Abstract
The genus Aspergillus contains several species that are important plant pathogens. Plant pathogenic Aspergillus spp. affect agricultural crops in the field as well as after harvest, often associated with corn ear rot, cotton boll rot, peanut yellow mold, black mold of onion and garlic, fruit rot on grapes, pomegranates, olives, citrus, and apples. Coffee berries and coffee beans as well as tree nuts are also frequently infected by Aspergillus spp. Some of the plant pathogenic Aspergillus spp. are also mycotoxigenic, produced mycotoxin in the plant tissues leading to contamination of agricultural products. Over the years, reports of plant diseases caused by Aspergillus in various crops have increased, suggesting they are commonly encountered plant pathogens. This review focuses on agricultural crops or cultivated plants infected by Aspergillus spp. The compilation of plant pathogenic Aspergillus spp. provides information to mycologists, particularly those involved in plant pathology and crop protection, with updated information on plant diseases caused by various species of Aspergillus. The updated information also includes the locality or location, province, state and the country. The knowledge on the prevalence and geographic distribution of plant pathogenic Aspergillus spp. is beneficial in the application of crop protection.
Keywords: Aspergillus, crops, corn ear rot, cotton boll rot, peanut yellow mold, black mold, fruit rot, mycotoxins
1. Introduction
Aspergillus species are ubiquitous, found in various types of substrates, and distributed across all geographic areas and climatic conditions worldwide. Worldwide distribution of Aspergillus contributes to the conidia, which are common constituents of air, moving or drifting via air currents and spreading across both short and long distances. When conidia are deposited on a suitable substrate, they germinate when the conditions are suitable [1], colonizing the substrates via the degradation process. Agricultural crops and products, particularly food and feed, are common substrates of Aspergillus, leading to rotting or spoilage of crops and produce [1].
In earlier studies of plant pathogenic Aspergillus, A. niger and A. flavus have often been implicated in diseases of agricultural crops. Over the years, particularly after the introduction of the one fungus, one name concept, and taxonomic revision of the genus Aspergillus [2,3], other species have been reported as plant pathogens.
Black Aspergillus (section Nigri) often causes postharvest diseases in fruit crops, tree nuts, and vegetables, and is often found on peanuts, corn, onions, coffee, and grapes [4]. It is easy to recognize black Aspergillus as masses of black conidia appear on the infected parts of plants [5]. These conidia contain melanin in the cell wall, which protects them against UV light, drought, and high salt concentrations [6]. Species of black Aspergillus reported as plant pathogens are A. niger, A. carbonarius, A. welwitschiae, A. ochraceus, A. awamori, A. aculeatus, A. tubingensis, A. japonicus, A. uvarum, A. foetidus, A. brasiliensis, A. aculeatinus, and A. sclerotiicarbonarius, which are mentioned in this manuscript. Some of these species are producers of ocharatoxin such as A. carbonarius, A. welwitschiae, and A. niger. In addition, A. niger is also a fumonisin producer [7].
Aspergillus section Flavi is associated with plant diseases including A. flavus, A. parasiticus, A. oryzae, A. tamarii, and A. minisclerotigenes. Among the species, A. flavus is a well-known aflatoxin producer that is often associated with cottonseed, maize, peanuts, and tree nuts in the field and postharvest. Aspergillus parasiticus is also an aflatoxin producer particularly associated with peanuts.
Other species of Aspergillus that have been reported to be associated with plant diseases are A. fumigatus (section Fumigati), A. westerdijkiae, and A. ostianus (section Circumdati), A. terreus (section Terrei), A. versicolor (section Versicolores), A. candidus (section Candidi), A. sulphureus (section Aspergillus), and A. ustus (section Usti), as mentioned in this manuscript.
Due to taxonomic revision of the genus Aspergillus, new species have been described [8,9] and may affect the identity of plant pathogenic species. As such, the information summarized in this work, including details on Aspergillus species associated with plant diseases, their occurrence, and geographic distribution, provides a valuable contribution that can assist professionals in this field in their efforts to address crop health and protection issues.
2. Pathogenicity of Aspergillus in Plants
Aspergillus species associated with plant diseases are generally opportunistic pathogens, and wounds or injuries are necessary for infection and colonization of the plant host [10]. Infection by Aspergillus usually occurs because of insect damage after drought or heat stress. For Aspergillus to cause disease, the conidia must germinate, followed by hyphal penetration and the colonization of the plant tissues. Subsequently, the plant host physiology is altered, and Aspergillus must adapt to the plant environment. After colonization and disease occurrence, conidia are produced and dispersed in the environment [10], and are an important factor for the survival of Aspergillus under hostile conditions [11].
The developmental stages of Aspergillus pathogenesis involve genes that enable infection and suppress resistance. The expression of the genes involved may be influenced by plant defense mechanisms and nutrient composition [12]. For Aspergillus, more data are available on pathogenesis in animals and humans than on pathogenesis in plants. However, according to Sexton and Howlett [10], fungal pathogenesis in animals, humans, and plants is similar, and information on pathogenesis in animals and humans can be applied to plants to understand disease mechanisms.
Conidial germination is an early stage of disease infection. Three morphological stages of conidial germination have been proposed: dormancy, isotropic growth, and polarized growth. Conidial dormancy is broken by several factors, including the presence of water and/or nutrients. Isotrophic growth is the swelling stage of conidia, which involves water uptake and the formation of new cell wall materials. The formation of a germ tube is known as polarized growth [11]. Ras protein and Cdc42/Rho GTPases are involved in fungal development and adaptation of fungal cells [13,14]. In polarized growth, RasA and RasB genes are essential in hyphal morphogenesis [13]. Detailed information on the genes and proteins involved in conidial germination and the formation of morphological stages of Aspergillus is provided in an earlier review by Baltussen et al. [11].
Germination of conidia leads to the formation of hyphae and mycelia, which subsequently enter and colonize the plant host. The conidia and hyphae are hydrophobic because they contain hydrophobins and globular proteins that are associated with pathogenicity, including hyphae attachment to plant tissues, the dispersal of conidia [15,16] and the increased longevity of aerial hyphae [17].
During the infection process, Aspergillus does not have access to the nutrients needed for its energy supply nor the biosynthesis of essential molecules to further colonize the plant tissues. To obtain the nutrients, the fungus depends on fatty acid metabolism, which is based on the glyoxylate cycle [18], which plays a role in fungal nutrition, and fungal virulence [19].
Lytic enzymes, such as proteases, are considered virulence factors in fungal pathogenesis as they are active in a wide pH range (pH 4–11) and have broad substrate specificity. Aspergillus also produces proteases for metabolism and pathogenicity [20,21]. Lytic enzymes are also useful for fungal colonization, nutrient uptake, adherence, and dissemination in plant tissues [22].
Melanin is a component of the fungal cell wall that confers resistance to UV light, protects against adverse environmental conditions, and contributes to fungal virulence [23]. Melanin also plays a role in conidial survival in plant hosts [10]. Furthermore, Aspergillus species have been reported to synthesize DHN melanin (1,8-dihydroxynaphthalene) and pyomelanin [24].
Superoxide dismutases (SOD) may also be virulence factors for the colonization of plant hosts by Aspergillus, acting together with other virulence factors [25]. Several species, such as A. niger, A. flavus, A. terreus, and A. nidulans, have been reported to produce SOD [25]. Reverberi et al. [26] demonstrated that A. flavus exhibited transcriptional changes in both primary and secondary metabolism genes, depending on the substrate colonized, as a result of the trophic shift from saprobic growth to invasive pathogenic colonization. Pathogenic growth of the fungus in living kernels led to the upregulation of oxidative stress response pathway genes. Oxidative stress conditions arise at the fungus-host interface due to the plant’s defense mechanisms, and fungal pathogens have evolved strategies to detect and mitigate ROS accumulation, such as through the secretion of SOD and catalase, which convert ROS into less reactive molecules. Antioxidant mutants of A. flavus showed impaired growth and produced less aflatoxins, highlighting oxidative stress responses as a key factor in the switch from saprobic to pathogenic behavior.
In colonization of a plant host, mycotoxin is also a virulence factor that kills host tissues. For example, cyclopiazonic acid has been reported to be the main pathogenic factor in the colonization by A. flavus [27]. As for aflatoxin, Mehl et al. [28] suggested that the production of the mycotoxin in the soil gives the fungus a better competing ability against soil organisms, instead of functioning as a pathogenicity factor for colonization in plant tissues.
Colonization creates favorable conditions for the growth and development of Aspergillus. The fungus reproduces asexually within the plant tissues and produces conidia. Then, lesions form on the surface of the plant [10].
3. Common Plant Diseases Caused by Aspergillus
3.1. Corn Ear Rot
Aspergillus flavus is one of the main fungal species causing corn ear rot, although A. parasiticus and A. niger have also been reported to be associated with this disease [29]. Many studies on Aspergillus ear rot in corn-producing countries worldwide have focused on A. flavus as it is an aflatoxin producer [30,31,32,33].
Aspergillus species that cause corn ear rot survive in the soil and remain in crop debris, which becomes a source of inoculum. The infection of the ears occurs through silk during pollination and grain filling. Conidia from sources of inoculum land on the silk and germinate, develop in the silk, and grow downwards to colonize the ears [30]. The infection of the kernels occurs once the kernels are mature [31]. The growth of this pathogen is favored by high temperatures (>28 °C) and the high-water activity found in kernels. Under these conditions, A. flavus tends to become the predominant pathogen in corn kernels and develops during postharvest.
Drought stress and insect damage contribute to the susceptibility of corn plants to Aspergillus infection [29]. Drought and heat conditions lead to poor kernel development, which is suitable for the rapid growth of A. flavus as well as mycotoxin production [31]. Wound or injury produced by earworms and corn borers provide a point of entry for conidial infection. Drought stress intensifies insect damage to husks, which expedites the transmission of A. flavus [32,33].
The endophytic infection of corn ear rot by black Aspergillus may occur as the fungus has been isolated from healthy kernels. Moreover, some species of black Aspergillus can occur as biotrophic endophytes in corn [7]. Endophytic A. flavus has also been previously recovered from healthy corn [34,35].
The appearance of black or green conidial masses on the kernels is an indication of Aspergillus ear rot, which occurs at wound areas or near the ear tip. Aspergillus flavus typically form olive-green conidial masses, while A. niger forms a black coloration [29]. Aspergillus infection can occur in the field in maturing or mature kernels, as well as during harvest, storage, and processing. Infection in corn does not necessarily imply aflatoxin occurrence but clearly indicates an increased risk of contamination. For A. flavus, aflatoxin production occurs at a water activity of 0.87 [36] and an optimum temperature of 27–30 °C [37]. Under suitable conditions, aflatoxin can be produced within 24 h after infestation [38].
Ochratoxin A was also detected in corn under field conditions, suggesting an association between black aspergilli, especially A. niger, and corn during crop growth [7,39,40]. Thus, in addition to aflatoxin, ochratoxin is another Aspergillus mycotoxin that has the potential to contaminate corn.
3.2. Peanut Crown Rot, Root Rot, and Yellow Mold
Peanuts (Arachis hypogaea) are one of the most important cash crops cultivated worldwide for food and oil. The production of peanuts is affected by various fungal diseases, of which soil-borne diseases caused by Aspergillus species are among the most common diseases, leading to substantial losses. Soil-borne diseases in peanuts associated with Aspergillus include crown/collar rot, root rot, and yellow mold (Table 1).
Table 1.
Aspergillus species associated with diseases of peanuts.
| Peanuts (Arachis hypogaea) |
Aspergillus spp. | Country | References |
|---|---|---|---|
| Diseases | |||
| Crown rot/collar rot | A. niger | Oklahoma, USA; Andhra Pradesh, Karnataka and Tamil Nadu, India; Jackson County, Florida, USA. | [41,42,43,44,45] |
| Yellow mold | A. flavus, A. parasiticus | Tropical and subtropical areas (country not stated) | [46,47,48,49,50] |
| Root rot | A. niger | Laizi District, Shandong Province, China | [51] |
3.2.1. Crown/Collar Rot
Crown rot, also known as collar rot, is caused by A. niger and occurs in all peanut-producing countries. Economic losses due to crown rot are difficult to evaluate because the affected plants are scattered throughout the field; however, in some infected fields, losses of 50% have been reported [41]. According to Pande and Rao [42], the annual worldwide loss of peanut crops due to this disease is more than 10%.
The most common symptoms of crown rot are pre-emergence, post-emergence seedling damping-off, and sudden wilting. Young plants and seedlings are more susceptible than mature plants, which can lead to higher mortality rates. Older plants may become infected from the mid- to late season of planting [43].
Peanut seeds are susceptible to pathogens in moist soil environments. When the seeds germinate, the elongated shoots become infected, causing the hypocotyl to become water-soaked. Sudden wilting of the seedlings can be observed, as well as a rotation in the hypocotyl and cotyledon. Once infected, the hypocotyl and rotting roots are covered by black masses of conidia and mycelia. As infection occurs rapidly, peanut plants often die within 30 days, although others may survive longer [41,44].
Aspergillus niger causing crown rot in peanuts can be either soil-borne or seedborne. The pathogen is often present in the soil where peanuts are planted and can also often be found in the peanut seeds. This pathogen is prevalent in soils in which peanuts have been planted, often serving as the primary inoculum. The sporulation and growth of the pathogen mainly occurs under warm and moist conditions [41,45]. Outbreaks of crown rot are sporadic, with poor seed quality, changes in soil moisture due to high temperature during the seedling stage, drought stress, seedling damage due to pesticides, and feeding by roots and stem borers among the factors contributing to disease incidence [41].
3.2.2. Yellow Mold
Yellow mold in peanuts is caused by yellow-green aspergilli, A. flavus, and A. parasiticus, which are saprophytes and facultative parasites in the soil, plant debris, rotting seeds, and peanut pods. These yellow-green aspergilli are also often found in healthy peanut pods. Both aflatoxigenic A. flavus and A. parasiticus infect and contaminate peanuts in the field. After harvest, during the drying and storage stages, aflatoxin is produced in the seeds, seedling stems, and pods. In the soil, A. flavus and A parasiticus occur as conidia and mycelia in plant debris and can infect the plant directly or when the plants are predisposed to several factors, such as damage by insects and nematodes, as well as dry weather [46].
During the preharvest infection and invasion of peanut seeds, A. flavus has been found to be more aggressive than A. parasiticus [46,47]. Excessive heat in the soil (27–30 °C) and lengthy drought periods (3–6 weeks) towards the end of the growing season favor Aspergillus invasion and aflatoxin production. During periods of drought, the leaf canopy recedes due to higher soil temperatures and soil moisture evaporation. These conditions disrupt the synthesis of phytoalexin, such that the growth of Aspergillus is no longer inhibited [46]. Severe drought causes permanent wilting, leaf shedding, and receding canopy, which leads to favorable conditions for the production of aflatoxin in peanut seeds [46].
The pre-emergence rotting of the seeds and seedlings are indicative of severe peanut infection. At this stage, necrotic lesions appear with sporulating A. flavus emerging on the hypocotyls, radicles, and cotyledons of both ungerminated and germinated seeds. This condition is known as yellow mold. When infected seedlings emerge, plant growth is stunted, the root system is poorly developed, and the leaves become chlorotic [46].
The contamination of peanuts with aflatoxin in the field increases during drought stress as the moisture in the seed is reduced, which can lead to pod damage caused by insects. These conditions also facilitate pathogen infection. Moreover, sucrose exudates from the roots and peanut pods contribute to the growth of A. flavus and A. parasiticus [48]. Insect damage in peanuts is favored by hot and dry conditions, and wounds on the pods encourage the penetration and colonization of pathogens. Infected seeds often display yellow-green discoloration, which may be associated with fungal sporulation. Seed infection may also occur without noticeable damage to the pod [46].
Aflatoxin contamination in peanuts is more prominent in tropical and subtropical regions [46]. Aflatoxins are commonly produced at moisture levels greater than 80% and temperatures exceeding 25 °C [49]. Inadequate drying favors fungal growth, and aflatoxins tend to accumulate in the plant seeds. Fungal growth can be controlled by drying peanut pods to 7% moisture and storing them at 25–27 °C at a relative humidity of 70% [50].
3.2.3. Root Rot
Aspergillus niger has been reported to cause root rot in peanuts in the Laizi District, Shandong Province, China [51]. During infection, early symptoms in the peanut plants, including brown spots, appeared on the root and stem base, as well as the plants showing leaf chlorosis, stunted growth, and sudden wilting. Later, as the disease progressed, rot symptoms were also visible in the infected stem and root tissues, and numerous brown and black conidia were observed on the surface of the infected parts [51]. In this case, the causal pathogen was recovered from the infected roots and the stem base.
3.3. Cotton Boll Rot
One of the most serious diseases of cotton (Gossypium herbaceum) is boll rot, caused by a complex of fungal pathogens, of which Aspergillus species are among the pathogens. Boll rot was first reported in the late 1920s in the southwestern states of the USA. Aspergillus niger was recovered from several parts of infected cotton, including bolls, young dying squares or fellow buds, discolored pedicels, and lesions formed on the bracts [52]. Boll rot was initially known as smut, and the symptoms appeared only in injured bolls, mainly due to infestation by insects. During a survey on cotton disease in California in 1957–1960, several boll-rotting fungi, including A. flavus and A. niger, were found on cotton [53]. Table 2 shows the Aspergillus species associated with cotton boll rot reported in the USA and Bangladesh.
Table 2.
Aspergillus species associated with cotton boll rot.
Cotton boll rot occurs in all cotton-producing countries and affects the yield and fiber quality of the resulting crop. Two species of Aspergillus, namely A. niger and A. flavus, are commonly associated with cotton boll rot [54]. However, most reports and publications have focused on A. flavus, possibly due to its aflatoxin contamination, which is the most notable problem related to the development of fibers and bolls. The contamination of cotton by aflatoxin has been reported in cotton-growing areas in the USA [55,56].
