Abstract
Salmonella is a significant zoonotic foodborne pathogen, and the global spread of multidrug-resistant (MDR) strains poses substantial challenges, necessitating alternatives to antibiotics. Among these alternatives, vaccines protect the community against infectious diseases effectively. This review aims to summarize the efficacy of developed Salmonella vaccines evaluated in various animal hosts and highlight key transitions for future vaccine studies. A total of 3221 studies retrieved from Web of Science, Google Scholar, and PubMed/Medline databases between 1970 and 2023 were evaluated. One hundred twenty-seven qualified studies discussed the vaccine efficacy against typhoidal and nontyphoidal serovars, including live-attenuated vaccines, killed inactivated vaccines, outer membrane vesicles, outer membrane complexes, conjugate vaccines, subunit vaccines, and the reverse vaccinology approach in different animal hosts. The most efficacious vaccine antigen candidate found was recombinant heat shock protein (rHsp60) with an incomplete Freund’s adjuvant evaluated in a murine model. Overall, bacterial ghost vaccine candidates demonstrated the highest efficacy at 91.25% (95% CI = 83.69–96.67), followed by the reverse vaccinology approach at 83.46% (95% CI = 68.21–94.1) across animal hosts. More than 70% of vaccine studies showed significant production of immune responses, including humoral and cellular, against Salmonella infection. Collectively, the use of innovative methods rather than traditional approaches for the development of new effective vaccines is crucial and warrants in-depth studies.
Keywords: bacterial vaccines, Salmonellosis, conventional vaccine technologies, reverse vaccinology, immunotherapy, infectious diseases
1. Introduction
Salmonellosis, caused by a wide range of Salmonella serovars, is one of the leading bacterial diseases in both humans and animals [1]. It is reported that Salmonella mainly consists of two species: Salmonella bongori and Salmonella enterica, with over 2600 serovars discovered so far [2]. These serovars can be grouped into typhoidal Salmonella (TS) and nontyphoidal Salmonella (NTS) based on their disease syndromes and host ranges [3,4]. Typhoidal Salmonella (TS) serovars are restricted to one host species, whereas NTS serovars have diverse hosts, including humans and animals with mild to moderate gastrointestinal syndromes [5]. Salmonella annually causes an estimated 1.35 million infections, 26,500 hospitalizations, and 420 deaths in the United States alone, leading to an estimated economic burden of over $3.7 billion. Globally, there are approximately 93 million NTS infections, and 155,000 deaths occur annually [6].
Salmonellosis is considered as the third leading cause of mortality among food-borne illnesses [7]. The majority of human salmonellosis cases are food-borne, mainly directly or indirectly linked to animal or human fecal contamination [8]. Infections can also spread through direct or indirect contact with animals and animal-associated food products [9,10,11]. In recent years, sub-Saharan Africa and southern Asia have witnessed a persistent rise in invasive NTS infections in both adults and children [12]. Notably, S. Typhimurium and Enteritidis were responsible for over 80% of invasive nontyphoidal Salmonella (iNTS) cases [13]. Therefore, the control of these specific Salmonella serovars at the animal interface is essential for preventing transmission of infections to humans. Antibiotics are commonly used to treat bacterial infections in humans as well as animals. Although the therapeutic use of antimicrobials has revolutionized modern medicine, the emergence of multidrug-resistant (MDR) bacterial strains has led to a significant antimicrobial resistance (AMR) crisis [10]. The high mortality and morbidity caused by MDR TS and NTS highlighted the urgent need for alternate therapies against Salmonella [14] dissemination and infection, such as vaccines, probiotics, and prebiotics.
Vaccines stimulate an immunological response in the host to combat infection. The field of vaccinology has produced several effective vaccines that have substantially reduced the burden of deadly pathogens, i.e., Salmonella in animals and humans [15]. Historically, vaccines have been produced using different methods, including live attenuated (weakened) or inactivated (killed). However, both strategies have their shortcomings. Conventional vaccinations (attenuated or killed) are typically costly to produce, require adjuvants (inactivated vaccines) and multiple doses (live attenuated and inactivated vaccine) to induce adequate immunity, can interfere with maternal antibodies (live attenuated, inactivated), and offer little or no protection. Considering all of these challenges, continuous research is needed for the development of effective and safe vaccines [16]. Therefore, conventional vaccines have undergone considerable improvements, including mutant-attenuated (live-attenuated) and subunit vaccines (a type of inactivated vaccine containing part of the bacteria or virus) over the years. Despite these advancements, only a few licensed commercial Salmonella vaccines are available (Table 1), which include live-attenuated vaccines, killed inactivated vaccines, and a few subunit vaccines [17]. Recently, the introduction of new biotechnological approaches in vaccine development generation has led to the development of potential next-generation vaccines, such as recombinant subunit vaccines, DNA vaccines, mRNA vaccines, bacterial ghost (BGs) vaccines, and reverse vaccinology [18]. DNA, bacterial ghost, and mRNA vaccines, when produced through recent developments in molecular biological techniques, induce robust immune responses against pathogens [19]. Another innovative approach to vaccine development is reverse vaccinology, which combines genomics, proteomics, and bioinformatics to identify new genes in pathogens that could elicit immune response [20]. However, the development of vaccines remains challenging due to the various Salmonella serovars and their unique pathogenic mechanisms.
Table 1.
A summary of commercial vaccines against salmonellosis in different animal models.
| Vaccine Name | Company Name | Animal | General Information |
|---|---|---|---|
| POULVAC® | Zoetis, Parsippany, NJ, USA | Chicken | Mutant-attenuated aroA-deleted Salmonella Enteritidis and S. Typhimurium |
| SALMOVAC® | IDT Bio, Coralville, IA, USA | Chicken | Freeze-dried live-attenuated S. Enteritidis |
| NOBILIS 9R Vac® | CEVA Animal Health, Lenexa, KS, USA | Chicken | Live-attenuated S. Gallinarum |
| Fowlvax | Kenya Vaccine Institute, Nairobi, Kenya | Chicken | Live-attenuated S. Gallinarum |
| LAYERMUNE® | CEVA Animal Health, Lenexa, KS, USA | Chicken | Live-attenuated S. Enteritidis |
| CORYMUNE® | CEVA Animal Health, Lenexa, KS, USA | Chicken | Killed inactivated S. Enteritidis |
| AviPro® Megan® Vac 1 | ELANCO, Greenfield, IN, USA | Chicken | Live metabolic drift mutant strain of S. Enteritidis |
| Nobilis® Salenvac T | MSD Animal Health, Rahway, NJ, USA | Chicken | Formalin-killed cells of S. Enteritidis PT4 & S. Typhimurium DT104 |
| Salmoporc® | CEVA Animal Health, Lenexa, KS, USA | Swine | Live-attenuated S. Typhimurium |
| Enterisol® Salmonella | Boehringer Ingelheim Animal Health, Ingelheim am Rhein, Germany | Swine | Live-attenuated S. Typhimurium and S. Choleraesuis |
| BIOSUIS SALM® | Animal Health Distributors, Carlow, Ireland | Swine | Formalin-killed S. Typhimurium, Derby, Infantis |
| ARGUS® | Merck Animal Health, Rahway, NJ, USA | Swine | Live-attenuated S. Choleraesuis |
| Autogenous Bio One Salmonella® | Armor Animal Health, Cortland, NY, USA | Swine | Killed S. Cerro, S. Heidelberg, S. Dublin, and S. Typhimurium |
| Salvexin®+B | MSD Animal Health, Rahway, NJ, USA | Sheep/cattle | Killed S. Bovismorbificans, S. Hindmarsh, S. Typhimurium and S. Brandenburg |
| Endovac-Bovi® | Animal Health Supply, El Paso, TX, USA | Sheep/cattle | Mutant-attenuated S. Typhimurium bacterin |
| Salmonella Vetovax™ SRP® | Veto quinol, Princeville, Canada | Sheep/cattle | killed S. Newport |
Therefore, this study provides a comprehensive overview of the developed vaccines for TS and NTS serovars in different animal hosts. Additionally, we used a systematic review approach to gather pertinent studies regarding the safety and efficacy of developed conventional and next-generation vaccines assessed in various animal hosts for this review, which is primarily intended for a broad scientific audience. This review will provide insights into the key issues that veterinary immunologists are currently facing.
2. Methods
2.1. Systematic Literature Search
A systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [21] to address key research questions. The key question focused on identifying potential vaccine antigens against diverse Salmonella serovars and determining the most effective, influential vaccine candidates. Three electronic databases, including Elsevier ScienceDirect, Scopus, and PubMed Central, were searched to identify relevant studies for this review, using the following terms: “Salmonella”, “Vaccine”, and “Animal Models”. The search was conducted in December 2023, and only English-language research articles published until 30 November 2023, were considered.
2.2. Selection Criteria
A two-step procedure consisting of primary and secondary inclusion/exclusion criteria was used to determine the eligibility of studies for inclusion in this review (Table 2). The review excluded evaluating vaccine effects in clinical settings due to variations in immune responses and colonization between animal models and human trials [22]. In cases where multiple sample types (i.e., ceca, cloaca, liver, and spleen) were assessed within a single trial, the cecal sample result was chosen for this study [9]. When necessary, if information on vaccine efficacy data was unavailable, the author (AS) emailed the corresponding author of the article to obtain the missing information.
Table 2.
