suspicious Recurrence of Glioma after Multimodal Treatment: Initial Results of a Retrospective Comparative Study. Nucl. Med. Mol. Imaging. 2010;44:45–54. doi: 10.1007/s13139-009-0007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jansen N.L., Suchorska B., Schwarz S.B., Eigenbrod S., Lutz J., Graute V., Bartenstein P., Belka C., Kreth F.W., Fougère C.L. [18F]Fluoroethyltyrosine–Positron Emission Tomography-Based Therapy Monitoring after Stereotactic Iodine-125 Brachytherapy in Patients with Recurrent High-Grade Glioma. Mol. Imaging. 2013;12:7290.2012.00027. doi: 10.2310/7290.2012.00027. [DOI] [PubMed] [Google Scholar]
- 62.Puranik A.D., Rangarajan V., Dev I.D., Jain Y., Purandare N.C., Sahu A., Choudhary A., Gupta T., Chatterjee A., Moiyadi A., et al. Brain FET PET tumor-to-white mater ratio to differentiate recurrence from post-treatment changes in high-grade gliomas. J. Neuroimaging. 2021;31:1211–1218. doi: 10.1111/jon.12914. [DOI] [PubMed] [Google Scholar]
- 63.Kertels O., Mihovilovic M.I., Linsenmann T., Kessler A.F., Tran-Gia J., Kircher M., Brumberg J., Monoranu C.M., Samnick S., Ernestus R.-I., et al. Clinical Utility of Different Approaches for Detection of Late Pseudoprogression in Glioblastoma with O-(2-[18F]Fluoroethyl)-l-Tyrosine PET. Clin. Nucl. Med. 2019;44:695–701. doi: 10.1097/RLU.0000000000002652. [DOI] [PubMed] [Google Scholar]
- 64.Verger A., Filss C.P., Lohmann P., Stoffels G., Sabel M., Wittsack H.-J., Kops E.R., Galldiks N., Fink G.R., Shah N.J., et al. Comparison of O-(2-18 F-Fluoroethyl)-L-Tyrosine Positron Emission Tomography and Perfusion-Weighted Magnetic Resonance Imaging in the Diagnosis of Patients with Progressive and Recurrent Glioma: A Hybrid Positron Emission Tomography/Magnetic Resonance Study. World Neurosurg. 2018;113:e727–e737. doi: 10.1016/j.wneu.2018.02.139. [DOI] [PubMed] [Google Scholar]
- 65.Pyka T., Hiob D., Preibisch C., Gempt J., Wiestler B., Schlegel J., Straube C., Zimmer C. Diagnosis of glioma recurrence using multiparametric dynamic 18F-fluoroethyl-tyrosine PET-MRI. Eur. J. Radiol. 2018;103:32–37. doi: 10.1016/j.ejrad.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 66.Werner J.-M., Weller J., Ceccon G., Schaub C., Tscherpel C., Lohmann P., Bauer E.K., Schäfer N., Stoffels G., Baues C., et al. Diagnosis of Pseudoprogression Following Lomustine-Temozolomide Chemoradiation in Newly Diagnosed Glioblastoma Patients Using FET-PET. Clin. Cancer Res. 2021;27:3704–3713. doi: 10.1158/1078-0432.CCR-21-0471. [DOI] [PubMed] [Google Scholar]
- 67.Galldiks N., Dunkl V., Stoffels G., Hutterer M., Rapp M., Sabel M., Reifenberger G., Kebir S., Dorn F., Blau T., et al. Diagnosis of pseudoprogression in patients with glioblastoma using O-(2-[18F]fluoroethyl)-l-tyrosine PET. Eur. J. Nucl. Med. Mol. Imaging. 2015;42:685–695. doi: 10.1007/s00259-014-2959-4. [DOI] [PubMed] [Google Scholar]
- 68.Werner J.-M., Stoffels G., Lichtenstein T., Borggrefe J., Lohmann P., Ceccon G., Shah N.J., Fink G.R., Langen K.-J., Kabbasch C., et al. Differentiation of treatment-related changes from tumour progression: A direct comparison between dynamic FET PET and ADC values obtained from DWI MRI. Eur. J. Nucl. Med. Mol. Imaging. 2019;46:1889–1901. doi: 10.1007/s00259-019-04384-7. [DOI] [PubMed] [Google Scholar]