Most cotton boll pathogens, including A. flavus, are unable to penetrate healthy plant tissues. However, the conidia can enter the boll through wounds or holes made by aphids and other insects, including pink boll worms, tobacco budworms, boll weevils, and cotton stainers [57,58]. The infection of inner tissues affects the seeds and lint, which rot as a result. Dry and blackened bolls with black or brown spots are indicative of infection [54]. Temperature and humidity are the main parameters that influence A. flavus colonization, as well as the production of aflatoxin. Moist lint resulting from the opening of the boll is susceptible to infection, which causes the lint to weaken and results in the discoloration of the fiber [58].
3.4. Black Mold in Onion and Garlic
The infection of onions (Allium cepa L.) and garlic (Allium sativum L.) with black mold results in the appearance of black conidial masses on the bulbs (Figure 1A,B). On onions, conidial masses are formed between or on the outer layer of the scale leaves. Rot develops at the neck of the infected bulb, resulting in a shriveling of the scales. On garlic, dark brown or black conidial masses are formed on the bulb, and dry rot develops [59]. Black mold often occurs along the bulb veins, and a larger portion of the bulb is enveloped by conidia. The occurrence of black mold on onions and garlic gives the bulbs a sooty appearance.
Figure 1.
Black mold of onion (A) and garlic (B).
Black Aspergillus, particularly A. niger, is often the causal pathogen of black mold in onion and garlic. Other Aspergillus species reported include A. awamori and A. ochraceus in garlic [60,61] and A. welwitschiae in onion [62,63,64] (Table 3).
Table 3.
Aspergillus species associated with black mold of onion and garlic.
| Onion (Allium cepa L.) and Garlic (Allium sativum L.) |
Aspergillus spp. | Country | References |
|---|---|---|---|
| Black mold | A. niger (onion) | Worldwide | [59] |
| A. niger, A. ochraceus (garlic) | USA, China | [60] | |
| A. awamori (garlic) | Korea | [61] | |
| A. welwitschiae (onion) | Taif region, Saudi Arabia; Stara Pazova, Serbia; Paraná State, Brazil. | [62,63,64] | |
| A. niger (onion) | Shambat, Sudan; Wellesbourne, UK | [65] | |
| A. awamori (onion) | Hungary | [66] |
The infection of onion and garlic bulbs by black mold can occur either in the field or during postharvest. Aspergillus species associated with black mold in onion and garlic are mainly saprophytes occupying plant debris and decaying organic matter and can turn into opportunistic pathogens by conidial infection. The conidia in the soil spread to the bulbs via the wind or rain. Conidia then enter the plants via wounds. Contaminated seeds are also sources of black mold inoculum [59]. In addition, endophytic A. niger has also been suggested as a vehicle of infection [7].
Black mold becomes apparent during storage, transportation, and sale. During the postharvest period, infection by black mold can cause significant losses, with bulbs becoming discolored and their tissues disintegrating. Black mold often occurs at high temperatures (27–30 °C) and humidity (70–80%), which can also lead to mycotoxin contamination [65]. As a result, the pathogens that cause black mold are widespread in hot and dry climates, but these can also be a problem in temperate areas when bulbs are stored at high temperatures and humidity levels. Moreover, the presence of black Aspergillus in onion seed samples has been reported to be prevalent in seeds grown or stored in warm climates.
Fumonisins (0.3 mg/kg) have been detected in onion samples from Hungary, albeit at low levels. In this case, the sample was contaminated with black Aspergillus, identified as A. awomori, which was found in the fleshy part and outer layer of the onion bulb [66]. Fumonisin B2 has also been detected in onion samples in Taif, Egypt, wherein A. welwitschiae was identified as a potential fumonisin producer [62]. Ochratoxin has yet to be detected in onion and garlic bulbs. However, under suitable conditions, such as the optimum temperatures and humidity levels, there is always the possibility that black Aspergillus produces ochratoxin.
3.5. Aspergillus Fruit Rot
Aspergillus infects various types of fruit crops worldwide. Aspergillus rot is one of the main postharvest diseases affecting fruit crops and infected fruits cultivated in tropical, subtropical, and temperate regions. Among these, Mediterranean fruit crops are susceptible to Aspergillus rot. Infection often occurs during the harvest period, and the most common Aspergillus species associated with fruit crops is black Aspergillus, especially A. niger, with other species including A. flavus, A. fumigatus, A. tubingensis, A. parasitus, A. awamori, A. terreus, A.welwitschiae, A. uvarum, and A. japonicus [67].
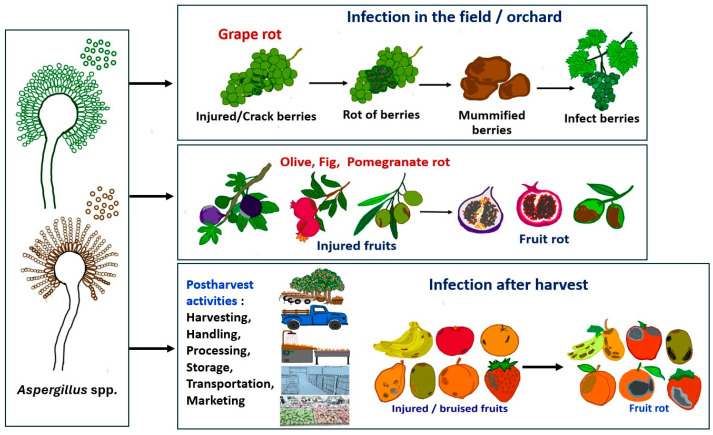
Figure 2 illustrates the infection of Aspergillus in fruits crops. The infection of fruit crops by Aspergillus occurs in the field, during harvest, and postharvest. In the field, when the sugar content increases during fruit maturation, the population of Aspergillus increases. When fruits are wounded, Aspergillus can easily infect these weakened fruits. Aspergillus also infects fruits during harvesting, handling, storage, washing, grading, packing, transportation, and sale, up until the product is bought by consumers [68]. Postharvest Aspergillus infection usually occurs via bruises, or other cuts on fruits, as well as through natural openings. Infection is favored by conditions of high temperatures and moisture, which promote conidial germination and fungal growth [68]. Moreover, wounds lead to the release of nutrients and water from the cells, providing suitable conditions for fungal growth. Postharvest fruit rot can lead to huge losses in storage and supply chains since fruits with rot symptoms are unmarketable and unsuitable for consumption.
Figure 2.
Infection of Aspergillus spp. on various fruit crops in the field and after harvest.
Aflatoxin and ochratoxin A produced by mycotoxigenic Aspergillus have been detected in grapes, figs, pomegranates, and olives, as well as products based on these fruit crops. Studies on mycotoxin contamination of these fruit crops have received much more attention compared to studies on mycotoxins in tropical fruit crops, which remain scarce.
3.5.1. Grapes Bunch Rot, Sour Rot, and Vine Canker
Grapes (Vitis vinifera) are one of the most important fruit crops in the world, and are mainly cultivated for wine production (71%). Only 27% of grapes are consumed fresh, while 2% are turned into dried fruits [69]. In vineyards, Aspergillus species infect grape berries, particularly during the summer when the conditions of high moisture and temperatures of 20–30 °C are prevalent [69,70]. During maturation, the rates of infection by Aspergillus spp. are higher, and black Aspergillus dominates at temperatures higher than 37 °C [71]. Occasionally, A. flavus and A. parasiticus have been isolated from grapes [72,73]. Some strains of pathogenic Aspergillus species are also mycotoxigenic, contaminating grapes, as well as their corresponding final products. In the postharvest period, grapes are processed according to their intended use. During these processes, contamination by Aspergillus, as well as other fungi, can occur [69].
Bunch rot, vine canker, and sour rot are diseases often associated with black Aspergillus in vineyards. The main sources of the inoculum of black Aspergillus in vineyards are soil and vine debris, from which wind-borne conidia are deposited onto the surface of the berries [74]. Black Aspergillus, which infects grape berries, is regarded as a secondary invader or opportunistic pathogen that causes infection when the berries are injured or wounded by insects or mechanical impact [75]. Prevalent black Aspergillus species found in infected grapes include A. niger, A. carbonarius, A. aculateus, A. japonicus, and A. uvarum [76,77], as well as occasionally A. tubingensis [77] and A. awamori [78]. These species are frequently reported to cause disease in grapes (Table 4).
Several black Aspergillus species are ochratoxin producers, and ochratoxin A is produced during veraison to ripening. Although A. carbonarius is the main producer of ochratoxin A, to a certain extent, A. niger, A. tubingensis, and A. awamori also contribute to ochratoxin A contamination in grape berries [79,80]. The contamination of ochratoxin A in wine was first reported by Zimmerli and Dick [80]. Subsequently, studies on ochratoxin A in wine and other grape products have increased [69,81,82,83,84,85].
Table 4.
Aspergillus spp. associated with diseases of grape berries.
| Grapes (Vitis vinifera) |
Aspergillus spp. | Country | References |
|---|---|---|---|
| Disease | |||
| Bunch rot | A. aculeatus | southwestern Ontario | [86] |
| A. niger | Chile | [87] | |
| A. carbonarius | Victoria, Australia | [88] | |
| A. niger, A. carbonarius | - | [89] | |
| A.tubingensis | Kimcheon-si, Gyeongbuk province, Korea | [90] | |
| Gimcheon, South Korea | [77] | ||
| Sour rot | A.carbonarius | Kern County, California | [91] |
| A. niger, A. aculeatus, A. oryzae | Yantai, Shandong Province, China | [92] | |
| A. niger, A. carbonarius | Rhodes, Greece | [93] | |
| Central and Southern Joaquin Valley, California | [94] | ||
| Vine canker | A. niger | San Joaquin Valley, California | [95] |
| A. niger | southeastern Sicily, Italy | [96] | |
| A. niger, A. tubingensis, A. carbonarius | Sicily, Italy | [97] | |
| A. niger and/or A. tubingensis | Fresno and Sonoma counties, California | [98] |
Aspergillus niger and A. awamori (now known as A. welwitschiae) are also fumonisin producers. Similar to ochratoxin A, fumonisin contamination has been reported in wine and other grape products [99,100,101,102,103]. According to Varga et al. [99], the accumulation of fumonisins can occur during the drying process, as mycotoxins are present before drying.
Bunch Rot
Bunch rot in grapes is caused by a range of fungi, including Aspergillus, which infect grape berries through wounds. Fungal pathogens that infect berries can sometimes be identified based on their conidial appearance. Aspergillus produces dark brown or black conidia, Botrytis produces gray conidia, and Penicillium produces green conidia [104]. Several Aspergillus species (Table 4) have been reported to be associated with grape bunch rot, including A. niger, A. carbonarius [87,88,89], A. aculateus [86], and A tubingensis [77,90].
Infection with bunch rot pathogens starts at the site of a wounded area and spreads rapidly to the entire grape cluster. Brown spots emerge on the berries, and as the disease progresses, the berries rot and black-to-dark brown conidia appear. Rotted berries become soft, shrivel, or collapse [104]. Bunch rot development is influenced by the wound on the berries and the compactness of the berry cluster. The sugar content increases as the fruits ripen, which increases the susceptibility of the wounded berries to infection by bunch rot pathogens [104]. Growth pressure on grape berry clusters leads to splitting or cracking. Bunch rot infection is favored by warm and wet conditions, with prolonged wet conditions leading to an increased rotting of berries [89,104]. Severe outbreaks of this disease can occur during periods of harvest under warm conditions [87].
Kazi et al. [88] studied the infection process of A. carbonarius in grape berries. Their findings showed that infection can occur at any stage of berry development if the inoculum is sufficient. Lower infection was found to occur when berries were small, green, and hard, which suggests that young berries are resistant to A. carbonarius infection. Infection was generally higher during veraison and harvest, which is similar to the findings reported by Battilani et al. [105] and Ponsone et al. [106]. Guzev et al. [107] also reported that infection was very low before veraison but often higher at harvest. The occurrence of black Aspergillus was also higher at harvest [105], which contributes to the incidence of bunch rot.
Sour Rot
Bunch rot often leads to sour rot, which causes the infected berries to appear wet due to leaking of juice or the oozing of the berry tissues, resulting in the cracking and collapse of the berries, which also enhances the growth of yeast and bacteria [104,108]. This disease is also known as summer bunch rot. Grape sour rot is a complex disease involving filamentous fungi, yeasts, acetic acid bacteria, and fruit flies. The disease is characterized by the smell of acetic acid or vinegar, as yeasts convert sugars to ethanol. Ethanol is then oxidized to acetic acid by the bacteria [109]. Fruit flies attracted to the sour smell act as vectors, spreading the filamentous fungi, yeasts, and acetic acid bacteria. Fruit flies may also cause injury to grape berries, which facilitates infection, particularly by fungi and bacteria [108,110]. Sour rot development is conducive to a high relative humidity and longer periods of wetness [94]. The main notable difference between bunch rot and sour rot is the vinegar-like smell caused by the accumulation of ethanol and acetic acid. Both diseases result in economic losses as they affect the berries, which in turn affects the final products.
Many filamentous fungi are involved in the sour rot of grapes, including Aspergillus, of which A. niger and A. carbonarius are frequently found on infected berries (Table 4). Both A. niger and A. carbonarius colonize wounded berries, causing bunch rot, followed by sour rot. Aspergillus niger and A. carbonarius have both been recovered from berries affected by sour grapes on the island of Rhodes, Greece [93]. Later, Rooney-Latham et al. [91] found that A. carbonarius was the main organism recovered from berries infected with sour rot in California. Findings by Gao et al. [92] indicated that A. niger, A. aculeatus, and A. oryzae are involved in sour rot in Yantai, Shandong Province, China.
Vine Canker
Grapevine canker is commonly associated with fungal pathogens in the families Botryosphaeriaceae, Diatrypaceae, and Diaporthaceae. Typical symptoms of grapevine canker include necrosis of the internal part of the trunk, indicating the formation of canker, the dieback of cordons or the whole vine, stunted shoot development, shoot death, rotting, and the dropping of berry clusters [98,111].
Aspergillus species causing vine canker have been reported in San Joaquin Valley, California, and southeastern Sicily, Italy. In California, Michailides et al. [95] reported A. niger as the causal pathogen of vine canker, in which the disease was detected in one-year-old cv. Redglobe vines. The disease was detected in the crotch, branching, and along shoots. Abundant black conidia were observed within the canker, as well as on the surface of the canker. Vitale et al. [97] identified A. niger, A. tubingensis, and A. carbonarius as pathogens of vine canker in Italy, of which the virulence was equal among the three species. Most canker lesions were detected at branch points and on the stems of young shoots, of which the infected tissues were discolored, and some were dead. Black powdery conidia are abundant and sometimes appear on the surface of lesions [96]. A recent study by Zhuang et al. [98] on vine canker in California indicated that A. niger and A. tubingensis, or both may be the causal pathogens of this disease (Table 4). Further studies on the species confirmations are currently underway.
Infection by Aspergillus causing vine canker occurs through wounds due to the removal of lateral shoots or leaves, particularly when the vine is topped to form cordons. Another method involves growth cracks that often occur in fast-growing one-year-old shoots [95,97]. Aspergillus causing vine canker usually forms black sporulations on the surface and underneath the affected bark tissues, which is the main characteristic differentiating this infection from other vine canker fungal pathogens. Moreover, multiple canker lesions appear on the cordon, spurs, and trunk of the vine, with visible brown discoloration in the xylem tissues. Infected tissues also typically show sporulation, necrosis, and black discoloration [98].
3.6. Fig Fruit Rot
Fig (Ficus carica) is mainly cultivated in the Mediterranean regions as the plant is well adapted to the Mediterranean climate, with its hot and dry summers and cold winters. Although fig is widely cultivated in this region, it can also be cultivated in humid tropical and subtropical regions [112]. For commercial purposes, fig fruits are converted into dried or preserved forms. Fig fruits are sold fresh for local consumption, as the fruits are easily perishable, and their shelf life is short [113]. Because the skin of the fruit is soft, it is easily wounded or damaged, and is thus susceptible to infection by fungi. Moreover, owing to their high sugar content, various fungi can grow on these fruits, which can lead to fruit rot [114].
Aspergillus fig rot is caused by several species, including A. flavus, A. parasiticus, A. fumigatus, A. niger, A. japonicus, and A. carbonarius [115,116,117], as shown in Table 5. Aspergillus causes rot in fresh fig fruits and smut in dried figs. Fig cultivars with larger ostioles are more susceptible, as the ostiole is a natural opening, which permits the fungi to enter the internal tissues of the fruit. When fruits ripen, abundant conidial masses are formed in the infected tissues [117]. Wounded or damaged fruits are also susceptible to Aspergillus infection, as the fungi can directly infect fruits.
Table 5.
Aspergillus spp. associated with fig fruit rot and dried fig.
Ripe and sun-dried fig fruits are susceptible to Aspergillus infection, which provides favorable conditions for mycotoxin production [118]. According to Buchanan et al. [118] and Iamanaka et al. [120], dried figs are susceptible to infection by A. flavus and A. parasiticus. Both species have often been recovered from dried figs [115,119]. Based on a study by Heperkan and Karbancioglu-Güler [121], A. flavus was found to be prevalent in dried fig, while A. parasiticus was not frequently isolated. Due to the presence of A. flavus and A. parasiticus, aflatoxins were detected, particularly in dried figs [121,123,124].
Mycotoxigenic black Aspergillus, particularly A. niger and A. carbonarius, have been isolated from diseased figs. Aspergillus niger and A. carbonarius are also prevalent during sun-drying, and both species are tolerant to ultraviolet rays, contributing to their prevalence during the drying process [71]. Similar to aflatoxins, ochratoxin A has been reported in dried figs [125,126,127,128,129].
Despite the susceptibility of fig to Aspergillus infection, as well as the contamination of fruit and fig products with aflatoxins and ochratoxin A, the level of contamination is generally low [118].