Inclusion and exclusion criteria in this study.
| Inclusion Criteria | Exclusion Criteria | |
|---|---|---|
| Process | Primary | Primary |
| Screening | Vaccine studies conducted in different animal models (mice, chicken, swine, bovines, and caprine) | Review articles and guidelines |
| Primary research studies containing vaccinated and unvaccinated groups | Non-vaccine studies, non-challenge studies or invitro studies, non-Salmonella Studies | |
| Information on conventional and reverse vaccinology approaches, vaccines and vaccination protocols provided | Non-English | |
| Evaluation and data of vaccine efficacy provided in | Unable to access the full text of papers | |
| English language | Vaccines conducted in clinical trials | |
| Eligibility | Vaccine studies conducted in different animal models | Studies evaluated immune response alone without an effect of Salmonella colonization after challenge |
| Studies described the levels of Salmonella loads in cecal and fecal contents after vaccination and challenge | Studies evaluated the adjuvant efficacy alone or non-Salmonella antigens | |
| Studies described immune responses after vaccination and challenge | Studies that were unable to estimate Salmonella loads |
Vaccine efficacy and study eligibility for this review were assessed based on reductions in Salmonella load in the intestine and other organs during postmortem examination, as commonly used methods to evaluate the effectiveness of different Salmonella control strategies in various animal models [22]. Host-adapted serovars are all evaluated in the respective animal hosts, whereas S. Typhi serovar is restricted to humans; humanized mice are used as model animals for preclinical studies. Consequently, studies reported vaccination efficacy by analyzing the prevalence or proportion of “diseased” (i.e., colonized) or decreased levels of Salmonella colonization in both vaccinated and unvaccinated groups after the Salmonella challenge.
2.3. Data Extraction and Analysis
All research publications relevant to Salmonella vaccine studies were imported into Microsoft Excel datasheets, where duplicate studies were manually removed. Initially, one author (AS) studied the article to determine whether they fulfilled the inclusion criteria. If the titles and abstracts matched the selection criteria, the complete text of each potential publication was examined for the final evaluation of eligible studies. The complete text was examined during this stage to classify the qualifying studies based on the vaccine-efficacy studies and extract relevant information. The final lists of eligible articles were entered into the EndnoteX9 application for storage and consolidation.
The extracted information of the eligible studies comprised article identification, information about animal models, vaccine candidates, Salmonella challenge serovars, and vaccine efficacy, safety, and immune responses among vaccinated subjects. In cases where multiple trials were conducted in a single study, we only evaluated the trials involving immune responses and proliferation assays. Vaccine candidates prepared using reverse vaccinology but not assessed in an in vivo model were excluded. Only three vaccine studies used the reverse vaccinology approach: two in the murine model and one in the chicken type. The extracted information was summarized in Microsoft Excel datasheets (Supplementary Table S1).
2.4. Data Analysis
The statistical analysis was performed using the GraphPad Prism v10.1.2 software. The pooled Efficacy of vaccines was calculated using the random-effects model with a 95% confidence interval (95% CI) with Metaprop order [23]. Descriptive statistics were imported into Microsoft Excel for graphic analysis. Statistical significance was determined at p-values < 0.05.
3. Results
3.1. An Assembly of Quantified Studies
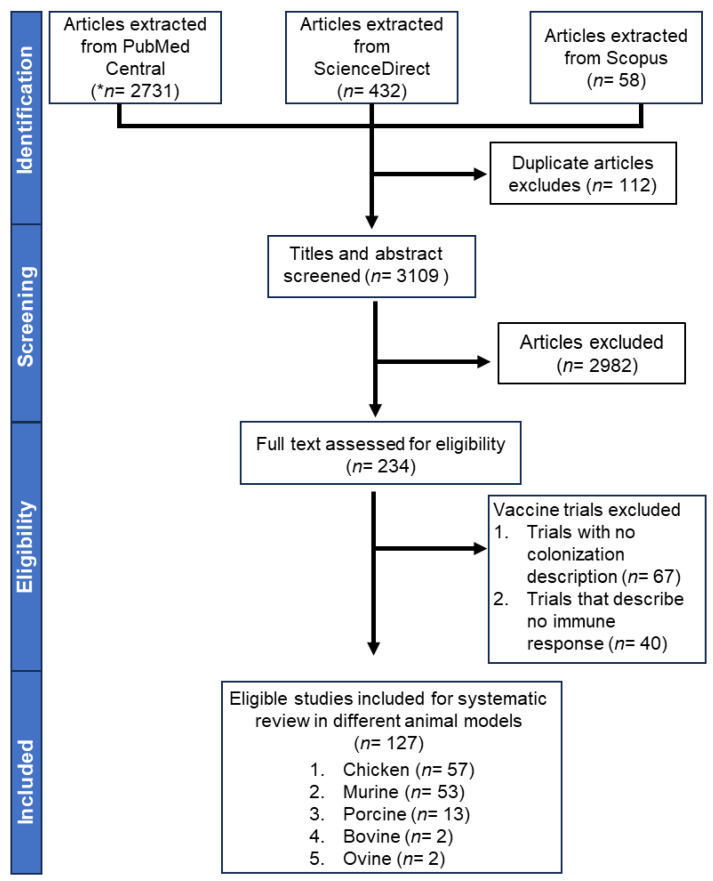
During the initial search, a total of 3221 publications were identified from three databases. After removing duplicates, the remaining 3109 articles (96.5%) were evaluated based on their abstracts. After reviewing the titles and abstracts, 2982 publications were eliminated (Figure 1). Among the excluded articles, 857 were review articles. Additionally, there were 372 articles related to control and pathogenesis of Salmonella. Moreover, 105 articles were related to clinical studies and knowledge, attitudes, and practice (KAPE) studies, 98 were related to other alternatives to antibiotics (probiotics, prebiotics, and natural products), and 75 were related to bacteriophages and parasites. Furthermore, 49 articles were related to bacteria other than Salmonella, 47 were related to only antigen preparation, 45 were related to One Health, and 41 were related to food safety issues. Additionally, there were 36 articles related to guidelines for the prevention and diagnosis of infections, 13 articles related to only methods for antigen preparation, and three articles lacking full text. A total of 1244 primary research studies did not meet the inclusion criteria: 466 were non-Salmonella studies, 421 were Salmonella studies but not related to vaccines, 245 were reverse-vaccinology vaccine candidate research without animal models, and 112 were Salmonella vaccines conducted in clinical trials, immunogenicity experiments, or articles with full-text unavailability. In the end, a total of 127 qualified studies that met the criteria were included in the systematic review.
Figure 1.
A simplified PRISMA diagram of methodology. A total of 3221 articles from different animal models were identified by our search strategy from different online databases (i.e., PubMed, ScienceDirect, and Google Scholar). * n is the no. of studies.
3.2. Animal Models
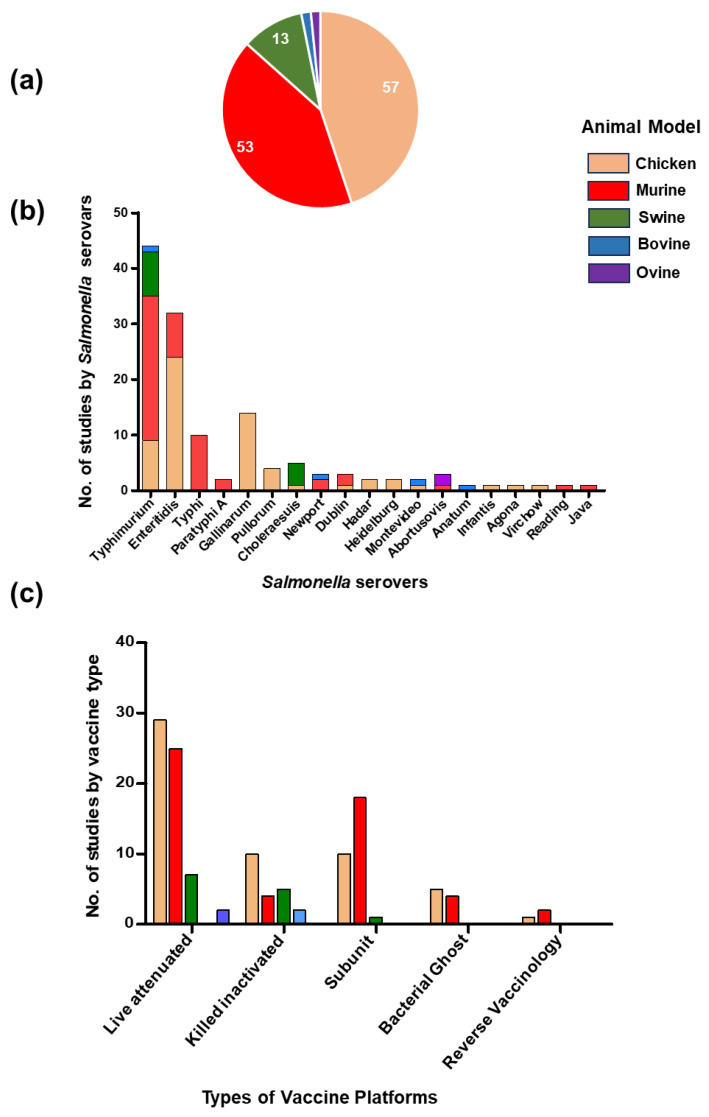
In this systematic review, five animal hosts (chicken, swine, bovine, ovine, and murine) were used to evaluate the efficacy and protective properties of conventional and reverse vaccinology approach vaccines in preventing Salmonella infections (Figure 2a). A total of 57 vaccine studies (59 trials) were conducted in the chicken model, 53 studies (55 trials) in the murine model, 13 studies (20 trials) in swine, two studies in bovine, and two studies in ovine. During our search, we identified 19 different serovars of Salmonella, encompassing both typhoidal and non-typhoidal, targeted by a vaccine. Notably, S. Typhimurium emerged as one of the most extensively researched serovars, serving as a challenge infection during vaccination trials across all animal models, followed by S. Enteritidis (Figure 2b).