- 69.Lohmann P., Elahmadawy M.A., Gutsche R., Werner J.-M., Bauer E.K., Ceccon G., Kocher M., Lerche C.W., Rapp M., Fink G.R., et al. FET PET Radiomics for Differentiating Pseudoprogression from Early Tumor Progression in Glioma Patients Post-Chemoradiation. Cancers. 2020;12:3835. doi: 10.3390/cancers12123835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kebir S., Fimmers R., Galldiks N., Schäfer N., Mack F., Schaub C., Stuplich M., Niessen M., Tzaridis T., Simon M., et al. Late Pseudoprogression in Glioblastoma: Diagnostic Value of Dynamic O-(2-[18F]fluoroethyl)-L-Tyrosine PET. Clin. Cancer Res. 2016;22:2190–2196. doi: 10.1158/1078-0432.CCR-15-1334. [DOI] [PubMed] [Google Scholar]
- 71.Rachinger W., Goetz C., Pöpperl G., Gildehaus F.J., Kreth F.W., Holtmannspötter M., Herms J., Koch W., Tatsch K., Tonn J.-C. Positron Emission Tomography with O-(2-[18F]fluoroethyl)-l-tyrosine versus Magnetic Resonance Imaging in the Diagnosis of Recurrent Gliomas. Neurosurgery. 2005;57:505–511. doi: 10.1227/01.NEU.0000171642.49553.B0. [DOI] [PubMed] [Google Scholar]
- 72.Lohmeier J., Bohner G., Siebert E., Brenner W., Hamm B., Makowski M.R. Quantitative biparametric analysis of hybrid 18F-FET PET/MR-neuroimaging for differentiation between treatment response and recurrent glioma. Sci. Rep. 2019;9:14603. doi: 10.1038/s41598-019-50182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bashir A., Mathilde Jacobsen S., Mølby Henriksen O., Broholm H., Urup T., Grunnet K., Andrée Larsen V., Møller S., Skjøth-Rasmussen J., Skovgaard Poulsen H., et al. Recurrent glioblastoma versus late posttreatment changes: Diagnostic accuracy of O-(2-[18F]fluoroethyl)-L-tyrosine positron emission tomography (18F-FET PET) Neuro Oncol. 2019;21:1595–1606. doi: 10.1093/neuonc/noz166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Steidl E., Langen K.-J., Hmeidan S.A., Polomac N., Filss C.P., Galldiks N., Lohmann P., Keil F., Filipski K., Mottaghy F.M., et al. Sequential implementation of DSC-MR perfusion and dynamic [18F]FET PET allows efficient differentiation of glioma progression from treatment-related changes. Eur. J. Nucl. Med. Mol. Imaging. 2021;48:1956–1965. doi: 10.1007/s00259-020-05114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pöpperl G., Götz C., Rachinger W., Schnell O., Gildehaus F.J., Tonn J.C., Tatsch K. Serial O-(2-[18F]fluoroethyl)-L-tyrosine PET for monitoring the effects of intracavitary radioimmunotherapy in patients with malignant glioma. Eur. J. Nucl. Med. Mol. Imaging. 2006;33:792–800. doi: 10.1007/s00259-005-0053-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Müller M., Winz O., Gutsche R., Leijenaar R.T.H., Kocher M., Lerche C., Filss C.P., Stoffels G., Steidl E., Hattingen E., et al. Static FET PET radiomics for the differentiation of treatment-related changes from glioma progression. J. Neurooncol. 2022;159:519–529. doi: 10.1007/s11060-022-04089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mehrkens J.H., Pöpperl G., Rachinger W., Herms J., Seelos K., Tatsch K., Tonn J.C., Kreth F.W. The positive predictive value of O-(2-[18F]fluoroethyl)-l-tyrosine (FET) PET in the diagnosis of a glioma recurrence after multimodal treatment. J. Neurooncol. 2008;88:27–35. doi: 10.1007/s11060-008-9526-4. [DOI] [PubMed] [Google Scholar]