3.7. Olive Fruit Rot
Aspergillus is also the most common fungal flora recovered from olive fruits (Olea europaea) and has been isolated from fruit rot lesions, as well as from fruits infested by fruit flies [130,131]. According to Lazzizera et al. [132], most fungi associated with olive fruit rot, including Aspergillus, are secondary invaders or saprophytes, as the fungi infect olive fruits through wound, unlike Colletotrichum and Botryosphaeriaceae fungi, which directly infect olive fruits. In a study by Chliyeh et al. [133], A. flavus was found to only infect olive fruits through wounded fruit epicarps.
Aspergillus species that have been isolated from olive fruit rot (Table 6) include A. ochraceus, A. fumigatus, A. flavus, and A. niger [130,133,134,135]. Aspergillus niger and A. tubingensis were isolated from olive fruits infested with olive fruit flies [136]. In fresh olive fruits, A. fumigatus, A. niger, and A. tubingensis have also been reported [131,137], which may indicate that these species are endophytes. Endophytic Aspergillus species have been recovered from the twigs and roots of olive trees [137,138]. The main concern regarding Aspergillus growth on olive fruits is contamination by mycotoxigenic aspergilli, which can affect the production of olive oil.
Table 6.
Aspergillus spp. associated with olive fruit rot and healthy fruits.
| Olive (Olea europaea) |
Aspergillus spp. | Country | References |
|---|---|---|---|
| Fruit rot | A. ochraceus | Tarom-Zanjan Province, Tabriz, Iran | [134] |
| A. fumigatus | Halkidiki, Kalamata, Athens | [135] | |
| A. niger | Karak, Jordan | [136] | |
| A. flavus | Gharb and Zoumi, Morocco | [133] | |
|
A. niger, A. fumigatus |
Sidi Kacem, Meknes, Fes, Taounate, Sefrou, Khenifra, Errachidia, Goulmima, and Marrakech, Morocco |
[130] | |
| Healthy fruits |
A. niger, A. tubingensis |
Canakkale province, Turkey | [136] |
As olives are stored after harvest, improper storage can promote the growth of mycotoxigenic Aspergillus, and the production of aflatoxin and ochratoxin A. The occurrence of mycotoxigenic fungi on olive fruits may lead to the contamination of olive oil with mycotoxin. In fact, the co-occurrence of aflatoxins and ochratoxin A has been reported in olive oil in southern Italy [139,140]. Ochratoxin A has also been reported in extra virgin oil [141] and in olive oil of Greek origin [142,143]. Aflatoxins have also been detected in olive oil in Greece [144], Iran [145], and Spain [146].
Although aflatoxins and ochratoxin A have been reported in olive fruits and olive oil, the level of contamination tends to be low, and it is believed to not affect consumers or cause any public health concerns. However, the continuous intake and exposure to contaminated products can pose a significant risk to consumers [140,147,148]. Moreover, the cumulative intake of these olive products may lead to health concerns.
3.8. Pomegranate Fruit Rot
Aspergillus rot of pomegranate (Punica granatum) is commonly associated with fruit rot and heart rot in pomegranates. This tends to start in the field during flowering and early fruit development, particularly after rainfall. Rot symptoms appear on the external part of the fruits near the calyx and manifest as discoloration of the rind, with the rind turning paler red or brownish red. Inside infected fruits, black powdery conidia are apparent, resulting in the rotting of the arils and the cracking of the fruit [149,150]. In a study by Ezra et al. [151], Aspergillus was found to cause fruit rot by penetrating the fruit through a damaged crown, resulting in the rotting of the fruit mesocarp tissue; however, rotting of the arils was not observed. Although pomegranate heart rot did not cause any noticeable symptoms on the rind, the arils rotted, and fungal mycelia were observed. As a result of the different stages of rot development, some arils exhibited brown/soft rot as well as black/dry rot [151].
In most cases, black aspergilli are associated with pomegranate fruit rot, which can occur both in the field and postharvest (Table 7). In an earlier study, A. variecolor, A. awamori, A. fumigatus, A. flavus, and A. niger were found to be causal pathogens of pomegranate fruit rot [152]. Pomegranate fruits in orchards near Cairo, Egypt, were found to be infected with A. niger, of which the fungus was isolated from the internal parts of the fruits. Aspergillus niger was also reported to cause the soft rot and dry rot of pomegranates in Shaanxi Province, China [153]. Infection by A. niger subsequently facilitates infection with bacteria and yeast [154].
Table 7.
Aspergillus spp. associated with pomegranate fruit rot.
| Pomegranate (Punica granatum) |
Aspergillus spp. | Country | References |
|---|---|---|---|
| Disease | |||
| Heart rot | A. niger | Cairo, Egypt | [154] |
| Fruit rot | A. niger | California, USA | [149] |
| A. tubingensis | China | [155] | |
| Soft rot and dry rot | A. niger | Shaanxi, China | [153] |
| Postharvest rot |
A. tubingensis, A. welwitschiae, A. uvarum, A. japonicus |
Southern Italy | [156,157] |
After the revision of the taxonomy and nomenclature of the genus Aspergillus, other species have been found to be associated with pomegranate fruit rot during the preharvest and postharvest periods. In southern Italy, A. tubingensis, A. welwitschiae, A. japonicus, and A. uvarum are associated with postharvest pomegranate fruit rot [156]. According to Mincuzzi et al. [157], A. tubingensis and A. welwitschiae were the main species causing pomegranate fruit rot, whereas A. uvarum and A. japonicus were minor species. Preharvest pomegranate fruit rot in Greece and Cyprus were found to be mainly caused by A. niger and A. tubingensis, although various fungal pathogens, including Alternaria, Colletotrichum, and Botrytis, were also associated with fruit rot [158]. Guo et al. [155] reported A. tubingensis as a causal pathogen of pomegranate fruit rot in China.
Pomegranate fruit rot not only reduces the yield and quality of the fruits but also contaminated fresh and processed fruits with ochratoxin and fumonisin. Kanetis et al. [158] reported approximately 20% of A. niger isolates associated with pomegranate fruit rot could produce ochratoxin A in vitro. Isolates of A. tubingensis (33%) from Greece also produced ochratoxin A. Only A. niger isolates were able to produce fumonisin B2 in vitro. The analysis of ocharatoxin A and fumonisin B2 in artificially inoculated pomegranate fruits indicated that only a small percentage of the isolates were mycotoxin-producing isolates.
3.9. Citrus Fruit Rot
Aspergillus rot affects citrus fruits (Citrus spp.), including oranges (C. sinensis), lemons (C. limon), grapefruits (C. paradisi), and lime (C. aurantiifolia), and can occur in the field, postharvest, during storage and sale. The most common species associated with citrus fruit rot is A. niger followed by A. flavus (Table 8). However, in a study by Tournas and Katsoudas [159], A. niger was only recovered from lemons and not from other citrus fruit samples. Other species associated with Aspergillus rot in citrus include A. westerdijkiae, A. aculeatus, and A. nidulans (Table 8).
Table 8.
Aspergillus spp. associated with citrus fruit rot.
| Citrus (Citrus spp.) |
Aspergillus spp. | Country | References |
|---|---|---|---|
| Lemon | A. niger | Washington D.C., USA | [159] |
| Lemon and grapefruit | A. niger | Islamabad, Rawalpindi, Taxila, and Wah districts Pakistan | [160] |
| Lemon, sweet lemon, lime, sweet orange | A. niger, A. flavus | Adamawa state, Nigeria | [161] |
| Lemon | A. flavus | Erzurum, Turkey | [162] |
| Orange | A. flavus, A. niger | Oyo State, Nigeria | [163] |
| A. niger | El-beida, Libya | [164] | |
| A. westerdijkiae | Italy | [165] | |
| A. niger, A. aculeatus, A. nidulans | Mexico | [166] | |
| A. niger | Nigeria | [167] |
Ochratoxin and aflatoxin have been reported in citrus infected by Aspergillus, as well as production of mycotoxins by the fungi. In a study by Marino et al. [165], A. westerdijkiae inoculated on the surface of an orange fruit was able to produce ochratoxin A and caused visible rot lesions. The production of ochratoxin A increases at temperatures higher than 26 °C, which is the optimum temperature for mycotoxin production [168]. Aflatoxin was detected in orange samples with a high incidence of A. flavus [163]. Aspergillus niger from oranges collected from orchards in Mexico was found to produce aflatoxin B1 and fumonisin B1 [166].
3.10. Tropical Fruit Crops
Aspergillus rot in banana (Musa spp.), mango (Mangifera indica), papaya (Carica papaya), pineapple (Ananas comosus), and guava (Psidium guajava) is mainly associated with A. niger and A. flavus. Other species, such as A. tamarii, A. fumigatus, A. terreus, A. ochraceous, and A. japonicus, have also been reported to cause fruit rot in these tropical fruits (Table 9). Aspergillus rot has also been reported in jackfruit (Artocarpus heterophyllus) and sapota (Manilkara zapota) (Table 9). During harvest and handling, it is vital to minimize fruit bruising and wounding since during storage, bruised and wounded fruits are susceptible to Aspergillus infection [169].
Table 9.
Aspergillus spp. associated with diseases of tropical fruit crops.
| Fruit Crop/Disease | Aspergillus spp. | Country | References |
|---|---|---|---|
| Banana (Musa spp.) |
|||
| Fruit rot | A. niger, A. flavus | Dhaka, Bangladesh | [170] |
| Aspergillus spp. | South Gujarat | [171] | |
| A. niger, A. flavus | Kono, Nigeria | [172] | |
|
A. niger, A. fumigatus, A. flavus |
Sokoto, Nigeria | [173] | |
| A. tamarii | Malaysia | [174] | |
| Crown rot | A. niger, A. flavus | Jimma town, Ethiophia | [175] |
| Aspergillus sp. | Kerala, India | [176] | |
| Mango (Mangifera indica) |
|||
| Fruit rot | A. niger | Sri Lanka, Iran | [177,178] |
| A. flavus, A. niger | Saudi Arabia, Faisalabad, Pakistan | [179,180] | |
| A. niger, A. oryzae | Nasarawa State, Nigeria | [181] | |
|
A.niger, A. flavus, A. fumigatus, A. terreus |
Dhaka, Bangladesh | [170] | |
| Pineapple (Ananas comosus) |
|||
| Fruit rot | A. flavus | Nigeria | [167] |
| A. flavus. A. niger | Osun State, Nigeria | [182] | |
| black Aspergillus | Anambra State, Nigeria | [183] | |
| Papaya (Carica papaya) |
|||
| Fruit rot |
A. niger, A. terreus, A. flavus, A. ochraceous, A. tamarii, A. fumigatus |
Gorakhpur, India | [184] |
| A. flavus | Maharashtra, India | [185] | |
| A. niger | Uttar Pradesh, India | [186] | |
| A. niger, A. flavus | Osun State, Nigeria | [182] | |
| Guava (Psidium guajava) |
|||
| Crown rot |
A. flavus, A. fumigatus, A. japonicus, A. niger, A. tamarii |
Nueva Ecija, Phillippines | [187] |
| Fruit rot | A. awamori | Lahore, Pakistan | [188] |
| Soft rot | A. niger var. awamori | India, Malaysia | [189,190,191] |
| Dry rot | A. fumigatus | Nigeria | [192] |
| A. niger | Ethiopia | [193] | |
| A. niger, A. flavus, A. parasiticus | Beheira, El-Sharkia and Qualubia governorates, Egypt | [194] | |
| A. niger, A. awamori | Aurangabad, India | [195] | |
| Jackfruit (Artocarpus heterophyllus) |
|||
| Fruit rot | A. niger | Nayarit, Mexico | [196] |
| Sapota (Manilkara zapota) |
|||
| Fruit rot | A. minisclerotigenes | Gujarat, India | [197] |
| A. niger | Maharashtra, India | [198] |
Unlike grapes, fig, olives, and pomegranates, data on the contamination of tropical fruit crops with Aspergillus mycotoxins is currently lacking. This may be because Aspergillus infection of many tropical fruit crops is a secondary infection.
3.11. Strawberry Fruit Rot
Strawberry fruits (Fragaria x ananassa) are fleshy and soft, which makes them highly perishable and have a limited shelf-life [199]. These factors contribute to the susceptibility of strawberries to postharvest pathogens that cause fruit rot. Although Botrytis cinerea is the main postharvest pathogen of strawberry, causing gray mold, Aspergillus spp. have also been identified as pathogens, causing strawberry postharvest rot. Aspergillus species reported to be associated with strawberry rot include A. niger, A. flavus, A. fumigatus, A. tubingensis, A. parasiticus, and A. terreus (Table 10).
In a study by Palmer et al. [200], A. tubingensis was identified as a causal pathogen of strawberry rot in a field in California. However, the disease is of minor significance as the fungus was isolated during hot weather that favors the growth of Aspergillus. Most reports on strawberry rot caused by Aspergillus occur after harvest, particularly during storage and sale [201,202,203,204].
Table 10.
Aspergillus spp. associated with strawberry fruit rot and fresh fruit.
| Strawberry (Fragaria x ananassa) |
Aspergillus spp. | Country | References |
|---|---|---|---|
| Fruit rot | A. flavus, A. niger | Qena city, Egypt | [203] |
| A. niger, A. fumigatus | Lahore, Pakistan | [201] | |
| A. tubingensis | California, USA | [200] | |
| A. terreus, Aspergillus sp. | Indonesia | [204] | |
| Fresh fruit and juice |
A. flavus, A. niger, A. parasiticus |
Saudi Arabia | [202] |
Mycotoxigenic A. flavus and A. parasiticus associated with strawberry fruit rot were able to produce aflatoxins, as reported by Saleem [202] and Hussein et al. [203]. Saleem [202] reported that 30–60% isolates of A. flavus and A. parasiticus recovered from diseased fruits could produce aflatoxin B at varying concentrations. Aspergillus niger and A. flavus isolated from strawberry rot were also found to produce ochratoxin and aflatoxin, respectively [203]. These findings highlight the susceptibility of strawberries and strawberry products to contamination with aflatoxin and ochratoxin.
3.12. Apple Fruit Rot
Fruit rot in apples (Malus domestica) is caused by a range of postharvest pathogens, including Aspergillus. Aspergillus is not only associated with apple fruit rot; several species have also recovered from healthy apple fruits. The species isolated from apple fruit rot include A. oryzae, A. flavus, A. niger, A. terreus, and A. versicolor (Table 11).
Table 11.
Aspergillus spp. associated with apple fruit rot.
Aspergillus infection often leads to the contamination of apple fruits with mycotoxins. Hasan [205] isolated A. flavus from 67% (from 100 samples) of rotted apples, with A. flavus being the most isolated fungus from healthy apples. Aflatoxins B1, B2, G1, and G2 were detected in the lesions of rotted apples. These findings demonstrate an association between A. flavus infection in apples and the occurrence of aflatoxins. Aspergillus versicolor isolated from rotten apples produced sterigmatocystin [209], which is a precursor of aflatoxin B1.
3.13. Peach, Cherry, and Kiwi Fruit Rot
Peach (Prunus persica), cherry (Prunus avium), and kiwi (Actinidia deliciosa) are also highly perishable and have short shelf-life, as well as being predisposed to Aspergillus infection (Table 12). In peaches, A. flavus, A. niger, and A. aculeatus are associated with peach rot [210,211,212]. Wounded peach fruits are more prone to infection by Aspergillus [213].
Aspergillus was the most dominant species recovered from postharvest sour cherries, and two species, A. niger and A. penicillioides, were identified [214]. Aspergillus niger was also reported as a causal pathogen of postharvest fruit rot in cherries in northern Greece [215].
Zhu et al. [216] isolated A. flavus from mature kiwifruit with brown lesions in southwestern Shaanxi, China, of which 15% of the fruits in the orchard exhibited soft rot symptoms. This study was the first to report A. flavus causing fruit rot in kiwis.
Table 12.
Aspergillus spp. associated with fruit rot of cherry, peach, and kiwi.
| Disease | Aspergillus spp. | Country | References |
|---|---|---|---|
| Cherry (Prunus avium) |
|||
| Postharvest fruit rot | A. niger | Imathia and Pella (northern Greece) |
[215] |
| A. niger, A. penicilioides | Lithuania | [214] | |
| Peach (Prunus persica) |
|||
| Soft rot | A. aculeatus | Shaanxi, China | [212] |
| Fruit rot | A. niger | Gansu, China | [213] |
| A. flavus | Imathia county, northern Greece | [210] | |
| A. niger | Jeddah, Saudi Arabia | [217] | |
| Postharvest rot | A. niger | Rawalpindi, Pakistan | [211] |
| Kiwi (Actinidia deliciosa) |
|||
| Soft rot | A. flavus | southwestern Shaanxi, China | [216] |
3.14. Tree Nuts
Common tree nuts are almonds (Amygdalus communis L.), Brazil nuts (Bertholletia excelsa), cashews (Anacardium occidentale), hazelnuts (Corylus avellana), pecans (Carya illinoinensis), pistachio nuts (Pistacia vera), macadamia (Macadamia ternifolia), and walnuts (Juglans regia) [218]. Among these, the most consumed tree nuts are almonds and walnuts, followed by pistachios, cashews, and hazelnuts [219].
Aspergillus infection of tree nuts occurs in the field, particularly in fruits wounded by insects as well as wounds caused during harvesting. Conidia are abundant as airborne inoculum, colonizing nuts and remaining present until their harvest, storage, and processing. These carry-over inoculums can remain in the produce until the processing and final product stages. When conditions favor fungal growth, the internal parts of nuts are often infected [220].
In a study on the occurrence of Aspergillus in pistachio, almond, and walnut, Bayman et al. [220] found that most common species of Aspergillus detected were A. niger, A. flavus, A. nidulans, A. tamarii, A. ochraceus, A. melleus, and A. fumigatus. Three species, A. candidus, A. parasiticus, and A. terreus were not common (less than 2% of the collected nuts). These results indicate that Aspergillus species are prevalent in tree nuts and suggest that the handling of nuts during harvest and postharvest has a major influence on the occurrence of mycoflora [220].