Figure 2.
Summary of vaccine studies used in this review. (a) Proportion of vaccine studies according to animal models; (b) types of different Salmonella serovars used in different vaccine studies; (c) types of vaccine strategies used in this study.
3.3. Vaccine Types
Overall, eight types of vaccine strategies (live-recombinant, mutant-attenuated, subunit, a combination of vaccines, killed whole-cell, cell lysate, crude-cell lysate, and bacterial ghost vaccines) and two types of reverse vaccinology approach vaccines (single-peptide, multi-epitope) (Table 3) were classified.
Table 3.
A detailed overview of Salmonella vaccine antigens identified in this review.
| Vaccine Type | Role of Antigen in Salmonella Colonization | References | |
|---|---|---|---|
| Bacterin | Killed-whole bacterial cells (multiple antigens) used for immunization | [24,25,26] | |
| Conventional Approach | histidine-adenine auxotrophic | Adhesion | [27,28] |
| surface-exposed lipoprotein A | Adhesion | [29,30] | |
| Outer membrane proteins | Adhesion and invasion | [31,32,33,34] | |
| Flagellar Proteins (fliC) | Motility and adherence | [35,36] | |
| Whole-cell lysate | Adhesion | [15,37,38] | |
| Capsular polysaccharide (CPS) | Serum resistance | [39,40] | |
| ABC-Type multidrug efflux pump (MacAB) | Multidrug efflux system | [41] | |
| flagellar hook-associated proteins (fliD) | Adhesion | [42,43,44] | |
| Hypothetical protein | Protein–Protein interactions | [45,46] | |
| Peptidoglycan Recognition Protein PGLYRP2 | Maintenance of cell wall | [30] | |
| Crude-cell lysate | Adhesion | [47,48] | |
| Reverse Vaccinology Approach | Multi epitope | Adhesion and invasion | [49,50] |
| Single peptide | Adhesion | [51,52] |
Based on the investigation of 127 studies analyzing Salmonella loads in the ceca, liver, or spleen of the vaccinated and non-vaccinated mice, chicken, swine, ovine, and bovine, live-attenuated vaccine candidates were the most frequently used (61 studies), followed by subunit vaccines (24 studies) and inactivated killed vaccine (12 studies) (Figure 2c). The majority of investigations in swine focused on the development of live-attenuated vaccination candidates (n = 12), highlighting the importance of assessing the efficacy of new-generation vaccinations in combating Salmonella infection within the swine industry. Bacterial ghost vaccines were also used in mice and chicken models to combat multidrug-resistant Salmonella serovars. Only three trials (one in the chicken model and two in the mouse model) were found in which antigens were prepared using a reverse vaccinology approach.
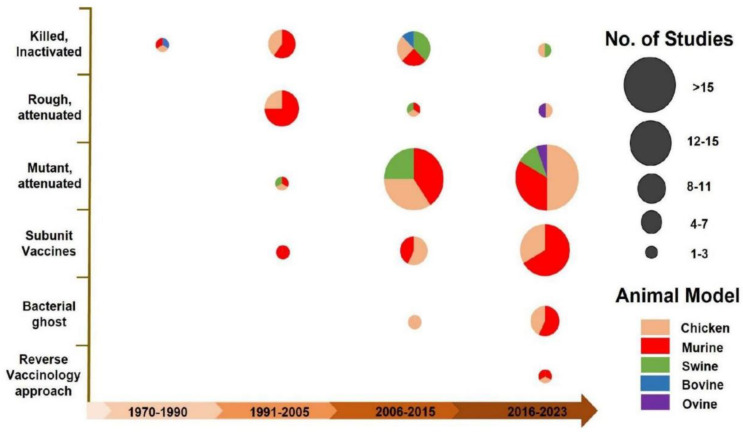
The shift in vaccination types in different animal models between 1970 and 2023 is shown (Figure 3). Initially, killed inactivated, rough attenuated, and few mutant-attenuated strains (galE mutant strain of S. Typhi, aroA and cya crp double-deletion strategy, and galE mutant strain of S. Gallinarum) vaccines were evaluated in mice, bovine, swine, and chickens before the year 2000. However, over the past decade, there has been a noticeable increase in the use of mutant-attenuated, subunit, and bacterial ghost vaccines in different animal models. Vaccine candidates prepared through the reverse vaccinology approach have also been evaluated in the chicken and murine models recently.
Figure 3.
Transition in the development of different vaccine candidates across various animal models from 1970 to 2023.
3.4. Vaccine Antigens
This review identified 13 antigens from selected vaccine studies using criteria related to vaccine efficacy (relative reduction of disease risk and mortality) (Supplementary Table S1). Live attenuated vaccine candidates were the most frequently assessed antigen in different animal hosts, including chickens (n = 22), mice (n = 25), swine (n = 10), and bovine (n = 2). Live-attenuated vaccine candidates (roughly attenuated or mutant-attenuated) were administered via oral, subcutaneous, or intramuscular (IM) routes as a single dose or booster. Metabolic mutations (Δasd, ΔrpoS, and ΔphoP) or amino acid (ΔaroA) were used for attenuation in different studies (n = 14). Flagellin proteins (fliA, fliB, fliC), used in eight trials (eight papers), were evaluated in two different vaccine types (mutant-attenuated and subunit vaccines) and different routes of administration (orally, intramuscularly, or subcutaneously with a booster). Formalin- and acetone-inactivated vaccines were used in seven trials in three animal models, including chickens (n = 3), mice (n = 2), and swine (n = 2) orally and subcutaneously.
Eleven studies were conducted using three antigens, including capsular polysaccharide, entire cell lysate, and crude cell lysate, to assess their effectiveness in homologous challenges across several animal models. The efficacy of core and O-polysaccharide (COPS) vaccine antigens, both with and without adjuvant, was assessed using subunit vaccination methods (oral, subcutaneous, and intramuscular with booster) and COPS vectored vaccines (oral with booster) after challenge with different Salmonella serovars. The antigenicity of both the total outer membrane proteins (OMP) and vesicles employed in the crude lysate vaccine was assessed in only two animal models, namely mice (n = 8) and chickens (n = 7), after a different Salmonella serovars challenge. The antigen was administered either as biodegradable and biocompatible nanoparticles (OMP-NP) or directly without encapsulation through oral or subcutaneous vaccinations, followed by a booster. Bacterial ghost vaccines (empty bacterial envelopes) using mutant-attenuated strains as antigens were tested in nine studies in two animal models. Chickens (n = 5) and mice (n = 4) were administered orally, intramuscularly, and subcutaneously.
Reverse vaccinology is an innovative method that combines immunology, computational biology, structural biology, and microbial genetics to identify and design vaccine antigens. Several studies have been conducted in which vaccine candidates were prepared and analyzed using bioinformatic tools, but they were not tested in animal models. Here, only three articles were identified in which vaccine antigens were identified and prepared using a reverse vaccinology approach and then tested in animal models; one study was conducted in chickens and two in mice models.
3.5. Vaccine Efficacy Evaluated by Organ Bacterial Colonization
This analysis found three different outcomes regarding the efficacy of vaccination, as reported in the 127 studies: no reduction in bacterial load in organs (intestine and liver) (n = 9), non-significant log10 bacterial load reductions (n = 27), and significant log10 bacterial load reductions (n = 96) (Supplementary Table S1).
Among 96 studies in which significant log10 bacterial load reductions occurred, 84 vaccine studies in different hosts, including mice (n = 39), chickens (n = 33), swine (n = 10), bovine (n = 1), and ovine (n = 1), exhibited significant log10 reductions ranging between 1.0-log10 and 4.0-log10 of Salmonella serovars loads in the intestine and liver after challenge with different Salmonella serovars. A subunit vaccine candidate, recombinant heat shock protein (rHsp60), derived from gram-negative bacteria with incomplete Freund’s adjuvant antigen, caused the most significant decrease in bacterial load in the intestine, with a reduction of 4.0-log10 after S. Enteritidis infection in mice model [37]. Furthermore, bacterial ghost cells carrying a heat-labile enterotoxin B component from S. Typhimurium result in a 3.7-log10 reduction in bacterial load in the caecum of chicken after S. Typhimurium challenges, compared to non-vaccinated animals. While all the other studies (n = 12) reported significant reductions (≤1 log10) in Salmonella loads after challenging. Moreover, there was just one study conducted on sheep to assess the effectiveness of the vaccine against S. Abortusovis, with results showing a 2.0-log10 reduction in feces after challenge infection. All three antigens identified through the reverse vaccinology approach significantly reduced the Salmonella load in both mouse and chicken models.
Twenty-seven Salmonella vaccine trials performed in swine, mice, sheep, and bovine showed a non-significant reduction upon challenge. These trials included live mutant or rough inactivated vaccines given orally with or without a booster, crude lysate vaccines with outer membrane protein (OMP), OMP-NP given orally with the booster, outer membrane vesicles (OMVs) given orally and subcutaneously, and formalin-killed with mineral oil adjuvant given orally and intramuscularly with and without a booster.