- 78.Galldiks N., Stoffels G., Filss C., Rapp M., Blau T., Tscherpel C., Ceccon G., Dunkl V., Weinzierl M., Stoffel M., et al. The use of dynamic O-(2-18F-fluoroethyl)-l-tyrosine PET in the diagnosis of patients with progressive and recurrent glioma. Neuro Oncol. 2015;17:1293–1300. doi: 10.1093/neuonc/nov088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pöpperl G., Götz C., Rachinger W., Gildehaus F.-J., Tonn J.-C., Tatsch K. Value of O-(2-[18F]fluoroethyl)-l-tyrosine PET for the diagnosis of recurrent glioma. Eur. J. Nucl. Med. Mol. Imaging. 2004;31:1464–1470. doi: 10.1007/s00259-004-1590-1. [DOI] [PubMed] [Google Scholar]
- 80.Müther M., Koch R., Weckesser M., Sporns P., Schwindt W., Stummer W. 5-Aminolevulinic Acid Fluorescence-Guided Resection of 18F-FET-PET Positive Tumor Beyond Gadolinium Enhancing Tumor Improves Survival in Glioblastoma. Neurosurgery. 2019;85:E1020–E1029. doi: 10.1093/neuros/nyz199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Suchorska B., Unterrainer M., Biczok A., Sosnova M., Forbrig R., Bartenstein P., Tonn J.-C., Albert N.L., Kreth F.-W. 18F-FET-PET as a biomarker for therapy response in non-contrast enhancing glioma following chemotherapy. J. Neurooncol. 2018;139:721–730. doi: 10.1007/s11060-018-2919-0. [DOI] [PubMed] [Google Scholar]
- 82.Galldiks N., Langen K.-J., Holy R., Pinkawa M., Stoffels G., Nolte K.W., Kaiser H.J., Filss C.P., Fink G.R., Coenen H.H., et al. Assessment of Treatment Response in Patients with Glioblastoma Using O-(2-18F-Fluoroethyl)-l-Tyrosine PET in Comparison to MRI. J. Nucl. Med. 2012;53:1048–1057. doi: 10.2967/jnumed.111.098590. [DOI] [PubMed] [Google Scholar]
- 83.Suchorska B., Jansen N.L., Linn J., Kretzschmar H., Janssen H., Eigenbrod S., Simon M., Pöpperl G., Kreth F.W., La Fougere C., et al. Biological tumor volume in 18FET-PET before radiochemotherapy correlates with survival in GBM. Neurology. 2015;84:710–719. doi: 10.1212/WNL.0000000000001262. [DOI] [PubMed] [Google Scholar]
- 84.Jansen N.L., Suchorska B., Wenter V., Eigenbrod S., Schmid-Tannwald C., Zwergal A., Niyazi M., Drexler M., Bartenstein P., Schnell O., et al. Dynamic 18F-FET PET in Newly Diagnosed Astrocytic Low-Grade Glioma Identifies High-Risk Patients. J. Nucl. Med. 2014;55:198–203. doi: 10.2967/jnumed.113.122333. [DOI] [PubMed] [Google Scholar]
- 85.Thon N., Kunz M., Lemke L., Jansen N.L., Eigenbrod S., Kreth S., Lutz J., Egensperger R., Giese A., Herms J., et al. Dynamic 18F-FET PET in suspected WHO grade II gliomas defines distinct biological subgroups with different clinical courses. Int. J. Cancer. 2015;136:2132–2145. doi: 10.1002/ijc.29259. [DOI] [PubMed] [Google Scholar]
- 86.Kunz M., Albert N.L., Unterrainer M., la Fougere C., Egensperger R., Schüller U., Lutz J., Kreth S., Tonn J.-C., Kreth F.-W., et al. Dynamic 18F-FET PET is a powerful imaging biomarker in gadolinium-negative gliomas. Neuro Oncol. 2019;21:274–284. doi: 10.1093/neuonc/noy098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ceccon G., Lohmann P., Werner J.-M., Tscherpel C., Dunkl V., Stoffels G., Rosen J., Rapp M., Sabel M., Herrlinger U., et al. Early Treatment Response Assessment Using 18F-FET PET Compared with Contrast-Enhanced MRI in Glioma Patients After Adjuvant Temozolomide Chemotherapy. J. Nucl. Med. 2021;62:918–925. doi: 10.2967/jnumed.120.254243. [DOI] [PubMed] [Google Scholar]