Several mycotoxigenic species have been reported, with aflatoxin and ochratoxin contamination also occurring in tree nuts. Tree nuts have a low sugar content, low moisture levels (particularly during storage and transportation), and high levels of water activity, which may contribute to the production of mycotoxins [221]. The majority of aflatoxin incidences have been reported in nuts damaged by insects or by the early splitting of the shell and hull [222]. However, mycotoxigenic Aspergillus species have also been found in nuts without insect damage or shell and hull splitting.
Aflatoxins have been detected at higher levels in several tree nuts, including almonds, Brazil nuts, pistachios, and walnuts [223,224,225]. According to Taniwaki et al. [226], the occurrence of aflatoxigenic A. flavus and other aflatoxigenic species on tree nuts is comparable to that on peanuts. The occurrence of ochratoxin A in almonds, hazel nuts, cashews, and walnuts was reported by Essawet et al. [227]. Although the contamination of tree nuts with ochratoxin A is often low, higher levels of mycotoxins have been detected occasionally [228].
3.15. Coffee Beans
Similar to other agricultural crops, coffee beans (Arabica and Robusta) are also infected by Aspergillus in the field and during storage, and are present at various production stages, including harvesting, postharvest, handling, processing, and transportation [229]. Black Aspergillus is the most commonly detected species in coffee beans. Black Aspergillus associated with coffee contamination include A. carbonarius, A. niger, A. sclerotioniger, A. lacticoffeatus, A. sclerotiicarbonarius, A. aculeatinus, A. tubingensis, and A. foetidus, among which some species are also ochratoxin A producers (Table 13). Other Aspergillus spp. recovered from coffee beans include A. westerdijkiae, A. candidus, A. sydowii, A. ochraceus, A. parasiticus, A. fumigatus, A. flavus, and A. versicolor (Table 13).
Table 13.
Aspergillus spp. associated with coffee cherry and coffee bean reported in several countries.
| Coffee (Coffea spp.) |
Aspergillus spp. | Country | References |
|---|---|---|---|
| Coffea arabica—cherries and beans | A. ochraceus (and possibly related species), A. carbonarius, A. niger | Alta Paulista, Sorocabana, Alta Mogiana, and Cerrado Mineiro, Brazil | [230] |
| Coffea arabica and Coffea canephora var. robusta |
A. melleus, A. sclerotiorum, A. steynii, A. westerdijkiae, A. aculeatinus, A. foetidus, A. niger, A. tubingensis |
Chiang Mai, Chumphon, Thailand | [231] |
| Green coffee bean (Robusta and Arabica) |
A. carbonarius, A. niger, A. ochraceus and related species in section Circumdati |
southern and central Vietnam | [232] |
| Coffee bean |
A. carbonarius, A. niger, A. ochraceus |
Paraná, São Paulo and Minas Gerais, Brazil | [233] |
| Coffea arabica, Coffea canephora var. Robusta, Coffea liberica, Coffea excelsea |
A. ochraceus, A. westerdijkiae, A. carbonarius, A. niger, A. japonicus |
Benguet, Ifugao; Abra, Cavite, Ifugao, Cavite, Philippines | [234] |
| Dry parchment, dry cherries and green coffee beans |
A. carbonarius, A. niger, A. ochraceus |
west region of Bafoussam and Dschang, Cameroon | [235] |
| Coffea arabica, Coffea canephora L. var. robusta (Robusta coffee) green coffee beans |
A. candidus, A. sydowii, A. niger, A. ochraceus, A. parasiticus, A. fumigatus, A. flavus, A. versicolor |
Brazil, Timor, Honduras, Angola, Vietnam, Costa Rica, Colombia, Guatemala, Nicaragua, India, and Uganda | [236] |
| Arabica—parchment and green coffee beans | A. niger A. tubingensis, A. foetidus | North Thailand | [237] |
| Robusta—dried coffee cherries and green coffee beans |
A. carbonarius, A. niger, A. westerdijkiae, A. aculeatinus, A. sclerotiicarbonarius |
South Thailand | [237] |
| Coffee beans |
A. brasiliensis, A. flavus, A. lanosus, A. niger, A. ochraceus A. oryzae, A. ostianus, A. sulphureus, A. tamarii, A. tubingensis |
Minas Gerais, Brazil |
[238] |
Infestation by the coffee berry borer (Hypothenemus hampei) has been found to increase the incidence of fungal contamination in coffee beans, as well as the levels of ochratoxin A [238]. Ochratoxin A produced during different stages of coffee processing reduces the quality of coffee and affects its taste [239]. Based on a study by Noonim et al. [231] on the production of ochratoxin by Aspergillus isolated from coffee beans in Thailand, A. carbonarius, A. westerdijkiae, and A. steynii were found to produce high amounts of ochratoxin A. An intermediate amount of ochratoxin A was produced by A. niger and A. sclerotiorum. Aspergillus carbonarius producing ochratoxin A with significant amount has been reported by Joosten et al. [240], Pardo et al. [241], and Leong et al. [232]. Although A. niger is among the most prevalent black Aspergillus contaminating coffee beans, the species is unlikely to be an important producer of ochratoxin A in coffee beans, as only a small percentage of A. niger isolates were able to produce the mycotoxin [230,231].
Different species of Aspergillus have been detected in coffee beans from coffee-producing countries. This suggests that the Aspergillus species depends on the climate of the geographic region, agricultural practices, pest infestation, and postharvest handling, including drying and storage [231,242]. In Brazil, A. niger, A. ochraceus, and A. carbonarius have been frequently isolated from coffee beans. Although A. niger was recovered at a higher percentage (63%), only 3% of the isolates produced ochratoxin A [230]. Three species, namely A. niger, A. ochraceus, and A. carbonarius, were also reported in coffee beans in Vietnam and Cameroon [232,235]. In Vietnam, Leong et al. [232] isolated A. westerdijkiae and A. steyni, but ochratoxin A was only produced by A. carbonarius., A. westerdijkiae, and A. steyni [232]. In the Phillipines, five species have been associated with the contamination of coffee, namely, A. ochraceus, A. westerdijkiae, A. carbonarius, A. niger, and A. japonicus, all of which were able to produce ochratoxin [234]. Four new species of black Aspergillus, A. sclerotiorum, A. lacticoffeatus, A. sclerotiicarbonarius, and A. aculeatinus, were identified in coffee beans in Thailand. Other species isolated from coffee beans included A. niger, A. tubingensis, A. foetidus, A. carbonarius, A. niger, and A. westerdijkiae. Among these, only A. carbonarius and A. niger were able to produce ochratoxins [237].
4. Other Plant Diseases Caused by Aspergillus spp.
Although Aspergillus species are typically weak or secondary pathogens, several species have been reported to cause foliar diseases, including leaf spot and leaf soft rot. Although Aspergillus are not common leaf spot pathogens, there are several reports that identify species of Aspergillus as leaf spot pathogens in several plants. Leaf spots appear as discolored spots or lesions on the leaf, with necrosis often occurring at the center of the lesion [243]. The spots on the leaf may coalesce and form irregular blight lesions. This disease usually occurs under conditions of continuous moisture and humidity. Most leaf spot pathogens including Aspergillus disseminate through conidia by rain splashing, irrigation, and wind dispersal [244].
Aspergillus niger has been reported to cause leaf spots in ginger (Zingiber officinale), in which severe infection caused defoliation [245] as well as leaf spot of avocado (Persea americana) [246]. Aspergillus niger has also been associated with soft rot of an ornamental plant, mother-in-law’s tongue (Dracaena trifasciata) [247], stem rot in lucky bamboo (Dracaena sanderiana) [248], and stem rot of Adenium obesum [246]. Another black Aspergillus, A. tubingensis, was reported to cause leaf spots on Jatropha curcas [249], Helleborus species [250], and cotton [251]. Aspergillus tubingensis has also been reported to cause black pods in tamarind (Tamarindus indica) [252], act as a pre-emergent pathogen of Phoenix dactylifera [253], and cause leaf rot in pak choi (Brassica rape spp. chinensis) [254]. Furthermore, three species, A. niger, A. ustus, and A. flavus, were identified as causal pathogens of foliar diseases in Terminalia catappa, a deciduous tropical tree [255]. Aspergillus fumigatus was also found to be a causal pathogen of marigold (Tagetes erecta and T. patula), causing foliage blight [256]. Recently, A. versicolor was identified as a pathogen causing severe fruit rot of tomato [257] and A. niger causing fruit rot of bilimbi (Averrhoa bilimbi) [258].
5. Control of Aspergillus Diseases
Integrated approaches are commonly employed to manage Aspergillus diseases both in the field and postharvest. Aspergillus infections in the field are often linked to wounds caused by insect infestations. The conidia, which reside in plant debris, soil, and mummified fruits, can be introduced into wounds through soil dust and rain splash. Therefore, maintaining sanitation in the field or orchard, which includes the removal of dead plant material and mummified fruits, is highly recommended [149].
Harvesting and postharvest activities predispose the crops and fruits to mechanical injury. Postharvest activities such as handling, sorting, grading, packing, and transportation require extensive operations, and often results in bruises, cracks, and cuts on the produce [259]. Minimizing injury during these activities reduces the risk of infection from fungal pathogens including Aspergillus.
Before packaging, individual fruits should be washed and cleaned in plenty of clean water to remove dirt and latex, as well as inoculum of pathogen that can cause rot disease during storage and transportation. Chlorine, chlorine dioxide, and hydrogen peroxide can serve as disinfectants for cleaning the fruits [260].
The use of fungicides is the main method of pre- and postharvest disease control. Fungicides should be applied during preharvest to prevent infection during postharvest storage and to control rot disease [261]. The use of fungicides to control grape berries infected with black Aspergillus and to reduce ochratoxin A levels was reviewed by Varga et al. [262]. Among the fungicides used were captan, fludioxonil, mepanipyrim, pyrimethanil, fluazinam, and iprodione mepanipyrim, pyrimethanil, fluazinam, and iprodione.
The potential of utilizing biological control methods for managing Aspergillus diseases has garnered significant interest. Among promising biocontrol agents tested is yeast to reduce infection, and mycotoxin production by different Aspergillus spp. Saccharomyces cerevisiae has the ability regulate production of aflatoxin by A. flavus during storage [263]. The growth of A. carbonarius can be inhibited by four yeast species, Pichia kluyveri, Hanseniaspora uvarum, Meyerozyma guilliermondii, and Hanseniaspora clermontiae, which is achieved through competition for available substrates [264].
Other potential methods to control Aspergillus growth and production of mycotoxin are utilizing essential oils and nanocoating. Essential oils extracted from thyme, cinnamon, basil, clove, mint, oregano, coriander, and anise have been reported to inhibit growth of Aspergillus [262]. Oregano and mint oils inhibited growth of A. westerdijkiae and ochratoxin production [265]. Nanocoating based on chitosan and propolis have been demonstrated to suppress growth of A. flavus and production of aflatoxins [266,267].
6. Conclusions
The compilation of different plant-pathogenic Aspergillus species along with the plant hosts demonstrated the genus/species global distribution. The plant pathogenic Aspergillus infection of a variety of crops may be due to a number of factors. Contributing factors might include the ability of Aspergillus species to inhabit agriculture environment, effective conidia dispersal by air, rapid adaptation to the host, growth and survival in a range of ecological conditions, and extensive use of chemicals in agricultural practices. However, there are still many scientific problems and knowledge gaps that need to be addressed, including the adaptation to various ecological areas, host-switching, and infection-causing mechanisms in various crops and plants.
Aspergillus is currently regarded as a potential emergent plant pathogen and probably will lead to future outbreaks of plant disease. In this situation, if plant pathogenic Aspergillus species that cause serious diseases are not detected and identified in a timely manner, and appropriate plant disease management approaches are not implemented, food safety could be adversely affected, which would have a significant economic impact.
Conflicts of Interest
The author declares no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Bennett J.W. An overview of the genus Aspergillus. In: Machida M., Gomi K., editors. Aspergilli. Genomics, Medical Aspects, Biotechnology, and Research Methods. Caister Academic Press; Norfolk, UK: 2010. pp. 1–17. [Google Scholar]
- 2.Lee J. Discovery of Aspergillus as a Human Pathogen. 1999. [(accessed on 20 December 2023)]. Available online: http://www.antimicrobe.org/hisphoto/history/Aspergillus-Human%20Pathogens.asp.
- 3.Thom C., Church M.B. The Aspergilli. Baillière, Tindall & Cox; London, UK: 1926. [Google Scholar]
- 4.Raper K.B., Fennell D.I. The Genus Aspergillus. Williams & Wilkins; Baltimore, MD, USA: 1965. [Google Scholar]
- 5.Raper K.B., Thom C. A Manual of the Penicillia. The Williams & Wilkins Company; Baltimore, MD, USA: 1949. [Google Scholar]
- 6.Benjamin C.R. Ascocarps of Aspergillus and Penicillium. Mycologia. 1955;47:669–687. doi: 10.1080/00275514.1955.12024485. [DOI] [Google Scholar]
- 7.Palencia E.R., Hinton D.M., Bacon C.W. The black Aspergillus species of maize and peanuts and their potential for mycotoxin production. Toxins. 2010;2:399–416. doi: 10.3390/toxins2040399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samson R.A., Visagie C.M., Houbraken J., Hong S.B., Hubka V., Klaassen C.H., Perrone G., Seifert K.A., Susca A., Tanney J.B., et al. Phylogeny, identification and nomenclature of the genus Aspergillus. Stud. Mycol. 2014;78:141–173. doi: 10.1016/j.simyco.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Houbraken J., Kocsubé S., Visagie C.M., Yilmaz N., Wang X.C., Meijer M., Kraak B., Hubka V., Bensch K., Samson R.A., et al. Classification of Aspergillus, Penicillium, Talaromyces and related genera (Eurotiales): An overview of families, genera, subgenera, sections, series and species. Stud Mycol. 2020;95:5–169. doi: 10.1016/j.simyco.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sexton A.C., Howlett B.J. Parallels in fungal pathogenesis on plant and animal hosts. Eukaryot. Cell. 2006;5:1941–1949. doi: 10.1128/EC.00277-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baltussen T.J., Zoll J., Verweij P.E., Melchers W.J. Molecular mechanisms of conidial germination in Aspergillus spp. Microbiol. Mol. Biol. Rev. 2020;84:e00049-19. doi: 10.1128/MMBR.00049-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfliegler W.P., Pócsi I., Győri Z., Pusztahelyi T. The Aspergilli and Their Mycotoxins: Metabolic Interactions with Plants and the Soil Biota. Front. Microbiol. 2020;10:2921. doi: 10.3389/fmicb.2019.02921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fillinger S., Chaveroche M.K., Shimizu K., Keller N., D’enfert C. cAMP and ras signalling independently control spore germination in the filamentous fungus Aspergillus nidulans. Mol. Microbiol. 2002;44:1001–1016. doi: 10.1046/j.1365-2958.2002.02933.x. [DOI] [PubMed] [Google Scholar]
- 14.Harris S.D. Cdc42/Rho GTPases in fungi: Variations on a common theme. Mol. Microbiol. 2011;79:1123–1127. doi: 10.1111/j.1365-2958.2010.07525.x. [DOI] [PubMed] [Google Scholar]