Nine trials from mice (n = 6) and chickens (n = 3) used rough and mutant-attenuated with and without a booster, outer membrane vesicles (OMVs), COPS, and the flagellar monomer protein (fliC) conjugate, and a recombinant fliC protein failed to reduce Salmonella load in organs.
3.6. Vaccine Safety, Efficacy, and Immune Responses
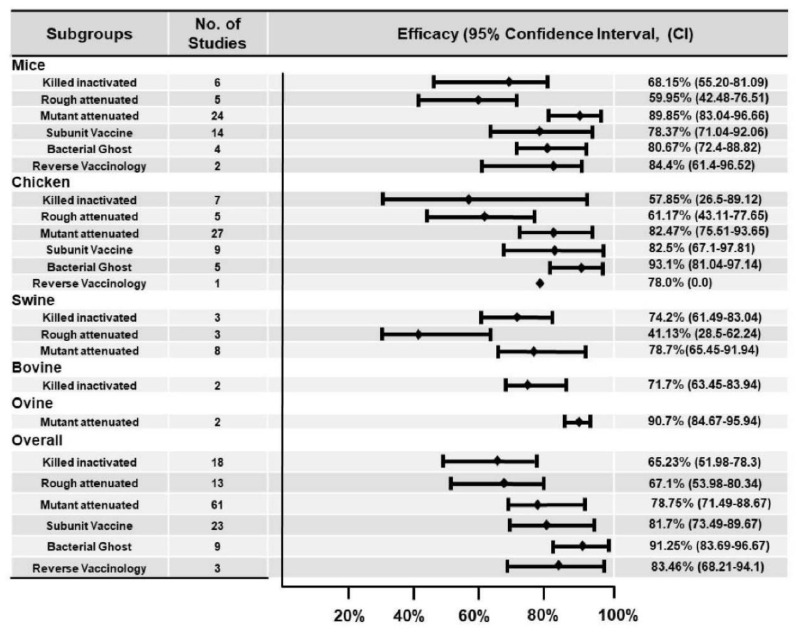
This study analyzed high-quality studies to evaluate vaccine efficacy by comparing mortality rates between vaccinated and unvaccinated groups. Additionally, immunological responses and vaccination safety were assessed. Various levels of vaccination efficacy were observed in vaccinated animals, ranging from 100% inhibition of Salmonella serovar colonization to no effect. The effectiveness of each vaccine candidate is presented (Supplementary Table S2). According to our results, out of 130 trials conducted in various studies, the majority (36 trials) were in mice, whereas 30 trials in chickens and 10 trials in swine used live recombinant mutant antigens and showed vaccine efficacy of >75%. Collectively, we observed efficacy of 89.85% (95% CI = 83.04–96.66) in mice, 82.47% (95% CI = 75.51–93.65) in chickens, and 78.7% (95% CI = 65.45–91.94) in swine for mutant-attenuated antigens (Figure 4). Only four investigations showed no adequate protection after vaccination: one from mice, using rough attenuated vaccines; two from chicken using killed inactivated vaccines; and one from swine, using rough attenuated vaccines. In the swine model, rough attenuated antigens had the lowest efficiency of 41.13% (95% CI = 28.5–62.24), followed by 57.85% (95% CI = 26.5–89.12) in the chicken model for killed inactivated antigens. Antigens generated using the reverse vaccinology approach were also utilized to examine vaccine efficacy, but two investigations on mice revealed vaccine efficacy of less than 70%, and one on poultry revealed vaccination efficacy of 75%. According to our study findings, the bacterial ghost vaccine strategy produced the most efficient vaccine candidates, 91.25% (95% CI = 83.69–96.67), followed by the reverse vaccinology approach (83.46% (95% CI = 68.21–94.10) and subunit vaccines (81.7% (95% CI = 73.49–89.67). The live mutant attenuated vaccine candidates also showed good efficacy (78.75%; 95% CI = 71.49–88.67) in different animal models.
Figure 4.
Efficacy of different vaccine candidates in various animal models. Here, the small square represents the reported efficacy after vaccination. All data were taken from the articles included in this systematic review. The 95% confidence intervals were not evenly distributed because the response levels for all vaccines were not the same. There is only one reverse vaccinology study in the chicken model, so we were not able to find the upper and lower limits of 95% CI; thus, it was not reported in the figure.
The immunological responses produced by vaccines played a crucial role in conferring protection against salmonellosis in various hosts. This systematic review article included 127 published studies on the immunogenicity of Salmonella vaccines that met the inclusion criteria. Overall, these trials found that more than 70% (n = 95) of vaccine studies showed significant production of antibodies against Salmonella infection. Only seven studies (rough attenuated = three, mutant attenuated = two, dead inactivated = two) (5.5%) found no effect on immune response against Salmonella infection. The ability of the Salmonella vaccines to induce cell-mediated immunity among different animal hosts was also assessed (Supplementary Table S2)
Vaccine safety is another important criterion for evaluating vaccine efficacy. Local effects, including pain and swelling at the vaccination site, and systemic effects, such as fever, fatigue, and diarrhea (mild and moderate adverse effects), were reported as the side effects of Salmonella vaccination. The majority of Salmonella vaccine antigens produced no symptoms or adverse effects in animals after vaccination. However, few vaccine trials conducted in mice, chickens, swine, and bovine using rough and mutant-attenuated strains revealed mild-to-moderate adverse effects on the hosts. Nevertheless, none of the antigens derived from reverse vaccinology exhibited adverse effects on the animal hosts.
4. Discussion
The emergence of MDR Salmonella serovars from animals significantly threatens public health. Effective vaccines are often used as an alternative to antibiotics to minimize the risk of Salmonella infections in different hosts [53]. This systematic literature review aimed to assess the efficacy of high-qualified Salmonella vaccine studies conducted in various animals and highlight the transition for future vaccine developments with the advancement of biotechnological approaches. The efficacy of Salmonella control measures is often assessed by measuring the decrease in the prevalence (in percentage) of Salmonella load in the intestine or other tissues after challenge [54,55]. Furthermore, the efficacy of vaccines is evaluated by assessing the immunological response and safety profile of vaccines tested in different animal hosts [56].
In this review, the majority of the studies indicate that vaccination leads to enhanced immune responses to different types of vaccine antigens and reduced bacterial burdens in the intestines and other organs in different animal hosts [35,38,44,52,57,58,59,60,61,62,63,64,65,66]. Numerous studies indicate significant decreases in Salmonella loads in mice, chickens, and swine; however, the extent of these reductions varies considerably. Divergence in vaccine response among various animal hosts can be attributed to their genetic background, physiological state, and immunological responses. Large animal species like pigs, cows, and sheep are physiologically and immunologically closer to humans and often are host to the same or closely related infections [67]. However, it is challenging to assess the possible effects of these investigations on the risk of Salmonella transmission to humans.
In total, 127 studies met the criteria for selection and were included. These trials evaluated different Salmonella serovars, including TS and NTS loads, in vaccinated mice [68,69,70,71], chickens [72,73], pigs [25,74,75], bovine [17,76], and sheep [4]. The reason for including a murine model here is that most therapeutic vaccines rely on sophisticated and extensive studies in mice [40], whereas other animals are employed as a food supply, and it is directly linked to Salmonella transmission to humans [77,78]. The present review focuses on the development of vaccine candidates from various Salmonella serovars through different approaches. For example, serovars Typhi and Paratyphi A/B/C are mainly adapted to the human host, Choleraesuis is mainly limited to swine, Abortusovis is mainly limited to goats and sheep, and Dublin is mainly limited to cows, while Gallinarum and Pullorum are restricted to poultry. These serovars are then employed to induce infection in different animals, allowing for the assessment of vaccination effectiveness. Since S. Typhi serovar is restricted to humans, humanized mice are used as model animals to study S. Typhi. S. Typhimurium was one of the most researched serovars as a challenge infection during vaccination trials in all animals, followed by S. Enteritidis and S. Typhi. According to previous reports, S. Typhimurium is one of the most prevalent serovars detected in animals and food, and it is a serovar well-adapted for transfer to humans, where it causes pathogenesis [10,71]. According to reports from sub-Saharan Africa, S. Typhimurium can cause invasive infections in humans known as invasive nontyphoidal salmonellae [iNTS] that are comparable to typhoid forms [79]. Salmonella serovars are commonly found in the gastrointestinal tracts of animals and can be transmitted to humans through feces and other organs [80]. As a result, MDR Salmonella must be controlled at the animal interface in order to prevent transmission to humans.
The differences in bacterial loads (log10) in the ceca, liver, or spleen between vaccinated and non-vaccinated animals were used to determine vaccine efficacy in these trials [71,81,82]. In this study, we found that numerous vaccine candidates demonstrated significant reductions in Salmonella loads in vaccinated animals’ intestines and other organs, ranging from 1.0 to 4.0-log10 reductions, when compared to non-vaccinated animals, as reported in various trials [72,83,84]. However, the efficacy of the vaccination was found to have no significant effect on the reduction of Salmonella colonization in a few studies [70,85,86], suggesting that the statistical data was insufficient to distinguish between vaccinated and non-vaccinated groups. Therefore, more clinical investigations are needed to identify the appropriate vaccine trial parameters in order to precisely determine reductions and their impact on the risk of human transmission. Defining these characteristics is critical for measuring the efficacy of Salmonella vaccines, which are likely to remain dependent on challenge tests. Several studies have revealed weak connections between immune responses and a decrease in the bacterial burdens in the intestines and other organs of animals during challenge studies [25,42,43].