- 88.Galldiks N., Dunkl V., Ceccon G., Tscherpel C., Stoffels G., Law I., Henriksen O.M., Muhic A., Poulsen H.S., Steger J., et al. Early treatment response evaluation using FET PET compared to MRI in glioblastoma patients at first progression treated with bevacizumab plus lomustine. Eur. J. Nucl. Med. Mol. Imaging. 2018;45:2377–2386. doi: 10.1007/s00259-018-4082-4. [DOI] [PubMed] [Google Scholar]
- 89.Carles M., Popp I., Starke M.M., Mix M., Urbach H., Schimek-Jasch T., Eckert F., Niyazi M., Baltas D., Grosu A.L. FET-PET radiomics in recurrent glioblastoma: Prognostic value for outcome after re-irradiation? Radiat. Oncol. 2021;16:46. doi: 10.1186/s13014-020-01744-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Suchorska B., Giese A., Biczok A., Unterrainer M., Weller M., Drexler M., Bartenstein P., Schüller U., Tonn J.-C., Albert N.L. Identification of time-to-peak on dynamic 18F-FET-PET as a prognostic marker specifically in IDH1/2 mutant diffuse astrocytoma. Neuro Oncol. 2018;20:279–288. doi: 10.1093/neuonc/nox153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wirsching H.-G., Roelcke U., Weller J., Hundsberger T., Hottinger A.F., von Moos R., Caparrotti F., Conen K., Remonda L., Roth P., et al. MRI and 18FET-PET predict survival benefit from bevacizumab plus radiotherapy in patients with IDH wild-type glioblastoma: Results from the randomized ARTE trial. Clin. Cancer Res. 2021;27:179–188. doi: 10.1158/1078-0432.CCR-20-2096. [DOI] [PubMed] [Google Scholar]
- 92.Sweeney R., Polat B., Samnick S., Reiners C., Flentje M., Verburg F.A. O-(2-[18F]fluoroethyl)-l-tyrosine uptake is an independent prognostic determinant in patients with glioma referred for radiation therapy. Ann. Nucl. Med. 2014;28:154–162. doi: 10.1007/s12149-013-0792-7. [DOI] [PubMed] [Google Scholar]
- 93.Pyka T., Gempt J., Ringel F., Hüttinger S., van Marwick S., Nekolla S., Wester H.-J., Schwaiger M., Förster S. Prediction of Glioma Recurrence Using Dynamic 18F-Fluoroethyltyrosine PET. AJNR Am. J. Neuroradiol. 2014;35:1924–1929. doi: 10.3174/ajnr.A3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wollring M.M., Werner J.-M., Bauer E.K., Tscherpel C., Ceccon G.S., Lohmann P., Stoffels G., Kabbasch C., Goldbrunner R., Fink G.R., et al. Prediction of response to lomustine-based chemotherapy in glioma patients at recurrence using MRI and FET PET. Neuro Oncol. 2022;25:984–994. doi: 10.1093/neuonc/noac229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bauer E.K., Stoffels G., Blau T., Reifenberger G., Felsberg J., Werner J.M., Lohmann P., Rosen J., Ceccon G., Tscherpel C., et al. Prediction of survival in patients with IDH-wildtype astrocytic gliomas using dynamic O-(2-[18F]-fluoroethyl)-l-tyrosine PET. Eur. J. Nucl. Med. Mol. Imaging. 2020;47:1486–1495. doi: 10.1007/s00259-020-04695-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Piroth M.D., Holy R., Pinkawa M., Stoffels G., Kaiser H.J., Galldiks N., Herzog H., Coenen H.H., Eble M.J., Langen K.J. Prognostic impact of postoperative, pre-irradiation 18F-fluoroethyl-l-tyrosine uptake in glioblastoma patients treated with radiochemotherapy. Radiother. Oncol. 