- 15.Talbot N.J., Ebbole D.J., Hamer J.E. Identification and characterization of MPG1, a gene involved in pathogenicity from the rice blast fungus Magnaporthe grisea. Plant Cell. 1993;5:1575–1590. doi: 10.1105/tpc.5.11.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jensen B.G., Andersen M.R., Pedersen M.H., Frisvad J.C., Søndergaard I. Hydrophobins from Aspergillus species cannot be clearly divided into two classes. BMC Res. Notes. 2010;3:344. doi: 10.1186/1756-0500-3-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai F., Zhao Z., Gao R., Chen P., Ding M., Jiang S., Fu Z., Xu P., Chenthamara K., Shen Q., et al. The pleiotropic functions of intracellular hydrophobins in aerial hyphae and fungal spores. PLoS Genet. 2021;17:e1009924. doi: 10.1371/journal.pgen.1009924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lorenz M.C., Bender J.A.G., Fink R. Transcriptional response of Candida albicans upon internalization by macrophages. Eukaryot. Cell. 2004;3:1076–1087. doi: 10.1128/EC.3.5.1076-1087.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ebel F., Schwienbacher M., Beyer J., Heesemann J., Brakhage A.A., Brock M. Analysis of the regulation, expression, and localisation of the isocitrate lyase from Aspergillus fumigatus, a potential target for antifungal drug development. Fungal Genet. Biol. 2006;43:476–489. doi: 10.1016/j.fgb.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 20.Kunert J., Kopeciek P. Multiple forms of the serine protease Alp of Aspergillus fumigatus. Mycoses. 2000;43:339–347. doi: 10.1046/j.1439-0507.2000.00586.x. [DOI] [PubMed] [Google Scholar]
- 21.Charles P., Devanathan V., Anbu P., Ponnuswamy M., Kalaichelvan P., Hur B.K. Purification, characterization and crystallization of an extracellular alkaline protease from Aspergillus nidulans HA-10. J. Basic Microbiol. 2008;48:347–352. doi: 10.1002/jobm.200800043. [DOI] [PubMed] [Google Scholar]
- 22.Ogawa H., Nozawa Y., Rojanavanich V., Tsuboi R., Yoshiike T., Banno Y., Takahashi M., Nombela C., Herreros E., Garcia-Saez M.I., et al. Fungal enzymes in the pathogenesis of fungal infections. J. Med. Vet. Mycol. 1992;30((Suppl. 1)):189–196. doi: 10.1080/02681219280000881. [DOI] [PubMed] [Google Scholar]
- 23.Nosanchuk J.D., Casadevall A. Impact of melanin on microbial virulence and clinical resistance to antimicrobial compounds. Antimicrob. Agents Chemother. 2006;50:3519–3528. doi: 10.1128/AAC.00545-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heinekamp T., Thywißen A., Macheleidt J., Keller S., Valiante V., Brakhage A.A. Aspergillus fumigatus melanins: Interference with the host endocytosis pathway and impact on virulence. Front. Microbiol. 2013;3:440. doi: 10.3389/fmicb.2012.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holdom M.D., Hay R.J., Hamilton A.J. The Cu,Zn superoxide dismutases of Aspergillus flavus, Aspergillus niger, Aspergillus nidulans, and Aspergillus terreus: Purification and biochemical comparison with the Aspergillus fumigatus Cu,Zn superoxide dismutase. Infect. Immun. 1996;64:3326–3332. doi: 10.1128/iai.64.8.3326-3332.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reverberi M., Punelli M., Scala V., Scarpari M., Uva P., Mentzen W.I., Dolezal A.L., Woloshuk C., Pinzari F., Fabbri A.A., et al. Genotypic and phenotypic versatility of Aspergillus flavus during maize exploitation. PLoS ONE. 2013;8:e68735. doi: 10.1371/journal.pone.0068735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chalivendra S.C., DeRobertis C., Chang P.K., Damann K.E. Cyclopiazonic acid is a pathogenicity factor for Aspergillus flavus and a promising target for screening germplasm for ear rot resistance. Mol. Plant Microbe Interact. 2017;30:361–373. doi: 10.1094/MPMI-02-17-0026-R. [DOI] [PubMed] [Google Scholar]
- 28.Mehl H.L., Jaime R., Callicott K.A., Probst C., Garber N.P., Ortega-Beltran A., Grubisha L.C., Cotty P.J. Aspergillus flavus diversity on crops and in the environment can be exploited to reduce aflatoxin exposure and improve health. Ann. N. Y. Acad. Sci. 2012;1273:7–17. doi: 10.1111/j.1749-6632.2012.06800.x. [DOI] [PubMed] [Google Scholar]
- 29.Rodrigo M., Jackson-Ziems T. Ear Rot Diseases Developing in Some Nebraska Corn Fields. Institute of Agriculture and Natural Resources (IANR), Cropwatch, University of Nebraska-Lincoln; Lincoln, NE, USA: 2017. [Google Scholar]
- 30.Robertson E. Aspergillus Ear Rot and Aflatoxin Production. [(accessed on 25 January 2024)];Integr. Crop Manag. News. 2012 :153. Available online: https://dr.lib.iastate.edu/handle/20.500.12876/17783. [Google Scholar]
- 31.Payne G.A. Process of contamination by aflatoxin-producing fungi and their impact on crops. In: Sinha K.K., Bhatnagar D., editors. Mycotoxins in Agriculture and Food Safety. Marcel Dekker; New York, NY, USA: 1998. pp. 279–306. [Google Scholar]
- 32.Rodriguez J.G., Paterson C.G., Potts M.F., Poneleit C.G., Beine R.L. Role of selected arthropods in the contamination of corn by Aspergillus flavus as measured by aflatoxin production. In: Diener U.L., Asquith R.L., Dickens J.W., editors. Aflatoxin and Aspergillus flavus in Corn. Volume 279 Auburn University; Auburn, AL, USA: 1983. Southern Cooperative Series Bulletin. [Google Scholar]
- 33.Wicklow D.T. Taxonomic features and ecological significance of sclerotia. In: Diener U.L., Asquith R.L., Dickens J.W., editors. Aflatoxin and Aspergillus flavus in Corn. Volume 279. Auburn University; Auburn, AL, USA: 1983. pp. 6–12. Southern Cooperative Series Bulletin. [Google Scholar]
- 34.Terna P.T., Mohamed Nor N.M.I., Zakaria L. Endophytic Aspergillus species from corn kernels in Peninsular Malaysia. IOP Conf. Ser. Earth Environ. Sci. 2021;711:01202. doi: 10.1088/1755-1315/711/1/012026. [DOI] [Google Scholar]
- 35.EL-Lebody K., Soliman M.S., EL-Metwally E.M., Abd-Elaziz M., Moustafa H.Z. Isolation and pathogenicity of endophytic fungi associated with some maize hybrids against certain Lepidoptera pests. Egypt. J. Agric. Res. 2021;99:49–60. doi: 10.21608/ejar.2021.58352.1073. [DOI] [Google Scholar]
- 36.Pitt J.I., Miscamble B.F. Water relations of Aspergillus flavus and closely related species. J. Food Protect. 1995;58:86–90. doi: 10.4315/0362-028X-58.1.86. [DOI] [PubMed] [Google Scholar]
- 37.Robertson A.E. Risk of aflatoxin contamination increases with hot and dry growing conditions. [(accessed on 25 January 2024)];Integr. Crop Manag. News. 2005 :1383. Available online: https://crops.extension.iastate.edu/encyclopedia/risk-aflatoxin-contamination-increases-hot-and-dry-growing-conditions. [Google Scholar]
- 38.Gwinner J., Harnisch R., Muck O. Manual of the Prevention of Post-Harvest Grain Losses. GTZ; Eschborn, Germany: 1996. p. 330. [Google Scholar]
- 39.Magnoli C., Hallak C., Astoreca A., Ponsone L., Chiacchiera S., Dalcero A.M. Occurrence of ochratoxin A-producing fungi in commercial corn kernels in Argentina. Mycopathologia. 2006;161:53–58. doi: 10.1007/s11046-005-0237-5. [DOI] [PubMed] [Google Scholar]
- 40.Yazid S.N., Ng W.J., Selamat J., Ismail S.I., Samsudin N.I. Diversity and toxigenicity of mycobiota in grain corn: A case study at pioneer grain corn plantations in Terengganu, Malaysia. Agriculture. 2021;11:237. doi: 10.3390/agriculture11030237. [DOI] [Google Scholar]
- 41.Damicone J. Soilborne Diseases of Peanuts. Oklahoma Cooperative Extension Fact Sheets. 2017. [(accessed on 28 August 2023)]. Available online: http://osufacts.okstate.edu.
- 42.Pande S., Rao J.N. Changing scenario of groundnut diseases in Andhra Pradesh, Karnataka and Tamil Nadu states of India. Int. Arachis Newsl. 2000;20:42–44. [Google Scholar]
- 43.Melouk H.A., Damicone J.P. Aspergillus crown rot. In: Kokalis-Burelle N., Porter D.M., Rodríguez-Kábana R., Smith D.H., Subrahmanyam P., editors. Compendium of Peanut Disease. American Phytopathological Society; St. Paul, MN, USA: 1997. [Google Scholar]
- 44.Garren K.H., Jackson C.R. Peanuts, Culture and Uses. American Peanut Research and Education Society; Stillwater, OK, USA: 1973. Peanut diseases; pp. 429–494. [Google Scholar]
- 45.Carter E. Crown Rot Creeps. 2016. [(accessed on 8 May 2022)]. Available online: https://nwdistrict.ifas.ufl.edu/phag/2016/07/08/crown-rot-creeps-in/
- 46.Horn B., Pitt J. Yellow Mold and Aflatoxin. In: Kokalis-Burelle N., Porter D.M., Rodríguez-Kábana R., Smith D.H., Subrahmanyam P., editors. Compendium of Peanut Diseases. American Phytopathological Society; Stillwater, OK, USA: 1997. [Google Scholar]
- 47.Pettit R.E. Yellow mold and aflatoxin. In: Porter D.M., Smith D.H., Rodeiguez-Kabana R., editors. Compendium of Peanut Diseases. American Phytopathological Society; St. Paul, MN, USA: 1984. pp. 35–36. [Google Scholar]
- 48.Sibakwe C.B., Kasambara-Donga T., Njoroge S.M.C., Msuku W.A.B., Mhango W.G., Brandenburg R.L., Jordan D.L. The role of drought stress on aflatoxin contamination in groundnuts (Arachis hypogea L.) and Aspergillus flavus population in the soil. Mod. Agric. Sci. Technol. 2017;3:22–29. doi: 10.15341/mast(2375-9402)/03.03.2017/005. [DOI] [Google Scholar]
- 49.Tola M., Kebedel B. Occurrence, importance and control of mycotoxins: A review. Cogent. Food Agric. 2016;2:1191103. doi: 10.1080/23311932.2016.1191103. [DOI] [Google Scholar]
- 50.Torres A.M., Barros G.G., Palacios S.A., Chulze S.N., Battilani P. Review on pre-and post-harvest management of peanuts to minimize aflatoxin contamination. Food Res. Int. 2014;62:11–19. doi: 10.1016/j.foodres.2014.02.023. [DOI] [Google Scholar]
- 51.Xu M.L., Yang J.G., Wu J.X., Chi Y.C., Xie L.H. First report of Aspergillus niger causing root rot of peanut in China. Plant Dis. 2001;99:284. doi: 10.1094/PDIS-05-14-0530-PDN. [DOI] [PubMed] [Google Scholar]
- 52.Shapovalov M. The two most common decays of cotton bolls in the Southwestern states. J. Agric. Res. 1927;35:307–312. [Google Scholar]
- 53.Halisky P., Schnathorst W., Erwin D. Distribution and control of cotton boll rots in California cotton growing areas. Calif. Agric. 1961;15:6–7. [Google Scholar]
- 54.Lutfunnessa R.J.F., Shamsi S. Fungal diseases of cotton plant Gossypium hirsutum L. in Bangladesh. Dhaka Univ. J. Bio. Sci. 2011;20:139–146. doi: 10.3329/dujbs.v20i2.8974. [DOI] [Google Scholar]
- 55.Guthrie D., Whitam K., Batson B., Crawford J., Jividen G. Boll rot. Cotton Physio. Today. 1994;8 Newsletter of the Cotton Physiology Education Program. [Google Scholar]
- 56.Bedre R., Rajasekaran K., Mangu V.R., Sanchez Timm L.E., Bhatnagar D., Baisakh N. Genome-Wide Transcriptome Analysis of Cotton (Gossypium hirsutum L.) Identifies Candidate Gene Signatures in Response to Aflatoxin Producing Fungus Aspergillus flavus. PLoS ONE. 2015;10:e0138025. doi: 10.1371/journal.pone.0138025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moreira P.H.R., Soares J.J., Busoli S.A., da Cruz V.R., Pimentel M.H.L., Pelinson G.J.B. Causas do apodrecimento de maçãs do algodoeiro. Pesq. Agropec. Bras. 1994;29:1503–1507. [Google Scholar]
- 58.Olsen M., Silvertooth J.C. Diseases and Production Problems of Cotton in Arizona. The University of Arizona Cooperative Extension; Prescott, AZ, USA: 2001. [Google Scholar]
- 59.Jackson G. Onion Black Mould. Pacific Pests, Pathogens and Weeds, Fact Sheets. 2017. [(accessed on 1 June 2024)]. Available online: https://apps.lucidcentral.org/ppp_v9/text/web_full/entities/onion_black_mould_187.htm.
- 60.Dugan F.M., Hellier B.C., Lupien S.L. Pathogenic fungi in garlic seed cloves from the United States and China, and efficacy of fungicides against pathogens in garlic germplasm in Washington State. J. Phytopathol. 2007;155:437–445. doi: 10.1111/j.1439-0434.2007.01255.x. [DOI] [Google Scholar]
- 61.Oh J.Y., Mannaa M., Han G.D., Chun S.C., Kim K.D. First report of Aspergillus awamori as a fungal pathogen of garlic (Allium sativum L.) Crop Prot. 2016;85:65–70. doi: 10.1016/j.cropro.2016.03.019. [DOI] [Google Scholar]
- 62.Gherbawy Y., Elhariry H., Kocsubé S., Bahobial A., Deeb B.E., Altalhi A., Varga J., Vágvölgyi C. Molecular characterization of black Aspergillus species from onion and their potential for ochratoxin A and fumonisin B2 production. Foodborne Pathog. Dis. 2015;12:414–423. doi: 10.1089/fpd.2014.1870. [DOI] [PubMed] [Google Scholar]
- 63.Vico I., Lazarevic M., Duduk N. Black mold of stored onion bulbs caused by Aspergillus welwitschiae. Acta Hortic. 2021;1325:67–72. doi: 10.17660/ActaHortic.2021.1325.11. [DOI] [Google Scholar]
- 64.Massi F.P., Iamanaka B.T., Barbosa R.L., Sartori D., Ferrranti L., Taniwaki M.H., Fungaro M. Molecular analysis of Aspergillus section Nigri isolated from onion samples reveals the prevalence of A. welwitschiae. Braz. J. Microbiol. 2021;52:387–392. doi: 10.1007/s42770-020-00390-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hayden N.J., Maude R.B., Proctor F.J. Studies on the biology of black mould (Aspergillus niger) on temperate and tropical onions. 1. A comparison of sources of the disease and tropical field crops. Plant Pathol. 1994;43:562–569. doi: 10.1111/j.1365-3059.1994.tb01591.x. [DOI] [Google Scholar]
- 66.Verga J., Kocsubé S., Szigeti G., Man V., Tóth B., Vágvölgyi C., Bartók T. Black Aspergilli and fumonisin contamination in onions purchased in Hungary. Acta Aliment. 2012;41:414–423. doi: 10.1556/AAlim.41.2012.4.3. [DOI] [Google Scholar]
- 67.Serra R., Braga A., Venâncio A. Mycotoxin-producing and other fungi isolated from grapes for wine production, with particular emphasis on ochratoxin A. Res. Microbiol. 2005;156:515–521. doi: 10.1016/j.resmic.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 68.Agrios G.N. Plant Pathology. 5th ed. Academic Press; Cambridge, MA, USA: 2018. [Google Scholar]
- 69.Somma S., Perrone G., Logrieco A.F. Diversity of black Aspergilli and mycotoxin risks in grape, wine and dried vine fruits. Phytopathol. Mediterr. 2012;51:131–147. [Google Scholar]
- 70.Pitt J.I., Hocking A.D. Fungi and Food Spoilage. 2nd ed. Blackie Academic and Professional; London, UK: 1997. [Google Scholar]
- 71.Belli N., Pardo E., Marin S., Farrè G., Ramos A.J., Sanchis V. Occurrence of ochratoxin A and toxigenic potential of fungal isolates from Spanish grapes. J. Sci. Food Agric. 2004;84:541–546. doi: 10.1002/jsfa.1658. [DOI] [Google Scholar]
- 72.Valero A., Begum M., Leong S.L., Hocking A.D., Ramos A.J., Sanchis V., Marin S. Effect of germicidial UVC light on fungi isolated from grapes and raisins. Lett. Appl. Microbiol. 2007;45:238–243. doi: 10.1111/j.1472-765X.2007.02175.x. [DOI] [PubMed] [Google Scholar]
- 73.Hocking A.D., Leong S.L., Kazi B.A., Emmett R.W., Scott E.S. Fungi and mycotoxins in vineyards and grape products. Int. J. Food Microbiol. 2007;119:84–88. doi: 10.1016/j.ijfoodmicro.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 74.Leong S.L., Hocking A.D., Pitt J.I., Kazi B.A., Emmett R.W., Scott E.S. Australian research on ochratoxigenic fungi and ochratoxin A. Int. J. Food Microbiol. 2006;111:10–17. doi: 10.1016/j.ijfoodmicro.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 75.Emmett R.W., Harris A.R., Taylor R.H., McGechan J.K. Grape diseases and vineyard protection. In: Coombe B.G., Dry P.R., editors. Viticulture, Volume 2. Practices. Winetitles; Adelaide, Australia: 1992. pp. 232–278. [Google Scholar]
- 76.Perrone G., Varga J., Susca A., Frisvad J.C., Stea G., Kocsube S., Toth B., Kozakiewicz Z., Samson R.A. Aspergillus uvarum sp. nov., an uniseriate black Aspergillus species isolated from grapes in Europe. Int. J. Syst. Evol. Microbiol. 2008;58:1032–1039. doi: 10.1099/ijs.0.65463-0. [DOI] [PubMed] [Google Scholar]
- 77.Lim Y., Hassan O., Kim M.-K., Chang T. First report of bunch rot caused by Aspergillus tubingensis of shine muscat grape in Korea. Plant Dis. 2019;103:2953. doi: 10.1094/PDIS-05-19-1104-PDN. [DOI] [Google Scholar]
- 78.Perrone G., Stea G., Epifani F., Varga J., Frisvad J.C., Samson R.A. Aspergillus niger contains the cryptic phylogenetic species A. awamori. Fungal Biol. 2011;115:1138–1150. doi: 10.1016/j.funbio.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 79.Medina A., Mateo R., Lopez-Ocana L., Valle-Algarra F.M., Jimenez M. Study of Spanish grape mycobiota and ochratoxin A production by isolates of Aspergillus tubingensis and other members of Aspergillus Section Nigri. Appl. Environ. Microbiol. 2005;71:4696–4702. doi: 10.1128/AEM.71.8.4696-4702.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zimmerli B., Dick R. Ochratoxin A in table wine and grape-juice: Occurrence and risk assessment. Food Addit. Contam. 1996;13:655–668. doi: 10.1080/02652039609374451. [DOI] [PubMed] [Google Scholar]
- 81.Varga J., Kozakiewicz Z. Ochratoxin A in grapes and grape-derived products. Trends Food Sci. Technol. 2006;17:72–81. doi: 10.1016/j.tifs.2005.10.007. [DOI] [Google Scholar]
- 82.Palumbo J.D., O’Keeffe T.L., Vasquez S.J., Mahoney N.E. Isolation and identification of ochratoxin A-producing Aspergillus section Nigri strains from California raisins. Lett. Appl. Microbiol. 2011;52:330–336. doi: 10.1111/j.1472-765X.2011.03004.x. [DOI] [PubMed] [Google Scholar]
- 83.Lucchetta G., Bazzo I., Cortivo G.D., Stringher L., Bellotto D., Borgo M., Angelini E. Occurrence of black aspergilli and ochratoxin A on grapes in Italy. Toxins. 2010;2:840–855. doi: 10.3390/toxins2040840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Akdeniz A.S., Ozden S., Alpertunga B. Ochratoxin A in dried grapes and grape-derived products in Turkey. Food Addit. Contam. Part B. 2013;6:265–269. doi: 10.1080/19393210.2013.814719. [DOI] [PubMed] [Google Scholar]