This study includes only three investigations in which potential antigens, identified through a reverse vaccinology approach, were tested in mice [87,88] and chicken [52], resulting in a significant reduction (p ≤ 0.05) in Salmonella load. However, most of the potential vaccine candidates identified through the reverse vaccinology approach were only validated by in silico analysis [87,89,90]. Therefore, there is a need for these potential vaccine candidates to be tested in animal models for evaluation of vaccine efficacy and safety. Another aspect critical for evaluating vaccine efficacy involves assessing immune responses within the host. This study provides a comprehensive evaluation of the existing literature on Salmonella vaccines in various animal hosts. More than 70% (n = 95) of vaccine studies produced significant antibodies against Salmonella infection. The appearance of signs and symptoms, including inflammation, lethargy, and diarrhea observed after vaccination, provides insights into the vaccine’s safety profile [91]. A broad spectrum of safety profile data was identified, ranging from the absence of adverse effects (AEs) to the occurrence of mild and moderate AEs in animal models after vaccination. The vaccination program has been continued with the development of new vaccine candidates against Salmonella serovars. Our findings indicate that ongoing surveillance and randomized animal research on potential vaccine candidates against emerging MDR Salmonella serovars are critical.
Currently, no commercially available Salmonella vaccine with antigens produced through a reverse vaccinology approach exists. Current options are limited to a few vaccine candidates, including live-attenuated, killed-inactivated, and subunit vaccines, which are licensed and commercially available. Vaccination is a complex procedure that necessitates an in-depth knowledge of the host’s genetic components, cellular defenses, and interconnections. Therefore, continuous research is ongoing for developing potential vaccine candidates through novel approaches against infectious diseases. However, new research has revealed that various vaccine candidates developed through reverse vaccinology, mutant-attenuated vaccination, subunit vaccination, and bacterial ghost vaccines, identified as potential antigens (with efficacy exceeding 80%), could pave the way for commercial vaccine preparation. Despite the potential efficacy of these vaccination strategies, further study on immunological pathways is necessary to develop a vaccine that may effectively achieve the intended outcome while avoiding serious adverse effects like chronic stress. Researchers should focus more on developing vaccines with long-lasting immune responses. Over the past decade, notable advancements in vaccination have been achieved, expedited by the response to the COVID-19 epidemic [19]. Therefore, our approach may reveal new Salmonella vaccine candidates with improved efficacy and commercial viability.
The primary merit of the study is that it used strict bias-reduction strategies to interpret the most accurate results by reviewing high-quality publications published in reputable journals. This systematic review included only data from vaccine studies in which bacterial load, immune responses, and vaccine safety were estimated between vaccinated and non-vaccinated groups, as well as randomized trials in animal hosts, for a complete evaluation of the efficacy of the Salmonella vaccines. A limited number of trials or a single animal host were included in previous systematic review research on Salmonella vaccinations against different serovars in animal hosts. [47]. We incorporated a large number of investigations from various animal hosts against different Salmonella serovars in our study, indicating more precise data than previously demonstrated. [47,48]. The main drawback of this research is its inability to demonstrate the long-term protective effect of Salmonella vaccinations in animal hosts due to a lack of relevant literature.
5. Conclusions
This systematic analysis comprehensively integrated the most recent data on developed vaccine efficacy, immunogenicity, and safety in different animal hosts against Salmonella serovars. Over the last decade, immense progress has been made in establishing novel strategies to develop potential Salmonella vaccine candidates. In conclusion, mutant-attenuated, subunit, and bacterial ghost vaccines, as well as antigens prepared through reverse vaccinology, showed higher efficacy in different animal hosts. These next-generation vaccines are able to expedite the development timeline and can rapidly advance to commercialization in order to combat the spread of antibiotic-resistant Salmonella. Therefore, next-generation vaccines present a prospective route for improving the efficacy and safety of vaccine candidates, ultimately leading to better public health outcomes. This study also provides a comprehensive baseline dataset on the efficacy and safety of Salmonella vaccines against typhoidal and nontyphoidal serovars for future research.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines12091067/s1. Table S1: Composition of vaccines, Salmonella serovars used as a challenge and effect of vaccines on bacterial load on organs after challenge infection; Table S2: Summary of the vaccine efficacy, safety, and immune responses from the eligible trials at the end of the study.
Author Contributions
Conceptualization, M.Y. and A.S.; methodology, A.S. and Z.W.; software, A.S. and C.J.; validation, H.Z., L.H. and B.W.; formal analysis, A.S. and A.E.-D.; investigation, Y.L.; resources, M.Y.; data curation, L.T. and Y.L.; writing—original draft preparation, A.S.; writing—review and editing, A.E.-D., L.T. and Y.L.; visualization, A.S. and H.Z.; supervision. M.Y.; funding acquisition, M.Y. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
All data relevant to this work are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
The project is supported by the National Program on Key Research Project of China (2022YFC2604201) and the European Union’s Horizon 2020 Research and Innovation Program (861917–SAFFI). Zhejiang Provincial Key R&D Program of China (2023C03045; 2022C02024), Zhejiang Provincial Natural Science Foundation of China (LZ24C180002) and Hainan Provincial Joint Project of Sanya Yazhou Bay Science and Technology City (2021JJLH0083).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Ferrari R.G., Rosario D.K., Cunha-Neto A., Mano S.B., Figueiredo E.E., Conte-Junior C.A. Worldwide epidemiology of Salmonella serovars in animal-based foods: A meta-analysis. Appl. Environ. Microbiol. 2019;85:e00591-19. doi: 10.1128/AEM.00591-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.García-Seco T., Montbrau C., Fontseca M., March R., Sitja M., Domínguez L., Bezos J. Efficacy of a Salmonella enterica serovar Abortusovis (S. abortusovis) inactivated vaccine in experimentally infected gestating ewes. Res. Vet. Sci. 2021;135:486–494. doi: 10.1016/j.rvsc.2020.11.017. [DOI] [PubMed] [Google Scholar]
- 3.Frost I., Sati H., Garcia-Vello P., Hasso-Agopsowicz M., Lienhardt C., Gigante V., Beyer P. The role of bacterial vaccines in the fight against antimicrobial resistance: An analysis of the preclinical and clinical development pipeline. Lancet Microbe. 2023;4:e113–e125. doi: 10.1016/S2666-5247(22)00303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uzzau S., Marogna G., Leori G.S., Curtiss R., III, Schianchi G., Stocker B.A., Rubino S. Virulence attenuation and live vaccine potential of aroA, crp cdt cya, and plasmid-cured mutants of Salmonella enterica serovar Abortusovis in mice and sheep. Infect. Immun. 2005;73:4302–4308. doi: 10.1128/IAI.73.7.4302-4308.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ke Y., Teng L., Zhu Z., Lu W., Liu W., Zhou H., Yu Q., Ye L., Zhu P., Zhao G., et al. Genomic investigation and nationwide tracking of pediatric invasive non-typhoidal Salmonella in China. mLife. 2024;1:156–160. doi: 10.1002/mlf2.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang L., Zhou H., Chen J., Jia C., Siddique A., Wu B., Wang H., Tang B., He F., Zhao G., et al. Impact of COVID-19-related Nonpharmaceutical Interventions on Diarrheal Diseases and Zoonotic Salmonella. hLife. 2024;5:246–256. doi: 10.1016/j.hlife.2024.03.005. [DOI] [Google Scholar]
- 7.Jia C., Wang Z., Huang C., Teng L., Zhou H., An H., Liao S., Liu Y., Huang L., Tang B., et al. Mobilome-driven partitions of the resistome in Salmonella. mSystems. 2023;6:e0088323. doi: 10.1128/msystems.00883-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y., Teng L., Xu X., Li X., Peng X., Zhou X., Du J., Tang Y., Jiang Z., Wang Z., et al. A non-typhoidal Salmonella serovar domestication accompanying enhanced niche adaptation. EMBO Mol. Med. 2022;11:e16366. doi: 10.15252/emmm.202216366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee Y.J., Kang M.S. Safety and efficacy of Salmonella gallinarum 9R vaccine in young laying chickens. Avian Pathol. 2005;34:362–366. doi: 10.1080/03079450500180895. [DOI] [PubMed] [Google Scholar]