2011;99:218–224. doi: 10.1016/j.radonc.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 97.Jansen N.L., Suchorska B., Wenter V., Schmid-Tannwald C., Todica A., Eigenbrod S., Niyazi M., Tonn J.-C., Bartenstein P., Kreth F.-W., et al. Prognostic Significance of Dynamic 18F-FET PET in Newly Diagnosed Astrocytic High-Grade Glioma. J. Nucl. Med. 2015;56:9–15. doi: 10.2967/jnumed.114.144675. [DOI] [PubMed] [Google Scholar]
- 98.Moller S., Law I., Munck Af Rosenschold P., Costa J., Poulsen H.S., Engelholm S.A., Engelholm S. Prognostic value of 18F-FET PET imaging in re-irradiation of high-grade glioma: Results of a phase I clinical trial. Radiother. Oncol. 2016;121:132–137. doi: 10.1016/j.radonc.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 99.Dissaux G., Basse V., Schick U., EL Kabbaj O., Auberger B., Magro E., Kassoul A., Abgral R., Salaun P.-Y., Bourhis D., et al. Prognostic value of 18F-FET PET/CT in newly diagnosed WHO 2016 high-grade glioma. Medicine. 2020;99:e19017. doi: 10.1097/MD.0000000000019017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Piroth M.D., Pinkawa M., Holy R., Klotz J., Nussen S., Stoffels G., Coenen H.H., Kaiser H.J., Langen K.J., Eble M.J. Prognostic Value of Early [18F]Fluoroethyltyrosine Positron Emission Tomography After Radiochemotherapy in Glioblastoma Multiforme. Int. J. Radiat. Oncol. Biol. Phys. 2011;80:176–184. doi: 10.1016/j.ijrobp.2010.01.055. [DOI] [PubMed] [Google Scholar]
- 101.Schneider F., Wolpert F., Stolzmann P., Albatly A.A., Kenkel D., Weller J., Weller M., Kollias S.S., Rushing E.J., Veit-Haibach P., et al. Prognostic value of O-(2-[18F]-fluoroethyl)-L-tyrosine PET in relapsing oligodendroglioma. Acta Oncol. 2020;59:1357–1364. doi: 10.1080/0284186X.2020.1787507. [DOI] [PubMed] [Google Scholar]
- 102.Kertels O., Kessler A.F., Mihovilovic M.I., Stolzenburg A., Linsenmann T., Samnick S., Brändlein S., Monoranu C.M., Ernestus R.-I., Buck A.K., et al. Prognostic Value of O-(2-[18F]Fluoroethyl)-L-Tyrosine PET/CT in Newly Diagnosed WHO 2016 Grade II and III Glioma. Mol. Imaging Biol. 2019;21:1174–1181. doi: 10.1007/s11307-019-01357-y. [DOI] [PubMed] [Google Scholar]
- 103.Floeth F.W., Pauleit D., Sabel M., Stoffels G., Reifenberger G., Riemenschneider M.J., Jansen P., Coenen H.H., Steiger H.-J., Langen K.-J. Prognostic Value of O-(2-18F-Fluoroethyl)-L-Tyrosine PET and MRI in Low-Grade Glioma. J. Nucl. Med. 2007;48:519–527. doi: 10.2967/jnumed.106.037895. [DOI] [PubMed] [Google Scholar]
- 104.Niyazi M., Jansen N., Ganswindt U., Schwarz S.B., Geisler J., Schnell O., Büsing K., Eigenbrod S., La Fougère C., Belka C. Re-irradiation in recurrent malignant glioma: Prognostic value of [18F]FET–PET. J. Neurooncol. 2012;110:389–395. doi: 10.1007/s11060-012-0980-7. [DOI] [PubMed] [Google Scholar]
- 105.Albert N.L., Galldiks N., Ellingson B.M., van den Bent M.J., Chang S.M., Cicone F., de Groot J., Koh E.-S., Law I., Rhun E.L., et al. PET-based response assessment criteria for diffuse gliomas (PET RANO 1.0): A report of the RANO group. Lancet Oncol. 2024;25:e29–e41. doi: 10.1016/S1470-2045(23)00525-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.