- 85.Chebil S., Rjiba-Bahri W., Oueslati S., Ben Ismail H., Ben-Amar A., Natskoulis P. Ochratoxigenic fungi and Ochratoxin A determination in dried grapes marketed in Tunisia. Ann. Microbiol. 2020;70:38 [Google Scholar]
- 86.Jarvis W.R. Bunch rot of grapes caused by Aspergillus aculeatus. Plant Dis. 1984;68:718–719. doi: 10.1094/PD-69-718. [DOI] [Google Scholar]
- 87.Latorre B.A., Viertel S.C., Spadaro I. Severe outbreaks of bunch rots caused by Rhizopus stolonifer and Aspergillus niger on table grapes in Chile. Plant Dis. 2002;86:815. doi: 10.1094/PDIS.2002.86.7.815C. [DOI] [PubMed] [Google Scholar]
- 88.Kazi B.A., Emmett R.W., Nancarrow N., Partington D.L. Berry infection and the development of bunch rot in grapes caused by Aspergillus carbonarius. Plant Pathol. 2008;57:301–307. doi: 10.1111/j.1365-3059.2007.01767.x. [DOI] [Google Scholar]
- 89.Hocking A.D. Encyclopedia of Food Microbiology. Elsevier; Amsterdam, The Netherlands: 2014. Spoilage Problems: Problems caused by fungi; pp. 471–481. [Google Scholar]
- 90.Kim Y.S., Kwon H.T., Hong S.-B., Jeon Y. Occurrence of bunch rot disease caused by Aspergillus tubingensis on shine Muscat grape. Res. Plant Dis. 2019;25:220–225. doi: 10.5423/RPD.2019.25.4.220. [DOI] [Google Scholar]
- 91.Rooney-Latham S., Janousek C.N., Eskalen A., Gubler W.D. First Report of Aspergillus carbonarius causing sour rot of table grapes (Vitis vinifera) in California. Plant Dis. 2008;92:651. doi: 10.1094/PDIS-92-4-0651A. [DOI] [PubMed] [Google Scholar]
- 92.Gao H., Yin X., Jiang X., Shi H., Yang Y., Wang C., Dai X., Chen Y., Wu X. Diversity and spoilage potential of microbial communities associated with grape sour rot in eastern coastal areas of China. PeerJ. 2020;8:e9376. doi: 10.7717/peerj.9376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tjamos S.E., Antoniou P.P., Kazantzidou A., Antonopoulos D.F., Papageorgiou I., Tjamos E.C. Aspergillus niger and Aspergillus carbonarius in Corinth Raisin and wine-producing vineyards in Greece: Population composition, ochratoxin a production and chemical control. J. Phytopathol. 2004;152:250–255. doi: 10.1111/j.1439-0434.2004.00838.x. [DOI] [Google Scholar]
- 94.Pisani C., Nguyen T.T., Gubler W.D. A novel fungal fruiting structure formed by Aspergillus niger and Aspergillus carbonarius in grape berries. Fungal Biol. 2015;119:784–790. doi: 10.1016/j.funbio.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 95.Michailides T.J., Peacock W., Christensen P., Morgan D.P., Felts D. First report of Aspergillus vine canker of table grapes caused by Aspergillus niger. Plant Dis. 2002;86:75. doi: 10.1094/PDIS.2002.86.1.75A. [DOI] [PubMed] [Google Scholar]
- 96.Vitale A., Castello I., Polizzi G. First Report of Aspergillus Vine canker on table grapes caused by Aspergillus niger in Europe. Plant Dis. 2008;92:1471. doi: 10.1094/PDIS-92-10-1471B. [DOI] [PubMed] [Google Scholar]
- 97.Vitale A., Cirvilleri G., Panebianco A., Epifani F., Perrone G., Polizzi G. Molecular characterisation and pathogenicity of Aspergillus Sect. Nigri causing Aspergillus vine canker of table grapes in Italy. Eur. J. Plant Pathol. 2012;132:483–487. doi: 10.1007/s10658-011-9906-z. [DOI] [Google Scholar]
- 98.Zhuang G., Eakalen A., Arreguin M., Alfar K., Bustamante M. Aspergillus Vine Canker: An Overlooked Canker Disease of Grapevine in California. Progressive Crop Consultant. 2022. [(accessed on 6 June 2023)]. Available online: https://progressivecrop.com/2022/05/aspergillus-vine-canker-an-overlooked-canker-disease-of-grapevine-in-california/
- 99.Varga J., Kocsubé S., Suri K., Szigeti G., Szekeres A., Varga M., Tóth B., Bartók T. Fumonisin contamination and fumonisin producing black Aspergilli in dried vine fruits of different origin. Int. J. Food Microbiol. 2010;143:143–149. doi: 10.1016/j.ijfoodmicro.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 100.Mogensen J.M., Frisvad J.C., Thrane U., Nielsen K.F. Production of fumonisin B2 and B4 by Aspergillus niger on grapes and raisins. J. Agric. Food Chem. 2010;58:954–958. doi: 10.1021/jf903116q. [DOI] [PubMed] [Google Scholar]
- 101.Mogensen J., Larsen T.O., Nielsen K.F. Widespread occurrence of the mycotoxin fumonisin B2 in wine. J. Agric. Food Chem. 2010;58:4853–4857. doi: 10.1021/jf904520t. [DOI] [PubMed] [Google Scholar]
- 102.Logrieco A., Ferracane R., Visconti A., Ritieni A. Natural occurrence of fumonisin B2 in red wine from Italy. Food Addit. Contam. 2010;27:1136–1141. doi: 10.1080/19440041003716547. [DOI] [PubMed] [Google Scholar]
- 103.Mikušová P., Caboň M., Melichárková A., Urík M., Ritieni A., Slovák M. Genetic diversity, Ochratoxin A and fumonisin profiles of strains of Aspergillus Section Nigri isolated from dried vine fruits. Toxins. 2020;12:592. doi: 10.3390/toxins12090592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schilder A. Management of Bunch rot Diseases in Grapes. Michigan State University Extension, Department of Plant Pathology; East Lansing, MI, USA: 2008. [Google Scholar]
- 105.Battilani P., Giorni P., Bertuzzi T., Formenti S., Pietri A. Black aspergilli and ochratoxin A in grapes in Italy. Int. J. Food Microbiol. 2006;111:53–60. doi: 10.1016/j.ijfoodmicro.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 106.Ponsone M.L., Combina M., Dalcero A., Chulze S. Ochratoxin A and ochratoxigenic Aspergillus species in Argentinean wine grapes cultivated under organic and nonorganic systems. Int. J. Food Microbiol. 2007;114:131–135. doi: 10.1016/j.ijfoodmicro.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 107.Guzev L., Danshin A., Ziv S., Lichter A. Occurrence of ochratoxin A producing fungi in wine and table grapes in Israel. Int. J. Food Microbiol. 2006;111:67–71. doi: 10.1016/j.ijfoodmicro.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 108.Vogel A., Breeden S., Brannen P., Blaauw B., Hickey C. Grape Sour Rot. UGA Cooperative Extension Circular 1212. 2020. [(accessed on 23 May 2023)]. Available online: https://secure.caes.uga.edu/extension/publications/files/pdf/C%201212_4.PDF.
- 109.Pinto L., Malfeito-Ferreira M., Quintieri L., Silva A.C., Baruzzi F. Growth and metabolite production of a grape sour rot yeast-bacterium consortium on different carbon sources. Int. J. Food Microbiol. 2019;296:65–74. doi: 10.1016/j.ijfoodmicro.2019.02.022. [DOI] [PubMed] [Google Scholar]
- 110.Oliva J., Navarro S., Navarro G., Camara M.A., Barba A. Integrated control of grape berry moth (Lobesia botrana), powdery mildew (Uncinula necator), downy mildew (Plasmopara viticola) and grapevine sour rot (Acetobacter spp.) Crop Prot. 1999;18:581–587. doi: 10.1016/S0261-2194(99)00064-2. [DOI] [Google Scholar]
- 111.Pearson R.C., Gohee A.C., editors. Compendium of Grape Diseases and Insects. American Phytopathological Society; St Paul, MN, USA: 1988. [Google Scholar]
- 112.Stover E., Aradhya M., Ferguson L., Crisosto C. The fig: Overview of an ancient fruit. HortScience. 2007;42:1083–1087. doi: 10.21273/HORTSCI.42.5.1083. [DOI] [Google Scholar]
- 113.Stover E., Aradhya M., Crisosto C., Ferguson L. Overview of the California fig Industry and New Interest in Varieties for Fresh Fruit. [(accessed on 1 June 2024)]. pp. 169–175. Available online: https://www.researchgate.net/publication/288261421_Overview_of_the_California_fig_industry_and_new_interest_in_varieties_for_fresh_fruit.
- 114.Venditti T., Molinu M.G., Dore A., D’hallewin G., Fiori P., Tedde M., Agabbio M. Treatments with GRAS compounds to keep fig fruit (Ficus carica L.) quality during cold storage. Commun. Agric. Appl. Biol. Sci. 2005;70:339. [PubMed] [Google Scholar]
- 115.Steiner W.E., Rieker R.H., Battaglia R. Aflatoxin contamination in dried figs: Distribution and association with fluorescence. J. Agric. Food Chem. 1988;36:88–91. doi: 10.1021/jf00079a022. [DOI] [Google Scholar]
- 116.Doster M.A., Michailides T.J. Fungal decay of first crop and main-crop figs. Plant Dis. 2007;91:1657–1662. doi: 10.1094/PDIS-91-12-1657. [DOI] [PubMed] [Google Scholar]
- 117.Michailides T.J., Ferguson L. UC IPM Pest Management Guidelines: Fig, Publication 3447. 2009. [(accessed on 15 June 2023)]. Available online: https://ipm.ucanr.edu/pdf/pmg/pmgfig.pdf.
- 118.Buchanan J.R., Sommer N.F., Fortlage R.J. Aspergillus flavus infection and aflatoxin production in fig fruits. Appl. Microbiol. 1975;30:238–241. doi: 10.1128/am.30.2.238-241.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Heperkan D. The importance of mycotoxins and a brief history of mycotoxin studies in Turkey, Special issue Mycotoxins: Hidden hazards in food. ARI Bull. Istanb. Tech. Univ. 2006;54:18–27. [Google Scholar]
- 120.Iamanaka B.T., Menezes H.C., Vincente E., Leite R.S.F., Taniwaki M.H. Aflatoxigenic fungi and aflatoxins occurence in sultanas and dried figs commercialized in Brazil. Food Control. 2007;18:454–457. doi: 10.1016/j.foodcont.2005.12.002. [DOI] [Google Scholar]
- 121.Heperkan D., Karbancioglu-Güler F. Determination of Aspergillus section Flavi and their aflatoxin and cyclopiazonic acid production patterns in naturally dried figs. In: Appell M., Kendra D.F., Trucksess M.W., editors. Mycotoxin Prevention and Control in Agriculture. Volume 1031. American Chemical Society; Washington, DC, USA: 2009. pp. 77–90. [Google Scholar]
- 122.Isman B., Biyik H. The aflatoxin contamination of fig fruits in Aydin City (Turkey) J. Food Saf. 2009;29:318–330. doi: 10.1111/j.1745-4565.2009.00159.x. [DOI] [Google Scholar]
- 123.Heperkan D., Moretti A., Dikmen C.D., Logrieco A.F. Toxigenic fungi and mycotoxin associated with figs in the Mediterranean area. Phytopathol. Mediterr. 2012;51:119–130. [Google Scholar]
- 124.Bircan C., Barringer S.A., Ulken U., Pehlivan R. Aflatoxin levels in dried figs, nuts and paprika for export from Turkey. Int. J. Food Sci. Technol. 2008;43:1492–1498. doi: 10.1111/j.1365-2621.2008.01726.x. [DOI] [Google Scholar]
- 125.Zohri A.A., Abdel-Gawad K.M. Survey of mycoflora and mycotoxins of some dried fruits in Egypt. J. Basic Microbiol. 1993;33:279–288. doi: 10.1002/jobm.3620330413. [DOI] [PubMed] [Google Scholar]
- 126.Iamanaka B.T., Taniwaki M.H., Menezes H.C., Vicente E., Fungaro M.H.P. Incidence of toxigenic fungi and ochratoxin A in dried fruits old in Brazil. Food Addit. Contam. 2005;22:1258–1263. doi: 10.1080/02652030500260447. [DOI] [PubMed] [Google Scholar]
- 127.Zinedine A., Soriano J.M., Juan C., Mojemmi B., Molto J.C., Bouklouze A., Cherrah Y., Idrissi L., El Aouad R., Manes J. Incidence of ochratoxin A in rice and dried fruits from Rabat and Sale area, Morocco. Food Addit. Contam. 2007;24:285–291. doi: 10.1080/02652030600967230. [DOI] [PubMed] [Google Scholar]
- 128.Karbancioglu-Güler F., Heperkan D. Natural occurrence of ochratoxin A in dried figs. Anal. Chim. Acta. 2008;617:32–36. doi: 10.1016/j.aca.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 129.Bircan C. Incidence of ochratoxin A in dried fruits and co-occurrence with aflatoxins in dried figs. Food Chem. Toxicol. 2009;47:1996–2001. doi: 10.1016/j.fct.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 130.Roussos S., Zaouia N., Salih G., Tantaoui-Elaraki A., Lamrani K., Cheheb M., Hassouni H., Verhé F., Perraud-Gaime I., Augur C., et al. Characterization of filamentous fungi isolated from Moroccan olive and olive cake: Toxinogenic potential of Aspergillus strains. Mol. Nutri. Food Res. 2006;50:500–506. doi: 10.1002/mnfr.200600005. [DOI] [PubMed] [Google Scholar]
- 131.Baffi M.A., Romo-Sanchez S., Ubeda-Iranzo J., Briones-Perez A.I. Fungi isolated from olive ecosystems and screening of their potential biotechnological use. New Biotechnol. 2012;29:451–456. doi: 10.1016/j.nbt.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 132.Lazzizera C., Frisullo S., Alves A., Lopes J., Phillips A.J.L. Phylogeny and morphology of Diplodia species on olives in southern Italy and description of Diplodia olivarum sp. nov. Fungal Divers. 2008;31:63–71. [Google Scholar]
- 133.Chliyeh M., Achbani E., Rhimini Y., Selmaoui K., Touhami A.O., Filali-Maltouf A., El Modafar C., Moukhli A., Oukabli A., Benkirane R., et al. Pathogenicity of four fungal species on fruits and leaves of the olive tree (Olea europaea L.) Int. J. Pure Appl. Biosci. 2014;2:1–9. [Google Scholar]
- 134.Torbati M., Arzanlou M., Azadmard-damirchi S., Babai-ahari A., Alijani S. Effect of fungal species involved in the olive fruit rot on the qualitative properties of olive oil. Arch. Phytopathol. Plant Prot. 2014;47:292–297. doi: 10.1080/03235408.2013.809183. [DOI] [Google Scholar]
- 135.Ghitakou S., Koutras K., Kanellou E., Markaki P. Study of aflatoxin B1 and ochratoxin A production by natural microflora and Aspergillus parasiticus in black and green olives of Greek origin. Food Microbiol. 2006;23:612–621. doi: 10.1016/j.fm.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 136.Al-Ameiri N.S., Karajeh M.R., Qaraleh S.Y. Molds associated with olive fruits infested with olive fruit fly (Bactrocera oleae) and their effects on oil quality. Jordan J. Biol. Sci. 2015;8:217–220. [Google Scholar]
- 137.Ozsoy N., Ozkilinc H., Pala C.U. Molecular characterization of natural fungal flora in black olives: From field to table. Turk. J. Agric.-Food Sci. Technol. 2017;5:944–949. doi: 10.24925/turjaf.v5i8.944-949.1255. [DOI] [Google Scholar]
- 138.Ferraro V., Conigliaro G., Torta L., Burruano S., Moschetti G. Preliminary investigation on the endophytic communities in Olea europaea in Sicily; Proceedings of the 7th International Conference Integrated Fruit Production; Avignon, France. 27–30 October 2008; pp. 459–463. [Google Scholar]
- 139.Ferracane R., Tafuri A., Logieco A., Galvano F., Balzano D., Ritieni A. Simultaneous determination of aflatoxin B1 and ochratoxin A and their natural occurrence in Mediterranean virgin olive oil. Food Addit. Contam. 2007;24:173–180. doi: 10.1080/02652030600986040. [DOI] [PubMed] [Google Scholar]
- 140.Cavaliere C., Foglia P., Samperi R., Laganà A. Olives and Olive Oil in Health and Disease Prevention. Academic Press; New York, NY, USA: 2010. Determination of aflatoxins and ochratoxin A in olive oil; pp. 645–652. [Google Scholar]
- 141.Finoli C., Vecchio A., Planeta D. Mycotoxin occurrence in extra virgin olive oils and in olives. Ind. Aliment. 2005;44:506–514. [Google Scholar]
- 142.Markaki P., Delpont-Binet C., Grosso F., Dragacci S. Determination of ochratoxin A in red wine and vinegar by immunoaffinity high-pressure liquid chromatogrpahy. J. Food Prot. 2001;64:533–537. doi: 10.4315/0362-028X-64.4.533. [DOI] [PubMed] [Google Scholar]
- 143.Papachristou A., Markaki P. Determination of ochratoxin A in virgin olive oils of Greek origin by immunoaffinity column clean-up and high-performance liquid chromatography. Food Addit. Contam. 2004;21:85–92. doi: 10.1080/02652030310001632547. [DOI] [PubMed] [Google Scholar]
- 144.Daradimos E., Marcaki P., Koupparis M. Evaluation and validation of two fluorometric HPLC methods for the determination of aflatoxin B1 in olive oil. Food Addit. Contamin. 2000;17 Part A:65–73. doi: 10.1080/026520300283603. [DOI] [PubMed] [Google Scholar]
- 145.Nabizadeh S., Shariatifar N., Shokoohi E., Shoeibi S., Gavahian M., Fakhri Y., Azari A., Khaneghah A.M. Prevalence and probabilistic health risk assessment of aflatoxins B1, B2, G1, and G2 in Iranian edible oils. Environ. Sci. Pollut. Res. 2018;25:35562–35570. doi: 10.1007/s11356-018-3510-0. [DOI] [PubMed] [Google Scholar]
- 146.Hidalgo-Ruiz J.L., Romero-Gonzalez R., Martinez Vidal J.L., Garrido Frenich A. A rapid method for the determination of mycotoxins in edible vegetable oils by ultra-high performance liquid chromatography-tandem mass spectrometry. Food Chem. 2019;288:22–28. doi: 10.1016/j.foodchem.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 147.Alamprese C. The Extra-Virgin Olive Oil Handbook. Wiley Online Library; Hoboken, NJ, USA: 2014. Extra-virgin olive oil contaminants; pp. 75–85. [DOI] [Google Scholar]
- 148.Agriopoulou S., Stamatelopoulou E., Varzakas T. Advances in occurrence, importance, and mycotoxin control strategies: Prevention and detoxification in foods. Foods. 2020;9:137. doi: 10.3390/foods9020137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Adaskaveg J.E., Michailides T.J. UC IPM Pest Management Guidelines: Pomegranate UC ANR Publication 3474. 2018. [(accessed on 24 August 2023)]. Available online: https://ipm.ucanr.edu/pdf/pmg/pmgpomegranate.pdf.