- 10.Wang X., Biswas S., Paudyal N., Pan H., Li X., Fang W., Yue M.J.F.i.m. Antibiotic resistance in Salmonella Typhimurium isolates recovered from the food chain through national antimicrobial resistance monitoring system between 1996 and 2016. Front. Microbiol. 2019;10:985. doi: 10.3389/fmicb.2019.00985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X., Kang X., Pan M., Wang M., Zhang J., Song H. Evaluation of the protective immune response induced by an rfbG-deficient Salmonella enterica serovar enteritidis strain as a live attenuated DIVA (differentiation of infected and vaccinated animals) vaccine in chickens. Microbiol. Spectr. 2022;10:e01574-22. doi: 10.1128/spectrum.01574-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J., Ed-Dra A., Zhou H., Wu B., Zhang Y., Yue M. Antimicrobial resistance and genomic investigation of non-typhoidal Salmonella isolated from outpatients in Shaoxing city, China. Front. Public Health. 2022;10:988317. doi: 10.3389/fpubh.2022.988317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang X., An H., Wang B., Huang L., Huang C., Huang Y., Wang Z., He F., Li Y., Yue M. Integrated OMICs approach reveals energy metabolism pathway is vital for Salmonella Pullorum survival within the egg white. mSphere. 2024;7:e00362-24. doi: 10.1128/msphere.00362-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou X., Kang X., Chen J., Song Y., Jia C., Teng L., Tang Y., Jiang Z., Peng X., Tao X. Genome degradation promotes Salmonella pathoadaptation by remodeling fimbriae-mediated proinflammatory response. Natl. Sci. Rev. 2023;10:nwad228. doi: 10.1093/nsr/nwad228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pollard A.J., Bijker E.M. A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2021;21:83–100. doi: 10.1038/s41577-020-00479-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kon E., Levy Y., Elia U., Cohen H., Hazan-Halevy I., Aftalion M., Ezra A., Bar-Haim E., Naidu G.S., Diesendruck Y. A single-dose F1-based mRNA-LNP vaccine provides protection against the lethal plague bacterium. Sci. Adv. 2023;9:eadg1036. doi: 10.1126/sciadv.adg1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dodd C.C., Renter D.G., Thomson D.U., Nagaraja T. Evaluation of the effects of a commercially available Salmonella Newport siderophore receptor and porin protein vaccine on fecal shedding of Salmonella bacteria and health and performance of feedlot cattle. Am. J. Vet. Res. 2011;72:239–247. doi: 10.2460/ajvr.72.2.239. [DOI] [PubMed] [Google Scholar]
- 18.Li Y., Ed-Dra A., Tang B., Kang X., Müller A., Kehrenberg C., Jia C., Pan H., Yang H., Yue M. Higher tolerance of predominant Salmonella serovars circulating in the antibiotic-free feed farms to environmental stresses. J. Hazard. Mater. 2022;438:129476. doi: 10.1016/j.jhazmat.2022.129476. [DOI] [PubMed] [Google Scholar]
- 19.Mba I.E., Sharndama H.C., Anyaegbunam Z.K.G., Anekpo C.C., Amadi B.C., Morumda D., Doowuese Y., Ihezuo U.J., Chukwukelu J.U., Okeke O.P. Vaccine development for bacterial pathogens: Advances, challenges and prospects. Trop. Med. Int. Health. 2023;28:275–299. doi: 10.1111/tmi.13865. [DOI] [PubMed] [Google Scholar]
- 20.Khan K., Burki S., Alsaiari A.A., Alhuthali H.M., Alharthi N.S., Jalal K. A therapeutic epitopes-based vaccine engineering against Salmonella enterica XDR strains for typhoid fever: A Pan-vaccinomics approach. J. Biomol. Struct. Dyn. 2023;14:1–15. doi: 10.1080/07391102.2023.2246587. [DOI] [PubMed] [Google Scholar]
- 21.Lee S.W., Koo M.J. PRISMA 2020 statement and guidelines for systematic review and meta-analysis articles, and their underlying mathematics: Life Cycle Committee Recommendations. Life Cycle. 2022;2:e9. doi: 10.54724/lc.2022.e9. [DOI] [Google Scholar]
- 22.Lin C.-S., Lu T.-L., Chen Y.-A., Yu H.-Y., Wu C.-Y., Yang W.-Y. Safety of bivalent live attenuated Salmonella vaccine and its protection against bacterial shedding and tissue invasion in layers challenged with Salmonella. Poult. Sci. 2022;101:101943. doi: 10.1016/j.psj.2022.101943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu S.-M., Chen T.H.-H., Wang C.-H. Efficacy of avian influenza vaccine in poultry: A meta-analysis. Avian Dis. 2010;54:1197–1209. doi: 10.1637/9305-031710-Reg.1. [DOI] [PubMed] [Google Scholar]
- 24.Gast R.K., Stone H.D., Holt P.S., Beard C. Evaluation of the efficacy of an oil-emulsion bacterin for protecting chickens against Salmonella enteritidis. Avian Dis. 1992;36:992–999. doi: 10.2307/1591560. [DOI] [PubMed] [Google Scholar]
- 25.Moura E.A.G.D.O., Silva D.G.D., Turco C.H., Sanches T.V.C., Storino G.Y., Almeida H.M.D.S., Mechler-Dreibi M.L., Rabelo I.P., Sonalio K., Oliveira L.G.d. Salmonella Bacterin Vaccination Decreases Shedding and Colonization of Salmonella Typhimurium in Pigs. Microorganisms. 2021;9:1163. doi: 10.3390/microorganisms9061163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Román B.S., Garrido V., Muñoz P.-M., Arribillaga L., García B., De Andrés X., Zabaleta V., Mansilla C., Farrán I., Lasa I. The extradomain a of fibronectin enhances the efficacy of lipopolysaccharide defective Salmonella bacterins as vaccines in mice. Vet. Res. 2012;43:31. doi: 10.1186/1297-9716-43-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Ridder L., Maes D., Dewulf J., Pasmans F., Boyen F., Haesebrouck F., Méroc E., Roels S., Leyman B., Butaye P. Effect of a DIVA vaccine with and without in-feed use of coated calcium-butyrate on transmission of Salmonella Typhimurium in pigs. BMC Vet. Res. 2013;9:243. doi: 10.1186/1746-6148-9-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peeters L., Dewulf J., Boyen F., Brossé C., Vandersmissen T., Rasschaert G., Heyndrickx M., Cargnel M., Mattheus W., Pasmans F. Evaluation of group vaccination of sows and gilts against Salmonella Typhimurium with an attenuated vaccine in subclinically infected pig herds. Prev. Vet. Med. 2020;182:104884. doi: 10.1016/j.prevetmed.2020.104884. [DOI] [PubMed] [Google Scholar]
- 29.Erova T.E., Kirtley M.L., Fitts E.C., Ponnusamy D., Baze W.B., Andersson J.A., Cong Y., Tiner B.L., Sha J., Chopra A.K. Protective immunity elicited by oral immunization of mice with Salmonella enterica serovar Typhimurium Braun lipoprotein (Lpp) and acetyltransferase (MsbB) mutants. Front. Cell. Infect. Microbiol. 2016;6:148. doi: 10.3389/fcimb.2016.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu T., König R., Sha J., Agar S.L., Tseng C.-T.K., Klimpel G.R., Chopra A.K. Immunological responses against Salmonella enterica serovar Typhimurium Braun lipoprotein and lipid A mutant strains in Swiss-Webster mice: Potential use as live-attenuated vaccines. Microb. Pathog. 2008;44:224–237. doi: 10.1016/j.micpath.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y., Jie K., Li B., Yu H., Ruan H., Wu J., Huang X., Liu Q. Immunization with outer membrane vesicles derived from major outer membrane protein-deficient Salmonella Typhimurium mutants for cross protection against Salmonella enteritidis and avian pathogenic Escherichia coli O78 infection in chickens. Front. Microbiol. 2020;11:588952. doi: 10.3389/fmicb.2020.588952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J., Qiu J., Huang Z., Liu T., Pan J., Zhang Q., Liu Q. Reverse vaccinology approach for the identifications of potential vaccine candidates against Salmonella. Int. J. Med. Microbiol. 2021;311:151508. doi: 10.1016/j.ijmm.2021.151508. [DOI] [PubMed] [Google Scholar]
- 33.Maiti S., Halder P., Banerjee S., Dutta M., Mukhopadhyay A.K., Dutta S., Koley H. Development of a novel trivalent invasive non-typhoidal Salmonella outer membrane vesicles based vaccine against salmonellosis and fowl typhoid in chickens. Immunobiology. 2022;227:152183. doi: 10.1016/j.imbio.2022.152183. [DOI] [PubMed] [Google Scholar]
- 34.Maiti S., Howlader D.R., Halder P., Bhaumik U., Dutta M., Dutta S., Koley H. Bivalent non-typhoidal Salmonella outer membrane vesicles immunized mice sera confer passive protection against gastroenteritis in a suckling mice model. Vaccine. 2021;39:380–393. doi: 10.1016/j.vaccine.2020.11.040. [DOI] [PubMed] [Google Scholar]