- 150.Munhuweyi K., Lennox C.L., Meitz-Hopkins J.C., Caleb O.J., Opara U.L. Major diseases of pomegranate (Punica granatum L.), their causes and management—A review. Sci. Hortic. 2016;211:126–139. doi: 10.1016/j.scienta.2016.08.016. [DOI] [Google Scholar]
- 151.Ezra D., Kirshner B., Hershcovich M., Shtienberg D., Kosto I. Heart Rot of Pomegranate: Disease etiology and the events leading to development of symptoms. Plant Dis. 2015;99:496–501. doi: 10.1094/PDIS-07-14-0707-RE. [DOI] [PubMed] [Google Scholar]
- 152.Sharma R.B., Roy A.N., Singh G. A new fruit rot of pomegranate caused by Aspergillus varicolor. Curr. Sci. 1982;51:318. [Google Scholar]
- 153.Li X., Lu X., He Y., Deng M., Lv Y. Identification the pathogens causing rot disease in pomegranate (Punica granatum l.) in China and the antifungal activity of aqueous garlic extract. Forests. 2019;11:34. doi: 10.3390/f11010034. [DOI] [Google Scholar]
- 154.Yehia H.M. Heart rot caused by Aspergillus niger through splitting in leathery skin of pomegranate fruit. Afr. J. Microbiol. Res. 2013;7:834–837. [Google Scholar]
- 155.Guo M.J., Wang Q.T., Cheng Y.H., Hou C.L. Identification of Aspergillus tubingensis causing pomegranate fruit rot in China. Australas. Plant Pathol. 2021;50:233–240. doi: 10.1007/s13313-020-00769-7. [DOI] [Google Scholar]
- 156.Mincuzzi A., Ippolito A., Montemurro C., Sanzani S.M. Characterization of Penicillium s.s. and Aspergillus sect. nigri causing postharvest rots of pomegranate fruit in Southern Italy. Int. J. Food Microbiol. 2020;314:108389. doi: 10.1016/j.ijfoodmicro.2019.108389. [DOI] [PubMed] [Google Scholar]
- 157.Mincuzzi A., Sanzani S.M., Palou L., Ragni M., Ippolito A. Postharvest rot of pomegranate fruit in Southern Italy: Characterization of the main pathogens. J. Fungi. 2022;8:475. doi: 10.3390/jof8050475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kanetis L., Testempasis S., Goulas V., Samuel S., Myresiotis C., Karaoglanidis G.S. Identification and mycotoxigenic capacity of fungi associated with pre-and postharvest fruit rots of pomegranates in Greece and Cyprus. Int. J. Food Microbiol. 2015;208:84–92. doi: 10.1016/j.ijfoodmicro.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 159.Tournas V.H., Katsoudas E. Mould and yeast flora in fresh berries, grapes and citrus fruits. Int. J. Food Microbiol. 2005;105:11–17. doi: 10.1016/j.ijfoodmicro.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 160.Liaquat F., Arif S., Ashraf M., Chaudhary H.J., Munis M.F.H., Farooq A.B.U. Aspergillus niger causes fruit rot of lemon and grapefruit in Pakistan. Plant Dis. 2016;100:1951. doi: 10.1094/PDIS-02-16-0199-PDN. [DOI] [Google Scholar]
- 161.Mohammed B., Fatima A.H., Musa Sale P. Postharvest fungal spoilage of some citrus fruits. Bioeng. Biosci. 2020;7:10–14. [Google Scholar]
- 162.Kotan R., Dikbas N., Bostan H. Biological control of postharvest disease caused by Aspergillus flavus on stored lemon fruits. Afr. J. Biotechnol. 2009;8:209–214. [Google Scholar]
- 163.Olaniran O., Ojo O.A., Odelade K.A. Survey of the post-harvest diseases and aflatoxin contamination of marketed orange fruit (Citrus sp.) in major cities in Oyo State, Nigeria. IOSR J. Agric. Vet. Sci. 2014;7:27–31. [Google Scholar]
- 164.Zahra I.G., Arwa M.H. Fungi associated with postharvest fruit rots of orange in local market of El-Beida City, Libya. J. Adv. Bot. Zool. 2017;5:1–4. [Google Scholar]
- 165.Marino A., Nostro A., Fiorentino C. Ochratoxin A production by Aspergillus westerdijkiae in orange fruit and juice. Int. J. Food Microbiol. 2009;132:185–189. doi: 10.1016/j.ijfoodmicro.2009.03.026. [DOI] [PubMed] [Google Scholar]
- 166.Sandoval-Contreras T., Villarruel-López A., Torres-Vitela R., Garciglia-Mercado C., Gómez-Anduro G., Velázquez-Lizárraga A.E., Sierra-Beltran A., Ascencio F. Mycotoxigenic potential of phytopathogenic moulds isolated from citrus fruits from different states of Mexico. Qual. Assur. Saf. Crops Foods. 2018;10:125–136. doi: 10.3920/QAS2016.0890. [DOI] [Google Scholar]
- 167.Onyemata E.K., Ibrahim R.O. Isolation and identification of fungi and pathogenicity assessment of some spoilt fruits sold in Wuse Market, Abuja, Nigeria. Int. J. Curr. Res. 2018;10:76256–76259. [Google Scholar]
- 168.Häggblom P. Production of ochratoxin A in barley by Aspergillus ochraceus and Penicillium viridicatum: Effect of fungal growth, time, temperature, and inoculum size. Appl. Environ. Microbiol. 1982;43:1205–1207. doi: 10.1128/aem.43.5.1205-1207.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Udoh I., Eleazar C., Ogeneh B., Ohanu M. Studies on fungi responsible for the spoilage/deterioration of some edible fruits and vegetables. Adv. Microbiol. 2015;5:285–290. doi: 10.4236/aim.2015.54027. [DOI] [Google Scholar]
- 170.Bashar M.A., Shamsi S., Hossain M.Z. Fungi associated with rotten fruits in Dhaka Metropolis. Bangladesh J. Bot. 2012;41:115–117. doi: 10.3329/bjb.v41i1.11090. [DOI] [Google Scholar]
- 171.Nath K., Ku S., Bala M. Management of banana (Musa paradisiaca) fruit rot diseases using fungicides. J. Plant Path. Microbiol. 2015;6:1–7. doi: 10.4172/2157-7471.1000298. [DOI] [Google Scholar]
- 172.Yahaya S.M., Abubakar Y., Ali M.U., Lawan M., Ajingi Y.S., Haruna M., Mardiyya A.Y. Fungal infection of banana (Musa sapientum) sold at Wudil and Yanlemo markets of Kano State. Dutse J. Pure Appl. Sci. 2018;4:254–262. [Google Scholar]
- 173.Sani M.A., Kasim M. Isolation and identification of fungi associated with postharvest deterioration of banana (Musa paradisiaca L.) Pharmacol. Online. 2019;2:347–354. [Google Scholar]
- 174.Latiffah Z., Chai Y.Y., Masratul Hawa M., NurAmalina K., Nurul Farizah A. Characterisation and pathogenicity of Aspergillus tamarii causing banana fruit rot. Trop. Life Sci. Res. 2021;32:179–187. doi: 10.21315/tlsr2021.32.3.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Alemu K. Importance and pathogen spectrum of crown rot of banana in Jimma Town, Southwestern Ethiopia. J. Biol. Agric. Healthc. 2014;4:106–111. [Google Scholar]
- 176.Chandran D.R., Thara S.S. Etiology of fungi causing postharvest crown rot of Robusta variety banana in Kerala. Trop. Agric. 2021;59:124–133. [Google Scholar]
- 177.Krishnapillai N., Wijeratnam R. Aspergillus rot of ripe mangoes (Mangifera indica L.) var. Ambalavi, Willard and Karuthakolumban. J. Nat. Sci. Found. Sri Lanka. 2013;41:69–70. doi: 10.4038/jnsfsr.v41i1.5335. [DOI] [Google Scholar]
- 178.Javadpour S., Golestani A., Rastegar S., Dastjer M. Postharvest control of Aspergillus niger in mangos by means of essential oils. Adv. Hortic. Sci. 2018;32:389–398. [Google Scholar]
- 179.Al-Najada A.R., Al-Suabeyl M.S. Isolation and classification of fungi associated with spoilage of post-harvest mango (Mangifera indica L.) in Saudi Arabia. Afr. J. Microbiol. Res. 2014;8:685–688. [Google Scholar]
- 180.Mohsan M., Intizar-ul-Hassan M., Ali L. Chemotheraptic management of Alternaria black spot (Alternaria alternata) in mango fruits. J. Agric. Res. 2011;49:499–506. [Google Scholar]
- 181.Anadi A.C., Abdulkarim B.M., Aliyu R.H. Moulds associated with deterioration of mango (Mangifera indica L.) and proximate analysis of infected fruits in Keffi, Nasarawa State, Nigeria. Dutse J. Pure Appl. Sci. 2020;6:18–24. [Google Scholar]
- 182.Akinro E.B., Adetuberu I.A., Efunwole O.O., Olakunle T.P. Isolation and identification of fungal species associated with the spoilage of some selected edible fruits in iree town of Boripe local government, Osun State, Nigeria. J. Res. Pharm. Sci. 2015;2:7–10. [Google Scholar]
- 183.Onuorah S.C., Udemezue O.I., Uche J.C., Okoli I.C. Fungi associated with the spoilage of pineapple fruits in Eke Awka market. Bioscientist. 2013;1:22–27. [Google Scholar]
- 184.Srivastava D., Misra N. Fungal spoilage of stored fruits of Carica papaya L. and Vitis vinifera L. and fungitoxicity of plants extracts. J. Plant Sci. Res. 2017;4:170. [Google Scholar]
- 185.Vivek K., Prasad B., Anuradha B., Sandhya S., Sourabh C., Sujit W., Rupali C., Kanade M.B. Studies on post-harvest fungal pathogens of papaya fruits (Carica papaya L.) Int. J. Curr. Microbiol. Appl. Sci. 2019;8:2176–2180. [Google Scholar]
- 186.Chaudhary M.M., Patel D., Chaudhary D.H., Dighule S.B. Isolation and characterization of fungi associated with deterioration of papaya fruits. J. Pharmacog. Phytochem. 2020;9:3434–3437. [Google Scholar]
- 187.Valentino M.J.G., Pineda F.G., Fandialan M.F. Phytopathogenicity of fungi associated with crown rot of guava (Psidium guajava) Plant Pathol. Quar. 2015;5:7–13. doi: 10.5943/ppq/5/1/2. [DOI] [Google Scholar]
- 188.Akhtar N., Hanif K., Shafiq M., Anwar W. New report of Aspergillus awamori fruit rot of guava in Pakistan. J. Anim. Plant Sci. 2018;28:1537–1541. [Google Scholar]
- 189.Lal B., Rai R.N., Arya A., Tewari D.K. A new soft rot of guava. Natl. Acad. Sci. Lett. 1980;3:259–260. [Google Scholar]
- 190.Utikar P.G., Shinde P.A., Soawane C.S. Influence of temperature and incubation period on fruit for initiation and development by postharvest fungi of guava. Curr. Res. Rep. Mahatma Phule Agric. Univ. 1986;2:209–211. [Google Scholar]
- 191.Lim T.K., Khoo K.C. Guava in Malaysia; Production, Pests and Diseases. Tropical Press; Kuala Lumpur, Malaysia: 1990. [Google Scholar]
- 192.Adisa V.A. Fruit rot diseases of guava (Psidium guajava) in Nigeria. Indian Phytopathol. 1985;38:427–430. [Google Scholar]
- 193.Amadi J.E., Nwaokike P., Olahan G.S. Isolation and identification of fungi involved in the post-harvest spoilage of guava (Psidium guajava) in Awka metropolis. Int. J. Pharm. Sci. Res. 2014;1:145–149. [Google Scholar]
- 194.Embaby E., Hassan M.K. Decay of guava fruit (Psidium guajava Linn.) quality caused by some mold fungi. Int. J. Agric. Technol. 2015;11:713–730. [Google Scholar]
- 195.Fatima S. Introduction to major post-harvest diseases of guava. J. Drug Deliv. Ther. 2019;9:591–593. doi: 10.22270/jddt.v9i4.3592. [DOI] [Google Scholar]
- 196.Ragazzo-Sánchez J.A., Gutiérrez-Escatel A., Luna-Solano G., Gómez-Leyva J.F., Calderón-Santoyo M. Molecular identification of the fungus causing postharvest rot in jackfruit. Rev. Mex. Micol. 2011;34:9–15. [Google Scholar]
- 197.Oza K., Jain B.K., Maitreya B. Isolation and identification of fungi from Kalipati variety of Sapota fruits. Int. J. Botany Stud. 2020;5:264–266. [Google Scholar]
- 198.Kolhe D.B., Chavan R.A., Sahane P.A., Udar R.B.V. Efficacy of plant extracts and chemicals against Aspergillus fruit rot of sapota. J. Pharmacog. Phytochem. 2021;10:967–970. [Google Scholar]
- 199.Kuchi V.S., Sharavani C.S.R. Fruit Physiology and Postharvest Management of Strawberry. In: Asao T., Asaduzzaman M., editors. Strawberry Pre- and Post-Harvest Management Techniques for Higher Fruit Quality. Intech Open; Rijeka, Croatia: 2019. [DOI] [Google Scholar]
- 200.Palmer M.G., Mansouripour S.M., Blauer K.A., Holmes G.J. First report of Aspergillus tubingensis causing strawberry fruit rot in California. Plant Dis. 2019;103:2948. doi: 10.1094/PDIS-05-19-0978-PDN. [DOI] [Google Scholar]
- 201.Umar K., Shumaila F., Maroof S., Sana S., Salik Nawaz K. Fungi associated with postharvest quality deterioration of strawberry at green markets of Lahore. Mycopath. 2017;15:67–69. [Google Scholar]
- 202.Saleem A.R. Mycobiota and molecular detection of Aspergillus flavus and A. parasiticus aflatoxin contamination of strawberry (Fragaria ananassa Duch.) fruits. Arch. Phytopathol. Plant Prot. 2017;50:982–996. doi: 10.1080/03235408.2017.1411155. [DOI] [Google Scholar]
- 203.Hussein M.A., El-Said A.H., Yassein A.S. Mycobiota associated with strawberry fruits, their mycotoxin potential and pectinase activity. Mycology. 2020;11:158–166. doi: 10.1080/21501203.2020.1759719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Dwihastuti M.E., Soesanto L., Aji T.G., Devy N.F. Biological control strategy for postharvest diseases of citrus, apples, grapes and strawberries fruits and application in Indonesia. Egypt. J. Biol. Pest Control. 2021;31:141 [Google Scholar]
- 205.Hasan H.A.H. Patulin and aflatoxin in brown rot lesion of apple fruits and their regulation. World J. Microbiol. Biotechnol. 2000;16:607–612. doi: 10.1023/A:1008982511653. [DOI] [Google Scholar]
- 206.Alwakeel S.S. Molecular identification of isolated fungi from stored apples in Riyadh, Saudi Arabia. Saudi J. Biol. Sci. 2013;20:311–317. doi: 10.1016/j.sjbs.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Khadega H.K., Al-Hussaini I.M. Diagnostic and environmental study of Aspergillus terreus isolated from various varieties of apples fruits. J. Contemp. Med. Sci. 2015;1:31–35. [Google Scholar]
- 208.Ewekeye T.S., Oke O.A., Esan O.O. Studies on postharvest rot of apple (Malus domestica Borkh) Indian J. Plant Sci. 2016;5:36–41. [Google Scholar]
- 209.Tančinová D., Barboráková Z., Kačinová J., Mašková Z., Volčková M. The occurrence of micromycetes in apples and their potential ability to produce mycotoxins. J. Microbiol. Biotechnol. Food Sci. 2021;2:1800–1807. [Google Scholar]
- 210.Michailides T., Thomidis T. First report of Aspergillus flavus causing fruit rots of peaches in Greece. Plant Pathol. 2007;56:352. doi: 10.1111/j.1365-3059.2007.01536.x. [DOI] [Google Scholar]
- 211.Naeem M., Irshad G., Naz F., Noorin S., Aslam F., Rafay A. Morphological identification and management of fungal post-harvest pathogens of peach (Prunus persica L) World J. Biol. Biotechnol. 2018;3:183–185. doi: 10.33865/wjb.003.01.0144. [DOI] [Google Scholar]
- 212.Kong Q., Yu X., Song D., Ren X. Effect of tricyclazole on morphology, virulence and gene expression of Aspergillus aculeatus for management of soft rot disease in peach. J. Appl. Microbiol. 2018;125:1827–1835. doi: 10.1111/jam.14076. [DOI] [PubMed] [Google Scholar]
- 213.Zhang S., Zheng Q., Xu B., Liu J. Identification of the fungal pathogens of postharvest disease on peach fruits and the control mechanisms of Bacillus subtilis JK-14. Toxins. 2019;11:322. doi: 10.3390/toxins11060322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Valiuškait A., Raudonis L., Survilien E. Analysis of micromycetes composition of sour cherry fruits in Lithuania. Phytopathol. Pol. 2005;35:197–201. [Google Scholar]
- 215.Thomidis T., Exadaktylou E. First report of Aspergillus niger causing postharvest fruit rot of cherry in the Prefectures of Imathia and Pella, Northern Greece. Plant Dis. 2012;96:458. doi: 10.1094/PDIS-07-11-0620. [DOI] [PubMed] [Google Scholar]
- 216.Zhu G.Y., Wang X., Chen T.M., Wang S.Y., Chen X., Song Z.W., Shi X.C., Laborda P. First report of Aspergillus flavus causing fruit rot on kiwifruit in China. Plant Dis. 2022;106:1990. doi: 10.1094/PDIS-08-21-1771-PDN. [DOI] [PubMed] [Google Scholar]
- 217.Khallaf H.H., Nawar L.S., Tawfiq F.H. Postharvest rot diseases of some stone fruits collected from Jeddah City, Saudi Arabia. IOSR J. Pharm. Biologic. Sci. 2017;12:29–37. doi: 10.9790/3008-1202022937. [DOI] [Google Scholar]
- 218.Robert M.-C. Food Allergens: Seafood, Tree Nuts, Peanuts. In: Melton L., Shahidi F., Varelis P., editors. Encyclopedia of Food Chemistry. Academic Press; Cambridge, MA, USA: 2019. pp. 460–467. Encyclopedia of Food Chemistry. [Google Scholar]
- 219.INC Nuts & Dried Fruits Statistical Yearbook 2021/22. The International Nut and Dried Fruit Council Foundation (INC) 2022. [(accessed on 13 August 2022)]. Available online: https://www.nutfruit.org.