- 35.Schuster O., Sears K.T., Ramachandran G., Fuche F.J., Curtis B., Tennant S.M., Simon R. Immunogenicity and protective efficacy against Salmonella C2-C3 infection in mice immunized with a glycoconjugate of S. Newport Core-O polysaccharide linked to the homologous serovar FliC protein. Hum. Vaccines Immunother. 2018;15:1436–1444. doi: 10.1080/21645515.2018.1483808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Senevirathne A., Hewawaduge C., Lee J.H. Immunization of chickens with Salmonella Gallinarium ghosts expressing Salmonella Enteritidis NFliC-FimAC and CD40LC fusion antigen enhances cell-mediated immune responses and protects against wild-type challenges with both species. Dev. Comp. Immunol. Microbiol. 2022;126:104265. doi: 10.1016/j.dci.2021.104265. [DOI] [PubMed] [Google Scholar]
- 37.Bajzert J., Gorczykowski M., Stefaniak T. Evaluation of the protective effect of immunization spf DBA/2J mice with selected bacterial, recombinant Hsp60 antigens during Salmonella Enteritidis challenge. Microb. Pathog. 2019;128:206–214. doi: 10.1016/j.micpath.2018.12.045. [DOI] [PubMed] [Google Scholar]
- 38.Renu S., Han Y., Dhakal S., Lakshmanappa Y.S., Ghimire S., Feliciano-Ruiz N., Senapati S., Narasimhan B., Selvaraj R., Renukaradhya G. Chitosan-adjuvanted Salmonella subunit nanoparticle vaccine for poultry delivered through drinking water and feed. Carbohydr. Polym. 2020;243:116434. doi: 10.1016/j.carbpol.2020.116434. [DOI] [PubMed] [Google Scholar]
- 39.Hajam I.A., Kim J.H., Lee J.H. Incorporation of membrane-anchored flagellin into Salmonella Gallinarum bacterial ghosts induces early immune responses and protection against fowl typhoid in young layer chickens. Dev. Vet. Immunol. Immunopathol. 2018;199:61–69. doi: 10.1016/j.vetimm.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 40.Honda-Okubo Y., Cartee R.T., Thanawastien A., Yang J.S., Killeen K.P., Petrovsky N. A typhoid fever protein capsular matrix vaccine candidate formulated with Advax-CpG adjuvant induces a robust and durable anti-typhoid Vi polysaccharide antibody response in mice, rabbits and nonhuman primates. Vaccine. 2022;40:4625–4634. doi: 10.1016/j.vaccine.2022.06.043. [DOI] [PubMed] [Google Scholar]
- 41.Hu M., Zhao W., Li H., Gu J., Yan Q., Zhou X., Pan Z., Cui G., Jiao X. Immunization with recombinant Salmonella expressing SspH2-EscI protects mice against wild type Salmonella infection. BMC Vet. Res. 2018;14:79. doi: 10.1186/s12917-018-1404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matulova M., Havlickova H., Sisak F., Rychlik I. Vaccination of chickens with SPI1-lon and SPI1-lon-fliC mutant of Salmonella enterica Serovar Enteritidis. PLoS ONE. 2013;8:e66172. doi: 10.1371/journal.pone.0066172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Methner U., Barrow P.A., Berndt A., Rychlik I. Salmonella Enteritidis with double deletion in phoP fliC—A potential live Salmonella vaccine candidate with novel characteristics for use in chickens. Vaccine. 2011;29:3248–3253. doi: 10.1016/j.vaccine.2011.02.030. [DOI] [PubMed] [Google Scholar]
- 44.Okamura M., Matsumoto W., Seike F., Tanaka Y., Teratani C., Tozuka M., Kashimoto T., Takehara K., Nakamura M., Yoshikawa Y. Efficacy of soluble recombinant FliC protein from Salmonella enterica serovar Enteritidis as a potential vaccine candidate against homologous challenge in chickens. Avian Dis. 2012;56:354–358. doi: 10.1637/9986-111011-Reg.1. [DOI] [PubMed] [Google Scholar]
- 45.Watson D.C., Robbins J., Szu S.C. Protection of mice against Salmonella typhimurium with an O-specific polysaccharide-protein conjugate vaccine. Infect. Immun. 1992;60:4679–4686. doi: 10.1128/iai.60.11.4679-4686.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wisner A.L., Desin T.S., Lam P.-K.S., Berberov E., Mickael C.S., Townsend H.G., Potter A.A., Köster W. Immunization of chickens with Salmonella enterica subspecies enterica serovar Enteritidis pathogenicity island-2 proteins. Vet. Microbiol. 2011;153:274–284. doi: 10.1016/j.vetmic.2011.05.041. [DOI] [PubMed] [Google Scholar]
- 47.De la Cruz M., Conrado I., Nault A., Perez A., Dominguez L., Alvarez J. Vaccination as a control strategy against Salmonella infection in pigs: A systematic review and meta-analysis of the literature. Res. Vet. Sci. 2017;114:86–94. doi: 10.1016/j.rvsc.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 48.Marchello C.S., Fiorino F., Pettini E., Crump J.A., Martin L.B., Breghi G., Canals R., Gordon M.A., Hanumunthadu B., Jacobs J. Incidence of non-typhoidal Salmonella invasive disease: A systematic review and meta-analysis. J. Infect. 2021;83:523–532. doi: 10.1016/j.jinf.2021.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Acevedo-Villanueva K., Akerele G., Al-Hakeem W., Adams D., Gourapura R., Selvaraj R. Immunization of broiler chickens with a killed chitosan nanoparticle Salmonella vaccine decreases Salmonella enterica serovar enteritidis load. Front. Physiol. 2022;13:920777. doi: 10.3389/fphys.2022.920777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akter T., Nooruzzaman M., Belal S.M.S.H., Ahammed M., Uddin A.J., Parvin R., Khan M.A.H.N.A., Islam M.A., Hossain M.M. Fowl typhoid live lyophilized vaccine applied at 3-month intervals protected layer chickens from Salmonella gallinarum infection and prevented cloacal shedding. J. Adv. Vet. Anim. Res. 2022;9:301. doi: 10.5455/javar.2022.i597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Esmailnia E., Amani J., Gargari S.L.M. Identification of novel vaccine candidate against Salmonella enterica serovar Typhi by reverse vaccinology method and evaluation of its immunization. Genomics. 2020;112:3374–3381. doi: 10.1016/j.ygeno.2020.06.022. [DOI] [PubMed] [Google Scholar]
- 52.Jiang Z., Kang X., Song Y., Zhou X., Yue M. Identification and Evaluation of Novel Antigen Candidates against Salmonella Pullorum Infection Using Reverse Vaccinology. Vaccines. 2023;11:865. doi: 10.3390/vaccines11040865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lamichhane B., Mawad A.M., Saleh M., Kelley W.G., Harrington P.J., Lovestad C.W., Amezcua J., Sarhan M.M., El Zowalaty M.E., Ramadan H. Salmonellosis: An Overview of Epidemiology, Pathogenesis, and Innovative Approaches to Mitigate the Antimicrobial Resistant Infections. Antibiotics. 2024;13:76. doi: 10.3390/antibiotics13010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Curtiss R., III Vaccines to control Salmonella in poultry. Avian Dis. 2024;67:427–440. doi: 10.1637/aviandiseases-D-23-99988. [DOI] [PubMed] [Google Scholar]
- 55.La Guidara C., Adamo R., Sala C., Micoli F. Vaccines and monoclonal antibodies as alternative strategies to antibiotics to fight antimicrobial resistance. Int. J. Mol. Sci. 2024;25:5487. doi: 10.3390/ijms25105487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moges E., Ushula B., Darcho I. Veterinary Vaccines: Unlocking the Power of Immunization for Livestock Health—A Review. J. Innov. Med. Res. 2024;3:1–13. doi: 10.56397/JIMR/2024.03.01. [DOI] [Google Scholar]
- 57.Guo Y., Xu Y., Kang X., Gu D., Jiao Y., Meng C., Tang P., Wang X., Huang C., Geng S. Immunogenic potential and protective efficacy of a sptP deletion mutant of Salmonella Enteritidis as a live vaccine for chickens against a lethal challenge. Int. J. Med. Microbiol. 2019;309:151337. doi: 10.1016/j.ijmm.2019.151337. [DOI] [PubMed] [Google Scholar]
- 58.Haque S., Sengupta S., Gupta D., Bhan M.K., Kumar R., Khan A., Jailkhani B.S. Typhi derived OmpC peptide conjugated with Vi-polysaccharide evokes better immune response than free Vi-polysaccharide in mice. Biologicals. 2019;62:50–56. doi: 10.1016/j.biologicals.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 59.Harada H., Nishikawa F., Higashi N., Kita E. Development of a mucosal complex vaccine against oral Salmonella infection in mice. Microbiol. Immunol. 2002;46:891–905. doi: 10.1111/j.1348-0421.2002.tb02778.x. [DOI] [PubMed] [Google Scholar]
- 60.Jawale C.V., Lee J.H. Characterization of a Salmonella Typhimurium ghost carrying an adjuvant protein as a vaccine candidate for the protection of chickens against virulent challenge. Avian Pathol. 2014;43:506–513. doi: 10.1080/03079457.2014.966303. [DOI] [PubMed] [Google Scholar]
- 61.Jawale C.V., Lee J.H. Evaluation of immunogenicity and protective efficacy of adjuvanted Salmonella Typhimurium ghost vaccine against salmonellosis in chickens. Vet. Q. 2016;36:130–136. doi: 10.1080/01652176.2016.1138248. [DOI] [PubMed] [Google Scholar]
- 62.Liu Q., Yi J., Liang K., Zhang X., Liu Q. Outer membrane vesicles derived from Salmonella Enteritidis protect against the virulent wild-type strain infection in a mouse model. J. Microbiol. Biotechnol. 2017;27:1519–1528. doi: 10.4014/jmb.1705.05028. [DOI] [PubMed] [Google Scholar]
- 63.Muniz E.C., Verdi R., Leão J.A., Back A., Nascimento V.P.d. Evaluation of the effectiveness and safety of a genetically modified live vaccine in broilers challenged with Salmonella Heidelberg. Avian Pathol. 2017;46:676–682. doi: 10.1080/03079457.2017.1348598. [DOI] [PubMed] [Google Scholar]
- 64.Ruan P., Xia X.-P., Sun D., Ojcius D.M., Mao Y.-F., Yue W.-Y., Yan J. Recombinant SpaO and H1a as immunogens for protection of mice from lethal infection with Salmonella paratyphi A: Implications for rational design of typhoid fever vaccines. Vaccine. 2008;26:6639–6644. doi: 10.1016/j.vaccine.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 65.Senevirathne A., Hewawaduge C., Lee J.H. Assessing an O-antigen deficient, live attenuated Salmonella Gallinarium strain that is DIVA compatible, environmentally safe, and protects chickens against fowl typhoid. Dev. Comp. Immunol. 2022;133:104433. doi: 10.1016/j.dci.2022.104433. [DOI] [PubMed] [Google Scholar]
- 66.Shin H., La T.-M., Lee H.-J., Kim T., Song S.-u., Park E., Park G.-H., Choi I.-S., Park S.-Y., Lee J.-B. Evaluation of Immune Responses and Protective Efficacy of a Novel Live Attenuated Salmonella Enteritidis Vaccine Candidate in Chickens. Vaccines. 2022;10:1405. doi: 10.3390/vaccines10091405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Feng Y., Pan H., Zheng B., Li F., Teng L., Jiang Z., Feng M., Zhou X., Peng X., Xu X., et al. An integrated nationwide genomics study reveals transmission modes of typhoid fever in China. mBio. 2023;5:e0133323. doi: 10.1128/mbio.01333-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee H.-Y., Cho S.-A., Lee I.-S., Park J.-H., Seok S.-H., Baek M.-W., Kim D.-J., Lee S.-H., Hur S.-J., Ban S.-J. Evaluation of phoP and rpoS mutants of Salmonella enterica serovar Typhi as attenuated typhoid vaccine candidates: Virulence and protective immune responses in intranasally immunized mice. FEMS Immunol. Med. Microbiol. 2007;51:310–318. doi: 10.1111/j.1574-695X.2007.00307.x. [DOI] [PubMed] [Google Scholar]
- 69.Ochoa-Repáraz J., García B., Solano C., Lasa I., Irache J.M., Gamazo C. Protective ability of subcellular extracts from Salmonella Enteritidis and from a rough isogenic mutant against salmonellosis in mice. Vaccine. 2005;23:1491–1501. doi: 10.1016/j.vaccine.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 70.Pati N.B., Vishwakarma V., Selvaraj S.K., Dash S., Saha B., Singh N., Suar M. Salmonella Typhimurium TTSS-2 deficient mig-14 mutant shows attenuation in immunocompromised mice and offers protection against wild-type Salmonella Typhimurium infection. BMC Microbiol. 2013;13:236. doi: 10.1186/1471-2180-13-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Piao H.H., Tam V.T.M., Na H.S., Kim H.J., Ryu P.Y., Kim S.Y., Rhee J.H., Choy H.E., Kim S.W., Hong Y. Immunological responses induced by asd and wzy/asd mutant strains of Salmonella enterica serovar Typhimurium in BALB/c mice. J. Microbiol. 2010;48:486–495. doi: 10.1007/s12275-010-0023-z. [DOI] [PubMed] [Google Scholar]
- 72.Berghaus R.D., Thayer S., Maurer J., Hofacre C. Effect of vaccinating breeder chickens with a killed Salmonella vaccine on Salmonella prevalences and loads in breeder and broiler chicken flocks. J. Food Prot. 2011;74:727–734. doi: 10.4315/0362-028X.JFP-10-542. [DOI] [PubMed] [Google Scholar]
- 73.Copper G.L., Venables L.M., Nicholas R.A., Cullen G.A., Hormaeche C.E. Vaccination of chickens with chicken-derived Salmonella enteritidis phage type 4 aroA live oral Salmonella vaccines. Vaccine. 1992;10:247–254. doi: 10.1016/0264-410X(92)90160-L. [DOI] [PubMed] [Google Scholar]
- 74.Gil C., Latasa C., García-Ona E., Lázaro I., Labairu J., Echeverz M., Burgui S., García B., Lasa I., Solano C. A DIVA vaccine strain lacking RpoS and the secondary messenger c-di-GMP for protection against salmonellosis in pigs. Vet. Res. 2020;51:3. doi: 10.1186/s13567-019-0730-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Theuß T., Ueberham E., Lehmann J., Lindner T., Springer S. Immunogenic potential of a Salmonella Typhimurium live vaccine for pigs against monophasic Salmonella Typhimurium DT 193. BMC Vet. Res. 2017;13:343. doi: 10.1186/s12917-017-1271-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Robertsson J., Carlsson H. ELISA for measurement of antibody response to a killed Salmonella typhimurium vaccine in cattle. Zentralblatt Für Veterinärmedizin Reihe B. 1980;27:28–35. doi: 10.1111/j.1439-0450.1980.tb01634.x. [DOI] [PubMed] [Google Scholar]
- 77.Fuche F.J., Jones J.A., Ramachandran G., Higginson E.E., Simon R., Tennant S.M. Deletions in guaBA and htrA but not clpX or rfaL constitute a live-attenuated vaccine strain of Salmonella Newport to protect against serogroup C2-C3 Salmonella in mice. Hum. Vaccines Immunother. 2018;15:1427–1435. doi: 10.1080/21645515.2018.1491499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Graziani C., Busani L., Dionisi A., Lucarelli C., Owczarek S., Ricci A., Mancin M., Caprioli A., Luzzi I. Antimicrobial resistance in Salmonella enterica serovar Typhimurium from human and animal sources in Italy. Vet. Microbiol. 2008;128:414–418. doi: 10.1016/j.vetmic.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 79.Wang Z., Zhou H., Liu Y., Huang C., Chen J., Siddique A., Yin R., Jia C., Li Y., Zhao G. Nationwide trends and features of human salmonellosis outbreaks in China. Emerg. Microbes Infect. 2024;13:2372364. doi: 10.1080/22221751.2024.2372364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lehti S.M., Andersen O., Leppäaho-Lakka J., Suominen E., Vainio A., Matsinen M., Kuronen H., Rimhanen-Finne R. Salmonella Typhimurium caused an unprecedentedly large foodborne outbreak in Finland in 2021. Zoonoses Public Health. 2024;71:560–567. doi: 10.1111/zph.13157. [DOI] [PubMed] [Google Scholar]
- 81.Mitra A., Loh A., Gonzales A., Łaniewski P., Willingham C., Curtiss R., III, Roland K.L.J.C. Safety and protective efficacy of live attenuated Salmonella Gallinarum mutants in Rhode Island Red chickens. Vaccine. 2013;31:1094–1099. doi: 10.1016/j.vaccine.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 82.Moon J., Kim S., Kim W., Rao Z., Park J., Park B., Hur J. Protective efficacy of the recombinant lysozyme-PMAP36 fusion protein-inactivated Salmonella Typhimurium vaccine candidate via oral immunization in a murine model. Can. J. Vet. Res. 2020;84:241–244. [PMC free article] [PubMed] [Google Scholar]
- 83.Hur J., Song S.O., Lim J.S., Chung I.K., Lee J.H. Efficacy of a novel virulence gene-deleted Salmonella Typhimurium vaccine for protection against Salmonella infections in growing piglets. Vet. Immunol. Immunopathol. 2011;139:250–256. doi: 10.1016/j.vetimm.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 84.Nandre R.M., Lee J.H. Comparative evaluation of safety and efficacy of a live Salmonella gallinarum vaccine candidate secreting an adjuvant protein with SG9R in chickens. Vet. Immunol. Immunopathol. 2014;162:51–58. doi: 10.1016/j.vetimm.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 85.Matsui H., Isshiki Y., Eguchi M., Ogawa Y., Shimoji Y. Evaluation of the live vaccine efficacy of virulence plasmid-cured, and phoP-or aroA-deficient Salmonella enterica serovar Typhimurium in mice. J. Vet. Med. Sci. 2015;77:181–186. doi: 10.1292/jvms.14-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Valentine P.J., Devore B.P., Heffron F. Identification of three highly attenuated Salmonella typhimurium mutants that are more immunogenic and protective in mice than a prototypical aroA mutant. Infect. Immun. 1998;66:3378–3383. doi: 10.1128/IAI.66.7.3378-3383.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vij S., Thakur R., Rishi P. Reverse engineering approach: A step towards a new era of vaccinology with special reference to Salmonella. Expert Rev. Vaccines. 2022;21:1763–1785. doi: 10.1080/14760584.2022.2148661. [DOI] [PubMed] [Google Scholar]
- 88.Sharma S., Solanki V., Tiwari V. Reverse vaccinology approach to design a vaccine targeting membrane lipoproteins of Salmonella typhi. J. Biomol. Struct. Dyn. 2023;41:954–969. doi: 10.1080/07391102.2021.2015443. [DOI] [PubMed] [Google Scholar]
- 89.Ullah N., Anwer F., Ishaq Z., Siddique A., Shah M.A., Rahman M., Rahman A., Mao X., Jiang T., Lee B.L. In silico designed Staphylococcus aureus B-cell multi-epitope vaccine did not elicit antibodies against target antigens suggesting multi-domain approach. J. Immunol. Methods. 2022;504:113264. doi: 10.1016/j.jim.2022.113264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Aghaie S.M., Tabatabaei M., Nazarian S. Bioinformatics design of recombinant chimeric protein containing SipD and LptD immunogens and evaluation of its immunogenicity against Salmonella Typhimurium. Microb. Pathog. 2023;175:105959. doi: 10.1016/j.micpath.2022.105959. [DOI] [PubMed] [Google Scholar]
- 91.Lee S., Lee K., Park J., Jeong Y.D., Jo H., Kim S., Woo S., Son Y., Kim H.J., Lee K. Global burden of vaccine-associated hepatobiliary and gastrointestinal adverse drug reactions, 1967–2023: A comprehensive analysis of the international pharmacovigilance database. J. Med. Virol. 2024;96:e29792. doi: 10.1002/jmv.29792. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to this work are available from the corresponding author upon request.