- 220.Bayman P., Baker J.L., Mahoney N.E. Aspergillus on tree nuts: Incidence and associations. Mycopathologia. 2002;155:161–169. doi: 10.1023/A:1020419226146. [DOI] [PubMed] [Google Scholar]
- 221.Kluczkovski A.M. Fungal and mycotoxin problems in the nut industry. Curr. Opin. Food Sci. 2019;29:56–63. doi: 10.1016/j.cofs.2019.07.009. [DOI] [Google Scholar]
- 222.Doster M.A., Michailides T.J. The relationship between date of hull splitting and decay of pistachio nuts by Aspergillus species. Plant Dis. 1995;79:766–769. doi: 10.1094/PD-79-0766. [DOI] [Google Scholar]
- 223.Pitt J.I., Wild C.P., Baan R.A., Gelderblom W.C.A., Miller J.D., Riley R.T., Wu F. Improving Public Health through Mycotoxin Control. IARC; Lyon, France: 2012. International Agency for Research on Cancer Nº 158. [Google Scholar]
- 224.Rodrigues P., Venâncio A., Lima N. Mycobiota and mycotoxins of almonds and chestnuts with special reference to aflatoxins. Food Res. Int. 2012;48:79–90. doi: 10.1016/j.foodres.2012.02.007. [DOI] [Google Scholar]
- 225.Calderari T.O., Iamanaka B.T., Frisvad J.C., Pitt J.I., Sartori D., Pereira J., Fungaro M.H.P., Taniwaki M.H. The biodiversity of Aspergillus section Flavi in brazil nuts: From rainforest to consumer. Int. J. Food Microbiol. 2013;160:267–272. doi: 10.1016/j.ijfoodmicro.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 226.Taniwaki M.H., Pitt J.I., Magan N. Aspergillus species and mycotoxins: Occurrence and importance in major food commodities. Curr. Opin. Food Sci. 2018;23:38–43. doi: 10.1016/j.cofs.2018.05.008. [DOI] [Google Scholar]
- 227.Essawet N., Abushahma H., Inbaia S., Najii A., Amra H.A. Natural incidence of aflatoxins and ochratoxin A nuts collected from local market in Tripoli. Int. J. Curr. Microbiol. Appl. Sci. 2017;6:1479–1486. [Google Scholar]
- 228.Palumbo J.D., O’Keeffe T.L., Ho Y.S., Santillan C.J. Occurrence of ochratoxin A contamination and detection of ochratoxigenic Aspergillus species in retail samples of dried fruit and nuts. J. Food Prot. 2015;78:836–844. doi: 10.4315/0362-028X.JFP-14-471. [DOI] [PubMed] [Google Scholar]
- 229.da Silva C.F., Schwan R.F., Dias Ë.S., Wheals A.E. Microbial diversity during maturation and natural processing of coffee cherries of Coffea arabica in Brazil. Int. J. Food Microbiol. 2000;60:251–260. doi: 10.1016/S0168-1605(00)00315-9. [DOI] [PubMed] [Google Scholar]
- 230.Taniwaki M.H., Pitt J.I., Teixeira A.A., Iamanaka B.T. The source of ochratoxin A in Brazilian coffee and its formation in relation to processing methods. Int. J. Food Microbiol. 2003;82:173–179. doi: 10.1016/S0168-1605(02)00310-0. [DOI] [PubMed] [Google Scholar]
- 231.Noonim P., Mahakarnchanakul W., Nielsen K.F., Frisvad J.C., Samson R.A. Isolation, identification and toxigenic potential of ochratoxin A-producing Aspergillus species from coffee beans grown in two regions of Thailand. Int. J. Food Microbiol. 2008;128:197–202. doi: 10.1016/j.ijfoodmicro.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 232.Leong S.L., Hien L.T., An T.V., Trang N.T., Hocking A.D., Scott E.S. Ochratoxin A-producing Aspergilli in Vietnamese green coffee beans. Lett. Appl. Microbiol. 2007;45:301–306. doi: 10.1111/j.1472-765X.2007.02189.x. [DOI] [PubMed] [Google Scholar]
- 233.Sartori D., Furlaneto M.C., Martins M.K., Ferreira de Paula M.R., Pizzirani-Kleiner A.A., Taniwaki M.H., Fungaro M.H. PCR method for the detection of potential ochratoxin-producing Aspergillus species in coffee beans. Res. Microbiol. 2006;157:350–354. doi: 10.1016/j.resmic.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 234.Alvindia D.G., de Guzman M.F. Survey of Philippine coffee beans for the presence of ochratoxigenic fungi. Mycotoxin Res. 2016;32:61–67. doi: 10.1007/s12550-016-0240-3. [DOI] [PubMed] [Google Scholar]
- 235.Nganou Donkeng N., Durand N., Tatsadjieu N.L., Metayer I., Montet D., Mbofung C.M. Fungal flora and ochratoxin a associated with coffee in Cameroon. Br. Microbiol. Res. J. 2014;4:1–17. doi: 10.9734/BMRJ/2014/4284. [DOI] [Google Scholar]
- 236.Viegas C., Pacífico C., Faria T., de Oliveira A.C., Caetano L.A., Carolino E., Gomes A.Q., Viegas S. Fungal contamination in green coffee beans samples: A public health concern. J. Toxicol. Environ. Health Part A. 2017;80:719–728. doi: 10.1080/15287394.2017.1286927. [DOI] [PubMed] [Google Scholar]
- 237.Perrone G., Susca A., Cozzi G., Ehrlich K., Varga J., Frisvad J.C., Meijer M., Noonim P., Mahakarnchanakul W., Samson R.A. Biodiversity of Aspergillus species in some important agricultural products. Stud. Mycol. 2012;72:53–66. doi: 10.3114/sim.2007.59.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.da Silva S.A., Pereira R.G.F.A., de Azevedo Lira N., da Gloria E.M., Chalfoun S.M., Batista L.R. Fungi associated to beans infested with coffee berry borer and the risk of ochratoxin A. Food Control. 2020;113:107204. doi: 10.1016/j.foodcont.2020.107204. [DOI] [Google Scholar]
- 239.Suárez-Quiroz M., González-Rios O., Barel M., Guyot B., Schorr-Galindo S., Guiraud J.P. Study of ochratoxin-A producing strains in coffee processing. Int. J. Food Sci. Technol. 2004;39:501–507. doi: 10.1111/j.1365-2621.2004.00810.x. [DOI] [Google Scholar]
- 240.Joosten H.M.L.J., Goetz J., Pittet A., Schellenberg M., Bucheli P. Production of ochratoxin A by Aspergillus carbonarius on coffee cherries. Int. J. Food Microbiol. 2001;65:39–44. doi: 10.1016/S0168-1605(00)00506-7. [DOI] [PubMed] [Google Scholar]
- 241.Pardo E., Marin S., Ramos A.J., Sanchis V. Occurrence of ochratoxigenic fungi and ochratoxin A in green coffee from different origins. Food Sci. Technol. Int. 2004;10:45–50. doi: 10.1177/1082013204041509. [DOI] [Google Scholar]
- 242.Paterson R.R.M., Lima N., Taniwaki M.H. Coffee, mycotoxins and climate change. Food Res. Int. 2014;61:1–15. doi: 10.1016/j.foodres.2014.03.037. [DOI] [Google Scholar]
- 243.Horst R. Westcott’s Plant Disease Handbook. 7th ed. Springer; Dordrecht, The Netherlands: 2008. [Google Scholar]
- 244.Lucas G., Campbell L. Introduction to Plant Diseases Identification and Management. 2nd ed. Springer; New York, NY, USA: 1992. [Google Scholar]
- 245.Pawar N.V., Patil V.B., Kamble S.S., Dixit G.B. First report of Aspergillus niger as a plant pathogen on Zingiber officinale from India. Plant Dis. 2008;92:1368. doi: 10.1094/PDIS-92-9-1368C. [DOI] [PubMed] [Google Scholar]
- 246.Kiyan V., Smagulova A., Kovenskiy A., Aiganym B., Uakhit R. First report of stem rot of Adenium obesum and leaf spot of Persea americana caused by Aspergillus niger in Kazakhstan. Plant Dis. 2023;10:1094. doi: 10.1094/PDIS-02-23-0202-PDN. [DOI] [PubMed] [Google Scholar]
- 247.Huang S., Zheng X., Yang D., An J., Wang L., Pang F., Tao A., Fu G. First report of soft rot caused by Aspergillus niger sensu lato on mother-in-law’s tongue in China. Plant Dis. 2021;105:703. doi: 10.1094/PDIS-03-20-0678-PDN. [DOI] [PubMed] [Google Scholar]
- 248.Abdel-Rahman T.F., El-Morsy S.A., Halawa A.E.A. Occurrence of stem and leaf Spots on Lucky bamboo (Dracaena sanderiana hort. ex. Mast.) plants in vase and its cure with safe means. J. Plant Prot. Pathol. 2020;11:705–713. doi: 10.21608/jppp.2020.170648. [DOI] [Google Scholar]
- 249.Guo J.W., Gao Y., Li C.Y., Yang L.F., Tian X.J., Hong L., Kong Q., Zhang Y.G., Li W.J. First report of leaf spot disease caused by Aspergillus tubingensis on Jatropha curcas in Yunnan, China. Plant Dis. 2017;101:505. doi: 10.1094/PDIS-08-16-1183-PDN. [DOI] [Google Scholar]
- 250.Liaquat F., Munis M.F.H., Arif S., Liu Q.L. Presence of Aspergillus tubingensis causing leaf spot disease of Helleborus species in Shanghai, China. Plant Dis. 2018;103:766. doi: 10.1094/PDIS-07-18-1226-PDN. [DOI] [Google Scholar]
- 251.Khizar M., Haroon U., Ali M., Arif S., Shah I.H., Chaudhary H.J., Munis M.F.H. Aspergillus tubingensis causes leaf spot of cotton (Gossypium hirsutum L.) in Pakistan. Phyton. 2020;89:103. doi: 10.32604/phyton.2020.08010. [DOI] [Google Scholar]
- 252.Meena C., Bhatnagar P., Meena R.R., Prahlad V.C., Kumar A. First report of black pod in tamarind due to Aspergillus niger from India. Int. J. Curr. Microbiol. App. Sci. 2018;7:1127–1130. doi: 10.20546/ijcmas.2018.704.123. [DOI] [Google Scholar]
- 253.Alomran M., Houbraken J., Newcombe G. Aspergillus tubingensis is a pre-emergent pathogen of date palm seedlings. Forests. 2020;11:1327. doi: 10.3390/f11121327. [DOI] [Google Scholar]
- 254.Arif S., Munis M.F.H., Liaquat F., Zhao L., Haroon U., Gulzar S., Shah I.H., Pan J., Zhang Y. Detection and characterization of Aspergillus tubingensis causing leaf rot disease in pak choi in China. Can. J. Plant Pathol. 2022;44:702–708. doi: 10.1080/07060661.2022.2067901. [DOI] [Google Scholar]
- 255.Nasir M.N. Management of Aspergillus leaf spot diseases on Terminalia catappa in Sokoto, Nigeria. Pac. Int. J. 2021;4:07–12. doi: 10.55014/pij.v4i1.26. [DOI] [Google Scholar]
- 256.Aktar M., Shamsi S. Blight of two species of marigold (Tagetes) caused by Aspergillus fumigatus Fresenius. Bangladesh J. Plant Pathol. 2015;31:1–6. [Google Scholar]
- 257.Chandra Mohana N., Narendra Kumar H.K., Mahadevakumar S., Sowmya R., Sridhar K.R., Satish S. First report of Aspergillus versicolor associated with fruit rot disease of tomato (Solanum lycopersicum) from India. Plant Dis. 2022;106:1300. doi: 10.1094/PDIS-07-21-1461-PDN. [DOI] [PubMed] [Google Scholar]
- 258.Mohd Zainudin N.A.I., Abd Murad N.B., Aris A., Hussain N.H. First report of Aspergillus niger causing fruit rot of bilimbi in Malaysia. Plant Dis. 2023;107:1227. doi: 10.1094/PDIS-06-22-1291-PDN. [DOI] [PubMed] [Google Scholar]
- 259.Montero C.R.S., Schwarz L.L., Dos Santos L.C., Andreazza C.S., Kechinski C.P., Bender R.J. Postharvest mechanical damage affects fruit quality of ‘Montenegrina’ and ‘Rainha’ tangerines. Pesq. Agropec. Bras. 2009;44:1636–1640. doi: 10.1590/S0100-204X2009001200011. [DOI] [Google Scholar]
- 260.Rahul S.N., Khilari K., Sagar S., Chaudhary S., Kumar S., Vihan N., Tomar A. Challenges in postharvest management of fungal diseases in fruits and vegetables—A review. South Asian J. Food Technol. Environ. 2015;1:126–130. doi: 10.46370/sajfte.2015.v01i02.04. [DOI] [Google Scholar]
- 261.Snowdon A. A Color Atlas of Post-Harvest Disease and Disorders of Fruits and Vegetables. Volume 2. Wolfe Scientific Ltd.; Barcelona, Spain: 1991. p. 416. [Google Scholar]
- 262.Varga J., Kocsube S., Peteri Z., Vagvolgyi C., Toth B. Chemical, physical and biological approaches to prevent ochratoxin induced toxicoses in humans and animals. Toxins. 2010;2:1718–1750. doi: 10.3390/toxins2071718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Abdel-Kareem M.M., Rasmey A.M., Zohri A.A. The action mechanism and biocontrol potentiality of novel isolates of Saccharomyces cerevisiae against the aflatoxigenic Aspergillus flavus. Lett. Appl. Microbiol. 2019;68:104–111. doi: 10.1111/lam.13105. [DOI] [PubMed] [Google Scholar]
- 264.Cordero-Bueso G., Mangieri N., Maghradze D., Foschino R., Valdetara F., Cantoral J.M., Vigentini I. Wild Grape-associated yeasts as promising biocontrol agents against Vitis vinifera fungal pathogens. Front. Microbiol. 2017;8:2025. doi: 10.3389/fmicb.2017.02025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Basilico M.Z., Basılico J.C. Inhibitory effects of some spice essential oils on Aspergillus ochraceus NRRL3174 growth and ochratoxin A production. Lett. Appl. Microbiol. 1999;29:238–241. doi: 10.1046/j.1365-2672.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- 266.Cortés-Higareda M., Ramos-García M.D.L., Correa-Pacheco Z.N., Del Río-García J.C., Bautista-Baños S. Nanostructured chitosan/propolis formulations: Characterization and effect on the growth of Aspergillus flavus and production of aflatoxins. Heliyon. 2019;5:1–7. doi: 10.1016/j.heliyon.2019.e01776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Aparicio-García P.F., Ventura-Aguilar R.I., del Río-García J.C., Hernández-López M., Guillén-Sánchez D., Salazar-Piña D.A., Ramos-García M.D.L., Bautista-Baños S. Edible chitosan/propolis coatings and their effect on ripening, development of Aspergillus flavus, and sensory quality in fig fruit, during controlled storage. Plants. 2021;10:112. doi: 10.3390/plants10010112. [DOI] [PMC free article] [PubMed] [Google Scholar]